Abstract
The retrotrapezoid nucleus (RTN) contains CO2-activated interneurons with properties consistent with central respiratory chemoreceptors. These neurons are glutamatergic and express the transcription factor Phox2b. Here we tested whether RTN neurons receive an input from slowly adapting pulmonary stretch receptors (SARs) in halothane-anaesthetized ventilated rats. In vagotomized rats, RTN neurons were inhibited to a variable extent by stimulating myelinated vagal afferents using the lowest intensity needed to inhibit the phrenic nerve discharge (PND). In rats with intact vagus nerves, RTN neurons were inhibited, also to a variable extent, by increasing positive end-expiratory pressure (PEEP; 2–6 cmH2O). The cells most sensitive to PEEP were inhibited during each lung inflation at rest and were instantly activated by stopping ventilation. Muscimol (GABA-A agonist) injection in or next to the solitary tract at area postrema level desynchronized PND from ventilation, eliminated the lung inflation-synchronous inhibition of RTN neurons and their steady inhibition by PEEP but did not change their CO2 sensitivity. Muscimol injection into the rostral ventral respiratory group eliminated PND but did not change RTN neuron response to either lung inflation, PEEP increases, vagal stimulation or CO2. Generalized glutamate receptor blockade with intracerebroventricular (i.c.v.) kynurenate eliminated PND and the response of RTN neurons to lung inflation but did not change their CO2 sensitivity. PEEP-sensitive RTN neurons expressed Phox2b. In conclusion, RTN chemoreceptors receive an inhibitory input from myelinated lung stretch receptors, presumably SARs. The lung input to RTN may be di-synaptic with inhibitory pump cells as sole interneurons.
The rat retrotrapezoid nucleus (RTN) is a cluster of CO2-sensitive glutamatergic neurons located at the ventral surface of the medulla oblongata within a region involved in respiratory chemoreception (Loeschcke, 1982; Eldridge et al. 1985; Akilesh et al. 1997; Ho et al. 2001; Nattie, 2001a; Okada et al. 2002; Feldman et al. 2003; Mulkey et al. 2004; Putnam et al. 2004; Ritucci et al. 2005; Stornetta et al. 2006). RTN neurons selectively innervate the pontomedullary regions that contain the respiratory pattern generator (Smith et al. 1989; Cream et al. 2002; Mulkey et al. 2004; Rosin et al. 2006). RTN neurons are activated by acidification in vitro (Mulkey et al. 2004, 2006; Ritucci et al. 2005; Guyenet et al. 2005a,b) and their main source of drive under anaesthesia appears to be their intrinsic response to the pH of the surrounding parenchyma (Mulkey et al. 2004; Guyenet et al. 2005a). RTN neurons can also be activated by peripheral chemoreceptor stimulation and they receive inhibitory inputs from several components of the respiratory pattern generator (Guyenet et al. 2005a; Takakura et al. 2006). The known anatomical and physiological properties of RTN neurons are thus consistent with the notion that these cells function as a chemosensory integrating centre that drives the ponto-medullary respiratory network.
Slowly adapting lung stretch receptors (SARs) influence a variety of respiratory and autonomic outflows and the activation of these receptors often opposes the cardiorespiratory effects produced by chemoreceptor stimulation (Hayashi et al. 1996; Coleridge & Coleridge, 2001; Vatner & Uemura, 2001; Kubin et al. 2006). Given that RTN neurons are excited both by brain PCO2 and by carotid body stimulation, it is reasonable to assume that these neurons could be subject to an inhibitory control by lung stretch afferents. The present study is designed to test this prediction and to define how the inputs from myelinated lung stretch afferents reach the RTN.
Methods
Animals
The experiments were performed on 56 male Sprague-Dawley rats (Taconic; Germantown, NY, USA) weighing 250–350 g. Procedures were in accordance with NIH Animal Care and Use Guidelines and were approved by the University of Virginia's Animal Care and Use Committee.
Surgery and anaesthesia
General anaesthesia was induced with 5% halothane in 100% oxygen. The rats received a tracheostomy and artificial ventilation with 1.4–1.5% halothane in 100% oxygen was maintained throughout surgery. All rats were subjected to the following previously described surgical procedures: femoral artery cannulation for arterial pressure (AP) measurement, femoral vein cannulation for administration of fluids and drugs, removal of the occipital plate to insert a recording electrode into the medulla oblongata via a dorsal transcerebellar approach, and skin incision over the lower jaw for placement of a bipolar stimulating electrode next to the mandibular branch of the facial nerve (Takakura et al. 2006). The phrenic nerve was accessed by a dorsolateral approach after retraction of the right shoulder blade. Fifteen rats were subjected to a bilateral vagotomy in the neck, and the central end of the left vagus nerve was mounted on bipolar electrodes for electrical stimulation.
Upon completion of surgical procedures, halothane concentration was adjusted (0.9–1%) for each animal to a level sufficient to abolish the corneal reflex and the retraction of distal phalanges to strong nociceptive stimulation of the hindpaw. All rats were ventilated with 100% oxygen throughout the experiment. Rectal temperature (maintained at 37°C) and end-expiratory CO2 were monitored throughout the experiment with a capnometer (Columbus Instruments, OH, USA) that was calibrated against a known CO2–N2 mix. This instrument provides end-expiratory CO2 values that approximate arterial PCO2 (Guyenet et al. 2005a). The adequacy of anaesthesia was monitored during a 20 min stabilization period by testing for absence of hindlimb withdrawal response, lack of AP change and lack of change in the mass discharge of the phrenic nerve (PND) rate or amplitude to firm toe pinch. After these criteria were satisfied, the muscle relaxant pancuronium was administered at the initial dose of 1 mg kg−1i.v. and the adequacy of anaesthesia was thereafter gauged solely by the lack of increase in AP and PND rate or amplitude to firm toe pinch. No adjustment of the halothane concentration was needed.
In vivo recordings of physiological variables and neuronal activity
AP, PND and tracheal CO2 were recorded as previously described (Mulkey et al. 2004; Takakura et al. 2006; Stornetta et al. 2006). Lung inflation was monitored by measuring tracheal pressure through a side port of the tracheal cannula. Single-unit recording and juxtacellular labelling of RTN neurons with biotinamide were done as previously described (Mulkey et al. 2004; Takakura et al. 2006; Stornetta et al. 2006). RTN units were encountered at a depth between 150 and 300 μm below the lower edge of the facial motor nucleus from 100 μm caudal to 300 μm rostral to the caudal boundary of this nucleus (Mulkey et al. 2004; Takakura et al. 2006; Stornetta et al. 2006). Prior single neuron labelling experiments have indicated that this region lies between coronal planes Bregma −11.6 mm and Bregma −11.2 mm of the Paxinos and Watson atlas (Paxinos & Watson, 1998; Mulkey et al. 2004; Guyenet et al. 2005a; Stornetta et al. 2006). The defining property of RTN neurons is a strong activation by hypercapnia (discharge threshold at 4–4.5% CO2 and firing rate of 6–14 Hz at 10% CO2). Their high sensitivity to hypercapnia and insensitivity to changes in blood pressure distinguish these neurons from the blood pressure-regulating presympathetic neurons that are the only other active neurons detected within this limited region of the brain under our experimental conditions (Mulkey et al. 2004). Before searching for RTN neurons, ventilation was adjusted to lower end-expiratory CO2 to 4% at steady-state (60–80 cycles s−1; tidal volume 1–1.2 ml (100 g)−1). Variable amounts of pure CO2 were then added to the breathing mixture to adjust end-expiratory CO2 to the desired level without changing ventilation parameters. Most recordings were made on the left side of the brain.
All analog data (end-expiratory CO2, PND, unit activity, AP) were stored on a microcomputer via a micro-1401 digitizer from Cambridge Electronics Design (CED, Cambridge, UK) and were processed off-line using version 5 of the Spike 2 software (CED). Processing included action potential discrimination and binning, neuronal discharge rate measurement, and PND ‘integration’ (iPND) consisting of rectification and smoothing (τ, 0.015 s). Neural minute × volume (mvPND, a measure of the total phrenic nerve discharge per unit of time) was determined by averaging iPND over 50 s in vagotomized rats or during 20 respiratory cycles in rats with intact vagus nerves and normalizing the result by assigning a value of 0 to the dependent variable recorded at low levels of end-expiratory CO2 (below PND threshold) and a value of 1 at the highest level of PCO2 investigated (between 9.5 and 10%). The CED software was also used for acquisition of peri-event histograms of neuronal activity and peri-event averages of iPND, tracheal CO2, or tracheal pressure. The peri-event histograms of neuronal single-unit activity were triggered either on iPND or on the tracheal pressure trace. Each histogram represents the summation of at least 100 central respiratory or ventilation cycles (350–800 action potentials per histogram).
