Abstract
The activation and function of Ca2+–calmodulin-dependent kinase II (CaMKII) in contracting rat skeletal muscle was examined. The increase in autonomous activity and phosphorylation at Thr287 of CaMKII of gastrocnemius muscle in response to contractions in situ was rapid and transient, peaking at 1–3 min, but reversed after 30 min of contractions. There was a positive correlation between CaMKII phosphorylation at Thr287 and autonomous CaMKII activity. In contrast to the rapid and transient increase in autonomous CaMKII activity, the phosphorylation of the putative CaMKII substrate trisk95/triadin was rapid and sustained during contractions. There were no changes in CaMKII activity and phosphorylation or trisk95 phosphorylation in the resting contralateral muscles during stimulation. When fast-twitch muscles were contracted ex vivo, CaMKII inhibition resulted in a greater magnitude of fatigue as well as blunted CaMKII and trisk95 phosphorylation, identifying trisk95 as a physiological CaMKII substrate. In summary, skeletal muscle CaMKII activation was rapid and sustained during exercise/contraction and is mediated by factors within the contracting muscle, probably through allosteric activation via Ca2+–CaM. CaMKII may signal through trisk95 to modulate Ca2+ release in fast-twitch rat skeletal muscle during exercise/contraction.
The rise in myoplasmic Ca2+ levels is a critical second messenger event in skeletal muscle excitation–contraction coupling (Melzer et al. 1995). Aside from this, Ca2+ signalling is proposed to play an important role in the regulation of metabolism (Lewis et al. 1982; Hargreaves & Richter, 1988; Spriet & Heigenhauser, 2002; Watt et al. 2003; Rose & Richter, 2005; Rose et al. 2005), gene transcription (Chin, 2004), and ion homeostasis (Sacchetto et al. 2005a) in skeletal muscle. As protein phosphorylation is a rapid, convenient, and flexible manner to alter the function of proteins (Cohen, 2000), Ca2+ signalling via kinases and phosphatases is likely to be an important mechanism of regulation of cellular responses in skeletal muscle. Calmodulin (CaM) is a Ca2+ receptor protein that undergoes conformational change when Ca2+ is bound and can allosterically modify several proteins (Hook & Means, 2001) among which include Ca2+–CaM-dependent phosphatases and kinases. Studies show that the Ca2+–CaM-dependent protein phosphatase 2B (PP2B/calcineurin) is activated during electrically induced muscle contractions which mimic slow motor nerve activity, as indexed by a translocation of the PP2B substrate NFAT2 to nuclei of adult muscle fibres (Liu et al. 2001; Tothova et al. 2006). However, whether PP2B has a role in plasticity of differentiated skeletal muscle is controversial (Parsons et al. 2004; Garcia-Roves et al. 2006; Huang et al. 2006).
Along with the unifunctional phosphorylase kinase, myosin light chain kinase and eukaryotic elongation factor 2 kinase, the multifunctional calmodulin-dependent protein kinases (i.e. CaMKI, II and IV) are likely to play functional roles in skeletal muscle (Chin, 2004). Of these, CaMKII is the best described (for a review see Sacchetto et al. 2005a), and was shown to be expressed in skeletal muscle more than 20 years ago (Campbell & MacLennan, 1982; Chiesi & Carafoli, 1982; Woodgett et al. 1982, 1983, 1984), and is likely to regulate gene transcription (Ojuka et al. 2002; Liu et al. 2005), ion homeostasis (Hawkins et al. 1994; Tavi et al. 2003) and metabolism (Watt et al. 2003; Wright et al. 2004; Singh et al. 2004; Sacchetto et al. 2005b). More recently, studies have demonstrated CaMKII expression in skeletal muscle of humans (Damiani et al. 1996; Margreth et al. 2000; Rose & Hargreaves, 2003; Rose et al. 2006).
The functional properties and regulation of skeletal muscle CaMKII have been reviewed in detail recently by Damiani and colleagues (Sacchetto et al. 2005a). Of note, despite different isoforms of CaMKII being expressed in skeletal muscle (Bayer et al. 1998; Rose & Hargreaves, 2003; Rose et al. 2006), the CaMKII of skeletal muscle displays properties similar to that of neural CaMKII (Woodgett et al. 1984; Pelosi & Donella-Deana, 2000; Rose et al. 2006). In particular, skeletal muscle CaMKII exists as a multimeric complex of 10–12 individual CaMKII enzymes (Woodgett et al. 1983), and upon rises in intracellular Ca2+ and Ca2+–CaM binding, undergoes a conformational change which relieves autoinhibition to increase catalytic activity (Colbran et al. 1989; Lengyel et al. 2001). In addition, skeletal muscle CaMKII can undergo autophosphorylation in response to prolonged exposure to Ca2+, which enables the kinase to be partially active in the absence of Ca2+–CaM (Pelosi & Donella-Deana, 2000; Rose et al. 2006).
Recently, it was shown that skeletal muscle CaMKII of humans was activated during exercise as indexed by higher autophosphorylation and autonomous activity as well as higher phosphorylation of a protein substrate phospholamban (Rose & Hargreaves, 2003; Rose et al. 2006). In particular, the activation of CaMKII in working skeletal muscle during exercise was rapid and sustained (Rose et al. 2006). However, in these studies, whether the activation was a result of local versus humoral factors during exercise could not be determined. Thus, the primary aims of the present study were to examine the time effect of contractions on skeletal muscle CaMKII and to gain further insight into the mechanisms as well as functional consequences of CaMKII activation in skeletal muscle.
Methods
Materials
All materials were from Sigma-Aldrich (USA) unless stated otherwise.
Animals
Male Sprague–Dawley rats were used for experimentation and were fed standard laboratory chow and consumed water ad libitum, and kept on a constant 12: 12 h light–dark cycle. All experiments were approved by the Danish Animal Experimental Inspectorate and complied with the European Convention for the Protection of Vertebrate Animals used for Experiments and other Scientific Purposes (council of Europe no. 123, Strasbourg, France, 1985).
In situ experiments
Rats (190–230 g) were anaesthetized by intraperitoneal injection of sodium pentobarbital (5 mg per 100 g body wt). With some animals (n = 8–12 per time-point), an in situ stimulation protocol was applied as a model of exercise, as previously described (Richter et al. 1984), to examine the time effect of contractions on signalling proteins. This protocol of muscle contraction was used rather than exercise as it results in recruitment of the entire fibre population of the stimulated muscles, the effect of local versus humoral factors can be accounted for by comparing the stimulated versus the resting contralateral hindlimb muscles, and it allows rapid collection of muscle tissue during the stimulation. In brief, the gastrocnemius muscles of both hindlimbs were exposed by surgically removing skin and connective tissues around these muscles. In addition, the sciatic nerve of the right hindlimb was carefully exposed. Afterwards, the rats were placed in a standard position by fixing the knee in a set position by inserting a needle under the patella tendon. A hook was then placed under the Achilles tendon of the same leg, and this was connected to a force transducer as previously described (Wojtaszewski et al. 1996). An electrode was placed around the sciatic nerve, and the hindlimb was stretched to a standard basal tension. The animals rested in this position for 10 min after which the gastrocnemius muscle was either freeze-clamped and dissected immediately (representing time 0), or was stimulated to contract isometrically via the sciatic nerve (train rate: 0.5 Hz; train duration: 200 ms; pulse rate: 100 Hz, pulse duration: 0.1 ms; 5–20 V; DISA Impulse Generator) for 10 s, 1, 3, 10 or 30 min and muscles were freeze-clamped during stimulation and dissected free. To ensure that all muscle fibres were recruited, the voltage was adjusted to a level where no further increase in tension was produced during the first few seconds of stimulation. Using this protocol, there was an initial 5–10% reduction in tension development during the initial 2–3 min of stimulation, with a further ∼5% fall after 10 min, after which tension development was stable during the remaining 20 min (data not shown). For the animals stimulated for 10 and 30 min, the gastrocnemius muscle from the contralateral leg was also sampled (30–45 s after sampling the other limb) to examine the possible effects of humoral stimuli resulting from the stimulation or surgery. In some animals, the gastrocnemius muscle was freeze-clamped immediately after surgery and dissected free. To control for the time effect of surgery and the small amount of tension placed on the hindlimb induced by the experimental set-up, some (n = 4) animals were placed in the set-up with the electrode on the nerve without stimulation for 40 min after which the muscle was freeze-clamped and dissected free. All muscle samples were placed in liquid nitrogen after dissection and later stored at −80°C until required. After experimentation, the animals were killed by cervical dislocation while unconscious.
