Abstract
Carotid body chemoreceptors respond to a decrease in arterial partial pressure of O2 with an increase in sinus nerve action potential (AP) activity which initiates a number of protective reflexes. The spike generation process is unresolved but is generally considered to be caused by a synaptic depolarizing potential (SDP) in the nerve endings caused by release of an excitatory transmitter from the glomus cell, which is a secretory cell that is presynaptic to the nerve terminals. To detect the purported SDPs, stimulating electrodes were placed at sites within the carotid body from which orthodromic APs could be evoked at low threshold currents. The probability of AP generation as a function of stimulus current was fitted well to a Boltzmann distribution. Subthreshold electrical stimuli which were expected to summate with subthreshold SDPs, failed, in all instances, to evoke APs at the expected probability. When the stimulus was gated to the occurrence of a spontaneous AP, no change in electrical threshold was observed as the delay between the spontaneous AP and electrical stimulus was increased, despite the presumed disappearance of an SDP in the post-AP period. Decreases in spontaneous AP generation rate, caused by hyperoxia, were associated with only slight changes in the mean orthodromic stimulus threshold, but with a significant increase in slope of the Boltzmann function, suggesting a decrease in the variance of nerve terminal excitability during hyperoxia. These results suggest that AP generation is not due to SDP events; rather, AP generation is likely to be due to a process endogenous to the nerve terminals that modulates the variability of nerve terminal excitability.
Carotid body chemoreceptors respond to a decrease in arterial partial pressure of O2 by increasing the rate of action potential (AP) generation in sinus nerve afferent fibres. These fibres terminate in the brainstem and initiate a number of protective reflexes, including increased breathing, arousal from sleep and increased blood pressure. The mechanism by which hypoxia leads to increased nerve AP generation is not well resolved. It is generally considered that the glomus cell – a secretory cell apposed to the afferent nerve fibres – is the initial site of hypoxia transduction. Hypoxia causes a rise in intracellular calcium level in the glomus cell, leading to fusion of dense-cored vesicles and release of a (presumed) excitatory transmitter(s). This transmitter release may cause synaptic depolarizing potentials (SDPs) in the afferent nerve endings which summate and eventually lead to AP generation.
Although this model of AP generation is widely accepted, experimental data supporting this mechanism are sparse, resting largely on the results of one experiment (Hayashida et al. 1980). These authors impaled nerve endings within the cat carotid body and reported the occurrence of SDPs lasting 46 ms, on average, which apparently summated to produce an AP. Because of difficulties in obtaining and maintaining the recordings, no attempt was made to pharmacologically identify the agent causing the SDP or even determine whether it originated through synaptic transmission. By contrast, we previously examined changes in afferent nerve excitability and in spontaneous AP generation rate caused by iso-osmotic reductions in the extracellular sodium level. Large changes in AP generation rate were associated with small changes in excitability – a result which led us to conclude that AP generation was linked to a high-event process (e.g. channel flicker) rather than a low-event process (e.g. SDP events) (Donnelly et al. 1998).
The present study was designed to more directly test the conjecture that SDP events occur in chemoreceptor nerve terminals. In the experiments we utilized supra-threshold and subthreshold electrical stimuli at points within the carotid body at which APs could be initiated with low-intensity stimuli. In all instances, subthreshold electrical stimuli failed to evoke APs, which would have been expected had SDPs been present during presentation of the stimulus. This suggests that the mechanism of AP generation may be an endogenous characteristic of the nerve terminals and this process may be slowly modulated by release of chemicals from the glomus cells.
Methods
Experimental preparation
All experimental procedures were approved by the Yale University School of Medicine Animal Experimentation and Use Committee. The chemoreceptor complexes were harvested from rats, aged 14–28 days, using methodology previously described in detail (Donnelly et al. 1998; Donnelly & Rigual, 2000). In brief, rats were deeply anaesthetized by placement in a chamber that was subsequently filled with 100% CO2. After cessation of breathing and other motor movements, rats were removed and decapitated. The carotid body–sinus nerve–glossopharyngeal nerve and petrosal ganglion were removed, intact, and placed in dilute solution of collagenase (0.8 mg ml−1, Boheringer type P) and protease (0.2 mg ml−1, Sigma Type IX) for 30 min at 37°C with gentle agitation. After this, the complex was removed, cleaned of connective tissue and transferred to a recording chamber mounted on the stage of an inverted microscope. The chamber was perfused with bicarbonate-buffered saline containing (mm): NaCl 120, NaHCO3 24, KCl 5, glucose 5, CaCl2 2, Na2HPO4 1 and MgSO4 1; equilibrated with 21% O2–5% CO2 and the balance N2 at 37°C.
Single-unit chemoreceptor recordings were obtained using an extracellular electrode advanced into the petrosal ganglion (Fig. 1). In order to facilitate unit identification, a stimulus electrode filled with 1 N NaCl (impedance, 1 MΩ) was placed in the carotid body and stimulated with a cathodal electrical stimulus (A-M Systems Stimulus Isolator, model 2200; –100 μA × 0.1 ms) in order to evoke an orthodromic AP. Once a single unit was localized, the stimulus electrode was moved in a matrix (50 μm grid) over the carotid body surface and the ability to evoke an orthodromic AP was tested at each point (5–100 μA × 0.1 ms). Points that showed a low stimulus threshold were re-probed with the stimulus electrode using smaller spacial increments to place the stimulus electrode at a point of lowest threshold current. These points were considered to be the presumed sites of spontaneous AP generation. In some cases, the characteristics of the stimulus point were tested by applying a continuous cathodal or anodal current to see whether its application could alter the rate of AP generation.
