Abstract
AMPA receptors (AMPARs) are the principal glutamate receptors mediating fast excitatory synaptic transmission in neurons. Aberrant extracellular glutamate has long been recognized as a hallmark phenomenon during neuronal excitotoxicity. Excessive glutamate triggers massive Ca2+ influx through NMDA receptors (NMDARs), which in turn can activate Ca2+-dependent protease, calpain. In the present study, we found that prolonged NMDA treatment (100 μm, 10 min) caused a sustained and irreversible suppression of AMPAR-mediated currents in cortical pyramidal neurons, which was largely blocked by selective calpain inhibitors. Biochemical and immunocytochemical studies demonstrated that in cortical cultures, prolonged glutamate or NMDA treatment reduced the level of surface and total GluR1, but not GluR2, subunits in a calpain-dependent manner. Consistent with the in vitro data, in animals exposed to transient ischaemic insults, calpain was strongly activated, and the AMPAR current density and GluR1 expression level were substantially reduced. Moreover, calpain inhibitors blocked the ischaemia-induced depression of AMPAR currents, and the NMDAR-induced, calpain-mediated depression of AMPA responses was occluded in ischaemic animals. Taken together, our studies show that overstimulation of NMDARs reduces AMPAR functions in cortical pyramidal neurons through activation of endogenous calpain, and calpain mediates the ischaemia-induced synaptic depression. The down-regulation of AMPARs by calpain provides a negative feedback to dampen neuronal excitability in excitotoxic conditions like ischaemia and epilepsy.
Glutamate is the major excitatory neurotransmitter in the brain. It plays a vital role in numerous neuronal events including short-term membrane excitability, long-term synaptic plasticity, dendritic sprouting, and regulation of gene expression (Collingridge & Lester, 1989; Carroll & Zukin, 2002). The AMPA receptor, a tetramer composed of subunits coded by GluR1, GluR2, GluR3 and GluR4 subunits, is the major type of glutamate receptor mediating synaptic transmission (Wisden & Seeburg, 1993; Hollmann & Heinemann, 1994). Each AMPAR subunit possesses an extracellular N-terminal domain, three transmembrane loops and a intracellular C-terminal domain (Song & Huganir, 2002). The C-terminal regions not only serve as the phosphorylation target for multiple kinases (Roche et al. 1996; Mammen et al. 1997), but also act as the docking site for many anchoring proteins (Dong et al. 1997; Leonard et al. 1998). Emerging evidence shows that protein–protein interactions at GluR1 and GluR2 C-termini are important for AMPAR trafficking and synaptic functions (Malinow & Malenka, 2002), suggesting that post-translational modification of the AMPAR C-terminal tail plays a crucial role in regulating AMPAR functions.
Proteolysis is one of the post-translational modifications often occurring at the protein's C-terminal regions. Calcium entry through NMDA receptors can activate the Ca2+-dependent protease, calpain (Siman et al. 1989; Adamec et al. 1998; Wu et al. 2005). Calpain-mediated proteolysis cleaves many downstream substrates, including cytoskeletal proteins, kinases and phosphatases, receptors and ion channels (Johnson & Guttmann, 1997; Goll et al. 2003), and is implicated in excitotoxicity-related diseases, such as hypoxia, ischaemia, epilepsy and Alzheimer's disease (Saido et al. 1993, 1994; Patrick et al. 1999; Chen et al. 2001). Biochemical studies have shown that the AMPAR GluR1 subunit is a calpain substrate in vitro (Bi et al. 1996; Lu et al. 2000) and calpain cleaves GluR1 at the C-terminal tail (Bi et al. 1997; Gellerman et al. 1997), suggesting that calpain-mediated truncation of AMPARs may alter the level of functional AMPARs.
Since the physiological impact of calpain on AMPAR functions in neurons is largely unknown, in this study we examined the calpain regulation of AMPAR currents in cortical pyramidal neurons. Our evidence indicated that prolonged stimulation of NMDARs activated endogenous calpain, which in turn caused a sustained inhibition of AMPAR-mediated ionic and synaptic currents via a mechanism involving calpain-mediated proteolysis of GluR1 subunits. Given the crucial role of AMPARs in excitatory synaptic transmission, the suppression of AMPAR functions suggests a potentially neuroprotective mechanism for calpain following excessive NMDAR stimulation.
Methods
Acute-dissociation procedure and primary neuronal culture
Cortical neurons from 3- to 4-week-old Sprague–Dawley rats (body mass: ∼120 g) were acutely dissociated as we have previously described (Yan & Surmeier, 1996; Yuen et al. 2005a). All experiments were performed with the approval of the Institutional Animal Care and Use Committee (IACUC) of the State University of New York at Buffalo, and our animal care procedures were in accordance with the IACUC guidelines under the Animal Welfare Act. In brief, rats were anaesthetized with halothane vapour before decapitation. Brain slices (400 μm) were incubated in a NaHCO3-buffered saline bubbled with 95% O2–5% CO2. The frontal cortical areas were dissected and digested in an oxygenated chamber consisted of papain (0.4 mg ml−1; Calbiochem) for 40 min at room temperature. Following washing, the tissue was mechanically dissociated with a graded series of fire-polished Pasteur pipettes. The isolated cells were then dispersed into a 35 mm Lux Petri dish positioned on the stage of a Nikon inverted microscope. Rat cortical cultures from E18 embryos were prepared with procedures similar to those described previously (Yuen et al. 2005a).
Whole-cell recordings
Whole-cell recordings of ion channel currents in acutely dissociated neurons employed standard voltage-clamp techniques as previously described (Yuen et al. 2005b). The external solution contained (mm): 127 NaCl, 20 CsCl, 1 MgCl2, 10 Hepes, 5 BaCl2, 12 glucose, 0.001 TTX, pH 7.3–7.4, 300–305 mosmol l−1. Recording electrodes (2–4 MΩ) were filled with the following internal solution (mm): 170 N-methyl-d-glucamine, 4 MgCl2, 40 Hepes, 0.5 BAPTA, 12 phosphocreatine, 3 Na2ATP, and 0.5 Na2GTP, pH 7.2–3, 265–270 mosmol l−1. Recordings were obtained with an Axon Instruments 200B patch clamp amplifier that was controlled and monitored with an IBM PC running pCLAMP (v. 8) with a DigiData 1320 series interface (Axon Instruments). Following seal rupture, series resistance (4–10 MΩ) was compensated (70–90%). AMPAR-mediated currents were recorded with glutamate (1 mm) or AMPA (100 μm) application for 2 s every 30 s in cells (held at −60 mV) exposed to an Mg2+-containing solution (to block NMDAR activation). For recording GABAAR-activated currents, GABA (100 μm) was applied for 2 s every 60 s with neurons held at 0 mV. During prolonged NMDA or glutamate treatment, NMDA (100 μm) or glutamate (100 or 500 μm) was continuously perfused for 10 min or sequentially applied for 20 s on, 20 s off for 10 min in the presence of 2 mm CaCl2, 20 μm glycine and Mg2+-free external solution. Drugs were applied with a gravity-fed ‘sewer pipe’ system. The array of application capillaries (approx. 150 μm i.d.) was positioned a few hundred micrometres from the cell under study. Solution changes were effected by the SF-77B fast-step solution stimulus delivery device (Warner Instruments, Hamden, CT, USA). Data were analysed with AxoGraph (Axon Instruments) and Kaleidagraph (Albeck Software).
