Abstract
Inhibitory and facilitatory intracortical pathways regulating motor cortical output can be studied non-invasively in humans with transcranial magnetic stimulation. These circuits include short-interval intracortical inhibition (SICI), long-interval intracortical inhibition (LICI) and intracortical facilitation (ICF). Stimulation of the motor cortex also inhibits the contralateral motor cortex (interhemispheric inhibition, IHI) at short (∼10 ms, IHI10) or long intervals (∼40 ms, IHI40). We investigated how SICI, ICF, and LICI influence IHI10 and IHI40. We hypothesize that intracortical circuits will have similar effects on IHI and cortical output neurons: SICI and LICI will decrease IHI, and ICF will increase it. Motor evoked potentials were recorded from the first dorsal interosseous muscles bilaterally in 10 healthy subjects. We compared IHI10 and IHI40 alone to IHI10 and IHI40 elicited in the presence of SICI, ICF, or LICI. Our results showed that SICI and LICI reduced IHI10, IHI40 and corticospinal output to a similar degree. ICF increased corticospinal output but had no effect on either IHI10 or IHI40. The different effects of ICF on corticospinal excitability and IHI suggest the transcallosal fibres mediating IHI and the corticospinal output system arise from different neuronal populations. SICI and LICI produce more global inhibition with similar effects on the transcallosal and descending corticospinal circuits.
Transcranial magnetic stimulation (TMS) can been used to study different inhibitory and excitatory circuits in the human motor cortex (Rothwell, 1997; Hallett, 2000; Chen, 2004). Two types of cortico-cortical inhibition are short-interval intracortical inhibition (SICI) and long-interval intracortical inhibition (LICI). SICI is elicited by a subthreshold conditioning stimulus (CS) followed by a test stimulus (TS) at interstimulus intervals (ISI) of 1–6 ms (Kujirai et al. 1993; Chen et al. 1998). LICI differs with respect to stimulus strength and ISI requiring a suprathreshold CS at an ISI of 50–200 ms (Valls-Sole et al. 1992; Wassermann et al. 1996). It is thought that SICI is mediated by GABAA receptors (Ziemann et al. 1996a; Hanajima et al. 1998) while LICI is mediated by GABAB receptors (Werhahn et al. 1999). In addition to inhibitory cortico-cortical circuits there are facilitatory pathways. One such circuit is termed intracortical facilitation (ICF) elicited by similar parameters to SICI, with a subthreshold CS, but at ISIs of 8–30 ms (Kujirai et al. 1993).
Motor cortex stimulation also inhibits the output of the contralateral motor cortex. This interhemispheric inhibition (IHI) may be responsible for suppressing activity of the contralateral hemisphere to achieve hemispheric dominance while executing motor tasks. IHI can be measured by a paired pulse paradigm, a CS delivered to the contralateral motor cortex preceding the TS by 6–50 ms (Ferbert et al. 1992; Gerloff et al. 1998; Hanajima et al. 2001; Chen et al. 2003), or by the ipsilateral silent period (iSP) (Kujirai et al. 1993; Meyer et al. 1995; Trompetto et al. 2003), which refers to interruption of ongoing voluntary electromyographic (EMG) activity following stimulation of the ipsilateral motor cortex. IHIs at ISIs of 10 ms (IHI10) and 40 ms (IHI40) are probably mediated by different mechanisms (Chen et al. 2003). The neurotransmitter systems responsible for IHI have not been established, but IHI40 may be related to LICI (Kukaswadia et al. 2005), which is probably due to GABAB mechanisms. Reductions in IHI10 have been demonstrated in several neurological and psychiatric disorders, such as schizophrenia (Daskalakis et al. 2002a), as well as in musicians who have trained from a young age (Ridding et al. 2000).
By characterizing the interactions between intracortical circuits and IHI, we can better understand the effects of abnormal intracortical circuits observed in disease states on activities of the contralateral hemisphere. Daskalakis et al. (2002b) studied how IHI interacts with intracortical circuits in the target hemisphere. It was found that IHI10 inhibits SICI and LICI reduces IHI10. Only one study examined how intracortical inhibitory circuits interact with transcallosal projections in the originating hemisphere. Trompetto et al. (2004) reported that SICI reduced the iSP area, suggesting that it suppresses the transcallosal motor output. However, the effects of SICI and ICF on IHI measured by the paired pulse method, and the effects of LICI have not been studied. We hypothesize that inhibitory and facilitatory circuits will produce widespread changes in the motor cortex, with similar effects on the corticospinal projections and the transcallosal projections.
Methods
Subjects
We studied 10 healthy volunteers (6 men and 4 women, mean age 35 years; range: 20–58 years). All subjects provided written informed consent. The experimental protocol was approved by the University Health Network Research Ethics Board in accordance with the Declaration of Helsinki on the use of human subjects in experiments.
EMG recording
Motor-evoked potentials (MEPs) were recorded by surface EMG from the left and right first dorsal interosseous (FDI) muscles using disposable disc electrodes in a belly tendon arrangement. The subjects in all experiments maintained relaxation and EMG activity was monitored using a computer screen and speakers at high gain. Trials with background muscle activity were rejected. When a constant contraction was required, the EMG passed through a leaky integrator and was displayed on an oscilloscope to provide visual feedback in addition to auditory feedback to the subject. The signal was amplified (Intronix Technologies, Bolton, Ontario, Canada), filtered (band-pass 2 Hz to 2.5 kHz), digitized at 5 kHz (Micro 1401, Cambridge Electronics Design, Cambridge, UK), and stored in a laboratory computer for off-line analysis (Signal 3.07 software).
Trnascranial magnetic stimulation setup
TMS was delivered to the left motor cortex using a figure-of-eight coil (mean diameter, 70 mm; maximum strength: 2.2 T) connected directly to a Magstim 200 stimulator (The Magstim Company, Dyfed, UK). The coil was placed over the scalp area optimal for eliciting MEPs in the right FDI, with the handle pointing posteriorly, approximately perpendicular to the central sulcus and about 45 deg to the mid-saggital line. This position was marked for reference. This orientation induced a posterior to anterior current (Werhahn et al. 1994).
TMS was delivered to the right motor cortex using a second figure-of-eight coil and four Magstim 200 stimulators connected to three BiStim Modules in a ‘pyramid’ arrangement. Each pair of Magstim 200 stimulators was connected to a BiStim Module and the output of the two BiStim Modules connected to a third BiStim Module that was connected to the TMS coil. This setup allowed the delivery of up to four pulses of different intensities to the right motor cortex at short intervals. This arrangement is associated with a power attenuation of about 15% (Sanger et al. 2001; Daskalakis et al. 2002b). The coil was placed over the scalp area optimal for eliciting MEPs in the left FDI. The coil orientation was the same as that for the left motor cortex.
To measure IHI, a suprathreshold contralateral CS (CCS) was delivered to the right motor cortex and a suprathreshold TS was delivered to the left motor cortex. ISIs of 10 ms (IHI10) and 40 ms (IHI40) were tested, as they represent different mechanisms (Chen et al. 2003). Both the CCS (right motor cortex) and the TS (left motor cortex) were set at the minimum intensity required to produce peak-to-peak MEP amplitude of 1 mV in five out of ten trials in the relaxed contralateral FDI muscle.
