Abstract
In adults, motoneurone pools of synergistic muscles that act around a common joint share a common presynaptic drive. Common drive can be revealed by both time domain and frequency domain analysis of EMG signals. Analysis in the frequency domain reveals significant coherence in the range 1–45 Hz, with maximal coherence in low (1–12 Hz) and high (16–32 Hz) ranges. The high-frequency range depends on cortical drive to motoneurones and is coherent with cortical oscillations at ∼20 Hz frequencies. It is of interest to know whether oscillatory drive to human motoneurone pools changes with development. In the present study we examined age-related changes in coherence between rectified surface EMG signals recorded from the short and long thumb abductor muscles during steady isometric contraction obtained while subjects abducted the thumb against a manipulandum. We analysed EMG data from 36 subjects aged between 4 and 14 years, and 11 adult subjects aged between 22 and 59 years. Using the techniques of pooled coherence analysis and the χ2 difference of coherence test we demonstrate that between the ages of 7 and 9 years, and 12 and 14 years, there are marked increases in the prevalence and magnitude of coherence at frequencies between 11 and 45 Hz. The data from subjects aged 12–14 years were similar to those obtained from adult controls. The most significant differences between younger children and the older age groups were detected at frequencies close to 20 Hz. We believe that these are the first reported results demonstrating significant late maturational changes in the ∼20 Hz common oscillatory drive to human motoneurone pools.
In the adult, analysis of motor unit firing recorded from pairs of different cocontracting muscles shows coherence at frequencies between 1 and 45 Hz (Farmer et al. 1993), with maxima in a low-frequency range, typically 1–12 Hz, and a higher frequency range, typically 16–32 Hz (maximal at ∼20 Hz). Similar results are obtained when the analysis is applied to pairs of rectified surface EMG signals (Mayston et al. 2001).
Studies of single motor unit coherence and electromyogram (EMG–EMG) coherence in stroke patients and patients with congenital mirror movements have demonstrated that the ∼20 Hz oscillations originate from activity in corticospinal pathways (Farmer et al. 1993, 2004; Mayston et al. 2001). Direct confirmation of this has come from studies of magnetoencephalogram–electromyogram (MEG–EMG) and electroencephalogram–electromyogram (EEG–EMG) coherence which show that the cortical signal recorded from over primary motor cortex interacts with the muscle signal at ∼20 Hz (Conway et al. 1995; Salenius et al. 1997; Halliday et al. (1998); Brown et al. 1998; Mima & Hallett, 1999).
The EEG in humans undergoes considerable change during development with slow rhythmic activity dominating early in life followed by an age-dependent increase in the frequency of background rhythmic activity (Gasser et al. 1988; Marshall et al. 2002). Recent studies of the development of the human EEG patterns show that relative to other frequencies, beta frequencies (13.5–20.5 Hz) increase between ages 8 and 12 years, with maturation being latest in frontal cortical areas (Clarke et al. 2001).
Given that EMG–EMG coherence analysis detects oscillatory drive from corticospinal pathways and EMG is coherent with sensorimotor cortex EEG and MEG, it is of interest to establish whether there are detectable differences in EMG–EMG coherence between different age groups. Thus in this study we examined changes in prevalence and strength of EMG–EMG coupling as a function of frequency (coherence estimates) and time (cumulant density estimates) in children between the ages of 4 and 14 years. Children were grouped in three age bands: 4–6, 7–9 and 12–14 years, and their results were compared with those of adult controls. Our data set comprises recordings previously obtained by Gibbs et al. (1997) and data more recently collected using the same experimental protocol. The EMG–EMG data have been analysed and represented using the methodology of pooled coherence and pooled cumulant analysis (Amjad et al. 1997; Halliday & Rosenberg, 2000). The results demonstrate that coherence between 1 and 45 Hz increases with age. Discrete coherence maxima in the frequency range 16–32 Hz (∼20 Hz) show the clearest increases with age, with marked changes between ages 7–9 and 12–14 years.
Methods
Subjects
Recordings were obtained with local ethical approval adhering to standards set out in the Declaration of Helsinki from 36 healthy child subject volunteers (21 male and 15 female) aged from 4 to 14 years recruited from local inner London schools, and 11 healthy adult subjects volunteers (6 male and 5 female) aged 22–59 years. Data from three adult subjects had been collected for a previous study by Gibbs et al. (1997). Data from an additional eight adult subjects were obtained recently using the same experimental protocol. Two subjects aged 14 years and 54 years were left-handed, and all other subjects were right-handed. Verbal and written consent was obtained from the subjects, and in the case of children consent was obtained from the parents. All of the subjects had a normal neurodevelopmental history. For the purposes of further analysis the subjects were divided into four age ranges: 4–6 years (n= 12), 7–9 years (n= 13), 12–14 years (n= 11) and 22–59 years (n= 11).
EMG recordings
Pairs of simultaneous EMG signals were recorded from the dominant hand using small bipolar surface electrodes. These were constructed by cutting down neonatal ECG electrodes (Pink ARBO H82V) to 5 mm × 5 mm, with the poles of the electrode pair placed 5 mm apart. The first EMG signal was obtained from bipolar electrodes attached to the skin overlying the long thumb abductor (abductor pollicis longus, APL), as this muscle passes around the lateral border of the radius in the distal forearm. The second EMG signal was obtained from a bipolar electrode pair over the short thumb abductor (abductor pollices brevis, APB) of the thenar eminence.
Subjects were seated with their arms resting in a semipronated position on a table. During the recordings the subjects were asked to extend and abduct both thumbs towards the midline against a firm metal strip that was inclined at 30 deg to the vertical. All other digits were held by subjects in a flexed posture to reduce EMG contamination from other digit extensor muscles. Using these recording sites and protocol Gibbs et al. (1997) demonstrated that the EMG recordings are highly specific for the short and long thumb muscles. Subjects were given feed back of their root mean square (RMS) EMG signals. Subjects were asked to produce steady levels of EMGs between 10 and 20% of the EMG associated with maximum voluntary contraction as measured by the RMS signal. This level of force is easily maintained without fatigue (both subjectively and through the maintenance during the 2 min of constant EMG levels) or discomfort and in addition produces EMG localized to the muscles under study.
Continuous recordings lasting for 2 min were made using a Medlec Sapphire 4ME EMG machine (Oxford Instruments Medical, Old Woking, Surrey, UK). The EMG signals were amplified (50–200 μV division−1) and filtered −3 dB at 150 Hz and 5 KHz. The data were stored on magnetic tape for further analysis.
