Abstract
Gallbladder smooth muscle (GBSM) exhibits spontaneous rhythmic electrical activity, but the origin and propagation of this activity are not understood. We used morphological and physiological approaches to determine whether interstitial cells of Cajal (ICC) are present in the guinea pig extrahepatic biliary tree. Light microscopic studies involving Kit tyrosine kinase immunohistochemistry and laser confocal imaging of Ca2+ transients revealed ICC-like cells in the gallbladder. One type of ICC-like cell had elongated cell bodies with one or two primary processes and was observed mainly along GBSM bundles and nerve fibres. The other type comprised multipolar cells that were located at the origin and intersection of muscle bundles. Electron microscopy revealed ICC-like cells that were rich in mitochondria, caveolae and smooth endoplasmic reticulum and formed close appositions between themselves and with GBSM cells. Rhythmic Ca2+ flashes, which represent Ca2+ influx during action potentials, were synchronized in any given GBSM bundle and associated ICC-like cells. Gap junction uncouplers (1-octanol, carbenoxolone, 18β-glycyrrhetinic acid and connexin mimetic peptide) eliminated or greatly reduced Ca2+ flashes in GBSM, but they persisted in ICC-like cells, whereas the Kit tyrosine kinase inhibitor, imanitib mesylate, eliminated or reduced action potentials and Ca2+ flashes in both cell types, as well as associated tissue contractions. This study provides morphological and physiological evidence for the existence of ICC-like cells in the gallbladder and presents data supporting electrical coupling between ICC-like and GBSM cells. The results support a role for ICC-like cells in the generation and propagation of spontaneous rhythmicity, and hence, the excitability of gallbladder.
Based on the presence or absence of spontaneous rhythmic activity, smooth muscle can be described as phasic or tonic. Phasic smooth muscle, such as that found in most regions of the gastrointestinal (GI) tract, generates basal tone with superimposed phasic contractions that correspond to cyclic depolarizations of the membrane potential that are referred to as slow waves (Horowitz et al. 1999). GI smooth muscle exhibits a broad range of electrical behaviours that underlie the excitation–contraction coupling events occurring in these cells during motor activities. Various ionic conductances and regulatory mechanisms are responsible for these behaviours (Horowitz et al. 1999; Ward et al. 2004; Kito et al. 2005; Zhu et al. 2005).
When compared to the GI tract, the gallbladder exhibits unique arrangement of smooth muscle cells and electrical events that underlie the excitation–contraction coupling. Gallbladder smooth muscle (GBSM) cells are arranged in interdigitated bundles orientated in multiple directions, which is in contrast with the sheet arrangement of smooth muscle fibres observed in the GI tract (Cai & Gabella, 1983; Balemba et al. 2006b). The gallbladder is a tonic organ, but GBSM cells exhibit spontaneous rhythmic action potentials consisting of a spike followed by a plateau phase. These action potentials and corresponding Ca2+ transients are shorter in duration and occur at higher frequency (about 0.3 Hz) (Zhang et al. 1993; Balemba et al. 2006b) than slow waves in the GI tract (Hirst & Edwards, 2006). Occasionally, subthreshold depolarizations, which resemble slow waves in shape occur among action potentials in GBSM (Balemba et al. 2006a). In the GI tract, only spikes of slow wave action potentials are eliminated by inhibiting voltage-dependent Ca2+ channels (VDCCs) whereas in the GBSM inhibition of VDCCs eliminates all membrane potential fluctuations (Zhang et al. 1993; Balemba et al. 2006b). However, like in the GI tract (van Helden et al. 2000; Ward et al. 2003) there appears to be an interdependent relationship between sarcoplasmic reticulum Ca2+ release via inositol 1,4,5-trisphosphate (InsP3) receptors (Ca2+ waves) and Ca2+ influx during action potentials (Ca2+ flashes) in GBSM (Balemba et al. 2006a).
The mechanisms for the generation and propagation of the excitation in GBSM are not understood. Whether generation, synchronization and coordination of action potentials and associated contractile activities in GBSM involve a pacemaker mechanism via specialized cells or an intrinsic property of GBSM cells has not been resolved.
In the GI tract, the presence of specialized pacemaker cells that generate oscillatory electrical activity has been described. These cells, identified as interstitial cells of Cajal (ICC), generate unique electrical patterns that drive slow waves in these cells. ICC form a network of electrically coupled cells that are linked to the smooth muscle syncytium. ICC conduct pacemaker slow waves into neighbouring smooth muscle cells causing membrane depolarization, opening of VDCCs, intracellular Ca2+ release and activation of the contractile apparatus (Ward, 2000; Ward et al. 2003, 2004; Hirst & Edwards, 2006; Hirst et al. 2006; Park et al. 2006; Sanders et al. 2006; Sanders & Ward, 2006). The loss of ICC is associated with lack of intestinal slow wave activity (Huizinga et al. 1995; Torihashi et al. 1995).
Although shapes of ICC vary amongst species, tissues and tissue layers, they have typical morphological characteristics by which they can be readily identified. At the ultrastructural level, ICC are described as myoid-like cells with multiple long interconnected processes that are rich in mitochondria, smooth endoplasmic reticulum and caveolae. They are intercalated between nerves and smooth muscle cells, and form direct appositions and/or gap junctions with surrounding cells (Faussone-Pellegrini & Thuneberg, 1999; Thuneberg, 1999; Huizinga & Faussone-Pellegrini, 2005; Popescu et al. 2005b). These cells can also be identified by immunostaining for the Kit receptor tyrosine kinase (Komuro & Zhou, 1996) and this receptor is required for their development and maintenance (Maeda et al. 1992; Torihashi et al. 1995).
While cytological and physiological studies of ICC have been conducted in the GI tract, ICC-like cells have been described in a number of organs, including urinary bladder (McCloskey & Gurney, 2002; Hashitani et al. 2004; Shafik et al. 2004; Lang & Klemm, 2005), vasculature (Harhun et al. 2005), pancreas (Popescu et al. 2005c), reproductive organs (Hashitani & Suzuki, 2004; Popescu et al. 2005a) and heart (Huizinga & Faussone-Pellegrini, 2005). The results of this study support the existence of specialized ICC-like cells in the gallbladder. Data reported here indicate that ICC-like cells are electrically coupled with GBSM cells suggesting that they could play a role in the generation and propagation of rhythmic electrical activity and gallbladder motility.
Methods
Tissue preparation
Male adult guinea pigs (200–350 g) were exsanguinated under deep halothane or isoflurane anaesthesia, according to a protocol approved by the Institutional Animal Care and Use Committee of the University of Vermont. The abdomen was opened and the biliary system, including the gallbladder, cystic duct, distal portions of the hepatic ducts, common bile duct, sphincter of Oddi and duodenum, were placed in an ice-cold Krebs’ solution (mm: 121 NaCl, 5.9 KCl, 2.5 CaCl2, 1.2 MgCl2, 25 NaHCO3, 1.2 NaH2PO4 and 8 glucose; pH 7.4). Tissues were cut open and pinned–stretched mucosa side up in a Sylgard-coated dish (Dow Corning, Midland, MI, USA). They were washed and either kept with intact mucosal layer (full thickness), or the mucosal layer was teased off with sharp forceps under stereoscopic microscopic observation to prepare whole-mounts without the mucosal layer (muscularis propria). The type of preparation and use of fixative depended on the nature of the experiment.
Immunohistofluorescence
Immunohistofluorescence was performed using whole-mounts of muscularis propria and cryostat sections of full thickness preparations. Tissues were fixed in 2% paraformaldehyde and 0.2% picric acid in 0.1 m sodium phosphate buffer pH 7.4, 24–48 h at 4°C. After fixation, tissues for cryostat sections were washed with 0.01 m phosphate buffered saline (PBS) pH 7.4 and stored in 30% sucrose phosphate buffer solution at least 24 h at 4°C. They were then embedded and frozen in Tissue Tek O.C.T. compound (Sakura Finetek USA Inc, Torrance, CA, USA). Sections 12–50 μm were cut in parallel or transverse planes to the mucosal surface using a cryostat (Microm Inc., Eden Prairie, MN, USA), and were collected on Colorfrost/Plus microscope slides (Fisher Scientific, Pittsburgh, PA, USA).
