Abstract
The Ca2+ release-activated Ca2+ (CRAC) channel is a plasma membrane Ca2+ entry pathway activated by endoplasmic reticulum (ER) Ca2+ store depletion. STIM1 proteins function as ER Ca2+ sensors and regulate CRAC channel activation. Recent studies have demonstrated that CRAC channels are encoded by the human Orai1 gene and a homologous Drosophila gene. C. elegans intestinal cells express a store-operated Ca2+ channel (SOCC) regulated by STIM-1. We cloned a full-length C. elegans cDNA that encodes a 293 amino acid protein, ORAI-1, homologous to human and Drosophila Orai1 proteins. ORAI-1 GFP reporters are co-expressed with STIM-1 in the gonad and intestine. Inositol 1,4,5-trisphosphate (IP3)-dependent Ca2+ signalling regulates C. elegans gonad function, fertility and rhythmic posterior body wall muscle contraction (pBoc) required for defecation. RNA interference (RNAi) silencing of orai-1 expression phenocopies stim-1 knockdown and causes sterility and prevents intestinal cell SOCC activation, but has no effect on pBoc or intestinal Ca2+ signalling. Orai-1 RNAi suppresses pBoc defects induced by intestinal expression of a STIM-1 Ca2+-binding mutant, indicating that the proteins function in a common pathway. Co-expression of stim-1 and orai-1 cDNAs in HEK293 cells induces large inwardly rectifying cation currents activated by ER Ca2+ depletion. The properties of this current recapitulate those of the native SOCC current. We conclude that C. elegans expresses bona fide CRAC channels that require the function of Orai1- and STIM1-related proteins. CRAC channels thus arose very early in animal evolution. In C. elegans, CRAC channels do not play obligate roles in all IP3-dependent signalling processes and ER Ca2+ homeostasis. Instead, we suggest that CRAC channels carry out highly specialized and cell-specific signalling roles and that they may function as a failsafe mechanism to prevent Ca2+ store depletion under pathophysiological and stress conditions.
Intracellular Ca2+ signals regulate a diverse array of physiological processes including secretion, muscle contraction, cell growth and gene expression (Berridge et al. 2003). Changes in cytoplasmic Ca2+ levels are brought about by altered flux across the plasma membrane and by release from intracellular compartments. The endoplasmic reticulum (ER) is a major cellular Ca2+ store. Calcium release from the ER is mediated by activation of inositol 1,4,5-trisphosphate (IP3)-regulated Ca2+ channels.
Store-operated Ca2+ entry (SOCE) is activated by depletion of ER Ca2+ stores and is a widely observed mechanism of plasma membrane Ca2+ influx (Parekh & Penner, 1997; Venkatachalam et al. 2002; Parekh & Putney, 2005). Store-operated Ca2+ channel (SOCC) activity was first identified in 1992 by patch-clamp electrophysiology (Hoth & Penner, 1992). This SOCC was termed the Ca2+ release-activated Ca2+ (CRAC) channel and is the most extensively characterized SOCE pathway (Parekh & Penner, 1997; Parekh & Putney, 2005). The mechanisms that regulate SOCE and the molecular identity of the CRAC channel have been the subjects of intense investigation for over 15 years.
Two recent discoveries have dramatically advanced our understanding of SOCE. RNA interference (RNAi) screening in Drosophila S2 cells first identified stromal interaction molecule 1 (STIM1) as an essential component of CRAC activation (Roos et al. 2005). Studies from several laboratories have established that Drosophila and human STIM1 homologues function as ER Ca2+ sensors (Liou et al. 2005; Zhang et al. 2005; Soboloff et al. 2006a; Spassova et al. 2006). In response to Ca2+ store depletion, STIM1 undergoes redistribution from a diffuse ER localization to a punctate localization (Liou et al. 2005; Zhang et al. 2005; Baba et al. 2006; Wu et al. 2006; Xu et al. 2006) that corresponds to sites of ER–plasma membrane contact (Wu et al. 2006). This redistribution in turn activates CRAC and SOCE (Zhang et al. 2005; Liou et al. 2005; Luik et al. 2006; Soboloff et al. 2006a,Spassova et al. 2006; Wu et al. 2006). The sites of punctate STIM1 localization also appear to be sites of localized Ca2+ influx and CRAC activity (Luik et al. 2006).
In an elegant study, Feske et al. (2006) used linkage analysis and a Drosophila S2 cell genome-wide RNAi screen to identify Orai1 as an essential component of the CRAC channel (see also Vig et al. 2006b; Zhang et al. 2006;). Work from several laboratories indicates that Orai1 homologues are essential components of the CRAC channel and probably function as pore subunits (Prakriya et al. 2006; Vig et al. 2006a; Yeromin et al. 2006). Co-expression of STIM1 and Orai1 homologues dramatically increases SOCE and CRAC channel activity (Mercer et al. 2006; Peinelt et al. 2006; Prakriya et al. 2006; Soboloff et al. 2006b,Vig et al. 2006a; Yeromin et al. 2006;). During SOCE/CRAC channel activation, Orai1 redistributes from a diffuse localization pattern in the plasma membrane and colocalizes with STIM1 puncta (Luik et al. 2006; Xu et al. 2006). Co-immunoprecipitation studies suggest that STIM1 and Orai1 homologues bind to each other directly or through intermediary proteins (Vig et al. 2006a; Yeromin et al. 2006). Together, these observations have led to the hypothesis that redistribution and subsequent co-association of STIM1 and Orai1 homologues in response to ER Ca2+ depletion activates CRAC channels and SOCE.
Depletion of ER Ca2+ stores in C. elegans intestinal cells activates a SOCC current with many of the same biophysical properties as ICRAC (Estevez et al. 2003). We demonstrated recently that a single STIM1 homologue encoding gene stim-1 is present in the worm genome. Green fluorescent protein (GFP)-tagged STIM-1 is expressed in several cell types including cells of the intestine and gonad (Yan et al. 2006). IP3-dependent Ca2+ signals control the contractile properties of gonad cells required for ovulation in C. elegans (Clandinin et al. 1998; Bui & Sternberg, 2002; Kariya et al. 2004; Yin et al. 2004). Oscillatory Ca2+ signals with a period of ∼50 s occur in the worm intestine and trigger rhythmic posterior body wall muscle contraction (pBoc) required for defecation (Dal Santo et al. 1999; Espelt et al. 2005; Teramoto & Iwasaki, 2006). SOCE is thought to be essential for maintenance of ER Ca2+ stores and intracellular Ca2+ signalling (Parekh & Penner, 1997; Venkatachalam et al. 2002; Parekh & Putney, 2005). RNA interference silencing of C. elegans stim-1 expression causes complete sterility and prevents activation of intestinal SOCCs but surprisingly has no effect on pBoc or intestinal Ca2+ oscillations (Yan et al. 2006). These and other findings suggest that SOCE is not essential for certain oscillatory Ca2+ signalling processes or for maintenance of store Ca2+ levels in C. elegans, and raise important questions regarding the function of SOCE and SOCCs under normal and pathophysiological conditions (Yan et al. 2006).
In an effort to further exploit C. elegans as a model system for characterizing SOCE, we conducted a BLAST search and identified a single gene (orai-1) that encodes a 293 amino acid protein with 55% overall similarity to human Orai1. ORAI-1::GFP and STIM-1::GFP reporters are co-expressed in specific cell and tissue types, and knockdown of orai-1 expression phenocopies the effect of stim-1 RNAi. Orai-1 RNAi suppresses pBoc defects induced by expression of a STIM-1 EF hand mutant in the worm intestine, indicating that the two proteins function together. Furthermore, co-expression of stim-1 and orai-1 cDNAs in HEK293 cells induces large inwardly rectifying cation currents activated by ER Ca2+ depletion. Our results demonstrate that C. elegans expresses bona fide CRAC channels and that, as in Drosophila and mammals, channel activity requires the function of STIM1 and Orai1-related proteins. STIM1 and Orai1 homologues have not yet been detected in plants and single-celled organisms, suggesting that CRAC channels arose very early in animal evolution and that they carry out conserved physiological functions. The present work and our previous studies (Estevez et al. 2003; Yan et al. 2006) underscore the utility of C. elegans as a model system for developing a detailed molecular and integrative physiological understanding of CRAC channel function and regulation.
Methods
Celegans strains
Nematodes were cultured using standard methods (Brenner, 1974). Wild-type worms were the Bristol N2 strain. The following mutant/transgenic strains were used: NL2098 [rrf-1(pk1417)], VP303 [rde-1(ne219); kbEx200] (Espelt et al. 2005), VP506 [kbIs15(Pstim-1::STIM-1(D55A; D57A)::GFP)] (Yan et al. 2006) and BC10427 [sEx10427(Porai-1::GFP)]. All worm strains were grown at 16–25°C.
Analysis of sheath cell contraction and ovulation
Young adult worms that had undergone no more than one ovulation attempt were anaesthetized for 30–40 mins in M9 solution containing 0.1% tricaine and 0.01% tetramisole, mounted onto 2% agarose pads (McCarter et al. 1999) and then imaged at room temperature (22–23°C) by differential interference contrast (DIC) microscopy using a Nikon (Melville, NY, USA) Eclipse TE2000 inverted microscope and a Superfluor 40×/1.3 NA oil immersion objective lens. Images were recorded at 30 frames s−1 on videotape using a DAGE-MTI (Michigan City, Indiana) CCD100 camera and analysed offline. Sheath contractions were counted in 1 min intervals.
Measurement of brood size
Brood size was quantified at 25°C by transferring single L4 larvae to new growth plates daily for 4 days. The number of progeny on each plate was counted 24–36 h after eggs hatched.
Characterization of pBoc cycle
Posterior body wall muscle contraction (pBoc) was monitored at room temperature (21–22°C) in 2-day-old adult worms. A minimum of 10 pBoc cycles were measured in each animal. Worms were imaged using a Zeiss Stemi SV11 M2BIO stereo dissecting microscope (Kramer Scientific Corp., Valley Cottage, NY, USA), equipped with a DAGE-MTI (Michigan City, IN, USA) DC2000 CCD camera. pBoc rhythmicity in individual worms was assessed by calculating the coefficient of variance (CV), which is the standard deviation expressed as a percentage of the mean.
Dissection and fluorescence imaging of intestines
Calcium oscillations were measured in isolated intestines as previously described (Espelt et al. 2005). Briefly, worms were placed in control saline (mm: 137 NaCl, 5 KCl, 1 MgCl2, 1 MgSO4, 0.5 CaCl2, 10 Hepes, 5 Glucose, 2 l-asparagine, 0.5 l-cysteine, 2 l-glutamine, 0.5 l-methionine, 1.6 l-tyrosine, 27 sucrose, pH 7.3, 340 mosol l−1) and cut behind the pharynx using a 26-gauge needle. The hydrostatic pressure in the worm spontaneously extruded the intestine, which remained attached to the rectum and the posterior end of the animal. Isolated intestines were incubated for 10 min in bath saline containing 5 μm fluo-4 AM and 1% bovine serum albumin (BSA). Imaging was performed at room temperature (21–22°C) using a Nikon TE2000 inverted microscope, a Superfluor 40×/1.3 NA oil objective lens, a Photometrics Cascade 512B cooled CCD camera (Roper Industries, Duluth, GA, USA) and MetaFluor software (Universal Imaging Corporation, Downingtown, PA, USA). Fluo-4 was excited using a 490–500 bp filter and a 523–547 bp filter was used to detect fluorescence emission. Changes in fluo-4 intensity were quantified using region-of-interest selection and MetaFluor software (Universal Imaging Corporation, Downingtown, PA, USA).
