Abstract
The transcription factor nuclear factor of activated T cells (NFAT)c1 has been shown to be involved in turning on slow skeletal muscle fibre gene expression. Previous studies from our laboratory have characterized the stimulation pattern-dependent nuclear import and resting shuttling of NFATc1–green fluorescent protein (GFP) in flexor digitorum brevis (FDB) muscle fibres from adult mouse. In this study, we use viral expression of the transcription factor NFATc1–GFP fusion protein to investigate the mechanisms underlying the nuclear export of the NFATc1–GFP that accumulated in the nuclei of cultured dissociated adult mouse FDB muscle fibres during slow-twitch fibre type electrical stimulation. In these studies, we found that inhibition of either glycogen synthase kinase 3β (GSK3β) or casein kinase 1 or 2 (CK1/2) markedly slowed the decay of nuclear NFATc1–GFP after cessation of muscle fibre electrical stimulation, whereas inhibition of casein kinase 1δ, p38 mitogen-activated protein kinase, c-Jun N-terminal kinase and protein kinase A had little effect. Simultaneous inhibition of GSK3β and CK1/2 completely blocked the nuclear export of NFATc1–GFP after muscle activity. We also developed a simplified model of NFATc1 phosphorylation/dephosphorylation and nuclear fluxes, and used this model to simulate the observed time courses of nuclear NFATc1–GFP with and without NFATc1 kinase inhibition. Our results suggest that GSK3β and CK1/2 are the major protein kinases that contribute to the removal of NFATc1 that accumulates in muscle fibre nuclei during muscle activity, and that GSK3β and CK1/2 are responsible for phosphorylating NFATc1 in muscle nuclei in a complementary or synergistic fashion.
The nuclear factor of activated T cells (NFAT) was first identified in T cells as a transcription factor (Shaw et al. 1988). The transcriptional activity of NFAT is regulated by its intracellular localization. NFAT harbours nuclear localization signals (NLSs) and nuclear export sequence (NES, Beals et al. 1997a; Klemm et al. 1997). In resting lymphocytes, NFAT is heavily phosphorylated in its regulatory domain, especially in the serine-rich region (SRR) and the serine–proline (SP) repeat regions (Beals et al. 1997a). Phosphorylation of serine residues on NFAT results in intramolecular masking of the NLSs, with consequent cytoplasmic sequestration of the transcription factor. It has previously been shown that NFAT functions as a working memory of calcium signals in decoding calcium oscillations (Tomida et al. 2003). When intracellular calcium concentration is increased, which activates the calcium- and calmodulin-dependent phosphatase calcineurin (CaN; Tavi et al. 2004), NFAT can be dephosphorylated by CaN, resulting in exposure of NLSs and nuclear import of the transcription factor, where intranuclear NFAT activates gene expression. When calcium signalling is terminated, NFAT can be rephosphorylated by protein kinases and relocated to cytoplasm via the nuclear export receptor chromosome region maintenance 1 (CRM1; Kehlenbach et al. 1998; Zhu & McKeon, 1999). Several protein kinases such as glycogen synthase kinase 3β (GSK3β; Beals et al. 1997b), casein kinase 1 (CK1; Zhu et al. 1998; Okamura et al. 2004), casein kinase 2 (CK2; Porter et al. 2000; Komarova et al. 2005), p38 mitogen-activated protein kinase (MAPK) (p38; Braz et al. 2003), c-Jun N-terminal kinase (JNK; Liang et al. 2003) and GSK3β priming kinase protein kinase A (PKA; Beals et al. 1997b; Sheridan et al. 2002) can rephosphorylate NFAT, causing the transcription factor to exit the nucleus and relocate to the cytoplasm in various cell types.
Four NFAT transcription factors have been identified which are regulated by CaN-mediated dephosphorylation: NFATc1, NFATc2, NFATc3 and NFATc4. NFATc1 is the primary isoform expressed at the mRNA level in human skeletal muscle (Hoey et al. 1995), and it appears to play a role in fast-twitch to slow-twitch skeletal muscle fibre transformation (Chin et al. 1998). In cultured resting single flexor digitorum brevis (FDB) muscle fibres from adult mouse, exogenously expressed NFATc1–green fluorescent protein (GFP) fusion protein delivered by adenovirus infection is predominantly located in the cytoplasm at the sarcomeric Z-line (Liu et al. 2001). Electrical stimulation with patterns typical of slow-twitch muscle (Hennig & Lomo, 1985) caused a CaN-dependent (cyclosporin A-sensitive) nuclear translocation of NFATc1–GFP in FDB muscle fibres. However, little is known about the protein kinases that regulate the nuclear export of NFATc1 after CaN-dependent entry in skeletal muscle fibres.
In contrast to previous studies that concentrated on characterizing and modelling activity-dependent activation of CaN (Tavi et al. 2004), here we focus our attention on the kinases necessary for NFATc1 nuclear export following muscle activity. We have used adenovirus infection to deliver NFATc1–GFP fusion protein in cultured adult skeletal muscle fibres. We have demonstrated that treatment of muscle fibres in culture with inhibitors of GSK3β and CK1 or 2 (CK1/2) strongly slows the nuclear export of NFATc1 after prior electrical stimulation, whereas inhibition of casein kinase 1δ (CK1δ), p38 or JNK activity has negligible effect on this process. We also show that inhibition of GSK3β priming kinase PKA has no significant effect on the nuclear export of NFATc1–GFP after electrical stimulation-induced nuclear accumulation. Taken together, these results provide new insights into the mechanisms involved in the regulation of NFATc1 localization in skeletal muscle fibres.
Methods
Construction of recombinant adenoviruses
The expression plasmid of NFATc1 cDNA (NFAT2 isoform α, 716 residues; Rao et al. 1997) was a gift from Dr Gerald R. Crabtree (Howard Hughes Medical Institute, Stanford, CA, USA). The construction of recombinant adenovirus of NFATc1–GFP was described in detail previously (Liu et al. 2001). The viral construct was confirmed by sequencing the viral DNA.
Chemicals and reagents
Alsterpaullone, anisomycin, cyclosporin A (CsA), KT5720, SB202190 and SP600125 were purchased from Sigma-Aldrich (St Louis, MO, USA). CKI-7 was obtained from Seikagaku America (Falmouth, MA, USA) and D4476 was purchased from Calbiochem (San Diego, CA, USA).
FDB fibre preparation and infection with recombinant adenoviruses
Experiments were performed on single skeletal muscle fibres enzymatically isolated from the FDB muscles of 4- to 5-week-old CD-1 mice. Animals were killed by CO2 exposure followed by cervical dislocation before removal of the muscles according to protocols approved by the University of Maryland Institutional Animal Care and Use Committee. Procedures for enzymatic isolation of single muscle fibres have been previously described (Liu et al. 1997). Isolated fibres were cultured on laminin-coated glass coverslips, each glued over a 10 mm diameter hole through the centre of a 35 mm plastic Petri dish. Fibres were cultured in minimum essential medium (MEM) containing 10% fetal bovine serum and 50 μg ml−1 gentamicin sulphate in 5% CO2 (37°C). Infection of muscle fibre cultures by recombinant adenoviruses was carried out about 20 h after the fibres were plated. Before infection, the FDB cultures were rinsed twice with MEM without serum. The recombinant adenoviruses were added to the culture dishes with MEM without serum. The cultures were kept in the 5% CO2 incubator for 1 h and the medium was then changed to MEM with serum and gentamicin for continuous culture (Liu et al. 2001).
Microscopy, image acquisition and fibre treatment
The microscopy and image acquisition were as described in our previous report (Shen et al. 2006). Briefly, approximately 48 h after NFATc1–GFP viral infection, culture medium was changed to Ringer solution containing (mm): NaCl 135, KCl 4, MgCl2 1, Hepes 10, glucose 10 and CaCl2 1.8; pH 7.4. The culture chamber was mounted on an Olympus IX70 inverted microscope equipped with an Olympus FLUOVIEW 500 laser scanning confocal imaging system, utilizing an excitation wavelength of 488 nm. Fibres were viewed with an Olympus 60×/1.2 NA water immersion objective and scanned at 3.0 × zoom using constant laser power and gain. Each confocal fluorescence image represents an individual optical section. For electrical stimulation, two platinum electrodes connected to a stimulator were placed into the fibre culture chamber to give field stimulation, and fibres were stimulated with a slow fibre type pattern of activity (a 5 s train of 10 Hz stimuli once every 50 s, Hennig & Lomo, 1985; Liu et al. 2001) for 1 h. The duration of the individual stimulating pulses was 1 ms. The stimulation voltage was adjusted to give microscopically observed fibre contraction in all cases. Most fibres remained attached to the laminin-coated coverslip throughout the period of fibre stimulation, and only such fibres were used to obtain the data reported here. Fibres were maintained and imaged at room temperature (24°C) every 10 min. After electrical stimulation for 1 h, fibres were treated with 1 μm CsA (to halt CaN-dependent dephosphorylation of NFATc1) together with either added solvent alone or different protein kinase inhibitors in the same amount of solvent. The nuclear export of NFATc1–GFP was recorded for the next 90 min following the application of chemical reagents (Table 1).
