Abstract
Agonists of protease-activated receptor 2 (PAR2) evoke hyperexcitability of dorsal root ganglia (DRG) neurons by unknown mechanisms. We examined the cellular mechanisms underlying PAR2-evoked hyperexcitability of mouse colonic DRG neurons to determine their potential role in pain syndromes such as visceral hyperalgesia. Colonic DRG neurons were identified by injecting Fast Blue and DiI retrograde tracers into the mouse colon. Using immunofluorescence, we found that DiI-labelled neurons contained PAR2 immunoreactivity, confirming the presence of receptors on colonic neurons. Whole-cell current-clamp recordings of acutely dissociated neurons demonstrated that PAR2 activation with a brief application (3 min) of PAR2 agonists, SLIGRL-NH2 and trypsin, evoked sustained depolarizations (up to 60 min) which were associated with increased input resistance and a marked reduction in rheobase (50% at 30 min). In voltage clamp, SLIGRL-NH2 markedly suppressed delayed rectifier IK currents (55% at 10 min), but had no effect on the transient IA current or TTX-resistant Na+ currents. In whole-cell current-clamp recordings, the sustained excitability evoked by PAR2 activation was blocked by the PKC inhibitor, calphostin, and the ERK1/2 inhibitor PD98059. Studies of ERK1/2 phosphorylation using confocal microscopy demonstrated that SLIGRL-NH2 increased levels of immunoreactive pERK1/2 in DRG neurons, particularly in proximity to the plasma membrane. Thus, activation of PAR2 receptors on colonic nociceptive neurons causes sustained hyperexcitability that is related, at least in part, to suppression of delayed rectifier IK currents. Both PKC and ERK1/2 mediate the PAR2-induced hyperexcitability. These studies describe a novel mechanism of sensitization of colonic nociceptive neurons that may be implicated in conditions of visceral hyperalgesia such as irritable bowel syndrome.
The irritable bowel syndrome (IBS) is a common disorder affecting daily living and quality of life (Lembo et al. 2005). Of those symptoms that characterize IBS (i.e. abdominal pain, altered bowel pattern such as constipation and diarrhoea, abdominal bloating and altered defecation), abdominal pain is reported to be most troublesome and best correlates with severity of illness (Sandler et al. 1984). The lack of available therapies to effectively manage this pain continues to stimulate studies of the mechanisms that underlie this pain and to define novel therapeutic targets.
Visceral hypersensitivity (i.e. an increased sensitivity to stimuli arising from the intestinal wall) is widely recognized to underlie abdominal pain in IBS, at least in a significant subset of patients (gado-Aros & Camilleri, 2005). Both central and peripheral mechanisms have been implicated and their relative role may be dependent on the clinical setting. In the periphery, sensitization of nociceptive nerve endings in the wall of the colon appears to be important. This may result from signalling from low levels of persisting cytokines in conditions such as post-infectious IBS (Spiller & Campbell, 2006), although there is also growing evidence that release of mast cell mediators may contribute to peripheral sensitization in IBS (Barbara et al. 2004).
Of the many substances released from mast cells, proteases such as tryptase may play a particularly important role in signalling to neurons (Barbara et al. 2004; Spiller & Campbell, 2006). The number of tryptase-containing mast cells is increased in intestinal tissues of patients with IBS (Barbara et al. 2004; 2006). Moreover, tryptase levels in tissues, and tryptase release from biopsies are increased in IBS patients (Barbara et al. 2004). Proteases such as tryptase can signal to enteric (Reed et al. 2003) and dorsal root ganglia (DRG) (Vergnolle et al. 2001) neurons by cleaving and activating protease-activated receptors (PARs) to induce sustained increases in their excitability. While hyperexcitability of enteric neurons could result in disturbances in intestinal secretion and motility, hyperexcitability of DRG neurons could be an important mechanism of peripheral sensitization in conditions such as IBS (Mawe et al. 2004; Beyak & Vanner, 2005; Lomax et al. 2006). Although whole-animal studies have demonstrated a role for mast cell proteases signalling to PARs in the genesis of visceral hypersensitivity (Vergnolle et al. 2001), the precise mechanism(s) by which this occurs remain unclear.
