Abstract
Despite numerous investigations on the corticospinal system there is only scant information on neuronal networks mediating actions of corticospinal neurones on ipsilateral motoneurones. We have previously demonstrated double crossed pathways through which pyramidal tract neurones can influence ipsilateral motoneurones, via contralaterally descending reticulospinal neurones and spinal commissural interneurones. The aim of the present study was to examine the effects of stimulation of pyramidal tract (PT) fibres mediated via ipsilaterally descending pathways and to find out which neurones relay these effects. This was done by using intracellular recordings from 96 lumbar motoneurones in deeply anaesthetized cats. To eliminate actions of fibres descending on the side contralateral to the location of the motoneurones, the spinal cords were hemisected on this side at a low-thoracic level. Stimuli that selectively activated ipsilateral PT fibres evoked EPSPs and/or IPSPs in 34/47 motoneurones tested. These PSPs were evoked at latencies indicating that the most direct coupling between PT neurones and motoneurones in uncrossed pathways is disynaptic. Occlusion and spatial facilitation between actions evoked by stimulation of ipsilateral PT and of reticulospinal tract fibres in the ipsilateral medial longitudinal fascicle (MLF) indicated that PT actions are mediated by reticulospinal neurones with axons in the MLF. However, after transection of the MLF in the caudal medulla, stimulation of the ipsilateral PT continued to evoke EPSPs and IPSPs with characteristics similar to when the MLF was intact (in 15/49 motoneurones) suggesting the existence of parallel disynaptic pathways via other relay neurones.
Actions of corticospinal neurones are much more potent on contralateral than on ipsilateral limb motoneurones, reflecting the predominant crossed projections of these neurones (see Phillips & Porter, 1977). However, varying proportions of uncrossed corticospinal tract fibres have been found in different species from about 5% in the rat (e.g. Brosamle & Schwab, 1997), up to 37% in the cat (Armand & Kuypers, 1980; also see Chambers & Liu, 1957; Nyberg-Hansen & Brodal, 1963; Flindt-Egebak, 1979), up to 23% in the cervical enlargement of the macaque (Porter & Lemon, 1993), about 10% in the lumbosacral enlargement in rhesus monkeys (Lacroix et al. 2004) and up to 25% in humans (for references see Nyberg-Hansen & Rinvik, 1963). Actions evoked by stimulation of the ipsilateral pyramidal tract (PT) were generally considered to be hardly detectable in the cat (e.g. Lance, 1954) but a few studies revealed both excitatory and inhibitory actions on hindlimb motoneurones (Van der Muelen & Ghez, 1970; Endo et al. 1975; Edgley et al. 2004). Ipsilateral PT actions were found on the upper extremities in monkeys (e.g. Bucy & Fulton, 1933) and in man (for references see Cauraugh & Summers, 2005) but to our knowledge no such actions were reported to be evoked on the lower extremities.
Ipsilateral PT neurones might, theoretically, affect lumbar motoneurones in three ways: via their uncrossed spinal projections, via ipsilaterally descending supraspinal neurones activated by PT neurones and via double-crossed pathways, including contralaterally descending reticulospinal (RS) neurones and commissural interneurones that re-cross at the level of the lumbosacral enlargement (Edgley et al. 2004; Jankowska et al. 2005; Jankowska & Edgley, 2006). Only actions evoked by double crossed pathways have so far been investigated in detail. The aim of the present study was therefore to analyse uncrossed actions of pyramidal tract neurones evoked by ipsilaterally descending PT fibres and/or by their similarly ipsilaterally descending relay neurones on lumbar motoneurones. As schematically indicated in Fig. 1, the most direct connections between corticospinal neurones and ipsilaterally located motoneurones have been hypothesized to be via uncrossed projections of PT neurones and ipsilaterally located premotor interneurones, or via ipsilaterally descending RS neurones. For the sake of simplicity, cell bodies of either ipsilaterally or contralaterally descending RS neurones are indicated on the left or right side but they could be located on both sides (Mitani et al. 1988). Actions of contralaterally descending PT and RS neurones and of their axon collaterals given off within the lumbo-sacral segments were prevented by hemisection of the spinal cord at a low thoracic level.
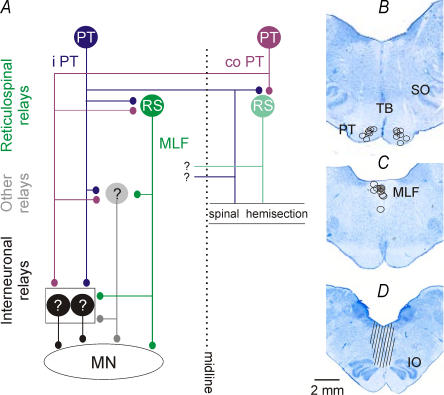
Figure 1. Diagram of neuronal pathways examined in the present study and the location of the stimulation sites.
A, diagram of the putative coupling in uncrossed pathways between ipsilaterally descending left pyramidal tract (PT) fibres and hindlimb motoneurones (MN) on the left side. Premotor interneurones in the lumbosacral enlargement (black circles) are targeted by ipsilateral PT neurones and reticulospinal (RS) neurones projecting via the ipsilateral medial longitudinal fascicle (MLF) and by other hypothetical relay neurones. The stimuli were applied at sites indicated by ipsilateral (i) PT, contralateral (co) PT and MLF. Note that hemisection of the spinal cord at a low thoracic level prevented any actions of crossed PT fibres within the lumbosacral enlargement. However, some actions of axon collaterals re-crossing at more rostral levels and targeting so far undefined neurones (indicated with ?) would be possible. B and C, reconstruction of stimulation sites in the ipsilateral and contralateral PT and the ipsilateral MLF in all of the experiments of the present series. They are displayed on a representative brainstem section in the plane of the insertion of the electrodes. D, reconstruction of the extent of the MLF transection in one of the animals. IO, inferior olive; SO, superior olive; TB, trapezoid body.
Methods
Preparation
The experiments were performed on eight deeply anaesthetized cats weighing 3.1–5.0 kg. All experimental procedures were approved by the local ethics committee (Göteborgs djurförsöksetiska nämnd) and followed NIH and EU guidelines for animal care. Anaesthesia was induced with sodium pentobarbital (40–44 mg kg−1, i.p.) and maintained with intermittent doses of α-chloralose (Rhône-Poulenc Santé, France; 5 mg kg−1; administered every 1–2 h, up to about 25 mg kg−1 and every 2–3 h up to about 55 mg kg−1, i.v.). Additional doses of α-chloralose were given when increases in blood pressure or heart rate, which were continuously monitored, were evoked by peripheral or central stimulation, or if the pupils dilated. During recordings, neuromuscular transmission was blocked by pancuronium bromide (Pavulon, Organon, Sweden; about 0.2 mg kg−1 h−1i.v.) and the animals were artificially ventilated. Mean blood pressure was kept at 100–130 mmHg and the end-tidal concentration of CO2 at about 4% by adjusting the parameters of artificial ventilation and the rate of a continuous infusion of a bicarbonate buffer solution with 5% glucose (1–2 ml h−1 kg−1). Core body temperature was kept at about 38°C by servo-controlled infrared lamps. The experiments were terminated by a lethal dose of anaesthetic and formalin perfusion resulting in cardiac arrest. The effectiveness of synaptic transmission was increased by intravenous application of 4-aminopyridine (4-AP) in doses 0.1–0.2 mg kg−1, i.v. Atropine (0.05–0.2 mg kg−1i.m.) and dexamethasone (1 mg kg−1i.m.; Oradexon, Organon, Holland) were given at the beginning of the surgery in most of the experiments.
The spinal cord was exposed by laminectomy from the third to the seventh lumbar (L3–L7) segments and at the level of the low thoracic (Th11–Th13) and upper cervical (C3 or C4) segments. The spinal cord was hemisected at the Th12–Th13 level on the right side (see Fig. 1) before the recording began. The hemisection was performed after opening the dura on the right side, transecting the dorsal columns and exposing the central canal under a dissection microscope. Using watchmaker's forceps the lateral and ventral funiculi on the right side were then torn apart intrapially over a distance of about 2–3 mm until the midline was reached. The gap between the transected funiculi was filled with a small piece of gelfoam to keep them separated. The completeness of the hemisection was verified after formalin perfusion and additional post fixation by splitting the two halves of the spinal cord about 1–2 cm away from the level of the hemisection and checking that no parts of the right half remained attached to the left side within the area of the hemisection. In order to verify that no damage to the left ventral funiculus (e.g. by pressure) occurred, descending volleys evoked by stimulation of the MLF were recorded both rostral and caudal to the hemisection and from the lumbar segments. The data from two experiments in which the hemisection was not complete, or the ipsilateral funiculi were damaged, have not been included.
