Abstract
The systemic renin–angiotensin system (RAS) plays an important role in regulating blood pressure, electrolyte and fluid homeostasis. However, local RASs also exist in diverse tissues and organs, where they play a multitude of autocrine, paracrine and intracrine physiological roles. The existence of a local RAS is now recognized in pancreatic acinar, islet, duct, endothelial and stellate cells, the expression of which is modulated in response to physiological and pathophysiological stimuli such as hypoxia, pancreatitis, islet transplantation, hyperglycaemia, and diabetes mellitus. This pancreatic RAS has been proposed to have important endocrine and exocrine roles in the pancreas, regulating local blood flow, duct cell sodium bicarbonate secretion, acinar cell digestive enzyme secretion, islet beta-cell (pro)insulin biosynthesis, and thus, glucose-stimulated insulin release, delta-cell somatostatin secretion, and pancreatic cell proliferation and differentiation. It may further mediate oxidative stress-induced cell inflammation, apoptosis and fibrosis. Further exploration of this system would probably offer new insights into the pathogenesis of pancreatitis, diabetes, cystic fibrosis and pancreatic cancer formation. New therapeutic targets and strategies might thus be suggested.
The renin–angiotensin system
The renin–angiotensin system (RAS) is classically known as a circulating or hormonal system regulating blood pressure, electrolyte and fluid homeostasis (Peach, 1977). Such an endocrine function is mediated largely by its potent effects on vascular smooth muscle and on the renal reabsorption of electrolytes as well as water via direct tubule actions, and via stimulation of aldosterone and vasopressin (Matsusaka & Ichikawa, 1997). This classical RAS consists of several key components: the hepatic derived precursor angiotensinogen, two critical enzymes, namely the renal synthesized renin and pulmonary-bound angiotensin-converting enzyme (ACE), and the physiologically active peptide, angiotensin II, as well as its receptors. The sequential actions of renin and ACE on plasma angiotensinogen produce the decapeptide angiotensin I and octapeptide angiotensin II, respectively. In addition, alternative enzymes to renin and ACE generate a number of bioactive peptides from angiotensin I and/or angiotensin II, such as angiotensin III, angiotensin IV and angiotensin (1–7). Such angiotensin-processing peptidases include, to name but a few, chymase, chymotrypsin, tonin, ACE2 (a homologue of ACE) and aminopeptidase A, as well as the aminopeptidase B/N. Angiotensin II, together with these bioactive peptides, mediates their specific functions via the respective cellular receptors of target tissue organs (Lavoie & Sigmund, 2003). The biological actions of the RAS are mediated by the angiotensin II type 1 and type 2 receptors, i.e. AT1 and AT2 receptors; most of the functions are, however, mediated by the AT1 receptor (De Gasparo et al. 2000).
The local RAS in organ systems
The presence of local tissue RASs has been increasingly recognized over the last 15 years, with mounting recognition of their clinical importance (Montgomery et al. 2003). These functional local RASs have been found in such diverse organ systems as the pancreas, heart, kidney, vasculature and adipose tissue as well as the nervous, reproductive and digestive systems (see review by Paul et al. 2006). Such local RASs operate in an autocrine, paracrine and/or intracrine manner and exhibit multiple physiological effects at the cellular level which adds to, and/or differs from, the circulating RAS. In addition to haemodynamic actions, the local RAS has multiple and novel functions including the regulation of cell growth, differentiation, proliferation and apoptosis, reactive oxygen species (ROS) generation, tissue inflammation and fibrosis, and hormonal secretion (Leung, 2004). Such a diversity of roles makes tissue RASs attractive therapeutic targets in diverse disease states. This topical review is undertaken to give a critical appraisal on the physiological roles of a local RAS in the pancreas and, additionally, to discuss its clinical relevance.
The existence of a local pancreatic RAS
The accumulated evidence supports the existence of a complete pancreatic RAS (where renin and ACE are involved in the biosynthetic pathway), although the data are somewhat conflicting. Thus, angiotensinogen and renin are expressed in rat pancreas (Leung et al. 1999), whilst neither angiotensin I nor renin activity have been identified in the dog pancreas (Chappell et al. 1991). On the other hand, binding sites for angiotensin II receptors have been characterized in the endocrine and exocrine cells of pancreas (Chappell et al. 1992, 1995; Ghiani & Masini, 1995). Indeed, AT1 and AT2 receptors and angiotensin II have been specifically localized to different cell types of the pancreas including endothelial, ductal, acinar and islet cells (Leung et al. 1997, 1998). Consistent with this finding, mRNA for the AT1 receptor subtypes (AT1a and AT1b) and the AT2 receptor has also been found in the rat pancreas (Leung et al. 1999). In the human pancreas, AT1 receptors and (pro)renin have been shown to be localized not only in exocrine cells but also the beta cells of the endocrine pancreas (Tahmasebi et al. 1999). The presence of a pancreatic RAS in the human pancreas is further substantiated by the expression and localization of angiotensinogen and AT1 receptors, notably in pancreatic islets and ducts (Lam & Leung, 2002).
