Abstract
Hypertrophic cardiomyocyte growth contributes substantially to the progression of heart failure. Activation of the plasma membrane Na+–H+ exchanger (NHE1) and Cl−–HCO3− exchanger (AE3) has emerged as a central point in the hypertrophic cascade. Both NHE1 and AE3 bind carbonic anhydrase (CA), which activates their transport flux, by providing H+ and HCO3−, their respective transport substrates. We examined the contribution of CA activity to the hypertrophic response of cultured neonatal and adult rodent cardiomyocytes. Phenylephrine (PE) increased cell size by 37 ± 2% and increased expression of the hypertrophic marker, atrial natriuretic factor mRNA, twofold in cultured neonatal rat cardiomyocytes. Cell size was also increased in adult cardiomyocytes subjected to angiotensin II or PE treatment. These effects were associated with increased expression of cytosolic CAII protein and the membrane-anchored isoform, CAIV. The membrane-permeant CA inhibitor, 6-ethoxyzolamide (ETZ), both prevented and reversed PE-induced hypertrophy in a concentration-dependent manner in neonate cardiomyocytes (IC50 = 18 μm). ETZ and the related CA inhibitor methazolamide prevented hypertrophy in adult cardiomyocytes. In addition, ETZ inhibited transport activity of NHE1 and the AE isoform, AE3, with respective EC50 values of 1.2 ± 0.3 μm and 2.7 ± 0.3 μm. PE significantly increased neonatal cardiomyocyte Ca2+ transient frequency from 0.33 ± 0.4 Hz to 0.77 ± 0.04 Hz following 24 h treatment; these Ca2+-handling abnormalities were completely prevented by ETZ (0.28 ± 0.07 Hz). Our study demonstrates a novel role for CA in mediating the hypertrophic response of cardiac myocytes to PE and suggests that CA inhibition represents an effective therapeutic approach towards mitigation of the hypertrophic phenotype.
Cardiac hypertrophy, which frequently leads to heart failure, results from the altered cardiac cell growth known as cardiomyocyte hypertrophy (CH) (Frey et al. 2004). Emerging evidence suggests that aberrant activity of pHi regulatory transporters contributes to the hypertrophic response. There are a number of pHi regulatory transporters in the cardiac cell. Briefly, in response to acid loading, Na+–H+ exchange (NHE) and Na+–HCO3− symport (NBC) activate to restore intracellular pH (pHi) (Sterling & Casey, 2002). Conversely, intracellular alkalosis stimulates Na+-independent Cl−–HCO3− exchangers (AE) to acidify cardiomyocytes through HCO3− efflux (Sterling & Casey, 2002). The predominant Cl−–HCO3− exchanger of myocardium was recently identified as Slc26a6, a Cl−–HCO3− and Cl−–OH− exchanger (Alvarez et al. 2004), while NHE1 is the dominant alkalinizing transporter of heart (Moor & Fliegel, 1999; Camillion De Hurtado et al. 2000).
Previous attention regarding the role of these transporters as contributors to hypertrophy has centred on NHE1, the cardiac-specific NHE isoform. NHE1 inhibition attenuates cardiac hypertrophy following myocardial infarction (Yoshida & Karmazyn, 2000; Kusumoto et al. 2001) as well as to cardiomyocyte hypertrophy in cells exposed to the hypertrophic aldosterone or phenylephrine (Ennis et al. 2003; Karmazyn et al. 2003). Consistent with a central role of NHE1 in hypertrophic growth, NHE1 activity is also stimulated in hypertrophic myocardium of spontaneously hypertensive rats and the hypertrophy is prevented by NHE1 inhibition (Perez et al. 1995; Ennis et al. 2003). Similarly, NHE1 activity dramatically increases in hearts of patients with end-stage heart failure (Yokoyama et al. 2000).
Although these data support a role for NHE1 in perpetuating hypertrophic growth, it is important to point out that NHE1 activity requires the presence of an acidifying pathway, such as Cl−–HCO3− exchange, since sustained NHE activity will alkalinize the cell resulting in NHE1 inactivation through a cytosolic modifier site (Slepkov & Fliegel, 2002). Interestingly, the hypertrophic myocardium of spontaneously hypertensive rat (SHR) manifests both elevated NHE1 and elevated Cl−–HCO3− exchange activities (Perez et al. 1995). Coactivation of these two transport systems results in no change of pHi, but induces accumulation of cytosolic NaCl (Perez et al. 2001; Cingolani & Camilion De Hurtado, 2002). Consistent with NHE1–Cl−–HCO3− exchanger coactivation, SHR myocardium has normal pHi, in spite of activated NHE1 (Perez et al. 1995). The observation that the AE3 is the only AE isoform activated by hypertrophic stimuli suggests that AE3 is the myocardial transporter working counter to NHE1 (Alvarez et al. 2001, 2004).
NHE1 and AE3 in the myocardium are functionally linked by carbonic anhydrase (CA), which catalyses the hydration of CO2: CO2 + H2O ↔ H2CO3 ↔ H+ + HCO3− to produce both the H+ and HCO3− substrates for transport by NHE1 and AE3 (Pastorekova et al. 2004). CAII is a near-ubiquitous cytosolic isoform, which was previously thought not to be expressed in adult rat cardiomyocytes (Geers et al. 1992) but was identified in embryonic and fetal hearts (Vuillemin & Pexieder, 1997). However, recent studies using DNA microarray analysis of adult human heart has identified CAII mRNA in these tissues (http://cardiogenomics.med.harvard.edu/home (2005)). Moreover, in this paper we present evidence for CAII expression in isolated mouse cardiomyocytes using immunoblotting. Expression of CAII in human ventricular samples has also been observed (B. V. Alvarez & J. R. Casey, unpublished observations). The adult myocardium also expresses significant amounts of CAIV, CAIX, CAXII and CAXIV, which have their catalytic sites anchored to the extracellular surface (Purkerson & Schwartz, 2005). CAII binds an acidic motif in the cytoplasmic C-terminal domains of both Cl−–HCO3− exchangers and Na+–HCO3− cotransporters, which activates their rate of HCO3− transport (McMurtrie et al. 2004). Similarly, an NHE1–CAII complex activates the rate of H+ efflux by NHE1 (Li et al. 2002).
Using molecular, cellular and pharmacological approaches, the present study examined the role of CA in mediating the hypertrophic response of cardiac myocytes.
Methods
Electrophoresis and immunoblot analysis
Protein samples were transferred to PVDF membranes and then incubated with rabbit anti-human SLC26A6 (raised against peptides corresponding to the N-terminal 20 amino acids of human SLC26A6; 1: 1000 dilution) (Lohi et al. 2000), or anti-hemagglutinin (HA) antibody (HA-probe Y-11; Santa Cruz Biotechnology, San Diego CA, USA), or anti-NHE1 antibody (rabbit NHE1 polyclonal; Chemicon, Temucula, CA, USA; 4 μg ml−1), AP3 polyclonal rabbit anti-AE3 antibody (1: 1000) (Sterling & Casey, 1999), anti-CAII antibody (rabbit polyclonal H-70; Santa Cruz Biotechnology; 1: 1000), anti-CAIV antibody (goat polyclonal N-16; Santa Cruz Biotechnology; 1: 500), or mouse monoclonal anti-β-actin antibody (Sigma, St Louis, MO, USA; 1: 1000), or anti-atrial natriuretic factor (anti-ANF) antibody (goat polyclonal N-20; Santa Cruz Biotechnology; 1: 200). Immunoblots were then incubated with donkey anti-rabbit IgG conjugated to horseradish peroxidase (Sterling et al. 2002), mouse anti-goat IgG conjugated to horseradish peroxidase, or sheep anti-mouse IgG conjugated to horseradish peroxidase (GE Healthcare, Little Chalfont, UK; 1: 2000), as appropriate. Blots were visualized and quantified using ECL reagent and a Kodak Image Station.
PCR primers
cDNA sequences were obtained from the public GenBank sequence database of the National Centre for Biotechnology Information (http://www.ncbi.nlm.nih.gov/), and primers were designed with the Oligo software of the DNA Star program (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3.cgi). In conventional RT-PCR, all primers generated only one amplification band visualized by agarose gel electrophoresis on 1% agarose gels stained with ethidium bromide, demonstrating specificity.
Real-time reverse transcription PCR
Real-time PCR was performed in an ABI Prism 7900H Sequence Detection System (Applied Biosystems). Each real-time RT-PCR reaction contained: 50 mm KCl, 3 mm MgCl2, 0.08% (v/v) glycerol, 0.001% (v/v) Tween 20, 0.02% (v/v) DMSO, 1/40 000 dilution SYBR Green (Invitrogen, Burlington, Canada), 0.03 U μl−1 Jumpstart Taq (Sigma), 3.2 μm of each primer, 5 μl of template diluted (0, 1/4, 1/16 and 1/64), and 1 mm Tris, pH 8.3. In each case the template was a reverse transcription reaction prepared from 2 μg total RNA and in a total of 20 μl (Alvarez et al. 2004). Replicate samples were pipetted into 384-well reaction plates (Axygen, Union City, CA, USA) using a Biomek Fx Pipetting Robot (Beckman Coulter). Cycle threshold values (Ct) were obtained for ANF, CAII and GAPDH. GAPDH, assumed not to vary between samples, was used to normalize for differences in the efficiency of mRNA isolation from the samples as follows. Ct values were corrected for each sample by addition or subtraction of cycles so that GAPDH Ct values were the same in each case. This same Ct correction was applied to each of the Ct values. Absolute differences in gene expression between samples were calculated using the relation that a difference of 1 cycle corresponds to a difference of twofold in abundance of template. Thus, relative transcript expression was anti-log2 of the difference in Ct relative to control. Primers used to detect messages were: (forward, followed by reverse primer in each case): GAPDH (5′CGTCTCATAGACAAGAT3′ and 5′TGATGGCAACAATGTCCACT3′), ANF (5′GGGGGTAGGATTGACAGGAT3′ and 5′GGATCTTTTGCGATCTGCTC3′), and CAII (5′ACCAGAGAACTGGCACAAGG and 5′ATGAGCAGAGGCTGTAGGGA3′).
Cell culture and protein expression
Expression constructs for human SLC26A6 (Lohi et al. 2003) HA-epitope tagged versions of rat AE3fl (Fujinaga et al. 2003) and α1a-adrenergic receptor (Stanasila et al. 2003) were expressed by transient transfection of HEK293 cells (Sterling & Casey, 1999), using the calcium phosphate method (Ruetz et al. 1993). Chinese hamster ovary cell line (AP1), which lacks endogenous NHE activity (Rotin et al. 1989), was grown in a humidified atmosphere of 5% CO2 and 95% air in α-MEM (Invitrogen, Burlington, Canada) medium supplemented with 10% (v/v) fetal bovine serum, 25 mm Hepes, 100 units ml−1 penicillin and 100 μg ml−1 streptomycin; pH was 7.4 at 37°C. Stable transfections were made and selected essentially as described earlier (Murtazina et al. 2001). The plasmid pYN4+ contains the HA-tagged NHE1 isoform of the human Na+–H+ exchanger (Murtazina et al. 2001), was behind the constitutively active cytomegalovirus (CMV) promoter and was used to stably transfect AP1 cells, as described previously (Murtazina et al. 2001).