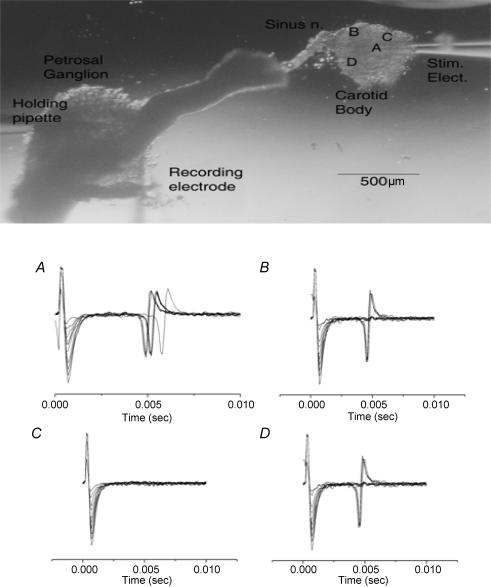
Figure 1. Effect of electrical stimulation at different points across the carotid body surface.
Top, rat chemoreceptor complex with a stimulus pipette placed in the carotid body and recording pipette in the petrosal ganglion. A–D, polygraphic recordings of response to orthodromic stimuli; 10 sweeps of increasing stimulus intensity (5, 10, 15 … 50 μA × 0.1 m). Low-threshold activation (< 10 μA) only occurred at electrode location shown in A. (Tracings are from a different preparation to that shown in the upper panel, but electrode positions (A–D), corresponding to A–D below, are accurately represented with respect to the sinus nerve entry zone.)
Orthodromic stimulation during normoxia
At a given stimulus point, the current intensity needed to evoke an AP about half of the time was estimated; this was considered the threshold stimulus. Individual trials were undertaken with stimulus intensities set approximately 20% below threshold and incrementally raised to 20% above threshold. At each stimulus intensity, 100 stimuli were delivered at 1–2 s−1, after which the stimulus intensity was increased or decreased and the protocol repeated.
In other experiments, the stimulus delivery was gated to the occurrence of a spontaneous AP. This was detected by a window discriminator (BAK Electronics DIS-1) which provided a logic pulse that initiated an electrical stimulus following a variable waiting period (pCLAMP9; Axon Instruments). Delay periods of about 5, 20, 50 and 100 ms were tested and repeated at six different stimulus intensities around the threshold stimulus. Four to 10 observations were made for each delay and for each stimulus intensity.
Orthodromic stimulation during hypoxia or hyperoxia
In order to alter the rate of spontaneous AP generation, the superfusate was switched to a reservoir equilibrated with 12% O2–5% CO2 and the balance N2 (hypoxia) or equilibrated with 95% O2–5% CO2 (hyperoxia). Once a steady-state discharge rate was achieved, electrical stimuli around the orthodromic threshold were delivered.
Data analysis
For each stimulus series, the probability of success of evoking an orthodromic AP was computed based on the success rate over 100 trials. The success rates were analysed using three methods. Firstly, the probability of successfully evoking an AP was plotted against the stimulus intensity and the relationship was fitted to a Boltzmann function:
Where x is the stimulus intensity, x0 is the stimulus intensity that yields a 50% success rate, and dx is an inverse slope factor. The variables, x0 and normalized, inverse slope factor (dx/x0), were compared between baseline and during changes in ongoing nerve activity produced by hypoxia or hyperoxia using Student's paired t test. A decrease in dx/x0 represents an increase in the slope of the fitting function. This fitting function represents an extension of a model originally developed by Gallego et al. (1979) in which AP generation was modelled as a chemical process in which rate = Ke(-U/RT), where K is the proportionality constant, E is the base of natural logarithms, U is energy of activation, R is the universal gas constant and T is temperature. Extension of this model to the present circumstances gives:
where I is the amount the activation energy that is reduced due to the electrical stimulus. Thus, the overall probability of success, Prob(AP)/(Prob(AP) + Prob(no AP)), is in the form of the Boltzmann function.
Secondly, to determine any trend that may have been present over the 100 trials, successes and failures were converted to a series of ones and zeros, respectively, and the series analysed using an autocorrelation function with lags set at 0.1. The autocorrelation value for the original series was compared against a population of values arising from repeated shuffling of the original series. After each shuffle, the autocorrelation value was computed and used to estimate the mean and variance of the shuffled population. If the original series differed by more than 1.7 × s.d. from the mean of the population arising from shuffling, then the original series was deemed to be significantly different from a random series.
Thirdly, the success rate observed at subthreshold stimulus levels was compared to that expected if SDPs were required to summate to produce an AP. Calculation of the probability that an SDP is occurring at the time of stimulus presentation is presented in the Appendix.
Results
Experiments were undertaken on 42 chemoreceptor complexes harvested from 32 rats.