Certain serine proteases that are generated and released during inflammation can signal to cells by cleaving protease-activated receptors (PARs), a family of four G-protein-coupled receptors. Cleavage exposes a tethered ligand domain that binds to and activates the cleaved receptors. Synthetic peptides that correspond to the tethered ligand domain of PAR1, PAR2, and PAR4 directly activate these receptors and are useful tools to probe receptor function. Of the four cloned PARs, tryptase selectively activates PAR2, and trypsins are also potent activators of this receptor (Saito & Bunnett, 2005). PAR2 is expressed by DRG and enteric neurons, and PAR2-selective activating peptides induce hyperexcitability of these neurons. Although the digestive tract is the richest source of proteases that can activate PAR2, such as tryptase and trypsins, the effect of PAR2 agonists on hyperexcitability of colonic nociceptive DRG neurons has not been examined. Moreover, although PAR2 agonists are known to cause hyperexcitability of DRG neurons (Amadesi et al. 2004), the mechanisms of this effect are unknown. In the present study we examined the effects of PAR2 activation with a selective agonist on the neuronal excitability of mouse colonic DRG nociceptive neurons. Our aims were to (1) determine if colonic DRG neurons express immunoreactive PAR2, using retrograde tracing and immunofluorescence; (2) examine whether activation of PAR2 results in hyperexcitability of these neurons, and to identify any effects upon voltage-gated potassium or sodium currents using electrophysiological approaches; (3) identify the kinases that mediate PAR2-induced hyperexcitability using pharmacological approaches; and (4) determine if activated kinases are appropriately localized to regulate the affected ion channels, using immunofluorescence and confocal microscopy. Small DRG neurons were studied because these neurons express properties associated with nociceptors (Gold et al. 1996a; Yoshimura & de Groat, 1999; Moore et al. 2002; Beyak & Vanner, 2005). We found that PAR2 activation evoked a sustained hyperexcitability of these colonic neurons, and sought to determine the mechanism(s) that underlies this action.
Methods
Drugs and reagents
Synthetic peptides corresponding to the tethered ligand of rat and mouse PAR2 (PAR2-activating peptide, PAR2-AP, SLIGRL-NH2) and the reverse peptide sequence that does not activate PAR2 (PAR2-reverse peptide, PAR2-RP, LRGILS-NH2, control) were from Sigma Genosys (The Woodlands, TX, USA). All other chemicals were obtained from Sigma, with the exception of TTX and PD98059 (Calbiochem). All agonists were applied to the bath using a fast-flow solution-switching system (VC6; Warner Instruments) and a three-barrel square glass (Harvard Apparatus). In electrophysiological studies involving PD98059, the cells were incubated with the drugs for a minimum of 2 h before the experiment began. Both PD98059 and calphostin C were included in the bath and pipette solutions.
Animals
Electrophysiological studies were conducted on CD-1 mice (Charles River Laboratories, Montreal, QC, Canada) of either sex, weighing 30–40 g. Immunohistochemical studies were conducted on C57Bl6 mice (6–8 weeks) from Jackson Laboratories (Bar Harbour, ME, USA) and Sprague-Dawley rats (male, 200–250 g) from Charles River Laboratories (Wilmington, MA, USA). The Queen's University Animal Care Committee and the UCSF Institutional Animal Care and Use Committees approved and monitored all procedures with these animals. At the end of experiments, animals were killed using sodium pentobarbitone (200 mg kg−1, i.p.) and bilateral thoracotomy.
Retrograde labelling of colonic neurons
Mice or rats were anaesthetized with isoflurane (5%) or a combination of ketamine hydrochloride (Pfizer; New York, NY, USA) 18.75 mg ml−1 and xylazine (Bayer; Etobicoke, ON, USA) 1.25 mg ml−1 injected i.p. (0.1 ml (10 g body weight)−1). The descending colon was exposed by a midline laparotomy. A 25 μl syringe (Hamilton, Reno, Nevada, USA) with a 30–32 gauge needle was used to inject Fast Blue (17 mg ml−1, volume = 1.0 μl per injection) or 1,1-dioctadecyl-3–3-3′3′-tetramethylindocarbocyanine (DiI) (5–17 mg ml−1 in DMSO) into 5–10 sites along the colon wall (volume = 10 μl per injection site), as previously described (Beyak et al. 2004). Excess dye was removed using a cotton swab to prevent the dye leaking to other tissues. The abdomen was irrigated with saline and sutured closed. After surgery, animals were allowed to recover on a warming blanket and were given free access to water and food. After recovery from anaesthesia, animals were monitored for signs of pain, feeding, and weight loss. Animals that displayed behaviour consistent with ongoing pain or failure to thrive were killed.
