Abstract
The magnocellular neurosecretory cells of the hypothalamus (MNCs) regulate water balance by releasing vasopressin (VP) and oxytocin (OT) as a function of plasma osmolality. Release is determined largely by the rate and pattern of MNC firing, but sustained increases in osmolality also produce structural adaptations, such as cellular hypertrophy, that may be necessary for maintaining high levels of neuropeptide release. Since increases in Ca2+ current could enhance exocytotic secretion, influence MNC firing patterns, and activate gene transcription and translation, we tested whether Ca2+ currents in MNCs acutely isolated from the supraoptic nucleus (SON) of the hypothalamus are altered by 16–24 h of water deprivation. A comparison of whole-cell patch-clamp recordings demonstrated that dehydration causes a significant increase in the amplitude of current sensitive to the L-type Ca2+ channel blocker nifedipine (from –56 ± 6 to –99 ± 10 pA; P < 0.001) with no apparent change in other components of Ca2+ current. Post-recording immunocytochemical identification showed that this increase in current occurred in both OT- and VP-releasing MNCs. Radioligand binding studies of tissue from the SON showed there is also an increase in the density of binding sites for an L-type Ca2+ channel ligand (from 51.5 ± 4.8 to 68.1 ± 4.1 fmol (mg protein)−1; P < 0.05), suggesting that there was an increase in the number of L-type channels on the plasma membrane of the MNCs or some other cell type in the SON. There were no changes in the measured number of binding sites for an N-type Ca2+ channel ligand. Dehydration was not associated with changes in the levels of mRNA coding for Ca2+ channel α1 subunits. These data are consistent with the hypothesis that a selective increase of L-type Ca2+ current may contribute to the adaptation that occurs in the MNCs during dehydration.
The magnocellular neurosecretory cells of the hypothalamus (MNCs) are responsible for the synthesis and controlled release of vasopressin (VP), and oxytocin (OT; Poulain & Wakerley, 1982; Bourque & Oliet, 1997). VP and OT are synthesized in the somata of the MNCs, most of which are located in the supraoptic nucleus (SON) and paraventricular nucleus of the hypothalamus, and released into the circulation from the MNC terminals in the neurohypophysis. The amount of release is primarily determined by the rate and pattern of action potentials generated in the MNC somata (Bicknell, 1988). Both VP- and OT-releasing MNCs fire irregularly and infrequently when plasma osmolality is near normal, and both fire more rapidly as the external osmolality increases (Poulain & Wakerley, 1982; Bourque & Oliet, 1997). VP-releasing MNCs also respond to increased osmolality by adopting a phasic pattern of firing, which is characterized by bursts of action potentials lasting tens of seconds followed by rest intervals of about the same length (Poulain & Wakerley, 1982). Most OT MNCs do not adopt a phasic pattern of firing in response to elevations of osmolality, but do fire rapid bursts during lactation and at parturition (Poulain & Wakerley, 1982). The adoption of a phasic pattern of firing is important physiologically because this maximizes hormone release (Bicknell, 1988).
The elevation in firing frequency that occurs during an increase in external osmolality involves both intrinsic and extrinsic mechanisms. There is an increase in excitatory drive from other cells, and the MNCs are themselves osmosensitive (Bourque & Oliet, 1997). MNCs become more susceptible to excitatory inputs due to a membrane depolarization mediated by stretch-inactivated cation channels (Oliet & Bourque, 1993), which sense changes in membrane tension caused by osmotic shrinkage or swelling and therefore become more active as the external osmolality increases (Zhang & Bourque, 2003). Phasic firing depends on currents that are activated by the Ca2+ influx that occurs during action potentials. Initiation of a burst requires a depolarizing afterpotential (DAP) that can summate into the plateau potential that underlies phasic firing in MNCs (Andrew & Dudek, 1983). The DAP may result from Ca2+-dependent activation of a non-selective cation current (Ghamari-Langroudi & Bourque, 1998, 2002) and/or the Ca2+-dependent inactivation of a K+ current (Li & Hatton, 1997). The rate of firing during a burst, and possibly also the termination of firing, are regulated by afterhyperpolarizations (AHPs) mediated by K+ currents (Bourque & Brown, 1987; Greffrath et al. 1998; Ghamari-Langroudi & Bourque, 2004). Changes in firing pattern may also depend on an increase in the amplitude of an osmosensitive slowly activating voltage-gated cation current (Liu et al. 2005).
Firing patterns are also influenced by the release of local modulators, including VP and OT, which are released in an autocrine fashion from the somatodendritic (SD) region of the MNCs (Ludwig & Pittman, 2003). SD release of VP may be important in regulating MNC firing patterns (Gouzenes et al. 1998) and SD release of OT modulates neurotransmitter release from synaptic inputs onto the MNCs (Kombian et al. 1997; Hirasawa et al. 2004). Other peptides, such as apelin (De Mota et al. 2004) and dynorphin (Brown & Bourque, 2004), are co-released with VP within the SON and can also regulate MNC firing.
While changes in external osmolality cause almost immediate changes in cell firing, the MNCs also undergo longer term changes that may be required to sustain or potentiate release of neuropeptides. Following 24 h of dehydration, and during lactation and parturition, structural changes occur in both the SON and in the neurohypophysis (Hatton, 1997; Theodosis et al. 2004). These changes include retraction of the glial processes surrounding the MNCs, hypertrophy of the MNC somata, and increased synaptic innervation (Tasker et al. 2002). Other longer term adaptations to dehydration include up-regulation of the genes coding for VP (Zingg et al. 1986) and other peptides (Ghorbel et al. 2003; Hindmarch et al. 2006), an increase in the expression of the V1a vasopressin receptor on the MNC membrane (Hurbin et al. 2002), translocation of the dynorphin receptor to the MNC plasma membrane (Shuster et al. 1999), and a change in MNC firing patterns toward shorter and faster bursts (Dyball & Pountney, 1973).
Ca2+ currents play important roles in both the somata and axon terminals of the MNCs (Fisher & Bourque, 1996). Although Ca2+ currents are not affected by acute changes in osmolality (Liu et al. 2005), it is possible that changes in one or more types of Ca2+ current during sustained dehydration could occur, with important physiological consequences. Several types of Ca2+ channels, including N- (mediated by the α1B Ca2+ channel subunit or CaV2.2), P/Q- (α1A or CaV2.1) and L-types (α1C and α1D or CaV1.2 and CaV1.3), have been identified in MNC somata using electrophysiological (Fisher & Bourque, 1995a; Foehring & Armstrong, 1996), immunocytochemical (Joux et al. 2001), and molecular biological techniques (Glasgow et al. 1999). N-, P/Q- and L-type Ca2+ channels have also been detected in recordings from isolated MNC terminals (Lemos & Nowycky, 1989; Fisher & Bourque, 1995b) and in immunoblots of neurohypophysial tissue (Fisher et al. 2000). Activation of Ca2+ channels in the MNC axon terminals trigger neuropeptide secretion into the systemic circulation, whereas those in the MNC somata may be involved in SD secretion, regulation of firing patterns (particularly due to activation of Ca2+-dependent currents such as the DAP and the AHPs), and regulation of gene transcription and translation (Fisher & Bourque, 1996). Ca2+ currents increase in OT-releasing MNCs during lactation (De Kock et al. 2003; Teruyama & Armstrong, 2005) and this could contribute to both an increase in SD release of OT (De Kock et al. 2003) and to an increase in the activation of Ca2+-dependent K+ currents (Teruyama & Armstrong, 2005).
We have therefore used whole-cell patch-clamp recording, radioligand binding assays, and RT-PCR to test the hypothesis that dehydration causes an increase in Ca2+ current in the MNCs. We report here that there is a selective increase in L-type Ca2+ current in MNC somata acutely isolated from rats exposed to 16–24 h of water deprivation. These changes were seen in both OT- and VP-releasing MNCs. Radioligand binding studies were used to show that dehydration causes an increase in the number of binding sites in the SON for L-type channel-selective ligands, suggesting that there is an increase in the number of channels on the plasma membrane of the MNCs or of another cell type. These data are consistent with the hypothesis that the increase in L-type current in the MNCs is caused by an increase in the number of functional channels on the MNC membrane. Although we cannot rule out the possibility that the increase in current is due to an increase in channel synthesis, we observed no change in the quantity of mRNA coding for L-type channels in the SON following dehydration. The selective increase in L-type Ca2+ current may play an important role in the physiological adaptation that occurs in MNCs during dehydration.