The cerebellum was exposed to allow insertion of stimulating electrodes into both PTs at the level of the superior olives (Fig. 1B) and the ipsilateral MLF just rostral to the inferior olive (Fig. 1C), as well as to enable a transection of the MLF. The MLF was transected in four experiments 3–6 mm caudal to the stimulating electrode, at the levels corresponding to the Horsley–Clarke planes P11–12, as described by Matsuyama & Jankowska (2004). This was done after aspiration of the posterior cerebellar vermis and exposure of the floor of the fourth ventricle. The transection was deepened until the descending volleys following MLF stimulation recorded at Th12 level disappeared. Reconstruction of one of the transections is shown in Fig. 1D.
A number of peripheral nerves on the left side were dissected free and mounted on stimulating electrodes. They included the quadriceps (Q) and sartorius branches of the femoral nerve mounted in subcutaneous cuff electrodes; the posterior biceps and semitendinosus (PBST), the anterior biceps and semimembranosus (SMAB), the gastrocnemius–soleus (GS), the plantaris, the flexor digitorum and hallucis longus and the deep peroneal including extensor digitorum longus and tibialis anterior nerves.
Stimulation and recording
Tungsten electrodes were placed in both medullary PTs at the level of the superior olive (SO) and in the ipsilateral MLF at the level of the inferior olive (IO) (at Horsley–Clarke's horizontal levels about −5 and −10.5, respectively). The electrodes were inserted through the cerebellum (at an angle of 35 deg, with the tip directed rostrally) and left at sites from which descending volleys were evoked at threshold stimulus intensities of 20 μA or less, and were maximal at 150–200 μA; the descending volleys were recorded transdurally from the C3 segment and from the Th12 segment caudal to the hemisection. The stimulation sites were marked with electrolytic lesions and verified histologically on transverse sections of the brainstem cut in the plane of insertion of the electrodes using a freezing microtome and counterstained with cresyl violet (Fig. 1B and C).
For activation of the corticospinal and reticulospinal tract fibres constant current cathodal stimuli (0.2 ms, 100–150 μA or less for PT, but up to 200 μA for MLF) were used. The risk of inadvertent activation of MLF fibres was estimated by comparing descending volleys evoked from the MLF, from within the PTs and from the areas dorsal to the PTs. As shown in panels A and B of Fig. 2 in Jankowska et al. (2006) even stimuli of 200 μA evoked hardly any volleys at the Th12 recording site when they were applied up to 1.8 mm above the PT.
The risk of activation of fibres in one of the PTs by stimuli applied in the other PT was estimated in the following way: stimuli were applied to the left and right PT separately and together at time intervals at which the second stimulus applied to the same fibres would be ineffective because of the refractory period after the first stimulus. The difference between the volleys evoked by the joint stimulation (ipsilateral PT and contralateral PT) and the sum of the volleys evoked by separate stimulation of the right and left PT (ipsilateral PT + contralateral PT) was therefore used as a measure of co-activation. As reported previously (Jankowska et al. 2006) spread of current has as a rule been found to occur at stimulus intensities exceeding 150 μA, but this was routinely verified after the electrodes had been placed and only effects of stimuli of 150 μA or less will be reported.
Near maximal stimuli applied in the MLF were expected to activate a large proportion of pontine and medullary reticulospinal tract fibres (see Krutki et al. 2003). Previous control tests have demonstrated that stimuli ≤ 200 μA applied in the dorsal and middle parts of the MLF would not activate fibres from the lateral vestibular (Deiter's) nucleus (Hongo et al. 1975), nor the more distantly located PT fibres, but they would activate vestibulospinal tract fibres arising from the medial vestibular nucleus that are intermingled with reticulospinal fibres in the MLF. However, these vestibulospinal fibres do not project caudally as far as the lumbar segments (Nyberg-Hansen & Mascitti, 1964). Any monosynaptically evoked effects of MLF stimuli in the lumbar segments could thus be attributed to reticulospinal fibres.
Glass micropipettes filled with a 2 m solution of potassium citrate (2–5 MΩ) were used for intracellular recording from α-motoneurones identified by antidromic activation following stimulation of a muscle nerve. Peripheral nerves were stimulated with constant voltage stimuli at intensities expressed in multiples of threshold for the activation of the most excitable fibres.
Analysis
Both original data and averages of 10–20 single records (with the time resolution of 30 or 40 μs per address) were stored online using software for sampling and analysis developed by E. Eide, T. Holmström & N. Pihlgren (Göteborg University). The latencies of the postsynaptic potentials evoked by stimulation of the PTs and the MLF were measured from the stimuli that were responsible for these potentials. The latencies of potentials evoked from the MLF were also measured from the descending volleys. Data are expressed as means ± s.e.m. Differences between data sets were assessed for statistical significance by using Student's t test for paired or unpaired samples.
Results
Evidence for di- or trisynaptic PSPs evoked via uncrossed pathways between ipsilateral corticospinal neurones and hindlimb motoneurones
Postsynaptic potentials evoked by ipsilateral PT neurones in hindlimb motoneurones were previously found to be very small or even marginal (about 100 μV; see Fig. 11C and F in Edgley et al. 2004) unless synaptic transmission was facilitated by 4-AP (Jankowska et al. 2005). In the present study we therefore analysed the uncrossed PT actions in preparations treated with 4-AP (see Methods). Under these conditions stimulation of the ipsilateral PT was found to evoke EPSPs and/or IPSPs in 34 of the 47 lumbar motoneurones tested. These PSPs were evoked at current intensities as low as 50 μA (Fig. 2B) and by single stimuli as well as by short trains of stimuli (Fig. 2A).
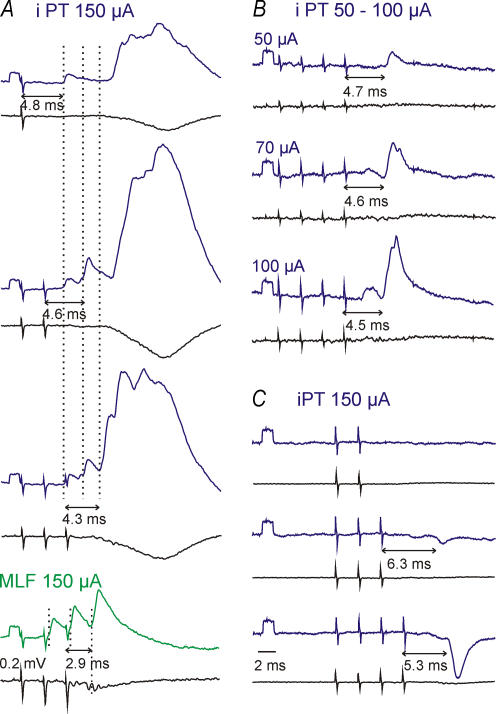
Figure 2. Excitatory and inhibitory postsynaptic potentials evoked by stimulation of the ipsilateral PT.
Averaged (n = 10–20) intracellular records (top traces) and records from the cord dorsum (bottom traces). A, B and C, records from 3 different motoneurones. A, comparison of EPSPs evoked by an increasing number of PT stimuli and of monosynaptic EPSPs from the MLF in a sartorius motoneurone. Three dotted lines and double headed horizontal arrows indicate early EPSPS evoked by the successive stimuli. B, comparison of EPSPs evoked by stimuli of increasing intensity in a posterior biceps–semitendinosus motoneurone. C, comparison of IPSPs evoked by increasing numbers of PT stimuli in a gastrocnemius–soleus motoneurone. The numbers below the arrows indicate latencies of the EPSPs or IPSPs from the stimuli that evoke them. Rectangular pulses at the beginning of the intracellular records are calibration pulses (0.2 mV). Time calibration in C is for all panels. In this and the following figures the negativity is down in intracellular records and up in records from the cord dorsum.