Immunohistochemical localization of PAR2
Ten to fifteen days following DiI injection, animals were killed using sodium pentobarbital (200 mg kg−1i.p.). DRG (T9–L1, T5 for control) were immersion fixed in 4% paraformaldehyde in 100 mm PBS pH 7.4 for 4 h at 4°C, washed with PBS and placed in a 25% sucrose solution overnight at 4°C. DRG were embedded in OCT compound (Miles, Elkhart, IN, USA) and sectioned at 25 μm. Sections were washed in PBS containing 1–10% NGS, 1% BSA and 0.3–0.5% Triton X-100, and incubated with primary antibodies to PAR2 (mouse DRG, A5, 1: 750; rat DRG, SAM11, 1: 250) overnight at 4°C. Sections were washed and incubated with goat anti-antirabbit or antimouse IgG conjugated to FITC (1: 200, room temperature, 2 h). Sections were washed and mounted in Prolong (Molecular Probes, Eugene, OR, USA). Mouse monoclonal antibodies to PAR2 (SAM11) were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Rabbit antibody to PAR2 (A5) was from Dr M. Hollenberg (Calgary University). Goat antimouse or antirabbit IgG conjugated to FITC was from Jackson ImmunoResearch (West Grove, PA, USA).
Isolation and culture of DRG neurons for ERK phosphorylation studies
Mice and rats were killed using sodium pentobarbital (200 mg kg−1i.p.). DRG from thoracic and lumbar spinal cord were minced in cold Hank's balance salt solution (HBSS) (Steinhoff et al. 2000; Amadesi et al. 2004). Mouse DRG were digested by incubating in DMEM containing 1 mg ml−1 collagenase type 1A and 0.8 mg ml−1 DNAse type IV, for 60 min at 37°C. This solution was removed and neurons were incubated for 45 min at 37°C with DMEM containing 0.25% trypsin. Rat DRG were digested by incubating in Dulbecco's modified Eagle's medium (DMEM) containing 0.5 mg ml−1 trypsin, 1 mg ml−1 collagenase type IA and 0.1 mg ml−1 DNAse type IV (all from Sigma, St Louis, MO, USA) for 60–90 min at 37°C. After digestion, soybean trypsin inhibitor (Sigma, 0.5%) was added to neutralize trypsin. Neurons were pelleted, suspended in DMEM containing 10% fetal bovine serum, 10% horse serum, 100 u ml−1 penicillin, 0.1 mg ml−1 streptomycin, 2 mm glutamine and 2.5 μg ml−1 DNAse type IV, plated on glass coverslips coated with Matrigel (BD Biosciences, Bedford, MA, USA), and cultured for 2–3 days.
ERK phosphorylation assays
Neurons in short-term culture (48–72 h) were incubated for 18 h at 37°C in DMEM containing 1% horse serum, 1% fetal bovine serum, 100 u ml−1 penicillin, 0.1 mg ml−1 streptomycin, 2 mm glutamine and 2.5 μg ml−1 DNAse IV. Neurons were washed and incubated for 30 min at 37°C in DMEM containing 0.1% BSA (protease-free). Neurons were challenged by addition of SLIGRL-NH2 (PAR2-AP) or LRGILS-NH2 (PAR2-RP) (both 100 μm) or PMA (1 μm) for 0–30 min. Neurons were immediately washed in ice-cold PBS (100 mm, pH 7.4), and were fixed in 4% paraformaldehyde in PBS for 20 min at room temperature. Neurons were incubated for 30 min at room temperature in PBS containing 1% NGS and 0.1% saponin, and were incubated overnight at 4°C with pERK antibody (1: 500). Neurons were washed and incubated with goat antimouse IgG conjugated to FITC (1: 200, room-temperature, 1 h). Slides were washed, fixed for 20 min in paraformaldehyde, and mounted in Vectashield with DAPI (Vector Laboratories, Burlingame, CA, USA). Mouse monoclonal antibodies to phosphorylated ERK1/2 (pERK1/2, E4) were from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Confocal microscopy
Specimens were observed using a Zeiss Axiovert microscope, a Bio-Rad MRC1000 confocal microscope, with a Zeiss Plan Apo ×40 (NA 1.4) objective for tissue sections and a Zeiss Plan Apo ×100 (NA 1.3) objective for cultured neurons. Images were collected at laser intensities of 3–30%, an iris of 2.5–3 mm and a zoom of 1–2, and typically 5–10 optical sections were taken at intervals of 0.5–1.0 μm. Images were processed to adjust contrast and brightness using Adobe Photoshop 7.0 (Adobe Systems, Mountain View, CA, USA). In experiments to localize pERK1/2 in cultured neurons, images were collected under identical conditions (laser intensity, iris and gain) for control and stimulated neurons.