Whole-mounts and cryostat sections were processed for immunohistofluorescence using the same protocol. Tissues were washed with PBS. After a 30 min preincubation in 10% normal goat serum, 0.5% Triton X-100 in PBS, the tissues were incubated with polyclonal rabbit anti-Kit (1: 1000) in 1% normal goat serum, 0.5% Triton X-100 in PBS overnight at room temperature (RT) or 2 nights at 4°C. Tissues were then washed with PBS, incubated with goat anti-rabbit IgG conjugated to Cy3 (1: 1000) as secondary antibody for 1 h at RT, followed by washes in PBS. Mouse anti-smooth muscle α-actin (SMA; 1: 20 000, overnight at RT) and mouse anti-protein gene product 9.5 (PGP9.5; 1: 1000, overnight at RT) were used to compare the distribution of Kit-immunoreactive cells with muscle bundles and neurons, respectively, and revealed using goat anti-mouse IgG coupled to fluorescein isothiocyanate (FITC; 1: 250, 1 h at RT). Tissues were then mounted using CitiFluor (Electron Microscopy Sciences Co., Ft Washington, PA, USA).
Tissues were analysed using an Olympus AX70 fluorescence microscope equipped with proper filter sets and MagnaFire CCD camera (Optronics, Goleta, CA, USA). Images were captured using MagnaFire imaging software (Optronics, Goleta, CA, USA).
Transmission electron microscopy
Tissues (full thickness) were placed in a fixative solution containing 4% paraformaldehyde and 3% glutaraldehyde in 0.1 m phosphate buffer pH 7.4 overnight at 4°C. Tissues were rinsed in sodium hydrogen maleate buffer and postfixed with 1% osmium tetroxide for 2 h at 4°C. Tissues were then washed in distilled water, followed by sodium hydrogen maleate buffer, stained with saturated uranyl acetate solution overnight at 4°C, dehydrated through ethanol and propylene oxide solutions, infiltrated with PO: Spurr’s Resin and embedded in fresh 100% Spurr’s Flat between two microscope slides that had been previously coated with liquid release agent (Electron Microscopy Sciences Co.) and allowed to polymerize overnight at 70°C. Ultrathin sections were cut using a Reichert Ultracut E microtome, mounted on nickel thin bar grids and contrasted with uranyl acetate and lead citrate before viewing under a JEOL 1210 transmission electron microscope.
Laser confocal imaging of Ca2+ transients
Laser confocal imaging of Ca2+ transients was perfomed mostly by using muscularis propria preparations as previously described (Balemba et al. 2006a,b). Briefly, tissues were washed with Hepes buffer (mm: 110 NaCl, 5.4 KCl, 1.8 CaCl2, 1.0 MgCl2, 20 Hepes, 5 glucose, 60 sucrose; pH 7.4) and pinned out, serosal surface up, between two Sylgard blocks (1.5 cm2). They were loaded at RT with 10 μm fluo-4 acetoxymethyl ester (fluo-4 AM) in Hepes buffer containing 2.5 μg ml−1 Pluronic acid for 1 h and then washed for 30 min to 1 h with Hepes buffer to allow for de-esterification. Tissues were studied using a 2 ml chamber maintained at 35–36°C by continuous superfusion with aerated (95% O2–5% CO2) re-circulating physiological saline solution (PSS) (mm: 119 NaCl, 7.5 KCl, 1.6 CaCl2, 1.2 MgCl2, 23.8 NaHCO3, 1.2 NaH2PO4, 0.023 EDTA, 11 glucose; pH 7.3) at a rate of 3 ml min−1. Laser confocal scanning was performed using an inverted Nikon TMD microscope (Nikon USA, Melville, NY, USA) with a 60× water immersion objective (1.2 NA) equipped with Noran Oz laser confocal system (Noran Instruments, Madison, WI, USA). Intervision software (Noran Instruments) on an Indy workstation (Silicon Graphics, Mountain View, CA, USA) was used to acquire digital movies (30 images s−1 for 20 s, 600 images per movie) of oscillating fluctuations of cytosolic Ca2+ concentration ([Ca2+]i) in intact GBSM bundles. Tissues were continuously superfused with drugs immediately after recording a movie file for basal data. Data studying the action of the drugs were collected after 5, 15, 25 and 35 min of exposure to these compounds.
Analysis of digital movie files
Movie files were analysed off line for the frequency of Ca2+ flashes and Ca2+ waves (Hz) by using custom software written in our laboratory (Dr A. D. Bonev) as previously described (Balemba et al. 2006a,b). In each movie file, the frequency of Ca2+ flashes was typically determined in 4–5 different GBSM cells. The frequency of Ca2+ transients in ICC-like cells were determined whenever these cells could be delineated clearly in the fields of observation and proven to be associated with the muscle bundles being evaluated. At the same time, movies were visually assessed for the discharge and propagation of Ca2+ events among cells and bundles, tissue movements and morphology of cells involved. Then, to reduce the variability between cells the frequency data were normalized to the basal frequency value of Ca2+ events from the same cells. Data were compared using statistical programs built into the GraphPad Prism software package (GraphPad Software Inc., San Diego, CA, USA). The basal frequency of Ca2+ flashes and Ca2+ waves typically ranged between 0.2 and 0.5 Hz. Ca2+ flashes and Ca2+ waves can occur together in GBSM cells (Balemba et al. 2006a). Tissues were considered for studying Ca2+ flashes if the frequency of these events was ≥ 0.14 Hz. They were considered for Ca2+ wave studies if they displayed Ca2+ waves with either no Ca2+ flashes or Ca2+ flashes occurring at a frequency of ≤ 0.09 Hz.
Intracellular recording
Gallbladder preparations were stretched on Sylgard in a recording chamber serosal side up and placed on the stage of a Nikon TMD inverted microscope (Nikon USA). Smooth muscle bundles were visualized with a 10× objective using Hoffman modulation contrast optics (Modulation Optics, Greenvale, NY, USA). The preparations were continuously superfused at a rate of 8–10 ml min−1 with a modified aerated (95% O2–5% CO2) Krebs’ solution (see composition above). Temperature in the recording chamber was maintained between 35 and 36°C. Glass microelectrodes used for intracellular recording were filled with 2 m KCl and had input tip resistances of 70–150 MΩ. A negative-capacity compensation amplifier (Axoclamp 2A, Axon Instruments, Union City, CA, USA) with bridge circuitry was used to record electrical activity. Transmembrane voltage recordings were analysed to evaluate changes of frequency and membrane potential by using PowerLab/4SP and Chart 5, v.5.01 software (AD Instruments Inc., Colorado Springs, CO, USA).
In the present study, the extent of stretch was not measured. Samples that were not immediately used were kept in ice-chilled Hepes or Krebs’ buffers for 3–6 h.
Statistical analysis
Student’s t test and one-way ANOVA with the Newman–Keuls test were performed using GraphPad Prism 4 (GraphPad Software Inc., San Diego, CA, USA). Data were expressed as the mean ±s.e.m. and the difference was considered statistically significant at P < 0.05. The n value and number of preparations represent tissues from different animals.
Antibodies, chemicals and drugs
The following antibodies were used in this study: rabbit Kit/CD117 (DakoCytomation Inc., Carpentaria, CA, USA) mouse PGP9.5 (Biogenesis Inc., Kingston, NH, USA) mouse SMA (Sigma-Aldrich, St Louis, MO, USA), goat anti-rabbit Cy3 and goat anti-mouse FITC (Jackson Laboratories, West Grove, PA, USA). Chemicals and drugs used in the present study include: fluo-4 AM and Pluronic acid (F-127) (Molecular Probes–Invitrogen, Eugene, Oregon, USA); 1-octanol, carbenoxolone and 18β-glycyrrhetinic acid (18β-GCA) (Sigma-Aldrich). Connexin mimetic peptide (connexin P 076; H-HN-SLSAVYTCKRDPCPHQ-COOH; Mr 1804; Kwak & Jongsma, 1999) was synthesized at the University of Vermont Protein Core Facility. For standard intracellular recording, carbenoxolone and 18β-GCA were dissolved in DMSO, while in Ca2+ studies, these compounds were dissolved in double distilled water. Imatinib mesylate (imatinib; Glivec®, a gift from Novartis Pharmaceuticals Corp., East Hanover, NJ, USA) was dissolved in double distilled water. Connexin P 076 and 1-octanol were directly reconstituted in Krebs’ solution or PSS.
