Abstract
5-Hydroxytryptamine (5-HT) activates colonic splanchnic afferents, a mechanism by which it has been implicated in generating symptoms in postinfectious and postinflammatory states in humans. Here we compared mechanisms of colonic afferent activation by 5-HT and mechanical stimuli in normal and inflamed rat colon, and after recovery from inflammation. Colonic inflammation was induced in rats by dextran sulphate sodium. Single-fibre recordings of colonic lumbar splanchnic afferents revealed that 58% of endings responded to 5-HT (10−4m) in controls, 88% in acute inflammation (P < 0.05) and 75% after 21 days recovery (P < 0.05 versus control). Maximal responses to 5-HT were also larger, and the estimated EC50 was reduced from 3.2 × 10−6 to 8 × 10−7m in acute inflammation and recovered to 2 × 10−6m after recovery. Responsiveness to mechanical stimulation was unaffected. 5-HT3 receptor antagonism with alosetron reduced responses to 5-HT in controls but not during inflammation. Responses to the mast cell degranulator 48/80 mimicked those to 5-HT in inflamed tissue but not in controls, and more 5-HT-containing mast cells were seen close to calcitonin gene-related peptide-containing fibres in inflamed serosa. We conclude that colonic serosal and mesenteric endings exhibit increased sensitivity to 5-HT in inflammation, with both an increase in proportion of responders and an increase in sensitivity, which is maintained after healing of inflammation. This is associated with alterations in the roles of 5-HT3 receptors and mast cells.
A large proportion of the 5-HT in the body is found in the gastrointestinal tract, primarily contained within enterochromaffin cells and mast cells (Yu et al. 1999). It is released by food, toxins and chemotherapeutic agents (Andrews et al. 1990; Bearcroft et al. 1998). 5-HT release is well known to activate vagal afferent endings in the upper gastrointestinal tract (Blackshaw & Grundy, 1993; Hillsley et al. 1998; Zhu et al. 2001). 5-HT also activates cutaneous nociceptive primary afferents contributing to a role in inflammatory pain (Zeitz et al. 2002). It was shown recently that high-threshold extrinsic primary afferents from the colon also respond to 5-HT (Hicks et al. 2002). 5-HT is thus implicated in mediating symptoms from the colon. Correspondingly, patients suffering from postinfectious irritable bowel syndrome (IBS) have increased sources of 5-HT in the form of increased numbers of enterochromaffin cells (Spiller et al. 2000) and increased mast cell populations (O'Sullivan et al. 2000; Barbara et al. 2004). They show increased postprandial 5-HT release (Bearcroft et al. 1998; Houghton et al. 2003) and a decrease in symptoms of IBS with 5-HT3 receptor antagonists (Camilleri et al. 2000; Camilleri, 2001). Metabolism of 5-HT may also be disrupted in both IBS and inflammatory bowel disease (IBD) (Coates et al. 2004). Patients with IBD in the recovery period between episodes of active inflammation may also have symptoms from the colon (Isgar et al. 1983).
It is well documented that inflammation can have a sensitizing effect on sensory nerves elsewhere in the body leading to increased sensitivity to chemical stimuli, but increased mechanical responsiveness remains controversial (Kocher et al. 1987; Schaible & Schmidt, 1988; Habler et al. 1990; Andrew & Greenspan, 1999; Koltzenburg et al. 1999). Regardless of the cause, increased afferent signals to the central nervous system are likely to result in false perception of the environment and pain. Our aim in the present study was to determine whether increased chemosensitivity or mechanosensitivity of colonic afferent fibres occurs during inflammation, and therefore to gain insight into the role of 5-HT in visceral hypersensitivity. We examined changes in afferent 5-HT sensitivity in a rat model of colonic inflammation. Colitis was induced by ingestion of dextran sulphate sodium (DSS) and this model was chosen for its reproducibility, its morphological similarities with human ulcerative colitis and its proven effectiveness in rat studies (Howarth et al. 1998). We determined the effects of 5-HT and the mast cell degranulator compound 48/80 on colonic afferents in normal rats and those with colitis using an isolated preparation in which the activity of lumbar splanchnic afferent fibres can be recorded during delivery of controlled stimuli to the colon (Lynn & Blackshaw, 1999). We also localized 5-HT-immunoreactive mast cells to the colonic serosa and mesentery and determined their relation to calcitonin gene-related peptide (CGRP)-immunoreactive nerve fibres; CGRP is an established marker for colonic afferents (Christianson et al. 2006).
Methods
Animals
A total of 98 adult Sprague-Dawley rats (180–200 g) were provided by the Institute of Medical and Veterinary Sciences (IMVS) (Adelaide, South Australia). Animals were housed in groups under standard laboratory conditions (22°C, 12 h light–dark cycle) until the study period for convenience of care and stress minimization. They were then transferred to individual metabolism cages, fed a powdered diet suitable for metabolism cage use, and allowed free access to water (Howarth et al. 1998). All experiments were carried out with the approval of, and according to the guidelines of, the animal ethics committees of the Institute of Medical and Veterinary Science and University of Adelaide.
Induction of colonic inflammation
50 animals were designated for induction of colonic inflammation, with 37 animals used for inflamed studies and 20 used for studies after recovery from inflammation. Animals were housed individually in Tecniplast ® metabolism cages and acclimatized for a period of 3 days. Colonic inflammation was induced by addition of 2%w/v dextran sulphate sodium (DSS) (MW 40000, ICN Biochemicals, Cleveland, Ohio) to the animal's drinking water for a period of 7 days. This produced mild symptoms as described in Table 1, but with no observable behavioural disturbances. 36 animals were designated for controls and were housed in identical conditions but without DSS treatment for the same duration. Recovery animals were allowed a 21-day recovery period post DSS treatment before subjection to the electrophysiological protocol. Afferent recordings were obtained from the majority of preparations, with numbers of fibres given for each protocol in Results.
Table 1.
