Abstract
Submucosal glands line the cartilaginous airways and produce most of the antimicrobial mucus that keeps the airways sterile. The glands are defective in cystic fibrosis (CF), but how this impacts airway health remains uncertain. Although most CF mouse strains exhibit mild airway defects, those with the C57Bl/6 genetic background have increased airway pathology and susceptibility to Pseudomonas. Thus, they offer the possibility of studying whether, and if so how, abnormal submucosal gland function contributes to CF airway disease. We used optical methods to study fluid secretion by individual glands in tracheas from normal, wild-type (WT) mice and from cystic fibrosis transmembrane conductance regulator (CFTR) knockout mice (Cftrm1UNC/Cftrm1UNC; CF mice). Glands from WT mice qualitatively resembled those in humans by responding to carbachol and vasoactive intestinal peptide (VIP), although the relative rates of VIP- and forskolin-stimulated secretion were much lower in mice than in large mammals. The pharmacology of mouse gland secretion was also similar to that in humans; adding bumetanide or replacement of HCO3− by Hepes reduced the carbachol response by ∼50%, and this inhibition increased to 80% when both manoeuvres were performed simultaneously. It is important to note that glands from CFTR knockout mice responded to carbachol but did not secrete when exposed to VIP or forskolin, as has been shown previously for glands from CF patients. Tracheal glands from WT and CF mice both had robust secretory responses to electrical field stimulation that were blocked by tetrodotoxin. It is interesting that local irritation of the mucosa using chili pepper oil elicited secretion from WT glands but did not stimulate glands from CF mice. These results clarify the mechanisms of murine submucosal gland secretion and reveal a novel defect in local regulation of glands lacking CFTR which may also compromise airway defence in CF patients.
Cystic fibrosis (CF) is a common fatal disease caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene (cftr). Many epithelial tissues are affected in CF; however, patients usually succumb to respiratory failure that results from chronic bacterial infection and inflammation in the airways (Pilewski & Frizzell, 1999; Sheppard & Welsh, 1999). While accumulation of abnormally thick mucus has long been considered to be a hallmark of the lung disease of CF, the events leading from CFTR mutations to airway pathology remain poorly understood (Pilewski & Frizzell, 1999; Gibson et al. 2003).
There is evidence that airway submucosal glands, which lie beneath the epithelium and are connected to the surface by ducts, play an important role in the pathophysiology of CF. They secrete mucus when stimulated by secretagogues such as acetylcholine (ACh) or vasoactive intestinal peptide (VIP). Dilatation of the submucosal gland ducts with gland mucus is among the earliest pathological changes observed in CF lungs (Oppenheimer & Esterly, 1975) and implicates the glands in pathogenesis before the onset of chronic infections that irrevocably alter the biology of the airways. Consistent with the role of CFTR in submucosal gland function, glands from CF patients have altered responses to secretagogues when compared to normal glands (Jayaraman et al. 2001; Joo et al. 2002a, 2006; Verkman et al. 2003; Wine & Joo, 2004; Salinas et al. 2005). Non-CF submucosal glands secrete fluid when stimulated with Ca2+-elevating agents (e.g. ACh or carbachol) and/or cAMP agonists (e.g. VIP or forskolin) whereas those from CF patients do not respond to VIP or forskolin (Joo et al. 2002a) and produce a mucus during stimulation with carbachol that is thicker (Jayaraman et al. 2001) and more acidic (Song et al. 2005).
Airway submucosal glands are complex structures that normally produce mucus in response to a wide range of different stimuli (Ballard & Inglis, 2004; Wine & Joo, 2004). Electrical stimulation of the superior laryngeal nerves elicits tracheal mucus secretion, and this provided early evidence for neuronal control of mucus secretion (Johnson, 1935). Submucosal gland secretion is controlled by parasympathetic and possibly sympathetic innervation as well as by local release of stimulatory signals from nociceptive sensory nerves comprising C- and Aδ-fibres (Barnes, 2001; Tai & Baraniuk, 2002; Widdicombe, 2003; Ballard & Inglis, 2004). Although the relative roles of local and central regulatory pathways in CF has not been investigated, airways from normal donors can remain functional and uninfected for many years when transplanted into CF patients despite their lack of central sympathetic and parasympathetic innervation. As central regulation is not essential for airway host defence, the clinical experience implicates abnormal local regulation of gland function in CF.
Submucosal glands have been studied using human biopsies, transplant samples or human volunteers, but an animal model would be useful. CF mice offer many potential advantages: a consistent supply of tissue for experimentation; control over bacterial infection and disease severity; and the possibility of crossing CF mice with other transgenic and knockout mice to gain new insights into roles of particular channels, transporters and signalling pathways in gland function. With these advantages in mind, we undertook a study of tracheal submucosal glands in an inbred congenic CFTR knockout mouse strain (C57BL/6J Cftrm1UNC/Cftrm1UNC (CF mouse); for reviews see Stotland et al. 2000; Davidson & Dorin, 2001). CF mice do not have gross lung disease under normal conditions and their gland phenotype is therefore a direct consequence of CFTR deficiency. It is important to note that when CFTR−/− C57BL/6J mice are challenged with Pseudomonas aeruginosa they are more susceptible to lung infection than their CFTR-expressing littermates (Gosselin et al. 1998; Tam et al. 1999; Sapru et al. 1999; Schroeder et al. 2001) and develop spontaneous and progressive lung disease of early onset (Kent et al. 1997; Durie et al. 2004), including abnormal mucociliary clearance (Cowley et al. 1997; Durie et al. 2004). Their airway phenotype includes postbronchiolar over-inflation (Durie et al. 2004), an increase in goblet cells, and decreased airway surface liquid in the nasal epithelium (Tarran et al. 2001). For these reasons, and to exploit the wealth of information available on rodent airway physiology, we examined submucosal gland fluid secretion in tracheas from wild-type (WT) and CF mice.
Our results show that glands from normal mice secrete fluid when stimulated by the muscarinic agonist carbachol or by the cAMP agonists VIP and forskolin, as has been reported previously for glands in larger species including humans. Carbachol-induced secretion was inhibited by the calcium-activated chloride channel blocker niflumic acid and was unaffected in CF mice, whereas the VIP–forskolin pathway was insensitive to this inhibitor and was not detectable in the CFTR knockout animals. Interestingly, local stimulation of airway sensory nerves by luminal application of an irritant (chili pepper oil) triggered fluid secretion by WT but not CF mouse submucosal glands, although glands from both WT and CF mice were responsive to electrical field stimulation (EFS). These results indicate that a local neuronal reflex controls airway submucosal glands and is greatly diminished in CF, and suggests that mouse airway submucosal glands provide a useful model for studying the regulation of normal and CF human glands.
Methods
Mice
Congenic C57BL/6J (B6) CFTR heterozygote and homozygous B6 CFTR−/− (CF) and B6 CFTR+/+ (WT litter mates) mice were kindly provided by Dr Christina Haston, Department of Medicine, McGill University (Haston et al. 2002). The tails were clipped at 18 days of age and genomic DNA was isolated for subsequent cftr genotyping using a PCR assay reported previously (Kent et al. 1997). Due to the risk of death from intestinal obstruction, all CF mice were maintained on a liquid diet (Peptamen) from the age of 18 days (Kent et al. 1996). WT mice of the same strain from Charles River Canada (Saint-Constant, Quebec, Canada) or the Veterinary Service Center (Stanford, CA, USA) were used to study the effects of ouabain, bumetanide, niflumic acid and replacement of HCO3− by Hepes on secretion by normal glands as well as the effect of electrical field and chili pepper oil stimulation. For all other experiments with CF mice, comparisons were made between the CFTR−/− mice and their CFTR+/+ littermates. Mice were housed in the animal facility at McGill University or the Department of Laboratory Animal Care facility, Department of Comparative Medicine at Stanford School of Medicine. All procedures followed Canadian Institutes of Health Research (CIHR) and National Institutes of Health (NIH) rules and were approved by the faculty Animal Care Committees at each institution.
