Abstract
The spinal cord is able to express use-dependent plasticity, as demonstrated in spinalized cats following treadmill training. In humans, spinal use-dependent plasticity is inferred from modifications in the size of H reflex, which are often more prominent after skilled motor training. Plasticity can develop at synaptic connections between afferent fibres and/or descending tracts and motoneurones or interneurones interposed in the spinal pathways. Here we explore whether skilled training induces a change in synaptic efficacy at the synapse between Ia afferents and soleus (Sol) motoneurones. Synaptic efficacy can be modulated presynaptically through changes of the probability of transmitter release (homosynaptic depression, HD). The frequency-related depression of the Sol H reflex, thought to reflect HD, was tested at rest, before and after one single skilled (14 subjects) or non-skilled (9 subjects) cycling training session. Performance improved in both groups but to a larger extent with skilled training, while HD increased immediately after and the day following skilled training in the absence of changes with non-skilled training. These results support the view that spinal cord function is able to encode a local motor memory.
Activity-dependent plasticity associated with performance improvements takes place at multiple sites in the central nervous system, including the spinal cord (Wolpaw & Tennissen, 2001). Cortical plasticity has been extensively documented during motor learning (Sanes & Donoghue, 2000), and the traditional view has been that most of the lasting changes in spinal cord function accompanying motor learning are driven by changes in descending inputs to spinal motor and interneurones (Schneider & Capaday, 2003; Perez et al. 2005). There is now growing evidence that the spinal cord is able to express use-dependent plasticity, as demonstrated in spinalized cats following treadmill training (de Leon et al. 1998). Functional improvements depend on the nature of the training regimens (de Leon et al. 1998). The timing of somatosensory input during training plays a prominent role in the development of plastic changes (Bouyer et al. 2001), as shown in the isolated spinal cord of cats, rats and dogs (Mendell & Wall, 1965; Kandel, 1977; Durkovic, 1985; Liu & Sandkuhler, 1997). Plasticity in the nervous system can be achieved through sprouting of new synapses or unmasking of silent ones, but also through activity-dependent changes in the efficacy of the synaptic transmission occurring either presynaptically (Zalutsky & Nicoll, 1990) or postsynaptically (Malinow & Malenka, 2002). On the presynaptic side, synaptic efficacy can be adapted through activity-dependent changes of the probability of transmitter release (short-term facilitation, homosynaptic depression (HD), post-tetanic facilitation; Kitago et al. 2004).
In humans, the existence of spinal use-dependent plasticity has been inferred from modifications in the size of H reflexes (soleus (Sol) or wrist flexors H reflexes (Nielsen et al. 1993; Voigt et al. 1998; Trimble & Harp, 1998; Kato et al. 2003; Motl et al. 2003). Motor skill training, as opposed to non-skilled or passive motor training, appears to be a critical factor in driving plastic reorganization at the spinal level and in the motor cortex (Perez et al. 2005, 2006). Consistent with these studies, Mazzocchio et al. (2006) have shown that skilful establishment of a constant cycling speed despite changing pedal resistance was associated with a persistent downregulation of the Sol H reflex which was absent after cycling at the same speed without change in pedal resistance.
Changes in H reflexes can be the consequence of modifications in synaptic connections between afferent fibres and/or descending tracts with motoneurones and interneurones interposed in the spinal pathways. The purpose of the present study was to determine whether encoding of a motor memory in spinal cord function relies on changes in the synaptic efficacy between Ia afferent fibres and motoneurones. We used the cycling task developed by Mazzocchio et al. (2006) and assessed HD at the synapse between Ia afferents and Sol motoneurones at rest, before and immediately after or later after one single cycling training session. We were interested in finding whether training-induced changes of synaptic efficacy could be expressed as persistent changes in HD at rest. To address the role of motor learning versus motor training in the development of changes in HD, we compared changes in HD after a complex and a simple cycling training task, as Mazzocchio et al. (2006) did for the Sol H reflex. To assess HD we used the frequency-related depression of the Sol H reflex (Kohn et al. 1997; Hultborn & Nielsen, 1998; Aymard et al. 2000). This method was designed from animal experiments. A frequency-related depression of the monosynaptic reflex discharge has been described in cats (Brooks et al. 1950) and ascribed (using intracellular recordings) to HD because the reduction of the monosynaptic Ia EPSP occurred without change in membrane potential or conductance (Hultborn et al. 1996). Homosynaptic depression results from changes in the probability of transmitter release at the synapse (Kuno, 1964) and depends on the preceding activity of Ia afferents. Homosynaptic depression is thought to minimize afferent input from stretch receptors during sustained or repeated muscle stretch and to concentrate the damping effect on lower threshold motoneurones (Floeter & Kohn, 1997).
Methods
Participants
The experiments were performed on 23 healthy volunteers. All subjects gave their written informed consent to the experimental procedure according to the Declaration of Helsinki, and the NINDS Institutional Review Board. The subjects were randomly allocated to two different motor training groups. One group (n= 14, 6 women, 8 men) was trained with a ‘complex’ visuo-motor cycling task (group C) and the other (n= 9, 5 women, 4 men) with a ‘simple’ visuo-motor cycling task (group S). The two groups were matched for age (group C, mean age, 33.2 ± 10 years; group S, mean age, 28.4 ± 5.6 years; Mann–Whitney U test P= 0.3) and for usual physical activity. To rate their usual physical activity, subjects filled out a questionnaire. They were asked how many times a week they trained and what kind of training they practiced (jogging, cycling, training with elliptical machine or treadmill). Subjects performed mild physical activities on average 2.3 ± 1.8 days (range 0–4) a week in group S and 2.07 ± 1.6 days (range 0–5) in group C (Mann–Whitney U test P= 0.5). Subjects were grouped as ‘sedentary’ or ‘active’ according to the frequency of exercising (less or more than twice a week). In group C, seven subjects were active and seven sedentary. In group S, five subjects were active and four sedentary. In addition, we used the mean heart rate at rest and during cycling to compute, for each subject, an objective measure of the difficulty of the task. Mean value of this ‘hardness coefficient’ was comparable in the two groups (0.4 ± 0.13 in group S; 0.35 ± 0.14 in group C; Mann–Whitney U test P= 0.5). Subjects were divided into two groups (easy versus difficult) according to the hardness coefficient (below or above 0.3). Results of one subject in group C were discarded from the H reflex analysis because it was impossible to keep a stable size of the H reflex at the 9 s interstimulus interval (ISI) throughout the experiment (see below).
Experimental schedule
All subjects participated in at least three experimental sessions. During the first session, they initially received baseline pretraining assessment of HD. Subsequently, they were tested on their ability to establish a constant cycling speed on a recumbent bicycle despite frequent changes in pedal resistance. The coefficient of variation of speed (CVspeed; Schabort et al. 1998) measured their ability to perform this task. Previous work demonstrated downregulation of the Sol H reflex after complex but not simple training (Perez et al. 2005; Mazzocchio et al. 2006). Our working hypothesis was therefore that HD increases (and may participate in a decrease of the Sol H reflex size) after complex training (establishment of a constant cycling speed on a recumbent bicycle exposed to frequent changes in pedal resistance, which requires calibrated compensatory action), but not after simple training (maintenance of a constant cycling speed against constant pedal resistance, which requires less compensatory action). These two types of training engage comparable physical activity, but different levels of skill acquisition during cycling.
After baseline determinations, subjects trained for 16 min (with either of the two tasks; see training section below). Immediately post-training, the day after (day 2) and 48 h later (day 4), HD and CVspeed were tested again.
Eight out of the 14 subjects (group C7) who had performed the complex task, came for a fourth visit a week after the training (day 7). Seven out of the 14 subjects (group C30) who had performed the complex task (not all the same as those who came on day 7) came for a follow-up fifth visit 30–45 days after the training. During visits on days 7 and 30, subjects underwent physiological recordings and measurement of CVspeed.
