Abstract
Pronounced multivesicular release (MVR) occurs at the ribbon synapses of sensory neurones that signal via graded potential changes. As MVR increases the likelihood of postsynaptic receptor saturation, it is of interest to consider how sensory synapses overcome this problem and use MVR to encode signals of widely varying intensities. Here, I discuss three postsynaptic mechanisms that permit three different retinal synapses to utilize MVR.
Multivesicular release (MVR), or the concomitant (or near-concomitant) exocytosis of multiple vesicles at the same active zone (AZ), occurs at many synapses. Consequently, it is of interest to consider whether its occurrence has any consequence for postsynaptic neurones. The answer depends primarily upon whether postsynaptic receptors are activated differentially by the contents of one vesicle and also whether postsynaptic receptors are saturated by the contents of a single vesicle.
This question is of particular importance when considering synaptic function in the retina. Photoreceptors and bipolar cells, the primary glutamatergic retinal neurones, exhibit high rates of exocytosis sustained by virtue of a specialized presynaptic AZ containing a structure called a ribbon. For example, capacitance measurements from goldfish retinal bipolar cells have demonstrated that axon terminals containing 45–65 AZs can release thousands of vesicles in only tens of milliseconds (von Gersdorff & Matthews, 1999). Although its precise function is uncertain, the ribbon is thought to promote MVR by organizing vesicles close to the synaptic cleft (Sterling & Matthews, 2005). Additionally, at retinal ribbon synapses, a complex geometry makes glutamate concentration (glu) vary throughout the cleft, and the strength and timing of postsynaptic responses depends upon the relative placement of release sites and postsynaptic receptors (DeVries et al. 2006).
At central synapses where postsynaptic receptors are not saturated, MVR may be a component of short-term synaptic plasticity. This appears to be the case at hippocampal Schaffer collateral–CA1 synapses, at which elevating release probability (PR) promotes MVR (Oertner et al. 2002). As postsynaptic glutamate receptors (GluRs) on CA1 neurones are not saturated by the contents of a single vesicle (Mainen et al. 1999), MVR can be encoded postsynaptically.
Where saturation occurs, MVR ensures that the postsynaptic neurone is insensitive to small changes in PR. At cerebellar climbing fibre–Purkinje cell synapses, an AZ releases two to four vesicles per action potential (Wadiche & Jahr, 2001). Although reducing PR generates large changes in cleft [Glu] (∼3 mm per vesicle; Wadiche & Jahr, 2001), the effect on postsynaptic currents is mitigated by AMPAR saturation (Foster et al. 2002; Harrison & Jahr, 2003). Thus, saturation arising from MVR can enhance the reliability of a synapse: in this case, by ensuring that action potentials in climbing fibres of varying PR evoke complex spikes in Purkinje cells (Foster et al. 2002; Harrison & Jahr, 2003).
Here, I discuss the relationship between MVR and saturation of glutamatergic signalling at three synapses occurring in series in the mammalian retinal circuit: those made by rods, rod bipolar cells, and ON cone bipolar cells (Fig. 1). As the rod light response is transferred between each one, a different postsynaptic mechanism either compensates for or takes advantage of saturation to enhance the signalling capacity of the synapse.
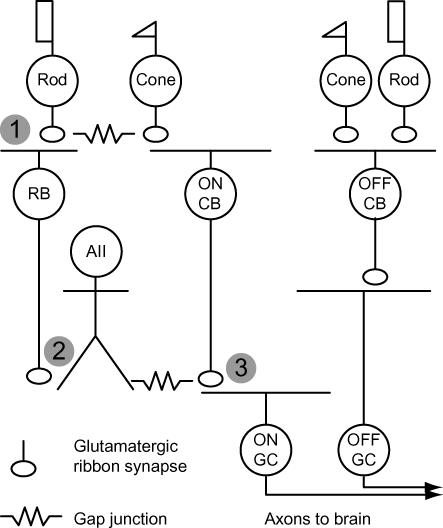
Figure 1. Basic glutamatergic circuitry of the mammalian retina.
Synapses that are considered in this mini-review occur sequentially in the retinal circuitry and are numbered (1–3). Cones make synapses with two classes of cone bipolar cell (CB): ON and OFF CBs. ON and OFF CBs make excitatory synapses with ganglion cells (GCs), the output cells of the retina. (1) Rods make synapses with an ON bipolar cell, the rod bipolar (RB), and with a subset of OFF CBs. Transmission between rods and RBs is mediated by metabotropic GluRs; ionotropic GluRs mediate transmission between rods and OFF CBs. (2) RBs contact AII amacrine cells, which are coupled by gap junctions to some types of ON CBs. RB output is transferred into ON CBs, thereby depolarizing their terminals. (3) ON CBs make synapses with ON GCs. See Field et al. (2004) for a more complete discussion.