Tracheal preparation
Mice (10–12 weeks old) were killed by exposure to 100% carbon dioxide and tracheas were immediately dissected and placed in ice-cold Krebs–Ringer bicarbonate buffer containing (mm): NaCl 115, K2HPO4 2.4, KH2PO4 0.4, CaCl2 1.2, MgCl2 1.2, NaHCO3 25 and glucose 10; pH 7.4, equilibrated to 95% O2–5% CO2. To minimize tissue exposure to endogenously generated prostaglandins during tissue preparation and mounting, 1.0 μm indomethacin was present in the bath throughout the experiment. The dissection was modified from the methods of Joo et al. (2001b): the trachea was cut dorsally along its length and placed in a custom-built chamber mucosal-side up so that the serosal side was bathed in ∼60 μl Krebs-Ringer solution and the mucosal side was exposed to air. The luminal surface was gently cleaned with absorbent paper, dried with a stream of air, and coated with ∼5 μl mineral oil (water saturated) between the first and third cartilage rings. To avoid any possibility of tissue damage that might trigger nociceptive responses, the luminal surface was not cleaned with absorbent paper during the experiments involving chili pepper oil. Droplets of mucus formed under oil during secretion by individual submucosal glands, as first described for insect Malpighian tubules by Ramsay (1954) and modified for trachea by Quinton (1979). The tissue was placed in a temperature-controlled system (TC-102, Medical Systems Corp., Greenvale, NY, USA), warmed to 37°C at a rate of ∼2°C min−1, and continuously exposed to warm, humidified 95% O2–5% CO2. Pharmacological agents were added to the serosal side.
EFS
Responses to EFS were investigated by passing current through platinum electrodes placed at the caudal and frontal end of the isolated trachea. Pulses (20 V, 5 Hz, 10 ms) were applied for 1 min using a Grass SD9 stimulator (Quincy, MA, USA), because those parameters yielded maximal and reproducible secretion rates in preliminary experiments.
Exposure to a chemical irritant
Airway responses to chemical irritants have been studied using a wide range of insults including ammonia vapour (Phipps & Richardson, 1976), cigarette smoke (Schultz et al. 1991), capsaicin (Schultz et al. 1985), ozone (McBride et al. 1991), nitrogen dioxide (Holroyd et al. 1997) and diesel exhaust (Ichinose et al. 1998; Miyabara et al. 1998). To measure secreted droplets, the tracheal preparation must be coated with oil, which precludes the use of air- or water-borne irritants. Therefore we used commercially available hot chili pepper cooking oil as a source of capsaicin and other chemical irritants (Melina's, Pepper Mill Imports, Carmel, CA, USA). After measuring basal secretion rate, a small volume (0.5 μl) of chili pepper oil was gently added to the 5 μl paraffin oil bathing the mucosal surface.
Calculation of secreted volume
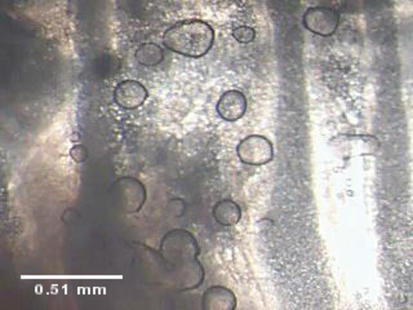
As with larger mammalian species, droplets of mucus formed at gland openings (Fig. 1). Images were taken at 30 s intervals using a digital camera (MiniVID, LW Scientific, Lawrenceville, GA, USA) mounted on a dissecting microscope and were stored for off-line analysis. Stored images were analysed using ImageJ 1.32J (NIH). Secretion volumes were calculated as previously described using the formula: V = 4/3π r3, where r is the radius (Joo et al. 2001b). To be included in the analysis, each droplet had to meet the following criteria: (a) circular outline so that a spherical shape could be assumed; (b) clear edges to allow accurate measurement of the radius; and (c) no fusion with neighbouring droplets. Viability was tested at the end of each experiment by measuring the response to carbachol and those glands that did not respond to carbachol (< 5%) were excluded from the analysis.
Figure 1. Fluid secretion by mouse airway submucosal glands.
Digital image of mucus droplets that form under paraffin oil following stimulation of isolated mouse trachea with carbachol. Mouse glands are smaller than those in humans but there are more per unit area in this region of the trachea.
The secretion rate was calculated by fitting the volume versus time plots with straight lines using linear regression and the slopes were taken as the secretion rates using at least three points. Rates were expressed in nl min−1 except when they were low and so would be clearer in pl min−1. The r2 value for such linear fits was > 0.8.
Reagents
Stock solutions of carbachol, adrenaline (epinephrine) and VIP were dissolved directly in Krebs–Ringer solution. Indomethacin, atropine, tetrodotoxin, forskolin, bumetanide, niflumic acid and CFTRinh172 stocks were prepared in DMSO. The final DMSO concentration did not exceed 0.1%, a concentration which did not affect fluid secretion in response to carbachol during control experiments (data not shown). The vehicle for stock solutions was Krebs–Ringer solution. Reagents were obtained from Sigma (St Louis, MO, USA) except for CFTRinh172, which was obtained from Calbiochem (Darmstadt, Germany).
Statistics
Data are presented as means ± s.e.m. and were analysed using ANOVA, Student's t test or F test as appropriate, with P < 0.05 considered significant. Linear regression was performed using SigmaPlot 9 (Systat Software, Point Richmond, CA, USA). Non-linear regressions were performed using GraphPad Prism 4 (GraphPad Software Inc., San Diego, CA, USA).
Results
Although most submucosal glands were quiescent immediately after isolation, about one-third of the glands began producing fluid spontaneously as they were warmed from room temperature and became quiescent by the time they reached 37°C. The initial fluid volume at 37°C was subtracted from subsequent measurements when calculating secretion rates.
Responses to cholinergic stimulation
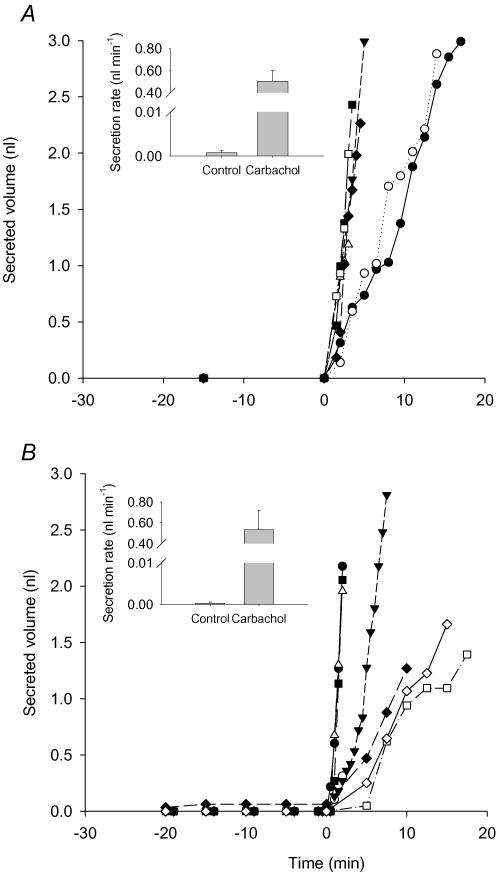
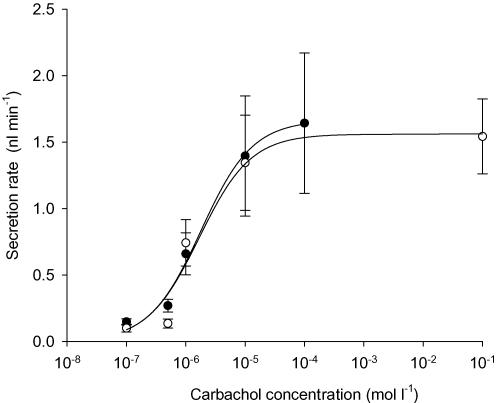
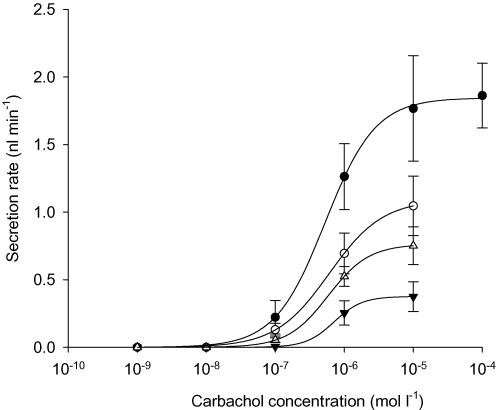
Ach activates muscarinic receptors and is a potent stimulus of fluid secretion by submucosal glands in humans and other large mammals. To examine the possible role of CFTR in cholinergically stimulated secretion we compared responses when glands from CF mice and their WT littermates were exposed to the muscarinic receptor agonist carbachol. At each carbachol concentration, we examined tracheas from at least two WT and CF mice (n = 2–4) in order to have at least six glands with secretion properties matching our inclusion criteria. Large stimulations were observed using tracheas from four WT (Fig. 2A) and three CF mice (Fig. 2B) exposed to 1 μm carbachol at t = 0. Similar numbers of glands from WT and CF mice responded to carbachol (WT, 15 ± 2 glands per trachea from a total of 61 glands; CF, 16 ± 1.5 glands per trachea from a total of 48 glands). Carbachol stimulation of CFTR−/− glands suggests involvement of another type of apical chloride channel besides CFTR. Experiments were performed at several carbachol concentrations from 100 nm to 100 μm and the results were fitted to a three-parameter logistic curve by non-linear regression (Fig. 3). Seven glands from each group met the criteria for quantifying secretion rate and were included in the dose–response curve. The best-fit curves for stimulation of glands from WT and CF animals did not differ significantly (F test, P > 0.8). Thus, the EC50 for carbachol was not altered in glands from CF mice (1.8 μm, 95% confidence interval at 0.3–8 μm) when compared with those from WT littermate controls (1.7 μm, 95% confidence interval at 0.8–4 μm), and the maximum secretion rate (Vmax) also did not differ significantly (1.66 ± 0.25 (n = 36) and 1.58 ± 0.16 nl min−1 (n = 55), respectively).
