Abstract
Neonatal maternal deprivation (NMD) increases gut paracellular permeability (GPP) through mast cells and nerve growth factor (NGF), and modifies corticotrophin-releasing factor (CRF) and corticosterone levels. CRF, corticosterone and mast cells are involved in stress-induced mucosal barrier impairment. Consequently, this study aimed to specify whether corticosteronaemia and colonic expression of both preproCRF and CRF are modified by NMD, and to determine if altered expression may participate in the elevated GPP in connection with NGF and mast cells. Male Wistar rat pups were either separated from postnatal days 2–14, or left undisturbed with their dam. At 12 weeks of age, adult rats were treated with mifepristone (an antagonist of corticoid receptors), α-helical CRF(9-41) (a non-specific CRF receptor antagonist), or SSR-125543 (CRF-R1 receptor antagonist). We also determined corticosteronaemia and both colonic preproCRF and CRF expression. Then, control rats were treated by CRF, doxantrazole (mast cell stabilizer), BRX-537A (a mast cell activator) and anti-NGF antibody. NMD did not modify colonic CRF level but increased colonic preproCRF expression and corticosteronaemia. Peripheral CRF, via CRF-R1 receptor, but not corticosterone, was involved in the elevated GPP observed in these rats, through a mast-cell-mediated mechanism, since the increase of GPP induced by exogenous CRF was abolished by doxantrazole. Anti-NGF antibody treatment also reduced the elevated GPP induced by CRF or BRX-537A. CRF acts through CRF-R1 receptors to stimulate NGF release from mast cells, which participates in the elevated GPP observed in NMD adult rats. This suggests that early traumatic experience induced neuro-endocrine dysfunction, involved in alterations of gut mucosal barrier.
There is evidence that early life trauma and ongoing psychological stress can affect the clinical course of intestinal disorders(Levenstein et al. 2000; Mayer et al. 2001) and also reactivate (Collins, 2001) or enhance (Gue et al. 1997) inflammation in experimental colitis. Thus, childhood adverse events, considered as potent stressors are often associated in humans with gastrointestinal diseases such as Crohn's disease (Ringel & Drossman, 2001) or irritable bowel syndrome (IBS) (Hislop, 1979; Lowman et al. 1987). In animal models, neonatal maternal deprivation (NMD) has been found to predispose to colonic barrier dysfunction (Barreau et al. 2004b, 2006) and to enhance mucosal response to a mild stress (Soderholm et al. 2002).
However, the mechanisms underlying the effects of neonatal stress on the induction or exacerbation of colonic mucosal barrier dysfunction remain largely unknown. In adult rats, acute or chronic stress induces short-term intestinal alterations, through mechanisms involving corticotrophin-releasing factor (CRF) (Santos et al. 1999; Saunders et al. 2002), glucocorticoids (Meddings & Swain, 2000) and mast cells (Santos et al. 2001). In agreement with the hypothesis of CRF involvement in stress-induced immediate gut permeability alterations, it has been reported that systemic administration of CRF increases gut permeability (Santos et al. 1999). Biological actions of CRF are exerted by interacting with two distinct CRF receptors subtypes, CRF-R1 and CRF-R2, both being expressed in the gastrointestinal tract (Chatzaki et al. 2004). These receptors belong to the family of G protein-coupled receptors signalling through cAMP synthesis (Hauger et al. 2003). Although CRF is largely produced in the hypothalamus, a peripheral synthesis has been detected in colonic mucosal cells in the neighbourhood of the base of the crypts (Kawahito et al. 1994), and its local release may play a role in the modulation of the intestinal immune system and/or other gastrointestinal functions, either basally or under stressful conditions. However, the central or peripheral origin of CRF-mediated effects of stress on the mucosal barrier is still under debate.
Among immune cells, mast cells play a critical role in the regulation of epithelial transport both in human (Crowe et al. 1997; Santos et al. 1998) and rodent intestine (Perdue et al. 1991; Berin et al. 1998), and it is widely accepted that nerve–mast cell interactions are involved in intestinal epithelial dysfunction (Argenzio, 1997; Coelho et al. 2000). Nerves and mast cells participate in the development of stress-induced increase of colonic paracellular permeability (Castagliuolo et al. 1998; Santos et al. 1999). Indeed, a mast cell stabilizer, doxantrazole, abolishes acute stress-induced ion secretion and permeability increase in the rat colon, and these effects are not observed in mast cell-deficient mice (Castagliuolo et al. 1998). However, it remains to be confirmed that mast cells are involved in the increased gut paracellular permeability (GPP) induced by CRF.
Concerning the effects of neonatal stress on mast cells, some investigations have reported that NMD increases the number of colonic mucosal mast cells (Barreau et al. 2004b), and that daily handling of new-born rats enhances the number of mast cells within thalamus nuclei in adult rats (Lafreniere et al. 2001). Moreover, we have previously shown that mast cell stabiliser doxantrazole and anti-NGF (nerve growth factor) antibody (Ab) treatment in adult rats abolished the increase in GPP induced by NMD (Barreau et al. 2004a). Thus, both mast cells and NGF play together a pivotal role in the elevated GPP observed in NMD adult rats. However, although NGF may stimulate mast cell proliferation and degranulation, the pathway involving NGF and mast cells in the regulation of GPP is unknown. Neonatal stress also affects the hypothalamic-pituitary-adrenal (HPA) axis, modifying the adrenocortical response to novelty in adult rats (Biagini et al. 1998). NMD increases supraspinal (brain) CRF expression (Husum & Mathe, 2002; Vazquez et al. 2003) associated with an altered expression of glucocorticoid receptors (Ogawa et al. 1994) in the central nervous system.
Taken together, these data suggest that even though NGF is involved, during the neonatal period, in the genesis of long-term effect of NMD, corticosterone, CRF, mast cells and NGF also play a pivotal role in the gut barrier alterations induced by NMD in adult rats. Consequently this study aimed (1) to assess in adult rats whether corticosterone and CRF are involved in the permanent alterations of GPP induced by NMD; (2) to determine the type of CRF receptor involved and; (3) to investigate whether CRF and mast cells are involved in cascade with NGF.
Methods
Animals
Primiparous pregnant female Wistar rats were individually housed in standard polypropylene cages containing 2.5 cm of wood chip bedding material. They were kept at a constant temperature (23 ± 1°C) and maintained on a 12: 12 h light: dark cycle (lights on at 7 am). Food (UAR pellets, Epinay, France) and water were available ad libitum. Mothers and their pups, and then the young rats after weaning on day 22, were kept in the same conditions.
