Abstract
The speed and force of myocardial contraction during systolic ejection is largely dependent on the intrinsic contractile properties of cardiac myocytes. As the myosin heavy chain (MHC) isoform of cardiac muscle is an important determinant of the contractile properties of individual myocytes, we studied the effects of altered MHC isoform expression in rat myocardium on the mechanical properties of skinned ventricular preparations. Skinned myocardium from thyroidectomized rats expressing only the β MHC isoform displayed rates of force redevelopment that were about 2.5-fold slower than in myocardium from hyperthyroid rats expressing only the α MHC isoform, but the amount of force generated at a given level of Ca2+ activation was not different. Because recent studies suggest that the stretch activation response in myocardium has an important role in systolic function, we also examined the effect of MHC isoform expression on the stretch activation response by applying a rapid stretch (1% of muscle length) to an otherwise isometrically contracting muscle fibre. Sudden stretch of myocardium resulted in a concomitant increase in force that quickly decayed to a minimum and was followed by a delayed redevelopment of force (i.e. stretch activation) to levels greater than prestretch force. β MHC expression dramatically slowed the overall rate of the stretch activation response compared to expression of α MHC isoform; specifically, the rate of force decay was ∼2-fold slower and the rate of delayed force development was ∼2.5-fold slower. In contrast, MHC isoform had no effect on the amplitude of the stretch activation response. Collectively, these data show that expression of β MHC in myocardium dramatically slows rates of cross-bridge recruitment and detachment which would be expected to decrease power output and contribute to depressed systolic function in end-stage heart failure.
At the cellular level, the ability of the heart to perform haemodynamic work is determined by the force generated during contraction and the velocity of myocardial shortening during the ejection phase of the cardiac cycle. At the myofilament level, cardiac muscle contraction is the result of cyclic interactions of myosin and actin molecules and the hydrolysis of ATP. In striated muscle, myosin is a hexameric protein composed of two myosin heavy chains (MHCs), two essential light chains (ELCs) and two regulatory light chains (RLCs) (Rayment et al. 1993). The C-terminal domain of the myosin molecule (S2 region), is incorporated into the backbone of the thick filament, and the N-terminus contains the globular catalytic domain (S1 region) which hydrolyses ATP, binds to actin and undergoes the conformational changes associated with the force-producing power stroke (Huxley, 1957; Lymn & Taylor, 1971).
In mammalian cardiac muscle, α and β isoforms of MHC have been identified (Hoh et al. 1979). The expression of the MHC genes is developmentally regulated (Lompre et al. 1984) such that in the ventricles the β isoform predominates in early development in all mammalian species. Expression of the β isoform decreases in rodents with increasing age so that the α isoform is dominant in adulthood (Lompre et al. 1984), whereas in humans the expression of the β isoform remains the dominant MHC isoform throughout life (Lompre et al. 1991). In adult human ventricles, the α MHC mRNA expression is believed to be ∼25–35% of the total ventricular MHC mRNA (Lowes et al. 1997; Nakao et al. 1997; Miyata et al. 2000), but the expression of the α MHC isoform is only ∼10% of the total ventricular MHC protein (Miyata et al. 2000). The functional importance of the small amount of α MHC isoform present in human ventricles is unclear but several studies show that during chronic heart failure α MHC mRNA expression decreases 15-fold while protein expression is virtually nil (Nakao et al. 1997; Miyata et al. 2000; Reiser et al. 2001) This suggests that down-regulation of the α MHC isoform may be a contributing factor to dysfunction in human heart failure. Furthermore, improved pump function of failing human hearts with β-adrenergic blockade has been shown to increase both α MHC mRNA and α MHC protein levels (Lowes et al. 2002).
Despite 93% amino acid identity between the α and β MHC isoforms (McNally et al. 1989), they are functionally distinct in that the α MHC isoform displays significantly faster contractile activity as evidenced by higher ATPase activity, faster shortening velocity and faster cross-bridge cycling (Schiaffino & Reggiani, 1996). Even small changes in the MHC profile of myocardium can have significant functional effects on contractile function at the myofilament level (Fitzsimons et al. 1998; Herron & McDonald, 2002; Rundell et al. 2005; Korte et al. 2005) as well as the whole organ level (Fitzsimons et al. 1999; Tardiff et al. 2000; Krenz et al. 2003; Korte et al. 2005). These studies predict that the relatively small decrease in α MHC isoform expression in human heart failure can have significant effects on cardiac contractile function.
Variable MHC isoform expression can also influence contractile properties of myocardium in ways that have not been systematically explored. For example, it has been shown that heart muscle exhibits a pronounced stretch activation response (Steiger, 1971) in that stretch of the muscle to a new length produces a delayed rise in force that eventually reaches a plateau at a force appropriate to the new muscle length. The physiological role of the stretch activation response in myocardium is not well understood but its underlying kinetics have been shown to be well matched to the heart rate of mammals across species (Steiger, 1977), and it is thought by some to contribute to ventricular ejection (Vemuri et al. 1999; Davis et al. 2001; Campbell & Chandra, 2006; Epstein & Davis, 2006; Stelzer et al. 2006a,c). Furthermore, we have recently shown (Stelzer et al. 2006c) that the amplitude and overall kinetics of the stretch activation response in myocardium varies with the level of activating Ca2+, suggesting that it contributes to the regulation of contractile function on a beat-to-beat basis. Because of the potential functional importance of MHC isoform shifts in development, hypertrophy or in diseases such as heart failure, this study was undertaken to examine the effects of altered MHC isoform expression on the stretch activation response at different levels of Ca2+ activation. We studied the stretch activation response in skinned ventricular preparations from thyroid-deficient rats that expressed only the β MHC isoform and hyperthyroid rats that expressed only the α MHC isoform. Our results show that at a given levels of Ca2+ activation, expression of the β MHC isoform was associated with significantly slowed kinetics of stretch activation response but did not affect the amplitude of the response. The slower stretch activation response with increased expression of the β MHC isoform would be expected to slow the rate of force generation during systole thereby decreasing power production and could contribute to depressed systolic function observed in end-stage human heart failure.
Methods
Experimental animals
Normal euthyroid and thyroidectomized female Sprague-Dawley rats (Harlan Laboratories, Madison, WI, USA) were housed in groups of two to three per cage in a light- and temperature-controlled (22–23°C) room. The rats (∼6–9 months of age) were divided into separate groups with each group fed a specific diet for a period of 5 weeks. All thyroidectomized rats (n = 5) were fed a diet of 6-propyl-2-thiouracil (PTU, 0.15 g PTU (100 g rat meal)−1, Sigma) which has been shown to eliminate any residual plasma triiodothyronine and thyroxine following thyroidectomy (Haddad et al. 1997). The hyperthyroid rats (n = 5) were fed a diet of thyroid extract (0.15 g thyroid gland (100 g rat meal)−1, Sigma) and normal euthyroid rats (n = 3) were fed rat meal (Sigma). All rats had access to food and water ad libitum. No animal showed any signs of distress throughout the feeding protocol and therefore no rats were excluded from the study. All animal usage was conducted under the strict guidelines established by the University of Wisconsin Animal Care and Use Committee. Rats were placed in an enclosed bell-jar which was pretreated with isoflurane (15% isoflurane in mineral oil, 0.03 ml (g body weight)−1). Inhalation of the isoflurane caused deep anaesthesia which was confirmed by loss of the pedal reflex and muscular tension of all limbs, and animals were killed by creating a pneumothorax.
Solutions
Solution compositions were calculated using the computer program of Fabiato Fabiato (1988) and the stability constants given by Godt & Lindley (1982) (corrected to pH 7.0 and 22°C). All solutions contained (mm): N,N-bis(2 hydroxy-ethyl)-2-aminoethanesulphonic acid (BES) 100, creatine phosphate 15, dithiothreitol 5, free Mg2+ 1 and MgATP 4. In addition, the solution with −log [Ca2+] of 9.0 (pCa 9.0 solution) contained 7 mm EGTA and 0.02 mm CaCl2, pCa 4.5 solution contained 7 mm EGTA and 7.01 mm CaCl2, and pre-activating solution contained 0.07 mm EGTA. Ionic strength of all solutions was adjusted to 180 mm with potassium propionate. Solutions containing different amounts of free [Ca2+] (i.e. pCa 6.1–5.5) were prepared by mixing appropriate volumes of stock solutions of pCa 9.0 and pCa 4.5.