Figure 2. Cumulative secreted volumes versus time from single glands.
Carbachol (1 μm) was added at time 0. Each curve indicates the fluid secreted by one gland. A, wild-type mice (n = 7 glands from four tracheas). B, CFTR−/− mice (n = 7 glands from three tracheas). Insets show the calculated secretion rates.
Figure 3. Mean concentration dependence of carbachol stimulation in wild-type (WT) and CFTR−/− knockout mouse glands.
Data from WT (○) and CFTR−/− (•) submucosal glands were fitted with a three-parameter logistic curve using non-linear regression (n = 6 glands for each point). The concentration giving half-maximal stimulation (EC50) was 1.7 μm for WT mice and 1.8 μm for CFTR−/− mice. Maximum secretion rates (Vmax) were also similar: 1.58 ± 0.16 nl min−1 (n = 55) and 1.66 ± 0.25 (n = 36), respectively.
Most fluid secretion by submucosal glands from sheep (Joo et al. 2001b), pigs (Inglis et al. 1997; Ballard et al. 1999, 2006; Trout et al. 2001; Joo et al. 2002b) and humans (Joo et al. 2002a) is driven by net Cl− and HCO3− transport (Ballard & Inglis, 2004). Cl−-driven fluid secretion involves basolateral entry of Cl−, K+ and Na+ via Na+–K+–2Cl− (NKCC1) cotransporters and apical exit through anion channels, with energy for the uphill chloride entry ultimately provided by the Na+–K+-ATPase pump (reviewed by Verkman et al. 2003). We tested this model in mouse submucosal glands by measuring the effects of known inhibitors on carbachol (50 nm)-induced fluid secretion. This concentration of carbachol was used to stimulate a low rate of secretion so that merging of neighbouring secreted droplets during long experiments would be minimized, although we recognize that the pattern of inhibition revealed by these low concentrations may differ from that seen with much higher concentrations, for a variety of reasons.
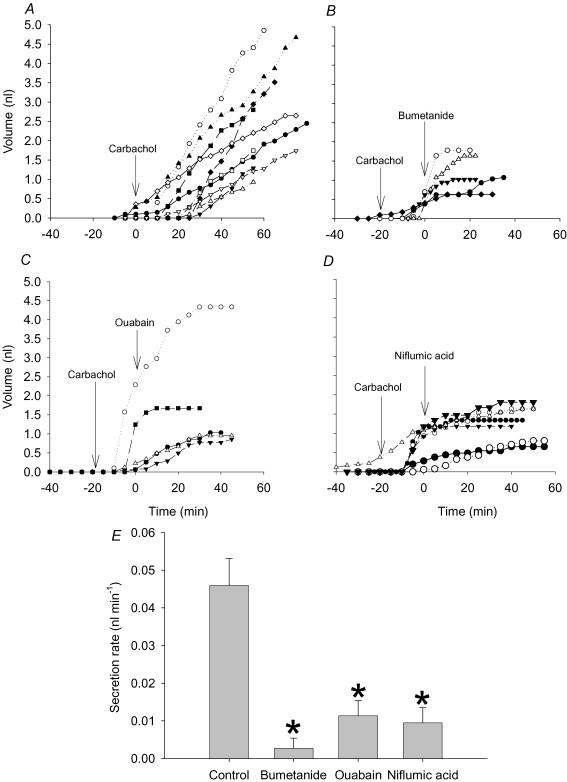
Figure 4A–D shows the net (cumulative) secretion by individual glands and Fig. 4E summarizes the mean rate under various conditions. All the transport inhibitors caused plateaux in the cumulative volume versus time plots as expected from the model for gland secretion. Adding the Na+–K+-ATPase inhibitor ouabain (1 mm) to the basolateral side reduced carbachol-stimulated secretion from 60 ± 10 pl min−1 (n = 10 glands from five tracheas) to 7.0 ± 4.0 pl min−1 within 35 min (n = 5 glands from three tracheas, one-way ANOVA, P < 0.05; Fig. 4C and E). Secretion rates measured between 15 and 35 min after the addition of bumetanide (10 μm) were also significantly lower than in control glands when stimulated by 50 nm carbachol for 35 min (one-way ANOVA, P < 0.05, n = 5 glands from three tracheas; Fig. 4B and E). To examine the possible role of Ca2+-activated Cl− channels in stimulation by carbachol, we tested the effect of niflumic acid on fluid secretion rate. Niflumic acid (10 μm) abolished carbachol-induced fluid secretion within 20 min (n = 8 glands from four tracheas, one-way ANOVA, P < 0.05; Fig. 4D and E).
Figure 4. Effect of bumetanide, ouabain and niflumic acid on carbachol stimulated fluid secretion by wild-type (WT) mouse submucosal glands.
A, effect of 50 nm carbachol on submucosal glands (n = 11 glands from five tracheas). B, effect of 10 μm bumetanide on fluid secretion (n = 5 glands from three tracheas). C, effect of 1 mm ouabain on fluid secretion (n = 5 glands from two tracheas). D, effect of 10 μm niflumic acid on fluid secretion by mouse submucosal glands (n = 8 glands from from tracheas). E, effect of bumetanide, ouabain and niflumic acid on carbachol-induced fluid secretion rate (mean + s.e.m.). The secretion rate during the time interval 15–35 min after addition of blocker was compared with that measured after 35–55 min stimulation with carbachol. *Significant differences (Student's t test, P < 0.05) between the control and experimental groups.
To investigate the roles of Cl− and HCO3− in mouse submucosal gland secretion we examined carbachol-induced responses after adding 40 μm bumetanide to block Cl− transport by the NKCC1 cotransporters, and after replacement of HCO3− by Hepes to eliminate HCO3− transport, and after both of these treatments in combination (Fig. 5). In the absence of inhibitors, the concentration dependence of the carbachol responses yielded an EC50 of 0.51 ± 0.03 μm and an average Vmax of 1.84 ± 0.02 nl min−1 (n = 68 glands from five tracheas). Although the EC50 was not affected significantly by the presence of 40 μm bumetanide (0.62 ± 0.03 μm, Student's t test, P > 0.2), Vmax was reduced by 40% to 1.1 ± 0.03 nl min−1 (n = 44 glands from five tracheas, Student's t test, P < 0.05). When glands were stimulated with carbachol in Hepes-buffered Krebs-Ringer solution lacking HCO3− and gassed with O2, the EC50 was again unaffected whereas secretion in response to carbachol was inhibited by 55% and the Vmax was reduced to 0.8 ± 0.1 nl min−1 (n = 52 glands from five tracheas, Student's t test, P < 0.05). Finally, with simultaneous addition of bumetanide and replacement of HCO3− by Hepes, Vmax was reduced by 80% to 0.39 nl min−1 (n = 36 glands from four tracheas, Student's t test, P < 0.01). We conclude that chloride and bicarbonate both contribute significantly to carbachol-induced secretion in mouse submucosal glands under these conditions.