For the right motor cortex we tested different intracortical circuits (SICI, ICF, LICI) with paired pulses. CS were delivered 2, 10, or 100 ms prior to the CCS and are termed CS2, CS10, and CS100. CS2 was found to consistently produce SICI (Kujirai et al. 1993; Chen et al. 1998), and was set at an intensity of 95% of the active motor threshold (AMT). AMT was the lowest stimulator output required to produce MEPs of >200 μV in five out of ten trials with a sustained contraction of 20% of maximal strength. CS10 was used to elicit ICF (Kujirai et al. 1993; Ridding et al. 1995b) and was also set at 95% AMT. CS2 and CS10 were set below the AMT to ensure that no descending corticospinal volleys were evoked (Nakamura et al. 1997; Di Lazzaro et al. 1998). CS100 was used to elicit LICI as it reduced cortical excitability (Chen et al. 1999) without affecting spinal excitability (Fuhr et al. 1991). It was set at the lowest intensity required to produce MEPs of 1 mV peak-to-peak amplitude in five out of ten trials with the muscle relaxed.
Experimental design
This study consisted of three experiments. The first experiment examined the effect of SICI on IHI, the second experiment tested the effect of ICF on IHI, and the third experiment tested the effect of LICI on IHI.
The CCS used to elicit IHI also served as the TS used to measure intracortical circuits in the right motor cortex. Thus, the ISIs for SICI, ICF, and LICI are in relation to the CCS. The CCS intensity needed to produce peak-to-peak MEP amplitudes of 1 mV is labelled ‘CCS1mV’ and IHI produced by CCS of this intensity would be termed IHI1mV. In some trials, we adjusted the CCS intensity to produce MEPs of ∼1 mV in the presence of another inhibitory or facilitatory circuit, and these conditions were identified by subscripts. For example, a CCS of intensity ‘CCS1mVCS2’ produced a 1 mV response in the presence of CS2 (SICI). IHI elicited by a CCS of this intensity would be termed IHI1mVCS2.
If the same CCS intensities were used in the presence and absence of an inhibitory or excitatory circuit, MEP amplitudes in response to CCS would be different. This was a concern as IHI increases with increasing CS intensity and MEP amplitude (Chen et al. 2003). Since MEP amplitude and stimulus intensity could not be matched in the same trial, we designed the experiments to match the CCS stimulus intensity and CCS evoked MEP amplitude in the presence and absence of an inhibitory or excitatory circuit in different trials. To match for MEP amplitude, the CCS intensity was increased or decreased to produce a 1 mV MEP response in the presence of an inhibitory or facilitatory mechanism. We then compared IHI in the presence of intracortical inhibitory or facilitatory circuits elicited by this adjusted CCS to IHI elicited by a CCS that produced a 1 mV MEP response in the absence of inhibitory or facilitatory circuits. In stimulus intensity-matched trials, the CCS intensities were identical.
Experiment 1: effects of SICI on IHI
This experiment investigated the effects of SICI on IHI. It consisted of 11 conditions (1A–1K, Table 1) of 10 trials each delivered in a randomized order for a total of 110 trials. Conditions 1A to 1F examined the interaction between SICI and IHI10, while conditions 1G to 1K examined the interaction between SICI and IHI40. Since SICI reduces the amplitude of the CCS, which may affect the amount of IHI, the CCS intensity in conditions 1E, 1F, 1J, and 1K were increased to produce a 1 mV MEP response in the presence of a CS2 pulse. The resulting IHI was termed IHI1mVCS2 and allowed matching of the CCS MEP amplitude, resulting in similar degrees of corticospinal activation in the presence and absence of SICI. The effect of SICI on IHI was tested with three pulses in conditions 1C, 1F, 1H and 1K. Conditions 1B and 1G were used to determine the amount of IHI with a 1 mV CCS (IHI101mV and IHI401mV). Conditions 1E and 1J were used to determine the amount of IHI with the stronger CCS intensity (IHI101mVCS2 and IHI401mVCS2). We compared IHI10 and IHI40 alone (IHI10: 1B/1A; IHI40: 1G/1 A) to IHI10 and IHI40 in the presence of SICI (IHI10: 1F/1A; IHI40: 1K/1A) matched for MEP amplitude produced by the CCS pulse (∼1 mV). We also compared IHI10 and IHI40 alone (IHI10: 1B/1A and 1E/1A; IHI40: 1G/1A and 1J/1A) to IHI10 and IHI40 in the presence of SICI matched for the stimulus intensity of the CCS pulse (IHI 10: 1C/1A and 1F/1A; IHI40: 1H/1A and 1K/1A). The stimulus intensities of the CCS pulses were either 1 mV (IHI10: 1B/1A versus 1C/1A; IHI40 1G/1A versus 1H/1A) or CCS1mVCS2 (IHI10: 1E/1A versus 1F/1A; IHI40: 1J/1A versus 1K/1A). To ensure the CS2 pulse was not eliciting IHI, it was paired with the IHI TS in the absence of a CCS10 (1D) or CCS40 (1I).
Table 1.
Test conditions for experiment 1
| Right motor cortex | ||||
|---|---|---|---|---|
| Condition | CS2 | CCS10 | CCS40 | Left motor cortex TS |
| 1A | 1 mV | |||
| 1B | 1 mV | 1 mV | ||
| 1C | 95% AMT | 1 mV | 1 mV | |
| 1D | 95% AMT | 1 mV | ||
| 1E | 1mVCS2 | 1 mV | ||
| 1F | 95% AMT | 1mVCS2 | 1 mV | |
| 1G | 1 mV | 1 mV | ||
| 1H | 95% AMT | 1 mV | 1 mV | |
| 1I | 95% AMT | 1 mV | ||
| 1J | 1mVCS2 | 1 mV | ||
| 1K | 95% AMT | 1mVCS2 | 1 mV | |
Stimulus intensities. CS2, conditioning stimulus delivered 2 ms before the CCS to elicit SICI; CCS10, contralateral conditioning stimulus delivered 10 ms before the test stimulus (TS) to elicit IHI10; CCS40, contralateral conditioning stimulus delivered 40 ms before the TS to elicit IHI40. All the above stimuli were applied to the right motor cortex. TS, test stimulus applied to the left motor cortex to elicit an MEP in the right FDI. See Methods for CCS intensity nomenclature.
Experiment 2: effects of ICF on IHI
Eleven conditions were tested as detailed in Table 2 (2A–2K). Ten trials of each condition were implemented in a randomized order, totalling 110 trials. Conditions 2A to 2F examined the interaction between ICF and IHI10, while conditions 2G to 2K examined the interaction between ICF and IHI40. The CCS intensities in conditions 2E, 2F, 2J, and 2K were decreased to produce a 1 mV MEP response in the presence of a CS10 pulse. The resulting IHI was termed IHI1mVCS10 and allowed matching of the CCS MEP amplitude resulting in similar degrees of corticospinal activation in the presence and absence of ICF. The effect of ICF on IHI was tested with three pulses in conditions 2C, 2F, 2H and 2K. Conditions 2B and 2G were used to determine the amount of IHI with a 1 mV CCS (IHI101mV and IHI401mV). Conditions 2E and 2J were used to determine the amount of IHI with the weaker CCS intensity (IHI101mVCS10 and IHI401mVCS10). We compared IHI10 and IHI40 alone (IHI10: 2B/2A; IHI40: 2G/2A) to IHI10 and IHI40 in the presence of ICF (IHI10: 2F/2A; IHI40: 2K/2A) matched for MEP amplitude produced by the CCS pulse (∼1 mV). We also compared IHI10 and IHI40 alone (IHI10: 2B/2A and 2E/2A; IHI40: 2G/2A and 2J/2A) to IHI10 and IHI40 in the presence of ICF matched for the stimulus intensity of the CCS pulse (IHI 10: 2C/2A and 2F/2A; IHI40: 2H/2A and 2K/2A). The stimulus intensities of the CCS pulses were either 1 mV (IHI10: 2B/2A versus 2C/2A; IHI40 2G/2A versus 2H/2A) or 1mVCS10 (IHI10: 2E/2A versus 2F/2A; IHI40: 2J/2A versus 2K/2A). To ensure the CS10 pulse was not eliciting IHI, it was paired with the IHI TS in the absence of a CCS10 (2D) or CCS40 (2I).
