Abstract
The factors responsible for control of glucose transport during exercise are not fully understood. We investigated the role of mechanical load in contraction-mediated glucose transport in an isolated muscle preparation. Mouse extensor digitorum longus muscles were stimulated with repeated contractions for 10 min with or without N-benzyl-p-toluene sulphonamide (BTS, an inhibitor of myosin II ATPase) to block crossbridge activity. BTS inhibited force production during repeated contraction to ∼5% of control. In contrast, BTS had little effect on glucose transport in the basal state (control = 0.55 ± 0.04; BTS = 0.47 ± 0.09 μmol (20 min)−1 ml−1) or after contraction (control = 2.27 ± 0.15; BTS = 2.10 ± 0.16 μmol (20 min)−1 ml−1). BTS did not significantly alter the contraction-mediated changes in high-energy phosphates, glutathione status (a measure of oxidant status) or AMP-activated protein kinase activity. In conclusion, these data show that mechanical load plays little role in contraction-mediated glucose transport. Instead, it is likely that the increased glucose transport during contraction is a consequence of the increase in myoplasmic Ca2+ and the subsequent alterations in metabolism, e.g. increased energy turnover and production of reactive oxygen species.
Exercise increases glucose uptake in skeletal muscle by inducing the translocation of glucose transport proteins to the cell surface. Skeletal muscle expresses two isoforms of glucose transporters, Glut 1 and Glut 4. Exercise and insulin enhance the recruitment of Glut 4 to the cell surface by independent mechanisms (Hayashi et al. 1997; Goodyear & Kahn, 1998; Rose & Richter, 2005). How contraction-mediated glucose uptake is regulated is unknown. Current evidence supports an important role for AMP-activated protein kinase (Winder & Hardie, 1999; Fujii et al. 2004; Hardie et al. 2006). However, in some instances activation of glucose transport and AMPK have been dissociated (Derave et al. 2000; Mu et al. 2001).
It has long been debated whether glucose transport is dependent on force production per se in skeletal muscle during exercise. Early research suggested that mechanical load was not an important component (Holloszy & Narahara, 1965). However, more recent studies have shown a direct correlation between muscle force production and glucose transport (Ihlemann et al. 2000; Fujii et al. 2005). To investigate the role of mechanical load in contraction-mediated glucose transport we used N-benzyl-p-toluene sulphonamide (BTS), which is a highly specific inhibitor of myosin II ATPase that does not interfere with skeletal muscle Ca2+ handling (Cheung et al. 2002; Young et al. 2003; Shaw et al. 2003; Bruton et al. 2006). The results demonstrate that although cross-bridges of BTS-treated muscles produced only ∼5% of control force, glucose transport was similar to control.
Methods
Materials
2-Deoxy-d-[1,2-3H]glucose (2-DG), carboxy-[14C]inulin and [γ-32P]ATP were from Amersham Biosciences. BTS was from Sigma. Antibodies against pan-α-AMPK, phosphorylated α-AMPK (T172), acetyl CoA carboxylase (ACC) and phosphorylated ACC (S79) were from Cell Signalling Technology. All other reagents were from either Sigma or Boehringer Mannheim. Male NMRI mice, weighing 25–30 g, and Wistar rats (85–90 g) were housed at room temperature with a 12 h: 12 h light–dark cycle. Food and water were provided ad libitum. Animals were killed by rapid cervical dislocation (following CO2 intoxication in rats) and the extensor digitorum longus (EDL) muscles were isolated. All procedures were approved by the Stockholm North local ethics committee.
Experimental design
For contraction studies, stainless steel hooks were tied with nylon thread to the tendons of the isolated muscles. Muscles were then transferred to a stimulation chamber and mounted between a force transducer and an adjustable holder (World Precision Instruments). The chamber temperature was set to 30°C with a water-jacketed circulation bath. Experiments were performed at 30°C since the characterization of BTS was performed at this temperature (Zhang et al. 2006a). Moreover, higher temperatures (e.g. 35°C) result in some deterioration of force production (Zhang et al. 2006b). The muscles were bathed in a Tyrode solution with the following composition (mm): NaCl, 121; KCl, 5; CaCl2, 1.8; NaH2PO4, 0.4; MgCl2, 0.5; NaHCO3, 24; EDTA, 0.1; glucose, 5.5; 0.1% fetal calf serum. The pH of the Tyrode solution was set to 7.4 by continuously gassing with 95% O2/5% CO2. Muscles were stimulated with current pulses (0.5 ms duration; ∼150% of the current required for maximum force response) delivered via plate electrodes lying parallel to the fibres. Muscles were set to the length at which tetanic force was maximum, and then bathed in a Tyrode solution with or without 25 μm BTS dissolved in DMSO (control = same volume of DMSO) for 60 min (Zhang et al. 2006a). Thereafter, the muscles were stimulated by repeated 70 Hz tetani (tetanic duration 100 ms, 2 trains s−1) for 10 min. For measurements of AMPK activity, AMPK and ACC protein phosphorylation, glutathione status and metabolites (see below), muscles were quick-frozen in liquid nitrogen, immediately after the last tetanus. For measurement of glucose uptake, muscles were immediately transferred to vials containing 1.5 ml Tyrode solution lacking glucose and containing 2 mm pyruvate ± BTS and incubated in a shaking water bath (110 oscillations min−1, 35°C, continuously gassed as above) for 40 min and frozen in liquid nitrogen. Radiolabelled 2-DG (1 mm; 1 mCi mmol−1) and inulin (0.2 μCi ml−1 medium) were added 20 min before freezing as described elsewhere (Shashkin et al. 1995).