Calcium oscillation period, rise time (RT) and fall time (FT) were quantified as previously described (Prakash et al. 1997; Espelt et al. 2005). Fluorescence images were typically acquired at 0.2 Hz, to avoid photobleaching and damage to the intestinal epithelium. However, when Ca2+ spike period, rise and fall times were quantified, images were acquired at 1–3 Hz.
C. elegans embryonic cell culture and patch-clamp electrophysiology
Embryo cells were isolated from rde-1(ne219);kbEx200 worms and cultured on 12 mm diameter acid-washed glass coverslips using methods previously described (Christensen et al. 2002; Estevez et al. 2003). Intestinal cells were identified in culture by their shape and the presence of highly refractile cytoplasmic granules (Fukushige et al. 1998; Estevez et al. 2003).
Coverslips with cultured embryo cells were placed in the bottom of a bath chamber (model R-26G; Warner Instrument Corp., Hamden, C, USA) that was mounted onto the stage of a Nikon TE2000 inverted microscope. Cells were visualized by video-enhanced DIC microscopy. Patch electrodes were pulled from soft glass capillary tubes (PG10165-4, World Precision Instruments, Sarasota, F, USA) that had been silanized with dimethyl-dichloro silane. Pipette resistance was 4–7 MΩ. Bath and pipette solutions contained (mm): 145 NaCl, 20 CaCl2, 10 Hepes, 20 Glucose, pH 7.2 (adjusted with NaOH), 345–350 mosmol l−1 and 147 sodium gluconate (Na-gluconate), 0.6 CaCl2, 6 MgCl2, 10 BAPTA, 10 Hepes, 10 μm IP3, pH 7.2 (adjusted with CsOH), 330 mosmol l−1, respectively.
Whole-cell currents were recorded using an Axopatch 200B (Axon Instruments, Foster City, CA, USA) patch-clamp amplifier. Command voltage generation, data digitization, and data analysis were carried out on a 1.6 GHz Pentium computer (Dimension 4400; Dell Computer Corp) using a Digidata 1322A AD/DA interface with pClamp 10 and Clampfit 10 software (Axon Instruments). Electrical connections to the amplifier were made using Ag/AgCl wires and 3 m KCl/agar bridges. Leak current was defined as the current observed immediately after obtaining whole-cell access, and was subtracted from all subsequent current records obtained in the cell.
Heterologous expression of ORAI-1 and STIM-1
HEK293 (human embryonic kidney) cells were cultured in 35 mm diameter tissue culture plates in Eagle's minimal essential medium (MEM; Invitrogen, Carlsbad, CA, USA), containing 10% fetal bovine serum (Invitrogen), non-essential amino acids, sodium pyruvate, 50 μ ml−1 penicillin and 50 μg ml−1 streptomycin. After reaching approximately 50% confluency, cells were transfected using FuGENE 6 Transfection Reagent (Roche, Indianapolis, IN, USA), with 1 or 2 μg GFP, 1 μg STIM-1 and/or 1 μg ORAI-1 cDNA ligated into pcDNA3.1/V5-His-TOPO (Invitrogen). The total amount of cDNA transfected into cells for all experiments was 3 μg.
HEK293 cells were incubated with cDNAs for ∼24 h. Two hours before initiating electrophysiological experiments, transfected cells were dissociated by exposure to 0.25% trypsin containing 1 mm EDTA (Gibco) for 45 s, and then plated onto poly l-lysine-coated coverslips. Plated coverslips were placed in a bath chamber mounted onto the stage of an inverted microscope. Cells were visualized by fluorescence and differential interference contrast microscopy.
Transfected cells were identified by GFP fluorescence and patch clamped using methods similar to those described above for embryo cells. Leak current was defined as the current observed immediately after obtaining whole-cell access, and was subtracted from all subsequent current records obtained in the cell. Standard bath and pipette solutions contained (mm): 135 NaCl, 1.2 MgCl2, 10 CaCl2, 10 Hepes and 10 glucose (pH 7.4, 300 mosmol l−1), and 110 NMDG-gluconate or Na-gluconate, 8 MgCl2, 0.6 CaCl2, 10 Hepes, 10 BAPTA and 10 μm IP3 (pH 7.2, 280 mosmol l−1), respectively. Divalent cation-free bath solution contained (mm): 145 NaCl, 10 Hepes, 10 glucose and 1 EDTA (pH 7.4, 300 mosmol l−1). To prevent ER Ca2+ store depletion, a pipette solution containing (mm): 105 NMDG-gluconate, 8 MgCl2, 5 CaCl2, 10 BAPTA, 10 Hepes and 2 ATP (pH 7.2, 280 mosmol l−1) was used.
Relative Cs+ permeability was determined using the Goldman–Hodgkin–Katz equation, and change in reversal potential (Erev) induced by replacing NaCl in the divalent cation-free bath with CsCl. Changes in liquid junction potential induced by this ion substitution were measured directly using a free-flowing 3 m KCl electrode. Reversal potentials were corrected for these changes.
Construction of transgenes and transgenic worms
A full-length orai-1 cDNA was cloned from a C. elegans cDNA library by PCR amplification. Primers were designed based on the predicted WormBase sequence of C09F5.2. Full-length translational GFP and DsRed reporters for ORAI-1 and STIM-1 (Yan et al. 2006), respectively, were generated using a PCR fusion-based method (Hobert, 2002) and inserted into Fire vector pPD95.77 (GFP) or a modified pPD95.77, pXHY2006.1, in which GFP was replaced with DsRed. GFP or DsRed were fused to the C-termini of the proteins. Expression of ORAI-1::GFP and STIM-1::DsRed were driven by 4 kb and 1.9 kb, respectively, of promoter sequence upstream of the orai-1 and stim-1 start codons. Promoter sequences were amplified by PCR from C. elegans N2 genomic DNA. Transgenic worms were generated by injecting wild-type worms with DNA as described by Mello et al. (1991). Rol-6 was used as a transformation marker, and co-injected with STIM-1 and ORAI-1 DNA.
An integrated line of worms expressing ORAI-1::GFP and STIM-1::DsRed was generated by exposing 50 P0 Porai-1::ORAI-1::GFP;Pstim-1::STIM-1::DsRed;rol-6(su1006) transgenic L4 animals to a dose of 30 000 μJ cm−2 of UV light that was generated with a UV crosslinker (Hoefer Scientific Instruments, San Francisco, CA, USA). Five hundred F1 roller offspring were isolated, and then two F2 roller offspring from each F1 worm were isolated. A single integrated line, KbIs18 (Porai-1::ORAI-1::GFP;Pstim-1::STIM-1::DsRed;rol-6(su1006)), was then isolated that segregated 100% GFP, DsRed and rol-6-positive animals.
RNA interference
RNA interference was induced by feeding worms bacteria producing double-stranded RNA (dsRNA) (e.g. Kamath et al. 2000; Rual et al. 2004). The orai-1 RNAi bacterial strain was obtained from the ORF-RNAi feeding library (Open Biosystems, Huntsville, AL, USA). GFP dsRNA-producing bacteria were engineered as previously described (Yin et al. 2004). The ORF-RNAi orai-1 bacterial feeding strain targeted the entire open reading frame of ORAI-1. BLAST searches of C. elegans genomic and EST databases failed to identify genes with nucleotide sequence homology to the orai-1 open reading frame, indicating that off-target effects of RNAi are unlikely. Bacterial strains were streaked to single colonies on agar plates containing 50 μg ml−1 ampicillin and 12.5 μg ml−1 tetracycline. Single colonies were used to inoculate LB media containing 50 μg ml−1 ampicillin, and cultures were grown at 37°C for 16–18 h with shaking. Four hundred microlitres of each bacterial culture were seeded onto 60 mm nematode growth medium (NGM) agar plates containing 1 mm IPTG and 50 μg ml−1 ampicillin. After seeding, plates were left at room temperature overnight. The effectiveness of orai-1 silencing was assessed by measuring whole-worm fluorescence in animals expressing ORAI-1::GFP, using a COPAS Biosort (Union Biometrica, Somerville, MA, USA).
For cell culture studies, an orai-1 DNA template was generated by PCR from the ORF-RNAi clone using T7 primers. dsRNA was synthesized from the DNA template by T7 polymerase reactions (MEGAscript kit, Ambion, Inc., Austin, TX, USA).
Isolated embryo cells were seeded onto glass coverslips in individual wells of four-well culture plates (Nalge Nunc International, Naperville, IL, USA). The cells were incubated initially with 100 μl of L-15 cell culture medium (Life Technologies, Grand Island, NY, USA) containing 15 μg ml−1 of orai-1 dsRNA. After 2 h, the culture medium volume was increased to 300 μl and the final dsRNA concentration diluted to 5 μg ml−1. An additional 100 μl of L-15 containing 5 μg ml−1orai-1 dsRNA was added on the second and third day of culture. Cells were patched clamp 2–3 days after seeding.
Microscopy
Fluorescence and DIC micrographs were obtained using a Zeiss M2BIO stereo dissecting microscope and DAGE-MTI DC2000 CCD camera or a Nikon TE2000 inverted microscope and a Micromax CCD-1300 camera (Princeton Instruments, Tucson, AZ, USA). Confocal imaging was performed using an LSM510 confocal microscope (Carl Zeiss MicroImaging, Inc., Thornwood, NY, USA).
Statistical analysis
Data are presented as means ± s.e.m. Statistical significance was determined using Student's two-tailed t test for unpaired means. When comparing three or more groups, statistical significance was determined by one-way analysis of variance. P values of ≤0.05 were taken to indicate statistical significance.
Results
A single Orai homologue is present in the C. elegans genome
BLAST searches of genomic and EST databases demonstrated that a single predicted Orai homologue (sequence name C09F5.2; accession number U22832) is present in the C. elegans genome. We cloned a full-length C09F5.2 cDNA that encoded a 293 amino acid protein, ORAI-1, with a sequence identical to that predicted by WormBase.