The threshold for orthodromic activation varies over the CB surface
Over the tested range of 0–100 μA, an orthodromic AP could be evoked at approximately half of the points tested (Fig. 1). However, at only one to two points across the surface could an AP be evoked at a stimulus intensity less than 10 μA amplitude (Fig. 1A). These points were often characterized by having several discrete conduction times as measured from the start of the stimulus artifact to the time of arrival of the orthodromic AP. As several discrete arrival times are apparent (Fig. 1A), it suggests that spike initiation was occurring at several different sites around the stimulus electrode. These points also showed the longest conduction time for an AP to arrive at the soma. For instance, for the unit pictured in Fig. 1, stimulation near the nerve entry point (Fig. 1D) evoked an AP with a conduction delay of 4.5 ms but the conduction time for the lower threshold points was about 5 ms (Fig. 1A).
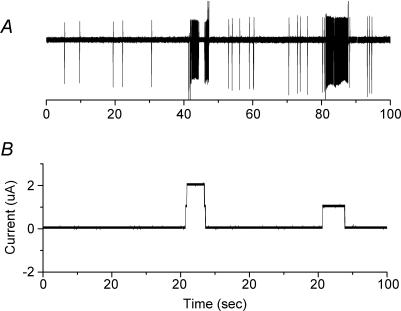
In order to determine whether a continuous electrical field could change the rate of AP generation, a small cathodal or anodal current was applied for several seconds. At points of low current threshold, continuous current application could evoke trains of APs which immediately terminated at the end of the stimulus current (Fig. 2). By contrast, a continuous current application at sites away from the low-threshold points failed to evoke these trains even if applied at current amplitudes 20–100 times greater (data not shown).
Figure 2. Application of DC currents at low-threshold stimulus points (e.g. A of Fig. 1) evoke multiple APs.
A, polygraphic recording of spontaneous and evoked APs from the soma of a petrosal neuron with projections to the carotid body. B, current passed through a stimulus electrode placed in the carotid body at which orthodromic AP could be evoked at low threshold.
Stimulus–response relationship during normoxia
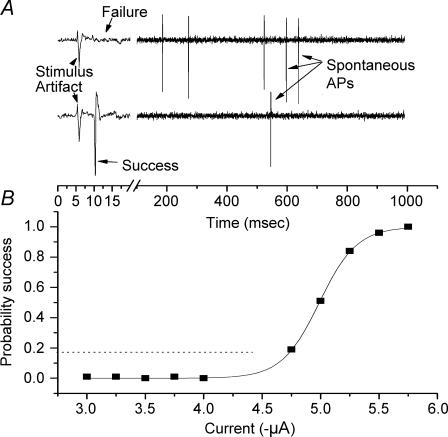
The stimulus current threshold was estimated once an electrode was placed at a low-threshold point and a series of 100 stimulus pulses (0.1 ms duration, 1–2 s−1) was delivered around threshold (Fig. 3). At stimulus intensities near threshold, successful orthodromic activation varied throughout the trial (Fig. 3). The relationship between the probabilities of successfully initiating an orthodromic AP in response to the stimulus current could be fitted well to a Boltzmann function (Fig. 3). It is important to note that stimuli delivered at intensities below 80% of threshold failed to evoke APs. Such stimuli would be expected to summate with an SDP, if present, and initiate an AP.
Figure 3. Stimulus–response relationship for electrical stimuli delivered.
Stimuli were delivered at 1 s−1 for 100 trials at each stimulus intensity. A, polygraphic recording for two trials at the same stimulus intensity (−5.0 μA, 0.1 ms). The stimulus delivered in the upper sweep failed to evoke an orthodromic AP whereas the lower sweep was successful. The remaining time of the interstimulus period was used for measurement of the rate of spontaneous APs. The rate of spontaneous APs was used to calculate the estimated probability of an ongoing SDP (see text) and used to anticipate a success probability of a summation between an SDP and subthreshold electrical stimulus. B, the success rate versus stimulus intensity could be fitted well to a Boltzmann function. For the example shown, probability of success = 1/(1 + e((x+4.9893)/0.1607))). An interaction between SDPs and electrical stimuli was anticipated to produce a higher success rate for subthreshold current intensities (dotted line).
Because SDPs could not be directly observed, the expected percentage of time that an SDP is present was calculated based on different models of spike generation (see Appendix). If two SDPs were required to overlap and, thus, summate to initiate an AP, the time that an SDP is present may be calculated. If the observed spontaneous spiking rate is 1 Hz and SDPs last 50 ms, an SDP would be present approximately 15–20% of the time. Thus, it is expected that the SDP would summate with the subthreshold electrical stimulus resulting in an AP about 20% of the time. However, this was never observed in any recording. The expected probability of success at 80% threshold was calculated and compared to the observed spike probability for six cells using a χ2 statistic. The observed probability of success was significantly lower than the expected probability (P < 0.001).
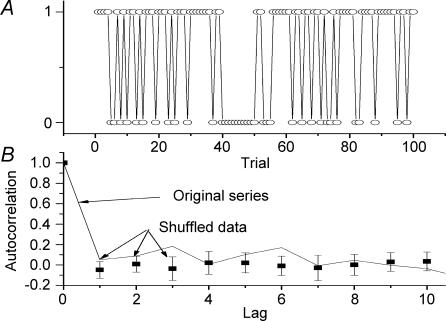
In order to determine whether trends were present within a series, the results of 100 trials were converted to a series of ones (success) and zeros (failure) and analysed using an autocorrelation function (Fig. 4). The autocorrelation values were compared against a population of values arising from shuffling of the original series. In all instances (n = 10), the autocorrelation value of the original series did not significantly differ from that arising from shuffling the series (Fig. 4). This suggests that the chance of successfully evoking an orthodromic AP was not dependent on the success or failure of previous trials.
