Abstract
Reflex increases in genioglossus (GG) muscle activity in response to negative pharyngeal pressure are important for maintenance of upper airway patency in humans. However, little is known of the central circuitry that mediates this negative pressure reflex (NPR). We used two approaches to determine which GG premotoneurons relay negative pressure-related information to the hypoglossal motor nucleus. First, to identify GG premotoneurons, we injected pseudorabies virus (PRV152) into the GG muscle. We found that medullary GG premotoneurons were concentrated mainly in the reticular formation adjacent to the hypoglossal motor nucleus. Second, in order to determine whether these perihypoglossal neurons were involved in the NPR, we quantified GG EMG responses to negative pressure applied to the isolated upper airway in anaesthetized rats before and after microinjection of muscimol (9 nl; 0.25 mm), a GABA-A receptor agonist, into the perihypoglossal premotor field. Pressures as low as −4 cmH2O increased inspiratory phase-related GG activity. The NPR was abolished following bilateral injections of muscimol into the perihypoglossal premotor field at and up to 500 μm rostral to the obex. Muscimol in this location also increased the amplitude of basal, unstimulated phasic GG activity. By contrast, inhibition of neurons caudal to the obex decreased phasic GG activity but had no impact on the NPR. These results suggest that perihypoglossal GG premotoneurons near the obex mediate the NPR and those caudal to the obex are important mediators of respiratory-related GG activity but are not involved in the NPR.
A growing body of evidence indicates that decreased pharyngeal dilator muscle activity during sleep is an important mechanism in the pathophysiology of obstructive sleep apnoea. A key muscle in this process is the genioglossus (GG) (Remmers et al. 1978; Brouillette & Thach, 1979), which forms a portion of the body of the tongue. Its origin is the mental spine of the mandible and its insertion is the dorsum of the tongue. Innervated by a subset of hypoglossal motoneurons, the GG depresses and protrudes the tongue. It has been hypothesized that reflex GG activation in response to negative airway pressure is a fundamental mechanism for maintaining airway patency and that failure of this reflex during sleep may be the proximate cause for obstructive sleep apnoea (Wheatley et al. 1993). Upper airway patency depends on the balance between the dilating force of pharyngeal muscles and the collapsing force of negative intraluminal pressure that is generated by respiratory ‘pump’ muscles (for review see White, 2005). The anatomical abnormality in obstructive sleep apnoea is a small, collapsible pharyngeal airway due to a variety of causes including excessive adipose tissue or abnormal jaw structure. This deficient anatomy requires activation of upper airway dilator muscles, including the GG, via the negative pressure reflex (NPR) to maintain airway patency (Mezzanotte et al. 1992). Thus, the precipitous decline in the NPR and consequently inadequate GG activity that occurs at sleep onset (Mezzanotte et al. 1996) can cause disordered breathing in anatomically susceptible individuals.
Animal studies have shown that GG muscle activity increases in response to negative pressure in the upper airway (Mathew et al. 1982; Hwang et al. 1984b; van Lunteren et al. 1984; Zhang & Bruce, 1998; Ryan et al. 2001) as it does in humans (Horner et al. 1991; Malhotra et al. 2000). Negative pressure applied to the larynx or to the nasal cavity causes reflex increases in GG activity although responses from the larynx predominate (Mathew et al. 1982; Hwang et al. 1984b; van Lunteren et al. 1984). The primary afferents innervating the larynx are carried by the superior laryngeal nerve and terminate in the interstitial nucleus of the solitary tract (NTSis; Furusawa et al. 1996). It is not known how laryngeal pressure information is conveyed from the NTS to the genioglossal motoneurons. Direct projections from the NTSis to the hypoglossal motor nucleus are sparse (Borke et al. 1983; Li et al. 1997; Cunningham & Sawchenko, 2000). However, the NTS does project heavily to the adjacent medullary reticular formation (Ross et al. 1985), which in turn has dense projections to the hypoglossal motor nucleus (Borke et al. 1983). Therefore, we hypothesized that hypoglossal premotoneurons in the medullary reticular formation that project to genioglossus motoneurons mediate the NPR. To test this hypothesis we performed two sets of experiments. First, we injected pseudorabies virus (PRV152) into the genioglossus muscle to identify locations of medullary hypoglossal premotoneurons that project to the GG motoneurons. Second, in a different set of rats, we examined the effect of inhibiting subsets of these neurons on GG activity and the NPR.
Methods
Every effort was made to minimize the number of animals used and prevent their suffering. All procedures involving animals were approved by the Institutional Animal Care and Use Committees at Harvard Medical School and Beth Israel Deaconess Medical Center. Seventy-one adult male Sprague-Dawley rats (300–400 g; Harlan Sprague Dawley, Indianapolis, IN, USA) were used in these studies.
PRV152 injections
The PRV152 recombinant strain of pseudorabies virus (provided by Dr Lynn Enquist) was constructed to express enhanced green fluorescent protein (GFP) and is otherwise isogenic with PRV Bartha (Smith et al. 2000). To determine the identity of neurons that project to GG motor neurons, 25 rats were anaesthetized with chloral hydrate (350 mg kg−1; i.p.). Absence of a withdrawal response to a noxious pinch applied to a hind limb was taken as adequate depth of anaesthesia. PRV152 (5–6 μl; final titre 9 × 108 PFU ml−1) was injected into one side of the GG muscle from a ventral approach. The superior cervical ganglion was removed ipsilateral to the PRV injection site to prevent CNS labelling via sympathetic innervation. Buprenorphine (0.05–0.1 mg kg−1, s.c., every 12 h) analgesia was administered postoperatively, and the rats were killed by formalin perfusion under deep chloral hydrate anaesthesia (500 mg kg−1; i.p.) 52–65 h later.