Data analysis
Data was played back from the magnetic tape and digitized at 1000 Hz (CED 1401, Cambridge Electronic Design, UK). Analysis of the present data was undertaken in three distinct steps: (1) analysis of individual records using time and frequency domain measures of correlation between paired EMG signals (120 s of data in each channel – producing 120 contiguous segments each of 1 s; sufficient data to produce low (coherence <0.05) 95% confidence levels when calculating the coherence, see (Fig. 2); (2) combination of all records within each subject group using pooled, or population, spectral parameters; and (3) comparison between different populations to characterize any changes that occur within EMG–EMG correlation.
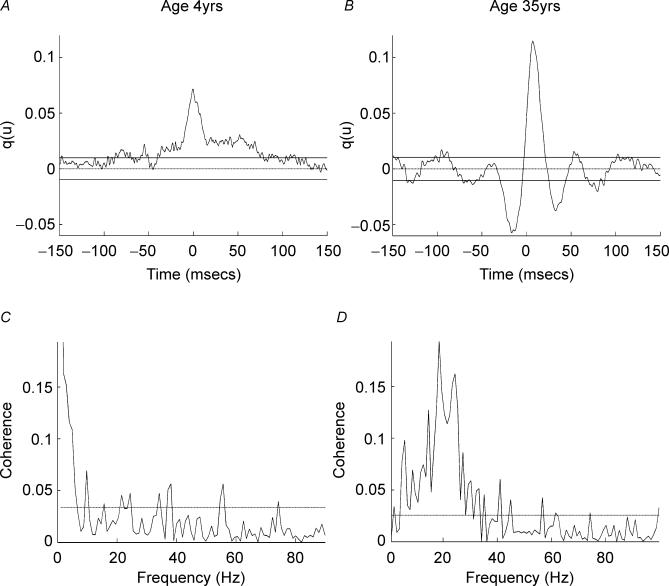
Figure 2. Individual subject EMG–EMG cumulant and coherence.
Cumulant density and coherence spectra calculated for 2 min of the EMG data sets shown in Fig. 1. A, cumulant density between APL and APB rectified EMGs in the subject aged 4 years. The horizontal dashed line shows the expected value of zero for uncorrelated EMG signals; the continuous horizontal lines indicate the upper and lower 95% confidence limits. The cumulant contains a central peak magnitude q(u) = 0.07, duration ±8 ms, on a asymmetric broad feature duration 200 ms. B, cumulant density between APL and APB rectified EMGs in the subject age 35 years; confidence limits as in A. The cumulant contains a central peak magnitude q(u) = 0.12, peak duration ±10 ms, troughs of decreased joint EMG firing probability are seen either side of the central feature, with small secondary peaks at ±50 and ±100 ms. C, coherence calculated between APL and APB EMGs of the 4-year-old subject associated with the cumulant in A. The dashed horizontal line denotes the upper 95% confidence limit based on the assumption of independence. Significant coherence is detected in the range 1–5 Hz (maximum at 1 Hz) with occasional crossings at higher frequencies. D, coherence calculated between APL and APB EMGs of the 35-year-old subject whose cumulant is shown in B, confidence limits as in C. Significant coherence is detected in the range 5–40 Hz, with maximal coherence of 0.19 at 19 Hz.
The basic time and frequency domain analysis of the data was undertaken using the methods set out in detail in Halliday et al. (1995). The standard practice of full wave rectification of surface EMG signals has been adopted in this study. This approach has been shown to maximize the information regarding timing of motor unit action potentials whilst suppressing information regarding waveform shape (Myers et al. 2003). In addition, as a precursor to undertaking population analysis of the data (Halliday & Rosenberg, 2000), all rectified EMG signals were normalized to have unit variance. Rectified and normalized EMG signals are assumed to be realizations of stationary zero mean time series, denoted by x and y. Auto spectra, fxx(λ) and fyy(λ), and cross spectra, fxy(λ), were estimated by averaging discrete Fourier transforms from non-overlapping segments of data taken from each signal. Time and frequency domain measures of correlation between the two signals were derived from the auto and cross spectra. Correlation as a function of frequency is assessed through estimates of the coherence function, |Rxy(λ)|2, analogous to a magnitude squared correlation coefficient, r2, in the case of ordinary random variables, except that coherence is interpreted as a function of frequency, λ. Like r2 values, estimates of the coherence function provide a normative measure of linear association between 0 and 1. Coherence spectra are defined and estimated from the magnitude squared of the cross spectrum, normalized by the product of the two auto spectra (Halliday et al. 1995), as shown in eqn (1).
| (1) |
For the present data, coherence estimates provide a measure of the fraction of the activity in one surface EMG signal that can be predicted by the activity in the second surface EMG signal. The reference signal was EMG from the more proximal muscle APL, the response signal was EMG from APB. In this way, coherence estimates quantify the strength and range of frequencies of common rhythmic synaptic inputs distributed across the motoneurone pool (Farmer et al. 1993; Rosenberg et al. 1998). Correlation in the time domain is assessed through estimates of the cumulant density function, qxy(u), at time lag u, which is defined and estimated as the inverse Fourier transform of the cross spectrum, fxy(λ) (Halliday et al. 1995). For two uncorrelated signals the cumulant has an expected value of zero; significant deviations from this indicate a correlation between the EMG signals at a particular time lag u. Rhythmic input correlation will induce additional oscillatory components in the cumulant (Perkel et al. 1967), and the frequency and strength of these components can be quantified from the corresponding coherence estimates. Cumulant density functions are analogous to, and have an interpretation similar to, cross correlation, or cross covariance functions (Halliday et al. 1995). Significance of individual coherence and cumulant density estimates are assessed by inclusion of an upper 95% confidence limit in coherence plots, and upper and lower 95% confidence limits in cumulant density plots, based on the assumption of independence. For details see Halliday et al. (1995).
Estimates of pooled coherence and pooled cumulant density functions are used to summarize the correlation structure in each group of subjects. Pooled coherence and cumulant functions provide single measures that summarize at the population level the correlation structure across several data sets (Amjad et al. 1997). Pooled coherence estimates, like individual coherence estimates, provide a normative measure of linear association on a scale from 0 to 1 (Halliday & Rosenberg, 2000). The interpretation of pooled estimates is similar to those for individual records, except any inferences relate to the population as a whole. In addition, the pooled coherence framework contains a χ2 extended difference of coherence test. This test is based on the hypothesis of equal coherence values within the population, where significant values of the χ2 variate would indicate that this null hypothesis does not provide a plausible interpretation of the data. Like coherence, the χ2 test is applied separately at each frequency of interest (see Amjad et al. 1997). The approach used here to calculate pooled coherence estimates is to pool individual coherency estimates (Halliday & Rosenberg, 2000). The individual coherency estimate for record i is denoted as Rxyi(λ), where this has been calculated from Li segments of data. The coherency function is a complex quantity; the corresponding coherence is the magnitude squared of this function. The pooled coherence across k records, at frequency λ is then:
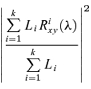 |
(2) |
Estimates of the above pooled coherence provide a single parameter describing the correlation structure, as a function of frequency, within the records in a single population, where the total number of records to be used is k (in the present study k equates to the number subjects for each group). This can be considered analogous to single coherence estimate calculated from 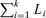 segments of data, where i denotes each individual record to be included in the pooled analysis. The pooled cumulant density is estimated by taking the inverse Fourier transform of the pooled cross-spectral estimate (Amjad et al. 1997). The pooled phase is calculated as the phase angle of the pooled cross spectral estimate. Also included in the plots are histograms showing how many individual coherence estimates exhibit significant values (above the estimated 95% confidence limit) at each frequency. All histograms are normalized to have range 0–1.