Table 1 summarizes the accumulated data on the evidence for the existence of a pancreatic RAS from different animal species and isolated cells as well as cell lines of the pancreas.
Table 1.
A summary of existence of a pancreatic RAS from previous studies as evidenced by immunocytochemistry (ICC), Western blot (WB), quantitative/semiquantitative polymerase chain reaction (PCR), in vitro autoradiography/binding studies (AR/BS), and high performance liquid chromatography/radioimmunoassay (HPLC/RIA)
| Species/isolated | Presence of RAS components as evidenced by: | Literature cited | ||||
|---|---|---|---|---|---|---|
| pancreatic cells/pancreatic cell lines | ICC | WB | PCR | AR/BS | HPLC/RIA | |
| Dog | – | – | – | + | + | Chappell et al. 1991, 1992) |
| Rat | + | + | + | + | – | Ghiani & Masini, 1995; Leung et al. 1997, 1999; Tikellis et al. 2004; Wong et al. 2004 |
| Mouse | + | – | – | – | – | Leung et al. 1988 1988, 1997 |
| Human | + | – | + | – | – | Tahmasebi et al. 1999; Lam & Leung, 2002 |
| Mouse isolated islets | + | + | + | – | – | Lau et al. 2004; Chu et al. 2006 |
| Human isolated islets | – | + | + | – | – | Lupi et al. 2006; Ramracheya et al. 2006 |
| Rat isolated acinar cells | – | – | + | – | – | Tsang et al. 2004a,Tsang et al. 2004b |
| Mouse MIN6 cells | – | + | + | – | – | Ramracheya et al. 2006 |
| Rat pancreatic AR42J cells | – | – | + | + | – | Chappell et al. 1995; Cheung et al. 1999 |
| Rat pancreatic stellate cells | – | – | + | – | – | Ko et al. 2006 |
Physiological and pathophysiological regulation
It is intriguing that the pancreatic RAS components are responsive to various physiological and pathophysiological stimuli, including hypoxia, pancreatitis, islet transplantation, hyperglycaemia, type 2 diabetes mellitus (T2DM), and pancreatic cancer (Leung, 2004).
In chronic hypoxia, the expression of several major components of the pancreatic RAS is increased (Chan et al. 2000). Of interest in this context is the reversibility and adaptability of the RAS activation by chronic hypoxia, further indicating its physiological relevance to the pancreas (Ip et al. 2003b). Hypoxia causes a decrease of blood flow (and the presence of ischaemia) in several tissues including the pancreas, and leads to enhanced tissue inflammation and injury (Kuwahira et al. 1993). The up-regulation of RAS by hypoxia could contribute to ischaemia via vasoconstriction of the pancreatic microcirculation (Carlsson et al. 1998). Acute pancreatitis is associated with increased expression of major components of the pancreatic RAS (Leung et al. 2000). On the other hand, expression of local RAS components are up-regulated in human pancreatic endocrine tumours (Lam & Leung, 2002) and ACE inhibition may also modulate mitosis and gene expression in pancreatic cancer cells (Reddy et al. 1995).
The pancreatic RAS is also subjected to regulation by such conditions as islet transplantation, diabetes mellitus, and hyperglycaemia. Of note, AT1 receptor and angiotensinogen expression are markedly up-regulated in islets retrieved from 4-week-old islet transplants (Lau et al. 2004) and in islets or pancreas from animal models of T2DM (Tikellis et al. 2004; Chu et al. 2006) as well as in islets or pancreatic stellate cells exposed to hyperglycaemia (Lupi et al. 2006; Ko et al. 2006). Taken together, the regulation of the pancreatic RAS by (patho)physiological conditions suggests that a functional RAS exists in the pancreas and that interference with such a local RAS may be promising in the prevention and treatment of pancreatic inflammation and disease.
Table 2 summarizes the regulated expression of RAS components by physiological and pathophysiological stimuli from various cells in the pancreas.
Table 2.