Negative pressure reflex testing protocol and muscimol injection
Anaesthesia was induced with isoflurane (1.5–3% in 100% oxygen) and maintained by intravenous Saffan (gift from Schering Plough Animal Health UK Ltd, Derbyshire, UK; composed of alfaxalone, 9 mg ml−1, and alfadolone acetate, 3 mg ml−1; 6–12 mg total steroid kg−1 h−1) in 27 rats. Saffan-anaesthetized rats spontaneously breathed an inspired oxygen concentration of 50%. Body temperature was measured with a rectal probe and maintained at 37 ± 1°C by a thermostatically controlled heating blanket (CWE, Inc., Ardmore, PA, USA). Electromyographic (EMG) recordings were made with insulated stainless steel wires (California Fine Wire Co., Grover Beach, CA, USA) inserted into the diaphragm and the GG, one on each side of the midline. EMG signals were led to differential amplifiers in a Grass Polygraph, filtered (100 Hz low pass, 10 kHz high pass), and digitized by a desktop computer equipped with Digidata A/D hardware and Axotape software (Axon Instruments, now Molecular Devices Corp., Sunnydale, CA, USA). The trachea was transected and cannulated distally with PE240 tubing through which the rat spontaneously breathed. A pressure-sensitive catheter (Millar Institute; Houston, TX, USA) was inserted into the proximal trachea and its tip placed in the larynx just below the level of the thyroid cartilage. A plastic cap was placed over the rat's muzzle and sealed with epoxy glue for application of negative pressure. Rats were placed in a Kopf stereotaxic device and the dorsal surface of the medulla was surgically exposed.
Airway pressure, diaphragm and GG EMGs signals were recorded continuously as negative pressure was delivered at the nose by gating a vacuum source with a solenoid valve. In each rat we applied a stimulus (−4 to −60 cmH2O; 4–5 s) that produced consistent changes in GG activity as monitored during the experiment by the moving time average (Fig. 1). Negative pressure was applied three times (every 60 s) before and after injecting muscimol (0.25 mm in 0.9% saline, 9 nl, containing 1% pontamine sky blue or fluorescent latex microspheres) into the medulla with glass pipettes. Injection volumes were monitored by measuring the movement of the fluid meniscus with an operating microscope equipped with an eyepiece reticule (3 nl per division). Injections were made using the calamus scriptorius as a reference point (0.5–1 mm rostral, 0.4–1.2 mm lateral, and 0.5–1.2 mm below the dorsal surface). In pilot studies inhibition of local medullary neurons with muscimol produced variable changes in phasic GG activity and the NPR, but exploratory data analysis indicated this variability could be accounted for by the anatomical location of the injection site. Therefore, we targeted subsequent injections of muscimol into one of three distinct sites: (1) an area adjacent to the hypoglossal nucleus at and up to 500 μm rostral to the level of the obex, which we termed the periobex perihypoglossal area; (2) an area adjacent to the hypoglossal nucleus 100–600 μm caudal to the obex which we termed the retroobex perihypoglossal area; or (3) the NTSis, which contains the termination zone of the superior laryngeal nerve. At the end of each experiment, anaesthesia was supplemented with chloral hydrate (350 mg kg−1; i.p.) and rats were perfused intracardially first with saline (0.9%) and then formalin (10% in 0.1 m phosphate-buffered saline, pH 7.4).
Figure 1. Effect of local inhibition of neurons rostral to the obex on phasic GG and its response to negative pharyngeal pressure.
A, example of increased phasic GG amplitude in response to negative pressure before application of muscimol (compare breaths 1–3 with 4–6). The dashed line shows the amplitude of baseline moving time averaged GG signal prior to application of negative pressure. B, example of increase in phasic GG amplitude and abolition of negative pressure reflex following bilateral injection of muscimol at the site shown in Fig. 2 (case 57). Scales for A and B are the same. The baseline airway pressure is zero.
Physiological data analysis
Voltage signals were digitized with Clampfit (Axon Instruments) and analysed with Clampfit, Igor Pro (WaveMetrics, Inc., Lake Oswego, OR, USA), Microsoft Excel, and/or Sigmastat software. Phasic GG activity was defined as the entire burst that occurred in phase with each single inspiration. To measure the amplitude of GG bursts, we digitally integrated the absolute value of the voltage signal. Interburst (expiratory) GG EMG levels, which included both electrical noise and tonic muscle activity, were subtracted. The change in GG amplitude during negative pressure was measured by taking the average of the second, third and fourth breaths after onset of negative pressure application, and comparing it to the average of the three breaths just prior to negative pressure application. The average response to three trials of negative pressure was determined in each rat before and after muscimol injection. Statistical significance was evaluated with a Student's t test, two-sample or paired as appropriate (P < 0.05). Data are expressed as mean ±s.e.m.