Electrophysiological recordings in slices
To measure AMPAR-mediated synaptic transmission, we performed the standard whole-cell recording techniques in cortical slices (Yuen et al. 2005a). Patch pipettes were filled with the internal solution containing (mm): 130 Cs-methanesulfonate, 10 CsCl, 4 NaCl, 1 MgCl2, 10 Hepes, 5 EGTA, 2.2 QX-314, 12 phosphocreatine, 5 MgATP, 0.5 Na2GTP, pH 7.2–7.3, 265–270 mosmol l−1. Cortical slices (300 μm) were perfused with artificial CSF (ACSF) containing bicuculline (10 μm) bubbled with 95% O2–5% CO2. The NMDAR antagonist d-aminophosphonovalerate (APV, 25 μm) was present throughout the recording except during the prolonged (10 min) NMDA treatment. Neurons were observed with a ×40 water-immersion lens and illuminated with near infrared (IR) light, and the image was captured with an IR-sensitive CCD camera. Recordings were performed on neurons (held at −70 mV) using a Multiclamp 700A amplifier (Axon Instruments). Tight seals (2–10 GΩ) were obtained by applying negative pressure. The membrane was disrupted with additional suction and the whole-cell configuration was obtained. The access resistances ranged from 13 to 18 MΩ and were compensated 50–70%. EPSCs were evoked by stimulating the neighbouring cortical neurons (50 μs pulse) with a bipolar tungsten electrode (FHC, Inc., Bowdoinham, ME, USA). Data analyses were performed with the Clampfit software (Axon Instruments).
Western blotting
After treatment, cultured cortical neurons (14 days in vitro (DIV)) were lysed with the lysis buffer containing: 1% SDS, 0.5% deoxycholic acid, 50 mm NaPO4, 150 mm NaCl, 2 mm EDTA, 50 mm NaF, 10 mm sodium pyrophosphate, 1 mm sodium orthovanadate, 1 mm PMSF. Cell lysates were centrifuged at 16 000 × g, and supernatant fractions were incubated with primary antibodies, which included GluR1 (C-terminal, last 15 amino acids (AAs), Upstate Biotechnology, Lake Placid, NY, USA), GluR2/3 (C-terminal, AAs 864–883, Upstate), GABAAR β2/3 subunits (Upstate) or α-spectrin (Chemicon, Temecula, CA, USA). After incubation with appropriate secondary antibodies conjugated with horseradish peroxidase (Sigma–Aldrich), positive bands were visualized using an enhanced chemiluminescence detection system (Amersham Biosciences). Quantification was obtained from densitometric measurements of immunoreactive bands on films.
Biochemical measurement of surface-expressed receptors
The surface AMPA receptors were detected as previously described (Wang et al. 2003). Briefly, after treatment, cortical cultures were incubated with ACSF containing 1 mg ml−1 Sulfo-NHS-LC-Biotin (Pierce Chemical Co., Rockford, IL, USA) for 20 min on ice. The cultures were then rinsed three times in TBS to quench the biotin reaction, followed by homogenization in 300 μl of modified radio-immunoprecipitation assay (RIPA) buffer (1% Triton X-100, 0.1% SDS, 0.5% deoxycholic acid, 50 mm NaPO4, 150 mm NaCl, 2 mm EDTA, 50 mm NaF, 10 mm sodium pyrophosphate, 1 mm sodium orthovanadate, 1 mm PMSF, and 1 mg ml−1 leupeptin). The homogenates were centrifuged at 14 000 × g for 15 min at 4°C. To measure total protein, 15 μg of protein were removed. For surface protein, 150 μg of protein were incubated with 100 μl 50% Neutravidin agarose (Pierce Chemical Co.) for 2 h at 4°C, and bound proteins were resuspended in 25 μl of SDS sample buffer and boiled. Quantitative Western blots were performed on both total and biotinylated (surface) proteins using antibodies against the N-terminus of GluR1 (1: 250, Santa Cruz), GluR1 (1: 250, Upstate), GluR2 (1: 250, Chemicon) and an antibody against GABAAR β2/3 subunits (1: 500, Upstate).
Immunocytochemical staining
Co-immunostaining of GluR1 and MAP2 in cortical cultures (14 DIV) was performed. After treatment with different agents, neurons were fixed in 4% paraformaldehyde for 30 min, then permeabilized with 0.1% Triton X-100 for 10 min and blocked with 5% BSA for 1 h at room temperature to eliminate the background signals. Next, neurons were incubated with a polyclonal anti-GluR1 (C-terminal, last 15 AAs, Upstate) and a monoclonal anti-MAP2 (Upstate) for 2 h at room temperature. After three washes, neurons were incubated with an FITC-conjugated anti-rabbit and a TRITC-conjugated anti-mouse secondary antibody (Invitrogen, Carlsbad, CA, USA) for 1 h at room temperature. After washing in PBS, the coverslips were mounted on slides with Vectashield mounting media (Vector Laboratories, Burlingame, CA, USA). Data analysis was performed using Image J software. All specimens were imaged under identical conditions and analysed using identical parameters. For detection of GluR1 and MAP2 fluorescence signals, images were captured using a ×100 objective with a cooled CCD camera mounted on a Nikon microscope. The fluorescence intensity at the soma (within a 15 μm × 15 μm area) and dendrites (50 μm length) were measured. Three to four independent experiments for each of the treatments were performed. Quantitative analyses were conducted blind to the experimental treatment.
Ischaemia model
Male Mongolian gerbils (6 months old, body mass ∼70 g) were used for the induction of ischaemia. In this species, the circle of Willis is incomplete and there is no connection between the basilary and internal carotid artery, thus global ischaemia can be produced by ligation of both common carotid arteries without additional occlusion of the vertebral arteries (Hossmann, 1998). Gerbils were anaesthetized by i.p. injection of pentobarbital (5 mg (100 g body weight)−1, Abbott Laboratories) before surgery. The level of anaesthesia was assessed by detecting the presence of eye and foot reflexes. A midline ventral incision was made in the neck and both common carotid arteries were occluded using non-traumatic aneurysm clips. After 10 min of occlusion, the clips were removed. In one group of animals, calpain inhibitor III (3 mg kg−1) was i.p. injected at 5 min after the onset of ischaemia, and the clips were removed 5 min following the injection. Gerbils did not receive analgesia, but were kept warm under light with water and food available after surgery. There were no indications that any animals should be humanely killed prematurely, and all animals (ischaemia versus sham) woke up 12 h after pentobarbital injection, and became active and alert. Following various recovery times, gerbils were anaesthetized by inhaling halothane vapour and decapitated, and the brains were sliced for electrophysiological or biochemical experiments. Sham-operated animals were subjected to the same surgical procedures except that the common carotid arteries were not occluded.