The steady-state relationship between RTN neuronal activity and end-expiratory CO2 was obtained by stepping the inspired CO2 level to various values for a minimum of 3 min and up to 5 min. The mean discharge rate of the neuron was measured during the last 30 s of each step at which time end-expiratory CO2 and the discharge of the neuron appeared to have reached equilibrium. End-expiratory CO2 was measured by averaging the maximum values recorded from 10 consecutive breaths at the midpoint of the time interval sampled.
In rats with intact vagus nerves, lung mechanoreceptors were activated by transiently elevating positive end-expiratory pressure (PEEP) (5–20 s) from a resting level of +1 cmH2O to +2, +4 or +6 cmH2O. In vagotomized rats, lung mechanoreceptor stimulation was approximated by stimulating the central end of the left vagus nerve for 5 s at 10 Hz with pulses of 0.1 ms. The short pulse duration was designed to favour activation of myelinated fibres (Hayashi et al. 1996). Current intensity was kept between threshold and twice threshold, the threshold being defined as the intensity necessary to produce approximately 30–60% reduction in PND amplitude but no central apnoea.
Pharmacological treatments
The GABA-mimetic drug muscimol (Sigma Chemicals Co., St Louis, MO, USA; 1.75 mm in sterile saline pH 7.4) was injected intraparenchymally while a single RTN neuron was being recorded. The muscimol solution contained a 5% dilution of fluorescent latex microbeads (Lumafluor, New City, NY, USA) for later histological identification of the injection sites (Moreira et al. 2006). Muscimol was pressure injected bilaterally through single-barrel glass pipettes with a 20 μm tip diameter. The injection volume (30 nl in 5 s; 50 pmol per side) was determined by following the downward movement of the meniscus within the cylindrical portion of the pipette using a microscope fitted with a calibrated eye-piece (estimated accuracy: ±5 nl). The glass pipettes used for drug injection allowed recording of multiunit neuronal activity. This property was used to direct the electrode tip to the rostral ventral respiratory group (rVRG) by locating inspiratory-related field potentials. This region was found 500 μm rostral to the calamus scriptorius, 1.8 mm lateral to midline and 1.9–2.2 mm below the dorsal surface of the brainstem using electrodes angled 20 deg forward. After directing the tip of the muscimol pipette to the rVRG, we searched for a suitable RTN unit. The first muscimol injection was made into the predetermined site while the RTN unit was being recorded. The second injection was placed 1–2 min later in the symmetric brain location based on the stereotaxic coordinates of the first one, also while the cell was recorded. The injections rarely lead to the loss of the recorded RTN unit and all reported units were kept for at least 20 min after drug injection. Due to the long duration of action of muscimol, a single RTN neuron was subjected to this experimental protocol in any given rat. The protocol used to test the effect of muscimol injection into the nucleus of the solitary tract (NTS) was the same except that the injections were made 0.5 mm below the surface of the brain using surface landmarks to identify the insertion point of the pipette (500 μm rostral to the calamus scriptorius, 0.8 mm lateral to midline).
Kynurenate, a broad-spectrum antagonist of glutamate ionotropic receptors (KYN; Sigma Chemicals Co.), was administered into the fourth ventricle while an RTN unit was being recorded. Unit recording was typically maintained during and for at least 30 min after drug injection. Kynurenate was prepared as a 0.5 m pH 7.3 stock solution and diluted 50% just before use with normal bicarbonate Ringer solution of the following composition (mm): 130 NaCl, 3 KCl, 2 MgCl2, 2 CaCl2, 1.25 NaH2PO4, 26 NaHCO3 and 10 glucose. The solution was slowly injected (1–2 min; 60 μl; 15 μmol) into the cerebrospinal fluid via a needle inserted through the atlanto-occipital membrane. The needle shaft was sealed to the membrane with cyanoacrylate prior to performing the injection. KYN injected in this manner initially bathes the lower and upper surfaces of the brainstem and upper spinal cord. How deep within the brain the drug eventually penetrates is unknown; however, within minutes, it eliminates PND, the respiratory discharge of neurons located deep within the ventrolateral medulla (Mulkey et al. 2004) and all known sensory inputs to the blood pressure-regulating neurons of the ventrolateral medulla (Sun et al. 1988). Note that if KYN had equilibrated within 2 cm3 of brain tissue the mean concentration of the drug would be 7.5 mm which is five times more than necessary to block all forms of ionotropic glutamate receptors in vitro.
Histology
At the end of the experiment the rat was deeply anaesthetized with halothane and perfused transcardially with heparinised phosphate-buffered saline (pH 7.4) followed by paraformaldehyde (4% in 0.1 m phosphate buffer, pH 7.4). All histochemical procedures were done using 30-μm-thick free-floating sections according to previously described protocols (Takakura et al. 2006; Stornetta et al. 2006). Cells labelled with biotinamide were identified by incubating the sections with streptavidin conjuguated with Alexa-488. The transcription factor Phox2b was detected as previously described using a rabbit polyclonal antibody (1: 800 for 48–72 h followed by a Cy3-tagged donkey anti-rabbit IgG at 1: 200; Jackson) (Stornetta et al. 2006). The antibody (a gift from J.-F. Brunet, Ecole Normale Supèrieure, Paris, France) was raised against the 14 amino acid C-terminal sequence of the Phox2b protein and its specificity has been previously established for both mouse and rat (Pattyn et al. 1997; Stornetta et al. 2006).
Cell mapping and imaging
The computer-assisted mapping technique designed to map the location of drug injection sites and biotinamide-labelled neurons has been described in detail previously (Stornetta & Guyenet, 1999). Section alignment between brains was done relative to a reference section. To align sections around the RTN level, the most caudal section containing an identifiable cluster of facial motor neurons was identified in each brain and assigned the level 11.6 mm caudal to Bregma (Bregma −11.6 mm) according to the atlas of Paxinos & Watson (1998). Levels rostral or caudal to this reference section were determined by adding a distance corresponding to the interval between sections multiplied by the number of intervening sections. The same method was also used to identify the Bregma level of the muscimol injections targeted to the rVRG. When the object of the experiment was to locate injection sites at the ventrolateral NTS level, the reference section used to align all others was the one closest to the mid-area postrema level (Bregma −13.8 mm).
The Neurolucida files (MicroBrightField, Williston, VT, USA) were exported to the Canvas 9 software drawing program (ACD Systems of America, Miami, FL, USA) for final modifications. Photographs were taken with a 12-bit colour CCD camera (CoolSnap, Roper Scientific, Tuscon, AZ, USA; resolution 1392 × 1042 pixels). IPLab software (Scanalytics, Rockville, MD, USA) was used for merging of colour channels in photographs of dual labelling experiments.
The neuroanatomical nomenclature is after Paxinos & Watson (1998).
Statistics
Statistical analysis was done with Sigma Stat version 3.0 (Jandel Corporation, Point Richmond, CA, USA). Data are reported as means ± standard error of the mean (s.e.m.). Paired t test, one- and two-way repeated measure parametric ANOVA followed by the Tukey multiple comparisons test were used as appropriate. Significance was set at P < 0.05.
Results
Effect of lung inflation or low-intensity stimulation of a vagus nerve on RTN neurons
Two standard protocols were used to determine whether RTN neurons receive input from lung stretch receptors (Hayashi et al. 1996). First, we tested the effect produced by increasing end-expiratory pressure on RTN neurons in rats with intact vagus nerves. Second, we examined the effect of low-intensity vagus nerve stimulation on RTN neurons in vagotomized rats. In all cases, RTN was identified by its location 150–300 μm below the caudal end of the facial motor nucleus. This region was identified by recording antidromic field potentials within this nucleus (Mulkey et al. 2004; Guyenet et al. 2005a). The only other criteria for inclusion in this study was that the cells be active under hypercapnia (6–14 Hz at 10% CO2) and silent in hypocapnia with a discharge threshold between 4 and 5% CO2 (Fig. 1A). The CO2 threshold of these neurons was always lower than that of the PND (Fig. 1A).