Electrophysiological studies
Neurons were acutely dissociated as previously described (Beyak et al. 2004). Briefly, tissue was incubated in collagenase (Worthington; Lakewood, NJ, USA) 1 mg ml−1 and dispase (Roche; Indianapolis, IN, USA) (4 mg ml−1) for 10 min at 37°C, titurated with a fire-polished Pasteur pipette, and incubated for an additional 5 min at 37°C. Dissociated neurons were plated onto vitrogen-coated coverslips and stored in a humidified incubator at 37°C, under 95% air–5% CO2 until retrieval (4–24 h) for electrophysiological studies. The incubating media was obtained from a stock solution containing 10 ml fetal bovine serum, 1 ml penicillin/streptomycin, 0.028 g glutamine, 0.6 g dextrose and 0.2 g NaHCO3, and made up to 100 ml with DMEM (pH 7.2–7.3).
Whole-cell or perforated-patch-clamp electrophysiological experiments were performed in current or voltage clamp at room temperature. Neurons adhering to the glass coverslip were placed in an RC-26 recording chamber (Warner Instruments; Hamden, CT, USA), which was mounted on the stage of an inverted microscope (Olympus IX70; Tokyo, Japan) fitted for both bright-field and fluorescence microscopy. With the use of the U-MWIG2 filter (Olympus) for Fast Blue, labelled colonic neurons were identified by their bright blue fluorescence. All cells were labelled with Fast Blue except in some latter experiments where a few unlabelled neurons were studied because of relatively lower numbers of labelled neurons. Almost all of these latter experimental groups had ∼50% or more labelled neurons, and no differences were observed between labelled and unlabelled neurons. Only small neurons (≤25 μm diameter and ≤40 pF capacitance) were studied, because these neurons express properties associated with nociceptors (i.e. capsaicin sensitivity, TTX-resistant action potentials) (Gold et al. 1996a; Yoshimura & de Groat, 1999; Moore et al. 2002; Beyak & Vanner, 2005) Recordings were obtained using G85165T-4 patch glass capillary tubing with flame-polished ends (Warner Instruments), pulled with a PP-830 micropipette puller from Narishige (East Meadow, NY, USA), and fire-polished by using an FP-830 fire polisher (Narishige). Final pipette resistance was between 2 and 5 MΩ for all experiments. Signals were amplified using an Axopatch 200B amplifier and digitized with a Digidata 1322A A/D converter from Axon Instruments (San Jose, CA, USA). Signals were low-pass filtered at 5 kHz, acquired at 20 kHz, and stored on disk using Clampex 8.2 (Axon Instruments).
Data were only analysed from recordings that exhibited the following properties. The starting seal resistance between the cell membrane and the electrode tip was ≥1 GΩ. The starting series resistance was ≤20 MΩ, and remained stable throughout the experiment. In current clamp, cells had a stable resting membrane potential more negative than −45 mV for more than 5 min before recording, and displayed overshooting action potentials (peak ≥ +50 mV) for the duration of the experiment. In voltage clamp, the leak current was ≤0.5 nA when tested at a holding potential of −100 mV throughout the experiment. All perforated-patch recordings were obtained using amphotericin B from Sigma (St Louis, MO, USA) as previously described (Rae et al. 1991). Briefly, amphotericin B stock solution was made by diluting 6 mg of powder into 100 μl dimethylsulfoxide (DMSO) (Sigma), followed by sonication and vortex-mixing. Immediately prior to experiments, 20 μl of stock solution were added into 5 ml of internal pipette solution. The solution was vortexed until a uniform light-yellowish tint developed, then covered with aluminium foil to prevent exposure to light. The final amphotericin B concentration was 240 μg ml−1. Due to the compound's photosensitivity and rate of breakdown upon exposure to light, pipette solutions with amphotericin B were replaced every 2 h. Pipette tips were dipped briefly into amphotericin-free internal pipette solution, then back-filled with unfiltered amphotericin-containing solution.
Current-clamp recordings were carried out in perforated-patch mode using the following solutions (mm): (pipette) 140 KCl, 10 Hepes, 5 EGTA, 4 Na2-ATP, 5 MgCl2, 2.5 CaCl2 with pH adjusted to 7.2 using KOH; (bath), 140 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 Hepes, and 10 d-glucose, with pH adjusted to 7.4 using NaOH.
The membrane potential was continuously monitored using Clampex 8.2. After a stable membrane potential was recorded for 5 min, membrane potential, rheobase, twice rheobase, and input resistance were recorded. Cells were then superfused with PAR2-AP or PAR2-RP (both 100 μm) for 3 min and the parameters were again measured at 3, 10, 30 and 60 min after application of the peptide. Rheobase was recorded using 180 ms steps in 0.01 nA increments, which continued until an action potential was fired and the current needed to generate the action potential was recorded. Input resistance was recorded using a single 180 ms hyperpolarizing step command equal to the negative value of the cell's rheobase, and varied between −0.1 and −0.3 nA. The membrane response to the PAR2 agonist trypsin (300 nm) was tested in a separate series of experiments.
