Abstract
Extracellular ATP and ADP trigger vasodilatatory and prothrombotic signalling events in the vasculature. Here, we tested the hypothesis that nucleotide turnover is activated in the bloodstream of exercising humans thus contributing to the enhanced platelet reactivity and haemostasis. Right atrial, arterial and venous blood samples were collected from endurance-trained athletes at rest, during submaximal and maximal cycle ergometer exercise, and after early recovery. ATP-specific bioluminescent assay, together with high-performance liquid chromatographic analysis, revealed that plasma ATP and ADP concentrations increased up to 2.5-fold during maximal exercise. Subsequent flow cytometric analysis showed that plasma from exercising subjects significantly up-regulated the surface expression of P-selectin in human platelets and these prothrombotic effects were diminished after scavenging plasma nucleotides with exogenous apyrase. Next, using thin layer chromatographic assays with [γ-32P]ATP and 3H/14C-labelled nucleotides, we showed that two soluble nucleotide-inactivating enzymes, nucleotide pyrophosphatase/phosphodiesterase and nucleoside triphosphate diphosphohydrolase, constitutively circulate in human bloodstream. Strikingly, serum nucleotide pyrophosphatase and hydrolase activities rose during maximal exercise by 20–25 and 80–100%, respectively, and then declined after 30 min recovery. Likewise, soluble nucleotidases were transiently up-regulated in the venous blood of sedentary subjects during exhaustive exercise. Human serum also contains 5′-nucleotidase, adenylate kinase and nucleoside diphosphate (NDP) kinase; however, these activities remain unchanged during exercise. In conclusion, intravascular ADP significantly augments platelet activity during strenuous exercise and these prothrombotic responses are counteracted by concurrent release of soluble nucleotide-inactivating enzymes. These findings provide a novel insight into the mechanisms underlying the enhanced risk of occlusive thrombus formation under exercising conditions.
Extracellular nucleotides mediate diverse vasodilatatory, immunomodulatory and prothrombotic responses in the vasculature and their turnover consists of ATP and ADP release, triggering of signalling events via G-protein-coupled P2Y and ligand-gated P2X receptors, and subsequent inactivation (Vassort, 2001; Burnstock, 2006). Vascular endothelial (Marcus et al. 2003) and lymphoid (Heptinstall et al. 2005) membrane-bound nucleoside triphosphate diphosphohydrolase (NTPDase; known as ecto-ATPDase, CD39) and ecto-5′-nucleotidase/CD73 are considered the major regulators of the duration and magnitude of purinergic signalling. Yet, a significant contribution of soluble nucleotide pyrophosphatases/phosphodiesterases (NPP), NTPDases and other purinergic enzymes into metabolism of circulating nucleotides has emerged recently (Birk et al. 2002; Yegutkin et al. 2003; Cauwenberghs et al. 2006).
The precise regulation of oxygen supply to contracting muscle is essential for the normal physiological response under various hypoxic and hypercapnic conditions, including strenuous exercise. Blood flow and its surrogate oxygen delivery regulation are generally thought to result from the interplay of neural, myogenic and metabolic signals that serve to meet the continually changing metabolic demands (Shepherd, 1983; Saltin et al. 1998; Rowell, 2004). Current models suggest that the erythrocytes, in addition to serving as efficient oxygen carriers, act as sensors and controllers of local blood flow via three mechanisms: (1) release of nitric oxide from S-nitroso haemoglobin (Hb) upon deoxygenation (McMahon et al. 2002), (2) reduction of nitrite to vasoactive nitric oxide by deoxy-Hb (Cosby et al. 2003), and (3) release of the vasodilator ATP in proportion to the degree of Hb deoxygenation (Ellsworth et al. 1995; González-Alonso et al. 2002; Ellsworth, 2004). The contracting skeletal muscle (Vassort, 2001) and shear stress-activated vascular endothelial cells (Yegutkin et al. 2000) can serve as other supplementary sources of intravascular ATP under exercising conditions. The released ATP subsequently induces a conducted vasodilatatory response upstream and regulates oxygen supply to contracting muscle via binding to the endothelial P2Y1/P2Y2 receptors and stimulation of vascular endothelium to release nitric oxide and arachidonic acid metabolites (Burnstock, 2006) and, concurrently, counteracts the effects of enhanced sympathetic activity (Rosenmeier et al. 2004).
Another important feature of strenuous exertion is the promotion of a prothrombotic state manifested in platelet activation and thrombocytosis in vivo and enhanced platelet reactivity in vitro (Li et al. 1999; Wang & Cheng, 1999; El-Sayed, 2002). Platelet activation and recruitment, followed by haemostatic plug formation, is generally initiated either via formation of thromboxane-A2 by cyclooxygenase or secretion of ADP from dense platelet granules. In turn, vascular endothelium controls platelet reactivity and prevents thrombus formation via three pathways, including nitric oxide and prostanglandin-I2 synthesis and ADP scavenging via NTPDase activity (Marcus et al. 2003; Jin et al. 2005). Inhibition of endogenous thromboxane synthesis by the most widely used antiplatelet agonist aspirin does not affect the prothrombotic responses to exercise (Li et al. 1999). By using the selective P2Y12-receptor antagonist clopidrogel, the same authors suggested later that prothrombotic responses to exercise were also little influenced by ADP (Perneby et al. 2004). However, this interpretation must be taken with certain caution since, along with P2Y12-, the platelets express another ADP-selective P2Y1-receptor which primarily mediates platelet shape and aggregation, especially at high shear rates (Gachet, 2006), and in addition, the increased platelet count in studies with clopidrogel does not exclude the possibility of acute mobilization of more active platelets from the reticuloendothelial system during exercise.
While a significant role of circulating ATP in exercise hyperaemia is now appreciated, the contribution of a purinergic signalling cascade to the acute prothrombotic responses still remains controversial. Here, we hypothesized that extracellular nucleotide turnover is activated in the bloodstream of exercising humans and in this way, affects the enhanced platelet reactivity and haemostasis. To accomplish this, we studied the whole spectrum of plasma nucleotides and serum purinergic activities in venous and arterial blood from the heart and the exercising limbs of endurance-trained athletes, and further examined the ability of plasma from exercising subjects to activate platelet P-selectin expression. Since physically inactive individuals are characterized by a higher risk of acute myocardial infarction and other cardiovascular complications following strenuous exercise (El-Sayed, 2002), we additionally measured plasma ATP level and soluble nucleotidases in the venous blood of sedentary subjects during exhaustive exercise.
Methods
Subjects
Twenty endurance-trained male subjects with a mean (±s.d.) age of 29 ± 3 years, body weight of 78.9 ± 8.2 kg, height of 180 ± 8 cm, maximal heart rate of 193 ± 6 beats min−1 and V̇o2,max of 4.7 ± 0.4 l min−1 participated in the first two studies. Seven sedentary subjects (3 males and 4 females) with age of 30 ± 5 years, body weight of 80.6 ± 23.1 kg, height of 170 ± 8 cm, maximal heart rate of 188 ± 10 beats min−1 and V̇o2,max of 2.4 ± 0.4 l min−1 participated in the third study. They were informed of any risks and discomforts associated with the experiments before giving their written consent to participate. The studies were approved by the Ethics committee of Copenhagen and Frederiksberg communities and were conducted in accordance with the guidelines of the Declaration of Helsinki.