Results
Prolonged NMDA treatment causes a persistent reduction of AMPAR-mediated ionic and synaptic currents in cortical pyramidal neurons
To examine the potential impact of prolonged NMDA treatment on AMPA receptor functions, we measured the whole-cell AMPAR-mediated currents elicited by glutamate or AMPA in an Mg2+-containing solution in acutely dissociated cortical pyramidal neurons (Cai et al. 2002). Application of glutamate (1 mm) or AMPA (100 μm) induced an inward current consisting of a fast-desensitizing peak and a non-desensitizing sustained part, which was completely blocked by the AMPAR/kainate receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 10 μm) (Cai et al. 2002), and largely blocked by the selective AMPAR antagonist GYKI 53655 (Paternain et al. 1995), indicating that it is mediated primarily by AMPA receptors. As shown in Fig. 1A, prolonged application of NMDA (100 μm, 10 min) markedly suppressed the subsequently recorded AMPAR currents. In parallel control experiments in which no NMDA was administered, AMPAR current amplitudes remained stable throughout the entire recording period (Fig. 1B). This effect of prolonged NMDA treatment on AMPAR currents was long-lasting and irreversible, maintaining at the suppressed level up to 100 min without recovery or further decline. Moreover, after being depressed after the first NMDA treatment, a second application of NMDA failed to further reduce AMPAR currents (n = 4, data not shown). As shown in Fig. 1C, different durations of NMDA treatment significantly decreased AMPAR currents to different extents (1 min: 24 ± 1.8%, n = 6; 3 min: 24.6 ± 1.8%, n = 4; 5 min: 34.3 ± 0.8%, n = 5; 10 min: 40.1 ± 2.9%, n = 10). Similar results were also observed in AMPAR currents of cultured cortical neurons following prolonged NMDA (100 μm, 10 min) treatment (47.8 ± 1.2%, n = 5). To examine the specificity of this effect, we also recorded GABAAR-mediated currents. As shown in Fig. 1D, prolonged NMDA treatment (100 μm, 10 min) failed to produce a significant reduction of GABA-evoked currents. These results indicate that prolonged NMDA receptor stimulation causes a persistent and specific down-regulation of glutamate receptor-mediated currents in neurons.
Figure 1. Prolonged activation of NMDA receptors irreversibly suppresses AMPAR-mediated ionic and synaptic currents in cortical pyramidal neurons.
A and B, plots of peak AMPAR currents (IAMPA) elicited by glutamate (1 mm, 2 s) in dissociated cortical neurons with (A) or without (B) a prolonged NMDA application (100 μm, 10 min). C, cumulative data (mean ± s.e.m.) showing the percentage reduction of AMPAR currents by different durations (1–10 min) of NMDA treatment (100 μm). The number of cells tested in each condition is shown in each column. *P < 0.001, ANOVA. D, plot of peak GABAAR currents elicited by GABA (100 μm, 2 s) in a dissociated cortical neuron with a prolonged NMDA application (100 μm, 10 min). Insets (A, B and D): representative traces taken at the indicated time points. Scale bars: 0.1 nA (A and B) or 0.5 nA (D), 1 s. E, plot of normalized peak AMPAR-mediated synaptic currents (AMPAR-EPSCs) with or without a prolonged NMDA application (100 μm, 10 min). Each point represents the average peak (mean ± s.e.m.) of three consecutive AMPAR-EPSCs. F, representative current traces (average of 3 trials) taken at the indicated time points. Scale bars: 100 pA, 10 ms.
To investigate whether prolonged NMDAR stimulation affects synaptic AMPA receptors, we recorded AMPAR-EPSCs in cortical slices. Application of prolonged NMDA (100 μm, 5 or 10 min) significantly reduced the amplitude of AMPAR-EPSCs (5 min: 40.8 ± 6.5%, n = 5; 10 min: 57.9 ± 4.3%, n = 9). Representative time courses and current traces are shown in Fig. 1E and F. The bigger effect found in slices compared to dissociated cells suggests that prolonged NMDA treatment may preferably modulate synaptic AMPA receptors.
To examine whether prolonged NMDAR stimulation changes AMPAR subunit composition, we measured the rectification of AMPA responses (ratio of the current amplitude at −60 mV to that at +40 mV; Malinow & Malenka, 2002; Zhu et al. 2002), since GluR2-containing AMPAR channels have no rectification, while GluR1 homomeric channels pass minimal current in the outward direction and have profound inward rectification (Verdoorn et al. 1991). As shown in Supplemental Fig. 1 (see online Supplemental material), the rectification of AMPAR-EPSCs was stable throughout the recording (control: 3.1 ± 0.13, after 10 min waiting, wash: 3.0 ± 0.15, n = 5), similar to the finding in CA1 hippocampal neurons (Hayashi et al. 2000), and it was not changed by prolonged NMDAR stimulation (control: 3.1 ± 0.12; after 10 min NMDA treatment, wash: 3.0 ± 0.04, n = 6). The rectification of AMPA (100 μm)-elicited ionic currents in dissociated cortical cultures was also unaltered by prolonged NMDAR stimulation (control: 1.49 ± 0.07; after 10 min NMDA treatment, wash: 1.45 ± 0.05, n = 6). These data suggest the lack of alterations in the AMPAR subunit composition in response to calpain activation.
Prolonged NMDA treatment suppresses AMPAR currents mainly through activation of calpain
We next examined the mechanism underlying the effect of prolonged NMDA treatment on AMPAR currents. NMDAR activation induces Ca2+ influx, which in turn activates diverse Ca2+-dependent molecules such as calpain (Siman et al. 1989), calcineurin (Klee et al. 1998; Wu et al. 2004) and Ca2+–calmodulin-dependent protein kinase II (CaMKII) (Hudmon & Schulman, 2002). Therefore, we first tested the Ca2+ dependence of this effect of NMDA on AMPAR currents. As shown in Fig. 2A, dialysis with the potent Ca2+ chelator BAPTA (10 mm) and perfusing with a Ca2+-free external solution prevented the suppression of AMPAR currents by prolonged NMDA (100 μm, 10 min) treatment (5.4 ± 2.4%, n = 5, Fig. 2C).
Figure 2. Prolonged NMDA treatment reduces AMPAR currents via calpain activation.
A and B, plot of IAMPA with a prolonged NMDA application (100 μm, 10 min) in cells dialysed with the Ca2+ chelator BAPTA (10 mm, A) or the specific calpain inhibitor CS peptide (5 μm, B). Insets: representative traces taken from the indicated times. Scale bars: 100 pA, 1 s. C, cumulative data (mean ± s.e.m.) summarizing the percentage reduction of AMPAR currents by prolonged NMDA treatment in the presence of BAPTA or various calpain inhibitors. *P < 0.001, ANOVA. D, plot of normalized peak AMPAR-EPSCs with a prolonged NMDA application (100 μm, 10 min) in cells dialysed with the CS peptide (5 μm) or a scrambled control peptide. E, representative traces (average of 3 trials) taken from the recordings used to construct D (at time points denoted by numbers). Scale bars: 100 pA, 10 ms. F, cumulative data (mean ± s.e.m.) showing the percentage reduction of AMPAR-EPSC amplitude by prolonged NMDA treatment in the presence of different peptides. *P < 0.001, ANOVA.