Nenonatal maternal deprivation
NMD was performed according to a previously validated method (Rosztoczy et al. 2003; Barreau et al. 2004b). Briefly, the litters were culled to 10 pups after delivery (day 1). NMD was performed daily for three consecutive hours (from 0900 to 1200 h), during which pups were removed from the home cage and kept in temperature-controlled cages at 28 ± 1°C, where bedding was changed every day. This procedure was applied between postnatal days 2 and 14. Control pups were left with their dam. From days 15–22, all control and maternally deprived pups were maintained with their dam. Weaning was performed on day 22, siblings were sex-matched, and males were selected.
Gut paracellular permeability
Assessment of GPP was performed using 51Cr-ethylenediaminetetraacetic acetic (EDTA) as a selective marker of paracellular permeation of tight junctions. Thus, 0.7 μCi of 51Cr-EDTA (Perkin Elmer Life Science, Paris, France) was diluted in 500 μl water and administered by oral route. Rats were then placed in metabolic cages, and faeces and urine were separately collected during 24 h. The radioactivity found in urine was measured by a gamma-counter (Cobra II; Packard, Meridien, CT, USA). Permeability to 51Cr-EDTA was expressed as a percentage of the total administered radioactivity.
PreproCRF immunohistochemistry
Under anaesthesia (i.p. administration of 0.6 mg kg−1 acepromazine (Vetoquinol, Lure, France) and 120 mg kg−1 ketamine (Rhone-Mérieux, Lyon, France)), rats were exsanguinated by beheading, and a 2 cm-long portion of the colon was excised and washed in sterile saline. The collected fragments were fixed in Duboscq-Brazil solution for 24 h, dehydrated in ethanol solution, embedded in paraffin blocks, and cut into 5 μm sections. Paraffin sections were rehydrated and submerged in antigen retrieval solution (citrate buffer, 10 mm, pH 6, 95°C, 3 min). After inhibition of endogenous peroxidases with 0.6% H2O2 in 100% methanol for 30 min, and incubation in blocking solution (phosphate-buffered saline containing 1% bovine serum albumin and 2% normal donkey serum), sections were incubated with goat anti-preproCRF antibody (Santa Cruz, Le Perray en Yvelines, France) (1/100; overnight, +4°C) followed by a biotinylated donkey anti-goat IgG immune serum (Interchim, Montluçon, France) 1/1000; 30 min, RT. Slides were subsequently incubated with ABC complexes coupled to peroxidase (Abcys, Paris, France). 3-3′diaminobenzidine (ICN Pharmaceuticals, Costa Mesa, CA, USA) was used as chromogen. Immunoreactivity was graded from 0 to 3 indicating the degree of preproCRF expression (0, none; 1, weak; 2, intermediate; 3, strong). Grading was done in a blind fashion.
Corticosterone assay
Plasma samples were stored at −20°C until assayed. Plasma corticosterone concentrations were determined using an adapted high-performance liquid chromatography (HPLC) after a solid/liquid extraction on C8 cartridge and in the presence of internal standard (flumethasone 2.5 μg ml−1). An Inertsil ODS 33 μm (150 × 4.0 mm) column was eluted by H2O/methanol (50/50; v/v) mixing, and a 0.5 ml min−1 rate of flow was used to separate cortisol and corticosterone. The corticosterone detection was performed at 254 nm and the quantification limit of the method was 25 ng ml−1.
Quantification of NGF release under CRF stimulation
Under anaesthesia (i.p. administration of 0.6 mg kg−1 acepromazine (Vetoquinol, Lure, France) and 120 mg kg−1 ketamine (Rhone-Mérieux, Lyon, France)), rats were exsanguinated by beheading and four segments (0.5 cm) of distal colon were immediately taken from each animal. Two colonic pieces were used to investigate the CRF-induced NGF release, and two others for basal NGF release. Each segment was incubated for 15 min at 37°C, in a Tyrode solution (composition, (mm): NaCl 136.9, KCl 2.68, CaCl2 1.8, MgCl2 1.05, NaHCO3 1.19, NaH2PO4 0.42 and glucose 0.55). Then Tyrode solution containing CRF (10 μg ml−1) or CRF-vehicle was added, and NGF released was determined for 1 h. NGF levels in the supernatant were measured by ELISA. Briefly, anti-NGF polyclonal Ab, which binds soluble NGF, was used at 100 μl well−1. The sample incubation was 6 h at room temperature. The captured NGF is bound by a second specific monoclonal antibody (mAb). After washing, the amount of specifically bound mAb is detected using an anti-rat IgG conjugated to HRP. After, incubation with a chromogenic substrate, the colour change is measured at 450 nm after reaction was stopped. All these products were obtained from Promega (Lyon, France). NGF levels were expressed in pmol (ml supernatant)−1.
Quantification of colonic mucosal CRF levels
Under anaesthesia (i.p. administration of 0.6 mg kg−1 acepromazine (Vetoquinol, Lure, France) and 120 mg kg−1 ketamine (Rhone-Mérieux, Lyon, France)), rats were exsanguinated by beheading, and segments (4 cm) of distal colon were immediately taken from each animal. Mucosa was recovered and homogenized in PBS buffer supplemented with anti-protease cocktail (Roche, Meylan, France). Sample protein content was determined by a modified Lowry-based assay (Bio-Rad, Marnes-La-Coquette, France). CRF levels were determined by ELISA (CosmoBio, Tokyo, Japan) according to the manufacturer's instructions. CRF was quantified against a CRF standard curve and expressed as ng (mg protein)−1.
Experimental protocol
All experiments were performed in 12-week-old-male rats.
In a first set of experiments, four groups of eight contol and eight NMD rats were used.
In a first group of rats, colonic preproCRF immuno-reactivity, mucosal colonic CRF level (distal segment) and corticosterone plasma level were determined. Rats (control and NMD) were both anaesthetized (10 AM) by i.p. administration of 0.6 mg kg−1 acepromazine (Vetoquinol, Lure, France) and 120 mg kg−1 ketamine (Rhone-Mérieux, Lyon, France), and both aortic blood and colonic samples were collected. Then rats were killed by cervical dislocation.
In a second group of rats, two administrations of mifepristone, a corticoid receptor antagonist, was administered s.c. (4 mg kg−1 in 0.2 ml) 6 h and 1 h before measurement of GPP.