Ex vivo experiments
Rats (45–55 g) were anaesthetized by intraperitoneal injection of sodium pentobarbital (5 mg per 100 g body wt). Extensor digitorum longus (EDL) muscles from both hindlimbs were carefully excised and placed in incubation chambers (multi myograph system organ bath 700MO; Danish Myo Technology A/S, Aarhus, Denmark) and suspended from the tendons by ligatures at resting tension (4–5 mN). After muscle excision, the rats were killed by cervical dislocation while unconscious and muscles were constantly bathed in Krebs–Ringer–Henseleit buffer (118.5 mm NaCl, 24.7 mm NaHCO3, 4.74 mm KCl, 1.18 mm MgSO3, 1.18 mm KH2PO4, and 2.5 mm CaCl2, pH 7.4) containing 2 mm pyruvate and 0.01% (w/v) BSA at 30°C which was gassed with 95% O2 and 5% CO2.
In one experiment (n = 6 per treatment), muscles were preincubated with 25 μm 1-[N,O-bis-(5-isoquinolinesulphonyl)-N-methyl-l-tyrosyl]-4-phenyl-piperazine (KN62; LC Laboratories, MA, USA) + 0.1% DMSO or 0.1% DMSO only for 1 h. In another experiment (n = 8 per treatment), muscles were preincubated for 2 h with 75 μm myristoylated autocamtide-2-related inhibitory peptide 2 (myr-AIP2; N-myr-KKKLRRQEAFDAL-OH; KJ Ross-Petersen ApS, Denmark). KN62 and AIP2 are allosteric (Hidaka & Yokokura, 1996) and competitive (Ishida et al. 1998) inhibitors of CaMKII, respectively. KN62 inhibits CaMKI, CaMKII and CaMKIV but presumably not other kinases (Hidaka & Yokokura, 1996; Davies et al. 2000) and has been used to inhibit CaMKII in rat skeletal muscle previously (Wright et al. 2004). AIP is a specific CaMKII inhibitor when compared with other multifunctional CaM kinases, cAMP-dependent protein kinase and protein kinase C (Ishida et al. 1995, 1998) and has been used in vitro previously (De Santiago et al. 2002). One muscle from the same animal served as the basal control while the other was electrically stimulated, via platinum electrodes in contact with the incubation buffer, to contract for 60 s following the preincubation period, with the same pattern described above except that the voltage was 50 V. Tension development was recorded at 0.2, 4.2, 20.2 and 58.2 s, and muscles were frozen at 60 s. A representative tension–time profile is shown in Fig. 1. Basal muscles were frozen within several minutes of stimulating the corresponding muscle, and samples were stored at −80°C until required. EDL was chosen as fast-twitch EDL muscles have higher expression of trisk95 than slow-twitch soleus muscle (Vassilopoulos et al. 2005; A. J. Rose & E. A. Richter, data not shown), and has similar fibre-type distribution as the gastrocnemius in that they are comprised mainly of fast-twitch muscle fibres (Armstrong & Phelps, 1984) and that there were no differences in CaMKII and trisk95 expression when comparing gastrocnemius and EDL muscles (n = 8; data not shown).
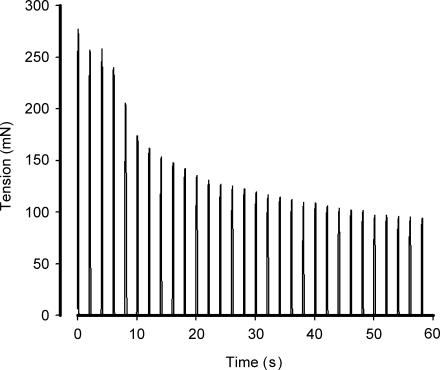
Figure 1.
Representative tension–time profile of ex vivo extensor digitorum longus muscle stimulation.
An experiment was conducted to test whether the muscles were viable after a 60 s period of electrical stimulation. To do this, EDL muscles from seven rats were excised and preincubated in Krebs–Ringer–Henseleit buffer. One muscle was preincubated for 1 h and then stimulation (same as above) was applied (A in Table 1) following which time the media was changed and stimulation was applied again after 1 h (B in Table 1). The contralateral muscle was preincubated for 2 h after which stimulation was applied (C in Table 1), with a media change at 1 h. Muscle tensions were recorded during the stimulations and are shown in Table 1. As shown in Table 1, despite a slightly lower tension following the first stimulation, there were no differences in the magnitude of fatigue. In addition, tensions from the prestimulated muscle at 2 h were comparable to a 2 h preincubation without stimulation. These findings indicate that the muscles were viable following 60 s stimulation, if allowed to recover. Furthermore, no further increase in tension was recorded when the voltage was increased from 50 to 75 V during the stimulus (n = 4).
Table 1.
Test of the viability of EDL muscles after electrical stimulation
| Initial | 4 s | 20 s | 60 s | |
|---|---|---|---|---|
| A | 271 ± 11 | 249 ± 14 | 180 ± 20 | 121 ± 7 |
| B | 255 ± 10* | 231 ± 13* | 155 ± 10* | 106 ± 7* |
| C | 266 ± 8 | 231 ± 5 | 171 ± 13 | 108 ± 6 |
Shown are muscle tensions in mN during a 60 s electrical stimulation. A, stimulation after 1 h preincubation. B, stimulation of same muscle after 1 h recovery. C, stimulation of contralateral muscle after 2 h preincubation. Data are mean ± s.e.m.
Different from A at corresponding time, P < 0.05.
Tissue preparation
The gastrocnemius muscle samples were mechanically powdered under liquid nitrogen to make each aliquot taken from a sample representative of the whole sample. After powdering, visible connective tissue was removed and separate aliquots were taken for protein extraction. For extraction, samples were homogenized while in an ice slurry (i.e. 0°C) in a buffer (15 μl per mg tissue) containing 50 mm Tris (pH 7.4), 150 mm NaCl, 10% (v/v) glycerol, 1 mm EDTA, 1 mm EGTA, 50 mm sodium fluoride, 5 mm sodium pyrophosphate, 2 mm sodium orthovanadate, 1 mm PMSF, 1 mm dithiothreitol, 1 mm benzamidine, 0.5% (v/v) protease inhibitor cocktail, and 1% (v/v) Nonidet P-40 using a polytron homogenizer (PT 1200, Kinematica) until no visible particles remained. The homogenates were mixed thoroughly by end-over-end rotation at 4°C for 30 min, and then spun at 6000 g for 10 min at 4°C. The clarified supernatant was taken and stored at −80°C until required. A small aliquot of each lysate was taken and diluted for protein concentration determination prior to storage.
Analytical techniques
Protein concentration of tissue extracts was determined in triplicate using the bicinchoninic acid (BCA) method using bovine serum albumin standards (Pierce, USA) and BCA assay reagents (Pierce, USA). A maximal coefficient of variance of 5% was accepted between replicates. Samples were immunoblotted for protein expression and phosphorylation was according to Rose et al. (2005). The primary antibodies used were anti-CaMKII (BD Biosciences-Pharmingen, USA; 612624), anti-phospho-Thr287-CaMKII (Cell Signalling Technology, Inc., USA; 3361), anti-phospho-Thr (Cell Signalling Technology, Inc., USA; 9381) and anti-triadin/trisk95 (Affinity Bioreagents, USA; MA3-927). Secondary antibodies were from DakoCytomation (Denmark). Band intensity was quantified by Kodak imaging software (Kodak 1D 3.5, USA). Preliminary experiments demonstrated that the amounts of protein loaded were within the dynamic range for the conditions used and the results obtained (data not shown). To measure kinase activity muscle extracts were analysed according to Rose & Hargreaves (2003). In brief, CaMKII activity of muscle extracts was measured in the presence (maximal activity) or absence (autonomous activity) of Ca2+–calmodulin with autocamtide-2 (Upstate Biotechnology, USA) as the peptide substrate. This assay has been shown to be specific for CaMKII as using a specific inhibitor for CaMKII reduces the majority of phosphotransfer activity by lysate proteins in the presence or absence of calmodulin (Rose & Hargreaves, 2003). To measure trisk95/triadin phosphorylation, 10 μl of anti-triadin antibody was mixed with 1 mg (gastrocnemius) or 0.5 mg (EDL) of tissue lysate protein with 40 μl 30% (v/v) protein-G-sepharose (Amersham Biosciences, Sweden) in a final volume of 500 μl and mixed overnight at 4°C. Immunocomplex pellets were washed several times and resuspended with 30 μl of 1 × sample buffer. Samples were denatured and immunoblotted using phospho-Thr antibodies. Following capture, membranes were stripped (Restore Western Blot Stripping Buffer, Pierce Biotech. Inc., USA) and re-probed with anti-trisk95 antibodies (Affinity Bioreagents, USA; MA3-927).