Neonatal rat cardiomyocyte isolation and culture
Neonatal rat cardiomyocytes were isolated and cultured, as previously described (Kovacic et al. 2003). All experimental protocols involving animals were carried out in accordance with policies of the Canadian Council on Animal Care. Neonatal rat pups (1–2 days old) were killed by decapitation with sharp scissors and hearts were isolated and placed in cold PBS solution. After hearts were collected, they were rinsed with PBS 2–3 times to remove debris. Atria were removed and the ventricles minced with scissors and placed in a small volume of 4°C PBS. Heart tissue was placed in a T-25 flask with 17 ml of cold PBS, after which 1 ml of sterile DNase (0.5% w/v), 1 ml collagenase (2% w/v), and 0.5 ml trypsin (2% w/v) were added to the flask. Flasks were agitated for 20 min at 80 r.p.m. at 37°C to homogenize tissue. After homogenization, 20 ml of DF20 medium, 20% fetal bovine serum, and 50 μg ml−1 gentamycin was added to myocytes. Cells were then centrifuged at 146 × g at 4°C for 1 min. Supernatant was discarded and the pellet was placed in a flask again for homogenization and centrifugation. After the second digestion, the tissue was again transferred into a 50 ml Falcon tube with 20 ml of DF20 medium and centrifuged at 146 × g for 1 min at 4°C. This step was repeated twice. After the final digestion, all supernatant fractions were combined and centrifuged at 740 × g for 7 min at 4°C. The resulting pellet was resuspended in 10 ml of plating medium (DF20 medium with 5% fetal bovine serum, 10% horse serum, 50 μg ml−1 gentamycin) and incubated at 37°C in a flask for 60 min. After 60 min, the supernatant was removed and placed in another flask for another 60 min. This step was repeated twice. After serial plating, the resulting pellet was resuspended in plating media. Cells were plated at a density of (1.8–2.0) × 106 cells per plate.
Adult mouse cardiomyocyte isolation and culture
The protocol was based upon a culture procedure previously reported (Sambrano et al. 2002). Mice (3–4 months old) were anaesthetized with pentobarbital sodium (100 mg kg−1, intraperitoneal injection). Hearts were quickly excised and retrogradely perfused at 37°C for ∼3 min with Ca2+-free perfusion solution, containing (mm): 120 NaCl, 5.4 KCl, 1.2 MgSO4, 1.2 NaH2PO4, 5.6 glucose, 20 NaHCO3, 10 2,3-butanedione monoxime (BMD, Sigma), and taurine (Sigma), gassed with 95% O2–5% CO2. Enzymatic digestion was initiated by adding to a final concentration in the perfusion solution collagenase type B (0.5 mg ml−1, Roche), collagenase type D (0.5 mg ml−1, Roche) and protease type XIV (0.02 mg ml−1; Sigma). After 3 min of digestion, CaCl2 in the cells was made up to 50 μm by addition of a 50 mm stock solution. Approximately 7–10 min later, left ventricles were quickly removed, cut in several pieces and further digested in a shaker (50–70 r.p.m.) for 10 min at 37°C in the same enzyme solution. Samples were triturated by repeated aspiration with a transfer pipette. Enzymatic digestion was stopped by addition of myocyte stopping buffer I (perfusion solution, containing 10% (v/v) fetal bovine serum and 50 μm CaCl2). Samples were allowed to settle under gravity for 10 min. Supernatants were collected and centrifuged at 400 g for 2 min. Cells collected by gravitational and centrifugational sedimentation were pooled, resuspended in 10 ml myocyte stopping buffer II (perfusion solution, containing 5% (v/v) fetal bovine serum, 50 μm CaCl2) and transferred to a 60 mm tissue culture dish. Calcium levels were increased by addition of 10 mm CaCl2 to achieve final concentrations of 62 μm, 112 μm, 212 μm, 500 μm and 1 mm. At each step of rising [Ca2+], cells were incubated for 4 min at room temperature (20-22°C). Cells were transferred to 15 ml tubes and allowed to sediment under gravity for 10 min. The supernatant was collected, centrifuged at 400 g for 2 min. Cells collected by gravitational and centrifugational sedimentation were pooled and resuspended in myocyte culture medium (MCM) (minimum essential medium (MEM), with Hank's Balanced Salt solution (Invitrogen, Burlington, Canada), supplemented with 10 mm BMD, 1% penicillin–streptomycin (Invitrogen), 0.1 mg ml−1 bovine serum albumin and 2 mml-glutamine (Invitrogen), containing 5% fetal bovine serum. Myocytes were plated at a density of (0.5–1) × 104 cells cm−2 onto 35 mm culture dishes, pre-coated for 2 h with 10 μg ml−1 mouse laminin (Invitrogen) in PBS containing 1% penicillin–streptomycin (Invitrogen). Cells were cultured at 37°C in a 5% CO2 incubator. Medium was replaced 1 h later with MCM containing 10 μg ml−1 insulin (Sigma), 5 ng ml−1 selinite (Sigma) and 5.5 μg ml−1 transferrin (Sigma). The entire culture procedure was performed in a laminar flow hood.
Measurement of hypertrophic growth in cultured cardiomyocytes
Adult or neonatal cardiomyocyte cultures prepared as described above were maintained in the appropriate culture medium, supplemented with solvent carrier (control), 10 μm phenylephrine (PE; Sigma) or 1 μm angiotensin II (AngII; Sigma). Cultures were treated with solvent carrier (control), 6-ethoxyzolamide (ETZ, 10 or 100 μm; Sigma) or methazolamide (MTZ, 100 μm; Sigma). ETZ or MTZ were added at the time of PE or AngII addition (early protocol) or 24 h later (late protocol). Hypertrophy was assayed by measurement of the cell surface area of cells. Studies of adult cardiomyocytes were performed blind. Cell surface area was measured before and after intervention with drugs. To measure cell surface area, cells were selected on the basis of morphology; in the case of adult cardiomyocytes, characteristic rod-shaped cells were selected. Images of cultured cardiomyocytes were collected with a QICAM fast cooled 12-bit colour camera (QImaging Corporation). Images were analysed with Image-Pro Plus software (Media Cybernetics) to measure cell surface area. In each group surface areas were measured for 49–188 cells. Cell surface area (% relative to control) = Surface area (after treatment)/Surface area (before treatment) × 100.
Anion exchange activity assay
HEK293 cells, grown on 7.5 × 11 mm glass poly l-lysine-coated coverslips (Erie Scientific Co, USA) in 60 mm dishes, were cotransfected with the human SLC26A6 and α1a-adrenergic receptor cDNAs, or cotransfected with the rat AE3fl and α1a-adrenergic receptor cDNAs, or transfected with the pcDNA3.1 cDNA (Invitrogen; empty vector). Anion exchange assays were performed as described (Alvarez et al. 2004). All solutions contained 1 mm amiloride (Sigma) to block Na+–H+ exchanger activity. The initial rates of change of pHi determined during the removal and re-addition of Cl− were then fitted to a straight line by linear least squares fit, using Kaleidagraph software (Synergy Software, Reading, PA, USA). All transport data have been corrected for background activity of HEK293 cells transfected with pcDNA3 vector alone. In some assays the α1a-adrenergic agonist PE (10 μm) or the carbonic anhydrase inhibitor ETZ (0.5–100 μm) was perfused through the cuvette for 10 min after a standard assay. Residual transport activity was then monitored in a standard assay with either PE or ETZ present in all buffers. Curves for transport inhibition by PE or ETZ were fitted with Kaleidagraph software.
NHE1 activity assay
The pHi recovery activity of either AP1 cells or AP1 cells stably transfected with NHE1-HA was measured during the recovery from transient intracellular acidification. Coverslips were mounted in a cuvette and perfused with Na+-free bicarbonate buffer solution (128.3 mm choline chloride, 4.5 mm KCl, 1.35 mm CaCl2, 20.23 mm choline bicarbonate, 1.05 MgSO4, 11 mm glucose; pH 7.40). NaCl and NaHCO3− were replaced by equimolar amounts of choline chloride and choline bicarbonate, respectively, in the normal bicarbonate buffer solution. Both solutions were equilibrated with 5% CO2–air. HCO3− transport activity of NHE1 (JH) was measured during the recovery from transient intracellular acidification. Cells were acid loaded using the NH4Cl prepulse method (Murtazina et al. 2001). After peak acidosis was reached, cells were perfused with Na+-free bicarbonate buffer for 2–3 min (plateau phase). The Na+-free bicarbonate buffer was then quickly replaced by normal bicarbonate buffer solution, and the cells perfused for a further 5–7 min (recovery phase). The initial rate of pHi recovery from an acid load was calculated by fitting a linear regression of the first 1 min of the pHi recovery (recovery phase) after maximum acidosis. In all cases the transport activity of AP1 cells was subtracted from the total rate, to ensure that these rates consisted only of NHE1 transport activity. For dose–response curves of NHE1 to ETZ, after a first NH4Cl pulse (control), cells were incubated with ETZ (0.5–100 mm) for 10 min and subjected to a second NH4Cl pulse.
Measurement of pHi in isolated adult mouse cardiac myocytes
Intracellular pH was measured using the pH-sensitive probe BCECF. Cardiomyocytes were loaded with the acetoxymethyl ester form of BCECF, 2 μm BCECF-AM, at 37°C for 30 min. Cells attached to laminin-coated glass coverslips were placed in an Attofluor cell chamber (Invitrogen) and then transferred to the stage of an inverted Leica DMIRB microscope. Cardiomyocytes were continuously perfused at 3.5 ml min−1 with HCO3− Ringer buffer solution containing 128.3 mm NaCl, 4.7 mm KCl, 1.35 mm CaCl2, 20.23 mm NaHCO3, 1.05 mm MgSO4 and 11 mm glucose; pH 7.40. Cardiomyocytes were treated with 10 μm PE, or 100 μm ETZ or a combination of 10 μm PE and 100 μm ETZ (10 min, 37°C). Solutions were bubbled with 5% CO2–balanced air. Experiments were conducted in the absence or presence of the β-adrenoceptor antagonist atenolol (10 μm, Sigma). pHi of individual cardiomyocytes was recorded by photometry at excitation wavelengths 502.5 nm and 440 nm with a Photon Technologies International (PTI, Lawrenceville, NJ, USA) Deltascan monochromator. Emission wavelength 528 nm was selected using a dichroic mirror and narrow range filter (Chroma Technology Corp., Rockingham, VT, USA) and was measured with a PTI D104 photometer.