Rod–rod bipolar synapses
The isomerization of a single rhodopsin molecule (Rh*) by a single photon generates a small (∼1 mV), hyperpolarizing voltage change sufficient to reduce the rate of ongoing exocytosis from a rod's terminal. During scotopic (i.e. night) vision, when few photons reach the retina, a rod bipolar cell must differentiate single photon responses (i.e. small changes in the release rate) at one or a few synapses from ongoing synaptic noise at the remainder (Field et al. 2005). These conditions do not favour reliable signal transfer, but a number of pre- and postsynaptic mechanisms at the rod–rod bipolar synapse improve its signal-to-noise ratio.
Presynaptically, the anatomy and physiology of the rod terminal minimize fluctuations in cleft [Glu]. The mammalian rod terminal possesses a deep invagination, at the top of which sits a single, large AZ (ribbon); apposed to the ribbon are processes from four separate neurones (Fig. 2A) (Rao-Mirotznik et al. 1995). Given the volume of the synaptic cleft and their distance (up to hundreds of nanometres) from the presynaptic AZ, it is likely that the postsynaptic metabotropic GluRs (mGluR6-containing) on the rod bipolar dendrites cannot sense individual release events but instead are exposed to a relatively low, steady-state [Glu].
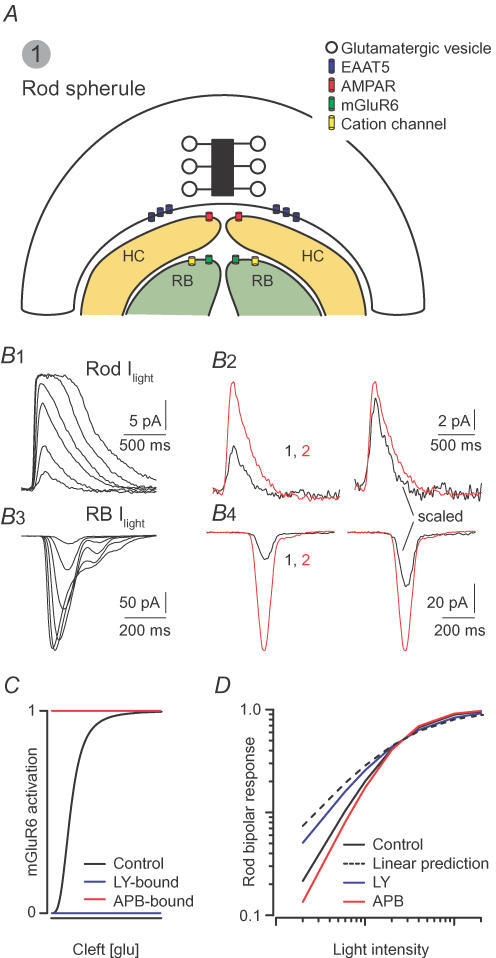
Figure 2. Saturation of postsynaptic glutamate receptor-mediated signalling underlies non-linear signal transfer at the rod–rod bipolar cell synapse.
A, the rod synapse: four postsynaptic processes are located within an invagination having a volume of ∼0.2 μm3 (Rao-Mirotznik et al. 1995). AMPARs on horizontal cell (HC) dendrites are apposed to release sites. Rod bipolar (RB) dendrites are located up to hundreds of nanometres from the presynaptic membrane. Their mGluR6-containing receptors sense a lower [Glu] than the AMPARs. B, light responses from rods and rod bipolars: B1, currents elicited in rods by light flashes (10 ms) of increasing intensity (beginning at 0.75 Rh*, doubling with each successive flash). B2, at left, currents elicited by 0.75 Rh* (black) and 1.5 Rh* (red); scaled responses at right: the response increases linearly with the number of Rh*. B3, currents elicited in rod bipolars by flashes of increasing intensity (beginning at 0.5 Rh*, doubling with each successive flash). B4, left: currents elicited by 0.5 Rh* (black) and 1 Rh* (red); scaled traces at right: doubling the flash strength produces a supra-linear increase in the rod bipolar current. C, schematic illustration of the experiments utilizing mGluR6 agonists and antagonists: LY341495 (antagonist) divides postsynaptic mGluR6 into unoccupied (available to bind glutamate and signal) and inactivated (bound to LY) populations. APB (agonist) also divides the receptors into two populations: unoccupied and activated constitutively (bound to APB). D, LY makes rod bipolar responses more linear, and APB amplifies the non-linearity. F. Rieke provided the traces in B; C and D are modelled on data presented by Sampath & Rieke (2004).