In vitro experiments
All phospho-specific antibodies used were tested for phosphospecificity as described by Rose et al. (2006). Extraction of rat muscle samples without phosphatase inhibitors and incubation with phosphatase completely removed or greatly reduced signal obtained when using the phospho-specific antibodies used in this study (data not shown).
To further examine the relationship between CaMKII autophosphorylation and autonomous activity, an in vitro experiment was performed to manipulate CaMKII autophosphorylation levels. This was achieved by taking aliquots of a rat skeletal muscle extract (final concentration: 2.4 μg μl−1) and incubating them with differing amounts of Ca2+ and CaM (Upstate Biotechnology, USA). All samples were reacted in quadruplicate in a buffer containing 10 mm Hepes (pH 7.2), 1 mm EGTA, 0.5 mm Na3VO4 and 400 μm ATP under four different conditions. One condition was without added Ca2+ or CaM, another was with 2 mm CaCl2, another with 2 mm CaCl2 + 0.12 μm CaM, and another with 2 mm CaCl2 + 1.2 μm CaM. These reaction mixes were preheated to 30°C, and the reaction was initiated by the addition of the sample. The reaction proceeded for 1 min at 30°C and was stopped by placing in an ice slurry (0°C) and the addition of 5 mm EGTA (final concentration). An aliquot of each sample was then taken for immunoblot analysis of CaMKII autophosphorylation. Aliquots of the remaining sample were taken and CaMKII was immunoprecipitated in duplicate as described above and these immunopreciptates were analysed for CaMKII activity (Rose & Hargreaves, 2003).
Calculations and statistics
Statistical analyses were performed using SPSS 13.0 for Windows. Initially, descriptive analyses were performed, and as all data was normally distributed, the data were analysed using parametric statistical tests. To test whether the data displayed equal variances, Levene's test for homogeneity of variance was used, and when variances were not equal, the data were transformed appropriately (Montgomery, 2001). For the time-course samples, an unpaired t test was performed initially to examine whether there was an effect of placing the muscle in the experimental set-up for 10 min and then sampling versus sampling immediately after surgery. One-way ANOVA was conducted to evaluate whether there were differences between time-points during stimulation. When the ANOVA indicated significance, Tukey's post hoc testing was used. To test for effects between the data from stimulated versus the non-stimulated muscle of the same animal, paired t tests were conducted. To test for the combined effect of surgery and placing in the experimental set-up for 40 min, data from samples taken immediately after surgery as well as after 40 min in the set-up without stimulation were compared using unpaired t tests. The strength of association between variables was analysed by Pearson correlations. For the ex vivo work, two-way ANOVA was conducted with post hoc testing using SigmaStat v. 3.1. Differences were considered to be significant when P was less than 0.05.
Results
Effect of contraction on CaMKII activity
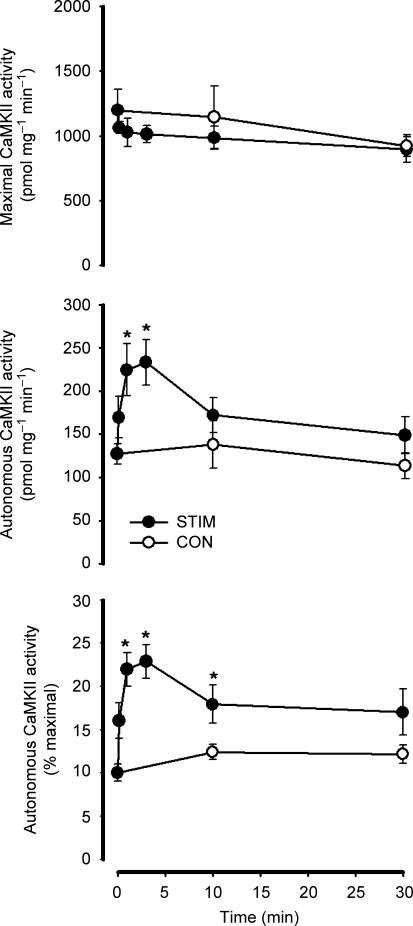
There was no effect of in situ contractions on maximal CaMKII activity or CaMKII expression (Figs 2 and 3). In contrast, there was a transient increase in Ca2+–calmodulin-independent (i.e. autonomous) CaMKII activity in response to contractions. More specifically, autonomous CaMKII activity was 10 ± 1% of maximal at time 0, and increased to 20–25% of maximal at 1–3 min of contractions (Fig. 2). Autonomous CaMKII activity was 18 ± 2% and 17 ± 3% of maximal at 10 and 30 min of contractions, respectively, but only 10 min was different from time 0 (Fig. 2). There were no differences in autonomous CaMKII activity between time 0, 10 and 30 min when compared with the unstimulated contralateral muscles (Fig. 2). Importantly, there were no significant differences in CaMKII expression and phosphorylation between samples taken at basal, time 0 or 40 min of placement in the experimental set-up, indicating that the effect of electrical stimulation was a real and not simply due to placement in the experimental set up (data not shown).
Figure 2. Effect of in situ contraction duration on skeletal muscle lysate Ca2+–calmodulin-dependent protein kinase II activity.
Skeletal muscle extracts were assayed in vitro for CaMKII activity in the presence (i.e. maximal activity; top panel) or absence (i.e. autonomous activity; middle panel) of Ca2+ and calmodulin. The bottom panel shows the autonomous activity expressed relative to the maximal activity (i.e. % maximal). STIM, electrically stimulated to contract: CON, contralateral control. Data are mean ± s.e.m., n = 7–8; *Different from time 0, P < 0.05.
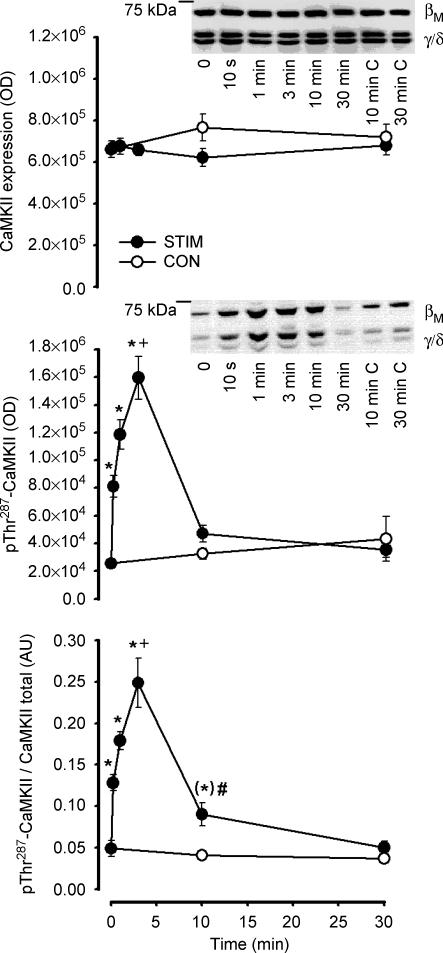
Figure 3. Effect of in situ contraction duration on skeletal muscle Ca2+–calmodulin-dependent protein kinase II expression and phosphorylation.
Skeletal muscle extract proteins were immunoblotted for CaMKII expression (top panel) and phospho-Thr287CaMKII (middle panel). The bottom panel is signal intensity for phospho-Thr287CaMKII relative to CaMKII expression. Representative immunoblots are shown and CaMKII isoforms are indicated; C, contralateral unstimulated muscle: STIM, electrically stimulated to contract, CON: contralateral muscle. Data are mean ± s.e.m., n = 8–12; *Different from time 0, P < 0.05; (*) borderline different compared with time 0, P = 0.069; +different from time 10 s, P < 0.05; #different from corresponding CON time.
As there was a positive correlation between the phosphorylation of CaMKII at Thr287 of βM and γ/δ isoforms (r2 = 0.73; P < 0.05; data no shown), the optical densities of both of these bands were summed. Similar to autonomous CaMKII activity, there was a rapid and transient increase in skeletal muscle CaMKII phosphorylation at Thr287 in response to contractions (Fig. 3). Specifically, there was a 1.5-fold increase in pThr287-CaMKII after 10 s of contractions compared with time 0, and was even higher (3- to 5-fold) after 3 min of contractions (Fig. 3). When expressed relative to CaMKII expression, CaMKII phosphorylation was also higher at 10 min of contractions when compared with time 0 or the resting contralateral muscle at this time (Fig. 3). At 30 min of contractions, there were no differences in CaMKII phosphorylation when compared with time 0 or the resting contralateral muscle at this time (Fig. 3).