Calcium transient recordings from neonatal rat cardiac myocytes
After 24 h of incubation under the appropriate test condition, neonatal rat cardiac myocytes were loaded for 30 min at room temperature and for 30 min at 37°C with the calcium-sensitive fluorescent probe Calcium Green-1AM (4 μm). After loading, the myocytes were rinsed and placed on slides for observation at ×200 magnification with an inverted microscope (Olympus, CK40), while being superfused with the appropriate test solution. Myocytes contracted spontaneously so no electrical pacing was applied. Data collected with a photomultiplier detection system (PTI) were analysed with Clampex 8.1 software (Axon Instruments, Union City, CA, USA). Calcium Green-1AM was excited at 480 nm, and the emitted intensity at 520 nm was recorded (Baczko et al. 2005). Experiments were conducted at 30 ± 1°C.
Statistics
Data are expressed as mean ± s.e.m. Statistical analysis was performed by Student's paired t test, or one-way ANOVA, as appropriate. Probability of null hypothesis < 0.05 was considered significant.
Results
Effect of 6-ethoxyzolamide (ETZ) on cardiomyocyte hypertrophy
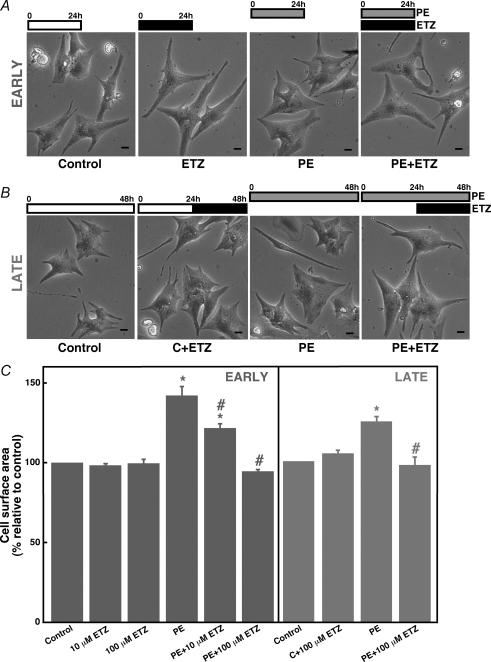
Since CAII binds NHE1 to activate NHE1-mediated H+ efflux rate (Li et al. 2002), we reasoned that inhibition of CAII could indirectly inhibit NHE1 and thus reduce hypertrophy in cardiomyocytes. To test this idea, hypertrophy was induced in cultured rat neonatal cardiomyocytes using the established hypertrophic adrenergic agonist phenylephrine (PE) (Omura et al. 2002). Cardiomyocytes were cultured in the presence or absence of the carbonic anhydrase inhibitor ETZ. ETZ was added concurrently with PE in an early intervention protocol, or 24 h after the onset of cell culture, in a late intervention protocol (Fig. 1). Increase of cell surface area, a key marker of hypertrophic cardiomyocyte growth, following treatment with PE was evident within 24 h of addition of PE (Fig. 1A). ETZ added concurrently with PE prevented the increase in cell surface area. Figure 1C quantifies the increase in average cell surface area following incubation with phenylephrine. ETZ alone (10 or 100 μm) did not affect cardiomyocyte cell size. In contrast, ETZ inhibited cardiomyocyte hypertrophy in a dose-dependent manner; 10 μm ETZ was sufficient to significantly reduce cardiomyocyte size and 100 μm ETZ fully blocked PE-induced hypertrophic growth.
Figure 1. Effect of phenylephrine and the membrane-permeant carbonic anhydrase inhibitor 6-ethoxyzolamide (ETZ) on cell surface area of cultured neonatal rat ventricular myocytes.
A and B, micrograph images of cultured rat neonatal myocytes treated with 100 μm ETZ, 10 μm phenylephrine (PE), or untreated (control). Scale bars are 10 μm. A, cells were treated with ETZ and/or PE at the onset of the experiment in an early intervention protocol (Early). Cells were imaged after 24 h treatment. B, in a late intervention protocol (Late) cells were grown under either control or 10 μm PE-treated conditions for a total of 48 h before the images were collected. ETZ (100 μm) was added to the culture medium at the 24 h point of the experiment in the indicated samples. C, cells were treated for 24 h under the ‘Early’ intervention (blue columns) or ‘Late’ intervention protocol (red columns). Cells were treated with 10 or 100 μm ETZ. Values are mean ±s.e.m., n = 6–7. *P < 0.05, compared with control group. #P < 0.05, compared with PE-treated group.
To examine whether ETZ could revert established hypertrophic growth, cardiomyocytes incubated with PE for 24 h were treated for an additional 24 h with or without ETZ (Fig. 1B and C). The increase of cell size induced by PE was slightly less after 48 h (Fig. 1C, late) than 24 h (early). Late addition of ETZ was, however, able to completely reverse the increase of cardiomyocyte cell size induced by PE.
We also tested the effect of the related sulphonamide carbonic anhydrase inhibitor acetazolamide (ACTZ) on cardiomyocyte hypertrophy. Surprisingly, 100 μm ACTZ did not affect cardiomyocyte hypertrophy induced by phenylephrine (data not shown).
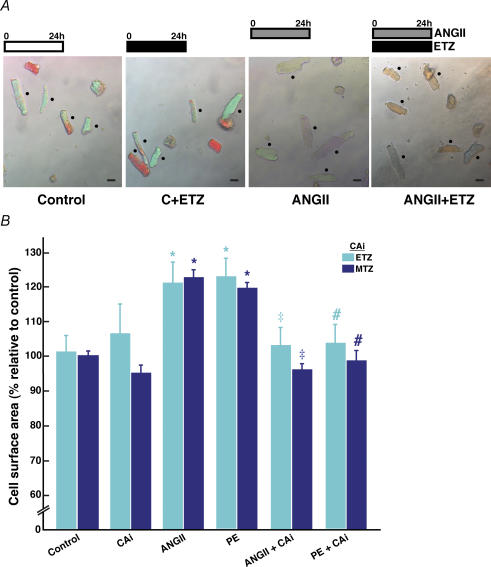
Effect of carbonic anhydrase inhibition on adult cardiomyocyte hypertrophy
We examined the effect of CA inhibitors on hypertrophied adult cardiomyocytes, since adults are more prone to the pathology of hypertrophy than neonates (Fig. 2A and B). Cultured adult cardiomyocytes significantly increased in size when treated with both PE and the hypertrophic hormone angiotensin II (AngII), but ETZ fully corrected this hypertrophy. Similarly, the membrane-permeant CA inhibitor methazolamide (MTZ, 100 μm) prevented the AngII-induced and PE-induced cardiomyocyte hypertrophy in cultured adult mouse cardiomyocytes (Fig. 2B). Cell surface area was not significantly affected by MTZ in cardiomyocytes incubated for 24 h with MTZ alone.
Figure 2. Effect of hypertrophic factors and carbonic anhydrase inhibitors on adult cardiomyocyte hypertrophy.
A, micrograph images of cultured adult mouse myocytes treated with 100 μm ETZ, 1 μm angiotensin II (AngII), or untreated (control, C) (scale bar, 30 μm). Cells were treated with ETZ and/or AngII for 24 h, and the images collected after 24 h treatment. Examples of cells used for cell surface area measurement are indicated by a black dot. B, cells were treated for 24 h with 100 μm ETZ or 100 μm MTZ, or for 24 h with either 1 μm AngII or 10 μm PE, or treated for 24 h with either 1 μm AngII or 10 μm PE in the presence of 100 μm ETZ or in the presence of 100 μm MTZ. Light blue bars and dark blue bars represent ETZ and MTZ treatment groups, respectively. Values are mean ±s.e.m., n = 3–4 trials (total cells analysed in each group, 49–188). *P < 0.05, compared with control group. #P < 0.05, compared with PE-treated group. ‡P < 0.05, compared with AngII-treated group.
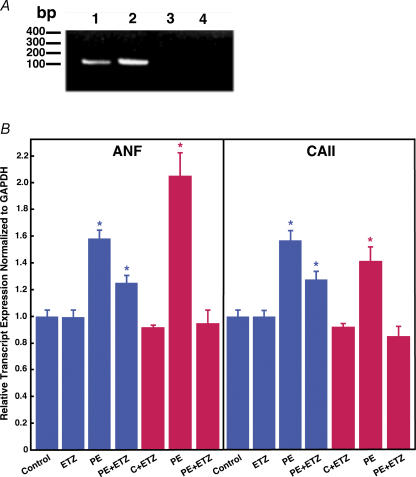
Altered gene expression during cardiomyocyte hypertrophy
To understand the molecular basis for the inhibition of hypertrophy by ETZ, we examined gene expression by real-time reverse transcription PCR. Atrial natriuretic factor (ANF) is a fetal gene whose expression is induced during hypertrophic cardiomyocyte growth. Both ANF and CAII messages could be detected by RT-PCR using rat neonatal cardiomyocyte mRNA as a template (Fig. 3A). Cardiomyocytes were cultured with PE and/or ETZ, with ETZ added at the onset of cultures (Blue) or after 24 h of culture (Red). Marked increases of ANF and CAII expression were evident in cultures grown with PE for either 24 or 48 h (Fig. 3B). The increase of ANF message is a second indication that PE treatment caused hypertrophic growth of the cardiomyocytes. ANF and CAII mRNA levels were reduced, but not normalized, when cardiomyocytes were treated with 100 μm ETZ along with PE. Surprisingly, the effect of ETZ was greater when added 24 h after the onset of PE incubation than when added at the same time as PE (Fig. 3B). ETZ reduced the expression of both CAII and ANF to control levels when added to the cultures 24 h after PE. ETZ alone, however, did not affect ANF or CAII expression, suggesting that the compound is not a direct modulator of the expression of these genes.
Figure 3. Expression of atrial natriuretic factor (ANF) and carbonic anhydrase II (CAII) transcripts in neonatal rat ventricular cardiomyocytes.
Rat neonatal cardiomyocytes were treated for 24 h under the ‘Early’ intervention (blue bars) or for 48 h in the ‘Late’ intervention protocol (red bars) with ETZ (100 μm), sham (control, C) or PE (10 μm), as indicated. A, RT-PCR analysis of ANF and CAII mRNA expression in control cardiomyocytes. Amplicons were analysed on 1% agarose–EtBr gels. PCR primers amplified ANF (1, 3) and CAII (2, 4). Template was either reverse transcribed control myocyte mRNA (1, 2) or mRNA prepared without reverse transcriptase (3, 4). B, ANF and CAII expression were quantified by real-time quantitative RT-PCR. Data were corrected for variation using GAPDH expression and results expressed as transcript expression normalized to GAPDH. Values are mean ±s.e.m., n = 4 for each treatment group. *P < 0.05, compared with control group.