Results
Cells with the morphological features of ICC exist in the gallbladder and extrahepatic biliary tree
Kit immunoreactivity in the extrahepatic biliary tree
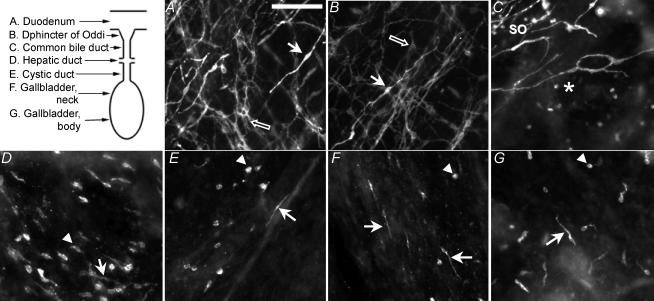
As an initial step in testing for the presence of ICC in the gallbladder, we evaluated Kit tyrosine kinase receptor immunoreactivity in whole-mount preparations of the duodenum, where Kit-immunoreactive ICC are known to exist (Komuro, 1999; Komuro et al. 1999), and of the entire extrahepatic biliary tree, including the sphincter of Oddi and the gallbladder. In the duodenum, Kit-positive cells were found in the longitudinal and circular muscle layers and at the level of the myenteric plexus. In the longitudinal and circular muscle layers, Kit-positive, elongated intramuscular ICC (Fig. 1A, white arrow) were typically orientated parallel to smooth muscle cells. At the level of the myenteric plexus, the myenteric ICC had round to triangular cell bodies from which emerged several branching processes (Fig. 1A, black arrow) that formed a network. In the sphincter of Oddi, Kit-immunopositive ICC were orientated parallel to smooth muscle cells in the longitudinal and circular muscle layers and formed a network overlying the ganglionated myenteric nerve plexus, which is similar to the organization of ICC in the duodenum (Fig. 1B). ICC were also observed in the region of the common bile duct, immediately adjacent to the sphincter of Oddi (Fig. 1C), but the density of these cells diminished rapidly within 1–2 mm of the distal end of the common bile duct. In the hepatic and cystic ducts and in the gallbladder, the majority of Kit-positive cells had morphologies reminiscent of mast cells, which also express the Kit receptor (Romert & Mikkelsen, 1998), but occasional elongated ICC-like cells were also observed (Fig. 1D–G).
Figure 1. Distribution of Kit-positive cells in the duodenum and extrahepathic biliary tree.
Upper left, schematic drawing of the biliary tree that illustrates the regions where photomicrographs were obtained. A and B, in the duodenum (A) and the sphincter of Oddi (B), elongated (white arrows) and multipolar (black arrows) ICC cells were observed in the muscular layers (longitudinal and circular) and in the myenteric plexus. C, the number of Kit-positive cells decreased abruptly at the junction of the sphincter of Oddi (SO) with the common bile duct (asterisk). C–E, in the rest of the common bile duct (C), hepatic duct (D) and cystic duct (E) the majority of Kit-positive cells had the morphological characteristics of mast cells (arrowheads) although a few isolated ICC-like cells were also noted (arrows). F–G, in the gallbladder neck (F) and body (G) a mixed population of ICC-like cells (arrows) and mast cells (arrowheads) were observed. Scale bar on A, 125 μm, valid for B–G.
In the gallbladder Kit-immunoreactive ICC-like cells and their long processes were easily visualized in whole-mount sections (Figs 1F–G and 2A–B). The ICC-like cells, although more abundant along muscle bundles and near major blood vessels, were scattered throughout the entire extent of the gallbladder, and did not appear to form a global network as has been described in other organs. Double immunolabelling of gallbladder preparations with Kit and smooth muscle α-actin (SMA) antibodies showed ICC-like cells in GBSM bundles (Fig. 2C) where they were orientated along the main axis and at the periphery of smooth muscle bundles. ICC-like cells were sometimes aligned with PGP9.5-positive nerve fibres, particularly those constituting the paravascular plexus (Fig. 2D). Kit-positive mast cells did not appear to be preferentially associated with GBSM bundles or nerve fibres.
Figure 2. Distribution of Kit-positive ICC-like cells along GBSM bundles and nerve fibres.
A–B, in the gallbladder, Kit-positive ICC-like cells (arrows) were elongated and had one or two main processes that were aligned along muscle bundles. Mast cells (arrowheads) were also observed. C, the ICC-like cells were aligned along SMA-positive cells mainly at the periphery of small GBSM bundles. D, elongated Kit-positive cells were sometimes observed adjacent to PGP9.5-positive neuronal processes. Abbreviation: BV, blood vessel. Scale bar on A, 250 μm, valid for B–D.
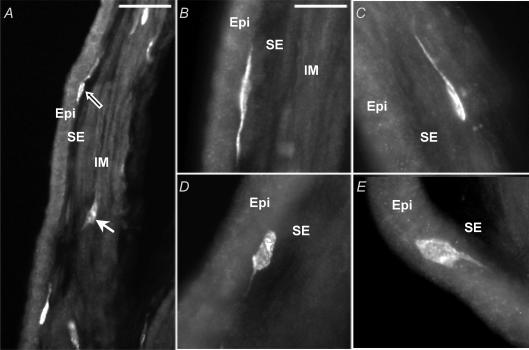
Cross-sections of gallbladder with intact mucosa (full thickness) showed Kit-positive ICC-like cells in the subepithelial connective tissue layer (SE, Fig. 3A and B) and also in the muscularis propria (IM, Fig. 3A and C). In both regions, ICC-like cells typically had elongated cell bodies (∼25 μm lengthwise) with one to two long primary processes (> 100 μm); secondary processes were sometimes observed. The processes of the ICC-like cells were generally orientated parallel to the epithelial surface or to the GBSM bundles. Kit-positive mast cells had larger round cell bodies with shorter processes (Fig. 3D and E).
Figure 3. Kit-positive ICC-like cells in the gallbladder.
In cryostat cross-sections of full-thickness gallbladder, Kit labelling was mainly observed in the subepithelial connective tissue layer (SE; A, black arrow; and B) and in the muscularis propria (IM; A, white arrow; and C). As observed in the wholemount preparation, Kit-positive cells were elongated and had one (C) or two (B) main processes. They can be differentiated from mast cells (D–E), which have larger, rounded cell body and shorter or absent processes. Abbreviation: Epi: epithelium. Scale bars: A, 50 μm; B–E, 25 μm.
Electron microscopy of ICC-like cells in the gallbladder
Transmission electron microscopy confirmed the presence of ICC-like cells in the subepithelial connective tissue layer and within the muscularis propria in the gallbladder. The distribution of ICC-like cells corresponded to that shown by light microscopy for Kit-immunoreactivity and laser confocal imaging of Ca2+ transients (see below). Elongated, electron dense, myoid-like cells that were rich in mitochondria, smooth endoplasmic reticulum and caveolae were observed along and within GBSM bundles (Fig. 4A–C). These cells were in direct apposition with GBSM cells (Fig. 4B–C) and with each other (Fig. 4D–E). Some ICC-like cells were located in close proximity to nerve fibres although direct contact between ICC-like cells and nerve fibres was not observed at the electron microscopic level (Fig. 4F). Direct appositions between adjacent GBSM cells were observed (Fig. 4G).
Figure 4. Ultrastrutural features of ICC-like cells in the gallbladder.
A, transmission electron microscopy of cross-sections of GBSM show electron dense ICC-like cells (ICC) within a GBSM bundle of the muscularis propria. At higher magnifications, wholemount gallbladder preparation revealed that ICC-like cells were rich in mitochondria (m) and caveolae (c) and formed direct contacts (curved arrows) with surrounding cells including GBSM (SM; B–C) and other ICC-like cells (ICC; D–E) and were sometimes found nearby nerve profiles (N; F) G, direct appositions (curved arrow) between GBSM cells were also observed (SM; G). Scale bars: A and E, 500 nm; B–D, 1 μm; F, 400 μm; G, 200 μm.
Laser confocal imaging of Ca2+ flashes and waves in ICC-like cells in the gallbladder muscularis
Rhythmic electrical activity and corresponding Ca2+ transients can be detected in intact GBSM by intracellular recording of the action potentials and Ca2+ imaging of Ca2+ flashes, respectively (Balemba et al. 2006b). In the GI tract, laser confocal imaging of rhythmic Ca2+ transients has been used to study rhythmic electrical activity among ICC and smooth muscle cells (Hennig et al. 2004; Park et al. 2006). We used laser confocal imaging of spontaneous Ca2+ flashes to examine whether ICC-like cells are involved in rhythmic electrical activity in the gallbladder.
In the muscularis propria, GBSM and ICC-like cells exhibited spontaneous Ca2+ flashes and Ca2+ waves (Fig. 5Ai–x; online Supplemental material Movies 1–2). The most abundant cell type was the spindle-shaped GBSM cells that constituted the vast majority of cells in the muscle bundles (mean diameter: 52.5 ± 12.0 μm; range: 14–150 μm; n= 16). Ca2+ flashes, or Ca2+ flashes and Ca2+ waves occurring together, clearly delineated the different shapes of ICC-like cells from GBSM cells. Compared to GBSM cells, ICC-like cells were more brightly loaded with fluo-4 AM (Fig. 5Aii, iv, v, vii, ix and x) and Ca2+ flashes in ICC-like cells exhibited higher intensity and longer durations. The ICC-like cells were less numerous than GBSM, and varied in shape, size and location. ICC-like cells exhibiting Ca2+ transients were either elongated or multipolar in shape. Elongated ICC-like cells with long cytoplasmic processes were closely associated with GBSM and were mainly observed in the periphery of GBSM bundles (Fig. 5Aix). Small clusters of multipolar ICC-like cells with short and long processes were usually larger in diameter than GBSM cells (Fig. 5Aiii–viii and x; Supplemental material Movies 1–2) and found at the intersections and edges of GBSM bundles (Fig. 5Av–viii), but these cells were occasionally observed adjacent to GBSM bundles (Fig. 5Ax). Both types of ICC-like cells formed close appositions with GBSM cells in the bundles (Fig. 5Aii–vii and x).