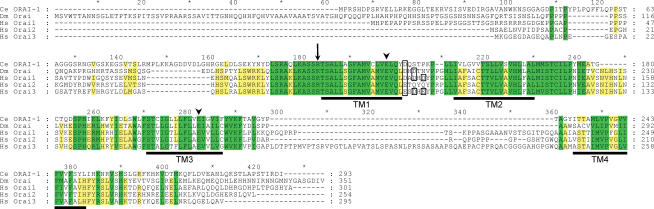
Sequence analysis indicated that ORAI-1 shares 34–38% and 54–59% amino acid identity and similarity, respectively, with Drosophila Orai and human Orai1, Orai2 and Orai3. Alignment of the amino acid sequences of ORAI-1, Drosophila Orai, and the three human Orai homologues is shown in Fig. 1. The four predicted transmembrane (TM) domains show strong conservation of primary structure in all five proteins. In addition, the predicted intracellular loop between TM2 and TM3 is highly conserved. Glutamate residues located in TM1 and TM3 have recently been shown to play key roles in controlling CRAC channel ion selectivity (Prakriya & Lewis, 2006; Vig et al. 2006a; Yeromin et al. 2006) and are fully conserved in worm, fly and human Orai homologues (arrowheads, Fig. 1). Mutation of an arginine residue at the beginning of TM1 in human Orai1 is responsible for the loss of CRAC channel activity in lymphocytes of a subset of severe combined immuno deficiency (SCID) patients (Feske et al. 2006). This residue is conserved in worm and human Orai proteins and exhibits a conserved substitution with lysine in fly Orai (arrow, Fig. 1).
Figure 1. Amino acid sequence alignment of C. elegans (Ce) ORAI-1 with Drosophila (Dm) Orai and human (Hs) Orai1, Orai2 and Orai3.
Yellow and green shading indicates sequence identity and conserved amino acid substitutions, respectively. Transmembrane (TM) domains of ORAI-1 predicted by TMpred (http://www.ch.embnet.org/software/TMPRED_form.html#) are underlined in black. A conserved arginine residue mutated in SCID patients lacking lymphocyte CRAC channel activity (Feske et al. 2006) is denoted by the arrow. Conserved glutamate residues located in TM1 and TM3 that contribute to channel ionic selectivity (Prakriya & Lewis, 2006; Vig et al. 2006a; Yeromin et al. 2006) are denoted by arrowheads. Alignment was performed using Vector NTI software (InforMax, Bethesda, MD, USA). Percentage identity and similarity of C. elegans ORAI-1 to Drosophila Orai, human Orai1, human Orai2 and human Orai3 were 34% and 55%, 34% and 54%, 38% and 59%, and 35% and 59%, respectively. Acidic residues in the extracellular loop between TM1 and TM2 are outlined in black. In Drosophila Orai (Yeromin et al. 2006) and human Orai1 (Vig et al. 2006a), these residues may function to attract polyvalent cations towards the channel pore and control channel selectivity.
The extracellular loop located between TM1 and TM2 contains two acidic amino acid residues in Drosophila Orai and three acidic residues in human Orai1 and Orai3 (see black boxes, Fig. 1). Mutagenesis studies on Drosophila Orai (Yeromin et al. 2006) and human Orai1 (Vig et al. 2006a) suggest that these residues may function to attract polyvalent cations towards the channel pore and control channel selectivity. Interestingly, only a single acidic residue is present in this region of C. elegans ORAI-1 and human Orai2 (Fig. 1), suggesting that these channels may exhibit selectivity properties distinct from other Orai homologues.
ORAI-1 is expressed in functionally diverse tissue types
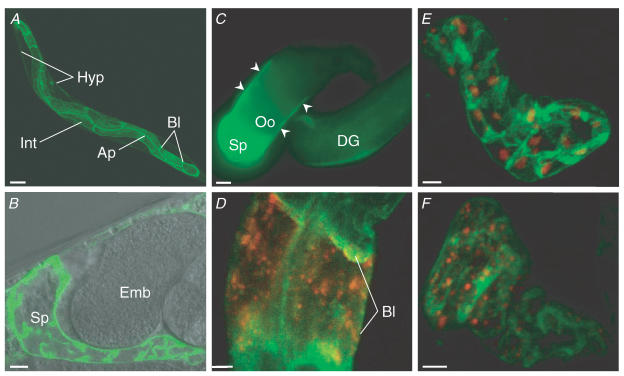
To identify cells in which ORAI-1 is expressed, we generated transgenic worms expressing full-length ORAI-1 fused to GFP. Expression was driven by 4 kb of the orai-1 promoter located immediately upstream of the start codon. Prominent expression of ORAI-1::GFP was detected in the spermatheca, intestine and hypodermis (Fig. 2A and B). Intestinal expression appeared to be localized to both apical and basolateral membrane regions (Fig. 2A).
Figure 2. ORAI-1 expression pattern.
A, low-magnification confocal 3D reconstruction of ORAI-1::GFP expression pattern in an intact worm. Expression was detected throughout the intestine (Int) and appeared to be localized to apical (Ap) and basolateral (Bl) membrane regions. Weaker fluorescence was also detected throughout the hypodermis (Hyp). Scale bar is 50 μm. B, combined DIC and fluorescence confocal micrographs showing ORAI-1::GFP expression in the spermatheca (Sp). Scale bar is 5 μm. Emb, embryo. C, fluorescence micrograph of an isolated gonad dissected from a worm expressing a transcriptional ORAI-1 GFP reporter. Porai-1::GFP expression is detected in the spermatheca (Sp) and gonadal sheath cells (arrowheads). Scale bar is 10 μm. Oo, oocyte; DG, distal gonad. D, confocal micrograph showing expression of ORAI-1::GFP and STIM-1::DsRed in two cells of the anterior intestine. Bl, basolateral membrane. Scale bar is 5 μm. E and F, confocal 3D reconstructions of ORAI-1::GFP and STIM-1::DsRed expression in the spermatheca. Scale bars are 5 μm.
The spermatheca is an accordian-like tube comprised of 24 cells. Apical and basal surfaces of the spermatheca are collapsed and highly folded in the absence of an oocyte. The circumferential ORAI-1::GFP localization pattern shown in Fig. 2B is consistent with expression in the basal plasma membrane. Non-circumferential localization is probably due to membrane folding and/or expression at lateral cell borders. The complicated morphology of the spermatheca precluded definitive localization of ORAI-1::GFP to the apical cell membrane.
In the intact animal, it was unclear whether gonadal sheath cells expressed ORAI-1::GFP, due to the intense fluorescence from the intestine and spermatheca. To examine sheath cell expression further, we imaged gonads dissected free from a worm strain (kindly provided by Dr David Baillie) expressing an orai-1 transcriptional GFP reporter. As shown in Fig. 2C, orai-1 is also expressed in both proximal and distal gonadal sheath cells.
In mammalian and Drosophila cells, STIM1 homologues function as ER Ca2+ sensors and trigger SOCE and CRAC activation in response to Ca2+ store depletion (Liou et al. 2005; Zhang et al. 2005; Soboloff et al. 2006a; Spassova et al. 2006). Our previous studies on C. elegans STIM-1 suggested that STIM-1::GFP is localized to an intracellular compartment in the intestine, spermatheca and gonadal sheath cells (Yan et al. 2006). To examine the spatial relationship between ORAI-1 and STIM-1, we generated full length STIM-1 fused at the C-terminus to DsRed, and co-expressed it in worms with ORAI-1::GFP. Strong expression of STIM-1::DsRed was detected primarily in the spermatheca and anterior and posterior intestine. Figure 2D shows STIM-1::DsRed and ORAI-1::GFP expression in two cells in the anterior intestine. STIM-1 is localized to intracellular puncta, whereas ORAI-1 is primarily localized to a plasma membrane region.
Two patterns of STIM-1::DsRed expression were observed in the spermatheca. In some worms, STIM-1::DsRed localized to large puncta, with individual spermatheca cells appearing to contain only a single site of STIM-1::DsRed expression (Fig. 2E). The spermatheca cells of other worms, in contrast, contained numerous smaller STIM-1::DsRed puncta (Fig. 2F).
ORAI–1 is required for Ca2+- and IP3-dependent contractile activity of sheath cells and the spermatheca
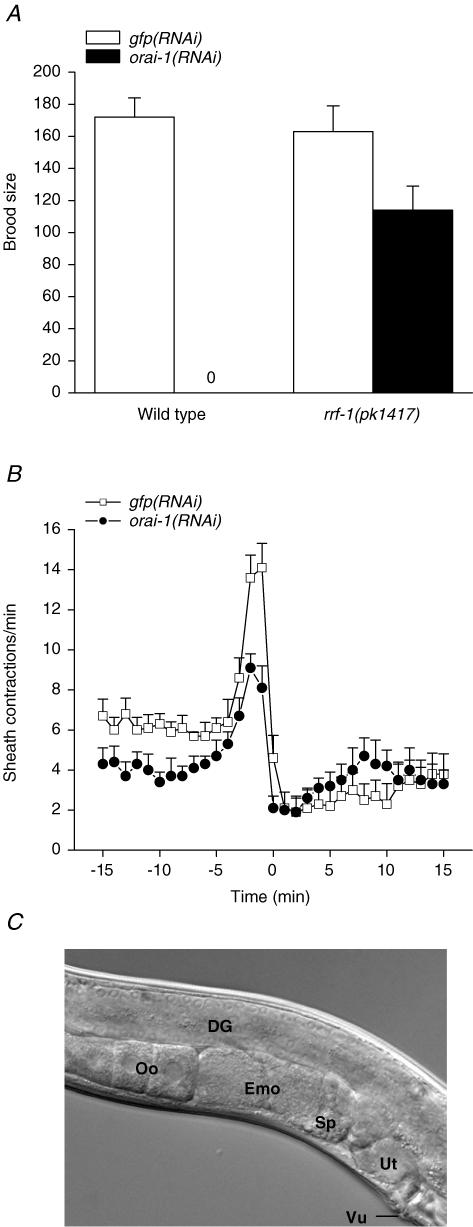
Knockdown of orai-1 expression by RNAi beginning at the L1 larval stage significantly (P < 0.0001) reduced mean ± s.e.m.. brood size from 145 ± 8 (n = 9) in control worms fed GFP dsRNA-producing bacteria to 18 ± 8 (n = 12) in worms fed orai-1 dsRNA. Continued feeding of orai-1 dsRNA-producing bacteria to the offspring of these worms caused complete sterility (Fig. 3A). Other than the fertility defect, young adult orai-1(RNAi) worms appeared healthy and exhibited no obvious defects in external morphology, movement or feeding behaviour.
Figure 3. Effect of orai-1 RNAi on fertility and ovulation.
A, brood size in wild-type and rrf-1(pk1417) mutant worms. Brood size is defined as the total number of progeny produced over four days. rrf-1 encodes an RNA-directed RNA polymerase homologue required for RNAi in somatic but not germ cells. pk1417 is a predicted rrf-1 null allele (Sijen et al. 2001). Worms were fed GFP or orai-1 dsRNA-producing bacteria for two generations beginning at the L1 larval stage. Values are means ± s.e.m. (n = 6–12). B, rates of sheath contraction during a single ovulatory cycle. Time 0 is defined as the time at which ovulation was completed in control worms. orai-1(RNAi) worms never ovulated (see Results). Therefore in these animals, time 0 is defined the first time point after peak sheath contraction rate was observed. Values are means ± s.e.m. (n = 6–7). Worms were fed dsRNA-producing bacteria for two generations. C, differential interference contrast micrograph of the gonad of an orai-1(RNAi) worm. The distal spermatheca fails to open during ovulation in orai-1(RNAi) worms, and oocytes are trapped in the proximal gonad arm where they undergo endomitosis. DG, Distal gonad; Oo, normal oocytes; Emo, endomitotic oocytes; Sp, spermatheca; Ut, uterus; Vu, vulva. Note that the uterus in the orai-1(RNAi) worm is empty due to failure of ovulation. worms fed dsRNA-producing bacteria for two generations.