Figure 5. Effect of 40 μm bumetanide, replacement of bicarbonate by Hepes, and both interventions simultaneously on carbachol-induced secretion.
Data were fitted to a three-parameter logistic curve using non-linear regression. Control carbachol responses (•) yielded EC50 of 0.51 ± 0.03 μm and maximal secretion rate (Vmax) of 1.84 ± 0.02 nl min−1 (68 glands from five tracheas). Vmax was decreased by 40% when glands were exposed to 40 μm bumetanide (○), by 55% by replacement of bicarbonate-containing Krebs-Ringer solution by Hepes-buffered solution lacking HCO3− and gassed with O2 (▵), and by 80% with the combined treatment (▿). EC50 values were not affected significantly by the treatments.
Responses to VIP-, forskolin- and adrenaline-induced stimulation
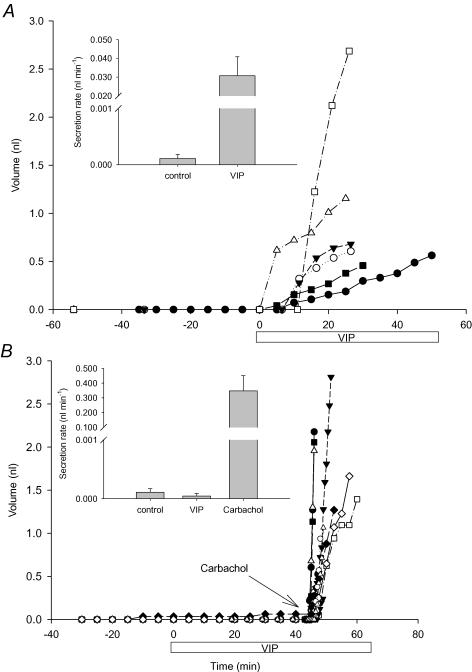
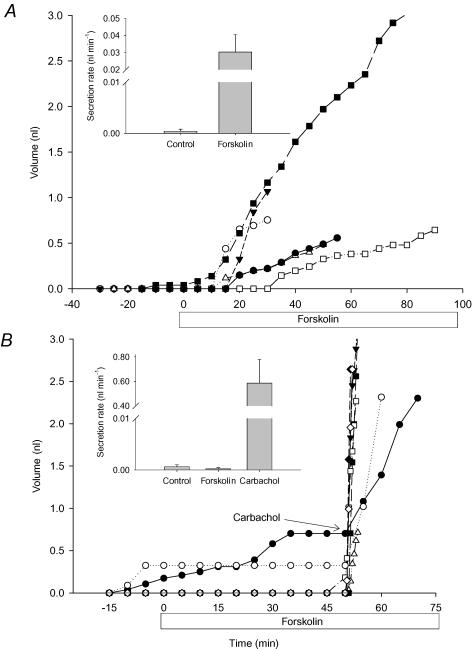
In humans, airway submucosal glands secrete in response to VIP and forskolin whereas those from CF patients do not (Joo et al. 2002a). We compared fluid secretion by WT and CF mouse glands during stimulation by VIP and forskolin, to see whether this CF phenotype is also manifested in mice. In glands from WT mice, VIP at 10 μm produced small responses with long and variable latencies, possibly because of physical barriers and abundant peptidases in the tissue. Increasing the serosal VIP concentration to 100 μm caused significant mucus secretion in half the WT mouse glands tested (21/42 glands from three tracheas); however, no secretion was detected using glands from CFTR−/− littermates (0/62 glands from six tracheas). The criteria for quantification were met by six WT (Fig. 6A) and 12 CF mice glands (Fig. 6B). Elevating cAMP with 10 μm forskolin also stimulated submucosal gland fluid secretion in tracheas from WT mice (72/102 glands from seven tracheas; Fig. 7A) but not in tracheas from their CF littermates (0/36 glands from three tracheas; Fig. 7B) although the CF mouse glands did respond vigorously to carbachol. The rates induced by VIP and forskolin were similar (40 ± 10 (n = 3 tracheas) and 30 ± 10 pl min−1, (n = 7 tracheas), respectively) and were insensitive to 10 μm niflumic acid and the muscarinic receptor antagonist atropine (10 μm) (data not shown). Thus VIP does not stimulate epithelial Ca2+-activated Cl− channels or indirectly stimulate muscarinic receptors in this complex tissue. In some species such as cat, α-adrenergic receptors stimulate copious submucosal gland secretion (Quinton, 1979; Joo et al. 2001a,b), whereas glands in other species such as sheep, pigs and humans are not responsive (Joo et al. 2001a,b) and β-adrenergic receptor activation stimulates only modest secretion (Leikauf et al. 1984; Joo et al. 2001b). However adrenaline, which should activate both α and β-adrenergic receptors, did not stimulate glands from WT mice when tested at concentrations up to 1 mm (data not shown).
Figure 6. Effect of VIP on cumulative fluid volumes and secretion rates.
VIP (100 μm) was added to glands at time 0. A, Wild-type (n = 6 glands from three tracheas) and B, CFTR−/− (n = 12 glands from five tracheas) mice. Carbachol (1 μm) was added to the glands from CFTR−/− mice shown in B to confirm their viability. The insets show the secretion rates.
Figure 7. Effect of forskolin (10 μm) on submucosal gland secretion.
A, wild-type (n = 6 glands from seven tracheas) and B, CFTR−/− (n = 7 glands from three tracheas) mice. Carbachol (1 μm) was added to the CFTR−/− knockout mouse glands as a positive control for viability. The insets show the secretion rates.
Gland responses to local (chili pepper oil) and to local and central (EFS) stimuli
Chemical irritants stimulate fluid secretion in mammalian airways in part through a local reflex that involves anterograde transmitter release by sensory neurons (Barnes, 2001; Widdicombe, 2003; De Swert & Joos, 2006), but it is not known whether this local reflex is affected in CF. We used tracheas from WT and CF mice to compare secretory responses to chili pepper oil, which is a rich source of the lipophilic capsaicinoid capsaicin (8-methyl-N-vanillyl-6-nonenamide) and related molecules such as di-hydrocapsaicin and nordihydrocapsaicin. Capsaicin depolarizes sensory neurons (C-fibres in airways) and other cell types that express the ionotropic vanilloid (VR1) receptor (Szallasi, 2001).
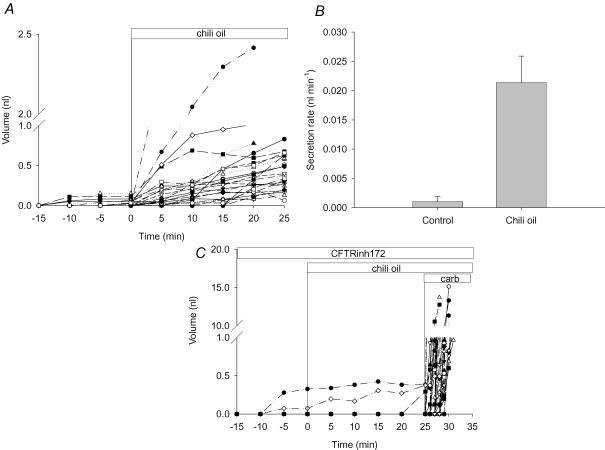
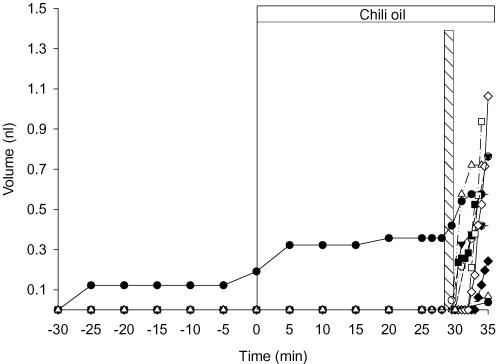
Adding chili pepper oil to the surface of WT mouse trachea consistently increased the rate of gland secretion from 1 ± 0.8 to 20 ± 4 pl min−1 (Student's t test, P < 0.05, n = 29 glands from eight tracheas). Chili pepper oil was added 60 min after initiation of the experiment shown in Fig. 8A and B. When tracheas were incubated for 60 min with the CFTR inhibitor CFTRinh172 (nominally 100 μm; Fig. 8C), fluid secretion in response to chili pepper oil was blocked. In WT mouse tracheas, 32 of 78 glands (41%) responded to chili pepper oil, but in seven tracheas preincubated with CFTRinh172, 0/54 glands responded to stimulation with chili pepper oil although they had robust responses to carbachol (Fig. 8C).