Figure 5. Demonstration of GBSM cells in the bundles and associated ICC-like cells revealed by laser confocal imaging of Ca2+ flashes with or without Ca2+ waves.
Ai–x, GBSM muscle bundles (arrows) with associated ICC-like cells (arrowheads). Notice cytoplasmic processes (iii–x) and appositions between ICC-like and GBSM cells (ii, iv and v). v-x, ICC-like cells (arrowheads) intimately associated with GBSM in bundles at the intersections (v–viii) or at the periphery (ix–x) of the bundles. Bi–iii, traces of Ca2+ flashes (arrows) and Ca2+ waves (arrowheads) generated from GBSM (indicated by blue circles) and ICC-like (indicated by red rectangles) cells in Ai, vi and ii, respectively. In Bi, traces of Ca2+ flashes and Ca2+ waves showing that Ca2+ flashes in a given bundle are synchronized, while Ca2+ waves are asynchronous. In Bii and iii, traces of Ca2+ flashes and Ca2+ waves showing electrical coupling between GBSM and ICC-like cells by synchronized Ca2+ flashes. Ca2+ waves are not synchronized. Scale bars: 10 μm.
Ca2+ flashes occurring in a given GBSM bundle were always synchronized with activities of nearby ICC-like cells. Furthermore, in a given field of observation, Ca2+ flashes in overlapping or intersecting GBSM bundles and associated ICC-like cells were typically synchronized (Fig. 5Bi–iii; Supplemental material Movies 1–2). We were unable to determine whether ICC-like cells initiated rhythmic Ca2+ flashes, or if Ca2+ waves occurring in ICC-like cells induced similar events in adjacent GBSM cells, due to tissue movements, which were caused by contractions that occurred after Ca2+ flashes.
Propagation and synchronization of action potentials and Ca2+ transients depend on gap junctions
In intact gallbladder muscularis, the synchronicity between Ca2+ flashes in GBSM bundles and associated ICC-like cells is strongly suggestive of electrical coupling. Many studies have shown that coupling of ICC and smooth muscle cells via gap junctions accounts for synchronization of slow waves, intracellular Ca2+ oscillations and muscle contractions (Rottingen & Iversen, 2000; van Helden et al. 2000; Hashitani et al. 2004; Hanani et al. 2005; Takeda et al. 2005; Park et al. 2006). Due to the multiple effects of the chemicals available for blocking gap junctions, including membrane depolarization and inhibition of ion channels (Takeda et al. 2005), we conducted parallel studies with a number of compounds known to block gap junctions. The effects of gap junction uncouplers 1-octanol (0.5–2 mm), carbenoxolone (50–200 μm) and 18β-GCA (40 μm), were tested on GBSM action potentials. Furthermore, the actions of these compounds, as well as connexin mimetic peptide (connexin P 076; 0.5 mm), on Ca2+ transients were studied in GBSM and ICC-like cells. 1-Octanol (0.5–2 mm), carbenoxolone (200 μm), and 18β-GCA (40 μm) reduced the frequency or eliminated action potentials in GBSM cells (Fig. 6A, Table 1) whereas vehicles (0.1% DMSO v/v and PSS) had no effect (Table 1). These compounds, as well as connexin P 076 (0.5 mm), also dramatically reduced the frequency of, or completely eliminated, Ca2+ flashes in GBSM cells in a time-dependent manner (Figs. 6B and 7; Supplemental material Movies 3).
Figure 6. Demonstration of the effects of the gap junction uncoupler, 1-octanol, on spontaneous action potentials and Ca2+ transients in GBSM cells.
Within minutes of initial exposure to 1-octanol, the frequencies of action potentials (A) as well of Ca2+ flashes and waves (B) were dramatically reduced in GBSM cells, and Ca2+ flashes were abolished in the cells represented in B. In B, data from 3 GBSM cells are shown and filled circles indicate synchronized Ca2+ flashes.
Table 1.
The effect of gap junction uncouplers on action potentials at 20 min
| Treatment | Hz (n-values) | P-values |
|---|---|---|
| PSS | 0.3 ± 0.01 (6) | >0.5 |
| DMSO (0.1%v/v) | 0.3 ± 0.02 (8) | >0.5 |
| 1-Octanol (2 mm) | 0.1 ± 0.04 (5) | 0.007 |
| Carbenoxolone (200 μm) | 0.1 ± 0.04 (5) | 0.002 |
| 18β-GCA (40 μm) | 0.2 ± 0.01 (4) | 0.0001 |
P < 0.05 indicates a statistically significant change caused by drug compared to vehicle control (either PSS, or DMSO). n= number of animals used.
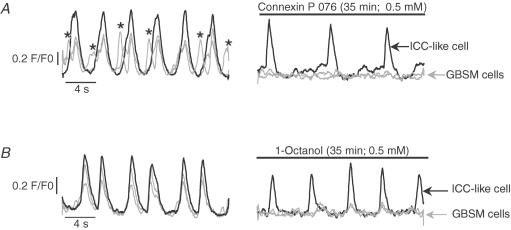
Figure 7. Demonstration of the differential effects of gap junction uncouplers on Ca2+ transients in ICC-like cellsversusGBSM cells.
After 35 min exposure to connexin P 076 (A) or 1-octanol (B), Ca2+ transients were abolished in GBSM cells (grey traces) but persisted in ICC-like cells (black traces). The asterisks in A indicate Ca2+ waves; all other Ca2+ transients in this figure are Ca2+ flashes.
In microscopic fields containing both GBSM and ICC-like cells, Ca2+ flashes persisted in the ICC-like cells (0.5 mm 1-octanol: ICC-like cell flashes persisted in all 3 preparations tested, 8 cells; 50 μm carbenoxolone: Ca2+ flashes persisted in ICC-like cells in both preparations tested, 4 cells; 0.5 mm connexin P 076: Ca2+ flashes persisted in ICC-like cells in both preparations tested, 3 cells; Fig. 7; Supplemental material Movies 3), whereas they were usually abolished, or in the remaining cases disrupted, in GBSM (1-octanol: GBSM flashes eliminated in all 3 preparations, 25 cells; carbenoxolone: GBSM flashes eliminated in 1 of 2 preparations, 14/20 cells; connexin P 076: GBSM flashes eliminated in 1 of 2 preparations, 9/21 cells). Collectively, these findings suggest that the propagation and synchronization of action potentials and Ca2+ flashes among GBSM and ICC-like cells involved intercellular conductivity via gap junctions.
The propagation of Ca2+ transients amongst adjacent cells depends on the diffusion of intracellular messengers InsP3, cyclic ADP-ribose and Ca2+, and electrical coupling across gap junctions (Churchill & Louis, 1998; Rottingen & Iversen, 2000; Hennig et al. 2002; Park et al. 2006). In the gallbladder muscularis propria, 1-octanol (0.5–2 mm), carbenoxolone (50–200 μm), 18β-GCA (40 μm), and connexin P 076 (0.5 mm) reduced or eliminated Ca2+ waves (Figs 6B and 7A) suggesting that syncytial communication via gap junctions is required for the generation of Ca2+ waves in GBSM bundles and ICC-like cells.
The Kit receptor tyrosine kinase inhibitor, imanitib disrupts the generation and propagation of spontaneous action potentials and Ca2+ transients
Studies using imanitib mesylate, a selective Kit tyrosine kinase inhibitor, have suggested that Kit receptor is involved in the generation of rhythmic excitation by ICC in the GI tract (Shimojima et al. 2005; Popescu et al. 2006) and ICC-like cells in the urinary tract (Kubota et al. 2004, 2006; Biers et al. 2006) and uterus (Popescu et al. 2006). We therefore investigated the effects of imatinib on rhythmic spontaneous action potentials and Ca2+ flashes in intact gallbladder preparations.