To prevent spontaneous pauses in exocytosis (arising from thermal isomerization of rhodopsin and spontaneous activation of phosphodiesterases in the outer segment; Field et al. 2005) from generating postsynaptic responses, the tonic release rate must be high (at least 40–80 Hz) to make the average inter-event interval shorter than the time course of the smallest postsynaptic response (Rao et al. 1994; van Rossum & Smith, 1998). Supporting this notion, capacitance measurements of exocytosis from isolated salamander rods have yielded tonic release rates of ∼55–80 Hz per AZ (∼400 Hz distributed over 5–7 AZs) (Rieke & Schwartz, 1996). A recently proposed model accounting for cleft geometry, diffusion, and glutamate transport suggests that release rates of 30–500 Hz from mouse rods generate a cleft [Glu] of 10–100 μM (within the operating range of mGluR6; EC50 ≈ 10 μM) (Hasegawa et al. 2006).
Postsynaptically, mGluR6 is coupled to non-specific cation channels by a second messenger cascade that is not elucidated fully; mGluR6 activation closes these channels, and decreased cleft [Glu] elicited by light reduces mGluR6 activity and allows them to open (Nawy & Jahr, 1990; Shiells & Falk, 1990). This signalling cascade is maximally activated in darkness, and saturation within it prevents small reductions in cleft [Glu] from eliciting channel opening (whether mGluRs themselves are saturated is uncertain) (Sampath & Rieke, 2004). Consequently, saturation allows the rod bipolar cell to apply a threshold to rod signals (van Rossum & Smith, 1998; Sampath & Rieke, 2004). This threshold forces the rod bipolar cell to sacrifice sensitivity for reliability: only the largest 25% of rod single photon responses lower cleft [Glu] sufficiently to elicit a postsynaptic rod bipolar response, but the rod bipolar cell never depolarizes in the absence of a rod response (i.e. false positive responses are eliminated) (Field & Rieke, 2002).
Since saturation biases postsynaptic responses toward larger rod responses, rod bipolars combine the outputs of multiple rods non-linearly (as postulated by Van Rossum & Smith, 1998): Field & Rieke (2002) demonstrated that the rod bipolar response to a light flash that generates two Rh* per rod is much larger than the sum of two single Rh* per rod responses (Fig. 2B). Consequently, the synaptic gain at the rod–rod bipolar synapse is higher for larger voltage changes. Sampath & Rieke (2004) built upon this work to demonstrate that the magnitude of the non-linearity is determined by the activity of the second messenger cascade rather than the number of receptors available to bind synaptically released glutamate (Fig. 2C and D).
Additional evidence that the locus of this non-linearity is the mGluR6-linked second messenger pathway comes from the work of Field & Rieke (2002), which demonstrated that mammalian OFF bipolar cells – in which glutamatergic neurotransmission is mediated by AMPA/kainate receptors (Attwell et al. 1987b; DeVries, 2000) – sum rod inputs linearly (over the same range of light intensities in which rod bipolar responses are non-linear). Interestingly, amphibian OFF bipolar cells exhibit a non-linearity that is larger for smaller rod voltage changes (Attwell et al. 1987a; Belgum & Copenhagen, 1988). Electrical coupling between amphibian rods may be responsible for at least part of the difference between mammalian and amphibian rod–OFF bipolar signal transfer (Attwell et al. 1987a), though other significant differences between mammalian and amphibian retinae (e.g. the amphibian retina lacks a dedicated rod bipolar cell) probably contribute to it.
Rod bipolar–AII amacrine cell synapses
Rod bipolar cells transfer the thresholded rod output to interneurones, including the AII amacrine (Fig. 3A). Under scotopic conditions, the AII faces the same signal-processing problem as the rod bipolar: how to separate sparse synaptic signals from synaptic noise. Field & Rieke (2002) suggested that it would be advantageous for the threshold generated at the rod–rod bipolar synapse to be preserved at the rod bipolar–AII synapse. MVR at the rod bipolar–AII synapse may serve this thresholding role by ensuring that rod bipolar signals are larger than quantal synaptic noise.
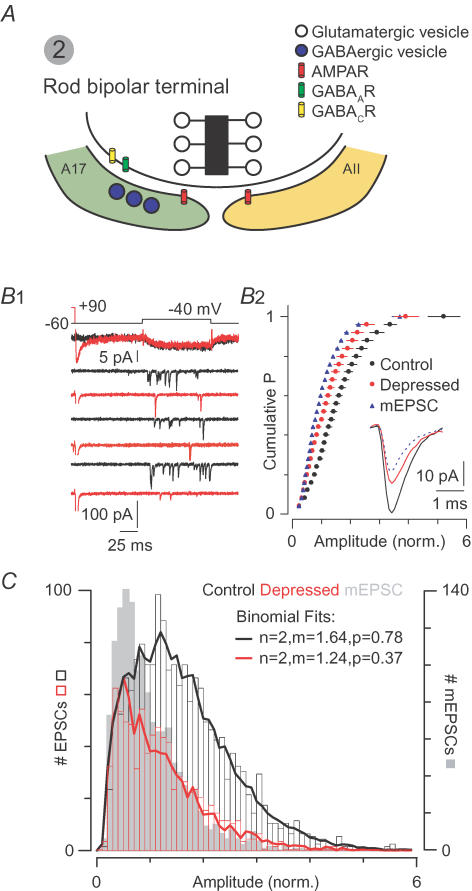
Figure 3. MVR at the rod bipolar–AII synapse.