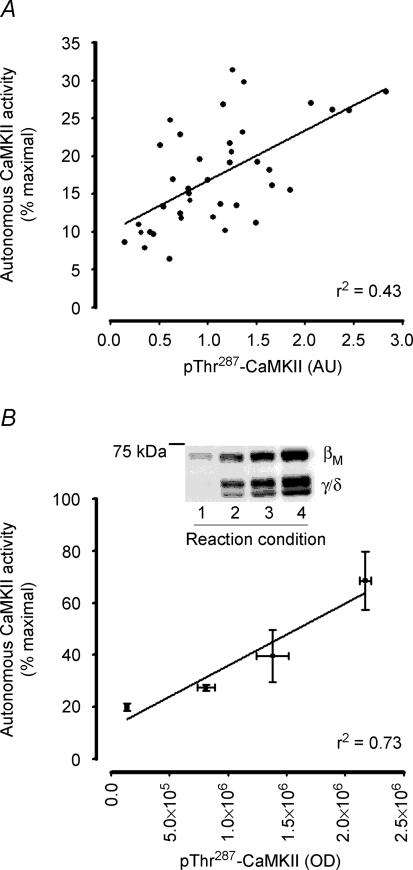
There was a positive-linear correlation between CaMKII phosphorylation at Thr287 and autonomous CaMKII activity (r2 = 0.43, P < 0.05; Fig. 4A). Furthermore, when the level of autophosphorylation was manipulated by prior incubation of skeletal muscle proteins with varying concentrations of free Ca2+ and CaM, there was also a positive-linear correlation between CaMKII phosphorylation and activity (r2 = 0.73, P < 0.05; Fig. 4B).
Figure 4. In vitro autonomous Ca2+–calmodulin-dependent protein kinase II activity is positively related to phosphorylation at Thr287.
Correlation between CaMKII phosphorylation at Thr287 and autonomous CaMKII activity from skeletal muscle samples taken before and during contractions (A; r2 = 0.43, P < 0.05, n = 39) and from basal skeletal muscle extracts manipulated to differing levels of phosphorylation by incubation with differing amounts of Ca2+ and calmodulin (B; r2 = 0.73, P < 0.05, n = 4). A representative immunoblot is shown.
Effect of contraction on trisk95
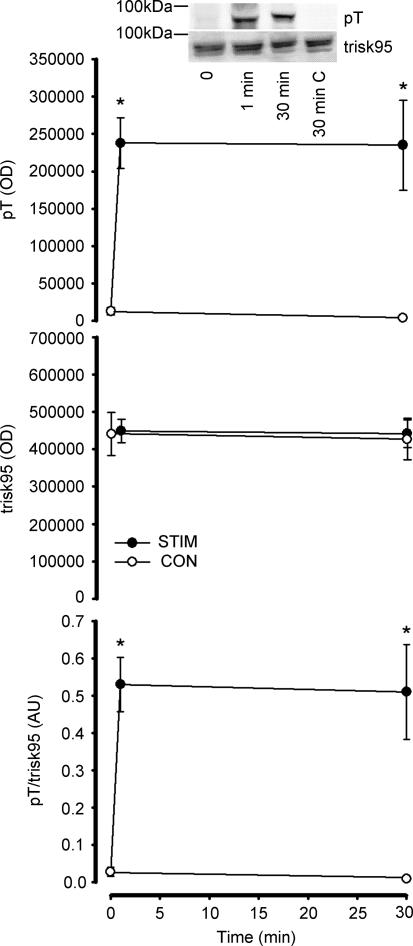
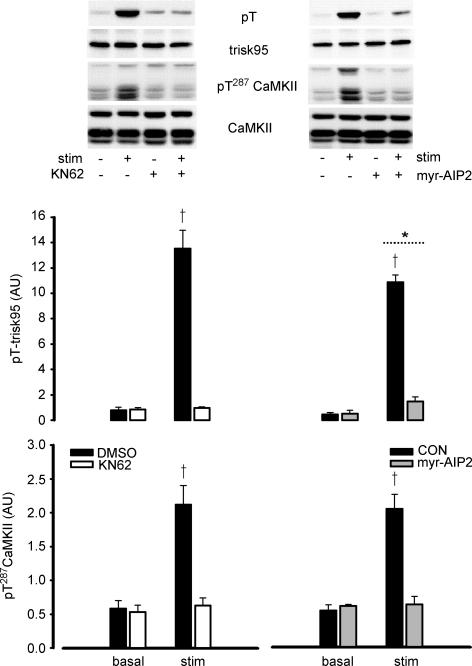
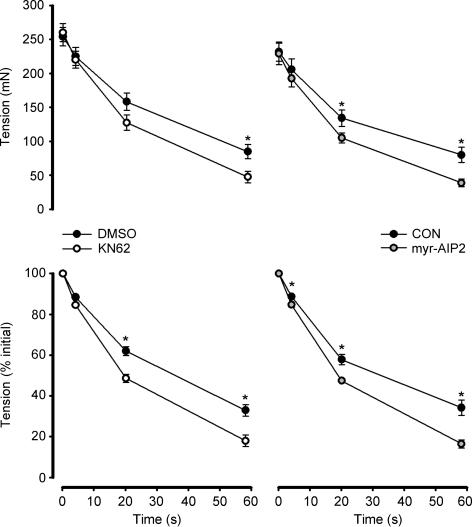
In response to contractions in situ, trisk95 phosphorylation at Thr residues was 20- to 30-fold higher at 1 and 30 min, when compared with time 0 (Fig. 5). Furthermore, trisk95 phosphorylation was higher in the contracting compared with the non-stimulated muscles at 30 min of stimulation, and there was no difference between trisk95 phosphorylation when comparing non-stimulated and time 0 muscles (Fig. 5). When EDL muscles were contracted ex vivo, there was a 15- to 20-fold increase in trisk95 Thr phosphorylation (Fig. 6). Taken together, these data indicate that the increase in trisk95 phosphorylation at Thr residues is due to local stimuli within the contracting muscle. Indeed, the increase in trisk95 phosphorylation with contraction was largely or completely blunted when muscles were preincubated with CaMKII inhibitors myr-AIP2 and KN62, respectively (Fig. 6). Importantly, the increase in skeletal muscle CaMKII phosphorylation by contraction was also blocked by CaMKII inhibition (Fig. 6). Furthermore, the magnitude of fatigue was exacerbated when muscles were preincubated with both CaMKII inhibitors (Fig. 7).
Figure 5. Effect of in situ contraction duration on skeletal muscle trisk95 Thr phosphorylation.
Trisk95 was immunoprecipitated from skeletal muscle extracts and immunoprecipitates were immunoblotted for phospho-Thr (top panel) and trisk95 (middle panel). The bottom panel is signal intensity for phospho-Thr relative to trisk95 expression. Representative immunoblots are shown; C, non-stimulated contralateral muscle: STIM, electrically stimulated to contract: CON, contralateral muscle. Data are mean ± s.e.m., n = 6; *different from time 0, P < 0.001.
Figure 6. Effect of CaMKII inhibition on contraction-mediated increases in trisk95 and CaMKII phosphorylation.
Rat extensor digitorum longus muscles were preincubated with or without CaMKII inhibitors KN62 (left panel) and myr-AIP2 (right panel) and electrically stimulated to contract ex vivo. Samples were analysed for trisk95 (top) and CaMKII (bottom) phosphorylation and adjusted to relative to expression. Representative immunoblots are shown. Stim, electrically stimulated to contract: CON, control; DMSO, pretreated with 0.1% DMSO only: KN62, pretreated with 25 μm KN62 + 0.1% DMSO; myr-AIP2, pretreated with 75 μm myristoylated CaMKII inhibitory peptide. Data are mean ± s.e.m., n = 6 (KN62), n = 8 (myr-AIP2); *main effect of stimulation, P < 0.01; †different from KN62 or myr-AIP2, P < 0.001.
Figure 7. Effect of CaMKII inhibition on contraction-mediated tension development.
Rat extensor digitorum longus muscles were preincubated with or without CaMKII inhibitors KN62 (left panel) and myr-AIP2 (right panel) and electrically stimulated to contract ex vivo. Force was recorded at selected time-points. CON, control; DMSO, pretreated with 0.1% DMSO only; KN62, pretreated with 25 μm KN62 + 0.1% DMSO; myr-AIP2, pretreated with 75 μm myristoylated CaMKII inhibitory peptide. Data are mean ± s.e.m., n = 6 (KN62), n = 8 (myr-AIP2); *different from corresponding time in KN62 or myr-AIP2, P < 0.05.