Experimental protocols
In the first study (n= 10), the catheters were placed under local anaesthesia into the brachial artery and antecubital vein using the Seldinger technique, with the latter catheter being advanced to the right atrium. Following 30 min of supine rest, the subjects performed 15 min of submaximal exercise (146 ± 6 W; < 50% V̇o2,max) on a cycle ergometer (Excalibur, Lode, the Netherlands) followed by a 3 min rest and a constant load maximal exercise bout (372 ± 11 W) that lasted 6.8 ± 0.5 min. During the protocol, blood was drawn from the brachial artery and right atrium at rest, during submaximal and maximal exercises and after 10 min of passive recovery. In the second study (n= 10), an additional catheter was inserted into the femoral vein 2 cm from the inguinal ligament to allow for blood sampling and measurement of leg blood flow. The workload was increased every minute to elicit 25, 50, 75, 90 and 100% of peak power. Blood was drawn from the femoral vein, femoral artery and right atrium at supine rest, at 90% of peak power output (350 ± 15 W), and during 30 min recovery period. In the third study (n= 7), sedentary subjects performed incremental cycle-ergometer exercise to exhaustion (peak power output 192 ± 31 W) while blood samples were drawn from an antecubital vein at rest, peak exercise and after 30 min of recovery.
Blood sampling
For quantification of plasma nucleotides, blood was collected using syringes containing EDTA (S-monovette, Sarstedt, Germany), as previously described (González-Alonso et al. 2002). The duration of the whole procedure from blood withdrawal to plasma separation was ∼90 s. Since potential ATP release from formed elements might occur during blood sampling (Gorman et al. 2003), blood gases, total haemoglobin (ABL700 analyser; Radiometer, Denmark) and plasma haemoglobin (Cripps, 1968) were measured. Plasma samples showing elevated haemoglobin were excluded from further analyses. For serum preparation, blood (5 ml) was allowed to clot before centrifugation (10 min at 2000 g). Supernatants were additionally centrifuged for 10 min at 15 400 g followed by freezing the resultant serum at –40°C. Serum was also passed through Millipore 0.22 μm filters or ultracentrifuged for 60 min at 100 000 g (Sorvall Ultra-Pro80, rotor TST-60.4, DuPont). For preparation of heparinized plasma, venous blood from resting volunteers was collected into heparin-containing tubes (Terumo, Belgium).
Systemic haemodynamics
Whole-body V̇o2 was measured online with a Medgraphics cardiopulmonary exercise testing system CPX/D (Saint Paul, MN, USA). Heart rate was obtained from an electrocardiogram while arterial blood pressure was monitored from the brachial artery (Pressure Monitoring Kit, Baxter). Cardiac output was estimated using the Fick principle (cardiac output = V̇o2/a-vO2 difference), assuming negligible differences in blood oxygenation between the right atrium and pulmonary artery (Edwards & Mayall, 1998). Leg blood flow was measured with the constant thermodilution method (Andersen & Saltin, 1985). Systemic O2 delivery was calculated by multiplying cardiac output by the arterial O2 content.
Flow-cytometric analysis of P-selectin expression on platelet surface
For platelet isolation, 7 ml blood from an antecubital vein of three healthy females (32 ± 3 years) was collected into EDTA-containing tubes, mixed with 3 ml of 0.6% (w/v) dextran T70 saline, and allowed to stay for 1 h. Platelet-rich plasma was centrifuged, washed with EDTA-containing citrate glucose saline and resuspended in 5 ml of 1% (w/v) ammonium oxalate to lyse the residual red cells. The resultant platelets were washed twice with Tyrode buffer and immediately activated by incubating 5 × 106 cells for 20 min at 22°C in a final volume of 600 μl RPMI-1640 without and with 1 μmol l−1 ADP or 260 μl plasma from exercising humans. Plasma was also pre-incubated for 30 min at 37°C in RPMI-1640 with 1 unit ml−1 soluble apyrase from potato (grade III, Sigma). Platelets were washed twice with PBS containing 2% fetal calf serum and 0.01% NaN3 and stained with anti-P-selectin mAb WAPS-12.2 (gift from Dr Eugene Butcher, Stanford University, CA, USA). Mouse 3G6 mAb against an antigen expressed on chicken peripheral T-cells was used as negative control antibody. Cells were then incubated with FITC-conjugated antimouse Ig (Dako, Glostrup, Denmark) and analysed using FACSCalibur with CellQuest-Pro software (Becton Dickinson, CA, USA).
Quantification of ATP and other nucleotides in human plasma
ATP concentration was determined in EDTA-containing plasma by the luciferin–luciferase technique as previously described (González-Alonso et al. 2002). Endogenous purines were also extracted from 300 μl of plasma by adding 60 μl 4 m perchloric acid. After centrifugation, the supernatant was adjusted to neutral pH by 4 n KOH (∼50 μl), clarified again by centrifugation and stored at –70°C until high-performance liquid chromatography (HPLC) analysis. The samples (90 μl) were injected onto a 4 μm, 150 mm × 4.6 mm, reverse-phase Synergi Hydro-RP 80A column (Phenomenex, Torrance, CA, USA) and separated as previously described (Yegutkin et al. 2003). Plasma AMP and ADP were monitored by absorption at 258 nm at retention times of 15 and 25 min, respectively, and quantified from the areas under the corresponding peaks using the authentic standards injected versus the observed chromatographic peak areas.
Measurement of soluble nucleotide converting activities
Purinergic activities were measured by incubating serum at 37°C in 60 μl RPMI-1640 medium containing 5 mmol l−1β-glycerophosphate, unlabelled nucleotides and tracer [2,8-3H]ADP (Perkin Elmer, Boston, MA, USA), [8-14C]ATP and [2-3H]AMP (Amersham, Little Chalfont, UK) as appropriate substrates. Specifically, for ADPase activity, serum (10 μl) was incubated with 50 μmol l−1[3H]ADP in the presence of adenylate kinase inhibitor Ap5A (80 μmol l−1) (Yegutkin et al. 2003). ATPase and 5′-nucleotidase were assayed by incubating 5 μl serum with 100 μmol l−1[14C]ATP and 300 μmol l−1[3H]AMP, respectively. For adenylate kinase and NDP kinase activities, serum (5 μl) was incubated with 500 μmol l−1[3H]AMP or [3H]ADP as respective phosphate acceptors and 800 μmol l−1 of γ-phosphate-donating ATP. Incubation times varied from 30 to 60 min so that the amount of converted nucleotides did not exceed 5–10% of the initially introduced substrate. Mixture aliquots were applied onto Alugram SIL G/UV254 sheets (Macherey-Nagel, Germany). Radiolabelled substrates and their products were separated by thin layer chromatography (TLC) and quantified by scintillation β-counting (Yegutkin et al. 2001).
Autoradiographic analysis of [γ-32P]ATP hydrolysis by human serum
Serum (10 μl) was incubated for 10–35 min at 37°C in 40 μl RPMI-1640 containing 1 mmol l−1β-glycerophosphate and 10 μmol l−1 ATP with tracer [γ-32P]ATP (3000 Ci mmol−1; Perkin Elmer). Aliquots of the mixture were spotted onto Polygram CEL-300 PEI sheets (Macherey-Nagel), separated by TLC with 0.75 mol l−1 KH2PO4 (pH 3.5) and developed by autoradiography. The images were acquired to the Gel Doc-2000 (Bio-Rad Laboratories) and intensities of the bands corresponding to [γ-32P]ATP-derived 32Pi and 32PPi were quantified using the accompanying Quantity One software.
Statistical analysis
One-way repeated measures analysis of variance (ANOVA) with Dunnett’s multiple comparison test was performed to test significance within the trials (GraphPad Prism, version 4.02, San Diego, CA, USA). Data are presented either as column bars (mean +s.e.m.) or as box-and-whiskers plots, where the box extends from the 25th to the 75th percentile with a horizontal line at the median and with whiskers showing the range of the data. The significance level was set at P < 0.05.