In a third group of rats, two i.p. administrations of α-helical CRF(9-41) (a non-selective CRF1/CRF2-receptor (CRF-R1/CRF-R2) antagonist; 250 μg kg−1 in 0.2 ml) were similarly performed 6 h and 1 h before permeability measurement.
A fourth group of rats received oral SSR-125543 (a selective CRF-R1 antagonist (Griebel et al. 2002); 10 mg kg−1 in 0.5 ml) 6 h and 1 h before assessing GPP. Another group of rats was used to evaluate the release of NGF from colonic strips in basal condition and under CRF stimulation.
In a second set of experiments, six groups of eight contol rats were used.
Group 1 received two i.p. administrations of CRF 1 h before and 6 h after starting the permeability measurement.
Group 2 was treated by two i.p. administrations of doxantrazole (mast cell stabilizer; 5 mg kg−1 in 0.2 ml; 6 h and 1 h before permeability measurement) and CRF (50 μg kg−1 in 0.2 ml; 1 h before and 6 h after starting the permeability measurement).
Group 3 received two administrations of doxantrazole (5 mg kg−1 in 0.2 ml; 6 h and 1 h before permeability measurement).
Group 4 was treated by two i.p. administrations of anti-NGF antibodies (15 μg kg−1 in 0.2 ml; 6 h and 1 h before permeability measurement) and CRF (50 μg kg−1 in 0.2 ml; 1 h before and 6 h after starting the permeability measurement).
Group 5 received two i.p. administrations of BRX-537A (2 mg kg−1 in 0.2 ml; 1 h before and 6 h after starting the permeability measurement).
Group 6 was treated by two i.p. administrations of anti-NGF antibodies (15 μg kg−1 in 0.2 ml; 6 h and 1 h before permeability measurement) and BRX-537A (2 mg kg−1 in 0.2 ml; 1 h before and 6 h after starting the permeability measurement).
All experimental protocols described in this study were approved by the local Institutional Animal Care and Use Committee.
Drugs
Doxantrazole was kindly supplied by Wellcome Research laboratories (Beckenham, UK), and was dissolved in 5% sodium bicarbonate. Mifepristone was a gift from Roussel-Uclaf (Paris, France), and was dissolved in olive oil. α-helical CRF(9-41) was purchased from Fisher (Illkirch, France), and was diluted in sterile saline solution. CRF was purchased from Calbiochem (VWR, Fontenay-sous-bois, France), and was diluted in saline solution. SSR-125543 was kindly supplied by Sanofi-Aventis, and was dissolved in DMSO 5% and cremophor-EL 5%. Anti-NGF Ab was purchased from R&D Systems (R&D, Lille, France), and was diluted in sterile saline solution. BrX-537A (bromolasalocid ethanolate) was kindly supplied by Roche Laboratories (London, UK) and was dissolved in DMSO.
Statistical analysis
Results are expressed as means ± s.e.m. Multiple groups were compared by Dunnett's test after one-way ANOVA. Single comparisons were performed by non-parametric test (unpaired Student's t test) for statistical analysis of CRF immunoreactivity and CRF effects on gut paracellular permeability. Differences were considered significant for P < 0.05.
Results
Corticosterone and maternal deprivation
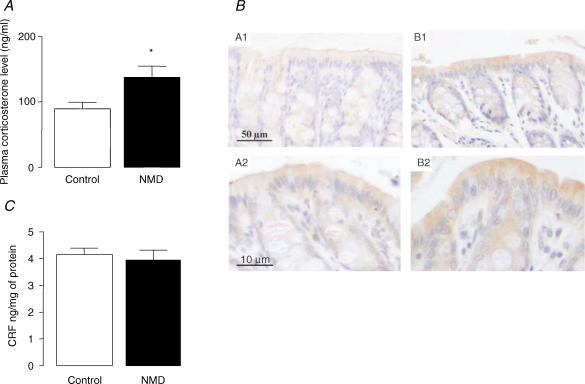
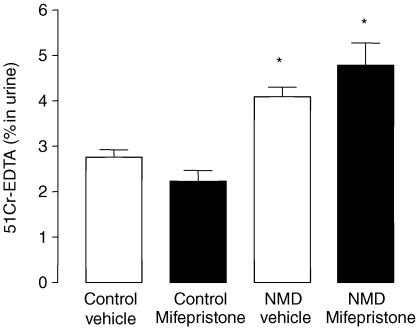
In control rats, the corticosterone plasma concentration was 90 ± 9 ng ml−1. In NMD rats, this value was significantly increased to 138 ± 17 ng ml−1 (P < 0.05) (Fig. 1A). In NMD rats, GPP was significantly increased in comparison with control rats (4.1 ± 0.2% versus 2.7 ± 0.3%; P < 0.05). However, mifepristone (4 mg kg−1; s.c.) did not modify GPP in either control or NMD rats (P > 0.05) (Fig. 2).
Figure 1. Effect of NMD on corticosterone plasma levels, and both prepro CRF and CRF colonic mucosa expression in adult rats (12 weeks).
A, corticosterone plasma level. B, preproCRF immunoreactivity (brown) in colonic sections of non-NMD (A1, A2) and NMD (B1, B2). A1, B1, bar = 50 μm, and A2, B2, bar = 10 μm. C, colonic CRF mucosa level. Control (open bars) and NMD rats (filled bars). Values are means ± s.e.m.; n = 8/group.*P < 0.05 versus controls.
Figure 2. Effect of mifepristone on the increased GPP induced by NMD in adult rats.
Control (left) and NMD rats (right) were treated by vehicle (open bars) or mifepristone (filled bars). Values are means ± s.e.m.; n = 8/group. *P < 0.05 versus vehicle controls.
PreproCRF, CRF and maternal deprivation
An intense preproCRF labelling of enterocytes was observed in both control and NMD rats (Fig. 1B), while labelling of colonic bottom crypts and submucosa was weaker. In NMD rats, preproCRF immunoreactivity was significantly increased (P < 0.05) in colonic mucosa, lamina propria layers and colonic bottom crypts, in comparison with control rats (Table 1 and Fig. 1B). Surprisingly, as presented in Fig. 1C, no difference of CRF colonic mucosal levels was observed between control and NMD rats (3.9 ± 0.4 ng (mg protein)−1versus 4.2 ± 0.2 ng (mg protein)−1; P > 0.05).
Table 1.