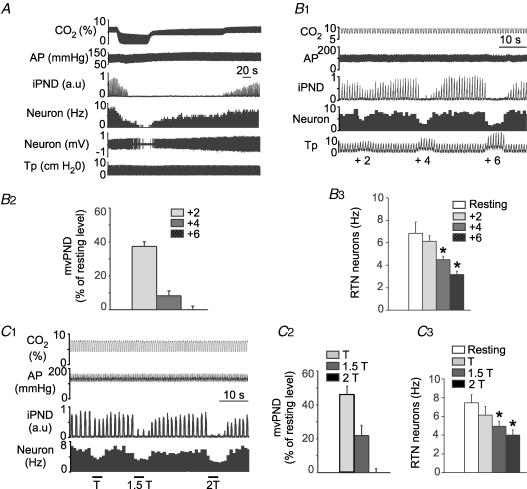
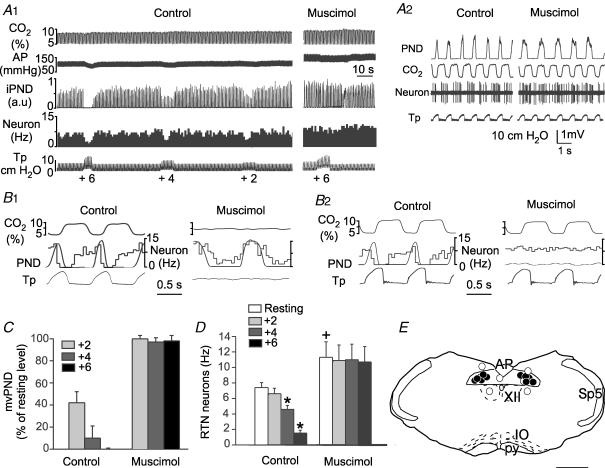
Figure 1. Inhibition of RTN CO2-sensitive neurons by lung inflation or low-intensity vagus nerve stimulation.
A, example of one RTN neuron exposed to various levels of end-expiratory CO2 in a rat with intact vagus nerves. B1, effect of lung inflation (+2, +4 and +6 cmH2O positive end-expiratory pressure, PEEP) on the neuron shown in A. B2, effect of lung inflation (+2, +4 and +6 cmH2O) on neural minute × volume (mvPND) in a group of 28 rats with intact vagus nerves. B3, average effect of lung inflation (+2, +4 and +6 cmH2O) on the discharge rate of 36 RTN CO2-activated neurons sampled in these rats (*P < 0.05 relative to resting; RM ANOVA). C1, example of one RTN neuron recorded on the side ipsilateral to the stimulated vagal nerve in a vagotomized rat. This RTN neuron was detectably inhibited at the threshold (T) for PND inhibition. C2, group data showing the effect of vagus nerve stimulation (T, 1.5T and 2T) on mvPND (neural minute × volume) in 15 vagotomized rats. C3, group data showing the effect of vagus nerve stimulation (T, 1.5T and 2T) on the discharge rate of 18 RTN neurons in 15 vagotomized rats (one-way RM ANOVA: *P < 0.05 compared with resting). Abbreviations: AP, arterial pressure; iPND, integrated phrenic nerve discharge; Tp, tracheal pressure.
Raising positive end-expiratory pressure (PEEP) from a resting level of +1 to +2, +4 and +6 cmH2O in rats with intact vagus nerves produced graded degrees of inhibition of PND and inhibited RTN neurons to a variable extent (0–100%) depending on the cell (Fig. 1B1 and B2). Figure 1B1 illustrates the case of an RTN neuron that had an above average response to a rise in PEEP. At the population level (36 neurons from 28 rats), RTN neurons were significantly inhibited by increasing PEEP and the magnitude of the inhibition increased with the amount of positive pressure applied (Fig. 1B3). The PEEP threshold for reduction of RTN unit activity and PND inhibition appeared similar but the percentage reduction in RTN unit activity was smaller on average than the reduction in PND (Fig. 1B2 and B3).
The effect produced by left vagus nerve stimulation was examined in 18 RTN cells from 15 bilaterally vagotomized rats. RTN neurons were recorded ipsilateral to the stimulated vagus nerve and end-expiratory PCO2 was set at a level that produced a robust activation of both PND and RTN neurons (7.5–8.5% CO2). Vagus nerve stimulation was normalized between animals using its effect on PND as reference. The response curve for PND inhibition was steep with only a twofold difference between the intensity that slowed PND and caused about 50% inhibition of its amplitude, defined here as threshold intensity, and the intensity that caused central apnoea (Fig. 1C1 and C2). This narrow range of intensity (typically 0.1–0.2 mA) was selectively used to examine the impact of vagus nerve stimulation on the discharge of RTN neurons. RTN neurons were inhibited to a variable degree by vagus nerve stimulation with detectable effects at the threshold for PND inhibition for the most sensitive neurons (Fig. 1C1). At the population level, inhibition of RTN neurons was significant and was a graded function of the stimulus intensity (18 cells; Fig. 1C3).
For comparative purposes, we examined the effect of lung inflation (+2, +4 and +6 cmH2O) on the barosensitive blood pressure-regulating neurons of the rostroventrolateral medulla (RVLM) (Guyenet, 2006) (10 neurons from 6 rats with intact vagus nerves) and on a variety of respiratory-related cells located in the rVRG (24 cells in 7 rats with intact vagus nerves). The BP-regulating neurons (N = 10) were unaffected by lung inflation up to 6 cmH2O but every respiratory-related neuron recorded within the rVRG region was profoundly impacted by lung inflation. Inspiratory-related neurons (4 inspiratory-augmenting, 3 inspiratory, 3 early inspiratory and 2 late-inspiratory) and expiratory-related neurons (3 expiratory-augmenting and 2 expiratory throughout) were inhibited to the same degree as the PND amplitude (data not shown). Both classes of cells and the PND were always silenced by 6 cmH2O PEEP (results not illustrated). As expected (Hayashi et al. 1996) post-inspiratory neurons (N = 7) were uniformly activated by lung inflation. Their activity remained phasic at low levels of PEEP and usually became tonic at 6 cmH2O (results not illustrated).
Sensitivity of RTN neurons to increased PEEP correlates with magnitude of phasic inhibition during each lung inflation at rest
Peri-event histograms of the neuronal discharge at rest (+ 1 cmH2O PEEP) were obtained in the 36 RTN neurons recorded in rats with intact vagus nerves to seek whether any particular feature of their respiratory modulation could predict the effect produced by increasing PEEP. The RTN cells that were most strongly inhibited by increasing PEEP had an especially marked reduction in discharge probability during each inflation of the lungs as monitored by the rise in tracheal pressure. In the most extreme cases the cells had a phasic (ON–OFF) discharge at rest (Fig. 2A1–3). Such cells were also strikingly and immediately activated by interrupting the ventilator indicating that their decreased discharge probability during each lung inflation was due to an inhibition of their activity (Fig. 2A1). Immediately after the ventilator was stopped, the discharge of these neurons became very regular (Fig. 2A1) and central respiratory generator entrainment was weak at best as is typically the case of most RTN neurons recorded in vagotomized rats (Takakura et al. 2006). This observation suggests that, although the rise in tracheal pressure coincides with the post-inspiratory phase of the central respiratory cycle in our preparation, the periodic inhibition of RTN neurons observed during central post-inspiration is primarily due to a lung afferent input rather than inhibition from the central pattern generator. The RTN neurons that were not detectably inhibited during the rise in tracheal pressure responded minimally or not at all to increases in end-expiratory pressure (Fig. 2B). Note that the neuron illustrated in Fig. 2B1 was inhibited only during the early inspiratory phase of the central respiratory cycle, and not during lung inflation (Fig. 2B2). A robust correlation was found between the magnitude of the phasic inhibition observed during each lung inflation at rest (1 cmH2O PEEP) and the percentage inhibition of the mean firing rate caused by raising PEEP to 6 cmH2O (r2, 0.76; F (1, 35) = 105.4; P < 0.001; 36 neurons; Fig. 2C. This correlation indicates that the RTN neurons that are most strongly inhibited by increasing PEEP also receive the strongest phasic inhibitory input from lung mechanoreceptors at rest. The variability in the strength of the lung input was cell-dependent, not preparation-dependent, because RTN neurons with and without marked tracheal pressure synchronous inhibition could be found in the same animals.
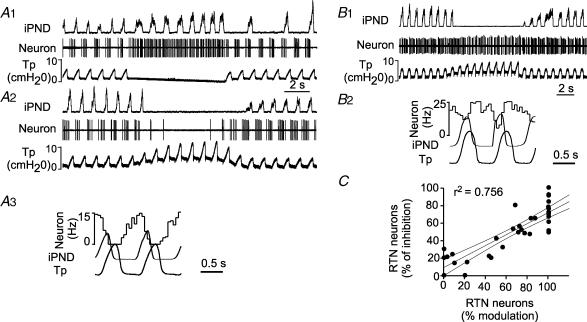
Figure 2. Respiratory pattern of RTN neurons inhibited by lung inflation.