Observations of disease activity in 25 inflamed and 20 recovered preparations, and histopathology in three of each during treatment with 2% dextran sulphate sodium and subsequent recovery after 21 days
| Disease activity | Histology | ||||||
|---|---|---|---|---|---|---|---|
| Days DSS | Index score | Stool consistency | Occult or gross bleeding | Mucosal ulceration | Basal cell hyperplasia | Oedema | Crypt destruction |
| 1 | 0 | Normal | Negative | − | − | − | − |
| 2 | 0 | Normal | Negative | − | − | − | − |
| 3 | 1 | Loose | Negative | − | − | − | − |
| 4 | 1 | Loose | Negative | + | + | + | + |
| 5 | 2 | Loose | Positive | ++ | ++ | + | + |
| 6 | 3 | Diarrhoea | Positive | ++ | ++ | ++ | ++ |
| 7 | 4 | Diarrhoea | Positive | ++ | ++ | +++ | +++ |
| Recovery | 0 | Normal/Loose | Negative | − | − | − | − |
Severity of histological findings is indicated as: −, +, mild; ++, moderate; +++, severe; according to standard diagnostic criteria. Disease activity index is calibrated as described in the Methods.
Validation of inflammation
Three animals from each of the groups were used for validation of the inflammatory protocol. Inflammation was validated by disease activity index score, histopathological assessment and myeloperoxidase (MPO) assay. The disease activity was scored on a severity scale of 0–4 based on physical observations of weight loss, stool consistency and colonic bleeding. Histopathological assessment using haematoxylin and eosin staining was used to show morphological changes associated with DSS treatment over the 7 day period and subsequent recovery after a further 21 days. Animals from control, inflamed and recovery groups were anaesthetized with sodium pentobarbitone (Nembutal, 60 mg kg−1i.p.) and the abdomen opened by midline incision. A 2 cm length of distal colon was removed and divided into 1 cm segments of which one was snap frozen in liquid nitrogen for MPO assay and the other fixed in 10% formaldehyde and processed for histopathological assessment (Department of Tissue Pathology, IMVS). Animals were then killed by exsanguination and excision of thoracic organs while under deep anaesthesia. Haematoxylin and eosin-stained sections were assessed for colonic damage, oedema and inflammatory cell infiltration. Neutrophil infiltration as a measure of inflammation was also assessed by MPO assay according to the protocol described by Morris et al. (1989).
Electrophysiological protocol
Dissection was carried out according to a previously described protocol (Lynn & Blackshaw, 1999). Rats from all groups were anaesthetized with sodium pentobarbitone (as above) and the abdomen opened by midline incision. Next, 4–5 cm of distal colon was removed with attached lumbar colonic nerves (LCN) and a bundle containing the inferior mesenteric artery, abdominal aorta, inferior mesenteric ganglion, intermesenteric nerve and lumbar splanchnic nerve (LSN) according to the classification of Baron et al. (1988), and placed in cold modified Krebs solution (bicarbonate buffer; 4°C). Rats were killed as in the histology protocols. The distal colon was opened longitudinally and pinned flat, mucosal side down, in an isolated perfused organ bath (Danz Instruments, Blackwood, South Australia) with the LCN insertions orientated to lie along the edge of the opened preparation. Connective tissue was dissected away from the neurovascular bundle which was then drawn through into a separate paraffin-filled recording chamber. The attached LSN was finely dissected from the aorta and de-sheathed from the transected nerve stump to expose the nerve fibres; these were divided into fine bundles to be placed onto fine platinum recording electrodes for single afferent fibre recording. Extraluminal receptive fields on the serosal surface and the mesentery were located by responses to blunt probing with a glass rod and discriminated as high-threshold serosal/mesenteric afferents by their lack of response to fine stroking with a calibrated von-Frey hair (10 mg) or circular stretch (8 mm) as previously described (Lynn & Blackshaw, 1999; Berthoud et al. 2001; Hicks et al. 2002). Signals from the recording electrode were differentially amplified, filtered and the analog signal sampled at a rate of 20 kHz via a 1401 data interface (CED, Cambridge, UK). This was recorded to a Pentium III notebook computer and analysed using Spike II software (CED). 5-HT (10−10–10−3m) was applied to the receptive fields via a 1 cm diameter stainless steel ring placed directly around the corresponding receptive field, after residual Krebs solution had been aspirated. Each concentration of 5-HT was applied for a period of 2–3 min followed by a 10 min washout. 5-HT (10−4m) was used to determine whether or not a fibre was responsive to 5-HT as this was shown previously to be a maximal concentration (Hicks et al. 2002). Responses to drugs were counted as having occurred if a reproducible > 25% increase in discharge occurred with the maximum concentration. For quantification of responses, activity induced by 5-HT was measured over the second minute of response. This period was selected to accurately reflect the peak of response and compensate for latency of response onset especially at lower concentrations. Concentration–response curves for 5-HT (10−10–10−3m) were constructed and analysed for changes in response between control and inflamed colons and colons after recovery from inflammation. No differences in 5-HT responsiveness or histological scores were seen when inflammation was induced instead by 5% DSS in male rats housed in wire-bottomed cages. Responses to probing were measured prior to testing of chemosensitivity as total spikes evoked by a 1 s probe application, and as mean peak instantaneous frequency. These responses were averaged over eight trials. No changes in probing responses were observed before and after treatment with 5-HT. Spontaneous discharge was measured over 1 min prior to testing of chemosensitivity. Mechanical thresholds were determined with 10, 50, 200 and 1000 mg von Frey hairs applied perpendicularly to the receptive field. In studies with antagonists, colons were incubated for 2 min with alosetron (2 × 10−7m) alone, followed by 2 min at the same concentration with 5-HT (10−4m). In three of these studies, the adenylate cyclase inhibitor MDL 12330 (30 μm) was added to alosetron and the response to 5-HT tested in the presence of both antagonists.
Localization of 5-HT- and CGRP-like immunoreactivity in normal and inflamed tissue
Distal colon was removed from rats after transcardial perfusion with 4% paraformaldehyde under deep anaesthesia with sodium pentobarbitone (60 mg kg−1i.p.). The region corresponding to that used for electrophysiological study, including the mesenteric attachment, was taken and fixed for 2 h at room temperature (RT, 20°C) in 4% paraformaldehyde, after which the tissue was washed three times for 20 min with phosphate-buffered saline (PBS) at pH 7.4 then cryoprotected with 30% sucrose in PBS for 24 h at 4°C. The colon was then frozen and 20 μm transverse sections cut. Sections were air dried at RT for 10 min, after which the tissue was washed with PBS (pH 7.4) including 0.1% Triton X-100 (PBST). Tissue was then blocked with 2% goat serum, 1% bovine serum albumin, 0.005% Tween 20 and 0.1% gelatin in PBST for 60 min at RT and subsequently incubated with monoclonal anti-5-HT (1 : 100; Dako, Sydney, Australia) and rabbit anti-CGRP (1 : 200; Calbiochem) in blocking solution in PBST overnight at 4°C. Tissue was then washed three times with PBST, then incubated a mixture of secondary antibodies: goat anti-rabbit Alexa Fluor 350 and donkey anti-mouse Alexa Fluor 555 (5 μg ml−1; Molecular Probes, USA) for 45 min at RT. The tissue was then washed three times with PBST and mounted with ProLong Antifade (Molecular Probes, USA). Slides were allowed to dry overnight before viewing with an Olympus BX51 epifluorescence microscope. Images were taken with a Photometrics CoolSnap fx monochrome camera then re-coloured. Negative controls were prepared as above with the primary antibody omitted. Both antibodies have been shown to be specific for their respective epitope in previous studies (Ito et al. 1986; Christianson et al. 2006).