Figure 4. No trend is evident in the distribution of successes and failures of orthodromic stimuli.
A, sequence of success/failure for a sequence of 100 stimuli delivered at the same intensity at 1 s−1. A success was given a value of one and failure a value of zero. An autocorrelation (solid line) failed to detect any correlation between successes and failures which would be expected if short-term modulatory effects prevailed (i.e. success tends to be followed by success or failure by another failure). Data (▪, and error bars) show mean ± s.d. of autocorrelation values computed on shuffled series.
Changes in orthodromic stimulus response following a spontaneous AP
If SDPs gave rise to spontaneous APs then the occurrence of a spontaneous AP would signal that SDP events had recently occurred. Because SDP events are speculated to last approximately 50 ms, nerve excitability would be altered by the SDP and recover over the subsequent 50 ms. This was tested by applying an orthodromic stimulus that was gated to the occurrence of a spontaneous AP (Fig. 5). Each stimulus intensity was tested at delays of 5, 20, 50 and 100 ms following the occurrence of a spontaneous AP and repeated four to 10 times. As shown in Fig. 5, there was no discernable difference in nerve excitability at different post-AP periods. For a population of six units, the threshold stimulus intensity was not a significant function of time between the occurrence of a spontaneous AP and delivery of the orthodromic stimulus (P = 0.22; ANOVA with repeated measures).
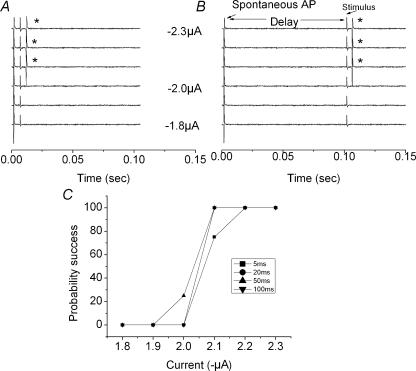
Figure 5. Gating of the orthodromic stimulus to the occurrence of a spontaneous AP.
A, polygraphic tracings of increasing stimulus currents delivered at 5 ms following the occurrence of a spontaneous AP. Threshold for AP generation was approximately −2.1 μA. B, similar tracings with delay between spontaneous AP and stimulus delivery set at 100 ms (at a time the presumed SDP would have dissipated). Note, threshold stimulus current was also −2.1 μA. C, summary of success percentage for four trials conducted at four different delays. Asterisk denotes success evoking orthodromic AP.
Orthodromic stimulus response during hypoxia and hyperoxia
Initial experiments were conducted to determine whether stimulation with hypoxia altered the threshold current for evoking an orthodromic AP. A typical recording is shown in Fig. 6 in which hypoxia (12% O2) increased the rate of spontaneous AP generation, but had only a small effect on the threshold current. In this case, hypoxia may have reduced the threshold current from 4.2 to 4.0 μA, but 4.2 μA, during hypoxia, failed to evoke an AP. Hypoxic stimulation of spontaneous APs also reduced the ability to discriminate between orthodromically evoked APs and those arising spontaneously. Thus, the effects of changes in partial pressure of oxygen were studied by switching from normoxia (21% O2) to hyperoxia (95% O2) which caused a large decrease in spontaneous AP generation rate.
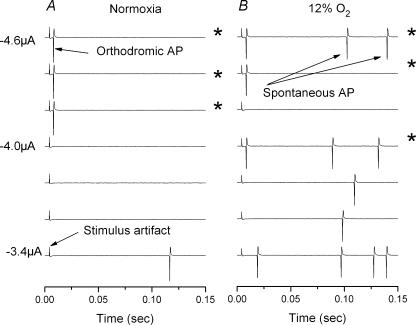
Figure 6. Stimulus threshold during normoxia and during hypoxia.
A, polygraphic tracings of increasing stimulus intensity during superfusion with saline equilibrated with 21% O2–5% CO2 and the balance N2. Stimulus threshold was approximately −4.2 μA. B, similar polygraphic recording obtained during superfusion with saline equilibrated with 12% O2–5% CO2 and the balance N2. Note increase in spontaneous AP generation rate. Stimulus threshold may have decreased slightly to −4.0 μA, but −4.2 μA failed to evoke an AP. Asterisk denotes success evoking orthodromic AP.
As observed during normoxia, the probability of successfully initiating an orthodromic AP as a function of stimulus intensity could be fitted well to a Boltzmann function. For the example shown in Fig. 7, the half-success stimulus current (x0) slightly increased during hyperoxia from 3.2 to 3.3 μA, but the slope of the relationship was increased during hyperoxia. Response of a population of fibres is shown in Fig. 8. Hyperoxia decreased the spontaneous AP rate from 1.06 ± 0.38 to 0.06 ± 0.02 Hz (n = 10). The half-success stimulus intensity normalized to the initial value during normoxia (x0/x0(normoxia)) was not significantly changed, but the normalized slope factor (dx/x0) was decreased by approximately half, from 0.024 ± 0.040 to 0.012 ± 0.002 (Fig. 8). The decrease in dx/x0 reflects an increase in the slope of the line of the Boltzmann fit, as shown in Fig. 7. These changes were reversed upon returning to normoxia.