segments of data, where i denotes each individual record to be included in the pooled analysis. The pooled cumulant density is estimated by taking the inverse Fourier transform of the pooled cross-spectral estimate (Amjad et al. 1997). The pooled phase is calculated as the phase angle of the pooled cross spectral estimate. Also included in the plots are histograms showing how many individual coherence estimates exhibit significant values (above the estimated 95% confidence limit) at each frequency. All histograms are normalized to have range 0–1.
The third step in the present analysis is a characterization of changes in the correlation structure between two different populations, achieved by undertaking a χ2 extended difference of coherence test on the two populations to be compared. In practice, this is achieved by undertaking a further pooled spectral analysis on the two sets of pooled spectra from each population of data. The only parameter considered from this step is the χ2 test, used in this case to indicate whether the null hypothesis of equal coherence estimates between the two populations is plausible. Significance is assessed through inclusion of an upper 95% confidence limit, based on the null hypothesis. In situations where the calculated value of the χ2 variate exceeds this confidence limit, further quantitative information is available from its magnitude. A larger difference in the two population coherence estimates will result in a larger value of the calculated χ2 variate, providing an indication of where the largest differences in the two population coherence estimates occur. In addition, visual inspection of this test is useful in highlighting where there are consistent differences in the two population coherence estimates across a range of frequencies.
Results
We believe that the data show for the first time a relationship between increasing age and increasing incidence and magnitudes of EMG–EMG coherence in the frequency range 3–45 Hz. This result was clear from visual inspection of individual coherence data from individuals of different ages, and these conclusions were confirmed through application of pooled data analysis, including the extended difference of coherence (χ2) test.
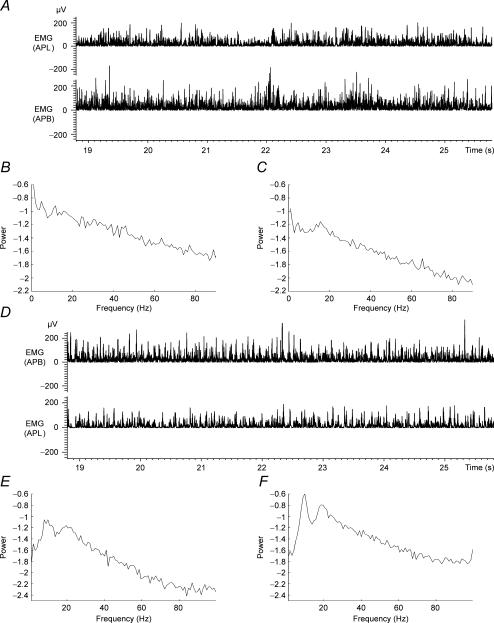
The quality of the EMG recordings from young children and adults was similar, and this is illustrated in Fig. 1A and D which shows typical examples of rectified surface EMG from short and long thumb abductor muscles recorded during steady abduction of the dominant thumb of a child aged 4 years (Fig. 1A) and adult subject aged 35 years (Fig. 1D). The corresponding auto-spectral densities are shown for each of the EMG signals in Fig. 1B, C, E and F. The auto-spectral densities show the range of frequencies detected within each of the EMG signals. They are included in this figure and in subsequent figures for completeness, so that the elements making up the analysis of coherence and cumulant can be seen together. Figure 2 shows the results of cumulant and coherence analysis performed on the data sets shown in Fig. 1.
Figure 1. Rectified EMG and auto-spectra from two subjects.
A, sections of rectified surface EMGs recorded from abductor pollices longus (APL) (top panel) and abductor pollices brevis (APB) (bottom panel) from the right hand of a healthy 4-year-old male subject during isometric extension/abduction of the thumb. B and C, autospectra (1–90 Hz) constructed from 2 min of continuous APL EMG data (B) and APB EMG data (C), same data as A. D, sections of rectified surface EMG recorded from APL (top panel) and abductor pollices brevis (APB) bottom panel from the right hand of a healthy 35-year-old male subject during isometric extension/abduction of the thumb. E and F, autospectra (1–90 Hz) constructed from 2 min of continuous APL EMG data (E) and APB EMG data (F), same data as D.
Figure 2A–D shows coherence and cumulant calculated between rectified EMGs from the short and long thumb abductors of the dominant hand from the two male subjects aged 4 and 35 years whose EMG and auto-spectral data are shown in Fig. 1. Figure 2A and C shows data from the 4-year-old subject, and Fig. 2B and D shows data from the subject aged 35 years. The data show clear differences in the coherence and cumulant functions. There is a central peak in the cumulant density constructed from the 4-year-old child's data indicating synchronization between the short and long thumb abductor EMGs. However, coherence analysis of the same data is flat at frequencies in excess of 4 Hz, with the only consistently significant coherence at 1–4 Hz, indicating low frequency common modulation of the two EMGs. In contrast, the data from the adult subject show significant coherence at frequencies in excess of 4 Hz with maxima at ∼20 Hz. There is also a large central cumulant peak indicating significant EMG–EMG synchrony. This is flanked by troughs and secondary cumulant peaks indicative of this oscillatory activity. These marked differences between adults and young children were evident in the individual coherence and cumulant measures. The differences between the measures of EMG–EMG correlation for all of age ranges (4–6, 7–9, 12–14 and 22–59 years) were identified in greater detail using pooled analysis.
The data have been expressed as pooled auto-spectra, coherence, phase and cumulant, along with a plot of the proportion of records that showed coherence at any given frequency in the range 1–90 Hz. In order to achieve robust estimates of the correlation parameters, one record from each of the subjects was entered into the pooled analysis. We chose four age ranges to analyse as groups, and the data were pooled for each group. The age ranges used were 4–6, 7–9 and 12–14 years, and adults (ages 22–59 years). The data illustrated were all obtained from the dominant hand.