Skinned myocardial preparations
Skinned ventricular myocardium for mechanical experiments was prepared as previously described (Patel et al. 2001; Stelzer et al. 2004). Following pneumothorax, the heart was excised and right and left ventricles were dissected at room temperature (22–23°C) in a relaxing solution containing (mm): KCl 100, imidazole 20, MgCl2 7, EGTA 2 and MgATP 4 (pH 7.0) and were then rapidly frozen in liquid nitrogen. Previous work in this laboratory (Patel et al. 2001; Stelzer et al. 2004) has shown that freezing in liquid nitrogen improves the quality and robustness of the muscle preparations perhaps by disrupting membranes or connective tissue. To prepare skinned myocardial preparations, frozen ventricles were thawed and homogenized in relaxing solution for ∼2 s using a Polytron (Kinematica), which yielded multicellular preparations of 100–250 μm × 600–900 μm. The homogenate was centrifuged at 120 g for 1 min and the resultant pellet was washed with fresh relaxing solution and re-suspended in relaxing solution containing 250 μg ml−1 saponin and 1% Triton X-100. After 30 min, the skinned preparations were washed with fresh relaxing solution and then dispersed in ∼50 ml relaxing solution in a glass Petri dish. The dish was kept on ice at all times except during the selection of preparations for mechanical experiments.
Experimental apparatus
Skinned preparations with smooth, well-defined edges were transferred from the Petri dish to a stainless steel experimental chamber containing relaxing solution. The ends of each preparation were attached to the arms of a motor (model 312B, Aurora Scientific Inc.) and force transducer (model 403; Aurora Scientific Inc.) in an experimental apparatus similar to one previously described (Moss, 1979). The chamber assembly was then placed on the stage of an inverted microscope (Carl Zeiss) fitted with a 40 × objective and a CCTV camera (model WV-BL600, Panasonic). Bitmap images of the preparations were acquired using an AGP 4X/2X graphics card and software (ATI Technologies Inc.) and were used to assess mean sarcomere length (SL) during the course of each experiment. Changes in force and motor position were sampled (16 bit resolution, DAP5216a, Microstar Laboratories, Bellevue, WA, USA) at rates of 2.0 kHz using SLControl software developed in our laboratory (Campbell & Moss, 2003) and saved to computer files for later analysis. Force during the experiments was also recorded on a digital oscilloscope (Nicolet Instrument Corporation).
Experimental protocols
Kinetics of force redevelopment
The rate constant of force redevelopment (ktr) in rat skinned myocardium was assessed for different compositions of MHC using a modification of the experimental protocol originally described by Brenner & Eisenberg (1986). Each preparation was transferred from relaxing to activating solutions of varying free [Ca2+] (pCa 6.1–4.5) and allowed to generate steady-state force. The preparation was rapidly (< 2 ms) slackened by 20% of its original length, resulting in a coincident reduction in force to near zero (i.e. < 5% of steady isometric force). This was followed by a brief period of unloaded shortening (10 ms) after which the preparation was rapidly restretched to its original length (Stelzer et al. 2006b). A ktr–relative force relationship was obtained by initially activating the preparation in solution of pCa 4.5 and also in a series of submaximally activating solutions between pCa 6.1 and 5.5 and then expressing the submaximal force (P) as a fraction of maximum force (Po). ktr was estimated by linear transformation of the half-time of force redevelopment (i.e. ktr = 0.693/t1/2), as previously described (Chase et al. 1994).
Force–pCa relationships
During the recordings of force redevelopment with different MHC composition, each preparation was allowed to develop steady force in solutions of varying free [Ca2+]. The difference between steady-state force and the force baseline obtained after the 20% slack step was measured as the total force at that pCa. Active force was calculated by subtracting Ca2+-independent force in solution of pCa 9.0 from the total force and was normalized to the cross-sectional area of the preparation, which was calculated from the width of the preparations assuming a cylindrical cross-section. Force–pCa relationships were derived by expressing P at each pCa as a fraction of Po determined at pCa 4.5 (P/Po) in the same preparation. Apparent cooperativity in the activation of force was inferred from the steepness of the force–pCa relationship and was quantified using a Hill plot transformation of the force–pCa data (Shiner & Solaro, 1984). The data were fitted with the equation: P/Po = [Ca2+]n/(kn + [Ca2+]n), where n is the Hill coefficient and k is the [Ca2+] required for half-maximal force (pCa50).
Stretch activation experiments
At the beginning of each experiment, the length of the preparation was set to obtain a sarcomere length of ∼2.1 μm in order to measure initial isometric force and for subsequent imposition of stretch. Fibres were activated at pCa values that yielded maximal force or submaximal forces of ∼50% and ∼25% maximal. When the fibre achieved steady-state force, a sudden stretch of 1% was imposed and held for 5 s before returning to solution of pCa 9.0. The speed of stretches (∼10 muscle lengths s−1) imposed in these experiments was designed to minimize changes in cross-bridge populations during stretch, such that the initial increase in force was presumably due to the elastic strain of the cross-bridges bound before the stretch was imposed (Stelzer et al. 2006c).
The method used for measuring the stretch activation variables have been described in detail (Stelzer et al. 2006c). Amplitudes were measured as follows: P1, measured from prestretch steady-state force to the peak of phase 1; P2, measured from prestretch steady-state force to the minimum force decay; P3, measured from prestretch steady-state force to the peak value of delayed force; and Pdf, difference between P3 and P2.
All amplitudes were normalized to prestretch Ca2+-activated isometric force to allow comparisons at different Ca2+ activation levels. Apparent rate constants were derived for phase 2 (krel, s−1) from the peak of phase 1 to the minimum of phase 2 and for phase 3 (kdf, s−1) from the point of force re-uptake following phase 2 to the completion of delayed force development.
Protein analysis
At the conclusion of each experiment, the cardiac preparation was cut free, placed in a microcentrifuge tube containing 10 μl SDS sample buffer (62.5 mm Tris, 75 mm dithiothreitol, 25% glycerol, 2% SDS and 0.01% bromophenol blue) and stored at −80°C. For analysis of MHC protein content by SDS-PAGE, microcentrifuge tubes were first heated for 3 min at 100°C and sample buffer containing proteins was then loaded onto polyacrylamide gels. MHC isoform content was assessed using acrylamide gels cross-linked with N-N1 diallyltartardiamide (DATD) (Warren & Greaser, 2003). Stacking gels contained 3% acrylamide (acrylamide:DATD ratio, 5.6: 1), 10% glycerol, 130 mm Tris (pH 6.8) and 0.1% SDS. Separating gels contained 6% acrylamide (acrylamide:DATD ratio, 37.5: 1), 10% glycerol, 0.37 m Tris (pH 8.8) and 0.1% SDS. Gels were run using a Bio-Rad Protean 3 unit with 0.1 mm spacers and a Bio-Rad PowerPac 300 power supply. The running buffer consisted of 50 mm Tris (base), 150 mm glycine and 0.1% SDS. The gels were run at 16 mA for 2 h at 4°C and then silver stained using Bio-Rad Silver Stain Plus kit. The relative proportions of α and β MHC isoforms were determined by densitometric analysis of silver-stained gels using LaserPix software (Bio-Rad Laboratories).
Data analysis
Analysis of stretch activation data was performed as previously described (Stelzer et al. 2006c). Briefly, rate constants of force decay (krel) were obtained by fitting a single exponential to the time course of decay:
y = a (1−exp(−k1x)), where a is the amplitude of the single exponential phase and k1 is the rate constant of decay. Rate constants of delayed force development in phase 3 were estimated either with a double exponential fit:
y = a exp(−k1x) + b exp(−k2x), where a is the amplitude of the first exponential phase rising with rate constant k1 and b is the amplitude of the second exponential phase rising with rate constant k2, or were estimated as a single composite rate constant by linear transformation of the half-time of force redevelopment (i.e. kdf = −ln 0.5 × (t1/2)−1; Stelzer et al. 2006c).
All data are reported as means ± s.e.m. Comparisons of steady-state force, ktr and stretch activation variables at different levels of Ca2+ activation for different MHC compositions were performed using a one-way analysis of variance (ANOVA) with Tukey's post hoc test for significance (P < 0.05).
Results
Thyroid status and rat ventricular MHC expression
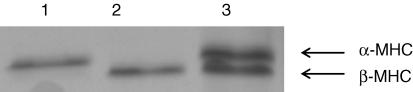
Surgical thyroidectomy of rats and subsequent dietary supplementation with PTU resulted in the expression of only the β MHC isoform, while dietary supplementation of euthyroid rats with thyroid extract resulted in the expression of only the α MHC isoform (Fig. 1). Manipulations of thyroid state in rats have been shown to elicit significant shifts in cardiac MHC isoforms expression without altering the expression of other thick and thin filament contractile proteins (Schiaffino & Reggiani, 1996; Fitzsimons et al. 1998) and thus provide a useful way to probe the specific effects of MHC expression on cardiac contractile function. Thus, in this study differences in contractile function between the two groups of rats were attributed to variable expression of MHC isoforms.