Methods
Male Long Evans rats (Charles River, QC, Canada) weighing 225–250 g, were housed in pairs with access to rodent chow and water ad libitum, under a 12 h light–dark cycle. To induce a dehydrated state, the water bottle was removed from the cage for a period of 16 or 24 h prior to killing. Water deprivation of this duration causes an increase in plasma osmotic pressure to 320–340 mosmol kg−1 (Wakerley et al. 1978) and leads to ultrastructural modifications in the SON (Tweedle & Hatton, 1977) and neurohypophysis (Tweedle & Hatton, 1980).
Rats were anaesthetized by halothane inhalation and decapitated using a small rodent guillotine, in accordance with a protocol approved by the University of Saskatchewan Animal Care Committee. The brain was removed from the cranial vault and placed with the inferior surface exposed. A tissue slice containing the two supraoptic nuclei (about 1.0 mm wide) was cut with a razor blade and two tissue blocks, each containing a portion of one SON, were dissected using a dissecting microscope. The neurohypophysis was separated from the adenohypophysis and intermediate lobe.
Electrophysiology
The enzymatic isolation of MNC somata was performed as previously described (Oliet & Bourque, 1992; Fisher & Bourque, 1995a). Briefly, tissue blocks containing the SON were harvested as above, but in Pipes buffer (composition: pipes 20 mm, NaCl 110 mm, KCl 5 mm, MgCl2 1 mm, pH 7.1) with glucose (25 mm) and CaCl2 (1 mm). Tissue blocks were placed in a glass tube containing buffer plus trypsin (Type XI, 0.6 mg ml−1) and bubbled with 100% O2 in a water bath at 34°C for 90 min. The tissue blocks were washed in trypsin-free buffer at room temperature and bubbled with 100% O2 for 30 min. Tissues were triturated through a series of fire-polished Pasteur pipettes of decreasing diameter, plated on untreated glass-bottom culture dishes, covered and left undisturbed at room temperature for a minimum of 30 min. Immunocytochemical identification of cells isolated using this technique has shown that MNCs can readily be differentiated from other neurons by their size (Oliet & Bourque, 1992) and we therefore used this method to identify MNCs for recording. Whole-cell patch-clamp recordings were performed at room temperature using an EPC-9 amplifier (HEKA) operated with Pulse software (HEKA). Electrodes were pulled from borosilicate capillary glass (1.2 mm outer diameter; A-M Systems Inc.) on a P-97 horizontal pipette puller (Sutter Instrument Company) and fire-polished using a microforge (Narashige). The external solution contained (mm); NMDG 90, CaCl2 2, glucose 10, Hepes 10, TTX 0.0002, TEA 20, 4-AP 4, pH 7.35. The recording electrodes (2.5–5 MΩ) were filled with a solution that contained (mm): Tris 110, MgCl2 1, EGTA 1.6, TEA 40, Na2ATP 2, phosphocreatine 14, pH 7.2. The osmolalities of all buffers were measured using a Vapro vapour pressure osmometer (Wescor), and were modified by the addition of mannitol. The external recording solutions and the isolation solutions were maintained at 295 mosmol kg−1 for MNCs isolated from control rats, but at 325 mosmol kg−1 for MNCs isolated from dehydrated rats, to prevent reversal of any changes caused by dehydration. The density of L-type current and whole-cell capacitance of MNCs isolated from control rats following an incubation in 325 mosmol kg−1 solutions for 1–3 h (–4.2 ± 0.7 pA pF−1 and 12.1 ± 1.0 pF, respectively; n = 8) were not increased relative to those of control MNCs (–4.5 ± 0.6 pA pF−1 and 13.4 ± 0.5 pF; see Results). The internal recording solutions had an osmolality of 290 mosmol kg−1. In some cells (n = 9 and 11 for control and dehydrated, respectively), currents were evoked by stepping from a holding potential of –100 mV to –10 mV for 400 ms. In others (n = 27 and 31 for control and dehydrated, respectively), current–voltage relationships were constructed by stepping the cells from a holding potential of –80 mV to a series of potentials between –60 and 20 mV for 400 ms every 1.5 s. Since total and nifedipine-sensitive currents evoked by stepping to –10 mV from holding potentials of –100 and –80 mV were not different, the data were pooled for the comparisons of evoked currents. Nifedipine was dissolved in DMSO (which had a final concentration of 0.1%) and used at a concentration of 10 μm. Administration of 0.1% DMSO had no significant effect on the amplitude of evoked Ca2+ currents (n = 3).
Immunocytochemistry
Cells were prepared for immunocytochemistry as described previously (Fisher et al. 1998). Following recording, the pipette was carefully detached from the cell. Cells were then fixed with 4% paraformaldehyde (20 min at room temperature) in phosphate-buffered saline (PBS; 0.1 m, pH 7.3), washed with PBS, treated with blocking solution (PBS with 4% normal donkey serum (Jackson Immunoresearch) and 0.02% Triton X-100), and incubated overnight at 4°C with goat antibodies (Santa Cruz Biotechnology) recognizing either neurophysin I (NP I, 1: 100) or neurophysin II (NP II, 1: 100) in blocking solution. NP I and II are precursor proteins for OT and VP, respectively. The following day, cells were rinsed in blocking solution, then incubated with a secondary antibody labelled with Cy3 (donkey anti-goat; Jackson Immunoresearch, 1: 500) for 30 min at room temperature. Cells were then rinsed, placed in mounting solution (Citifluor, Marico Inc.) and covered with a glass coverslip. Differential contrast interference (DIC) and fluorescence images were obtained using appropriate filter sets and a cooled CCD camera attached to a Zeiss Axiovert 200 microscope with a 40 × objective. MNCs were identified as being VP-releasing if they were immunopositive for NP II or immunonegative for NP I (n = 12 and 10, respectively) or as being OT-releasing if they were immunopositive for NP I or immunonegative for NP II (n = 4 and 5, respectively). Experiments using no primary antibody resulted in the absence of specific labelling.
125I-ω-CTX GVIA binding
SON and neurohypophysial tissues were prepared in buffer A (Hepes 10 mm, BSA 0.02%, NaCl 100 mm, pH 7.3) with glucose (25 mm), one Complete Mini Protease Inhibitor Tablet (Roche) and pepstatin (1.5 μm). Tissue from four rats was pooled for each experiment and manually homogenized in 600 μl of buffer A, until the buffer appeared cloudy and no distinct tissue pieces were visible. A Bio-Rad protein assay was performed to determine the protein content of the samples based on a BSA standard curve. Saturation binding curves were generated using concentrations of 125I-ω-conotoxin-GVIA (125I-ω-CTX GVIA; PerkinElmer) from 0.005 to 0.6 nm in a total volume of 200 μl (5–10 μg protein per assay). Non-specific binding was determined by preincubating the tissue homogenates for 30 min with 0.1 μm of the non-iodinated ω-CTX GVIA (Alomone Laboratories) at room temperature. The specific activity of the ligand was decreased from 2200 to 550 Ci mmol−1 by a 1: 3 dilution in ω-CTX GVIA. Following a 90 min incubation at room temperature, the tubes containing the samples were placed in ice water to stop the reaction. Samples were then vacuum-filtered through Whatman GF/A glass fibre filters that had been soaked in 0.3% polyethyleneimine, washed 3 times with buffer A, and placed in glass counting tubes. Radioactivity on the filters was measured using a LKB Wallac 1271 Riagamma Automatic Gamma Counter. The equilibrium dissociation constant (KD) and the density of binding sites (BMAX) of the binding reaction was determined using GraphPad Prism3 software.
3H-isradipine binding
The specific activity of the 3H-isradipine is much lower (86 Ci mmol−1) than that of 125I-ω-CTX GVIA, which means that a much higher number of binding sites is required to obtain a reliable signal. It was therefore not practical to obtain full saturation binding curves for 3H-isradipine using homogenates of the SON and neurohypophysis. We instead decided to use a saturating concentration of 3H-isradipine to estimate BMAX. Homogenates of whole rat brain were used to determine the KD for 3H-isradipine under our conditions. Approximately a quarter of a rat brain was placed in 2 ml of Tris-HCl buffer (Tris-HCl 50 mm, glucose 25 mm, 1 Complete Mini Protease Inhibitor Tablet, pepstatin 1.5 μm, and CaCl2 1 mm). The tissue was finely diced using scissors, and homogenized in 3 ml buffer with a polytron homogenizer. The homogenate was then transferred to a plastic centrifuge tube and centrifuged at 35 000 g for 15 min, at 4°C. The supernatant was discarded and the pellet was re-suspended in 3 ml Tris-HCl buffer. The total volume of each assay was 1.0 ml with total protein ∼80 μg and concentrations of 3H-isradipine from 0.05 to 1.6 nm. Glass culture tubes containing the samples were incubated for 90 min under minimal light conditions. Non-specific binding was determined by preincubating samples for 30 min with 1.5 μm nifedipine (in 0.15% DMSO). Following the incubation, tubes were placed in ice water to stop the reaction. Samples were vacuum filtered as described above, and washed 3 times with ice-cold Tris buffer. Filters were placed in liquid scintillation vials, 5 ml of Ready Gel (Beckman Coulter) was added, and the vials were capped and shaken. The samples were left at room temperature overnight in minimal light, and then counted in a β-counter (Beckman LS5000TA). The BMAX and KD were determined using GraphPad Prism3 software.