Discussion
In response to muscle contractions, there was a rapid and transient increase in autonomous CaMKII activity and CaMKII phosphorylation at Thr287 of gastrocnemius muscle, with no changes in the contralateral resting muscle during stimulation (Figs 2 and 3). This rapid and transient increase in skeletal muscle CaMKII autonomous activity and autophosphorylation has also been observed in exercising humans (Rose et al. 2006). Given that the increase in CaMKII activity was restricted to the contracting muscle during exercise indicates that the mechanism for activation originates from local factors within the contracting muscle. Indeed, it is well established that the major mechanism for activation of CaMKII is a rise in intracellular Ca2+ levels, subsequent binding of Ca2+ to calmodulin, and allosteric activation of CaMKII by Ca2+–CaM binding (Hudmon & Schulman, 2002), and the increase in Ca2+ to levels that activate CaMKII would only be expected to occur in the stimulated muscle. The activation of CaMKII via increases in intracellular Ca2+ is also intuitively obvious as a large proportion of CaMKII is localized to the site of Ca2+ release in fast-twitch muscles (Sacchetto et al. 2005a), and skeletal muscle CaMKII is activated at Ca2+ levels that occur during a muscle twitch (Pelosi & Donella-Deana, 2000; Chin, 2004). The transient nature of the increase in autonomous activity of the stimulated muscle was unexpected, as Ca2+ release would be expected to be high at all times during stimulation in that tension development was sustained throughout the entire stimulation period, and Ca2+–CaM binding is a requirement for on-going autophosphorylation (Hanson et al. 1994). However, it may be that there was a transient increase in Ca2+ release rate during stimulation (Westerblad & Allen, 1991) which may have led to the transient nature of CaMKII autophosphorylation.
As shown in Figs 2 and 3, there is a similar pattern between the changes in CaMKII autonomous activity and CaMKII phosphorylation at Thr287 during contractions and there was a significant positive correlation between these two variables (Fig. 4A). In addition, when CaMKII Thr287 phosphorylation level was artificially manipulated by incubation of skeletal muscles lysates in vitro with variable amounts of Ca2+ and CaM, there was a stronger positive correlation (Fig. 4B). Taken together, these findings suggest that the increases in CaMKII autonomous activity during contractile activity are mediated by the increases in CaMKII phosphorylation at Thr287. Indeed, expression of CaMKII that has a non-phosphorylatable residue at this site displays a lack of increase in autonomous activity when exposed to stimuli that increase Ca2+ (Hanson et al. 1989). However, only 43% of variance of CaMKII autonomous activity could be accounted for by Thr287 phosphorylation (Fig. 5A), suggesting that there may be additional factors involved. These factors might include phosphorylation of CaMKII on other sites (Colbran, 1993; Migues et al. 2006) or localization and binding to substrate proteins (Bayer & Schulman, 2001).
The mechanism by which skeletal muscle CaMKII becomes phosphorylated during exercise probably occurs via autophosphorylation. Indeed, similar to neuronal CaMKII (Hudmon & Schulman, 2002), skeletal muscle CaMKII probably exists as a multimeric complex of 12 individual CaMKII subunits (Woodgett et al. 1983, 1984), and upon rises in cytosolic [Ca2+] and Ca2+–CaM binding, the CaMKII multimeric complex undergoes intersubunit phosphorylation at Thr287 (Fig. 4B; Hudmon & Schulman, 2002; Rose et al. 2006). In terms of the functional consequences of CaMKII autophosphorylation, the affinity for calmodulin is greatly enhanced (Meyer et al. 1992), and thus the rapid autophosphorylation may be a mechanism to accelerate the activation of CaMKII with contractions. In addition, autophosphorylation may allow CaMKII to remain partially active between Ca2+ transients by disrupting autoinhibition (Hudmon & Schulman, 2002), thereby allowing persistent phosphorylation of downstream substrates. However, it should be noted that the increase in autonomous activity was only 15–25% of Ca2+–CaM-stimulated activity during the majority of contraction time (Fig. 2), meaning that the rise in activity during the Ca2+ spike may have been quantitatively more important in the overall increase in CaMKII activity. Indeed, the patterns of CaMKII phosphorylation (Fig. 3) and phosphorylation of the putative CaMKII substrate trisk95 (Fig. 5), which may be indicative of in vivo CaMKII activity during contraction (discussed below), were remarkably different. The transient nature of the increase in autophosphorylation with contraction (Fig. 3) is difficult to explain, but may be related to differences in Ca2+ uptake and release kinetics by the sarcoplasmic reticulum and thus Ca2+–CaM binding, or a differential action of CaMKII phosphatases on CaMKII over time. Indeed, it has been observed that there is a transient increase in stimulus-induced intracellular Ca2+ during repeated tetanic contraction of single muscle fibres (Westerblad & Allen, 1991). Also, skeletal muscle CaMKII may indirectly regulate its own dephosphorylation by increasing PP1c activity through phosphorylation of the PP1c-targeting subunit GM (Sacchetto et al. 2005b). This could be the case as dephosphorylation of glycogen synthase was higher in contracting rat skeletal muscle in situ using the same protocol (J. N. Nielsen & J. F. P. Wojtaszewski, unpublished observations), and PP1c-GM mediates glycogen synthase dephosphorylation by contraction (Aschenbach et al. 2001).
To gain further insight into the regulation of skeletal muscle CaMKII activity with exercise as well as functional consequences of CaMKII activation, an examination of the phosphorylation of putative downstream substrates was conducted. Unlike humans and other mammals (Briggs et al. 1992; Damiani et al. 2000; Margreth et al. 2000; Rose et al. 2006), rat skeletal muscle does not express the CaMKII substrate phospholamban (A. J. Rose & E. A. Richter, unpublished observation; Vangheluwe et al. 2005), and thus it could not be examined in the present study. On the other hand, endogenously expressed CaMKII can phosphorylate another SR-associated protein trisk95, presumably at Thr37 (Damiani et al. 1995, 2003; Colpo et al. 2001), and trisk95 is expressed in rat (Carl et al. 1995; Vassilopoulos et al. 2005; Fig. 5) but not human (Thevenon et al. 2003) skeletal muscle. The majority of trisk95 is embedded within the lumen of the SR, but a small part of the C-terminus is cytoplasmic (Marty, 2004). CaMKII is anchored to the cytoplasmic side of junctional and longitundinal SR membranes (Sacchetto et al. 2005a), and can phosphorylate triadin at the cytoplasmic C-terminal domain (Colpo et al. 2001). In response to contractions in situ, trisk95 phosphorylation at Thr residues was rapidly increased, and was sustained during the 30 min stimulation period, with no change in the contralateral muscle during stimulation (Fig. 5). Furthermore, trisk95 phosphorylation was higher in EDL muscles electrically stimulated to contract ex vivo, a response that was greatly blunted by treatment of muscles with chemical inhibitors of CaMKII activity (Fig. 6), further indicating that trisk95 is a substrate of CaMKII. This is not inconceivable as CaMKII is localized to the junctional SR (Chu et al. 1990; Damiani et al. 2003), and endogenous CaMKII inhibition blunts Ca2+–CaM-induced trisk95 phosphorylation in SR vesicles from rabbit fast-twitch muscle (Damiani et al. 2003). Taken together, similar to exercising humans (Rose et al. 2006), these data show that the activation of skeletal muscle CaMKII is rapid and sustained during contractions, and occurs via a local mechanism within the contracting muscle, probably via allosteric activation by Ca2+–CaM.
Trisk95 is an integral membrane protein within the junctional SR in skeletal muscle and physically interacts with calsequestrin and the ryanodine receptor (RyR1; SR Ca2+ release channel; Ohkura et al. 1998; Groh et al. 1999) and inhibits excitation-induced Ca2+ release (Rezgui et al. 2005); probably by decreasing RyR1 open probability (Groh et al. 1999). It has been hypothesized (Sacchetto et al. 2005a) that phosphorylation of trisk95 (Figs 5 and 6) can relieve this inhibitory action on the RyR1 to potentiate Ca2+ release rate during repeated muscle contractions. Indeed, although controversial (Hain et al. 1994), CaMKII can increase RyR1 activity (Dulhunty et al. 2001), and inhibition of CaMKII leads to a blunting of force and Ca2+ release, but not Ca2+ uptake, during twitches of murine fast-twitch muscle (Tavi et al. 2003). In support of this, there was a greater magnitude of fatigue during stimulation of isolated whole rat EDL muscles when CaMKII was inhibited (Fig. 7). It is important to highlight that this effect was seen when CaMKII was inhibited by two structurally unrelated inhibitors which affect CaMKII activity via different mechanisms. This effect of CaMKII on the RyR1 and Ca2+ release is probably mediated by trisk95, as RyR1 is probably not a substrate of endogenously expressed CaMKII in skeletal muscle (Sacchetto et al. 2005a). Furthermore, these data suggest that the activation of CaMKII may play a protective role in the development of fatigue during strenuous contractile activity, a phenomenon that is not entirely inconceivable given that failure of Ca2+ release from the SR is a likely cause of fatigue (Dahlstedt & Westerblad, 2001; Dutka et al. 2005). However, as CaMKII is a mulitifunctional kinase, and probably affects other substrates that are involved in excitation–contraction coupling (Sacchetto et al. 2005a) as well as catabolism (Wright et al. 2004, 2005; Singh et al. 2004; Sacchetto et al. 2005b), it is possible that CaMKII affects skeletal muscle fatigue rate by multiple mechanisms, perhaps not involving trisk95. Clearly, further work is required to examine the precise role and mechanism of CaMKII-mediated phosphorylation on skeletal muscle Ca2+ release and fatigue.