Effect of neonatal maternal deprivation on colonic preproCRF immunohistochemistry in adult rats
| Control | NMD | |
|---|---|---|
| Sub mucosa | 0.6 ± 0.2 | 0.9 ± 0.2 |
| Lamina propria | 0.2 ± 0.2 | 1.1 ± 0.3* |
| Enterocytes | 1.1 ± 0.3 | 2.4 ± 0.4* |
| Colonic bottom crypts | 0.6 ± 0.4 | 1.5 ± 0.2* |
| Total CRF | 2.5 ± 0.6 | 5.9 ± 0.7* |
Results were obtained from at least six different rats from control and NMD groups. Data (mean ± s.e.m.) are expressed as the total scoring, graded from 0 to 3 (0, no immunoreactivity; 1, weak immunoreactivity; 2, intermediate immunoreactivity; 3, strong immunoreactivity).
P < 0.05 from control (Student's t test).
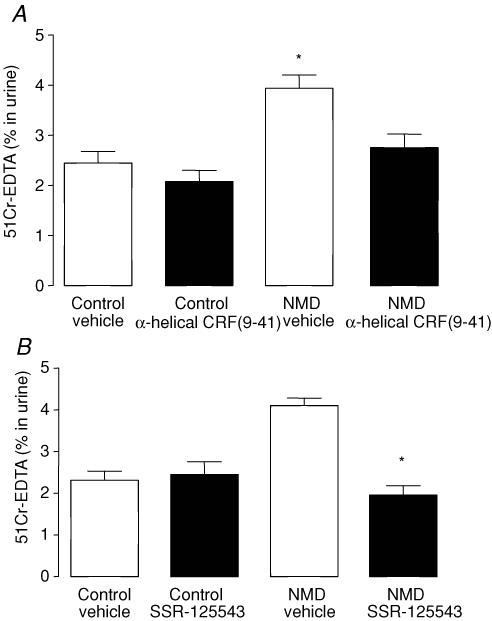
In NMD rats, an increased GPP was observed (3.9 ± 0.3% versus 2.4 ± 0.2%; P < 0.05). both α-helical CRF(9-41) and SSR-125543 suppressed the increase in GPP induced by maternal deprivation (P < 0.05) (Fig. 3A and B). In control rats, GPP was not modified by either α-helical CRF(9-41) or SS-125543 treatment (P > 0.05).
Figure 3. Involvement of CRF in the regulation of elevated GPP induced by NMD in adult rats.
A, effect of α-helical CRF(9-41) on the elevated GPP induced by NMD in adult rats. Control (left) and NMD rats (right) were treated by vehicle (open bars) or α-helical CRF(9-41) (filled bars). B, effect of SSR-125543A (CRF-R1 antagonist) on the increased GPP induced by NMD in adult rats. Control (left) and NMD-rats (right) were treated by vehicle (open bars) or SSR-125543A (filled bars). Values are means ± s.e.m.; n = 8/group. *P < 0.05 versus vehicle controls.
CRF and mast cells
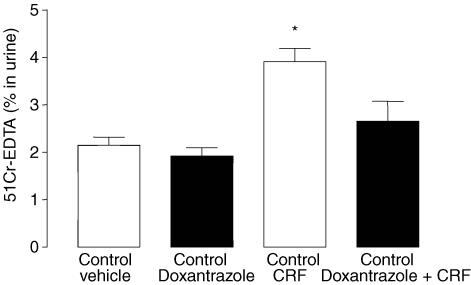
In control rats, CRF (50 μg kg−1) increased GPP (3.9 ± 0.3% versus 2.1 ± 0.2%; P < 0.05). Doxantrazole treatment abolished the increase of GPP induced by CRF (2.6 ± 0.4% versus 3.9 ± 0.3%; P < 0.05), but had no effect under basal conditions (1.9 ± 0.2% versus 2.1 ± 0.2%; P > 0.05) (Fig. 4).
Figure 4. Role of mast cell degranulation on the increased GPP induced by CRF treatment in control rats.
Values are means ± s.e.m.; n = 8/group. *P < 0.05 versus Vehicle.
NGF, CRF and mast cells
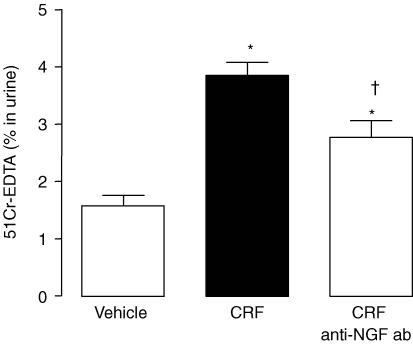
In control rats, CRF (50 μg kg−1) increased GPP (3.9 ± 0.3% versus 1.6 ± 0.2%; P < 0.05) (Fig. 5). Anti-NGF Ab treatment reduced significantly the increase of GPP induced by CRF administration (2.8 ± 0.3% versus 3.9 ± 0.3%; P < 0.05). However, the GPP observed after CRF plus anti-NGF Ab treatments, remained elevated compared with control rats (2.8 ± 0.3% versus 1.6 ± 0.3%; P < 0.05) (Fig. 5). Moreover, anti-NGF-neutralizing antibody treatment did not modify the GPP in adult control rat (data not shown; P > 0.05).
Figure 5. Involvement of NGF on the increased GPP induced by CRF treatment in control rats.
Values are means ± s.e.m.; n = 8/group. *P < 0.05 versus vehicle controls, †P < 0.05 versus CRF.
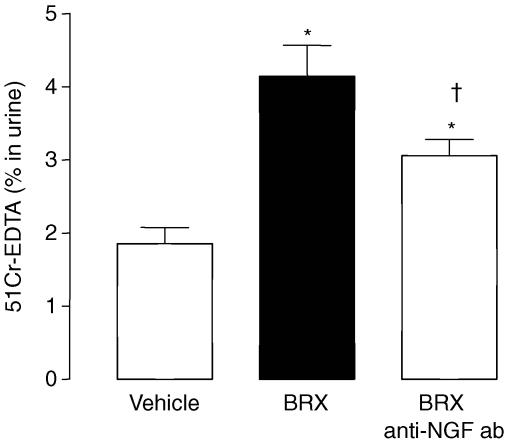
Similarly, mast cell stimulation by BRX-537A increased GPP (4.1 ± 0.4% versus 1.8 ± 0.2%; P < 0.05) (Fig. 6). Anti-NGF Ab treatment reduced the increase of GPP induced by BRX-537A (3.1 ± 0.4% versus 4.8 ± 0.4%; P < 0.05). However, the GPP still remained higher than that observed in control rat (3.1 ± 0.4% versus 1.8 ± 0.2%; P < 0.05) (Fig. 6).