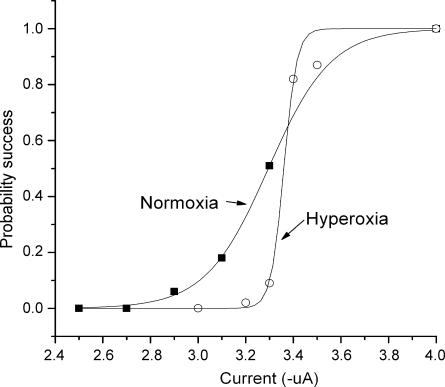
Figure 7. Afferent nerve excitability during normoxia and hyperoxia.
Each stimulus point was tested 100 times (1 s−1) at the specified stimulus intensity and the probability of a successful orthodromic AP was calculated at each point, during normoxia and during lower spontaneous AP activity during hyperoxia. The stimulus–response points were fit to Boltzmann function. Normoxia: Probability of success = 1/(1 + e(3.295 –x)/0.131)). Hyperoxia: Probability of success = 1/(1 + e(3.360 – x)/0.027)). Note there was only a slight rightward shift in half-activation current during hyperoxia but a pronounced increase in slope.
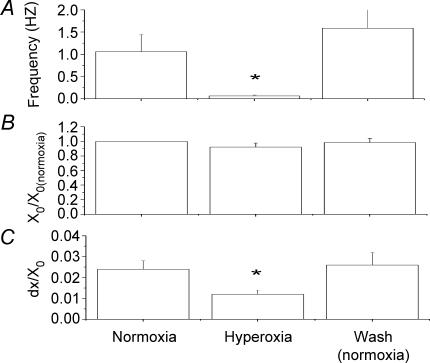
Figure 8. Average change in nerve activity and Boltzmann fits of stimulus–response relationship for nerve excitability.
A, spontaneous spiking rate during normoxia and hyperoxia, and on return to normoxia. B, half-activation current normalized to half-activation current during normoxia. Note that hyperoxia caused no significant change in the half-activation current. C, normalized, inverse slope factor (dx/x0) was significantly decreased during hyperoxia, representing an increase in slope and a reduction in the variability of excitability. *Significantly different than normoxia (P < 0.05, Student's paired t test).
Discussion
The major conclusion from the present work is that chemoreceptor spike generation is not dependent on SDP-like events occurring in nerve terminals. Rather, AP generation and the rate of AP generation appear to be linked to a process endogenous to the nerve terminals which is manifest by increased variability in terminal excitability during stimulation by hypoxia.
Although SDP events leading to AP generation is a widely accepted model of how spike generation comes about, evidence for this model is limited. In only one study have direct recordings been obtained from nerve terminals, in cat carotid body, and this study appeared to show SDP-like events, which summated and gave rise to APs (Hayashida et al. 1980). However, these recordings were difficult to obtain and could only be held for short periods, making it impossible to test pharmacological blockers or to discern whether they arose from calcium-dependent release processes. SDP-like events were also observed in petrosal neurons which were co-cultured with glomus cells, producing a reconstructed carotid body synapse (Zhong et al. 1997). These SDPs were reduced by lowering extracellular calcium concentration or by application of cholinergic blockers (Zhong et al. 1997), but whether this reconstructed synapse reproduces the normal carotid body synapses remains speculation.
If SDP events do occur in chemoreceptor nerve terminals, they would be expected to have characteristics readily detectable using the present methodology. Subthreshold events would be expected to summate with subthreshold electrically induced depolarizations leading to AP generation, but subthreshold electrical stimuli failed, in all instances, to evoke APs, although the expected success rate should have been about 15–20% of the trials (Fig. 9). An SDP, as recorded by Hayashida et al. (1980), lasts about 50 ms which is considerably longer than the AP conduction period between the carotid body and soma. Thus, gating the stimulus to an AP would be equivalent to gating the stimulus to the occurrence of an SDP, and changes in electrical excitability caused by the SDP should have been apparent as a function of time in the post-spike period. Again, no experimental result has shown that such a change was taking place. Taken together, the results are consistent in indicating that SDP-like events are not the cause for AP generation in the afferent fibres.
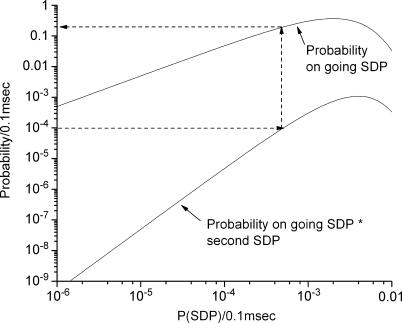
Figure 9. Theoretical probability of an ongoing SDP and spiking rate produced by summation of two SDPs to reach spike threshold.
Probability is expressed as probability per 0.1 ms, which is the duration of the electrical stimulus. The normal spontaneous AP frequency during normoxia is approximately 1 Hz, which is a spike probability of 0.0001 (0.1 ms)−1. The probability that an SDP was initiated within the last 50 ms is given on the upper tracing and is approximately 0.18 when the spontaneous AP rate is 1 Hz (dotted arrow lines). If subthreshold electrical stimuli summated with subthreshold SDPs to produce an AP, the success probability of subthreshold SDP would also be 0.18.