Stimulation of the ipsilateral PT evoked early PSPs accompanied by later PSPs (Figs 2A, and 4A and C), or only early PSPs (Figs 2B and C, and 4B). None of the early EPSPs fulfilled the criteria of monosynaptically evoked EPSPs because they all displayed marked temporal facilitation which characterizes disynaptically or polysynaptically but not monosynaptically evoked EPSPs (for discussion of features of disynaptic PSPs and differences between them and monosynaptically evoked PSPs see, e.g. Jankowska et al. 2003). Appearance of distinct EPSPs following successive stimuli (with the onset indicated by dotted lines in Fig. 2A) differentiated them from more fused (see top panel in Fig. 2A), most likely polysynaptically evoked later PSPs, but there were no reliable criteria to differentiate between disynaptically and trisynaptically evoked PSPs.
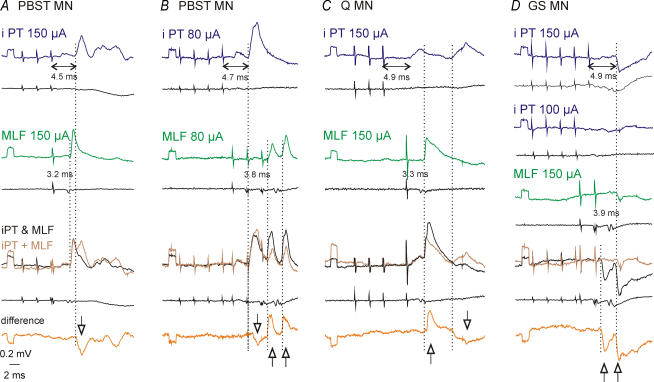
Figure 4. Evidence for mediation of EPSPs and IPSPs evoked by ipsilateral PT fibres via RS neurones.
Averaged (n = 10–20) intracellular records from 4 motoneurones and records from the cord dorsum (bottom traces). A–C, records in the first two rows show effects of separate stimulation of the ipsilateral PT and the ipsilateral MLF. Records in the third row show effects of their joint stimulation and the sum of the effects evoked by separate stimulation of PT or MLF; the bottom traces show the differences between them. D, records in the first row show IPSPs evoked by five stimuli delivered to the ipsilateral PT (at 150 μA), records in the second and third rows show effects of four weaker stimuli (at 100 μA) and two stimuli delivered to the ipsilateral MLF, respectively. Records in the fourth row show effects of their joint stimulation and the sum of their effects evoked by separate stimulation; the differences between them are shown in the bottom (fifth) row. Note the predominant occlusion (indicated by downward arrows) of EPSPs in A and the predominant facilitation (indicated by upward arrows) of EPSPs in C and of IPSPs in D, and both a decrease and an increase of EPSPs in B. MLF stimuli evoked monosynaptic EPSPs in A, monosynaptic and disynaptic EPSPs in C, disynaptic EPSPs in B and disynaptic IPSPs in D. Note that disynaptic PSPs from the MLF were evoked after the 2nd and 3rd but not 1st stimuli and that their latencies from both the stimuli and the first components of the descending MLF volleys were longer. Vertical dotted lines indicate onset of the facilitated or occluded PSPs evoked by PT and MLF stimuli.
Temporal facilitation of EPSPs and IPSPs was expressed as an increase in the size and a shortening of latency (Fig. 2A–C). For six motoneurones in which EPSPs were evoked by single stimuli, the amplitudes of EPSPs increased on average from 0.65 ± 0.47 mV after the 1st stimulus to 1.53 ± 0.50 and 2.37 ± 0.51 mV after the 2nd and 3rd stimuli, respectively. EPSPs evoked by the 2nd or later stimuli were evoked in a larger proportion of motoneurones than EPSPs evoked by single stimuli. For 21 out of 34 motoneurones in which the effects of different numbers of stimuli were compared, these proportions were 6/21 (29%) for the 1st stimulus, 12/21 (57%) for the 2nd stimulus, 20/21 (95%) for the 3rd stimulus and all 21 motoneurones for the 4th stimulus. For the whole sample, the mean amplitude of shortest latency EPSPs evoked by the 3rd stimulus was 0.73 ± 1.05 mV (n = 20). Linking of PSPs evoked from the ipsilateral PT to individual stimuli in a train was done by comparing those evoked by various numbers of stimuli, as illustrated in Fig. 2A and C.
In order for temporally facilitated EPSPs with characteristics of those illustrated in Fig. 2A and B to be evoked disynaptically from the PT, they should be evoked at appropriate latencies. Because latencies of the earliest of these EPSPs were 4.3–5.0 ms from the PT stimuli (diamonds in Fig. 3A), they would require axonal conduction velocities of 100–120 m s−1, allowing for about 3 ms conduction time along the distance of 300–320 mm between the caudal part of the medulla and the lumbosacral enlargement and about 1 ms per one synaptic relay. The PSPs with latencies of about 5–6 ms would be similarly compatible with disynaptic coupling if the neurones mediating them conducted at 80–100 m s−1 (considering about 4 ms conduction time for the same distance) but could also be evoked trisynaptically by faster conducting neurones.
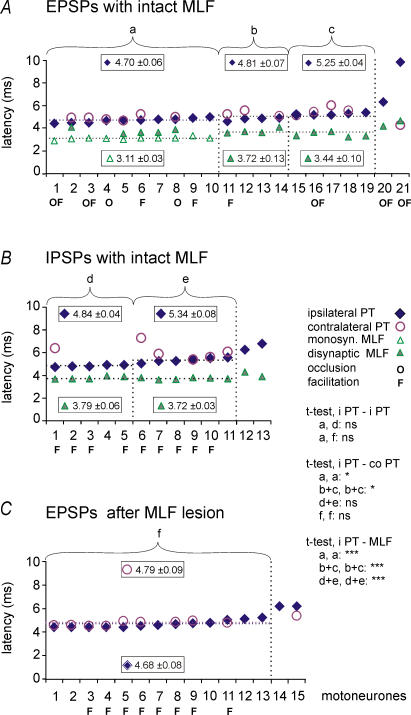
Figure 3. Minimal latencies of EPSPs and IPSPs evoked by stimulation of the ipsilateral PT, the contralateral PT and/or the ipsilateral MLF in the same motoneurones.
Latencies of EPSPs (A and C) and IPSPs (B) evoked by stimulation of the ipsilateral and contralateral PT at 100–150 μA and of the ipsilateral MLF at 150–200 μA. The latencies were measured from stimulus artefacts evoked by the effective stimuli. A, data points for 3 samples of PT excited motoneurones, a, b and c, separated by vertical dotted lines (each ranked in order of increasing latency). They were first subdivided on the basis of the presence (sample a) or absence (samples b and c) of monosynaptic EPSPs from the MLF. Data included in samples b and c were then subdivided taking into account differences between latencies of disynaptic EPSPs from the MLF and latencies of EPSPs of PT origin; ≤ 1.2 ms (in sample b) and > 1.2 ms (in sample c) and would not or would be compatible with mediation of PT actions by RS neurones (see text). B, data points for two samples of PT inhibited motoneurones, d and e, separated by a vertical line, each ranked in order of increasing latency. They were subdivided taking into account differences between latencies of disynaptic IPSPs from the MLF and latencies of IPSPs of PT origin (≤ 1.2 ms in sample d and > 1.2 ms in sample e) for the same reasons as in the case of samples b and c. C, comparison of latencies of EPSPs evoked by stimulation of the ipsilateral and the contralateral PTs after transection of the MLF. In all panels, horizontal dotted lines indicate mean latencies of PSPs evoked from the ipsilateral PT and from the MLF for the data to the left of the vertical dotted lines. ‘O’and ‘F’ below the abscissa indicate motoneurones in which occlusion and/or facilitation was found between synaptic actions of the ipsilateral PT and MLF in A and B or between actions of the ipsilateral and contralateral PT in C. Statistically significant differences, calculated using Student's t test for unpaired iPT–iPT and paired iPT–coPT and iPT–MLF samples, are indicated (***P < 0.001; *P < 0.05; ns, not significant). The longest latencies in A, B and C (to the right of the last vertical dotted lines) appeared to reflect PT actions evoked by more complex polysynaptic actions and were not included for the statistical comparisons.