Figure 6. NGF release from mast cells participated on the increased GPP induced by BRX-537A in control rats.
Values are means ± s.e.m.; n = 8/group. *P < 0.05 versus vehicle, †P < 0.05 versus BRX-537A.
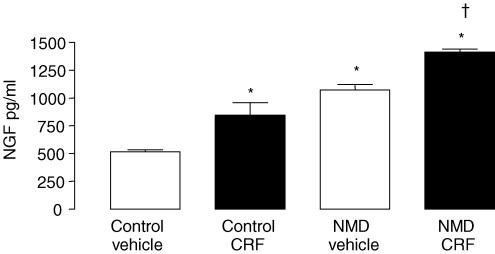
In basal condition, the spontaneous release of NGF from colonic strips of NMD and control rats was measured in the cleared supernatant obtained after 1 h of incubation (Fig. 7). The NGF amount released by colon sample from NMD rats was significantly higher (1073.2 ± 48.4 ng ml−1versus 514.3 ± 20.6 ng ml−1; P < 0.01) than that of control rats. On colonic strips, CRF stimulated NGF release with a greater response in NMD than in control rats (1413.2 ± 26.1 ng ml−1versus 843.3 ± 113.7 ng g−1; P < 0.001) (Fig. 7).
Figure 7. Effect of NMD on spontaneous and CRF-induced NGF release from colonic mucosal mast cells in adult rats.
Control (left) and NMD rats (right) were treated by vehicle (open bars) or CRF (filled bars).Values are means ± s.e.m.; n = 5/group. *P < 0.01 versus vehicle controls, †P < 0.001 versus CRF controls.
Discussion
This report demonstrates that NMD both increases corticosteronaemia and preproCRF immunoreactivity in the different colonic mucosa areas, but does not modify colonic mucosal levels of CRF in adult NMD rats. Moreover, CRF but not corticosterone plays a major role in long-term alterations of the gut epithelial barrier triggered by NMD. This is supported by our data showing that the effects of NMD are suppressed by treatment of adult rats with α-helical CRF(9-41) or a CRF-R1 receptor antagonist, but not by the glucocorticoid/progesterone receptor antagonist mifepristone. We demonstrate for the first time that CRF favours the release of NGF from colonic mast cells, which in turn is responsible for the increase of GPP. Indeed, doxantrazole treatment abolishes the increased GPP induced by CRF, and anti-NGF Ab reduces the CRF or BRX-537A-induced increase of GPP. This result is confirmed by the observed greater release of NGF in response to CRF in adult NMD rats.
The neonatal period, roughly extending in rats from birth to day 14, is often referred to as a stress hyporesponsive period characterized by a diminished ACTH (adrenocorticotrophic hormone) and corticosterone response to most stressors (Rosenfeld et al. 1991). Although NMD is known to affect the HPA axis function (Levine, 2001), the effects of NMD on plasma corticoid levels are in opposition. These differences may be explained by the rodent strain (Ellenbroek & Cools, 2000), sex (Wigger & Neumann, 1999; Barna et al. 2003), or the period where NMD (Van Oers et al. 1998) is applied (chronic or acute). In this study we have shown that NMD increased plasma corticosterone in adult rats. Several hypotheses may be considered. Firstly, NMD induced NGF overexpression in colonic tissue of adult rats (Cirulli, 2001; Barreau et al. 2004a). Since NGF promotes adrenal gland hypertrophy (Bigi et al. 1992; Alleva & Santucci, 2001) and glucocorticoid secretion (Otten et al. 1979, 1981), NGF overexpression may also stimulate corticosterone synthesis and accumulation in the blood. Secondly, NMD rats exhibited hyperresponsiveness to psychological stressors as compared to controls (Caldji et al. 2000; Ladd et al. 2000). This hyperresponsiveness results from a resistance to glucocorticoid-mediated negative feedback (Caldji et al. 2000; Ladd et al. 2000), which may participate in the elevated corticosteronemia. Thirdly, since NMD triggers bacterial translocation (Barreau et al. 2004b), leading to a downstream activation of the HPA axis (Ando et al. 2000), bacterial translocation induced by NMD may participate to increase corticosteronaemia in NMD rats. Recently, it was shown that acute stress increases plasma corticosterone, which in turn increases GPP (Meddings & Swain, 2000). However, in NMD rats, glucocorticoid receptor antagonist treatment failed to affect GPP. Consequently, a direct involvement of corticoids in the increased GPP can be excluded.
By contrast with glucocorticoids, the effects of NMD on CRF release and CRF receptor expression were extensively investigated in the central nervous system. Maternal deprivation induces a significant increase of CRF, CRF-R1 and CRF-R2 mRNA expression in the cortex, and a decrease of CRF-R1 and CRF-R2 mRNA expression in amygdala (Vazquez et al. 2003). Although CRF secretion is mainly localized in the central nervous system, a colonic CRF production has been detected in the mucosal cells in the neighbourhood of the base of the colonic crypts (Kawahito et al. 1994), and an elevated colonic CRF expression has been observed in biopsies of patients with ulcerative colitis (Kawahito et al. 1995). In agreement with these previous studies, in the present study we have reported that both preproCRF and mature CRF are present within the colonic mucosa. We have also shown for the first time that NMD increases preproCRF expression in several colonic mucosal areas, while it does not modify colonic mucosal CRF content in adult rats. This differential effect of NMD on colonic mucosa preproCRF and CRF levels may be explained by the fact that NMD may only alter preproCRF synthesis without modifying the rate of maturation of preproCRF into CRF. In addition, colonic content of CRF may result from both central and peripheral production of CRF. Consequently, this can explain the lack of relationship between preproCRF expression and tissue CRF concentration.