Data analysis
Electrophysiological data are presented as means ± s.e.m., and are compared by one-way ANOVA with Dunnet's post hoc test or by two-way ANOVA for concentration–response curves. Proportions of fibres responding were compared with a Fisher's exact test. Statistical analyses, EC50 values and curve fits were all determined using Graphpad Prism Software (v 3.02; San Diego, CA, USA) according to standard pharmacological methods. Associations between CGRP-containing fibres and 5-HT-containing mast cells were counted from 15 randomly selected sections from four rats.
Results
Inflammatory response to DSS
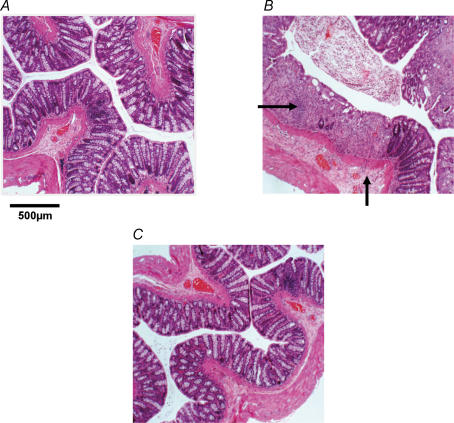
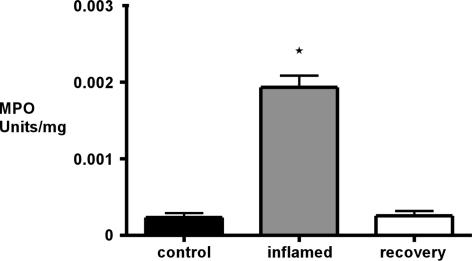
The DSS model of colitis has been used extensively in rats. We re-validated this model in our laboratory in both active and recovery phases of inflammation. Disease activity by morphological examination showed a progressive deterioration of the colonic mucosa over the 7 day DSS treatment programme and subsequent recovery 21 days after DSS (Table 1). Ulceration of the colonic mucosa and infiltration of inflammatory cells were first detected by light microscopy at day 4 of DSS treatment and increased in severity until day 7. This was accompanied by submucosal oedema and destruction of colonic crypts. After recovery, 21 days after DSS treatment, morphological changes had reversed; morphology was indistinguishable from controls (Fig. 1). Blood was detected in the stool from day 4 and gross colonic bleeding was apparent after 7 days. MPO assay for neutrophil infiltration (Fig. 2) showed a greater than 500% increase in inflamed animals at day 7 compared with controls (n = 3). MPO levels after recovery from inflammation had returned to basal levels.
Figure 1. Examples of disease progression and regression in haematoxylin and eosin-stained colonic sections (20 μm).
A, control with normal colonic morphology. B, inflamed colon of a dextran sulphate sodium (DSS)-treated (7 days) animal. The field of view shows examples of crypt destruction, ulceration, oedema and polymorph infiltration in the mucosa and submucosa (arrows), whereas outer muscle and serosa appear unchanged. C, animal 21 days after DSS treatment exhibiting recovery to morphology as in controls.
Figure 2. Myeloperoxidase (MPO) assay.
Results showed a significant increase from 2.34 ± 0.57 × 10−4 in controls to 1.93 ± 0.15 × 10−3 U mg−1 in inflamed colons. MPO levels in animals after recovery had returned to basal levels of 2.56 ± 0.63 × 10−4 U mg−1 (P < 0.05, n = 3, one-way ANOVA with Dunnet's post hoc test).
Afferent fibre responses to 5-HT and mechanical stimulation
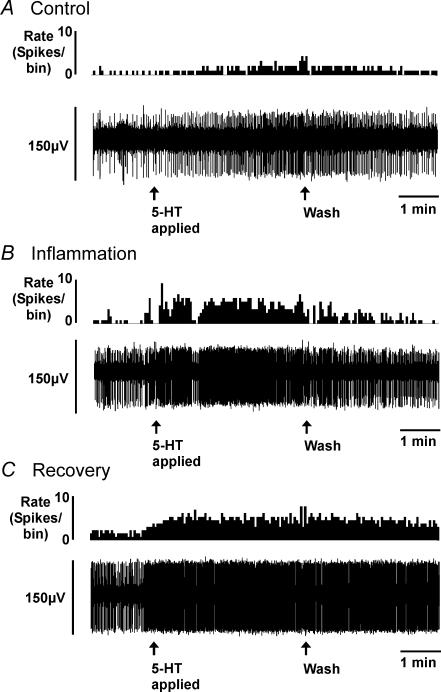
As we have reported previously (Lynn & Blackshaw, 1999; Berthoud et al. 2001; Hicks et al. 2002), serosal/mesenteric afferents were located throughout the length of the preparation and were clustered towards the mesenteric border with receptive fields located on or near blood vessels. Many colonic serosal/mesenteric afferents had multiple receptive fields in both the mesentery and serosal surface. Responses to 5-HT were maintained throughout the period of superfusion, showing little if any adaptation during exposure, and normally continued for approximately 2 min after washout before discharge returned to basal levels (Fig. 3), as we have found previously (Hicks et al. 2002).
Figure 3. Examples of serosal/mesenteric afferent responses on application of 5-HT (10 μm) for 3 min in spontaneously active fibres.
Control (A), a dextran sulphate sodium (DSS)-treated animal (B) and animal following recovery (C). A–C, upper traces show rate of nerve firing (bin size, 2 s). The lower traces show a raw record of nerve activity, with one active fibre evident. 5-HT was introduced over the period indicated by first arrows, and was washed out at the point shown by second arrows. Note increase in size of response in B and C compared with control (A).