Evidence for the earliest ipsilateral PT actions being evoked via reticulospinal neurones with axons in the MLF
In view of previous observations that stimulation of ipsilateral PT fibres facilitates synaptic actions evoked from the ipsilateral MLF (Figs 9 and 11 in Edgley et al. 2004) and that MLF stimuli evoke both monosynaptic and disynaptic PSPs in hindlimb motoneurones (Grillner & Lund, 1968; Grillner et al. 1971; Peterson et al. 1979; Takakusaki et al. 1989; Floeter et al. 1993) a substantial contribution of RS neurones to the earliest PSPs evoked by PT stimuli might be expected.
In order to verify this we first of all compared the effects of stimulation of the ipsilateral PT and of the MLF. The comparison showed that whenever EPSPs or IPSPs were evoked by PT stimuli they were evoked by MLF stimuli as well. We also used tests for either collision or spatial facilitation of effects of stimuli applied to the ipsilateral PT and the MLF.
If PSPs evoked by PT stimuli were mediated by RS neurones, a collision should occur between effects of suprathreshold stimuli at time intervals at which MLF fibres would be refractory after their preceding activation. Occlusion of PSPs due to collision between disynaptic PT and monosynaptic MLF actions is illustrated in Fig. 4A but it was also found between later actions of MLF and PT stimuli, e.g. in Fig. 4C. It might also have occurred between disynaptic actions of both PT and MLF stimuli (Fig. 4B, downward arrow) even though the illustrated decrease of the first EPSPs might also have been due to inhibitory interactions at a pre-motoneuronal level. Motoneurones in which occlusion was observed are indicated by ‘O’ below the abscissa in Fig. 3A.
In the case of submaximal ipsilateral PT and MLF stimuli, an opposite effect was expected, i.e. facilitation of their actions on subliminally excited RS or spinal neurones that mediated them. Examples of facilitation of EPSPs are shown in Fig. 4B and C and of IPSPs in Fig. 4D. The PSPs evoked upon joint actions of ipsilateral PT and MLF stimuli were larger than the sums of PSPs evoked when these stimuli were applied separately, as indicated by the upward arrows in the difference traces. Similar cases of facilitation in other motoneurones are indicated by ‘F’ below the abscissa in Fig. 3A and B.
Records from 10 motoneurones in which EPSPs with characteristics of disynaptic EPSPs from the ipsilateral PT were associated with monosynaptic EPSPs from the MLF are particularly well in keeping with actions from the ipsilateral PT being relayed via RS neurones. Latencies of these EPSPs, represented by data points of sample ‘a’ in Fig. 3A, ranged between 4.3 and 5.0 ms. They exceeded latencies of monosynaptic EPSPs evoked in the same motoneurones from the MLF by 1.3–1.9 ms, the difference corresponding to the previously found 1.1–1.6 ms or longer latencies with which PT stimuli activate RS neurones (Fig. 6 in Jankowska et al. 2006). Longer latency EPSPs and IPSPs of ipsilateral PT origin (in samples ‘c’ and ‘e’ in Fig. 3A and B for motoneurones in which PSPs from the MLF were evoked disynaptically) would also be compatible with PSPs relayed by RS neurones if these were mediated by RS neurones with indirect actions on motoneurones. Because latencies of PSPs evoked by PT stimuli in samples ‘c’ and ‘e’ exceeded latencies of disynaptic EPSPs from the MLF to the same extent as in the sample ‘a’ (with monosynaptic EPSPs from the MLF), they would be in keeping with trisynaptic rather than disynaptic PT actions. Therefore, an arbitrary border line between latencies of EPSPs disynaptically or trisynaptically evoked from the ipsilateral PT (to the left and right of the second dotted line, respectively) has been put at about 5 ms.
Indications for ipsilateral PT actions evoked via non-MLF pathways
Comparison between latencies of EPSPs evoked from the ipsilateral PT and from the MLF has suggested that some of the disynaptically evoked EPSPs of PT origin might not have been mediated by RS neurones because they were matched by disynaptically and not by monosynaptically evoked EPSPs from the MLF. These are EPSPs in sample ‘b’ in Fig. 3A; it will be noted that their range overlaps with the range of latencies of EPSPs evoked from the ipsilateral PT in sample ‘a’. EPSPs of sample ‘b’ might thus have been evoked disynaptically via other neurones.
A comparison between latencies of IPSPs evoked from the ipsilateral PT in sample ‘d’ in Fig. 3B and latencies of EPSPs attributable to disynaptic PT actions similarly puts in doubt that these IPSPs could be mediated by RS neurones. Because no statistically significant differences have been found between these latencies there is no reason to doubt that the IPSPs were also evoked disynaptically. The earliest IPSPs of PT origin mediated by RS neurones should, on the other hand, be evoked trisynaptically, in view of the lack of evidence for direct inhibitory actions of RS neurones on lumbar motoneurones (Wilson & Yoshida, 1968; Peterson et al. 1979).
Opposite late actions evoked by MLF and PT stimulation provide further indications that some of the PT actions on ipsilateral motoneurones are mediated via non-MLF pathways. Such opposite actions were found in nine motoneurones in which MLF stimulation evoked no actions or late IPSPs while PT stimulation evoked late EPSPs (similar to those illustrated in Fig. 2A).
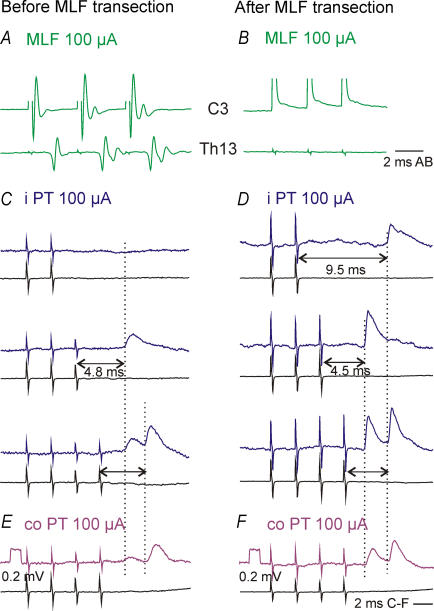
In order to verify that uncrossed PT actions might be relayed not only by RS neurones with axons in the ipsilateral MLF but also by other neurones, effects of stimulation of the ipsilateral PT were tested after having eliminated synaptic actions relayed via the MLF by its transection in the caudal part of the medulla resulting in abolition of MLF descending volleys at both the C3–4 and Th12–Th13 levels as illustrated in Fig. 5B. The common finding was that ipsilateral PT stimuli continued to evoke EPSPs with characteristics similar to when the MLF was intact, although less frequently as EPSPs were found in only 15 of the 49 motoneurones tested in four experiments. They were also smaller; the average amplitude of EPSPs evoked by the 4th ipsilateral PT stimulus after the MLF transection (0.36 ± 0.06 mV) was about half the size of the EPSPs evoked by the 3rd stimulus in preparations with an intact MLF (see above). There were, on the other hand, no statistically significant differences between latencies of these EPSPs (see Fig. 3). After transection of the MLF, they ranged from 4.4 to 6.2 ms with a mean of 4.68 ± 0.08 ms for the whole sample (Fig. 3C). There were no indications that any of these EPSPs were evoked by monosynaptic actions of PT fibres, even in the three motoneurones in which they followed not only trains but also single stimuli (at latencies of 4.5, 11.7 and 12.0 ms) because all of these EPSPs showed temporal facilitation. Mean amplitudes of EPSPs evoked in the same motoneurones by the 3rd stimuli were larger (0.43 ± 0.11 mV) than those evoked by the 2nd stimuli (0.31 ± 0.05 mV). They were also evoked in a larger proportion of motoneurones (93%) than EPSPs induced by the 2nd stimuli (60%).
Figure 5. Similar actions of ipsilaterally descending PT fibres before and after transection of the MLF.
A and B, averaged recordings (n = 10) from the cord dorsum at the cervical (C3, top) and thoracic (Th13, bottom) levels following stimulation of the ipsilateral MLF at 100 μA before (A) and after (B) transection of the MLF 1–2 mm rostral to the obex and 3–6 mm caudal to the MLF stimulation site which is indicated by the filled circle in Fig. 1C. Note the lack of descending MLF volleys after the lesion. C–F, averaged (n = 30) intracellular recordings from two PBST motoneurones and cord dorsum recordings (lower traces). They were obtained before (C and E) and after (D and F) transection of the MLF (upper traces) by stimulation of the ipsilateral and contralateral PT as indicated. Dotted lines indicate the onset of the EPSPs and the double headed arrows show the effective stimuli evoking them.