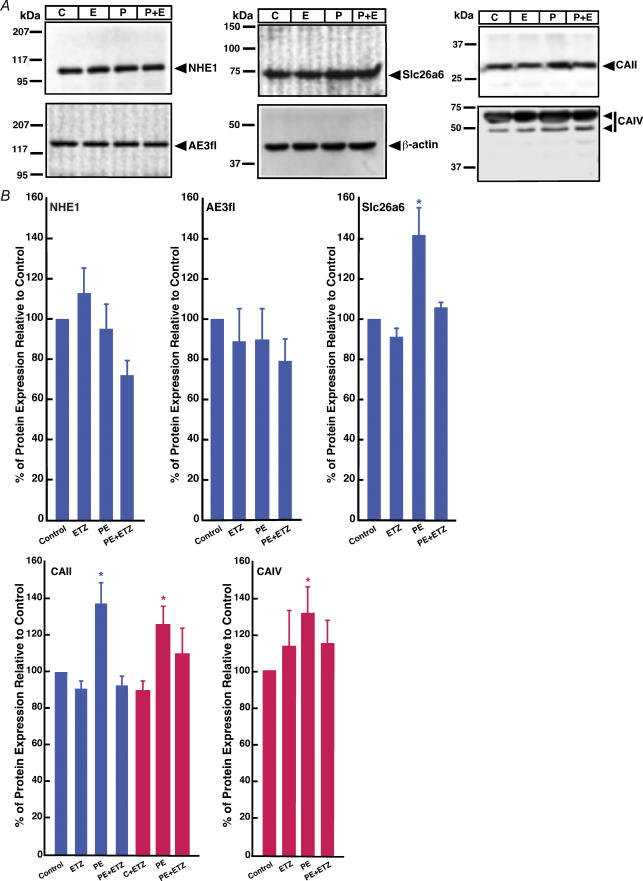
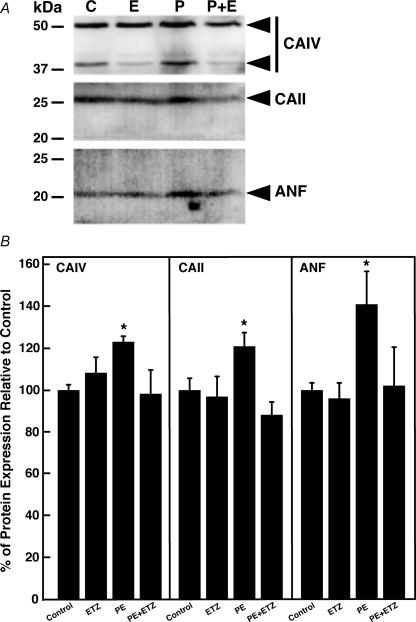
Immunoblots revealed the expression levels of CAs and transporters, which might be altered upon PE/ETZ treatment. Expression was quantified by densitometry of the immunoblots and values were corrected for loading differences by normalization to β-actin levels. Each of the proteins could be clearly identified on immunoblots with a migration position consistent with the known molecular weight of the protein (Fig. 4A). NHE1 and AE3fl protein levels did not change significantly upon treatment with PE or ETZ. In contrast, CAII expression was unaffected by ETZ (100 μm), but expression was significantly increased upon treatment with 10 μm PE (Fig. 4B, blue). CAIV expression was markedly increased in response to PE, but this response was blunted by ETZ (red). Similarly, PE induced expression of Slc26a6 protein. Importantly, expression of both CAII and Slc26a6 proteins (by convention Slc26a6 refers to non-human protein; SLC26A6 is human) returned to control levels in cells co-treated with PE and ETZ.
Figure 4. Effect of phenylephrine and 6-ethoxyzolamide on transporter and carbonic anhydrase protein levels, in neonate cardiomyocytes.
A, cellular lysates were prepared from neonatal rat ventricular myocytes, which were untreated controls (C), treated with 10 μm ETZ (E), treated with 10 μm phenylephrine (P, PE) or treated with PE and ETZ (P + E). Immunoblots of the myocardial lysates were probed with antibody against NHE1, AE3, Slc26a6, CAII, CAIV and β-actin antibody, as indicated. B, expression levels of transporters and carbonic anhydrases, quantified by densitometry, were normalized to β-actin expression. Cells were treated for 24 h under the ‘Early’ intervention (blue bars) or for 48 h in the ‘Late’ intervention protocol (red bars) with ETZ (100 μm), sham (control) or PE (10 μm). Values are mean ±s.e.m., n = 4–7. *P < 0.05, compared to control.
The effects seen in neonatal cardiomyocytes were mirrored in adult cardiomyocytes treated with PE. Immunoblots showed that ETZ alone had no significant effect on levels of protein expression for CAIV, CAII, or ANF. In contrast, CAIV and CAII levels increased upon treatment with PE (123 ± 2% of control, and 121 ± 6% of control, respectively). In addition, protein levels of the hypertrophic marker, ANF, rose upon treatment with PE (141 ± 14% of control). The increase in CAIV, CAII and ANF protein levels was fully blocked by treatment with ETZ (Fig. 5A and B).
Figure 5. Effect of phenylephrine and 6-ethoxyzolamide on carbonic anhydrase and ANF protein levels in adult cardiomyocytes.
A, cellular lysates were prepared from adult mouse ventricular myocytes, which were untreated (controls, C), treated with 100 μm 6-ethoxyzolamide (E, ETZ), treated with 10 μm phenylephrine (P, PE) or treated with phenylephrine and 6-ethoxyzolamide (P + E). Immunoblots of the cardiomyocyte lysates were probed with antibody against CAII, CAIV and ANF, as indicated. C, expression levels of carbonic anhydrases and ANF were quantified by densitometry. Cells were treated for 24 h with ETZ (100 μm), sham (control, C) or PE (10 μm). Values are mean ±s.e.m., n = 4. *P < 0.05, compared to control.
Isoform-specific activation of Cl−–HCO3− exchange by phenylephrine
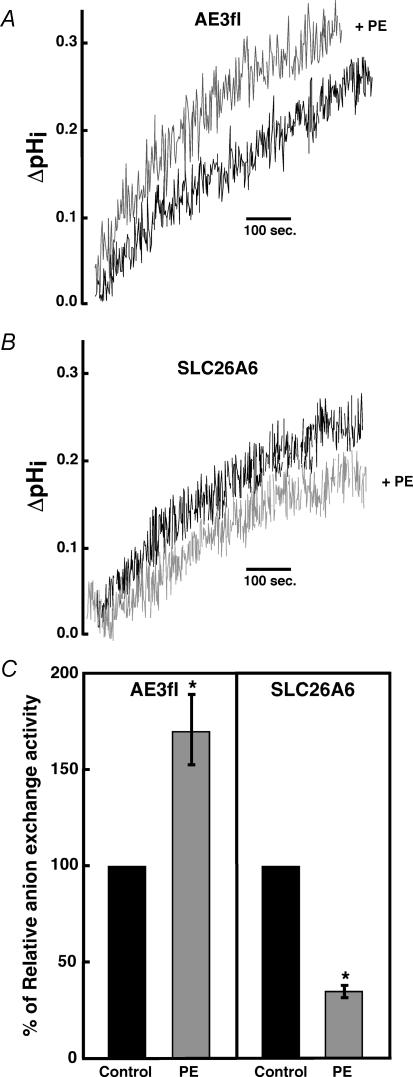
We examined whether AE3fl or SLC26A6 responded to stimulation of the α1a-adrenergic signalling pathway. HEK293 cells were transfected with α1a-adrenergic receptor cDNA along with AE3fl or SLC26A6 cDNA and the effect of phenylephrine treatment was assessed. Cl−–HCO3− exchange assays were performed on SLC26A6 and AE3fl-transfected HEK293 cells before and after 10 min incubation with 10 μm PE. Immunoblots revealed that untransfected HEK293 cells do not express functionally measurable amounts of Cl−–HCO3− exchanger protein (not shown). The rate of pHi increase in these cells following removal of extracellular Cl− from the medium (Fig. 6A and B) is thus attributable to the influx of HCO3− coupled to Cl− efflux, resulting from the transporter transfected into the cells. Strikingly, 10 μm phenylephrine activated AE3fl transport activity by 70 ± 9%, whereas SLC26A6 activity was inhibited by 65 ± 3% (Fig. 6C). Since AE3fl is activated by phenylephrine, it may be the Cl−–HCO3− exchanger that works counter to NHE1 in response to α1a-adrenergic stimulation.
Figure 6. Effect of phenylephrine treatment on Cl−–HCO3− exchange activity.
HEK293 cells were cotransfected with α1a-adrenergic receptor, and with AE3fl (A) or SLC26A6 (B) cDNAs. Forty-eight hours after transfection, Cl−–HCO3− exchange assays were performed on these cells before (dark trace) and 10 min after (light trace) exposure to PE (10 μm). Initial rates of change of pHi during the first 100 s were estimated from the slope of the line fitted by the least squares method before and after treatment. Traces were superimposed to compare slopes. C, summary of the effect of PE (10 μm, 10 min) on transport activity of HEK293 cells co-expressing α1a-adrenergic receptor with AE3fl (n = 3), or SLC26A6 (n = 4). *P < 0.05, compared with control group.
Effects of CAII inhibition on AE and NHE transport activity
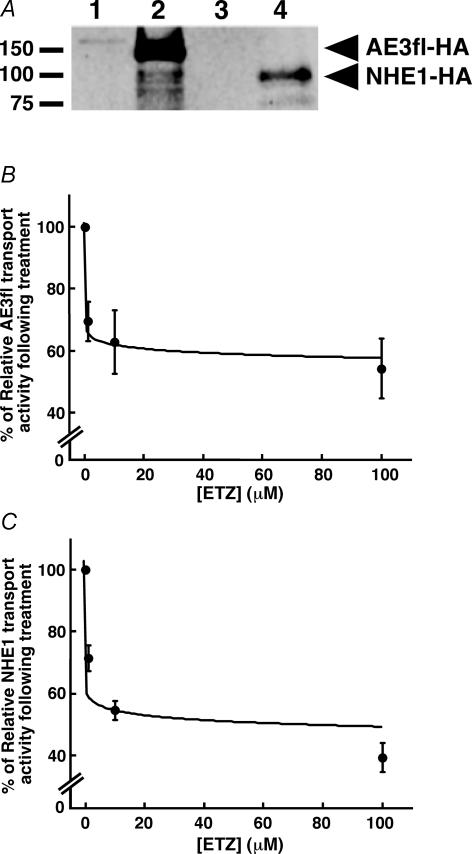
These data suggested that the profound reduction in ETZ-induced cardiomyocyte hypertrophy resulted from a reduction in the combined action of NHE1 and Cl−–HCO3− exchange. We thus examined the response of NHE1 and AE3fl transport activity to treatment with ETZ. ETZ inhibits CA enzymatic activity without direct effect on anion exchange (Cousin & Motais, 1976). Transport activity was examined in HEK293 cells transfected with AE3fl cDNA and in AP1 cells stably transfected with NHE1. Haemagglutinin-epitope tagged NHE1 and AE3fl were detected at the appropriate migration position, only in cells transfected with their respective cDNAs (Fig. 7A). NHE1 expression was not detectable in AP1 cells, which do not express NHE1, but immunoreactivity was found in samples from AP1/pYN4+ cells, which stably express NHE1-HA (Fig. 7A). Anion exchange and NHE1 transport rates were determined by linear regression of the initial slopes of curves produced as pHi changes. Transport activity was measured, and then cells were incubated for 10 min with varied concentrations of ETZ (1–100 μm). ETZ will not covalently react with CA, so all buffers used subsequent to the incubation also contained the appropriate concentration of ETZ. Importantly both HEK293 cells and AP1 cells express sufficient levels of CAII endogenously to maximally activate Cl−–HCO3− exchange and NHE activity (Sterling et al. 2001; Li et al. 2002).