Somatic cells of rrf-1(pk1417) mutant worms are resistant to dsRNA, but their germline shows a normal RNAi response (Sijen et al. 2001). The sterility observed in worms fed orai-1 dsRNA-producing bacteria was rescued by the rrf-1(pk1417) mutation. Worms fed orai-1 dsRNA-producing bacteria beginning at the L1 larval stage had a mean ± s.e.m. brood size of 138 ± 13 (n = 11) that was not significantly (P > 0.4) different from that of gfp(RNAi) worms (mean ± s.e.m. brood size = 157 ± 16; n = 6). Brood size in the F1 offspring of orai-1(RNAi); rrf-1(pk1417) worms was slightly but significantly (P < 0.05) reduced compared to control animals (Fig. 3A). The rescue of fertility in orai-1(RNAi) worms by the rrf-1(pk1417) mutation indicates that orai-1 RNAi-induced sterility is due largely to dysfunction of somatic cells.
Adult C. elegans hermaphrodites possess two U-shaped gonad arms connected via spermatheca to a common uterus. Oocytes form in the proximal gonad arm and accumulate in a single-file row of graded developmental stages. Developing oocytes remain in diakinesis of prophase I until they reach the most proximal position in the gonad arm, where they undergo meiotic maturation and are then ovulated into the spermatheca for fertilization (reviewed by Hubbard & Greenstein, 2000).
Oocytes are surrounded by myoepithelial sheath cells (Hall et al. 1999). Prior to ovulation, sheath cells contract weakly and slowly (McCarter et al. 1999). Release of the EGF-like protein LIN-3 from the maturing oocyte induces ovulation by increasing the rate and force of sheath cell contractions and by triggering opening of the distal spermatheca (Iwasaki et al. 1996; McCarter et al. 1999; Yin et al. 2004). The contractile activity of both the sheath cells (Yin et al. 2004) and spermatheca (Clandinin et al. 1998; Bui & Sternberg, 2002; Kariya et al. 2004) is regulated by IP3 and Ca2+ signalling. In addition, we have recently shown that a C. elegans homologue of human STIM1 is required for sheath cell and spermatheca function (Yan et al. 2006).
Given that orai-1 RNAi causes sterility by disrupting somatic cell function (Fig. 3A), we examined the contractile activity of the sheath cells and spermatheca in dsRNA-fed worms. Figure 3B demonstrates that orai-1 RNAi reduces sheath cell contractions under basal conditions and during ovulation. The mean rates of basal sheath cell contraction measured at –10 min in gfp(RNAi) control and orai-1(RNAi) worms were 6.3 contractions min−1 and 3.4 contractions min−1, respectively. During ovulation, sheath contraction increased significantly (P < 0.0001) to a peak rate of 14.2 contractions min−1 in control worms and 9.1 contractions min−1 in orai-1(RNAi) animals. Both basal and peak rates of sheath contraction were significantly (P < 0.004) reduced by silencing of orai-1 expression.
Spermatheca function was also defective in orai-1(RNAi) worms. During ovulation, the distal spermatheca opens allowing contracting sheath cells to pull the spermatheca over the maturing oocyte (Hubbard & Greenstein, 2000). In 35 young adult orai-1(RNAi) worms examined, the distal spermatheca failed to open during ovulation attempts. Maturing oocytes were therefore trapped in the proximal gonad arm where they underwent endomitosis (Fig. 3C). Two worms examined showed what appeared to be incomplete spermatheca opening. Maturing oocytes partially entered the spermatheca but were then pinched off and broken into two pieces, one of which remained trapped in the proximal gonad arm.
ORAI-1 is required for SOCC activity in intestinal epithelial cells but plays no role in IP3-dependent oscillatory Ca2+ signalling
Defecation in C. elegans is an ultradian rhythm mediated by sequential contraction of the posterior body wall muscles, anterior body wall muscles and enteric muscles (Iwasaki & Thomas, 1997). Posterior body wall muscle contraction (pBoc) is controlled by IP3-dependent Ca2+ oscillations in intestinal epithelial cells (Dal Santo et al. 1999; Espelt et al. 2005; Teramoto & Iwasaki, 2006). Oscillatory Ca2+ signalling is thought to be critically dependent on SOCE (Parekh & Penner, 1997; Venkatachalam et al. 2002; Parekh & Putney, 2005). However, we have shown previously that oscillatory Ca2+ signalling in the intestine is unaffected by silencing of C. elegans stim-1 (Yan et al. 2006).
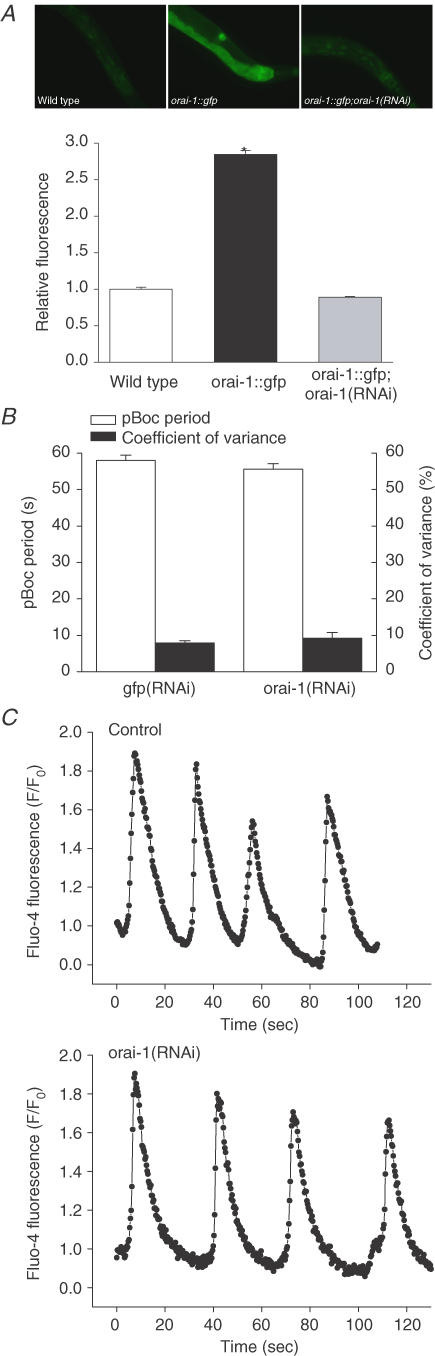
To further examine the role of SOCE in intestinal Ca2+-signalling events, we quantified pBoc in wild-type worms fed orai-1 dsRNA-producing bacteria for two generations. The effectiveness of orai-1 RNAi was first assessed by quantifying whole-worm ORAI-1::GFP fluorescence. As shown in Fig. 4A, orai-1 RNAi dramatically and significantly (P < 0.001) reduced ORAI-1::GFP expression. Whole-worm fluorescence in ORAI-1::GFP worms was increased 2.85-fold relative to wild-type animals. When these worms were fed orai-1 dsRNA-producing bacteria for one generation, total fluorescence was reduced to ∼90% of wild-type worm background autofluorescence levels. However, despite this strong knockdown, pBoc period and rhythmicity in orai-1(RNAi) worms were not significantly (P > 0.3) different from control animals fed GFP dsRNA-producing bacteria (Fig. 4B).
Figure 4. Effect of orai-1 RNAi on ORAI-1 expression, pBoc and intestinal Ca2+ signalling.
A, effect of orai-1 RNAi on ORAI-1::GFP expression. Top: fluorescence micrographs of wild-type and ORAI-1::GFP-expressing worms fed bacteria containing the empty RNAi feeding empty RNAi feeding vector and ORAI-1::GFP expressing worms fed orai-1 dsRNA producing bacteria [orai-1:: gfp; orai-1 (RNAi)]. Fluorescence in wild-type worms is background autofluorescence. Bottom: relative whole-animal fluorescence in wild-type, orai-1:gfp and orai-1::gfp; orai-1(RNAi) worms. Wild-type and orai-1:gfp worms were fed bacteria containing the empty feeding vector. Values are mean ± s.e.m. (n = 164–532). *P < 0.001 compared to wild-type and orai-1::gfp animals. Whole-worm fluorescence was quantified using a COPAS Biosort and normalized to time-of-flight (i.e. fluorescence/time-of-flight), which is a measure of worm size. RNAi feeding was carried out for one generation. GFP-expressing worms were the integrated strain KbIs18 (Porai-1::ORAI-1::GFP; Pstim-1::STIM-1::DsRed; rol-6(su1006)). B, effect of orai-1 RNAi on pBoc period and coefficient of variance, which is a measure of cycle rhythmicity. Wild-type worms were fed GFP or orai-1 dsRNA-producing bacteria for two generations. Values are means ± s.e.m. (n = 9–15). C, examples of Ca2+ oscillations observed in intestines isolated from rde-1(ne219);kbEx200 worms fed bacteria containing the empty feeding vector (control) or orai-1 dsRNA-producing bacteria for two generations. Fluorescent images were acquired at 3 Hz.
We also directly assessed the effect of orai-1 RNAi on Ca2+ oscillations in isolated intestines (Espelt et al. 2005). To maximize ORAI-1 knockdown, intestines were dissected from rde-1(ne219);kbEx200 worms fed orai-1 dsRNA-producing bacteria for 2–5 generations. rde-1 (RNAi defective) encodes a protein involved in translation initiation (Tabara et al. 1999; Fagard et al. 2000) and rde-1 loss-of-function mutants are strongly resistant to RNAi induced by dsRNA injection, feeding or expression (Tabara et al. 1999). Rde-1 is rescued selectively in the intestines of rde-1(ne219);kbEx200 worms (Espelt et al. 2005), thus allowing us to knockdown intestinal gene expression while maintaining normal fertility. As shown in Fig. 4C and Table 1, Ca2+ oscillation periods and oscillation rise and fall times were not significantly (P > 0.06) different in control and orai-1(RNAi) worms.
Table 1.
Effect of orai-1 RNAi on intestinal Ca2+ oscillations
| Experiment | Oscillation period (s) | Oscillation rise time (s) | Oscillation fall time (s) |
|---|---|---|---|
| Empty vector | 33 ± 2 (14) | 5 ± 0.3 (22) | 17 ± 1 (20) |
| Orai-1 RNAi | 27 ± 2 (15) | 5 ± 0.3 (34) | 15 ± 1 (33) |
Calcium oscillations were measured in rde-1(ne219); kbEx200 worms fed orai-1 dsRNA-producing bacteria or bacteria containing the empty feeding vector for 2–5 generations. Values are means ± s.e.m. Number of observations (n) is shown in parentheses.