Figure 1. Myosin heavy chain (MHC) composition of rat skinned myocardium.
MHC isoform content was determined using 6% SDS-PAGE. The relative proportions of α and β MHC isoforms were determined by densitometric analysis gels following a silver-staining protocol. In this gel it can be seen that hyperthyroid rats expressed only cardiac α-MHC (lane 1), whereas thyroidectomized rats treated with 6-n-propyl-2-thiouracil expressed only cardiac β-MHC (lane 2). Lane 3 shows the MHC composition (55%α-MHC and 45%β-MHC) of control euthyroid rat myocardium.
Effects of altered MHC expression on steady-state mechanical properties of rat skinned myocardium
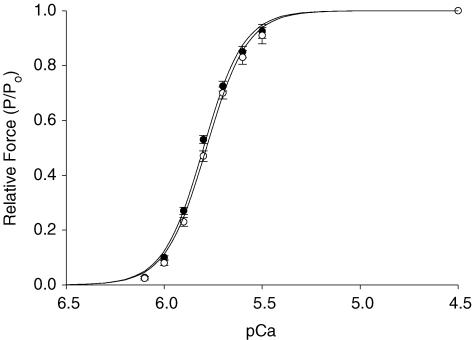
The effects of α or β MHC expression on steady-state mechanical properties of skinned myocardium are summarized in Table 1. As shown in Fig. 2, skinned preparations expressing α or β MHC displayed similar force–pCa relationships; that is, there were no differences in the amount of force produced by cardiac preparations at each activating [Ca2+], and no differences in the steepness of the force–pCa relationship (Hill coefficient, nH). The lack of effect of MHC isoform expression on force–pCa relationships in this study is in agreement with some previous studies (Fitzsimons et al. 1998; Herron et al. 2001; Rundell et al. 2004, 2005) though not all (Gibson et al. 1992; Metzger et al. 1999). Expression of α or β MHC isoforms also had no significant effect on maximal Ca2+-activated force at pCa 4.5, confirming that there are no inherent differences in the force-producing capabilities of different cardiac MHC isoforms (VanBuren et al. 1995; Sugiura et al. 1998; Palmiter et al. 1999).
Table 1.
Effect of MHC composition on steady-state mechanical properties of rat myocardium
| %α MHC | n | Fmin (mN mm−2) | Fmax (mN mm−2) | pCa50 | nH |
|---|---|---|---|---|---|
| 0 | 10 | 0.8 ± 0.3 | 22.1 ± 1.3 | 5.80 ± 0.01 | 4.24 ± 0.28 |
| 100 | 10 | 0.7 ± 0.2 | 20.5 ± 1.1 | 5.78 ± 0.01 | 4.11 ± 0.25 |
Data are means ±s.e.m.n, number of cardiac preparations; Fmin, Ca2+-independent force at pCa 9.0; Fmax, maximal Ca2+-activated force at pCa 4.5; pCa50, pCa required for half-maximal force generation; nH, Hill coefficient for total force–pCa relationship.
Figure 2. Lack of effect of MHC composition on force–pCa relationships.
Thyroidectomized myocardium (0%α-MHC, •, n= 10) and hyperthyroid myocardium (100%α -MHC, ○, n= 10) displayed similar force–pCa relationships. Data points are means ±s.e.m. Forces measured at submaximal free [Ca2+] were expressed relative to the maximal force obtained at pCa 4.5. The smooth lines were fitted using the Hill equation: P/Po=[Ca2+]n/(kn+[Ca2+]n)], where P is the force measured at submaximal free [Ca2+], Po is the force measured at maximal free [Ca2+] (pCa 4.5), nH is the Hill coefficient, and k is the Ca2+ concentration required for half-maximal activation.
Effect of MHC composition on the kinetics of force redevelopment
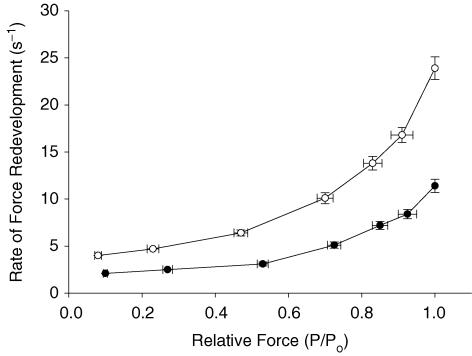
The effect of MHC isoform expression on the rate constant of force development (ktr) was investigated in this study using a modified release–restretch protocol (Brenner & Eisenberg, 1986). Measurements of ktr provide an estimate of the rate of transitions of cross-bridges between weak-binding, non-force-generating states and strong-binding, force-generating states. Expression of the β MHC isoform had a significant effect on ktr at all levels of activation, as indicated by ∼2.5-fold slowing of ktr compared to the α MHC isoform (Fig. 3). The 2.5-fold difference in the rate of force redevelopment with α versus β MHC isoform expression in this study is similar to results obtained in previous studies (Fitzsimons et al. 1998; Rundell et al. 2005). Furthermore, our data show that expression of β MHC isoform slowed ktr to a similar extent at all levels of Ca2+ activation, suggesting that depressed rates of cross-bridge cycling have the potential to impair cardiac contractile function not only in situations where increased pump function is required, such as exercise, but also during resting conditions when myocardium usually operates far below maximal capacity (Katz, 1992).
Figure 3. Effects of MHC composition on the rate constant of force redevelopment in rat skinned myocardium.
The rate constant of force redevelopment (ktr) was measured as a function of isometric force (P/Po) in myocardium from thyroidectomized (0%α-MHC, •, n= 10) and hyperthyroid (100%α-MHC, ○, n= 10) rats. Data are means ±s.e.m.
Effect of MHC composition on stretch activation
The effects of MHC composition on the stretch activation response in rat skinned myocardium was studied by imposing stretches of 1% of initial muscle length during maximal and submaximal Ca2+ activations. Variable expression of MHC isoform resulted in significant changes to the stretch activation response of rat skinned myocardium as shown in Fig. 4, where a stretch of 1% of initial muscle length was imposed at different levels of Ca2+ activation. To facilitate comparisons of stretch activation at different activation levels, the phases of the stretch activation response were normalized to the isometric force preceding the application of the stretch which in the stretch activation figures is indicated as an arbitrary ‘zero’ baseline: P2, the minimum force at the end of phase 2; Pdf, the trough-to-peak excursion of force in phase 3; and P3, the amplitude of phase 3 measured from the prestretch isometric force. The amplitude of phase 1 (P1) was not assessed in this work.
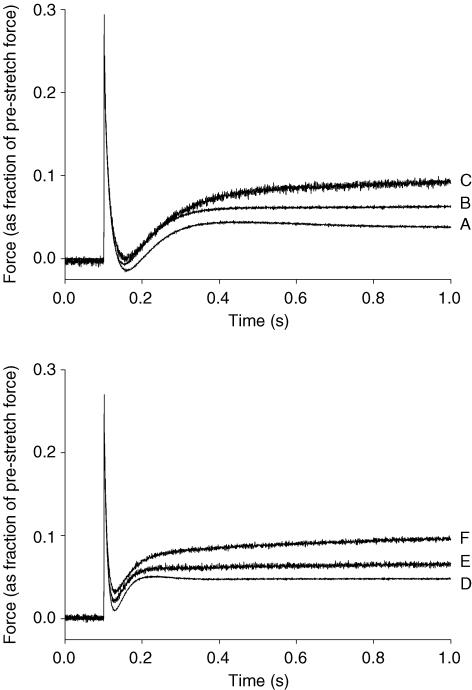
Figure 4. Effect of MHC composition on the stretch activation response of rat myocardium recorded at various levels of Ca2+ activation.
The force responses following a stretch of 1% of muscle length from thyroidectomized (top panel) and hyperthyroid (bottom panel) rat myocardium. In each case, the force responses were normalized to the prestretch isometric force (corresponding to the zero baseline) recorded at the same level of activation; that is, the prestretch force baseline corresponds to: P/Po= 1.00 (A and D; maximal activation); 0.53 (B); 0.48 (E; intermediate activation); 0.27 (C); and 0.23 (F; low activation).