A, example of an RTN neuron that was strongly inhibited by increasing PEEP. A1, the neuron was instantly activated by interrupting the ventilator and became tonically active. A2, the neuron was 100% inhibited by lung inflation (+6 cmH2O PEEP). A3, peri-event histogram triggered on the tracheal pressure shows that the discharge probability of the neuron is zero at peak lung inflation. B, example of a different RTN neuron that was almost unaffected by increasing end-expiratory pressure. B1, a 6 cm increase in end-expiratory pressure produces little effect on this cell. B2, peri-event histogram triggered on the tracheal pressure showing absence of periodic slowing of the cell during lung inflation. C, correlation between the magnitude of the inhibition observed during each inflation cycle at rest (% modulation; abscissa) and the percentage reduction in mean firing rate caused by raising PEEP to 6 cmH2O (ordinate; 36 neurons). The first parameter (% modulation) was derived from peri-event-triggered histograms such as shown in A3. The value represents 1 minus the ratio between the discharge rate of the neuron at the peak of the tracheal pressure and the maximum discharge rate wherever it occurs during the respiratory cycle. For example the ratio was 1 (100%) in histogram A3 and 0 in histogram B2. The histograms were obtained at normal tidal volume and in the presence of 1 cm end-expiratory pressure. The 95% confidence intervals are also shown on the graph.
Inhibition of RTN neurons by lung inflation or vagus nerve stimulation persists after muscimol injection into rVRG
These experiments were designed to test whether the inhibition of RTN neurons by phasic lung inflation, PEEP increase or low-intensity vagal stimulation requires that the central respiratory pattern generator be active. To that effect, we determined whether bilateral injection of muscimol into rVRG changes the inhibition of RTN neurons caused by lung inflation (rats with intact vagus nerves) or vagus nerve stimulation (vagotomized rats). The experiment relies on the likely assumption that such injections silence the central respiratory pattern generator (CPG). In all cases the experiment consisted of testing the property of the same RTN neuron before and after bilateral injection of muscimol. All neuronal recordings were kept for at least 30 min after the injections.
The effect of lung inflation was tested in eight rats with intact vagus nerves (subset of the rats described in the first paragraph of the Results). End-expiratory CO2 was preset at a level that produced a robust activation of both PND and RTN neurons (7.5–9.5% CO2) and left at that level for the rest of the experiment. The neurons were initially selected for their robust response to lung inflation (> 50% inhibition at 6 cmH2O; Fig. 3A, top) and were also markedly inhibited during each lung inflation at rest (Fig. 3A, bottom). As expected, bilateral injection of muscimol into rVRG eliminated the PND and elevated blood pressure (Takakura et al. 2006). However, muscimol did not change the resting discharge of the RTN neurons significantly (Fig. 3A and D) nor their response to raising end-expiratory pressure (Fig. 3A and D). The phasic inhibition of these RTN neurons during each lung inflation also persisted after muscimol injection (Fig. 3A and B). Prior to muscimol injection, lung inflation inhibited PND as usual in these eight rats (Fig. 3C). The location of the muscimol injection sites are shown in coronal projection in Fig. 3E and their rostro-caudal scatter is shown in Fig. 3F. The fluorescent beads co-injected with the muscimol extended for 250–300 μm on either side of the injection centre. The area of diffusion of these beads may be an underestimate of the region where neurons are silenced by muscimol. Although the exact region of influence of the drug could not be accurately determined, it is unlikely to have extended rostrally as far as the Bötzinger section of the ventral respiratory column because injection of muscimol at this level would have decreased blood pressure, not increased it. The centre of the injections was located where the bulbospinal inspiratory-augmenting neurons that define the rVRG are most concentrated in rats of the same size (Stornetta et al. 2003).
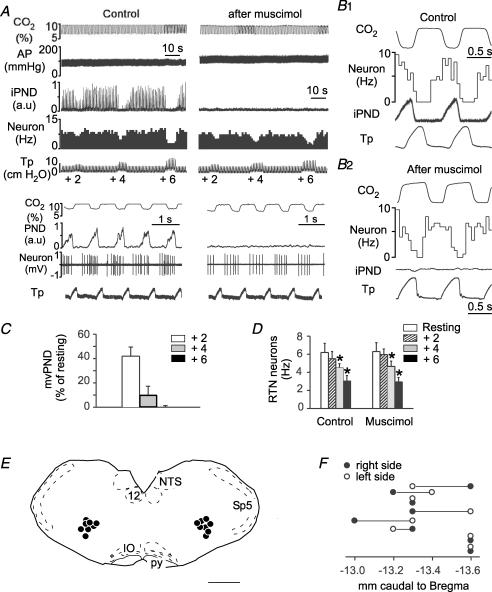
Figure 3. Bilateral injection of muscimol into the rostral ventral respiratory group (rVRG) does not change the effect of lung inflation on RTN neurons.
A, this single RTN neuron was inhibited in a graded fashion by raising end-expiratory pressure to +2, +4 and +6 cmH2O. Bilateral injection of muscimol into the rVRG eliminated PND, raised arterial pressure (AP) but did not change markedly the inhibition of the RTN by lung inflation. Lower traces with expanded time scales indicate that muscimol did not change the phasic inhibition of the cell during lung inflation. B1, peri-event histogram of the discharge of the neuron shown in A. PND served as trigger. B2, peri-event histogram of the same RTN neuron after muscimol injection. In this case end-expiratory CO2 served as trigger. Note that the cell is still inhibited during the rise in tracheal pressure which corresponds to lung inflation. C, group data showing the effect of lung inflation (+2, +4 and +6 cmH2O PEEP) on mvPND before muscimol. D, group data showing the effect of lung inflation (+2, +4 and +6 cmH2O PEEP; 1 cm at rest) on the discharge rate of 8 RTN neurons before and after muscimol injection into the rVRG (2-way RM ANOVA; *P < 0.05 relative to resting level before or after muscimol; muscimol had no effect on the activity of the cells at rest or on the effect of PEEP). E, computer-assisted plots of the centre of the muscimol injection sites revealed by the presence of fluorescent microbeads included in the injectate. All sites projected on a single section (Bregma −13.3 mm). This level corresponds to the rVRG. Scale bar, 1 mm. F, rostro-caudal scatter of the muscimol injection into the ventrolateral medulla.
The experiments on vagotomized rats were also carried out in eight rats (subset of the animals described in the first paragraph of the Results). We selected in each case an RTN neuron that was robustly inhibited by vagus nerve stimulation. As usual, muscimol eliminated PND and raised BP (Fig. 4A). Consistent with the results obtained in rats with intact vagus nerves, the basal discharge of RTN neurons was unaffected by muscimol (Fig. 4A and C) and the inhibitory effect of vagus nerve stimulation on RTN neurons was also unchanged (Fig. 4A and C). The location of the eight muscimol injection sites is shown in a representative coronal section in Fig. 4D and their rostro-caudal scatter is shown in Fig. 4E. The injection sites were in the same general location as in the experiments performed in rats with intact vagus nerves.
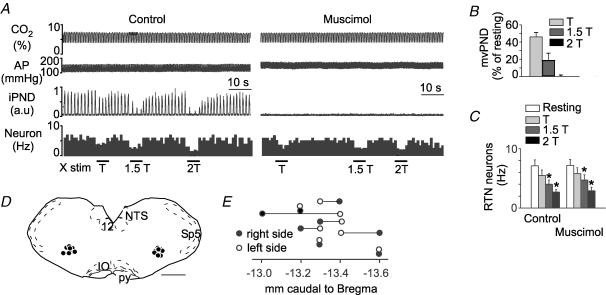
Figure 4. Bilateral injection of muscimol into the rostral ventral respiratory group (rVRG) does not change the inhibition of chemosensitive RTN neurons by low-intensity vagus nerve stimulation.
A, a single RTN neuron was exposed to various levels of vagus nerve stimulation (T, threshold). Muscimol was then injected into the ventrolateral medulla on both sides causing a disappearance of PND. Muscimol had no effect on the resting discharge of the neuron and did not change its inhibition by vagus nerve stimulation. B, group data showing the effect of vagus stimulation on mvPND before muscimol (n = 8 rats). C, group data showing the effect of vagus stimulation on the discharge rate of the RTN neurons before and after muscimol injection into rVRG (n = 8 rats; 2-way RM ANOVA; *P < 0.05 relative to resting level before or after muscimol; muscimol had no effect on the activity of the cells at rest or on the effect of vagus nerve stimulation). D, computer-assisted plots of the centre of the muscimol injection sites revealed by the presence of fluorescent microbeads included in the injectate (coronal projection on plane Bregma −13.3 mm of the Paxinos atlas (Paxinos & Watson, 1998)). Scale bar, 1 mm. E, rostro-caudal scatter of muscimol injection into the ventrolateral medulla.
These results demonstrate that the resting activity of the RTN neurons with the most pronounced lung mechanoreceptor input is unaffected by silencing neurons within the rVRG region. The data also suggest that RTN neuron inhibition by lung mechanoreceptors does not require an active respiratory pattern generator and therefore could be due to a direct inhibitory input from the NTS.