Figure 7. Effect of carbonic anhydrase inhibition on AE3fl and NHE1 transport activity.
A, lysates prepared from HEK293 cells transfected with pcDNA3 (lane 1, empty vector) or AE3fl-HA cDNA (lane 2), or AP1 cells (lane 3), or AP1 cell line stably transfected with the pYN4+ plasmid containing HA-tagged NHE1 (lane 4) were immunoblotted and probed for the expression of AE3fl-HA or NHE1-HA. B and C, dose–response curves of the ETZ effect on AE3fl and NHE1 transport activity. HEK293 cells transfected with AE3fl-HA were subjected to anion exchange assays (B), and AP1 cells expressing NHE1-HA, subjected to NHE1 activity assay (C), in the absence and presence of varied concentrations of ETZ.
Figure 7B is a dose–response curve for the effect of ETZ on bicarbonate influx. The effect of ETZ on AE3fl-HA activity was measured at similar pHi values: 7.21 ± 0.08, 7.24 ± 0.14, 7.19 ± 0.14 and 7.18 ± 0.11 for ETZ concentrations of 0, 1, 10 and 100 μm, respectively. The apparent IC50 for the effect of ETZ on AE3fl-mediated bicarbonate transport was 1.2 ± 0.3 μm. The in vitro IC50 values of human CAII for sulphonamides such as ETZ, dorzolamide and acetazolamide, range between 8 and 90 nm, respectively (Landolfi et al. 1997; Garaj et al. 2004).
We examined NHE1 sensitivity to ETZ in NHE1-expressing AP1/pYN4+ cells. Cells were exposed to 30 mm NH4Cl pulses, and the recovery after acid load, in AP1 cells lacking NHE1, showed little pHi recovery (not shown). AP1/pYN4+ cells, however, had much greater pHi recovery rate, attributable to NHE1 (not shown). The effect of ETZ was examined at similar acidic pHi values: 6.81 ± 0.20, 6.88 ± 0.17, 6.78 ± 0.16 and 6.73 ± 0.17, for control, 1, 10 and 100 μm ETZ, respectively (Fig. 7C). The apparent IC50 for the NHE1 transport activity inhibition was 2.7 ± 0.3 μm. Differences in the IC50 of the inhibitory effect of ETZ on AE3fl-HA and NHE1 transport activity may reflect differences in the substrate availability (HCO3− and H+) at the transport site of the transporters.
Effects of CA inhibition on pHi in isolated adult mouse cardiomyocytes
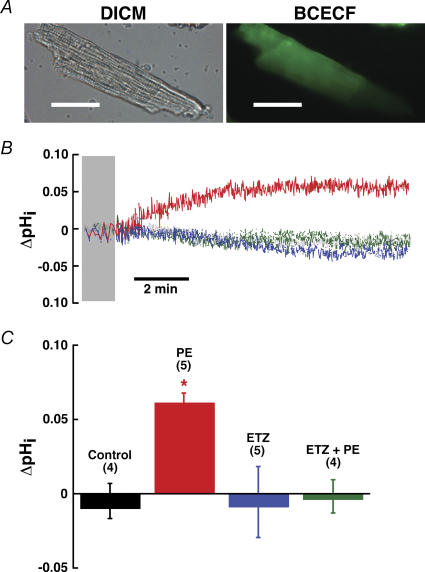
What effect does PE have on cardiomyocyte pH? Steady-state pHi was studied in single adult cardiac ventricular myocytes loaded with the pH-sensitive dye BCECF-AM (Fig. 8A). Cells were stabilized for ∼5 min without evident ‘run-down’. After stabilization, cells were stimulated with the α1a-adrenergic agonist phenylephrine (PE, 10 μm) for a period of 10 min. Experiments were performed in the presence of the β-adrenoreceptor antagonist atenolol (10 μm) to be sure that the observed effects were mediated through α1a-adrenergic receptors (Terzic et al. 1992). Experiments in the absence of atenolol gave the same results (not shown). For example, stimulation with PE without atenolol produced an alkalinization of 0.055 pH units over untreated control, while cells treated with PE and atenolol elicited an alkalinization (Fig. 8B), reaching a maximal rise of 0.061 ± 0.006 pH units above the baseline in 10 min. No change of pHi was observed in cells perfused for 10 min with Ringer buffer solution alone (control, −0.010 ± 0.007 pH units) or with ETZ (100 μm) alone (pHi change, −0.009 ± 0.026 pH units). Interestingly, ETZ prevented the PE-induced intracellular alkalinization of cardiomyocytes (pH change, −0.006 ± 0.018 pH units). Changes of pHi were studied at similar initial pHi for the four groups: 7.21 ± 0.08, 7.17 ± 0.05, 7.25 ± 0.04 and 7.26 ± 0.04, for control, PE, ETZ, and PE + ETZ, respectively. We conclude that PE causes a rapid rise in cardiomyocyte steady-state pHi; this rise is blocked upon co-treatment with ETZ and PE.
Figure 8. Effect of carbonic anhydrase inhibition on basal pHi in single mouse cardiomyocytes stimulated with the α1-adrenergic agonist phenylephrine.
A, example of a single mouse myocyte used for pHi measurement experiments. Cell was imaged with differential interference contrast microscopy (DICM). Cardiac myocytes were loaded with BCECF pH-sensitive fluorescent dye to measure pHi. Scale bar, 30 μm. B, changes in steady-state pHi (ΔpHi) observed in myocytes treated with the α1-adrenergic agonist, phenylephrine (PE, 10 μm; red trace), treated with the CA inhibitor, 6-ethoxyzolamide (ETZ, 100 μm; blue trace), or a combination of phenylephrine and 6-ethoxyzolamide (ETZ + PE, green trace), for 10 min in Ringer buffer solution containing 25 mm NaHCO3−. For control experiments, cardiomyocytes were incubated for 10 min in Ringer solution containing 25 mm NaHCO3− (black trace). Gray shading highlights period before treatment. C, summary of cardiomyocyte pH unit changes (ΔpHi) after 10 min of no treatment (control), or 10 min with 10 μm PE, 100 μm ETZ, or 10 μm PE + 100 μm ETZ. *P value < 0.05, versus control group. Colours of columns correspond to colour coding in B. Number of trials is shown in parentheses.
Calcium transient recordings
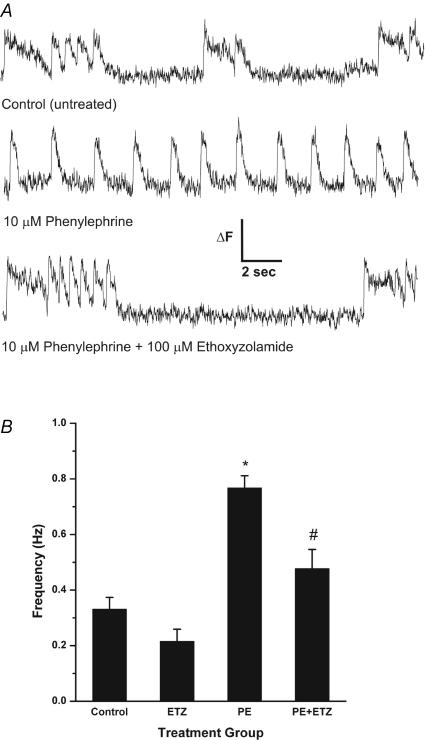
To determine whether the hypertrophic effect of PE was associated with altered Ca2+ handling, we examined Ca2+ transients and spontaneous contractile activity of cultured neonatal cardiomyocytes and determined the effect of ETZ. Untreated neonatal cardiomyocytes exhibited slow non-rhythmic spontaneous calcium transients (SCT) with accompanying contractions with a frequency of 0.33 ± 0.04 Hz (Fig. 9). ETZ treatment for 24 h caused no significant change in the occurrence of SCT when compared to control (not shown, 0.22 ± 0.04 Hz). In contrast, 24 h exposure to PE resulted in a significant increase in SCT frequency (0.77 ± 0.04 Hz). Co-exposure of neonatal cardiac myocytes to PE + ETZ significantly decreased SCT frequency (0.48 ± 0.07 Hz) when compared to PE alone (Fig. 9). PE increased SCT and associated contractions, which were both prevented if cardiomyocytes were treated with ETZ. We conclude therefore that ETZ corrects the alterations of Ca2+ handling that occur in cardiomyocytes during development of PE-induced hypertrophy.
Figure 9. Cytosolic Ca2+ and contractile activity in rat neonatal cardiomyocytes.
A, representative traces of calcium transient recordings of rat neonatal cardiac myocytes after 24 h treatments with either 10 μm phenylephrine alone or 10 μm phenylephrine with 100 μm ethoxyzolamide. ΔF represents change in 525 nm fluorescence signal, which is a measure of cytosolic [Ca2+]. B, frequency of rat neonatal cardiac myocyte contractions after 24 h treatment with either 10 μm phenylephrine (PE), 100 μm ethoxyzolamide (ETZ) or 10 μm phenylephrine with 100 μm ethoxyzolamide (PE + ETZ). *P value < 0.05, control group versus PE group. #P value < 0.05, PE group versus PE + ETZ group. n = 8–9 trials for all treatment groups.
Discussion
Hypertrophic heart growth is central to the downward spiral of heart failure. Activation of NHE1 by hormonal and other pathways induces hypertrophy. Conversely, treatment with NHE1-specific inhibitors prevents hypertrophy and blocks heart failure in genetic models, or animal models of infarction (Yoshida & Karmazyn, 2000; Karmazyn, 2001; Karmazyn et al. 2001; Engelhardt et al. 2002; Chen et al. 2004). The present report focused on the availability of substrate for NHE1 activity as a target to inhibit NHE1. Sustained NHE1 activity requires an acid load, since NHE1 self-inhibits at alkaline cytosolic pH (Slepkov & Fliegel, 2002). The primary acidifying transport proteins of the myocardium are Cl−–HCO3− exchangers, which acidify by efflux of HCO3− (Sterling & Casey, 2002). Cl−–HCO3− exchangers and NHE1 bind the cytosolic enzyme CAII, which produces the HCO3− and H+ substrates for transport by Cl−–HCO3− exchangers (Sterling et al. 2001) and NHE1 (Li et al. 2002). We thus reasoned that inhibition of CAII could limit NHE1 activity through limiting substrate availability. Inhibition of CAII with either ETZ or MTZ prevented the cardiomyocyte hypertrophy induced by adrenergic stimulation. Adrenergic stimulation activated the AE3fl isoform of Cl−–HCO3− exchanger, suggesting a role in hypertrophy. Expression of the hypertrophic marker ANF, which was induced by adrenergic stimulation, was nearly normalized upon ETZ treatment. Similarly, CAII expression substantially increased upon adrenergic stimulation, but was corrected upon ETZ treatment. We conclude that carbonic anhydrase is a novel candidate for anti-hypertrophic therapy. As the carbonic anhydrase inhibitors acetazolamide (Diamox), methazolamide (Neptazane) and ETZ (Cardrase) have been used as diuretics and anti-glaucoma drugs since the 1950s (Friedberg et al. 1953; Moyer & Ford, 1958; Becker, 1960), anti-CAII therapy might be readily adopted in treatment of CH.