Histology
Brains and spinal cords from PRV152-injected rats were removed and submerged in sucrose (20%) for at least 12 h. Frozen sections through the entire brain (4 series of 40 μm coronal sections) and spinal cord (3 series of 50 μm; horizontal orientation) were cut with a microtome and stored in phosphate-buffered saline (PBS; pH 7.4) containing sodium azide until staining. To stain PRV152-infected neurons, tissue was incubated in primary antisera (rabbit anti-GFP; Molecular Probes; 1: 20 000 in PBS containing 0.25% Triton X-100) overnight with gentle agitation at room temperature. The next day tissue was incubated in biotinylated donkey anti-rabbit antiserum (Jackson; 1: 1000 in PBS containing Triton X-100; 1 h), streptavidin peroxidase (Vector ABC elite kit; 1: 500 in PBS; 1 h), and finally diaminobenzidine (DAB; 0.05%) and hydrogen peroxide (0.01%) for 10 min with multiple rinses in PBS between all steps. Tissue from four of the PRV152-injected rats was stained for GFP in combination with tyrosine hydroxylase (TH) to visualize noradrenergic PRV152-infected neurons. This tissue was incubated overnight in mouse anti-TH (DiaSorin; 1: 30 000) in combination with rabbit anti-GFP in PBS containing 0.25% Triton X-100. The next day tissue was incubated in donkey anti-mouse conjugated to CY3 and donkey anti-rabbit conjugated to Alexo-Fluor 488 (both from Jackson and diluted 1: 1000 in PBS) for 1 h. Sections were rinsed in PBS and mounted on gelatin-coated glass microscope slides. DAB-stained tissue was counterstained with thionin (for Nissl substance). Both DAB and fluorescent-labelled tissue were dehydrated, cleared in xylene, and coverslipped. GFP-immunoreactive neurons were viewed, photographed and quantified with a Zeiss Axioplan microscope and Axiovision image acquisition and analysis software. The intermediolateral cell column of the spinal cord was examined from each PRV152-injected rat. Labelled cells were found in two rats, suggesting that despite prior removal of the ipsilateral superior cervical ganglion, some uptake and transport occurred via sympathetic innervation of the tongue. Therefore those two cases were excluded from further analysis.
To localize muscimol injection sites formalin-fixed brains were removed and submerged overnight in sucrose (20%). Frozen 50 μm coronal sections were cut through the medulla oblongata with a microtome and mounted immediately on glass microscope slides. Muscimol injection sites were photographed under brightfield (pontamine sky blue) or fluorescence (latex microspheres) optics (Fig. 2). Coverslips were then removed by immersion in xylene and the tissue counterstained with thionin. The slides were recoverslipped and injection sites rephotographed and the two sets of photographs compared to localize the muscimol injections with respect to cytoarchitectonic anatomy (Fig. 2). The rostro-caudal level of each injection was determined with respect to the obex, defined as the ependyma-lined junction of the 4th ventricle where it closes to form the central canal. Digital summary drawings of multiple cases were constructed by importing image files into Canvas software (ACD Systems; Miami, FL, USA). Digital overlays were used to map the positions of muscimol injection sites with respect to locations of PRV152-labelled GG premotoneurons in different rats (Fig. 3).
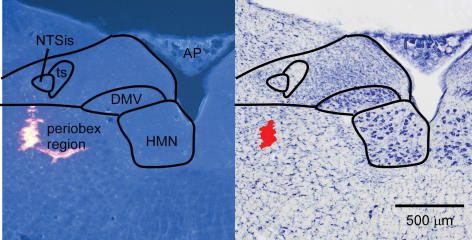
Figure 2. Photomicrographs of a muscimol injection site in the periobex region.
Left, fluorescence photomicrograph showing latex beads. Some beads entered a blood vessel branching medially. Right, brightfield photomicrograph following Nissl stain of the same section. Red area was digitally added to show injection site. AP, area postrema; DMV, dorsal motor nucleus of the vagus; HMN, hypoglossal motor nucleus; NTSis interstitial subnucleus of the nucleus of the solitary tract; ts, solitary tract.
Figure 3. Anatomical distribution of muscimol injection sites compared to GG premotoneurons.
Orange, pink and red dots indicate positions of GFP-ir neurons in three representative rats 63–64 h following PRV152 injection into the right side of the GG. Small dots are hypoglossal motoneurons and larger dots are premotoneurons. Each drawing includes PRV-labelled neurons from three 40 μm sections in a 1: 4 series (i.e. every fourth section). Numbers indicate histologically verified locations where muscimol was injected in a separate series of rats. A, injection sites at levels 0–500 μm rostral to the obex (periobex). B, injection sites −100 to −600 μm caudal to the obex (retroobex). Numbers in blue type indicate NTS injections. AP, area postrema; DMV, dorsal motor nucleus of the vagus; HMN, hypoglossal motor nucleus. Scalebar is 500 μm.
Results
Locations of GG motoneurons and premotoneurons
PRV152 injection into the GG muscle labelled neurons mainly in the ventral and ventrolateral portions of the hypoglossal motor nucleus (Figs 3 and 4A). This distribution reflects the myotopic organization of the hypoglossal nucleus and matches published records of hypoglossal motoneuron labelling following horseradish peroxidase injection into the GG (Aldes, 1995; McClung & Goldberg, 2002). With survival times ranging from 52 to 56 h, first-order retrogradely infected, GFP-labelled neurons were confined to the hypoglossal motor nucleus (n = 5). To assess the distribution of second-order transsynaptically labelled GG premotoneurons, 16 rats were perfused 60–65 h following injection of PRV152. In 3 rats there was very meager labelling (fewer than 3 immunoreactive GFP-ir neurons in one series of sections through the hypoglossal nucleus and nothing in the rest of the brain) indicating unsuccessful infections. In the 13 remaining cases the densest labelling was in the reticular formation lateral to the hypoglossal motor nucleus with about twice as many labelled neurons rostral versus caudal to the obex (Figs 3 and 4A and B). We refer to this pattern of labelling as perihypoglossal. Numerous labelled neurons were also found in the pontine parvocellular reticular formation (PCRt) dorsal to the facial motor nucleus with a predominance at its rostral pole (Fig. 4F). Many reticular neurons were also labelled in the ventral medulla dorsal to the pyramids extending caudally from the level of the facial motor nucleus (Fig. 4C and D) to the level of the inferior olive just rostral to the hypoglossal motor nucleus (Fig. 4E). Small numbers of labelled neurons, which were confirmed as noradrenergic by TH immunoreactivity, consistently appeared in the locus coeruleus and/or subcoeruleus, A5 and A7 noradrenergic cell groups (Fig. 5). Labelling also occurred in the lateral hypothalamus (in the orexin field) and tuberomammillary nucleus (Fig. 5). The lowest density of labelled neurons was seen in the periaquaductal grey in the vicinity of the dorsal raphe nucleus, the supratrigeminal, intertrigeminal and spinal trigeminal nuclei, the ventrolateral medulla and the NTS. Occasionally a few neurons were found in the pedunculopontine and laterodorsal tegmental nucleus. All labelling was predominant ipsilateral to the injection site.