A summary of contemporary research on the physiological and pathophysiological conditions that regulate the RAS components in different pancreatic cell types
| (Patho)physiological condition | RAS components subject to regulation | Pancreatic cell types | Literature cited |
|---|---|---|---|
| Chronic hypoxia | Angiotensinogen, ACE, | All cell types | Chan et al. 2000; Ip et al. 2003b |
| AT1a and AT1b receptor subtypes, | |||
| AT2 receptor | |||
| Acute pancreatitis | Angiotensinogen, ACE, | Acinar cell, duct cell, | Leung et al. 2000; Tsang et al. 2003; |
| AT1a and AT1b receptor subtypes, | endothelial cell | Ip et al. 2003a; Tsang et al. 2004a,Tsang et al. 2004b | |
| AT2 receptor | |||
| Diabetes mellitus | Angiotensinogen, ACE, ACE2, | Islet cell (beta cell) | Ko et al. 2004; Tikellis et al. 2004; |
| AT1 receptor, AT2 receptor | Shao et al. 2006; Chu et al. 2006 | ||
| Hyperglycaemia | Angiotensinogen, ACE, | Islet cell, stellate cell | Lupi et al. 2006; Ko et al. 2006 |
| AT1 receptor | |||
| Islet transplantation | Angiotensinogen, ACE, | Islet cell | Lau et al. 2004; Kamp et al. 2005 |
| AT1 receptor, | |||
| AT2 receptor | |||
| Pancreatic cancer | Angiotensinogen, AT1 receptor, | Islet cell, duct cell | Lam & Leung 2002 |
| AT2 receptor |
Physiological roles in the exocrine pancreas and clinical relevance
Recent studies have suggested novel roles for the pancreatic RAS in the regulation of pancreatic duct cell and acinar cell secretion in the exocrine pancreas. In pancreatic duct cells, angiotensin II influences anion secretion via the mediation of AT1 receptors. By using a short-circuit current (ISC) technique, angiotensin II was found to dose-dependently increase the ISC in a cystic fibrosis pancreatic cell line, which effect is completely abolished by losartan, an AT1 receptor blocker (Chan et al. 1997). This AT1 receptor-mediated anion secretion is dependent on Ca2+ and cAMP as its signal transduction events (Cheng et al. 1999). In isolated dog pancreatic epithelial cells and cystic fibrosis pancreatic cell cultures, AT1 receptor activation of calcium channels is involved in bicarbonate secretion (Fink et al. 2002). Interestingly, captopril reduces secretin-induced bicarbonate output in conscious dogs (Howard-McNatt & Fink, 2002).
In pancreatic acinar cells, AT1 receptors have been identified in rat pancreatic AR42J cells and angiotensin II mediates a dose-dependent increase in amylase secretion and inositol 1,3,4-triphosphate production (Chappell et al. 1995; Cheung et al. 1999). This effect can be inhibited by losartan (an AT1 receptor antagonist), but not by CGP42112 (an AT2 receptor antagonist). More recently, several key RAS components (AT1 and AT2 receptors and angiotensinogen) have been found to be expressed in isolated rat pancreatic acinar cells (Tsang et al. 2004a). Exogenous addition of angiotensin II stimulates a dose-dependent release of digestive enzyme secretion (amylase and lipase) from these isolated pancreatic acini. Administration of losartan significantly inhibits acinar digestion enzyme secretion while the specific AT2 receptor blocker PD123319 did not exhibit any such suppressive effect (Tsang et al. 2004a).
All the convergent data indicate that the pancreatic RAS plays a physiological role in regulating pancreatic ductal bicarbonate and acinar digestive enzyme secretion. What, then, are its roles in diseases of the exocrine pancreas, and in pancreat it is ? To address this issue, the differential effects of RAS blockade on pancreatitis-induced inflammation and injury have been studied. Intriguingly, saralasin, a non-specific antagonist of AT1/AT2 receptors, is effective in improving pancreatitis-induced injury in the pancreas (Tsang et al. 2003), perhaps through an inhibition of RAS activation of ROS (Ip et al. 2003a). Prophylactic and therapeutic treatment with losartan, but not PD123319, also reduces the pancreatitis-induced oxidative stress, presumably by improving pancreatic microcirculation and by diminishing AT1 receptor-mediated NADPH oxidase-dependent production of ROS (Tsang et al. 2004b). Furthermore, AT1 receptor antagonism has been shown to be protective against acute pancreatitis and its associated pulmonary injury (Chan & Leung, 2006).