Since both MTZ and ETZ prevented hypertrophic cardiomyocyte growth it is likely that they work by targeting the same cellular process; both compounds are CA inhibitors, so it is likely that the effect is through CA inhibition. Both PE and AngII ultimately act through downstream activation of PKC. Neonatal cardiomyocytes increased in size by about 37% following 24 h treatment with PE. This response is smaller than that seen with larger PE doses (i.e. 90% increase in cardiomyocyte size upon treatment with 100 μm PE (Jeong et al. 2006), but we used the lower dose to mimic a pathologically significant response. We found that ETZ prevented hypertrophy in both neonatal and adult cardiomyocytes. This is important since the two developmental stages of the cardiomyocyte express a different complement of proteins. Moreover, hypertrophy is more significant in a pathological setting for the adult than for the neonatal myocardium. The ability to revert established hypertrophy opens the door to intervention in established hypertrophy through treatment with CA inhibitors.
Molecular analysis provided insight into the hypertrophic processes. The ability of ETZ to prevent and revert the rise of ANF mRNA and protein following PE/AngII treatment provides evidence that ETZ directly targets hypertrophic mechanisms. Also, both CAII and CAIV expression increased under hypertrophic conditions, but ETZ reversed the effect. This suggests that there is a pathological feed-forward mechanism where active CA enzymes provide substrate for NHE1 and AE3fl. Similar to our findings, DNA microarray data from hearts hypertrophic secondary to either angiotensinogen over-expression (mice), or hypertrophic cardiomyopathy (human) showed that CAII and CAIV are significantly over-expressed (Domenighetti et al. 2004; http://cardiogenomics.med.harvard.edu/home (2005)). The activity of AE3fl and NHE1 promotes hypertrophy and the hypertrophic programme increases expression of the CA enzymes. ETZ intervenes in the feed-forward cascade. Expression of Slc26a6 also responded to PE by a significant increase, which was blocked by ETZ. The increased expression of Slc26a6 is important because Slc26a6 was recently identified as the most abundant Cl−–HCO3− exchanger of mouse myocardium (Alvarez et al. 2004). The PE-induced increase in Slc26a6 expression (Fig. 4) is nearly identical in magnitude to the decrease in turnover induced by PE (Fig. 6B and C). The overall effect is that PE will have no net effect on the total bicarbonate flux carried by Slc26a6.
The profile of CA inhibitors that were effective in inhibiting hypertrophy suggests the CA isoform that is responsible. EC50 values for inhibition of AE3fl and NHE1 by ETZ were, respectively, 1.2 ± 0.3 μm and 2.7 ± 0.3 μm. Interestingly, the ETZ concentration estimated to give half-maximal effect in reducing cardiomyocyte hypertrophy was 18 μm, which should be sufficient to achieve near-maximal inhibition of both NHE1 and AE3fl (Fig. 4). Given that pharmacological doses of ETZ are 125–500 mg day−1 (Moyer & Ford, 1958), it is likely that circulating ETZ levels in ETZ-treated humans would be sufficient to reduce cardiac hypertrophy (CH). The concentration of ETZ required to inhibit AE3fl, NHE1 and CH is substantially above the in vitro Ki for CAII inhibition (0.3 nm) (Siffert & Gros, 1984). This may reflect the limited permeability of ETZ to the cells and the fact that CAII, with a catalytic turnover rate of 106 s−1, must be inhibited to a substantial degree before effects on NHE1 transport rate would manifest. ETZ is commonly used at concentrations of 100 μm or greater to inhibit carbonic anhydrases (Geers & Gros, 1995; Wetzel et al. 2002; Lankat-Buttgereit et al. 2004). We found that relatively membrane-impermeant ACTZ did not inhibit hypertrophy, while more permeant ETZ and MTZ did prevent and revert cardiomyocyte hypertrophy. Similarly, in rat skeletal muscle ETZ, but not acetazolamide, slowed Ca2+ transients, which the authors suggested might result from reduced H+ availability for sarcoplasmic reticulum H+ counter-transport by the Ca2+-ATPase (Wetzel et al. 2002). Taken together, it is unlikely that cardiac CA isoforms with catalytic sites outside the cell (CAIV, CAXII, CAXIV and CAIX, Purkerson & Schwartz, 2005; Scheibe et al. 2006) mediate the ETZ/MTZ effect. Rather a cytosolic isoform is most likely responsible. CAII is a near-ubiquitous cytosolic isoform, which associates with bicarbonate transporters and NHE1 at the plasma membrane surface (Sterling et al. 2001; Li et al. 2002), making it a likely cytosolic target of ETZ and MTZ.
Experiments measuring steady-state pHi in adult cardiomyocytes treated with PE suggest that CA inhibitors act on hypertrophy by targeting NHE1 substrate availability. PE caused a rapid rise in cardiomyocyte pHi, which rapidly reached a new steady-state value about 0.06 pH units above resting values. This observation is fully consistent with the earlier finding that PE induced a rise of 0.1 pH units in HCO3−-free Hepes medium and 0.07 pH units in HCO3−-containing medium (Terzic et al. 1992). The observation that a new steady state was reached indicates either that the alkalinizing pathway inactivated after reaching the new steady state, or a parallel acidifying pathway begins to balance the alkalinization to maintain the new steady state. PE-induced cardiomyocyte alkalinization was previously found to result from NHE1 activation, since the amiloride derivative EIPA suppressed the pHi rise (Terzic et al. 1992; Vila-Petroff et al. 1996). The ability of ETZ to suppress PE-induced pHi rise thus strongly suggests that ETZ effectively targets NHE1 through limiting the supply of the H+ produced by CA activity. NHE1 has a cytosolic pH-sensing site that shuts down transport activity at pHi values above resting pH (Putney et al. 2002). The observation that pHi is not restored to its original steady-state value upon PE treatment suggests that a new equilibrium is established. That is, NHE1 works against an acidifying pathway, with the elevated steady-state pHi value established by the combined activated states of the acidifier and NHE1 activities. The observation that PKC activates only the AE3fl isoform (data in this paper and Alvarez et al. 2001) of the Cl−–HCO3− exchanger, combined with the ETZ sensitivity of AE3-mediated Cl−–HCO3− exchange, suggests that AE3fl is the parallel acidifying pathway.
Together our data suggest a possible mechanism by which ETZ exerts an anti-hypertrophic effect. Myocardial NHE1 is activated by an array of hypertrophic signals (Moor & Fliegel, 1999). Further, direct inhibition of NHE1 prevents CH (Yoshida & Karmazyn, 2000; Kusumoto et al. 2001; Engelhardt et al. 2002; Ennis et al. 2003; Kilic et al. 2005). We have previously shown that NHE1 is functionally activated by physical interaction with the cytosolic enzyme CAII (Li et al. 2002). Activation of NHE1 through association is explained by the ability of CAII to produce HCO3− and the NHE1 substrate H+, by catalysis of CO2. Thus, localization of CAII to the cytosolic surface of NHE1 maximizes the local concentration of H+ at the surface of NHE1, thereby activating the transport flux (Li et al. 2006). ETZ and MTZ inhibit CAII catalytic activity, reducing H+ availability for NHE1 and reducing its transport rate (Fig. 7). The effect of ETZ is potentiated by its ability to inhibit Cl−–HCO3− exchange activity. NHE1 is inactivated by alkaline cytosolic pH (Slepkov & Fliegel, 2002); thus, NHE1 can only maintain its activity if working against an acid load. Cl−–HCO3− exchange is the primary acidifying pathway of cardiomyocytes. It is therefore significant that CAII also activates the transport of Cl−–HCO3− exchangers and inhibition of CAII inactivates their transport (Sterling et al. 2001). Inhibition of CAII will thus inhibit Cl−–HCO3− exchange, blocking this acid-producing transport system, which is necessary for NHE1 activity (Fig. 10). The mechanism by which NHE1 hyperactivity promotes hypertrophy is not absolutely established, but may be related to the role of the protein in cytosolic sodium accumulation. Elevated sodium levels activate PKC (Hayasaki-Kajiwara et al. 1999), resulting in feed-forward stimulation. Further, the plasma membrane Ca2+ efflux transporter, NCx, is impaired by elevation of cytosolic Ca2+, resulting in a rise in cytosolic Ca2+ levels (Karmazyn, 2001). In turn elevation of cytosolic Ca2+ is the central hypertrophic signal in cardiomyocytes (Frey & Olson, 2003).
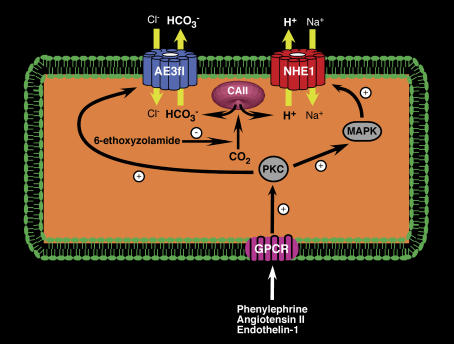
Figure 10. Proposed model for anti-hypertrophic action of 6-ethoxyzolamide.
G protein-coupled receptors (GPCR), including receptors for AngII, endothelin-1 and α1a-R, are coupled to activation of protein kinase C (PKC) in cardiomyocytes. PKC directly activates AE3 by phosphorylation of Ser-67 (Alvarez et al. 2001) and also activates the MAP kinase signalling pathway (MAPK), which in turn activates NHE1. AE3 and NHE1 physically associate with cytosolic carbonic anhydrase II (CAII), which activates their transport activity by supplying HCO3− and H+ for their respective transport functions. Co-activation of AE3 and NHE1 is pathological, since the acid generated by AE3 efflux of bicarbonate is in turn removed by NHE1. Resulting hyperactivity of NHE1 causes cellular sodium loading. Sodium activates PKC (Hayasaki-Kajiwara et al. 1999), resulting in feed-forward activation of PKC. Elevated cytosolic sodium levels reduce activity of the plasma membrane sodium–calcium exchanger, which is normally required to maintain low calcium levels. Activated PKC and elevated cytosolic calcium levels will contribute to hypertrophic growth in the myocardium (Karmazyn, 2001; Frey et al. 2004). 6-Ethoxyzolamide may arrest the hypertrophic programme directly by inhibition of carbonic anhydrase and indirectly by limiting substrates for transport by NHE1 and AE3fl.