Bilateral injection of muscimol into the region of the NTS that contains inhibitory pump cells eliminates the effect of lung inflation on RTN neurons
Pump cells are the second-order neurons that relay inputs from SARs to the ventral respiratory column and the pons. These cells are located close to the tractus solitarius at area postrema level whereas the cells that respond to rapidly adapting lung stretch receptors are predominantly located in the commissural nucleus (Hayashi et al. 1996; Ezure et al. 2002; Ezure & Tanaka, 2004; Kubin et al. 2006). To further support the notion that SARs are responsible for the inhibition of RTN neurons by lung inflation, muscimol injections were targeted bilaterally to the region of the solitary tract nucleus (NTS) that contains the pump cells. The expectation was that, only if correctly placed, these injections should have the following three effects: to dissociate the central respiratory generator from the ventilation cycle, to eliminate the pump-related rhythm of RTN neurons and to eliminate the effect of increasing PEEP on PND and on the activity of RTN neurons. Results conforming to these expectations were obtained in 6 out of 9 rats (1 neuron per rat). The three unsuccesful cases were due to misplaced injections.
In each case, we selected an RTN unit that was markedly inhibited by lung inflation (Fig. 5A1) and, accordingly, the units were also markedly inhibited during each inflation of the lungs (Fig. 5A2 and B2). In the six successful cases, the PND became dissociated from ventilation after muscimol injection into the NTS (Fig. 5A2, B1 and B2). Respiratory frequency decreased (from 1.17 ± 0.02 to 0.79 ± 0.1 Hz; P < 0.01) and PND duration increased (from 0.34 ± 0.04 to 0.63 ± 0.1 s; P < 0.01) but PND amplitude remained approximately the same (98 ± 4% of control; NS). In the same six cases, muscimol eliminated the response of PND and RTN neurons to lung inflation (Fig. 5A1; group data in Fig. 5C and D). Muscimol also raised the resting discharge rate of the sampled neurons by 46 ± 3% (P < 0.05; Fig. 5A1 and D). This rise was presumably due to the loss of phasic inhibition from the lung afferents since after muscimol, RTN neurons were no longer periodically inhibited by lung inflation at rest (Fig. 5A2 and B2). Most RTN neurons (5/6) still retained some form of central respiratory modulation after muscimol injection in the NTS (Fig. 5A2 and B1). In these six successful cases, the centres of the muscimol injections were symmetrically placed in close proximity of the tractus solitarius (Fig. 5E, •). In the remaining three cases, one or both injections were misplaced by up to 500 μm (Fig. 5E, ○). In each of these three cases, muscimol failed to dissociate PND from the ventilation cycle and the effect of lung inflation on PND and the RTN neurons persisted.
Figure 5. Bilateral injection of muscimol close to the tractus solitarius eliminates the effect of lung inflation on RTN neurons.
A1, this RTN neuron was exposed to various levels of lung inflation (+2, +4 and +6 cmH2O). Muscimol injection next to the tractus solitarius on both sides raised the arterial pressure (AP) and eliminated the inhibition produced by lung inflation on PND and RTN neurons. A2, the RTN neuron had a clear pump rhythm before muscimol injection into the NTS. Muscimol injection into NTS dissociated the central respiratory generator from the ventilation cycle and eliminated the pump-related rhythm of the RTN neuron. B1, PND-triggered activity histogram of the RTN neuron shown in A, before and after muscimol was injected in the NTS. B2, tracheal pressure-triggered activity histogram of the same RTN neuron before and after muscimol injection. C, group data showing the effect of lung inflation (+2, +4 and +6 cmH2O PEEP) on mvPND before and after muscimol into the NTS. Lung inflation had no effect after muscimol (RM ANOVA). D, group data showing the effect of lung inflation (+2, +4 and +6 cmH2O) on the discharge rate of RTN neurons before and after muscimol into the NTS. The effect of lung inflation was significant before muscimol but not after (2-way RM ANOVA; *P < 0.05 relative to resting; +P < 0.05 relative to resting discharge before muscimol). E, computer-assisted plots of the centre of the muscimol injection sites revealed by the presence of fluorescent microbeads included in the injectate. All succesful injections were within or in close proximity of the solitary tract. Scale bar, 1 mm.
The disappearance of the lung inflation-related oscillations of RTN neuron discharges after muscimol injection into the NTS demonstrates that these oscillations could not have been caused by a mechanical artifact associated with respiratory movements. The result also suggests that the lung inflation-related changes in RTN neuronal activity are unlikely to be caused by ventilation-synchronous proprioceptive inputs from the thorax and neck region since this type of input would presumably not relay through the NTS. The most logical interpretation of the data is that lung inflation inhibits RTN neurons by activating NTS pump cells.
Kynurenate i.c.v. blocks the effect of lung inflation on RTN neurons but spares the effect of CO2
The last two series of experiments were designed to determine whether the CO2-sensitive neurons of RTN with prominent input from lung stretch receptors possess two additional characteristics previously attributed to RTN chemoreceptors at large, namely whether their sensitivity to CO2 resists glutamate ionotropic receptor blockade within the pontomedullary region (Mulkey et al. 2004) and whether they express the transcription factor Phox2b (Stornetta et al. 2006).
In four rats, the broad-spectrum glutamate receptor antagonist kynurenate (KYN) was administered into the fourth ventricle while recording the response of a single RTN neuron to hypercapnia and lung inflation. The four RTN neurons selected for study were phasically inhibited during lung inflation at rest (3 totally inhibited, Fig. 6B1, and one partially inhibited) and were markedly inhibited by increasing PEEP to 6 cmH2O (Fig. 6A and B1). Each neuron responded in the manner illustrated in Fig. 6. As shown before (Mulkey et al. 2004), KYN eliminated PND within minutes after injection, regardless of the CO2 level (Fig. 6A). KYN slightly increased the RTN neuron's resting discharge rate, blocked its inhibitory response to increasing PEEP (Fig. 6A) and eliminated the periodic inhibition of the cell coinciding with each inflation of the lungs (Fig. 6A and B). The steady-state response of the RTN neuron to graded levels of CO2 was changed in the manner shown in Fig. 6C. The CO2 threshold was not noticeably affected but the cell discharged at a higher rate at all levels of CO2. The CO2 threshold was similarly unaffected in the other three cases. At high CO2 the discharge rate was elevated above control only in two of the three other cells. Figure 6D shows the discharge rate of the four cells at two representative levels of CO2. The increase in discharge rate at high CO2 was not statistically significant.
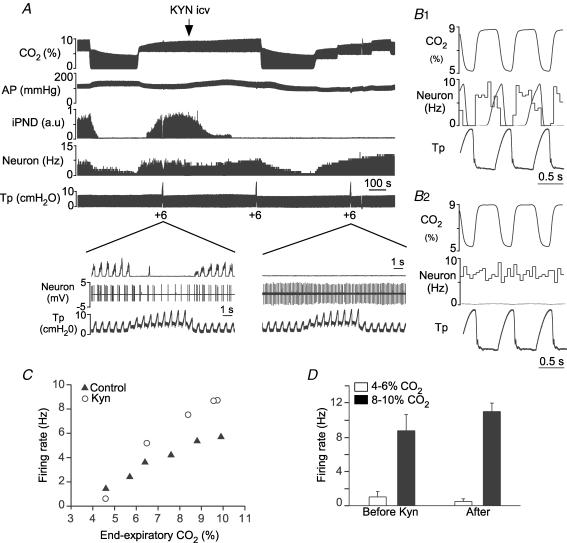
Figure 6. Kynurenate blocks the response to lung inflation but not the CO2 sensitivity of RTN neurons.
A, example of one RTN neuron recorded before and after i.c.v. injection of 15 μmol of kynurenate (KYN). KYN eliminated PND, reduced BP, eliminated the neuron's response to lung inflation but had minimal effect on the cell's response to CO2. The expanded scale insets show the discharge pattern of the neuron before and after kynurenate. B1, peri-event histogram triggered on tracheal pressure demonstrating that, before kynurenate, the cell stops firing during each inflation of the lungs at rest (1 cmH2O PEEP). B2, similar histogram done after kynurenate showing that the cell is no longer inhibited by lung inflation. C, steady-state relationship between the activity of the cell shown in A and B and end-expiratory CO2 before and after KYN. D, resting discharge rate of 4 RTN neurons recorded at two levels of end-expiratory CO2 before and after i.c.v. kynurenate.
RTN neurons sensitive to lung inflation express Phox2b
In vagotomized rats, generic CO2-activated neurons of RTN express the transcription factor Phox2b (Stornetta et al. 2006). The final experiments were designed to test whether the RTN neurons that display a pronounced pump modulation in rats with intact vagus nerves also express this marker. Five such cells were labelled juxtacellularly with biotinamide and each labelled neuron had a Phox2b-immunoreactive (ir) nucleus (Fig. 7A–D). The labelled neurons (Fig. 7E) were generally found within the dorsal half of the cluster of Phox2b-immunoreactive nuclei. These results suggest that the RTN neurons with pronounced lung stretch receptor input belong to the same general group of CO2-sensitive neurons as the cells that we previously characterized in vagotomized rats (Stornetta et al. 2006).