A series of glucose uptake experiments was also performed in resting muscle preparations. These muscles were incubated in 1.5 ml of the pyruvate-supplemented Tyrode solution at 35°C ± BTS in the shaking water bath for a total of 80 min; 2-DG and inulin were present during the last 20 min. To study the effects of BTS on glutathione status, AMPK activity, AMPK and ACC protein phosphorylation under resting conditions, muscles were incubated in 1.5 ml of the glucose-supplemented Tyrode ± BTS in the shaking bath at 30°C for a total of 70 min and then frozen.
Analysis
Force was sampled online and stored on a desktop computer for subsequent analysis. Tetanic force was measured as the peak force during the 100 ms of stimulation.
2-DG uptake
Frozen muscles were added to preweighed Eppendorf tubes containing 0.5 ml of 1 m NaOH. The muscles were weighed and then digested at 70°C for 15 min. The tubes were cooled on ice and centrifuged at 23 000 g for 5 min. Aliquots of the supernatant were added to scintillation cocktail and counted for 14C and 3H as described earlier (Shashkin et al. 1995).
Glutathione
A kit was used to measure glutathiones (Biooxytech GSH/GSSG-412, Oxis Health Products). Muscles were freeze-dried, dissected free of non-muscle constituents, powdered and thoroughly mixed. The powders were divided into two aliquots, which were homogenized in ground-glass homogenizers containing ice-cold 5% metaphosphoric acid (80 μl (mg dry weight)−1) with and without 1-methyl-2-vinyl-pyridinium trifluoromethane sulphonate (M2VP, 10% v/v), a scavenger of reduced glutathione (GSH). The homogenates were centrifuged at 23 000 g for 15 min at 4°C. The pellets were digested with 1 m NaOH (60°C) and assayed for protein with the Bio-Rad assay (BIO-RAD). For measurement of GSH + oxidized glutathione (GSSG; TGSH = GSH + GSSG), 4 μl supernatant were mixed with 96 μl assay buffer. For GSSG estimation, 5 μl supernatant (+M2VP) were mixed with 95 μl GSSG assay buffer. For both assays, the samples were mixed with 300 μl of chromogen, glutathione reductase and NADPH, and absorbance (reduction of dithiobis-2-nitrobenzoic acid at 412 nm) was measured after 4.5 min in a spectrophotometer. Preliminary experiments demonstrated that the assays were linear with respect to the extract volume used and reaction time, and GSH was not detectable in the presence of M2VP (data not shown).
AMPK
AMPK activity was analysed based on a method reported elsewhere following the incorporation of radiolabelled phosphate from ATP into SAMS peptide (Winder & Hardie, 1996) with some modifications (Sandström et al. 2006). Briefly, freeze-dried muscles were homogenized in ice-cold buffer (100 μl (mg dry weight)−1) consisting of (mm): Tris, 10; sucrose, 250; NaF, 50; EDTA, 1; α-mercaptoethanol, 10; and 1 tablet protease inhibitor cocktail (Roche) per 50 ml of buffer, pH 7.5. The homogenate was centrifuged at 23 000 g for 30 min at 4°C. The supernatant was divided into two aliquots. One was assayed for protein. The other was diluted with seven volumes of homogenization buffer, and 10 μl of the diluted extract was mixed with 30 μl reaction buffer, resulting in the following final concentrations (mm): Hepes (pH 7.0), 40; SAMS peptide, 0.2; NaCl, 80; EDTA, 0.8; AMP, 0.2; DTT, 0.8; MgCl2, 5; ATP, 0.2; [γ-32P]ATP, 2 μCi; and glycerol, 8% (v/v);. The assay was performed at 37°C for 10 min. Thereafter, 30 μl of the mixture were spotted onto Whatman P81 discs, washed in 1% phosphoric acid, dried and counted. Blanks consisted of mixtures spotted without incubation. The assay was linear with respect to the used extract volume and reaction time, and no activity was detected in the absence of SAMS peptide (data not shown).