A, the rod bipolar synapse: AII and A17 amacrine cells express Ca2+-permeable AMPARs; NMDARs are not present. The A17 makes a reciprocal GABAergic synapse with the bipolar terminal. B, coordinated MVR during desynchronized release: B1, small presynaptic voltage steps elicit Ca2+ currents (top) and EPSCs. A depressing stimulus (Ca2+ tail current, red) precedes the step in alternate trials and makes the EPSCs smaller and less frequent. B2, depression reduces EPSC amplitudes almost to those of quantal mEPSCs (normalized cumulative probability distributions pooled from 7 experiments; inset: average EPSCs). C, MVR obeys binomial statistics, indicating that quanta are summed linearly: normalized amplitude distributions for quantal mEPSCs (grey bars), small EPSCs (black bars), and depressed EPSCs (red bars) pooled from 7 experiments. Lines are predicted distributions generated by combining mEPSCs at random in a Monte Carlo simulation. B and C are modelled on data from Singer et al. (2004).
The rod bipolar terminal is capable of highly synchronized MVR (2–4 vesicles ms−1 AZ−1, like the climbing fibre synapse) (Singer et al. 2004). Importantly, the postsynaptic AMPARs (NMDARs are absent from this synapse) are well-suited to encode MVR: they have low affinity for glutamate, exhibit rapid deactivation kinetics, and desensitize slowly relative to deactivation (Morkve et al. 2002; Veruki et al. 2003). Consequently, despite high release rates, receptor desensitization does not play a significant role in shaping transmission at this synapse (Singer et al. 2004; Singer & Diamond, 2006).
To examine the interactions between quanta during MVR at rod bipolar synapses, Singer et al. (2004) evoked small multiquantal EPSCs using stimuli that desynchronized release. These EPSCs arose from the virtually simultaneous release of, on average, two vesicles from a single AZ (Fig. 3B). Similar coordinated, multivesicular EPSCs have been observed at the salamander rod–OFF bipolar cell synapse (Suryanarayanan & Slaughter, 2006) and at a non-retinal ribbon synapse: the cochlear hair cell synapse (Glowatzki & Fuchs, 2002). A quantal analysis of the EPSCs recorded in AIIs demonstrated that they arise from the linear summation of quanta, implying that the postsynaptic AMPARs are far from saturation (Fig. 3C). Additional evidence for linear summation and absence of saturation at the rod bipolar–AII synapse comes from experiments demonstrating that EPSC amplitude varies linearly with the number of vesicles released: in paired-pulse experiments, when the magnitude of the first (depressing) stimulus was varied widely, the quantal content of the second (depressed) EPSC was reduced by almost exactly the number of quanta released to generate the first EPSC (Singer & Diamond, 2006). Thus, AIIs can encode MVR effectively, thereby expanding the rod bipolar synapse's operating range.
Cone bipolar–ganglion cell synapses
Ultimately, photoreceptor-derived signals are transferred to ganglion cells (GCs), the output cells of the retina, by cone bipolar cells (Fig. 4A). MVR saturates postsynaptic AMPARs at ON cone bipolar cell synapses, but nonetheless can be encoded by the postsynaptic neurones because they express peri-synaptic NMDARs that sense a relatively low [Glu] resulting from glutamate spillover out of the cleft (Matsui et al. 2001; Chen & Diamond, 2002; Sagdullaev et al. 2006).
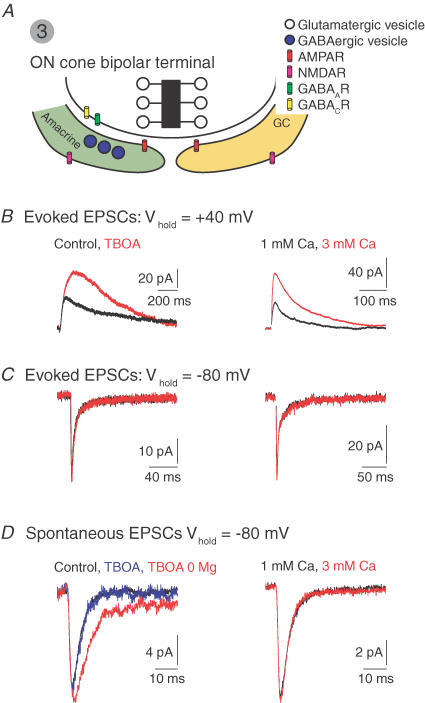
Figure 4. MVR at the ON cone bipolar–ganglion cell synapse.