To measure 3H-isradipine binding to SON and neurohypophysial tissues, samples were treated as for 125I-ω-CTX GVIA, but with the following modifications. A single concentration of 3H-isradipine 20 times the KD determined from the saturation binding curve in the whole brain (1.6 nm) was used. Samples were prepared in a total volume of 80 μl Tris buffer with ∼50 μg protein per assay. Specific binding was estimated as total binding minus non-specific binding of the mean of triplicate assays. Each assay required the pooling of tissue from four rats.
RT-PCR
The expression levels of mRNA encoding various Ca2+ channel α1 subunits in the SON were compared in control and water-deprived rats. Tissue samples were extracted from one control and one dehydrated rat and run in parallel. Levels of expression were compared with that of a control gene that is expected to be expressed at a constant level (Murphy et al. 1990); in our case we used GAPDH. Total RNA was extracted from supraoptic nuclei from control or water-deprived rats using Trizol (Invitrogen, USA), as described by the manufacturer. The RNA was dissolved in 20 μl of RNAse-free water and stored at –70°C until it was used for reverse transcription. First-strand cDNA synthesis was performed according to the manufacture's protocol (SuperScript First Strand Synthesis System, Invitrogen). Oligonucleotide primers for Ca2+ channel α1B, α1C and α1D subunits, VP, and GAPDH (Glasgow et al. 1999) were synthesized by the University Core DNA Services, University of Calgary (Table 1) and used for PCR. The reaction mixtures contained 1.5 mm MgCl2, 2 Units of Taq DNA polymerase, 2 pmol of each primer, 10 μmol dNTP and 1 × PCR buffer (Invitrogen). The initial denaturation occurred at 95°C for 3 min and this was followed by cycles of 95°C for 40 s, 58°C for 1 min and 72°C for 1.5 min. All reactions were performed in triplicate and negative controls (without cDNA) were run with each reaction. For each reaction, tubes were run for different numbers of cycles (in 3 cycle intervals) to ensure that the reaction used to measure PCR products was in the exponential phase. Tubes were removed at cycles 31–37 for α1B, 30–36 for α1D and 23–29 for GAPDH. Bands for α1C were visible in some experiments but not in others, even after 40 cycles, for both control and dehydrated rats. PCR products were analysed on 1.5% agarose gels stained with ethidium bromide. Gels were visualized under UV light (302 nm), photographed, and the band intensities were measured using Image J software (National Institutes of Health, USA). As a positive control (Zingg et al. 1986), we compared the quantity of mRNA for the VP precursor in SON from control and dehydrated rats and found that, in the mean of two experiments, there was a 3.9-fold increase in expression in dehydrated rats.
Table 1.
Primer sequences used
| mRNA species | Sequence | 5′-position | PCR product (bp) |
|---|---|---|---|
| α1B F | 5′-GAAGTAGCTGAAGTCAGCC-3′ | 2221 | 501 |
| α1B R | 5′-CTTGTGTGTCAGCCCCTGGA-3′ | 2722 | — |
| α1C F | 5′-TCGTGGGTTTCGTCATTGTCA-3′ | 4236 | 474 |
| α1C R | 5′-CCTCTGCACTCATAGAGGGAGAGG-3′ | 4710 | — |
| α1D F | 5′-GAGCCTGCATTAGTATAGTGAATTG-3′ | 873 | 217 |
| α1D R | 5′-AGGATGCAGCAGCAGTCCGTA-3′ | 1090 | — |
| GAPDH F | 5′-GGACATTGTTGCCATCAACGAC-3′ | 149 | 441 |
| GAPDH R | 5′-ATGAGCCCTTCCACGATGCCAAAG-3′ | 589 | — |
Data analysis
Data were entered in a GraphPad Prism or Excel worksheet and Student's unpaired t test was used to determine statistical significance, which was defined as P < 0.05. Data are shown as mean ± s.e.m.
Results
Electrophysiology
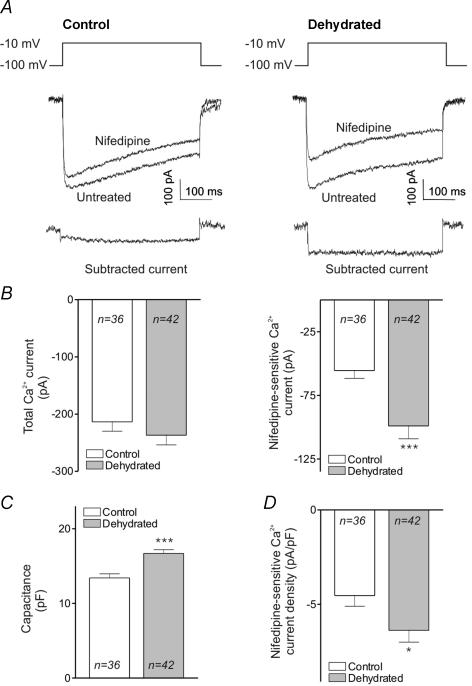
MNCs were isolated from the SON of rats that were normally hydrated (control) and from rats that were deprived of water for 24 h (dehydrated). Whole-cell patch-clamp experiments were performed to measure total Ca2+ current and Ca2+ current sensitive to the selective L-type Ca2+ channel blocker nifedipine. Figure 1A shows traces of typical currents evoked by steps to –10 mV in MNCs isolated from control and dehydrated rats, before and after addition of nifedipine. The subtracted current, which represents the currents sensitive to nifedipine, are shown immediately below. Figure 1B, left, shows that there was no significant difference between the total Ca2+ current evoked in control and dehydrated MNCs (–214 ± 16 and –237 ± 17 pA, respectively). Figure 1B, right, however, shows that the nifedipine-sensitive current was significantly larger in dehydrated rats. While the control cells had an amplitude of –56 ± 6 pA, the cells from dehydrated rats had an amplitude of –99 ± 10 pA (P < 0.001), which corresponds to an 80% increase. The L-type current expressed as a percentage of total current has therefore increased from 26% to 42%. There was, however, no significant change in the amplitude of the current insensitive to nifedipine (data not shown). These data suggest that dehydration causes a selective increase in a nifedipine-sensitive current.
Figure 1. Nifedipine-sensitive Ca2+ currents in MNCs isolated from control and dehydrated rats.
A. whole-cell recordings showing representative Ca2+ currents evoked by stepping from −100 to −10 mV in the absence and presence of nifedipine. ‘Control’ refers to currents evoked in MNCs isolated from normally hydrated rats while ‘dehydrated’ refers to currents evoked in MNCs isolated from rats deprived of water for 24 h. The subtracted currents show the nifedipine-sensitive currents for the two conditions. B, left, a bar graph showing the mean amplitudes of total Ca2+ current evoked under these conditions. These values are not statistically different. B, right, a bar graph showing the amplitude of nifedipine-sensitive Ca2+ current evoked under these conditions. There is a highly significant difference between the current amplitude in control and dehydrated rats (P < 0.001). C, a bar graph comparing the whole-cell capacitance of MNCs isolated from control and dehydrated rats (P < 0.001). D, a bar graph of the density of nifedipine-sensitive Ca2+ currents in control and dehydrated rats (P < 0.05).
Since dehydration causes MNC hypertrophy (Tweedle & Hatton, 1977), we measured the membrane capacitance of the MNCs and used these values to calculate current density. A comparison of the capacitance of MNCs isolated from control and dehydrated rats is shown in Fig. 1C. These data show that the MNCs isolated from dehydrated rats had a cell capacitance about 25% larger than that of control cells (16.7 ± 0.5 versus 13.4 ± 0.5 pF; P < 0.001). The bar graphs in Fig. 1D show the density of Ca2+ current in the two groups. The density of nifedipine-sensitive current was significantly larger in MNCs isolated from dehydrated rats (–6.4 ± 0.6 versus –4.5 ± 0.6 pA pF−1; P < 0.05).