CRF acts within the brain and pituitary to coordinate the overall endocrine and behavioural stress response. CRF is known to be involved in various stress-induced abnormalities in the gastrointestinal tract (Castagliuolo et al. 1996; Santos et al. 1999; Tache et al. 2001). A peripheral injection of CRF mimics stress-induced changes in colonic function regarding mucin release (Castagliuolo et al. 1996), ion secretion and permeability (Santos et al. 1999). Moreover, physical and psychological stress in rats enhanced colonic epithelial permeability via peripheral CRF (Saunders et al. 2002). In this context, in this study we have shown that CRF plays an important role in the elevated GPP induced by NMD through peripheral and/or central CRF-R1 receptor. Intraperitoneal α-helical CRF(9-41) or SSR-125543 administration suppress the stress-induced increase of GPP. Furthermore, the suppressive effect of doxantrazole treatment on the increased GPP induced by CRF points out the involvement of mast cells. Because we have previously shown that colonic mucosa from adult NMD animals exhibit an elevated number of mast cells (Barreau et al. 2004b), we can also hypothesize that for a similar CRF colonic content, more mast cells may be activated and, consequently, larger amounts of mediators participating in the elevated GPP induced by NMD can be released.
As mast cells synthesize and release NGF (Leon et al. 1994), and NGF is known to be involved in the elevated GPP induced by NMD (Barreau et al. 2004a), we investigated CRF, NGF and mast cell interplay in the regulation of GPP. Firstly, we have shown that NGF release from mast cells under CRF and BRX-537A (mast cell activator) stimulation participates in the increased GPP in control rats. Indeed, anti-NGF Ab treatment significantly reduces the increase of GPP induced by CRF and BRX-537A. Secondly, in this study we have shown that CRF stimulation triggers a greater NGF release from colon biopsies of NMD rats than in control animals. Consequently, these data also support that NGF release from mast cells under CRF stimulation participates in the increased GPP in rats. However, the NGF receptor subtype (TrKa and P-75) (Vega et al. 2003) and cell type (nerve ending, epithelial cell, lymphocyte…) (Vega et al. 2003) involved in NGF-mediated increase of GPP remain unclear. Indeed, NGF released from mast cell may directly interfere with receptors located on epithelial cells or on nerve endings, stimulating the release of mediators or stimulating immune cells (lymphocytes) (Ferrier et al. 2003), which in turn increase GPP. Nevertheless, since anti-NGF Ab only partially lower the increase of GPP induced by CRF or BRX-537A, it can be suggested that other mediators such as rat mast cell protease II, cytokines (IL-4, IFNγ) or neuromediators (substance P) are also involved.
In summary, this study shows that NMD promotes long-term alterations of colonic preproCRF expression and corticosteronaemia level in adult NMD rats. CRF may interact with the CRF-R1 receptor to stimulate mast cell release of NGF, which in turn participates in the increased GPP in adult NMD rats. These results also provide evidence that adverse experiences in early life can induce neuroendocrine changes (HPA axis, NGF) associated with alterations of gut mucosa physiology in adult rats, and could have implications in the development of intestinal disorders such as IBS.
Acknowledgments
This work was supported by Institut National de la Recherche Agronomique (INRA), France. We thank Bernard Joseph for technical assistance.
References
- Alleva E, Santucci D. Psychosocial vs. ‘physical’ stress situations in rodents and humans: role of neurotrophins. Physiol Behav. 2001;73:313–320. doi: 10.1016/s0031-9384(01)00498-x. [DOI] [PubMed] [Google Scholar]
- Ando T, Brown RF, Berg RD, Dunn AJ. Bacterial translocation can increase plasma corticosterone and brain catecholamine and indoleamine metabolism. Am J Physiol Regul Integr Comp Physiol. 2000;279:R2164–R2172. doi: 10.1152/ajpregu.2000.279.6.R2164. [DOI] [PubMed] [Google Scholar]
- Argenzio RA. Neuro-immune pathobiology of infectious enteric disease. Adv Exp Med Biol. 1997;412:21–29. doi: 10.1007/978-1-4899-1828-4_2. [DOI] [PubMed] [Google Scholar]
- Barna I, Balint E, Baranyi J, Bakos N, Makara GB, Haller J. Gender-specific effect of maternal deprivation on anxiety and corticotropin-releasing hormone mRNA expression in rats. Brain Res Bull. 2003;62:85–91. doi: 10.1016/s0361-9230(03)00216-8. [DOI] [PubMed] [Google Scholar]
- Barreau F, Cartier C, Ferrier L, Fioramonti J, Bueno L. Nerve growth factor mediates alterations of colonic sensitivity and mucosal barrier induced by neonatal stress in rats. Gastroenterology. 2004a;127:524–534. doi: 10.1053/j.gastro.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Barreau F, De Lahitte JD, Ferrier L, Frexinos J, Bueno L, Fioramonti J. Neonatal maternal deprivation promotes Nippostrongylus brasiliensis infection in adult rats. Brain Behav Immun. 2006;20:254–260. doi: 10.1016/j.bbi.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Barreau F, Ferrier L, Fioramonti J, Bueno L. Neonatal maternal deprivation triggers long term alterations in colonic epithelial barrier and mucosal immunity in rats. Gut. 2004b;53:501–506. doi: 10.1136/gut.2003.024174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berin MC, Kiliaan AJ, Yang PC, Groot JA, Kitamura Y, Perdue MH. The influence of mast cells on pathways of transepithelial antigen transport in rat intestine. J Immunol. 1998;161:2561–2566. [PubMed] [Google Scholar]
- Biagini G, Pich EM, Carani C, Marrama P, Agnati LF. Postnatal maternal separation during the stress hyporesponsive period enhances the adrenocortical response to novelty in adult rats by affecting feedback regulation in the CA1 hippocampal field. Int J Dev Neurosci. 1998;16:187–197. doi: 10.1016/s0736-5748(98)00019-7. [DOI] [PubMed] [Google Scholar]