Figure 4. Photomicrographs of GFP-ir neurons following PRV152 injection.
A, hypoglossal motoneurons and periobex premotoneurons. B, higher magnification of boxed area in A. C, ventrally located reticular neurons medial to the facial motor nucleus (7) and dorsal to the pyramids. D, higher magnification of boxed area in C. E, reticular neurons dorsal to the inferior olive (IO). F, pontine parvocellular reticular neurons (PCRt) dorsal to 7 and slightly rostral to the section in C. Scalebars: A, 500 μm; B, 200 μm.
Figure 5. Photomicrographs of GFP-ir neurons in aminergic, histaminergic and orexinergic cell groups.
A, LC (locus coeruleus); B, A7 noradrenergic cell group; C, orexin field; arrowheads show 3 of the labelled neurons; D, tuberomammillary nucleus. E, digitally merged image of GFP (F) and TH (G) staining in LC. A–D. E–G, arrows show double-labelled neurons; case 44. Medial is to the left.
Effects of neuronal inhibition with muscimol on GG responses to negative pressure
Based on the results of our PRV152 experiments we hypothesized that perihypoglossal GG premotoneurons were likely to relay negative pressure sensory information from the NTS to the hypoglossal motoneurons. This group of cells was the most heavily labelled with PRV152 indicating a dense synaptic projection to GG motoneurons. Moreover, of all the labelled cell groups, those of the perihypoglossal are the only ones known to receive a substantial input from the NTS (Ross et al. 1985). Therefore, we examined the effects of their inhibition with muscimol on the reflex. As pilot studies indicated a rostro-caudal functional organization, we subdivided injection targets into an area centred in the premotor field 0–500 μm rostral to the obex (periobex) and a corresponding area −100 to −600 μm caudal to the obex (retroobex). Figure 3 shows the relationship between these targets and the location of GG premotoneurons labelled by PRV152 injection into the GG muscle. We also targeted the NTSis with the expectation (based on pilot studies) that blocking the central neurons postsynaptic to the afferents that mediate the reflex would abolish the reflex and thereby serve as a positive control. In 19 rats we successfully injected muscimol into one of these three histologically verified (Figs 2 and 3) targets and measured the effects on the GG. The distance of periobex and retroobex muscimol injections from the NTSis averaged 498 ± 26 μm (n = 7) and 518 ± 46 μm (n = 9), respectively. The magnitudes of negative pressure used to assess the reflex in the periobex (41 ± 6 cmH2O; n = 7) and retroobex (37 ± 7 cmH2O; n = 9) groups were not statistically different. The pressures used in the NTS group were slightly less (25 ± 6 cmH2O; n = 3).
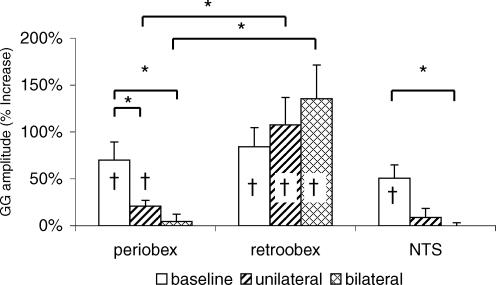
Following unilateral muscimol injections into the periobex perihypoglossal medullary reticular formation, negative pressure responses were significantly attenuated (but still present; n = 7; Fig. 6). Bilateral inhibition of neurons in the periobex region abolished GG responses to negative pressure (n = 6; Figs 1 and 6). In contrast to the periobex injections, muscimol delivered into the more caudal retroobex region adjacent to the hypoglossal motor nucleus had no effect on the GG responses to negative pressure (Fig. 6). Muscimol injected into the NTSis abolished GG responses to negative pressure (n = 3; Fig. 6).
Figure 6. Effect of local inhibition of neurons with muscimol on the NPR.
Shown are the mean (±s.e.m.) changes in GG amplitude in 3 groups of rats during negative pressure under baseline conditions and following unilateral and bilateral muscimol injection. † Negative pressure application significantly increased GG amplitude (NPR). Note that muscimol injection into the periobex and NTS but not into the retroobex region significantly decreased the NPR (*P < 0.05).
Effects of neuronal inhibition with muscimol on phasic unstimulated GG activity
Bilateral inhibition of periobex perihypoglossal neurons increased respiratory-related GG activity (Figs 1 and 7). In contrast to periobex injections, muscimol in the retroobex region decreased phasic GG activity (Fig. 7). Inhibition of NTS neurons also tended to decrease respiratory-related GG activity (Fig. 7).
Figure 7. Effects of local neuronal inhibition on phasic GG amplitude Changes in phasic GG amplitude.
from baseline following unilateral and bilateral muscimol injection into the periobex and retroobex perihypoglossal reticular formation and the NTS. Muscimol injection in the periobex increased phasic GG activity whereas its injection in the retroobex decreased GG activity (**P < 0.01, *P < 0.1).