In a subset of MNCs (n = 27 and 31 for control and dehydrated, respectively), the current–voltage relationship of the total and nifedipine-sensitive Ca2+ current was determined. Figure 2A shows the comparison of total peak Ca2+ current. There is a significant increase in current in dehydrated rats only at 0 mV (P < 0.01). Figure 2B shows the comparison of nifedipine-sensitive current, which shows a significant increase in current in dehydrated rats at all voltages between –20 and +10 mV (P < 0.05 at –20 and +10 mV, P < 0.01 at –10 mV, and P < 0.001 at 0 mV). These data suggest that there is a selective increase in a high-threshold, nifedipine-sensitive current in MNCs isolated from dehydrated rats.
Figure 2. The current–voltage relationship for total and nifedipine-sensitive Ca2+ currents in MNCs isolated from control and dehydrated rats.
Cells were stepped from a holding potential of −80 mV to the indicated potentials in the absence and presence of 10 μm nifedipine. A, the current–voltage relationship of peak Ca2+ currents evoked by stepping to the indicated voltages in control (○; n = 27) and dehydrated rats (•; n = 31). B, the current–voltage relationship of nifedipine-sensitive Ca2+ currents evoked by stepping to the indicated voltages in control (○; n = 27) and dehydrated rats (•; n = 31).
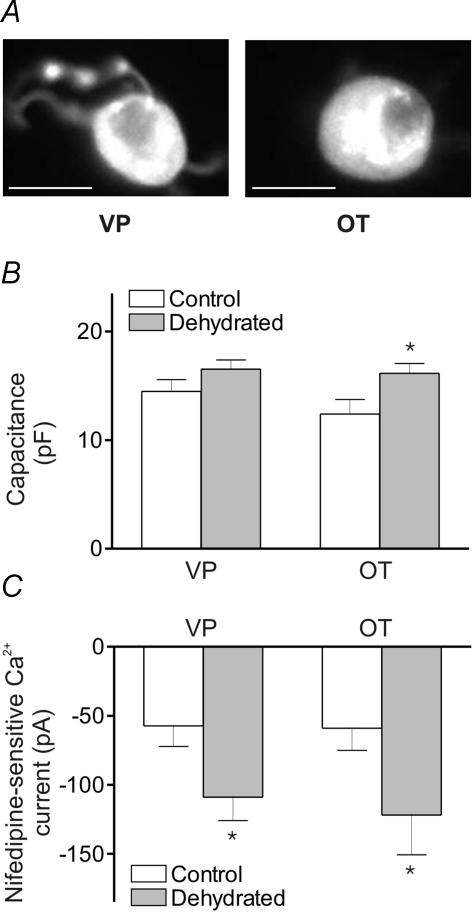
We used post-recording immunocytochemical identification to determine whether the increases in nifedipine-sensitive currents occur in both types of MNCs. Figure 3A, left, shows an isolated MNC that was immunoreactive for VP, while Fig. 3A, right, shows an isolated MNC that was immunoreactive for OT. Increases in capacitance were observed following dehydration both in VP-releasing (14.5 ± 1.1 and 16.5 ± 0.8 pF; n = 8 and 14, respectively) and OT-releasing MNCs (12.4 ± 1.3 and 16.2 ± 0.9 pF, n = 5 and 4, respectively), although only the latter difference was significant (probably due to the relatively small number of cells sampled). The increase in nifedipine-sensitive Ca2+ current, however, was significant both for VP-releasing (–57 ± 15 and –109 ± 17 pA) and OT-releasing MNCs (–59 ± 16 and –122 ± 29 pA).
Figure 3. Capacitance measurements and nifedipine-sensitive Ca2+ currents in identified VP and OT MNCs.
A, immunofluorescence images of MNCs after whole-cell recording showing immunoreactivity to anti-NP II (left) and anti-NP I (right). The scale bar is 15 μm. B, a bar graph showing the whole-cell capacitance of MNCs isolated from control and dehydrated rats that were immunoreactive for VP (n = 8 and 14, respectively) and OT (n = 5 and 4, respectively; P < 0.05). C, a bar graph showing the nifedipine-sensitive Ca2+ current amplitude in MNCs isolated from control and dehydrated rats that were immunoreactive for VP (n = 8 and 14, respectively; P < 0.05) and OT (n = 5 and 4, respectively; P < 0.05).
Radioligand binding studies
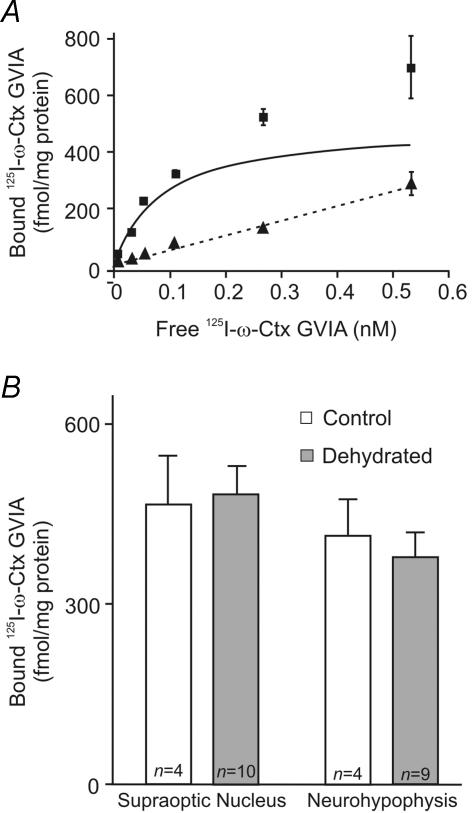
Radioligand binding studies were used to test whether dehydration induces a change in Ca2+ channel density in the MNCs. Tissue homogenates of supraoptic nuclei and neurohypophyses from control and dehydrated rats were probed with radioactively labelled ligands that bind specifically to extracellular domains of either N- or L-type Ca2+ channels. The N-type channel mediates the largest component of Ca2+ current in both MNC somata and terminals (Fisher & Bourque, 1996). We measured the density of N-type channels using the peptide toxin 125I-ω-CTX GVIA, which is derived from a marine mollusk and has high specificity and affinity for the N-type Ca2+ channels (Olivera et al. 1985). Saturation binding curves were generated using concentrations of 125I-ω-CTX GVIA ranging from 0.005 to 0.6 nm. An example of such a binding curve is shown in Fig. 4A. Non-specific binding was determined by performing the binding reaction in the presence of 0.1 μm of non-iodinated ω-CTX GVIA. GraphPad software was used to analyse the resultant data to determine the KD of the interaction between ligand and channel and BMAX. The results for BMAX, expressed as fmol (mg protein)−1, are summarized in Fig. 4B. The BMAX in the SON of control rats was 477 ± 89 fmol (mg protein)−1, while in the dehydrated rats it was 483 ± 54 fmol (mg protein)−1. These values are not significantly different. Similarly, the values for BMAX in the neurohypophysis for the two conditions (413 ± 61 and 379 ± 56 fmol (mg protein)−1) were not significantly different. The KD for 125I-ω-CTX GVIA binding was not different between control and dehydrated rats or between the SON and neurohypophysis (data not shown). The combined KD was 0.079 ± 0.008 nm, which is similar to a reported value for rat brain membranes (Ichida et al. 1993).
Figure 4. Saturation binding of 125I-ω-CTX GVIA in tissue homogenates of the SON and neurohypophysis from control and dehydrated rats.
A, a saturation binding curve for 125I-ω-CTX GVIA in a homogenate of SON tissue. ▪, the amount of bound 125I-ω-CTX GVIA at the indicated concentrations of free 125I-ω-CTX GVIA. ▴ and dashed line show the amount of non-specific binding at the same concentrations, which were obtained by performing the binding reaction in the presence of 0.1 μm of the non-iodinated ω-CTX GVIA. The continuous line shows the calculated binding isotherm for 125I-ω-CTX GVIA. B, the bar graphs show the calculated BMAX for 125I-ω-CTX GVIA in the SON and neurohypophysis from control and dehydrated rats. These values are not significantly different for either the SON or neurohypophysis. The n values shown represent the number of experiments performed, each of which used pooled tissue from 4 rats.