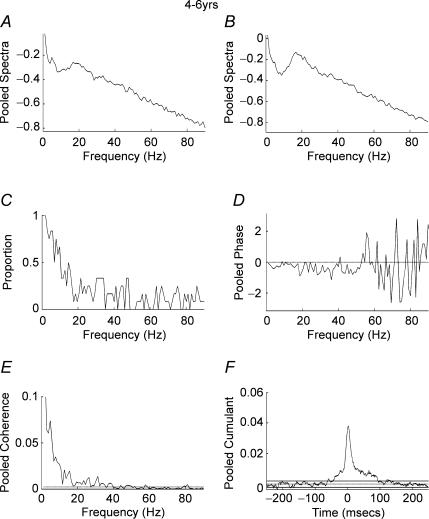
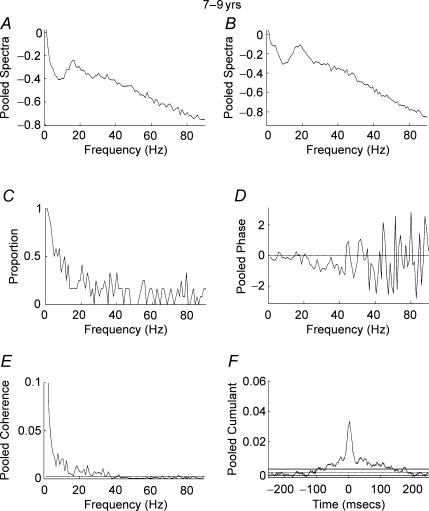
Figure 3A–F shows the results of pooled autospectra, phase, coherence, coherence incidence and cumulant for the dominant hand of 12 subjects aged 4–6 years. The pooled cumulant data show a cumulant estimate with a peak close to time zero sitting on a broad base indicating EMG–EMG synchrony (Fig. 3F). The pooled coherence spectra show coherence at low (1–8 Hz) frequencies, with maximal coherence at 1 Hz, but little coherence at frequencies above 8 Hz (Fig. 3E). Plotting the proportion of records that showed significant coherence at any given frequency between 1 and 90 Hz (P < 0.05) further emphasizes these results (Fig. 3C). From this plot it can be seen that a low proportion of records showed significant coherence at frequencies above 10 Hz. Similar results were obtained for children aged 7–9 years (Fig. 4A–F). A significant central peak sitting on a broad base was detected in the pooled cumulant (Fig. 4F) with pooled coherence between 1 and 10 Hz but little coherence at frequencies in excess of 10 Hz (Fig. 4E) and a low proportion of records showing significant (P < 0.05) coherence at frequencies in excess of 10 Hz.
Figure 3. Pooled analysis, ages 4–6 years.
Pooled autospectra, phase, coherence and cumulant calculated from records of APL and APB rectified EMGs recorded from the dominant hand of 12 subjects aged 4–6 years. A, pooled autospectra from APL. B, pooled autospectra from APB. C, plot of proportion of records (n= 12) that showed significant (P < 0.05) coherence at frequencies between 1 and 90 Hz. D, pooled phase for frequencies between 1 and 90 Hz. E, pooled coherence (n= 12) between 1 and 90 Hz. The coherence is significant between 1 and 18 Hz, with some crossings of the 95% confidence limit up to 40 Hz. F, pooled cumulant density plotted ±250 ms; the cumulant contains a central peak of magnitude q(u) = 0.04. Confidence limits for coherence and cumulant density estimates as in Fig. 2.
Figure 4. Pooled analysis, ages 7–9 years.
Pooled autospectra, phase, coherence and cumulant calculated from records of APL and APB rectified EMGs recorded from the dominant hand of 13 subjects aged 7–9 years. A, pooled autospectra from APL. B, pooled autospectra from APB. C, plot of proportion of records (n= 13) that showed significant (P < 0.05) coherence at frequencies between 1 and 90 Hz. D, pooled phase for frequencies between 1 and 90 Hz. E, pooled coherence (n= 13) between 1 and 90 Hz. The coherence is significant between 1 and 14 Hz with additional significant coherence up to 40 Hz. F, pooled cumulant density plotted ±250 ms; the cumulant contains a central peak magnitude q(u) = 0.037. Confidence limits for coherence and cumulant density estimates as in Fig. 2.
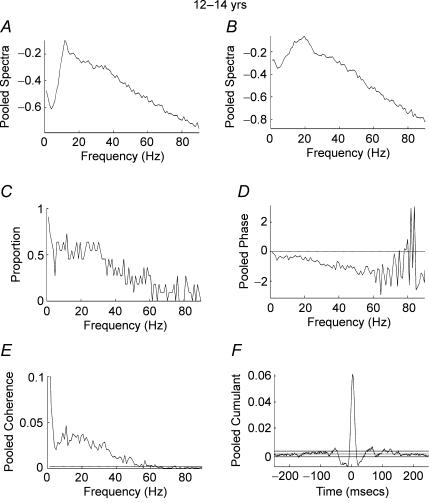
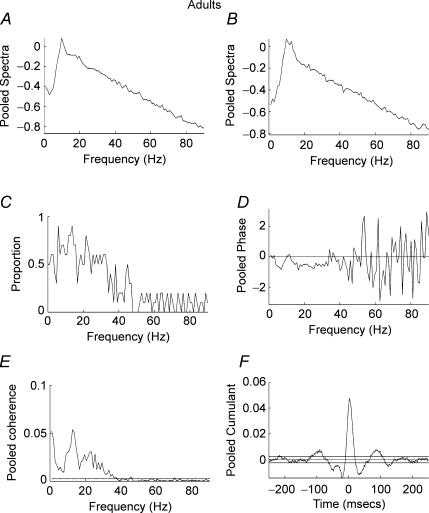
Figure 5A–F shows pooled data from subjects in the age range 12–14 years. In contrast to the two younger age groups, significant coherence was detected between the two EMG signals at frequencies between 1 and 45 Hz. A high proportion of records showed significant coherence (P < 0.05) at frequencies between 5 and 45 Hz (Fig. 5C). The pooled cumulant shows a significant peak close to time zero, indicating EMG–EMG synchronization flanked by troughs and secondary peaks (Fig. 5F). The pooled phase estimate (Fig. 5D) is well fitted with a straight line over the frequencies of significant coherence and is consistent with the 4 ms offset of the central peak towards positive lags. Due to small peripheral conduction delays between APL (used as the reference EMG) and APB (used as response EMG) calculation of the slope of the phase which gives the timing differences indicates that the APB EMG lags the APL EMG by 4 ms. This was the maximum temporal displacement between the signals of all the groups studied. For Figs 3, 4 and 6 the delays were 2, 3 and 2 ms, respectively. Taking into account 2–4 ms peripheral delays, which can be attributed to the more proximal location of APL compared with APB, the pooled phase measures shown in Figs 3–6 indicate that that the significant EMG–EMG coherences arise as the result of synchronous oscillatory drive to motoneurone pools. The results from adult subjects (ages 22–59 years) were similar to those obtained for subjects aged 12–14 years (Fig. 6A–F). It can be seen that there is high pooled coherence with maxima at ∼10 and ∼22 Hz (Fig. 6E) with a high proportion of records showing significant (P < 0.05) coherence in the range 5–35 Hz (Fig. 6C). The pooled cumulant structure is similar to that of the subjects aged 12–14 years with a central peak flanked by troughs and secondary peaks (Fig. 6F).