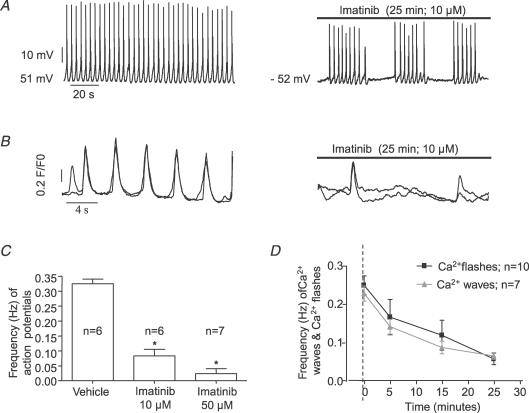
Imatinib (10–50 μm) decreased the frequency and disrupted the rhythmic pattern of action potentials in GBSM in a concentration-and time-dependent manner (Fig. 8A and C). However, non-specific actions of experimental compounds are a necessary concern in pharmacological studies. It has been shown that higher concentrations of imatinib (50–100 μm) block inward (VDCC) and outward currents in guinea pig detrussor (Kubota et al. 2004). These currents are not affected by 10 μm imatinib (Kubota et al. 2004, 2006; Biers et al. 2006). For this reason we used this concentration in most experiments. Imatinib (10 μm) decreased the frequency and disrupted the rhythmic pattern of action potentials by causing clustered, irregularly occurring action potentials separated by quiescent periods (Fig. 8A). Washout for 5–15 min restored the basal pattern and frequency of action potentials (n= 2).
Figure 8. Demonstration of the effects of imatinib on action potentials, Ca2+ flashes and Ca2+ waves.
Traces showing that imatinib (10 μm) disrupts the discharge and rhythmic pattern and decreases the frequency of action potentials(A) and Ca2+ flashes (B) in intact guinea pig gallbladder muscularis. The action of imatinib on the frequency of action potentials (C 25 min, P < 0.0007), Ca2+ flashes and Ca2+ waves (D) in GBSM cells is concentration and time dependent.
Imatinib did not affect the amplitude (mV) (basal: 61.9 ± 5.5 versus 10 μm imatinib: 53.4 ± 2.1, n= 5, P > 0.05; 25 min), or the average slope (mV s−1) of the rapid upstroke depolarization (basal: 0.008 ± 0.001 versus 10 μm imatinib: 0.006 ± 0.002, n= 5, P= 0.112; 25 min). Imatinib did not affect the duration (s) of action potentials (basal: 1.06 ± 0.13, n= 7 versus 10 μm imatinib: 1.075 ± 0.100, n= 5, P= 0.9; 25 min) and the duration of the spikes of action potentials (measured between the first component of the upstroke depolarization and the complete repolarization of the spike; basal: 0.37 ± 0.01 versus 10 μm imatinib: 0.48 ± 0.05, n= 4, P= 0.4; 25 min). In addition, the duration of the plateau and the shape of action potentials were not affected by imatinib (10 μm). Furthermore, imatinib, even at a higher concentration (50 μm), did not affect the resting membrane potential (basal: –47.05 ± 2.00 versus 50 μm imatinib: –46.7 ± 3.0, n= 8; P= 0.5; 25 min).
The effects of imatinib on Ca2+ flashes corresponded to its effects on action potentials in GBSM cells. Furthermore, imatinib had comparable effects on Ca2+ flashes in both GBSM and ICC-like cells. Imatinib (10 μm) reduced the frequency and disrupted the rhythmic pattern of Ca2+ flashes in GBSM and ICC-like cells in a time-dependent fashion (Fig. 8B and D) but did not eliminate Ca2+ flashes. In the digital movies, imatinib caused marked reduction of, or completely eliminated, tissue movement after 15–25 min.
Ca2+ waves play a fundamental role in the gallbladder excitability (Balemba et al. 2006a). We therefore tested the effect of imatinib on Ca2+ waves in GBSM and associated ICC-like cells in intact gallbladder muscularis propria preparations and found that imatinib (10 μm) decreased the frequency of Ca2+ waves in a time-dependent manner (Fig. 8D).
Discussion
The aims of this investigation were to determine whether ICC exist in the gallbladder, and if so, to test whether they play a role in rhythmic, spontaneous electrical activity in GBSM. We found ICC in the sphincter of Oddi and the adjacent region of the common bile duct and ICC-like cells in the gallbladder and other regions of the extrahepatic biliary tract. In the gallbladder, ultrastructural examination revealed that ICC-like cells were in direct contact with GBSM cells, and Ca2+ imaging studies demonstrated that the rhythmic activities of these two cell types were synchronized. Gap junction blockers eliminated or greatly disrupted spontaneous rhythmic Ca2+ flashes in GBSM, but they persisted in ICC-like cells. Furthermore, spontaneous rhythmic electrical activity in the gallbladder musclularis was disrupted or abolished by an inhibitor of Kit receptor tyrosine kinase. These observations strongly suggest that ICC-like cells play a role in the generation and propagation of rhythmic excitation in gallbladder muscularis.
The classical identification of ICC in the GI tract is based on their ultrastructural characteristics (Faussone-Pellegrini & Thuneberg, 1999; Komuro et al. 1999; Thuneberg, 1999; Huizinga & Faussone-Pellegrini, 2005). Recently Kit receptor tyrosine kinase immunolocalization has been used as a reliable marker of ICC in the GI tract and ICC-like cells in other organs (Maeda et al. 1992; Faussone-Pellegrini & Thuneberg, 1999; Komuro, 1999; Hashitani & Suzuki, 2004; Huizinga & Faussone-Pellegrini, 2005; Popescu et al. 2005c; Sanders & Ward, 2006). In this study, immunohistochemistry, transmission electron microscopy and laser confocal imaging were used to identify ICC-like cells. We describe for the first time, networks of Kit-positive ICC in the longitudinal and circular muscular layers and at the level of the myenteric plexus in sphincter of Oddi and adjacent region of the common bile duct. We have found that ICC-like cells in the gallbladder resemble ICC in the GI muscularis (Komuro, 1999), the sphincter of Oddi (this study), and ICC-like cells in other organs (McCloskey & Gurney, 2002), but they do not form a global network. Instead, they are typically located along GBSM bundles and nerve fibres.
Morphological characteristics of ICC are species and tissue dependent, and ICC have been divided in different classes according to their location in the GI tract (Burns et al. 1997; Komuro, 1999; Komuro et al. 1999). Based on their distribution across the gallbladder wall, ICC-like cells were divided into subepithelial and intramuscular cells. A brief report suggests a similar distribution of kit-positive ICC in the murine gallbladder wall (Sun et al. 2006). The morphologies of ICC-like cells in both of these regions were comparable, with primary processes being orientated parallel to GBSM bundles and nerve fibres. ICC with similar morphological characteristics have been observed in the GI tract (Burns et al. 1997; Komuro, 1999) as well as the urinary bladder (McCloskey & Gurney, 2002).
Laser confocal imaging of Ca2+ flashes is a useful complementary tool to study ICC-like cells and electrical coupling in the gallbladder muscularis. Ca2+ imaging revealed small clusters of multipolar ICC-like cells at the intersections and the distal ends of GBSM bundles, as well as elongated bipolar ICC-like cells at the edges of GBSM bundles. The clear delineation of ICC-like cells from GBSM cells corresponded to descriptions of these cell types in the GI tract (Yamazawa & Iino, 2002; Hennig et al. 2004; Park et al. 2006).
In the gallbladder, ICC-like cells appear to be electrically coupled with GBSM cells. We have previously shown that in any given GBSM bundle, intracellular Ca2+ flashes are synchronized amongst all of the smooth muscle cells. They originate at one end, and propagate along the longitudinal axis towards the far end of a bundle (Balemba et al. 2006b). Similarly, action potentials in GBSM cells within a bundle are synchronized (O. B. Balemba & G. M. Mawe, personal observations from dual intracellular recording expriments). Furthermore, when neurobiotin is intracellularly injected into GBSM cells, it spreads in a longitudinal direction into other smooth muscle cells in the same bundle (O. B. Balemba & G. M. Mawe, personal observations). In the present study, we found that spontaneous rhythmic activity in smooth muscle cells is synchronized with the activity of ICC-like cells in a given bundle, which indicates electrical coupling between GBSM and ICC-like cells. Direct appositions among GBSM cells have been previously demonstrated in guinea pig gallbladder (Cai & Gabella, 1983). In addition to appositions between GBSM cells, we provide in this study novel findings for direct appositions between ICC-like cells and also between GBSM and ICC-like cells. Although unambiguous gap junctions were not identified morphologically, the observations of direct appositions, synchronized Ca2+ flashes, and disruption or elimination of electrical activity and Ca2+ flashes by gap junction uncouplers in GBSM cells whereas Ca2+ flashes persist in ICC-like cells strongly suggest that electrical coupling occur between GBSM and ICC-like cells. This is consistent with data from other organs (Kwak & Jongsma, 1999; Schultz et al. 2003; Chaytor et al. 2005; Takeda et al. 2005; Matchkov et al. 2006; Park et al. 2006).