Training
Each subject participated in a single training session. The subjects sat on a recumbent cycle ergometer (Cateye EC 3700, VacuMed, Inc., Ventura, CA, USA). The subject was asked to cycle for 16 min at a constant speed (60 r.p.m.). The cycle display provided continuous feedback of instantaneous speed. In the complex training session, pedal resistance (range 0.5–1.6 kg) changed every 15 s in a preprogrammed sequence. In the simple training session, pedal resistance was fixed at the mean overall torque level (0.9 kg) of the complex training session.
Performance
Performance was assessed by the subject's ability to maintain a constant speed of 60 r.p.m. during a 4 min cycling session in the presence of variable pedal resistance. Performance was quantified by the coefficient of variation of speed (CVspeed). The coefficient of variation is defined by Schabort et al. (1998) as the within-subject variation, expressed as a percentage of the subject's mean (CV =s.d./mean). The CVspeed was calculated before and immediately after training, and also at day 2, 4, 7 and 30 in the absence of any further training.
Task difficulty
Heart rate was continuously monitored during each measurement of CVspeed and during the training session using an earlobe sensor. The hardness coefficient was defined as:
 |
Heart rate in exercise was defined as the mean heart rate during the 4 min cycling preceding the training session (calculation of baseline performance). Maximum heart rate was calculated by the standard formula (220 minus age; Karvonen et al. 1957; American College of Sports Medicine Position Stand, 1998).
Physiological recordings
Soleus H reflex
Elecromyogram was recorded from the right Sol muscle through a pair of surface electrodes. The recording electrode was on the mid-line of the calf, 4 cm below the inferior margin of the gastrocnemius muscle, and the reference electrode was over the Achilles tendon. The signal was amplified and filtered (5 Hz to 1 kHz) by a Dantec EMG machine, and then digitized at 4 kHz by a PC-based data acquisition and processing system (Labview, National Instruments, Austin, TX, USA). Electrical stimulation (pulse width, 1 ms) to evoke a Sol H reflex was delivered to the posterior tibial nerve with the cathode in the popliteal fossa and the anode over the patella, from a Grass S88 stimulator (Grass Instruments). Physiological recordings started by calculating the amplitude of the Sol maximal M wave (Mmax) after a supramaximal stimulation of the posterior tibial nerve. Peak-to-peak amplitudes of the H reflexes were measured off-line and expressed as a percentage of the Mmax amplitude.
Homosynaptic depression (HD) measurements
The experimental set-up was based on that developed by Crone & Nielsen (1989) and Aymard et al. (2000). Homosynaptic depression at the level of the synapse between Sol Ia afferents and motoneurones was assessed by comparing the effects of different stimulus rates on H reflex size. Here we used ISIs of 9, 8, 6, 3 and 1 s. At the beginning of the pretraining physiological recordings, the intensity of the stimulation was adjusted so that the amplitude of the Sol H reflex at the 9 s ISI (HD is considered to be absent or minimal at this frequency) was between 10 and 30% of Mmax. At the beginning of the following recordings (post-training, day 2, 4, 7 and 30), the intensity of the stimulation was carefully adjusted so that the size of the H reflex at the 9 s ISI was the same as that during the pretraining recordings. It was verified throughout the experiment that the amplitude of the reflex at the 9 s ISI remained constant. If this amplitude changed, the intensity of the stimulation was adjusted. After averaging 20 reflexes using a 9 s ISI, four other series of 20 reflexes were obtained, using in each of them a different ISI (1, 3, 6 and 8 s). The sequence of stimulus rates randomly varied.
A curve of the size of the Sol H reflex against the ISIs (1, 3, 6 and 8 s) was drawn (size–ISI curve). The mean size of the 20 reflexes obtained at each ISI tested was expressed as a percentage of the mean size of the 20 reflexes obtained at the 9 s ISI.
To ascribe a unique value to each size–ISI curve, we also calculated the area under each curve between 1 and 8 s on the abscissa (AUC).
Calculation of AUC
Each curve used to calculate AUC had four points. Abscissas of these points were always 1, 3, 6 and 8 s ISIs, and the corresponding ordinates were the mean size (n= 20) of the Sol H reflex at 1 (y1), 3 (y3) 6 (y6) and 8 s ISIs (y8). The curve fitting these points was described by the function P:
with:
The area (A) between the curve and the two vertical lines with x= 1 and x= 8 as abscissas was calculated as follows:
Control experiments
To eliminate a confounding effect of a training-induced change inside the Sol motoneuronal pool (‘recruitment gain’; see Discussion), recruitment curves of the Sol M wave and H reflex were built up before and after training. To that end, the intensity of the posterior tibial nerve stimulation was increased by steps of 0.1MT, from 0.5 to 1.5–2.0MT (MT = intensity evoking a Sol M wave of 5% of Mmax). Fifteen reflexes were evoked at each intensity.
To be sure that we compared over the time the H reflexes elicited by equivalent afferent volleys, the recruitment curves of M waves were exactly matched between pre- and post-training as well as at days 2, 4 and 7. The steepest part of the ascending limb of the recruitment curve was fitted to a linear regression function. The slope of the fitted line was taken as witness of the ‘recruitment gain’ (Kernell & Hultborn, 1990). If training induced any damaging effect on Sol muscle fibres or a change in the neuromuscular transmission, it would mean that M waves of same size would not reflect activation of a same number of motor units. To eliminate such a bias, we recorded, in parallel with Sol M wave and H reflex, the M wave (and H reflex when it existed) of plantar foot muscles (mainly flexor digitorum brevis), which was considered to be ‘non-trained’ muscle but is also innervated by the posterior tibial nerve.
The recruitment curves were built up in eight different experiments: (1) in four subjects, the curves were constructed in parallel with two different ISIs (1 s, where the HD is prominent, and 12 s, where the HD is absent) pre- and post-training, and at days 2 and 4 (also day 7 in 2 subjects); (2) in two subjects, the curves were built while evoking the reflexes at the 10 s ISI (a frequency at which HD is absent or minimal) pre- and post-training, and at day 2; (3) in two subjects, the curves were built up while evoking the reflex at 4 s ISI only pre- and post-training.
Statistics
The effect of training on CVspeed and on AUC was assessed using a repeated-measures ANOVA with the four measures of CVspeed and AUC (time: pre- and post-training, days 2 and 4) forming the repeats. A fifth measure was added (day 7 or 30) when looking at the duration of the effects. The effect of training on frequency-related depression of the H reflex was assessed by a two-level repeated ANOVA with the 16 sizes of the Sol H reflex forming the repeats (four different ISIs: 1, 3, 6 and 8 s; and four different times: pretraining, immediate post-training, days 2 and 4).
To compare the effect of simple versus complex training on changes in CVspeed and AUC we performed a two-group repeated-measures ANOVA with the three measures of the normalized CVspeed or normalized AUC (time: post-/pretraining, day 2/pretraining, day 4/pretraining) forming the repeats and assignment to S or C training forming the two groups.
The effect of physical activity on changes in CVspeed and AUC was tested separately in group C and group S by using a two-group factorial ANOVA (assignment to ‘sedentary’ or ‘active’ forming the 2 groups). For this analysis, the three values of the normalized CVspeed or normalized AUC (post-/pretraining, day 2/pretraining, day 4/pretraining) were grouped, forming a unique variable. Fisher's test was used for the post hoc analysis.
Age, number of training days a week (regular physical activity), coefficient of hardness and baseline CVspeed were compared between the group C and the group S using a non-parametric Mann–Whitney U test.