Figure 8. Effect of chili pepper oil (capsaicinoids) on fluid secretion by submucosal glands from wild-type mice.
A, secreted volume (32 glands from eight tracheas) before and after addition of chili pepper oil. Note the break in the ordinate. B, secretion rates calculated under control conditions and after exposure to chili pepper oil (n = 8 tracheas). C, chili pepper oil response measured in the presence of CFTRinh172 (nominally 100 μm; n = 53 glands from seven tracheas). Carbachol (1 μm) was added to the CFTRinh172-treated glands as a positive control for viability.
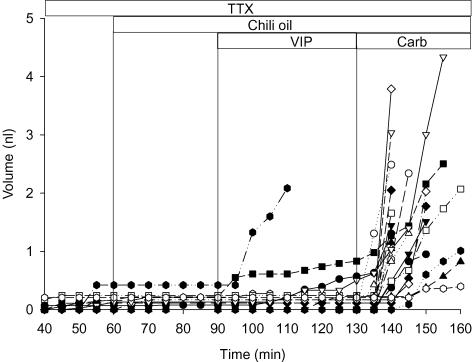
To examine whether capsaicinoids act directly on the glands or through a local reflex, chili pepper oil was applied to WT tracheas that had been pretreated with tetrodotoxin (TTX; 1 μm) for 60 min to block neuronal voltage-gated sodium channels (Fig. 9). The secretory response to chili pepper oil was abolished under these conditions (24 glands in three tracheas) whereas responses to VIP (100 μm) and carbachol (1 μm) were unaffected. These results suggest that chemical stimulation by capsaicin is mediated by a local nerve reflex whereas VIP and carbachol act directly on gland receptors. The ability of intratracheal nerves to stimulate vigorous mucus secretion in this preparation was confirmed by using EFS. Mean secretion rate increased from 1 ± 0.3 to 80 ± 20 pl min−1 after application of voltage pulses as described in the Methods (Student's t test, P < 0.05, n = 12 glands from three WT tracheas; Fig. 10). We conclude that local regulation by nociceptive stimuli involves TTX-sensitive neurons. The stronger secretory response to EFS may result, in part, from activation of both local sensory afferent nerves and surviving central parasympathetic preganglionic axons that release Ach and perhaps other agonists onto the gland cells.
Figure 9. Effect of tetrodotoxin (TTX) on responses to chili pepper oil, VIP (100 μm) and carbachol (1 μm).
The first time point shown is at 40 min; however, TTX (1 μm) was added at t = 0, then glands were sequentially exposed to chili pepper oil, VIP and carbachol (n = 24 glands from three tracheas).
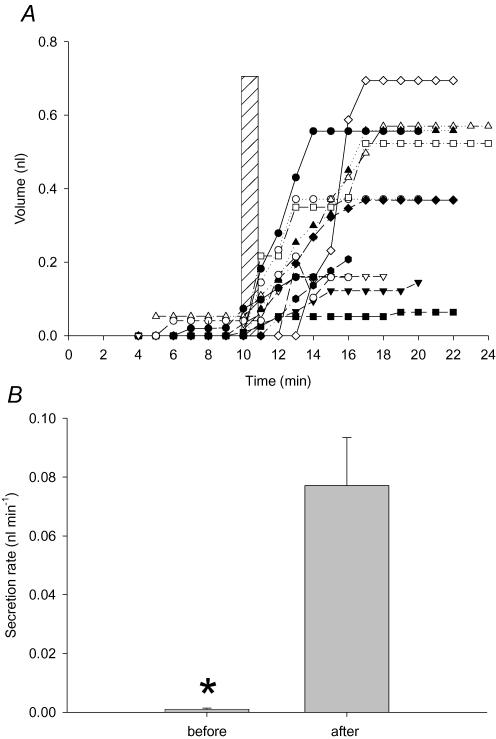
Figure 10. Effect of electrical field stimulation (EFS) on fluid secretion by submucosal glands from wild-type mice.
A, cumulative fluid secretion by individual glands before and after EFS (duration indicated by hatched vertical bar). B, mean secretion rates calculated immediately before and after EFS. *Significant difference between the control and experimental groups (12 glands from two tracheas).
The role of CFTR in mediating chili pepper oil-induced fluid secretion was assessed by studying its effect in tracheas from CF mice. In marked contrast to submucosal glands from normal mouse, those from CF mouse did not respond to chili pepper oil stimulation, indicating a profound defect in the local control of gland function (12 glands from four tracheas; Fig. 11). Although CF mouse glands did not respond to chili pepper oil, they still had robust secretion during EFS. This result is consistent with retention of the cholinergic response in CF mouse glands and confirms that insensitivity to irritants was not caused by a loss of viability (Fig. 11).
Figure 11. Effect of chili pepper oil and electrical field stimulation (EFS) on fluid secretion by submucosal glands from CFTR−/− knockout mice.
Note insensitivity to capsaicinoids but strong regulation by EFS to mimic central nervous system stimulation (12 glands from four tracheas). Most glands were non-secreting until stimulated by EFS (duration indicated by hatched vertical bar), despite the presence of chili pepper oil.
Discussion
The present findings suggest that the isolated mouse trachea is useful for studying the physiological control of normal and CF airway submucosal glands. Although the absolute rate of cAMP-stimulated gland secretion in mice was much lower than in humans or pigs, functional properties of the glands were qualitatively similar. It is interesting that the secretory response to local irritants is lost in CF mice, a feature of the CF phenotype that has not been reported previously. This has implications for host defence in human disease because local regulatory mechanisms may mediate responses to minor insults that are normally innocuous in healthy airways but become severe in CF. Whether these local mechanisms are activated by the opportunistic pathogen Pseudomonas aeruginosa remains to be established.
Advantages and disadvantages of the mouse submucosal gland model
Although it is sometimes erroneously stated that mice lack airway glands or have only one or two, we observed duct openings for 15–20 airway glands in the upper mouse trachea. Glands are more abundant in CFTR−/− animals and extend further down the trachea than in WT mice (Borthwick et al. 1999). The present results indicate that the functional properties of mouse glands that we tested are qualitatively similar to those in humans and other large mammals. As in other species, carbachol caused robust secretion, with an EC50 of ∼1 μm and Vmax of ∼1.6 nl min−1, although detailed comparisons of EC50 and Vmax values are not possible because concentration–response curves for carbachol have not been reported for other animals. The secretion rates at 10 μm carbachol were 16 nl min1 in sheep (Joo et al. 2001) and 12 nl min−1 in pigs (Joo et al. 2002), which are much higher values and may reflect larger gland size.
Aspects of the model for submucosal gland secretion were explored and found to parallel those in human glands. The prevailing model for serous cell ion transport involves Cl− uptake by basolateral NKCC1 cotransporters, which is driven by the inward sodium gradient generated by Na+–K+-ATPase pumps, and passive Cl− efflux through apical CFTR or Ca2+-activated channels (Singh et al. 1997; Lee et al. 1998; Devor et al. 1999; Inglis et al. 2002). Our pharmacological results are consistent with this scheme in that bumetanide, a blocker of the NKCC1 cotransporter, and ouabain, a Na+–K+-ATPase inhibitor that would diminish the favourable sodium gradient, both strongly inhibited carbachol-induced fluid secretion. Bicarbonate replacement also reduced carbachol responses by ∼50%, suggesting that muscarinic secretion by mouse submucosal glands is mediated by Cl− and HCO3− as reported for glands from sheep (Joo et al. 2001a,b), pigs (Inglis et al. 1997; Ballard et al. 1999, 2006; Trout et al. 2001; Joo et al. 2002) and humans (Joo et al. 2001a,b, 2002).