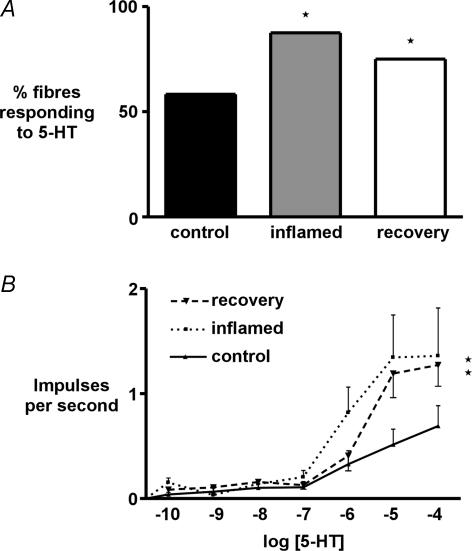
We used 12 animals from each group to determine the proportion of colonic afferent fibres responding to 5-HT (Fig. 4A). Seven out of 12 fibres tested (58.3%) responded to 10−4m 5-HT in controls compared with 14 out of 16 (87.5%) in inflammation and nine out of 12 (75%) in recovery (both P < 0.05, Fisher's exact test). It was possible to determine concentration–response relationships over a range of 5-HT concentrations (10−10–10−4m) in most 5-HT-responsive fibres from each group (Fig. 4B). This showed a significant increase in sensitivity to 5-HT in both the inflamed and recovered state (P < 0.01, two-way ANOVA). The response at maximum concentration, indicative of efficacy, evoked on application of 5-HT was significantly larger during inflammation and this significance was sustained in animals after recovery at 10−5 and 10−4m 5-HT (P < 0.05). EC50 was estimated from non-linear regression of the concentration–response curves. Because a plateau was not reached, an accurate quantification of EC50 was not possible. Estimated EC50 for 5-HT was reduced from 3.2 × 10−6 in controls to 8.2 × 10−7 during inflammation and increased to 2.0 × 10−6m in animals after recovery. In order to investigate whether other aspects of chemosensitivity or general excitability were affected during inflammation, the response to a maximal concentration of capsaicin (3 × 10−6m), which is a very potent activator of these fibres (Berthoud et al. 2001), was compared between three controls and five inflamed preparations, and there was a comparable 5-fold mean increase in discharge in responsive fibres from both groups, indicating that the increase in chemosensitivity of afferents was relatively specific to 5-HT.
Figure 4. Proportion of responders and size of response to 5-HT.
A, percentage of high threshold serosal/mesenteric afferent fibres responding to 5-HT was 58.3% in controls and significantly increased to 87.5% in inflamed and 75% in recovered preparations (P < 0.05, n = 12 each, Fisher's exact test). B, concentration–response relationships to 5-HT were performed in serosal/mesenteric afferents showed a significant increase of activity in inflamed and recovered preparations (P < 0.01, n = 7–9, two-way ANOVA). Activity was averaged over the first 60 s of response. From the concentration–response curves an estimate of curve fit was performed using Prism software, from which EC50 for 5-HT in both groups was estimated. EC50 was 3.2 × 10−6m 5-HT in controls, 8.2 × 10−7m 5-HT in inflamed tissue and 2.0 × 10−6m after recovery.
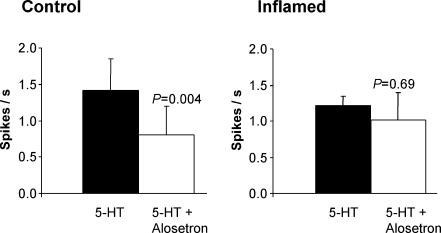
The effects of 5-HT3 receptor antagonism on afferent responses to 5-HT was tested in five control and inflamed preparations (Fig. 5). As we showed in our previous study (Hicks et al. 2002), a maximally effective concentration of alosetron significantly reduced responses to 5-HT in controls. Administration of the adenylate cyclase inhibitor MDL 12330 (30 μm) in addition to alosetron completely abolished the remaining response to 5-HT in three preparations. In inflamed preparations, alosetron had no significant effect on the response to 5-HT (Fig. 5).
Figure 5. Effects of 5-HT3 receptor antagonism on serosal/mesenteric afferent responses to 5-HT (100 μm) in control and inflamed tissue.
Alosetron (200 nm) significantly reduced responses to 5-HT in controls (n = 5), whereas it had no significant effect in the inflamed group (n = 5).
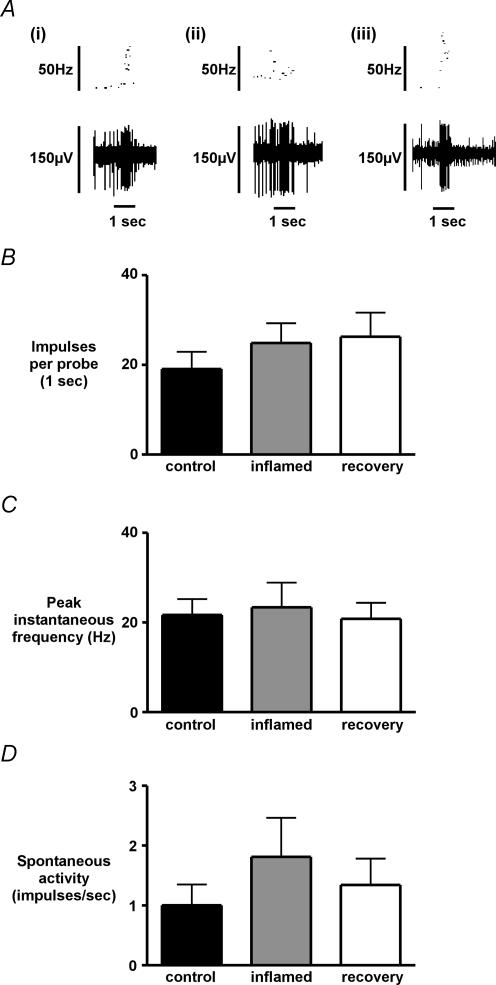
In nine preparations from each group, mechanical sensitivity to a 1 s standardized blunt probe of the receptive field was assessed systematically (Fig. 6A), and showed no significant difference between control and inflamed preparations in terms of total spikes evoked per stimulus (Fig. 6B), or in terms of mean peak instantaneous frequency (Fig. 6C). Spontaneous discharge of serosal/mesenteric afferent endings was not significantly altered in inflamed conditions, or following recovery (Fig. 6D). During the investigation of mechanically evoked responses, no changes were observed either in the size or the distribution of receptive fields. The von Frey threshold of serosal/mesenteric afferents in control colons was reproducibly 200 mg when tested with 10, 50, 200 and 1000 mg stimuli and this was unchanged in both inflammation and recovery.