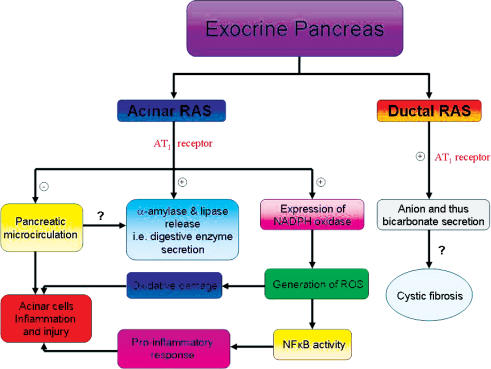
Using a rat model of obstructive pancreatitis, the involvement of angiotensin II might be due to AT1 receptor-mediated NADPH oxidase-dependent NFκB-dependent activation, and thus angiotensin II ultimately promotes pro-inflammatory actions during the development of pancreatitis (Y. C. Chan & P. S. Leung, unpublished data). On the other hand, a recent study has shown that ACE inhibition attenuates chronic pancreatitis-induced injury and pancreatic fibrosis, possibly via the prevention of pancreatic stellate cell activation (Kuno et al. 2003). In this regard, losartan also prevents apoptosis of pancreatic acinar cell by AT1 receptor-mediated pancreatic cell fibrosis (Wang et al. 2004). All the available data support the potential clinical value of RAS blockade in protecting and treating pancreatitis and fibrosis. Figure 1 summarizes the physiological roles of pancreatic RAS in the exocrine pancreas and the clinical implications.
Figure 1. A summary of physiological functions of pancreatic RAS and clinical implications in the exocrine pancreas.
+ denotes stimulatory action; – denotes inhibitory action; ? denotes the action has yet to be determined.
Physiological roles in the endocrine pancreas and clinical relevance
In the endocrine pancreas, islet RASs play a novel role in regulating islet glucose-stimulated insulin secretion and thus glucose homeostasis. In this context, pancreatic blood flow and preferentially islet blood flow is significantly impaired by locally generated angiotensin II, as demonstrated by perfused rat pancreas; this inhibitory effect was rescued by RAS antagonists (Carlsson et al. 1998). Captopril and irbesartan (an AT1 receptor antagonist) selectively enhance pancreatic islet blood flow, insulin secretion and glucose tolerance (Huang et al. 2006, 2007). Interestingly, angiotensin II dose-dependently inhibits insulin release from isolated mouse islets in response to a high glucose concentration (Lau et al. 2004). This inhibitory action is due, at least in part, to a decreased (pro)insulin biosynthesis which is preventable by losartan treatment. These data from isolated islets rule out the possibility that the AT1 receptor-mediated effect on glucose-stimulated insulin secretion is exclusively attributable to changes in pancreatic islet blood flow (Carlsson et al. 1998; Lau et al. 2004).
Although the AT2 receptor has been expressed in isolated mouse islets, angiotensin II does not affect glucose-stimulated insulin secretion via this receptor subtype, as shown by PD123319 treatment (Lau et al. 2004). In contrast, AT2 receptor has also been localized to the outer region of islets and colocalizes with somatostatin-secreting cells in the endocrine pancreas and in the immortalized rat pancreatic cell lines RIN-m and RIN-14B (Wong et al. 2004). In RIN-14B cells, angiotensin II stimulates somatostatin secretion in a dose-dependent manner. This action seems to be mediated by AT2 receptors, as CGP42112 treatment abolishes the response to angiotensin II (Wong et al. 2004). These data prompt us to conclude that the pancreatic islet RAS has a functional role in regulating pancreatic islet insulin and somatostatin secretion and thus physiologically involved in glucose homeostasis.
A large body of clinical studies has provided evidence indicating that pharmacological RAS blockade reduces the incidence of new-onset T2DM cases in high-risk patients with cardiovascular disease. In this respect, a recent meta-analysis of clinical trials of ACE inhibitors and AT1 receptor blockers has been conducted in order to evaluate these protective effects (Abuissa et al. 2005). This meta-analysis concluded that the mean risk for developing T2DM was reduced by 27% with ACE inhibitor treatments, 23% with AT1 receptor treatments, and 25% overall in a pooled analysis of these two RAS blockers. Nevertheless, the exact mechanism(s) for the protective effect of RAS blockade in T2DM remain ambiguous. Of note, recent identification of an islet RAS in the pancreas and its emerging role in islet function might provide a novel and alternative explanation for the reduced incidence of T2DM observed in these clinical trials (Lau et al. 2004).
In order to delineate this issue, several animal models of T2DM have recently been developed for this topical research. For the long-term effect (24 weeks) of ACE inhibitor on pancreatic islets, ramipril is administered to Otsuka Long-Evans Tokushima fatty (OLETF) rats; ramipril treatment can prevent islet destruction by fibrosis in these diabetic rats, as shown by the expression profile of TGF-β and its downstream signalling molecules (Ko et al. 2004). Using another rat model of T2DM, Zucker diabetic fatty rats, chronic treatment (10 weeks) of either an ACE inhibitor (perindopril) or AT1 receptor blocker (irbesartan) attenuates islet fibrosis as well as apoptosis and oxidative stress (Tikellis et al. 2004). These data suggest that islet RAS activation may be involved in oxidative stress-induced islet apoptosis and fibrosis, and thus islet dysfunction is observed in T2DM. Interestingly, hyperglycaemia as observed in T2DM has also recently been shown to activate the RAS in pancreatic islet and stellate cells; RAS blockade ameliorates the angiotensin II-induced pancreatic inflammation and fibrosis aggravated by chronic exposure to high glucose levels (Lupi et al. 2006; Ko et al. 2006).