Table 2.
Test conditions for experiment 2
| Right motor cortex | ||||
|---|---|---|---|---|
| Condition | CS10 | CCS10 | CCS40 | Left motor cortex TS |
| 2A | 1 mV | |||
| 2B | 1 mV | 1 mV | ||
| 2C | 95% AMT | 1 mV | 1 mV | |
| 2D | 95% AMT | 1 mV | ||
| 2E | 1mVCS10 | 1 mV | ||
| 2F | 95% AMT | 1mVCS10 | 1 mV | |
| 2G | 1 mV | 1 mV | ||
| 2H | 95% AMT | 1 mV | 1 mV | |
| 2I | 95% AMT | 1 mV | ||
| 2J | 1mVCS10 | 1 mV | ||
| 2K | 95% AMT | 1mVCS10 | 1 mV | |
Stimulus intensities. CS10, conditioning stimulus delivered 10 ms before the CCS to elicit ICF; CCS10, contralateral conditioning stimulus delivered 10 ms before the TS to elicit IHI10; CCS40, contralateral conditioning stimulus delivered 40 ms before the TS to elicit IHI40. The above stimuli were delivered to the right motor cortex. TS, test stimulus applied to the left motor cortex to elicit an MEP in the right first dorsal interosseous (FDI). See Methods for CCS intensity nomenclature.
Experiment 3: effects of LICI on IHI
Eleven conditions were tested as detailed in Table 3 (3A–3K). Ten trials of each condition were implemented in a randomized order, totalling 110 trials. Conditions 3A to 3F looked at the interaction between LICI and IHI10, while conditions 3G to 3K looked at the interaction between LICI and IHI40. In conditions 3E, 3F, 3J, and 3K, the CCS pulse was increased to produce a 1 mV MEP response in the presence of a CS100 pulse. The resulting IHI was termed IHI1mVCS100 and allowed matching of the CCS MEP amplitude, resulting in similar degrees of corticospinal activation in the presence and absence of LICI. The effect of LICI on IHI was tested with three pulses in conditions 3C, 3F, 3H and 3K. Conditions 3B and 3G were used to determine the amount of IHI with a 1 mV CCS (IHI101mV and IHI401mV). Conditions 3E and 3J were used to determine the amount of IHI with the stronger CCS intensity (IHI101mVCS100 and IHI401mVCS100). We compared IHI10 and IHI40 alone (IHI10: 3B/3A; IHI40: 3G/3A) to IHI10 and IHI40 in the presence of LICI (IHI10: 3F/3A; IHI40: 3K/3A) matched for MEP amplitude produced by the CCS pulse (∼1 mV). We also compared IHI10 and IHI40 alone (IHI10: 3B/3A and 3E/3A; IHI40: 3G/3A and 3J/3A) to IHI10 and IHI40 in the presence of LICI matched for the stimulus intensity of the CCS pulse (IHI 10: 3C/3A and 3F/3A; IHI40: 3H/3A and 3K/3A). The stimulus intensities of the CCS pulses were either 1 mV (IHI10: 3B/3A versus 3C/3A; IHI40 3G/3A versus 3H/3A) or CCS1mVCS100 (IHI10: 3E/3A versus 3F/3A; IHI40: 3J/3A versus 3K/3A). To determine whether the CS100 pulse elicited IHI, it was paired with the IHI TS in the absence of a CCS10 (3D) or CCS40 (3I).
Table 3.
Test conditions for experiment 3
| Right motor cortex | ||||
|---|---|---|---|---|
| Condition | CS100 | CCS10 | CCS40 | Left motor cortex TS |
| 3A | 1 mV | |||
| 3B | 1 mV | 1 mV | ||
| 3C | 1 mV | 1 mV | 1 mV | |
| 3D | 1 mV | 1 mV | ||
| 3E | 1mVCS100 | 1 mV | ||
| 3F | 1 mV | 1mVCS100 | 1 mV | |
| 3G | 1 mV | 1 mV | ||
| 3H | 1 mV | 1 mV | 1 mV | |
| 3I | 1 mV | 1 mV | ||
| 3J | 1mVCS100 | 1 mV | ||
| 3K | 1 mV | 1mVCS100 | 1 mV | |
Stimulus intensities. CS100, conditioning stimulus delivered 100 ms before the CCS to elicit LICI; CCS10, contralateral conditioning stimulus delivered 10 ms before the TS to elicit IHI10; CCS40, contralateral conditioning stimulus delivered 40 ms before the TS to elicit IHI40. These stimuli were delivered to the right motor cortex. TS, test stimulus applied to the left motor cortex to elicit a MEP in the right FDI. See Methods for CCS intensity nomenclature.
Data analysis
The peak-to-peak MEP amplitude for each trial was measured offline. MEP amplitudes were expressed as a ratio of the mean unconditioned MEP amplitude (condition A) for each subject. Ratios below one represent inhibition and ratios above one represent facilitation. Values are expressed as the mean ± standard deviation (s.d.).
For experiment 1, the effect of SICI on IHI was determined by repeated-measures ANOVA with test condition for IHI as the repeated measure (IHI alone elicited by CCS of 1 mV, IHI alone elicited by CCS adjusted to produce a 1 mV MEP in the presence of SICI, IHI elicited by the adjusted CCS in the presence of SICI tested through the triple pulse configuration) and ISI for IHI (IHI10 and IHI40) as an independent variable. If the main effect was significant, Fisher's protected least significant difference (PLSD) post hoc test was used. In experiments 2 (effects of ICF on IHI) and 3 (effects of LICI on IHI), similar repeated-measures ANOVA and Fisher's PLSD post hoc test were used. For experiments 1 and 3, correlation between the strength of SICI and LICI to the change in IHI was tested using Pearson product-moment correlation coefficients. The threshold for significance was set at P < 0.05.
Results
Experiment 1: effects of SICI on IHI
All 10 subjects participated in this experiment. The TS delivered to the left motor cortex was set at 44.6 ± 5.2% of the stimulator output to produce a 1 mV MEP response in the right FDI. The mean TS MEP amplitude in the right FDI was 1.34 ± 0.4 mV (Table 1: condition 1A), which was reduced to 0.80 ± 0.4 mV by IHI101mV (condition 1B) and to 0.56 ± 0.4 mV by IHI401mV (condition 1G). With the stronger CCS pulse (denoted CCS1mVCS2), the resulting inhibition was greater with IHI101mVCS2 producing a 0.50 ± 0.4 mV response (condition 1E) and IHI401mVCS2 producing a 0.41 ± 0.3 mV response (condition 1J).
IHI10 and IHI40 were elicited by CCS at 63.1 ± 11.3% of the stimulator output to produce a 1 mV MEP response in the left FDI and 79.1 ± 16.7% of the stimulator output to produce a 1 mV MEP response in the presence of SICI. The mean CCS1mV MEP amplitude in the left FDI was 1.0 ± 0.2 mV (conditions 1B and 1G) and increased to 2.3 ± 1.1 mV with the stronger CCS1mVCS2 pulse (conditions 1E and 1 J). CS2 at 42.3 ± 8.8% of stimulator output elicited SICI which reduced the MEP produced by the CCS1mV pulse to 0.5 ± 0.3 mV (conditions 1C and 1H) and the CCS1mVCS2 pulse to 1.0 ± 0.3 mV (conditions 1F and 1K). Therefore, conditions 1B and 1F and conditions 1G and 1K were matched for MEP amplitude in the left FDI muscle.
