Abstract
Ageing is associated with increased leg vascular resistance and reductions in leg blood flow during rest and exercise, potentially predisposing older adults to a host of functional and cardiovascular complications. The purpose of these studies was to examine the effects and possible mechanisms of ageing and exercise training on arteriolar adrenergic vasoreactivity. Young and old male Fischer 344 rats were divided into young sedentary (YS), old sedentary (OS), young exercise-trained (YT) or old exercise-trained (OT) groups, where training consisted of chronic treadmill exercise. Isolated soleus (SOL) and gastrocnemius (GAS) muscle arterioles were studied in vitro. Responses to noradrenaline in endothelium-intact and endothelium-denuded arterioles, as well as during nitric oxide synthase (NOS) inhibition were determined. Vasodilator responses to isoproterenol and forskolin were also determined. Results: Noradrenaline-mediated vasoconstriction was increased in SOL arterioles with ageing, and exercise training in old rats attenuated α-adrenergic vasoconstriction in arterioles from both muscle types. Removal of the endothelium and NOS inhibition eliminated these ageing and training effects. Isoproterenol-mediated vasodilatation was impaired with ageing in SOL and GAS arterioles, and exercise training had little effect on this response. Forskolin-induced vasodilatation was not affected by age. The data demonstrate that ageing augments α-adrenergic vasoconstriction while exercise training attenuates this response, and both of these alterations are mediated through an endothelial α-receptor-NOS-signalling pathway. In contrast, ageing diminishes β-receptor-mediated vasodilatation, but this impairment is specific to the smooth muscle. These studies indicate that α- and β-adrenergic mechanisms may serve to increase systemic vascular resistance with ageing, and that the effects of exercise training on adrenergic vasomotor properties could contribute to the beneficial effects of exercise on cardiovascular disease.
Recent studies have shown a lower resting leg blood flow (25–30%) in aged humans that is independent of skeletal muscle mass (Meneilly et al. 1995; Dinenno et al. 1999, 2001a; Moreau et al. 2003), but is the result of an ∼50% higher leg vascular resistance in older males and females (Dinenno et al. 1999, 2001b; Moreau et al. 2003). In addition, leg blood flow is lower and vascular resistance higher during exercise in most populations of older male and female subjects (Beere et al. 1999; Proctor et al. 1998, 2003; Lawrenson et al. 2003; Poole et al. 2003). Such age-associated alterations in the control of leg blood flow and vascular resistance could adversely affect regulation of many critical physiological variables associated with the development of cardiovascular disease, including arterial blood pressure, glucose disposal, insulin sensitivity, oxygen delivery, and clearance of metabolic by products.
Previous work has shown that chronically augmented α-adrenergic vasoconstriction in older adult males accounts for the majority of the reduction in blood flow and elevation in vascular resistance at rest (Dinenno et al. 2001b). This increased α-adrenergic vasoconstriction in healthy older men could be due to several factors, including increased muscle sympathetic nerve activity (Seals & Esler, 2000) or increased resistance vessel responsiveness or sensitivity to noradrenaline (NA), due to greater α-adrenergic gene expression (Rudner et al. 1999). In addition, the effects of ageing on β-adrenergic vasodilator responsiveness remains unclear, with one study showing no age-related changes in forearm blood flow in response to β-agonists (DeSouza et al. 2002), and others demonstrating age-associated reductions in β-mediated vasodilatation in the arm and leg (Ford et al. 1995; van Brummelen et al. 1981). Thus, the effects of ageing on the responsiveness of the skeletal muscle resistance vasculature to adrenergic vasoconstrictor and vasodilator stimuli are not completely understood.
Chronic endurance exercise training programmes have been shown to ameliorate the attenuated blood flow response during exercise among the aged (Beere et al. 1999). This effect could be due to a training-induced attenuation of α-adrenergic vasoconstriction, as has been reported to occur in large-conduit arteries of young exercise-trained rats (Delp, 1995; Spier et al. 1999), or to a putative augmentation of β-adrenergic-mediated vasodilatation. To date the effects of chronic aerobic exercise training on the adrenergic vasoreactivity of the resistance vasculature in skeletal muscle from senescent animals has not been determined. Therefore, the primary purpose of this study was to determine whether ageing and exercise training alter adrenergic vasoconstrictor and vasodilator responses of arterioles from two skeletal muscles composed of different fibre types. We hypothesized that ageing augments α-adrenergic vasoconstrictor responses to NA and diminishes β-adrenergic vasodilator responses to isoproterenol (ISO), and that these ageing effects would be attenuated or reversed by chronic exercise training. The secondary purpose of this study was to determine the mechanism(s) of ageing- and training-induced alterations in skeletal muscle arteriolar responsiveness.
Methods
All animal procedures were approved by the Texas A&M University Laboratory Animal Care Committee, and complied by the guidelines of the National Research Council Guide for the Care and Use of Laboratory Animals (NIH Publication no. 85-23, revised 1996).
Animal characteristics
Young (4–6 months) and old (24–26 months) male Fischer 344 rats were obtained (Harlan Inc), housed in a temperature controlled (23 ± 2°C) room with a 12: 12 h light–dark cycle, and were provided with water and rat chow ad libitum. These ages represent young adulthood and senescence (∼50% mortality rate) (Yu et al. 1985). The Fischer 344 rat model is supported by the National Institute on Ageing because these rats can be used to study the effects of old age on the cardiovascular system in the absence of overt cardiovascular disease (Maeda et al. 1985).
Exercise training
All rats were habituated to treadmill exercise, during which time each rat walked on a motor-driven treadmill at 15 m min−1 (0° incline), 5 min day−1 for 3 days. At the end of the habituation period young and old rats were randomly assigned to a young sedentary (YS), old sedentary (OS), young exercise-trained (YT) or old exercise-trained (OT) group. Exercise-trained rats performed treadmill running at 15 m min−1 up a 15° incline, 1 h day−1, 5 days week−1, for 10–12 weeks as previously described (Delp et al. 1993; Spier et al. 2004). Vascular responses were determined 48 h after the last exercise bout in the exercise-trained rats.