Figure 5. Pooled analysis, ages 12–14 years.
Pooled autospectra, phase, coherence and cumulant calculated from records of APL and APB rectified EMGs recorded from the dominant hand of 11 subjects aged 12–14 years. A, pooled autospectra from APL. B, pooled autospectra from APB. C, plot of proportion of records (n= 11) that showed significant (P < 0.05) coherence at frequencies between 1 and 90 Hz. D, pooled phase for frequencies between 1 and 90 Hz. E, pooled coherence (n= 11) between 1 and 90 Hz. The coherence is significant between 1 and 50 Hz. F, pooled cumulant density plotted ±250 ms; the cumulant contains a central peak magnitude q(u) = 0.062. Confidence limits for coherence and cumulant density estimates as in Fig. 2.
Figure 6. Pooled analysis, ages 22–59 years.
Pooled autospectra, phase, coherence and cumulant calculated from records of APL and APB rectified EMGs recorded from the dominant hand of 11 adult subjects aged 22–59 years. A, pooled autospectra from APL. B, pooled autospectra from APB. C, plot of proportion of records (n= 11) that showed significant (P < 0.05) coherence at frequencies between 1 and 90 Hz. D, pooled phase for frequencies between 1 and 90 Hz. E, pooled coherence (n= 11) between 1 and 90 Hz. The coherence is significant between 1 and 38 Hz, with maxima at 1, 12 and 22 Hz. F, pooled cumulant density plotted ±250 ms; the cumulant contains a central peak magnitude q(u) = 0.05. Confidence limits for coherence and cumulant density estimates as in Fig. 2.
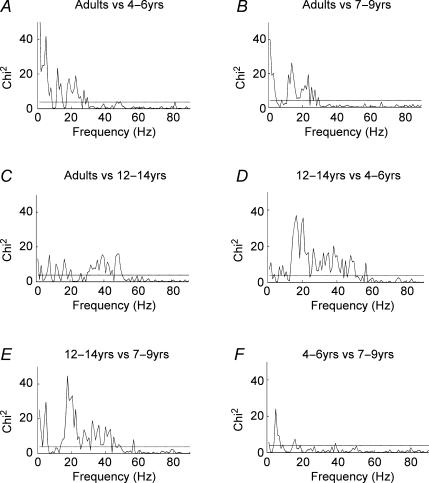
Figure 7 shows the results of the χ2 extended difference of coherence tests applied as pair-wise comparisons between each of the age ranges studied. When the calculated value of the χ2 variate exceeds the 95% confidence limit further quantitative information is available from its magnitude. A larger difference in the two population coherence estimates results in a larger value of the calculated χ2 variate, providing an indication of where the largest differences in the two population coherence estimates occur. It can be seen from Fig. 7 that there are large differences between the older subjects and the younger subjects for frequencies between 10 and 40 Hz. Figure 7A and B shows strong χ2 differences between adults and the 4–6 and 7–9 years age groups. The two groups of younger children showed greater low frequency common modulation at 1–3 Hz frequencies (see also Figs 3 and 4). In contrast, the adults showed greater high frequency (10–30 Hz) coherence (compare also Figs 3 and 4 with Fig. 6).
Figure 7. Statistical comparison of coherence at each frequency between different age groups.
χ2 extended difference of coherence test showing frequencies of maximum coherence difference. The dashed horizontal line in all plots denotes the 95% confidence limit (χ(0.05,1)2= 3.84). A, adult subjects versus subjects aged 4–6 years. B, adult subjects versus subjects aged 7–9 years. C, adult subjects versus subjects aged 12–14 years. D, subjects aged 12–14 years versus subjects aged 7–9 years. E, subjects aged 12–14 years versus subjects aged 4–6 years. F, subjects aged 7–9 years versus subjects aged 4–6 years.
It can be seen from Fig. 7C that there are differences between subjects aged 12–14 years and adults at around 40 Hz that may suggest a difference in gamma frequency drive to the two motoneurone pools in favour of the 12–14 years age group (see also Figs 5 and 6). However, there are no significant differences between these subject groups at ∼20 Hz frequencies. There are small differences at lower (1–17 Hz) frequencies with the 12- to 14-year-old subjects showing more low frequency (∼1 Hz EMG comodulation) than adults.
Figure 7D and E shows striking χ2 differences between subjects aged 12–14 years and the younger children ages 7–9 and 4–6 years. The maximal differences are between 15 and 50 Hz, with peak difference at ∼20 Hz in favour of the 12–14 years age group (compare also Figs 3 and 4 with Fig. 5). At low (1–3 Hz) frequencies the 4–6 years olds showed greater low frequency common EMG modulation compared with the 12–14 years age group. However, a difference was not apparent in the 1–3 Hz range when the comparison was made with the 7- to 9-year-old group. The χ2 difference of coherence test between children aged 4–6 and 7–9 years revealed an isolated difference at 5 Hz but otherwise no significant differences for coherence at other frequencies.
The χ2 difference of coherence test emphasizes the frequency bands that differ between the age groups, with the most striking differences being the increased coherence between 10 and 45 Hz (peaking at ∼20 Hz) in the older subjects.
Discussion
During childhood, there is an increase in the amount of synchrony of firing between motor units recorded from the short and long thumb abductor muscles, with adult values being reached between 12 and 15 years of age, indicating an increase in the amount of common synaptic drive shared between motoneurones of these synergistic muscles (Gibbs et al. 1997).
In the present study, between the ages of 7–9 and 12–14 years, we have found an increase in the prevalence and magnitude of coherence between EMG signals recorded from these same muscles at frequencies between 11 and 45 Hz. Little coherence is detectable at ages 4–6 and 7–9 years. The data from subjects aged 12–14 years were similar to those obtained from adult controls. The most significant differences between younger children and the older age groups were detected at frequencies close to 20 Hz.
We believe that these are the first data to show an increase in ∼20 Hz EMG–EMG coherence with maturity. Thus during motor development, viewed in both the time and frequency domain, the synergistic action of these separate muscles becomes gradually more closely linked by an increase in shared synaptic drive.
Methodological considerations
Two methodological factors may have influenced the observed age-related differences in EMG–EMG coherence: EMG cross-talk and the performance of the motor task.