A, the ON cone bipolar synapse. Postsynaptic amacrine and GCs express synaptic AMPARs and peri-synaptic NMDARs. The amacrine makes a reciprocal GABAergic synapse with the terminal. B, blocking glutamate uptake with DL-threo-β-benzyloxyaspartate (TBOA, left) or increasing PR (elevating external [Ca2+], right), potentiates evoked NMDAR- but not AMPAR-mediated EPSCs C. D, spontaneous AMPAR-mediated EPSCs are unaffected by TBOA and elevated [Ca2+]. In the absence of Mg2+ (permitting NMDARs activation at negative potentials), TBOA induces an NMDAR-mediated component of the sEPSC. 0 Mg2+ alone does not reveal an NMDAR-mediated component. J. Diamond provided the illustrated traces.
Evoked EPSCs recorded in ON GCs (and in some ON amacrine cells) exhibit pronounced NMDAR-mediated components, but quantal mEPSCs recorded in these neurones are mediated exclusively by AMPARs (even under experimental conditions favouring NMDAR activation) (Chen & Diamond, 2002). Thus, AMPARs and NMDARs cannot be co-localized postsynaptically. Rather, NMDARs are peri-synaptic, and they are exposed to glutamate only after it has diffused some distance from the release site. This has been demonstrated in electrophysiological (Chen & Diamond, 2002) and anatomical (Zhang & Diamond, 2006) studies. A peri-synaptic location allows NMDARs to respond to released glutamate when synaptic AMPARs are saturated by MVR. Supporting the notion that AMPARs are saturated, increasing PR or blocking glutamate uptake potentiates NMDAR- but not AMPAR-mediated synaptic currents (Fig. 4B–D) (Chen & Diamond, 2002).
Tonic inhibition mediated by activation of GABACRs in the bipolar cell terminal regulates PR (and MVR) and, by extension, AMPAR occupancy and peri-synaptic NMDAR activation (Matsui et al. 2001; Sagdullaev et al. 2006). Matsui et al. (2001) found that a GABACR antagonist induced an NMDAR-mediated component in the light-evoked EPSC recorded in ON amacrine cells without affecting the AMPAR-mediated one.
Sagdullaev et al. (2006) demonstrated directly that GABACR activation reduces MVR: in the presence of low-affinity AMPAR antagonist (which relieves AMPAR saturation because antagonist efficacy varies inversely with cleft [Glu]), lowering PR affected spontaneous EPSC (sEPSC) amplitude only when GABACRs were blocked. Thus, in the absence of GABACR-mediated inhibition, changing PR changed cleft [Glu]. This indicates that sEPSCs were multiquantal in the absence of GABACR-mediated inhibition and suggests that MVR can saturate postsynaptic AMPARs at this synapse.
In keeping with the results of Matsui et al. (2001) and Chen & Diamond (2002), Sagdullaev et al. (2006) also demonstrated that NMDARs on ON GCs are exposed to lower [Glu] than AMPARs. Interestingly, however, these authors found that NMDARs on OFF GCs are activated by the release of a single glutamatergic vesicle, giving rise to the hypothesis that postsynaptic NMDARs at OFF bipolar cell synapses are co-localized with AMPARs. This hypothesis, however, is not supported by electron micrographic studies of NMDAR distribution (Zhang & Diamond, 2006), and consequently, the mechanism underlying the differences between NMDAR activation by single quanta at ON and OFF bipolar cell synapses remain uncertain. The fact that GABACRs are found primarily in ON bipolar cell terminals, though, raises interesting questions about the role of tonic inhibition in setting the dynamic range of signalling (perhaps by controlling MVR) in ON and OFF pathways.
Synaptic mechanisms underlying MVR in the retina
MVR at conventional inhibitory synapses made by cerebellar interneurons exhibits the same degree of asynchrony as does univesicular release, indicating that the activity of individual release sites does not need to be coordinated to permit MVR (Auger et al. 1998). Rather, MVR at some conventional synapses may be simply a consequence of elevated PR (Tong & Jahr, 1994; Wadiche & Jahr, 2001) although high PR does not result necessarily in MVR (Silver et al. 2003). The extent of coordination of MVR at retinal ribbon synapses, though, indicates that MVR does not arise simply by chance.