Microvessel preparation
Animals were anaesthetized with sodium pentobarbital (85 mg kg−1, i.p.) and killed by exsanguination. The gastrocnemius–plantaris–soleus muscle complex was carefully excised from each leg and placed in cold (4°C) physiological saline solution (PSS) containing (mm): 145.0 NaCl, 4.7 KCl, 2.0 CaCl2, 1.17 MgSO4, 1.2 NaH2PO4, 5.0 glucose, 2.0 pyruvate, 0.02 EDTA, 3.0 MOPS buffer and 1 g (100 ml)−1 BSA at pH 7.4. Soleus and gastrocnemius muscle first-order (1A) arterioles were then isolated with the aid of a dissecting microscope (Olympus SVH10) as previously described (McCurdy et al. 2000; Muller-Delp et al. 2002a). In soleus muscle, 1A arterioles were defined as the first branch that occurred after the feed artery entered the muscle tissue. In gastrocnemius muscle, 1A arterioles were defined as the first branch off the feed artery that runs over the superficial portion of the muscle. The arterioles (length, 0.5–1.0 mm; maximal inner diameter: soleus muscle, 60–159 μm; gastrocnemius muscle, 84–220 μm) were cleared of surrounding muscle fibres, removed from the muscle and placed in Lucite chambers containing MOPS-buffered PSS equilibrated to room air. The arterioles were cannulated on both ends to micropipettes, and secured with nylon monofilament suture (Alcon 11-0). After cannulation, the chambers were transferred to the stage of an inverted microscope (Olympus IX70) equipped with a video camera (Panasonic BP310), video caliper (Microcirculation Research Institute, Texas A&M), and data acquisition system (MacLab) for recording of luminal diameter. Intraluminal pressure was set according to the arteriolar internal diameter to coincide with pressures measured in vivo (Meininger et al. 1984; Joyner & Davis, 1987) and as used in previous in vitro studies of skeletal muscle arterioles (Aaker & Laughlin, 2002; Donato et al. 2005), i.e. 60–125 μm at 44 mmHg, 126–150 μm at 49 mmHg, 151–175 μm at 54 mmHg, and 176–200 μm at 60 mmHg. Leaks were detected by pressurizing the vessel and then closing lines to the fluid reservoirs to determine whether vessel diameter was maintained. Arterioles that exhibited leaks were discarded. Arterioles free of leaks were warmed to 37°C and allowed to develop spontaneous tone during a 30–60 min equilibration period. The arterioles were allowed to equilibrate and were discarded unless at least 20% baseline tone was achieved prior to addition of vasoactive agents. In a previous study where baseline tone was not used as an exclusion criterion, baseline tone was significantly lower in old animal arterioles (Muller-Delp et al. 2002a). That more vessels from old sedentary rats were discarded because of insufficient baseline tone is consistent with this previous finding. Following active responses, vessels were incubated in calcium-free PSS, to determine maximal lumen diameter. Sensitivity of the arterioles to agonists was assessed by calculating the dose eliciting 50% of the maximal vasoconstrictor or vasodilator response (EC50 or IC50, respectively).
Study design
Study 1
To determine whether adrenergic vasomotor function of the skeletal muscle arterioles is altered with ageing or exercise training, responses of 1A arterioles were determined to the cumulative addition of either ISO (10−9 to 10−5m) or NA (10−9 to 10−4m) in the presence of the β-blocker propranolol (10−5m). In preliminary studies it was determined that 10−5m propranolol blocked the vasodilator effects of 10−6m ISO in soleus and gastrocnemius muscle arterioles.
Study 2
When differences in the reactivity to the pharmacological agonists were found, a second series of studies was performed to determine whether these differences were mediated through the vascular endothelium. For these studies, the endothelium was denuded from arterioles by passing 3–5 ml of air through the lumen of the vessel as previously described (Donato et al. 2005). To ensure full removal of the endothelium, arterioles were exposed to the endothelium-dependent vasodilator acetylcholine (3 × 10−5m). Any vessel that exhibited vasodilatation >5% was excluded. Following the acetylcholine test, the vessels were washed several times with PSS and allowed to establish spontaneous tone. A dose–response relation to either the cumulative addition of NA (10−9 to 10−4m) with 10−5m propranolol or ISO (10−9 to 10−5m) was then performed.
Study 3
To determine whether the nitric oxide synthase (NOS)-signalling mechanism contributed to the altered α-adrenergic vasoconstrictor function with ageing and exercise training, arterioles were incubated for 20 min with the NOS inhibitor NG-nitro-l-arginine methyl ester (l-NAME, 10−5m) (Muller-Delp et al. 2002b; Spier et al. 2004). Responses of 1A arterioles to the cumulative addition of NA (10−9 to 10−4m) in the presence of both 10−5m propranolol and 10−5ml-NAME were then determined. Following active responses, vessels were incubated in calcium-free PSS to determine maximal lumen diameter.
Study 4
A final series of studies was done to examine whether the age-related changes in vascular smooth muscle β2-receptor-mediated vasodilatation might be due to the downstream signalling. Arterioles were denuded of the endothelium as described above, and after stable tone was achieved, vessels underwent a dose–response to forskolin (FOR, 10−9 to 10−5m), which directly stimulates adenylate cyclase to increase cytosolic cAMP. Following active responses, vessels were incubated in calcium-free PSS to determine maximal lumen diameter.
Muscle oxidative enzyme activity
Sections of the soleus and gastrocnemius muscles from each animal were stored at −80°C for determination of citrate synthase activity (Srere, 1969), a measure of muscle oxidative capacity, to determine the efficacy of the training regimen.
Solutions and stocks
Stock solutions of drugs were prepared in PSS and frozen. Fresh dilutions of these stocks were prepared daily. All drugs were purchased from Sigma Chemical.
Data presentation and statistical analysis
Responses were recorded as actual diameters and expressed as a percentage of possible vasoconstriction or vasodilatation according to the following formulae:
where Ds is the steady-state inner diameter recorded after addition of agonist, Db is the initial baseline inner diameter before the first addition of a pharmacological agonist, and Dm is maximal inner diameter obtained in Ca2+-free PSS. Comparison of data as a percentage of the maximal vasoconstriction or vasodilatation normalizes for potential differences in maximal diameter or spontaneous tone among vessels. Spontaneous tone is expressed at a percentage of maximal intraluminal diameter according to the formula:
Repeated measures analysis of variance (ANOVA) was used to determine differences among young and old, sedentary and exercise-trained groups. Planned comparisons of YS versus OS, YS versus YT, OS versus OT, and YT versus OT were tested where appropriate by a Fisher's protected least significant difference post hoc test. A one-way ANOVA was used to determine the significance of differences among groups in animal mass, muscle masses, muscle citrate synthase activity and vessel characteristics. All data are presented as mean ± s.e.m. Significance was set at P = 0.05.
Results
Animals
Body mass increased with age, and exercise training resulted in decreased body mass in old rats but not in young rats (Table 1). The left ventricle-to-body mass ratio was not different as a function of age, but was greater in trained rats compared to age-matched controls (Table 1). Soleus and gastrocnemius muscle mass-to-body mass ratios were lower with ageing and greater with exercise training (Table 1). Citrate synthase activity was higher in both muscles of young and old trained rats relative to the sedentary control groups (soleus muscle: YS 16 ± 1, OS 15 ± 1, YT 20 ± 1, OT 19 ± 2 μmol min−1 (g wet weight)−1; gastrocnemius muscle: YS 16 ± 1, OS 13 ± 1, YT 18 ± 1; OT 19 ± 1 μmol min−1 (g wet weight)−1), confirming the efficacy of the exercise training regimen.
Table 1.