EMG cross-talk
In the present study we used small (5 mm) closely spaced (5 mm inter pole distance) bipolar surface EMG electrodes and a low-frequency filter setting of −3 dB at 150 Hz. Under these conditions the recorded EMG signal is dominated by motor units with muscle fibres lying within a few millimetres of the electrodes (Fuglevand et al. 1992). Together with our task protocol and weak muscle contraction (10–20% MVC), these conditions provide restricted sampling of EMG and recordings similar to those obtained from multiunit concentric needle recordings (Gibbs et al. 1997, see their Figs 1 and 2). EMG–EMG cross-talk, when present, produces very narrow and very large synchronization peak present in one or at most two central 1 ms bins of the cross-correlogram/cumulant. It may be also appreciated during visual inspection of the raw EMGs when a large motor unit appears in both channels. Recordings from the APB–APL muscle pair, which are widely spaced anatomically, rarely produced cross-talk even in the youngest subjects, and data where cross-talk was suspected through either visual inspection of the raw EMG signals or cross-correlation/cumulant was excluded from the analysis. Furthermore, EMG cross-talk produces high levels of coherence across a wide range of frequencies (1–250 Hz) no data was observed with these properties. Importantly, it is to be expected that there will be an increased risk of EMG cross-talk in subjects where the electrodes are closer together, i.e. the younger children. If this were the case we would have found high levels of EMG–EMG coherence, whereas in fact very little coherence was detected in these age groups.
Performance of the motor task
Although the youngest children required more encouragement to maintain a steady motor output, they performed the task with ease, albeit with greater fluctuation. This is reflected by increased low frequency ∼1–3 Hz coherence between EMGs in children compared with adults. In all subject groups in the present study, isometric thumb abduction was maintained against a fixed support. This is a relevant consideration because it has been demonstrated that muscle shortening and limb movement results in abolition of ∼20 Hz EMG–EMG and cortex–muscle coherence (Kilner et al. 1999). In a previous study of coherence between pairs of single motor units in which the effects of low frequency common modulation were studied explicitly during isometric contraction, the presence of voluntarily produced low frequency (1–3 Hz) common modulation was not found to affect coherence at ∼20 Hz (Farmer et al. 1993). In the present experiments the observed differences between adults and children in low frequency common modulation of EMGs is analogous to this earlier experiment of Farmer et al. (1993). Furthermore, it can be seen from Figs 3–5 that high frequency coherence emerges in the 12–14 years group despite levels of low frequency coherence that are equivalent to the younger children. This is further emphasized by the χ2 difference of coherence test (Fig. 7), which shows strong differences between the 12–14 years age group and the younger children at ∼20 Hz, with smaller or no difference at low frequencies (1–3 Hz). The fact that there are clear differences in ∼20 Hz coherence without differences in 1–3 Hz coherence indicates that low-frequency modulation at different ages does not influence the likelihood of detecting strong differences in ∼20 Hz coherence between different ages.
The pooled coherence spectrum
In contrast to previous studies when individual subject data were used (for example, Farmer et al. 1993), in the present study the pooled coherence shows less discrete frequency ranges of interest. There are a number of possible explanations for this difference. First, in contrast to previous single unit studies, the time and frequency domain correlations were calculated from the rectified surface EMGs with inevitable loss of temporal precision. Second, the pooling technique will blur discrete frequency ranges, as there are small differences in the frequency and ranges of maximal coherence between individuals whose coherence data contribute to the pool. Third, there are no previous studies of EMG–EMG coherence between short and long thumb abductors, thus there are no other coherence data from this muscle pair for comparison. Finally, we noted in some subjects the emergence of ∼10 Hz physiological tremor which in some records produced significant ∼10 Hz coherence, thus contributing to the lack of a discrete cut-off between the two frequency ranges previously described. It is important to note that despite pooled coherence analysis in adults and subjects aged 12–14 years showing a broad range of coherence from 1 to 45 Hz, the χ2 difference of coherence measure picked out discrete ranges of maximal difference between these older age groups and the younger subjects corresponding to the ∼20 Hz band described in previous studies of EMG–EMG and EEG/MEG–EMG coherence.
EMG–EMG coherence and synchrony
The previous study of the development of motor unit synchronization by Gibbs et al. (1997) showed an increase in the prevalence and magnitude of the central peak in a cross-correlogram constructed between the times of occurrence of multiunit EMGs from the short and long thumbs abductor muscles between the ages 4 and 12 years. From the age of 10 years, the prevalence and magnitude of EMG–EMG synchronization reached adult values. Coherence analysis explicitly measures frequency components that are common to the coactivated EMG signals. It is of great interest therefore that little coherence is detectable at ages 4–6 and 7–9 years, and that there is a clear emergence of coherence in the age range 12–14 years that reaches adult values.
In the present study, the pooled EMG–EMG cumulant, although showing an increase in prevalence and magnitude with age, in keeping with the findings of Gibbs et al. (1997), does not show such clear age differences as the coherence data. Because of the use of pooled cumulant and coherence some of the fine structure of developmental changes may have been sacrificed, and this may account for the failure to observe subtle increases in synchronization strength. However, the pooled analysis treats the cumulant and coherence in the same way and it is clear that the coherence changes with age are by far the most striking. The cumulant/cross-correlation function is a measure of the strength of common input to pools of neurons. Cumulant measures will detect common input even if there is no frequency structure in the common input, e.g. it is Poisson. In contrast, coherence is highly sensitive to oscillations and in the case of a Poisson distribution of common input interspike intervals the coherence is flat (Farmer et al. 1993). These considerations lead us to suggest that common inputs to pools of spinal motoneurones, as evidenced by significant peaks in the EMG–EMG cross-correlation/cumulant, are present in children of 9 years and younger, but they are not transmitting oscillatory synchrony.
The change in pooled coherence magnitude between age ranges 7–9 and 12–14 years can be understood in basic neurophysiological terms. EMG–EMG coherence has been shown to reflect oscillatory synchronization between common inputs to spinal motoneurones (Farmer et al. 1993) and originates from synchronous oscillations within networks of corticospinal neurons that are then transmitted to spinal motoneurones via fast-conducting corticospinal pathways (Conway et al. 1995; Salenius et al. 1997; Baker et al. 1997; Halliday et al. 1998; Brown et al. 1998; Mima et al. 1999; Farmer et al. 2004). Thus we conclude that the emergence of EMG–EMG coherence in the 12–14 years age group reflects a change in the oscillatory corticospinal common input to the motoneurone pools of short and long thumb abductors.
General considerations
There is strong evidence that ∼20 Hz coherence and motor unit synchrony reflect important common drive that leads to coactivation of closely related muscles. For example, in conditions such as pathological obligatory mirror movements there is strong abnormal coherence and synchrony between homologous left and right hand muscles, suggesting that it is this common drive that is responsible for obligatory coactivation of EMGs (Mayston et al. 2001; Farmer et al. 2004). Whether in children improvements in synergies involving different digits are reflected in changes in coherence and synchrony between muscles needs further study, as do the potential effects of motor learning in children on motor synergies, synchrony and coherence.