We cannot rule out the possibility that ETZ and MTZ are anti-hypertrophic through a target other than CAII. In other cell types intriguing effects on cell growth and transcriptional regulation have been observed with carbonic anhydrase inhibitors. ETZ inhibited the growth of three different cell lines by 5–20% at 100 μm and about 80% inhibition at 1 mm (Lankat-Buttgereit et al. 2004). The authors suggested that the effect could be through CAII inhibition and concomitant reduction of HCO3− availability since HCO3− is required for the formation of the pyrimidines and amino acids associated with cell growth (Lankat-Buttgereit et al. 2004). In studies somewhat parallel to those presented here, 200 μm ETZ suppressed clonal expansion of cultured adipocytes, reduced the expression of the CAIII isoform of carbonic anhydrase and decreased expression of the transcription factors C/EBPβ and PPARγ (Takahata et al. 2004). Consistent with the present report, 200 μm acetazolamide had no effect on cell growth (Takahata et al. 2004). The consistent failure of acetazolamide to affect cell growth in the same way as ETZ suggests that either ETZ acts through a pathway other than inhibition of CAII, or that the greater membrane permeability of ETZ is required for its biological activity. The basis for the effect of ETZ on cell growth, however, remains uncertain, although neither of these examples is inconsistent with the NHE1-based model for ETZ function that we have proposed (Fig. 9), especially given that NHE1 is universally expressed in mammalian cells (Slepkov & Fliegel, 2002).
Inhibition of NHE1 limits hypertrophic cardiomyocyte growth (Yoshida & Karmazyn, 2000; Kusumoto et al. 2001). We began with the premise that inhibition of cytosolic carbonic anhydrase could thus be anti-hypertrophic by limiting substrate H+ availability for NHE1. Consistent with this we found that the membrane-permeant carbonic anhydrase inhibitors 6-ethoxyzolamide and methazolamide prevented and reverted cardiomyocyte hypertrophy induced by phenylephrine. Transport activity of NHE1 and the AE3 isoform Cl−–HCO3− exchanger were both inhibited by 6-ethoxyzolamide, with a concentration dependency similar to the effect on cardiomyocyte hypertrophy. Testing of CA inhibitors with more relevant models of cardiac hypertrophy is needed before we can conclude that cytosolic carbonic anhydrase represents a novel target for therapy in CH. The availability of existing drugs that inhibit carbonic anhydrase does, however, make this an attractive clinical possibility.
Acknowledgments
J.R.C and P.E.L. are supported by the Alberta Heritage Foundation for Medical Research. M.K. holds a Canada Research Chair in Experimental Cardiology. B.V.A. was supported by a Canadian Cystic Fibrosis Foundation Fellowship. This work was also supported by the Heart and Stroke Foundation of Alberta (J.R.C) and Canadian Institutes of Health Research (M.K). We thank Dr Susanna Cotecchia for the α1a-R construct.
References
- Alvarez BV, Fujinaga J, Casey JR. Molecular basis for angiotensin II-induced increase of chloride/bicarbonate exchange in the myocardium. Circ Res. 2001;89:1246–1253. doi: 10.1161/hh2401.101907. [DOI] [PubMed] [Google Scholar]
- Alvarez BV, Kieller DM, Quon AL, Markovich D, Casey JR. Slc26a6: a cardiac chloride/hydroxyl exchanger and predominant chloride/bicarbonate exchanger of the heart. J Physiol. 2004;561:721–734. doi: 10.1113/jphysiol.2004.077339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baczko I, Jones L, McGuigan CF, Manning Fox JE, Gandhi M, Giles WR, Clanachan AS, Light PE. Plasma membrane KATP channel-mediated cardioprotection involves posthypoxic reductions in calcium overload and contractile dysfunction: mechanistic insights into cardioplegia. FASEB J. 2005;19:980–982. doi: 10.1096/fj.04-3008fje. [DOI] [PubMed] [Google Scholar]
- Becker B. Use of methazolamide (neptazane) in the therapy of glaucoma; comparison with acetazolamide (diamox) Am J Ophthalmol. 1960;49:1307–1311. doi: 10.1016/0002-9394(60)91346-5. [DOI] [PubMed] [Google Scholar]
- Camilion de Hurtado MC, Alvarez BV, Ennis IL, Cingolani HE. Stimulation of myocardial Na+-independent Cl−-HCO3− exchanger by angiotensin II is mediated by endogenous endothelin. Circ Res. 2000;86:622–627. doi: 10.1161/01.res.86.6.622. [DOI] [PubMed] [Google Scholar]
- Chen L, Chen CX, Gan XT, Beier N, Scholz W, Karmazyn M. Inhibition and reversal of myocardial infarction-induced hypertrophy and heart failure by NHE-1 inhibition. Am J Physiol Heart Circ Physiol. 2004;286:H381–H387. doi: 10.1152/ajpheart.00602.2003. [DOI] [PubMed] [Google Scholar]
- Cingolani HE, Camilion de Hurtado MC. Na+-H+ exchanger inhibition: a new antihypertrophic tool. Circ Res. 2002;90:751–753. doi: 10.1161/01.res.0000016836.24179.ae. [DOI] [PubMed] [Google Scholar]
- Cousin JL, Motais R. The role of carbonic anhydrase inhibitors on anion permeability into ox red blood cells. J Physiol. 1976;256:61–80. doi: 10.1113/jphysiol.1976.sp011311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenighetti AA, Ritchie M, Smyth G, Pedrazzini T, Proietto J, Delbridge LMD. Gene expression profiling reveals distinct sets of genes altered during hormonally and metabolically induced cardiac hypertrophies. J Mol Cell Cardiol. 2004;37:303. [Google Scholar]
- Engelhardt S, Hein L, Keller U, Klambt K, Lohse MJ. Inhibition of Na+-H+ exchange prevents hypertrophy, fibrosis, and heart failure in β1-adrenergic receptor transgenic mice. Circ Res. 2002;90:814–819. doi: 10.1161/01.res.0000014966.97486.c0. [DOI] [PubMed] [Google Scholar]
- Ennis IL, Escudero EM, Console GM, Camihort G, Dumm CG, Seidler RW, Camilion de Hurtado MC, Cingolani HE. Regression of isoproterenol-induced cardiac hypertrophy by Na+/H+ exchanger inhibition. Hypertension. 2003;41:1324–1329. doi: 10.1161/01.HYP.0000071180.12012.6E. [DOI] [PubMed] [Google Scholar]
- Frey N, Katus HA, Olson EN, Hill JA. Hypertrophy of the heart: a new therapeutic target? Circulation. 2004;109:1580–1589. doi: 10.1161/01.CIR.0000120390.68287.BB. [DOI] [PubMed] [Google Scholar]
- Frey N, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol. 2003;65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243. [DOI] [PubMed] [Google Scholar]
- Friedberg CK, Taymor R, Minor JB, Halpern M. The use of diamox, a carbonic anhydrase inhibitor, as an oral diuretic in patients with congestive heart failure. N Engl J Med. 1953;248:883–889. doi: 10.1056/NEJM195305212482102. [DOI] [PubMed] [Google Scholar]
- Fujinaga J, Loiselle FB, Casey JR. Transport activity of chimaeric AE2-AE3 chloride/bicarbonate anion exchange proteins. Biochem J. 2003;371:687–696. doi: 10.1042/BJ20030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaj V, Puccetti L, Fasolis G, Winum JY, Montero JL, Scozzafava A, Vullo D, Innocenti A, Supuran CT. Carbonic anhydrase inhibitors: synthesis and inhibition of cytosolic/tumor-associated carbonic anhydrase isozymes I, II, and IX with sulfonamides incorporating 1,2,4-triazine moieties. Bioorg Med Chem Lett. 2004;14:5427–5433. doi: 10.1016/j.bmcl.2004.07.087. [DOI] [PubMed] [Google Scholar]
- Geers C, Gros G. Contractile function of papillary muscles with carbonic anhydrase inhibitors. Life Sci. 1995;57:591–597. doi: 10.1016/0024-3205(95)00309-t. [DOI] [PubMed] [Google Scholar]
- Geers C, Kruger D, Siffert W, Schmid A, Bruns W, Gros G. Carbonic anhydrase in skeletal and cardiac muscle from rabbit and rat. Biochem J. 1992;282:165–171. doi: 10.1042/bj2820165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaki-Kajiwara Y, Kitano Y, Iwasaki T, Shimamura T, Naya N, Iwaki K, Nakajima M. Na+ influx via Na+/H+ exchange activates protein kinase C isozymes delta and epsilon in cultured neonatal rat cardiac myocytes. J Mol Cell Cardiol. 1999;31:1559–1572. doi: 10.1006/jmcc.1999.0993. [DOI] [PubMed] [Google Scholar]
- Jeong D, Cha H, Kim E, Kang M, Yang DK, Kim JM, Yoon PO, Oh JG, Bernecker OY, Sakata S, Le TT, Cui L, Lee YH, Kim Do H, Woo SH, Liao R, Hajjar RJ, Park WJ. PICOT inhibits cardiac hypertrophy and enhances ventricular function and cardiomyocyte contractility. Circ Res. 2006;99:307–314. doi: 10.1161/01.RES.0000234780.06115.2c. [DOI] [PubMed] [Google Scholar]
- Karmazyn M. Therapeutic potential of Na-H exchange inhibitors for the treatment of heart failure. Expert Opin Invest Drugs. 2001;10:835–843. doi: 10.1517/13543784.10.5.835. [DOI] [PubMed] [Google Scholar]
- Karmazyn M, Liu Q, Gan XT, Brix BJ, Fliegel L. Aldosterone increases NHE-1 expression and induces NHE-1-dependent hypertrophy in neonatal rat ventricular myocytes. Hypertension. 2003;42:1171–1176. doi: 10.1161/01.HYP.0000102863.23854.0B. [DOI] [PubMed] [Google Scholar]
- Karmazyn M, Sostaric JV, Gan XT. The myocardial Na+/H+ exchanger: a potential therapeutic target for the prevention of myocardial ischaemic and reperfusion injury and attenuation of postinfarction heart failure. Drugs. 2001;61:375–389. doi: 10.2165/00003495-200161030-00006. [DOI] [PubMed] [Google Scholar]
- Kilic A, Velic A, De Windt LJ, Fabritz L, Voss M, Mitko D, Zwiener M, Baba HA, van Eickels M, Schlatter E, Kuhn M. Enhanced activity of the myocardial Na+/H+ exchanger NHE-1 contributes to cardiac remodeling in atrial natriuretic peptide receptor-deficient mice. Circulation. 2005;112:2307–2317. doi: 10.1161/CIRCULATIONAHA.105.542209. [DOI] [PubMed] [Google Scholar]