Figure 6. Mechanically-evoked and spontaneous activity of serosal/mesenteric afferent fibres.
Examples of mechanical stimulation with a blunt probe (A) in colonic afferent fibres from control (a), inflamed (b) and recovered (c) preparations. Impulses evoked by a 1 s calibrated blunt probe (B) and mean peak instantaneous frequency of the probe response (C) showed no significant changes between colonic afferent fibres from control, inflamed or recovered preparations (P > 0.05, n = 9, one-way ANOVA with Dunnet's post hoc test). Spontaneous discharge in responsive fibres was not significantly altered in inflammation and recovery.
Afferent fibre responses to mast cell degranulation
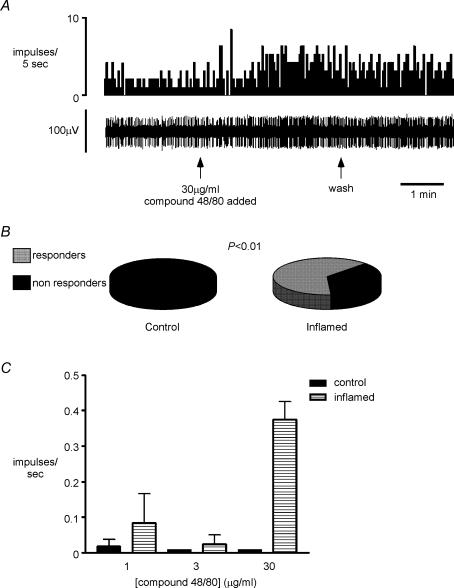
In separate experiments to those described above (n = 6 controls and n = 7 inflamed preparations), the afferent response to the mast cell degranulator, compound 48/80 (1–30 μg ml−1), was tested. No significant increases in afferent discharge were recorded in afferents from control animals after application of the maximum concentration. In inflamed tissue, four out of seven endings responded to compound 48/80 with an increase of discharge greater than 25% (P < 0.01, Fisher's exact test, Fig. 7). Proportions of responsive endings and magnitude of response are therefore similar to that seen on application of 5-HT.
Figure 7. Colonic afferent responsiveness to mast cell degranulation in control and inflamed tissue.
A, original record of response to compound 48/80 (30 μg ml−1) added to the ring around the receptive field for approximately 3 min from a splanchnic afferent fibre innervating the inflamed colon. B, occurrence of responders and non-responders to 48/80 in control (n = 6) and inflamed (n = 7) tissue, showing significantly more responders (n = 4) in inflamed than control tissue (n = 0; P < 0.01). C, group data showing concentration–response relationship for compound 48/80 in all fibres tested in control and inflamed tissue. At the maximum dose (30 μg kg−1), there was a significant increase in response in inflamed tissue (P < 0.01, one-way ANOVA with Dunnet's post hoc test), which was not seen in controls.
Localization of 5-HT-like immunoreactivity in normal and inflamed tissue
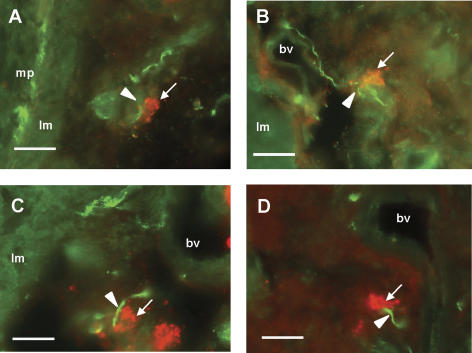
Tissue from five control and five inflamed preparations revealed different immunolabelling for 5-HT and for the sensory neural marker CGRP, which labels a large proportion of rat colonic splanchnic afferents (Christianson et al. 2006). DSS-treated colon showed both a higher level of background 5-HT-like immunoreactivity and an increased number of 5-HT-containing cells in the mucosa and submucosa (data not shown), which were of comparable size and distribution to those previously shown to be mast cells in inflamed colon (Oshima et al. 1999; O'Hara et al. 2004). 5-HT-immunoreactive cells in thick sections of serosa and mesentery were observed in both inflamed and normal tissue (Fig. 8), which corresponded in morphology to mast cells when examined under Nomarsky optics (Yu et al. 1999; Nagata et al. 2001). In this location, no other 5-HT-immunoreactive structures were seen. Mast cells were found close to blood vessels before entering the gut wall, often in pairs or threes, and were particularly closely associated with CGRP-immunoreactive fibres, which normally followed a circuitous path around the blood vessel and mast cell(s). The distance between mast cells and CGRP fibres was measured and the number of mast cells that were within 25 μm of CGRP fibres was counted, although previous data indicate that these associations may be much closer (Stead et al. 1987). In inflamed specimens, the number of mast cells per section close to fibres was significantly greater (34.5 ± 6.3%) than in non-inflamed specimens (17.0 ± 4.9%, data from 15 random sections each, P < 0.05, t test).
Figure 8. Immunohistochemistry for 5-HT and CGRP in rat colon in the region of the mesenteric border.
Sections were taken from dextran sulphate sodium (DSS)-treated rats. A–D, arrowheads indicate calcitonin gene-related peptide-immunoreactive fibres (green) in association with 5-HT-immunoreactive cells (red, arrows). Section in A shows CGRP labelling also in myenteric plexus (mp). Section in D is taken from a region of mesentery several millimetres from the colonic wall. lm, longitudinal muscle; bv, serosal/mesenteric blood vessel. Scale bar, 50 μm.