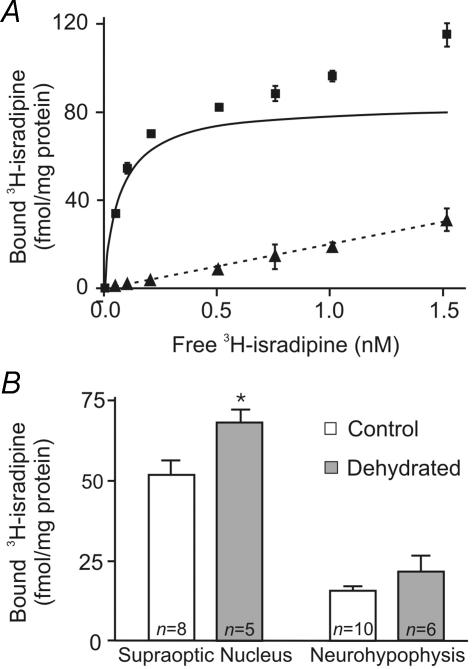
3H-isradipine has been used to quantify L-type Ca2+ channel density in rat brain and other tissues (Hirota & Lambert, 1997; Nakayama et al. 1999). Figure 5A shows a saturation binding curve for 3H-isradipine in a rat brain homogenate. Total binding at each concentration of 3H-isradipine (0.05–1.6 nm) is shown as squares, and the non-specific binding, determined in the presence of 1.5 μm nifedipine, is shown as triangles. The BMAX for rat brain homogenates was estimated to be 84.0 ± 0.2 fmol (mg protein)−1 and the KD was 0.089 ± 0.001 nm, which is similar to the KD determined in rat brain cortical homogenates (Hirota & Lambert, 1997). Since the specific activity of 3H-isradipine is much lower than that of 125I-ω-CTX GVIA (86 Ci mmol−1versus 2200 Ci mmol−1) and since the number of L-type channels in the membrane is also smaller, a full binding curve such as that in Fig. 5A would have required a prohibitively large number of supraoptic nuclei or neurohypophyses. We therefore chose a single saturating concentration of 3H-isradipine, equal to 20 times the determined KD (1.6 nm), to determine the number of L-type channels present in the tissue. These experiments also required pooled tissue from four rats. Non-specific binding was determined by including 1.5 μm nifedipine in the binding reaction mixture. The results of these experiments are shown in Fig. 5B. 3H-isradipine binding in the SON of control rats was 51.5 ± 4.8 fmol (mg protein)−1, while in the dehydrated rats this value was increased to 68.1 ± 4.1 fmol (mg protein)−1. This represents a 32% increase in channel density and is statistically significant (P < 0.05). In neurohypophysial tissue the corresponding values were 15.6 ± 1.7 and 21.6 ± 5.0 fmol (mg protein)−1; this increase is not statistically significant.
Figure 5. Saturation binding of 3H-isradipine in rat brain homogenates and measurement of binding in homogenates from the SON and neurohypophysis from control and dehydrated rats.
A, saturation binding curves were obtained for 3H-isradipine (0.05–1.6 nm) in rat brain homogenates. ▪, the amount of bound 3H-isradipine at the indicated concentrations of free 3H-isradipine. ▴, the amount of non-specific binding at the same concentrations, obtained by performing the binding reaction in the presence of 1.5 μm nifedipine, and the dashed line shows the calculated best fit linear regression. The continuous line shows the calculated binding isotherm for 3H-isradipine. B, the bar graph shows the specific binding for 3H-isradipine in the SON and neurohypophysis from control and dehydrated rats. The binding reaction was performed with 1.6 nm3H-isradipine and the non-specific binding was determined in the presence of 1.5 μm nifedipine. Specific 3H-isradipine binding was 51.5 ± 4.8 fmol (mg protein)−1 in control rats and 68.1 ± 4.1 fmol (mg protein)−1 in dehydrated rats, which is significantly different (P < 0.05). Specific 3H-isradipine binding in the neurohypophyses of control and dehydrated rats was not significantly different. The n values shown represent the number of experiments performed, each of which used pooled tissue from 4 rats.
RT-PCR
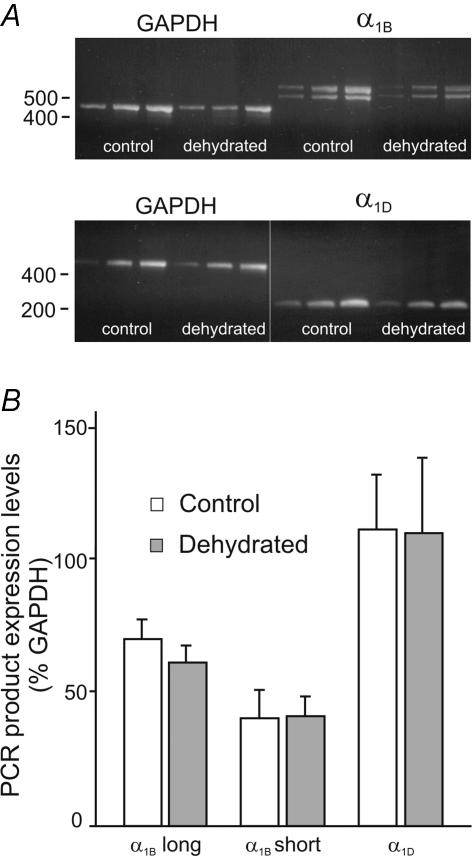
To test whether the change in L-type Ca2+ channel density could be explained by an increase in channel synthesis, RT-PCR was used to quantify the mRNA coding for Ca2+ channel α1 subunits. mRNA was extracted from the SON and neurohypophyses of control and dehydrated rats and first-strand cDNA synthesis was performed. The resultant cDNA was probed with primers for the α1B, α1C, and α1D subunits in parallel with those of GAPDH, a housekeeping gene. Each sample was prepared in triplicate and the PCR reactions stopped at 3-cycle intervals to ensure that the middle band showed the results of the PCR reaction in the exponential phase. These bands were then used for comparisons (see Fig. 6A). Two distinct bands were observed for α1B. The smaller, lower band represents the expected PCR product (Glasgow et al. 1999), while the larger, upper one is an alternatively spliced variant resulting from the insertion of 21 amino acids in the II–III cytoplasmic loop of the channel protein (Pan & Lipscombe, 2000). The identity of the larger band was confirmed by sequencing of the PCR product (data not shown). The possibility that expression of one or other variant could be influenced by the hydration status of the animal was considered. Figure 6B shows a comparison of the intensity of the bands as expressed as a percentage of the GAPDH band. No significant differences were found between control and dehydrated rats with either the larger (70.1 ± 7.4 versus 61.4 ± 6.4; n = 4) or smaller (40.6 ± 10.0 versus 41.4 ± 7.2; n = 4) molecular weight bands for the α1B subunit, or with the bands for the α1D subunit (111.0 ± 21.0 versus 109.8 ± 28.4; n = 5). Although expression of α1B, α1D and GAPDH was consistently found in all samples tested, expression of α1C was undetectable in many experiments even after 40 PCR cycles. We observed a band for α1C in only 2 of 5 control rats and 2 of 5 dehydrated rats. Although the reason for this inconsistency is unclear, we concluded that an increase in the synthesis of α1C was not likely to explain the increase in L-type Ca2+ channel density in response to dehydration.
Figure 6. Quantification of mRNA coding for α1B and α1D Ca2+ channel subunits in control and dehydrated rats using RT-PCR.
A, PCR reactions with primers for GAPDH and α1B and α1D Ca2+ channel subunits were run on RNA isolated from the SON of control and dehydrated rats. Reactions were stopped at 3 cycle intervals and the mid-points were used to compare the intensity of the Ca2+ channel bands with that of the GAPDH band using NIH Image J software. Note that two PCR products were expressed using the primers for α1B. B, the bar graph shows the intensity of the bands obtained for each of the Ca2+ channel α1 subunit primer sets for control and dehydrated conditions, as compared with the intensity of the appropriate GAPDH bands. No differences were observed for any of the PCR products between control and dehydrated rats.
Discussion
The MNCs and their surrounding glia undergo a remarkable structural and functional reorganization in response to prolonged water deprivation (Hatton, 1997). The data presented here suggest that part of this adaptive transformation may involve an increase in the amplitude of L-type voltage-gated Ca2+ current. Whole-cell patch-clamp recording were used to demonstrate that 16–24 h of water deprivation causes a marked (80%) increase in L-type Ca2+ current in MNCs, with no significant changes in the nifedipine-insensitive current. These data suggest that the hypertrophy associated with dehydration is not associated with a scaled increase in the amplitude of all Ca2+ currents, but rather that the amplitudes of most currents remain unchanged while that of the L-type current is selectively increased. Immunocytochemistry was used to show that this increase is observed in both OT- and VP-releasing MNCs.