To record potassium currents, perforated patch was first established using the current-clamp bath solution described above. After perforation was complete, sodium-free bath solution was perfused onto the cell to isolate potassium currents. The composition (mm) was: 140 NMDG, 4 KCl, 1.8 Hepes, 1 d-glucose, 1 CaCl2, 1 MgCl2, pH adjusted to 7.4 using HCl. Pipette solution composition was (mm): 110 K-aspartate, 30 KCl, 10 EGTA, 10 Hepes, 2 Na2-ATP, 1 MgCl2, pH adjusted to 7.2 using KOH.
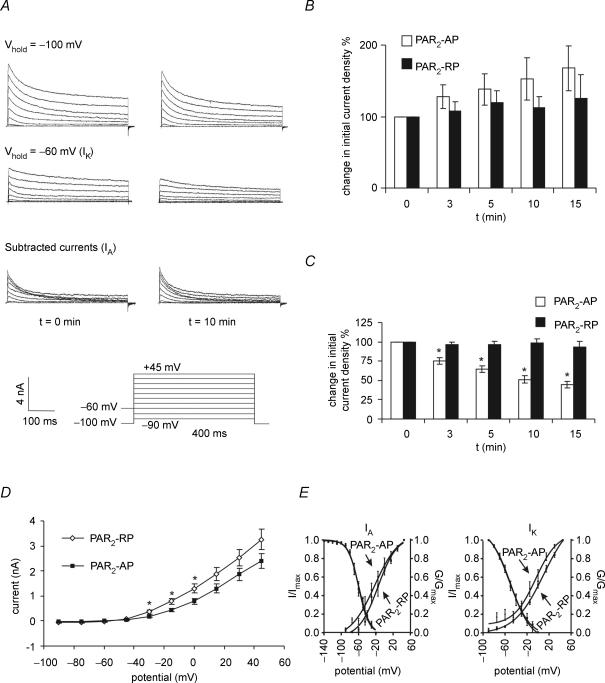
To study the effect of PAR2 activation on IA and IK currents, neurons were superfused for 3 min with PAR2-AP or PAR2-RP (both 100 μm). IA and IK currents were separated using voltage protocols, as previously described (Stewart et al. 2003). Briefly, IK was isolated using 400 ms depolarizing steps in 15 mV increments from −90 mV to +45 mV at a holding potential of −60 mV, with the sustained IK measured isochronally, 400 ms after the onset of the pulse, at which time IA was largely inactivated, minimizing contamination by this current. The IA was isolated by subtracting the sustained IK from the total potassium current recorded using 400 ms depolarizing steps in 15 mV increments from −90 mV to +45 mV from a holding potential of −100 mV. Peak IA was measured as the peak of the transient component of this subtracted current. Recordings were made at the beginning of the experiment and at 3, 5, 10 and 15 min following the application of the peptide.
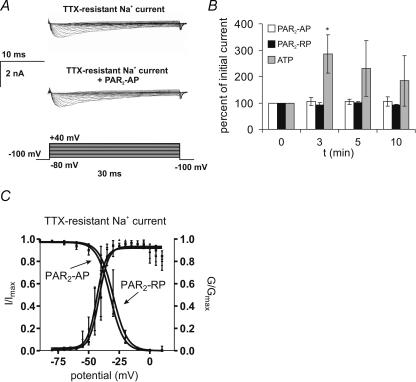
Voltage-clamp studies of Na+ currents were carried out in whole-cell mode. All recordings were obtained with the following solutions (mm): (pipette) 110 CsCl, 1 MgCl2, 11 EGTA, 10 Hepes, 10 NaCl, pH adjusted to 7.2 with CsOH; (bath), 55 NaCl, 80 cholineCl, 2 CaCl2, 1 MgCl2, 10 Hepes, and 5 d-glucose, pH adjusted to 7.4 using NaOH. The low extracellular sodium concentration was used to reduce the size of the sodium currents, enabling them to be accurately clamped. Na+ currents were recorded in voltage clamp using 30 ms depolarizing steps in 5 mV increments from −80 to +40 mV at a holding membrane potential of −100 mV. TTX-resistant Na+ currents were isolated with TTX (1 μm). The PAR2 agonist SLIGRL-NH2 (100 μm) or LRGILS-NH2 (100 μm) was then superfused for 3 min, and voltage protocols in the presence of TTX repeated at 3, 5, and 10 min following application of the peptide.
Conductance was determined using the relation:
where G is the conductance, I is the measured membrane current, Vm is the command voltage, and EX is the equilibrium potential, which was calculated to be −84 mV for potassium and +89.82 mV for sodium in control solutions. Normalized average conductances were plotted against membrane potential and the resultant curve was fitted to a Boltzmann function of the form:
where G is the conductance, Gmax is the fitted maximal conductance, V50 is the membrane potential for half-activation, Vm is the command potential, and k is the slope factor.