Notwithstanding the presentation of this persuasive data, however, the precise mechanism(s) by which a local pancreatic RAS is involved in islet dysfunction has yet to be elucidated. More recently, a mouse model of T2DM, i.e. an obesity-induced db/db mouse, has been employed to test the hypothesis that there is a change in AT1 receptor expression in T2DM which enables endogenous levels of angiotensin II to impair islet function (Chu et al. 2006). These data clearly conclude that the pancreatic islet AT1 receptors, though not having any obvious effects on normal islet function, become up-regulated during T2DM; this up-regulation thus has negative effects on islet glucose-stimulated insulin secretion and (pro)insulin biosynthesis, and islet blood flow (Chu et al. 2006). These findings provide a novel and, at least, partial explanation for the reduced incidence of T2DM that has been observed in a number of clinical trials applying AT1 receptor blockade to individuals at high risk for this disease.
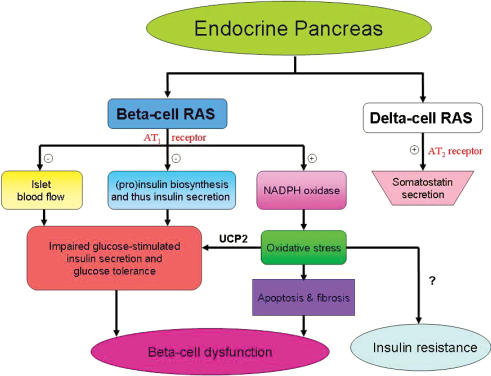
Using the db/db mouse model, it has been further shown that AT1 receptor antagonism (for 8 weeks) attenuates NADPH oxidase-induced oxidative stress; this, in turn, results in a down-regulation of uncoupling protein 2 (UCP2) expression, which is associated with improved beta-cell insulin secretion and reduces apoptosis-induced beta-cell mass loss (Chu & Leung, 2007). In keeping with these findings, chronic AT1 receptor antagonism improves islet cell function and structure, an effect that is apparently mediated by NADPH oxidase-induced oxidative stress in animal models of T2DM, i.e. OLETF rats and db/db mice (Nakayama et al. 2005; Shao et al. 2006). Taking all these data together, it is plausible to propose that AT1 receptor activation by T2DM mediates UCP2-driven oxidative stress and thus leads to pancreatic islet beta-cell dysfunction. Figure 2 summarizes the physiological roles of pancreatic RAS in endocrine pancreas and the clinical implications.
Figure 2. A summary of physiological functions of pancreatic RAS and clinical implications in the endocrine pancreas.
+ denotes stimulatory action; – denotes inhibitory action; ? denotes the action has yet to be determined.
Conclusions
Local RASs exist in major cell types of the endocrine and exocrine pancreas, the activities of which are subjected to regulation by physiological and pathophysiological stimuli. These functional RASs have important roles in the regulation of islet, acinar and duct cell secretion and their activation is involved in AT1 receptor-mediated oxidative stress-induced cell inflammation, apoptosis and fibrosis. Inhibition of the pancreatic RAS activation may shed new light on the prevention as well as treatment of pancreatic inflammation and diabetes mellitus.
Acknowledgments
The author gratefully acknowledges the financial support provided by the Competitive Earmarked Research Grant from the Research Grants Council of Hong Kong (Project No. CUHK4537/05M and CUHK4364/04M), awarded to P.S.L.