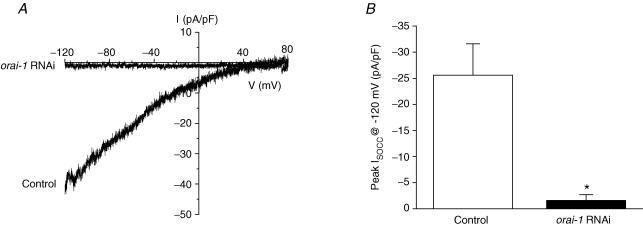
In Drosophila and mammalian cells, the Orai and Orai1 genes are thought to encode store-operated CRAC channels (Prakriya et al. 2006; Vig et al. 2006a; Yeromin et al. 2006). Cultured intestinal cells express a SOCC current with many of the same biophysical characteristics as ICRAC (Estevez et al. 2003). We therefore examined the effect of orai-1 RNAi on SOCC activity using whole-cell patch clamp. To maximize orai-1 knockdown, we cultured intestinal cells from rde-1(ne219);kbEx200 worms fed orai-1 dsRNA-producing bacteria for three generations, and included orai-1 dsRNA in the culture medium at the time of cell plating. Figure 5A shows current–voltage relationships of whole-cell currents measured after 5 min of intracellular Ca2+ store depletion in a control cell and a cell exposed to orai-1 dsRNA. Inwardly rectifying store-operated Ca2+ current is detected in the control intestinal cell but not in the orai-1 dsRNA-treated intestinal cell. Mean whole-cell SOCC current density measured at –120 mV in control cells was –25.6 pA pF−1. orai-1 RNAi significantly (P < 0.003) reduced whole-cell SOCC current density to –1.6 pA pF−1 (Fig. 5B). The inhibitory effect of orai-1 RNAi is comparable to that of stim-1 RNAi reported by us previously (Yan et al. 2006). Taken together, data in Figs 4 and 5 and Table 1 suggest that SOCC activity is not required for generation of Ca2+ oscillations in intestinal epithelial cells. An alternative interpretation is that the small amounts of channel activity that remain after RNAi treatment are sufficient to maintain ER Ca2+ store levels and normal cytoplasmic Ca2+ signals.
Figure 5. Effect of orai-1 RNAi on SOCC activity in cultured intestinal cells.
A, examples of whole-cell current–voltage relationships of SOCC currents induced by 5 min of store depletion in a control cell and a cell treated with orai-1 dsRNA. Store depletion was induced using a pipette solution containing 10 μm IP3, 10 mm BAPTA and 18 nm free-Ca2+. Currents were elicited by ramping membrane voltage from –120 mV to +80 mV at 200 mV s−1 every 5 s. B, effect of orai-1 dsRNA on peak ISOCC measured 5 min after obtaining whole-cell access. Values are means ± s.e.m. (n = 7–10). *P < 0.003 compared to control. Intestinal cells were cultured from rde-1(ne219);kbEx200 worms (Espelt et al. 2005) fed orai-1 dsRNA-producing bacteria for three generations. orai-1 dsRNA was also included in the culture medium beginning at the time of cell plating. Cells were patch clamped 2–3 days after isolation from worm embryos.
ORAI-1 and STIM-1 interact functionally and mediate SOCC activity
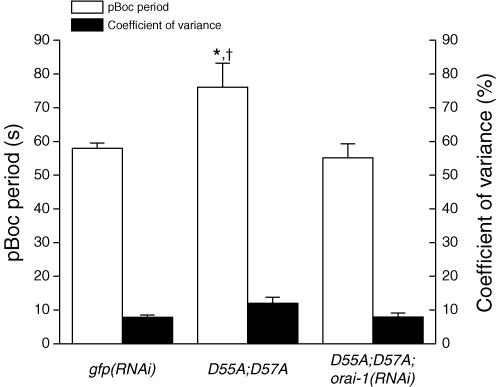
Mutation of the EF hand domain of Drosophila and human STIM homologues constitutively activates SOCE and ICRAC (Zhang et al. 2005; Liou et al. 2005; Soboloff et al. 2006a; Spassova et al. 2006). Transgenic worms expressing a C. elegans stim-1 EF hand mutant tagged with GFP (i.e. STIM-1(D55A; D57A::GFP)) are sterile and exhibit an increased pBoc period and pBoc arrhythmia (Yan et al. 2006). To determine whether ORAI-1 functions together with STIM-1, we fed stim-1(D55A; D57A)::gfp worms orai-1 dsRNA-producing bacteria. As shown in Fig. 6, the increased pBoc period induced by STIM-1(D55A; D57A)::GFP expression was suppressed completely (P < 0.01) by orai-1 RNAi, indicating that orai-1 and stim-1 function together in a common pathway. Unlike our previous studies (Yan et al. 2006), we found that pBoc rhythmicity, as measured by the coefficient of variance, was not significantly (P > 0.05) different in control and stim-1(D55A;D57A)::gfp worms. Knockdown of orai-1 expression in stim-1(D55A;D57A)::gfp animals had no significant (P > 0.05) effect on CV.
Figure 6. Effect of orai-1 RNAi on pBoc period and coefficient of variance in transgenic worms expressing the GFP-tagged STIM-1 EF hand mutant protein STIM-1(D55A; D57A)::GFP.
Data on wild-type worms fed GFP dsRNA-producing bacteria (gfp(RNAi)) are reproduced from Fig. 4B. D55A; D57A worms were fed bacteria containing the empty RNAi feeding vector or bacteria producing orai-1 dsRNA. RNAi feeding was carried out over two generations. Values are means ± s.e.m. (n = 13–15). *P < 0.05 compared to gfp(RNAi) worms. †P < 0.01 compared to D55A;D57A worms fed orai-1 dsRNA-producing bacteria. pBoc periods in gfp(RNAi) and D55A; D57A; orai-1(RNAi) worms were not significantly (P > 0.05) different. Coefficients of variance in all three groups of worms were not significantly (P > 0.05) different.
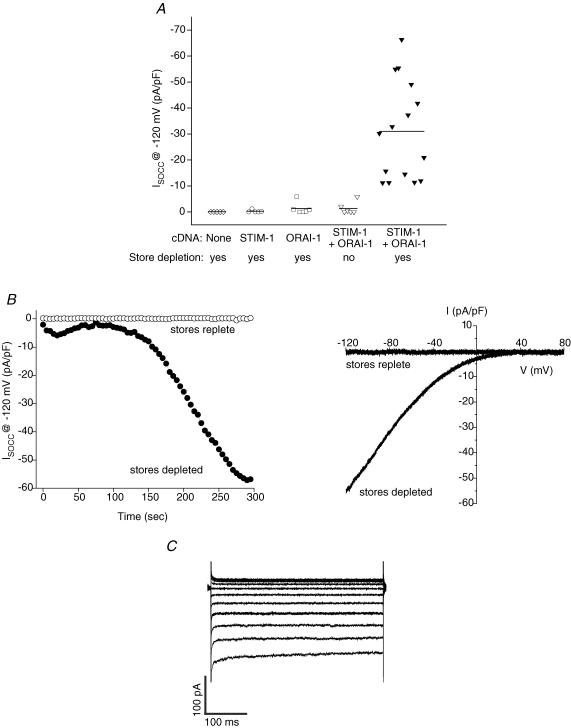
Co-transfection of cells with human or Drosophila STIM and Orai1 homologues dramatically increases SOCE and ICRAC (Mercer et al. 2006; Peinelt et al. 2006; Prakriya & Lewis, 2006; Soboloff et al. 2006b; Vig et al. 2006a; Yeromin et al. 2006). To further examine the relationship between C. elegans ORAI-1 and STIM-1 then, we co-expressed the proteins in HEK293 cells and performed whole-cell patch-clamp measurements. Intracellular Ca2+ stores were depleted by dialysing cells with a solution containing 10 μm IP3, 10 mm BAPTA and a free Ca2+ concentration of ∼18 nm. SOCC currents were undetectable in HEK293 cells expressing GFP alone, and in cells co-expressing STIM-1 and GFP or ORAI-1 and GFP (Fig. 7A). However, co-expression of STIM-1 and ORAI-1 dramatically increased whole-cell current (Fig. 7A and B). The current showed strong inward rectification (Fig. 7B) and was not gated by membrane voltage (Fig. 7C). Current density was quite variable from cell-to-cell, and ranged between –11 pA pF−1 and –66 pA pF−1. Mean current density at –120 mV observed 5 min after whole-cell access was obtained was –31.0 pA pF−1 (n = 15). No current was detected in STIM-1/ORAI-1 co-transfected cells in the absence of store depletion (Fig. 7A and B).
Figure 7. Effect of heterologous expression of ORAI-1 and STIM-1 in HEK293 cells on store-operated currents.
A, whole-cell currents in individual HEK293 cells transfected with GFP, STIM-1 and GFP, ORAI-1 and GFP, or STIM-1, ORAI-1 and GFP cDNAs. Solid lines are the mean currents for each of the groups shown. Currents were elicited by ramping membrane voltage from –120 mV to +80 mV at 200 mV s−1 every 5 s. Holding potential was 0 mV. The currents shown were those observed 5 min after obtaining whole-cell access and are leak current subtracted. Mean whole-cell current in STIM-1/ORAI-1-expressing cells with depleted stores was significantly (P < 0.001) different from the other four groups shown. Store depletion was induced by dialysing cells with a pipette solution containing 10 mm BAPTA, 10 μm IP3 and ∼18 nm free-Ca2+. Depletion of stores was prevented using a pipette solution containing 10 mm BAPTA, 2 mm ATP and ∼140 nm free-Ca2+. B, examples of time-dependent changes in current activity (left panel) and current–voltage relationships (right panel) of currents observed in an ORAI-1/STIM-1–expressing cell with replete stores and in an ORAI-1/STIM-1-expressing cell in which stores were depleted. Current–voltage relationships were plotted for currents measured 5 min after obtaining whole-cell access. Leak current was subtracted from the current induced by store depletion. C, example of store-operated whole-cell currents elicited by stepping membrane voltage in 20 mV steps from –120 mV to +80 mV in an ORAI-1/STIM-1-expressing cell. Holding potential was 0 mV.
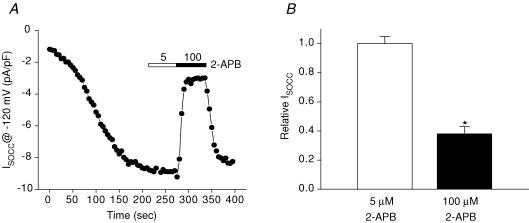
The C. elegans intestinal cell SOCC current shares many similarities with mammalian ICRAC. However, the current also exhibits some distinct differences. Unlike Drosophila and mammalian ICRAC, C. elegans ISOCC is not activated by low concentrations of 2-APB, has a relatively high Cs+ permeability and shows much slower rundown or ‘depotentiation’ when exposed to divalent cation-free (DVF) extracellular solution (Estevez et al. 2003; Yeromin et al. 2004). To determine whether heterologous expression of STIM-1 and ORAI-1 recapitulates the properties of the endogenous intestinal ISOCC, we characterized 2-APB sensitivity, Cs+ permeability and depotentiation. As shown in Figs 8A and B, 5 μm 2-APB had no significant (P > 0.9) effect on current amplitude, while exposure to 100 μm 2-APB inhibited the current ∼60% (P < 0.0002).