Results
Influence of training type on motor performance (Fig. 1)
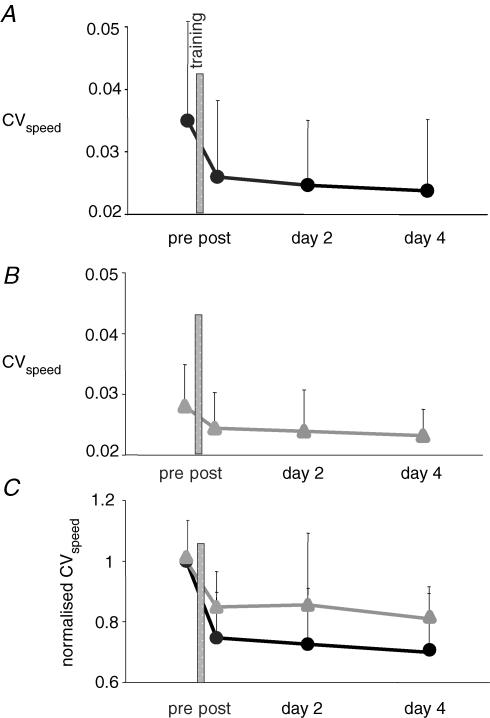
Figure 1. Improvement of performance after training.
Performance was assessed by the CVspeed. In A and B, CVspeed is plotted against time, pretraining, immediately post-training, 24 and 96 h after training. In A, subjects were trained with the complex cycling task. Each dot represents the mean of 14 values. In B, subjects were trained with the simple cycling task. Each triangle represents the mean of 9 values. In C, the normalized value of CVspeed (CVspeed/pretraining CVspeed) is plotted against time. Improvement of performance was better after complex training (•) than after simple training (▴).
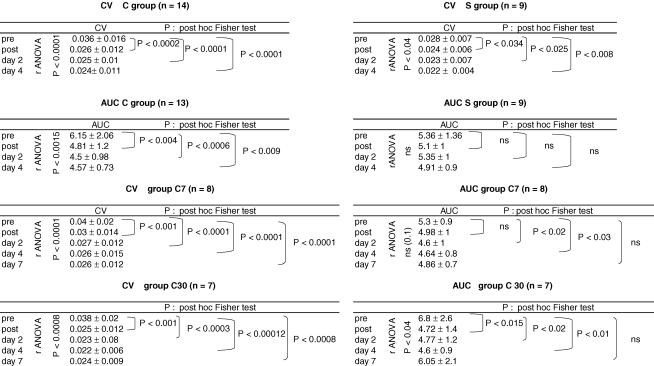
The two groups (C and S) had comparable mean baseline performance levels (mean ±s.d.; CVspeed= 0.036 ± 0.016 in group C and 0.028 ± 0.007 in group S; Mann–Whitney U test P= 0.2). In both groups, performance improved significantly over time. The CVspeed decreased by 24% in group C immediately after training (Fig. 1A and Table 1) and by 15% in group S (Fig. 1B and Table 1). Performance improved slightly in the absence of further training between post-training and day 2 and between days 2 and 4 (Fig. 1A and B and Table 1). A repeated-measure ANOVA was performed with the type of the training (complex versus simple) as interindividual variable. This analysis was performed after normalizing the CVspeed values to control for differences in mean baseline performance between the two groups. Improvement of performance was larger in group C than in group S (Fig. 1C) but the difference did not reach statistical significance (P= 0.06).
Table 1.
Effect of time on performance (CV) and HD (AUC)
Influence of training type on HD (Fig. 2)
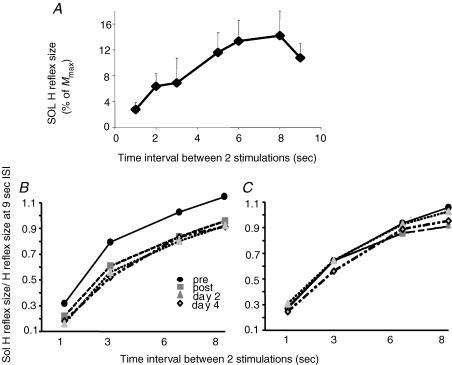
Figure 2.
Changes in homosynaptic depression of the soleus H reflex A, homosynaptic depression in one representative subject. Homosynaptic depression was assessed by the frequency-related depression of the Sol H reflex, as the decrease of size of the H reflex when decreasing the time interval between two consecutive stimulations (here from 9 to 1 s). In this subject, the curve reached a plateau at 8 s ISI. Each point represents the mean of 20 reflexes. B and C, modulation of homosynaptic depression of the Sol H reflex by the training in the two groups: subjects performing the complex cycling task (B); and subjects performing the simple cycling task (C). For each time (pre- and post-training, 24 and 96 h after training), sizes of the Sol H reflexes were normalized to the size of the reflex at the 9 s ISI. To compare the amount of HD at the different times, the intensity of the stimulation was adjusted in order that the size of the reflex at the 9 s ISI was the same during the three post-training measurements as during the pretraining one. B, after complex training, HD was increased and maintained the same level at days 2 and 4. Each point represents the mean of 260 reflexes. C, after simple training, HD was not modified. Each point represents the mean of 180 reflexes.
Baseline HD (Fig. 2A)
In the baseline condition (22 subjects) Sol H reflex size exhibited a frequency-related depression (ANOVA effect of ISI P < 0.0001, Fisher's post hoc test 1 s/3 s P < 0.0001, 3 s/6 s P < 0.0001, 6 s/8 s n.s.). A typical example is shown in Fig. 2A. In this subject, the mean size (n= 20) of the Sol H reflex was adjusted to be around 10% of Mmax at the 9 s ISI. Sol H reflex size was maximal (around 13% of Mmax) when the interval between two consecutive reflexes (ISI) was 8 s. As ISI decreased from 8 to 1 s, Sol H reflex size dropped progressively to 2.8% of Mmax at the 1 s ISI. Out of the 22 subjects tested for HD, the curve reached a plateau at the 8 s ISI in 15 of them, at the 6 s ISI in one, and had not yet reached its plateau at the 9 s ISI in the six remaining subjects.
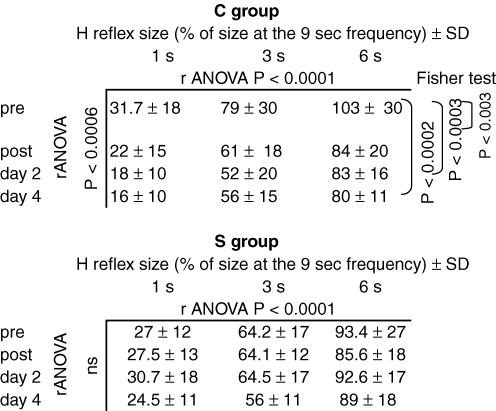
Effect of training on HD (Fig. 2B and C)
Training with the complex task had a significant effect on HD (Fig. 2B and Table 2). Immediately after training, HD increased, which means that at a given interval of stimulation the mean size of the reflex was smaller after the training than before, and it was true at all tested ISIs. The most striking result of this study was the unexpectedly long duration of the training-induced increase of HD, in that 24 h after the training (day 2) but also 3 days after (day 4) HD was still increased, keeping about the same level as immediately after training (see the statistical significance of Fisher's post hoc test in Table 2).
Table 2.
Effect of stimulation frequency and time on Sol H reflex size
Results were similar when using the AUC to assess HD (see Table 1 and Fig. 3B and D).
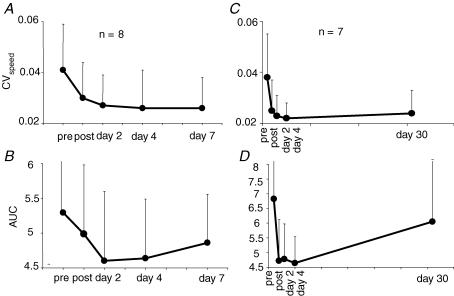
Figure 3.