There are numerous reports documenting changes in manual dexterity with age (Denckla, 1973). Of particular relevance to the present study is that of Gibbs et al. (1997). These authors demonstrated that the speed of repetitive finger movements increases with chronological age and that this is associated with an increase in the magnitude of motor unit synchrony, between the short and long thumb abductor muscles, up to 12 years of age. Evidence suggests that maturational functional developments are associated with changes in the corticospinal system. Anatomical evidence suggests that monosynaptic corticospinal projections are established prenatally, and that activity in them may play a part in shaping motor pathway connectivity during motor development (Eyre et al. 2000). Conduction delays following transcutaneous magnetic stimulation (TMS) stimulation of the motor cortex decrease between 34 weeks gestation and 2 years, at which point they reach adult values, suggesting that myelination of the corticospinal tract is complete by age 2 years (Eyre et al. 1991). However, the threshold for evoking a hand muscle response to TMS is high in young subjects; it reduces to adult values by age 16 years (Eyre et al. 1991). Thus the corticospinal tract is present and rapidly conducts externally applied impulses at an early age although it becomes easier to excite using TMS with increasing age. But the normal physiological behaviour of these pathways during voluntary muscle activation is not accessed with this technique. During motor development it is possible that the patterning of the corticospinal drive to spinal motoneurones may change as a result of alterations in central connectivity and physiology. The present study provides evidence for this hypothesis. In this study, we did not explicitly examine the relationship between speed of fine finger movements and coherence. However, from the Gibbs et al. (1997) study we know that the older subjects had the fastest and most accurate independent fine finger movements. It has been postulated on the basis of theoretical modelling that a ∼20 Hz common oscillatory drive provides a more efficient mechanism for motor unit recruitment than that provided by non-oscillatory drive (Baker et al. 1999). We hypothesize that the emergence of a central ∼20 Hz oscillatory drive to motoneurone pools during development is associated with more efficient motor unit recruitment and increased speed and accuracy of performance of a motor task.
The relatively late emergence of oscillatory drive, i.e. at 12–14 years, suggests maturational changes continue into the early teenage years. There are a number of studies that demonstrate changes in brain structure through to adolescence (see Paus, 2005 for review). Structural MRI studies, using longitudinal data, have revealed increases in grey matter volume in the parietal and frontal lobes of children up to the age of 12 years followed by a steep reduction of grey matter density beginning in the sensorimotor areas around puberty (Gogtay et al. 2004). It is not known whether this ‘loss’ of grey matter reflects activity-dependent pruning of neurons based on Hebbian principals. Changes in central white matter pathway connectivity may also occur around the same age. Whilst sexual maturity was not recorded in the present study it is striking that EMG–EMG coherence emerges between the 7–9 and 12–14 years age groups. Further studies are required to investigate the fine structure of the emergence of EMG–EMG coherence and its relation to function and sexual maturity.
On the basis of modelling studies it has been proposed that the emergence of oscillatory synchronization is associated with inhibition within neural networks (see Pauluis et al. 1999). Animal data show that GABA-mediated inhibition changes during neural development and may influence the cortical network structure. For example, in the visual system the normal development of the columnar architecture of the neocortex depends on correlated neural activity (circuits that fire together wire together) and this early experience-dependent plasticity may be disrupted through interference with GABA-mediated neural transmission (Hensch & Stryker, 2004 and Fagiolini et al. 2004). A developmental change in frequency of oscillations can be seen in mouse medullary preparations with the increase in frequency (17 Hz to 38 Hz) being dependent on the time course of synaptic GABAergic currents (Sebe et al. 2006). In man, the GABA agonist diazepam increases EEG power in the beta range (20 Hz); interestingly this was not reflected in a corresponding increase in EEG–EMG coherence suggesting that the phase coupling between the EEG and EMG signals can be dissociated from power increases (Baker & Baker, 2003). Indirect evidence for the role of GABA during maturational changes of the motor cortex in the human comes from a recent TMS study, which showed that intracortical inhibition is lower in children (Mall et al. 2004). The most striking differences in intracortical inhibition in this study were between children (median age 8 years) and adolescents (median age 15 years) with less marked differences between adolescents and adults.
In conclusion, we have shown that synchronous activity to motoneurone pools, indicating the presence of a common synaptic drive, can be detected at 4 years of age, but this drive does not become oscillatory until the early teenage years. Given that the emergence of neural oscillations in animals has a developmental profile that may depend on activity in inhibitory circuits and that EMG–EMG ∼20 Hz coherence is dependent on an oscillatory synchronizing corticospinal drive to motoneurones; then the late emergence of ∼20 Hz EMG–EMG coherence in humans, shown for the first time in the present study, may reflect an important developmental change in the central nervous system with implications for central nervous system plasticity. We propose that this age-dependent ‘fixing’ of the frequency of rhythmic drive to motoneurones may underlie important reductions in the capacity to learn new motor skills as adulthood is reached.