Among many Ca2+-dependent enzymes initiated by NMDAR activation, the protease calpain exerts a rapid and irreversible action on its downstream targets through proteolysis. One possibility is that prolonged NMDAR stimulation activates endogenous calpain, which in turn truncates AMPA receptors and thus down-regulates the number of functional AMPA receptors. To test this hypothesis, we injected neurons with the CS peptide, which is derived from the endogenous calpain inhibitor calpastatin and contains the sequence of TIPPKYR that is critical for inhibiting calpain activity (Kawasaki et al. 1989; Maki et al. 1989). As shown in Fig. 2B, intracellular application of the CS peptide (5 μm) substantially blocked the effect of prolonged NMDA treatment on AMPAR currents (11.4 ± 2.1%, n = 8, Fig. 2C). Several other calpain inhibitors gave similar results, including ALLN (25 μm, 16.4 ± 1.8%, n = 5, Fig. 2C) and calpain inhibitor III (10 μm, 7.7 ± 2.6%, n = 8, Fig. 2C). These data suggest that the prolonged NMDA-mediated reduction of AMPAR currents is mainly through activated calpain.
To further investigate the role of calpain in regulating synaptic AMPAR functions, we examined the effect of prolonged NMDA treatment on AMPAR-EPSCs in cortical slices in the presence of calpain inhibitors. As shown in Fig. 2D and E, dialysis of the CS peptide (5 μm) markedly abolished the suppression of AMPAR-EPSCs by NMDA (100 μm, 10 min), whereas the NMDA effect was intact in the presence of a scrambled peptide. As summarized in Fig. 2F, prolonged NMDA treatment induced a slight reduction of AMPAR-EPSCs in neurons loaded with the CS peptide (10.4 ± 1.9%, n = 7), which was significantly different from the NMDA effect in cells dialysed with the scrambled control peptide (57.9 ± 4.3%, n = 9; P < 0.001, ANOVA).
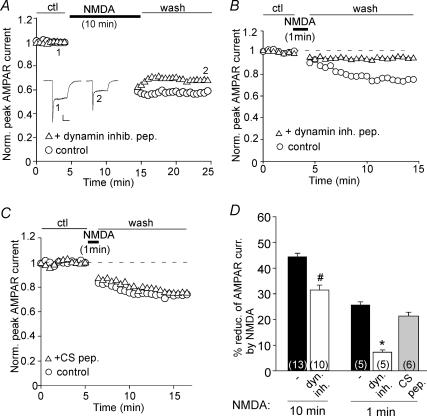
Previous studies have shown that a brief activation of NMDA receptors reduces the surface number of AMPA receptors through increasing their clathrin/dynamin-mediated endocytosis (Carroll et al. 1999; Beattie et al. 2000). To determine whether AMPAR endocytosis is involved in the regulation of AMPAR currents by prolonged NMDA treatment, we dialysed neurons with the dynamin inhibitory peptide, which competes with dynamin for binding to amphiphysin and thus blocks the clathrin-dependent endocytosis (Gout et al. 1993). As shown in Fig. 3A, under the condition in which AMPAR endocytosis was prevented by the dynamin inhibitory peptide (50 μm), prolonged NMDA treatment (100 μm, 10 min) caused a smaller reduction of AMPAR currents. In contrast, the decline of AMPAR currents by a short NMDA application (100 μm, 1 min) was eliminated in cells dialysed with the dynamin inhibitory peptide (Fig. 3B), consistent with the previous finding that brief NMDAR activation induces AMPAR endocytosis (Carroll et al. 1999; Beattie et al. 2000). Injecting the CS peptide (5 μm) to inhibit calpain failed to block the decline of AMPAR currents by a short NMDA application (100 μm, 1 min, Fig. 3C), suggesting the lack of involvement of calpain in this regulation. As summarized in Fig. 3D, prolonged NMDA treatment decreased AMPAR currents by 44.4 ± 1.7% (n = 13), which was partially attenuated by the dynamin inhibitory peptide (32.0 ± 2.1%, n = 10; P < 0.01, ANOVA), whereas a short NMDA application reduced AMPAR currents by 25.3 ± 1.3% (n = 4), which was largely blocked by the dynamin inhibitory peptide (7 ± 0.8%, n = 5; P < 0.001, ANOVA), but not by the CS peptide (21.3 ± 1.4%, n = 6). These results suggest that distinct cellular mechanisms underlie the effects of prolonged versus transient NMDAR stimulation on AMPAR currents. A prolonged NMDA treatment has dual effects on AMPA receptors: a short-term effect (∼25%) leading to clathrin/dynamin-dependent endocytosis and a long-term effect (∼75%) leading to calpain-mediated proteolysis, while a brief NMDA treatment only triggers clathrin-mediated AMPAR internalization.
Figure 3. Dynamin-mediated endocytosis of AMPA receptors is differently involved in the regulation of AMPAR currents by prolonged versus short NMDA treatment.
A and B, plots of IAMPA showing the effect of a prolonged (A) or short (B) NMDA application (100 μm, 10 or 1 min) in cells dialysed with the dynamin inhibitory peptide (50 μm). Inset in A: representative current traces at indicated time points. Scale bars: 100 pA, 1 s. C, plot of IAMPA showing the effect of a short NMDA application (100 μm, 1 min) in cells dialysed with or without the CS peptide (5 μm). D, cumulative data (mean ± s.e.m.) summarizing the percentage reduction of AMPAR currents by NMDA treatment (100 μm, 10 or 1 min) in the absence or presence of different peptides. *P < 0.001, #P < 0.01; ANOVA.
Prolonged glutamate treatment of cortical cultures induces calpain cleavage of GluR1 subunits
To provide further evidence to complement our electrophysiological studies, we performed biochemical experiments to test whether overstimulation of NMDARs can indeed activate calpain and induce the cleavage of AMPAR subunits. To detect the full-length (uncleaved) AMPAR GluR1 subunit, we used an antibody against the C-terminal end of GluR1, which is downstream of the putative sites for calpain proteolysis (Bi et al. 1996). As shown in Fig. 4A, prolonged glutamate stimulation (100 μm, 10 min) caused a profound reduction of the full-length GluR1, as well as the cleavage of α-spectrin (240 kDa), a well-defined substrate of calpain (Saido et al. 1993), into two fragments (150 kDa and 145 kDa). This effect was substantially eliminated in neurons pre-incubated with the specific calpain inhibitor ALLN (25 μm) or calpeptin (20 μm).
Figure 4. Activation of calpain causes cleavage of GluR1 subunits in cortical cultures treated with glutamate or NMDA, which leads to the loss of surface and total levels of GluR1.
A, immunoblots of GluR1 (detected with the C-terminal antibody) and α-spectrin in lysates of cortical cultures following glutamate treatment (100 μm, 10 min, collected after 60 min of washing) in the absence or presence of different calpain inhibitors (added 10 min before glutamate treatment), including ALLN (25 μm) and calpeptin (20 μm). B, immunoblots of AMPAR subunits in lysates of cultured cortical neurons following NMDA (100 μm, 10 min) or glutamate (500 μm, 10 min) treatment in the absence or presence of APV (25 μm, 10 min pretreatment). Cells were collected after 10 min of washing. GluR1 and GluR2 were detected with antibodies against their N-terminal, which labelled both the cleaved and uncleaved subunits (top panel: anti-GluR1 N-terminal from Santa Cruz; middle panel: anti-GluR1 N-terminal from Upstate). C, quantification analysis (mean ± s.e.m.) showing the GluR1 and GluR2 levels under different treatments. *P < 0.001, ANOVA. D, immunoblots of the surface and total GluR1, GluR2 and GABAAR β2/3 subunits in lysates of cortical cultures treated with glutamate (500 μm, 10 min, collected after 10 min of washing). Both GluR1 and GluR2 were detected with N-terminal antibodies. E, quantification analysis (mean ± s.e.m.) showing the surface and total levels of GluR1 and GluR2 with or without glutamate treatment. *P < 0.001, ANOVA.