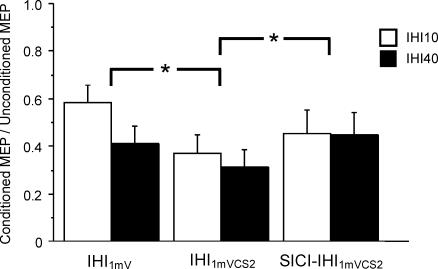
Figure 1 illustrates the interaction between SICI and IHI40 in a representative subject. The CCS40 pulse elicited IHI40 that reduced the MEP amplitude evoked by the TS (Fig. 1A and B) in the right FDI muscle. With the addition of a CS2 pulse to produce SICI, the MEP elicited by the CCS40 pulse in the left FDI muscle was decreased (Fig 1D and E) as expected. However, the addition of the CS2 pulse increased the MEP amplitude in the right FDI muscle when matched for stimulus intensity (Fig. 1B and C). The grouped data for IHI1mV (1B/1A, 1G/1A), IHI1mVCS2 (1E/1A, 1 J/1A), and IHI1mVCS2 in the presence of SICI (1F/1A, 1K/1A) for IHI10 and IHI40 are shown in Fig. 2 and Table 4. Repeated measures ANOVA indicated a significant effect of test conditions (P = 0.0005) but no significant effect of ISI (10 or 40 ms) on IHI. The test condition–ISI interaction was not significant. Post hoc tests revealed that the reduction in IHI10 and IHI40 by SICI was significant when matched for stimulus intensity (P = 0.0056) (Fig. 2, second and third column) but not when matched for MEP amplitude (Fig. 2, first and third column). In the other stimulus intensity-matched conditions, 1G and 1H (not shown in Fig. 2), the inhibition of IHI40 by SICI approached significance (P = 0.067). IHI elicited using the stronger CCS1mVCS2 pulse resulted in significantly greater inhibition of the test MEP (Fig. 2, first and second column, post hoc test, P = 0.0001). The CS2 pulse alone in conditions 1D and 1I did not produce IHI: the MEP ratios were 1.02 ± 0.06 for 1D/1A and 0.96 ± 0.09 for 1I/1A. The strength of IHI10 and IHI40 inhibition by SICI (1C/1A–1B/1 A, 1H/1A–1G/1A) did not correlate with the strength of SICI (left FDI, 1C/1B or 1H/1G) when the CCS were matched for stimulus intensity (r = 0.33, P = 0.35). Similarly, no correlation (r = 0.28, P = 0.24) was found for conditions matched for the higher CCS intensity (IHI10: 1F/1A–1E/1A versus 1F/1E, IHI40: 1K/1A–1J/1A versus 1K/1J).
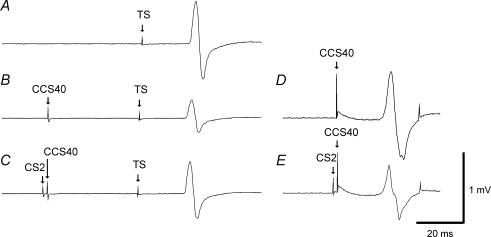
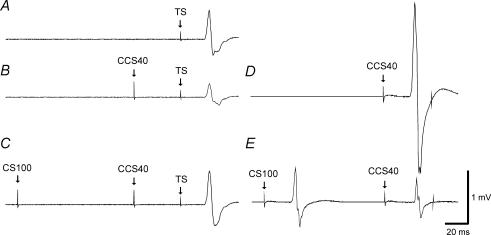
Figure 1. Effects of SICI on IHI40 in a representative subject.
Each trace represents the average of 10 trials. Traces A, B and C are recordings from the right FDI muscle and traces D and E are concurrent recordings from the left FDI muscle. A, response to 1 mV TS alone (condition 1A). B, IHI401mVCS2 alone: the adjusted CCS401mVCS2 pulse inhibited the TS MEP (condition 1J). C, IHI401mVCS2 in the presence of SICI: the CS2 pulse preceding the CCS401mVCS2 pulse led to reduction of IHI as shown here by an increase in MEP amplitude (condition 1K). The presence of SICI was confirmed by a decrease in the MEP amplitude in the left FDI from D (condition 1J) to E (condition 1K), due to the CS2 pulse.
Figure 2. Effects of SICI on IHI10 and IHI40 data from 10 subjects.
IHI1mV represents IHI with a CCS of 1 mV (IHI10: condition 1B/1A, IHI40: condition 1G/1A). IHI1mVCS2 represents IHI with intensity of the CCS adjusted to produce a 1 mV MEP in the left FDI muscle if preceded by a CS2 pulse (IHI10: condition 1E/1A, IHI40: condition 1J/1A). SICI-IHI1mVCS2 is IHI in the presence of SICI (IHI10: condition 1F/1A, IHI40: condition 1K/1A). SICI significantly reduced both IHI10 and IHI40 when matched for stimulus intensity (IHI1mVCS2 versus SICI-IHI1mVCS2), but not when matched for MEP amplitude (IHI1mV versus SICI-IHI1mVCS2). Inhibition or facilitation is expressed as a ratio of the conditioned to unconditioned MEPs. Ratios above 1 represent facilitation and ratios below 1 represent inhibition. Error bars represent s.e.m. *Significant difference (P < 0.05).
Table 4.
Values for SICI, ICF, LICI and IHI10, IHI40 in experiments 1–3
| Left FDI | Right FDI | |||||||
|---|---|---|---|---|---|---|---|---|
| Expt | IHI ISI | SICI | ICF | LICI | IHI | CS–IHI | IHI (adjusted CCS) | CS-IHI (adjusted CCS) |
| 1 | 10 | 49 ± 17 | 58 ± 22 | 65 ± 29 | 37 ± 25 | 45 ± 32 | ||
| (1C/1B, | — | — | (1B/1A) | (1C/1A) | (1E/1A) | (1F/1A) | ||
| 40 | 1H/1G) | 41 ± 24 | 51 ± 29 | 31 ± 22 | 45 ± 31 | |||
| (1G/1A) | (1H/1A) | (1J/1A) | (1K/1A) | |||||
| 2 | 10 | 158 ± 49 | 56 ± 21 | 54 ± 22 | 68 ± 24 | 69 ± 19 | ||
| — | (2C/2B, | — | (2B/2A) | (2C/2A) | (2E/2A) | (2F/2A) | ||
| 40 | 2H/2G) | 53 ± 25 | 59 ± 27 | 68 ± 27 | 71 ± 26 | |||
| (2G/2A) | (2H/2A) | (2J/2A) | (2K/2A) | |||||
| 3 | 10 | 29 ± 12 | 52 ± 23 | 86 ± 53 | 33 ± 19 | 55 ± 44 | ||
| — | — | (3C/3B, | (3B/3A) | (3C/3A) | (3E/3A) | (3F/3A) | ||
| 40 | 3H/3G) | 49 ± 19 | 73 ± 36 | 33 ± 19 | 60 ± 33 | |||
| (3G/3A) | (3H/3A) | (3J/3A) | (3K/3A) | |||||
SICI, ICF, and LICI recorded from the left FDI muscle are expressed as percentages of the response to the CCS alone. IHI, recorded from the right FDI muscle, are expressed as percentages of the response to the test stimulus alone. IHI: elicited by CCS1mV; CS–IHI: IHI1mV in the presence of SICI, ICF, or LICI; IHI (adjusted CCS): elicited by a CCS adjusted to produce a 1 mV MEP response in the presence of intracortical inhibition or facilitation; CS–IHI (adjusted CCS): IHI1mVCS2 in the presence of SICI, IHI1mVCS10 in the presence of ICF, or IHI1mVCS100 in the presence of LICI. The corresponding conditions are listed in parentheses.