What synaptic mechanism, then, underlies MVR at retinal ribbon synapses? It is probable that MVR occurs when multiple, closely spaced release sites sense the same elevation in presynaptic [Ca2+]. Spatiotemporally restricted [Ca2+] microdomains could arise from the opening of one or a few Ca2+ channels. At cochlear hair cell ribbon synapses, where MVR resembles that observed at bipolar synapses (Glowatzki & Fuchs, 2002), exocytosis of a single vesicle is regulated by the opening of one or a few Ca2+ channels located within nanometres of a release site (Brandt et al. 2005). Experimental outcomes of two studies of retinal ribbon synapses, however, argue against this mechanism of MVR: both in AIIs and in OFF bipolar cells postsynaptic to salamander rods, EPSCs reflecting coordinated MVR were recorded during asynchronous release, driven by residual intraterminal [Ca2+], hundreds of milliseconds following the closure of presynaptic Ca2+ channels (Singer et al. 2004; Suryanarayanan & Slaughter, 2006).
The [Ca2+] elevation driving MVR instead may arise from Ca2+-induced Ca2+ release (CICR). At one conventional synapse (the cerebellar basket–Purkinje cell synapse), spontaneous MVR is driven by localized [Ca2+] transients mediated by RyR activation (Llano et al. 2000). The recent work of Suryanarayanan & Slaughter (2006) demonstrated clearly that RyR-mediated CICR contributes substantially to exocytosis and probably is responsible for coordinating MVR from salamander rod terminals (Suryanarayanan & Slaughter, 2006). Although the mechanism of MVR at other retinal (and non-retinal) ribbon synapses is not understood fully, CICR may play an important role in transmission at these synapses. Supporting this notion, RyRs have been shown to modulate exocytosis from frog vestibular hair cells (Lelli et al. 2003).
MVR and AMPAR saturation in the retina
Where MVR occurs, changes in presynaptic activity alter the cleft [Glu]. At rod synapses, as at climbing fibre synapses in the cerebellum, MVR saturates postsynaptic GluR-mediated signalling, generating a safety factor that makes synaptic transmission more reliable and less sensitive to small changes in glutamate release. In contrast, both AII amacrine cells and ON GCs encode MVR effectively, thereby expanding their dynamic ranges, but they accomplish this in two different ways.
AII amacrine cells express low-affinity AMPARs that are not saturated even by high cleft [Glu] and can sum linearly the contents of at least four quanta. Postsynaptic AMPARs in ON GCs, however, are saturated by the contents of a single vesicle, but these cells express peri-synaptically high-affinity NMDARs that are activated by glutamate spillover. As they sense a relatively low peri-synaptic [Glu], these NMDARs can encode MVR without becoming saturated.
Why should the postsynaptic neurones at rod bipolar and ON cone bipolar synapses use different strategies to encode MVR? I suggest that the difference reflects the roles that the two cells play in the retinal circuitry. Although they do possess voltage-gated Na+ channels that can generate small sodium spikelets, AIIs are not spiking neurones in any classical sense. The postsynaptic depolarization generated in AIIs by rod bipolar input is passed through electrical synapses to ON cone bipolar cell terminals. As both postsynaptic AMPARs and these gap junctions are located in close proximity to each other in the distal dendrites of the AIIs, it is likely that a significant amount of the rod bipolar signal propagates passively into the ON cone bipolar terminal. Linear summation of quanta at the rod bipolar–AII synapse may permit the depolarization of the ON cone bipolar terminal to vary directly with the extent of MVR.
In contrast to the AII, GCs encode bipolar cell output as action potentials. Owing to the highly non-linear interactions between synaptic conductances and spike-generating conductances, a large, fast AMPAR-mediated conductance is not a good modulator of spike frequency. Rather, the primary role of AMPARs at GC synapses may be to generate a postsynaptic depolarization sufficient to remove the Mg2+ block of peri-synaptic NMDARs, as has been suggested (Diamond & Copenhagen, 1993). Activation of these NMDARs by glutamate spillover will generate a slow depolarizing conductance that varies fairly linearly with the cone bipolar cell activity and could allow the GC to encode presynaptic membrane potential in spike frequency.
Acknowledgments
I am particularly grateful to Dr J. Diamond for numerous discussions on the subjects of multivesicular release and receptor saturation; many of the ideas presented in this manuscript were developed during those talks. I thank Drs S. deVries, J. Diamond, W. Li, F. Rieke and A.P. Sampath for helpful criticism, and I thank Drs Diamond and Rieke for providing the data illustrated in Figs 2 and 4.