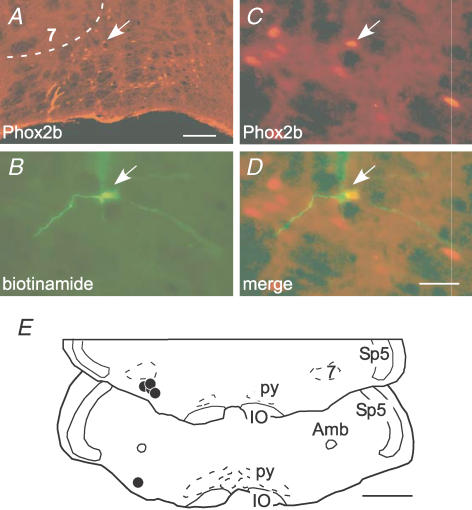
Figure 7. RTN neurons sensitive to lung inflation express Phox2b.
A, cluster of Phox2b-ir nuclei that defines the RTN. The facial motor nucleus, 7, lacks detectable Phox2b immunoreactivity in adult rats. The ventral medullary surface is visible at the bottom. The y-shaped object at the lower left of the RTN is a blood vessel artifact. Scale bar, 100 μm. B, a single recorded cell labelled with biotinamide (arrow). C, Phox2b-ir nucleus of the labelled neuron (the arrow in A points to the same cell). D, merged image between B and C. Scale bar, 30 μm, applies to B–D. E, location of the five cells labelled juxtacellularly with biotinamide. Each cell had a Phox2b-ir nucleus. Scale bar, 1 mm. Other abbreviations: Amb, ambiguus nucleus; IO, inferior olive; py, pyramidal tract; Sp5, spinal trigeminal tract.
Discussion
The present study demonstrates that the CO2-activated neurons of RTN receive inhibitory inputs from some form of lung stretch receptors, probably the myelinated slowly adapting receptors (SARs). Lung stretch afferents may inhibit RTN neurons via a direct projection from inhibitory pump cells located next to the tractus solitarius at intermediate levels of the NTS (Fig. 8).
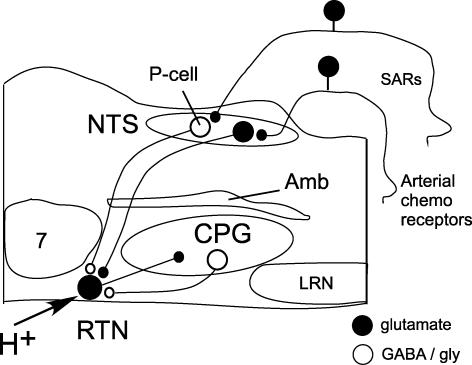
Figure 8. Presumed role of the RTN in chemosensory integration.
RTN neurons are activated by CO2 via their intrinsic pH sensitivity and via inputs from the carotid bodies (Mulkey et al. 2004; Takakura et al. 2006). RTN neurons target various components of the CPG and are presumed to play a key role in breathing automaticity during anaesthesia and sleep. RTN in turn receives a feedback inhibition from the CPG represented here by a single inhibitory neuron located in the ventral respiratory column but known to consist of multiple types of cells (Guyenet et al. 2005a). Here, we propose that RTN neurons are inhibited by lung inflation via SAR activation and that the effect of SARs is mediated by GABAergic pump cells located close to the tractus solitarius at area postrema level. Abbreviations: Amb, nucleus ambiguus; CPG, respiratory pattern generator; LRN, lateral reticular nucleus; NTS, nucleus of the solitary tract; P-cell (pump cell), RTN, retrotrapezoid nucleus; SAR, slowly adapting pulmonary stretch receptors; 7, facial motor nucleus.
The inhibitory input from SARs to RTN is interpreted as a feedback control of central chemoreceptors. We propose that this inhibitory input and the previously demonstrated feedback from the CPG serve to reduce the contribution of central chemoreceptors to breathing when the respiratory pattern generator is being sufficiently activated by unrelated CNS inputs. These feedbacks may explain why the central chemical drive is more critical to breathing under anaesthesia and during sleep than in the waking state and during exercise.
RTN neurons receive an inhibitory input from presumably slowly adapting myelinated lung stretch receptors, SARs
This interpretation is supported by the following evidence. The RTN neurons that were inhibited by increasing PEEP also had a reduced discharge probability at rest during each inflation of the lungs and the two characteristics were highly correlated. Second, the effect of lung inflation was eliminated by inhibiting the NTS region that harbours the pump cells, i.e. the second-order neurons that are phasically activated by slowly adapting lung stretch receptors during each inflation of the lungs (Otake et al. 2001; Ezure et al. 2002; Ezure & Tanaka, 2004; Kubin et al. 2006). Third, the RTN neurons that were strongly inhibited by lung inflation were also activated by interrupting ventilation. The instantaneous nature of this activation excludes the possibility that it might have been due to hypercarbia or hypoxia. Fourth, the discharge of the RTN neurons became markedly more regular after the interruption of ventilation indicating that their reduced discharge probability during lung inflation was not due to an inhibitory input from the central respiratory pattern generator. Fifth, the animals had a tracheostomy, therefore ventilation is unlikely to have resulted in periodic activation of mechanoreceptors located elsewhere along the respiratory system than in the lungs. Finally, in vagotomized rats, many RTN neurons were inhibited by stimulating the vagus nerve using short-duration pulses and intensities commensurate with those sufficient to inhibit PND. The short pulses and low intensity required to inhibit PND and RTN neurons suggest that myelinated axons must have been selectively activated. Consistent with this interpretation, our vagal stimulations produced no change in blood pressure. Under anaesthesia, changes in blood pressure are known to require vagal C fibre activation (Sun & Guyenet, 1987).
A contribution of rapidly adapting receptors (RARs) to the response of RTN neurons to vagal nerve stimulation cannot be completely eliminated because these lung receptors are also myelinated (Coleridge & Coleridge, 2001), including in rats (Ho et al. 2001). Also, rat RARs have a phasic discharge that is synchronized with tracheal pressure at normal levels of lung inflation (Ho et al. 2001); therefore their pattern of activity is theoretically appropriate to trigger a lung-inflation synchronous inhibition of RTN neurons. Yet, two observations reduce the plausibility that RARs are major contributors to the inhibition of RTN neurons by lung inflation. The first one is the sustained nature of RTN neuron inhibition caused by increasing PEEP (> 5 s). The second one is that the NTS neurons that receive RAR input are located in the commissural nucleus (Kubin et al. 2006). These neurons would presumably not have been affected by the muscimol injections that we targeted to the more rostral and lateral region of the NTS where the SAR second-order neurons (pump cells) reside.
In conclusion, the data favour the interpretation that SARs, not RARs, are the main form of lung stretch receptor responsible for inhibiting RTN during lung inflation.
Relationship with prior studies
The existence of lung afferent inputs to neurons located in the region of the cat RTN has not been reported previously (Connelly et al. 1990; Nattie et al. 1993; Bodineau et al. 2000a,b). In our earliest studies of the RTN performed in rats with intact vagus nerves (Mulkey et al. 2004; Guyenet et al. 2005a), we did encounter CO2-sensitive RTN cells with respiratory-modulated discharge patterns similar to those reported in the present study. However, in this work, the cells with the most phasic discharge pattern (ON–OFF) at rest were passed over, in truth because such cells did not fit our preconceived ideas of what central chemoreceptor neurons should look like. Cells with marked but still incomplete reduction of firing during one phase of the respiratory cycle were studied and these cells were usually found to have their discharge nadir during the post-inspiratory period (Guyenet et al. 2005a). We assumed that this pattern was caused by an input from post-inhibitory neurons that are part of the central respiratory pattern generator because post-inspiratory inhibition is also observed in a subset of RTN neurons recorded in vagotomized rats (Guyenet et al. 2005a). However, since tracheal pressure was not measured in our prior studies, we missed the fact that lung inflation coincides with the central post-inspiratory phase in ventilated rats with intact vagus nerves. In view of the present results, we conclude that, in artificially ventilated rats with intact vagus nerves, the RTN neurons with the most pronounced inhibition during the period that immediately follows the PND derive this characteristic primarily from a lung afferent input.