References
- Abuissa H, Jones PG, Marso SP, O'Keefe JH. Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers for prevention of type 2 diabetes. J Am Coll Cardiol. 2005;46:821–826. doi: 10.1016/j.jacc.2005.05.051. [DOI] [PubMed] [Google Scholar]
- Carlsson PO, Berne C, Jansson L. Angitoensin II and the endocrine pancreas: effects on islet blood flow and insulin secretion in rats. Diabetologia. 1998;41:127–133. doi: 10.1007/s001250050880. [DOI] [PubMed] [Google Scholar]
- Chan HC, Law SH, Leung PS, Wong PYD. Angiotensin II receptor type I-regulated anion secretion in cystic fibrosis pancreatic duct cells. J Memb Biol. 1997;156:241–250. doi: 10.1007/s002329900204. [DOI] [PubMed] [Google Scholar]
- Chan WP, Fung ML, Nobiling R, Leung PS. Activation of local renin-angiotensin system by chronic hypoxia in rat pancreas. Mol Cell Endocrinol. 2000;160:107–114. doi: 10.1016/s0303-7207(99)00258-0. [DOI] [PubMed] [Google Scholar]
- Chan YC, Leung PS. AT1 receptor antagonism ameliorates acute pancreatitis-associated pulmonary injury. Regul Pept. 2006;134:46–53. doi: 10.1016/j.regpep.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Chappell MC, Diz DI, Jacobsen DW. Pharmacological characterization of angiotensin II binding sites in the canine pancreas. Peptides. 1992;13:311–318. doi: 10.1016/0196-9781(92)90114-i. [DOI] [PubMed] [Google Scholar]
- Chappell MC, Jacobsen DW, Tallant EA. Characterization of angiotensin II receptor subtypes in pancreatic acinar AR42J cells. Peptides. 1995;16:741–747. doi: 10.1016/0196-9781(95)00044-k. [DOI] [PubMed] [Google Scholar]
- Chappell MC, Milsted A, Diz DI, Brosnihan KB, Ferrario CM. Evidence for an intrinsic angiotensin system in the canine pancreas. J Hypertens. 1991;9:751–759. doi: 10.1097/00004872-199108000-00008. [DOI] [PubMed] [Google Scholar]
- Cheng HS, So SC, Law SH, Chan HC. Angiotensin II-mediated signal transduction in cystic fibrosis pancreatic cells. Biochem Biophysica Acta. 1999;1449:254–260. doi: 10.1016/s0167-4889(99)00017-8. [DOI] [PubMed] [Google Scholar]
- Cheung WT, Yeung SY, Yiu AKL, Ip TM, Wan DCC, Luk SKS, Ho WKK. Characterization of a functional AT1A angiotensin receptor in pancreatoma AR4-2J cells. Peptides. 1999;20:829–836. doi: 10.1016/s0196-9781(99)00069-8. [DOI] [PubMed] [Google Scholar]
- Chu KY, Lau T, Carlsson PO, Leung PS. Angiotensin II type 1 receptor blockade improves beta-cell function and glucose tolerance in a mouse model of type 2 diabetes. Diabetes. 2006;55:367–374. doi: 10.2337/diabetes.55.02.06.db05-1022. [DOI] [PubMed] [Google Scholar]
- Chu KY, Leung PS. Angiotensin II type 1 receptor antagonism mediates uncoupling protein 2-driven oxidative stress and ameliorates pancreatic islet β-cell function in young type 2 diabetic mice. Antioxid Redox Signal. 2007 doi: 10.1089/ars.2007.1590. in press. [DOI] [PubMed] [Google Scholar]
- De Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415–472. [PubMed] [Google Scholar]
- Lupi R, Del Guerra S, Bugliani M, Boggi U, Mosca F, Torri S, Del Prato S, Marchetti P. The direct effects of the angiotensin-converting enzyme inhibitors, zofenoprilat and enalaprilat, on isolated human pancreatic islets. Eur J Endocrinol. 2006;154:355–361. doi: 10.1530/eje.1.02086. [DOI] [PubMed] [Google Scholar]
- Fink AS, Wang Y, Mendez T, Worrell RT, Eaton D, Nguyen TD, Lee SP. Angiotensin II evokes calcium-mediated signaling events in isolated dog pancreatic epithelial cells. Pancreas. 2002;25:290–295. doi: 10.1097/00006676-200210000-00012. [DOI] [PubMed] [Google Scholar]
- Ghiani BU, Masini MA. Angiotensin II binding sites in the rat pancreas and their modulation after sodium loading and depletion. Comp Biochem Physiol A Physiol. 1995;111:439–444. doi: 10.1016/0300-9629(95)00030-b. [DOI] [PubMed] [Google Scholar]
- Howard-McNatt M, Fink AS. Captopril inhibits secretin-induced pancreatic bicarbonate output. J Surg Res. 2002;103:96–99. doi: 10.1006/jsre.2001.6336. [DOI] [PubMed] [Google Scholar]