Discussion
The results of the present study show that excitation of colonic afferents by 5-HT is greater after a period of inflammation in three ways: in terms of the proportion of fibres responding; in the size of the response; and in the reduced threshold for response. Furthermore, most of the changes in 5-HT sensitivity are maintained after healing of the mucosa as assessed histologically and biochemically – a situation closely resembling postinfectious or postinflammatory hypersensitivity in humans (Spiller, 2003). A particularly striking feature of the changes we observed is the fact that the afferent endings that were affected are located in the serosa and mesentery away from the site of inflammation. This means that mediators released at the site of inflammation may evoke changes in afferent endings after they have diffused over some distance or reached the endings via the venous outflow. We often noticed that the endings we studied were located at the junctions of mesenteric blood vessels, which would place them in an optimal location to be influenced by mediators released into the venous outflow from the colon or arriving via its arterial supply. There are a number of candidates for mediators causing changes in afferent function, probably the best characterized of which is nerve growth factor, which has been shown to be responsible for increases in chemo- and thermosensitivity in skin afferents (Bevan & Winter, 1995; Koltzenburg et al. 1999).
In contrast to the changes in sensitivity to 5-HT we observed in lumbar splanchnic serosal and mesenteric afferent endings, their responsiveness to mechanical stimuli or capsaicin was not affected by inflammation. This is contrary to the findings of a recent report of sacral pelvic distension-sensitive fibres in rat colon after trinitrobenzene sulphonic acid (TNBS) colitis (Wynn et al. 2004), which found increases in mechanosensitivity that were associated with increased purinoceptor expression. Together, these findings suggest that endings closer to the site of inflammation may undergo changes in mechanosensitivity, whereas those more distant (or in a different pathway) may not. Our findings also indicate that increases in responsiveness are relatively confined to serotonergic responses, and are not a consequence of general increases in excitability, although this is evident in other disease models (Beyak et al. 2004). In a previous study, we showed that mechanical sensitivity of oesophageal mucosal afferents was in fact slightly reduced during oesophageal inflammation in ferrets, but it was indirectly increased during chemical activation (Page et al. 2000). In active colonic inflammation in humans, mechanical sensitivity may also be decreased (Chang et al. 2000), but may be increased paradoxically during periods of remission (Isgar et al. 1983).
Many reports describing inflammation-induced changes in afferent sensitivity have emerged from studies of cutaneous afferents. In the skin, mechanical hyperalgesia in inflammation is obvious, yet changes in mechanical responsiveness of primary afferents is controversial (Reeh et al. 1986; Kocher et al. 1987; Andrew & Greenspan, 1999; Koltzenburg et al. 1999; Schlegel et al. 2004), and reports of increased mechanosensitivity show that this is restricted to a small subpopulation of high-threshold afferents (Reeh et al. 1986; Kocher et al. 1987). However, there are much broader changes in chemical sensitivity, and these may prime afferent endings before a mechanical stimulus, and thereby contribute indirectly to the phenomenon of mechanical hyperalgesia (Blackshaw & Grundy, 1993; Koltzenburg et al. 1999; Banik et al. 2001). All this evidence point to the importance of increased chemosensitivity as a cause of increased apparent mechanosensitivity. Once afferent signals converge in the central nervous system, the potentiating effect of chemosensory pathways on mechanosensory inputs can be observed on second order neurons or their subsequent connections. We found, during activation of chemosensory pathways with bile (Lynn & Blackshaw, 1999), that an increase in spinal dorsal horn responses to colonic distension could be detected in vivo (Andrew & Blackshaw, 2001). Because serosal and mesenteric afferents are unlikely to be activated by physiological levels of distension, and are activated only by intense or rapid stretch (Lynn & Blackshaw, 1999; Brierley et al. 2004), the chemosensory role we have found for them may therefore be of importance in colouring the overall sensory input from the colon in the whole animal.
The present study was conducted in vitro without vascular perfusion, which makes it unlikely that appreciable levels of endogenous 5-HT release from the mucosa would reach endings. In vivo, on the other hand, circulating concentrations of 5-HT would exceed the threshold for activation of these endings, in particular, levels in the plasma seen after meals in symptomatic IBS patients (mean 6 × 10−8m 3 h after a meal (Houghton et al. 2003) up to 1.4 × 10−6m (Bearcroft et al. 1998)). The relevance of this is highlighted by the lowered threshold concentration of 5-HT we observed in inflammation. The lack of ongoing endogenous 5-HT action in vitro may also explain why we saw no significant change in spontaneous afferent fibre activity in inflammation. Increases in spontaneous discharge in vagal mucosal afferents are readily observed after mucosal deterioration or injury in vivo, and may be blocked by a 5-HT3 receptor antagonist (Blackshaw & Grundy, 1993).
We saw robust responses to mast cell degranulation only in inflamed preparations, which may be attributable to 5-HT release, although there are several other candidate mediators. In any case these data indicate greater likelihood of functional associations between sensory nerves and mast cells in the diseased state, as has already been shown anatomically in IBS (Barbara et al. 2004). Because the response to mast cell degranulation is not reproducible (presumably as a result of depletion of mast cell contents), we did not have the opportunity to investigate its pharmacology. However the response to 48/80 closely mimicked the response to 5-HT, and there was an increased association of 5-HT-containing cells with CGRP-immunoreactive fibres in the region of colon where we found receptive fields, as previously shown in a study of rat DSS colitis (Oshima et al. 1999). This would suggest that 5-HT is involved in the responses to 48/80, but not exclusively.
Our previous data on afferent sensitivity to 5-HT in non-inflamed colon show that the 5-HT-induced excitation was significantly reduced by the 5-HT3 receptor antagonist alosetron (Hicks et al. 2002), at a concentration that abolished excitation by a 5-HT3 receptor agonist. We confirmed the effect of alosetron on 5-HT responses in controls, and went on to determine the relative contribution of 5-HT3 receptors to the increased responses in inflammation. We found that reduction in the response to 5-HT was no longer observable after application of alosetron. This indicates that other excitatory 5-HT receptors are likely to play a larger role either because of downregulation of 5-HT3 receptors, or upregulation of others, or both. Alternatively, changes in relative expression of the two 5-HT3 receptor subunits in colonic afferents, or their phosphorylation state, may account for this result (e.g. Zeitz et al. 2002). Our findings with the adenylate cyclase inhibitor MDL 12330, which blocked alosetron-resistant responses, would indicate that the other subtypes involved signal via a pathway involving the G-protein GS and are therefore most likely to be 5-HT4, 6 or 7 subtypes (Gershon et al. 1990; Hoyer et al. 2002).
In conclusion, we have described a marked sensitization to 5-HT of lumbar splanchnic afferents from the colon in acute inflammation that is maintained after healing of the mucosa. This sensitization is sufficient to potentially underlie persistent sensory disturbances in humans after a bout of colonic infection or inflammation.