The lack of an SDP-like event is broadly consistent with anatomical observations of nerve–glomus cell endings within the carotid body. Gronblad (1983) examined exocytotic profiles of dense-cored vesicles around glomus cells following fixation in high-potassium fixative. Almost all fusion events occurred in non-synaptic regions, and no events were observed in the area between glomus cells and nerve endings ‘despite careful scrutiny’. Verna (1997) also failed to observe fusion profiles in association with nerve terminals. He stated: ′…I wonder if it is possible to say that the existence of a chemical synapse between glomus cells and sensory nerve endings is really demonstrated! … I would suggest the term ‘junction’ rather than ‘synapse’ to discuss the relationships between glomus cells and sensory nerve endings!′ Thus, the lack of evidence to support the existence of SDP events is consistent with anatomical observations.
The present results do not, however, bring into question the role of the glomus cell or the importance of any chemical transmitters it may release. Destruction of glomus cells by freezing ablates the nerve response to hypoxia, although some AP activity is still present on the sinus nerve (Verna et al. 1975). The glomus cell also possesses several unique oxygen-sensitive K+ currents (Lopez-Barneo et al. 1988; Peers, 1990; Buckler, 1997) with suppression by hypoxia leading to a rise in intracellular calcium levels and secretion of catecholamines. Postnatal enhancement of the calcium and secretory responses has the same developmental profile as the postnatal enhancement of nerve activity (Donnelly & Doyle, 1994; Sterni et al. 1995; Rigual et al. 2000). Although causality is not established, it is logical that organ function is dependent on such a hypoxia-responsive cell. However, the nature of the coupling between glomus cell and nerve ending remains unresolved. Glomus cells may release neurotransmitters, such as acetylcholine and ATP which are currently believed to be of importance in carotid body function (Rong et al. 2003; Zhang et al. 2000; Iturriaga & Alcayaga, 2004), but may (potentially) modulate nerve excitability by release of simple chemicals such as K+ and H+. The present results shed no light on the issue other than that this modulation occurs slowly, so as to not change excitability on a millisecond time scale.
If AP generation is not linked to SDP-events then the question of how APs are generated is relevant. Modelling studies of small nerve fibres indicate that channel noise developed by K+ and Na+ channel flicker may give rise to APs if the surface area is small (Chow & White, 1996). Such a mechanism was implicated in an earlier study in our laboratory which examined changes in spontaneous AP activity during iso-osmotic reductions in Na+ concentration (Donnelly et al. 1998). Large changes in nerve activity produced by reductions in Na+ concentration were correlated with small changes in excitability as judged using an orthodromic electrical stimulus. The result was inconsistent with SDP events but was consistent with small, fast transitions, such as those produced by channel flicker, leading to AP generation. One source of channel flicker is episodic transitions from the inactive to open states of the Na+ channel which is postulated to generate a ‘persistent sodium current’ around resting potential and contribute to cellular excitability (Raman et al. 1997). Recently, we demonstrated that riluzole, a drug that stabilizes the Na channel in the inactive state, greatly reduces AP activity in isolated chemoreceptors and reduces the ventilatory response to hypoxia (but not to hypercapnia) when given in vivo (Faustino & Donnelly, 2006). Thus, a reasonable hypothesis is that chemoreceptor AP generation is due to channel noise associated with a persistent sodium current and that this process is modulated chemically or by other means by the glomus cell.
The variability in nerve terminal excitability, observed in this study, is similar to that previously described for the node of Ranvier (Rubinstein, 1995; Hales et al. 2004). Success in evoking an AP following an electrical stimulus varied as a function of stimulus strength around threshold, due to stochastic characteristics of sodium channels (Rubinstein, 1995). In the node, the variance of excitability was decreased as the number of channels was increased, as the node was hyperpolarized and as the amount of persistent sodium current was reduced (Rubinstein, 1995; Hales et al. 2004). Electrically induced depolarization of the node increased the variance of excitability but also reduced the threshold current (Hales et al. 2004). If the same process occurs within the carotid body during hypoxic excitation, an increase in variability of nerve excitability would be expected (which was observed) along with a decrease in threshold current (which was not). This suggests that the increase in variability was not due to depolarization but due to other factors such as an increase in persistent sodium current, which, by itself, will increase variability (Hales et al. 2004). Hypoxia increases persistent sodium current in heart (Ju et al. 1996), hippocampus (Hammarstrom & Gage, 2000) and hypothalamus (Horn & Waldrop, 2000). If a similar modulation occurs in chemoreceptor nerve terminals, it could be a significant contributor to hypoxia transduction. Alternatively, depolarization may not necessarily decrease threshold current as sodium channels are rapidly inactivated around resting potential in chemoreceptor neurons (Cummins et al. 2002), and this decrease may, potentially, balance any increase in excitability caused by depolarization.