Next, we performed experiments with two different antibodies against the N-terminal of GluR1 that recognize both the cleaved and uncleaved GluR1 subunit. Cultured cortical neurons were treated with either NMDA or glutamate. As shown in Fig. 4B and C, cells exposed to prolonged NMDA (100 μm, 10 min) or glutamate (500 μm, 10 min) treatment had a significantly reduced level of GluR1 (NMDA: 49.5 ± 5% of control; glutamate: 45.0 ± 2% of control, n = 4; P < 0.001, ANOVA). Moreover, these treatments induced a small gel shift of GluR1 from 106 kDa to 98 kDa, which was consistent with the calpain-induced cleavage of GluR1 in a previous in vitro study (Bi et al. 1997). The effect of prolonged glutamate treatment on GluR1 was blocked by the NMDAR antagonist d-aminophosphonovalerate (APV; 25 μm, 102.3 ± 2% of control, n = 4), suggesting that the effect of glutamate is mediated by NMDA receptors. In contrast, prolonged NMDA or glutamate treatment failed to affect the level of AMPAR GluR2 subunit detected with an antibody against the GluR2 N-terminal (Fig. 4B and C, NMDA: 94.7 ± 5% of control; glutamate: 100 ± 5.3% of control, n = 4). Similarly, no change was observed in response to prolonged glutamate treatment with an antibody against the GluR2/3 C-terminal (data not shown).
What is the fate of calpain-cleaved GluR1 subunits? One possibility is that they remain on the surface but become less functional, since C-terminal truncated GluR1 have five times smaller currents compared to wild-type GluR1 in transfected cell lines (Suzuki et al. 2005). Alternatively, they get internalized from the surface and are degraded inside neurons. To test this, we performed surface biotinylation experiments to measure levels of surface AMPAR subunits in cortical cultures. Surface proteins were first labelled with Sulfo-NHS-LC-Biotin, and then biotinylated surface proteins were separated from non-labelled intracellular proteins by reaction with Neutravidin beads. Surface and total proteins were subjected to eletrophoresis and probed with antibodies against the N-terminal domain of GluR1 and GluR2. As shown in Fig. 4D and E, prolonged glutamate treatment (500 μm, 10 min) markedly reduced both the surface and the total levels of the GluR1 subunit (surface GluR1: 28.8 ± 2.1% of control; total GluR1: 44.3 ± 5.5% of control, n = 4; P < 0.001, ANOVA), but not the GluR2 subunit (surface GluR2: 99.7 ± 5.3% of control; total GluR2: 89.4 ± 7.2% of control, n = 4). The surface and the total GABAAR β2/3 levels were also unaltered. Since the uncleaved full-length GluR1 should remain intact on the surface, these data suggest that calpain-cleaved GluR1 subunits were internalized and degraded.
To examine the cellular distribution of AMPA receptors following prolonged NMDAR stimulation, we performed immunocytochemical studies in cortical culture with the antibody against GluR1 C-terminal end residues. The dendritic marker MAP2 was used in co-staining experiments to assess the morphological integrity of neurons following glutamate exposure. As shown in Fig. 5A, prolonged glutamate treatment (100 μm, 10 min) profoundly reduced the abundance of GluR1 in both neuronal soma and dendrites, as compared to untreated control cells, while the level of MAP2 was not affected. Application of the specific calpain inhibitor ALLN (25 μm) abolished the glutamate-induced reduction of GluR1 staining (Fig. 5B). Quantitative analyses of the full-length (uncleaved) GluR1 fluorescence intensity is summarized in (Fig. 5C). Prolonged glutamate treatment decreased the GluR1 level by 50.8 ± 4.1% in soma (n = 23) and 55.8 ± 4.3% in dendrites (n = 26). This effect was significantly attenuated in the presence of ALLN (soma: 10.6 ± 6.2%, n = 8; dendrite: 8.3 ± 6.9%, n = 8). There were no significant changes in GluR1 staining by ALLN itself. Taken together, these data provide direct evidence showing that prolonged glutamate treatment induces calpain-mediated proteolysis of AMPAR GluR1 subunits in cultured cortical neurons, which may lead to the decrease of AMPAR currents.
Figure 5. Prolonged glutamate treatment reduces the level of full-length GluR1 on neuronal soma and dendrites through a calpain-mediated mechanism.
A, immunocytochemical images showing the co-staining of full-length GluR1 and MAP2 in cultured cortical neurons (14 DIV) treated without (control) or with glutamate (100 μm, 10 min, collected after 60 min of washing). B, immunocytochemical images demonstrating the full-length GluR1 staining in neurons treated without (control) or with glutamate (100 μm, 10 min, collected after 60 min of washing) in the absence or presence of the calpain inhibitor ALLN (25 μm, pre-incubated for 20 min). C, quantification of GluR1 fluorescence intensity at the neuronal soma and dendrites in cells under various treatments. Values are presented as a percentage of the full-length GluR1 level in untreated control cells. *P < 0.001, ANOVA.
In animals after transient forebrain ischaemia, AMPAR current density and GluR1 expression are substantially reduced and calpain is concomitantly activated
To test the physiological relevance of the effect of calpain on AMPA receptors induced by prolonged NMDA treatment in vitro, we examined whether cerebral ischaemia can indeed activate calpain and thus cause the down-regulation of AMPA receptors in vivo. First, we measured AMPAR currents in cortical pyramidal neurons isolated from gerbils exposed to transient ischaemic insults following various recovery times (TR: 0, 4, 24 h). As shown in Fig. 6A, the AMPAR current density was markedly smaller in cells from ischaemic animals (TR = 0 h: 10.8 ± 3.1 pA pF−1, n = 9; TR = 4 h: 16.0 ± 3.5 pA pF−1, n = 9; TR = 24 h: 16.2 ± 3.4 pA pF−1, n = 8), compared to those from sham-operated animals (37.9 ± 5.2 pA pF−1, n = 12). In contrast, the GABAAR current density was not significantly different (Fig. 6B, sham: 63.3 ± 2.9 pA pF−1, n = 6; ischaemia TR = 0 h: 61.5 ± 1.7 pA pF−1, n = 7; TR = 4 h: 60.0 ± 1.8 pA pF−1, n = 8; TR = 24 h: 65.2 ± 2.1 pA pF−1, n = 8). These data suggest that AMPA receptors are selectively down-regulated after forebrain ischaemia. Moreover, the cortical neurons from ischaemic animals with calpain inhibitor III injected (3 mg kg−1, i.p. at 5 min after the onset of ischaemia) showed normal AMPAR current density (TR = 4 h: 35.2 ± 1.6 pA pF−1, n = 11, Fig. 6A), indicating that inhibition of calpain blocks the ischaemia-induced synaptic depression.
Figure 6. AMPAR current density and GluR1 expression are reduced and calpain is concomitantly activated in animals after transient forebrain ischaemia.