RTN neurons with strong or weak lung afferent input are both subsets of central chemoreceptors
The present evidence suggests that the CO2-activated neurons of RTN with pronounced lung afferent input are simply a subset of RTN chemoreceptors. Like the rest of the CO2-activated neurons, the RTN neurons with pronounced stretch afferent input are active below the phrenic apnoea threshold, their resting activity is unaffected by bilateral injection of muscimol into the rVRG region (Takakura et al. 2006) and they express Phox2b (Stornetta et al. 2006). This latter result also strongly suggests that the RTN neurons with pronounced lung stretch afferent input are also glutamatergic since all Phox2b-expressing neurons within the RTN contain VGLUT2 mRNA (Stornetta et al. 2006). Finally, like the rest of the RTN neurons, the neurons with pronounced lung stretch afferent input develop a remarkably regular discharge after attenuation of excitatory transmission with i.c.v. injection of kynurenate and, in the presence of this blocker, they continue to encode PCO2 in a manner consistent with what would be expected of neurons that derive their CO2 sensitivity from an intrinsic sensitivity to acid (Mulkey et al. 2004, 2006; Guyenet et al. 2005a). Based on these similarities, we conclude that the RTN neurons with pronounced lung afferent input are most probably a subset of RTN chemoreceptors. Given the correlation shown in Fig. 2C, we assume that most RTN neurons receive a lung stretch afferent input although the strength of this input clearly varies from cell to cell.
Pathway responsible for RTN inhibition by lung stretch afferents
SARs drive second-order neurons called pump cells that reside close to the tractus solitarius at the level of the area postrema (Ezure & Tanaka, 1996, 2004; Otake et al. 2001; Ezure et al. 2002). At least two-thirds of the pump cells located in this region are GABAergic or have a mixed GABA/glycine phenotype (Ezure & Tanaka, 2004). Generic pump cells (GABAergic and presumably others) project extensively to the ipsilateral ventrolateral medulla and occasionally to the level of the facial nucleus or to the pons (Ezure & Tanaka, 1996; Ezure et al. 2002).
A direct GABAergic input from NTS inhibitory pump cells to the RTN provides a plausible explanation of the present observations for the following three reasons. First, the effect of lung inflation on RTN neurons was eliminated by muscimol injections targeted to the region where these pump cells reside whereas even slightly mistargeted injections were ineffective. Second, GABA receptor blockade in RTN stimulates breathing in rats (Nattie et al. 2001). Third, muscimol injection into the rVRG region did not change the inhibitory effect of lung inflation nor the effect of low-intensity vagus nerve stimulation on RTN.
An alternative but less plausible explanation would be that RTN inhibition by lung inflation is mediated by the activation of inhibitory post-inspiratory neurons located somewhere in the ventrolateral medulla (Richter et al. 1987; Hayashi et al. 1996). The first difficulty with this interpretation is that the mechanism responsible for the activation of post-inspiratory cells by SARs is obscure given that the existence of excitatory NTS pump cells with projection to the ventrolateral medulla lacks definitive neurochemical support (Kubin et al. 2006). Second, the fact that muscimol injection into the rVRG did not change the effect of lung inflation on RTN neurons argues to some degree against the involvement of ventrolateral medullary post-inspiratory neurons. The argument is obviously not flawless because the critical post-inspiratory cells could reside very rostrally in the ventral respiratory column (e.g. in the Bötzinger region) or even in the pons, therefore out of reach of the injected muscimol (Song et al. 2006).
In summary (Fig. 8), a direct projection from NTS inhibitory pump cells to RTN neurons provides an anatomically and physiologically plausible explanation of the effects of lung inflation on RTN neurons but this interpretation is not yet definitive.
Functional significance of the pulmonary receptor input to RTN
The RTN seems to provide a pH-regulated excitatory drive to the central respiratory pattern generator, including its inspiratory component (Nattie & Li, 2000; Feldman et al. 2003; Onimaru & Homma, 2003; Takakura et al. 2006). The RTN is very important to maintain breathing automaticity under anaesthesia (Nattie & Li, 1994; Takakura et al. 2006) and may also be essential to breathing automaticity during sleep given that its cells express the gene responsible for the central congenital hypoventilating syndrome (Stornetta et al. 2006). To date no other excitatory input to RTN has been identified besides that from peripheral chemoreceptors (Takakura et al. 2006). In contrast, the activity of the nucleus is restrained by numerous types of inhibitory CPG neurons (Guyenet et al. 2005a). These inhibitory inputs may serve as regulatory feedbacks designed to limit the excitatory drive from RTN under conditions when the CPG is sufficiently activated by physical exercise, emotions or miscellaneous cortical influences (Guyenet et al. 2005a). Here we propose that the lung mechanoreceptor input serves as an additional form of feedback on the RTN which, under specific circumstances, contributes to reduce the influence of central chemoreceptors on the CPG.
The extent to which RTN inhibition contributes to PND reduction during increases in PEEP cannot be determined from the present experiments. RTN neurons as a group were only partially inhibited by lung inflation under conditions when PND was completely eliminated. Yet, PND appears highly dependent on RTN activity under anaesthesia (Nattie & Li, 2000; Takakura et al. 2006) and a partial inhibition of these cells could produce a proportionately greater decrease in PND. Specifically, the CO2 threshold of RTN neurons is typically 15 Torr lower than that of PND, which suggests that, under anaesthesia, RTN neurons may need to reach a relatively high level of activity to jump-start the central respiratory oscillator. On the other hand, RTN is not the exclusive nor even dominant target of NTS pump cells (Ezure & Tanaka, 1996; Ezure et al. 2002) and, for this reason, it seems more reasonable to propose that RTN is but one of several sources of drive to the CPG that are targeted by the pump cells to promote a reduction in PND.
Although there is plentiful anatomical and physiological evidence that RTN neurons, as a group, up-regulate the inspiratory network (Dobbins & Feldman, 1994; Nattie, 2001b; Feldman et al. 2003; Takakura et al. 2006), other experimental results suggest that the RTN region may regulate other components of the respiratory system such as the parasympathetic bronchial outflow (Perez Fontan & Velloff, 1997), expiratory muscles and facial motor neurons (Onimaru & Homma, 2003; Janczewski & Feldman, 2005; Onimaru et al. 2006). Much of the latter data were obtained in neonate rodents and do not specifically implicate the cells that we have defined as RTN neurons in the adult. In the neonate, the RTN region contains a neural oscillator or part of an oscillator suspected to control expiratory muscles selectively (Janczewski & Feldman, 2005; Feldman & Del Negro, 2006). However, RTN as we defined it, is unlikely to be part of such an expiratory oscillator because expiratory activity ought to be increased by SAR activation (Hayashi et al. 1996; Feldman & Del Negro, 2006) whereas RTN neurons were exclusively inhibited by this stimulus.
In conclusion, the RTN contains a population of glutamatergic, Phox2b-expressing interneurons that are activated by local changes in pH and receive excitatory inputs from peripheral chemoreceptors. RTN neurons are also inhibited by the activation of myelinated lung stretch receptors, most probably of the slowly adapting variety. The SAR input may reach RTN via a direct projection from the inhibitory pump cells of the ventrolateral NTS. The SAR input to RTN, along with the feedback from the CPG, may serve to reduce the contribution of central chemoreceptors to breathing when the pattern generator is sufficiently activated by other sources of input.
Acknowledgments
This research was supported by grants from the National Institutes of Health to P.G.G. (HL 74011 and HL 28785) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior to A.C.T. (BEX 4402/05-7).