Table 1.
The experimental conditions
| Rest (10 min) | Stimulation (60 min) | Recovery (90 min) | |
|---|---|---|---|
| Conditions | Control | Control | + CaN inhibitor |
| ± kinase inhibitor | |||
| Ca2+ | Resting | Repeated transients | Resting |
| CaN | Not active | Active | Inhibited |
| NFATc1 location | In sarcomere (not in nucleus) | Nuclear accumulation | Nuclear exit |
CaN, calcineurin.
Analysis of translocation of NFATc1 protein in muscle fibres
The average pixel fluorescence within user-specified areas of interest (AOI) of both cytoplasm and nucleus in each image was quantified by software custom-written in the IDL programming language (Research Systems, Boulder, CO, USA). To avoid the variations caused by differences in expression level, we normalized the fluorescence of pixels for the nucleus to the average cytoplasmic pixel fluorescence as previously described (Shen et al. 2006). Briefly, the mean pixel fluorescence values of the nucleus for the AOI at each time point were divided by the mean pixel fluorescence values in the cytoplasmic AOI at the corresponding time point in the same fibre. After normalization with respect to the average cytoplasmic pixel fluorescence, the nuclear NFATc1–GFP was further normalized as the percentage of the value at 60 min stimulation. Results are expressed as the mean ± s.e.m.
Immunocytochemistry of p38 activity
Cultured FDB muscle fibres were incubated with or without 20 μm p38 inhibitor SB202190 for 60 min followed by incubation with 10 μg ml−1 anisomycin for 30 min. Fibres were then fixed with 4% paraformaldehyde at room temperature for 10 min, and permeabilized with 0.2% Triton X-100 in PBS for 20 min. Non-specific binding sites were blocked by incubation with 5% normal goat serum. Subsequently, the fibres were incubated for 48 h at 4°C with polyclonal antibody against phospho-cAMP response element-binding protein (CREB) at Ser-133 (1: 100, Cell Signalling Technology, Danvers, MA, USA) followed by overnight incubation with Alexa 488 goat anti-rabbit secondary antibody (Molecular Probes, Carlsbad, CA, USA). The fluorescence of the stained fibres was visualized and quantified using the confocal laser scanning imaging system described above.
Simulation model
The NFATc1 phosphorylation and dephosphorylation processes, together with NFATc1 influx to and efflux from the nucleus, were described by a set of three differential equations, and by three exponential functions defining the time courses of the phosphorylation and dephosphorylation coefficients in the nucleus and cytosol (see Results). The equations were solved numerically using the Euler method (Scarborough, 1966). Briefly, small increments of the four parameters describing the four possible NFATc1 conditions (phosphorylated or dephosphorylated, and in the cytosol or the nucleus) were calculated by multiplying the numerical values of the time differentials (calculated based on the analytical formulas provided by the three differential equations) by the time unit (1 min in our study). These increments were then summed to estimate the time courses of the four dynamic parameters. The calculations were performed by a custom-written VBA program that runs under Microsoft Excel. The resulting time courses of the four dynamic parameters and the three time-dependent coefficients were then plotted either in Excel or in SigmaPlot 8.0 using custom-written macros. The model parameters were manually adjusted until the simulated curves and the experimental data were similar.
Results
Inhibition of GSK3β slows the nuclear export of NFATc1–GFP after electrical stimulation
Nuclear translocation of NFATc1–GFP has been detected within 10 min after the start of electrical stimulation (Liu et al. 2001; Shen et al. 2006). As shown in Fig. 1A and B, stimulation of muscle fibres with a slow fibre type activity induced a continuous increase of nuclear NFATc1–GFP fluorescence during the 60 min period of fibre electrical stimulation. After cessation of the stimulation, the nuclear fluorescence of NFATc1–GFP decreased much faster in the control fibre (Fig. 1A) than in the fibre treated with 20 μm of alsterpaullone, which inhibits GSK3β activity (Fig. 1B). Figure 2A shows the average changes of nuclear NFATc1–GFP fluorescence and Fig. 2B and C shows the percentage changes of the nuclear NFATc1–GFP before, during and after cessation of stimulation (after normalization to the 60 min stimulation value) for recovery in the presence of 1 μm CsA to inhibit CaN activity together with either solvent or GSK3β inhibitors alsterpaullone and LiCl. It is noteworthy that in the presence of alsterpaullone (Fig. 2A and B) or LiCl (Fig. 2C), the decline of nuclear NFATc1–GFP fluorescence after cessation of electrical stimulation required considerably more time than in the control experiment. Inhibition of GSK3β by alsterpaullone or LiCl resulted in a significant increase in the percentage of the NFATc1–GFP which had accumulated in the nucleus at the end of stimulation that still remained in the nucleus 90 min after cessation of stimulation (Fig. 2D; 69 ± 6% of the level at end of stimulation for alsterpaullone compared to its control of 26 ± 2%, and 62 ± 6% for LiCl compared to its control of 18 ± 2%), indicating that GSK3β is one of the protein kinases that contributes to the removal of accumulated nuclear NFATc1–GFP, presumably by NFATc1–GFP rephosphorylation after CaN-dependent dephosphorylation and nuclear entry during electrical stimulation (Liu et al. 2001; Shen et al. 2006).
Figure 1. Images of two fibres during stimulation and followed by recovery with or without GSK3β inhibitor.
A and B, fibres expressing NFATc1–GFP are shown in Ringer solution at room temperature 10 min prior to (−10), 60 min during (stimulation, 0–60) and 90 min after (recovery, 10–90) electrical stimulation for recovery in the presence of either the solvent DMSO (A) or 20 μm GSK3β inhibitor alsterpaullone (B). Scale bar, 20 μm.
Figure 2. Effect of GSK3β inhibition on NFATc1–GFP nuclear export.
A, time courses of NFATc1–GFP nuclear translocation resulting from electrical stimulation and nuclear export in the presence of either the solvent DMSO (eight nuclei from eight fibres) or the GSK3β inhibitor alsterpaullone (17 nuclei from 15 fibres). B and C, percentage changes of the nuclear NFATc1–GFP before, during and after cessation of stimulation (normalized to the 60 min stimulation value) in the presence of either DMSO or the GSK3β inhibitor alsterpaullone (B) or either 20 mm NaCl (six nuclei from six fibres) or 20 mm GSK3β inhibitor LiCl (eight nuclei from seven fibres; C). D, percentage of the NFATc1–GFP nuclear fluorescence still remaining in the nucleus 90 min after cessation of stimulation (normalized to the 60 min stimulation value) in the absence (control) or presence of the GSK3β inhibitors.