Metabolites
Muscles were freeze-dried, dissected free of non-muscle constituents (e.g. connective tissue), powdered, thoroughly mixed and aliquoted. For analysis of glycogen, aliquots of powder were digested with hot 1 m KOH and hydrolysed enzymatically to free glucose. Glucose was then analysed enzymatically with a fluorometric technique (Lowry & Passonneau, 1972). For analysis of metabolites, ice-cold 0.5 m perchloric acid was added to aliquots of muscle powder. The extract was kept in an ice bath for 15 min while agitating with a vortex mixer and then centrifuged (10 000 g). The supernatant was neutralized with 2.2 m KHCO3 and again centrifuged. The latter supernatant was assayed for phosphocreatine (PCr), creatine (Cr), ATP, glucose-6-P and lactate, with enzymatic techniques (changes in NAD[P]H) adapted for fluorometry (Lowry & Passonneau, 1972). To adjust for variability in solid non-muscle constituents, metabolite values were divided by the sum of PCr + Cr (total Cr) and then multiplied by the mean total creatine content for the whole material. BTS did not significantly affect total creatine under any condition (data not shown).
Western blots
Blots were performed for phosphorylated and total AMPK and ACC. Briefly, 20 μg (for total and phosphorylated ACC) or 25 μg (for total and phosphorylated AMPK) of supernatant protein (prepared as for AMPK activity, see above) were separated by SDS-PAGE (4–12% Bis-Tris gels, Invitrogen) and transferred onto polyvinylidine fluoride membranes. Membranes were blocked in 5% (w/v) non-fat milk Tris-buffered saline containing 0.05% Tween 20, followed by incubation with primary antibody, made up in 5% (w/v) bovine serum albumin (all at 1: 1000 dilution), overnight at 4°C. Membranes were then washed and incubated for 1 h at room temperature with secondary antibody (donkey-anti-rabbit at 1: 2000 dilution). Immunoreactive bands were visualized using enhanced chemiluminiscence (Super Signal, Pierce). Band densities were analysed with Image J (NIH, USA; http//rsb.info.nih.gov/j/).
Statistics
Significant differences between means ± BTS were determined with the Student's t test for paired samples. P < 0.05 was regarded as significant. Values are presented as means ± s.e.m.
Results
BTS inhibits force production in EDL muscles
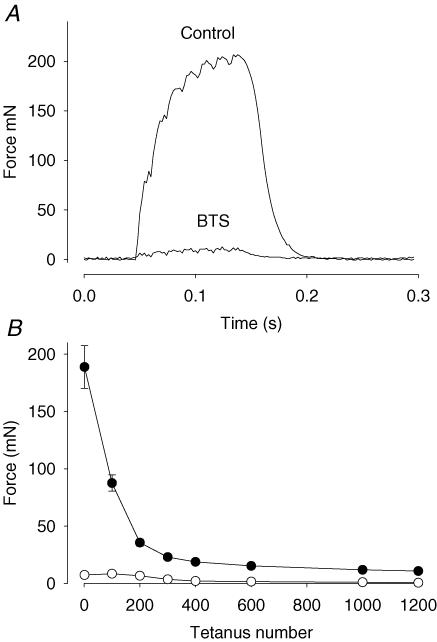
EDL muscles were stimulated with repeated 70 Hz tetani for 10 min in the absence or presence of BTS. Previous studies using fast-twitch muscles stimulated in the presence of BTS show almost no force production due to the inhibition of myosin II ATPase (Dentel et al. 2005; Zhang et al. 2006a). Accordingly, muscles treated with BTS produced only ∼5% of the force compared to the control muscles during the initial contractions, which is less than the control muscles produced at the end of the stimulation protocol (Fig. 1).
Figure 1. BTS inhibits force production in EDL muscle.
A, representative force records during tetanic stimulation (70 Hz, 100 ms) of unfatigued EDL muscles in the absence (Control) and presence of BTS (25 μm). B, mean (± s.e.m.) values of the force produced in 6 paired muscles during repeated tetanic stimulation for 10 min. •, control; ○, BTS.
BTS has little effect on contraction-mediated glucose transport
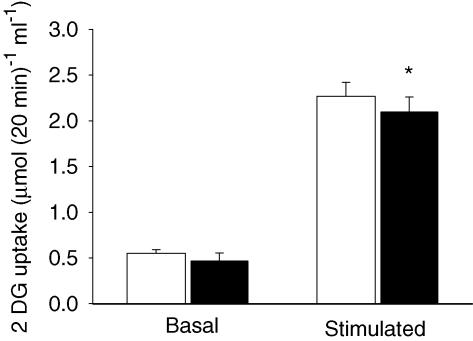
BTS had no effect on basal glucose transport but resulted in a glucose transport that was slightly decreased after repeated contractions (Fig. 2). The small decrease may be associated with the smaller degree of ATP turnover during contractions in the presence of BTS (see below). Nevertheless, glucose transport was increased ∼4-fold after contraction in BTS-treated and control muscles. Thus, glucose transport after the series of contractions was similar in the two groups, despite the low force produced in the muscles treated with BTS.
Figure 2. Contraction-mediated glucose transport is similar in BTS-treated and control muscles.
Values are means ± s.e.m. for 5 basal and 6 stimulated muscles. Unfilled bars, control; filled bars, BTS. *P < 0.05 versus control.