- Bigi S, Maestripieri D, Aloe L, Alleva E. NGF decreases isolation-induced aggressive behavior, while increasing adrenal volume, in adult male mice. Physiol Behav. 1992;51:337–343. doi: 10.1016/0031-9384(92)90150-z. [DOI] [PubMed] [Google Scholar]
- Caldji C, Diorio J, Meaney MJ. Variations in maternal care in infancy regulate the development of stress reactivity. Biol Psychiatry. 2000;48:1164–1174. doi: 10.1016/s0006-3223(00)01084-2. [DOI] [PubMed] [Google Scholar]
- Castagliuolo I, Lamont JT, Qiu B, Fleming SM, Bhaskar KR, Nikulasson ST, Kornetsky C, Pothoulakis C. Acute stress causes mucin release from rat colon: role of corticotropin releasing factor and mast cells. Am J Physiol Gastrointest Liver Physiol. 1996;271:G884–G892. doi: 10.1152/ajpgi.1996.271.5.G884. [DOI] [PubMed] [Google Scholar]
- Castagliuolo I, Wershil BK, Karalis K, Pasha A, Nikulasson ST, Pothoulakis C. Colonic mucin release in response to immobilization stress is mast cell dependent. Am J Physiol Gastrointest Liver Physiol. 1998;274:G1094–G1100. doi: 10.1152/ajpgi.1998.274.6.G1094. [DOI] [PubMed] [Google Scholar]
- Chatzaki E, Crowe PD, Wang L, Million M, Tache Y, Grigoriadis DE. CRF receptor type 1 and 2 expression and anatomical distribution in the rat colon. J Neurochem. 2004;90:309–316. doi: 10.1111/j.1471-4159.2004.02490.x. [DOI] [PubMed] [Google Scholar]
- Cirulli F. Role of environmental factors on brain development and nerve growth factor expression. Physiol Behav. 2001;73:321–330. doi: 10.1016/s0031-9384(01)00456-5. [DOI] [PubMed] [Google Scholar]
- Coelho AM, Fioramonti J, Bueno L. Systemic lipopolysaccharide influences rectal sensitivity in rats: role of mast cells, cytokines, and vagus nerve. Am J Physiol Gastrointest Liver Physiol. 2000;279:G781–G790. doi: 10.1152/ajpgi.2000.279.4.G781. [DOI] [PubMed] [Google Scholar]
- Collins SM. Stress and the gastrointestinal tract IV. Modulation of intestinal inflammation by stress: basic mechanisms and clinical relevance. Am J Physiol Gastrointest Liver Physiol. 2001;280:G315–G318. doi: 10.1152/ajpgi.2001.280.3.G315. [DOI] [PubMed] [Google Scholar]
- Crowe SE, Luthra GK, Perdue MH. Mast cell mediated ion transport in intestine from patients with and without inflammatory bowel disease. Gut. 1997;41:785–792. doi: 10.1136/gut.41.6.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbroek BA, Cools AR. The long-term effects of maternal deprivation depend on the genetic background. Neuropsychopharmacology. 2000;23:99–106. doi: 10.1016/S0893-133X(00)00088-9. [DOI] [PubMed] [Google Scholar]
- Ferrier L, Mazelin L, Cenac N, Desreumaux P, Janin A, Emilie D, Colombel JF, Garcia-Villar R, Fioramonti J, Bueno L. Stress-induced disruption of colonic epithelial barrier: role of interferon-gamma and myosin light chain kinase in mice. Gastroenterology. 2003;125:795–804. doi: 10.1016/s0016-5085(03)01057-6. [DOI] [PubMed] [Google Scholar]
- Griebel G, Simiand J, Steinberg R, Jung M, Gully D, Roger P, Geslin M, Scatton B, Maffrand JP, Soubrie P. 4-(2-chloro-4-methoxy-5-methylphenyl)-N-[(1S)-2-cyclopropyl-1-(3-fluoro-4-methylphenyl)ethyl]5-methyl-N-(2-propynyl)-1,3-thiazol-2-amine hydrochloride (SSR125543A), a potent and selective corticotrophin-releasing factor (1) receptor antagonist. II. Characterization in rodent models of stress-related disorders. J Pharmacol Exp Ther. 2002;301:333–345. doi: 10.1124/jpet.301.1.333. [DOI] [PubMed] [Google Scholar]
- Gue M, Bonbonne C, Fioramonti J, More J, Del Rio-Lacheze C, Comera C, Bueno L. Stress-induced enhancement of colitis in rats: CRF and arginine vasopressin are not involved. Am J Physiol Gastrointest Liver Physiol. 1997;272:G84–G91. doi: 10.1152/ajpgi.1997.272.1.G84. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Grigoriadis DE, Dallman MF, Plotsky PM, Vale WW, Dautzenberg FM. International Union of Pharmacology. XXXVI. Current status of the nomenclature for receptors for corticotropin-releasing factor and their ligands. Pharmacol Rev. 2003;55:21–26. doi: 10.1124/pr.55.1.3. [DOI] [PubMed] [Google Scholar]
- Hislop IG. Childhood deprivation: an antecedent of the irritable bowel syndrome. Med J Aust. 1979;1:372–374. doi: 10.5694/j.1326-5377.1979.tb126963.x. [DOI] [PubMed] [Google Scholar]
- Husum H, Mathe AA. Early life stress changes concentrations of neuropeptide Y and corticotropin-releasing hormone in adult rat brain. Lithium treatment modifies these changes. Neuropsychopharmacology. 2002;27:756–764. doi: 10.1016/S0893-133X(02)00363-9. [DOI] [PubMed] [Google Scholar]
- Kawahito Y, Sano H, Kawata M, Yuri K, Mukai S, Yamamura Y, Kato H, Chrousos GP, Wilder RL, Kondo M. Local secretion of corticotropin-releasing hormone by enterochromaffin cells in human colon. Gastroenterology. 1994;106:859–865. doi: 10.1016/0016-5085(94)90743-9. [DOI] [PubMed] [Google Scholar]
- Kawahito Y, Sano H, Mukai S, Asai K, Kimura S, Yamamura Y, Kato H, Chrousos GP, Wilder RL, Kondo M. Corticotropin releasing hormone in colonic mucosa in patients with ulcerative colitis. Gut. 1995;37:544–551. doi: 10.1136/gut.37.4.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Prog Brain Res. 2000;122:81–103. doi: 10.1016/s0079-6123(08)62132-9. [DOI] [PubMed] [Google Scholar]
- Lafreniere GF, Persinger MA, Lafreniere RF. Effects of permanent residence with foster mothers and new siblings upon numbers of mast cells within the thalamus of preweaned rats. Psychol Rep. 2001;88:625–626. doi: 10.2466/pr0.2001.88.3.625. [DOI] [PubMed] [Google Scholar]
- Leon A, Buriani A, Dal Toso R, Fabris M, Romanello S, Aloe L, Levi-Montalcini R. Mast cells synthesize, store, and release nerve growth factor. Proc Natl Acad Sci U S A. 1994;91:3739–3743. doi: 10.1073/pnas.91.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenstein S, Prantera C, Varvo V, Scribano ML, Andreoli A, Luzi C, Arca M, Berto E, Milite G, Marcheggiano A. Stress and exacerbation in ulcerative colitis: a prospective study of patients enrolled in remission. Am J Gastroenterol. 2000;95:1213–1220. doi: 10.1111/j.1572-0241.2000.02012.x. [DOI] [PubMed] [Google Scholar]