Comparison of the time courses of performance and HD after a bout of complex cycle training A and C, time courses of the performance (assessed by the CVspeed). B and D, time courses of the HD (assessed by the AUC, area under the curve size/ISI). The results of two different groups of subjects are presented in A and B and in C and D, respectively. A and B, CVspeed was still improved 6 days after a single bout of complex cycle training while the AUC was still decreased, indicating an increase in HD. C and D, CVspeed was still improved 30 days after a single bout of complex cycle training while the AUC was almost back to its pretraining control value. In A and B, each dot represents the mean of 8 values. In C and D, each dot represents the mean of 7 values.
Training with the simple task did not modify HD (Fig. 2C and Table 2), either immediately after training or in the following days. Results were the same using either the size–ISI curves (Table 2) or the AUC (Table 1).
After normalizing the AUC values to the AUC pretraining value, the effect of the complex training on AUC was compared with that of the simple training using an ANOVA with training as interindividual variable. The effect of complex training was significantly different from that of the simple training (ANOVA: effect of training on AUC P < 0.05).
Time course of modifications of CV and HD (Fig. 3)
Eight out of the 14 subjects trained with the complex task came for an additional experiment a week after the training, and seven out of the 14 came for a last session 30–45 days after the training. Statistical analysis was performed for each group (groups C7 and C30) separately.
In groups C7 and C30, as in the whole group, CVspeed and AUC dropped immediately after training and slightly decreased further at days 2 and 4 (Fig. 3A–D and Table 1). In group C7, at day 7 performance was still better than before training and not significantly different from that at day 4 (Fig. 3A and Table 1). Homosynaptic depression was still increased at day 7 compared with the baseline level, yet the difference did not reach statistical significance (Fig. 3B). In group C30, at day 30, performance was still improved compared with the baseline level, almost the same as at day 4 (Fig. 3C and Table 1) but there was then a clear dissociation between the decreased CVspeed and the AUC, which was almost back to its baseline level (Fig. 3D and Table 1).
Effect of fitness level and hardness of the task on change in performance and HD (Fig. 4)
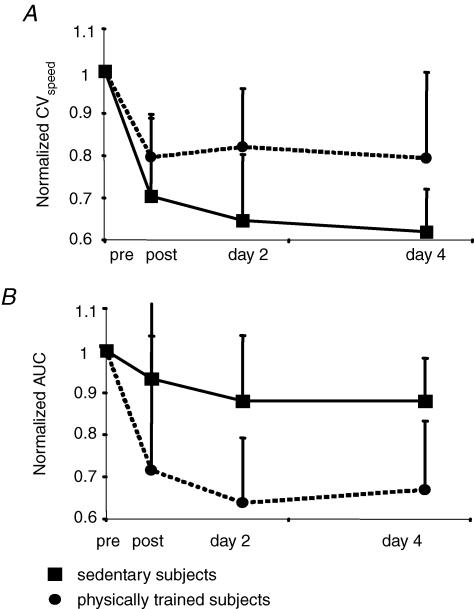
Figure 4.
Comparison of changes in performance and HD modulation between sedentary versus physically trained subjects after a bout of complex cycle training A, changes in performance were assessed by the normalized CVspeed (CVspeed/pretraining CVspeed). B, changes in HD were assessed by the normalized area under the curve (AUC/pretraining AUC). Sedentary subjects (▪) improved their performance better than physically trained ones (•). In contrast, HD was less increased in the sedentary group than in the physically trained one. In A and B, each dot represents the mean of 6 values and each square represents the mean of 7 values.
Baseline performance
Performance measured in the baseline condition (23 subjects) differed according to the fitness level, in that active subjects (n= 13, CVspeed= 0.027 ± 0.006) performed better than sedentary ones (n= 10, CVspeed= 0.04 ± 0.02; Mann–Whitney U test, P < 0.007). Performance was not influenced by the difficulty of the task by itself (easy, n= 9, CVspeed= 0.03 ± 0.007; difficult, n= 14, CVspeed= 0.035 ± 0.016; Mann–Whitney U test, P = 0.6).
Effect of complex training on performance improvement and HD (Fig. 4)
The fitness level significantly influenced the training-induced changes in performance and HD. Immediately after the complex training but also at days 2 and 4, active subjects improved their performance less than sedentary subjects (Fig. 4A; ANOVA effect of activity on CVspeed post-/pretraining + day 2/pretraining + day 4/pretraining, P= 0.007).
Fitness level also influenced the modulation of HD after training but in the opposite way, in that normalized AUC decreased more in active than in sedentary subjects (Fig. 4B; ANOVA effect of activity on AUC post-/pretraining + day 2/pretraining + day 4/pretraining, P < 0.005). This means that, despite a better improvement of performance, HD increased less in the sedentary subjects than in the physically trained ones.
Control experiments: assessment of the gain in the soleus motoneurone pool
The recruitment curves of the Sol M wave and H reflex (ISI 12 s) of a representative subject are presented in Fig. 5A and B. Only the ascending part of the H reflex recruitment curve is presented because, in this part, motoneurones are recruited in an orderly sequence by the Ia input from the smallest to the largest according to Henneman's size principle (Henneman & Mendell, 1981). In the descending part, owing to the collision in the axons of the largest motoneurones, only the small motoneurones are responding (Pierrot-Deseilligny & Mazevet, 2000). These curves were repeated before training (dark blue lines), immediately after training (pink), at day 2 (red), day 4 (light blue) and day 7 (violet). Soleus M waves were correctly matched (Fig. 5A), and it was verified that foot plantar muscle responses also matched over time. It is clear that the slope of the ascending part of the recruitment curve of the H reflex (Fig. 5B) was not increased after training (see Discussion) and was probably even slightly decreased immediately after training. The same results were obtained in the eight experiments shown in Table 3. Owing to the small number of experiments and the different ISIs used, statistical evaluation of the result has not been done.
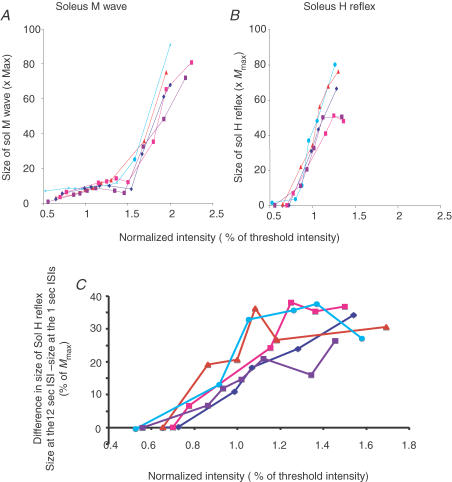
Figure 5.
Effect of training on Sol H reflex recruitment curve A and B, effect of training on the recruitment curves of the Sol M wave (A) and H reflex (B) in one representative subject at different times: at day 1 before training (dark blue lines), immediately after training (pink lines), at day 2 (red lines), day 4 (light blue lines) and day 7 (violet lines). Stimulation frequency was 12 s. C, effect of changing the stimulation frequency (ISI) from 12 to 1 s on the Sol H reflex recruitment curve at different times: pretraining, post-training, and at days 2, 4 and 7. Same colour code and subject as in A and B. Size of the Sol M waves (A), of the Sol H reflexes (B), and differences in size of the Sol H reflex at the 12 and 1 s ISIs are expressed as a percentage of Mmax and plotted against the intensity used to stimulate the posterior tibial nerve. Intensity is expressed as a percentage of the intensity evoking an M response of 5% of Mmax (threshold intensity). A, the Sol M waves matched correctly at the different times, a prerequisite for comparison of the H reflexes. B, there was no significant increase of the slope of the ascending part of the recruitment curve of the Sol H reflex at any time after training. The slope is slightly decreased immediately post-training (compare dark blue and pink lines). C, the difference between the size of the Sol H reflex at a 12 s ISI and at a 1 s ISI is enhanced immediately after training, at day 2 and at day 4, and is back to the pretraining level at day 7. This shows that homosynaptic depression at the synapses between Ia afferents and Sol motoneurones was enhanced after training until day 4.