References
- Akilesh MR, Kamper M, Li A, Nattie EE. Effects of unilateral lesions of retrotrapezoid nucleus on breathing in awake rats. J Appl Physiol. 1997;82:469–479. doi: 10.1152/jappl.1997.82.2.469. [DOI] [PubMed] [Google Scholar]
- Bodineau L, Frugière A, Marlot D, Wallois F. Connections between retrotrapezoid nucleus and nucleus tractus solitarii in cat. Neurosci Lett. 2000a;280:111–114. doi: 10.1016/s0304-3940(00)00770-9. [DOI] [PubMed] [Google Scholar]
- Bodineau L, Frugiere A, Marlot D, Wallois F. Effect of hypoxia on the activity of respiratory and non-respiratory modulated retrotrapezoid neurons of the cat. Auton Neurosci. 2000b;86:70–77. doi: 10.1016/S1566-0702(00)00237-X. [DOI] [PubMed] [Google Scholar]
- Coleridge HM, Coleridge JC. Afferent innervation of lungs, airways, and pulmonary artery. In: Zucker IH, Gilmore JP, editors. Reflex Control of the Circulation. Boca Raton: CRC Press; 2001. pp. 579–607. [Google Scholar]
- Connelly CA, Ellenberger HH, Feldman JL. Respiratory activity in retrotrapezoid nucleus in cat. Am J Physiol. 1990;258:L33–L44. doi: 10.1152/ajplung.1990.258.2.L33. [DOI] [PubMed] [Google Scholar]
- Cream C, Li A, Nattie E. The retrotrapezoid nucleus (RTN): local cytoarchitecture and afferent connections. Respir Physiol Neurobiol. 2002;130:121–137. doi: 10.1016/s0034-5687(01)00338-3. [DOI] [PubMed] [Google Scholar]
- Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol. 1994;347:64–86. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- Eldridge FL, Kiley JP, Millhorn DE. Respiratory responses to medullary hydrogen ion changes in cats: different effects of respiratory and metabolic acidoses. J Physiol. 1985;358:285–297. doi: 10.1113/jphysiol.1985.sp015551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezure K, Tanaka I. Pump neurons of the nucleus of the solitary tract project widely to the medulla. Neurosci Lett. 1996;215:123–126. [PubMed] [Google Scholar]
- Ezure K, Tanaka I. GABA, in some cases together with glycine, is used as the inhibitory transmitter by pump cells in the Hering-Breuer reflex pathway of the rat. Neurosci. 2004;127:409–417. doi: 10.1016/j.neuroscience.2004.05.032. [DOI] [PubMed] [Google Scholar]
- Ezure K, Tanaka I, Saito Y, Otake K. Axonal projections of pulmonary slowly adapting receptor relay neurons in the rat. J Comp Neurol. 2002;446:81–94. doi: 10.1002/cne.10185. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Mulkey DK, Stornetta RL, Bayliss DA. Regulation of ventral surface chemoreceptors by the central respiratory pattern generator. J Neurosci. 2005a;25:8938–8947. doi: 10.1523/JNEUROSCI.2415-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bayliss DA, Mulkey DK. Retrotrapezoid nucleus: a litmus test for the identification of central chemoreceptors. Exp Physiol. 2005b;90:247–253. doi: 10.1113/expphysiol.2004.029637. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Coles SK, McCrimmon DR. Respiratory neurons mediating the Breuer-Hering reflex prolongation of expiration in rat. J Neurosci. 1996;16:6526–6536. doi: 10.1523/JNEUROSCI.16-20-06526.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho CY, Gu Q, Lin YS, Lee LY. Sensitivity of vagal afferent endings to chemical irritants in the rat lung. Respir Physiol. 2001;127:113–124. doi: 10.1016/s0034-5687(01)00241-9. [DOI] [PubMed] [Google Scholar]
- Janczewski WA, Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol. 2005;570:407–420. doi: 10.1113/jphysiol.2005.098848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol. 2006;101:618–627. doi: 10.1152/japplphysiol.00252.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeschcke HH. Central chemosensitivity and the reaction theory. J Physiol. 1982;332:1–24. doi: 10.1113/jphysiol.1982.sp014397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira TS, Takakura AC, Colombari E, Guyenet PG. Central chemoreceptors and sympathetic vasomotor outflow. J Physiol. 2006;577:369–386. doi: 10.1113/jphysiol.2006.115600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Mistry AM, Guyenet PG, Bayliss DA. Purinergic P2 receptors modulate excitability but do not mediate pH sensitivity of RTN respiratory chemoreceptors. J Neurosci. 2006;26:7230–7233. doi: 10.1523/JNEUROSCI.1696-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, et al. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Nattie EE. Central chemosensitivity, sleep, and wakefulness. Respir Physiol. 2001a;129:257–268. doi: 10.1016/s0034-5687(01)00295-x. [DOI] [PubMed] [Google Scholar]
- Nattie EE. Chemoreception and tonic drive in the retrotrapezoid nucleus (RTN) region of the awake rat: bicuculline and muscimol dialysis in the RTN. Adv Exp Med Biol. 2001b;499:27–32. doi: 10.1007/978-1-4615-1375-9_4. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Fung ML, Li A, St John WM. Responses of respiratory modulated and tonic units in the retrotrapezoid nucleus to CO2. Respir Physiol. 1993;94:35–50. doi: 10.1016/0034-5687(93)90055-f. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. Retrotrapezoid nucleus lesions decrease phrenic activity and CO2 sensitivity in rats. Respir Physiol. 1994;97:63–77. doi: 10.1016/0034-5687(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Nattie E, Li A. Muscimol dialysis in the retrotrapezoid nucleus region inhibits breathing in the awake rat. J Appl Physiol. 2000;89:153–162. doi: 10.1152/jappl.2000.89.1.153. [DOI] [PubMed] [Google Scholar]
- Nattie E, Shi J, Li A. Bicuculline dialysis in the retrotrapezoid nucleus (RTN) region stimulates breathing in the awake rat. Respir Physiol. 2001;124:179–193. doi: 10.1016/s0034-5687(00)00212-7. [DOI] [PubMed] [Google Scholar]
- Okada Y, Chen Z, Jiang W, Kuwana S, Eldridge FL. Anatomical arrangement of hypercapnia-activated cells in the superficial ventral medulla of rats. J Appl Physiol. 2002;93:427–439. doi: 10.1152/japplphysiol.00620.2000. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci. 2003;23:1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Kumagawa Y, Homma I. Respiration-related rhythmic activity in the rostral medulla of newborn rats. J Neurophysiol. 2006;96:55–61. doi: 10.1152/jn.01175.2005. [DOI] [PubMed] [Google Scholar]
- Otake K, Nakamura Y, Tanaka I, Ezure K. Morphology of pulmonary rapidly adapting receptor relay neurons in the rat. J Comp Neurol. 2001;430:458–470. doi: 10.1002/1096-9861(20010219)430:4<458::aid-cne1043>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Pattyn A, Morin X, Cremer H, Goridis C, Brunet JF. Expression and interactions of the two closely related homeobox genes Phox2a and Phox2b during neurogenesis. Development. 1997;124:4065–4075. doi: 10.1242/dev.124.20.4065. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1998. [Google Scholar]
- Perez Fontan JJ, Velloff CR. Neuroanatomic organization of the parasympathetic bronchomotor system in developing sheep. Am J Physiol. 1997;273:R121–R133. doi: 10.1152/ajpregu.1997.273.1.R121. [DOI] [PubMed] [Google Scholar]
- Putnam RW, Filosa JA, Ritucci NA. Cellular mechanisms involved in CO2 and acid signaling in chemosensitive neurons. Am J Physiol Cell Physiol. 2004;287:C1493–C1526. doi: 10.1152/ajpcell.00282.2004. [DOI] [PubMed] [Google Scholar]
- Richter DW, Ballantyne D, Remmers JE. The differential organization of medullary post-inspiratory activities. Pflugers Arch. 1987;410:420–427. doi: 10.1007/BF00586520. [DOI] [PubMed] [Google Scholar]
- Ritucci NA, Erlichman JS, Leiter JC, Putnam RW. The Response of membrane potential (Vm) and intracellular pH (pHi) to hypercapnia in neurons and astrocytes from rat retrotrapezoid nucleus (RTN) Am J Physiol Regul Integr Comp Physiol. 2005;289:R851–R861. doi: 10.1152/ajpregu.00132.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol. 2006;499:64–89. doi: 10.1002/cne.21105. [DOI] [PubMed] [Google Scholar]
- Smith JC, Morrison DE, Ellenberger HH, Otto MR, Feldman JL. Brainstem projections to the major respiratory neuron populations in the medulla of the cat. J Comp Neurol. 1989;281:69–96. doi: 10.1002/cne.902810107. [DOI] [PubMed] [Google Scholar]
- Song G, Yu Y, Poon CS. Cytoarchitecture of pneumotaxic integration of respiratory and nonrespiratory information in the rat. J Neurosci. 2006;26:300–310. doi: 10.1523/JNEUROSCI.3029-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stornetta RL, Guyenet PG. Distribution of glutamic acid decarboxylase mRNA-containing neurons in rat medulla projecting to thoracic spinal cord in relation to monoaminergic brainstem neurons. J Comp Neurol. 1999;407:367–380. [PubMed] [Google Scholar]
- Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, et al. Selective expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci. 2006;26:10305–10314. doi: 10.1523/JNEUROSCI.2917-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stornetta RL, Sevigny CP, Guyenet PG. Inspiratory augmenting bulbospinal neurons express both glutamatergic and enkephalinergic phenotypes. J Comp Neurol. 2003;455:113–124. doi: 10.1002/cne.10486. [DOI] [PubMed] [Google Scholar]
- Sun MK, Guyenet PG. Arterial baroreceptor and vagal inputs to sympathoexcitatory neurons in rat medulla. Am J Physiol Regul Integr Comp Physiol. 1987;252:R699–R709. doi: 10.1152/ajpregu.1987.252.4.R699. [DOI] [PubMed] [Google Scholar]
- Sun MK, Hackett JT, Guyenet PG. Sympathoexcitatory neurons of rostral ventrolateral medulla exhibit pacemaker properties in the presence of a glutamate-receptor antagonist. Brain Res. 1988;438:23–40. doi: 10.1016/0006-8993(88)91320-0. [DOI] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol. 2006;572:503–523. doi: 10.1113/jphysiol.2005.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatner SF, Uemura N. Integrative cardiovascular control by pulmonary inflation reflexes. In: Zucker IH, Gilmore JP, editors. Reflex Control of the Circulation. Boca Raton: CRC Press; 2001. pp. 609–626. [Google Scholar]