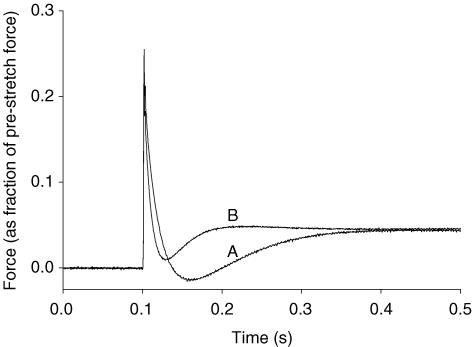
As reported previously (Stelzer et al. 2006a,c), increases in activating [Ca2+] accelerated the overall rate of the stretch activation response and increased its absolute amplitude but reduced its amplitude normalized to prestretch isometric force. Expression of β MHC slowed the overall rate of the stretch activation response at all levels of Ca2+ activation, such that peak delayed force following stretch occurred later (Fig. 4), and significantly increased the overall amplitude of phase 3 (delayed force rise) as indicated by a larger Pdf value, but did not alter the peak delayed force achieved following stretch (P3). The effect of MHC on the stretch activation response can be better appreciated in Fig. 5, where the stretch activation response of fibres expressing only α or β MHC at maximal Ca2+ activation are superimposed.
Figure 5. Effects of MHC composition on the normalized stretch activation response of rat skinned myocardium.
The force responses following a stretch of 1% of muscle length from thyroidectomized (0%α-MHC; A) and hyperthyroid (100%α-MHC; B) rat myocardium are superimposed. In each trace, the force response was normalized to the prestretch isometric force (corresponding to the zero baseline). In this case the level of Ca2+ activation was maximal (i.e. P/Po= 1.00 for both A and B).
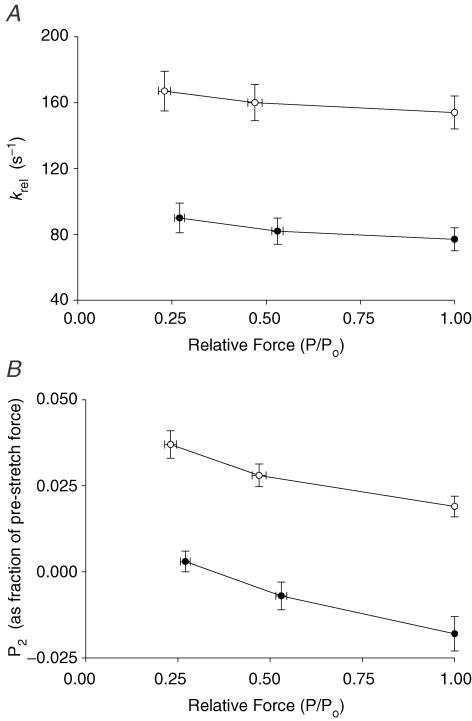
Regardless of MHC isoform content, increased prestretch activation slightly decelerated the rapid force decay in phase 2 (krel) (Fig. 6A) and increased the amount of force decay (i.e. decreased the value of P2; Fig. 6B), following stretch. At all levels of activation, myocardium expressing β MHC isoform exhibited greater cross-bridge detachment following stretch as indicated by decreased values of P2 (Fig. 6B) which were often negative (i.e. less than prestretch isometric force). Expression of β MHC also slowed krel ∼2-fold (Fig. 6A) indicating a decrease in the apparent rate of cross-bridge detachment.
Figure 6. Effects of MHC composition on krel and P2 at different levels of activation.
krel (A) and P2 values (B) (normalized to prestretch isometric force) were measured in myocardium from thyroidectomized (0%α-MHC, •, n= 10) and hyperthyroid (100%α-MHC, ○, n= 10) rats at different levels of prestretch isometric force (P/Po). The data shown were obtained from force responses to stretches of 1% of muscle length. Data are means ±s.e.m.
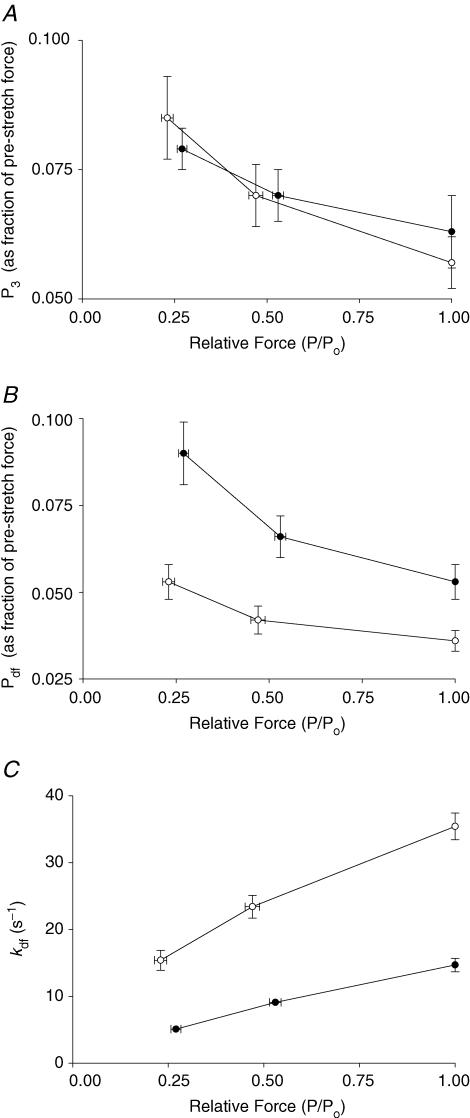
Because the amplitude of the delayed force response (phase 3) varies with prestretch isometric force (Linari et al. 2004; Stelzer et al. 2006c), the number of cross-bridges recruited by stretch can be estimated from the amplitude of the delayed force response (P3). The amplitude (normalized to prestretch isometric force) of the delayed force response (P3) was inversely proportional to activating [Ca2+] because at high levels of activating [Ca2+] a greater proportion of cross-bridges are initially bound to actin, leaving fewer cross-bridges available for recruitment by stretch (Fig. 7A). At all levels of activation, Pdf was also increased with β MHC expression (Fig. 7B), in part because P2 was decreased (Fig. 6B), such that the overall trough-to-peak amplitude of phase 3 was increased.
Figure 7. Effects of MHC composition on Pdf and kdf at different levels of activation.
P3 values normalized to prestretch isometric force (A), Pdf values normalized to prestretch isometric force (B) and kdf values (C) were measured in myocardium from thyroidectomized (0%α-MHC, •, n= 10) and hyperthyroid (100%α-MHC, ○, n= 10) rats at different levels of prestretch isometric force (P/Po). The data shown were obtained from force responses to stretches of 1% of muscle length. Data are means ±s.e.m.
As previously reported (Linari et al. 2004; Stelzer et al. 2006c), kdf following stretch, which represents the kinetics of recruitment of cross-bridges into strongly bound states (Piazzesi et al. 1997; Campbell et al. 2004; Stelzer et al. 2006c), is not well fitted by a single exponential equation, especially at low levels of activation. In order to facilitate comparisons of phase 3 rates of force development at different levels of activation, a composite apparent rate constant (kdf) of delayed force development was calculated from the half-time of force development. Consistent with earlier reports in murine myocardium (Stelzer et al. 2006a,c), kdf in rat myocardium was accelerated by increasing the level of Ca2+ activation (Fig. 7C). MHC isoform content had a dramatic effect on kdf, with values 2.5-fold less for fibres expressing the β MHC isoform at all levels of Ca2+ activation (Fig. 7C and Table 2). To assess whether the effect of MHC isoform expression on kdf was linear, we performed additional experiments on control euthyroid skinned myocardium that expressed ∼60% α MHC. As can be seen in Table 2, kdf values from euthyroid myocardium were intermediate to those from myocardium expressing 100% α or 100% β MHC at all levels of Ca2+ activation, suggesting that kdf varies in proportion with MHC isoform expression.
Table 2.
MHC and activation dependence of phase 3 delayed force development in rat myocardium
| %α MHC | Activation level (P/P0) | kdf (s−1) | a | k1 (s−1) | b | k2 (s−1) |
|---|---|---|---|---|---|---|
| 0 | 1.00 | 14.7 ± 1.0 | 1.00 | 15.5 ± 1.2 | — | — |
| 59 ± 6 | 1.00 | 25.3 ± 2.7* | 1.00 | 26.7 ± 2.9* | — | — |
| 100 | 1.00 | 35.4 ± 2.0** | 1.00 | 34.7 ± 2.0** | — | — |
| 0 | 0.53 ± 0.03 | 9.1 ± 0.8 | 0.85 ± 0.04 | 11.3 ± 0.7 | 0.15 ± 0.02 | 1.4 ± 0.3 |
| 59 ± 6 | 0.49 ± 0.05 | 15.5 ± 2.0* | 0.79 ± 0.06 | 16.8 ± 1.5* | 0.21 ± 0.04 | 2.2 ± 0.4* |
| 100 | 0.48 ± 0.02 | 23.4 ± 1.7** | 0.82 ± 0.03 | 26.9 ± 1.5** | 0.18 ± 0.02 | 3.2 ± 0.5** |
| 0 | 0.27 ± 0.02 | 5.1 ± 0.5 | 0.68 ± 0.03 | 8.7 ± 0.7 | 0.32 ± 0.03 | 1.0 ± 0.2 |
| 59 ± 6 | 0.22 ± 0.04 | 9.5 ± 0.9* | 0.63 ± 0.05 | 11.8 ± 0.9* | 0.37 ± 0.04 | 1.5 ± 0.2* |
| 100 | 0.23 ± 0.02 | 15.4 ± 1.5** | 0.66 ± 0.03 | 19.9 ± 1.3** | 0.34 ± 0.02 | 2.3 ± 0.4** |
Rate and amplitude data were obtained from force transients in response to stretches of 1% of muscle length at each of the indicated levels of activation (adjusted by varying free [Ca2+]). Data in each case are reported as means ±s.e.m. from 10 thyroidectomized and hyperthyroid preparations and seven euthyroid preparations. As described in the Methods, the apparent rate constants for delayed force recovery were obtained by fitting each record with a double exponential equation: y=a exp(−k1×x) +b exp(−k2×x), where a is the amplitude of the first exponential phase with rate constant k1 and b is the amplitude of the second exponential phase with rate constant k2.