Table 3.
Effect of time on the mean (± S.D.) slope of the ascending part of the Sol H reflex recruitment curve
| Slope | |||||
|---|---|---|---|---|---|
| n | ISI | Pre training | Post training | Day 2 | Day 4 |
| 4 | 12 s | 2.42 ± 1.6 | 2.27 ± 1.5 | 2 ± 1.4 | 2.37 ± 1.4 |
| 4 | 1 s | 2.13 ± 1.6 | 2.17 ± 1.9 | 1.83 ± 0.9 | 2.07 ± 1.7 |
| 2 | 10 s | 1.4 ± 0.1 | 1.35 ± 0.07 | 1.65 ± 0.1 | — |
| 2 | 4 s | 1.15 ± 0.07 | 1.15 ± 0.2 | — | — |
The Sol H reflex was downregulated after training (compare light blue and pink lines in Fig. 5B) and upregulated at days 2 and 4 (compare dark blue line with red and light blue lines in Fig. 5B). This result has been confirmed in the whole of group C using a calibrated stimulation of the posterior tibial nerve (results not shown). The downregulation of the H reflex immediately post-training was described by Mazzocchio et al. (2006) and correlated with improvement in performance.
At the 12 s ISI, HD is absent or minimal, while it is prominent at the 1 s ISI. Comparison of the recruitment curves obtained at 12 and 1 s ISIs over time is another way to display the increase of frequency-related depression of Sol H reflex after training. This is done for one representative subject in Fig. 5C. The difference in size of H reflexes obtained at 12 and 1 s ISIs is plotted against the stimulation intensity and compared before training (dark blue lines), immediately after training (pink), at day 2 (red), day 4 (light blue) and day 7 (violet). The difference in size of the H reflexes at 1 and 12 s ISIs was enhanced immediately after training as well as at days 2 and 4 and was almost back to its pretraining value at day 7. Similar results were found in three out of the four subjects tested. One subject did not show any difference in size of the H reflex when evoked at the 12 or 1 s ISIs either in the baseline condition or after training.
Discussion
Here we show that a brief period of training, consisting of keeping a constant cycling speed against frequent changes in pedal resistance, led to enduring changes of the frequency-related depression of the Sol H reflex, in that frequency-related depression of the Sol H reflex was increased, which can be interpreted as a decreased synaptic efficacy at the level of the synapse between Ia afferent and Sol motoneurone (HD). The same cycling task, but against a constant pedal resistance, did not induce any modification of the frequency-related depression of the Sol H reflex. The amount of training-induced change of HD depended on the degree of usual physical training and not on the difficulty of the task by itself.
Frequency-related depression of Sol H reflex in the baseline condition reflects homosynaptic depression at the level of the synapse between Ia afferent and Sol motoneurone
In the cat, depression of the size of the monosynaptic reflex discharge induced by repetitive activation of the synapse between Ia fibre and motoneurone has been shown to occur without concomitant changes in membrane potential or conductance (Hultborn et al. 1996) and has been ascribed to a presynaptic mechanism. This presynaptic mechanism (called postactivation depression or homosynaptic depression) differs from the classical presynaptic inhibition (transmitted through GABAergic axo-axonal synapses; see review by Hultborn & Nielsen, 1998; and Wood et al. 1996). Homosynaptic depression regulates the synaptic transmitter release, as presynaptic inhibition does. Cellular mechanisms supporting HD are not yet fully understood and several candidates have been put forward: depletion of releasable vesicles; failure of action potential conduction at axonal branches (Brody & Yue, 2000); decrease of presynaptic quantal size (Chen et al. 2004); reduced release probability of readily releasable pool (Schneggenburger et al. 2002); and adaptation of exocytosis machinery (Hsu et al. 1996). Even postsynaptic influence may play a role in regulating HD, which would be exerted through retrograde signalling (Davis & Murphey, 1994). This fits with the recent view that the degree of HD may depend on the type of group I afferents activated and even for the same afferents on the type of the target neurons (Hammar et al. 2002). The level of HD depends on the level of preceding activity of the afferent fibres. Since HD is decreased in spastic patients (Faist et al. 1994; Nielsen et al. 1995), it has been suggested that HD might also be susceptible to descending suprasegmental influence. Hultborn & Nielsen (1998) stressed that the decreased HD seen in patients with a spinal or brain lesion may be secondary to the disuse of motoneurones and Ia fibres that follows the lesion and may not result from the interruption of descending pathways alone, since: (1) reduction of HD does not immediately follow the lesion but develops with the transition from flaccid to spastic paralysis, even though reduced HD precedes clinically observable spasticity (Schindler-Ivens & Shields, 2000); (2) contrary to several other electrophysiological changes explored, HD is unchanged on the unaffected side of patients with hemiplegia (Aymard et al. 2000); and (3) synaptic efficacy of primary afferents can be up- and downregulated by disuse or use of synapses, respectively (Gallego et al. 1979).
In practice, two methods have been used to assess homosynaptic depression at the synapse between Ia fibre and motoneurone in humans: the frequency-related depression of the Sol (Kohn et al. 1997; Hultborn & Nielsen, 1998) and wrist flexors H reflexes (Rossi-Durand et al. 1999; Aymard et al. 2000) or the Sol H reflex depression following passive stretch of the test muscle (Hultborn et al. 1996). The main characteristics of HD, as described in cat experiments using intracellular recordings, have also been found in human experiments: long duration (several seconds up to 10 s); restriction to synapses previously activated; occurrence without concomitant decreased motoneuronal excitability; involvement of a different mechanism from that supporting ‘classical’ presynaptic inhibition (Hultborn et al. 1996; Wood et al. 1996); and different degree according to the type of the group I afferent or the postsynaptic cells (Lamy et al. 2005).
According to data in the literature, frequency-related depression (ISIs of 1–9 s) of the Sol H reflex observed in the naïve subjects before training in this study can be ascribed to homosynaptic depression at the level of the synapse between Ia afferents and Sol motoneurones. At short ISIs (less than 1 s), mechanisms other than HD could participate in the reflex depression (Taborikova & Sax, 1969). In the next section we will discuss whether training-induced modifications of the frequency-related depression of the Sol H reflex reflect true adaptative changes of the HD after training or are contaminated by experimental bias or by other pre- or postsynaptic events induced by the training.
Do changes in frequency-related depression of the H reflex after training faithfully reflect changes in homosynaptic depression?
Background contraction
A possible confounding factor in the interpretation of post-training modulation of HD would be different levels of background Sol activity over recording sessions. Indeed, HD is decreased during voluntary contraction of the tested muscle (Hultborn & Nielsen, 1998). Special care was taken to ensure that the Sol muscle was at complete rest during testing (see Methods). It cannot be completely ruled out that small remote muscle activity was not picked up by the surface recording electrodes. If such activity existed, its role in modulating HD was probably marginal.
Increased γ activity
Increased HD could result from increased traffic in Ia afferent axons at rest after training. There are converging arguments suggesting that, in humans, there is little, if any, activity in static γ motoneurones innervating resting muscles. Could training induce a change in the descending control to γ motoneurones and an increase in the static γ activity? It is still uncertain whether γ drive plays a role in the acquisition of a new motor skill. Attempts to produce spindle activation in advance of EMG by providing a warning cue or in learning paradigms have been unsuccessful so far (Vallbo & Al-Falahe, 1990). Therefore, while unlikely, it is possible that a post-training increase in Ia traffic was caused by an increase of the static γ drive.