Because recent studies on the role of mechanical stress on glucose transport were performed on isolated rat muscle (Ihlemann et al. 1999, 2000; Fujii et al. 2005), we performed an additional series of BTS experiments in rat EDL muscle. BTS did not significantly affect glucose transport in the basal state (control = 0.31 ± 0.05 μmol (20 min)−1 ml−1; BTS = 0.31 ± 0.03 μmol (20 min)−1 ml−1; n = 6; P > 0.05) or after contraction (control = 0.71 ± 0.04 μmol (20 min)−1 ml−1; BTS = 0.58 ± 0.04 μmol (20 min)−1 ml−1; n = 5; P > 0.05). BTS decreased initial force during the fatigue run to 28% of control (control = 365 ± 42 mN; BTS = 102 ± 15 mN). The lack of complete inhibition of force by BTS in rat EDL may be at least partly related to the presence of type 1 fibres, which do not express myosin II ATPase (5–10%) (Soukup et al. 2002). In contrast, mouse EDL muscle expresses solely myosin II ATPase (Marechal et al. 1995). Thus, the effects of BTS on glucose uptake were similar in mouse and rat muscle, and all subsequent experiments were performed on mouse EDL muscle.
BTS has limited effect on metabolite changes
Metabolites associated with energy consumption in stimulated EDL muscles in the absence and presences of BTS were measured (Table 1). The decrease in glycogen during stimulation in the presence of BTS was ∼30% less than the decrease during control. However, the changes in high-energy phosphates and lactate during contraction were similar in both groups. This indicates that ATP turnover was decreased ∼30% during stimulation in the presence of BTS, which is consistent with our earlier findings obtained under similar conditions (Zhang et al. 2006a).
Table 1.
Effect of BTS on metabolites in isolated EDL
| Glycogen | ATP | Lactate | G6P | PCr | Creatine | |
|---|---|---|---|---|---|---|
| Basal | ||||||
| Control | 78.3 ± 8.2 | 23.8 ± 0.5 | 0.2 ± 0.1 | 0.2 ± 0.1 | 70.2 ± 1.3 | 21.1 ± 1.3 |
| BTS | 75.3 ± 5.6 | 23.8 ± 0.6 | 0.4 ± 0.2 | 0.3 ± 0.1 | 74.0 ± 0.7** | 17.3 ± 0.7** |
| Stimulated | ||||||
| Control | 21.2 ± 3.6 | 10.5 ± 0.9 | 41.9 ± 4.0 | 1.4 ± 0.5 | 15.7 ± 1.6 | 92.3 ± 1.4 |
| BTS | 34.8 ± 1.1*** | 13.0 ± 0.4 | 45.2 ± 2.1 | 2.2 ± 0.6 | 17.7 ± 1.4 | 90.6 ± 1.5 |
Values are means ± s.e.m. for 5–6 muscles and are given in μmol (g dry weight)−1, with the exception of glycogen (μmol glucosyl units (g dry weight)−1). Basal data are from Zhang et al. (2006a).
P < 0.01
P < 0.001 versus control.
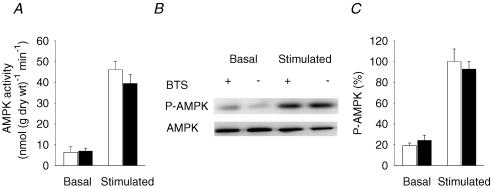
AMPK activity and phosphorylation are not affected by BTS
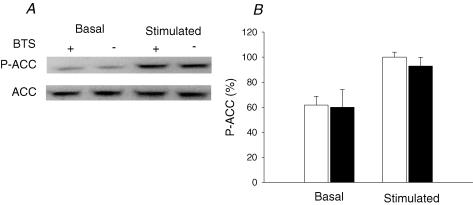
AMPK is considered an important signalling molecule for the regulation of glucose transport in contracting skeletal muscle (Winder & Hardie, 1999; Fujii et al. 2004; Hardie et al. 2006). Electrical stimulation increased AMPK activity in both BTS-treated muscles and control muscles (Fig. 3A). There was no significant difference in AMPK activity between the two groups in the basal state or after stimulation. We also used Western blots to measure phosphorylated AMPK (P-AMPK) levels (Fig. 3B and C). In line with the AMPK activity measurements, BTS had no significant effect on P-AMPK before or after stimulation. The amount of total AMPK was not affected by BTS under any condition. We also measured ACC phosphorylation, which is a downstream target of AMPK. Phosphorylated AMPK phosphorylates ACC, which leads to a decreased activity of ACC and increased fatty acid oxidation (Winder et al. 1997). There was no significant difference in the P-ACC levels between control and BTS in the basal state or after stimulation (Fig. 4). The amount of total ACC was also not affected by BTS under any condition.
Figure 3. BTS does not inhibit AMPK activity or phosphorylation during electrical stimulation.