IPSPs evoked after transection of the MLF were found in only 3/15 motoneurones, with an example in Fig. 6B. They were evoked at latencies of 5.44, 6.50 and 7.10 ms, overlapping with, or exceeding the two longest latencies of IPSPs evoked in preparations with the MLF intact (Fig. 3B).
Figure 6. Mutual facilitation of EPSPs and of IPSPs evoked by uncrossed ipsilateral and crossed contralateral PT fibres.
Averaged (n = 20–30) intracellular records from motoneurones (top traces) and cord dorsum recordings (bottom traces). A, mutual facilitation of EPSPs from the two PTs in a motoneurone after transection of the MLF. B and C, mutual facilitation of IPSPs evoked in two motoneurones, with the MLF intact and after transection of the MLF, respectively. Vertical dotted lines indicate the onset of the PSPs that were facilitated by stimulating both PTs.
Comparison of synaptic actions evoked from the ipsilateral and the contralateral PT
In preparations with the MLF intact, EPSPs evoked by stimulation of the contralateral PT were observed in the majority (16/19) of motoneurones with EPSPs from the ipsilateral PT and generally they were similar, as illustrated in Fig. 5. Furthermore, Table 1 shows that only minor differences were found in the distribution of EPSPs evoked from the two PTs in flexor and extensor motoneurones, although EPSPs from the ipsilateral PT occurred in a greater proportion of flexor and bifunctional than of extensor motoneurones. However, the samples of these neurones were too small to allow a meaningful comparison between them. IPSPs evoked by stimulation of the contralateral PT were observed in a smaller number of the motoneurones than IPSPs evoked from the ipsilateral PT and their distribution was more differentiated than that of EPSPs. No IPSPs at latencies ≤ 6 ms were evoked by stimulation of either the contralateral or ipsilateral PT in flexor motoneurones. In extensor and bifunctional motoneurones IPSPs at such latencies appeared to be more frequently evoked from the ipsilateral than from the contralateral PT (Table 1).
Table 1.
Distribution of PSPs evoked by stimulation of the ipsilateral (i PT) and contralateral pyramidal tract (co PT) and medial longitudinal fascicle (MLF) in different types of motoneurones
| Types of motoneurone | ||||
|---|---|---|---|---|
| Source | Input | Flexor | Extensor | Bifunctional |
| MLF | EPSP | 5 | 6 | 13 |
| IPSP | 1 | 13 | 15 | |
| i PT | EPSP | 5 | 4 | 11 |
| IPSP | 0 | 8 | 7 | |
| n = 5 | n = 13 | n = 16 | ||
| co PT | EPSP | 4 | 4 | 6 |
| IPSP | 0 | 2 | 2 | |
| n = 5 | n = 12 | n = 14 | ||
The proportions of motoneurones in which EPSPs and IPSPs were evoked at latencies ≤ 6.0 ms (measured from the effective stimulus) are shown for different types of motoneurones from preparations with intact MLF. The flexor motoneurones include deep peroneal, the extensor motoneurones include Semimembranosus–Anterior Biceps (SMAB) and gastrocnemius and the bifunctional motoneurones include sartorius, posterior biceps–semitendinosus, quadriceps and flexor digitorum hallucis and longus.
EPSPs from the ipsilateral PT were not matched by EPSPs from the contralateral PT in three motoneurones; in one of these IPSPs were evoked instead, and in two, no postsynaptic potentials followed the contralateral PT stimuli. IPSPs from the ipsilateral PT were not matched by IPSPs from the contralateral PT in six motoneurones; in four motoneurones EPSPs were evoked instead and in two motoneurones the contralateral PT stimuli failed to evoke any postsynaptic actions. Differences were also found in the latencies of disynaptic EPSPs and disynaptic or trisynaptic IPSPs evoked from the ipsilateral and the contralateral PT. In the same motoneurones latencies of EPSPs (in samples a + b + c in Fig. 3A) and of IPSPs (in samples d + e in Fig. 3A) from the ipsilateral PT (4.87 ± 0.06 ms and 5.20 ± 0.10 ms, respectively) were somewhat shorter than latencies of EPSPs and of IPSPs evoked from the contralateral PT (5.14 ± 0.12 ms and 5.62 ± 0.15 ms, respectively).
Mutual facilitation between excitatory actions evoked by stimulation of the ipsilateral and the contralateral PTs was examined only in preparations with the MLF transected. It was found to occur in all (n = 8) motoneurones tested (‘F’ in Fig. 3C), one of which is illustrated in Fig. 6A. Combined stimulation of both PTs resulted in EPSPs following the 1st contralateral PT stimulus at 4.8 ms (at the level of the dotted line) that were not present when ipsilateral and contralateral PT stimuli were applied separately, unless longer trains of stimuli were applied (bottom pair of records in Fig. 6A).
Mutual facilitation of inhibitory actions from the ipsilateral and contralateral PT was found when the MLF was intact as well as when it was transected. In preparations with the MLF intact, combined stimulation of the ipsilateral and contralateral PT fibres at appropriate intervals resulted in both occlusion and facilitation in one motoneurone and in facilitation in another; the latter is illustrated in Fig. 6B. In this motoneurone IPSPs were evoked only after the 4th ipsilateral PT stimuli (see the bottom pair of records). When three stimuli were applied to the ipsilateral and the contralateral PT, IPSPs were evoked only when these stimuli were applied together. The amplitude of these IPSPs was about half of those evoked by the 4th ipsilateral PT stimulus. The IPSPs were evoked at a latency of 5.31 ms from the 3rd ipsilateral PT stimulus or 4.35 ms from the 3rd contralateral PT stimulus, being compatible with a disynaptic effect. Similarly effective facilitation was also found after transection of the MLF. As illustrated in Fig. 6C, the facilitated IPSP was almost twice as large as that evoked after the 4th contralateral PT stimulus alone.
Discussion
The main results of this study include (i) demonstration of the disynaptic and trisynaptic actions of ipsilateral PT neurones on hindlimb motoneurones evoked via uncrossed pathways, (ii) evidence that these actions are to a great extent mediated by ipsilaterally projecting RS neurones with axons in the MLF, (iii) indications that they are also mediated by other neurones with a similar total conduction time, and (iv) demonstration of similar actions of ipsilateral and contralateral PT neurones.
Disynaptic and trisynaptic actions of PT neurones evoked by uncrossed pathways
PT neurones could be predicted to affect hindlimb motoneurones via uncrossed pathways in view of ipsilateral projections of PT fibres to the lumbosacral enlargement (see Introduction) and of the strong coupling between PT neurones and ipsilaterally descending RS neurones (He & Wu, 1985; Mitani et al. 1988; Canedo & Lamas, 1993; Jankowska et al. 2006) which in turn act on motoneurones (e.g. Grillner et al. 1968, 1971; Peterson et al. 1979; Floeter et al. 1993). The reasons why so little attention has been paid to ipsilateral PT actions in studies on animals, even after they have been repeatedly demonstrated in man (e.g. Wassermann et al. 1991; Turton et al. 1996; Muller et al. 1997; Feydy et al. 2002; Strens et al. 2003; for further references see Cauraugh & Summers, 2005) are obscure. However, they might be related to the failure to disclose these actions in earlier studies under pentobarbital anaesthesia and/or to a greater interest in the monosynaptic than in the polysynaptic actions of PT neurones on motoneurones. Another reason might be that the previously investigated uncrossed actions in humans (see Introduction) were generally weaker than crossed actions. In the present study the disclosure of the uncrossed actions greatly benefited from the use of the less depressive chloralose anaesthesia and of administration of 4-AP to enhance synaptic transmission (Jankowska et al. 2005).
Both the general characteristics and the latencies of the earliest EPSPs and IPSPs evoked in motoneurones were compatible with disynaptic actions of PT fibres, i.e. with only a single relay neurone between PT neurones and ipsilateral motoneurones. Nevertheless, EPSPs and IPSPs with similar characteristics, i.e. evoked by 2nd, 3rd or 4th stimuli of a train with only marginally longer latencies and with a similar degree of temporal facilitation, might be also compatible with trisynaptic actions if transmission between ipsilateral PT fibres and their relay neurones is very efficient. Both EPSPs and IPSPs recorded in some motoneurones (samples ‘c’ and ‘e’ in Fig. 3) might represent such PSPs. This possibility should also be kept in mind in view of disynaptic actions of PT neurones on spinal interneurones reported in the accompanying paper (Jankowska & Stecina, 2007).