Inactivation curves were measured using a two-pulse protocol, as previously described (Yoshimura & de Groat, 1999; Stewart et al. 2003). IA inactivation curve studies used a 1 s prepulse varying between −120 and 0 mV, followed by a 400 ms test pulse of +50 mV. IK inactivation curves were generated using a two-pulse protocol: an 8 s prepulse varying between −80 and 0 mV, followed by a 1 s test pulse of +50 mV. TTX-R sodium current inactivation curves were generated using a two-pulse protocol: a 1 s prepulse between −120 and 0 mV, followed by a 30 ms test pulse to 0 mV. Residual current amplitudes were normalized and plotted against conditioning pulse potential, and the continuous line in Fig. 4E is an average of fits to a negative Boltzmann function:
where I is the current, Imax is the maximal current, V50 is the membrane potential for half-activation, Vm is the command potential, and k is the slope factor.
Figure 4. PAR2 activation suppressed.
IK currents A, representative recordings of transient (IA) and sustained (IK) K+ currents before (left traces) and 10 min following the application of PAR2-AP (100 μm) (right traces). Currents were measured in voltage clamp from a holding membrane potential of −100 mV or −60 mV using 400 ms steps of 15 mV from −90 mV to +45 mV. IA was measured by subtracting recordings obtained at a holding membrane potential of −60 mV from recordings obtained at a holding membrane potential of −100 mV. IA was measured as the peak current in the subtracted recordings. IK was measured from the trace obtained at a holding membrane potential of −60 mV at t = 400 ms. B and C, summary of the mean current densities following PAR2 activation for IA and IK, respectively. IA peak current density was not altered, but the IK peak current density was suppressed. Neurons were superfused for 3 min with PAR2-AP (n = 7) or PAR2-RP (n = 8) (100 μm). *P < 0.05. D, current–voltage relationship for delayed rectifier IK current showing onset of the PAR2 effect at ∼−45 mV *P < 0.05. E, PAR2-AP did not significantly alter IA or IK activation (G/Gmax) or inactivation kinetics (I/Imax). Activation curves for IA and IK were measured using 5 mV steps from −80 to +50 mV, and for TTX-R sodium currents from −80 to +10 mV. Normalized conductance (G/Gmax) was plotted against test pulse voltage and fitted to a Boltzmann function. While the activation curves for PAR2-AP and PAR2-RP do not overlap, the differences at the various voltage steps are not statistically significant. IA inactivation curves were generated using a two-pulse protocol: a 1 s prepulse varying between −120 and 0 mV, followed by a 400 ms test pulse of +50 mV. IK inactivation curves were generated using a two-pulse protocol: an 8 s prepulse varying between −80 and 0 mV followed by a 1 s test pulse of +50 mV.
Statistical analysis
Electrophysiological recordings were analysed using Clampfit 8.2 software (Axon Instruments). Linear leak subtraction was used for all experiments with the P/N Clampfit protocol. Values obtained at various time points were expressed as a difference (absolute or percentage) from the resting membrane potential at t = 0 min. Data are expressed as means ± s.e.m. A two-way analysis of variance (ANOVA) with a Bonferroni post hoc test for significance was carried out to compare the results at every time point, and data values were deemed statistically significant if P < 0.05. Data from Fast Blue and non-labelled cells were also compared at every time point and were always found to be statistically insignificant (P > 0.05). Fitting of data was done with the least squares method using the fit function in Prism 4.0 (Graphpad Software). Voltages of half-activation (V50), time constants, and slope factors were obtained from means of the individual Boltzmann curve fits.