Animal and vessel characteristics
| Sedentary | Trained | |||
|---|---|---|---|---|
| Young | Old | Young | Old | |
| n | 26 | 24 | 25 | 21 |
| Body mass (g) | 335 ± 5 | 430 ± 5* | 337 ± 5 | 391 ± 6‡ |
| Heart mass/body mass ratio (mg g−1) | 2.76 ± 0.16 | 2.76 ± 0.02 | 3.09 ± 0.15† | 3.03 ± 0.13 |
| LV mass/body mass ratio (mg g−1) | 1.88 ± 0.04 | 1.90 ± 0.02 | 2.00 ± 0.02† | 2.08 ± 0.04‡ |
| Soleus muscle mass/body mass ratio (mg g−1) | 0.44 ± 0.01 | 0.36 ± 0.01* | 0.48 ± 0.01† | 0.42 ± 0.01‡ |
| Gastrocnemius muscle mass/body mass ratio (mg g−1) | 4.90 ± 0.06 | 3.80 ± 0.05* | 5.10 ± 0.10† | 4.10 ± 0.07 |
| Maximal soleus muscle arteriole lumen diameter (μm) | 116 ± 3 | 118 ± 3 | 101 ± 4† | 113 ± 4 |
| Maximal gastrocnemius muscle arteriole lumen diameter (μm) | 151 ± 3 | 153 ± 4 | 171 ± 5† | 186 ± 4‡ |
| Soleus muscle arteriole spontaneous tone (%) | 45 ± 3 | 46 ± 4 | 41 ± 4 | 32 ± 4‡ |
| Gastrocnemius muscle arteriole spontaneous tone (%) | 33 ± 2 | 31 ± 3 | 31 ± 3 | 26 ± 2 |
Values are means ±s.e.m.; n= number of rats; LV, left ventricle.
Significant difference between young sedentary and old sedentary
significant difference between young sedentary and young trained
significant difference between old sedentary and old trained, P < 0.05.
Adrenergic vasoconstrictor responses
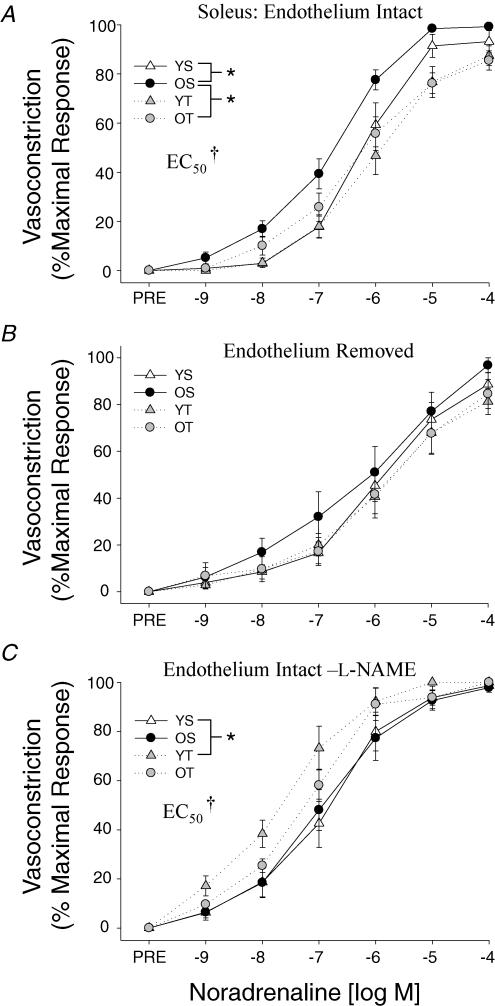
The maximal α-adrenergic vasoconstrictor responsiveness and sensitivity (EC50) of soleus muscle arterioles to NA were greater in old sedentary animals relative to young sedentary controls (Fig. 1A). Although chronic exercise training did not alter NA-induced α-adrenergic vasoconstriction in the young rats, exercise training abolished the enhanced vasoconstrictor response and sensitivity that occurred in the old sedentary rat soleus muscle arterioles (Fig. 1A). Removal of the vascular endothelial cell layer eliminated all ageing and exercise training-related changes in α-adrenergic vasoconstriction found in the soleus muscle arterioles (Fig. 1B). Likewise, NOS inhibition with l-NAME eliminated the ageing difference in NA-mediated vasoconstriction in soleus muscle arterioles from sedentary rats, and abolished the training adaptation in arterioles from old trained versus old sedentary rats (Fig. 1C). However, NOS inhibition resulted in an augmented α-adrenergic vasoconstriction of soleus muscle arterioles in young trained rats relative to young sedentary control animals (Fig. 1C).
Figure 1. Effects of ageing and exercise training on normalized vasoconstrictor responses to noradrenaline in the presence of the β-blocker propranolol (10−5m) in soleus muscle arterioles with A, an intact endothelium; B, the endothelium removed; and C, an intact endothelium with the NOS inhibitorl-NAME.
Values are means ±s.e.m., n= 8–13 per group. *Indicates responses are significantly different between groups (P < 0.05). †Indicates arteriolar sensitivity (EC50) is significantly greater in OS versus YS, YT and OT groups (A) and YT is greater than YS and OS (C) (P < 0.05). YS, young sedentary; OS, old sedentary; YT, young exercise-trained; OT, old exercise-trained.
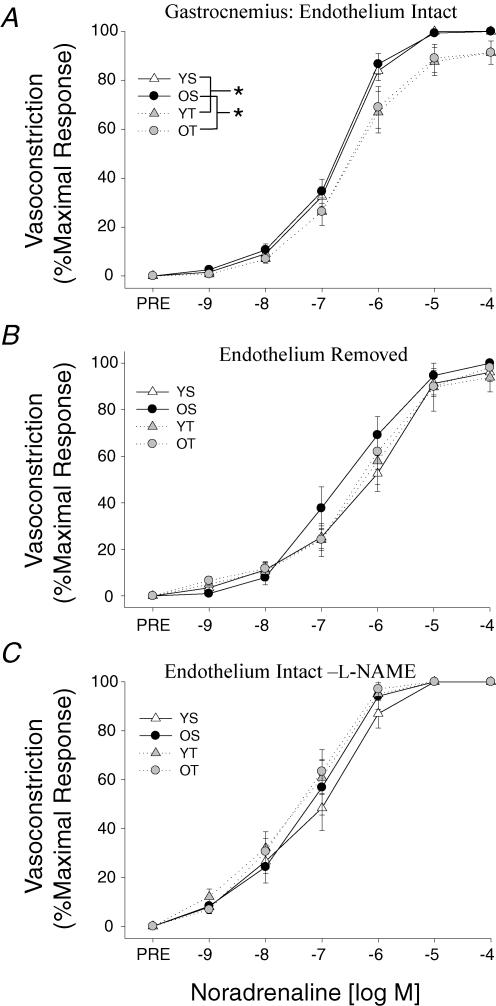
Gastrocnemius muscle arterioles exhibited no age-related differences in α-adrenergic vasoconstriction, but exercise training attenuated the vasoconstrictor responsiveness to NA in arterioles from young and old rats (Fig. 2A). Both removal of the endothelium (Fig. 2B) and NOS inhibition with l-NAME (Fig. 2C) abolished the exercise training effect in the gastrocnemius muscle arterioles.