Inhibition of p38 or JNK has negligible effect on the nuclear export of NFATc1–GFP after electrical stimulation
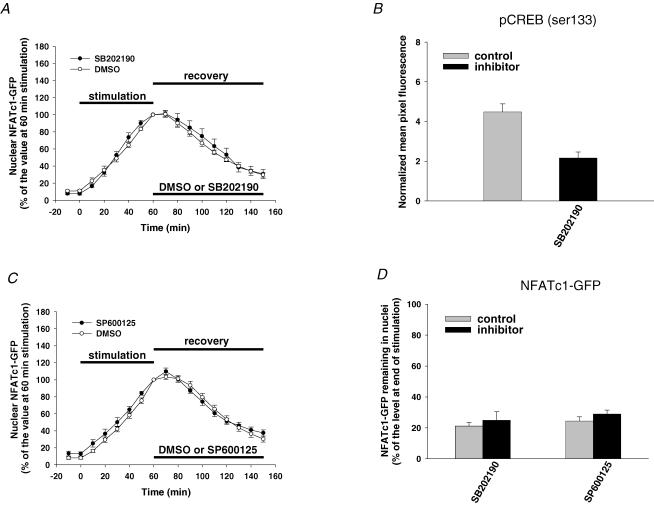
We next examined the roles of the MAPK family members p38 and JNK in the nuclear export of NFATc1–GFP in muscle fibres. These two protein kinases, when overexpressed, were found to negatively regulate NFATc1 nuclear localization in cultured cardiomyocytes (Braz et al. 2003; Liang et al. 2003). Muscle fibres were first electrically stimulated for 60 min to induce the nuclear translocation of NFATc1–GFP and then treated with 1 μm CsA to inhibit CaN and to stop the further nuclear translocation of NFATc1–GFP after cessation of stimulation. Fibres were also treated with either specific protein kinase inhibitor or the solvent (control) after cessation of stimulation, and examined for the next 90 min. Figure 3A and C shows the time courses of normalized nuclear NFATc1–GFP fluorescence changes before, during and after stimulation in the presence of either solvent or protein kinase inhibitors after cessation of stimulation. Clearly, exposure to either 20 μm p38 inhibitor SB202190 (Fig. 3A) or 30 μm JNK inhibitor SP600125 (Fig. 3C) had no effect on the decay of nuclear NFATc1–GFP after electrical stimulation. Upon treatment with the p38 inhibitor SB202190, the NFATc1–GFP that still remained in the nucleus 90 min after cessation of stimulation was 25 ± 5% of the level at end of stimulation compared to 21 ± 2% for its control (Fig. 3D). Likewise, in the presence of the JNK inhibitor SP600125, the nuclear level of NFATc1–GFP 90 min after cessation of stimulation was 29 ± 3% of the level at end of stimulation, compared to 24 ± 3% for its control (Fig. 3D). We tested for the intranuclear effectiveness of the p38 inhibitor used in Fig. 3A by examining its effect on the level of nuclear phospho-CREB. Muscle fibres were incubated with or without 20 μm p38 inhibitor SB202190 for 60 min followed by incubation with 10 μg ml−1 anisomycin for 30 min. Anisomycin has been shown to increase CREB phosphorylation by activating p38 in skeletal muscle (Geiger et al. 2005). We found that pretreatment with 20 μm SB202190 for 60 min clearly decreased the nuclear phospho-CREB (Fig. 3B), indicating that this inhibitor was effective at blocking p38 in the nuclei of adult mouse skeletal muscle fibres, as shown previously for rat fibres (Geiger et al. 2005). A similar concentration (20 μm) of the JNK inhibitor SP600125 as used here (30 μm) has been previously shown to significantly inhibit interleukin-1β-mediated activation of c-Jun by inhibition of JNK activity in rat smooth muscle nuclei (Zhai et al. 2004). Together, these data suggest that p38 and JNK have little effect on removal of nuclear NFATc1–GFP after electrical stimulation in skeletal muscle fibres.
Figure 3. Effect of p38 and JNK inhibition on NFATc1–GFP nuclear export.
A, time courses of NFATc1–GFP nuclear translocation resulting from electrical stimulation and nuclear export with either the solvent DMSO (five nuclei from five fibres) or the p38 inhibitor SB202190 (six nuclei from five fibres). B, effect of the p38 inhibitor SB202190 on nuclear phospho-CREB in muscle fibres (eight nuclei from eight fibres for each group). C, time courses of NFATc1–GFP nuclear translocation resulting from electrical stimulation and nuclear export with either the solvent DMSO (six nuclei from six fibres) or the JNK inhibitor SP600125 (six nuclei from six fibres). D, percentage of the NFATc1–GFP nuclear fluorescence still remaining in the nucleus 90 min after cessation of stimulation (normalized to the 60 min stimulation value) in the absence (control) or presence of inhibitors.
Inhibition of casein kinases slows the nuclear export of NFATc1–GFP after electrical stimulation
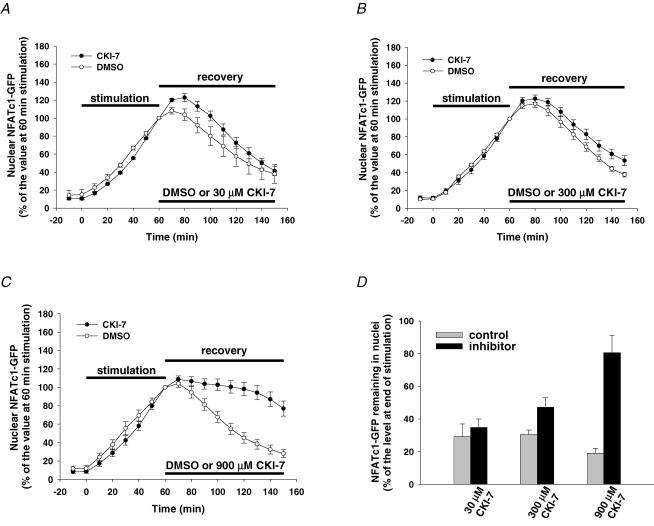
It has been demonstrated previously that casein kinases, including CK1 and CK2, can bind the N-terminus of NFAT and phosphorylate functionally important amino acid residues, thereby regulating NFAT nuclear export in various cell types (Zhu et al. 1998; Porter et al. 2000). Recently, Okamura et al. (2004) reported that incubation of T cells with CKI-7, a selective inhibitor for both CK1 and CK2 (Chijiwa et al. 1989), could significantly delay the nuclear export of NFATc2 after ionomycin treatment. Here, we tested the effect of CKI-7 on the nuclear export of NFATc1–GFP in FDB muscle fibres after electrical stimulation. Muscle fibres were first electrically stimulated for 60 min to induce the nuclear translocation of NFATc1–GFP and then treated with 1 μm CsA (to inhibit CaN) together with either CKI-7 or the solvent DMSO after cessation of stimulation. Figure 4A shows that in the presence of 30 μm CKI-7, the nuclear export pattern of NFATc1–GFP was similar to that of the control. The NFATc1–GFP that still remained in the nucleus 90 min after cessation of stimulation in the presence of 30 μm CKI-7 was 35 ± 5% of the level at the end of stimulation, compared to its control of 29 ± 8% (Fig. 4D). However, increasing the concentration of CKI-7 to 300 μm (Fig. 4B) and further to 900 μm (Fig. 4C) increased the percentage of the nuclear NFATc1–GFP that still remained in the nucleus 90 min after cessation of stimulation to 47 ± 6% in the presence of 300 μm CKI-7 (compared to its control of 30 ± 3%) and to 81 ± 11% in 900 μm CKI-7 (compared to its control of 19 ± 3%, Fig. 4D). CKI-7 inhibits partially purified CK1 and CK2 from rabbit skeletal muscle with IC50 values of 9.5 and 90 μm, respectively (Chijiwa et al. 1989); significant inhibition of nuclear export of NFATc1–GFP by CKI-7 required much high concentrations (300 and 900 μm), possibly because of the need for a higher concentration to block the enzyme in muscle fibres than needed for the isolated enzyme or, alternatively, possibly suggesting that CK2 is likely to be the main isoform of casein kinases involved in regulating NFATc1–GFP nuclear export after electrical stimulation in muscle fibres. However, the high doses of CKI-7 used here might also have non-specifically inhibited other kinases, such as ribosomal S6 kinase (S6K) or mitogen-and stress-activated protein kinase-1 (MSK1) (Rena et al. 2004).
Figure 4. Effect of CK inhibition on NFATc1–GFP nuclear export.
A–C, time courses of NFATc1–GFP nuclear translocation resulting from electrical stimulation and nuclear export in the presence of the solvent DMSO or different doses of CKI-7 (A, 30 μm, seven nuclei from seven fibres; B, 300 μm, six nuclei from six fibres; C, 900 μm, 10 nuclei from 10 fibres). D, percentage of the NFATc1–GFP nuclear fluorescence still remaining in the nucleus 90 min after cessation of stimulation (normalized to the 60 min stimulation value) in the absence (control) or presence of CK inhibitor.