Discussion
The present experiments were designed to determine the location of GG premotoneurons that mediate the negative pressure reflex. We used transsynaptic labelling with PRV152 to determine the anatomical distribution of medullary premotoneurons that control the GG muscle, and examined the consequences of inhibiting subsets of these neurons on the NPR. We have thereby identified a key region in the medullary reticular formation adjacent to the hypoglossal nucleus at and rostral to the level to the obex (termed periobex) that is critical for GG responsiveness to upper airway negative pressure. We have further shown that perihypoglossal neurons caudal to the obex (termed retroobex) contribute to respiratory-related GG activity, but not to negative pressure responses. Our finding that the NPR can be abolished by inhibition of neurons in the NTS near the termination zone of the superior laryngeal nerve is in accord with previous reports that section of this nerve abolishes the NPR in rats and confirms that the NPR in this species is entirely vagally mediated (Ryan et al. 2001).
Technical considerations
An initial goal of our study was to identify neurons with relatively strong, direct inputs to GG motoneurons in order to narrow the search for candidate mediators of the GG response to negative pressure. Thus we chose to use PRV152 injections into the GG muscle rather than the hypoglossal nerve or retrograde tracer injections into the hypoglossal motor nucleus that would additionally label premotor inputs to non-GG tongue muscles. Neurons are infected with PRV152 in a time-dependent manner with first-, second- and third-order synaptically connected neurons becoming infected with a delay of several hours between each synapse (Card et al. 1990). We found that first-order neurons became labelled after 52–56 h, and that second-order neurons (labelled neurons aside from the hypoglossal motor neurons) began to appear after 60 h. We confined our analysis to survival times ranging from 60 to 65 h. For comparison, a previous study using an isogenic virus, PRV Bartha, found that following hypoglossal nerve application first-order neurons appeared in 32–36 h, second-order neurons began to appear in 48 h and third-order not until 72 or more hours (Dobbins & Feldman, 1995). Therefore it is unlikely that third-order or higher neurons were labelled in our study. Thus, the neurons (outside of the hypoglossal motor nucleus) infected with PRV152 60–65 h following its injection into the GG muscle are likely to make monosynaptic connections with GG motoneurons. A theoretical source of false-positives is transsynaptic labelling of parasympathetic motor and premotoneurons as we did not section the nerves carrying parasympathetic innervation of the tongue. A theoretical source of false negatives is possible resistance of some neurons to PRV infection.
The main goal of muscimol injection experiments was to test our hypothesis that neurons in the perihypoglossal medullary reticular formation participate in the NPR. For the sake of anatomical precision, we limited our muscimol injection volumes to 9 nl. In theory, it is possible that following injections of perihypoglossal targets a low concentration of muscimol may have reached the NTS by diffusion. However, it is unlikely that this accounts for the effects on phasic GG activity and NPR observed with muscimol injections in the periobex region, as periobex and NTS injections had opposite effects on baseline phasic GG amplitude. Moreover, periobex and retroobex injections made at similar distances from the NTSis (roughly 0.5 mm) had markedly different effects on respiratory-related GG activity and responsiveness to negative airway pressure.
Locations of GG premotoneurons
The anatomical distribution of GG premotoneurons that we identified completely overlaps with that from earlier studies using conventional retrograde tracers injected into the hypoglossal motor nucleus (Travers & Norgren, 1983; Zhang & Luo, 2003) or PRV injected into the tongue or applied to the hypoglossal nerve (Dobbins & Feldman, 1995; Fay & Norgren, 1997). The labelling we observed in the medullary reticular formation was densest lateral to the HMN (Figs 3 and 4), and roughly twice as many neurons were labelled in the periobex region as in the retroobex perihypoglossal region. The rostrally predominant distribution may reflect a relatively rostral distribution of GG premotoneurons, and/or the portions of the GG muscle where we injected the virus. Surprisingly, we found no labelled neurons in the Kolliker-Fuse nucleus (KF), which projects to the hypoglossal motor nucleus and, when stimulated, can activate or suppress hypoglossal motor output (Kuna & Remmers, 1999; Gestreau et al 2005). If, as indicated by our studies, there is a sparse or non-existent projection from KF neurons to the subset of hypoglossal motoneurons that innervate the GG, the KF modulation of the GG muscle must be mediated via interneurons.
Effect of premotor inhibition on respiratory-related GG activity
It is likely that respiratory-related GG activity is tonically inhibited. The medullary reticular formation contains GABAergic and glycinergic neurons that project to the hypoglossal motor nucleus (Li et al. 1997; O'Brien et al. 2004; Travers et al. 2005) and local infusion of GABA or glycine receptor antagonists into the hypoglossal motor nucleus increases respiratory-related GG activity (Morrison et al. 2003a, b). The possibility that some or all of this tonic inhibition arises from periobex perihypoglossal GG premotoneurons is suggested by our finding that local inhibition of these neurons caused increases in phasic GG activity (Fig. 7).
Hypothesized neuronal circuit for the NPR
Our hypothesized negative pressure reflex circuit (Fig. 8) is based on the following observations: (1) Negative pharyngeal pressure increases GG activity. (2) Effects of negative pharyngeal pressure on GG activity are mediated by the superior laryngeal nerve which projects to the NTS. (3) Negative pharyngeal pressure inhibits tonic superior laryngeal nerve activity in the rat (Ryan et al. 2001). (4) Inhibition of NTS neurons (muscimol) decreases respiratory-related GG activity and abolishes the NPR. (5) Inhibition of periobex neurons increases baseline phasic GG activity and blocks the NPR. (6) To our knowledge, all of the primary afferent projections to NTS so far reported are excitatory; none of them monosynaptically inhibit secondary sensory neurons in the NTS. The hypothesized circuit is a simple pathway that incorporates all of the above findings. Implicit in the proposed model is the presence of ongoing respiratory-related excitatory drive to periobex inhibitory neurons. There must also be tonic, although not necessarily respiratory-related, input to the NTS output neurons (b in Fig. 8) that project to the periobex. Not included in the model are additional connections that are not critical for the reflex per se but may be physiologically important such as those that underlie sleep/wake modulatory influences on the NPR.