Experiment 2: effects of ICF on IHI
Nine subjects participated in this experiment as ICF could not be elicited in one subject. A TS of intensity 45.8 ± 7.3% of the stimulator output was delivered to the left motor cortex to produce TS MEP amplitude of 1.1 ± 0.4 mV in the right FDI (Table 2: condition 2A), which decreased to 0.7 ± 0.4 mV due to IHI101mV (condition 2B) and 0.62 ± 0.4 mV due to IHI401mV (condition 2G). The weaker CCS pulse used to match for MEP amplitude (denoted CCS1mVCS10) caused less inhibition with IHI101mVCS10, producing a 0.82 ± 0.4 mV response (condition 2E) and IHI401mVCS10, producing a 0.79 ± 0.4 mV response (condition 2J).
The mean CCS1mV MEP amplitude in the left FDI was 1.3 ± 0.3 mV (conditions 2B and 2G, stimulus intensities 60.9 ± 11.7% of stimulator output) and decreased to 0.9 ± 0.5 mV with the weaker CCS1mVCS10 pulse (conditions 2E and 2J, stimulus intensities 56.2 ± 9.9% of stimulator output). CS10 elicited ICF as it increased the amplitude of the MEP response produced by the CCS1mV to 1.9 ± 0.4 mV (conditions 2C and 2H) and the MEP evoked by the CCS1mVCS10 pulse to 1.2 ± 0.2 mV (conditions 2F and 2K). Conditions 2B and 2F, and conditions 2G and 2K, were matched for MEP amplitude.
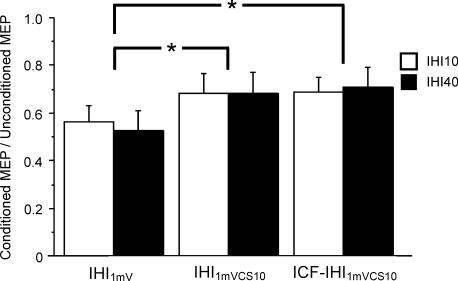
The interaction between ICF and IHI40 in a representative subject is shown in Fig. 3. The CCS40 pulse produced IHI40, which decreased the TS MEP amplitude (Fig. 3A and B). Addition of a CS10 pulse that preceded both CCS40 had no effect on TS MEP amplitude when conditions were matched for stimulus intensity (Fig. 3B and C), although the CS10 pulse led to ICF in the left FDI muscle (Fig. 3D and E). The data for all nine subjects for IHI1mV (2B/2A, 2G/2A), IHI1mVCS10 (2E/2A, 2J/2A), and IHI1mVCS10 in the presence of ICF (2F/2A, 2K/2A) for IHI10 and IHI40 are shown in Fig. 4 and Table 4. Repeated measures ANOVA demonstrated a significant effect of test condition on IHI10 and IHI40 (P = 0.0009) but no significant effect of ISI. The test condition–ISI interaction was not significant. Post hoc tests revealed that ICF significantly reduced IHI10 and IHI40 when matched for MEP amplitude (Fig. 4, first and third column, P = 0.0006) but had no effect when matched for stimulus intensity (Fig. 4, second and third column). IHI elicited with the weaker CCS1mVCS10 resulted in significantly less inhibition (Fig. 4, first and second column, P = 0.0017). The CS10 pulse alone did not elicit any IHI (MEP ratios were 1.05 ± 0.01 for 2D/2A and 1.01 ± 0.01 for 2I/2A).
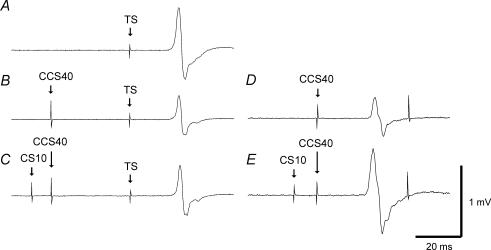
Figure 3. Effects of ICF on IHI40 in a representative subject.
Traces represent the average of 10 trials. Traces A, B and C are from the right FDI muscle, and traces D and E are concurrent recordings from the left FDI muscle. A, 1 mV TS MEP response (condition 2A). B, IHI401mVCS10 alone: the TS MEP was inhibited by the CCS401mVCS10 pulse (condition 2J). C, IHI401mVCS10 in the presence of ICF: the CS10 pulse preceding the CCS401mVCS10 pulse had no effect on IHI and there was no significant change in MEP amplitude (condition 2K). The presence of ICF was confirmed by the increase in MEP amplitude from D (condition 2J) to E (condition 2K) due to the CS10 pulse.
Figure 4. Effects of ICF on IHI10 and IHI40 data from nine subjects.
IHI1mV represents IHI with a CCS of 1 mV (IHI10: condition 2B/2A, IHI40: condition 2G/2A). IHI1mVCS10 represents IHI with intensity of the CCS adjusted to produce a 1 mV MEP in the left FDI if preceded by a CS10 pulse (IHI10: condition 2E/2A, IHI40: condition 2J/2A). ICF-IHI1mVCS10 is IHI in the presence of ICF (IHI10: condition 2F/2A, IHI40: condition 2K/2A). Both IHI10 and IHI40 were significantly reduced by ICF when matched for MEP amplitude (IHI1mV versus ICF-IHI1mVCS10), but not when matched for stimulus intensity (IHI1mVCS10 versus ICF-IHI1mVCS10). Inhibition or facilitation is expressed as a ratio of the conditioned to unconditioned MEPs. Ratios above 1 represent facilitation and ratios below 1 represent inhibition. Error bars represent s.e.m. *Significant difference (P < 0.05).
Experiment 3: effects of LICI on IHI
This experiment was conducted on nine subjects as IHI could not be elicited in one subject. A TS of intensity 44.9 ± 7.3% of the stimulator output was delivered to the left motor cortex to produce TS MEP amplitude of 1.14 ± 0.3 mV in the right FDI (Table 3: condition 3A), which was reduced to 0.71 ± 0.5 mV by IHI101mV (condition 3B) and 0.72 ± 0.6 mV by IHI401mV (condition 3G). With the stronger CCS pulse used to match for MEP amplitude (denoted CCS1mVCS100), the resulting inhibition was greater for IHI101mVCS100 producing a 0.53 ± 0.7 mV response (condition 3E) and IHI401mVCS100 producing a 0.52 ± 0.5 mV response (condition 3J).
The mean MEP amplitude in the left FDI evoked by the CCS1mV pulse was 1.2 ± 0.3 mV (conditions 3B and 3G, 60.7 ± 11.6% stimulator output) which increased to 2.6 ± 0.9 mV (conditions 3E and 3J, 74.4 ± 16.8% stimulator output) with the stronger CCS1mVCS100 pulse. LICI is demonstrated by reduction in the MEP amplitude produced by the CCS1mV pulse to 0.2 ± 0.2 mV (conditions 3C and 3H) and the CCS1mVCS100 pulse to 1.1 ± 0.3 mV (conditions 3F and 3K) in the presence of CS100. Thus, conditions 3B and 3F and conditions 3G and 3K were matched for MEP amplitude.