Results
Plasma from exercising subjects up-regulates P-selectin level on human platelets
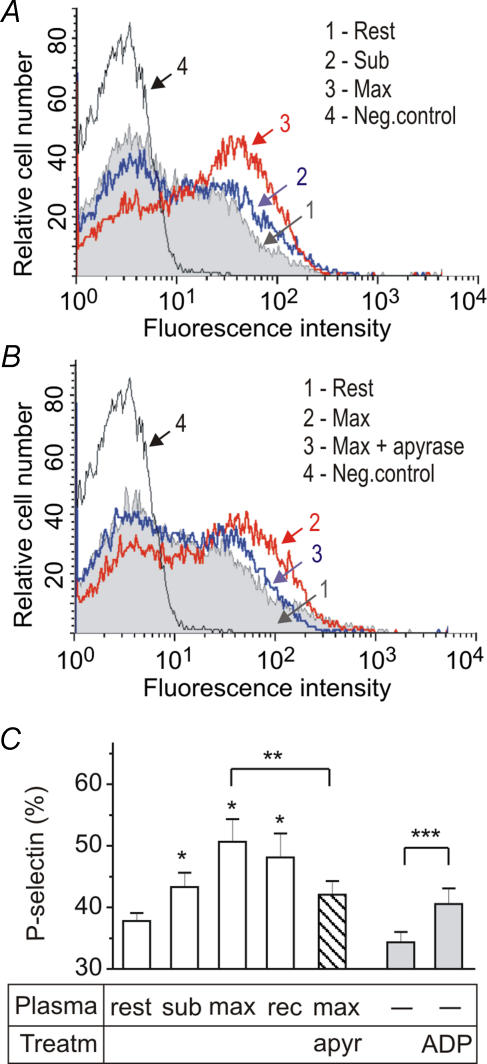
In the first study, 10 endurance-trained subjects performed constant intensity exercise until exhaustion. As expected, O2 delivery and systemic V̇o2 increased with increasing exercise intensity, accompanied by elevations in cardiac output, heart rate, stroke volume and mean arterial pressure. After 10 min of recovery, cardiac output, systemic O2 delivery and V̇o2 were still elevated above resting levels (Table 1). To examine whether circulating nucleotides and other soluble factors can be released under exercising conditions and contribute to the platelet reactivity, venous blood was collected from the right atrium at rest, during submaximal and maximal exercise and after early recovery. The obtained plasma samples were then co-incubated with platelets from resting volunteers while platelet activation was monitored by flow cytometry. Figure 1 shows that, unlike resting controls, plasma from exercising athletes significantly up-regulated the expression level of the platelet activation marker P-selectin and these prothrombotic effects were abolished after scavenging circulating nucleotides by exogenous apyrase. Interestingly, exogenous ADP (1 μmol l−1) also up-regulated the expression level of membrane-bound P-selectin, although not as efficiently as plasma from exercising subjects (Fig. 1C).
Table 1.
Systemic haemodynamic variables during rest, exercise and recovery
| Exercise protocols | Cardiac output (l min−1) | Heart rate (beats min−1) | Systemic O2 delivery (l min−1) | Systemic a-vO2 difference (ml l−1) | V̇O2(l min−1) | Mean arterial pressure (mmHg) |
|---|---|---|---|---|---|---|
| Study 1. Constant intensity exercise | ||||||
| Supine rest | 8.6 ± 0.6 | 64 ± 2 | 1.7 ± 0.1 | 38 ± 2 | 0.3 ± 0.0 | 87 ± 3 |
| Submaximal exercise | 18.8 ± 1.1* | 128 ± 2* | 3.9 ± 0.2* | 116 ± 6* | 2.2 ± 0.1* | 100 ± 4* |
| Maximal exercise | 26.4 ± 1.0* | 188 ± 2* | 5.6 ± 0.2* | 179 ± 4* | 4.7 ± 0.1* | 129 ± 7* |
| Recovery | 12.5 ± 0.7* | 105 ± 5* | 2.6 ± 0.1 | 63 ± 3* | 0.8 ± 0.0* | 83 ± 3 |
| Study 2. Incremental exercise | ||||||
| Seated rest | 10.4 ± 1.2 | 63 ± 2 | 2.2 ± 0.2 | 60 ± 5 | 0.6 ± 0.0 | 101 ± 2 |
| 90% peak power | 26.1 ± 1.3* | 188 ± 2* | 5.7 ± 0.2* | 173 ± 4* | 4.5 ± 0.1* | 134 ± 3* |
| Recovery | 9.8 ± 0.8 | 65 ± 3 | 2.2 ± 0.2 | 44 ± 6 | 0.4 ± 0.1 | 90 ± 3 |
Constant intensity exercise data (Study 1) were collected at rest, after 10 min of submaximal and 5–7 min of maximal upright cycle ergometer exercise (146 ± 6 and 372 ± 11 W, respectively), and after 10 min of recovery. Incremental exercise data (Study 2) were collected at 90% of peak power (350 ± 15 W) and after 30 min of recovery. Data are mean ±s.e.m. from 6 to 10 subjects for each protocol.
Significantly higher than rest, P < 0.05.
Figure 1. Plasma from exercising humans stimulates P-selectin expression on platelet surface.
Human platelets from healthy volunteers were pretreated for 20 min with plasma samples collected from the right atrium of trained athletes at rest, after submaximal (sub) and maximal (max) exercise and after 10 min recovery (rec). A and B, the platelets were then stained with anti-P-selectin mAb WAPS-12.2 (histograms 1, 2 and 3) or with negative control mAb 3G6 (Neg. control) and analysed by flow-cytometry. The abscissa represents the logarithmic scale of fluorescence intensity and the ordinate shows the relative cell number. C, the percentage of P-selectin expression after platelet-plasma co-incubation was determined from the above histograms (mean +s.e.m.; *P < 0.05 as compared with rest; n= 8). Plasma from exercising subjects was pretreated with apyrase (1 unit ml−1) prior to addition to platelets (**P < 0.05 as compared with non-treated plasma; n= 4). Platelets were also incubated in RPMI-1640 with and without 1 μmol l−1 ADP (***P < 0.05 as compared with medium alone; n= 3).
Effect of exercise on circulating adenine nucleotide levels in human plasma
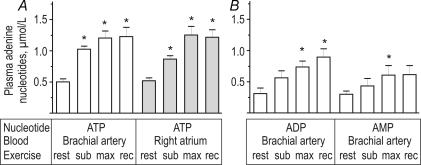
The above data suggest that strenuous exercise might significantly augment platelet activity via acute shifts in circulating nucleotide levels. This hypothesis was further verified by direct quantification of nucleotide concentrations in plasma samples collected from venous blood from the right atrium and arterial blood from the brachial artery. Use of an ATP-specific bioluminescent assay, in combination with reverse-phase HPLC, revealed that human plasma contains high nanomolar concentrations of ATP, ADP and AMP (Fig. 2). Importantly, plasma ATP (Fig. 2A) and ADP (Fig. 2B) increased with exercise, remaining elevated after the ensuing 10 min recovery. AMP also increased during maximal exercise (Fig. 2B). The observed increases in nucleotide levels were not due to elevated haemolysis, since plasma haemoglobin remained unchanged throughout the exercise and recovery (range 2–4 mg dl−1).
Figure 2. Effect of exercise on adenine nucleotide levels in blood plasma.