Figure 8. Effect of 2-APB on ORAI-1/STIM-1-induced store-operated currents in HEK293 cells.
A, example of the effect of 5 μm (open bar) and 100 μm (filled bar) 2-APB on store-operated current in an ORAI-1/STIM-1-expressing cell. B, effect of 5 μm and 100 μm 2-APB on relative whole-cell current in HEK293 cells co-expressing ORAI-1 and STIM-1. Values are means ± s.e.m. (n = 4–5). *P < 0.0002 compared to control without 2-APB.
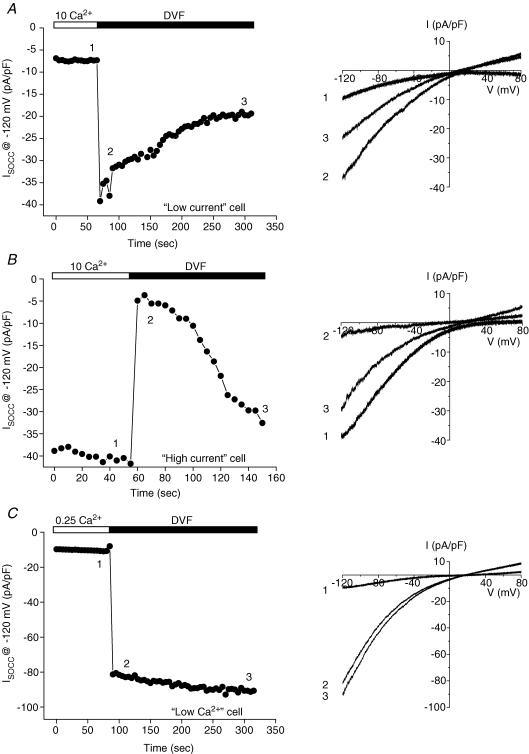
The effect of DVF bath on whole-cell current was complex. Out of 30 successful recordings, we observed only two cells that exhibited the typical response to DVF bath where current amplitude increased rapidly and then underwent slow depotentiation upon removal of extracellular divalent cations (Fig. 9A). Rates of current depotentiation in the two cells were –11% min−1 and –19% min−1. These values are comparable to the mean rate of depotentiation of –13% min−1 seen in cultured C. elegans intestinal cells (Estevez et al. 2003). Both cells had measurable outward currents and reversal potentials of 8.8 mV and 14.6 mV in the DVF bath. In one cell, Na+ in the bath solution was successfully replaced with Cs+, which shifted Erev by –9.1 mV. The calculated Cs+ permeability relative to Na+ (i.e. PCs/PNa) was 0.7, which is similar to the mean intestinal SOCC PCs/PNa of 0.6 (Estevez et al. 2003).
Figure 9. Examples of the effects of divalent cation-free (DVF) bath on store-operated whole-cell currents in different ORAI-1/STIM-1-expressing cells.
‘Low-current’ (A) and ‘high-current’ (B) cells had current densities of 6–7 pA pF−1 and 30–60 pA pF−1, respectively. The control bath solution for low- and high-current cells contained 10 mm Ca2+. ‘Low-Ca2+’ (C) cells were bathed in an extracellular solution containing 0.25 mm Ca2+ before exposure to the DVF bath. Data shown are for single cells. Current–voltage relationships for currents observed in each of the three groups of cells are shown in the right panels. Numbering of the traces corresponds to the times at which the current–voltage relationships were obtained (i.e. left panel).
The two cells that exhibited the typical response to DVF bath had current densities of 5.9 pA pF−1 and 7.3 pA pF−1. We refer to these cells as ‘low-current’ cells. All other cells in which these DVF experiments were performed successfully had current densities of 30–60 pA pF−1, and we refer to these as ‘high-current’ cells. The response to DVF bath in high-current cells contrasted sharply with that of low-current cells. In 28 out of 28 high-current cells, removal of bath divalent cations rapidly and nearly completely inhibited whole-cell current. Current inhibition was followed by slow current reactivation (Fig. 9B). The reactivated current showed both inward and outward components, and reversed at a mean ± s.e.m. value of 12.0 ± 2.4 mV (n = 5). Replacement of bath Na+ with Cs+ shifted Erev by a mean ± s.e.m. value of –31.6 ± 3.2 mV. The mean ± s.e.m.PCs/PNa calculated from this shift was 0.3 ± 0.04 (n = 5).
The different response to DVF bath of low- and high-current cells suggested that channel expression level and whole-cell current amplitude somehow alter channel regulation. Attempts to increase the proportion of low-current cells were unsuccessful. Reducing transfection time to 3 h, or transfecting cells with 10-fold less (i.e. 0.1 μg) ORAI-1 and STIM-1 cDNA did not increase the frequency of low-current cells, but instead increased the number of cells lacking measurable SOCC currents.
Interestingly, in two high-current cells, we were able to switch back to a 10 mm Ca2+ bath after an exposure to DVF medium of several minutes. When these cells were exposed a second time to DVF bath, current amplitude increased rapidly as expected (data not shown). Regulation of CRAC channels by intracellular Ca2+ concentration has been well described (e.g. Zweifach & Lewis, 1995a, 1995b). Our observation thus suggested the possibility that the anomalous response of high-current cells to extracellular divalent cation removal may be due to high rates of Ca2+ influx and intracellular Ca2+-dependent channel regulatory mechanisms. To test this possibility, cells were bathed in an extracellular solution containing 0.25 mm Ca2+ before being exposed to DVF medium. We refer to these cells as ‘low-Ca2+’ cells. In 10 out of 10 low-Ca2+ cells, exposure to DVF bath caused an immediate increase in whole-cell current (Fig. 9C). This current typically continued to activate slowly and then stabilized (e.g. Fig. 9C). Rapid rundown or ‘depotentiation’ of the current was never observed.
The mean ± s.e.m.Erev in the DVF bath was 22.6 ± 4.0 mV (n = 5). Replacement of bath Na+ with Cs+ shifted Erev to more negative values. The mean ± s.e.m. Cs+-induced shift in Erev and calculated PCs/PNa were –50.0 ± 6.8 mV and 0.2 ± 0.05 (n = 5), respectively. The shift in Erev was significantly (P < 0.04) different from that observed in high-current cells bathed with 10 mm Ca2+. However, even though there was a difference in the mean PCs/PNa between the two groups of cells, this difference did not achieve statistical significance (P > 0.08). Interestingly, both high-current cells bathed with 10 mm Ca2+ and low-Ca2+ cells had relative Cs+ permeabilities that were considerably lower than that of the single low-current cell in which we were able to measure this parameter. Taken together, the effects of low bath Ca2+ concentration suggest that normal channel regulation may be altered by high levels of channel expression and concomitant Ca2+ influx. This in turn affects the response of the channel, via unknown mechanisms, to removal of extracellular divalent cations. High levels of channel expression may also alter the permeability properties of the channel.
Discussion
The current paper extends our previous findings demonstrating that C. elegans possesses a STIM-1 homologue required for SOCC activity and some IP3-dependent Ca2+ signalling processes (Yan et al. 2006). As we demonstrate here, a single Orai homologue is also present in the worm genome. orai-1 and stim-1 are co-expressed in various cell types (Fig. 2) and RNAi silencing of either gene gives rise to identical phenotypes, namely, loss of intestinal cell SOCC function (Fig. 5) as well as sterility due primarily to disruption of IP3-dependent spermatheca contractility (Fig. 3 and Results) (Yan et al. 2006). ORAI-1 knockdown suppresses the pBoc defect induced by expression of a STIM-1 EF hand Ca2+-binding mutant in the worm intestine (Fig. 6 and Yan et al. 2006), indicating that the proteins function in a common signalling pathway. In addition, heterologous co-expression of ORAI-1 and STIM-1 gives rise to robust SOCC activity (Fig. 7).
The properties of the native C. elegans intestinal SOCC that distinguish it from Drosophila and mammalian CRAC channels are lack of stimulation by low concentrations of 2-APB, slow depotentiation in a DVF bath and relatively high Cs+ permeability (reviewed by Yeromin et al. 2004). The ORAI-1/STIM-1-induced SOCC is unaffected by 5 μm 2-APB (Fig. 8) and has a PCs/PNa 2–7-fold higher than that reported for other CRAC channels. In addition, whole-cell currents that showed a normal response to DVF bathing underwent depotentiation at a rate remarkably similar to that of native SOCC (Fig. 9A). We conclude from these results and studies on Drosophila and human Orai homologues (Prakriya et al. 2006; Vig et al. 2006a; Yeromin et al. 2006), that C. elegans possesses a bona fide CRAC channel encoded by orai-1 and regulated by STIM-1.
The rapid inhibitory effect of bath divalent cation removal observed in cells with high levels of channel activity (Fig. 9B) is intriguing. Reducing bath Ca2+ concentration from 10 mm to 0.25 mm prevented this inhibitory effect (Fig. 9C). This observation suggests that high rates of Ca2+ influx alter CRAC channel function and regulation. The underlying mechanism by which this occurs is unclear at present. However, further study of this phenomenon may shed light on the mechanisms by which both extracellular and intracellular Ca2+ regulate CRAC channel activity. Our findings also raise a cautionary note. We are unaware of studies showing that native CRAC channels undergo rapid inhibition in response to DVF extracellular solutions. Thus, it is likely that heterologous overexpression alters channel structure/function relationships and/or regulation. Conclusions drawn from heterologous expression studies on CRAC channel function, and in particular regulation, should be tempered by these concerns.
ORAI-1 shares 34–38% amino acid sequence identity with Drosophila and human Orai homologues. Not surprisingly, predicted transmembrane domains show considerable sequence homology across widely divergent species that are separated by many hundreds of millions of years of evolution. In addition, there is strong sequence conservation in the intracellular loop located between TM2 and TM3 (Fig. 1). This domain may function critically in channel regulation, perhaps as a site for proposed functional interactions with STIM proteins (Vig et al. 2006a; Yeromin et al. 2006). Conserved proline residues located in the intracellular N-terminus (Fig. 1) probably also play important functional roles.