Reticulospinal neurones as relay neurones in uncrossed pathways between PT neurones and motoneurones
Our results indicate that RS neurones mediate the earliest actions of ipsilateral PT neurones because occlusion and mutual facilitation of effects evoked from the ipsilateral PT and the ipsilateral MLF were found in all of the motoneurones tested. Most compatible with actions relayed by RS neurones are disynaptic EPSPs in motoneurones in which monosynaptic EPSPs were evoked from the MLF (sample ‘a’ in Fig. 3A) and in which the time difference between the EPSPs of PT and MLF origin was of the same order as the latency of activation of RS neurones by PT fibres (1.1–1.6 ms, Jankowska et al. 2006; 0.9–2.0 ms, T. Drew, personal communication). Also compatible are EPSPs and IPSPs evoked at latencies that were 1–2 ms longer than latencies of disynaptic EPSPs and IPSPs evoked by MLF stimuli (samples ‘c’ in Fig. 3A and ‘e’ in Fig. 3B) because they might have been evoked trisynaptically (see above) via RS neurones and neurones that mediate disynaptic RS actions. Latencies of these PSPs (5.25 ± 0.04 for EPSPs and 5.34 ± 0.08 for IPSPs) correspond to latencies of trisynaptically evoked PSPs in commissural interneurones (5.95 ± 0.43 ms for EPSPs and 5.65 ± 0.17 ms for IPSPs, see Jankowska et al. 2006).
The RS neurones mediating PT actions might be located in ipsilateral pontine and dorso-rostral medullary reticular nuclei from which both monosynaptic and disynaptic EPSPs were found to be evoked in motoneurones (e.g. Grillner et al. 1968, 1971; Lund & Pompeiano, 1968; Peterson et al. 1979; Floeter et al. 1993) and which were reported to be both monosynaptically and disynaptically excited by PT fibres (He & Wu, 1985; McCarley et al. 1987; Canedo & Lamas, 1993; Matsuyama & Drew, 1997). Whether the same or distinct RS neurones might mediate the disynaptic and the tri- or polysynaptic PT actions has not yet been established. One of the problems to be considered in this respect might be that the reported latencies of earliest IPSPs following spike potentials of individual RS neurones located within the nucleus reticularis magnocellularis were about 5 ms (Takakusaki et al. 1989, 1994, 2001), that is, more than 1 ms longer than latencies of disynaptic IPSPs evoked from the MLF in the present study (Fig. 3B).
Indications for other relay neurones in uncrossed pathways between PT neurones and motoneurones
As shown in the Results section, not all of the PSPs evoked by PT stimulation are likely to be relayed by RS neurones, at least not by those with axons descending in the MLF. The strongest indications to this end are similar effects of stimuli applied in the ipsilateral PT before and after transection of the MLF. Similar latencies of EPSPs and IPSPs evoked under these two conditions show in addition that the overall conduction time in uncrossed disynaptic pathways between PT neurones and ipsilateral motoneurones via RS and other relay neurones is similar and that the two pathways may be used in parallel. These indications are corroborated by a number of others. Disynaptic EPSPs found in motoneurones in which no monosynaptic EPSPs were evoked from the MLF (sample ‘b’ in Fig. 3A) appear to be incompatible with their mediation by RS neurones. IPSPs (sample ‘d’ in Fig. 3B) evoked at the same latencies as disynaptic EPSPs appear to be similarly incompatible, while IPSPs mediated by RS neurones and inhibitory premotor interneurones excited by them should be evoked trisynaptically. Late actions evoked by PT stimuli that have been sometimes found to differ from those evoked by MLF stimuli likewise suggest that they were evoked via other neurones.
Alternative relay neurones of uncrossed PT actions might include RS neurons projecting outside of the MLF and two categories of spinal neurones: long propriospinal neurones and segmental interneurones (Fig. 1A).
Here we will discuss only the possibility of contribution of other RS neurones to PT actions, since the spinal relay neurones of PT actions are the subject of the accompanying paper. Our MLF lesions (see Fig. 1D) should have involved transection of axons of RS neurones with cell bodies located in pontine and rostro-dorsal medullary nuclei, which descended both within and just outside of the MLF (Mitani et al. 1988; Matsuyama et al. 1988). However, fibres running in the brainstem more laterally than 1 mm from the MLF border and axons of more caudally located RS neurones projecting via the lateral reticulospinal tract (Peterson et al. 1979) and/or joining the MLF within the most caudal part of the medulla could have remained intact. Some RS neurones thus could have continued to relay actions of ipsilateral PT fibres even after MLF lesions. Of these, neurones located in the nucleus reticularis ventralis and the ventrocaudal part of nucleus reticularis gigantocellularis would be unlikely to relay disynaptic excitatory actions of ipsilateral PT neurones to motoneurones given that stimuli applied in these nuclei were reported to evoke polysynaptic rather than monosynaptic EPSPs in hindlimb motoneurones (Peterson et al. 1979) and IPSPs with about 1 ms longer latencies (Takakusaki et al. 2001) than minimal latencies of IPSPs recorded in this study. They could nevertheless contribute to trisynaptic or later excitatory and inhibitory actions of ipsilateral PT neurones. RS neurones from pontine and rostral medullary reticular nuclei would neither be likely to relay the earliest disynaptic actions of ipsilateral PT neurones since medium size pontine reticuloreticular neurones do not project to the spinal cord and axons of medium sized RS neurones from the bulbar nucleus gigantocellularis that descended outside the MLF were reported to be of small diameters (Mitani et al. 1988).
Are synaptic actions of ipsilateral and contralateral PT neurones on feline hindlimb motoneurones relayed by the same neurones?
Our results provide indications for some distinct relay neurones of crossed and uncrossed PT actions as there were differences in synaptic actions of the two PTs in the same motoneurones (e.g. EPSPs from one PT and IPSPs from the other) and in latencies of IPSPs evoked from the ipsilateral and the contralateral PT (see Results). However, the synaptic actions evoked from the PTs in the majority of the motoneurones examined in our study suggest that the uncrossed and crossed actions of PT fibres are generally similar. This is indicated by the predominant inhibition of extensor motoneurones and of the predominant excitation of both flexor and bifunctional motoneurones of our sample. These observations are in keeping with previous reports on the most frequent actions of the contralateral PT neurones in anaesthetized preparations (Lundberg et al. 1962; Uemura & Preston, 1965; Laursen & Wiesendanger, 1966; Aoki & McIntyre, 1975) and during locomotor activity in decerebrate (Orlovsky, 1972) as well as in intact (Bretzner & Drew, 2005) cats. Similar actions of the ipsilateral and the contralateral PT neurones relayed by RS neurones in preparations with the MLF intact are in keeping with the evidence that RS neurones are co-excited by left and right PT neurones (He & Wu, 1985; Matsuyama & Drew, 1997). Similar actions evoked after transection of the MLF and mutual facilitation and occlusion of synaptic actions evoked from the two PTs provide further evidence that at least some of these relay neurones are shared.
The use of shared relay neurones by left and right PT neurones will have several functional consequences. One of these would be that any sufficiently strong uncrossed PT action would involve the same motoneurones as the crossed PT action and not be restricted to more proximal muscles as previously suggested on the basis of transcranial stimulation in humans (see, e.g. Turton et al. 1996). Another consequence would be that basic patterns of movements induced by PT neurones on both sides of the body might be symmetrical rather than opposite. The extreme cases of such symmetrical movements are mirror movements associated with synchronous activity of cortical neurones, including PT neurones, in both hemispheres (for a recent review see Carson, 2005). Mirror movements were most often considered in terms of abnormalities in pyramidal decussations but occur normally in children and under certain conditions may reappear in adults (for references see Vulliemoz et al. 2005). Symmetrical PT actions may also be the basis of normal coordination in bi-manual tasks (for references see Cauraugh & Summers, 2005) and at least some cortical neurones serving bilateral movements have been identified (e.g. Brinkman & Kuypers, 1973; Aizawa et al. 1990). If crossed direct actions of PT neurones in each hemisphere are as a rule associated with symmetrical bilateral indirect actions, some neuronal systems would have to be used to replace these symmetrical actions by patterns of asymmetrical ones or of alternating activation of left and right limbs. Some bilateral PT actions could also be evoked via crossed axon collaterals of contralaterally descending PT fibres, given off within the cervical and thoracic segments. However, the possibility that such axon collaterals descend to the lumbar segments appears rather unlikely. At least, there are no reports of distant collateral projections and crossed collaterals of either corticospinal or reticulospinal fibres have been only demonstrated to branch within a few millimetres length of the spinal cord (see, e.g. Matsuyama et al. 1999; Li & Martin, 2002). For these reasons we feel it is justified to attribute the ipsilateral actions of PT neurones described in this and in the accompanying paper (Jankowska & Stecina, 2007) to uncrossed actions of PT neurones and/or of their ipsilaterally descending relay neurones.