Acceptance of the present result is critically dependent on placement of the stimulus electrode near the point of normal AP generation in the carotid body and on the nerve being stimulated directly rather than as a result of synaptic release of transmitter. An evoked synaptic release is unlikely because previously we demonstrated that the orthodromic response is little altered by blocking calcium influx with extracellular cobalt (Donnelly et al. 1998). Regarding electrode placement, if the electrode were, for instance, along the conducting axon then little or no interaction with the presumed SDP would be expected or detected. Although there are no definitive data to indicate that this is the case, several indirect observations argue for it. Firstly, despite probing the entire carotid body surface, only one or two sites could be identified at which an orthodromic AP could be initiated with a low-threshold stimulus. If nerve fibres were stimulated en passant, it would be expected that many sites would show such characteristics along the course of the axon. Secondly, a constant current applied to the stimulus electrode could often change the discharge rate of the afferent axon. Such modulatory characteristics are not characteristics of axons of passage which do not generate multiple action potentials during a constant current stimulus and DC currents generally result in blockade of axonal conduction (Bhadra & Kilgore, 2004). Thirdly, the excitability characteristics of the sites are altered by hypoxia or hyperoxia. This primarily manifests as an increase in variability of excitability as oxygen is decreased with only slight changes in the mean level of excitability. How increased variability is translated into increased AP generation is, at present, unclear; however, it may be linked to increased time in a state of higher excitability (lower left portion of the Boltzmann curve).
In conclusion, the present results suggest that glomus cell modulation of afferent nerve endings is not mediated by SDP events, but rather by a slow modulation of nerve terminal excitability. This modulation of excitability takes the form of an increase in variability of excitability of nerve terminals which leads to increased AP generation through a process endogenous to the nerve terminal. If this conjecture is correct, AP generation following destruction of glomus cells (perhaps using selective neurotoxins) may be expected to continue. Although this has not yet been tested experimentally, it is interesting to note that little change in AP generation was observed following treatment with reserpine (Donnelly, 1996) which impairs synthesis in the dense-cored granule – the primary site of purported transmitter storage in glomus cells.
Acknowledgments
This work was supported by a grant from National Institutes of Health, HL-073500.
Appendix
In a portion of this work, the possible interaction between subthreshold electrical stimuli and presumed subthreshold SDP events has been examined. The following is a calculation of the probability that there is an ongoing SDP event at the time of presentation of electrical stimulus. The electrical stimulus lasts 0.1 ms, and that will be the time unit considered. If the probability of an SDP event per 0.1 ms is p and the SDP lasts for 50 ms, the probability of a single, on-going SDP will be the summated probability of an SDP over the previous 50 ms (500 time units).
Probability (ongoing SDP) = ∑
Probability(SDP starting at time t) = p0 × (1− p)1 × (1− p)2 × …. (1− p)499
Probability(SDP starting at time t−1) = (1− p)0 × p1 ×(1− p)2 × (1− p)3 × …. (1− p)499
Probability(SDP starting at time t−499) = (1− p)0 × (1− p)1 × (1− p)2 × …. p499
Probability (ongoing SDP) = 500 × p × (1 − p)499
The probability of a second SDP occurring at t = 0 at the same time that an SDP event is in progress is:
500 × p × p × (1− p)499
If two SDPs summate to produce a spontaneous AP then this would be the probability of spontaneous AP generation.
Thus, a spontaneous AP rate of about 1 Hz leads to a probability of spontaneous AP of 0.0001 (0.1 ms)−1. For this AP probability, the probability of an ongoing SDP event is 0.15. If an ongoing SDP interacted with a subthreshold electrical stimulus to reach threshold, the success rate should be about 0.15.
References
- Bhadra N, Kilgore KL. Direct current electrical conduction block of peripheral nerve. IEEE Trans Neural Syst Rehabil Eng. 2004;12:313–324. doi: 10.1109/TNSRE.2004.834205. [DOI] [PubMed] [Google Scholar]
- Buckler KJ. A novel oxygen-sensitive potassium current in rat carotid body type I cells. J Physiol. 1997;498:649–662. doi: 10.1113/jphysiol.1997.sp021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow CC, White JA. Spontaneous action potentials due to channel fluctuations. Biophys J. 1996;71:3013–3021. doi: 10.1016/S0006-3495(96)79494-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins TR, Dib-Hajj SD, Waxman SG, Donnelly DF. Characterization and developmental changes of Na+ currents of petrosal neurons with projections to the carotid body. J Neurophysiol. 2002;88:2993–3002. doi: 10.1152/jn.00350.2002. [DOI] [PubMed] [Google Scholar]
- Donnelly DF. Chemoreceptor nerve excitation may not be proportional to catecholamine secretion. J Appl Physiol. 1996;81:657–664. doi: 10.1152/jappl.1996.81.2.657. [DOI] [PubMed] [Google Scholar]