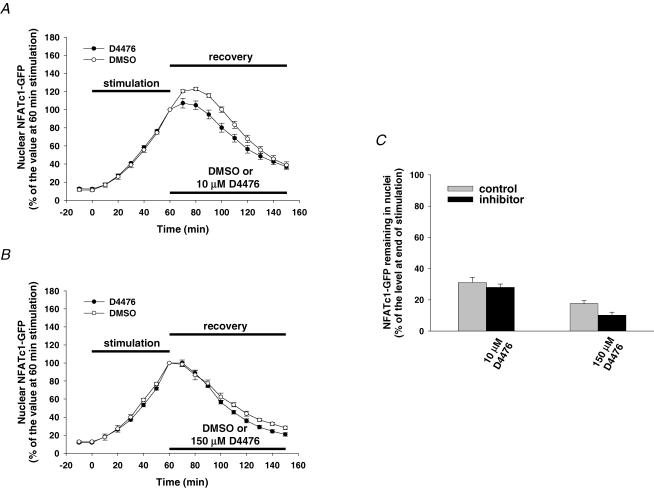
CK1 is a multigene family responsible for the expression of isoforms CK1α, CK1δ and CK1ɛ (Gross & Anderson, 1998). Using a recently developed CK1 inhibitor, D4476, which is found to be more highly potent against CK1δ than CKI-7 (Rena et al. 2004), we further investigated whether CK1δ regulates the nuclear export of NFATc1–GFP in muscle fibres after electrical stimulation. Muscle fibres were first electrically stimulated for 60 min to induce the nuclear translocation of NFATc1–GFP and then treated with 1 μm CsA (to inhibit CaN) together with either D4476 or the solvent DMSO after cessation of stimulation. D4476 at 10 μm, a concentration that inhibits the activity of purified CK1δ by more than 90% (Rena et al. 2004), had negligible effect on the dissipation of nuclear NFATc1–GFP following cessation of muscle fibre stimulation (Fig. 5A). Because D4476 is an ATP-competitive inhibitor of CK1δ, and because the ATP concentration should be higher in muscle fibres than used in in vitro (0.1 mm, Rena et al. 2004), we increased the concentration of D4476 (to 150 μm, the maximum concentration that could be used in our study because of the limit of solubility), as we did for CKI-7 experiments. We found that even at the higher concentration (150 μm), D4476 still had no effect on the nuclear export of NFATc1–GFP after prior muscle activity (Fig. 5B). The accumulated nuclear NFATc1–GFP remaining 90 min after cessation of stimulation was 29 ± 2% of the level at end of stimulation in the presence of 10 μm D4476 (compared to its control of 35 ± 3%, Fig. 5C), and 10 ± 2% in the presence of 150 μm D4476 (compared to its control of 18 ± 2%, Fig. 5C). Our negative results with D4476 can only rule out isoforms of CK1 that are sensitive to D4476 (i.e. CK1δ). Other CK1 isoforms (such as CK1α or ɛ) may still possibly be involved in NFATc1 nuclear phosphorylation after activity. Taken together, our results suggest that the nuclear export of NFATc1–GFP after electrical stimulation is probably regulated by CK2 and/or CK1 isoforms insensitive to D4476 such as CK1α or ɛ, but does not appear to be regulated by CK1δ.
Figure 5. Effect of CK1δ inhibition on NFATc1–GFP nuclear export.
A and B, time courses of NFATc1–GFP nuclear translocation resulting from electrical stimulation and nuclear export in presence of the solvent DMSO or different doses of the CK1δ-selective inhibitor D4476 (A, 10 μm, six nuclei from six fibres; B, 150 μm, eight nuclei from six fibres). C, percentage of the NFATc1–GFP nuclear fluorescence still remaining in the nucleus 90 min after cessation of stimulation (normalized to the 60 min stimulation value) in the absence (control) or presence of CK1δ inhibitor.
Simultaneous inhibition of GSK3β and CK1/2 completely blocks the nuclear export of NFATc1–GFP after electrical stimulation
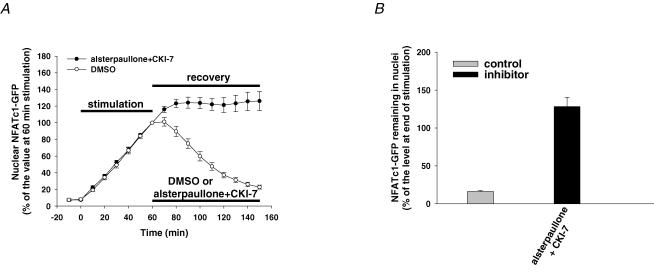
As we were able to show that GSK3β and CK1/2 are involved in the regulation of the nuclear export of NFATc1–GFP after electrical stimulation, we next wished to examine whether GSK3β and CK1/2 have synergistic effects on the nuclear removal of NFATc1–GFP. After 60 min electrical stimulation to induce the nuclear accumulation of NFATc1–GFP, fibres were incubated with 1 μm CsA together with either the solvent DMSO or GSK3β and CK1/2 inhibitors (20 μm alsterpaullone plus 900 μm CKI-7, Fig. 6A) for the next 90 min recovery period. Our results clearly showed that after inhibition of GSK3β and CK1/2, the nuclear export of NFATc1–GFP as a result of prior electrical stimulation was completely blocked (Fig. 6A). The percentage of accumulated nuclear NFATc1–GFP that still remained in the nucleus 90 min after cessation of stimulation was 128 ± 12% of the level at end of stimulation in the presence of alsterpaullone plus CKI-7 compared to 16 ± 1% for its control group (Fig. 6B). These data indicate that GSK3β and CK1/2 are the major protein kinases that phosphorylate nuclear NFATc1–GFP after electrical stimulation-induced nuclear accumulation and synergistically promote NFATc1–GFP nuclear export after cessation of muscle activity. Note that in the presence of the combined inhibitors, NFATc1–GFP removal from the nucleus is completely blocked. In this case, the additional NFATc1–GFP that enters the nucleus before CaN is completely deactivated is also retained, resulting in more than 100% of the immediate post-stimulation nuclear NFATc1–GFP being in the nucleus 90 min after cessation of fibre stimulation (Fig. 6B).
Figure 6. Effect of simultaneous GSK3β and CK1/2 inhibition on NFATc1–GFP nuclear export.
A, time courses of NFATc1–GFP nuclear translocation resulting from electrical stimulation and nuclear export in presence of the solvent DMSO (control, eight nuclei from seven fibres) or 20 μm GSK3β inhibitor alsterpaullone plus 900 μm CK1/2 inhibitor CKI-7 (10 nuclei from 10 fibres). B, percentage of the NFATc1–GFP nuclear fluorescence still remaining in the nucleus 90 min after cessation of stimulation (normalized to the 60 min stimulation value) in the absence (control) or presence of inhibitors.
Inhibition of putative GSK3β priming kinase PKA had no effect on the nuclear export of NFATc1–GFP after electrical stimulation
It has been reported previously that PKA exhibited a priming kinase activity for GSK3β-mediated phosphorylation of NFATc1 (Beals et al. 1997b; Sheridan et al. 2002). Thus, we investigated the possible role of PKA on the nuclear export of NFATc1 accumulated after electrical stimulation of skeletal muscle fibres. After 60 min electrical stimulation to induce the nuclear accumulation of NFATc1–GFP, fibres were incubated with 1 μm CsA together with either 5 μm PKA inhibitor KT5720 or its solvent methanol (Fig. 7A). The nuclear export of NFATc1–GFP was monitored for the next 90 min recovery period. Inhibition of PKA did not alter the decay of nuclear NFATc1–GFP during export after cessation of electrical stimulation (Fig. 7A). The percentage of the nuclear NFATc1–GFP at the end of stimulation that still remained in the nucleus 90 min after cessation of stimulation in the presence of the PKA inhibitor KT5720 was not significantly different from that of its control (29 ± 5% with KT5720 compared to 19 ± 2% with methanol control, Fig. 7C). Furthermore, simultaneous inhibition of PKA with 5 μm KT5720, and of GSK3β with 20 μm alsterpaullone, after muscle activity (Fig. 7B) showed a similar effect to that resulting from the inhibition of GSK3β alone (Fig. 2B). The percentage of the nuclear NFATc1–GFP at the end of stimulation that still remained in the nucleus 90 min after cessation of stimulation was 69 ± 6% with GSK3β inhibitor alone (Fig. 2D) compared to 78 ± 6% with both GSK3β and PKA inhibitors (Fig. 7C). These results suggest that PKA is not involved in the regulation of NFATc1–GFP nuclear export after electrical stimulation of muscle fibres.
Figure 7. Effect of PKA inhibition on NFATc1–GFP nuclear export.
A and B, time courses of NFATc1–GFP nuclear translocation resulting from electrical stimulation and nuclear export in presence of 5 μm PKA inhibitor KT5720 with (B, 13 nuclei from 12 fibres) or without (A, six nuclei from four fibres) 20 μm GSK3β inhibitor alsterpaullone. C, percentage of the NFATc1–GFP nuclear fluorescence still remaining in the nucleus 90 min after cessation of stimulation (normalized to the 60 min stimulation value) in the absence (control) or presence of inhibitors.
A simplified mathematical model for simulating the time course of nuclear NFATc1
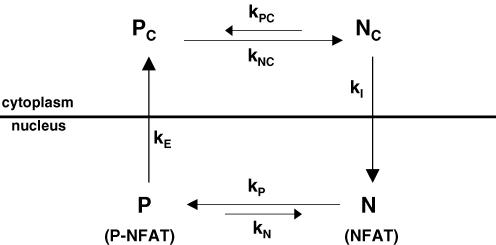
We have developed a simplified mathematical model for NFATc1 nuclear movements in order to more rigorously interpret the effects of various kinase inhibitors on the decay of nuclear NFATc1 after build up of nuclear NFATc1 by prior activity. The model (Fig. 8) includes dephosphorylation of phosphorylated cytosolic NFATc1 (PC) with rate constant kNC, nuclear influx of dephosphorylated NFATc1 (NC) at rate kI, the phosphorylation of intranuclear NFATc1 (N) with rate constant kP and the efflux of phosphorylated nuclear NFATc1 (P) from the nucleus at rate constant kE, as well as the possible phosphorylation of cytosolic dephosphorylated NFATc1 with rate constant kPC and dephosphorylation of phosphorylated NFATc1 within the nucleus with rate constant kN. The model is a useful first approximation, as shown below in the interpretation of the effects of kinase inhibitors on nuclear NFATc1. However, it should be noted that this model considers only a single phosphorylation site on NFATc1, whereas in reality there are 21 phosphorylatable serine residues on the NFATc1 SRR and the SP repeat regions (Beals et al. 1997a; Neal & Clipstone, 2001), each of which may influence the rate of NFATc1 nuclear efflux, possibly exhibiting sequential phosphorylation and interacting in a cooperative manner for NFAT translocation (Salazar & Höfer, 2003). For simplicity, we have also not included the possibility of cooperative interaction between phosphorylation sites in the conversion between two states, one with NLS exposed and another with NES exposed (Salazar & Höfer, 2003). Nevertheless, the simplified model does provide a first approach to quantifying the effects of kinase inhibitors on the nuclear movements of NFATc1.