We conducted BLAST searches using all available genome sequences, and identified STIM-1 and ORAI-1 homologues only in animals. Estimates of the evolutionary origin of nematodes range from 600 to 1300 million years ago (reviewed by Coghlan, 2005). CRAC channels are thus a very ancient animal innovation. Despite their presence in organisms as diverse as roundworms, fruit flies and humans, and their widespread expression in functionally diverse mammalian cell types (Parekh & Penner, 1997; Venkatachalam et al. 2002; Parekh & Putney, 2005), the physiological roles of CRAC channels are largely unknown. It is widely stated in the literature that CRAC channels and SOCE are essential for generation of IP3-dependent Ca2+ signals, and for maintenance of ER Ca2+ levels during Ca2+ signalling events (Parekh & Penner, 1997; Venkatachalam et al. 2002; Parekh & Putney, 2005). However, in most cell types, direct evidence supporting this notion is lacking. Furthermore, our previous studies on STIM-1 (Yan et al. 2006) and the data presented in this paper suggest that CRAC channel activity is not required for maintenance of ER Ca2+ homeostasis or for oscillatory Ca2+ signalling in the C. elegans intestine (Fig. 4 and Table 1) (Yan et al. 2006).
If CRAC channels are not essential components of all Ca2+-signalling pathways, why are they so widely observed and why have the channel's functional/structural properties been conserved from worms to humans? We have suggested previously that a primary function of SOCE may be to provide cells with a failsafe mechanism for protecting store Ca2+ levels during pathophysiological insults and exposure to cellular stressors. Bacterial toxins (Bryant et al. 2003; Saha et al. 2005), viral proteins (Tian et al. 1995; van Kuppeveld et al. 1997) ischaemia (Lehotsky et al. 2003) and oxidants (Henschke & Elliott, 1995; Pariente et al. 2001) induce store Ca2+ loss and depletion. Failure to maintain store Ca2+ levels under pathophysiological and stress conditions can exacerbate injury by disrupting ER protein synthesis and processing, and lead ultimately to cell death (Rao et al. 2004; Schroder & Kaufman, 2005).
CRAC channels clearly play critical signalling roles in some cell types. An important role for CRAC channels in immune cell signalling is well established (reviewed by Lewis, 2001), and they are essential for gonad function and fertility in C. elegans (Fig. 3 and Yan et al. 2006). The functional properties of CRAC channels are probably specialized for certain signalling mechanisms. CRAC channels have a very high Ca2+ selectivity and thus will have little effect on membrane potential when they are active and mediating Ca2+ influx. While CRAC channels are not gated directly by voltage, membrane potential will alter Ca2+ flux by changing the electrical driving force for Ca2+. In addition, CRAC channels have been reported to undergo slow voltage-dependent changes in macroscopic conductance (reviewed by Lewis, 2001; Parekh & Putney, 2005). Thus, variable patterns of CRAC channel-mediated Ca2+ influx can be induced by the activity of other plasma membrane ion channels that affect membrane potential. This in turn increases the complexity and information content of Ca2+ signals, as well as the rate of store refilling.
Calcium influx through CRAC channels appears to occur at discrete membrane locations where STIM1 proteins accumulate in response to store depletion (Luik et al. 2006). Such compartmentalization of Ca2+ entry would provide a mechanism for specifically regulating downstream cellular and ER signalling molecules that colocalize with STIM1 proteins and CRAC channels. Regulation of CRAC channel activity by store Ca2+ depletion also provides a way to coordinate compartmentalized Ca2+ influx with Ca2+ efflux through IP3 receptors and signalling pathways that control levels of IP3 and associated lipid second messengers.
Our findings suggesting that CRAC is not an essential component of C. elegans intestinal Ca2+ signalling imply that depletion of ER Ca2+ stores does not necessarily occur pari passu with generation of IP3-dependent Ca2+ signals. In most cell types, SOCE and CRAC channel activation has been observed only under conditions of extreme store depletion experimentally induced by SERCA inhibition, supraphysiological IP3 receptor activation, exposure to high concentrations of ionomycin and/or increases in cytoplasmic Ca2+ buffering (e.g. Parekh et al. 1997; Golovina et al. 2001; Machaca, 2003). Direct measurements of store Ca2+ levels during physiologically relevant Ca2+ signalling events are lacking. In the one detailed study conducted to date, little or no change in store Ca2+ levels was detected during acetylcholine-induced Ca2+ oscillations in pancreatic acinar cells. Only during stimulation with supraphysiological acetylcholine concentrations was store depletion observed (Park et al. 2000).
It has been suggested that global ER Ca2+ levels are unaffected during normal Ca2+ signalling events, and that instead Ca2+ depletion probably occurs in ER microdomains or cisternae located close to the plasma membrane (e.g. Berridge, 2002, 2004; Penner & Fleig, 2004). However, photobleaching and Ca2+-uncaging experiments suggest that in acinar cells at least, the ER is a continuous compartment and that Ca2+ loss from microdomains is rapidly replenished by Ca2+ in the bulk ER (Park et al. 2000).
The emerging model of CRAC channel regulation by STIM1 homologues also argues against localization of Ca2+ depletion to ER microdomains close to the plasma membrane. In Ca2+-replete stores, STIM1 proteins show a diffuse localization in the ER and redistribute to puncta during store depletion (Liou et al. 2005; Zhang et al. 2005; Wu et al. 2006). Elegant studies by Wu et al. (2006) have demonstrated that these puncta correspond to ER–plasma membrane contact sites. It is widely accepted that STIM1 homologues function as ER Ca2+ sensors (Liou et al. 2005; Zhang et al. 2005; Soboloff et al. 2006a; Spassova et al. 2006). Since the protein localizes to ER microdomains close to the plasma membrane only during CRAC channel activation, the activating signal cannot be Ca2+ depletion in these regions. Thus, STIM1 is either sensing global ER Ca2+ levels or Ca2+ levels in an as yet to be defined ER microdomain.
Clearly, a complete understanding of the physiological roles of CRAC channels requires direct measurements of ER Ca2+ store levels in a variety of cell types both under physiologically relevant conditions and during pathophysiological insults. In immune cells and C. elegans sheath and spermatheca cells, ER Ca2+ stores presumably become depleted during normal Ca2+-signalling events. Direct measurement of the dynamics of store depletion and refilling in these cell types would be valuable. Do Ca2+ stores in immune cells and worm sheath and spermatheca cells become depleted because they have a limited volume and/or because rates of ER Ca2+ uptake are slow relative to total store capacity combined with rates of passive Ca2+ leak and efflux through activated IP3 receptors? It is likely that the functional properties of the ER Ca2+ stores are specifically tailored to the signalling requirements of the cell and the role of CRAC channels in those signalling pathways.
In summary, we have identified a C. elegans Orai1 homologue that encodes a CRAC channel regulated by STIM-1. The C. elegans CRAC channel is the evolutionarily oldest example of this highly specialized channel type that has been described to date. Our current studies along with our previous work on C. elegans STIM-1 (Yan et al. 2006) argue that CRAC channels and SOCE are not obligate components of all IP3-dependent Ca2+-signalling pathways. Instead, we suggest that CRAC channels carry out specialized signalling functions that are tailored to the physiological requirements of the cell, and that they also may function to protect cells from pathophysiological insults and stressors that disrupt ER Ca2+ homeostasis. The identification of Orai1 and STIM1 proteins has now made it possible to precisely define the physiological roles and regulation of CRAC channels and to determine whether they will be useful targets for treatment of human disease.
Acknowledgments
This work was supported by NIH grants R01 GM74229 and R01 DK51610 to K.S. We thank Dr David Baillie for providing the BC10427 worm strain. Some C. elegans strains used in this work were provided by the Caenorhabditis Genetics Center (University of Minnesota, Minneapolis, MN). Confocal microscopy was performed in the Vanderbilt University Medical Center Cell Imaging Shared Resource, which is supported by NIH grants CA68485, DK20593, DK58404, HD15052, DK59637 and EY08126.
References
- Baba Y, Hayashi K, Fujii Y, Mizushima A, Watarai H, Wakamori M, Numaga T, Mori Y, Iino M, Hikida M, Kurosaki T. Coupling of STIM1 to store-operated Ca2+ entry through its constitutive and inducible movement in the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2006;103:16704–16709. doi: 10.1073/pnas.0608358103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium. 2002;32:235–249. doi: 10.1016/s0143416002001823. [DOI] [PubMed] [Google Scholar]
- Berridge M. Conformational coupling: a physiological calcium entry mechanism. Sci STKE. 2004;2004:e33. doi: 10.1126/stke.2432004pe33. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant AE, Bayer CR, Hayes-Schroer SM, Stevens DL. Activation of platelet gpIIbIIIa by phospholipase C from Clostridium perfringens involves store-operated calcium entry. J Infect Dis. 2003;187:408–417. doi: 10.1086/367964. [DOI] [PubMed] [Google Scholar]
- Bui YK, Sternberg PW. Caenorhabditis elegans inositol 5-phosphatase homolog negatively regulates inositol 1,4,5-triphosphate signaling in ovulation. Mol Biol Cell. 2002;13:1641–1651. doi: 10.1091/mbc.02-01-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen M, Estevez AY, Yin XM, Fox R, Morrison R, McDonnell M, Gleason C, Miller DM, Strange K. A primary culture system for functional analysis of C. elegans neurons and muscle cells. Neuron. 2002;33:503–514. doi: 10.1016/s0896-6273(02)00591-3. [DOI] [PubMed] [Google Scholar]
- Clandinin TR, DeModena JA, Sternberg PW. Inositol trisphosphate mediates a RAS-independent response to LET-23 receptor tyrosine kinase activation in C. elegans. Cell. 1998;92:523–533. doi: 10.1016/s0092-8674(00)80945-9. [DOI] [PubMed] [Google Scholar]
- Coghlan A. Nematode genome evolution In Wormbook. 2005 doi: 10.1895/wormbook.1.15.1. http://www.wormbook.org/chapters/www_genomevol/genomevol.html. [DOI] [PMC free article] [PubMed]
- Dal Santo P, Logan MA, Chisholm AD, Jorgensen EM. The inositol trisphosphate receptor regulates a 50-second behavioral rhythm in C. elegans. Cell. 1999;98:757–767. doi: 10.1016/s0092-8674(00)81510-x. [DOI] [PubMed] [Google Scholar]