Carbachol responses were similar in glands from WT and CF mice indicating the involvement of other, non-CFTR Cl− channels. Ca2+-activated Cl− channels are the most likely candidates because low-level carbachol stimulation was abolished by niflumic acid, which does not inhibit voltage-dependent chloride channels (Suzuki et al. 2006). This conclusion is supported by the patch-clamp studies of Griffin et al. (1996), who found that Ca2+-activated Cl− channels in cultured ovine submucosal gland cells are stimulated by 20 μm methacholine and blocked by 10 μm niflumic acid. The carbachol-stimulated Cl− channels apparently have little role in cAMP-stimulated secretion because responses to VIP and forskolin were insensitive to niflumic acid and atropine, and were completely absent in glands from CF mice. Although the second messenger pathways and channels that mediate VIP and muscarinic responses in mouse submucosal glands appear distinct in WT mice, we cannot rigorously exclude the possibilty of muscarinic activation of CFTR in WT mouse glands because it would probably be obscured by the much larger carbachol response and would therefore not differ significantly from the response in CF mouse glands.
About half the glands from WT mice responded to VIP and forskolin, although it was necessary to use very high VIP concentrations despite the high affinity of VPAC receptors for VIP (EC50 in the low nanomolar range). The cAMP-dependent secretion rates were only ∼2% of those produced by stimulation with carbachol, in marked contrast to pig and human glands where forskolin- and VIP-induced secretion is about ∼25% that of maximum carbachol-induced rates (Joo et al. 2001a,b, 2002a,b; Trout et al. 2001). Because small responses were also produced by forskolin, they probably reflect a reduced capacity for cAMP-mediated secretion in mouse glands rather than lower receptor expression. In summary, submucosal glands from normal mice have low rates of VIP- and forskolin-induced secretion, but as in human glands this response requires CFTR (Joo et al. 2001a,b, 2002a,b).
CFTR-dependent response of the glands to mucosal irritants
Mucus secretion is stimulated by many chemical irritants and has been studied using exposure to ammonia vapour in cat tracheas (Phipps & Richardson, 1976), cigarette smoke and capsaicin in dog (Schultz et al. 1985, 1991) and ozone in ferret (McBride et al. 1991). Nociception is mediated by C- and Aδ-fibres that are situated in the airway wall and have terminals within the surface epithelium (e.g. Hunter & Undem, 1999; reviewed by Widdicombe, 2003). Stimulation of C- and Aδ-fibres causes local anterograde release of the tachykinin substance P (SP), neurokinin A (NKA) and calcitonin gene-related peptide (CGRP) (McDonald, 1987; Németh et al. 2003; Widdicombe, 2003) which, like Ach and VIP, trigger secretion when applied directly to the submucosal gland (Webber et al. 1991; Trout et al. 2001; Barnes, 2001; Phillips et al. 2003; Ballard & Inglis, 2004). In rodents, there is strong evidence that airway sensory neurons stimulate mucus secretion by triggering a local reflex and antidromic release of SP, NKA and CGRP in addition to a reflex mediated by the central nervous system (McDonald, 1987; Solway & Leff, 1991; Barnes, 2001).
It has been suggested that SP-induced fluid secretion may involve CFTR and be defective in CF (Trout et al. 2001); however, the role of local reflexes and SP in CF mouse gland secretion has not previously been examined. We found that exposing the tracheal epithelium to chili pepper oil, which is rich in capsaicinoids that should stimulate transient receptor channels, triggered mucus secretion by submucosal glands from WT but not CF mice. Because all central innervation of the trachea was severed during isolation, this regulation is presumably triggered locally by sensory neurons. Neuronal involvement was confirmed when the chili pepper oil response was abolished by TTX. Submucosal glands from CF mice trachea that failed to secrete when exposed to chili pepper oil still responded strongly to EFS. These results suggested that VIP, neurokinins and other mediators of local reflexes have the same effector (CFTR), which is distinct from the central regulation mediated by ACh.
Relevance to airways disease in CF
To our knowledge this is the first report of a defect in gland responses to local stimulation in CF mouse trachea. It has implications for CF patients in that local regulation by ‘sensory–efferent’ pathways may be crucial for detecting noxious stimuli and responding appropriately (De Swert & Joos, 2006). One such stimulus could be the inhalation of bacteria such as Pseudomonas aeruginosa. Normal airways are kept sterile by innate immune defences such as lysozyme, lactoferrin, defensins, bicarbonate and other antimicrobial factors, which act synergistically to kill inhaled bacteria or inhibit their growth (Verkman et al. 2003; Song et al. 2005; Salinas et al. 2005; Kreda et al. 2005). Many of these factors are produced in the submucosal glands, therefore loss of locally activated gland secretion described here may contribute to the early impaction of the glands and reduced delivery of antimicrobial factors to the airway surface. More research is needed to determine whether the CFTR-dependent local pathways we elicited with a capsacinoid stimulus have a role in the early pathophysiology of airway disease in CF.
The mouse submucosal gland preparation is useful for studying physiological regulation of human, normal and CF, airway submucosal glands because of the nearly perfect qualitative correspondence to human gland physiology and the great potential for analysis of the molecular mechanisms afforded by transgenic and knockout animals. In addition, extensive background knowledge of mouse airways has been generated in previous studies directed towards asthma (Tamachi et al. 2006), neurogenic inflammation (Tripp et al. 2000; Barnes, 2001; Widdicombe, 2003), bacterial infection (Chu et al. 2003; Yoon & Hassett, 2004), submucosal gland morphogenesis (Filali et al. 2002) and various aspects of airway development, injury and repair. This information and the genetic tractability of mice should greatly facilitate further studies of abnormal gland function in CF.
Acknowledgments
We thank Dr Christina Haston and Melanie Lafleur for their help with the mice, and members of the Hanrahan and Wine laboratories for their useful criticisms. J.P.I. was supported by a Fellowship from the Canadian Cystic Fibrosis Foundation (CCFF) and J.Y.C. by Cystic Fibrosis Research Inc. The work was supported by the CCFF and Canadian Institutes of Health Research, the basic research and therapy program, and by grants from the Cystic Fibrosis Foundation (USA) and by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (DK-51817).