The basic characteristics of Ca2+ flashes, and thus spontaneous rhythmic electrical activity, in GBSM and ICC-like cells are similar. Like rhythmic Ca2+ transients in ICC and smooth muscle cells in the GI tract (Yamazawa & Iino, 2002; Hennig et al. 2004; Park et al. 2006), Ca2+ flashes in GBSM and ICC-like cells occurred with the same frequency and were synchronized. Ca2+ flashes are eliminated by VDCC inhibitors in intact gallbladder muscularis (Balemba et al. 2006b). Furthermore, Ca2+ flashes in GBSM and ICC-like cells are equally affected by the Kit tyrosine kinase inhibitor, imatinib. These observations suggest that a common cellular mechanism is responsible for the generation and propagation of electrical activity in these two cell types, although the ionic conductances and molecular entities may be different. The basis for potential differences are the new findings that gap junction uncouplers eliminate spontaneous rhythmic Ca2+ flashes in GBSM, but these events persist in ICC-like cells, which suggests a pacing role for the ICC-like cells. In addition, Ca2+ flashes in ICC-like cells had higher intensity and lasted longer than in GBSM cells, and ICC-like cells were more brightly loaded with Fluo-4 AM than GBSM cells. These later findings are similar to previous observations in intestinal tissues (Yamazawa & Iino, 2002; Hennig et al. 2004; Park et al. 2006).
It is now generally accepted that in the GI tract, ICC generate pacemaking slow waves and corresponding rhythmic Ca2+ transients (Ca2+ flashes), and that they transmit these electrical signals to gastrointestinal smooth muscle. In the GI tract, ICC are also involved in stretch perception, inhibitory and excitatory neurotransmission, and possibly afferent signalling (Hennig et al. 2004; Ward et al. 2004; Hirst & Edwards, 2006; Hirst et al. 2006; Sanders et al. 2006; Sanders & Ward, 2006). ICC-like cells have also been proposed to play a role as pacemakers in other organs, including the urinary bladder (Kubota et al. 2004), the penis (Hashitani & Suzuki, 2004) and the uterus (Popescu et al. 2006). However, others have suggested that ICC-like cells in the urinary bladder are more likely to modulate the transmission of Ca2+ transients originating from smooth muscle cells rather than serving as pacemakers (Hashitani et al. 2004). Morphological and functional data included in the current study support the concept that ICC-like cells are involved in pacing electrical rhythmicity in the gallbladder. However, the exact roles of ICC-like cells in the gallbladder have not yet been resolved. For example, we have not definitively determined whether ICC-like cells generate pacemaker activity, we still do not know whether different classes of ICC-like cells have different roles, and we do not yet know whether gallbladder ICC-like cells play a role in mediating neuromuscular response. However, now that ICC-like cells have been identified in the gallbladder, these issues will be the focus of future investigations.
Based on the results of the current study, Kit receptor tyrosine kinase appears to be involved in the discharge and propagation of excitability in gallbladder musculature. Among cell types with smooth muscle cell-like phenotype, Kit tyrosine kinase is exclusively expressed in ICC (Maeda et al. 1992; Torihashi et al. 1995). The inhibition of Kit tyrosine kinase by imatinib is a widely accepted procedure for treating GI stromal tumours, which arise from ICC (Druker et al. 1996; Peng et al. 2005) and are common in the gallbladder (Ortiz-Hidalgo et al. 2000; Mendoza-Marin et al. 2002; Park et al. 2004; Furihata et al. 2005). By using imatinib, we have shown that Kit receptor tyrosine kinase regulates excitability of GBSM, and thus gallbladder motility, by modulating the generation and rhythmic pattern of electrical activity in ICC-like and GBSM cells. Our findings are consistent with observations on spontaneous mechanical activity in the intestine (Popescu et al. 2005a; Shimojima et al. 2005) and uterus (Popescu et al. 2006), and on ion currents, action potentials and associated contractions in the urinary bladder (Kubota et al. 2004, 2006; Biers et al. 2006). However the use of Kit neutralizing antibody takes many days to block electrical rhythmicity in ICC (Ward et al. 2006). The reason for the difference in time course between the actions of neutralizing antibodies and imatinib as shown here and in other studies (Shimojima et al. 2005) is not clear. The actions of imatinib on the excitability of gallbladder muscularis are similar to those of compounds that inhibit sarcoplasmic reticulum Ca2+ release via ryanodine and InsP3 receptors (Balemba et al. 2006b). It is possible that imatinib may act at least in part by interfering with sarcoplasmic reticulum Ca2+ release mechanisms downstream, impeding both sarcoplasmic reticulum and mitochondrial function for the production of rhythmic activity.
The guinea pig gallbladder contains many Kit-positive mast cells. Morphological features and distribution of Kit-positive mast cells correspond to previous observations in the gut (Hemming et al. 2000) and other organs (Romert & Mikkelsen, 1998; McCloskey & Gurney, 2002; Hashitani et al. 2004; Shafik et al. 2004). Although mast cells are abundant in the wall of the guinea pig gallbladder, spontaneous rhythmic activity does not depend on histamine from mast cells (Hemming et al. 2000). Similar findings have been reported in small intestine and uterus (Popescu et al. 2006). Therefore, the actions of imatinib on mast cells do not appear to account for the observations in spontaneous electrical activity reported here.
In intact gallbladder muscularis, Kit receptor tyrosine kinase signalling and syncytial integrity amongst GBSM and ICC-like cells are crucial for optimal intracellular Ca2+ wave activity. In this study, gap junction uncouplers and imatinib reduced the frequency of Ca2+ waves suggesting that these compounds affected the discharge or re-activation of the propagation of Ca2+ waves, or both. These findings support the observation of Hennig et al. (2004) in the guinea pig antrum in which the occurrence of intracellular Ca2+ waves in intramuscular ICC was influenced by pacemaker activity in myenteric ICC. Our observations are also consistent with our theory that Ca2+ waves play a pivotal role in the excitability of GBSM cells, and represent a fundamental unit of spontaneous electrical activity (Balemba et al. 2006a). However, there is still some question with regard to the specificity of the actions of imanitib and the gap junction blockers used in the current study. Imatinib’s effects on the functions of ICC and GBSM were relatively rapid given that Kit is a tyrosine kinase receptor. Furthermore, while several gap junction inhibitors were used in the current study in an effort to validate the results, the available compounds are notorious for their non-specific actions. Clearly, the mechanisms for the entrainment of intracellular Ca2+ release in GBSM cells and associated ICC-like cells still need to be elucidated.
In summary, ICC exist in the sphincter of Oddi with organization similar to that of ICC in the duodenum. The reminder of the extrahepatic biliary tract including the gallbladder contains Kit-positive ICC-like cells, which do not form a global network. In the gallbladder, ICC-like cells are found in the muscularis propria and the subepithelial connective tissue layer and ultrastucturally resemble ICC. Data reported here support that ICC-like cells and GBSM cells are electrically coupled, and that ICC-like cells are likely involved in generating and pacing spontaneous electrical activity in gallbladder muscularis propria. The propagation and synchronization of rhythmic electrical activity in GBSM and ICC-like cells depend on gap junctions and Kit receptor tyrosine kinase. Additional studies will be required to resolve the precise role(s) of ICC-like cells in gallbladder function.
Acknowledgments
The authors wish to thank Dr S. A. Locknar for assistance with imaging, N. Bishop and Y. Bayguinov for assistance with transmission electron microscopy, Dr G. V. Petkov, Dr J. D. Thompkins, Dr L. A. Meriam, E. Krauter, B. A. Young and D. S. Strong for their help with tissue acquisition. We are grateful to Dr D. Eckman for helping to develop the Ca2+ imaging techniques and to Dr A. D. Bonev for developing the software used to analyse Ca2+ events. This work was funded by NIH grant NS26995 to G.M. and NIH grants DK53832 and DK065947 to M.T.N. The COBRE imaging-Physiology core facility is funded by NIH grant NCRR P20 RR16435.
Supplemental material
The online version of this paper can be accessed at:
DOI: 10.1113/jphysiol.2006.122861
http://jp.physoc.org/cgi/content/full/jphysiol.2006.122861/DC1 and contains supplemental material consisting of three movies.
Demonstration of synchronous Ca2+ flashes in GBSM cells in the bundles (arrows) and nearby ICC-like cells (asterisks). ICC-like cells are fewer and appear to be located at a different topographical location. Notice processes of ICC-like cells coursing into a GBSM bundle.
Demonstration of synchronous Ca2+ flashes between multipolar ICC-like cells (asterisks) and nearby GBSM cells in the bundles (arrows). Ca2+ waves propagate slowly and are asynchronous. GBSM bundles appear to converge and appose to ICC-like cells.