The identity of the α1 subunit responsible for the dehydration-induced increase in L-type Ca2+ current is not known. Ca2+ channel α1C and α1D subunits have been identified in the MNCs both by single cell RT-PCR (Glasgow et al. 1999) and by immunocytochemistry (Joux et al. 2001). The observed increase may be due to an increase in both subtypes or to a selective increase in one of the two. In the latter case the percentage increase of that one subtype might be much higher than 80%. The MNCs display a low-threshold nifedipine-sensitive current (Fisher & Bourque, 1995a; see also Fig. 5). Since α1D has a lower threshold of activation than α1C (see discussion in Fisher & Bourque, 1996; Lipscombe et al. 2004), it was proposed that the low-threshold L-type current in MNCs might be mediated by channels constructed of α1D subunits (Fisher & Bourque, 1995a). If this is the case, the increase in high-threshold nifedipine-sensitive current in MNCs caused by water deprivation might be due to an increase in currents mediated by the α1C subunit. Our experiments are also not able to resolve where the increase in L-type current is occurring within the somatodendritic area. Most of the cells used for these recordings consisted of MNC somata with short stretches of proximal dendrite (see Fig. 3) and immunocytochemical evidence suggests that α1C and α1D are distributed on MNC somata and dendrites (Joux et al. 2001). If the increase in current occurs primarily in the dendrites, the isolated MNC preparation might underestimate the total increase.
Although the physiological significance of the increase is not yet clear, the robust enhancement of a single component of Ca2+ current may enable the selective activation of one or more Ca2+-activated pathways without causing a large increase in total Ca2+ influx. The L-type channels that are augmented may, through selective localization or association with specific proteins, carry out specific physiological functions. Activation of such functions may be important for the adaptation of the MNCs to maintain increased release of OT and VP for long periods. The nature of these functions in MNCs is not known, but there are several possibilities. One possible role for increased L-type Ca2+ currents is to cause an enhancement of gene transcription and translation. L-type Ca2+ channels are particularly important in the signalling machinery that triggers activity-dependent changes in gene expression, in part because the C-termini of the α1C (Weick et al. 2003) and α1D (Zhang et al. 2005) subunits both contain specific sequences that localize the channels to allow specific activation of the cAMP response element binding protein (CREB), a transcription factor. L-type Ca2+ current augmentation could therefore be important in the complex regulation of gene expression that occurs in the MNCs during water deprivation (Burbach et al. 2001).
A second possibility is that the increased density of L-type Ca2+ channels could boost the SD release of VP, OT and/or other possible autocrine regulators such as apelin (De Mota et al. 2004) and dynorphin (Brown & Bourque, 2004). A specific role for L-type Ca2+ channels in the triggering of SD exocytotic release of VP would be consistent with what is known about exocytotic release from the somata of other cell types (Fisher & Bourque, 2001). L-type Ca2+ currents, for example, were found to mediate dynorphin release from the dendrites, but not axons, of hippocampal granule cells (Simmons et al. 1995). An increase in the SD secretion of neuropeptides could regulate MNC function, and an increase in somatic L-type Ca2+ current could be important in enhancing this release. The increase in Ca2+ current that occurs during lactation has been proposed to lead to an increase in evoked secretion of OT as measured by changes in membrane capacitance (De Kock et al. 2003).
A third possibility is that the increased density leads to the regulation of MNC firing patterns through an enhanced activation of Ca2+-dependent potentials. Ca2+-dependent currents such as the DAP, and slow and medium AHPs (sAHP and mAHP) are important regulators of MNC firing patterns (Roper et al. 2004) and an elevation of L-type current could cause an increase in their activation. It has been shown that long-term water deprivation leads to a change in MNC firing pattern, with the bursts becoming shorter and faster (Dyball & Pountney, 1973), and it is possible that an increase in the activation of Ca2+-dependent K+ currents could contribute to these changes.
Although the extent of similarity of the electrophysiological changes that occur during dehydration and lactation is not known, some parallels are apparent. Increases in MNC membrane capacitance (see Fig. 1) are also seen in OT-releasing MNCs during lactation (De Kock et al. 2003; Teruyama & Armstrong, 2005). Lactation is also associated with an increase in total Ca2+ current amplitude. It is not known whether increases in L-type current contribute to this increase, but both groups reported increases in peak and sustained Ca2+ currents, which could include L-type currents. OT-releasing MNCs also show an increase in the sAHP and mAHP during lactation (Teruyama & Armstrong, 2002, 2005). The mechanism for this increase is also not known, but it is possible that an increase in a specific subtype of Ca2+ current, such as L-type, could be responsible for this increase. If L-type current is specifically involved in activation of the AHPs, a selective increase in this current could cause a marked increase in Ca2+-dependent AHPs while causing only a small increase in total current. A specific association between L-type Ca2+ channels and Ca2+-dependent K+ currents has been reported for hippocampal neurons, in which activation of L-type Ca2+ channels is more likely to trigger opening of small-conductance Ca2+-dependent K+ channels than is the opening of N-type Ca2+ channels, apparently because the L-type Ca2+ channels are physically closer (Marrion & Tavalin, 1998).
The mechanism underlying the increase in the density of L-type Ca2+ channels remains unclear. The observed increase in 3H-isradipine binding suggests that there is an increase in the total number of L-type channels on the plasma membrane of the MNCs or some other cell type within the SON. Since the concentration of 3H-isradipine that we chose should have saturated the channels (based on our results from whole rat brain; see Fig. 4A), the increase in binding could not be explained by an increase in binding affinity, but only an increase in the number of channels available for binding. Although we cannot rule out the possibility that the observed increase in the density of binding sites for dihydropyridines occurs independently of the increase in nifedipine-sensitive current in MNC somata (i.e. there may also be an increase in the L-type channels in other neurons, glia or endothelial cells in the SON), the most parsimonious explanation is that an increase in the number of L-type channels on MNC somata explains both observations. The fact that the increase in binding sites (32%) was much lower than the increase in nifedipine-sensitive current in MNCs (80%) may be due to the fact that there are other cells in the SON that express L-type currents that are unaffected by dehydration.
If dehydration does cause an increase in the number of functional L-type channels on the plasma membrane of the MNCs, there are at least three possible mechanisms. One possibility is that water deprivation leads to an increase in L-type current amplitude by increasing the synthesis of L-type channels. Our PCR data suggest that there is no increase in the levels of mRNA coding for either of the subunits that mediate L-type currents. This is consistent with the results of a gene microarray study that did not list Ca2+ channel genes among those altered in the SON of rats following water deprivation (Hindmarch et al. 2006). These results, however, cannot rule out the possibility that synthesis of the L-type Ca2+ channel α1 subunit has increased, either because dehydration causes an increase mRNA in MNCs that was not detected by our method or because there is an elevation in protein synthesis that occurs independently of an increase in mRNA. A second possibility is that post-translational modification of L-type channels in the MNC plasma membrane results in an increase in channel activity as well as their availability for binding with extracellular ligands. A third possibility is that dehydration causes translocation of L-type Ca2+ channels from an intracellular store to the plasma membrane. Such a mechanism has been proposed to explain an increase in dynorphin receptors in MNCs during dehydration (Shuster et al. 1999). Recruitment of Ca2+ channels from internal stores has been described in the bag cell neurons of the marine mollusk Aplysia californica (Strong et al. 1987; White & Kaczmarek, 1997). In these neuroendocrine cells, stimulation of neuropeptide release is associated with the activation of protein kinase C and the subsequent translocation of a specific Ca2+ channel type from cytoplasmic vesicles to the plasma membrane (Strong et al. 1987; White & Kaczmarek, 1997). Immunoreactivity for Ca2+ channel α1 subunits has been observed in internal large dense core vesicle-like compartments in the MNC cytoplasm (Fisher et al. 2000), which is consistent with the idea that Ca2+ channels could be translocated to the MNC plasma membrane when the need for neuropeptide release is high.
Our studies provide evidence for a novel form of osmoregulation of Ca2+ current in the MNCs. The increase of L-type Ca2+ current in the MNC somata may be an important component of the adaptation that occurs in these cells during sustained dehydration.