References
- Ballard ST, Inglis SK. Liquid secretion properties of airway submucosal glands. J Physiol. 2004;556:1–10. doi: 10.1113/jphysiol.2003.052779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard ST, Trout L, Bebok Z, Sorscher EJ, Crews A. CFTR involvement in chloride, bicarbonate, and liquid secretion by airway submucosal glands. Am J Physiol Lung Cell Mol Physiol. 1999;277:L694–L699. doi: 10.1152/ajplung.1999.277.4.L694. [DOI] [PubMed] [Google Scholar]
- Ballard ST, Trout L, Garrison J, Inglis SK. Ionic mechanism of forskolin-induced liquid secretion by porcine bronchi. Am J Physiol Lung Cell Mol Physiol. 2006;290:L97–L104. doi: 10.1152/ajplung.00159.2005. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Neurogenic inflammation in the airways. Respir Physiol. 2001;125:145–154. doi: 10.1016/s0034-5687(00)00210-3. [DOI] [PubMed] [Google Scholar]
- Borthwick DW, West JD, Keighren MA, Flockhart JH, Innes BA, Dorin JR. Murine submucosal glands are clonally derived and show a cystic fibrosis gene-dependent distribution pattern. Am J Respir Cell Mol Biol. 1999;20:1181–1189. doi: 10.1165/ajrcmb.20.6.3475. [DOI] [PubMed] [Google Scholar]
- Chu HW, Campbell JA, Harbeck RJ, Martin RJ. Effects of inhaled fluticasone on bronchial hyperresponsiveness and airway inflammation in Mycoplasma pneumoniae-infected mice. Chest. 2003;123:427S. [PubMed] [Google Scholar]
- Cowley EA, Wang CG, Gosselin D, Radzioch D, Eidelman DH. Mucociliary clearance in cystic fibrosis knockout mice infected with Pseudomonas aeruginosa. Eur Respir J. 1997;10:2312–2318. doi: 10.1183/09031936.97.10102312. [DOI] [PubMed] [Google Scholar]
- Davidson DJ, Dorin JR. The CF mouse: an important tool for studying cystic fibrosis. Expert Rev Mol Med. 2001;2001:1–27. doi: 10.1017/S1462399401002551. [DOI] [PubMed] [Google Scholar]
- De Swert KO, Joos GF. Extending the understanding of sensory neuropeptides. Eur J Pharmacol. 2006;533:171–181. doi: 10.1016/j.ejphar.2005.12.066. [DOI] [PubMed] [Google Scholar]
- Devor DC, Singh AK, Lambert LC, DeLuca A, Frizzell RA, Bridges RJ. Bicarbonate and chloride secretion in Calu-3 human airway epithelial cells. J Gen Physiol. 1999;113:743–760. doi: 10.1085/jgp.113.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durie PR, Kent G, Phillips MJ, Ackerley CA. Characteristic multiorgan pathology of cystic fibrosis in a long-living cystic fibrosis transmembrane regulator knockout murine model. Am J Pathol. 2004;164:1481–1493. doi: 10.1016/S0002-9440(10)63234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filali M, Liu X, Cheng N, Abbott D, Leontiev V, Engelhardt JF. Mechanisms of submucosal gland morphogenesis in the airway. Novartis Found Symp. 2002;248:38–45. [PubMed] [Google Scholar]
- Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med. 2003;168:918–951. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- Gosselin D, Stevenson MM, Cowley EA, Griesenbach U, Eidelman DH, Boule M, Tam MF, Kent G, Skamene E, Tsui LC, Radzioch D. Impaired ability of Cftr knockout mice to control lung infection with Pseudomonas aeruginosa. Am J Respir Crit Care Med. 1998;157:1253–1262. doi: 10.1164/ajrccm.157.4.9702081. [DOI] [PubMed] [Google Scholar]
- Griffin A, Newman TM, Scott RH. Electrophysiological and ultrastructural events evoked by methacholine and intracellular photolysis of caged compounds in cultured ovine trachea submucosal gland cells. Exp Physiol. 1996;81:27–43. doi: 10.1113/expphysiol.1996.sp003917. [DOI] [PubMed] [Google Scholar]
- Haston CK, McKerlie C, Newbigging S, Corey M, Rozmahel R, Tsui LC. Detection of modifier loci influencing the lung phenotype of cystic fibrosis knockout mice. Mamm Genome. 2002;13:605–613. doi: 10.1007/s00335-002-2190-7. [DOI] [PubMed] [Google Scholar]
- Holroyd KJ, Eleff SM, Zhang LY, Jakab GJ, Kleeberger SR. Genetic modeling of susceptibility to nitrogen dioxide-induced lung injury in mice. Am J Physiol Lung Cell Mol Physiol. 1997;273:L595–L602. doi: 10.1152/ajplung.1997.273.3.L595. [DOI] [PubMed] [Google Scholar]
- Hunter DD, Undem BJ. Identification and substance P content of vagal afferent neurons innervating the epithelium of the guinea pig trachea. Am J Respir Crit Care Med. 1999;159:1943–1948. doi: 10.1164/ajrccm.159.6.9808078. [DOI] [PubMed] [Google Scholar]
- Ichinose T, Takano H, Miyabara Y, Sagai M. Long-term exposure to diesel exhaust enhances antigen-induced eosinophilic inflammation and epithelial damage in the murine airway. Toxicol Sci. 1998;44:70–79. doi: 10.1006/toxs.1998.2459. [DOI] [PubMed] [Google Scholar]
- Inglis SK, Corboz MR, Taylor AE, Ballard ST. Effect of anion transport inhibition on mucus secretion by airway submucosal glands. Am J Physiol Lung Cell Mol Physiol. 1997;272:L372–L377. doi: 10.1152/ajplung.1997.272.2.L372. [DOI] [PubMed] [Google Scholar]
- Inglis SK, Finlay L, Ramminger SJ, Richard K, Ward MR, Wilson SM, Olver RE. Regulation of intracellular pH in Calu-3 human airway cells. J Physiol. 2002;538:527–539. doi: 10.1113/jphysiol.2001.012806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman S, Joo NS, Reitz B, Wine JJ, Verkman AS. Submucosal gland secretions in airways from cystic fibrosis patients have normal [Na+] and pH but elevated viscosity. Proc Natl Acad Sci U S A. 2001;98:8119–8123. doi: 10.1073/pnas.131087598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. Effect of superior laryngeal nerves on tracheal mucus. Ann Surg. 1935;101:494–499. doi: 10.1097/00000658-193501000-00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo NS, Irokawa T, Robbins RC, Wine JJ. Hyposecretion, not hyperabsorption, is the basic defect of cystic fibrosis airway glands. J Biol Chem. 2006;281:7392–7398. doi: 10.1074/jbc.M512766200. [DOI] [PubMed] [Google Scholar]
- Joo NS, Irokawa T, Wu JV, Robbins RC, Whyte RI, Wine JJ. Absent secretion to vasoactive intestinal peptide in cystic fibrosis airway glands. J Biol Chem. 2002a;277:50710–50715. doi: 10.1074/jbc.M208826200. [DOI] [PubMed] [Google Scholar]
- Joo NS, Krouse ME, Wu JV, Saenz Y, Jayaraman S, Verkman AS, Wine JJ. HCO3- transport in relation to mucus secretion from submucosal glands. JOP. 2001a;2:280–284. [PubMed] [Google Scholar]
- Joo NS, Saenz Y, Krouse ME, Wine JJ. Mucus secretion from single submucosal glands of pig. Stimulation by carbachol and vasoactive intestinal peptide. J Biol Chem. 2002b;277:28167–28175. doi: 10.1074/jbc.M202712200. [DOI] [PubMed] [Google Scholar]
- Joo NS, Wu JV, Krouse ME, Saenz Y, Wine JJ. Optical method for quantifying rates of mucus secretion from single submucosal glands. Am J Physiol Lung Cell Mol Physiol. 2001b;281:L458–L468. doi: 10.1152/ajplung.2001.281.2.L458. [DOI] [PubMed] [Google Scholar]
- Kent G, Iles R, Bear CE, Huan LJ, Griesenbach U, McKerlie C, et al. Lung disease in mice with cystic fibrosis. J Clin Invest. 1997;100:3060–3069. doi: 10.1172/JCI119861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent G, Oliver M, Foskett JK, Frndova H, Durie P, Forstner J, Forstner GG, Riordan JR, Percy D, Buchwald M. Phenotypic abnormalities in long-term surviving cystic fibrosis mice. Pediatr Res. 1996;40:233–241. doi: 10.1203/00006450-199608000-00008. [DOI] [PubMed] [Google Scholar]
- Kreda SM, Mall M, Mengos A, Rochelle L, Yankaskas J, Riordan JR, Boucher RC. Characterization of wild-type and deltaF508 cystic fibrosis transmembrane regulator in human respiratory epithelia. Mol Biol Cell. 2005;16:2154–2167. doi: 10.1091/mbc.E04-11-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MC, Penland CM, Widdicombe JH, Wine JJ. Evidence that Calu-3 human airway cells secrete bicarbonate. Am J Physiol Lung Cell Mol Physiol. 1998;274:L450–L453. doi: 10.1152/ajplung.1998.274.3.L450. [DOI] [PubMed] [Google Scholar]