- Espelt MV, Estevez AY, Yin X, Strange K. Oscillatory Ca2+ signaling in the isolated Caenorhabditis elegans intestine: role of the inositol-1,4,5-trisphosphate receptor and phospholipases C β and γ. J Gen Physiol. 2005;126:379–392. doi: 10.1085/jgp.200509355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estevez AY, Roberts RK, Strange K. Identification of store-independent and store-operated Ca2+ conductances in Caenorhabditis elegans intestinal epithelial cells. J General Physiol. 2003;122:207–223. doi: 10.1085/jgp.200308804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagard M, Boutet S, Morel JB, Bellini C, Vaucheret H. AGO1, QDE-2, and RDE-1 are related proteins required for post-transcriptional gene silencing in plants, quelling in fungi, and RNA interference in animals. Proc Natl Acad Sci U S A. 2000;97:11650–11654. doi: 10.1073/pnas.200217597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- Fukushige T, Hawkins MG, McGhee JD. The GATA-factor elt-2 is essential for formation of the Caenorhabditis elegans intestine. Dev Biol. 1998;198:286–302. [PubMed] [Google Scholar]
- Golovina VA, Platoshyn O, Bailey CL, Wang J, Limsuwan A, Sweeney M, Rubin LJ, Yuan JX. Upregulated TRP and enhanced capacitative Ca2+ entry in human pulmonary artery myocytes during proliferation. Am J Physiol Heart Circ Physiol. 2001;280:H746–H755. doi: 10.1152/ajpheart.2001.280.2.H746. [DOI] [PubMed] [Google Scholar]
- Hall DH, Winfrey VP, Blaeuer G, Hoffman LH, Furuta T, Rose KL, Hobert O, Greenstein D. Ultrastructural features of the adult hermaphrodite gonad of Caenorhabditis elegans: relations between the germ line and soma. Dev Biol. 1999;212:101–123. doi: 10.1006/dbio.1999.9356. [DOI] [PubMed] [Google Scholar]
- Henschke PN, Elliott SJ. Oxidized glutathione decreases luminal Ca2+ content of the endothelial cell ins (1,4,5) P3-sensitive Ca2+ store. Biochem J. 1995;312:485–489. doi: 10.1042/bj3120485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobert O. PCR fusion-based approach to create reporter gene constructs for expression analysis in transgenic C. elegans. Biotechniques. 2002;32:728–730. doi: 10.2144/02324bm01. [DOI] [PubMed] [Google Scholar]
- Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- Hubbard EJ, Greenstein D. The Caenorhabditis elegans gonad: a test tube for cell and developmental biology. Dev Dyn. 2000;218:2–22. doi: 10.1002/(SICI)1097-0177(200005)218:1<2::AID-DVDY2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Iwasaki K, McCarter J, Francis R, Schedl T. emo-1, a Caenorhabditis elegans Sec61p gamma homologue, is required for oocyte development and ovulation. J Cell Biol. 1996;134:699–714. doi: 10.1083/jcb.134.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki K, Thomas JH. Genetics in rhythm. Trends Genet. 1997;13:111–115. doi: 10.1016/s0168-9525(97)01059-7. [DOI] [PubMed] [Google Scholar]
- Kamath RS, Martinez-Campos M, Zipperlen P, Fraser AG, Ahringer J. Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol. 2000;2:2.1–2.2.10. doi: 10.1186/gb-2000-2-1-research0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariya K, Kim BY, Gao X, Sternberg PW, Kataoka T. Phospholipase Cε regulates ovulation in Caenorhabditis elegans. Dev Biol. 2004;274:201–210. doi: 10.1016/j.ydbio.2004.06.024. [DOI] [PubMed] [Google Scholar]
- Lehotsky J, Kaplan P, Babusikova E, Strapkova A, Murin R. Molecular pathways of endoplasmic reticulum dysfunctions: possible cause of cell death in the nervous system. Physiol Res. 2003;52:269–274. [PubMed] [Google Scholar]
- Lewis RS. Calcium signaling mechanisms in T lymphocytes. Annu Rev Immunol. 2001;19:497–521. doi: 10.1146/annurev.immunol.19.1.497. [DOI] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER–plasma membrane junctions. J Cell Biol. 2006;174:815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machaca K. Ca2+-calmodulin-dependent protein kinase II potentiates store-operated Ca2+ current. J Biol Chem. 2003;278:33730–33737. doi: 10.1074/jbc.M305023200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter J, Bartlett B, Dang T, Schedl T. On the control of oocyte meiotic maturation and ovulation in Caenorhabditis elegans. Dev Biol. 1999;205:111–128. doi: 10.1006/dbio.1998.9109. [DOI] [PubMed] [Google Scholar]
- Mello CC, Kramer JM, Stinchcomb D, Ambros V. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 1991;10:3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer JC, Dehaven WI, Smyth JT, Wedel B, Boyles RR, Bird GS, Putney JW., Jr Large store-operated calcium-selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor, Stim1. J Biol Chem. 2006;281:24979–24990. doi: 10.1074/jbc.M604589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh AB, Fleig A, Penner R. The store-operated calcium current ICRAC: nonlinear activation by InsP3 and dissociation from calcium release. Cell. 1997;89:973–980. doi: 10.1016/s0092-8674(00)80282-2. [DOI] [PubMed] [Google Scholar]
- Parekh AB, Penner R. Store depletion and calcium influx. Physiol Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- Parekh AB, Putney JW. Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- Pariente JA, Camello C, Camello PJ, Salido GM. Release of calcium from mitochondrial and nonmitochondrial intracellular stores in mouse pancreatic acinar cells by hydrogen peroxide. J Membr Biol. 2001;179:27–35. doi: 10.1007/s002320010034. [DOI] [PubMed] [Google Scholar]
- Park MK, Petersen OH, Tepikin AV. The endoplasmic reticulum as one continuous Ca2+ pool: visualization of rapid Ca2+ movements and equilibration. EMBO J. 2000;19:5729–5739. doi: 10.1093/emboj/19.21.5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinelt C, Vig M, Koomoa DL, Beck A, Nadler MJ, Koblan-Huberson M, Lis A, Fleig A, Penner R, Kinet JP. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nat Cell Biol. 2006;8:771–773. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner R, Fleig A. Store-operated calcium entry: a tough nut to CRAC. Sci. STKE. 2004:e38. doi: 10.1126/stke.2432004pe38. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Kannan MS, Sieck GC. Regulation of intracellular calcium oscillations in porcine tracheal smooth muscle cells. Am J Physiol Cell Physiol. 1997;272:C966–C975. doi: 10.1152/ajpcell.1997.272.3.C966. [DOI] [PubMed] [Google Scholar]
- Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- Prakriya M, Lewis RS. Regulation of CRAC channel activity by recruitment of silent channels to a high open-probability gating mode. J Gen Physiol. 2006;128:373–386. doi: 10.1085/jgp.200609588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RV, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ. 2004;11:372–380. doi: 10.1038/sj.cdd.4401378. [DOI] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rual JF, Ceron J, Koreth J, Hao T, Nicot AS, Hirozane-Kishikawa T, Vandenhaute J, Orkin SH, van den Hill DE, HS & Vidal M. Toward improving Caenorhabditis elegans phenome mapping with an ORFeome-based RNAi library. Genome Res. 2004;14:2162–2168. doi: 10.1101/gr.2505604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Gupta DD, Chakrabarti MK. Involvement of phospholipase C in Yersinia enterocolitica heat stable enterotoxin (Y-STa) mediated rise in intracellular calcium level in rat intestinal epithelial cells. Toxicon. 2005;45:361–367. doi: 10.1016/j.toxicon.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- Sijen T, Fleenor J, Simmer F, Thijssen KL, Parrish S, Timmons L, Plasterk RH, Fire A. On the role of RNA amplification in dsRNA-triggered gene silencing. Cell. 2001;107:465–476. doi: 10.1016/s0092-8674(01)00576-1. [DOI] [PubMed] [Google Scholar]
- Soboloff J, Spassova MA, Hewavitharana T, He LP, Xu W, Johnstone LS, Dziadek MA, Gill DL. STIM2 is an inhibitor of STIM1-mediated store-operated Ca2+ entry. Curr Biol. 2006a;16:1465–1470. doi: 10.1016/j.cub.2006.05.051. [DOI] [PubMed] [Google Scholar]
- Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W, Gill DL. Orai1 and STIM reconstitute store-operated calcium channel function. J Biol Chem. 2006b;281:20665. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- Spassova MA, Soboloff J, He LP, Xu W, Dziadek MA, Gill DL. STIM1 has a plasma membrane role in the activation of store-operated Ca2+ channels. Proc Natl Acad Sci U S A. 2006;103:4040–4045. doi: 10.1073/pnas.0510050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabara H, Sarkissian M, Kelly WG, Fleenor J, Grishok A, Timmons L, Fire A, Mello CC. The rde-1 gene, RNA interference, and transposon silencing in C. elegans. Cell. 1999;99:123–132. doi: 10.1016/s0092-8674(00)81644-x. [DOI] [PubMed] [Google Scholar]
- Teramoto T, Iwasaki K. Intestinal calcium waves coordinate a behavioral motor program in C. elegans. Cell Calcium. 2006;40:319–327. doi: 10.1016/j.ceca.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Tian P, Estes MK, Hu Y, Ball JM, Zeng CQ, Schilling WP. The rotavirus nonstructural glycoprotein NSP4 mobilizes Ca2+ from the endoplasmic reticulum. J Virol. 1995;69:5763–5772. doi: 10.1128/jvi.69.9.5763-5772.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kuppeveld FJ, Hoenderop JG, Smeets RL, Willems PH, Dijkman HB, Galama JM, Melchers WJ. Coxsackievirus protein 2B modifies endoplasmic reticulum membrane and plasma membrane permeability and facilitates virus release. EMBO J. 1997;16:3519–3532. doi: 10.1093/emboj/16.12.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam K, van Rossum DB, Patterson RL, Ma HT, Gill DL. The cellular and molecular basis of store-operated calcium entry. Nat Cell Biol. 2002;4:E263–E272. doi: 10.1038/ncb1102-e263. [DOI] [PubMed] [Google Scholar]
- Vig M, Beck A, Billingsley JM, Lis A, Parvez S, Peinelt C, Koomoa DL, Soboloff J, Gill DL, Fleig A, Kinet JP, Penner R. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr Biol. 2006a;16:2073–2079. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006b;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174:803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Lu J, Li ZYuX, Chen L, Xu T. Aggregation of STIM1 underneath the plasma membrane induces clustering of Orai1. Biochem Biophys Res Commun. 2006;350:969–976. doi: 10.1016/j.bbrc.2006.09.134. [DOI] [PubMed] [Google Scholar]
- Yan X, Xing J, Lorin-Nebel C, Estevez AY, Nehrke K, Lamitina T, Strange K. Function of a STIM1 homologue in C. elegans: evidence that store-operated Ca2+ entry is not essential for oscillatory Ca2+ signaling and ER Ca2+ homeostasis. J Gen Physiol. 2006;128:443–459. doi: 10.1085/jgp.200609611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeromin AV, Roos J, Stauderman KA, Cahalan MD. A store-operated calcium channel in Drosophila S2 cells. J Gen Physiol. 2004;123:167–182. doi: 10.1085/jgp.200308982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeromin AV, Zhang SL, Jiang WYuY, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Gower NJ, Baylis HA, Strange K. Inositol 1,4,5-trisphosphate signaling regulates rhythmic contractile activity of smooth muscle-like sheath cells in the nematode Caenorhabditis elegans. Mol Biol Cell. 2004;15:3938–3949. doi: 10.1091/mbc.E04-03-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SLYuY, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SL, Yeromin AV, Zhang XHYuY, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc Natl Acad Sci U S A. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifach A, Lewis RS. Rapid inactivation of depletion-activated calcium current (ICRAC) due to local calcium feedback. J Gen Physiol. 1995a;105:209–226. doi: 10.1085/jgp.105.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifach A, Lewis RS. Slow calcium-dependent inactivation of depletion-activated calcium current. Store-dependent and -independent mechanisms. J Biol Chem. 1995b;270:14445–14451. doi: 10.1074/jbc.270.24.14445. [DOI] [PubMed] [Google Scholar]