Figure 8. A simplified chemical reaction scheme for simulating the time course of nuclear NFATc1 in skeletal muscle fibres.
Nc and Pc represent dephosphorylated and phosphorylated cytosolic NFATc1, respectively. N and P represent dephosphorylated and phosphorylated nuclear NFATc1, respectively. The rate constants for phosphorylation of NC(KPC), dephosphorylation of PC(kNC), phosphorylation of N(kP), dephosphorylation of p(kN), nuclear influx of NC(kI) and nuclear efflux of P(kE).
As the cytoplasmic compartment provides an essentially infinite pool of phosphorylated NFATc1–GFP, the model assumes PC to be constant over the entire simulation period. The effect of fibre stimulation on nuclear influx of dephosphorylated NFATc1 was simulated by setting kNC to zero prior to stimulation, and then linearly increasing the value of kNC with time throughout the simulated 60 min stimulation period. After a brief (or no) delay during which the value of kNC was held constant, the reversal of CaN activation during stimulation was simulated by assuming the value of kNC to decrease exponentially to zero (with time constant τD). With these assumptions, NC(t) (and thus the rate of NFATc1 nuclear influx, which is given by (kI)NC(t)), rises approximately exponentially during stimulation, and returns approximately exponentially to zero at the end of stimulation. To simulate the effect of inhibition of NFATc1 kinase(s) within the nucleus after the stimulation period, the value of kP was assumed to decline from its initial value to a final value with time constant τ2starting at the end of the stimulation period. For the system in Fig. 8, the time courses for the rate of change of concentrations of dephosphorylated cytosolic NFATc1 (NC), dephosphorylated nuclear NFATc1 (N) and phosphorylated nuclear NFATc1 (P) are given by:
| (1) |
| (2) |
| (3) |
The equations were solved numerically (see Methods) to give the time courses NC(t), N(t) and P(t) of cytosolic and intranuclear dephosphorylated NFATc1 and intranuclear phosphorylated NFATc1, respectively. Because our experimental nuclear fluorescence time course measurements cannot distinguish phosphorylated from dephosphoryoated NFATc1, we compared the time course of N(t) + P(t) to the time course of total NFATc1 nuclear fluorescence observed experimentally.
Observed and simulated NFATc1 nuclear time courses with and without kinase inhibition
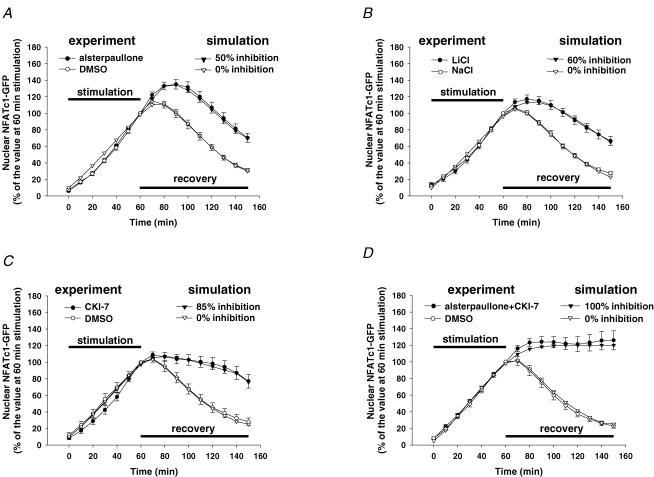
Each graph in Fig. 9 presents the observed and calculated time courses of NFATc1 nuclear fluorescence for 1 h of repetitive electrical stimulation followed by application of CsA either with (closed symbols) or without (open symbols) one or more kinase inhibitor, as indicated. The theoretical time courses of nuclear NFATc1 fluorescence were simulated using the model in Fig. 8 (as discussed in the preceding section), with the model parameter values set as specified in Table 2. The open triangle traces in each graph give the theoretical time course of nuclear NFATc1 fluorescence calculated for the control conditions for a 60 min period of stimulation followed by recovery in the presence of CsA to inhibit further CaN activity, but in the absence of kinase inhibitors, and closely resembles the corresponding time course recorded experimentally under control conditions (open circles) in the corresponding experiments. Note that the experimentally observed control records in Fig. 9A and B exhibit slightly higher rise after cessation of stimulation than the observed control records in Fig. 9C and D. This was simulated in Fig. 9A and B by including a 10 min or 3 min delay before turning off kNC, respectively. No delay was included in Fig. 9C or D, because the corresponding observed control records (Fig. 9C and D) exhibited much less rise after cessation of stimulation than in the observed control records in Fig. 9A and B.
Figure 9. Simulation of the build up of nuclear NFATc1 during 1 h of repetitive electrical stimulation and the effects of kinase inhibitors on the decline of nuclear NFATc1 following stimulation.
The observed (○ and •) effects of the GSK3β inhibitor alsterpaullone (A, 20 μm), LiCl (B, 20 mm), the CK1/2 inhibitor CKI-7 (C, 900 μm) and the combined application of alsterpaullone and CKI-7 (D, same concentrations as in A and C) on the time course of decline of nuclear NFATc1 were simulated (∇ and ▾) by assuming the NFATc1 kinase rate constant kP in the nucleus to be inhibited by 50% (A), 60% (B), 85% (C) and 100% (D) during the period following electrical stimulation. The time constant for onset of inhibition after cessation of fibre stimulation was 3, 12, 5 or 2 min in A–D, respectively. These and the other parameter values are listed in Table 2.
Table 2.
Parameter values for model simulation of NFATc1 nuclear translocation with or without various kinase inhibitors
| *Panel Inhibitor | A alsterpaullone | B LiCl | C CKI-7 | D alsterpaullone + CKI-7 |
|---|---|---|---|---|
| Inhibition (%) | 50 | 60 | 85 | 100 |
| τ2 (min) | 3 | 12 | 5 | 2 |
| Δt (min) | 10 | 3 | 0 | 0 |
| τD (min) | 30 | 30 | 12 | 10 |
| kPC (min−1) | 1.4 | 1.4 | 0.7 | 0.2 |
| kP (min−1) | 0.08 | 0.08 | 0.1 | 0.1 |
| kE (min−1) | 0.15 | 0.15 | 0.05 | 0.05 |
| kNC (min−1) | 1.0 | 1.0 | 0.3 | 0.3 |
| kI (min−1) | 0.1 | 0.1 | 0.1 | 0.2 |
| kN (min−1) | 0.1 | 0.1 | 0.1 | 0.1 |
| kN,shift (min−1) | 0.02 | 0.02 | 0.02 | 0.02 |
Refers to panels A-D in Fig. 9.
The filled triangles in Fig. 9A present the calculated effect of a 50% block of intranuclear kinase activity during the post-stimulation recovery period as predicted by the model with all other parameters having the same values as in the control simulation (Fig. 9A, open triangles). Relative to the control time course, the calculated time course shown by the filled triangles in Fig. 9A almost perfectly reproduces the effect of the GSK3β inhibitor alsterpaullone on the NFATc1 nuclear fluorescence time course (Fig. 9A, filled circles). In both the observed and simulated records, the fluorescence reached a higher peak value in the presence of GSK3β inhibitor, and then declined with time. Figure 9B (filled triangles) simulates the effect of a 60% block of kinase activity during the recovery period after stimulation, and reproduces the observed effect of the GSK3β inhibitor LiCl (Fig. 9B, filled circles). It is interesting to note when comparing the simulations of the effects of the GSK3β inhibitors alsterpaullone and LiCl that the LiCl simulation required a relatively long (12 min) time constant for development of inhibition (Fig. 9B), consistent with time for this salt to enter the fibres, whereas a time constant of only 3 min was used for the simulation of the effect of the hydrophobic inhibitor alsterpaullone (Fig. 9A).