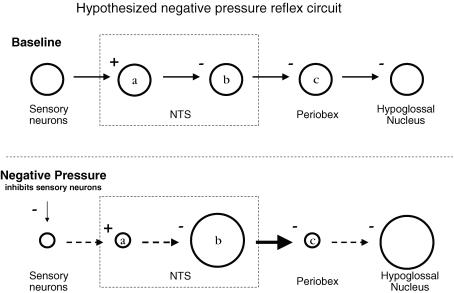
Figure 8. Proposed neural pathway for negative pressure GG activation.
Depicted is the proposed sequence of events (left to right) following negative pharyngeal pressure. Under baseline conditions (zero transmural pressure; top), sensory neurons are tonically active and excite neurons in the NTS (a) that in turn inhibit NTS output neurons (b) that otherwise would inhibit periobex neurons. Periobex and hypoglossal motoneurons are active in phase with inspiration. Negative pressure (bottom) inhibits sensory neurons, which reduces activity of secondary sensory NTS neurons (a) which releases NTS output neurons (b) from inhibition. Periobex neuronal activity is reduced, thus disinhibiting hypoglossal motoneurons. Plus and minus signs indicate synaptic excitation and inhibition, respectively. Small and large circles indicate reductions and increases, respectively, in neuron firing rate in response to negative pressure.
There is a precedent for a neural circuit like the one we have proposed; our model bears some similarities to another major NTS-mediated pressure reflex, the baroreceptor reflex pathway, which also includes inhibitory relays. Stretch of arterial baroreceptors activates NTS neurons, which excite neurons in the caudal ventrolateral medulla (CVL). CVL neurons, in turn, tonically inhibit the sympathoexcitatory neurons in the rostral ventrolateral medulla (RVL) (for review see Aicher et al. 2000). We propose that inhibitory neurons in the periobex region tonically suppress GG output in a manner similar to the tonic inhibition of RVL neurons by CVL input; CVL lesions cause hypertension and periobex inhibition increase GG activity via disinhibition of their downstream targets. In both pathways, vagal stretch receptors convey the sensory information to the NTS.
One major difference is that our pathway appears to have an inhibitory relay within the NTS itself. This is based upon our observation that muscimol injections into the NTS decreased GG activity (whereas similar injections increase blood pressure). We therefore hypothesized the existence of inhibitory output neurons in the NTS (b in Fig. 8) that receive input from the secondary sensory neurons that respond to upper airway pressure (a in Fig. 8).
Species differences and the role of other muscles in the NPR
There are species differences in laryngeal afferent responses to negative pressure. In rats and rabbits nerve activity is decreased by negative pressure (Tsubone et al. 1987; Sekizawa & Tsubone, 1991; Ryan et al. 2001) whereas activity is increased in dogs, and both types are seen in cats (Sant'Ambrogio et al. 1983; Hwang et al. 1984a; Sekizawa & Tsubone, 1991). A minor addition to our model could accommodate the presence of different or mixed types of pressure mechanoreceptors; we would speculate that sensory neurons that respond to negative pressure with an increase in activity may project directly to NTS output neurons (b in Fig. 8).
The proposed model attempts to account for the negative pressure-produced increases in GG activity and does not address the possibility that other tongue or upper airway muscles also respond to negative pressure (Fuller et al. 1999; Bailey et al. 2006). A complete understanding of the NPR will require study of the neural mechanisms that mediate other airway and respiratory pump muscle responses to airway obstruction and negative pressure.
Physiological implications
Upper airway patency is dependent on the balance between static pharyngeal mechanics, neuromuscular activity, and intraluminal pressure (White, 2005). In humans this balance is susceptible to disturbances of anatomical and physiological integrity. Anatomical pathology can be compensated for by an increase in GG activity during wakefulness and sometimes during sleep (Mezzanotte et al. 1992; Younes, 2004; Katz et al. 2006). GG activation protrudes the tongue, resulting in increased size (Kobayashi et al. 1996) and decreased collapsibility (Oliven et al. 2003) of the airway. Thus, neuromuscular reflexes are probably an important determinant of airway patency and stable breathing. We believe that improvements in our understanding of the neuroanatomy and neurochemistry of upper airway control are critical to advancing our understanding of sleep apnoea pathogenesis. By defining the relevant circuitry mediating the negative pressure reflex, the possibility now exists for modulating this reflex in the animal model using pharmacological interventions such as microinjections of selective receptor ligands. Since augmenting the negative pressure reflex is likely to protect pharyngeal patency during sleep, our studies may open new targets for the treatment of sleep apnoea.
Acknowledgments
We thank Drs Clifford Saper, Amy S. Jordan, Tom Scammell, and Andy Strassman for helpful discussions and advice and Quan Ha for excellent technical support. This work was supported by P50-HL60292, AG024837-01 and HL73146-01.