Human blood was collected from the trained athletes at rest, after submaximal (sub) and maximal (max) exercise and after 10 min recovery (rec). A, plasma ATP was assayed using luciferin–luciferase luminometry. B, HPLC analysis of ADP and AMP concentrations in plasma prepared from the brachial artery blood of exercising humans. Data are mean +s.e.m. (n= 8–10). *P < 0.05 as compared with rest.
Effect of exercise on soluble nucleotidases and other purinergic enzymes in human serum
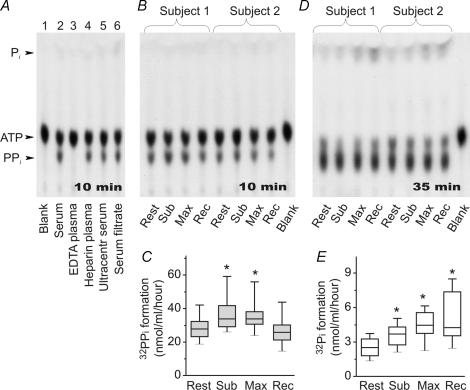
Next, knowing that human blood contains an extensive network of soluble purinergic enzymes (Yegutkin et al. 2003), we wanted to evaluate the impact of exercise on the pattern of nucleotide metabolism. Addition of 10 μmol l−1[γ-32P]ATP to human serum (Fig. 3A, lane 2) or heparinized plasma (lane 4), but not to EDTA-containing plasma (lane 3), caused significant formation of 32PPi and, to a lesser extent, 32Pi. Additional ultracentrifugation (Fig. 3A, lane 5) or filtration (lane 6) of serum samples did not affect their ability to hydrolyse [γ-32P]ATP. These data indicate that cation-dependent nucleotidases primarily circulate in the human bloodstream as ‘true’ soluble enzymes.
Figure 3. Pattern of [γ-32P]ATP metabolism in human plasma and serum.
A, serum and EDTA-containing or heparinized plasma from venous blood were incubated with 10 μmol l−1[γ-32P]ATP, separated using TLC and developed by autoradiography. Serum was additionally ultracentrifuged or filtered through 0.22 μm filters prior to adding [γ-32P]ATP. B and D, serum from right atrial blood of exercising trained subjects was incubated with [γ-32P]ATP for 10–35 min. Arrows indicate the positions of ATP, inorganic phosphorus (Pi) and pyrophosphate (PPi). Blanks show the radiochemical purity of [γ-32P]ATP. C, serum NPPs were quantified by acquiring the images shown in B and measuring the band intensities corresponding to [γ-32P]ATP-derived 32PPi. E, likewise, NTPDase activities were determined from the autoradiography in D by the rate of 32Pi cleavage from [γ-32P]ATP. Data are presented as box-and-whiskers plots (n= 10). *P < 0.05 as compared with rest.
Serum was then prepared from the blood of exercising subjects for subsequent measurement of soluble activities. Short-term incubation of [γ-32P]ATP with serum caused its predominant conversion into AMP and 32PPi (Fig. 3B) and further quantification of pyrophosphatase activity revealed that soluble NPP increased by 20–25% during exercise and then declined (Fig. 3C). Increasing the incubation time from 10 to 35 min additionally elicited a slight production of 32Pi (Fig. 3D). Quantitative analysis of these autoradiographic data showed a gradual rise in soluble NTPDase up to 2-fold during submaximal and maximal exercise, remaining markedly elevated during recovery (Fig. 3E). Noteworthy, contribution of NTPDase to the bulk serum ATP-hydrolysing pool (measured as relative intensity of 32Pi band with respect to the sum of [γ-32P]ATP-derived 32PPi and 32Pi) raised from 8.6 ± 1.1% in the resting blood up to 16.1 ± 1.6% during early recovery after maximal exercise (n= 10; P < 0.05).
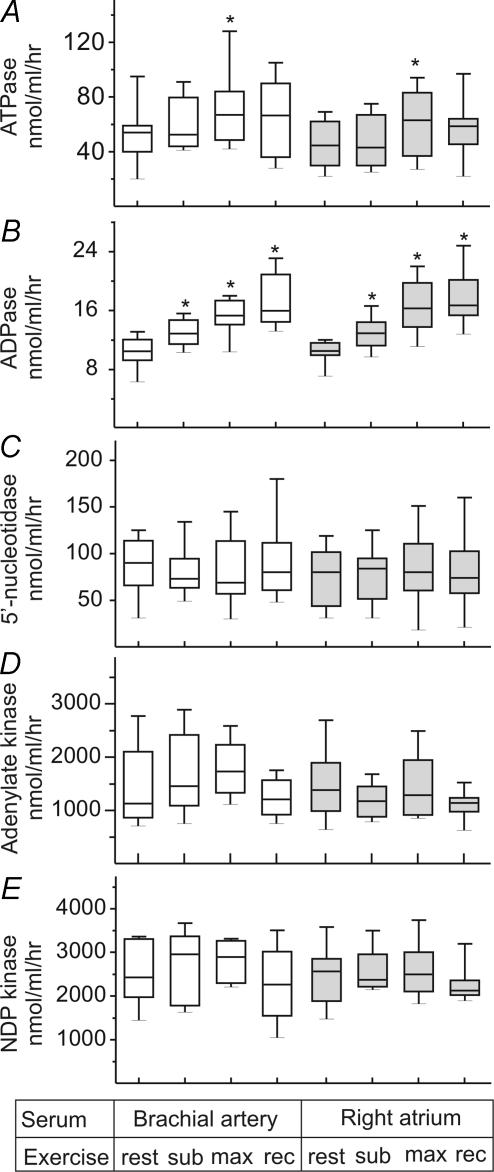
Measurement of the rate of [14C]ATP hydrolysis into 14C-metabolites (quantified as pooled ADP, AMP and nucleoside fractions) also demonstrated significant elevation of ‘bulk’ ATP-hydrolysing capacity of human serum during maximal exercise (Fig. 4A). Moreover, the employment of [3H]ADP as another preferred substrate for NTPDase confirmed the presence of moderate ADPase activity in human serum and its gradual activation with increasing exercise intensity and during early recovery (Fig. 4B). Further AMP breakdown into adenosine is mediated by another soluble enzyme 5′-nucleotidase. During exercise, however, we did not observe any increases in the rate of [3H]AMP hydrolysis (Fig. 4C). The counteracting nucleotide-phosphorylating pathway was also evaluated in human serum. Both adenylate kinase (Fig. 4D) and NDP kinase (Fig. 4E) activities remained unchanged during exercise test.
Figure 4. Effect of exercise on nucleotide-converting activities in human serum.
Serum was prepared at rest, during submaximal (sub) and maximal (max) exercise, and after ensuing 10 min recovery. ATPase (A), ADPase (B), and 5′-nucleotidase (C) were assayed by TLC with 100 μmol l−1[14C]ATP, 50 μmol l−1[3H]ADP and 300 μmol l−1[3H]AMP as respective substrates. Nucleotide-phosphorylating enzymes, adenylate kinase (D) and NDP kinase (E), were assayed using γ-phosphate donating ATP (800 μmol l−1) and 500 μmol l−1[3H]AMP or [3H]ADP as respective phosphate acceptors. Enzymatic activities are presented as box-and-whiskers plots expressed as nanomoles of nucleotide substrates hydrolysed (A–C) or phosphorylated (D and E) by 1 ml serum per hour (n= 10).