Figure 2. Effects of ageing and exercise training on normalized vasoconstrictor responses to noradrenaline in the presence of the β-blocker propranolol (10−5m) in gastrocnemius muscle arterioles with A, an intact endothelium; B, the endothelium removed; and C, an intact endothelium with the NOS inhibitor l-NAME.
Values are means ±s.e.m., n= 7–16 per group. *Indicates responses are significantly different between groups (P < 0.05). YS, young sedentary; OS, old sedentary; YT, young exercise-trained; OT, old exercise-trained.
Adrenergic vasodilator responses
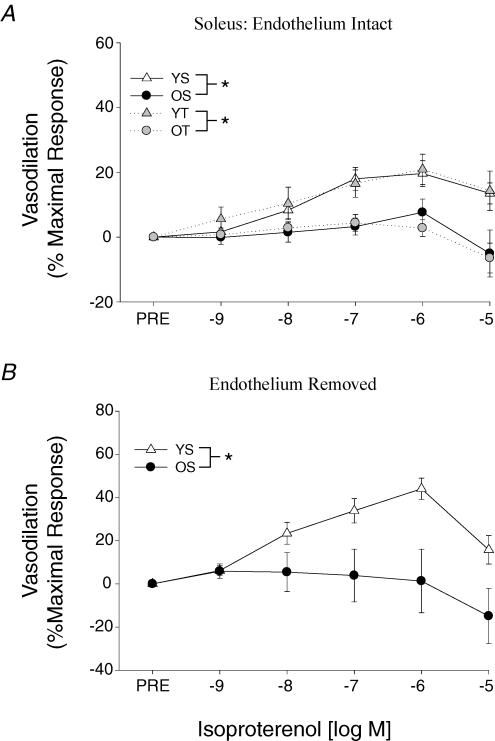
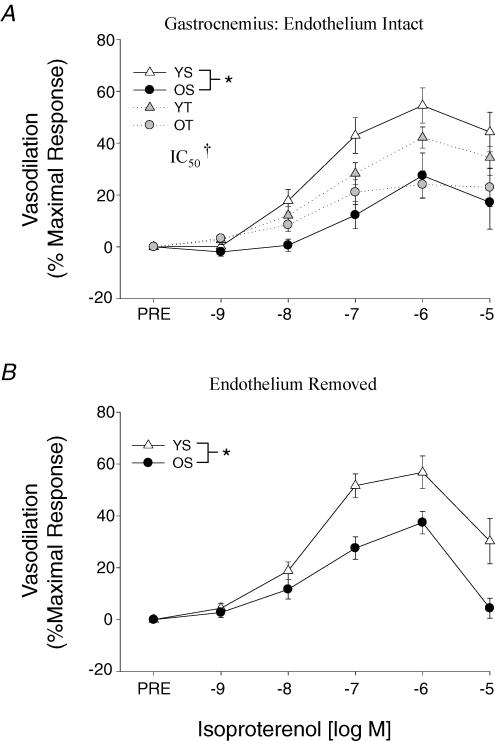
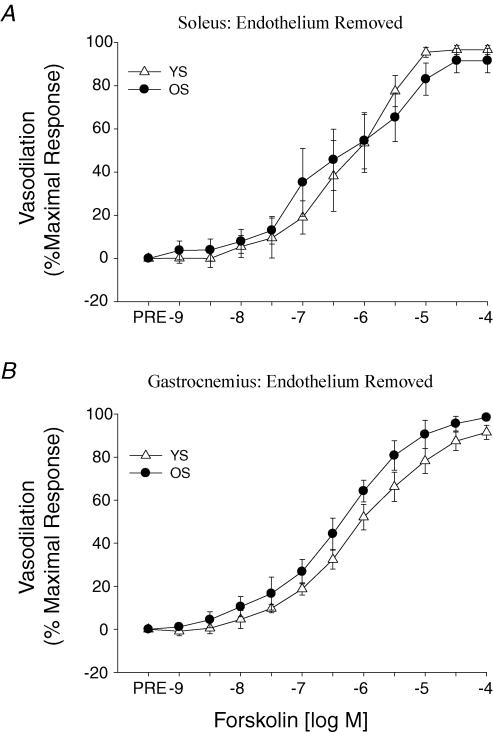
β-Adrenergic vasodilatation to ISO was reduced with ageing in both soleus (Fig. 3A) and gastrocnemius (Fig. 4A) muscle arterioles. Exercise training did not affect the β-mediated vasodilatation in the soleus muscle arterioles in either the young or old rats (Fig. 3A). However, gastrocnemius muscle arterioles exhibited an enhanced sensitivity (i.e. lower IC50) to ISO in the old trained rats (Fig. 4A), whereas the training in young rats induced a tendency toward a reduction in β-adrenergic vasodilatation (P = 0.1). Old-age-associated reductions in ISO-mediated vasodilatation persisted following the removal of the endothelium in both soleus (Fig. 3B) and gastrocnemius (Fig. 4B) muscle arterioles. Forskolin-mediated vasodilatation was not different between young and old arterioles from either the soleus (Fig. 5A) or gastrocnemius (Fig. 5B) muscles.
Figure 3. A, effects of ageing and exercise training on vasodilator responses to isoproterenol in soleus muscle arterioles with an intact endothelium. B, effects of ageing on vasodilator responses to isoproterenol in soleus muscle arterioles with the endothelium removed.
Values are means ±s.e.m., n= 7–13 per group. *Indicates responses are significantly different between groups (P < 0.05). YS, young sedentary; OS, old sedentary; YT, young exercise-trained; OT, old exercise-trained.
Figure 4. A, effects of ageing and exercise training on vasodilator responses to isoproterenol in gastrocnemius muscle arterioles with an intact endothelium. B, effects of ageing on vasodilator responses to isoproterenol in gastrocnemius muscle arterioles with the endothelium removed.
Values are means ±s.e.m., n= 9–17 per group. *Indicates responses are significantly different between groups (P < 0.05). †Indicates arteriolar sensitivity (IC50) is significantly greater in OT versus OS groups (P < 0.05). YS, young sedentary; OS, old sedentary; YT, young exercise-trained; OT, old exercise-trained.
Figure 5. Effects of ageing on vasodilator responses induced by forskolin in A, soleus and B, gastrocnemius muscle arterioles with the endothelium removed.
Values are means ±s.e.m., n= 6–8 per group. YS, young sedentary; OS, old sedentary.