Increased recruitment gain
It has been shown that the recruitment gain of the human corticospinal pathway can be durably modified by motor training (Carroll et al. 2002). This is also true for the monosynaptic reflex. A significantly lower Sol H reflex gain (decrease of slope) during standing was demonstrated in highly trained dancers compared with non-trained subjects (Mynark & Koceja, 1997); recently a depression of the Sol H reflex recruitment curve [decrease of the maximal H reflex value (Hmax)/Mmax ratio and a decrease of the slope] has been described immediately after a single visuo-motor skill training session involving ankle muscles (Perez et al. 2005). An increase in the slope of the input–output relationship for the Sol motoneurone pool after training might induce an apparent increase in the frequency-related depression of the Sol H reflex even in the absence of any change of the relative efficacy of the Ia afferent volley. To further assess whether increase in frequency-related depression of the Sol H reflex after training resulted from a shift of the slope of the input–output curve towards the left or reflected genuine modifications of HD, we looked at the effect of the training on the slope of the Sol H reflex recruitment curve. From results presented in Table 3, it appears that the slope of the ascending part of the recruitment curve was not increased by training but even slightly decreased immediately after training.
By adjusting the stimulus intensity to obtain H reflexes of similar sizes at the 9 s ISI, in order that a comparable proportion of the motoneuronal pool was activated at the different times, we tacitly assumed that the 9 s frequency was slow enough to induce little, if any, HD. This was not verified in all our subjects. In six out of the 22 subjects, the size of the reflex had not reached yet a plateau at the 9 s frequency. Between the pre- and immediately post-training recordings we had to increase the stimulus intensity; indeed, there was a depression of the H reflex evoked by a calibrated stimulation (data not shown). Since it has been shown that ‘reflexes whose postactivation depression was adjusted by increasing the stimulus intensity exhibited less depression that reflexes of the same size without a recent history of activity’ (Floeter & Kohn, 1997), our adjustment can certainly not explain an increase of HD but, in contrast, leads to an underestimation of training effects on HD.
We suggest that the changes in frequency-related depression of the Sol H reflex that we describe after complex training do reflect adaptative changes in HD at the level of the synapse between Ia afferents and Sol motoneurones, with a training-induced decrease in the efficacy of the synapse.
Synaptic plasticity induced by the complex task
It is difficult to appreciate exactly for how long after a single 16 min bout of training the synaptic efficacy maintained a decreased level, since the small trainings (4 min) done on days 2 and 4, to assess the performance, probably acted as reinforcements. Decreased synaptic efficacy lasted for at least 24 h after the first training, since HD maintained the same decreased level at day 2 in the absence of any new training. These enduring modifications of HD observed outside any movement are in keeping with the view that a synaptic spinal plasticity has developed. We can only speculate about mechanisms possibly supporting such a long-term modification of the amount of transmitter release. This might result from structural modifications of the synapse, such as modification of the density of the synaptic boutons or desensitization of postsynaptic receptors.
Source of HD modifications
Progressive adaptation of H reflexes during acquisition of various motor learning tasks in humans and animals has been mainly attributed to changes in the descending control exerted on spinal circuits (Wolpaw & Tennissen, 2001; Schneider & Capaday, 2003). Here we did not find any correlation between the overall level of excitability of Sol motoneurones as assessed by the size of Sol H reflex and the HD level, in that immediately post-training HD was increased and the Sol H reflex was depressed, but later on HD remained stable (days 2 and 4) while the Sol H reflex became slightly facilitated (see Fig. 5). Furthermore, there is no anatomical evidence for a descending control of HD. Taken together, these observations do not fit with the view that modulation of HD after complex cycle training could originate in descending drive. Instead, we suggest that HD modulation may be related to the pattern of incoming sensory inputs, since the pattern and magnitude of the incoming proprioceptive inputs were very different between the two tasks. There is now considerable evidence from animal experiments that, after loss of supraspinal inputs to spinal circuits, the sensory feedback can lead to a reorganization of spinal networks which plays a crucial role in the process of functional recovery (Muir, 1999). In humans recovering from spinal cord injury, progressively increasing weight bearing improves stepping ability (Harkema et al. 1997). There is evidence for restoration of H reflex frequency-dependent depression by an exercise regimen (using a motorized bicycle trainer) in an animal model with complete spinal cord transection (Skinner et al. 1996; Reese et al. 2006) and in humans with complete spinal cord injury (Schindler-Ivens & Shields, 2000; Kiser et al. 2005). Even in the presence of intact descending tracts, repetition of a patterned sensory feedback is able to induce adaptive changes in the spinal pathway of reciprocal Ia inhibition (Perez et al. 2003). At a neural level, the pattern of Ia stimulation has been shown to influence the amplitude of monosynaptic Ia EPSPs. In anaesthetized cats, Koerber & Mendell (1991) used three different patterns of stimulation of Ia afferents derived from spindles discharging in association with specific tasks: ‘paw shake’, ‘burst’ and ‘stepping’. During high-frequency stimulation the greatest decrease in amplitude of the EPSP was observed for the ‘paw shake’, i.e. the task associated with the highest frequency of spindle discharge. Taken together, all these findings support the hypothesis that an intrasegmental reorganization after skilful training, induced by highly differentiated somatosensory input, is possible.
Functional significance
Based on the following points, we propose that modulation of HD does not participate in acquisition of the motor task but rather in the encoding of a motor memory in the spinal cord, probably in frame of habituation mechanisms. (1) Modulation of HD is not directly related to the performance by itself. Subjects improved their performance in both the simple and the complex task, while HD was only modified by the complex task; performance slightly but continuously improved between post-training and later measurements while HD kept the same decreased level; when comparing sedentary and active subjects, the former had a better improvement of performance but a smaller decrease of HD than the latter. (2) Modulation of HD outlasted the training session for around 24 h and, during the following weeks, in adapted subjects, returned immediately to its increased level after few minutes of training. (3) Subjects who were used to exercising using a treadmill, elliptical or stepping machines exhibited a larger adaptation of HD after training than sedentary people. (4) The prevailing view, from animal studies, has been that HD of primary sensory afferents underlies short-term habituation (Bailey & Chen, 1988; Bristol & Carew, 2005), a simple form of learning. Long-term modifications of transmitter release have also been demonstrated and suggested to contribute to the storage of memory lasting one or more days. By means of microcultures (in which single sensory and motor neurons of Aplysia were plated together) and of quantal analysis (in which the size of the miniature excitatory postsynaptic potential released spontaneously from the presynaptic terminal was used as a reference), it has been demonstrated that long-term facilitation induced by repeated applications of 5-hydroxytryptamine was caused by an increase in the number of transmitter quanta released by the presynaptic neuron (Dale et al. 1988).
During acquisition of a new visuo-motor skill involving ankle muscles, ‘classical’ presynaptic inhibition of Sol Ia afferents is increased (Perez et al. 2005) without concomitant decrease of somatosensory evoked potentials. The role of such a gating of sensory input to spinal motoneurones but not to supraspinal centres during skill learning is unclear. Perez et al. (2005) propose that such a gating might optimize the integration at the cortical level of visual and sensory feedbacks and devote the control of muscle activity to the cortex. Increased presynaptic inhibition probably participates in the downregulation of Sol H reflex immediately post-training (Perez et al. 2005). Both increased presynaptic inhibition and downregulation of the H reflex are short lasting after the end of training. Plasticity in the efficacy of HD in relation to the pattern of incoming sensory input could have the functional effect of improving contrast during behaviourally relevant changes in afferent activity (Frerking & Ohliger-Frerking, 2006). Both presynaptic inhibition and HD would contribute to the gating of sensory input during skill learning but with different time scales, in that presynaptic inhibition would be involved in the acquisition of the task while HD would be involved in its retention.
Acknowledgments
We thank Professor Emmanuel Pierrot-Deseilligny for critical review of the manuscript. Dr Meunier was supported by funds from INSERM (Institut National de la Sante et de la Recherche Medicale, France) and by the intramural program of NINDS.