When comparing the in situ and ex vivo models it is apparent that there are stark differences in the tension–time profiles. In particular, even though the stimulation protocols were the same, there was a small decline (i.e. 10–15%) in tension development during the 30 min in situ hindlimb stimulation whereas there was up to a 70% decline in tension development after 1 min of EDL stimulation ex vivo. This is likely to be largely explained by the dependence of diffusion for O2 transport when muscles are incubated ex vivo in that the large fall in tension during stimulation can be attributed to the development of hypoxia due to inadequate O2 diffusion (Barclay, 2005). Indeed, when single muscle fibres are contracted ex vivo, O2 diffusion distances are relatively negligible, and substantial falls in tension are not observed until after several minutes of stimulation (Westerblad & Allen, 1991).
It is questionable as to whether trisk95 phosphorylation has a physiological role during all types of exercise. For instance, it was observed that there was a large increase in phosphorylation of trisk95 with no recorded increase in tension when muscles were contracted in situ. Furthermore, stimulus-induced intracellular Ca2+ levels increase in the early phase of repetitive tetanic contractions, and this phenomenon may be explained by trisk95 phosphorylation; however, the putative increase in Ca2+ release during this situation may be physiologically irrelevant as this increase may be supramaximal relative to the sensitivity of the myofibrils to Ca2+ (Westerblad & Allen, 1991). Thus, it may be that only when the stimulation of muscle is severe so that fatigue develops, that trisk95 phosphorylation becomes physiologically relevant to excitation–contraction coupling.
In summary, the activation of skeletal muscle CaMKII is rapid and sustained during contractions. Trisk95 is a substrate of CaMKII in predominantly fast-twitch skeletal muscle, and CaMKII may function to modulate Ca2+ release and fatigue via phosphorylation of Trisk95.
Acknowledgments
The financial support from the Copenhagen Muscle Research Centre, the Danish Medical and Natural Science Research Council, an Integrated Project (contract number LSHM-CT-2004-005272) from the European Union, as well as the Novo-Nordisk Research and Lundbeck Foundations, is also acknowledged. A.J.R. was supported by a postdoctoral fellowship from the Carlsberg Foundation and from the European Union. A. J. R. wishes to thank Thomas E. Jenson for excellent instruction on dissecting and incubating muscles for ex vivo work.
References
- Armstrong RB, Phelps RO. Muscle fiber type composition of the rat hindlimb. Am J Anat. 1984;171:259–272. doi: 10.1002/aja.1001710303. [DOI] [PubMed] [Google Scholar]
- Aschenbach WG, Suzuki Y, Breeden K, Prats C, Hirshman MF, Dufresne SD, et al. The muscle-specific protein phosphatase PP1G/RGL (GM) is essential for activation of glycogen synthase by exercise. J Biol Chem. 2001;276:39959–39967. doi: 10.1074/jbc.M105518200. [DOI] [PubMed] [Google Scholar]
- Barclay CJ. Modelling diffusive O2 supply to isolated preparations of mammalian skeletal and cardiac muscle. J Muscle Res Cell Motil. 2005;26:225–235. doi: 10.1007/s10974-005-9013-x. [DOI] [PubMed] [Google Scholar]
- Bayer KU, Harbers K, Schulman H. αKAP is an anchoring protein for a novel CaM kinase II isoform in skeletal muscle. EMBO J. 1998;17:5598–5605. doi: 10.1093/emboj/17.19.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer KU, Schulman H. Regulation of signal transduction by protein targeting: the case for CaMKII. Biochem Biophys Res Commun. 2001;289:917–923. doi: 10.1006/bbrc.2001.6063. [DOI] [PubMed] [Google Scholar]
- Briggs FN, Lee KF, Wechsler AW, Jones LR. Phospholamban expressed in slow-twitch and chronically stimulated fast-twitch muscles minimally affects calcium affinity of sarcoplasmic reticulum Ca2+-ATPase. J Biol Chem. 1992;267:26056–26061. [PubMed] [Google Scholar]
- Campbell KP, MacLennan DH. A calmodulin-dependent protein kinase system from skeletal muscle sarcoplasmic reticulum. Phosphorylation of a 60,000-dalton protein. J Biol Chem. 1982;257:1238–1246. [PubMed] [Google Scholar]
- Carl SL, Felix K, Caswell AH, Brandt NR, Brunschwig JP, Meissner G, Ferguson DG. Immunolocalization of triadin, DHP receptors, and ryanodine receptors in adult and developing skeletal muscle of rats. Muscle Nerve. 1995;18:1232–1243. doi: 10.1002/mus.880181104. [DOI] [PubMed] [Google Scholar]
- Chiesi M, Carafoli E. The regulation of Ca2+ transport by fast skeletal muscle sarcoplasmic reticulum. Role of calmodulin and of the 53,000-dalton glycoprotein. J Biol Chem. 1982;257:984–991. [PubMed] [Google Scholar]
- Chin ER. The role of calcium and calcium/calmodulin-dependent kinases in skeletal muscle plasticity and mitochondrial biogenesis. Proc Nutr Soc. 2004;63:279–286. doi: 10.1079/PNS2004335. [DOI] [PubMed] [Google Scholar]
- Chu A, Sumbilla C, Inesi G, Jay SD, Campbell KP. Specific association of calmodulin-dependent protein kinase and related substrates with the junctional sarcoplasmic reticulum of skeletal muscle. Biochemistry. 1990;29:5899–5905. doi: 10.1021/bi00477a003. [DOI] [PubMed] [Google Scholar]
- Cohen P. The regulation of protein function by multisite phosphorylation – a 25 year update. Trends Biochem Sci. 2000;25:596–601. doi: 10.1016/s0968-0004(00)01712-6. [DOI] [PubMed] [Google Scholar]
- Colbran RJ. Inactivation of Ca2+/calmodulin-dependent protein kinase II by basal autophosphorylation. J Biol Chem. 1993;268:7163–7170. [PubMed] [Google Scholar]
- Colbran RJ, Smith MK, Schworer CM, Fong YL, Soderling TR. Regulatory domain of calcium/calmodulin-dependent protein kinase II. Mechanism of inhibition and regulation by phosphorylation. J Biol Chem. 1989;264:4800–4804. [PubMed] [Google Scholar]
- Colpo P, Nori A, Sacchetto R, Damiani E, Margreth A. Phosphorylation of the triadin cytoplasmic domain by CaM protein kinase in rabbit fast-twitch muscle sarcoplasmic reticulum. Mol Cell Biochem. 2001;223:139–145. doi: 10.1023/a:1017987015807. [DOI] [PubMed] [Google Scholar]
- Dahlstedt AJ, Westerblad H. Inhibition of creatine kinase reduces the rate of fatigue-induced decrease in tetanic [Ca2+]i in mouse skeletal muscle. J Physiol. 2001;533:639–649. doi: 10.1111/j.1469-7793.2001.00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiani E, Angelini C, Pelosi M, Sacchetto R, Bortoloso E, Margreth A. Skeletal muscle sarcoplasmic reticulum phenotype in myotonic dystrophy. Neuromuscul Disord. 1996;6:33–47. doi: 10.1016/0960-8966(95)00016-x. [DOI] [PubMed] [Google Scholar]
- Damiani E, Picello E, Saggin L, Margreth A. Identification of triadin and of histidine-rich Ca2+-binding protein as substrates of 60 kDa calmodulin-dependent protein kinase in junctional terminal cisternae of sarcoplasmic reticulum of rabbit fast muscle. Biochem Biophys Res Commun. 1995;209:457–465. doi: 10.1006/bbrc.1995.1524. [DOI] [PubMed] [Google Scholar]