Discussion
The purpose of the present study was to determine (1) whether ageing alters adrenergic receptor-mediated vasoreactivity of skeletal muscle arterioles, and (2) whether exercise training modulates adrenergic vascular responses in young and old animals. The results of this study provide several new insights into the effects of ageing and exercise training on the vasomotor responses mediated through α- and β-adrenergic receptors of resistance arteries from skeletal muscles composed of different fibre types. First, the results demonstrate that ageing is associated with a greater α-adrenergic vasoconstriction in arterioles from high-oxidative soleus muscle, and this enhanced vasoconstriction occurs as a result of alterations in the endothelium-dependent NOS-signalling mechanism. Second, the present findings demonstrate that ageing is associated with a lower β-adrenergic vasodilatation in high-oxidative soleus and low-oxidative gastrocnemius muscles. This effect appears to be upstream of adenylate cyclase activity, and may be due to an alteration in the smooth muscle β-receptor number or sensitivity. Finally, the results demonstrate that exercise training attenuates α-adrenergic vasoconstriction in skeletal muscle arterioles from young and old rats through an endothelium-dependent NOS-signalling mechanism, whereas training increases the sensitivity of β-receptor-mediated vasodilatation in gastrocnemius muscle arterioles from old rats.
Ageing and α-adrenergic vasoconstriction
In the present study, ageing increased arteriolar responsiveness and sensitivity to NA in the soleus muscle (Fig. 1A), which is composed predominantly of high-oxidative type I fibres (Delp & Duan, 1996). In contrast, ageing did not alter NA-mediated vasoconstriction in arterioles from the superficial portion of the gastrocnemius muscle (Fig. 2A), which is composed primarily of low-oxidative glycolytic type IIB fibres (Delp & Duan, 1996). Such a muscle fibre-specific change in α-adrenergic vasoconstrictor responsiveness is consistent with the work of Musch et al. (2004) demonstrating that muscle blood flow in old rats is lower during moderate-intensity exercise to high-oxidative muscles, such as the soleus muscle, but not to low-oxidative muscles. The mechanism for the enhanced α-adrenergic vasoconstrictor responsiveness in highly oxidative muscle arterioles with old age appears to be through an alteration in a signalling pathway(s) associated with the endothelial cell layer and not the smooth muscle, since removal of the vascular endothelium abolished differences in NA-mediated vasoconstriction between soleus muscle arterioles from young and old rats (Fig. 1B). Previous work has demonstrated that stimulation of endothelial α2-adrenoreceptors causes the release of several endothelium-derived vasodilator substances, including nitric oxide, prostacyclin, and hyperpolarizing factor(s) (Vanhoutte, 2001). Because we have previously shown that endothelium-dependent vasodilatation in response to acetylcholine is reduced with old age in soleus muscle arterioles, but not gastrocnemius muscle arterioles, and that this reduction occurs as the result of an impairment of the NOS-signalling mechanism (Muller-Delp et al. 2002b; Spier et al. 2004), we tested the possibility that the enhanced α-adrenergic vasoconstriction may also occur through an impairment of the NOS-signalling pathway. Results from these studies demonstrate that the age-associated difference in NA-mediated vasoconstriction is no longer present with NOS inhibition in soleus muscle arterioles from young and old rats (Fig. 1C). Collectively, these data indicate that vasoconstrictor responses mediated through smooth muscle α-receptors are not altered by old age (Fig. 1B), but rather the vasodilator response mediated through the endothelial cell nitric oxide pathway via α-receptors is impaired by old age. The net effect of no change in smooth muscle constriction and diminished endothelium-mediated relaxation through endothelial α-receptors is an enhanced vasoconstrictor response in soleus muscle arterioles.
Dinenno et al. (2001b) demonstrated that infusion of the α-adrenergic blocker phentolamine abolished differences in basal leg blood flow and vascular resistance between young and old subjects, indicating the ageing effect to decrease blood flow and increase vascular resistance was the result of several possible mechanisms associated with adrenergic vasoconstrictor tone, including elevated adrenergic vasoconstrictor sensitivity of the leg vasculature in older men. Studies investigating in vivo and in vitro adrenergic vasoconstrictor responses, however, have generally found no change or diminished vascular responsiveness to adrenergic stimulation (Docherty, 1990; Hogikyan & Supiano, 1994; Delp et al. 1995; Davy et al. 1998; Dinenno et al. 2002). Such decrements in adrenergic vasoconstrictor responsiveness have generally been attributed to augmented sympathetic nerve activity and NA release in humans (Dinenno et al. 1999) and rats (Eikenburg, 1991; Irwin et al. 1992; Buchholz et al. 1998; Robert et al. 1998), leading to vascular desensitization to adrenergic simulation with ageing. Results from the present study demonstrate no change in adrenergic vasoconstrictor responsiveness of gastrocnemius muscle arterioles (Fig. 2A) and enhanced NA-mediated vasoconstriction of soleus muscle arterioles (Fig. 1A) with old age. Several possible factors could account for this apparent discrepancy with previous work. First, previous in vitro studies have used conduit arteries to study age-related changes in adrenergic responsiveness, whereas the present study is of resistance arteries from within skeletal muscle. Undoubtedly the local chemical milieu surrounding arterioles within metabolically active skeletal muscles such as the soleus muscle differs from that of conduit arteries and arterioles from generally inactive muscles (e.g. gastrocnemius muscle), which in turn could differentially affect vasomotor properties. A second possibility is that there are regional differences in the effects of ageing on the arterial vasculature. Although most human studies indicate that ageing reduces adrenergic vasoconstriction under resting conditions, these studies have predominantly been of the forearm (Hogikyan & Supiano, 1994; Davy et al. 1998; Dinenno et al. 2002). Previous work has shown that vasomotor responses can differ between the arms and legs (Newcomer et al. 2004), including greater adrenergic vasoconstriction in the legs than the arms (Gidding et al. 1985; Pawelczyk & Levine, 2002). Thus, age-associated changes in vascular responsiveness may not necessarily be homogeneous in humans. Data from the present study demonstrate that age-associated changes in α-adrenergic vascular responses are not homogeneous among skeletal muscles (e.g. soleus versus gastrocnemius muscle arterioles).
Contrary to the findings in old humans that adrenergic vasoconstrictor responsiveness is diminished in the forearm under resting conditions, during exercise it appears that adrenergic vasoconstriction is enhanced (Koch et al. 2003; Fadel et al. 2004). To further clarify the mechanism of this old-age-associated blunting of sympathetically mediated vasoconstriction during exercise, Dinenno et al. (2005) reported an impaired functional sympatholysis during the infusion of adrenergic receptor agonists in old men while performing rhythmic handgrip exercise. Koch et al. (2003), Fadel et al. (2004) and Dinenno et al. (2005) proposed that deficient bioavailability of a substance such as nitric oxide may mediate the enhanced adrenergic vasoconstriction in old subjects during exercise, since nitric oxide has been shown to attenuate sympathetic vasoconstrictor responses during exercise (Thomas & Victor, 1998; Chavoshan et al. 2002), and ageing impairs endothelium-dependent vasodilatation and nitric oxide signalling in the skeletal muscle vasculature (DeSouza et al. 2000; Taddei et al. 2001; Muller-Delp et al. 2002b; Eskurza et al. 2004). Results from the present study are the first to demonstrate that ageing-induced increases in adrenergic vasoconstriction of resistance arteries occur as a result of vascular dysfunction of the endothelial adrenoreceptor–NOS signal transduction pathway; this may serve to enhance adrenergic-mediated vasoconstriction and, correspondingly, limit skeletal muscle blood flow during exercise in the elderly.