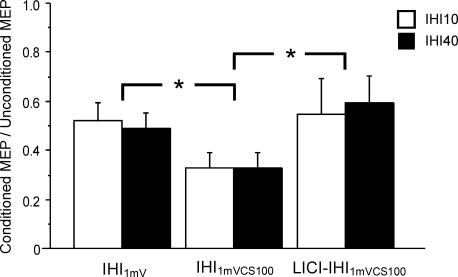
Figure 5 illustrates the interaction between LICI and IHI40 in a representative subject. The CCS40 pulse elicited IHI40 that reduced the MEP amplitude evoked by the TS (Fig. 5A and B). Addition of the CS100 pulse increased the MEP amplitude in the right FDI muscle when matched for stimulus intensity (Fig. 5B and C). As expected, the CS100 pulse produced LICI, which decreased the MEP elicited by the CCS40 pulse in the left FDI muscle. Grouped data from all subjects for IHI1mV (3B/3A, 3G/3A), IHI1mVCS100 (3E/3A, 3J/3A), and IHI1mVCS100 in the presence of LICI (3F/3A, 3K/3A) for IHI10 and IHI40 are shown in Fig. 6 and Table 4. Repeated measures ANOVA indicated a significant effect of test conditions (P = 0.0012) but no significant effect of ISI (10 or 40 ms) on IHI. The test condition–ISI interaction was not significant. Post hoc tests revealed that the reduction in IHI10 and IHI40 by LICI was significant when matched for stimulus intensity (P = 0.0004) (Fig. 6, second and third column) but not when matched for MEP amplitude (Fig. 6, first and third column). For conditions 3G and 3H that are also matched for test stimulus intensity (not shown in Fig. 6), the inhibition of IHI40 by LICI was also significant (P = 0.03). IHI elicited by the stronger CCS1mVCS100 produced significantly greater inhibition (Fig. 6, first and second column, P = 0.0074). The CS100 pulse alone in conditions 3D and 3I did not produce any IHI (MEP ratios 1.13 ± 0.09 for 3D/3A and 1.02 ± 0.1 for 3I/3A. The magnitude of IHI10 and IHI40 inhibition by LICI (3C/3A–3B/3A, 3H/3A–3G/3A) did not correlate with the strength of LICI (left FDI, 3C/3B or 3H/3G) when the CCS were matched for stimulus intensity (r = 0.07, P = 0.79). Similarly, no correlation (r = 0.09, P = 0.74) was found for conditions matched for the higher CCS intensity (IHI10: 3F/3A–3E/3A versus 3F/3E, IHI40: 3K/3A–3J/3A versus 3K/3J).
Figure 5. Effects of LICI on IHI40 in a representative subject.
Waveforms are the average of 10 trials. Traces A, B and C are from the right FDI muscle and traces D and E are concurrent recordings from the left FDI muscle. A, response to 1 mV TS alone (condition 3A). B, IHI401mVCS100 alone: the CCS401mVCS100 pulse reduced the amplitude of the TS MEP (condition 3J). C, IHI401mVCS100 in the presence of LICI: the CS100 pulse preceding the CCS401mVCS100 pulse led to inhibition of IHI resulting in an increase in MEP amplitude (condition 3K). The presence of LICI in the left FDI muscle was demonstrated by reduction in the MEP amplitude from D (condition 3J) to E (condition 3K), due to the CS100 pulse.
Figure 6. Effects of LICI on IHI10 and IHI40 data from nine subjects.
IHI1mV represents IHI with a CCS of 1 mV (IHI10: condition 3B/3A, IHI40: condition 3G/3A). IHI1mVCS100 represents IHI with intensity of the CCS adjusted to produce a 1 mV MEP in the left FDI muscle if preceded by a CS100 pulse (IHI10: condition 3E/3A, IHI40: condition 3J/3A). LICI-IHI1mVCS100 is IHI in the presence of LICI (IHI10: condition 3F/3A, IHI40: condition 3K/3A). LICI significantly reduced both IHI10 and IHI40 when matched for stimulus intensity (IHI1mVCS100 versus LICI-IHI1mVCS100), but not when matched for MEP amplitude (IHI1mV versus LICI-IHI1mVCS100). Inhibition or facilitation is expressed as a ratio of the conditioned to unconditioned MEPs. Ratios above 1 represent facilitation and ratios below 1 represent inhibition. Error bars represent s.e.m. *Significant difference (P < 0.05).
Discussion
SICI and LICI inhibit IHI
Several observations suggest that IHI is mainly mediated by transcallosal fibres, although subcortical circuits may also contribute (Gerloff et al. 1998). IHI at short ISIs of about 10 ms are consistent with transcallosal conduction times (Ferbert et al. 1992). A CCS applied to one motor cortex reduced the size of descending corticospinal volleys from the contralateral motor cortex (Di Lazzaro et al. 1999) but did not attenuate the TS response evoked by an anodal electrical stimulus (Ferbert et al. 1992). This interhemispheric inhibition is probably transmitted through the corpus callosum (Meyer et al. 1995; 1998; Hoppner et al. 1999). Since GABAergic neurons are predominantly found in local circuits (Somogyi et al. 1998), it is likely that IHI is due to excitatory transcallosal fibres from the originating hemisphere that synapse to inhibitory interneurons in the target hemisphere to attenuate descending corticospinal output.
We found that both SICI and LICI significantly reduced IHI10 and IHI40 when matched for CCS intensity. It is likely that the CS2 and CS100 pulses inhibited the effects of the CCS pulse through reduced activation of transcallosal fibres that mediate IHI (Ferbert et al. 1992; Meyer et al. 1998). In the MEP amplitude (left FDI muscle) matched conditions, SICI or LICI produced no change in either IHI10 or IHI40. By increasing the intensity of the CCS pulse to match for a similar degree of corticospinal activation, the inhibitory influence of a preceding CS2 or CS100 was also compensated for, and produced similar degrees of transcallosal fibre activation as the CCS1mV pulse alone. Therefore, SICI and LICI produced similar degrees of inhibition for the corticospinal and transcallosal output systems.
It has been reported that SICI reduces iSP area (Trompetto et al. 2004). The similar effect of SICI on iSP and IHI40 is consistent with the suggestion that they may be related (Chen et al. 2003). Since IHI10 and IHI40 are mediated by different mechanisms (Chen et al. 2003), both SICI and LICI appear to produce widespread cortical inhibition affecting several transcallosal circuits as well as the corticospinal output system.
The interactions between SICI and IHI10 are different for the originating hemisphere for IHI examined in this study compared to the target (contralateral) hemisphere, where IHI10 was found to inhibit SICI (Daskalakis et al. 2002b). However, LICI appears to inhibit IHI10 in both the originating and target hemispheres (Daskalakis et al. 2002b). The interactions between SICI, LICI and IHI40 in the target hemisphere have not been studied.
No interaction between ICF and IHI
In contrast to SICI and LICI, ICF had no effect on IHI10 and IHI40 when matched for the intensity of the CCS pulse. Although ICF reduced IHI10 and IHI40 when matched for MEP amplitude (Figs 3 and 4), this is probably because the CCS intensity was reduced in these trials to account for the facilitatory effect of ICF on MEP amplitude, and this resulted in reduced activation of the inhibitory transcallosal mechanism. These findings suggest that ICF does not interact with either IHI10 or IHI40, as the degree of IHI was solely contingent on the intensity of the CCS pulse irrespective of the presence of ICF. In the target hemisphere, a previous study also found no interaction between ICF and IHI10 (Daskalakis et al. 2002b).