References
- Attwell D, Borges S, Wu SM, Wilson M. Signal clipping by the rod output synapse. Nature. 1987a;328:522–524. doi: 10.1038/328522a0. [DOI] [PubMed] [Google Scholar]
- Attwell D, Mobbs P, Tessier-Lavigne M, Wilson M. Neurotransmitter-induced currents in retinal bipolar cells of the axolotl, Ambystoma mexicanum. J Physiol. 1987b;387:125–161. doi: 10.1113/jphysiol.1987.sp016567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger C, Kondo S, Marty A. Multivesicular release at single functional synaptic sites in cerebellar stellate and basket cells. J Neurosci. 1998;18:4532–4547. doi: 10.1523/JNEUROSCI.18-12-04532.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgum JH, Copenhagen DR. Synaptic transfer of rod signals to horizontal and bipolar cells in the retina of the toad (Bufo marinus) J Physiol. 1988;396:225–245. doi: 10.1113/jphysiol.1988.sp016960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt A, Khimich D, Moser T. Few CaV1.3 channels regulate the exocytosis of a synaptic vesicle at the hair cell ribbon synapse. J Neurosci. 2005;25:11577–11585. doi: 10.1523/JNEUROSCI.3411-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Diamond JS. Synaptically released glutamate activates extrasynaptic NMDA receptors on cells in the ganglion cell layer of rat retina. J Neurosci. 2002;22:2165–2173. doi: 10.1523/JNEUROSCI.22-06-02165.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries SH. Bipolar cells use kainate and AMPA receptors to filter visual information into separate channels. Neuron. 2000;28:847–856. doi: 10.1016/s0896-6273(00)00158-6. [DOI] [PubMed] [Google Scholar]
- DeVries SH, Li W, Saszik S. Parallel processing in two transmitter microenvironments at the cone photoreceptor synapse. Neuron. 2006;50:735–748. doi: 10.1016/j.neuron.2006.04.034. [DOI] [PubMed] [Google Scholar]
- Diamond JS, Copenhagen DR. The contribution of NMDA and non-NMDA receptors to the light-evoked input-output characteristics of retinal ganglion cells. Neuron. 1993;11:725–738. doi: 10.1016/0896-6273(93)90082-3. [DOI] [PubMed] [Google Scholar]
- Field GD, Rieke F. Nonlinear signal transfer from mouse rods to bipolar cells and implications for visual sensitivity. Neuron. 2002;34:773–785. doi: 10.1016/s0896-6273(02)00700-6. [DOI] [PubMed] [Google Scholar]
- Field GD, Sampath AP, Rieke F. Retinal processing near absolute threshold: from behavior to mechanism. Annu Rev Physiol. 2005;67:491–514. doi: 10.1146/annurev.physiol.67.031103.151256. [DOI] [PubMed] [Google Scholar]
- Foster KA, Kreitzer AC, Regehr WG. Interaction of postsynaptic receptor saturation with presynaptic mechanisms produces a reliable synapse. Neuron. 2002;36:1115–1126. doi: 10.1016/s0896-6273(02)01106-6. [DOI] [PubMed] [Google Scholar]
- Glowatzki E, Fuchs PA. Transmitter release at the hair cell ribbon synapse. Nat Neurosci. 2002;5:147–154. doi: 10.1038/nn796. [DOI] [PubMed] [Google Scholar]
- Harrison J, Jahr CE. Receptor occupancy limits synaptic depression at climbing fiber synapses. J Neurosci. 2003;23:377–383. doi: 10.1523/JNEUROSCI.23-02-00377.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa J, Obara T, Tanaka K, Tachibana M. High-density presynaptic transporters are required for glutamate removal from the first visual synapse. Neuron. 2006;50:63–74. doi: 10.1016/j.neuron.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Lelli A, Perin P, Martini M, Ciubotaru CD, Prigioni I, Valli P, Rossi ML, Mammano F. Presynaptic calcium stores modulate afferent release in vestibular hair cells. J Neurosci. 2003;23:6894–6903. doi: 10.1523/JNEUROSCI.23-17-06894.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano I, Gonzalez J, Caputo C, Lai FA, Blayney LM, Tan YP, Marty A. Presynaptic calcium stores underlie large-amplitude miniature IPSCs and spontaneous calcium transients. Nat Neurosci. 2000;3:1256–1265. doi: 10.1038/81781. [DOI] [PubMed] [Google Scholar]
- Mainen ZF, Malinow R, Svoboda K. Synaptic calcium transients in single spines indicate that NMDA receptors are not saturated. Nature. 1999;399:151–155. doi: 10.1038/20187. [DOI] [PubMed] [Google Scholar]
- Matsui K, Hasegawa J, Tachibana M. Modulation of excitatory synaptic transmission by GABAC receptor-mediated feedback in the mouse inner retina. J Neurophysiol. 2001;86:2285–2298. doi: 10.1152/jn.2001.86.5.2285. [DOI] [PubMed] [Google Scholar]