An important consequence of the use of shared relay neurones by ipsilateral and contralateral PT neurones would be that they may contribute to the compensation of missing crossed actions by uncrossed PT actions. The uncrossed actions have been found to be much weaker and more difficult to evoke by either transcranial magnetic stimulation or by electrical stimulation of the motor cortex and/or of the medullary pyramids than the crossed ones. Nevertheless, early effects of the ipsilateral and contralateral PT stimulation found in this study were comparable. Neuronal networks that mediate them could thus be used to induce both crossed and uncrossed PT actions provided that they are effectively activated. How to enhance uncrossed PT actions is another question and the most effective means must vary from case to case, depending on the extent of injuries in individual patients and also depending on which of the various PT relay neurones may be used to this end. In the accompanying paper we will report results of the follow-up study on spinal interneurones which relay some of the uncrossed PT actions and relate them to this question. We hope that better knowledge of neuronal networks that contribute to the uncrossed PT actions would provide a basis for designing strategies for a more effective use of these networks to replace the missing crossed PT actions and assist in the recovery of motor functions.
Acknowledgments
We wish to thank Drs S. Edgley and I. Hammar for their helpful comments and Mrs Rauni Larsson for her invaluable assistance. The study was supported by grants from NINDS/NIH (R01 NS040863) and the Swedish Research Council (15393-01A).
References
- Aizawa H, Mushiake H, Inase M, Tanji J. An output zone of the monkey primary motor cortex specialized for bilateral hand movement. Exp Brain Res. 1990;82:219–221. doi: 10.1007/BF00230856. [DOI] [PubMed] [Google Scholar]
- Aoki M, McIntyre AK. Cortical and long spinal actions on lumbosacral motoneurones in the cat. J Physiol. 1975;251:569–587. doi: 10.1113/jphysiol.1975.sp011109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armand J, Kuypers HG. Cells of origin of crossed and uncrossed corticospinal fibres in the cat. Exp Brain Res. 1980;40:23–34. doi: 10.1007/BF00236659. [DOI] [PubMed] [Google Scholar]
- Bretzner F, Drew T. Contribution of the motor cortex to the structure and the timing of hindlimb locomotion in the cat: a microstimulation study. J Neurophysiol. 2005;94:657–672. doi: 10.1152/jn.01245.2004. [DOI] [PubMed] [Google Scholar]
- Brinkman J, Kuypers HG. Cerebral control of contralateral and ipsilateral arm, hand and finger movements in the split-brain rhesus monkey. Brain. 1973;96:653–674. doi: 10.1093/brain/96.4.653. [DOI] [PubMed] [Google Scholar]
- Brosamle C, Schwab M. Cells of origin, course and termination patterns of the ventral, uncrossed component of the mature rat corticospinal tract. J Comp Neurol. 1997;386:293–303. doi: 10.1002/(sici)1096-9861(19970922)386:2<293::aid-cne9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Bucy PC, Fulton JF. Ipsilateral representation in the motor and premotor cortex of monkeys. Brain. 1933;56:318–342. [Google Scholar]
- Canedo A, Lamas JA. Pyramidal and corticospinal synaptic effects over reticulospinal neurones in the cat. J Physiol. 1993;463:475–489. doi: 10.1113/jphysiol.1993.sp019606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson RG. Neural pathways mediating bilateral interactions between the upper limbs. Brain Res Rev. 2005;49:641–662. doi: 10.1016/j.brainresrev.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Cauraugh JH, Summers JJ. Neural plasticity and bilateral movements: a rehabilitation approach for chronic stroke. Prog Neurobiol. 2005;75:309–320. doi: 10.1016/j.pneurobio.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Chambers WW, Liu C-N. Cortico-spinal tract of the cat. J Comp Neurol. 1957;108:23–55. doi: 10.1002/cne.901080103. [DOI] [PubMed] [Google Scholar]
- Edgley S, Jankowska E, Hammar I. Ipsilateral actions of feline corticospinal tract neurons on limb motoneurons. J Neurosci. 2004;24:7804–7813. doi: 10.1523/JNEUROSCI.1941-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo K, Araki T, Kawai Y. Contra- and ipsilateral cortical and rubral effects on fast and slow spinal motoneurones of the cat. Brain Res. 1975;88:91–98. doi: 10.1016/0006-8993(75)90953-1. [DOI] [PubMed] [Google Scholar]
- Feydy A, Carlier R, Roby-Brami A, Bussel B, Cazalis F, Pierot L, Burnood Y, Maier MA. Longitudinal study of motor recovery after stroke: recruitment and focusing of brain activation. Stroke. 2002;33:1610–1617. doi: 10.1161/01.str.0000017100.68294.52. [DOI] [PubMed] [Google Scholar]
- Flindt-Egebak P. The corticofugal projections from the sensorimotor cortex to the spinal cord. A neuroanatomical and autoradiographical study in the cat with some methodological comments. J Hirnforsch. 1979;20:363–373. [PubMed] [Google Scholar]
- Floeter MK, Sholomenko GN, Gossard J-P, Burke RE. Disynaptic excitation from the medial longitudinal fasciculus to lumbosacral motoneurones: modulation by repetitive activation, descending pathways and locomotion. Exp Brain Res. 1993;92:407–419. doi: 10.1007/BF00229029. [DOI] [PubMed] [Google Scholar]
- Grillner S, Hongo T, Lund S. Reciprocal effects between two descending bulbospinal systems with monosynaptic connections to spinal motoneurones. Brain Res. 1968;10:477–480. doi: 10.1016/0006-8993(68)90221-7. [DOI] [PubMed] [Google Scholar]
- Grillner S, Hongo T, Lund S. Convergent effects on alpha motoneurones from the vestibulospinal tract and a pathway descending in the medial longitudinal fasciculus. Exp Brain Res. 1971;12:457–479. doi: 10.1007/BF00234243. [DOI] [PubMed] [Google Scholar]
- Grillner S, Lund S. The origin of a descending pathway with monosynaptic action on flexor motoneurones. Acta Physiol Scand. 1968;74:274–284. doi: 10.1111/j.1748-1716.1968.tb04236.x. [DOI] [PubMed] [Google Scholar]
- He XW, Wu CP. Connections between pericruciate cortex and the medullary reticulospinal neurones in the cat: an electrophysiological study. Exp Brain Res. 1985;61:109–116. doi: 10.1007/BF00235626. [DOI] [PubMed] [Google Scholar]
- Hongo T, Kudo N, Tanaka R. The vestibulospinal tract: crossed and uncrossed effects on hindlimb motoneurones in the cat. Exp Brain Res. 1975;24:37–55. doi: 10.1007/BF00236016. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Cabaj A, Pettersson LG. How to enhance ipsilateral actions of pyramidal tract neurones. J Neurosci. 2005;25:7401–7405. doi: 10.1523/JNEUROSCI.1838-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Edgley SA. How can corticospinal tract neurones contribute to ipsilateral movements? A question with implications for recovery of motor functions. Neuroscientist. 2006;12:67–79. doi: 10.1177/1073858405283392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Slawinska U, Maleszak K, Edgley SA. Neuronal basis of crossed actions from the reticular formation upon feline hindlimb motoneurons. J Neurosci. 2003;23:1867–1878. doi: 10.1523/JNEUROSCI.23-05-01867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Stecina K. Uncrossed actions of feline corticospinal tract motoneurones on lumbar interneurones evoked via ipsilaterally descending pathways. J Physiol. 2007;580:133–147. doi: 10.1113/jphysiol.2006.122739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Stecina K, Cabaj A, Pettersson LG, Edgley SA. Neuronal relays in double crossed pathways between feline motor cortex and ipsilateral hindlimb motoneurones. J Physiol. 2006;575:527–541. doi: 10.1113/jphysiol.2006.112425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krutki P, Jankowska E, Edgley SA. Are crossed actions of reticulospinal and vestibulospinal neurons on feline motoneurons mediated by the same or separate commissural neurons? J Neurosci. 2003;23:8041–8050. doi: 10.1523/JNEUROSCI.23-22-08041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix S, Havton LA, McKay H, Yang H, Brant A, Roberts J, Tuszynski MH. Bilateral corticospinal projections arise from each motor cortex in the macaque monkey: a quantitative study. J Comp Neurol. 2004;473:147–161. doi: 10.1002/cne.20051. [DOI] [PubMed] [Google Scholar]