Mean values (± s.e.m., n = 6 in each group) of the AMPK activity (A) and relative AMPK phosphorylation (C) in rested (Basal) and stimulated muscles. Unfilled bars, control; filled bars, BTS. The mean value of the stimulated controls is set to 100% in C and all other values are expressed as a percentage of this value. B, representative immunoblots of phosphorylated AMPK (P-AMPK) and total AMPK (AMPK).
Figure 4. BTS does not inhibit ACC phosphorylation during electrical stimulation.
A, representative immunoblots of phosphorylated ACC (P-ACC) and total ACC (ACC). B, mean (± s.e.m., n = 6 in each group) values of the relative ACC phosphorylation in rested (Basal) and stimulated muscles. Unfilled bars, control; filled bars, BTS. The mean value of the stimulated controls is set to 100%, and all other values are expressed as a percentage of this value.
BTS does not affect the changes in glutathione oxidation
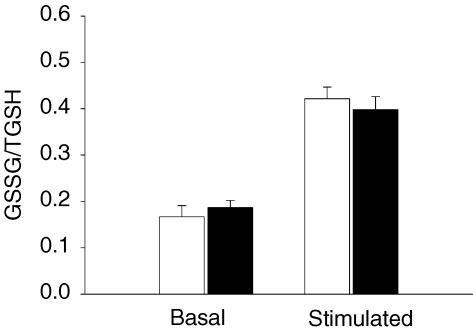
The formation of reactive oxygen species (ROS) in stimulated muscles was recently shown to play a significant role in the activation of AMPK and glucose transport (Sandström et al. 2006). We therefore investigated if BTS had any effect on glutathione status, which reflects the oxidant state of the muscles (Sandström et al. 2006). Hydrogen peroxide formation increases in the muscle cells during contraction leading to oxidation of glutathione, a reaction catalysed by glutathione peroxidase as follows: H2O2 + 2GSH → GSSG + 2H2O. We found no difference in the sum of GSH + GSSG (TGSH) levels between the control and the BTS-treated muscles under basal conditions (3.10 ± 0.11 versus 3.21 ± 0.32 μmol (g dry weight)−1) or after repeated tetani (2.57 ± 0.03 versus 2.41 ± 0.32 μmol (g dry weight)−1). The concentration of GSSG was similar in the two groups in the basal state (1.06 ± 0.12 versus 1.16 ± 0.05 μmol (g dry weight)−1) and after contraction (2.17 ± 0.12 versus 1.89 ± 0.09 μmol (g dry weight)−1). Thus the GSSG/TGSH ratio was not affected by BTS under any condition (Fig. 5).
Figure 5. BTS does not alter the glutathione status during electrical stimulation.
Values are means ± s.e.m. of 5 basal and 6 stimulated muscles. Unfilled bars, control; filled bars, BTS. The GSSG/TGSH ratio was calculated in GSH equivalents.
Discussion
We investigated the role of mechanical load in contraction-mediated glucose transport in isolated mouse fast-twitch muscles exposed to BTS, which is a highly specific inhibitor of cross-bridge force production that does not interfere with intracellular Ca2+ handling (Cheung et al. 2002; Young et al. 2003; Shaw et al. 2003; Bruton et al. 2006). Furthermore, we have recently shown that cross-bridge inhibition with BTS only has a limited effect on the rate of ATP consumption during submaximal contractions of mouse fast-twitch muscle (Zhang et al. 2006a). Thus, this novel model makes it possible to study the effect of mechanical load without inducing major changes in other factors associated with muscle contraction. Our results show that force production per se has little effect on contraction-mediated glucose transport.
An early study showed that force production per se was not correlated to glucose transport (Holloszy & Narahara, 1965), whereas more recent studies reported that there is a linear relationship between force generation and glucose transport (Ihlemann et al. 1999; Fujii et al. 2005). Thus, our current results are in agreement with the earlier results (Holloszy & Narahara, 1965). In the recent studies that investigated the relationship between mechanical load and glucose transport, the techniques used differed from that of the present study. In some studies muscles were shortened prior to stimulation to create reduced force (Ihlemann et al. 1999, 2000). Using this technique, Ihlemann et al. (1999) showed that contraction-mediated glucose transport was closely related to force development. A shortened muscle loses its ability to generate force, presumably because of inappropriate actomyosin interactions. However, this method encounters the potential problem of insufficient sarcoplasmic reticulum (SR) Ca2+ release, because action potentials may not reach the centre of the muscle (Taylor & Rudel, 1970). The observation that glucose transport was also closely related to AMPK activity supports the view that the muscle underwent a smaller degree of activation in the shortened state. Another approach previously used was to decrease the voltage during the electrical stimulation to induce different force levels (Goodyear, 2000). This, however, will result in fewer muscle fibres being activated. Furthermore, rested muscles stretched to the same tension as during contraction have an increased glucose transport but only to about 40% of that seen during contraction (Ihlemann et al. 1999). Passive stretch, however, does not increase AMPK activity or alter other metabolites (Ihlemann et al. 1999). Moreover, it has been shown that there is no increase in global Ca2+ in muscles during passive stretch (Balnave & Allen, 1996). These differences suggest that passive stretch and contraction activate glucose transport via separate pathways. The current findings suggest that changes associated with increased energy metabolism are primarily responsible for activation of glucose transport during contraction. Consistent with this view is the finding that exposure of resting muscle to hypoxia, which alters metabolism to a degree similar to that seen with contraction, also activates glucose transport in skeletal muscle (Ren et al. 1992; Katz & Westerblad, 1995).