- Morkve SH, Veruki ML, Hartveit E. Functional characteristics of non-NMDA-type ionotropic glutamate receptor channels in AII amacrine cells in rat retina. J Physiol. 2002;542:147–165. doi: 10.1113/jphysiol.2002.020305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawy S, Jahr CE. Suppression by glutamate of cGMP-activated conductance in retinal bipolar cells. Nature. 1990;346:269–271. doi: 10.1038/346269a0. [DOI] [PubMed] [Google Scholar]
- Oertner TG, Sabatini BL, Nimchinsky EA, Svoboda K. Facilitation at single synapses probed with optical quantal analysis. Nat Neurosci. 2002;5:657–664. doi: 10.1038/nn867. [DOI] [PubMed] [Google Scholar]
- Rao R, Buchsbaum G, Sterling P. Rate of quantal transmitter release at the mammalian rod synapse. Biophys J. 1994;67:57–63. doi: 10.1016/S0006-3495(94)80454-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao-Mirotznik R, Harkins AB, Buchsbaum G, Sterling P. Mammalian rod terminal: architecture of a binary synapse. Neuron. 1995;14:561–569. doi: 10.1016/0896-6273(95)90312-7. [DOI] [PubMed] [Google Scholar]
- Rieke F, Schwartz EA. Asynchronous transmitter release: control of exocytosis and endocytosis at the salamander rod synapse. J Physiol. 1996;493:1–8. doi: 10.1113/jphysiol.1996.sp021360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagdullaev BT, McCall MA, Lukasiewicz PD. Presynaptic inhibition modulates spillover, creating distinct dynamic response ranges of sensory output. Neuron. 2006;50:923–935. doi: 10.1016/j.neuron.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Sampath AP, Rieke F. Selective transmission of single photon responses by saturation at the rod-to-rod bipolar synapse. Neuron. 2004;41:431–443. doi: 10.1016/s0896-6273(04)00005-4. [DOI] [PubMed] [Google Scholar]
- Shiells RA, Falk G. Glutamate receptors of rod bipolar cells are linked to a cyclic GMP cascade via a G-protein. Proc Biol Sci. 1990;242:91–94. doi: 10.1098/rspb.1990.0109. [DOI] [PubMed] [Google Scholar]
- Silver RA, Lubke J, Sakmann B, Feldmeyer D. High-probability uniquantal transmission at excitatory synapses in barrel cortex. Science. 2003;302:1981–1984. doi: 10.1126/science.1087160. [DOI] [PubMed] [Google Scholar]
- Singer JH, Diamond JS. Vesicle depletion and synaptic depression at a mammalian ribbon synapse. J Neurophysiol. 2006;95:3191–3198. doi: 10.1152/jn.01309.2005. [DOI] [PubMed] [Google Scholar]
- Singer JH, Lassova L, Vardi N, Diamond JS. Coordinated multivesicular release at a mammalian ribbon synapse. Nat Neurosci. 2004;7:826–833. doi: 10.1038/nn1280. [DOI] [PubMed] [Google Scholar]
- Sterling P, Matthews G. Structure and function of ribbon synapses. Trends Neurosci. 2005;28:20–29. doi: 10.1016/j.tins.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Suryanarayanan A, Slaughter MM. Synaptic transmission mediated by internal calcium stores in rod photoreceptors. J Neurosci. 2006;26:1759–1766. doi: 10.1523/JNEUROSCI.3895-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong G, Jahr CE. Multivesicular release from excitatory synapses of cultured hippocampal neurons. Neuron. 1994;12:51–59. doi: 10.1016/0896-6273(94)90151-1. [DOI] [PubMed] [Google Scholar]
- van Rossum MC, Smith RG. Noise removal at the rod synapse of mammalian retina. Vis Neurosci. 1998;15:809–821. doi: 10.1017/s0952523898155037. [DOI] [PubMed] [Google Scholar]
- Veruki ML, Morkve SH, Hartveit E. Functional properties of spontaneous EPSCs and non-NMDA receptors in rod amacrine (AII) cells in the rat retina. J Physiol. 2003;549:759–774. doi: 10.1113/jphysiol.2003.039982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gersdorff H, Matthews G. Electrophysiology of synaptic vesicle cycling. Annu Rev Physiol. 1999;61:725–752. doi: 10.1146/annurev.physiol.61.1.725. [DOI] [PubMed] [Google Scholar]
- Wadiche JI, Jahr CE. Multivesicular release at climbing fiber-Purkinje cell synapses. Neuron. 2001;32:301–313. doi: 10.1016/s0896-6273(01)00488-3. [DOI] [PubMed] [Google Scholar]
- Zhang J, Diamond JS. Distinct perisynaptic and synaptic localization of NMDA and AMPA receptors on ganglion cells in rat retina. J Comp Neurol. 2006;498:810–820. doi: 10.1002/cne.21089. [DOI] [PMC free article] [PubMed] [Google Scholar]