Evidence for transient activation of soluble NTPDase during exercise
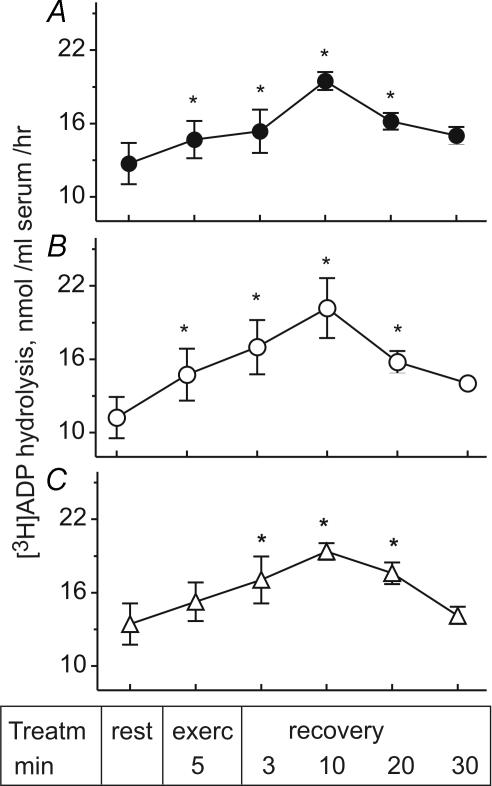
Ten subjects participated in the second study, in which they performed incremental cycling exercise to exhaustion. Systemic and leg haemodynamics, O2 transport and V̇o2 reached maximal or near-maximal values when cycling at 90% of peak power, and returned to baseline values after 30 min of recovery (Tables 1 and 2). As for constant intensity exercise, the rates of [3H]ADP hydrolysis by serum samples taken from the femoral artery (Fig. 5A) and right atrium (Fig. 5B) increased during incremental exercise, reaching peak values after 10 min of recovery. Moreover, this study revealed a similar pattern of soluble NTPDase activation in the blood drawn from the femoral vein of exercising humans (Fig. 5C), and additionally showed that ADPase activity in all tested samples gradually decayed to baseline level 30 min after completing exercise.
Table 2.
Leg haemodynamic variables during rest, incremental exercise and recovery
| Exercise protocol | Leg blood flow (l min−1) | Leg vascular conductance (ml min−1 mmHg−1) | Leg O2 delivery (l min−1) | Leg a-vO2 difference (ml l−1) | Leg V̇O2(l min−1) |
|---|---|---|---|---|---|
| Seated rest | 1.4 ± 0.2 | 14 ± 2 | 0.29 ± 0.05 | 102 ± 7 | 0.15 ± 0.03 |
| 90% peak power | 10.4 ± 0.8* | 83 ± 7* | 2.25 ± 0.16* | 176 ± 3* | 1.86 ± 0.13* |
| Recovery | 0.7 ± 0.1 | 8 ± 1 | 0.12 ± 0.01 | 55 ± 9 | 0.04 ± 0.00 |
Incremental cycle ergometer exercise data (Study 2) were collected at 90% of peak power (350 ± 15 W) whereas the recovery data were collected after 30 min of recovery. Data are mean ±s.e.m. from 10 subjects.
Significantly higher than seated rest, P < 0.05.
Figure 5. Hydrolysis of [3H]ADP by human serum during exercise and recovery.
Blood was prepared from the femoral artery (A), right atrium (B) and femoral vein (C) of the trained athletes at rest, during incremental exercise, and ensuing 30 min recovery. Serum NTPDase was assayed by TLC using 50 μmol l−1[3H]ADP as substrate. Data are mean ±s.e.m. (n= 6). *P < 0.05 as compared with rest.
Effect of exercise on intravascular nucleotide turnover in sedentary subjects
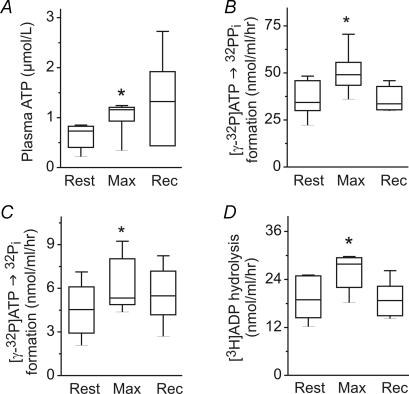
In a third study, seven sedentary subjects performed incremental exercise to exhaustion. During exercise, systemic V̇o2 and heart rate increased to 2.4 ± 0.4 l min−1 and 188 ± 10 beats min−1, respectively, and returned to baseline values after 30 min of recovery. Bioluminescent analysis revealed a significant exercise-mediated increase in circulating ATP (Fig. 6A), whereas plasma haemoglobin remained unchanged (1.9 ± 0.4, 1.9 ± 0.6 and 2.0 ± 0.4 mg dl−1 during rest, exercise and recovery, respectively). Enzyme assays with [γ-32P]ATP (Fig. 6B and C) and [3H]ADP (Fig. 6D) as substrates additionally demonstrated that soluble NPP and NTPDase activities transiently increased in the blood of exercising unfit subjects. The activities of serum 5′-nucleotidase and adenylate kinase remained unchanged during exercise (data not shown). Similar dynamics of transiently activated intravascular nucleotide turnover occurring both in endurance trained and sedentary exercising subjects reasonably allow us to generalize this phenomenon as a characteristic feature of strenuous exertion. Interestingly, basal serum nucleotidase activities in unfit subjects were higher compared with trained athletes (compare Fig. 6 with Figs 3–5). However, despite this apparent trend, more thorough studies are required to validate this interesting observation.
Figure 6. ATP level and nucleotidase activities in the blood of sedentary subjects.
Venous blood was collected at rest, during maximal exercise (max) and after 30 min recovery (rec). A, plasma ATP was assayed using luciferin–luciferase luminometry. B and C, serum was incubated with 10 μmol l−1[γ-32P]ATP for 10–35 min and separated by TLC. NPP and NTPDase activities were quantified from the autoradiographic images by measuring the band intensities corresponding to [γ-32P]ATP-derived 32PPi and 32Pi, as shown in Fig. 3. D, NTPDase was assayed by TLC using 50 μmol l−1[3H]ADP as substrate. Data are presented as box-and-whiskers plots (n= 7). *P < 0.05 as compared with rest.
Discussion
By simultaneously investigating plasma nucleotide levels and serum purinergic activities, here we showed that the pattern of nucleotide turnover is acutely disturbed both in cardiac and skeletal muscle circulations of exercising humans, and further identified a link between purinergic signalling and prothrombotic responses. The major findings of this study are that: (1) along with ATP, plasma level of ADP also rises during exercise and in this way, augments platelet activity; (2) serum nucleotide-inactivating enzymes NTPDase and NPP are transiently up-regulated with the increasing exercise intensity.
A salient finding of this work is the demonstration that plasma from exercising humans, but not from resting controls samples up-regulates the expression of the platelet activation marker P-selectin and these prothrombotic effects can be attenuated after scavenging nucleotides by exogenous apyrase (Fig. 1). Subsequent reverse-phase HPLC analysis directly confirmed significant increase in circulating ADP during exercise (Fig. 2B). Likewise, simultaneous increases in plasma ADP and platelet activity have been shown recently during myocardial infarction in humans (Borna et al. 2005). This work also corroborates recent findings from ours (González-Alonso et al. 2002) and other (Farias et al. 2005) laboratories on a significant rise in circulating ATP levels under exercising conditions. Since this elevated ATP level presumably occurs due to nucleotide release from the red blood cells in response to low oxygen tension and Hb deoxygenation (Ellsworth et al. 1995; Ellsworth, 2004), it would be expected that exercise would be accompanied by a more efficient increment in venous ATP compared with arterial blood. However, plasma ATP rose to the same extent both in the brachial artery and right atrium of exercising subjects (Fig. 2A). The reason for this discrepancy might be that arterial–venous differences in ATP level can be detected only within a confined region, during erythrocyte diffusion from the small venule to the paired arteriole, while within larger vessels these variations are somehow diminished, especially under exercising conditions with markedly increased blood flow.