Significantly different from thyroidectomized (P < 0.05)
significantly different from thyroidectomized and euthyroid (P < 0.05).
Analysis of the kinetics of delayed force development using a double exponential fit yielded fast and slow rate constants (k1 and k2) and their corresponding amplitudes (a and b), as shown in Table 2. As previously reported in murine myocardium (Stelzer et al. 2006a,c), increasing the level of activation progressively reduced the amplitude (b) of the slower rate process (thought to be indicative of cooperative recruitment of cross-bridges) and accelerated its apparent rate (k2), such that at maximal activations delayed force development following stretch proceeded as a single process with amplitude (a). Increased expression of β MHC isoform slowed both the fast (k1) and slow (k2) rate constants of force development by ∼2.5-fold at all levels of activation, but did not alter the relative amplitudes of the fast and slow processes (a and b) at any activation level (Table 2). Therefore, the deceleration of kdf in fibres expressing increased amounts of the β MHC isoform was due to similar proportional decelerations of k1 and k2 rather than a decrease in the amplitude (a) of the fast kinetic phase.
Discussion
Lack of effect of MHC isoform expression on Ca2+ sensitivity of force in rat myocardium
Consistent with earlier results (Fitzsimons et al. 1998; Herron et al. 2001; Rundell et al. 2004, 2005), we observed in the present study that MHC isoform expression does not alter Ca2+-activated force production in rat skinned myocardium, suggesting that the intrinsic ability of rat myocardium to produce force is independent of MHC isoform. By contrast, increased expression of β MHC with PTU treatment has been shown to decrease (Metzger et al. 1999) or increase (Gibson et al. 1992) the Ca2+ sensitivity of force production of rat skinned myocardium. The mechanisms for the shifts in the Ca2+ sensitivity of force in these studies are unclear but may be due to differences in experimental protocols, the length of time rats were treated with PTU, or the type of cardiac preparations employed (single myocytes or multicellular preparations). We have previously shown (Fitzsimons et al. 1998) that thyroid status does not alter thin filament protein composition or phosphorylation state, which is consistent with the lack of effect of thyroid status on the Ca2+ sensitivity of force observed in the present study. Also consistent with the observation that MHC isoform does not alter the Ca2+ sensitivity of force production in myocardium are studies of single-molecule properties of α and β MHC isoforms which have shown similar unitary displacements and force as assessed by the laser trap technique (Sugiura et al. 1998; Palmiter et al. 1999) and in vitro motility assays (VanBuren et al. 1995). These results imply that myosin molecules composed of either the α or β MHC isoforms have similar lever arm lengths and rotation during the power stroke (reviewed by Tyska & Warshaw, 2002; Palmer, 2005), suggesting that the functional differences observed between the two myosin isoforms are mostly determined by differences in the rates of cross-bridge attachment and force development and rates of cross-bridge detachment and force relaxation.
Effects of MHC isoform expression on the stretch activation response in rat myocardium
Stretching an active muscle to a new isometric length results in a multiphase force response, beginning with an increase in force that is coincident with the stretch (phase 1) and is due to an elastic response mediated by attached cross-bridges (Huxley & Simmons, 1971). The increased strain in cross-bridges as a result of stretch promotes a redistribution of cross-bridges to the early states of force generation, transiently shifting the equilibrium distribution farther from the power stroke (Lombardi & Piazzesi, 1990; Piazzesi et al. 1992). This is followed by a relatively rapid decay or relaxation of force that has been attributed to detachment of strained cross-bridges and re-attachment of unstrained cross-bridges (Davis & Rodgers, 1995; Piazzesi et al. 1997), with a net reduction in the total number bound. The subsequent delayed rise in force is the hallmark of stretch activation and is due to an increase in the number of force-generating cross-bridges (Linari et al. 2000) arising from cooperative recruitment of cross-bridges into force-generating states (Linari et al. 2004; Campbell et al. 2004; Stelzer et al. 2006c) and establishment of a new steady state.
In contrast to the lack of effect of MHC isoform expression on steady-state force production in rat skinned myocardium, expression of β MHC significantly slowed the overall rates of phases 2 and 3 of the stretch activation response (Fig. 5), as indicated by decreased rates of cross-bridge detachment (krel) and recruitment of cross-bridges into force-generating states (kdf). Reduced rates of cross-bridge detachment with increased expression of β MHC have been consistently shown by others using various methods (VanBuren et al. 1995; Fitzsimons et al. 1998; Weisberg & Winegrad, 1998; Galler et al. 2002; Korte et al. 2005; Rundell et al. 2005). The effects have been attributed to decreased rates of ADP dissociation and slowed cross-bridge cycling (Siemankowski et al. 1985). The deceleration of krel in this study was accompanied by an increase in the total numbers of cross-bridges that detach during phase 2 such that the force minimum following stretch (P2) often dipped below prestretch isometric force (Fig. 6B). In this case, changes in the rates of force decay (krel) and force recovery (kdf) may govern the amplitude of P2, which is the transition point at which the cross-bridge recruitment phase begins to dominate the cross-bridge detachment phase (Campbell et al. 2004). Slowed rates of cross-bridge recruitment and transitions to force-generating states (kdf) with increased expression of β MHC may contribute to increased force decay (P2) following stretch because replacement of detached cross-bridges following stretch with new force-generating cross-bridges would be delayed resulting in greater force deficits as evidenced by decreased P2 values in β MHC myocardium.
Decreased values of P2 in skeletal fibres expressing the slow MHC isoform have also been interpreted as indicating an increased sensitivity of cross-bridges to strain so that they are more easily detached from actin (Davis & Epstein, 2003). Increased strain-induced cross-bridge detachment has been accounted for in terms of a decrease in the probability of the forward force-generating power stroke (Cooke, 1997) and an increase in the probability of reversal of force-producing steps in response to stretch (Davis & Epstein, 2003) so that the reversal of steps such as the phosphate release step are more favoured. Such a mechanism would theoretically decrease ATP utilization in response to strain and may contribute to an increased economy of contraction in β MHC-containing myocardium such that isometric force production per ATP expended is elevated and tension cost is decreased (Rundell et al. 2004, 2005). However, increased cross-bridge reversibility in β MHC-containing myocardium would tend to reduce the time cross-bridges are attached to actin. This is inconsistent with the reported prolonged duty cycle of cross-bridges expressing the β MHC isoform (Palmiter et al. 1999), suggesting that in this case, increased cross-bridge reversibility is not likely to be the dominant mechanism that contributes to an increased economy of contraction. On the other hand, slowed rates of cross-bridge detachment in β MHC-containing myocardium would prolong the duty cycle of cross-bridges because they are in force-generating states for longer times consequently reducing ATP expenditure thereby contributing to increased contractile economy.
While slowed rates of cross-bridge detachment (krel) with increased β MHC expression may reduce tension cost and increase contractile efficiency they may contribute to diminished rates of force relaxation following systole which would slow the rate of diastolic filling. Impaired diastolic filling could compromise systolic function because less blood is available for ejection during the subsequent heart beat. This would therefore diminish ejection fraction during systole such that the ability to augment cardiac output is impaired, especially during times of increased circulatory demands due to physiological stress.
Previous studies have suggested (Vemuri et al. 1999; Davis et al. 2001; Stelzer et al. 2006a,c) that both the rate of force development (kdf) and the amplitude of the additional force recruited by stretch activation (P3) are associated with modulation of force generation and power output which are important determinants of systolic function. As we have previously shown (Stelzer et al. 2006c), the amplitude of delayed force development (in absolute force units) is related to prestretch isometric force such that increased activating [Ca2+] (and force) prior to muscle stretch produces a proportionally larger delayed force response amplitude thus contributing to greater force generation during systole. When normalized to prestretch isometric force, the amplitude of the delayed force development response (P3) is inversely proportional to activation level because at high levels of Ca2+ activation fewer cross-bridges are available for recruitment by stretch due to the increased numbers of cross-bridges already attached to actin, thereby diminishing P3. In this respect, expression of β MHC in the present study did not alter P3 (Fig. 7A) suggesting that the number of cross-bridges recruited by stretch at a given level of activation was similar in β and α MHC-containing myocardium.