The findings from the present study of an enhanced vasoconstrictor response to NA in soleus muscle arterioles with ageing are in contrast to previously published results in which we reported no difference in NA-induced vasoconstriction between young and old rats (Muller-Delp et al. 2002a). Several factors could contribute to these disparate findings. First, in the present study, the NA response was limited to α-receptors by blocking β-adrenergic receptors with propranolol. In our previous study no attempt was made to block β-receptors, so age-associated differences in β-receptor-mediated vasodilatation may have influenced the NA response. Second, one of the goals of our previous work was to determine whether ageing altered the development of spontaneous tone and myogenic vasoconstriction. Therefore, arterioles were included in the study even if they did not develop significant spontaneous tone and, consequently, arterioles from old animals had less tone at the initiation of the NA dose–response test. In the present study, arterioles that did not develop at least 20% tone were excluded from study. Thus in the present investigation, age-associated differences in spontaneous tone were largely factored out, so the specific effect of ageing on NA-mediated vasoconstriction could be ascertained.
Ageing and β-adrenergic vasodilatation
The present study demonstrates that ageing differentially affects the α-adrenergic and β-adrenergic vascular responses. The β2-adrenergic receptors mediate vasodilatation via both endothelium-dependent and -independent mechanisms (Vanhoutte, 2001). The present results demonstrate that there is an age-associated reduction in β-adrenergic vasodilatation in arterioles from both the soleus (Fig. 3A) and gastrocnemius (Fig. 4A) muscles, and that this impairment in vasodilatation is specifically associated with the smooth muscle (Figs 3B and 4B). Furthermore, responses to the adenylate cyclase agonist forskolin were not different between skeletal muscle arterioles from young and old animals (Fig. 5), indicating the activity of adenylate cyclase, formation of cAMP and relaxation induced by cAMP are not adversely affected by old age. Therefore, these data suggest that the impairment of β-adrenergic vasodilatation is the result of an alteration upstream of adenylate cyclase, such as a decrement in the smooth muscle β-receptor number or sensitivity.
The reduced β-adrenergic vasodilatation with ageing reported in the present study agrees with previous findings in the human leg showing β-adrenergic vasodilatation is reduced with old age (van Brummelen et al. 1981; Ford et al. 1995). Our data extend these previous observations by demonstrating that decreased β-adrenergic vasodilatation with age is not due to reductions in endothelial function, but is the result of a smooth muscle cell β-receptor dysfunction.
Exercise training and adrenergic vasomotor responses
Previous work in young animals has shown that chronic exercise training reduces vasoconstrictor responsiveness to NA in large-conduit arteries (e.g. abdominal aorta) through an upregulation of the NOS-signalling pathway (Delp et al. 1993; Delp & Laughlin, 1997; Spier et al. 1999). Results from the present study demonstrate that although ageing increases α-receptor-mediated vasoconstriction in the resistance vasculature of high-oxidative muscle, exercise training was able to reverse this ageing effect (Fig. 1A). In addition, exercise training attenuated NA-mediated vasoconstriction in resistance arterioles from low-oxidative glycolytic muscle from young and old animals where no ageing-induced enhancement of α-adrenergic vasoconstriction existed (Fig. 2A). In both muscle types, the training effect was abolished by removal of the endothelium or inhibition of the NOS signalling mechanism, indicating the training-induced attenuation of NA-mediated vasoconstriction occurs through an enhancement of the endothelial α-receptor-NOS signal transduction pathway. In fact, NOS inhibition augmented NA-mediated vasoconstriction in soleus muscle arterioles from YT rats (Fig. 1C), indicating the contribution of nitric oxide to endothelial α-receptor-mediated vasodilatation becomes greater with training in young animals. In support of these observations, we have previously reported that exercise training upregulates endothelial NOS mRNA expression and protein content, as well as endothelium-dependent vasodilatation in soleus and gastrocnemius muscle arterioles from young and old rats (Spier et al. 2004). The stimulus for the upregulation of the NOS-signalling mechanism appears to be exercise-induced elevations in blood flow and vascular shear stress (Delp, 1998; Spier et al. 2004), which has been shown to increase endothelial NOS mRNA and protein expression (Nadaud et al. 1996). Another possible stimulus for the training-induced decrease in vascular responsiveness to NA is the chronic elevation in muscle sympathetic nerve activity during exercise and a concomitant desensitization or downregulation of smooth muscle α-receptors. This possibility, however, seems unlikely due to the lack of a training effect on the arteriolar smooth muscle response to NA when the endothelial cell layer was removed (Fig. 1B).
The present study also demonstrates that there is an age-associated reduction in β-adrenergic vasodilatation and that exercise training fails to augment maximal vasodilatation in skeletal muscle arterioles from either young or old rats. However, training increased the sensitivity of gastrocnemius muscle arterioles to β-adrenergic stimulation in old rats (Fig. 4A), indicating that training elevates β-adrenergic vasodilatation in fast-twitch glycolytic skeletal muscles.
Conclusions
In summary, adrenergic receptor-mediated vascular responses shift toward a more proconstrictor phenotype in skeletal muscle arterioles during healthy ageing. This is brought about by an enhanced α-adrenergic vasoconstriction in high-oxidative slow-twitch muscle arterioles and a more generalized reduction in β-adrenergic vasodilatation in skeletal muscle arterioles. The increases in α-adrenergic vasoconstriction in soleus muscle arterioles with ageing is not the result of changes in smooth muscle cell responsiveness to NA, but rather is due to an impairment of the endothelial cell α-receptor-NOS signalling mechanism. Exercise training augmented endothelial α-adrenoreceptor-nitric oxide vasodilator function, normalizing the vasoconstrictor responses of arterioles from old animals to levels equivalent to that in young rats. The diminished β-mediated vasodilatation with old age does not appear to be due to a diminished endothelial function or impairment of the vascular smooth muscle adenylate cyclase activity, but may be related to a reduction in the number or sensitivity of smooth muscle β-receptors. Collectively, these studies indicate that adrenergic mechanisms may serve to increase systemic vascular resistance and diminish limb perfusion with ageing, particularly during exercise. Moreover, the effect of exercise training to enhance endothelial α-adrenergic NOS-mediated vasodilatation and arteriolar sensitivity to β-adrenergic stimulation could contribute to the beneficial effects of exercise on cardiovascular disease.
Acknowledgments
This work was supported by National Aeronautics and Space Administration grants NAG2-1340 and NCC2-1166.