Acknowledgments
This work was funded by the National Health and Medical Research Council Grant no. 104814 and 298942; J.R.C. and K.S. were supported by University of Adelaide Postgraduate Scholarships and Sigma Xi grant in aid of research (J.R.C.); L.A.B. was supported by an National Health and Medical Research Council (NHMRC) Senior Research Fellowship. We thank the staff of the Department of Tissue Pathology of the IMVS for their help with scoring of histopathological features and processing of specimens. Helen Bougesis of the Women's and Children's hospital assisted with animal-related work and Chandra Kirana of Commonwealth Scientific and Industrial Research Organisation (CSIRO) with biochemical assay procedures. Chris Martin of the Nerve-Gut Research Laboratory assisted with the antagonist experiments.
References
- Andrew D, Greenspan JD. Mechanical and heat sensitization of cutaneous nociceptors after peripheral inflammation in the rat. J Neurophysiol. 1999;82:2649–2656. doi: 10.1152/jn.1999.82.5.2649. [DOI] [PubMed] [Google Scholar]
- Andrew LK, Blackshaw LA. Colonic mechanoreceptor inputs to rat lumbo-sacral dorsal horn neurones: distribution, thresholds and chemosensory modulation. Neurogastroenterol Motil. 2001;13:333–337. doi: 10.1046/j.1365-2982.2001.00272.x. [DOI] [PubMed] [Google Scholar]
- Andrews PL, Davis CJ, Bingham S, Davidson HI, Hawthorn J, Maskell L. The abdominal visceral innervation and the emetic reflex: pathways, pharmacology, and plasticity. Can J Physiol Pharmacol. 1990;68:325–345. doi: 10.1139/y90-047. [DOI] [PubMed] [Google Scholar]
- Banik RK, Kozaki Y, Sato J, Gera L, Mizumura K. B2 receptor-mediated enhanced bradykinin sensitivity of rat cutaneous C-fiber nociceptors during persistent inflammation. J Neurophysiol. 2001;86:2727–2735. doi: 10.1152/jn.2001.86.6.2727. [DOI] [PubMed] [Google Scholar]
- Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate AM, Grady EF, Bunnett NW, Collins SM, Corinaldesi R. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693–702. doi: 10.1053/j.gastro.2003.11.055. [DOI] [PubMed] [Google Scholar]
- Baron R, Janig W, Kollmann W. Sympathetic and afferent somata projecting in hindlimb nerves and the anatomical organization of the lumbar sympathetic nervous system of the rat. J Comp Neurol. 1988;275:460–468. doi: 10.1002/cne.902750310. [DOI] [PubMed] [Google Scholar]
- Bearcroft CP, Perrett D, Farthing MJ. Postprandial plasma 5-hydroxytryptamine in diarrhoea predominant irritable bowel syndrome: a pilot study. Gut. 1998;42:42–46. doi: 10.1136/gut.42.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR, Lynn PA, Blackshaw LA. Vagal and spinal mechanosensors in the rat stomach and colon have multiple receptive fields. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1371–R1381. doi: 10.1152/ajpregu.2001.280.5.R1371. [DOI] [PubMed] [Google Scholar]
- Bevan S, Winter J. Nerve growth factor (NGF) differentially regulates the chemosensitivity of adult rat cultured sensory neurons. J Neurosci. 1995;15:4918–4926. doi: 10.1523/JNEUROSCI.15-07-04918.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyak MJ, Ramji N, Krol KM, Kawaja MD, Vanner SJ. Two TTX-resistant Na+ currents in mouse colonic dorsal root ganglia neurons and their role in colitis-induced hyperexcitability. Am J Physiol Gastrointest Liver Physiol. 2004;287:G845–G855. doi: 10.1152/ajpgi.00154.2004. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Grundy D. Effects of 5-hydroxytryptamine on discharge of vagal mucosal afferent fibres from the upper gastrointestinal tract of the ferret. J Auton Nerv Syst. 1993;45:41–50. doi: 10.1016/0165-1838(93)90360-7. [DOI] [PubMed] [Google Scholar]
- Brierley SM, Jones RC, 3rd, Gebhart GF, Blackshaw LA. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology. 2004;127:166–178. doi: 10.1053/j.gastro.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Camilleri M. Management of the irritable bowel syndrome. Gastroenterology. 2001;120:652–668. doi: 10.1053/gast.2001.21908. [DOI] [PubMed] [Google Scholar]
- Camilleri M, Northcutt AR, Kong S, Dukes GE, McSorley D, Mangel AW. Efficacy and safety of alosetron in women with irritable bowel syndrome: a randomised, placebo-controlled trial. Lancet. 2000;355:1035–1040. doi: 10.1016/S0140-6736(00)02033-X. [DOI] [PubMed] [Google Scholar]
- Chang L, Munakata J, Mayer EA, Schmulson MJ, Johnson TD, Bernstein CN, Saba L, Naliboff B, Anton PA, Matin K. Perceptual responses in patients with inflammatory and functional bowel disease. Gut. 2000;47:497–505. doi: 10.1136/gut.47.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JA, Traub RJ, Davis BM. Differences in spinal distribution and neurochemical phenotype of colonic afferents in mouse and rat. J Comp Neurol. 2006;494:246–259. doi: 10.1002/cne.20816. [DOI] [PubMed] [Google Scholar]
- Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM, Moses PL. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657–1664. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Gershon MD, Wade PR, Kirchgessner AL, Tamir H. 5-HT receptor subtypes outside the central nervous system. Roles in the physiology of the gut. Neuropsychopharmacology. 1990;3:385–395. [PubMed] [Google Scholar]