In view of the diverse roles of ATP and ADP in the haemostasis, simultaneous release of these nucleotides may provide an efficient two-tier control system regulating tissue O2 delivery and prothrombotic state during vigorous exercise. Since ATP acts as a competitive antagonist limiting the extent of ADP-induced platelet aggregation (Gachet, 2006), ADP provides a negative feedback pathway for ATP release from the red blood cells, most likely via interaction with Gi-protein coupled P2Y13 receptors on the erythrocyte surface and inhibition of cAMP formation (Wang et al. 2005). Taking into account the redundancy between multiple controllers (Clifford & Hellsten, 2004), we believe that other local substances (e.g. nitric oxide, potassium, adenosine, hydrogen ion and some eicosanoids) contribute, along with adenine nucleotides, to the exercise hyperaemia and thrombosis in certain compensatory or synergistic manners. Particularly, nitric oxide level can also be increased during exercise (McMahon et al. 2002; Clifford & Hellsten, 2004). The released nitric oxide, in turn, prevents further ATP release from the red blood cells (Olearczyk et al. 2004) and, in conjunction with soluble NTPDase, inhibits platelet deposition in a synergistic manner (Ramamurthi et al. 2001).
Surprisingly, exogenous ADP (1 μmol l−1) was less efficient in activating platelet P-selectin compared with plasma from exercising subjects (Fig. 1C). While this apparent discrepancy could be partially explained by a concurrent rise in circulating ATP (Fig. 2A) and its binding to ATP-selective P2X1-receptors on platelet surface (Gachet, 2006), it seems more likely that other soluble plasma factor(s) potentiate the prothrombotic effects of ADP during exercise. For example, strenuous exercise also increases plasma epinephrine (adrenaline) and enhances α2-adrenergic agonist-potentiated platelet adhesiveness on fibrinogen-coated surfaces (Wang & Cheng, 1999). However, epinephrine is not, by itself, an activating agent and its ability to stimulate platelet aggregation requires the presence of nanomolar concentrations of ADP (Steen et al. 1993; Wang & Cheng, 1999). Moreover, various chemokines may be released from endothelial cells (stromal cell-derived factor-1) and leucocytes (macrophage-derived and thymus activation-regulated chemokines) under hyperaemia conditions and, in combination with low subthreshold ADP concentrations (0.05–0.25 μmol l−1), potentiate the platelet aggregation, adhesion and P-selectin expression (Gear et al. 2001). The ability of the ADP-scavenging system to block or reduce epinephrine-(Steen et al. 1993) and chemokine-induced (Gear et al. 2001) platelet aggregation further point to a role of ADP in platelet function beyond its immediate activity as a primary agonist. Probably, the simultaneous release of ADP and other factors, in conjunction with the increased shear forces, would provide the stimuli for platelet activation during exercise.
Since the level of circulating purines represents a net balance between nucleotide release and inactivation, we then evaluated the pattern of nucleotide metabolism in human serum. By using a radio-TLC assay with [γ-32P]ATP and 3H/14C-labelled nucleotides, we were able to distinguish between nucleotide pyrophosphatase and hydrolase families which constitutively circulate in human blood; and further demonstrated transient release of these soluble enzymes during exercise. Human serum also contains 5′-nucleotidase, adenylate kinase and NDP kinase (Yegutkin et al. 2003); however, these purinergic activities remain unchanged during exercise. The up-regulation of serum NTPDase revealed here might represent a novel and currently unappreciated effector system for termination of acute prothrombotic effects of ADP. In addition, concurrent exercise-mediated activation of another nucleotidase, NPP, may allow by-passing of the generation of a principal platelet-recruiting agent ADP, via direct conversion of ATP into AMP and PPi. In this context, it is pertinent to note that ATP-and ADP-degrading activities in human plasma prevent desensitization of purinergic receptors in stored platelets and are essential for the shear-dependent formation of aggregates and thrombi (Birk et al. 2002; Cauwenberghs et al. 2006). In sedentary subjects, exercise was accompanied by similar dynamics of transiently elevated nucleotidase activities (Fig. 6), although the basal NTPDase was higher than in trained athletes. Probably, the thromboregulatory role of soluble NTPDase is even more relevant for patient populations who display the increased platelet aggregability at rest and especially during exercise, and in addition suffer from endothelial dysfunction and insufficient release of antiplatelet compounds (El-Sayed, 2002; Coppola et al. 2005).
The finding of secreted nucleotidases in a biological system is not a unique phenomenon. NTPDase/CD39 and other purinergic enzymes can be released from the nerve terminals of guinea-pig vas deferens during electric stimulation (Todorov et al. 1997), rat pancreatic acini upon stimulation with cholecystokinin octapeptide-8 (Sorensen et al. 2003; Yegutkin et al. 2006), human airway epithelial cells and nasal lavage after stimulating submucosal gland secretion (Donaldson et al. 2002) and human endothelial cells under conditions of shear stress (Yegutkin et al. 2000). Since vigorous exercise increases blood flow (González-Alonso et al. 2002; also Table 2), it would be anticipated that shear rate in vivo also activates the shedding of membrane-bound NTPDases during exercise. However, this seems unlikely since even a 10-fold increase in leg blood flow by injection of ATP into the femoral artery of resting subjects does not affect serum nucleotidase activities (authors unpublished observation).
Assessment of the relative importance of soluble versus membrane-bound enzymes is another important issue. Under basal conditions, vascular endothelial NTPDase maintains homeostasis via rapid inactivation of the released nucleotides (Marcus et al. 2003). Endothelial metabolism generally prevails in the microcirculation, whereas within larger vessels the capacity to metabolize nucleotides resides approximately equally at the luminal surface of the vessel wall and in the flowing blood (Coade & Pearson, 1989). Among cellular elements of the blood, the leucocytes, but not erythrocytes and platelets, contribute to the catabolism of nucleotides (Coade & Pearson, 1989; Heptinstall et al. 2005). Notably, flow cytometric analysis of venous blood from physically active men showed moderate exercise-mediated increase in CD39 expression on B-lymphocytes (from 46 ± 11 to 59 ± 11%, respectively) (Coppola et al. 2005). Vascular NTPDase can be diminished under conditions of inflammation or oxidant stress, thereby causing profound shifts in vascular permeability and local procoagulant responses (Robson et al. 2005). The strategies to offset the reduced effectiveness of endothelial nucleotidases include NTPDase expression in the injured vessels (Furukoji et al. 2005) or administration of soluble NTPDase/CD39 for antiplatelet therapy (Marcus et al. 2003; Atkinson et al. 2006). The recombinant soluble form of human CD39 is currently considered a promising aspirin-insensitive antithrombotic drug, which inhibits platelet reactivity under experimental prothrombotic conditions, including ischaemia/reperfusion murine models of cerebral shock (Pinsky et al. 2002) and hepatic injury (Robson et al. 2005), a rat model of focal cerebral ischaemia (Belayev et al. 2003), a porcine model of balloon arterial injury (Buergler et al. 2005), and acute and chronic models of transplantation (Robson et al. 2005).
In conclusion, here we show that strenuous exercise significantly augments platelet activity via transient ADP release, and these prothrombotic responses are partially counteracted by concurrent up-regulation of soluble nucleotide-inactivating enzymes. These findings provide a novel insight into the purinergic mechanisms underlying the enhanced risk of occlusive thrombus formation under exercising conditions. Furthermore, data on the constitutive presence of soluble NTPDase in human blood may open up further research for future therapeutic applications of this principal ADP-inactivating nucleotidase as a ‘natural antithrombotic enzyme’ for antiplatelet therapy in hypoxia-associated and other vascular diseases.