References
- Aaker A, Laughlin MH. Diaphragm arterioles are less responsive to alpha 1- adrenergic constriction than gastrocnemius arterioles. J Appl Physiol. 2002;92:1808–1816. doi: 10.1152/japplphysiol.01152.2001. [DOI] [PubMed] [Google Scholar]
- Beere PA, Russell SD, Morey MC, Kitzman DW, Higginbotham MB. Aerobic exercise training can reverse age-related peripheral circulatory changes in healthy older men. Circulation. 1999;100:1085–1094. doi: 10.1161/01.cir.100.10.1085. [DOI] [PubMed] [Google Scholar]
- Buchholz J, Sexton P, Hewitt CW. Impact of age on modulation of norepinephrine release from sympathetic nerves in the rat superior mesenteric artery. Life Sci. 1998;62:679–686. doi: 10.1016/s0024-3205(97)01163-6. [DOI] [PubMed] [Google Scholar]
- Chavoshan B, Sander M, Sybert TE, Hansen J, Victor RG, Thomas GD. Nitric oxide-dependent modulation of sympathetic neural control of oxygenation in exercising human skeletal muscle. J Physiol. 2002;540:377–386. doi: 10.1113/jphysiol.2001.013153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy KP, Seals DR, Tanaka H. Augmented cardiopulmonary and integrative sympathetic baroreflexes but attenuated peripheral vasoconstriction with age. Hypertension. 1998;32:298–304. doi: 10.1161/01.hyp.32.2.298. [DOI] [PubMed] [Google Scholar]
- Delp M. Effects of exercise training on endothelium-dependent peripheral vascular responsiveness. Med Sci Sports Exerc. 1995;27:1152–1157. [PubMed] [Google Scholar]
- Delp M. Differential effects of training on the control of skeletal muscle perfusion. Med Sci Sports Exerc. 1998;30:361–374. doi: 10.1097/00005768-199803000-00005. [DOI] [PubMed] [Google Scholar]
- Delp MD, Brown M, Laughlin MH, Hasser EM. Rat aortic vasoreactivity is altered by old age and hindlimb unloading. J Appl Physiol. 1995;78:2079–2086. doi: 10.1152/jappl.1995.78.6.2079. [DOI] [PubMed] [Google Scholar]
- Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol. 1996;80:261–270. doi: 10.1152/jappl.1996.80.1.261. [DOI] [PubMed] [Google Scholar]
- Delp M, Laughlin M. Time course of enhanced endothelium-mediated dilation in aorta of trained rats. Med Sci Sports Exerc. 1997;29:1454–1461. doi: 10.1097/00005768-199711000-00011. [DOI] [PubMed] [Google Scholar]
- Delp MD, McAllister RM, Laughlin MH. Exercise training alters endothelium-dependent vasoreactivity of rat abdominal aorta. J Appl Physiol. 1993;75:1354–1363. doi: 10.1152/jappl.1993.75.3.1354. [DOI] [PubMed] [Google Scholar]
- DeSouza CA, Clevenger CM, Greiner JJ, Smith DT, Hoetzer GL, Shapiro LF, Stauffer BL. Evidence for agonist-specific endothelial vasodilator dysfunction with ageing in healthy humans. J Physiol. 2002;542:255–262. doi: 10.1113/jphysiol.2002.019166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Dietz NM, Joyner MJ. Aging and forearm postjunctional α-adrenergic vasoconstriction in healthy men. Circulation. 2002;106:1349–1354. doi: 10.1161/01.cir.0000028819.64790.be. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Jones PP, Seals DR, Tanaka H. Limb blood flow and vascular conductance are reduced with age in healthy humans: relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation. 1999;100:164–170. doi: 10.1161/01.cir.100.2.164. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Masuki S, Joyner MJ. Impaired modulation of sympathetic α-adrenergic vsoconstriction in contracting forearm muscle of ageing men. J Physiol. 2005;567:311–321. doi: 10.1113/jphysiol.2005.087668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinenno FA, Seals DR, Desouza CA, Tanaka H. Age-related decreases in basal limb blood flow in humans: time course, determinants and habitual exercise effects. J Physiol. 2001a;531:573–579. doi: 10.1111/j.1469-7793.2001.0573i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinenno FA, Tanaka H, Stauffer BL, Seals DR. Reductions in basal limb blood flow and vascular conductance with human ageing: role for augmented alpha-adrenergic vasoconstriction. J Physiol. 2001b;536:977–983. doi: 10.1111/j.1469-7793.2001.00977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty JR. Cardiovascular responses to ageing: a review. Pharmacol Rev. 1990;42:103–125. [PubMed] [Google Scholar]
- Donato AJ, Lesniewski LA, Delp MD. The effects of aging and exercise training on endothelin-1 vasoconstrictor responses in rat skeletal muscle arterioles. Cardiovasc Res. 2005;66:393–401. doi: 10.1016/j.cardiores.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Eikenburg DC. Age-related changes in vascular sympathetic neurotransmission in the rat kidney. J Pharmacol Exp Ther. 1991;259:176–181. [PubMed] [Google Scholar]
- Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol. 2004;556:315–324. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel PJ, Wang Z, Watanabe H, Arbique D, Vongpatanasin W, Thomas GD. Augmented sympathetic vasoconstriction in exercising forearms of postmenopausal women is reversed by oestrogen therapy. J Physiol. 2004;561:893–901. doi: 10.1113/jphysiol.2004.073619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford GA, Dachman WD, Blaschke TF, Hoffman BB. Effect of aging on beta 2-adrenergic receptor-stimulated flux of K+, PO4, FFA, and glycerol in human forearms. J Appl Physiol. 1995;78:172–178. doi: 10.1152/jappl.1995.78.1.172. [DOI] [PubMed] [Google Scholar]
- Gidding SS, Rocchini AP, Moorehead C, Schork MA, Rosenthal A. Increased forearm vascular reactivity in patients with hypertension after repair of coarctation. Circulation. 1985;71:495–499. doi: 10.1161/01.cir.71.3.495. [DOI] [PubMed] [Google Scholar]
- Hogikyan RV, Supiano MA. Arterial alpha-adrenergic responsiveness is decreased and SNS activity is increased in older humans. Am J Physiol Endocrinol Metab. 1994;266:E717–E724. doi: 10.1152/ajpendo.1994.266.5.E717. [DOI] [PubMed] [Google Scholar]
- Irwin M, Hauger R, Brown M. Central corticotropin releasing hormone activates the sympathetic nervous system and reduces immune function: increased responsivity of the aged rat. Endocrinol. 1992;131:1047–1053. doi: 10.1210/endo.131.3.1505449. [DOI] [PubMed] [Google Scholar]
- Joyner W, Davis M. Pressure profile along the microvascular network and its control. Fed Proc. 1987;46:266–269. [PubMed] [Google Scholar]