Increases in [Ca2+]i have long been recognized as important in mediating the effect of contraction on glucose transport (Holloszy, 2003). Recently, evidence was presented that Ca2+ could signal increases in glucose transport by leading to activation of calmodulin-dependent protein kinase (CaMK) II (Wright et al. 2004). Since Ca2+ transients were probably similar in BTS and control muscles during repeated contractions (Bruton et al. 2006), the current findings (similar glucose uptake in BTS and control conditions) are consistent with the idea that increases in [Ca2+]i mediate the activation of glucose transport during contraction. Current evidence also implicates AMPK in the regulation of glucose transport during exercise (Winder & Hardie, 1999; Fujii et al. 2004; Hardie et al. 2006). It is now established that AMPK is activated not only by changes in high-energy phosphates but also by ROS (Toyoda et al. 2004; Quintero et al. 2006; Sandström et al. 2006). The production of ROS increases in skeletal muscle during contraction (Murrant & Reid, 2001; Zuo & Clanton, 2005; Sandström et al. 2006). Recently, we provided evidence to support the idea that ROS play an important role in contraction-mediated glucose transport, and that ROS work through an AMPK-dependent pathway (Sandström et al. 2006). The current findings, showing similar glutathione changes in BTS and control muscles during contraction, are thus consistent with a ROS involvement in contraction-mediated activation of AMPK and glucose transport.
Another potential regulator of AMPK activity is the level of glycogen. Several studies have shown an inverse relationship between levels of glycogen and the activation of AMPK after contraction (Derave et al. 2000; Wojtaszewski et al. 2002; Wojtaszewski et al. 2003). Whether this is due to a direct inhibition of AMPK by glycogen is somewhat unclear (Polekhina et al. 2003). In the present study we found no relationship between glycogen levels and AMPK activity after contraction, since muscles treated with BTS showed the same AMPK activity despite higher glycogen levels. Possibly, the relatively low glycogen levels after electrical stimulation in both groups exceed the range in which the relationship between glycogen and AMPK exists.
In conclusion, our data show that mechanical load plays little role in contraction-mediated glucose transport in mouse fast-twitch muscle. Instead, it is likely that the increased glucose transport during contraction is a consequence of the increase in myoplasmic Ca2+ and the subsequent alterations in metabolism, e.g. increased energy turnover and production of reactive oxygen species.
Acknowledgments
The present study was supported by the Swedish Research Council, the Swedish National Center for Sports Research, the Swedish Diabetes Foundation, Funds at the Karolinska Institutet and Stiftelsen Lars Hiertas Minne.
References
- Balnave CD, Allen DG. The effect of muscle length on intracellular calcium and force in single fibres from mouse skeletal muscle. J Physiol. 1996;492:705–713. doi: 10.1113/jphysiol.1996.sp021339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton J, Pinniger GJ, Lannergren J, Westerblad H. The effects of the myosin-II inhibitor N-benzyl-p-toluene sulphonamide on fatigue in mouse single intact toe muscle fibres. Acta Physiol. 2006;186:59–66. doi: 10.1111/j.1748-1716.2005.01499.x. [DOI] [PubMed] [Google Scholar]
- Cheung A, Dantzig JA, Hollingworth S, Baylor SM, Goldman YE, Mitchison TJ, Straight AF. A small-molecule inhibitor of skeletal muscle myosin II. Nat Cell Biol. 2002;4:83–88. doi: 10.1038/ncb734. [DOI] [PubMed] [Google Scholar]
- Dentel JN, Blanchard SG, Ankrapp DP, McCabe LR, Wiseman RW. Inhibition of cross-bridge formation has no effect on contraction-associated phosphorylation of p38 MAPK in mouse skeletal muscle. Am J Physiol Cell Physiol. 2005;288:C824–C830. doi: 10.1152/ajpcell.00500.2004. [DOI] [PubMed] [Google Scholar]
- Derave W, Ai H, Ihlemann J, Witters LA, Kristiansen S, Richter EA, Ploug T. Dissociation of AMP-activated protein kinase activation and glucose transport in contracting slow-twitch muscle. Diabetes. 2000;49:1281–1287. doi: 10.2337/diabetes.49.8.1281. [DOI] [PubMed] [Google Scholar]