References
- Aicher SA, Milner TA, Pickel VM, Reis DJ. Anatomical substrates for baroreflex sympathoinhibition in the rat. Brain Res Bull. 2000;51:107–110. doi: 10.1016/s0361-9230(99)00233-6. [DOI] [PubMed] [Google Scholar]
- Aldes LD. Subcompartmental organization of the ventral (protrusor) compartment in the hypoglossal nucleus of the rat. J Comp Neurol. 1995;353:89–108. doi: 10.1002/cne.903530109. [DOI] [PubMed] [Google Scholar]
- Bailey EF, Huang YH, Fregosi RF. Anatomic consequences of intrinsic tongue muscle activation. J Appl Physiol. 2006;101:1377–1385. doi: 10.1152/japplphysiol.00379.2006. [DOI] [PubMed] [Google Scholar]
- Borke RC, Nau ME, Ringler RL., Jr Brain stem afferents of hypoglossal neurons in the rat. Brain Res. 1983;269:47–55. doi: 10.1016/0006-8993(83)90961-7. [DOI] [PubMed] [Google Scholar]
- Brouillette RT, Thach BT. A neuromuscular mechanism maintaining extrathoracic airway patency. J Appl Physiol. 1979;46:772–779. doi: 10.1152/jappl.1979.46.4.772. [DOI] [PubMed] [Google Scholar]
- Card JP, Rinaman L, Schwaber JS, Miselis RR, Whealy ME, Robbins AK, Enquist LW. Neurotropic properties of pseudorabies virus: uptake and transneuronal passage in the rat central nervous system. J Neurosci. 1990;10:1974–1994. doi: 10.1523/JNEUROSCI.10-06-01974.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham ET, Jr, Sawchenko PE. Dorsal medullary pathways subserving oromotor reflexes in the rat: implications for the central neural control of swallowing. J Comp Neurol. 2000;417:448–466. [PubMed] [Google Scholar]
- Dobbins EG, Feldman JL. Differential innervation of protruder and retractor muscles of the tongue in rat. J Comp Neurol. 1995;357:376–394. doi: 10.1002/cne.903570305. [DOI] [PubMed] [Google Scholar]
- Fay RA, Norgren R. Identification of rat brainstem multisynaptic connections to the oral motor nuclei using pseudorabies virus. III. Lingual muscle motor systems. Brain Res Brain Res Rev. 1997;25:291–311. doi: 10.1016/s0165-0173(97)00028-3. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Williams JS, Janssen PL, Fregosi RF. Effect of co-activation of tongue protrudor and retractor muscles on tongue movements and pharyngeal airflow mechanics in the rat. J Physiol. 1999;519:601–613. doi: 10.1111/j.1469-7793.1999.0601m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusawa K, Yasuda K, Okuda D, Tanaka M, Yamaoka M. Central distribution and peripheral functional properties of afferent and efferent components of the superior laryngeal nerve: morphological and electrophysiological studies in the rat. J Comp Neurol. 1996;375:147–156. doi: 10.1002/(SICI)1096-9861(19961104)375:1<147::AID-CNE9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Gestreau C, Dutschmann M, Obled S, Bianchi AL. Activation of XII motoneurons and premotor neurons during various oropharyngeal behaviors. Respir Physiol Neurobiol. 2005;147:159–176. doi: 10.1016/j.resp.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Horner RL, Innes JA, Murphy K, Guz A. Evidence for reflex upper airway dilator muscle activation by sudden negative airway pressure in man. J Physiol. 1991;436:15–29. doi: 10.1113/jphysiol.1991.sp018536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JC, St John WM, Bartlett D., Jr Afferent pathways for hypoglossal and phrenic responses to changes in upper airway pressure. Respir Physiol. 1984a;55:341–354. doi: 10.1016/0034-5687(84)90056-2. [DOI] [PubMed] [Google Scholar]
- Hwang JC, St John WM, Bartlett D., Jr Receptors responding to changes in upper airway pressure. Respir Physiol. 1984b;55:355–366. doi: 10.1016/0034-5687(84)90057-4. [DOI] [PubMed] [Google Scholar]
- Katz ES, Marcus CL, White DP. Influence of airway pressure on genioglossus activity during sleep in normal children. Am J Respir Crit Care Med. 2006;173:902–909. doi: 10.1164/rccm.200509-1450OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi I, Perry A, Rhymer J, Wuyam B, Hughes P, Murphy K, Innes JA, McIvor J, Cheesman AD, Guz A. Inspiratory coactivation of the genioglossus enlarges retroglossal space in laryngectomized humans. J Appl Physiol. 1996;80:1595–1604. doi: 10.1152/jappl.1996.80.5.1595. [DOI] [PubMed] [Google Scholar]
- Kuna ST, Remmers JE. Premotor input to hypoglossal motoneurons from Kolliker-Fuse neurons in decerebrate cats. Respir Physiol. 1999;117:85–95. doi: 10.1016/s0034-5687(99)00058-4. [DOI] [PubMed] [Google Scholar]
- Li YQ, Takada M, Kaneko T, Mizuno N. Distribution of GABAergic and glycinergic premotor neurons projecting to the facial and hypoglossal nuclei in the rat. J Comp Neurol. 1997;378:283–294. doi: 10.1002/(sici)1096-9861(19970210)378:2<283::aid-cne10>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- McClung JR, Goldberg SJ. Organization of the hypoglossal motoneurons that innervate the horizontal and oblique components of the genioglossus muscle in the rat. Brain Res. 2002;950:321–324. doi: 10.1016/s0006-8993(02)03240-7. [DOI] [PubMed] [Google Scholar]
- Malhotra A, Fogel RB, Edwards JK, Shea SA, White DP. Local mechanisms drive genioglossus activation in obstructive sleep apnea. Am J Respir Crit Care Med. 2000;161:1746–1749. doi: 10.1164/ajrccm.161.5.9907109. [DOI] [PubMed] [Google Scholar]