Demonstration of the differential effects of gap junction uncouplers on spontaneous Ca2+ transients in ICC-like cells versus GBSM cells. After 35 min exposure to connexin P 076 (0.5 mm), Ca2+ flashes and Ca2+ waves were abolished in GBSM cells (arrows) but persisted in ICC-like cell (asterisk). As shown in Movie 2, Ca2+ flashes are the synchronized, rapidly occurring Ca2+ transients; Ca2+ waves are the slowly propagating, asynchronous Ca2+ transients.
This material can also be found as part of the full-text HTML version available from http://www.blackwell-synergy.com
References
- Balemba OB, Heppner TJ, Bonev AD, Nelson MT, Mawe GM. Calcium waves in intact guinea pig gallbladder smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2006 a;291:G717–G727. doi: 10.1152/ajpgi.00035.2006. [DOI] [PubMed] [Google Scholar]
- Balemba OB, Salter MJ, Heppner TJ, Bonev AD, Nelson MT, Mawe GM. Spontaneous electrical rhythmicity and the role of the sarcoplasmic reticulum in the excitability of guinea pig gallbladder smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2006 b;290:G655–G664. doi: 10.1152/ajpgi.00310.2005. [DOI] [PubMed] [Google Scholar]
- Biers SM, Reynard JM, Doore T, Brading AF. The functional effects of a c-kit tyrosine inhibitor on guinea-pig and human detrusor. BJU Int. 2006;97:612–616. doi: 10.1111/j.1464-410X.2005.05988.x. [DOI] [PubMed] [Google Scholar]
- Burns AJ, Herbert TM, Ward SM, Sanders KM. Interstitial cells of Cajal in the guinea-pig gastrointestinal tract as revealed by c-Kit immunohistochemistry. Cell Tissue Res. 1997;290:11–20. doi: 10.1007/s004410050902. [DOI] [PubMed] [Google Scholar]
- Cai WQ, Gabella G. The musculature of the gall bladder and biliary pathways in the guinea-pig. J Anat. 1983;136:237–250. [PMC free article] [PubMed] [Google Scholar]
- Chaytor AT, Bakker LM, Edwards DH, Griffith TM. Connexin-mimetic peptides dissociate electrotonic EDHF-type signalling via myoendothelial and smooth muscle gap junctions in the rabbit iliac artery. Br J Pharmacol. 2005;144:108–114. doi: 10.1038/sj.bjp.0706046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill GC, Louis CF. Roles of Ca2+, inositol trisphosphate and cyclic ADP-ribose in mediating intercellular Ca2+ signaling in sheep lens cells. J Cell Sci. 1998;111:1217–1225. doi: 10.1242/jcs.111.9.1217. [DOI] [PubMed] [Google Scholar]
- Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S, Zimmermann J, Lydon NB. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2:561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- Faussone-Pellegrini MS, Thuneberg L. Guide to the identification of interstitial cells of Cajal. Microsc Res Tech. 1999;47:248–266. doi: 10.1002/(SICI)1097-0029(19991115)47:4<248::AID-JEMT4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Furihata M, Fujimori T, Imura J, Ono Y, Furihata T, Shimoda M, Kato M, Kita J, Ohkura Y, Kubota K. Malignant stromal tumor, so called ‘gastrointestinal stromal tumor’, with rhabdomyomatous differentiation occurring in the gallbladder. Pathol Res Pract. 2005;201:609–613. doi: 10.1016/j.prp.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Hanani M, Farrugia G, Komuro T. Intercellular coupling of interstitial cells of Cajal in the digestive tract. Int Rev Cytol. 2005;242:249–282. doi: 10.1016/S0074-7696(04)42006-3. [DOI] [PubMed] [Google Scholar]
- Harhun MI, Pucovsky V, Povstyan OV, Gordienko DV, Bolton TB. Interstitial cells in the vasculature. J Cell Mol Med. 2005;9:232–243. doi: 10.1111/j.1582-4934.2005.tb00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Suzuki H. Identification of interstitial cells of Cajal in corporal tissues of the guinea-pig penis. Br J Pharmacol. 2004;141:199–204. doi: 10.1038/sj.bjp.0705622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Yanai Y, Suzuki H. Role of interstitial cells and gap junctions in the transmission of spontaneous Ca2+ signals in detrusor smooth muscles of the guinea-pig urinary bladder. J Physiol. 2004;559:567–581. doi: 10.1113/jphysiol.2004.065136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemming JM, Guarraci FA, Firth TA, Jennings LJ, Nelson MT, Mawe GM. Actions of histamine on muscle and ganglia of the guinea pig gallbladder. Am J Physiol Gastrointest Liver Physiol. 2000;279:G622–G630. doi: 10.1152/ajpgi.2000.279.3.G622. [DOI] [PubMed] [Google Scholar]
- Hennig GW, Hirst GD, Park KJ, Smith CB, Sanders KM, Ward SM, Smith TK. Propagation of pacemaker activity in the guinea-pig antrum. J Physiol. 2004;556:585–599. doi: 10.1113/jphysiol.2003.059055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig GW, Smith CB, O’Shea DM, Smith TK. Patterns of intracellular and intercellular Ca2+ waves in the longitudinal muscle layer of the murine large intestine in vitro. J Physiol. 2002;543:233–253. doi: 10.1113/jphysiol.2002.018986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GD, Edwards FR. Electrical events underlying organized myogenic contractions of the guinea pig stomach. J Physiol. 2006;576:659–665. doi: 10.1113/jphysiol.2006.116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GD, Garcia-Londono AP, Edwards FR. Propagation of slow waves in the guinea-pig gastric antrum. J Physiol. 2006;571:165–177. doi: 10.1113/jphysiol.2005.100735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz B, Ward SM, Sanders KM. Cellular and molecular basis for electrical rhythmicity in gastrointestinal muscle. Annu Rev Physiol. 1999;61:19–43. doi: 10.1146/annurev.physiol.61.1.19. [DOI] [PubMed] [Google Scholar]
- Huizinga JD, Faussone-Pellegrini MS. About the presence of interstitial cells of Cajal outside the musculature of the gastrointestinal tract. J Cell Mol Med. 2005;9:468–473. doi: 10.1111/j.1582-4934.2005.tb00372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- Kito Y, Ward SM, Sanders KM. Pacemaker potentials generated by interstitial cells of Cajal in the murine intestine. Am J Physiol Cell Physiol. 2005;288:C710–C720. doi: 10.1152/ajpcell.00361.2004. [DOI] [PubMed] [Google Scholar]
- Komuro T. Comparative morphology of interstitial cells of Cajal: ultrastructural characterization. Microsc Res Tech. 1999;47:267–285. doi: 10.1002/(SICI)1097-0029(19991115)47:4<267::AID-JEMT5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Komuro T, Seki K, Horiguchi K. Ultrastructural characterization of the interstitial cells of Cajal. Arch Histol Cytol. 1999;62:295–316. doi: 10.1679/aohc.62.295. [DOI] [PubMed] [Google Scholar]
- Komuro T, Zhou DS. Anti-c-kit protein immunoreactive cells corresponding to the interstitial cells of Cajal in the guinea-pig small intestine. J Auton Nerv Syst. 1996;61:169–174. doi: 10.1016/s0165-1838(96)00078-1. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Biers SM, Kohri K, Brading AF. Effects of imatinib mesylate (Glivec) as a c-kit tyrosine kinase inhibitor in the guinea-pig urinary bladder. Neurourol Urodyn. 2006;25:205–210. doi: 10.1002/nau.20085. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Kajioka S, Biers SM, Yokota E, Kohri K, Brading AF. Investigation of the effect of the c-kit inhibitor Glivec on isolated guinea-pig detrusor preparations. Auton Neurosci. 2004;115:64–73. doi: 10.1016/j.autneu.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Kwak BR, Jongsma HJ. Selective inhibition of gap junction channel activity by synthetic peptides. J Physiol. 1999;516:679–685. doi: 10.1111/j.1469-7793.1999.0679u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang RJ, Klemm MF. Interstitial cell of Cajal-like cells in the upper urinary tract. J Cell Mol Med. 2005;9:543–556. doi: 10.1111/j.1582-4934.2005.tb00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H, Yamagata A, Nishikawa S, Yoshinaga K, Kobayashi S, Nishi K. Requirement of c-kit for development of intestinal pacemaker system. Development. 1992;116:369–375. doi: 10.1242/dev.116.2.369. [DOI] [PubMed] [Google Scholar]
- Matchkov VV, Rahman A, Bakker LM, Griffith TM, Nilsson H, Aalkjaer C. Analysis of the effects of connexin-mimetic peptides in rat mesenteric small arteries. Am J Physiol Heart Circ Physiol. 2006;291:H357–367. doi: 10.1152/ajpheart.00681.2005. [DOI] [PubMed] [Google Scholar]