References
- American College of Sports Medicine Position Stand. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness and flexibility in healthy adults. Med Sci Sports Exerc. 1998;30:975–991. doi: 10.1097/00005768-199806000-00032. [DOI] [PubMed] [Google Scholar]
- Aymard C, Katz R, Lafitte C, Lo E, Penicaud A, Pradat-Diehl P, Raoul S. Presynaptic inhibition and homosynaptic depression: a comparison between lower and upper limbs in normal human subjects and patients with hemiplegia. Brain. 2000;123:1688–1702. doi: 10.1093/brain/123.8.1688. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Chen M. Long-term sensitization in Aplysia increases the number of presynaptic contacts onto the identified gill motor neuron L7. Proc Natl Acad Sci U S A. 1988;85:9356–9359. doi: 10.1073/pnas.85.23.9356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouyer LJ, Whelan PJ, Pearson KG, Rossignol S. Adaptive locomotor plasticity in chronic spinal cats after ankle extensors neurectomy. J Neurosci. 2001;21:3531–3541. doi: 10.1523/JNEUROSCI.21-10-03531.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristol AS, Carew TJ. Differential role of inhibition in habituation of two independent afferent pathways to a common motor output. Learn Mem. 2005;12:52–60. doi: 10.1101/lm.83405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody DL, Yue DT. Release-independent short-term synaptic depression in cultured hippocampal neurons. J Neurosci. 2000;20:2480–2494. doi: 10.1523/JNEUROSCI.20-07-02480.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks CM, Downman CB, Eccles JC. After-potentials and excitability of spinal motoneurones following antidromic activation. J Neurophysiol. 1950;13:9–38. doi: 10.1152/jn.1950.13.1.9. [DOI] [PubMed] [Google Scholar]
- Carroll TJ, Riek S, Carson RG. The sites of neural adaptation induced by resistance training in humans. J Physiol. 2002;544:641–652. doi: 10.1113/jphysiol.2002.024463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Harata NC, Tsien RW. Paired-pulse depression of unitary quantal amplitude at single hippocampal synapses. Proc Natl Acad Sci U S A. 2004;101:1063–1068. doi: 10.1073/pnas.0307149101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C, Nielsen J. Methodological implications of the post activation depression of the soleus H-reflex in man. Exp Brain Res. 1989;78:28–32. doi: 10.1007/BF00230683. [DOI] [PubMed] [Google Scholar]
- Dale N, Schacher S, Kandel ER. Long-term facilitation in Aplysia involves increase in transmitter release. Science. 1988;239:282–285. doi: 10.1126/science.2892269. [DOI] [PubMed] [Google Scholar]
- Davis GW, Murphey RK. Long-term regulation of short-term transmitter release properties: retrograde signaling and synaptic development. Trends Neurosci. 1994;17:9–13. doi: 10.1016/0166-2236(94)90028-0. [DOI] [PubMed] [Google Scholar]
- de Leon RD, Roy RR, Edgerton VR. Locomotor capacity attributable to step training versus spontaneous recovery after spinalization in adult cats. J Neurophysiol. 1998;79:1329–1340. doi: 10.1152/jn.1998.79.3.1329. [DOI] [PubMed] [Google Scholar]
- Durkovic RG. Retention of a classically conditioned reflex response in spinal cat. Behav Neural Biol. 1985;43:12–20. doi: 10.1016/s0163-1047(85)91440-2. [DOI] [PubMed] [Google Scholar]
- Faist M, Mazevet D, Dietz V, Pierrot-Deseilligny E. A quantitative assessment of presynaptic inhibition of Ia afferents in spastics. Differences in hemiplegics and paraplegics. Brain. 1994;117:1449–1455. doi: 10.1093/brain/117.6.1449. [DOI] [PubMed] [Google Scholar]
- Floeter MK, Kohn AF. H-reflexes of different sizes exhibit differential sensitivity to low frequency depression. Electroencephalogr Clin Neurophysiol. 1997;105:470–475. doi: 10.1016/s0924-980x(97)00032-5. [DOI] [PubMed] [Google Scholar]
- Frerking M, Ohliger-Frerking P. Functional consequences of presynaptic inhibition during behaviorally relevant activity. J Neurophysiol. 2006;96:2139–2143. doi: 10.1152/jn.00243.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego R, Kuno M, Nunez R, Snider WD. Disuse enhances synaptic efficacy in spinal mononeurones. J Physiol. 1979;291:191–205. doi: 10.1113/jphysiol.1979.sp012807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammar I, Slawinska U, Jankowska E. A comparison of postactivation depression of synaptic actions evoked by different afferents and at different locations in the feline spinal cord. Exp Brain Res. 2002;145:126–129. doi: 10.1007/s00221-002-1098-5. [DOI] [PubMed] [Google Scholar]
- Harkema SJ, Hurley SL, Patel UK, Requejo PS, Dobkin BH, Edgerton VR. Human lumbosacral spinal cord interprets loading during stepping. J Neurophysiol. 1997;77:797–811. doi: 10.1152/jn.1997.77.2.797. [DOI] [PubMed] [Google Scholar]
- Henneman E, Mendell LM. Functional organization of motoneurone pool and its input. In: Brookhart JM, Mountcastle VB, Brooks VB, Geiger SR, editors. Handbook of Physiology, The Nervous System, Motor Control. 1. II. Bethesda, MD USA: American Physiological Society; 1981. pp. 423–507. section I. [Google Scholar]
- Hsu SF, Augustine GJ, Jackson MB. Adaptation of Ca2+-triggered exocytosis in presynaptic terminals. Neuron. 1996;17:501–512. doi: 10.1016/s0896-6273(00)80182-8. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Illert M, Nielsen J, Paul A, Ballegaard M, Wiese H. On the mechanism of the post-activation depression of the H-reflex in human subjects. Exp Brain Res. 1996;108:450–462. doi: 10.1007/BF00227268. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Nielsen J. Modulation of transmitter release from Ia afferents by their preceding activity – a ‘postactivation depression’. In: Rudomin P, Mendell L, editors. Presynaptic inhibition & Neural Control. New York: Oxford University Press; 1998. pp. 178–191. [Google Scholar]
- Kandel ER. Neuronal plasticity and the modification of behavior. In: Brookhart JM, Mountcastle VB, Kandel ER, Geiger SR, editors. Handbook of Physiology, section I, The Nervous System, vol. I, Cellular Biology of Neurons. Bethesda: American Physiological Society MD, USA; 1977. pp. 1137–1182. [Google Scholar]
- Karvonen MJ, Kentala E, Mustala O. The effects of training on heart rate; a longitudinal study. Ann Med Exp Biol Fenn. 1957;35:307–315. [PubMed] [Google Scholar]
- Kato T, Takeda Y, Tsuji T, Kasai T. Further insights into post-exercise effects on H-reflexes and motor evoked potentials of the flexor carpi radialis muscles. Motor Control. 2003;7:82–99. doi: 10.1123/mcj.7.1.82. [DOI] [PubMed] [Google Scholar]
- Kernell D, Hultborn H. Synaptic effects on recruitment gain: a mechanism of importance for the input-output relations of motoneurone pools? Brain Res. 1990;507:176–179. doi: 10.1016/0006-8993(90)90542-j. [DOI] [PubMed] [Google Scholar]
- Kiser TS, Reese NB, Maresh T, Hearn S, Yates C, Skinner RD, Pait TG, Garcia-Rill E. Use of a motorized bicycle exercise trainer to normalize frequency-dependent habituation of the H-reflex in spinal cord injury. J Spinal Cord Med. 2005;28:241–245. doi: 10.1080/10790268.2005.11753818. [DOI] [PubMed] [Google Scholar]