In contrast to the lack of effect of MHC isoform expression on P3, increased expression of β MHC reduced the rate constant of delayed force development (kdf) at all levels of activation (Fig. 7C). The deceleration of kdf in β MHC-containing myocardium was due to a decrease in the apparent rates of the fast (k1) and slow (k2) components of the delayed force development phase but not to a change in the relative contributions of the corresponding fast and slow amplitudes (a and b) to the overall amplitude of the delayed force transient. This result is consistent with X-ray diffraction studies which show that at a given [Ca2+] the cardiac β MHC isoform has a longer delay from the onset of cross-bridge attachment to force generation (i.e. a slower transition from the weakly bound state to the strongly bound state), but the total number of cross-bridges bound to actin recruited at peak force is similar for the two MHC isoforms (Yagi et al. 2001). The deceleration in krel and the reduction in P2 (greater force relaxation in phase 2) following stretch also mean that a greater number of cross-bridges need be recruited in β MHC-containing myocardium to achieve similar P3 values, as indicated by the greater amplitude of Pdf in β MHC-containing myocardium (Fig. 7B), which will also act to slow the overall rate of delayed force development (see model by Campbell, 1997).
Effects of MHC isoform expression on the rate of force redevelopment in rat myocardium
Although not the primary focus of this study, we also examined the effects of MHC isoform on the rate constant of force redevelopment (ktr) induced using a stretch and release protocol modified from the methods of Brenner & Eisenberg (1986). ktr is thought to be the sum of the forward (fapp) and reverse (gapp) rate constants describing the transitions between force-generating and non-force-generating states (Brenner & Eisenberg, 1986), and thus deceleration of ktr is due to a decrease in one or both rate constants. Consistent with other studies (Fitzsimons et al. 1998, 1999; Regnier et al. 2000; Rundell et al. 2005), we found that the rate of force development was significantly decelerated with expression of β MHC at all levels of activation. This observation is consistent with our stretch activation results which show that the rate of cross-bridge recruitment and force generation (as indicated by kdf), and force decay and the transition to non-force generating states (as indicated by krel), are slowed with β MHC expression. Furthermore, the magnitude of the difference in ktr (representing the sum of fapp and gapp) between α and β MHC isoforms (∼2.5-fold) is in good agreement with differences in kdf (∼2.5-fold) and krel (∼2-fold) for the two isoforms, suggesting that at least some of the processes measured by the different protocols are similar (Campbell et al. 2004).
The combination of the mechanical differences between α and β MHC isoforms described above can be predicted to have a significant effect on cardiac systolic and diastolic function in vivo. Indeed, it has been shown that even small shifts in the MHC profile of myocardium can have considerable functional effects on contractile function of the myofilament (Fitzsimons et al. 1998; Herron & McDonald, 2002; Rundell et al. 2005; Korte et al. 2005) as well as at the whole organ level (Fitzsimons et al. 1999; Tardiff et al. 2000; Krenz et al. 2003; Korte et al. 2005). Although in the present study we mainly studied extreme differences in MHC isoform content (∼100% α versus ∼100% β MHC), the linear nature of the relationship between MHC isoform content in isolated skinned myocardium and whole-heart preparations and contractile function (Fitzsimons et al. 1998, 1999; Rundell et al. 2005; Korte et al. 2005) allows for extrapolation of our data to predict the functional effects of smaller shifts in MHC isoform content as may be expected in the case of human heart failure. In this respect, the slowing of the overall rate of the stretch activation response observed in the present study would also predict a decrease in power generation during systolic ejection due to slowed rates of cross-bridge transitions from non-force-generating states to force-generating states. This is consistent with previous results showing that increased expression of β MHC significantly depresses power output both in isolated rat skinned myocardium and isolated whole-heart preparations (Korte et al. 2005). Furthermore, slowed rates of cross-bridge detachment (krel) may reduce tension cost and increase contractile efficiency but could ultimately decrease diastolic filling so that less blood is available for ejection during each beat.
Physiological relevance of MHC isoform expression on cardiac function
There is growing evidence that implicates the loss of α MHC as a contributing factor in human chronic heart failure because there are significant reductions in the expression of ventricular α MHC mRNA and protein levels (Nakao et al. 1997; Miyata et al. 2000; Reiser et al. 2001). It has been suggested that the increased expression of β MHC in the failing human ventricle may serve as a compensatory mechanism to increase contractile efficiency by reducing ATP expenditure and decreasing the tension cost of contraction. However, it has been shown that improved pump function in failing animal and human hearts is associated with increases in ventricular α MHC mRNA and protein levels (Abraham et al. 2002; Lowes et al. 2002; James et al. 2005) suggesting that decreased expression of α MHC is detrimental to cardiac function rather than beneficial. Results from the present study are consistent with this interpretation because a slowing of the stretch activation response with increased β MHC expression would significantly slow the rate of force development during systole and diminish both stroke volume and work production during ventricular ejection. Furthermore, decreased rates of oscillatory work have been associated with impaired cardiac function in human heart failure (Ruf et al. 1998), suggesting that the slowing of cross-bridge cycling kinetics and the stretch activation response may contribute to the functional manifestations of this disease.
It is interesting to note that a gradient of MHC expression exists in both rodent (Carnes et al. 2004) and human (Bouvagnet et al. 1989) ventricles with the highest levels of β MHC found in the endocardium and the lowest levels in the epicardium. There is also a gradient of electrical and mechanical activation across the ventricular wall such that during systole the endocardium is the first part of the ventricle to be activated while the epicardium is the last (Buckberg et al. 2006). Therefore, to prevent asynchronous contraction of the endocardial and epicardial fibres during the systolic ejection phase, which may decrease contractile efficiency, the increased expression of β MHC in the endocardium would slow the rate of force generation so that peak force is reached with similar timing to the faster contracting epicardial fibres. In this way, the ventricular MHC gradient in the heart may play an important role in modulating the timing of force generation across the ventricular wall as has been suggested for the ventricular gradient of regulatory light chain phosphorylation (Davis et al. 2001). Here we show that the overall rate of the stretch activation response is tuned to the MHC isoform such that it can contribute to the timing of force generation and work production across the ventricular wall.
Acknowledgments
This study was HL- supported by National Heart, Lung and Blood Institute Grant 47053 (to R.L.M.) and a grant from the American Heart Association (to J.E.S.).