A and B, dot plots showing the AMPAR (A) or GABAAR (B) current density in cortical pyramidal neurons freshly isolated from sham-operated versus ischaemic animals following different recovery times (TR: 0, 4 and 24 h). In one group of ischaemic animals, calpain inhibitor III (3 mg kg−1) was i.p. injected at 5 min after the onset of ischaemia. Inset: representative AMPAR current traces (evoked by 1 mm glutamate, A) or GABAAR current traces (evoked by 100 μm GABA, B). Scale bars: 0.1 nA (A) or 0.5 nA (B), 1 s. C, plot of normalized peak AMPAR-EPSCs with a prolonged NMDA application (100 μm, 10 min) in cortical pyramidal neurons from sham-operated versus ischaemic animals. Each point represents the average peak (mean ± s.e.m.) of three consecutive AMPAR-EPSCs. Inset: representative traces taken at the indicated time points. Scale bars: 50 pA, 10 ms. D, Western blot analysis of GluR1 and GluR2 (both detected with the N-terminal antibody), and α-spectrin in cortical slices from sham-operated versus ischaemic animals with various recovery times (TR: 0, 4 and 24 h). E, quantification (mean ± s.e.m.) analysis showing the normalized GluR1 and GluR2 levels in sham versus ischaemic animals. *P < 0.001, ANOVA.
Next, we examined whether the NMDAR-induced, calpain-mediated depression is occluded in ischaemic animals. Compared to sham animals, similar presynaptic stimulation evoked AMPAR-EPSCs with much smaller amplitudes in ischaemic animals. Thus, we increased the intensity of presynaptic stimulation to evoke AMPAR-EPSCs in ischaemic animals with similar sizes to those in sham animals. As shown in Fig. 6C, prolonged NMDA application (100 μm, 10 min) induced a potent reduction of AMPAR-EPSCs in cortical neurons from sham animals (50.8 ± 3.5%, n = 6), but failed to do so in cells from ischaemic animals (7.8 ± 0.9%, n = 5). It suggests that calpain mediates the ischaemia-induced synaptic depression.
Finally, we measured the level of AMPAR GluR1 and GluR2 subunits (detected with N-term antibodies) in cortical slices from ischaemic animals. As shown in Fig. 6D and E, the GluR1 level was substantially lower in animals exposed to ischaemic insults (TR = 0 h: 54 ± 8.5% of control; TR = 4 h: 17.2 ± 5.0% of control; TR = 24 h: 34 ± 7.7% of control, n = 4; P < 0.001, ANOVA), compared to sham-operated animals. In contrast, the GluR2 level was unchanged among all ischaemic groups (TR = 0 h: 119 ± 6.9% of control; TR = 4 h: 118 ± 9.7% of control; TR = 24 h: 117 ± 7.4% of control, n = 4). α-Spectrin was cleaved into two fragments in ischaemic animals (Fig. 6D), confirming that calpain was strongly activated after transient forebrain ischaemia. Taken together, these results suggest that calpain proteolysis of AMPAR GluR1 subunits leads to down-regulation of AMPAR function in ischaemic conditions.
Discussion
Calpain, the Ca2+-activated protease, regulates a wide variety of substrates, many of which have been implicated in excitotoxicity-induced apoptosis (Huang & Wang, 2001). For instance, calpain-induced cleavage of the Na+–Ca2+ exchanger causes it to lose the function of extruding intracellular Ca2+, therefore contributing to neuronal cell death (Bano et al. 2005). Calpain cleavage of some substrates, such as the cyclin-dependent kinase 5 activator p35 and calcineurin, converts them to the hyperactive form, which also leads to excitotoxic neurodegeneration (Kishimoto et al. 1989; Lee et al. 2000; Wu et al. 2004). Calpain is primarily expressed at dendritic shaft and spines of cortical pyramidal neurons (Rami, 2003), where glutamate receptors are enriched, suggesting that calpain may also be able to regulate neuronal functions by modulating glutamatergic transmission. Despite previous biochemical evidence showing that calpain cleaves glutamate receptors in vitro (Bi et al. 1996, 1998), the physiological impact of calpain on glutamatergic transmission is largely unknown. Here, we provide direct evidence showing that calpain proteolysis of GluR1 markedly suppresses AMPAR functions in cortical pyramidal neurons.
Since calpain activation requires micromolar [Ca2+]i (Glading et al. 2002; Goll et al. 2003), we used NMDA treatment (100 μm, 10 min) to maximize Ca2+ influx and calpain activation. Our electrophysiological results showed that the prolonged NMDA application produced a persistent and irreversible reduction of AMPAR-mediated ionic and synaptic currents, which was blocked by inhibition of calpain activity. Our Western blotting analysis and immunocytochemical studies indicated that the full-length (uncleaved) AMPAR GluR1 subunit was substantially reduced in cortical cultures exposed to prolonged glutamate treatment, which was also eliminated by calpain inhibitors. Furthermore, our biochemical data demonstrated that the calpain-cleaved, C-terminal truncated GluR1 subunits were more readily lost from the surface, probably because they have lost the binding proteins important for stabilizing their anchorage on the membrane (Malinow & Malenka, 2002). Although we used the stimuli mimicking excitotoxic conditions, no cell death occurred during the experimental period, as indicated by the stable GABAAR current recording and the intact MAP2 staining following prolonged NMDAR activation. These results indicate that endogenous calpain, which is activated by excessive NMDAR stimulation, causes the proteolysis of GluR1, leading to the decreased number of GluR1 subunits and the ensuing reduction of AMPAR currents.
It has been reported that a brief application of NMDA (50 μm, 1 min) induces the clathrin/dynamin-mediated AMPAR endocytosis through a calcineurin-dependent mechanism, which is important for long-term depression (LTD), a form of synaptic plasticity (Beattie et al. 2000). This result was confirmed by our experiments showing that inhibition of AMPAR endocytosis abolished the reduction of AMPAR currents by a short NMDA (100 μm, 1 min) treatment. However, we found that a large portion (∼75%) of the suppressing effect on AMPAR current by prolonged NMDA (100 μm, 10 min) treatment was not affected by inhibition of clathrin/dynamin-dependent AMPAR endocytosis. These results suggest that different degrees of NMDAR activation may preferentially couple to distinct Ca2+-activated enzymes (e.g. calcineurin versus calpain) to regulate AMPAR function via different mechanisms (e.g. endocytosis versus proteolysis). Because the dynamin inhibitory peptide we used only inhibits the function of dynamin I, the calpain-cleaved AMPA receptors are internalized either by other members of the dynamin superfamily (Praefcke & McMahon, 2004) or through dynamin-independent endocytosis (Zhang et al. 2004; Bonazzi et al. 2005) before being degraded.
The studies in ischaemia animal models have confirmed the in vitro results. After transient forebrain ischaemia, calpain was strongly activated, which caused the loss of AMPAR GluR1 expression and AMPAR current density, suggesting that calpain-mediated down-regulation of AMPAR function is indeed happening under in vivo pathological conditions, such as ischaemia and stroke.
In summary, our studies show that prolonged NMDAR stimulation, by activating calpain, suppresses AMPAR functions in cortical pyramidal neurons. This regulation is mediated by calpain proteolysis of GluR1. This finding suggests that calpain-induced AMPAR cleavage may serve as an emergency rescuer to dampen overstimulated glutamate receptors. Although most AMPARs are not permeable to Ca2+, overstimulated AMPARs can be neurotoxic by inducing excessive membrane depolarization and causing Ca2+ influx through Ca2+-permeable channels like voltage-gated calcium channels and NMDARs (Syntichaki & Tavernarakis, 2003), which could ultimately lead to neuronal death (Choi, 1995; Lee et al. 1999). In response to excessive intracellular Ca2+, activated calpain cleaves GluR1, resulting in the down-regulation of AMPAR functions. Therefore, it provides a potential mechanism underlying the protective role of calpain in controlling neuronal excitability under conditions associated with excitotoxicity-related disorders.