- Damiani E, Sacchetto R, Margreth A. Variation of phospholamban in slow-twitch muscle sarcoplasmic reticulum between mammalian species and a link to the substrate specificity of endogenous Ca2+-calmodulin-dependent protein kinase. Biochim Biophys Acta. 2000;1464:231–241. doi: 10.1016/s0005-2736(00)00153-x. [DOI] [PubMed] [Google Scholar]
- Damiani E, Sacchetto R, Salviati L, Margreth A. Two splice variants of CaMKII-anchoring protein are present in the sarcoplasmic reticulum of rabbit fast-twitch muscle. Biochem Biophys Res Commun. 2003;302:73–83. doi: 10.1016/s0006-291x(03)00110-4. [DOI] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santiago J, Maier LS, Bers DM. Frequency-dependent acceleration of relaxation in the heart depends on CaMKII, but not phospholamban. J Mol Cell Cardiol. 2002;34:975–984. doi: 10.1006/jmcc.2002.2034. [DOI] [PubMed] [Google Scholar]
- Dulhunty AF, Laver D, Curtis SM, Pace S, Haarmann C, Gallant EM. Characteristics of irreversible ATP activation suggest that native skeletal ryanodine receptors can be phosphorylated via an endogenous CaMKII. Biophys J. 2001;81:3240–3252. doi: 10.1016/S0006-3495(01)75959-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutka TL, Cole L, Lamb DG. Calcium phosphate precipitation in the sarcoplasmic reticulum reduces action potential-mediated Ca2+ release in mammalian skeletal muscle. Am J Physiol Cell Physiol. 2005;289:C1502–C1512. doi: 10.1152/ajpcell.00273.2005. [DOI] [PubMed] [Google Scholar]
- Garcia-Roves PM, Huss J, Holloszy JO. Role of calcineurin in exercise-induced mitochondrial biogenesis. Am J Physiol Endocrinol Metab. 2006;290:E1172–E1179. doi: 10.1152/ajpendo.00633.2005. [DOI] [PubMed] [Google Scholar]
- Groh S, Marty I, Ottolia M, Prestipino G, Chapel A, Villaz M, Rojnat M. Functional interaction of the cytoplasmic domain of triadin with the skeletal ryanodine receptor. J Biol Chem. 1999;274:12278–12283. doi: 10.1074/jbc.274.18.12278. [DOI] [PubMed] [Google Scholar]
- Hain J, Nath S, Mayrleitner M, Fleischer S, Schlindler H. Phosphorylation modulates the function of the calcium release channel of sarcoplasmic reticulum from skeletal muscle. Biophys J. 1994;67:1823–1833. doi: 10.1016/S0006-3495(94)80664-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson PI, Kapiloff MS, Lou LL, Rosenfeld MG, Schulman H. Expression of a multifunctional Ca2+/calmodulin-dependent protein kinase and mutational analysis of its autoregulation. Neuron. 1989;3:59–70. doi: 10.1016/0896-6273(89)90115-3. [DOI] [PubMed] [Google Scholar]
- Hanson PI, Meyer T, Stryer L, Schulman H. Dual role of calmodulin in autophosphorylation of multifunctional CaM kinase may underlie decoding of calcium signals. Neuron. 1994;12:943–956. doi: 10.1016/0896-6273(94)90306-9. [DOI] [PubMed] [Google Scholar]
- Hargreaves M, Richter EA. Regulation of skeletal muscle glycogenolysis during exercise. Can J Sport Sci. 1988;13:197–203. [PubMed] [Google Scholar]
- Hawkins C, Xu A, Narayanan N. Sarcoplasmic reticulum calcium pump in cardiac and slow twitch skeletal muscle but not fast twitch skeletal muscle undergoes phosphorylation by endogenous and exogenous Ca2+/calmodulin-dependent protein kinase. Characterization of optimal conditions for calcium pump phosphorylation. J Biol Chem. 1994;269:31198–31206. [PubMed] [Google Scholar]
- Hidaka H, Yokokura H. Molecular and cellular pharmacology of a calcium/calmodulin-dependent protein kinase II (CaM kinase II) inhibitor, KN-62, and proposal of CaM kinase phosphorylation cascades. Adv Pharmacol. 1996;36:193–219. doi: 10.1016/s1054-3589(08)60583-9. [DOI] [PubMed] [Google Scholar]
- Hook SS, Means AR. Ca2+/CaM-dependent kinases: from activation to function. Annu Rev Pharmacol Toxicol. 2001;41:471–505. doi: 10.1146/annurev.pharmtox.41.1.471. [DOI] [PubMed] [Google Scholar]
- Huang YC, Dennis RG, Baar K. Cultured slow vs. fast skeletal muscle cells differ in physiology and responsiveness to stimulation. Am J Physiol Cell Physiol. 2006;291:C11–C17. doi: 10.1152/ajpcell.00366.2005. [DOI] [PubMed] [Google Scholar]
- Hudmon A, Schulman H. Structure-function of the multifunctional Ca2+/calmodulin-dependent protein kinase II. Biochem J. 2002;364:593–611. doi: 10.1042/BJ20020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida A, Kameshita I, Okuno S, Kitani T, Fujisawa H. A novel highly specific and potent inhibitor of calmodulin-dependent protein kinase II. Biochem Biophys Res Commun. 1995;212:806–812. doi: 10.1006/bbrc.1995.2040. [DOI] [PubMed] [Google Scholar]
- Ishida A, Shigeri Y, Tatsu Y, Uegaki K, Kameshita I, Okuno S, Kitani T, Yumoto N, Fujisawa H. Critical amino acid residues of AIP, a highly specific inhibitory peptide of calmodulin-dependent protein kinase II. FEBS Lett. 1998;427:115–118. doi: 10.1016/s0014-5793(98)00405-0. [DOI] [PubMed] [Google Scholar]
- Lengyel I, Nairn A, McCluskey A, Toth G, Penke B, Rostas J. Auto-inhibition of Ca2+/calmodulin-dependent protein kinase II by its ATP-binding domain. J Neurochem. 2001;76:1066–1072. doi: 10.1046/j.1471-4159.2001.00139.x. [DOI] [PubMed] [Google Scholar]
- Lewis SE, Anderson P, Goldspink DF. The effects of calcium on protein turnover in skeletal muscles of the rat. Biochem J. 1982;204:257–264. doi: 10.1042/bj2040257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Cseresnyes Z, Randall WR, Schneider MF. Activity-dependent nuclear translocation and intranuclear distribution of NFATc in adult skeletal muscle fibers. J Cell Biol. 2001;155:27–39. doi: 10.1083/jcb.200103020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Randall WR, Schneider MF. Activity-dependent and -independent nuclear fluxes of HDAC4 mediated by different kinases in adult skeletal muscle. J Cell Biol. 2005;168:887–897. doi: 10.1083/jcb.200408128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margreth A, Pallanca A, Damiani E. Calmodulin kinase-mediated phosphorylation of phospholamban in skeletal muscle sarcoplasmic reticulum. A critical reappraisal of the state of the problem at the light of new findings with human normal and diseased muscle. Basic Appl Myol. 2000;10:151–157. [Google Scholar]
- Marty I. Triadin: a multi-protein family for which purpose? Cell Mol Life Sci. 2004;61:1850–1853. doi: 10.1007/s00018-004-4196-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer W, Herrmann-Frank A, Luttgau HC. The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibres. Biochim Biophys Acta. 1995;1241:59–116. doi: 10.1016/0304-4157(94)00014-5. [DOI] [PubMed] [Google Scholar]
- Meyer T, Hanson PI, Stryer L, Schulman H. Calmodulin trapping by calcium-calmodulin-dependent protein kinase. Science. 1992;256:1199–1202. doi: 10.1126/science.256.5060.1199. [DOI] [PubMed] [Google Scholar]
- Migues PV, Lehmann IT, Fluechter L, Cammarota M, Gurd JW, Sim AT, Dickson PW, Rostas JA. Phosphorylation of CaMKII at Thr253 occurs in vivo and enhances binding to isolated postsynaptic densities. J Neurochem. 2006;98:289–299. doi: 10.1111/j.1471-4159.2006.03876.x. [DOI] [PubMed] [Google Scholar]
- Montgomery DC. Design and Analysis of Experiments. 5. New York: John Wiley & Sons Inc.; 2001. [Google Scholar]
- Ohkura M, Furukawa K, Fujimori H, Kuruma A, Kawano S, Hiraoka M, Kuniyasu A, Nakayama H, Ohizumi Y. Dual regulation of the skeletal muscle ryanodine receptor by triadin and calsequestrin. Biochemistry. 1998;37:12987–12993. doi: 10.1021/bi972803d. [DOI] [PubMed] [Google Scholar]