Acknowledgments
We give special thanks to Professor Niels H. Secher, Drs Rasmus Damsgaard, Ellen A. Dawson, Chie C. Yoshiga and Ms Sari Mäki for their help in these studies. We are grateful to Professor Eugene C. Butcher for providing us with the anti-P-selectin antibody.
We also thank the volunteer subjects for their enthusiasm. This study was supported by the Finnish Academy, Sigrid Juselius Foundation (G.G.Y. and S.J.) and the Copenhagen Hospital System, Novo Nordisk Foundation (J.G.-A.).
References
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson B, Dwyer K, Enjyoji K, Robson SC. Ecto-nucleotidases of the CD39/NTPDase family modulate platelet activation and thrombus formation: Potential as therapeutic targets. Blood Cells Mol Dis. 2006;36:217–222. doi: 10.1016/j.bcmd.2005.12.025. [DOI] [PubMed] [Google Scholar]
- Belayev L, Khoutorova L, Deisher TA, Belayev A, Busto R, Zhang Y, Zhao W, Ginsberg MD. Neuroprotective effect of SolCD39, a novel platelet aggregation inhibitor, on transient middle cerebral artery occlusion in rats. Stroke. 2003;34:758–763. doi: 10.1161/01.STR.0000056169.45365.15. [DOI] [PubMed] [Google Scholar]
- Birk AV, Broekman MJ, Gladek EM, Robertson HD, Drosopoulos JH, Marcus AJ, Szeto HH. Role of extracellular ATP metabolism in regulation of platelet reactivity. J Laboratory Clin Med. 2002;140:166–175. doi: 10.1067/mlc.2002.126719. [DOI] [PubMed] [Google Scholar]
- Borna C, Lazarowski E, Van Heusden C, Ohlin H, Erlinge D. Resistance to aspirin is increased by ST-elevation myocardial infarction and correlates with adenosine diphosphate levels. Thromb J. 2005;3:10. doi: 10.1186/1477-9560-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buergler JM, Maliszewski CR, Broekman MJ, Kaluza GL, Schulz DG, Marcus AJ, Raizner AE, Kleiman NS, Ali NM. Effects of SolCD39, a novel inhibitor of platelet aggregation, on platelet deposition and aggregation after PTCA in a porcine model. J Thromb Thrombolysis. 2005;19:115–122. doi: 10.1007/s11239-005-1381-y. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol Rev. 2006;58:58–86. doi: 10.1124/pr.58.1.5. [DOI] [PubMed] [Google Scholar]
- Cauwenberghs S, Feijge MA, Hageman G, Hoylaerts M, Akkerman JW, Curvers J, Heemskerk JW. Plasma ectonucleotidases prevent desensitization of purinergic receptors in stored platelets: importance for platelet activity during thrombus formation. Transfusion. 2006;46:1018–1028. doi: 10.1111/j.1537-2995.2006.00837.x. [DOI] [PubMed] [Google Scholar]
- Clifford PS, Hellsten Y. Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol. 2004;97:393–403. doi: 10.1152/japplphysiol.00179.2004. [DOI] [PubMed] [Google Scholar]
- Coade SB, Pearson JD. Metabolism of adenine nucleotides in human blood. Circ Res. 1989;65:531–537. doi: 10.1161/01.res.65.3.531. [DOI] [PubMed] [Google Scholar]
- Coppola A, Coppola L, dalla Mora L, Limongelli FM, Grassia A, Mastrolorenzo L, Gombos G, Lucivero G. Vigorous exercise acutely changes platelet and B-lymphocyte CD39 expression. J Appl Physiol. 2005;98:1414–1419. doi: 10.1152/japplphysiol.00315.2004. [DOI] [PubMed] [Google Scholar]
- Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, 3rd, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [see comment also] [DOI] [PubMed] [Google Scholar]
- Cripps CM. Rapid method for the estimation of plasma haemoglobin levels. J Clin Path. 1968;24:110–112. doi: 10.1136/jcp.21.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson SH, Picher M, Boucher RC. Secreted and cell-associated adenylate kinase and nucleoside diphosphokinase contribute to extracellular nucleotide metabolism on human airway surfaces. Am J Respir Cell Mol Biol. 2002;26:209–215. doi: 10.1165/ajrcmb.26.2.4650. [DOI] [PubMed] [Google Scholar]
- Edwards JD, Mayall RM. Importance of the sampling site for measurement of mixed venous oxygen saturation in shock. Crit Care Med. 1998;26:1356–1360. doi: 10.1097/00003246-199808000-00020. [DOI] [PubMed] [Google Scholar]
- Ellsworth ML. Red blood cell-derived ATP as a regulator of skeletal muscle perfusion. Med Sci Sports Exerc. 2004;36:35–41. doi: 10.1249/01.MSS.0000106284.80300.B2. [DOI] [PubMed] [Google Scholar]
- Ellsworth ML, Forrester T, Ellis CG, Dietrich HH. The erythrocyte as a regulator of vascular tone. Am J Physiol Heart Circ Physiol. 1995;269:H2155–H2161. doi: 10.1152/ajpheart.1995.269.6.H2155. [DOI] [PubMed] [Google Scholar]
- El-Sayed MS. Exercise and training effects on platelets in health and disease. Platelets. 2002;13:261–266. doi: 10.1080/0953770021000007221. [DOI] [PubMed] [Google Scholar]
- Farias M, 3rd, Gorman MW, Savage MV, Feigl EO. Plasma ATP during exercise: possible role in regulation of coronary blood flow. Am J Physiol Heart Circ Physiol. 2005;288:H1586–H1590. doi: 10.1152/ajpheart.00983.2004. [DOI] [PubMed] [Google Scholar]
- Furukoji E, Matsumoto M, Yamashita A, Yagi H, Sakurai Y, Marutsuka K, Hatakeyama K, Morishita K, Fujimura Y, Tamura S, Asada Y. Adenovirus-mediated transfer of human placental ectonucleoside triphosphate diphosphohydrolase to vascular smooth muscle cells suppresses platelet aggregation in vitro and arterial thrombus formation in vivo. Circulation. 2005;111:808–815. doi: 10.1161/01.CIR.0000155239.46511.79. [DOI] [PubMed] [Google Scholar]
- Gachet C. Regulation of platelet functions by P2 receptors. Annu Rev Pharmacol Toxicol. 2006;46:277–300. doi: 10.1146/annurev.pharmtox.46.120604.141207. [DOI] [PubMed] [Google Scholar]
- Gear AR, Suttitanamongkol S, Viisoreanu D, Polanowska-Grabowska RK, Raha S, Camerini D. Adenosine diphosphate strongly potentiates the ability of the chemokines MDC, TARC, and SDF-1 to stimulate platelet function. Blood. 2001;97:937–945. doi: 10.1182/blood.v97.4.937. [DOI] [PubMed] [Google Scholar]
- González-Alonso J, Olsen DB, Saltin B. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res. 2002;91:1046–1055. doi: 10.1161/01.res.0000044939.73286.e2. [DOI] [PubMed] [Google Scholar]
- Gorman MW, Marble DR, Ogimoto K, Feigl EO. Measurement of adenine nucleotides in plasma. Luminescence. 2003;18:173–181. doi: 10.1002/bio.721. [DOI] [PubMed] [Google Scholar]