Acknowledgments
This work was supported by grants from the American Heart Association and National Institute of Health to Z.Y. We would like to thank Dr Qian Jiang and Xiaoqing Chen for their technical support.
Supplemental material
The online version of this paper can be accessed at:
DOI: 10.1113/jphysiol.2006.122754
http://jp.physoc.org/cgi/content/full/jphysiol.2006.122754/DC1 and contains supplemental material consisting of a figure entitled:
Prolonged NMDAR stimulation does not change AMPAR subunit composition as indicated by the unaltered rectification of AMPA responses (ratio of the AMPAR-mediated current amplitude at −60 mV to that at +40 mV).
This material can also be found as part of the full-text HTML version available from http://www.blackwell-synergy.com
References
- Adamec E, Beermann ML, Nixon RA. Calpain I activation in rat hippocampal neurons in culture is NMDA receptor selective and not essential for excitotoxic cell death. Brain Res Mol Brain Res. 1998;54:35–48. doi: 10.1016/s0169-328x(97)00304-5. [DOI] [PubMed] [Google Scholar]
- Bano D, Young KW, Guerin CJ, Lefeuvre R, Rothwell NJ, Naldini L, Rizzuto R, Carafoli E, Nicotera P. Cleavage of the plasma membrane Na+/Ca2+ exchanger in excitotoxicity. Cell. 2005;120:275–285. doi: 10.1016/j.cell.2004.11.049. [DOI] [PubMed] [Google Scholar]
- Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, von Zastrow M, Malenka RC. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat Neurosci. 2000;3:1291–1300. doi: 10.1038/81823. [DOI] [PubMed] [Google Scholar]
- Bi X, Chang V, Molnar E, McIlhinney RA, Baudry M. The C-terminal domain of glutamate receptor subunit 1 is a target for calpain-mediated proteolysis. Neuroscience. 1996;73:903–906. doi: 10.1016/0306-4522(96)00157-1. [DOI] [PubMed] [Google Scholar]
- Bi X, Chen J, Dang S, Wenthold RJ, Tocco G, Baudry M. Characterization of calpain-mediated proteolysis of GluR1 subunits of α-amino-3-hydroxy-5-methylisoxazole-4-propionate receptors in rat brain. J Neurochem. 1997;68:1484–1494. doi: 10.1046/j.1471-4159.1997.68041484.x. [DOI] [PubMed] [Google Scholar]
- Bi X, Rong Y, Chen J, Dang S, Wang Z, Baudry M. Calpain-mediated regulation of NMDA receptor structure and function. Brain Res. 1998;790:245–253. doi: 10.1016/s0006-8993(98)00067-5. [DOI] [PubMed] [Google Scholar]
- Bonazzi M, Spano S, Turacchio G, Cericola C, Valente C, Colanzi A, Kweon HS, Hsu VW, Polishchuck EV, Polishchuck RS, Sallese M, Pulvirenti T, Corda D, Luini A. CtBP3/BARS drives membrane fission in dynamin-independent transport pathways. Nat Cell Biol. 2005;7:570–580. doi: 10.1038/ncb1260. [DOI] [PubMed] [Google Scholar]
- Cai X, Gu Z, Zhong P, Ren Y, Yan Z. Serotonin 5-HT1A receptors regulate AMPA receptor channels through inhibiting Ca2+/calmodulin-dependent kinase II in prefrontal cortical pyramidal neurons. J Biol Chem. 2002;277:36553–36562. doi: 10.1074/jbc.M203752200. [DOI] [PubMed] [Google Scholar]
- Carroll RC, Beattie EC, Xia H, Luscher C, Altschuler Y, Nicoll RA, Malenka RC, von Zastrow M. Dynamin-dependent endocytosis of ionotropic glutamate receptors. Proc Natl Acad Sci U S A. 1999;96:14112–14117. doi: 10.1073/pnas.96.24.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll RC, Zukin RS. NMDA-receptor trafficking and targeting: implications for synaptic transmission and plasticity. Trends Neurosci. 2002;25:571–577. doi: 10.1016/s0166-2236(02)02272-5. [DOI] [PubMed] [Google Scholar]
- Chen M, He H, Zhan S, Krajewski S, Reed JC, Gottlieb RA. Bid is cleaved by calpain to an active fragment in vitro and during myocardial ischemia/reperfusion. J Biol Chem. 2001;276:30724–30728. doi: 10.1074/jbc.M103701200. [DOI] [PubMed] [Google Scholar]
- Choi DW. Calcium: still center-stage in hypoxic-ischemic neuronal death. Trends Neurosci. 1995;18:58–60. [PubMed] [Google Scholar]
- Collingridge GL, Lester RA. Excitatory amino acid receptors in the vertebrate central nervous system. Pharmacol Rev. 1989;41:143–210. [PubMed] [Google Scholar]
- Dong H, O'Brien RJ, Fung ET, Lanahan AA, Worley PF, Huganir RL. GRIP: a synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature. 1997;386:279–284. doi: 10.1038/386279a0. [DOI] [PubMed] [Google Scholar]
- Gellerman DM, Bi X, Baudry M. NMDA receptor-mediated regulation of AMPA receptor properties in organotypic hippocampal slice cultures. J Neurochem. 1997;69:131–136. doi: 10.1046/j.1471-4159.1997.69010131.x. [DOI] [PubMed] [Google Scholar]
- Glading A, Lauffenburger DA, Wells A. Cutting to the chase: calpain proteases in cell motility. Trends Cell Biol. 2002;12:46–54. doi: 10.1016/s0962-8924(01)02179-1. [DOI] [PubMed] [Google Scholar]
- Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- Gout I, Dhand R, Hiles ID, Fry MJ, Panayotou G, Das P, Truong O, Totty NF, Hsuan J, Booker GW, et al. The GTPase dynamin binds to and is activated by a subset of SH3 domains. Cell. 1993;75:25–36. [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Hossmann KA. Experimental models for the investigation of brain ischemia. Cardiovascular Res. 1998;39:106–120. doi: 10.1016/s0008-6363(98)00075-3. [DOI] [PubMed] [Google Scholar]
- Huang Y, Wang KK. The calpain family and human disease. Trends Mol Med. 2001;7:355–362. doi: 10.1016/s1471-4914(01)02049-4. [DOI] [PubMed] [Google Scholar]
- Hudmon A, Schulman H. Neuronal Ca2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annu Rev Biochem. 2002;71:473–510. doi: 10.1146/annurev.biochem.71.110601.135410. [DOI] [PubMed] [Google Scholar]
- Johnson GV, Guttmann RP. Calpains: intact and active? Bioessays. 1997;19:1011–1018. doi: 10.1002/bies.950191111. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Emori Y, Imajoh-Ohmi S, Minami Y, Suzuki K. Identification and characterization of inhibitory sequences in four repeating domains of the endogenous inhibitor for calcium-dependent protease. J Biochem. 1989;106:274–281. doi: 10.1093/oxfordjournals.jbchem.a122844. [DOI] [PubMed] [Google Scholar]
- Kishimoto A, Mikawa K, Hashimoto K, Yasuda I, Tanaka S, Tominaga M, Kuroda T, Nishizuka Y. Limited proteolysis of protein kinase C subspecies by calcium-dependent neutral protease (calpain) J Biol Chem. 1989;264:4088–4092. [PubMed] [Google Scholar]