- Fujii N, Aschenbach WG, Musi N, Hirshman MF, Goodyear LJ. Regulation of glucose transport by the AMP-activated protein kinase. Proc Nutr Soc. 2004;63:205–210. doi: 10.1079/PNS2004340. [DOI] [PubMed] [Google Scholar]
- Fujii N, Hirshman MF, Kane EM, Ho RC, Peter LE, Seifert MM, Goodyear LJ. AMP-activated protein kinase alpha2 activity is not essential for contraction- and hyperosmolarity-induced glucose transport in skeletal muscle. J Biol Chem. 2005;280:39033–39041. doi: 10.1074/jbc.M504208200. [DOI] [PubMed] [Google Scholar]
- Goodyear LJ. AMP-activated protein kinase: a critical signaling intermediary for exercise-stimulated glucose transport? Exerc Sport Sci Rev. 2000;28:113–116. [PubMed] [Google Scholar]
- Goodyear LJ, Kahn BB. Exercise, glucose transport, and insulin sensitivity. Annu Rev Med. 1998;49:235–261. doi: 10.1146/annurev.med.49.1.235. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Hawley SA, Scott JW. AMP-activated protein kinase – development of the energy sensor concept. J Physiol. 2006;574:7–15. doi: 10.1113/jphysiol.2006.108944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Wojtaszewski JF, Goodyear LJ. Exercise regulation of glucose transport in skeletal muscle. Am J Physiol Endocrinol Metab. 1997;273:E1039–E1051. doi: 10.1152/ajpendo.1997.273.6.E1039. [DOI] [PubMed] [Google Scholar]
- Holloszy JO. A forty-year memoir of research on the regulation of glucose transport into muscle. Am J Physiol Endocrinol Metab. 2003;284:E453–E467. doi: 10.1152/ajpendo.00463.2002. [DOI] [PubMed] [Google Scholar]
- Holloszy JO, Narahara HT. Studies of tissue permeability. X. Changes in permeability to 3-methylglucose associated with contraction of isolated frog muscle. J Biol Chem. 1965;240:3493–3500. [PubMed] [Google Scholar]
- Ihlemann J, Ploug T, Hellsten Y, Galbo H. Effect of tension on contraction-induced glucose transport in rat skeletal muscle. Am J Physiol Endocrinol Metab. 1999;277:E208–E214. doi: 10.1152/ajpendo.1999.277.2.E208. [DOI] [PubMed] [Google Scholar]
- Ihlemann J, Ploug T, Hellsten Y, Galbo H. Effect of stimulation frequency on contraction-induced glucose transport in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2000;279:E862–E867. doi: 10.1152/ajpendo.2000.279.4.E862. [DOI] [PubMed] [Google Scholar]
- Katz A, Westerblad H. Insulin-mediated activation of glycogen synthase in isolated skeletal muscle: role of mitochondrial respiration. Biochim Biophys Acta. 1995;1244:229–232. doi: 10.1016/0304-4165(95)00050-l. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Passonneau JV. A Flexible System of Enzymatic Analysis. New York: Academic Press; 1972. [Google Scholar]
- Marechal G, Coulton GR, Beckers-Bleukx G. Mechanical power and myosin composition of soleus and extensor digitorum longus muscles of ky mice. Am J Physiol Cell Physiol. 1995;268:C513–C519. doi: 10.1152/ajpcell.1995.268.2.C513. [DOI] [PubMed] [Google Scholar]
- Mu J, Brozinick JT, Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell. 2001;7:1085–1094. doi: 10.1016/s1097-2765(01)00251-9. [DOI] [PubMed] [Google Scholar]
- Murrant CL, Reid MB. Detection of reactive oxygen and reactive nitrogen species in skeletal muscle. Microsc Res Tech. 2001;55:236–248. doi: 10.1002/jemt.1173. [DOI] [PubMed] [Google Scholar]
- Polekhina G, Gupta A, Michell BJ, van Denderen B, Murthy S, Feil SC, Jennings IG, Campbell DJ, Witters LA, Parker MW, Kemp BE, Stapleton D. AMPK beta subunit targets metabolic stress sensing to glycogen. Curr Biol. 2003;13:867–871. doi: 10.1016/s0960-9822(03)00292-6. [DOI] [PubMed] [Google Scholar]
- Quintero M, Colombo SL, Godfrey A, Moncada S. Mitochondria as signaling organelles in the vascular endothelium. Proc Natl Acad Sci U S A. 2006;103:5379–5384. doi: 10.1073/pnas.0601026103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren JM, Gulve EA, Cartee GD, Holloszy JO. Hypoxia causes glycogenolysis without an increase in percent phosphorylase a in rat skeletal muscle. Am J Physiol Endocrinol Metab. 1992;263:E1086–E1091. doi: 10.1152/ajpendo.2006.263.6.E1086. [DOI] [PubMed] [Google Scholar]
- Rose AJ, Richter EA. Skeletal muscle glucose uptake during exercise: how is it regulated? Physiology. 2005;20:260–270. doi: 10.1152/physiol.00012.2005. [DOI] [PubMed] [Google Scholar]