- Mathew OP, Abu-Osba YK, Thach BT. Genioglossus muscle responses to upper airway pressure changes: afferent pathways. J Appl Physiol. 1982;52:445–450. doi: 10.1152/jappl.1982.52.2.445. [DOI] [PubMed] [Google Scholar]
- Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism) J Clin Invest. 1992;89:1571–1579. doi: 10.1172/JCI115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzanotte WS, Tangel DJ, White DP. Influence of sleep onset on upper-airway muscle activity in apnea patients versus normal controls. Am J Respir Crit Care Med. 1996;153:1880–1887. doi: 10.1164/ajrccm.153.6.8665050. [DOI] [PubMed] [Google Scholar]
- Morrison JL, Sood S, Liu H, Park E, Liu X, Nolan P, Horner RL. Role of inhibitory amino acids in control of hypoglossal motor outflow to genioglossus muscle in naturally sleeping rats. J Physiol. 2003a;552:975–991. doi: 10.1113/jphysiol.2003.052357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JL, Sood S, Liu H, Park E, Nolan P, Horner RL. GABAA receptor antagonism at the hypoglossal motor nucleus increases genioglossus muscle activity in NREM but not REM sleep. J Physiol. 2003b;548:569–583. doi: 10.1113/jphysiol.2002.033696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien JA, Sebe JY, Berger AJ. GABAB modulation of GABAA and glycine receptor-mediated synaptic currents in hypoglossal motoneurons. Respir Physiol Neurobiol. 2004;141:35–45. doi: 10.1016/j.resp.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Oliven A, O'Hearn DJ, Boudewyns A, Odeh M, De Backer W, van de Heyning P, Smith PL, Eisele DW, Allan L, Schneider H, Testerman R, Schwartz AR. Upper airway response to electrical stimulation of the genioglossus in obstructive sleep apnea. J Appl Physiol. 2003;95:2023–2029. doi: 10.1152/japplphysiol.00203.2003. [DOI] [PubMed] [Google Scholar]
- Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol. 1978;44:931–938. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- Ross CA, Ruggiero DA, Reis DJ. Projections from the nucleus tractus solitarii to the rostral ventrolateral medulla. J Comp Neurol. 1985;242:511–534. doi: 10.1002/cne.902420405. [DOI] [PubMed] [Google Scholar]
- Ryan S, McNicholas WT, O'Regan RG, Nolan P. Reflex respiratory response to changes in upper airway pressure in the anaesthetized rat. J Physiol. 2001;537:251–265. doi: 10.1111/j.1469-7793.2001.0251k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sant'Ambrogio G, Mathew OP, Fisher JT, Sant'Ambrogio FB. Laryngeal receptors responding to transmural pressure, airflow and local muscle activity. Respir Physiol. 1983;54:317–330. doi: 10.1016/0034-5687(83)90075-0. [DOI] [PubMed] [Google Scholar]
- Sekizawa S, Tsubone H. The respiratory activity of the superior laryngeal nerve in the rat. Respir Physiol. 1991;86:355–368. doi: 10.1016/0034-5687(91)90106-s. [DOI] [PubMed] [Google Scholar]
- Smith BN, Banfield BW, Smeraski CA, Wilcox CL, Dudek FE, Enquist LW, Pickard GE. Pseudorabies virus expressing enhanced green fluorescent protein: a tool for in vitro electrophysiological analysis of transsynaptically labeled neurons in identified central nervous system circuits. Proc Natl Acad Sci U S A. 2000;97:9264–9269. doi: 10.1073/pnas.97.16.9264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers JB, Norgren R. Afferent projections to the oral motor nuclei in the rat. J Comp Neurol. 1983;220:280–298. doi: 10.1002/cne.902200303. [DOI] [PubMed] [Google Scholar]
- Travers JB, Yoo JE, Chandran R, Herman K, Travers SP. Neurotransmitter phenotypes of intermediate zone reticular formation projections to the motor trigeminal and hypoglossal nuclei in the rat. J Comp Neurol. 2005;488:28–47. doi: 10.1002/cne.20604. [DOI] [PubMed] [Google Scholar]
- Tsubone H, Mathew OP, Sant'Ambrogio G. Respiratory activity in the superior laryngeal nerve of the rabbit. Respir Physiol. 1987;69:195–207. doi: 10.1016/0034-5687(87)90027-2. [DOI] [PubMed] [Google Scholar]
- van Lunteren E, Van de Graaff WB, Parker DM, Mitra J, Haxhiu MA, Strohl KP, Cherniack NS. Nasal and laryngeal reflex responses to negative upper airway pressure. J Appl Physiol. 1984;56:746–752. doi: 10.1152/jappl.1984.56.3.746. [DOI] [PubMed] [Google Scholar]
- Wheatley JR, Mezzanotte WS, Tangel DJ, White DP. Influence of sleep on genioglossus muscle activation by negative pressure in normal men. Am Rev Respir Dis. 1993;148:597–605. doi: 10.1164/ajrccm/148.3.597. [DOI] [PubMed] [Google Scholar]
- White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med. 2005;172:1363–1370. doi: 10.1164/rccm.200412-1631SO. [DOI] [PubMed] [Google Scholar]
- Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med. 2004;169:623–633. doi: 10.1164/rccm.200307-1023OC. [DOI] [PubMed] [Google Scholar]
- Zhang J, Luo P. Ultrastructural features of synapse from dorsal parvocellular reticular formation neurons to hypoglossal motoneurons of the rat. Brain Res. 2003;963:262–273. doi: 10.1016/s0006-8993(02)04046-5. [DOI] [PubMed] [Google Scholar]
- Zhang X, Bruce EN. Response of breathing pattern to flow and pressure in the upper airway of rats. Respir Physiol. 1998;113:191–200. doi: 10.1016/s0034-5687(98)00065-6. [DOI] [PubMed] [Google Scholar]