- Habler HJ, Janig W, Koltzenburg M. Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder in the cat. J Physiol. 1990;425:545–562. doi: 10.1113/jphysiol.1990.sp018117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks GA, Coldwell JR, Schindler M, Ward PA, Jenkins D, Lynn PA, Humphrey PP, Blackshaw LA. Excitation of rat colonic afferent fibres by 5-HT3 receptors. J Physiol. 2002;544:861–869. doi: 10.1113/jphysiol.2002.025452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillsley K, Kirkup AJ, Grundy D. Direct and indirect actions of 5-hydroxytryptamine on the discharge of mesenteric afferent fibres innervating the rat jejunum. J Physiol. 1998;5062:551–561. doi: 10.1111/j.1469-7793.1998.551bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton LA, Atkinson W, Whitaker RP, Whorwell PJ, Rimmer MJ. Increased platelet depleted plasma 5-hydroxytryptamine concentration following meal ingestion in symptomatic female subjects with diarrhoea predominant irritable bowel syndrome. Gut. 2003;52:663–670. doi: 10.1136/gut.52.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth GS, Xian CJ, Read LC. Insulin-like growth factor-I partially attenuates colonic damage in rats with experimental colitis induced by oral dextran sulphate sodium. Scand J Gastroenterol. 1998;33:180–190. doi: 10.1080/00365529850166923. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Isgar B, Harman M, Kaye MD, Whorwell PJ. Symptoms of irritable bowel syndrome in ulcerative colitis in remission. Gut. 1983;24:190–192. doi: 10.1136/gut.24.3.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Hata J, Oda N, Miyamori S, Tahara E. Serotonin in tubular adenomas, adenocarcinomas and endocrine tumours of the stomach. An immunohistochemical study. Virchows Arch A Pathol Anat Histopathol. 1986;410:239–245. doi: 10.1007/BF00710830. [DOI] [PubMed] [Google Scholar]
- Kocher L, Anton F, Reeh PW, Handwerker HO. The effect of carrageenan-induced inflammation on the sensitivity of unmyelinated skin nociceptors in the rat. Pain. 1987;29:363–373. doi: 10.1016/0304-3959(87)90051-0. [DOI] [PubMed] [Google Scholar]
- Koltzenburg M, Bennett DL, Shelton DL, McMahon SB. Neutralization of endogenous NGF prevents the sensitization of nociceptors supplying inflamed skin. Eur J Neurosci. 1999;11:1698–1704. doi: 10.1046/j.1460-9568.1999.00590.x. [DOI] [PubMed] [Google Scholar]
- Lynn PA, Blackshaw LA. In vitro recordings of afferent fibres with receptive fields in the serosa, muscle and mucosa of rat colon. J Physiol. 1999;518:271–282. doi: 10.1111/j.1469-7793.1999.0271r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803. [PubMed] [Google Scholar]
- Nagata K, Fujimiya M, Sugiura H, Uehara M. Intracellular localization of serotonin in mast cells of the colon in normal and colitis rats. Histochem J. 2001;33:559–568. doi: 10.1023/a:1014960026247. [DOI] [PubMed] [Google Scholar]
- O'Hara JR, Ho W, Linden DR, Mawe GM, Sharkey KA. Enteroendocrine cells and 5-HT availability are altered in mucosa of guinea pigs with TNBS ileitis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G998–G1007. doi: 10.1152/ajpgi.00090.2004. [DOI] [PubMed] [Google Scholar]
- O'Sullivan M, Clayton N, Breslin NP, Harman I, Bountra C, McLaren A, O'Morain CA. Increased mast cells in the irritable bowel syndrome. Neurogastroenterol Motil. 2000;12:449–457. doi: 10.1046/j.1365-2982.2000.00221.x. [DOI] [PubMed] [Google Scholar]
- Oshima S, Fujimura M, Fukimiya M. Changes in number of serotonin-containing cells and serotonin levels in the intestinal mucosa of rats with colitis induced by dextran sodium sulfate. Histochem Cell Biol. 1999;112:257–263. doi: 10.1007/s004180050445. [DOI] [PubMed] [Google Scholar]
- Page AJ, O'Donnell TA, Blackshaw LA. P2X purinoceptor-induced sensitization of ferret vagal mechanoreceptors in oesophageal inflammation. J Physiol. 2000;523:403–411. doi: 10.1111/j.1469-7793.2000.00403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeh PW, Kocher L, Jung S. Does neurogenic inflammation alter the sensitivity of unmyelinated nociceptors in the rat? Brain Res. 1986;384:42–50. doi: 10.1016/0006-8993(86)91217-5. [DOI] [PubMed] [Google Scholar]
- Schaible HG, Schmidt RF. Time course of mechanosensitivity changes in articular afferents during a developing experimental arthritis. J Neurophysiol. 1988;60:2180–2195. doi: 10.1152/jn.1988.60.6.2180. [DOI] [PubMed] [Google Scholar]
- Schlegel T, Sauer SK, Handwerker HO, Reeh PW. Responsiveness of C-fiber nociceptors to punctate force-controlled stimuli in isolated rat skin: lack of modulation by inflammatory mediators and flurbiprofen. Neurosci Lett. 2004;361:163–167. doi: 10.1016/j.neulet.2003.12.073. [DOI] [PubMed] [Google Scholar]
- Spiller RC. Postinfectious irritable bowel syndrome. Gastroenterology. 2003;124:1662–1671. doi: 10.1016/s0016-5085(03)00324-x. [DOI] [PubMed] [Google Scholar]
- Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, Neal KR. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804–811. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead RH, Tomioka M, Quinonez G, Simon GT, Felten SY, Bienenstock J. Intestinal mucosal mast cells in normal and nematode-infected rat intestines are in intimate contact with peptidergic nerves. Proc Natl Acad Sci U S A. 1987;84:2975–2979. doi: 10.1073/pnas.84.9.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn G, Ma B, Ruan HZ, Burnstock G. Purinergic component of mechanosensory transduction is increased in a rat model of colitis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G647–G657. doi: 10.1152/ajpgi.00020.2004. [DOI] [PubMed] [Google Scholar]
- Yu PL, Fujimura M, Okumiya K, Kinoshita M, Hasegawa H, Fujimiya M. Immunohistochemical localization of tryptophan hydroxylase in the human and rat gastrointestinal tracts. J Comp Neurol. 1999;411:654–665. doi: 10.1002/(sici)1096-9861(19990906)411:4<654::aid-cne9>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Zeitz KP, Guy N, Malmberg AB, Dirajlal S, Martin WJ, Sun L, Bonhaus DW, Stucky CL, Julius D, Basbaum AI. The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. J Neurosci. 2002;22:1010–1019. doi: 10.1523/JNEUROSCI.22-03-01010.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JX, Zhu XY, Owyang C, Li Y. Intestinal serotonin acts as a paracrine substance to mediate vagal signal transmission evoked by luminal factors in the rat. J Physiol. 2001;530:431–442. doi: 10.1111/j.1469-7793.2001.0431k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]