- Huang Z, Jansson L, Sjoholm A. Pancreatic islet blood flow is selectively enhanced by captopril, irbesartan and pravastatin, and suppressed by palmitate. Biochem Biophys Res Comm. 2006;346:26–32. doi: 10.1016/j.bbrc.2006.05.144. [DOI] [PubMed] [Google Scholar]
- Huang Z, Jansson L, Sjoholm A. Vasoactive drugs enhance pancreatic islet blood flow, augment insulin secretion and improve glucose tolerance in female rats. Clin Sci. 2007;112:69–76. doi: 10.1042/CS20060176. [DOI] [PubMed] [Google Scholar]
- Ip SP, Tsang SW, Wong TP, Che CT, Leung PS. Saralasin, a non-specific angiotensin II receptor antagonist, attenuates oxidative stress and tissue injury in cerulien-induced acute pancreatitis. Pancreas. 2003a;26:224–229. doi: 10.1097/00006676-200304000-00003. [DOI] [PubMed] [Google Scholar]
- Ip SP, Wong TP, Tsai SJ, Leung PS. The recovery of some components of the renin-angiotensin system in the rat pancreas after chronic exposure to hypoxic condition. J Mol Endocrinol. 2003b;31:563–571. doi: 10.1677/jme.0.0310563. [DOI] [PubMed] [Google Scholar]
- Kamp C, Lau T, Olsson R, Leung PS, Carlsson PO. Angiotensin II type 1 receptor inhibition markedly improves the blood perfusion, oxygen tension and first phase of glucose-stimulated insulin secretion in revascularised syngeneic mouse islet grafts. Diabetologia. 2005;48:1159–1167. doi: 10.1007/s00125-005-1761-z. [DOI] [PubMed] [Google Scholar]
- Ko SH, Hong OK, Kim JW, Ahn YB, Song KH, Cha BY, Son HY, Kim MJ, Jeong IK, Yoon KH. High glucose increases extracellular matrix production in pancreatic stellate cells by activating the renin-angiotensin system. J Cell Biochem. 2006;98:343–355. doi: 10.1002/jcb.20797. [DOI] [PubMed] [Google Scholar]
- Ko SH, Kwon HS, Kim SR, Moon SD, Ahn YB, Song KH, Son HS, Cha BY, Lee KW, Son HY, Kang SK, Park CG, Lee IK, Yoon KH. Ramipril treatment suppresses islet fibrosis in Otsuka Long-Evans Tokushima fatty rats. Biochem Biophys Res Commum. 2004;316:114–122. doi: 10.1016/j.bbrc.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Kuno A, Yamada T, Kasuda K, Ogawa K, Sogawa M, Nakamura S, Ohara H, Nomura T, Joh T, Shirai T, Itoh M. Angiotensin-converting enzyme inhibitor attenuates pancreatic inflammation and fibrosis in male Wistar Bonn/Kobori rats. Gastroenterology. 2003;124:1010–1010. doi: 10.1053/gast.2003.50147. [DOI] [PubMed] [Google Scholar]
- Kuwahira I, Gonzalez NC, Heisler N, Piipet J. Changes in regional blood flow distribution and oxygen supply during hypoxia in conscious rats. J Appl Physiol. 1993;74:211–214. doi: 10.1152/jappl.1993.74.1.211. [DOI] [PubMed] [Google Scholar]
- Lam KY, Leung PS. Regulation and expression of renin-angiotensin system in human pancreas and pancreatic endocrine tumours. Eur J Endocrinol. 2002;146:567–572. doi: 10.1530/eje.0.1460567. [DOI] [PubMed] [Google Scholar]
- Lau T, Carlsson PO, Leung PS. Evidence for a local angiotensin-generating system and dose-dependent inhibition of glucose-stimulated insulin release by angiotensin II in isolated pancreatic islets. Diabetologia. 2004;47:240–248. doi: 10.1007/s00125-003-1295-1. [DOI] [PubMed] [Google Scholar]
- Lavoie JL, Sigmund CD. Overview of the renin-angiotensin system: an endocrine and paracrine system. Endocrinology. 2003;144:2179–2183. doi: 10.1210/en.2003-0150. [DOI] [PubMed] [Google Scholar]
- Leung PS. The peptide hormone angiotensin II: its new functions in tissues and organs. Curr Protein Pept Sci. 2004;5:267–273. doi: 10.2174/1389203043379693. [DOI] [PubMed] [Google Scholar]
- Leung PS, Chan HC, Fu LXM, Wong PYD. Localization of angiotensin II receptor subtypes AT1 and AT2 in the pancreas of rodents. J Endocrinol. 1997;153:269–274. doi: 10.1677/joe.0.1530269. [DOI] [PubMed] [Google Scholar]
- Leung PS, Chan WP, Nobiling R. Regulated expression of pancreatic renin-angiotensin system in experimental pancreatitis. Mol Cell Endocrinol. 2000;166:121–128. doi: 10.1016/s0303-7207(00)00275-6. [DOI] [PubMed] [Google Scholar]