Figure 9C (filled triangles) shows the simulation of the effect of a 85% block of kinase activity developing with a time constant of 5 min during the recovery period after stimulation, and reproduces the observed effect of the CK1/2 inhibitor CKI-7 (Fig. 9C, filled circles). Finally, in the combined presence of the GSK3β and CK1/2 inhibitors alsterpaullone and CKI-7 during the recovery period after stimulation, the nuclear fluorescence observed experimentally increased and then reached an essentially constant level (Fig. 9D, filled circles). This behaviour is best reproduced in the theoretical calculations by the model using 100% inhibition of kinase activity (i.e. kP = 0) during the recovery period (Fig. 9D, filled triangles). Thus, the mathematical model can reproduce the general features of the time courses of nuclear NFATc1 export under control conditions, and during partial as well as during full inhibition of nuclear NFATc1 kinase activity during the recovery period. Furthermore, the simulations provide a measure of the extent of NFATc1 kinase inhibition under each simulated condition. Of note, the model simulations indicate that the sum of the percentage of block by each of the two inhibitors applied individually is greater than 100%, indicating that one or both inhibitors may exhibit partial non-specific inhibition of the other kinase (see Discussion for further consideration of modelling results and alternative interpretations).
Discussion
The mechanism underlying the regulation of nucleo-cytoplasmic shuttling of NFATc1 in skeletal muscle fibres remains incompletely understood. In particular, it is not known which protein kinases regulate the nuclear export of NFATc1 in skeletal muscle after CaN-dependent entry as a result of muscle activity. In this study, using various inhibitors specific to different protein kinases, we demonstrate that GSK3β and CK1/2 are the primary protein kinases that rephosphorylate NFATc1 in muscle nuclei and promote the exit of this transcription factor from the nucleus after muscle activity due to a slow-twitch fibre type electrical stimulation.
Inhibition of GSK3β
GSK3β is a serine/threonine kinase which can directly phosphorylate NFATc1 on the SP repeat regions, resulting in NFATc1 nuclear export (Beals et al. 1997b). Using the GSK3β inhibitors alsterpaullone and LiCl, we have shown here that the nuclear export of NFATc1–GFP that accumulated during prior slow-twitch fibre type electrical stimulation was markedly delayed. The percentage of the nuclear NFATc1–GFP accumulated at the end of stimulation that still remained in the nucleus 90 min after cessation of stimulation is significantly higher after inhibition of GSK3β than the percentage after control treatment with solvent alone. In agreement with our findings that GSK3β can regulate NFATc1 nuclear export in skeletal muscle, a previous study in hippocampal neurons showed that overexpression of GSK3β increased the nuclear export of NFATc4 about 3-fold, whereas expression of a dominant negative form of GSK3β slowed the rate of nuclear export of NFATc4 (Graef et al. 1999). In cardiac myocytes, overexpression of GSK3βA9, a mutant kinase which is resistant to inhibition by Ser 9 kinases, accelerated NFAT export from the nucleus (Haq et al. 2000). Thus, GSK3β can modulate the nuclear–cytoplasmic partitioning of NFAT family members in various cell types, and thereby regulate NFAT activity-dependent signalling pathways. Supporting this notion is the recent report that inhibition of GSK3β activity was sufficient to induce the gene expression of slow myosin heavy chain 2 in non-innervated avian medial adductor muscle, indicating the role of GSK3β in the repression of the slow muscle fibre phenotype (Jiang et al. 2005). GSK3β is constitutively active in unstimulated cells and becomes inactivated following stimulation of a variety of types of cells by agents such as insulin, growth factors or phorbol esters (Woodgett, 1994; Welsh et al. 1996). GSK3β is localized both in cytoplasm and the nucleus in T cells (Neilson et al. 2001) and in cardiac myocytes (Morisco et al. 2001), and it is also found in the nucleus in neurons (Graef et al. 1999). In cardiac myocytes, stimulation with isoprenaline (isoproterenol) inactivated GSK3β and caused GSK3β nuclear exit (Morisco et al. 2001). The subcellular distribution of GSK3β in skeletal muscle fibres and whether the distribution can be modulated by slow-twitch fibre type stimulation or other interventions provide interesting questions for future studies.
Inhibition of casein kinases
CK1 and CK2 are another group of ubiquitous serine/threonine protein kinases, and both exhibit constitutive kinase activity. Previous studies have shown that CK1 can directly bind and phosphorylate NFATc2 and NFATc3, resulting in the inhibition of nuclear translocation of both NFAT isoforms (Zhu et al. 1998; Okamura et al. 2004). CK2 has also been found to bind the N-terminus of NFATc1 and phosphorylate functionally important amino acid residues including the SP repeat-associated motif that appears to be important for regulating NFATc1 nuclear export (Porter et al. 2000). Mutation of the CK2 binding sites of NFATc1 expressed in COS cells resulted in much slower nuclear export of NFATc1 (Porter et al. 2000), and inhibition of CK2 significantly increased NFATc1 nuclear accumulation in resting osteoclasts (Komarova et al. 2005). Here we demonstrated that CKI-7, a selective inhibitor for CK1/2 and having greater affinity for CK1 than for CK2, significantly blocked the nuclear export of NFATc1 at high concentrations (300 and 900 μm), possibly indicating the involvement of CK2 rather than CK1. The CK inhibitor D4476, which is about 10-fold more potent than CKI-7 for CK1 and is CK1δ selective, had little effect on NFATc1 nuclear export, indicating that other isoforms of CK1, such as CK1α and CK1ɛ, and/or CK2 are likely to be the relevant kinases for NFATc1 rephosphorylation in skeletal muscle fibres. However, the high doses of CKI-7 used here might have non-specific effects on other kinases, such as S6K or MSK1 (Rena et al. 2004). Alternative future approaches to CK1 or CK2 inhibition using more selective molecular tools such as small interfering RNA may provide better specificity.
Inhibition of both GSK3β and casein kinase
In our experiments, neither block of GSK3β alone nor block of CK1/2 alone was sufficient to completely eliminate NFATc1 nuclear efflux after muscle activity. However, an important finding of the present study is that simultaneous inhibition of GSK3β and CK1/2 did completely block the exit of exogenously expressed NFATc1–GFP from the nucleus after stimulation, suggesting that GSK3β and CK1/2 can synergistically regulate nuclear export of NFATc1 in skeletal muscle fibres.
Simulations of kinase inhibition
From our numerical simulations of the effects of kinase inhibitors on the efflux of NFATc1 from muscle fibre nuclei after cessation of fibre stimulation using our simplified mathematical model, we estimated that the GSK3β inhibitors alsterpaullone and LiCl produced 50% and 60% inhibition of kinase activity towards intranuclear NFATc1, respectively. It is interesting that the simulation required a considerably longer time constant for development of inhibition by LiCl (12 min) compared with alsterpaullone (3 min), consistent with the anticipated slower entry of the ionic GSK3β inhibitor Li+ compared with the presumably more membrane-permeable hydrophobic inhibitor alsterpaullone. The CK1/2 inhibitor CKI-7 produced 85% inhibition and the combination of the two inhibitors, alsterpaullone and CKI-7, produced 100% inhibition. In a simple model of two kinases phosphorylating NFATc1 in parallel at a single class of sites, the observed sum of the separate inhibitor effects being greater than 100% would indicate possible non-specific suppression of one or both of the kinases by the inhibitor of the other kinase. However, in view of the many (21) phosphorylatable serine residues in the NFATc1 regulatory region (Beals et al. 1997a; Neal & Clipstone, 2001), the possibility of sequential phosphorylation (Slazar & Höfer, 2003) of particular serine residues preferentially mediated by one or the other of the two kinases could possibly result in the sum of the separate percentage inhibitions of each kinase adding up to over 100%, as found with our simplified model simulations of block of a single assumed NFATc1 kinase rate constant. As more information becomes available concerning possible specificity of GSK3β or CK1/2 for particular serine residues in the NFATc1 regulatory domains, we may attempt to model the more complete enzymatic reaction sequence for the intranuclear NFATc1 phosphorylation system.
In our modelling of NFATc1 nuclear fluxes, we have assumed that cytosolic CaN activity increases monotonically throughout the 60 min period of chronic electrical stimulation with a 5 s train of stimuli at 10 Hz applied once every 50 s. This stimulation protocol results in experimentally measured rapid rise and subsequent fall in cytosolic Ca2+ concentration for each stimulus during the 5 s train of stimuli, followed by a steady decline of Ca2+ concentration in the 45 s between trains (Liu et al. 2005). This pattern of rising and falling Ca2+ concentration would give corresponding increases and decreases in CaN activation, which have been shown in experiments and models to give rise to stimulation pattern-dependent CaN activation and NFAT nuclear influx (Tomida et al. 2003). In our model we have not considered these factors, which are important for NFATc1 cytosolic dephosphorylation and consequent NFATc1 nuclear influx, because we were concerned here primarily with the mechanisms underlying NFATc1 nuclear efflux following cessation of fibre stimulation.