This is consistent with the work of Trompetto et al. (2004) who found no change in iSP with ICF, although only three subjects were tested. The mechanisms underlying ICF remains unclear. Epidural recordings found no increase in the amplitude of descending corticospinal volleys associated with ICF, although MEP amplitudes were increased (Di Lazzaro et al. 2006). However, there was also no evidence for changes in spinal excitability to account for ICF (Ziemann et al. 1996b; Di Lazzaro et al. 2006). If ICF is a cortical phenomenon, its influence is much more focused compared to SICI and LICI.
Different neuronal populations mediate IHI and corticospinal output
While it is known that corticospinal neurons originate from layer V of the cortex, the location of transcallosal neurons in the motor cortex remains controversial. Jacobson & Trojanowski (1974) used retrograde labelling and reported that cells of origin of the callosal system are found in layers II to VI, predominately in layers III and V. Casterman-Beerevoets et al. (1980) reported that retrogradely labelled callosal neurons are present in all layers except layer I. Jones et al. (1979) found that callosal projection cells arise from layer IIIB, but the study was conducted in the primary somatosensory cortex. By injecting retrograde tracers into the sensorimotor cortex and ipsilateral corticospinal tract at the C2 spinal segment of anaesthetized rats, Catsman-Berrevoets et al. (1980) showed that the transcallosal fibres and the descending corticospinal tract are of different origin as there were no double-retrogradely labelled neurons in layer V of the non-injected hemisphere. They also found that layer V neurons of the sensorimotor cortex which responded to antidromic stimulation of the corticospinal tract could not be activated by antidromic stimulation of the corpus callosum. Thus, these animal studies suggest that transcallosal and corticospinal projecting cells are distinct.
Two of our findings support the suggestion that the situation is similar in humans, that transcallosal fibres are not collaterals of corticospinal fibres, and transcallosal and corticospinal projection neurons belong to distinct populations. The first is that ICF facilitated the corticospinal output but had no effect on interhemispheric inhibition. The second is the lack of correlation between MEP inhibition and IHI inhibition by SICI and LICI. This is consistent with greater stimulus intensity required to produce iSP than MEPs (Trompetto et al. 2003), the suppression of iSP in subjects who failed to demonstrate SICI (Trompetto et al. 2004), and the different effects of the direction of induced currents on iSP and MEP latencies (Meyer et al. 1996).
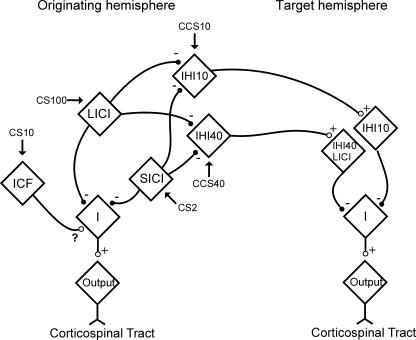
A model of how intracortical inhibitory and facilitatory circuits interact with the corticospinal and transcallosal output systems that is consistent with the previous and the current observations is shown in Fig. 7. The corticospinal output and IHI are shown as mediated by different neuronal populations. IHI10 and IHI40 are also shown as due to different mechanisms, based on the results of previous studies (Chen et al. 2003; Kukaswadia et al. 2005). Both SICI and LICI inhibit IHI10 and IHI40, in addition to inhibition of the corticospinal output. However, ICF only affects the corticospinal output system and the question mark indicates that the nature of this interaction remains unclear (Di Lazzaro et al. 2006).
Figure 7. Model of interactions between intracortical and interhemispheric circuits.
The diamonds represent neuronal circuits mediating SICI, LICI, ICF, IHI10 and IHI40. Arrows point to the circuits which the corresponding pulse activates. Excitatory connections are labelled by + and ○, while inhibitory connections are labelled by – and •. The interaction between ICF and descending I-waves is labelled ?, as the mechanism underlying ICF has not been firmly established. In both the originating and target hemispheres for IHI, the ‘I’ diamond represents neurons that lead to descending I-waves, and the ‘output’ diamond represents corticospinal neurons. IHI10 and IHI40 are represented by different neuronal populations based on the results of previous studies (Chen et al. 2003; Kukaswadia et al. 2005).
The IHI circuits probably send excitatory connections across the corpus callosum to activate inhibitory interneurons in the contralateral motor cortex. IHI10 and IHI40 are shown as separate in the target (contralateral) hemisphere, based on a previous study which also suggests that an overlapping population of interneurons mediate LICI and IHI40 (Kukaswadia et al. 2005).
Implications for findings in diseases
Disruptions in intracortical and transcallosal circuits are seen in various diseases, and our findings may provide a possible explanation for some of these observations. For example, reduction in SICI in the unaffected hemisphere (Shimizu et al. 2002; Butefisch et al. 2003) in stroke patients can potentially explain the increased IHI from the unaffected to the affected hemisphere before movement onset (Murase et al. 2004). In Parkinson's disease, decreased SICI (Ridding et al. 1995a) may be related to increased IHI in patients without mirror movements (Li et al. 2007). Our findings may also have implications in studies of plasticity and motor learning where there are changes in intracortical and transcallosal inhibition.
Conclusions
Separate neuronal populations give rise to transcallosal fibres and the descending corticospinal output. SICI and LICI generate diffuse cortical inhibition that affects both the corticospinal output and transcallosal systems to a similar extent, whereas ICF influences only the corticospinal output system.
Acknowledgments
This study was supported by the Canadian Institutes of Health Research, Canada Foundation for Innovation, Ontario Innovation Trust, University Health Network Krembil Family Chair in Neurology, the Catherine Manson Chair in Movement Disorders, and Institute of Medical Sciences at the University of Toronto.