- Leung PS, Chan HC, Wong PYD. Immunohistochemical localization of angiotensin II in the mouse pancreas. Histochem J. 1998;30:21–25. doi: 10.1023/a:1003210428276. [DOI] [PubMed] [Google Scholar]
- Leung PS, Chan WP, Wong TP, Sernia C. Expression and localization of the renin-angiotensin system in the rat pancreas. J Endocrinol. 1999;160:13–19. doi: 10.1677/joe.0.1600013. [DOI] [PubMed] [Google Scholar]
- Matsusaka T, Ichikawa I. Biological functions of angiotensin and its receptors. Ann Rev Physiol. 1997;59:395–412. doi: 10.1146/annurev.physiol.59.1.395. [DOI] [PubMed] [Google Scholar]
- Montgomery H, Humphries SE, Leung PS. Renin-angiotensin system: the new frontier. Int J Biochem Cell Biol. 2003;35:758. [Google Scholar]
- Nakayama M, Inoguchi T, Sonta T, Maeda Y, Sasaki S, Sawada F, Tsubouchi H, Sonoda N, Kobayashi K, Sumimoto H, Nawata H. Increased expression of NAD(P)H oxidase in islets of animal models of Type 2 diabetes and its improvement by an AT1 receptor antagonist. Biochem Biophys Res Commun. 2005;332:927–933. doi: 10.1016/j.bbrc.2005.05.065. [DOI] [PubMed] [Google Scholar]
- Paul M, Mehr AP, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- Peach M. Renin-angiotensin system: biochemistry and mechanism of action. Physiol Rev. 1977;57:313–370. doi: 10.1152/physrev.1977.57.2.313. [DOI] [PubMed] [Google Scholar]
- Ramracheya RD, Muller DS, Wu Y, Whitehouse BJ, Huang GC, Amiel SA, Karalliedde J, Viberti G, Jones PM, Persaud SJ. Direct regulation of insulin secretion by angiotensin II in human islets of Langerhans. Diabetologia. 2006;49:321–331. doi: 10.1007/s00125-005-0101-7. [DOI] [PubMed] [Google Scholar]
- Reddy MK, Baskaran K, Moiteni A. Inhibitors of angiotensin-converting enzyme modulate mitosis and gene expression in pancreatic cancer cells. Proc Soc Exp Biol Medical. 1995;210:221–226. doi: 10.3181/00379727-210-43942. [DOI] [PubMed] [Google Scholar]
- Shao J, Iwashita N, Ikeda F, Ogihara T, Uchida T, Shimizu T, Uchino H, Hirose T, Kawamori R, Watada H. Beneficial effects of candesartan, an angiotensin II type 1 receptor blocker, on beta-cell function and morphology in db/db mice. Biochem Biophys Res Commun. 2006;344:1224–1233. doi: 10.1016/j.bbrc.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Tahmasebi M, Puddefoot JR, Inwang ER, Vinsion GP. The tissue renin-angiotensin system in human pancreas. J Endocrinol. 1999;161:317–322. doi: 10.1677/joe.0.1610317. [DOI] [PubMed] [Google Scholar]
- Tikellis C, Wookey PJ, Candido R, Andrikopoulos S, Thomas MC, Cooper ME. Improved islet morphology after blockade of the renin-angiotensin system in the ZDF rat. Diabetes. 2004;53:989–997. doi: 10.2337/diabetes.53.4.989. [DOI] [PubMed] [Google Scholar]
- Tsang SW, Cheng CHK, Leung PS. The role of the pancreatic renin-angiotensin system in acinar digestive enzyme secretion and in acute pancreatitis. Regul Pept. 2004a;119:213–219. doi: 10.1016/j.regpep.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Tsang SW, Ip SP, Leung PS. Prophylactic and therapeutic treatments with AT1 and AT2 receptor antagonists and their effects on changes in the severity of pancreatitis. Int J Biochem Cell Biol. 2004b;36:330–339. doi: 10.1016/s1357-2725(03)00257-7. [DOI] [PubMed] [Google Scholar]
- Tsang SW, Ip SP, Wong TP, Che CT, Leung PS. Differential effects of saralasin and ramiprilat, the inhibitors of renin-angiotensin system, on cerulein-induced acute pancreatitis. Regul Pept. 2003;111:47–53. doi: 10.1016/s0167-0115(02)00226-4. [DOI] [PubMed] [Google Scholar]
- Wang XP, Zhang R, Wu K, Wu L, Dong Y. Angiotensin II mediates acinar cell apoptosis during the development of rat pancreatic fibrosis by AT1R. Pancreas. 2004;29:264–270. doi: 10.1097/00006676-200411000-00004. [DOI] [PubMed] [Google Scholar]
- Wong PF, Lee SS, Cheung WT. Immunohistochemical colocalization of type II angiotensin receptors with somatostatin in rat pancreas. Regul Pept. 2004;117:195–205. doi: 10.1016/j.regpep.2003.10.019. [DOI] [PubMed] [Google Scholar]