- Donnelly DF, Doyle TP. Developmental changes in hypoxia-induced catecholamine release from rat carotid body, in vitro. J Physiol. 1994;475:267–275. doi: 10.1113/jphysiol.1994.sp020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly DF, Panisello JM, Boggs D. Effect of sodium perturbations on rat chemoreceptor spike generation: implications for a Poisson model. J Physiol. 1998;511:301–311. doi: 10.1111/j.1469-7793.1998.301bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly DF, Rigual R. Single-unit recordings of arterial chemoreceptors from mouse petrosal ganglia in vitro. J Appl Physiol. 2000;88:1489–1495. doi: 10.1152/jappl.2000.88.4.1489. [DOI] [PubMed] [Google Scholar]
- Faustino EV, Donnelly DF. An important functional role of persistent sodium current in carotid body hypoxia transduction. J Appl Physiol. 2006;101:1076–1084. doi: 10.1152/japplphysiol.00090.2006. [DOI] [PubMed] [Google Scholar]
- Gallego R, Eyzaguirre C, Monti-Bloch L. Thermal and osmotic responses of arterial receptors. J Neurophysiol. 1979;42:665–680. doi: 10.1152/jn.1979.42.3.665. [DOI] [PubMed] [Google Scholar]
- Gronblad M. Improved demonstration of exocytotic profiles in glomus cells of rat carotid body after perfusion with glutaraldehyde fixative containing a high concentration of potassium. Cell Tissue Res. 1983;229:627–637. doi: 10.1007/BF00207702. [DOI] [PubMed] [Google Scholar]
- Hales JP, Lin CS, Bostock H. Variations in excitability of single human motor axons, related to stochastic properties of nodal sodium channels. J Physiol. 2004;559:953–964. doi: 10.1113/jphysiol.2004.068726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarstrom AKM, Gage PW. Oxygen-sensing persistent sodium channels in rat hippocampus. J Physiol. 2000;529:107–118. doi: 10.1111/j.1469-7793.2000.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida Y, Koyano H, Eyzaguirre C. An intracellular study of chemosensory fibers and endings. J Neurophysiol. 1980;44:1077–1088. doi: 10.1152/jn.1980.44.6.1077. [DOI] [PubMed] [Google Scholar]
- Horn EM, Waldrop TG. Hypoxic augmentation of fast-inactivating and persistent sodium currents in rat caudal hypothalamic neurons. J Neurophysiol. 2000;84:2572–2581. doi: 10.1152/jn.2000.84.5.2572. [DOI] [PubMed] [Google Scholar]
- Iturriaga R, Alcayaga J. Neurotransmission in the carotid body: transmitters and modulators between glomus cells and petrosal ganglion nerve terminals. Brain Res Brain Res Rev. 2004;47:46–53. doi: 10.1016/j.brainresrev.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Ju YK, Saint DA, Gage PW. Hypoxia increases persistent sodium current in rat ventricular myocytes. J Physiol. 1996;497:337–347. doi: 10.1113/jphysiol.1996.sp021772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline DD, Yang T, Huang PL, Prabhakar NR. Altered respiratory responses to hypoxia in mutant mice deficient in neuronal nitric oxide synthase. J Physiol. 1998;511:273–287. doi: 10.1111/j.1469-7793.1998.273bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Barneo J, Lopez-Lopez JR, Urena J, Gonzalez C. Chemotransduction in the carotid body: K current modulated by pO2 in type I chemoreceptor cells. Science. 1988;241:580–582. doi: 10.1126/science.2456613. [DOI] [PubMed] [Google Scholar]
- Peers C. Hypoxic suppression of K+ currents in type I carotid body cells: selective effect on the Ca2+-activated K+ current. Neurosci Lett. 1990;119:253–256. doi: 10.1016/0304-3940(90)90846-2. [DOI] [PubMed] [Google Scholar]
- Raman IM, Sprunger LK, Meisler MH, Bean BP. Altered subthreshold sodium currents and disrupted firing patterns in Purkinje neurons of Scn8a mutant mice. Neuron. 1997;19:881–891. doi: 10.1016/s0896-6273(00)80969-1. [DOI] [PubMed] [Google Scholar]
- Rigual R, Almaraz L, Gonzalez C, Donnelly DF. Developmental changes in chemoreceptor nerve activity and catecholamine secretion in rabbit carotid body: possible role of Na+ and Ca2+ currents. Pflugers Arch. 2000;439:463–470. doi: 10.1007/s004249900198. [DOI] [PubMed] [Google Scholar]
- Rong W, Gourine AV, Cockayne DA, Xiang Z, Ford APDW, Spyer KM, Burnstock G. Pivotal role of nucleotide P2X2 receptor subunit of the ATP-gated ion channel mediating ventilatory responses to hypoxia. J Neurosci. 2003;23:11315–11321. doi: 10.1523/JNEUROSCI.23-36-11315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein JT. Threshold fluctuations in an N sodium channel model of the node of Ranvier. Biophys J. 1995;68:779–785. doi: 10.1016/S0006-3495(95)80252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterni LM, Bamford OS, Tomares SM, Montrose MH, Carroll JL. Developmental changes in intracellular Ca2+ response of carotid chemoreceptor cells to hypoxia. Am J Physiol Lung Cell Mol Physiol. 1995;268:L801–L808. doi: 10.1152/ajplung.1995.268.5.L801. [DOI] [PubMed] [Google Scholar]
- Verna A. The mammalian carotid body: morphological data. In: Gonzalez C, editor. The Carotid Body Chemoreceptors. Heidelberg, Germany: Springer; 1997. pp. 1–29. [Google Scholar]
- Verna A, Roumy M, Leitner LM. Loss of chemoreceptive properties of the rabbit carotid body after destruction of the glomus cells. Brain Res. 1975;100:13–23. doi: 10.1016/0006-8993(75)90239-5. [DOI] [PubMed] [Google Scholar]
- Zhang M, Zhong H, Vollmer C, Nurse CA. Co-release of ATP and ACh mediates hypoxic signalling at rat carotid body chemoreceptors. J Physiol. 2000;525:143–158. doi: 10.1111/j.1469-7793.2000.t01-1-00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Zhang M, Nurse CA. Synapse formation and hypoxic signalling in co-cultures of rat petrosal neurones and carotid body type 1 cells. J Physiol. 1997;503:599–612. doi: 10.1111/j.1469-7793.1997.599bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]