References
- Amjad AM, Halliday D, Rosenberg JR, Conway BA. An extended difference of coherence test for comparing and combining several independent coherence estimates: theory and application to the study of motor units and physiological tremor. J Neurosci Methods. 1997;73:69–79. doi: 10.1016/s0165-0270(96)02214-5. [DOI] [PubMed] [Google Scholar]
- Baker MR, Baker SN. The effect of diazepam on motor cortical oscillations and corticomuscular coherence studied in man. J Physiol. 2003;546:931–942. doi: 10.1113/jphysiol.2002.029553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SN, Kilner JM, Pinches EM, Lemon RN. The role of synchrony and oscillations in the motor output. Exp Brain Res. 1999;128:109–117. doi: 10.1007/s002210050825. [DOI] [PubMed] [Google Scholar]
- Baker SN, Olivier E, Lemon RN. Coherent oscillations in monkey motor cortex and hand muscle EMG show task-dependent modulation. J Physiol. 1997;501:225–241. doi: 10.1111/j.1469-7793.1997.225bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P, Salenius S, Rothwell JC, Hari R. Cortical correlate of the Piper rhythm in humans. J Neurophysiol. 1998;80:2911–2917. doi: 10.1152/jn.1998.80.6.2911. [DOI] [PubMed] [Google Scholar]
- Clarke AR, Barry RJ, McCarthy R, Selikowitz M. Age and sex effects in the EEG: development of the normal child. Clin Neurophysiol. 2001;112:806–814. doi: 10.1016/s1388-2457(01)00488-6. [DOI] [PubMed] [Google Scholar]
- Conway BA, Halliday DM, Farmer SF, Shahani U, Maas P, Weir AI, Rosenberg JR. Synchronization between motor cortex and spinal motoneuronal pool during the performance of a maintained motor task in man. J Physiol. 1995;489:917–924. doi: 10.1113/jphysiol.1995.sp021104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denckla MB. Development of speed in repetitive and successive finger movements in normal children. Dev Med Child Neurol. 1973;15:635–645. doi: 10.1111/j.1469-8749.1973.tb05174.x. [DOI] [PubMed] [Google Scholar]
- Eyre JA, Miller S, Clowry GJ, Conway EA, Watts C. Functional corticospinal projections are established prenatally in the human foetus permitting involvement in the development of spinal motor centres. Brain. 2000;123:51–64. doi: 10.1093/brain/123.1.51. [DOI] [PubMed] [Google Scholar]
- Eyre JA, Miller S, Ramesh V. Constancy of central conduction delays during development in man: investigation of motor and somatosensory pathways. J Physiol. 1991;434:441–452. doi: 10.1113/jphysiol.1991.sp018479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolini M, Fritschy JM, Low K, Mohler H, Rudolph U, Hensch TK. Specific GABAA circuits for visual cortical plasticity. Science. 2004;303:1681–1683. doi: 10.1126/science.1091032. [DOI] [PubMed] [Google Scholar]
- Farmer SF, Bremner FD, Halliday DM, Rosenberg JR, Stephens JA. The frequency content of common synaptic inputs to motoneurones studied during voluntary isometric contraction in man. J Physiol. 1993;470:127–155. doi: 10.1113/jphysiol.1993.sp019851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer SF, Harrison LM, Mayston MJ, Parekh A, James LM, Stephens JA. Abnormal cortex–muscle interactions in subjects with X-linked Kallmann's syndrome and mirror movements. Brain. 2004;127:385–397. doi: 10.1093/brain/awh047. [DOI] [PubMed] [Google Scholar]
- Fuglevand AJ, Winter DA, Patla AE, Sashuk D. Detection of motor unit potentials with surface electrodes: influence of electrode size and spacing. Biol Cybern. 1992;67:143–153. doi: 10.1007/BF00201021. [DOI] [PubMed] [Google Scholar]
- Gasser T, Verleger P, Bacher P, Sroka L. Development of the EEG of school age children and adolescents. I. Analysis of band power. Electroencephogr Clin Neurophysiol. 1988;69:91–99. doi: 10.1016/0013-4694(88)90204-0. [DOI] [PubMed] [Google Scholar]
- Gibbs J, Harrison LM, Stephens JA. Cross-correlation analysis of motor unit activity recorded from seperate thumb muscles in man. J Physiol. 1997;499:255–266. doi: 10.1113/jphysiol.1997.sp021924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, III, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday DM, Conway BA, Farmer SF, Rosenberg JR. Using electroencephalography to study functional coupling between cortical activity and electromyograms during voluntary contractions in humans. Neurosci Lett. 1998;241:5–8. doi: 10.1016/s0304-3940(97)00964-6. [DOI] [PubMed] [Google Scholar]
- Halliday DM, Rosenberg JR. On the application, estimation and interpretation of coherence and pooled coherence. J Neurosci Methods. 2000;100:173–174. doi: 10.1016/s0165-0270(00)00267-3. [DOI] [PubMed] [Google Scholar]
- Halliday DM, Rosenberg JR, Amjad AM, Breeze P, Conway BA, Farmer SF. A framework for the analysis of mixed time series/point process data – theory and application to the study of physiological tremor, single motor unit discharges and electromyograms. Prog Biophys Mol Biol. 1995;64:237–278. doi: 10.1016/s0079-6107(96)00009-0. [DOI] [PubMed] [Google Scholar]
- Hensch TK, Stryker MP. Columnar architecture sculpted by GABA circuits in developing cat visual cortex. Science. 2004;303:1678–1681. doi: 10.1126/science.1091031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner JM, Baker SN, Salenius S, Jousmaki V, Hari R, Lemon RN. Task-dependent modulation of 15–30 Hz coherence between rectified EMGs from human hand and forearm muscles. J Physiol. 1999;516:559–570. doi: 10.1111/j.1469-7793.1999.0559v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mall V, Berweck S, Feitzek UM, Glocker FX, Oberhuber U, Walther M, Schessl J, Schulte-Monting J, Korinthenberg R, Heinen F. Low level of intracortical inhibition in children by transcortical magnetic stimulation. Neuropediatrics. 2004;35:120–125. doi: 10.1055/s-2004-815834. [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Bar-Haim Y, Fox NA. Development of the EEG from 5 months to 4 years of age. Clin Neurophysiol. 2002;113:1199–1208. doi: 10.1016/s1388-2457(02)00163-3. [DOI] [PubMed] [Google Scholar]
- Mayston MJ, Harrison LM, Stephens JA, Farmer SF. Physiological tremor in human subjects with X-linked Kallmann's syndrome and mirror movements. J Physiol. 2001;530:551–563. doi: 10.1111/j.1469-7793.2001.0551k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mima T, Hallett M. Electroencephalographic analysis of cortico-muscular coherence: reference effect, volume conduction and generator mechanism. Clin Neurophysiol. 1999;110:1892–1899. doi: 10.1016/s1388-2457(99)00238-2. [DOI] [PubMed] [Google Scholar]
- Myers LJ, Lowery M, O'Malley M, Vaughan CL, Heneghan C, St Clair GA, Harley YX, Sreenivasan R. Rectification and non-linear pre-processing of EMG signals for cortico-muscular analysis. J Neurosci Methods. 2003;124:157–165. doi: 10.1016/s0165-0270(03)00004-9. [DOI] [PubMed] [Google Scholar]
- Pauluis Q, Baker SN, Olivier E. Emergent oscillations in a realistic network: the role of inhibition and the effect of the spatiotemporal distribution of the input. J Comput Neurosci. 1999;6:289–310. [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Perkel DH, Gerstein GL, Moore GP. Neuronal spike trains and stochastic point processes. II. Simultaneous spike trains. Biophys J. 1967;7:419–440. doi: 10.1016/S0006-3495(67)86597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg JR, Halliday DM, Breeze P, Conway BA. Identification of patterns of neuronal connectivity – partial spectra, partial coherence, and neuronal interactions. J Neurosci Methods. 1998;83:57–72. doi: 10.1016/s0165-0270(98)00061-2. [DOI] [PubMed] [Google Scholar]
- Salenius S, Portin K, Kajola M, Salmelin R, Hari R. Cortical control of human motoneuron firing during isometric contraction. J Neurophysiol. 1997;77:3401–3405. doi: 10.1152/jn.1997.77.6.3401. [DOI] [PubMed] [Google Scholar]
- Sebe JY, van Brederode JF, Berger AJ. Inhibitory synaptic transmission governs inspiratory motoneuron synchronization. J Neurophysiol. 2006;96:391–403. doi: 10.1152/jn.00086.2006. [DOI] [PubMed] [Google Scholar]