References
- Butefisch CM, Netz J, Wessling M, Seitz RJ, Homberg V. Remote changes in cortical excitability after stroke. Brain. 2003;126:470–481. doi: 10.1093/brain/awg044. [DOI] [PubMed] [Google Scholar]
- Catsman-Berrevoets CE, Lemon RN, Verburgh CA, Bentivoglio M, Kuypers HGJM. Absence of callosal collaterals dervied from rat corticospinal neurons: a study using fluorescent retrograde tracing and electrophysiological techniques. Exp Brain Res. 1980;39:433–440. doi: 10.1007/BF00239308. [DOI] [PubMed] [Google Scholar]
- Chen R. Interactions between inhibitory and excitatory circuits in the human motor cortex. Exp Brain Res. 2004;154:1–10. doi: 10.1007/s00221-003-1684-1. [DOI] [PubMed] [Google Scholar]
- Chen R, Lozano AM, Ashby P. Mechanism of the silent period following transcranial magnetic stimulation. Evidence from epidural recordings. Exp Brain Res. 1999;128:539–542. doi: 10.1007/s002210050878. [DOI] [PubMed] [Google Scholar]
- Chen R, Tam A, Bütefisch C, Corwell B, Ziemann U, Rothwell JC, Cohen LG. Intracortical inhibition and facilitation in different representations of the human motor cortex. J Neurophysiol. 1998;80:2870–2881. doi: 10.1152/jn.1998.80.6.2870. [DOI] [PubMed] [Google Scholar]
- Chen R, Yung D, Li J-Y. Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. J Neurophysiol. 2003;89:1256–1264. doi: 10.1152/jn.00950.2002. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Chen R, Fitzgerald PB, Zipursky RB, Kapur S. Evidence for impaired cortical inhibition in schizophrenia using transcranial magnetic stimulation. Arch Gen Psychiatry. 2002a;59:347–354. doi: 10.1001/archpsyc.59.4.347. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Roshan L, Chen R. The mechanisms of interhemispheric inhibition in the human motor cortex. J Physiol. 2002b;543:317–326. doi: 10.1113/jphysiol.2002.017673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P, Rothwell JC. Direct demonstration of interhemispheric inhibition of the human motor cortex produced by transcranial magnetic stimulation. Exp Brain Res. 1999;124:520–524. doi: 10.1007/s002210050648. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Oliviero A, Dileone M, Saturno E, Mazzone P, Insola A, Profice P, Ranieri F, Capone F, Tonali PA, Rothwell JC. Origin of facilitation of motor-evoked potentials after paired magnetic stimulation: direct recording of epidural activity in conscious humans. J Neurophysiol. 2006;96:1765–1771. doi: 10.1152/jn.00360.2006. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Magnetic transcranial stimulation at intensities below active motor threshold activates inhibitory circuits. Exp Brain Res. 1998;119:265–268. doi: 10.1007/s002210050341. [DOI] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhr P, Agostino R, Hallett M. Spinal motor neuron excitability during the silent period after cortical stimulation. Electroencephalogr Clin Neurophysiol. 1991;81:257–262. doi: 10.1016/0168-5597(91)90011-l. [DOI] [PubMed] [Google Scholar]
- Gerloff C, Cohen LG, Floeter MK, Chen R, Corwell B, Hallett M. Inhibitory influence of the ipsilateral motor cortex on responses to stimulation of the human cortex and pyramidal tract. J Physiol. 1998;510:249–259. doi: 10.1111/j.1469-7793.1998.249bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation and the human brain. Nature. 2000;406:147–150. doi: 10.1038/35018000. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Machii K, Mochizuki H, Terao Y, Enomoto H, Furubayashi T, Shiio Y, Uesugi H, Kanazawa I. Interhemispheric facilitation of the hand motor area in humans. J Physiol. 2001;531:849–859. doi: 10.1111/j.1469-7793.2001.0849h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Sakai K, Furubayashi T, Machii K, Kanazawa I. Paired-pulse magnetic stimulation of the human motor cortex: differences among I waves. J Physiol. 1998;509:607–618. doi: 10.1111/j.1469-7793.1998.607bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppner J, Kunesch E, Buchmann J, Hess A, Grossmann A, Benecke R. Demyelination and axonal degeneration in corpus callosum assessed by analysis of transcallosally mediated inhibition in multiple sclerosis. Clin Neurophysiol. 1999;110:748–756. doi: 10.1016/s1388-2457(98)00075-3. [DOI] [PubMed] [Google Scholar]
- Jacobson S, Trojanowski JQ. The cells of origin of the corpus callosum in rat, cat and rhesus monkey. Brain Res. 1974;74:149–155. doi: 10.1016/0006-8993(74)90118-8. [DOI] [PubMed] [Google Scholar]
- Jones EG, Coulter JD, Wise SP. Commissural columns in the sensory-motor cortex of monkeys. J Comp Neurol. 1979;188:113–135. doi: 10.1002/cne.901880110. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukaswadia S, Wagle-Shukla A, Morgante F, Gunraj C, Chen R. Interactions between long latency afferent inhibition and interhemispheric inhibitions in the human motor cortex. J Physiol. 2005;563:915–924. doi: 10.1113/jphysiol.2004.080010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J-Y, Espay AJ, Gunraj CA, Pal PK, Cunic DI, Lang AE, Chen R. Interhemispheric and ipsilateral connections in Parkinson's disease: relation to mirror movements. Mov Disord. 2007 doi: 10.1002/mds.21386. DOI: 10.1002/mds.21386. [DOI] [PubMed] [Google Scholar]
- Meyer BU, Kuehn A, Roericht S. Influence of the direction of induced currents on callosally and corticospinally mediated electromyographic responses following magnetic motor cortex stimulation in man. J Physiol. 1996;497.P:34–35P. [Google Scholar]
- Meyer BU, Roricht S, Grafin von Einsiedel H, Kruggel F, Weindl A. Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with abnormalities of the corpus callosum. Brain. 1995;118:429–440. doi: 10.1093/brain/118.2.429. [DOI] [PubMed] [Google Scholar]
- Meyer BU, Roricht S, Woiciechowsky C. Topography of fibers in the human corpus callosum mediating interhemispheric inhibition between the motor cortices. Ann Neurol. 1998;43:360–369. doi: 10.1002/ana.410430314. [DOI] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55:400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. J Physiol. 1997;498:817–823. doi: 10.1113/jphysiol.1997.sp021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridding MC, Brouwer B, Nordstrom MA. Reduced interhemispheric inhibition in musicians. Exp Brain Res. 2000;133:249–253. doi: 10.1007/s002210000428. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Inzelberg R, Rothwell JC. Changes in excitability of motor cortical circuitry in patients with Parkinson's disease. Ann Neurol. 1995a;37:181–188. doi: 10.1002/ana.410370208. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Taylor JL, Rothwell JC. The effect of voluntary contraction on cortico-cortical inhibition in human motor cortex. J Physiol. 1995b;487:541–548. doi: 10.1113/jphysiol.1995.sp020898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell JC. Techniques and mechanisms of action of transcranial stimulation of the human motor cortex. J Neurosci Meth. 1997;74:113–122. doi: 10.1016/s0165-0270(97)02242-5. [DOI] [PubMed] [Google Scholar]
- Sanger TD, Garg RR, Chen R. Interactions between two different inhibitory systems in the human motor cortex. J Physiol. 2001;530:307–317. doi: 10.1111/j.1469-7793.2001.0307l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Hosaki A, Hino T, Sato M, Komori T, Hirai S, Rossini PM. Motor cortical disinhibition in the unaffected hemisphere after unilateral cortical stroke. Brain. 2002;125:1896–1907. doi: 10.1093/brain/awf183. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Tamas G, Lujan R, Buhl EH. Salient features of synaptic organisation in the cerebral cortex. Brain Res Brain Res Rev. 1998;26:113–135. doi: 10.1016/s0165-0173(97)00061-1. [DOI] [PubMed] [Google Scholar]
- Trompetto C, Bove M, Marinelli L, Avanzino L, Buccolieri A, Abbruzzese G. Suppression of the transcallosal motor output: a transcranial magnetic stimulation study in healthy subjects. Exp Brain Res. 2004;158:133–140. doi: 10.1007/s00221-004-1881-6. [DOI] [PubMed] [Google Scholar]
- Trompetto C, Buccolieri A, Marchese R, Marinelli L, Michelozzi G, Abbruzzese G. Impairment of transcallosal inhibition in patients with corticobasal degeneration. Clin Neurophysiol. 2003;114:2181–2187. doi: 10.1016/s1388-2457(03)00213-x. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Pascual-Leone A, Wassermann EM, Hallett M. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol. 1992;85:355–364. doi: 10.1016/0168-5597(92)90048-g. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Samii A, Mercuri B, Ikoma K, Oddo D, Grill SE, Hallett M. Responses to paired transcranial magnetic stimuli in resting, active, and recently activated muscle. Exp Brain Res. 1996;109:158–163. doi: 10.1007/BF00228638. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Fong JKY, Meyer BU, Priori A, Rothwell JC, Day BL, Thompson PD. The effect of magnetic coil orientation on the latency of surface EMG and single motor unit responses in the first dorsal interosseous muscle. Electroencephalogr Clin Neurophysiol. 1994;93:138–146. doi: 10.1016/0168-5597(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol. 1999;517:591–597. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Lönnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol. 1996a;40:367–378. doi: 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol. 1996b;496:873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]