Results
PAR2 is expressed in colonic neurons
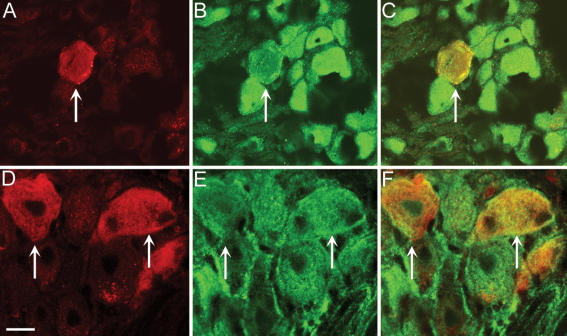
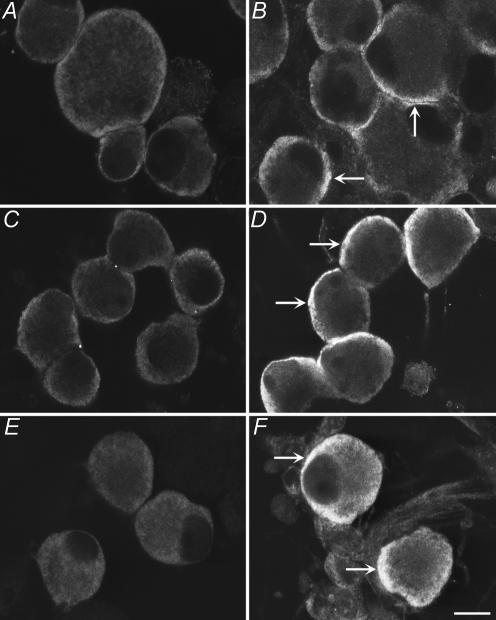
We have previously reported that nociceptive neurons of rat DRG that express TRPV1, CGRP and SP also express PAR2 (Steinhoff et al. 2000; Amadesi et al. 2004). To determine if those DRG neurons innervating the colon contain PAR2, we used retrograde tracing. When the tracer DiI was injected into the distal colon of mice, labelled DRG neurons could be detected in DRG from T9–L1 after 10–15 days (Fig. 1A). These neurons expressed immunoreactive PAR2 (Fig. 1B and C). Since we have previously reported that PAR2 is expressed by nociceptive DRG neurons in rats, and because intracolonic administration of PAR2 activators causes hyperalgesia to distension of the rat colon, we also determined if DRG neurons innervating the colon express PAR2 in this species. When DiI was injected into the distal colon of rats, labelled DRG neurons were detected in DRG from T9–L1 after 10–15 days (Fig. 1D). These neurons expressed immunoreactive PAR2 (Fig. 1E and F). Together, these results show that DRG neurons that innervate the distal colon of mice and rats express PAR2.
Figure 1. Localization of immunoreactive PAR2 in retrogradely labelled DRG from mouse (A–C) and rat (D–F) DRG.
DiI was injected into the wall of the distal colon and PAR2 was localized in DRG (T9–L1) 10–15 days later. A and D, DiI; B and E, immunoreactive PAR; C and F, merge. DiI-labelled neurons innervating the colon expressed PAR2 immunoreactivity. Representative images are shown from experiments on n = 3 animals. Scale bar = 20 μm. Arrows show labelled neuron.
PAR2 activation on mouse colonic DRG neurons evokes sustained hyperexcitability
Current-clamp recordings were obtained from 64 DRG neurons. Only small neurons were chosen for study (cell diameter ≤25 μm and a capacitance ≤40 pF) because these small cells have been shown to have properties exhibited by nociceptors (Beyak & Vanner, 2005). The mean resting membrane potential was −51.6 ± 1.6 mV. The mean input resistance was 585.29 ± 65.80 MΩ. All action potentials obtained displayed a shoulder on the repolarizing phase of the action potential, characteristic of TTX-resistant action potentials on nociceptive DRG neurons (Beyak & Vanner, 2005). Fewer than 10% of cells displayed spontaneous action potentials.
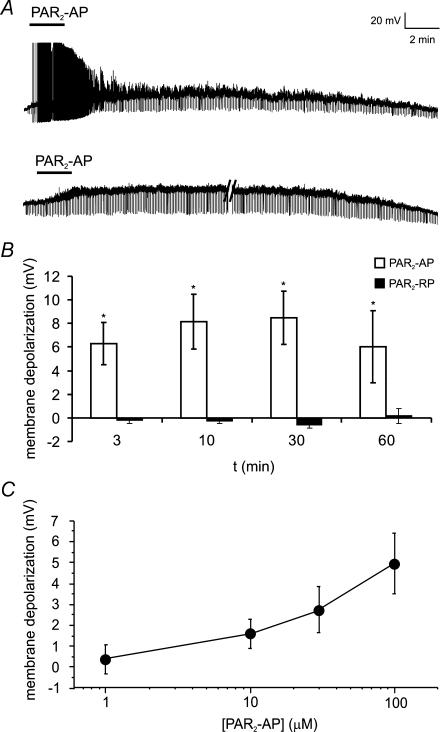
We have previously shown that PAR2 activation on guinea-pig submucosal (Reed et al. 2003) and rat DRG neurons (Amadesi et al. 2004) caused a sustained decrease in rheobase and in rat DRGs a prolonged membrane depolarization. To evaluate the effect of PAR2 activation on mouse colonic DRG neurons, Fast Blue-labelled neurons were superfused for 3 min with the PAR2-AP (100 μm) and changes in passive and active membrane properties were compared to baseline for 60 min. Superfusion of PAR2-AP (n = 5) caused a significant membrane potential depolarization at 3 min (mean = 6.8 mV; range: 2.1–12.4 mV) in all neurons tested, which peaked by 10 min (mean = 7.8 mV; range: 2–15.2 mV) (Fig. 2). This depolarization, although smaller, was still evident at 60 min. In contrast, superfusion with the reverse peptide PAR2-RP (100 μm;n = 6 Fast Blue-labelled) had no effect on membrane potential.