References
- Abraham WT, Gilbert EM, Lowes BD, Minobe WA, Larrabee P, Roden RL, et al. Coordinate changes in myosin heavy chain isoform gene expression are selectively associated with alterations in dilated cardiomyopathy phenotype. Mol Med. 2002;8:750–760. [PMC free article] [PubMed] [Google Scholar]
- Bouvagnet P, Mairhofer H, Leger JO, Puech P, Leger JJ. Distribution pattern of alpha and beta myosin in normal and diseased human ventricular myocardium. Basic Res Cardiol. 1989;84:91–102. doi: 10.1007/BF01907006. [DOI] [PubMed] [Google Scholar]
- Brenner B, Eisenberg E. Rate of force generation in muscle: correlation with actomyosin ATPase activity in solution. Proc Natl Acad Sci U S A. 1986;83:3542–3546. doi: 10.1073/pnas.83.10.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckberg GD, Mahajan A, Jung B, Markl M, Hennig J, Ballester-Rodes M. MRI myocardial motion and fiber tracking: a confirmation of knowledge from different imaging modalities. Eur J Cardiothorac Surg. 2006;29:S165–S177. doi: 10.1016/j.ejcts.2006.02.064. [DOI] [PubMed] [Google Scholar]
- Campbell K. Rate constant of muscle force redevelopment reflects cooperative activation as well as cross-bridge kinetics. Biophys J. 1997;72:254–262. doi: 10.1016/S0006-3495(97)78664-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KB, Chandra M. Functions of stretch activation in heart muscle. J Gen Physiol. 2006;127:89–94. doi: 10.1085/jgp.200509483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KB, Chandra M, Kirkpatrick RD, Slinker BK, Hunter WC. Interpreting cardiac muscle force-length dynamics using a novel functional model. Am J Physiol Heart Circ Physiol. 2004;286:H1535–H1545. doi: 10.1152/ajpheart.01029.2003. [DOI] [PubMed] [Google Scholar]
- Campbell KS, Moss RL. SL Control: PC-based data acquisition and analysis for muscle mechanics. Am J Physiol Heart Circ Physiol. 2003;285:H2857–H2864. doi: 10.1152/ajpheart.00295.2003. [DOI] [PubMed] [Google Scholar]
- Carnes CA, Geisbuhler TP, Reiser PJ. Age-dependent changes in contraction and regional myocardial myosin heavy chain isoform expression in rats. J Appl Physiol. 2004;97:446–453. doi: 10.1152/japplphysiol.00439.2003. [DOI] [PubMed] [Google Scholar]
- Chase PB, Martyn DA, Hannon JD. Isometric force redevelopment of skinned muscle fibers from rabbit activated with and without Ca2+ Biophys J. 1994;67:1994–2001. doi: 10.1016/S0006-3495(94)80682-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R. Actomyosin interaction in striated muscle. Physiol Rev. 1997;77:671–697. doi: 10.1152/physrev.1997.77.3.671. [DOI] [PubMed] [Google Scholar]
- Davis JS, Epstein ND. Kinetic effects of fiber type on the two subcomponents of the Huxley-Simmons phase 2 in muscle. Biophys J. 2003;85:390–401. doi: 10.1016/S0006-3495(03)74483-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JS, Hassanzadeh S, Winitsky S, Lin H, Satorius C, Vemuri R, Aletras AH, Wen H, Epstein ND. The overall pattern of cardiac contraction depends on a spatial gradient of myosin regulatory light chain phosphorylation. Cell. 2001;107:631–641. doi: 10.1016/s0092-8674(01)00586-4. [DOI] [PubMed] [Google Scholar]
- Davis JS, Rodgers ME. Indirect coupling of phosphate release to de novo tension generation during muscle contraction. Proc Natl Acad Sci U S A. 1995;92:10482–10486. doi: 10.1073/pnas.92.23.10482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein ND, Davis JS. When is a fly in the ointment a solution and not a problem? Circ Res. 2006;98:1110–1112. doi: 10.1161/01.RES.0000223888.99864.3c. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Fitzsimons DP, Patel JR, Moss RL. Role of myosin heavy chain composition in kinetics of force development and relaxation in rat myocardium. J Physiol. 1998;513:171–183. doi: 10.1111/j.1469-7793.1998.171by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimons DP, Patel JR, Moss RL. Aging-dependent depression in the kinetics of force development in rat skinned myocardium. Am J Physiol Heart Circ Physiol. 1999;276:H1511–H1519. doi: 10.1152/ajpheart.1999.276.5.H1511. [DOI] [PubMed] [Google Scholar]
- Galler S, Puchert E, Golsch B, Schmid D, Pette D. Kinetic properties of cardiac myosin heavy chain isoforms in rat. Pflugers Arch. 2002;445:218–223. doi: 10.1007/s00424-002-0934-6. [DOI] [PubMed] [Google Scholar]
- Gibson LM, Wendt IR, Stephenson DG. Contractile activation properties of ventricular myocardium from hypothyroid, euthyroid, and juvenile rats. Pflugers Arch. 1992;422:16–23. doi: 10.1007/BF00381508. [DOI] [PubMed] [Google Scholar]
- Godt RE, Lindley BD. Influence of temperature upon contractile activation and isometric force production in mechanically skinned muscle fibers of the frog. J Gen Physiol. 1982;80:279–297. doi: 10.1085/jgp.80.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad F, Masatsugu M, Bodell PW, Qin A, McCue SA, Baldwin KM. Role of thyroid hormone and insulin in control of cardiac isomyosin expression. J Mol Cell Cardiol. 1997;29:559–569. doi: 10.1006/jmcc.1996.0299. [DOI] [PubMed] [Google Scholar]
- Herron TJ, Korte FS, McDonald KS. Loaded shortening and power output in cardiac myocytes are dependent on myosin heavy chain isoform expression. Am J Physiol Heart Circ Physiol. 2001;281:H1217–H1222. doi: 10.1152/ajpheart.2001.281.3.H1217. [DOI] [PubMed] [Google Scholar]
- Herron TJ, McDonald KS. Small amounts of α-myosin heavy chain isoform expression significantly increase power output of rat cardiac myocytes fragments. Circ Res. 2002;90:1150–1152. doi: 10.1161/01.res.0000022879.57270.11. [DOI] [PubMed] [Google Scholar]
- Hoh JFY, Yeoh GPS, Thomas MAW, Higgenbottom L. Structural differences in the heavy chains of rat ventricular myosin isoenzymes. FEBS Lett. 1979;97:330–334. doi: 10.1016/0014-5793(79)80115-5. [DOI] [PubMed] [Google Scholar]
- Huxley AF, Simmons RM. Proposed mechanism of force generation in striated muscle. Nature. 1971;233:533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Huxley HE. The double array of filaments in cross-striated muscle. Biophys Biochem Cytol. 1957;3:631–648. doi: 10.1083/jcb.3.5.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James J, Martin L, Krenz M, Quatman C, Jones F, Klevitsky R, Gulick J, Robbins J. Forced expression of α-myosin heavy chain in the rabbit ventricle results in cardioprotection under cardiomyopathic conditions. Circulation. 2005;111:2339–2346. doi: 10.1161/01.CIR.0000164233.09448.B1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz AM. Physiology of the Heart. New York: Raven Press; 1992. [Google Scholar]
- Korte FS, Herron TJ, Rovetto MJ, McDonald KS. Power output is linearly related to MyHC content in rat skinned myocytes and isolated working hearts. Am J Physiol Heart Circ Physiol. 2005;289:H801–H812. doi: 10.1152/ajpheart.01227.2004. [DOI] [PubMed] [Google Scholar]
- Krenz M, Sanbe A, Bouyer-Dalloz F, Gulick J, Klevitsky R, Hewett TE, et al. Analysis of myosin heavy chain functionality in the heart. J Biol Chem. 2003;278:17466–17474. doi: 10.1074/jbc.M210804200. [DOI] [PubMed] [Google Scholar]
- Linari M, Lucii L, Reconditi M, Casoni ME, Amenitsch H, Bernstorff S, Piazzesi G, Lombardi V. A combined mechanical and X-ray diffraction study of stretch potentiation in single frog muscle fibres. J Physiol. 2000;526:589–596. doi: 10.1111/j.1469-7793.2000.00589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linari M, Reedy MK, Reedy MC, Lombardi V, Piazzesi G. Ca-activation and stretch-activation in insect flight muscle. Biophys J. 2004;87:1101–1111. doi: 10.1529/biophysj.103.037374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi V, Piazzesi G. The contractile response during steady lengthening of stimulated frog muscle fibres. J Physiol. 1990;431:141–171. doi: 10.1113/jphysiol.1990.sp018324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lompre AM, Mercadier JJ, Schwartz K. Changes in gene expression during cardiac growth. Int Rev Cytol. 1991;124:137–186. doi: 10.1016/s0074-7696(08)61526-0. [DOI] [PubMed] [Google Scholar]
- Lompre AM, Nadal-Ginard B, Mahdavi V. Expression of the cardiac alpha- and beta-myosin heavy chain genes is developmentally and hormonally regulated. J Biol Chem. 1984;259:6437–6446. [PubMed] [Google Scholar]
- Lowes BD, Gilbert EM, Abraham WT, Minobe W, Larrabee P, Ferguson D, et al. Myocardial gene expression in dilated cardiomyopathy treated with beta-blocking agents. N Engl J Med. 2002;346:1357–1365. doi: 10.1056/NEJMoa012630. [DOI] [PubMed] [Google Scholar]