- Koch DW, Leuenberger UA, Proctor DN. Augmented leg vasoconstriction in dynamically exercising older men during acute sympathetic stimulation. J Physiol. 2003;551:337–344. doi: 10.1113/jphysiol.2003.042747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrenson L, Poole JG, Kim J, Brown C, Patel P, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol. 2003;285:H1023–H1031. doi: 10.1152/ajpheart.00135.2003. [DOI] [PubMed] [Google Scholar]
- Maeda H, Gleiser C, Masoro E, Murata I, Mcmahan C&B. Nutritional influences on aging of Fischer 344 rats. II. Pathology. J Gerontol. 1985;40:671–688. doi: 10.1093/geronj/40.6.671. [DOI] [PubMed] [Google Scholar]
- McCurdy MR, Colleran PN, Muller-Delp J, Delp MD. Physiology of a microgravity environment: selected contribution: effects of fiber composition and hindlimb unloading on the vasodilator properties of skeletal muscle arterioles. J Appl Physiol. 2000;89:398–405. doi: 10.1152/jappl.2000.89.1.398. [DOI] [PubMed] [Google Scholar]
- Meininger G, Harris P, Joshua I. Distributions of microvascular pressure in skeletal muscle of one- kidney, one clip, two-kidney, one clip, and deoxycorticosterone-salt hypertensive rats. Hypertension. 1984;6:27–34. doi: 10.1161/01.hyp.6.1.27. [DOI] [PubMed] [Google Scholar]
- Meneilly G, Elliot T, Bryer-Ash M, Floras J. Insulin-mediated increase in blood flow is impaired in the elderly. J Clin Endocrinol Metab. 1995;80:1899–1903. doi: 10.1210/jcem.80.6.7775638. [DOI] [PubMed] [Google Scholar]
- Moreau KL, Donato AJ, Tanaka H, Jones PP, Gates PE, Seals DR. Basal leg blood flow in healthy women is related to age and hormone replacement therapy status. J Physiol. 2003;547:309–316. doi: 10.1113/jphysiol.2002.032524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol. 2002b;283:H1662–H1672. doi: 10.1152/ajpheart.00004.2002. [DOI] [PubMed] [Google Scholar]
- Muller-Delp J, Spier SA, Ramsey MW, Lesniewski LA, Papadopoulos A, Humphrey JD, Delp MD. Effects of aging on vasoconstrictor and mechanical properties of rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol. 2002a;282:H1843–H1854. doi: 10.1152/ajpheart.00666.2001. [DOI] [PubMed] [Google Scholar]
- Musch TI, Eklund KE, Hageman KS, Poole DC. Altered regional blood flow responses to submaximal exercise in older rats. J Appl Physiol. 2004;96:81–88. doi: 10.1152/japplphysiol.00729.2003. [DOI] [PubMed] [Google Scholar]
- Nadaud S, Philippe M, Arnal J-F, Michel J-B, Soubrier F. Sustained increase in aortic endothelial nitric oxide synthase expression in vivo in a model of chronic high blood flow. Circ Res. 1996;79:857–863. doi: 10.1161/01.res.79.4.857. [DOI] [PubMed] [Google Scholar]
- Newcomer SC, Leuenberger UA, Hogeman CS, Handly BD, Proctor DN. Different vasodilator responses of human arms and legs. J Physiol. 2004;556:1001–1011. doi: 10.1113/jphysiol.2003.059717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelczyk JA, Levine BD. Heterogeneous responses of human limbs to infused adrenergic agonists: a gravitational effect? J Appl Physiol. 2002;92:2105–2113. doi: 10.1152/japplphysiol.00979.2001. [DOI] [PubMed] [Google Scholar]
- Poole JG, Lawrenson L, Kim J, Brown C, Richardson RS. Vascular and metabolic response to cycle exercise in sedentary humans: effect of age. Am J Physiol Heart Circ Physiol. 2003;284:H1251–H1259. doi: 10.1152/ajpheart.00790.2002. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Koch DW, Newcomer SC, Le KU, Leuenberger UA. Impaired leg vasodilation during dynamic exercise in healthy older women. J Appl Physiol. 2003;95:1963–1970. doi: 10.1152/japplphysiol.00472.2003. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Shen PH, Dietz NM, Eickhoff TJ, Lawler LA, Ebersold EJ, Loeffler DL, Joyner MJ. Reduced leg blood flow during dynamic exercise in older endurance-trained men. J Appl Physiol. 1998;85:68–75. doi: 10.1152/jappl.1998.85.1.68. [DOI] [PubMed] [Google Scholar]
- Robert A, Tran NN, Giummelly P, Atkinson J, Capdevelle-Atkinson C. Sensitivity of norepinephrine evoked vasoconstriction to pertussis toxin in the old rat. Am J Physiol Regul Integr Comp Physiol. 1998;274:R1604–R1612. doi: 10.1152/ajpregu.1998.274.6.R1604. [DOI] [PubMed] [Google Scholar]
- Rudner XL, Berkowitz DE, Booth JV, Funk BL, Cozart KL, D'Amico EB, El-Moalem H, Page SO, Richardson CD, Winters B, Marucci L, Schwinn DA. Subtype specific regulation of human vascular α1-adrenergic receptors by vessel bed and age. Circulation. 1999;100:2336–2343. doi: 10.1161/01.cir.100.23.2336. [DOI] [PubMed] [Google Scholar]
- Seals DR, Esler MD. Human ageing and the sympathoadrenal system. J Physiol. 2000;528:407–417. doi: 10.1111/j.1469-7793.2000.00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spier SA, Delp MD, Meininger CJ, Donato AJ, Ramsey MW, Muller-Delp JM. Effects of ageing and exercise training on endothelium-dependent vasodilatation and structure of rat skeletal muscle arterioles. J Physiol. 2004;556:947–958. doi: 10.1113/jphysiol.2003.060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spier SA, Laughlin MH, Delp MD. Effects of acute and chronic exercise on vasoconstrictor responsiveness of rat abdominal aorta. J Appl Physiol. 1999;87:1752–1757. doi: 10.1152/jappl.1999.87.5.1752. [DOI] [PubMed] [Google Scholar]
- Srere PA. Citrate synthase. Meth Enzymol. 1969;13:3–5. [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of no availability and oxidative stress in humans. Hypertension. 2001;38:274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- Thomas GD, Victor RG. Nitric oxide mediates contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J Physiol. 1998;506:817–826. doi: 10.1111/j.1469-7793.1998.817bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Brummelen P, Buhler F, Kiowski W, Amann F. Age-related decrease in cardiac and peripheral vascular responsiveness to isoprenaline: studies in normal subjects. Clin Sci. 1981;60:571–577. doi: 10.1042/cs0600571. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. Endothelial adrenoceptors. J Cardiovasc Pharmacol. 2001;38:796–808. doi: 10.1097/00005344-200111000-00016. [DOI] [PubMed] [Google Scholar]
- Yu B, Masoro E, Mcmahan C. Nutritional influences on aging of Fischer 344 rats. I. Physical, metabolic, and longevity characteristics. J Gerontol. 1985;40:657–670. doi: 10.1093/geronj/40.6.657. [DOI] [PubMed] [Google Scholar]