- Sandström ME, Zhang SJ, Bruton J, Silva JP, Reid MB, Westerblad H, Katz A. Role of reactive oxygen species in contraction-mediated glucose transport in mouse skeletal muscle. J Physiol. 2006;575:251–262. doi: 10.1113/jphysiol.2006.110601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shashkin P, Koshkin A, Langley D, Ren JM, Westerblad H, Katz A. Effects of CGS 9343B (a putative calmodulin antagonist) on isolated skeletal muscle. Dissociation of signaling pathways for insulin-mediated activation of glycogen synthase and hexose transport. J Biol Chem. 1995;270:25613–25618. doi: 10.1074/jbc.270.43.25613. [DOI] [PubMed] [Google Scholar]
- Shaw MA, Ostap EM, Goldman YE. Mechanism of inhibition of skeletal muscle actomyosin by N-benzyl-p-toluenesulfonamide. Biochemistry. 2003;42:6128–6135. doi: 10.1021/bi026964f. [DOI] [PubMed] [Google Scholar]
- Soukup T, Zacharova G, Smerdu V. Fibre type composition of soleus and extensor digitorum longus muscles in normal female inbred Lewis rats. Acta Histochem. 2002;104:399–405. doi: 10.1078/0065-1281-00660. [DOI] [PubMed] [Google Scholar]
- Taylor SR, Rudel R. Striated muscle fibers: inactivation of contraction induced by shortening. Science. 1970;167:882–884. doi: 10.1126/science.167.3919.882. [DOI] [PubMed] [Google Scholar]
- Toyoda T, Hayashi T, Miyamoto L, Yonemitsu S, Nakano M, Tanaka S, Ebihara K, Masuzaki H, Hosoda K, Inoue G, Otaka A, Sato K, Fushiki T, Nakao K. Possible involvement of the alpha1 isoform of 5′AMP-activated protein kinase in oxidative stress-stimulated glucose transport in skeletal muscle. Am J Physiol Endocrinol Metab. 2004;287:E166–E173. doi: 10.1152/ajpendo.00487.2003. [DOI] [PubMed] [Google Scholar]
- Winder WW, Hardie DG. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol Endocrinol Metab. 1996;270:E299–E304. doi: 10.1152/ajpendo.1996.270.2.E299. [DOI] [PubMed] [Google Scholar]
- Winder WW, Hardie DG. AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol Endocrinol Metab. 1999;277:E1–E10. doi: 10.1152/ajpendo.1999.277.1.E1. [DOI] [PubMed] [Google Scholar]
- Winder WW, Wilson HA, Hardie DG, Rasmussen BB, Hutber CA, Call GB, Clayton RD, Conley LM, Yoon S, Zhou B. Phosphorylation of rat muscle acetyl-CoA carboxylase by AMP-activated protein kinase and protein kinase A. J Appl Physiol. 1997;82:219–225. doi: 10.1152/jappl.1997.82.1.219. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, Jorgensen SB, Hellsten Y, Hardie DG, Richter EA. Glycogen-dependent effects of 5-aminoimidazole-4-carboxamide (AICA)-riboside on AMP-activated protein kinase and glycogen synthase activities in rat skeletal muscle. Diabetes. 2002;51:284–292. doi: 10.2337/diabetes.51.2.284. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, MacDonald C, Nielsen JN, Hellsten Y, Hardie DG, Kemp BE, Kiens B, Richter EA. Regulation of 5′AMP-activated protein kinase activity and substrate utilization in exercising human skeletal muscle. Am J Physiol Endocrinol Metab. 2003;284:E813–E822. doi: 10.1152/ajpendo.00436.2002. [DOI] [PubMed] [Google Scholar]
- Wright DC, Hucker KA, Holloszy JO, Han DH. Ca2+ and AMPK both mediate stimulation of glucose transport by muscle contractions. Diabetes. 2004;53:330–335. doi: 10.2337/diabetes.53.2.330. [DOI] [PubMed] [Google Scholar]
- Young IS, Harwood CL, Rome LC. Cross-bridge blocker BTS permits direct measurement of SR Ca2+ pump ATP utilization in toadfish swimbladder muscle fibers. Am J Physiol Cell Physiol. 2003;285:C781–C787. doi: 10.1152/ajpcell.00025.2003. [DOI] [PubMed] [Google Scholar]
- Zhang SJ, Andersson DC, Sandström ME, Westerblad H, Katz A. Cross-bridges account for only 20% of total ATP consumption during submaximal isometric contraction in mouse fast-twitch skeletal muscle. Am J Physiol Cell Physiol. 2006a;291:C147–C154. doi: 10.1152/ajpcell.00578.2005. [DOI] [PubMed] [Google Scholar]
- Zhang SJ, Bruton JD, Katz A, Westerblad H. Limited oxygen diffusion accelerates fatigue development in mouse skeletal muscle. J Physiol. 2006b;572:551–559. doi: 10.1113/jphysiol.2005.104521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo L, Clanton TL. Reactive oxygen species formation in the transition to hypoxia in skeletal muscle. Am J Physiol Cell Physiol. 2005;289:C207–C216. doi: 10.1152/ajpcell.00449.2004. [DOI] [PubMed] [Google Scholar]