- Kovacic S, Soltys CL, Barr AJ, Shiojima I, Walsh K, Dyck JR. Akt activity negatively regulates phosphorylation of AMP-activated protein kinase in the heart. J Biol Chem. 2003;278:39422–39427. doi: 10.1074/jbc.M305371200. [DOI] [PubMed] [Google Scholar]
- Kusumoto K, Haist JV, Karmazyn M. Na+/H+ exchange inhibition reduces hypertrophy and heart failure after myocardial infarction in rats. Am J Physiol Heart Circ Physiol. 2001;280:H738–H745. doi: 10.1152/ajpheart.2001.280.2.H738. [DOI] [PubMed] [Google Scholar]
- Landolfi C, Marchetti M, Ciocci G, Milanese C. Development and pharmacological characterization of a modified procedure for the measurement of carbonic anhydrase activity. J Pharmacol Toxicol Meth. 1997;38:169–172. doi: 10.1016/s1056-8719(97)00095-6. [DOI] [PubMed] [Google Scholar]
- Lankat-Buttgereit B, Gregel C, Knolle A, Hasilik A, Arnold R, Goke R. Pdcd4 inhibits growth of tumor cells by suppression of carbonic anhydrase type II. Mol Cell Endocrinol. 2004;214:149–153. doi: 10.1016/j.mce.2003.10.058. [DOI] [PubMed] [Google Scholar]
- Li X, Alvarez B, Casey JR, Reithmeier RAF, Fliegel L. Carbonic anhydrase II binds to and enhances activity of the Na+/H+ exchanger. J Biol Chem. 2002;277:36085–36091. doi: 10.1074/jbc.M111952200. [DOI] [PubMed] [Google Scholar]
- Li X, Liu Y, Alvarez BV, Casey JR, Fliegel L. A novel carbonic anhydrase II binding site regulates NHE1 activity. Biochemistry. 2006;45:2414–2424. doi: 10.1021/bi051132d. [DOI] [PubMed] [Google Scholar]
- Lohi H, Kujala M, Kerkela E, Saarialho-Kere U, Kestila M, Kere J. Mapping of five new putative anion transporter genes in human and characterization of SLC26A6, a candidate gene for pancreatic anion exchanger. Genomics. 2000;70:102–112. doi: 10.1006/geno.2000.6355. [DOI] [PubMed] [Google Scholar]
- Lohi H, Lamprecht G, Markovich D, Heil A, Kujala M, Seidler U, Kere J. Isoforms of SLC26A6 mediate anion transport and have functional PDZ interaction domains. Am J Physiol Cell Physiol. 2003;284:C769–C779. doi: 10.1152/ajpcell.00270.2002. [DOI] [PubMed] [Google Scholar]
- McMurtrie HL, Cleary HJ, Alvarez BV, Loiselle FB, Sterling D, Morgan PE, Johnson DE, Casey JR. The bicarbonate transport metabolon. J Enzyme Inhib Med Chem. 2004;19:231–236. doi: 10.1080/14756360410001704443. [DOI] [PubMed] [Google Scholar]
- Moor AN, Fliegel L. Protein kinase-mediated regulation of the Na+/H+ exchanger in the rat myocardium by mitogen-activated protein kinase-dependent pathways. J Biol Chem. 1999;274:22985–22992. doi: 10.1074/jbc.274.33.22985. [DOI] [PubMed] [Google Scholar]
- Moyer JH, Ford RV. Laboratory and clinical observations on ethoxzolamide (cardrase) as a diuretic agent. Am J Cardiol. 1958;1:497–504. doi: 10.1016/0002-9149(58)90121-8. [DOI] [PubMed] [Google Scholar]
- Murtazina R, Booth BJ, Bullis BL, Singh DN, Fliegel L. Functional analysis of polar amino-acid residues in membrane associated regions of the NHE1 isoform of the mammalian Na+/H+ exchanger. Eur J Biochem. 2001;268:4674–4685. doi: 10.1046/j.1432-1327.2001.02391.x. [DOI] [PubMed] [Google Scholar]
- Omura T, Yoshiyama M, Yoshida K, Nakamura Y, Kim S, Iwao H, Takeuchi K, Yoshikawa J. Dominant negative mutant of c-Jun inhibits cardiomyocyte hypertrophy induced by endothelin 1 and phenylephrine. Hypertension. 2002;39:81–86. doi: 10.1161/hy0102.100783. [DOI] [PubMed] [Google Scholar]
- Pastorekova S, Parkkila AK, Pastorek J, Supuran C. Carbonic anhydrases: current state of the art, therapeutic applications and future prospects. J Enzyme Inhib Med Chem. 2004;19:199–229. doi: 10.1080/14756360410001689540. [DOI] [PubMed] [Google Scholar]
- Perez NG, Alvarez BV, Camilion de Hurtado MC, Cingolani HE. pHi regulation in myocardium of the spontaneously hypertensive rat. Compensated enhanced activity of the Na+-H+ exchanger. Circ Res. 1995;77:1192–1200. doi: 10.1161/01.res.77.6.1192. [DOI] [PubMed] [Google Scholar]
- Perez NG, de Hurtado MC, Cingolani HE. Reverse mode of the Na+-Ca2+ exchange after myocardial stretch: underlying mechanism of the slow force response. Circ Res. 2001;88:376–382. doi: 10.1161/01.res.88.4.376. [DOI] [PubMed] [Google Scholar]
- Purkerson JM, Schwartz GJ. Expression of membrane-associated carbonic anhydrase isoforms IV, IX, XII, and XIV in the rabbit: induction of CA IV and IX during maturation. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1256–R1263. doi: 10.1152/ajpregu.00735.2004. [DOI] [PubMed] [Google Scholar]
- Putney LK, Denker SP, Barber DL. The changing face of the Na+/H+ exchanger, NHE1: structure, regulation, and cellular actions. Annu Rev Pharmacol Toxicol. 2002;42:527–552. doi: 10.1146/annurev.pharmtox.42.092001.143801. [DOI] [PubMed] [Google Scholar]
- Rotin D, Steele-Norwood D, Grinstein S, Tannock I. Requirement of the Na+/H+ exchanger for tumor growth. Cancer Res. 1989;49:205–211. [PubMed] [Google Scholar]
- Ruetz S, Lindsey AE, Kopito RR. Function and biosynthesis of erythroid and nonerythroid anion exchangers. Soc Gen Physiol Ser. 1993;48:193–200. [PubMed] [Google Scholar]
- Sambrano GR, Fraser I, Han H, Ni Y, O'Connell T, Yan Z, Stull JT. Navigating the signalling network in mouse cardiac myocytes. Nature. 2002;420:712–714. doi: 10.1038/nature01306. [DOI] [PubMed] [Google Scholar]
- Scheibe RJ, Gros G, Parkkila S, Waheed A, Grubb JH, Shah GN, Sly WS, Wetzel P. Expression of membrane-bound carbonic anhydrases IV, IX, and XIV in the mouse heart. J Histochem Cytochem. 2006;54:1379–1391. doi: 10.1369/jhc.6A7003.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siffert W, Gros G. Carbonic anhydrase in human platelets. Biochem J. 1984;217:727–730. doi: 10.1042/bj2170727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slepkov E, Fliegel L. Structure and function of the NHE1 isoform of the Na+/H+ exchanger. Biochem Cell Biol. 2002;80:499–508. doi: 10.1139/o02-151. [DOI] [PubMed] [Google Scholar]
- Stanasila L, Perez JB, Vogel H, Cotecchia S. Oligomerization of the α1a- and α1b-adrenergic receptor subtypes. Potential implications in receptor internalization. J Biol Chem. 2003;278:40239–40251. doi: 10.1074/jbc.M306085200. [DOI] [PubMed] [Google Scholar]
- Sterling D, Alvarez BV, Casey JR. The extracellular component of a transport metabolon: Extracellular loop 4 of the human AE1 Cl−/HCO3− exchanger binds carbonic anhydrase IV. J Biol Chem. 2002;277:25239–25246. doi: 10.1074/jbc.M202562200. [DOI] [PubMed] [Google Scholar]
- Sterling D, Casey JR. Transport activity of AE3 chloride/bicarbonate anion-exchange proteins and their regulation by intracellular pH. Biochem J. 1999;344:221–229. [PMC free article] [PubMed] [Google Scholar]
- Sterling D, Casey JR. Bicarbonate transport proteins. Biochem Cell Biol. 2002;80:483–497. doi: 10.1139/o02-152. [DOI] [PubMed] [Google Scholar]
- Sterling D, Reithmeier RA, Casey JR. A transport metabolon. Functional interaction of carbonic anhydrase II and chloride/bicarbonate exchangers. J Biol Chem. 2001;276:47886–47894. doi: 10.1074/jbc.M105959200. [DOI] [PubMed] [Google Scholar]
- Takahata T, Kumano T, Ookawa K, Hayakari M, Kakizaki I, Tsuchida S. Inhibition of 3T3-L1 adipocyte differentiation by 6-ethoxyzolamide: repressed peroxisome proliferator-activated receptor γ mRNA and enhanced CCAAT/enhancer binding protein β mRNA levels. Biochem Pharmacol. 2004;67:1667–1675. doi: 10.1016/j.bcp.2003.12.039. [DOI] [PubMed] [Google Scholar]
- Terzic A, Puceat M, Clement O, Scamps F, Vassort G. α1-Adrenergic effects on intracellular pH and calcium and on myofilaments in single rat cardiac cells. J Physiol. 1992;447:275–292. doi: 10.1113/jphysiol.1992.sp019002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JA, Buchsbaum RN, Zimniak A, Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979;18:2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Vila-Petroff M, Perez GN, Alvarez B, Cingolani HE, Mattiazzi A. Mechanism of negative lusitropic effect of α1-adrenoceptor stimulation in cat papillary muscles. Am J Physiol Heart Circ Physiol. 1996;270:H701–H709. doi: 10.1152/ajpheart.1996.270.2.H701. [DOI] [PubMed] [Google Scholar]
- Vuillemin M, Pexieder T. Carbonic anhydrase II expression pattern in mouse embryonic and fetal heart. Anat Embryol (Berl) 1997;195:267–277. doi: 10.1007/s004290050046. [DOI] [PubMed] [Google Scholar]
- Wetzel P, Kleinke T, Papadopoulos S, Gros G. Inhibition of muscle carbonic anhydrase slows the Ca2+ transient in rat skeletal muscle fibers. Am J Physiol Cell Physiol. 2002;283:C1242–C1253. doi: 10.1152/ajpcell.00106.2002. [DOI] [PubMed] [Google Scholar]
- Yokoyama H, Gunasegaram S, Harding SE, Avkiran M. Sarcolemmal Na+/H+ exchanger activity and expression in human ventricular myocardium. J Am Coll Cardiol. 2000;36:534–540. doi: 10.1016/s0735-1097(00)00730-0. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Karmazyn M. Na+/H+ exchange inhibition attenuates hypertrophy and heart failure in 1-wk postinfarction rat myocardium. Am J Physiol Heart Circ Physiol. 2000;278:H300–H304. doi: 10.1152/ajpheart.2000.278.1.H300. [DOI] [PubMed] [Google Scholar]