Finally, we have not made any attempt to rigorously adjust parameter values in the model to produce an actual ‘best fit’ of the model to the observed NFATc1 nuclear time courses, nor have we carried out a systematic pattern search approach to obtain the ‘best’ set of parameter values. Rather the parameter values reported here have been selected by intuition and subsequent adjustment to obtain simulated time courses that are reasonably close to the observed time courses (Fig. 9). Thus, our values could correspond to a parameter set giving a ‘local minimum’ in terms of goodness of fit of model to the data, and conceivably rather different parameter value sets could give similar or even slightly improved simulation of the observed time courses. Future studies, possibly with additional experimental conditions and the corresponding restrictions on parameter values, may be used for a more rigorous fit of either the present model, or a more detailed model, to these expanded experimental results.
Priming kinase
By using a concentration that is much higher than that previously shown to be effective in the inhibition of PKA activity in mouse skeletal muscle (Kumar et al. 2002), the PKA inhibitor KT5720 had little effect on NFATc1 nuclear export after prior muscle activity. Although we show here that PKA does not appear to be a priming kinase for NFATc1 nuclear phosphorylation in adult skeletal muscle fibres, the possible role of other potential GSK3β priming kinases, such as dual-specifity tyrosine-phosphorylation regulated kinase 1A (DYRK1A) (Arron et al. 2006; Gwack et al. 2006), remains to be determined in future studies.
Other kinases
JNK and p38 have been shown to be capable of directly phosphorylating NFATc1 in its highly conserved SP-rich repeat motifs, which appear to be important residues involved in the regulation of NFATc1 subcellular localization (Porter et al. 2000). Overexpression of JNK and p38 was found to efficiently block ionomycin-induced NFATc1 nuclear translocation in COS cells (Porter et al. 2000). NFATc1 was found constitutively in nuclei of resting T cells in JNK knock-out mice and was associated with hyperproliferation of T cells and preferential differentiation to type 2 helper T cells (Dong et al. 1998). JNK and p38 function to antagonize the cardiac hypertrophic response through inhibition of CaN–NFAT signalling (Molkentin, 2004). In cultured neonatal cardiomyocytes, inhibition of p38 by adenoviral-mediated expression of dominant negative p38α significantly enhanced co-infected NFATc1–GFP nuclear translocation (Braz et al. 2003). Similarly, adenoviral-mediated overexpression of JNKs in the presence of MAPK kinase 7 antagonized CaN-induced NFATc1–GFP nuclear translocation (Liang et al. 2003). However, in our studies, we did not find any effect on NFATc1–GFP nuclear export by inhibition of either p38 or JNK in cultured adult skeletal muscle fibres. Ineffectiveness of the p38 and JNK inhibitors used here is highly unlikely, as our results show that antibody staining for phospho-CREB was suppressed by the p38 inhibitor SB202190 in cultured adult FDB muscle fibres. Moreover, previous studies using similar concentrations of SB202190 or SP600125 have reported that these inhibitors were effective in inhibition of p38 or JNK, respectively, in the nuclei of rat muscle fibres (Zhai et al. 2004; Geiger et al. 2005). Taken together, these results suggest that the regulation of NFATc1 nuclear export by p38 and JNK may vary among cell types or differ according to the status of cells. It is not known whether p38 or JNK regulate NFATc1 nuclear export in skeletal muscle under other conditions such as hypertrophy as is the case in the cardiomyocyte.
NFATc1 phosphorylation during muscle activity
It is important to note that all experiments described here to detect kinases that may phosphorylate NFATc1, and thus conteract nuclear accumulation, were carried out by applying kinase inhibitors during the period of recovery after termination of a period of electrical stimulation which caused the accumulation of NFATc1 within the nucleus. Because we exclusively examined the effects of various kinase inhibitors in slowing, or completely stopping, the efflux of the previously accumulated nuclear NFATc1 during a period of recovery without any applied electrical stimulation, our studies could not reveal which kinase(s), if any, may have been active during the period of muscle fibre stimulation. Such putative kinases would have opposed the dephosphorylation and consequent nuclear accumulation of NFATc1 during muscle activity. Future experiments, involving kinase inhibition during the period of electrical stimulation, will be important to determine whether any kinase activity may provide significant opposition to NFATc1 dephosphorylation and consequent nuclear accumulation during periods of fibre stimulation.
In conclusion, in the present study we identified GSK3β and CK1/2 as major protein kinases that regulate NFATc1 nuclear export after muscle activity. Selective inhibitors of these protein kinases significantly delayed the nuclear export of NFATc1 accumulated after slow fibre type electrical stimulation of muscle fibres, whereas the inhibitors of CK1δ, p38, JNK or PKA had little effect. Simultaneous inhibition of GSK3β and CK1/2 completely blocked the nuclear export of NFATc1 in muscle fibres. It is therefore concluded that both GSK3β and CK1/2 are responsible for phosphorylating NFATc1 in muscle nucleus in a complementary or synergistic fashion.
Acknowledgments
We thank Dr Gerald R. Crabtree for providing NFATc1 cDNA and Carrie Wagner for technical assistance. This work was supported by National Institutes of Health (NIH) grant R01-NS33578 from the National Institute of Neurological Disorders and Stroke. T.S. was supported by NIH Training Grant T32-AR-07592 to the Interdisciplinary Program in Muscle Biology, University of Maryland School of Medicine.
References
- Arron JR, Winslow MM, Polleri A, Chang C, Wu H, Gao X, et al. NFAT dysregulation by increased dosage of DSCR1 and DYRK1A on chromosome 21. Nature. 2006;441:595–600. doi: 10.1038/nature04678. [DOI] [PubMed] [Google Scholar]
- Beals CR, Clipstone NA, Ho SN, Crabtree GR. Nuclear localization of NF-ATc by a calcineurin-dependent, cyclosporin-sensitive intramolecular interaction. Genes Dev. 1997a;11:824–834. doi: 10.1101/gad.11.7.824. [DOI] [PubMed] [Google Scholar]
- Beals CR, Sheridan CM, Turck CW, Gardner P, Crabtree GR. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science. 1997b;275:1930–1934. doi: 10.1126/science.275.5308.1930. [DOI] [PubMed] [Google Scholar]
- Braz JC, Bueno OF, Liang Q, Wilkins BJ, Dai YS, Parsons S, et al. Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-NFAT signaling. J Clin Invest. 2003;111:1475–1486. doi: 10.1172/JCI17295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chijiwa T, Hagiwara M, Hiroyoshi H. A newly synthesized selective casein kinase I inhibitor, N-(2-aminoethyl)-5-chloroisoquinoline-8-sulfonamide and affinity purification of casein kinase I from bovine testis. J Biol Chem. 1989;264:4924–4927. [PubMed] [Google Scholar]
- Chin ER, Olson EN, Richardson JA, Yang Q, Humphries C, Shelton JM, Wu H, Zhu W, Bassel-Duby R, Williams RS. A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes Dev. 1998;12:2499–2509. doi: 10.1101/gad.12.16.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Yang DD, Wysk M, Ahitmarsh RJ, Davis RJ, Flavell RA. Defective T cell differentiation in the absence of JNK1. Science. 1998;282:2092–2095. doi: 10.1126/science.282.5396.2092. [DOI] [PubMed] [Google Scholar]
- Geiger PC, Wright DC, Han D, Holloszy JO. Activation of p38 MAP kinase enhances sensitivity of muscle glucose transport to insulin. Am J Physiol Endocrinol Metab. 2005;288:E782–E788. doi: 10.1152/ajpendo.00477.2004. [DOI] [PubMed] [Google Scholar]
- Graef IA, Mermelstein PG, Stankunas K, Neilson JR, Deisseroth K, Tsien RW, Crabtree GR. L-type calcium channels and GSK-3 regulate the activity of NF-ATc4 in hippocampal neurons. Nature. 1999;401:703–708. doi: 10.1038/44378. [DOI] [PubMed] [Google Scholar]