- Leikauf GD, Ueki IF, Nadel JA. Autonomic regulation of viscoelasticity of cat tracheal gland secretions. J Appl Physiol. 1984;56:426–430. doi: 10.1152/jappl.1984.56.2.426. [DOI] [PubMed] [Google Scholar]
- McBride RK, Oberdoerster G, Marin MG. Effects of ozone on the cholinergic secretory responsiveness of ferret tracheal glands. Environ Res. 1991;55:79–90. doi: 10.1016/s0013-9351(05)80142-2. [DOI] [PubMed] [Google Scholar]
- McDonald DM. Neurogenic inflammation in the respiratory tract: actions of sensory nerve mediators on blood vessels and epithelium of the airway mucosa. Am Rev Respir Dis. 1987;136:S65–S72. doi: 10.1164/ajrccm/136.6_Pt_2.S65. [DOI] [PubMed] [Google Scholar]
- Miyabara Y, Ichinose T, Takano H, Lim HB, Sagai M. Effects of diesel exhaust on allergic airway inflammation in mice. J Allergy Clin Immunol. 1998;102:805–812. doi: 10.1016/s0091-6749(98)70021-1. [DOI] [PubMed] [Google Scholar]
- Németh J, Helyes Z, Oroszi G, Jakab B, Pinter E, Szilvassy Z, Szolcsanyi J. Role of voltage-gated cation channels and axon reflexes in the release of sensory neuropeptides by capsaicin from isolated rat trachea. Eur J Pharmacol. 2003;458:313–318. doi: 10.1016/s0014-2999(02)02794-2. [DOI] [PubMed] [Google Scholar]
- Oppenheimer EH, Esterly JR. Pathology of cystic fibrosis review of the literature and comparison with 146 autopsied cases. Perspect Pediatr Pathol. 1975;2:241–278. [PubMed] [Google Scholar]
- Phillips JE, Hey JA, Corboz MR. Tachykinin NK3 and NK1 receptor activation elicits secretion from porcine airway submucosal glands. Br J Pharmacol. 2003;138:254–260. doi: 10.1038/sj.bjp.0705029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipps RJ, Richardson PS. The effects of irritation at various levels of the airway upon tracheal mucus secretion in the cat. J Physiol. 1976;261:563–581. doi: 10.1113/jphysiol.1976.sp011574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilewski JM, Frizzell RA. Role of CFTR in airway disease. Physiol Rev. 1999;79:S215–S255. doi: 10.1152/physrev.1999.79.1.S215. [DOI] [PubMed] [Google Scholar]
- Quinton PM. Composition and control of secretions from tracheal bronchial submucosal glands. Nature. 1979;279:551–552. doi: 10.1038/279551a0. [DOI] [PubMed] [Google Scholar]
- Ramsay JA. Active transport of water by the Malpighian tubules of the stick insect, Dixippus morotus (Orthoptera, Phasmidae) J Exp Biol. 1954;31:104–113. [Google Scholar]
- Salinas D, Haggie PM, Thiagarajah JR, Song Y, Rosbe K, Finkbeiner WE, Nielson DW, Verkman AS. Submucosal gland dysfunction as a primary defect in cystic fibrosis. FASEB J. 2005;19:431–433. doi: 10.1096/fj.04-2879fje. [DOI] [PubMed] [Google Scholar]
- Sapru K, Stotland PK, Stevenson MM. Quantitative and qualitative differences in bronchoalveolar inflammatory cells in Pseudomonas aeruginosa-resistant and -susceptible mice. Clin Exp Immunol. 1999;115:103–109. doi: 10.1046/j.1365-2249.1999.00762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder TH, Reiniger N, Meluleni G, Grout M, Coleman FT, Pier GB. Transgenic cystic fibrosis mice exhibit reduced early clearance of Pseudomonas aeruginosa from the respiratory tract. J Immunol. 2001;166:7410–7418. doi: 10.4049/jimmunol.166.12.7410. [DOI] [PubMed] [Google Scholar]
- Schultz HD, Davis B, Coleridge HM, Coleridge JC. Cigarette smoke in lungs evokes reflex increase in tracheal submucosal gland secretion in dogs. J Appl Physiol. 1991;71:900–909. doi: 10.1152/jappl.1991.71.3.900. [DOI] [PubMed] [Google Scholar]
- Schultz HD, Roberts AM, Bratcher C, Coleridge HM, Coleridge JC, Davis B. Pulmonary C-fibers reflexly increase secretion by tracheal submucosal glands in dogs. J Appl Physiol. 1985;58:907–910. doi: 10.1152/jappl.1985.58.3.907. [DOI] [PubMed] [Google Scholar]
- Sheppard DN, Welsh MJ. Structure and function of the CFTR chloride channel. Physiol Rev. 1999;79:S23–S45. doi: 10.1152/physrev.1999.79.1.S23. [DOI] [PubMed] [Google Scholar]
- Singh M, Krouse M, Moon S, Wine JJ. Most basal I(SC) in Calu-3 human airway cells is bicarbonate-dependent Cl− secretion. Am J Physiol Lung Cell Mol Physiol. 1997;272:L690–L698. doi: 10.1152/ajplung.1997.272.4.L690. [DOI] [PubMed] [Google Scholar]
- Solway J, Leff AR. Sensory neuropeptides and airway function. J Appl Physiol. 1991;71:2077–2087. doi: 10.1152/jappl.1991.71.6.2077. [DOI] [PubMed] [Google Scholar]
- Song Y, Salinas D, Nielson DW, Verkman AS. Hyperacidity of secreted fluid from submucosal glands in early cystic fibrosis. Am J Physiol Cell Physiol. 2005;290:C741–C749. doi: 10.1152/ajpcell.00379.2005. [DOI] [PubMed] [Google Scholar]
- Stotland PK, Radzioch D, Stevenson MM. Mouse models of chronic lung infection with Pseudomonas aeruginosa: models for the study of cystic fibrosis. Pediatr Pulmonol. 2000;30:413–424. doi: 10.1002/1099-0496(200011)30:5<413::aid-ppul8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Morita T, Iwamoto T. Diversity of Cl− channels. Cell Mol Life Sci. 2006;63:12–24. doi: 10.1007/s00018-005-5336-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szallasi A. Vanilloid receptor ligands: hopes and realities for the future. Drugs Aging. 2001;18:561–573. doi: 10.2165/00002512-200118080-00001. [DOI] [PubMed] [Google Scholar]
- Tai CF, Baraniuk JN. Upper airway neurogenic mechanisms. Curr Opin Allergy Clin Immunol. 2002;2:11–19. doi: 10.1097/00130832-200202000-00003. [DOI] [PubMed] [Google Scholar]
- Tam M, Snipes GJ, Stevenson MM. Characterization of chronic bronchopulmonary Pseudomonas aeruginosa infection in resistant and susceptible inbred mouse strains. Am J Respir Cell Mol Biol. 1999;20:710–719. doi: 10.1165/ajrcmb.20.4.3223. [DOI] [PubMed] [Google Scholar]
- Tamachi T, Maezawa Y, Ikeda K, Iwamoto I, Nakajima H. Interleukin 25 in allergic airway inflammation. Int Arch Allergy Immunol. 2006;140:59–62. doi: 10.1159/000092713. [DOI] [PubMed] [Google Scholar]
- Tarran R, Grubb BR, Parsons D, Picher M, Hirsh AJ, Davis CW, Boucher RC. The CF salt controversy: in vivo observations and therapeutic approaches. Mol Cell. 2001;8:149–158. doi: 10.1016/s1097-2765(01)00286-6. [DOI] [PubMed] [Google Scholar]
- Tripp RA, Moore D, Winter J, Anderson LJ. Respiratory syncytial virus infection and G and/or SH protein expression contribute to substance P, which mediates inflammation and enhanced pulmonary disease in BALB/c mice. J Virol. 2000;74:1614–1622. doi: 10.1128/jvi.74.4.1614-1622.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trout L, Corboz MR, Ballard ST. Mechanism of substance P-induced liquid secretion across bronchial epithelium. Am J Physiol Lung Cell Mol Physiol. 2001;281:L639–L645. doi: 10.1152/ajplung.2001.281.3.L639. [DOI] [PubMed] [Google Scholar]
- Verkman AS, Song Y, Thiagarajah JR. Role of airway surface liquid and submucosal glands in cystic fibrosis lung disease. Am J Physiol Cell Physiol. 2003;284:C2–C15. doi: 10.1152/ajpcell.00417.2002. [DOI] [PubMed] [Google Scholar]
- Webber SE, Lim JC, Widdicombe JG. The effects of calcitonin gene-related peptide on submucosal gland secretion and epithelial albumin transport in the ferret trachea in vitro. Br J Pharmacol. 1991;102:79–84. doi: 10.1111/j.1476-5381.1991.tb12135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdicombe JG. Overview of neural pathways in allergy and asthma. Pulm Pharmacol Ther. 2003;16:23–30. doi: 10.1016/S1094-5539(02)00178-5. [DOI] [PubMed] [Google Scholar]
- Wine JJ, Joo NS. Submucosal glands and airway defense. Proc Am Thorac Soc. 2004;1:47–53. doi: 10.1513/pats.2306015. [DOI] [PubMed] [Google Scholar]
- Yoon SS, Hassett DJ. Chronic Pseudomonas aeruginosa infection in cystic fibrosis airway disease: metabolic changes that unravel novel drug targets. Expert Rev Anti Infect Ther. 2004;2:611–623. doi: 10.1586/14787210.2.4.611. [DOI] [PubMed] [Google Scholar]