- Klee CB, Ren H, Wang X. Regulation of the calmodulin-stimulated protein phosphatase, calcineurin. J Biol Chem. 1998;273:13367–13370. doi: 10.1074/jbc.273.22.13367. [DOI] [PubMed] [Google Scholar]
- Lee MS, Kwon YT, Li M, Peng J, Friedlander RM, Tsai LH. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature. 2000;405:360–364. doi: 10.1038/35012636. [DOI] [PubMed] [Google Scholar]
- Lee JM, Zipfel GJ, Choi DW. The changing landscape of ischaemic brain injury mechanisms. Nature. 1999;399:A7–A14. doi: 10.1038/399a007. [DOI] [PubMed] [Google Scholar]
- Leonard AS, Davare MA, Horne MC, Garner CC, Hell JW. SAP97 is associated with the α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR1 subunit. J Biol Chem. 1998;273:19518–19524. doi: 10.1074/jbc.273.31.19518. [DOI] [PubMed] [Google Scholar]
- Lu X, Rong Y, Bi R, Baudry M. Calpain-mediated truncation of rat brain AMPA receptors increases their Triton X-100 solubility. Brain Res. 2000;863:143–150. doi: 10.1016/s0006-8993(00)02112-0. [DOI] [PubMed] [Google Scholar]
- Maki M, Bagci H, Hamaguchi K, Ueda M, Murachi T, Hatanaka M. Inhibition of calpain by a synthetic oligopeptide corresponding to an exon of the human calpastatin gene. J Biol Chem. 1989;264:18866–18869. [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Mammen AL, Kameyama K, Roche KW, Huganir RL. Phosphorylation of the α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR1 subunit by calcium/calmodulin-dependent kinase II. J Biol Chem. 1997;272:32528–32533. doi: 10.1074/jbc.272.51.32528. [DOI] [PubMed] [Google Scholar]
- Paternain AV, Morales M, Lerma J. Selective antagonism of AMPA receptors unmasks kainate receptor-mediated responses in hippocampal neurons. Neuron. 1995;14:185–189. doi: 10.1016/0896-6273(95)90253-8. [DOI] [PubMed] [Google Scholar]
- Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402:615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- Praefcke GJ, McMahon HT. The dynamin superfamily: universal membrane tubulation and fission molecules? Nature Rev. 2004;5:133–147. doi: 10.1038/nrm1313. [DOI] [PubMed] [Google Scholar]
- Rami A. Ischemic neuronal death in the rat hippocampus: the calpain-calpastatin-caspase hypothesis. Neurobiol Dis. 2003;13:75–88. doi: 10.1016/s0969-9961(03)00018-4. [DOI] [PubMed] [Google Scholar]
- Roche KW, O'Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–1188. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- Saido TC, Sorimachi H, Suzuki K. Calpain: new perspectives in molecular diversity and physiological-pathological involvement. Faseb J. 1994;8:814–822. [PubMed] [Google Scholar]
- Saido TC, Yokota M, Nagao S, Yamaura I, Tani E, Tsuchiya T, Suzuki K, Kawashima S. Spatial resolution of fodrin proteolysis in postischemic brain. J Biol Chem. 1993;268:25239–25243. [PubMed] [Google Scholar]
- Siman R, Noszek JC, Kegerise C. Calpain I activation is specifically related to excitatory amino acid induction of hippocampal damage. J Neurosci. 1989;9:1579–1590. doi: 10.1523/JNEUROSCI.09-05-01579.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song I, Huganir RL. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci. 2002;25:578–588. doi: 10.1016/s0166-2236(02)02270-1. [DOI] [PubMed] [Google Scholar]
- Suzuki E, Kessler M, Arai AC. C-terminal truncation affects kinetic properties of GluR1 receptors. Mol Cell Neurosci. 2005;29:1–10. doi: 10.1016/j.mcn.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Syntichaki P, Tavernarakis N. The biochemistry of neuronal necrosis: rogue biology? Nat Rev Neurosci. 2003;4:672–684. doi: 10.1038/nrn1174. [DOI] [PubMed] [Google Scholar]
- Verdoorn TA, Burnashev N, Monyer H, Seeburg PH, Sakmann B. Structural determinants of ion flow through recombinant glutamate receptor channels. Science. 1991;252:1715–1718. doi: 10.1126/science.1710829. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhong P, Gu Z, Yan Z. Regulation of NMDA receptors by dopamine D4 signalling in prefrontal cortex. J Neurosci. 2003;23:9852–9861. doi: 10.1523/JNEUROSCI.23-30-09852.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W, Seeburg PH. Mammalian ionotropic glutamate receptors. Curr Opin Neurobiol. 1993;3:291–298. doi: 10.1016/0959-4388(93)90120-n. [DOI] [PubMed] [Google Scholar]
- Wu HY, Tomizawa K, Oda Y, Wei FY, Lu YF, Matsushita M, Li ST, Moriwaki A, Matsui H. Critical role of calpain-mediated cleavage of calcineurin in excitotoxic neurodegeneration. J Biol Chem. 2004;279:4929–4940. doi: 10.1074/jbc.M309767200. [DOI] [PubMed] [Google Scholar]
- Wu HY, Yuen EY, Lu YF, Matsushita M, Matsui H, Yan Z, Tomizawa K. Regulation of N-methyl-D-aspartate receptors by calpain in cortical neurons. J Biol Chem. 2005;280:21588–21593. doi: 10.1074/jbc.M501603200. [DOI] [PubMed] [Google Scholar]
- Yan Z, Surmeier DJ. Muscarinic (m2/m4) receptors reduce N- and P-type Ca2+ currents in rat neostriatal cholinergic interneurons through a fast, membrane-delimited, G-protein pathway. J Neurosci. 1996;16:2592–2604. doi: 10.1523/JNEUROSCI.16-08-02592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Jiang Q, Chen P, Gu Z, Feng J, Yan Z. Serotonin 5-HT1A receptors regulate NMDA receptor channels through a microtubule-dependent mechanism. J Neurosci. 2005a;25:5488–5501. doi: 10.1523/JNEUROSCI.1187-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Jiang Q, Feng J, Yan Z. Microtubule regulation of N-methyl-D-aspartate receptor channels in neurons. J Biol Chem. 2005b;280:29420–29427. doi: 10.1074/jbc.M504499200. [DOI] [PubMed] [Google Scholar]
- Zhang C, Xiong W, Zheng H, Wang L, Lu B, Zhou Z. Calcium- and dynamin-independent endocytosis in dorsal root ganglion neurons. Neuron. 2004;42:225–236. doi: 10.1016/s0896-6273(04)00189-8. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Qin Y, Zhao M, Van Aelst L, Malinow R. Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell. 2002;110:443–455. doi: 10.1016/s0092-8674(02)00897-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Prolonged NMDAR stimulation does not change AMPAR subunit composition as indicated by the unaltered rectification of AMPA responses (ratio of the AMPAR-mediated current amplitude at −60 mV to that at +40 mV).