Acknowledgments
This research was supported by the Heart and Stroke Foundation of Canada through a research grant to T.E.F. T.E.F. was the recipient of a New Investigator Award from the Canadian Institutes of Health Research and the Saskatchewan Health Research Foundation through the CIHR Regional Partnership Program. W.Z. is supported by a Personnel Award from the Heart and Stroke Foundation of Saskatchewan. The authors wish to thank Xuan Vo for his excellent technical assistance.
References
- Andrew RD, Dudek FE. Burst discharge in mammalian neuroendocrine cells involves an intrinsic regenerative mechanism. Science. 1983;221:1050–1052. doi: 10.1126/science.6879204. [DOI] [PubMed] [Google Scholar]
- Bicknell RJ. Optimizing release from peptide hormone secretory nerve terminals. J Exp Biol. 1988;139:51–65. doi: 10.1242/jeb.139.1.51. [DOI] [PubMed] [Google Scholar]
- Bourque CW, Brown DA. Apamin and d-tubocurarine block the afterhyperpolarization of rat supraoptic neurosecretory neurons. Neurosci Lett. 1987;82:185–190. doi: 10.1016/0304-3940(87)90127-3. [DOI] [PubMed] [Google Scholar]
- Bourque CW, Oliet SH. Osmoreceptors in the central nervous system. Annu Rev Physiol. 1997;59:601–619. doi: 10.1146/annurev.physiol.59.1.601. [DOI] [PubMed] [Google Scholar]
- Brown CH, Bourque CW. Autocrine feedback inhibition of plateau potentials terminates phasic bursts in magnocellular neurosecretory cells of the rat supraoptic nucleus. J Physiol. 2004;557:949–960. doi: 10.1113/jphysiol.2004.063818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbach JP, Luckman SM, Murphy D, Gainer H. Gene regulation in the magnocellular hypothalamo-neurohypophysial system. Physiol Rev. 2001;81:1197–1267. doi: 10.1152/physrev.2001.81.3.1197. [DOI] [PubMed] [Google Scholar]
- De Kock CP, Wierda KD, Bosman LW, Min R, Koksma JJ, Mansvelder HD, Verhage M, Brussaard AB. Somatodendritic secretion in oxytocin neurons is upregulated during the female reproductive cycle. J Neurosci. 2003;23:2726–2734. doi: 10.1523/JNEUROSCI.23-07-02726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mota N, Reaux-Le Goazigo A, El Messari S, Chartrel N, Roesch D, Dujardin C, Kordon C, Vaudry H, Moos F, Llorens-Cortes C. Apelin, a potent diuretic neuropeptide counteracting vasopressin actions through inhibition of vasopressin neuron activity and vasopressin release. Proc Natl Acad Sci U S A. 2004;101:10464–10469. doi: 10.1073/pnas.0403518101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyball RE, Pountney PS. Discharge patterns of supraoptic and paraventricular neurones in rats given a 2 per cent NaCl solution instead of drinking water. J Endocrinol. 1973;56:91–98. doi: 10.1677/joe.0.0560091. [DOI] [PubMed] [Google Scholar]
- Fisher TE, Bourque CW. Voltage-gated calcium currents in the magnocellular neurosecretory cells of the rat supraoptic nucleus. J Physiol. 1995a;486:571–580. doi: 10.1113/jphysiol.1995.sp020835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher TE, Bourque CW. Distinct ω-agatoxin-sensitive calcium currents in somata and axon terminals of rat supraoptic neurones. J Physiol. 1995b;489:383–388. doi: 10.1113/jphysiol.1995.sp021059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher TE, Bourque CW. Calcium-channel subtypes in the somata and axon terminals of magnocellular neurosecretory cells. Trends Neurosci. 1996;19:440–444. doi: 10.1016/0166-2236(96)10034-5. [DOI] [PubMed] [Google Scholar]
- Fisher TE, Bourque CW. The function of Ca2+ channel subtypes in exocytotic secretion: new perspectives from synaptic and non-synaptic release. Prog Biophys Mol Biol. 2001;77:269–303. doi: 10.1016/s0079-6107(01)00017-7. [DOI] [PubMed] [Google Scholar]
- Fisher TE, Carrion-Vazquez M, Fernandez JM. Intracellular Ca2+ channel immunoreactivity in neuroendocrine axon terminals. FEBS Lett. 2000;482:131–138. doi: 10.1016/s0014-5793(00)02043-3. [DOI] [PubMed] [Google Scholar]
- Fisher TE, Voisin DL, Bourque CW. Density of transient K+ current influences excitability in acutely isolated vasopressin and oxytocin neurones of rat hypothalamus. J Physiol. 1998;511:423–432. doi: 10.1111/j.1469-7793.1998.423bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foehring RC, Armstrong WE. Pharmacological dissection of high-voltage-activated Ca2+ current types in acutely dissociated rat supraoptic magnocellular neurons. J Neurophysiol. 1996;76:977–983. doi: 10.1152/jn.1996.76.2.977. [DOI] [PubMed] [Google Scholar]
- Ghamari-Langroudi M, Bourque CW. Caesium blocks depolarizing after-potentials and phasic firing in rat supraoptic neurones. J Physiol. 1998;510:165–175. doi: 10.1111/j.1469-7793.1998.165bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghamari-Langroudi M, Bourque CW. Flufenamic acid blocks depolarizing afterpotentials and phasic firing in rat supraoptic neurones. J Physiol. 2002;545:537–542. doi: 10.1113/jphysiol.2002.033589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghamari-Langroudi M, Bourque CW. Muscarinic receptor modulation of slow afterhyperpolarization and phasic firing in rat supraoptic nucleus neurons. J Neurosci. 2004;24:7718–7726. doi: 10.1523/JNEUROSCI.1240-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorbel MT, Sharman G, Leroux M, Barrett T, Donovan DM, Becker KG, Murphy D. Microarray analysis reveals interleukin-6 as a novel secretory product of the hypothalamo-neurohypophyseal system. J Biol Chem. 2003;278:19280–19285. doi: 10.1074/jbc.M209902200. [DOI] [PubMed] [Google Scholar]
- Glasgow E, Kusano K, Chin H, Mezey E, Young WS, 3, Gainer H. Single cell reverse transcription-polymerase chain reaction analysis of rat supraoptic magno-cellular neurons: neuropeptide phenotypes and high voltage-gated calcium channel subtypes. Endocrinology. 1999;140:5391–5401. doi: 10.1210/endo.140.11.7136. [DOI] [PubMed] [Google Scholar]
- Gouzenes L, Desarmenien MG, Hussy N, Richard P, Moos FC. Vasopressin regularizes the phasic firing pattern of rat hypothalamic magnocellular vasopressin neurons. J Neurosci. 1998;18:1879–1885. doi: 10.1523/JNEUROSCI.18-05-01879.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greffrath W, Martin E, Reuss S, Boehmer G. Components of after-hyperpolarization in magnocellular neurones of the rat supraoptic nucleus in vitro. J Physiol. 1998;513:493–506. doi: 10.1111/j.1469-7793.1998.493bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton GI. Function-related plasticity in hypothalamus. Annu Rev Neurosci. 1997;20:375–397. doi: 10.1146/annurev.neuro.20.1.375. [DOI] [PubMed] [Google Scholar]
- Hindmarch C, Yao S, Beighton G, Paton J, Murphy D. A comprehensive description of the transcriptome of the hypothalamoneurohypophyseal system in euhydrated and dehydrated rats. Proc Natl Acad Sci U S A. 2006;103:1609–1614. doi: 10.1073/pnas.0507450103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa M, Schwab Y, Natah S, Hillard CJ, Mackie K, Sharkey KA, Pittman QJ. Dendritically released transmitters cooperate via autocrine and retrograde actions to inhibit afferent excitation in rat brain. J Physiol. 2004;559:611–624. doi: 10.1113/jphysiol.2004.066159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K, Lambert DG. A comparative study of L-type voltage sensitive Ca2+ channels in rat brain regions and cultured neuronal cells. Neurosci Lett. 1997;223:169–172. doi: 10.1016/s0304-3940(97)13434-6. [DOI] [PubMed] [Google Scholar]
- Hurbin A, Orcel H, Alonso G, Moos F, Rabie A. The vasopressin receptors colocalize with vasopressin in the magnocellular neurons of the rat supraoptic nucleus and are modulated by water balance. Endocrinology. 2002;143:456–466. doi: 10.1210/endo.143.2.8643. [DOI] [PubMed] [Google Scholar]