- Lance JW. Pyramidal tract in spinal cord of cat. J Neurophysiol. 1954;17:253–270. doi: 10.1152/jn.1954.17.3.253. [DOI] [PubMed] [Google Scholar]
- Laursen AM, Wiesendanger M. Pyramidal effect on alpha and gamma motoneurons. Acta Physiol Scand. 1966;67:165–172. doi: 10.1111/j.1748-1716.1966.tb03297.x. [DOI] [PubMed] [Google Scholar]
- Li Q, Martin JH. Postnatal development of connectional specificity of corticospinal terminals in the cat. J Comp Neurol. 2002;447:57–71. doi: 10.1002/cne.10203. [DOI] [PubMed] [Google Scholar]
- Lund S, Pompeiano O. Monosynaptic excitation of alpha motoneurones from supraspinal structures in the cat. Acta Physiol Scand. 1968;73:1–21. doi: 10.1111/j.1748-1716.1968.tb04075.x. [DOI] [PubMed] [Google Scholar]
- Lundberg A, Norsell U, Voorhoeve P. Pyramidal effects on lumbo-sacral interneurones activated by somatic afferents. Acta Physiol Scand. 1962;56:202–229. doi: 10.1111/j.1748-1716.1962.tb02497.x. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Ito K, Rodrigo-Angulo ML. Physiological studies of brainstem reticular connectivity. II. Responses of mPRF neurons to stimulation of mesencephalic and contralateral pontine reticular formation. Brain Res. 1987;409:111–127. doi: 10.1016/0006-8993(87)90746-3. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Drew T. Organization of the projections from the pericruciate cortex to the pontomedullary brainstem of the cat: A study using the anterograde tracer Phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1997;389:617–641. doi: 10.1002/(sici)1096-9861(19971229)389:4<617::aid-cne6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Jankowska E. Coupling between feline cerebellum (fastigial neurones) and motoneurones innervating hindlimb muscles. J Neurophysiol. 2004;91:1183–1192. doi: 10.1152/jn.00896.2003. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Mori F, Kuze B, Mori S. Morphology of single pontine reticulospinal axons in the lumbar enlargement of the cat: a study using the anterograde tracer PHA-L. J Comp Neurol. 1999;410:413–430. [PubMed] [Google Scholar]
- Matsuyama K, Ohta Y, Mori S. Ascending and descending projections of the nucleus reticularis gigantocellularis in the cat demonstrated by the anterograde neural tracer, Phaseolus vulgaris leucoagglutinin (PHA-L) Brain Res. 1988;460:124–141. doi: 10.1016/0006-8993(88)91212-7. [DOI] [PubMed] [Google Scholar]
- Mitani A, Ito K, Mitani Y, McCarley RW. Descending projections from the gigantocellular tegmental field in the cat: cells of origin and their brainstem and spinal cord projections. J Comp Neurol. 1988;268:546–566. doi: 10.1002/cne.902680406. [DOI] [PubMed] [Google Scholar]
- Muller K, Kass-Iliya F, Reitz M. Ontogeny of ipsilateral corticospinal projections: a developmental study with transcranial magnetic stimulation. Ann Neurol. 1997;42:705–711. doi: 10.1002/ana.410420506. [DOI] [PubMed] [Google Scholar]
- Nyberg-Hansen R, Brodal A. Sites of termination of corticospinal fibers in the cat: An experimental study with silver impregnation methods. J Comp Neurol. 1963;120:369–391. doi: 10.1002/cne.901200302. [DOI] [PubMed] [Google Scholar]
- Nyberg-Hansen R, Mascitti TA. Sites and mode of termination of fibers of the vestibulospinal tract in the cat. An experimental study with silver impregnation methods. J Comp Neurol. 1964;122:369–383. doi: 10.1002/cne.901220307. [DOI] [PubMed] [Google Scholar]
- Nyberg-Hansen R, Rinvik E. Some comments on the pyramidal tract, with special reference to its individual variations in man. Acta Neurol Scand. 1963;39:1–30. [Google Scholar]
- Orlovsky GN. The effect of different descending systems on flexor and extensor activity during locomotion. Brain Res. 1972;40:359–371. doi: 10.1016/0006-8993(72)90139-4. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Pitts NG, Fukushima K. Reticulospinal connections with limb and axial motoneurones. Exp Brain Res. 1979;36:1–20. doi: 10.1007/BF00238464. [DOI] [PubMed] [Google Scholar]
- Phillips CG, Porter R. Corticospinal Neurones: Their Role in Movement. London: Academic Press; 1977. [PubMed] [Google Scholar]
- Porter R, Lemon RN. Corticospinal Function and Voluntary Movement. Oxford: Clarendon Press; 1993. [Google Scholar]
- Strens LH, Fogelson N, Shanahan P, Rothwell JC, Brown P. The ipsilateral human motor cortex can functionally compensate for acute contralateral motor cortex dysfunction. Curr Biol. 2003;13:1201–1205. doi: 10.1016/s0960-9822(03)00453-6. [DOI] [PubMed] [Google Scholar]
- Takakusaki K, Kohyama J, Matsuyama K, Mori S. Medullary reticulospinal tract mediating the generalized motor inhibition in cats: parallel inhibitory mechanisms acting on motoneurons and on interneuronal transmission in reflex pathways. Neuroscience. 2001;103:511–527. doi: 10.1016/s0306-4522(00)00586-8. [DOI] [PubMed] [Google Scholar]
- Takakusaki K, Ohta Y, Mori S. Single medullary reticulospinal neurones exert postsynaptic inhibitory effects via inhibitory interneurons upon alpha-motoneurons innervating cat hindlimb muscles. Exp Brain Res. 1989;74:11–23. doi: 10.1007/BF00248276. [DOI] [PubMed] [Google Scholar]
- Takakusaki K, Shimoda N, Matsuyama K, Mori S. Discharge properties of medullary reticulospinal neurons during postural changes induced by intrapontine injections of carbachol, atropine and serotonin, and their functional linkages to hindlimb motoneurons in cats. Exp Brain Res. 1994;99:361–374. doi: 10.1007/BF00228973. [DOI] [PubMed] [Google Scholar]
- Turton A, Wroe S, Trepte N, Fraser C, Lemon RN. Contralateral and ipsilateral EMG responses to transcranial magnetic stimulation during recovery of arm and hand function after stroke. Electroencephalogr Clin Neurophysiol. 1996;101:316–328. doi: 10.1016/0924-980x(96)95560-5. [DOI] [PubMed] [Google Scholar]
- Uemura K, Preston JB. Comparison of motor cortex influences upon various hind-limb motoneurones in pyramidal cats and primates. J Neurophysiol. 1965;28:398–412. doi: 10.1152/jn.1965.28.2.398. [DOI] [PubMed] [Google Scholar]
- Van der Muelen JP, Ghez C. The effects of stimulation of the cerebral cortex on alpha and gamma motor units in cat hindlimb. Arch Ital Biol. 1970;108:538–563. [PubMed] [Google Scholar]
- Vulliemoz S, Raineteau O, Jabaudon D. Reaching beyond the midline: why are human brains cross wired? Lancet Neurol. 2005;4:87–99. doi: 10.1016/S1474-4422(05)00990-7. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Fuhr P, Cohen LG, Hallett M. Effects of transcranial magnetic stimulation on ipsilateral muscles. Neurology. 1991;41:1795–1799. doi: 10.1212/wnl.41.11.1795. [DOI] [PubMed] [Google Scholar]
- Wilson VJ, Yoshida M. Vestibulospinal and reticulospinal effects on hindlimb, forelimb, and neck alpha motoneurons of the cat. Proc Natl Acad Sci. 1968;60:836–840. doi: 10.1073/pnas.60.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]