- Lowes BD, Minobe W, Abraham T, Rizeq MN, Bohlmeyer TJ, Quaife RA, et al. Changes in gene expression in the intact human heart: downregulation of α-myosin heavy chain in hypertrophied, failing ventricular myocardium. J Clin Invest. 1997;100:2315–2324. doi: 10.1172/JCI119770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lymn RW, Taylor EW. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry. 1971;10:4617–4624. doi: 10.1021/bi00801a004. [DOI] [PubMed] [Google Scholar]
- McNally EM, Kraft R, Bravo-Zehnder M, Taylor DA, Leinwand LA. Full-length rat alpha and beta cardiac myosin heavy chain sequences. J Mol Biol. 1989;210:665–671. doi: 10.1016/0022-2836(89)90141-1. [DOI] [PubMed] [Google Scholar]
- Metzger JM, Wahr PA, Michele DE, Albayya F, Westfall MV. Effects of myosin heavy chain isoform on Ca2+-activated tension development in single adult cardiac myocytes. Circ Res. 1999;84:1310–1317. doi: 10.1161/01.res.84.11.1310. [DOI] [PubMed] [Google Scholar]
- Miyata S, Minobe W, Bristow MR, Leinwand LA. Myosin heavy chain isoform expression in the failing and nonfailing human heart. Circ Res. 2000;86:386–390. doi: 10.1161/01.res.86.4.386. [DOI] [PubMed] [Google Scholar]
- Moss RL. Sarcomere length-tension relations of frog skinned muscle fibers during calcium activation at short lengths. J Physiol. 1979;292:177–202. doi: 10.1113/jphysiol.1979.sp012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao K, Minobe W, Roden R, Bristow MR, Leinwand LA. Myosin heavy chain expression in human heart failure. J Clin Invest. 1997;100:2362–2370. doi: 10.1172/JCI119776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer BM. Thick filament proteins and performance in human heart failure. Heart Fail Rev. 2005;10:187–197. doi: 10.1007/s10741-005-5249-1. [DOI] [PubMed] [Google Scholar]
- Palmiter KA, Tyska MJ, Dupuis DE, Alpert NR, Warshaw DM. Kinetic differences at the single molecule level account for the functional diversity of rabbit cardiac myosin isoforms. J Physiol. 1999;519:669–678. doi: 10.1111/j.1469-7793.1999.0669n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel JR, Fitzsimons DP, Buck SH, Muthuchamy M, Wieczorek DF, Moss RL. PKA accelerates rate of force development in murine skinned myocardium expressing alpha- or beta-tropomyosin. Am J Physiol Heart Circ Physiol. 2001;280:H2732–H2739. doi: 10.1152/ajpheart.2001.280.6.H2732. [DOI] [PubMed] [Google Scholar]
- Piazzesi G, Francini F, Linari M, Lombardi V. Tension transients during steady lengthening of tetanized muscle fibres of the frog. J Physiol. 1992;445:659–711. doi: 10.1113/jphysiol.1992.sp018945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazzesi G, Linari M, Reconditi M, Vanzi F, Lombardi V. Cross-bridge detachment and attachment following a step stretch imposed on active single frog muscle fibres. J Physiol. 1997;498:3–15. doi: 10.1113/jphysiol.1997.sp021837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayment I, Rypniewski WR, Schmidt-Base K, Smith R, Tomchick DR, Benning MM, Winkelmann DA, Wesenberg G, Holden HM. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993;261:50–58. doi: 10.1126/science.8316857. [DOI] [PubMed] [Google Scholar]
- Regnier M, Rivera AJ, Chen Y, Chase PB. 2-deoxy-ATP enhances contractility of rat cardiac muscle. Circ Res. 2000;86:1211–1217. doi: 10.1161/01.res.86.12.1211. [DOI] [PubMed] [Google Scholar]
- Reiser PJ, Portman MA, Ning XH, Moravec CS. Human cardiac myosin heavy chain isoforms in fetal and failing adult atria and ventricles. Am J Physiol Heart Circ Physiol. 2001;280:H1814–H1820. doi: 10.1152/ajpheart.2001.280.4.H1814. [DOI] [PubMed] [Google Scholar]
- Ruf T, Schulte-Baukloh H, Lüdemann J, Posival H, Beyersdorf F, Just H, Holubarsch C. Alterations in cross-bridge kinetics in human atrial and ventricular myocardium. Cardiovasc Res. 1998;40:580–590. doi: 10.1016/s0008-6363(98)00164-3. [DOI] [PubMed] [Google Scholar]
- Rundell VL, Geenen DL, Buttrick PM, deTombe PP. Depressed cardiac tension cost in experimental diabetes is due to altered myosin heavy chain isoform expression. Am J Physiol Heart Circ Physiol. 2004;287:H408–H413. doi: 10.1152/ajpheart.00049.2004. [DOI] [PubMed] [Google Scholar]
- Rundell VL, Manaves MV, Martin AF, deTombe PP. Impact of β-myosin heavy chain isoform expression on cross-bridge cycling kinetics. Am J Physiol Heart Circ Physiol. 2005;288:H896–H903. doi: 10.1152/ajpheart.00407.2004. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev. 1996;76:371–423. doi: 10.1152/physrev.1996.76.2.371. [DOI] [PubMed] [Google Scholar]
- Shiner JS, Solaro RJ. The Hill coefficient for the Ca2+-activation of striated muscle contraction. Biophys J. 1984;46:541–543. doi: 10.1016/S0006-3495(84)84051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemankowski RF, Wiseman RO, White HD. ADP dissociation from actomyosin subfragment 1 is sufficiently slow to limit the unloaded shortening velocity in vertebrate muscle. Proc Natl Acad Sci U S A. 1985;82:658–662. doi: 10.1073/pnas.82.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger GJ. Stretch activation and myogenic oscillation of isolated contractile structures of heart muscle. Pflugers Arch. 1971;330:347–361. doi: 10.1007/BF00588586. [DOI] [PubMed] [Google Scholar]
- Steiger GJ. Stretch activation and tension transients in cardiac, skeletal, and insect flight muscle. In: Treager RT, editor. Insect Flight Muscle. Amsterdam: North-Holland; 1977. pp. 221–268. [Google Scholar]
- Stelzer JE, Dunning SB, Moss RL. Ablation of cardiac myosin binding protein-C accelerates stretch activation in murine skinned myocardium. Circ Res. 2006a;98:1212–1218. doi: 10.1161/01.RES.0000219863.94390.ce. [DOI] [PubMed] [Google Scholar]
- Stelzer JE, Fitzsimons DP, Moss RL. Ablation of myosin binding protein-C accelerates force development in mouse myocardium. Biophys J. 2006b;90:4119–4127. doi: 10.1529/biophysj.105.078147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzer JE, Larsson L, Fitzsimons DP, Moss RL. Activation dependence of stretch activation in mouse skinned myocardium: implications for ventricular function. J Gen Physiol. 2006c;127:95–107. doi: 10.1085/jgp.200509432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzer JE, Patel JR, Olsson MC, Fitzsimons DP, Leinwand LA, Moss RL. Expression of cardiac troponin T with COOH-terminal truncation accelerates cross-bridge interaction kinetics in mouse myocardium. Am J Physiol Heart Circ Physiol. 2004;287:H1756–H1761. doi: 10.1152/ajpheart.00172.2004. [DOI] [PubMed] [Google Scholar]
- Sugiura S, Kobayama N, Fujita H, Yamashita H, Momomura S, Chaen S, Omata M, Sugi H. Comparison of unitary displacements and forces between two cardiac myosin isoforms by the optical trap technique. Circ Res. 1998;82:1029–1034. doi: 10.1161/01.res.82.10.1029. [DOI] [PubMed] [Google Scholar]
- Tardiff JC, Hewett TE, Factor SM, Vikstrom KL, Robbins J, Leinwand LA. Expression of the beta (slow)-isoform of MHC in the adult mouse heart causes dominant-negative functional effects. Am J Physiol Heart Circ Physiol. 2000;278:H412–H419. doi: 10.1152/ajpheart.2000.278.2.H412. [DOI] [PubMed] [Google Scholar]
- Tyska MJ, Warshaw DM. The myosin power stroke. Cell Motil Cytoskeleton. 2002;51:1–15. doi: 10.1002/cm.10014. [DOI] [PubMed] [Google Scholar]
- VanBuren P, Harris DE, Alpert NR, Warshaw DM. Cardiac V1 and V3 myosins differ in their hydrolytic and mechanical activities in vitro. Circ Res. 1995;77:439–444. doi: 10.1161/01.res.77.2.439. [DOI] [PubMed] [Google Scholar]
- Vemuri R, Lankford EB, Poetter K, Hassanzadeh S, Takeda K, Yu ZX, Ferrans VJ, Epstein ND. The stretch-activation response may be critical to the proper functioning of the mammalian heart. Proc Natl Acad Sci U S A. 1999;96:1048–1053. doi: 10.1073/pnas.96.3.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren CM, Greaser ML. Method for cardiac myosin heavy chain separation by sodium dodecyl sulfate gel electrophoresis. Anal Biochem. 2003;320:149–151. doi: 10.1016/s0003-2697(03)00350-6. [DOI] [PubMed] [Google Scholar]
- Weisberg A, Winegrad S. Relation between crossbridge structure and actomyosin ATPase activity in rat heart. Circ Res. 1998;83:60–72. doi: 10.1161/01.res.83.1.60. [DOI] [PubMed] [Google Scholar]
- Yagi N, Saeki Y, Ishikawa T, Kurihara S. Cross-bridge movement and calcium behavior in ferret papillary muscle in different thyroid states. Jpn J Physiol. 2001;51:319–326. doi: 10.2170/jjphysiol.51.319. [DOI] [PubMed] [Google Scholar]