- McCloskey KD, Gurney AM. Kit positive cells in the guinea pig bladder. J Urol. 2002;168:832–836. [PubMed] [Google Scholar]
- Mendoza-Marin M, Hoang MP, Albores-Saavedra J. Malignant stromal tumor of the gallbladder with interstitial cells of Cajal phenotype. Arch Pathol Laboratory Med. 2002;126:481–483. doi: 10.5858/2002-126-0481-MSTOTG. [DOI] [PubMed] [Google Scholar]
- Ortiz-Hidalgo C, De Leon Bojorge B, Albores-Saavedra J. Stromal tumor of the gallbladder with phenotype of interstitial cells of Cajal: a previously unrecognized neoplasm. Am J Surg Pathol. 2000;24:1420–1423. doi: 10.1097/00000478-200010000-00013. [DOI] [PubMed] [Google Scholar]
- Park JK, Choi SH, Lee S, Min KO, Yun SS, Jeon HM. Malignant gastrointestinal stromal tumor of the gallbladder. J Korean Med Sci. 2004;19:763–767. doi: 10.3346/jkms.2004.19.5.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KJ, Hennig GW, Lee HT, Spencer NJ, Ward SM, Smith TK, Sanders KM. Spatial and temporal mapping of pacemaker activity in interstitial cells of Cajal in mouse ileum in situ. Am J Physiol Cell Physiol. 2006;290:C1411–C1427. doi: 10.1152/ajpcell.00447.2005. [DOI] [PubMed] [Google Scholar]
- Peng B, Lloyd P, Schran H. Clinical pharmacokinetics of imatinib. Clin Pharmacokinet. 2005;44:879–894. doi: 10.2165/00003088-200544090-00001. [DOI] [PubMed] [Google Scholar]
- Popescu LM, Ciontea SM, Cretoiu D, Hinescu ME, Radu E, Ionescu N, Ceausu M, Gherghiceanu M, Braga RI, Vasilescu F, Zagrean L, Ardeleanu C. Novel type of interstitial cell (Cajal-like) in human fallopian tube. J Cell Mol Med. 2005 a;9:479–523. doi: 10.1111/j.1582-4934.2005.tb00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu LM, Gherghiceanu M, Cretoiu D, Radu E. The connective connection: interstitial cells of Cajal (ICC) and ICC-like cells establish synapses with immunoreactive cells. Electron microscope study in situ. J Cell Mol Med. 2005 b;9:714–730. doi: 10.1111/j.1582-4934.2005.tb00502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu LM, Hinescu ME, Ionescu N, Ciontea SM, Cretoiu D, Ardelean C. Interstitial cells of Cajal in pancreas. J Cell Mol Med. 2005 c;9:169–190. doi: 10.1111/j.1582-4934.2005.tb00347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu LM, Vidulescu C, Curici A, Caravia L, Simionescu AA, Ciontea SM, Simion S. Imatinib inhibits spontaneous rhythmic contractions of human uterus and intestine. Eur J Pharmacol. 2006;546:177–181. doi: 10.1016/j.ejphar.2006.06.068. [DOI] [PubMed] [Google Scholar]
- Romert P, Mikkelsen HB. c-kit immunoreactive interstitial cells of Cajal in the human small and large intestine. Histochem Cell Biol. 1998;109:195–202. doi: 10.1007/s004180050218. [DOI] [PubMed] [Google Scholar]
- Rottingen J, Iversen JG. Ruled by waves? Intracellular and intercellular calcium signalling. Acta Physiol Scand. 2000;169:203–219. doi: 10.1046/j.1365-201x.2000.00732.x. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Koh SD, Ward SM. Interstitial cells of Cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006;68:307–343. doi: 10.1146/annurev.physiol.68.040504.094718. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Ward SM. Interstitial cells of Cajal: a new perspective on smooth muscle function. J Physiol. 2006;576:721–726. doi: 10.1113/jphysiol.2006.115279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz T, Daniel V, Daniel EE. Does ICC pacing require functional gap junctions between ICC and smooth muscle in mouse intestine? Neurogastroenterol Motil. 2003;15:129–138. doi: 10.1046/j.1365-2982.2003.00401.x. [DOI] [PubMed] [Google Scholar]
- Shafik A, El-Sibai O, Shafik AA, Shafik I. Identification of interstitial cells of Cajal in human urinary bladder: concept of vesical pacemaker. Urology. 2004;64:809–813. doi: 10.1016/j.urology.2004.05.031. [DOI] [PubMed] [Google Scholar]
- Shimojima N, Nakaki T, Morikawa Y, Hoshino K, Kitajima M. Imatinib blocks spontaneous mechanical activities in the adult mouse small intestine: possible inhibition of c-Kit signaling. Pharmacology. 2005;74:95–99. doi: 10.1159/000084021. [DOI] [PubMed] [Google Scholar]
- Sun X, Yu B, Xu L, Dong W, Luo H. Interstitial cells of Cajal in the murine gallbladder. Scand J Gastroenterol. 2006;41:1218–1226. doi: 10.1080/00365520600708800. [DOI] [PubMed] [Google Scholar]
- Takeda Y, Ward SM, Sanders KM, Koh SD. Effects of the gap junction blocker glycyrrhetinic acid on gastrointestinal smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2005;288:G832–G841. doi: 10.1152/ajpgi.00389.2004. [DOI] [PubMed] [Google Scholar]
- Thuneberg L. One hundred years of interstitial cells of Cajal. Microsc Res Tech. 1999;47:223–238. doi: 10.1002/(SICI)1097-0029(19991115)47:4<223::AID-JEMT2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Torihashi S, Ward SM, Nishikawa S, Nishi K, Kobayashi S, Sanders KM. c-kit-dependent development of interstitial cells and electrical activity in the murine gastrointestinal tract. Cell Tissue Res. 1995;280:97–111. doi: 10.1007/BF00304515. [DOI] [PubMed] [Google Scholar]
- van Helden DF, Imtiaz MS, Nurgaliyeva K, von der Weid P, Dosen PJ. Role of calcium stores and membrane voltage in the generation of slow wave action potentials in guinea-pig gastric pylorus. J Physiol. 2000;524:245–265. doi: 10.1111/j.1469-7793.2000.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM. Interstitial cells of Cajal in enteric neurotransmission. Gut. 2000;47(Suppl. 4):40–43. doi: 10.1136/gut.47.suppl_4.iv40. discussion iv 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Baker SA, de Faoite A, Sanders KM. Propagation of slow waves requires IP3 receptors and mitochondrial Ca2+ uptake in canine colonic muscles. J Physiol. 2003;549:207–218. doi: 10.1113/jphysiol.2003.040097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Dixon RE, de Faoite A, Sanders KM. Voltage dependent calcium entry underlies propagation of slow waves in canine gastric antrum. J Physiol. 2004;561:793–810. doi: 10.1113/jphysiol.2004.076067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, McLaren GJ, Sanders KM. Interstitial cells of Cajal in the deep muscular plexus mediate enteric motor neurotransmission in the mouse small intestine. J Physiol. 2006;573:147–159. doi: 10.1113/jphysiol.2006.105189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazawa T, Iino M. Simultaneous imaging of Ca2+ signals in interstitial cells of Cajal and longitudinal smooth muscle cells during rhythmic activity in mouse ileum. J Physiol. 2002;538:823–835. doi: 10.1113/jphysiol.2001.013045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Bonev AD, Nelson MT, Mawe GM. Ionic basis of the action potential of guinea pig gallbladder smooth muscle cells. Am J Physiol Cell Physiol. 1993;265:C1552–C1561. doi: 10.1152/ajpcell.1993.265.6.C1552. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Mucci A, Huizinga JD. Inwardly rectifying chloride channel activity in intestinal pacemaker cells. Am J Physiol Gastrointest Liver Physiol. 2005;288:G809–G821. doi: 10.1152/ajpgi.00301.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Demonstration of synchronous Ca2+ flashes in GBSM cells in the bundles (arrows) and nearby ICC-like cells (asterisks). ICC-like cells are fewer and appear to be located at a different topographical location. Notice processes of ICC-like cells coursing into a GBSM bundle.
Demonstration of synchronous Ca2+ flashes between multipolar ICC-like cells (asterisks) and nearby GBSM cells in the bundles (arrows). Ca2+ waves propagate slowly and are asynchronous. GBSM bundles appear to converge and appose to ICC-like cells.
Demonstration of the differential effects of gap junction uncouplers on spontaneous Ca2+ transients in ICC-like cells versus GBSM cells. After 35 min exposure to connexin P 076 (0.5 mm), Ca2+ flashes and Ca2+ waves were abolished in GBSM cells (arrows) but persisted in ICC-like cell (asterisk). As shown in Movie 2, Ca2+ flashes are the synchronized, rapidly occurring Ca2+ transients; Ca2+ waves are the slowly propagating, asynchronous Ca2+ transients.