- Ichida S, Wada T, Sekiguchi M, Kishino H, Okazaki Y, Akimoto T. Characteristics of specific 125I-omega-conotoxin GVIA binding in rat whole brain. Neurochem Res. 1993;18:1137–1144. doi: 10.1007/BF00978364. [DOI] [PubMed] [Google Scholar]
- Joux N, Chevaleyre V, Alonso G, Boissin-Agasse L, Moos FC, Desarmenien MG, Hussy N. High voltage-activated Ca2+ currents in rat supraoptic neurones: biophysical properties and expression of the various channel α1 subunits. J Neuroendocrinol. 2001;13:638–649. doi: 10.1046/j.1365-2826.2001.00679.x. [DOI] [PubMed] [Google Scholar]
- Kombian SB, Mouginot D, Pittman QJ. Dendritically released peptides act as retrograde modulators of afferent excitation in the supraoptic nucleus in vitro. Neuron. 1997;19:903–912. doi: 10.1016/s0896-6273(00)80971-x. [DOI] [PubMed] [Google Scholar]
- Lemos JR, Nowycky MC. Two types of calcium channels coexist in peptide-releasing vertebrate nerve terminals. Neuron. 1989;2:1419–1426. doi: 10.1016/0896-6273(89)90187-6. [DOI] [PubMed] [Google Scholar]
- Li Z, Hatton GI. Reduced outward K+ conductances generate depolarizing after-potentials in rat supraoptic nucleus neurones. J Physiol. 1997;505:95–106. doi: 10.1111/j.1469-7793.1997.095bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscombe D, Helton TD, Xu W. L-type calcium channels: the low down. J Neurophysiol. 2004;92:2633–2641. doi: 10.1152/jn.00486.2004. [DOI] [PubMed] [Google Scholar]
- Liu XH, Zhang W, Fisher TE. A novel osmosensitive voltage gated cation current in rat supraoptic neurones. J Physiol. 2005;568:61–68. doi: 10.1113/jphysiol.2005.093773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig M, Pittman QJ. Talking back: dendritic neurotransmitter release. Trends Neurosci. 2003;26:255–261. doi: 10.1016/S0166-2236(03)00072-9. [DOI] [PubMed] [Google Scholar]
- Marrion NV, Tavalin SJ. Selective activation of Ca2+-activated K+ channels by co-localized Ca2+ channels in hippocampal neurons. Nature. 1998;395:900–905. doi: 10.1038/27674. [DOI] [PubMed] [Google Scholar]
- Murphy LD, Herzog CE, Rudick JB, Fojo AT, Bates SE. Use of the polymerase chain reaction in the quantitation of mdr-1 gene expression. Biochemistry. 1990;29:10351–10356. doi: 10.1021/bi00497a009. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Fukuta Y, Kiyoshi A, Iwatsuki Y, Ishii K, Ishikawa T, Iida M, Iwata H, Enomoto M. (+)-[3H]isradipine and [3H]glyburide bindings to heart and lung membranes from rats with monocrotaline-induced pulmonary hypertension. Jpn J Pharmacol. 1999;81:176–184. doi: 10.1254/jjp.81.176. [DOI] [PubMed] [Google Scholar]
- Oliet SH, Bourque CW. Properties of supraoptic magnocellular neurones isolated from the adult rat. J Physiol. 1992;455:291–306. doi: 10.1113/jphysiol.1992.sp019302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliet SH, Bourque CW. Mechanosensitive channels transduce osmosensitivity in supraoptic neurons. Nature. 1993;364:341–343. doi: 10.1038/364341a0. [DOI] [PubMed] [Google Scholar]
- Olivera BM, Gray WR, Zeikus R, McIntosh JM, Varga J, Rivier J, De Santos V, Cruz LJ. Peptide neurotoxins from fish-hunting cone snails. Science. 1985;230:1338–1343. doi: 10.1126/science.4071055. [DOI] [PubMed] [Google Scholar]
- Pan JQ, Lipscombe D. Alternative splicing in the cytoplasmic II–III loop of the N-type Ca channel α1B subunit: functional differences are β subunit- specific. J Neurosci. 2000;20:4769–4775. doi: 10.1523/JNEUROSCI.20-13-04769.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulain DA, Wakerley JB. Electrophysiology of hypothalamic magnocellular neurones secreting oxytocin and vasopressin. Neuroscience. 1982;7:773–808. doi: 10.1016/0306-4522(82)90044-6. [DOI] [PubMed] [Google Scholar]
- Roper P, Callaway J, Armstrong W. Burst initiation and termination in phasic vasopressin cells of the rat supraoptic nucleus: a combined mathematical, electrical, and calcium fluorescence study. J Neurosci. 2004;24:4818–4831. doi: 10.1523/JNEUROSCI.4203-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster SJ, Riedl M, Li X, Vulchanova L, Elde R. Stimulus-dependent translocation of κ opioid receptors to the plasma membrane. J Neurosci. 1999;19:2658–2664. doi: 10.1523/JNEUROSCI.19-07-02658.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons ML, Terman GW, Gibbs SM, Chavkin C. L-type calcium channels mediate dynorphin neuropeptide release from dendrites but not axons of hippocampal granule cells. Neuron. 1995;14:1265–1272. doi: 10.1016/0896-6273(95)90273-2. [DOI] [PubMed] [Google Scholar]
- Strong JA, Fox AP, Tsien RW, Kaczmarek LK. Stimulation of protein kinase C recruits covert calcium channels in Aplysia bag cell neurons. Nature. 1987;325:714–717. doi: 10.1038/325714a0. [DOI] [PubMed] [Google Scholar]
- Tasker JG, Di S, Boudaba C. Functional synaptic plasticity in hypothalamic magnocellular neurons. Prog Brain Res. 2002;139:113–119. doi: 10.1016/s0079-6123(02)39011-3. [DOI] [PubMed] [Google Scholar]
- Teruyama R, Armstrong WE. Changes in the active membrane properties of rat supraoptic neurones during pregnancy and lactation. J Neuroendocrinol. 2002;14:933–944. doi: 10.1046/j.1365-2826.2002.00844.x. [DOI] [PubMed] [Google Scholar]
- Teruyama R, Armstrong WE. Enhancement of calcium-dependent afterpotentials in oxytocin neurons of the rat supraoptic nucleus during lactation. J Physiol. 2005;566:505–518. doi: 10.1113/jphysiol.2005.085985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodosis DT, Piet R, Poulain DA, Oliet SH. Neuronal, glial and synaptic remodeling in the adult hypothalamus: functional consequences and role of cell surface and extracellular matrix adhesion molecules. Neurochem Int. 2004;45:491–501. doi: 10.1016/j.neuint.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Tweedle CD, Hatton GI. Ultrastructural changes in rat hypothalamic neurosecretory cells and their associated glia during minimal dehydration and rehydration. Cell Tissue Res. 1977;181:59–72. doi: 10.1007/BF00222774. [DOI] [PubMed] [Google Scholar]
- Tweedle CD, Hatton GI. Evidence for dynamic interactions between pituicytes and neurosecretory axons in the rat. Neuroscience. 1980;5:661–671. doi: 10.1016/0306-4522(80)90063-9. [DOI] [PubMed] [Google Scholar]
- Wakerley JB, Poulain DA, Brown D. Comparison of firing patterns in oxytocin- and vasopressin-releasing neurones during progressive dehydration. Brain Res. 1978;148:425–440. doi: 10.1016/0006-8993(78)90730-8. [DOI] [PubMed] [Google Scholar]
- Weick JP, Groth RD, Isaksen AL, Mermelstein PG. Interactions with PDZ proteins are required for L-type calcium channels to activate cAMP response element-binding protein-dependent gene expression. J Neurosci. 2003;23:3446–3456. doi: 10.1523/JNEUROSCI.23-08-03446.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White BH, Kaczmarek LK. Identification of a vesicular pool of calcium channels in the bag cell neurons of Aplysia californica. J Neurosci. 1997;17:1582–1595. doi: 10.1523/JNEUROSCI.17-05-01582.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Maximov A, Fu Y, Xu F, Tang TS, Tkatch T, Surmeier DJ, Bezprozvanny I. Association of CaV1.3, L-type calcium channels with Shank. J Neurosci. 2005;25:1037–1049. doi: 10.1523/JNEUROSCI.4554-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Bourque CW. Osmometry in osmosensory neurons. Nat Neurosci. 2003;6:1021–1022. doi: 10.1038/nn1124. [DOI] [PubMed] [Google Scholar]
- Zingg HH, Lefebvre D, Almazan G. Regulation of vasopressin gene expression in rat hypothalamic neurons. Response to osmotic stimulation. J Biol Chem. 1986;261:12956–12959. [PubMed] [Google Scholar]