- Gross SD, Anderson RA. Casein kinase I: spatial organization and positioning of a multifunctional protein kinase family. Cell Signal. 1998;10:699–711. doi: 10.1016/s0898-6568(98)00042-4. [DOI] [PubMed] [Google Scholar]
- Gwack Y, Sharma S, Nardone J, Tanasa B, Iuga A, Srikanth S, Okamura H, Bolton D, Feske S, Hogan1 PG, Rao A. A genome-wide Drosophila RNAi screen identifies DYRK-family kinases as regulators of NFAT. Nature. 2006;441:646–650. doi: 10.1038/nature04631. [DOI] [PubMed] [Google Scholar]
- Haq S, Choukroun G, Kang ZB, Ranu H, Matsui T, Rosenzweig A, et al. Glycogen synthase kinase-3β is a negative regulator of cardiomyocyte hypertrophy. J Cell Biol. 2000;151:117–129. doi: 10.1083/jcb.151.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig R, Lomo T. Firing patterns of motor units in normal rats. Nature. 1985;314:164–166. doi: 10.1038/314164a0. [DOI] [PubMed] [Google Scholar]
- Hoey T, Sun YL, Williamson K, Xu X. Isolation of two new members of the NF-AT gene family and functional characterization of the NF-AT proteins. Immunity. 1995;2:461–472. doi: 10.1016/1074-7613(95)90027-6. [DOI] [PubMed] [Google Scholar]
- Jiang H, Li H, Dimario JX. Control of slow myosin heavy chain 2 gene expression by glycogen synthase kinase activity in skeletal muscle fibers. Cell Tissue Res. 2005;323:489–494. doi: 10.1007/s00441-005-0007-1. [DOI] [PubMed] [Google Scholar]
- Kehlenbach RH, Dickmanns A, Gerace L. Nucleocytoplasmic shuttling factors including Ran and CRM1 mediate nuclear export of NFAT in vitro. J Cell Biol. 1998;141:863–874. doi: 10.1083/jcb.141.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm JD, Beals CR, Crabtree GR. Rapid targeting of nuclear proteins to the cytoplasm. Curr Biol. 1997;7:638–644. doi: 10.1016/s0960-9822(06)00290-9. [DOI] [PubMed] [Google Scholar]
- Komarova SV, Pereverzev A, Shum JW, Sims SM, Dixon SJ. Covergent signaling by acidosis and receptor activator of NF-kB ligand (RANKL) on the calcium/calcineurin/NFAT pathway in osteoclasts. Proc Natl Acad Sci U S A. 2005;102:2643–2648. doi: 10.1073/pnas.0406874102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Chaudhry I, Reid MB, Boriek AM. Distinct signaling pathways are activated in response to mechanical stress applied axially and transversely to skeletal muscle fibers. J Biol Chem. 2002;277:46493–46503. doi: 10.1074/jbc.M203654200. [DOI] [PubMed] [Google Scholar]
- Liang Q, Bueno OF, Wilkins BJ, Kuan CY, Xia Y, Molkentin JD. c-Jun N-terminal kinases (JNK) antagonize cardiac growth through cross-talk with calcineurin-NFAT signaling. EMBO J. 2003;22:5079–5089. doi: 10.1093/emboj/cdg474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Carroll SL, Klein MG, Schneider MF. Calcium transients and calcium homeostasis in adult mouse fast-twitch skeletal muscle fibers in culture. Am J Physiol Cell Physiol. 1997;272:C1919–C1927. doi: 10.1152/ajpcell.1997.272.6.C1919. [DOI] [PubMed] [Google Scholar]
- Liu Y, Cseresnyés Z, Randall WR, Schneider MF. Activity-dependent nuclear translocation and intranuclear distribution of NFATc in adult skeletal muscle fibers. J Cell Biol. 2001;155:27–39. doi: 10.1083/jcb.200103020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Randall WR, Schneider MF. Activity-dependent and -independent nuclear fluxes of HDAC4 mediated by different kinases in adult skeletal muscle. J Cell Biol. 2005;168:887–897. doi: 10.1083/jcb.200408128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molkentin JD. Calcineurin-NFAT signaling regulates the cardiac hypertrophic response in coordination with the MAPKs. Cardiovasc Res. 2004;63:467–475. doi: 10.1016/j.cardiores.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Morisco C, Seta K, Hardt SE, Lee Y, Vatner SF, Sadoshima J. Glycogen synthase kinase 3β regulates GATA4 in cardiac myocytes. J Biol Chem. 2001;276:28586–28597. doi: 10.1074/jbc.M103166200. [DOI] [PubMed] [Google Scholar]
- Neal JW, Clipstone NA. Glycogen synthase kinase-3 inhibits the DNA binding activity of NFATc. J Biol Chem. 2001;276:3666–3673. doi: 10.1074/jbc.M004888200. [DOI] [PubMed] [Google Scholar]
- Neilson J, Stankunas K, Crabtree GR. Monitoring the duration of antigen-receptor occupancy by calcineurin/glycogen-synthase-kinase-3 control of NF-AT nuclear shuttling. Curr Opin Immunol. 2001;13:346–350. doi: 10.1016/s0952-7915(00)00225-9. [DOI] [PubMed] [Google Scholar]
- Okamura H, Garcia-Rodriguez C, Martinson H, Qin J, Virshup DM, Rao A. A conserved docking motif of CK1 binding controls the nuclear localization of NFAT1. Mol Cell Biol. 2004;24:4184–4195. doi: 10.1128/MCB.24.10.4184-4195.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter CM, Havens MA, Clipstone NA. Indentification of amino acid residues and protein kinases involved in the regulation of NFATc subcellular localization. J Biol Chem. 2000;275:3543–3551. doi: 10.1074/jbc.275.5.3543. [DOI] [PubMed] [Google Scholar]
- Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- Rena G, Bain J, Elliott M, Cohen P. D4476, a cell-permeant inhibitor of CK1, suppresses the site-specific phosphorylation and nuclear exclusion of FOXO1a. EMBO Rep. 2004;5:60–65. doi: 10.1038/sj.embor.7400048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar C, Höfer T. Allosteric regulation of the transcription factor NFAT1 by multiple phosphorylation sites: a mathematical analysis. J Mol Biol. 2003;327:31–45. doi: 10.1016/s0022-2836(03)00085-8. [DOI] [PubMed] [Google Scholar]
- Scarborough JB. Numerical Mathematical Analysis. 6. Baltimore, MD: Johns Hopkins Press; 1966. [Google Scholar]
- Shaw JP, Utz PJ, Durand DB, Toole JJ, Emmel EA, Crabtree GR. Identification of a putative regulator of early T cell activation genes. Science. 1988;241:202–205. [PubMed] [Google Scholar]
- Shen T, Liu Y, Cseresnyés Z, Hawkins A, Randall WR, Schneider MF. Activity- and calcineurin-independent nuclear shuttling of NFATc1, but not NFATc3, in adult skeletal muscle fibers. Mol Biol Cell. 2006;17:1570–1582. doi: 10.1091/mbc.E05-08-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan CM, Heist EK, Beals CR, Crabtree GR, Gardner P. Protein kinase A negatively modulates the nuclear accumulation of NF-ATc1 by priming for subsequent phosphorylation by glycogen synthase kinase-3. J Biol Chem. 2002;277:48664–48676. doi: 10.1074/jbc.M207029200. [DOI] [PubMed] [Google Scholar]
- Tavi P, Pikkaraine S, Ronkainen J, Niemelä P, Ilves M, Weckström M, Vuolteenaho O, Bruton J, Westerblad H, Ruskoaho H. Pacing-induced calcineurin activation controls cardiac Ca2+ signaling and gene expression. J Physiol. 2004;554:309–320. doi: 10.1113/jphysiol.2003.053579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomida T, Hirose K, Takizawa A, Shibasaki F, Iino M. NFAT functions as a working memory of Ca2+ signals in decoding Ca2+ oscillation. EMBO J. 2003;22:3825–3832. doi: 10.1093/emboj/cdg381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh GI, Miyamotoi S, Price NT, Saferi B, Proud CG. T-cell activation leads to rapid stimulation of translation initiation factor eIF2B and inactivation of glycogen synthase kinase-3. J Biol Chem. 1996;271:11410–11413. doi: 10.1074/jbc.271.19.11410. [DOI] [PubMed] [Google Scholar]
- Woodgett JR. Regulation and functions of the glycogen synthase kinase-3 subfamily. Semin Cancer Biol. 1994;5:269–275. [PubMed] [Google Scholar]
- Zhai W, Eynott PR, Oltmanns U, Leung SY, Chung KF. Mitogen-activated protein kinase signaling pathways in IL-1β-dependent rat airway smooth muscle proliferation. Br J Pharmacol. 2004;143:1042–1049. doi: 10.1038/sj.bjp.0705971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, McKeon F. NF-AT activation requires suppression of Crm1-dependent export by calcineurin. Nature. 1999;398:256–260. doi: 10.1038/18473. [DOI] [PubMed] [Google Scholar]
- Zhu J, Shibasaki F, Price R, Guillemot JC, Yano T, Dötsch V, Wagner G, Ferrara P, McKeon F. Intramolecular masking of nuclear import signal on NF-AT4 by casein kinase I and MEKK1. Cell. 1998;93:851–861. doi: 10.1016/s0092-8674(00)81445-2. [DOI] [PubMed] [Google Scholar]