- Heptinstall S, Johnson A, Glenn JR, White AE. Adenine nucleotide metabolism in human blood – important roles for leukocytes and erythrocytes. J Thromb Haemost. 2005;3:2331–2339. doi: 10.1111/j.1538-7836.2005.01489.x. [DOI] [PubMed] [Google Scholar]
- Jin RC, Voetsch B, Loscalzo J. Endogenous mechanisms of inhibition of platelet function. Microcirculation. 2005;12:247–258. doi: 10.1080/10739680590925493. [DOI] [PubMed] [Google Scholar]
- Li N, Wallen NH, Hjemdahl P. Evidence for prothrombotic effects of exercise and limited protection by aspirin. Circulation. 1999;100:1374–1379. doi: 10.1161/01.cir.100.13.1374. [DOI] [PubMed] [Google Scholar]
- McMahon TJ, Moon RE, Luschinger BP, Carraway MS, Stone AE, Stolp BW, Gow AJ, Pawloski JR, Watke P, Singel DJ, Piantadosi CA, Stamler JS. Nitric oxide in the human respiratory cycle. Nat Med. 2002;8:711–717. doi: 10.1038/nm718. [see comments also] [DOI] [PubMed] [Google Scholar]
- Marcus AJ, Broekman MJ, Drosopoulos JH, Islam N, Pinsky DJ, Sesti C, Levi R. Heterologous cell–cell interactions: thromboregulation, cerebroprotection and cardioprotection by CD39 (NTPDase-1) J Thromb Haemost. 2003;1:2497–2509. doi: 10.1111/j.1538-7836.2003.00479.x. [DOI] [PubMed] [Google Scholar]
- Olearczyk JJ, Ellsworth ML, Stephenson AH, Lonigro AJ, Sprague RS. Nitric oxide inhibits ATP release from erythrocytes. J Pharmacol Exp Ther. 2004;309:1079–1084. doi: 10.1124/jpet.103.064709. [DOI] [PubMed] [Google Scholar]
- Perneby C, Wallen NH, Hu H, Li N, Hjemdahl P. Prothrombotic responses to exercise are little influenced by clopidogrel treatment. Thromb Res. 2004;114:235–243. doi: 10.1016/j.thromres.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Pinsky DJ, Broekman MJ, Peschon JJ, Stocking KL, Fujita T, Ramasamy R, Connolly ES, Jr, Huang J, Kiss S, Zhang Y, Choudhri TF, McTaggart RA, Liao H, Drosopoulos JH, Price VL, Marcus AJ, Maliszewski CR. Elucidation of the thromboregulatory role of CD39/ectoapyrase in the ischemic brain. J Clin Invest. 2002;109:1031–1040. doi: 10.1172/JCI10649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamurthi A, Robson SC, Lewis RS. Effects of nitric oxide (NO) and soluble nucleoside triphosphate diphosphohydrolase (NTPDase) on inhibition of platelet deposition in vitro. Thromb Res. 2001;102:331–341. doi: 10.1016/s0049-3848(01)00244-4. [DOI] [PubMed] [Google Scholar]
- Robson SC, Wu Y, Sun X, Knosalla C, Dwyer K, Enjyoji K. Ectonucleotidases of CD39 family modulate vascular inflammation and thrombosis in transplantation. Semin Thromb Hemost. 2005;31:217–233. doi: 10.1055/s-2005-869527. [DOI] [PubMed] [Google Scholar]
- Rosenmeier JB, Hansen J, González-Alonso J. Circulating ATP-induced vasodilatation overrides sympathetic vasoconstrictor activity in human skeletal muscle. J Physiol. 2004;558:351–365. doi: 10.1113/jphysiol.2004.063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell LB. Ideas about control of skeletal and cardiac muscle blood flow (1876–2003): cycles of revision and new vision. J Appl Physiol. 2004;97:384–392. doi: 10.1152/japplphysiol.01220.2003. [DOI] [PubMed] [Google Scholar]
- Saltin B, Radegran G, Koskolou MD, Roach RC. Skeletal muscle blood flow in humans and its regulation during exercise. Acta Physiol Scand. 1998;162:421–436. doi: 10.1046/j.1365-201X.1998.0293e.x. [DOI] [PubMed] [Google Scholar]
- Shepherd JT. Circulation to skeletal muscle. In: Shepherd JT, Abboud FM, editors. Handbook of Physiology,The Cardiovascular System, Peripheral Circulation and Organ Blood Flow. III. Bethesda, MD, USA: American Physiological Society; 1983. pp. 319–370. section 2. [Google Scholar]
- Sorensen CE, Amstrup J, Rasmussen HN, Ankorina-Stark I, Novak I. Rat pancreas secretes particulate ecto-nucleotidase CD39. J Physiol. 2003;551:881–892. doi: 10.1113/jphysiol.2003.049411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen VM, Holmsen H, Aarbakke G. The platelet-stimulating effect of adrenaline through alpha 2-adrenergic receptors requires simultaneous activation by a true stimulatory platelet agonist. Evidence that adrenaline per se does not induce human platelet activation in vitro. Thrombosis Haemostasis. 1993;70:506–513. [PubMed] [Google Scholar]
- Todorov LD, Mihaylova-Todorova S, Westfall TD, Sneddon P, Kennedy C, Bjur RA, Westfall DP. Neuronal release of soluble nucleotidases and their role in neurotransmitter inactivation. Nature. 1997;387:76–79. doi: 10.1038/387076a0. [DOI] [PubMed] [Google Scholar]
- Vassort G. Adenosine 5′-triphosphate: a P2-purinergic agonist in the myocardium. Physiol Rev. 2001;81:767–806. doi: 10.1152/physrev.2001.81.2.767. [DOI] [PubMed] [Google Scholar]
- Wang JS, Cheng LJ. Effect of strenuous, acute exercise on alpha2-adrenergic agonist-potentiated platelet activation. Arteriosclerosis, Thrombosis Vascular Biol. 1999;19:1559–1565. doi: 10.1161/01.atv.19.6.1559. [DOI] [PubMed] [Google Scholar]
- Wang L, Olivecrona G, Gotberg M, Olsson ML, Winzell MS, Erlinge D. ADP acting on P2Y13 receptors is a negative feedback pathway for ATP release from human red blood cells. Circ Res. 2005;96:189–196. doi: 10.1161/01.RES.0000153670.07559.E4. [DOI] [PubMed] [Google Scholar]
- Yegutkin G, Bodin P, Burnstock G. Effect of shear stress on the release of soluble ecto-enzymes ATPase and 5′-nucleotidase along with endogenous ATP from vascular endothelial cells. Br J Pharmacol. 2000;129:921–926. doi: 10.1038/sj.bjp.0703136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yegutkin GG, Henttinen T, Jalkanen S. Extracellular ATP formation on vascular endothelial cells is mediated by ecto-nucleotide kinase activities via phosphotransfer reactions. FASEB J. 2001;15:251–260. doi: 10.1096/fj.00-0268com. [DOI] [PubMed] [Google Scholar]
- Yegutkin GG, Samburski SS, Jalkanen S. Soluble purine-converting enzymes circulate in human blood and regulate extracellular ATP level via counteracting pyrophosphatase and phosphotransfer reactions. FASEB J. 2003;17:1328–1330. doi: 10.1096/fj.02-1136fje. [DOI] [PubMed] [Google Scholar]
- Yegutkin GG, Samburski SS, Jalkanen S, Novak I. ATP-consuming and ATP-generating enzymes secreted by pancreas. J Biol Chem. 2006;281:29441–29447. doi: 10.1074/jbc.M602480200. [DOI] [PubMed] [Google Scholar]