- Kitago T, Mazzocchio R, Liuzzi G, Cohen LG. Modulation of H-reflex excitability by tetanic stimulation. Clin Neurophysiol. 2004;115:858–861. doi: 10.1016/j.clinph.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Koerber HR, Mendell LM. Modulation of synaptic transmission at Ia-afferent connections on motoneurons during high-frequency afferent stimulation: dependence on motor task. J Neurophysiol. 1991;65:1313–1320. doi: 10.1152/jn.1991.65.6.1313. [DOI] [PubMed] [Google Scholar]
- Kohn AF, Floeter MK, Hallett M. Presynaptic inhibition compared with homosynaptic depression as an explanation for soleus H-reflex depression in humans. Exp Brain Res. 1997;116:375–380. doi: 10.1007/pl00005765. [DOI] [PubMed] [Google Scholar]
- Kuno M. Mechansim of facilitation and depression of the excitatory synaptic potential in spinal motoneurones. J Physiol. 1964;175:100–112. doi: 10.1113/jphysiol.1964.sp007505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamy JC, Wargon I, Baret M, Ben Smail D, Milani P, Raoul S, Penicaud A, Katz R. Post-activation depression in various group I spinal pathways in humans. Exp Brain Res. 2005;166:248–262. doi: 10.1007/s00221-005-2360-4. [DOI] [PubMed] [Google Scholar]
- Liu X, Sandkuhler J. Characterization of long-term potentiation of C-fiber-evoked potentials in spinal dorsal horn of adult rat: essential role of NK1 and NK2 receptors. J Neurophysiol. 1997;78:1973–1982. doi: 10.1152/jn.1997.78.4.1973. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Mazzocchio R, Kitago T, Liuzzi G, Wolpaw JR, Cohen LG. Plastic changes in the human H-reflex pathway at rest following skillful cycling training. Clin Neurophysiol. 2006;11:1682–1691. doi: 10.1016/j.clinph.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Mendell LM, Wall PD. Responses of single dorsal cord cells to peripheral cutaneous unmyelinated fibres. Nature. 1965;206:97–99. doi: 10.1038/206097a0. [DOI] [PubMed] [Google Scholar]
- Motl RW, Knowles BD, Dishman RK. Acute bouts of active and passive leg cycling attenuate the amplitude of the soleus H-reflex in humans. Neurosci Lett. 2003;347:69–72. doi: 10.1016/s0304-3940(03)00652-9. [DOI] [PubMed] [Google Scholar]
- Muir GD. Locomotor plasticity after spinal injury in the chick. J Neurotrauma. 1999;16:705–711. doi: 10.1089/neu.1999.16.705. [DOI] [PubMed] [Google Scholar]
- Mynark RG, Koceja DM. Comparison of soleus H-reflex gain from prone to standing in dancers and controls. Electroencephalogr Clin Neurophysiol. 1997;105:135–140. doi: 10.1016/s0924-980x(96)96096-8. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Crone C, Hultborn H. H-reflexes are smaller in dancers from The Royal Danish Ballet than in well-trained athletes. Eur J Appl Physiol Occup Physiol. 1993;66:116–121. doi: 10.1007/BF01427051. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Petersen N, Crone C. Changes in transmission across synapses of Ia afferents in spastic patients. Brain. 1995;118:995–1004. doi: 10.1093/brain/118.4.995. [DOI] [PubMed] [Google Scholar]
- Perez MA, Field-Fote EC, Floeter MK. Patterned sensory stimulation induces plasticity in reciprocal Ia inhibition in humans. J Neurosci. 2003;23:2014–2018. doi: 10.1523/JNEUROSCI.23-06-02014.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MA, Lundbye-Jensen J, Nielsen JB. Changes in corticospinal drive to spinal motoneurones following visuo-motor skill learning in humans. J Physiol. 2006;573:843–855. doi: 10.1113/jphysiol.2006.105361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MA, Lungholt BK, Nielsen JB. Presynaptic control of group Ia afferents in relation to acquisition of a visuo-motor skill in healthy humans. J Physiol. 2005;568:343–354. doi: 10.1113/jphysiol.2005.089904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Mazevet D. The monosynaptic reflex: a tool to investigate motor control in humans. Interest and limits. Neurophysiol Clin. 2000;30:67–80. doi: 10.1016/s0987-7053(00)00062-9. [DOI] [PubMed] [Google Scholar]
- Reese NB, Skinner RD, Mitchell D, Yates C, Barnes CN, Kiser TS, Garcia-Rill E. Restoration of frequency-dependent depression of the H-reflex by passive exercise in spinal rats. Spinal Cord. 2006;44:28–34. doi: 10.1038/sj.sc.3101810. [DOI] [PubMed] [Google Scholar]
- Rossi-Durand C, Jones KE, Adams S, Bawa P. Comparison of the depression of H-reflexes following previous activation in upper and lower limb muscles in human subjects. Exp Brain Res. 1999;126:117–127. doi: 10.1007/s002210050721. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Donoghue JP. Plasticity and primary motor cortex. Annu Rev Neurosci. 2000;23:393–415. doi: 10.1146/annurev.neuro.23.1.393. [DOI] [PubMed] [Google Scholar]
- Schabort EJ, Hawley JA, Hopkins WG, Mujika I, Noakes TD. A new reliable laboratory test of endurance performance for road cyclists. Med Sci Sports Exerc. 1998;30:1744–1750. doi: 10.1097/00005768-199812000-00014. [DOI] [PubMed] [Google Scholar]
- Schindler-Ivens S, Shields RK. Low frequency depression of H-reflexes in humans with acute and chronic spinal-cord injury. Exp Brain Res. 2000;133:233–241. doi: 10.1007/s002210000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneggenburger R, Sakaba T, Neher E. Vesicle pools and short-term synaptic depression: lessons from a large synapse. Trends Neurosci. 2002;25:206–212. doi: 10.1016/s0166-2236(02)02139-2. [DOI] [PubMed] [Google Scholar]
- Schneider C, Capaday C. Progressive adaptation of the soleus H-reflex with daily training at walking backward. J Neurophysiol. 2003;89:648–656. doi: 10.1152/jn.00403.2002. [DOI] [PubMed] [Google Scholar]
- Skinner RD, Houle JD, Reese NB, Berry CL, Garcia-Rill E. Effects of exercise and fetal spinal cord implants on the H-reflex in chronically spinalized adult rats. Brain Res. 1996;729:127–131. [PubMed] [Google Scholar]
- Taborikova H, Sax DS. Conditioning of H-reflexes by a preceding subthreshold H-reflex stimulus. Brain. 1969;92:203–212. doi: 10.1093/brain/92.1.203. [DOI] [PubMed] [Google Scholar]
- Trimble MH, Harp SS. Postexercise potentiation of the H-reflex in humans. Med Sci Sports Exerc. 1998;30:933–941. doi: 10.1097/00005768-199806000-00024. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Al-Falahe NA. Human muscle spindle response in a motor learning task. J Physiol. 1990;421:553–568. doi: 10.1113/jphysiol.1990.sp017961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt M, Chelli F, Frigo C. Changes in the excitability of soleus muscle short latency stretch reflexes during human hopping after 4 weeks of hopping training. Eur J Appl Physiol Occup Physiol. 1998;78:522–532. doi: 10.1007/s004210050455. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Tennissen AM. Activity-dependent spinal cord plasticity in health and disease. Annu Rev Neurosci. 2001;24:807–843. doi: 10.1146/annurev.neuro.24.1.807. [DOI] [PubMed] [Google Scholar]
- Wood SA, Gregory JE, Proske U. The influence of muscle spindle discharge on the human H reflex and the monosynaptic reflex in the cat. J Physiol. 1996;497:279–290. doi: 10.1113/jphysiol.1996.sp021767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalutsky RA, Nicoll RA. Comparison of two forms of long-term potentiation in single hippocampal neurons. Science. 1990;248:1619–1624. doi: 10.1126/science.2114039. [DOI] [PubMed] [Google Scholar]