- Ojuka EO, Nolte LA, Holloszy JO. Regulation of GLUT4 biogenesis in muscle: evidence for involvement of AMPK and Ca2+ Am J Physiol Endocrinol Metab. 2002;282:E1008–E1013. doi: 10.1152/ajpendo.00512.2001. [DOI] [PubMed] [Google Scholar]
- Parsons SA, Millay DP, Wilkins BJ, Bueno OF, Tsika GL, Neilson JR, Liberatore CM, Yutzey KE, Crabtree GR, Tsika RW, Molkentin JD. Genetic loss of calcineurin blocks mechanical overload-induced skeletal muscle fiber type switching but not hypertrophy. J Biol Chem. 2004;279:26192–26200. doi: 10.1074/jbc.M313800200. [DOI] [PubMed] [Google Scholar]
- Pelosi M, Donella-Deana A. Localization, purification, and characterization of the rabbit sarcoplasmic reticulum associated calmodulin-dependent protein kinase. Biochemistry (Mosc) 2000;65:259–268. [PubMed] [Google Scholar]
- Rezgui SS, Vassilopoulos S, Brocard J, Platel JC, Bouron A, Arnoult C, Oddoux S, Garcia L, De Waard M, Marty I. Triadin (Trisk 95) overexpression blocks excitation-contraction coupling in rat skeletal myotubes. J Biol Chem. 2005;280:39302–39308. doi: 10.1074/jbc.M506566200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter EA, Garetto LP, Goodman MN, Ruderman NB. Enhanced muscle glucose metabolism after exercise: modulation by local factors. Am J Physiol. 1984;246:E476–E482. doi: 10.1152/ajpendo.1984.246.6.E476. [DOI] [PubMed] [Google Scholar]
- Rose AJ, Broholm C, Kiillerich K, Finn SG, Proud CG, Rider MH, Richter EA, Kiens B. Exercise rapidly increases eukaryotic elongation factor 2 phosphorylation in skeletal muscle of men. J Physiol. 2005;569:223–228. doi: 10.1113/jphysiol.2005.097154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AJ, Hargreaves M. Exercise increases Ca2+–calmodulin-dependent protein kinase II activity in human skeletal muscle. J Physiol. 2003;553:303–309. doi: 10.1113/jphysiol.2003.054171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AJ, Kiens B, Richter EA. Ca2+–calmodulin-dependent protein kinase expression and signalling in skeletal muscle during exercise. J Physiol. 2006;574:889–903. doi: 10.1113/jphysiol.2006.111757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AJ, Richter EA. Skeletal muscle glucose uptake during exercise: how is it regulated? Physiology. 2005;20:260–270. doi: 10.1152/physiol.00012.2005. [DOI] [PubMed] [Google Scholar]
- Sacchetto R, Bovo E, Damiani E. The Ca2+-calmodulin dependent protein kinase II system of skeletal muscle sarcoplasmic reticulum. Basic Appl Myol. 2005a;15:5–17. [Google Scholar]
- Sacchetto R, Bovo E, Donella-Deana A, Damiani E. Glycogen- and PP1c-targeting subunit GM is phosphorylated at Ser48 by sarcoplasmic reticulum-bound Ca2+-calmodulin protein kinase in rabbit fast twitch skeletal muscle. J Biol Chem. 2005b;280:7147–7155. doi: 10.1074/jbc.M413574200. [DOI] [PubMed] [Google Scholar]
- Singh P, Salih M, Leddy JJ, Tuana BS. The muscle-specific calmodulin-dependent protein kinase assembles with the glycolytic enzyme complex at the sarcoplasmic reticulum and modulates the activity of glyceraldehyde-3-phosphate dehydrogenase in a Ca2+/calmodulin-dependent manner. J Biol Chem. 2004;279:35176–35182. doi: 10.1074/jbc.M402282200. [DOI] [PubMed] [Google Scholar]
- Spriet LL, Heigenhauser GJ. Regulation of pyruvate dehydrogenase (PDH) activity in human skeletal muscle during exercise. Exerc Sport Sci Rev. 2002;30:91–95. doi: 10.1097/00003677-200204000-00009. [DOI] [PubMed] [Google Scholar]
- Tavi P, Allen DG, Niemela P, Vuolteenaho O, Weckstrom M, Westerblad H. Calmodulin kinase modulates Ca2+ release in mouse skeletal muscle. J Physiol. 2003;551:5–12. doi: 10.1113/jphysiol.2003.042002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevenon D, Smida-Rezgui S, Chevessier F, Groh S, Henry-Berger J, Beatriz Romero N, Vilaz M, De Waard M, Marty I. Human skeletal muscle triadin: gene organization and cloning of the major isoform, Trisk 51. Biochem Biophys Res Commun. 2003;303:669–675. doi: 10.1016/s0006-291x(03)00406-6. [DOI] [PubMed] [Google Scholar]
- Tothova J, Blaauw B, Pallfacchina G, Rudolf R, Argentini C, Reggani C, Schiaffino S. NFATc1 nucleocytoplasmic shuttling is controlled by nerve activity in skeletal muscle. J Cell Sci. 2006;119:1604–1611. doi: 10.1242/jcs.02875. [DOI] [PubMed] [Google Scholar]
- Vangheluwe P, Schuermans M, Zador E, Waelkens E, Raeymaekers L, Wuytack F. Sarcolipin and phospholamban mRNA and protein expression in cardiac and skeletal muscle of different species. Biochem J. 2005;389:151–159. doi: 10.1042/BJ20050068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilopoulos S, Thevenon D, Rezgui SS, Brocard J, Chapel A, Lacampagne A, Lunardi J, De Waard M, Marty I. Triadins are not triad-specific proteins: two new skeletal muscle triadins possibly involved in the architecture of sarcoplasmic reticulum. J Biol Chem. 2005;280:28601–28609. doi: 10.1074/jbc.M501484200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt MJ, Steinberg GR, Heigenhauser GJ, Spriet LL, Dyck DJ. Hormone-sensitive lipase activity and triacylglycerol hydrolysis are decreased in rat soleus muscle by cyclopiazonic acid. Am J Physiol Endocrinol Metab. 2003;285:E412–E419. doi: 10.1152/ajpendo.00023.2003. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. Changes of myoplasmic calcium concentration during fatigue in single mouse muscle fibers. J General Physiol. 1991;98:615–635. doi: 10.1085/jgp.98.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtaszewski JF, Hansen BF, Ursø B, Richter EA. Wortmannin inhibits both insulin- and contraction-stimulated glucose uptake and transport in rat skeletal muscle. J Appl Physiol. 1996;81:1501–1509. doi: 10.1152/jappl.1996.81.4.1501. [DOI] [PubMed] [Google Scholar]
- Woodgett JR, Cohen P, Yamauchi T, Fujisawa H. Comparison of calmodulin-dependent glycogen synthase kinase from skeletal muscle and calmodulin-dependent protein kinase-II from brain. FEBS Lett. 1984;170:49–54. doi: 10.1016/0014-5793(84)81366-6. [DOI] [PubMed] [Google Scholar]
- Woodgett JR, Davison MT, Cohen P. The calmodulin-dependent glycogen synthase kinase from rabbit skeletal muscle. Purification, subunit structure and substrate specificity. Eur J Biochem. 1983;136:481–487. doi: 10.1111/j.1432-1033.1983.tb07766.x. [DOI] [PubMed] [Google Scholar]
- Woodgett JR, Tonks N, Cohen P. Identification of a calmodulin-dependent glycogen synthase kinase in rabbit skeletal muscle, distinct from phosphorylase kinase. FEBS Lett. 1982;148:5–11. doi: 10.1016/0014-5793(82)81231-3. [DOI] [PubMed] [Google Scholar]
- Wright DC, Geiger PC, Holloszy JO, Han DH. Contraction- and hypoxia-stimulated glucose transport is mediated by a Ca2+-dependent mechanism in slow-twitch rat soleus muscle. Am J Physiol Endocrinol Metab. 2005;288:E1062–E1066. doi: 10.1152/ajpendo.00561.2004. [DOI] [PubMed] [Google Scholar]
- Wright DC, Hucker KA, Holloszy JO, Han DH. Ca2+ and AMPK both mediate stimulation of glucose transport by muscle contractions. Diabetes. 2004;53:330–335. doi: 10.2337/diabetes.53.2.330. [DOI] [PubMed] [Google Scholar]