- Levine S. Primary social relationships influence the development of the hypothalamic–pituitary–adrenal axis in the rat. Physiol Behav. 2001;73:255–260. doi: 10.1016/s0031-9384(01)00496-6. [DOI] [PubMed] [Google Scholar]
- Lowman BC, Drossman DA, Cramer EM, McKee DC. Recollection of childhood events in adults with irritable bowel syndrome. J Clin Gastroenterol. 1987;9:324–330. doi: 10.1097/00004836-198706000-00017. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Naliboff BD, Chang L, Coutinho SV. V. Stress and irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2001;280:G519–G524. doi: 10.1152/ajpgi.2001.280.4.G519. [DOI] [PubMed] [Google Scholar]
- Meddings JB, Swain MG. Environmental stress-induced gastrointestinal permeability is mediated by endogenous glucocorticoids in the rat. Gastroenterology. 2000;119:1019–1028. doi: 10.1053/gast.2000.18152. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Mikuni M, Kuroda Y, Muneoka K, Mori KJ, Takahashi K. Periodic maternal deprivation alters stress response in adult offspring: potentiates the negative feedback regulation of restraint stress-induced adrenocortical response and reduces the frequencies of open field-induced behaviors. Pharmacol Biochem Behav. 1994;49:961–967. doi: 10.1016/0091-3057(94)90250-x. [DOI] [PubMed] [Google Scholar]
- Otten U, Baumann JB, Girard J. Stimulation of the pituitary-adrenocortical axis by nerve growth factor. Nature. 1979;282:413–414. doi: 10.1038/282413a0. [DOI] [PubMed] [Google Scholar]
- Otten U, Goedert M, Baumann JB, Girard J. Stimulation of the pituitary-adrenocortical axis and induction of tyrosine hydroxylase by nerve growth factor are not dependent on mouse submaxillary gland isorenin. Brain Res. 1981;217:207–211. doi: 10.1016/0006-8993(81)90202-x. [DOI] [PubMed] [Google Scholar]
- Perdue MH, Masson S, Wershil BK, Galli SJ. Role of mast cells in ion transport abnormalities associated with intestinal anaphylaxis. Correction of the diminished secretory response in genetically mast cell-deficient W/Wv mice by bone marrow transplantation. J Clin Invest. 1991;87:687–693. doi: 10.1172/JCI115047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringel Y, Drossman DA. Psychosocial aspects of Crohn's disease. Surg Clin North Am. 2001;81:231–252. doi: 10.1016/s0039-6109(05)70283-8. x. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P, Gutierrez YA, Martin AM, Mallett HA, Alleva E, Levine S. Maternal regulation of the adrenocortical response in preweanling rats. Physiol Behav. 1991;50:661–671. doi: 10.1016/0031-9384(91)90001-5. [DOI] [PubMed] [Google Scholar]
- Rosztoczy A, Fioramonti J, Jarmay K, Barreau F, Wittmann T, Bueno L. Influence of sex and experimental protocol on the effect of maternal deprivation on rectal sensitivity to distension in the adult rat. Neurogastroenterol Motil. 2003;15:679–686. doi: 10.1046/j.1350-1925.2003.00451.x. [DOI] [PubMed] [Google Scholar]
- Santos J, Saperas E, Nogueiras C, Mourelle M, Antolin M, Cadahia A, Malagelada JR. Release of mast cell mediators into the jejunum by cold pain stress in humans. Gastroenterology. 1998;114:640–648. doi: 10.1016/s0016-5085(98)70577-3. [DOI] [PubMed] [Google Scholar]
- Santos J, Saunders PR, Hanssen NP, Yang PC, Yates D, Groot JA, Perdue MH. Corticotropin-releasing hormone mimics stress-induced colonic epithelial pathophysiology in the rat. Am J Physiol Gastrointest Liver Physiol. 1999;277:G391–G399. doi: 10.1152/ajpgi.1999.277.2.G391. [DOI] [PubMed] [Google Scholar]
- Santos J, Yang PC, Soderholm JD, Benjamin M, Perdue MH. Role of mast cells in chronic stress induced colonic epithelial barrier dysfunction in the rat. Gut. 2001;48:630–636. doi: 10.1136/gut.48.5.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders PR, Santos J, Hanssen NP, Yates D, Groot JA, Perdue MH. Physical and psychological stress in rats enhances colonic epithelial permeability via peripheral CRH. Dig Dis Sci. 2002;47:208–215. doi: 10.1023/a:1013204612762. [DOI] [PubMed] [Google Scholar]
- Soderholm JD, Yates DA, Gareau MG, Yang PC, MacQueen G, Perdue MH. Neonatal maternal separation predisposes adult rats to colonic barrier dysfunction in response to mild stress. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1257–G1263. doi: 10.1152/ajpgi.00314.2002. [DOI] [PubMed] [Google Scholar]
- Tache Y, Martinez V, Million M, Wang L. Stress and the gastrointestinal tract III. Stress-related alterations of gut motor function: role of brain corticotropin-releasing factor receptors. Am J Physiol Gastrointest Liver Physiol. 2001;280:G173–G177. doi: 10.1152/ajpgi.2001.280.2.G173. [DOI] [PubMed] [Google Scholar]
- Van Oers HJ, De Kloet ER, Levine S. Early vs. late maternal deprivation differentially alters the endocrine and hypothalamic responses to stress. Brain Res Dev Brain Res. 1998;111:245–252. doi: 10.1016/s0165-3806(98)00143-6. [DOI] [PubMed] [Google Scholar]
- Vazquez DM, Eskandari R, Phelka A, Lopez JF. Impact of maternal deprivation on brain corticotropin-releasing hormone circuits: prevention of CRH receptor-2 mRNA changes by desipramine treatment. Neuropsychopharmacology. 2003;28:898–909. doi: 10.1038/sj.npp.1300126. [DOI] [PubMed] [Google Scholar]
- Vega JA, Garcia-Suarez O, Hannestad J, Perez-Perez M, Germana A. Neurotrophins and the immune system. J Anat. 2003;203:1–19. doi: 10.1046/j.1469-7580.2003.00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigger A, Neumann ID. Periodic maternal deprivation induces gender-dependent alterations in behavioral and neuroendocrine responses to emotional stress in adult rats. Physiol Behav. 1999;66:293–302. doi: 10.1016/s0031-9384(98)00300-x. [DOI] [PubMed] [Google Scholar]