Figure 2. PAR2-AP evokes sustained depolarization of mouse colonic DRG neurons.
A, representative traces showing PAR2-AP (SLIGRL; 100 μm) superfusion for 3 min caused a sustained depolarization of mouse DRG neurons for up to 60 min. This was associated with action potential discharge in a few neurons (upper trace) and an increase in input resistance (brief downward deflections evoked by constant hyperpolarizing pulse). Parallel lines (lower trace) represent 16 min break in recording. Each neuron was recorded for 5 min prior to application of the PAR2-AP (not shown) to ensure a stable resting membrane potential. B, summary of mean depolarization at each time point following superfusion of PAR2-AP (100 μm). The reverse peptide, which lacks biological activity at the PAR2 (PAR2-RP, LRGILS; 100 μm), had no effect on membrane potential. n = 5 or more for each time point. C, PAR2-AP (1–100 μm) evoked a dose-dependent depolarization. n = 5 or more neurons at each point.
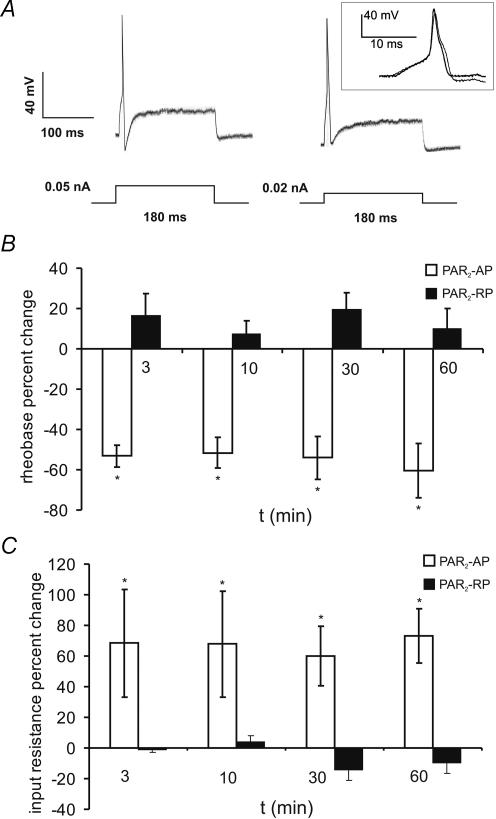
PAR2-AP (100 μm) also caused a decrease in rheobase at 3 min (mean = 55.5%; range: 33.3–71.4%), which was maximal by 60 min (mean = 73.3%; range: 56.2–100%) (Fig. 3B). Changes in membrane potential were controlled for by manually applying DC current to return the membrane potential to the resting value at each time point before retesting the rheobase. PAR2-RP had no effect on rheobase. Changes in input resistance were monitored with constant hyperpolarizing pulses (see trace in Fig. 2A). PAR2 activation caused a significant increase in input resistance at 3 min (mean = 68.3%; range: 5.44–201.94%), which was maximal by 60 min (73.3%; range: 49.4–108%) (Fig. 3C). Conversely, the reverse peptide had no effect on input resistance. Most neurons fired one or two action potentials when depolarized at 2× rheobase. These numbers did not change following activation of PAR2 receptors (data not shown).
Figure 3. PAR2-AP-evoked sustained hyperexcitability of mouse colonic DRG neurons.
A, representative recording showing rheobase was markedly reduced 10 min (right trace) following a 3 min superfusion of PAR2-AP (100 μm) compared to time 0 (left trace). The membrane potential was returned to the resting value by injecting depolarizing current at each time point before testing for changes in rheobase. Inset shows that the action potential duration was increased following PAR2 activation. Rheobase was determined using a series of 0.01 current steps (not shown) and recording the current necessary to elicit an action potential. B, summary of percentage decrease in rheobase over time showing change was sustained up to 60 min. In contrast, the PAR2-RP has no effect. n = 5 Fast Blue-labelled neurons at each time point. *P < 0.05 C, summary of mean changes in input resistance at each time point showing sustained increase in input resistance. PAR2-RP had no effect. n = 5 Fast Blue-labelled neurons at each time point. *P < 0.05.
In a separate series of experiments, the PAR2-AP (1–100 μm) displayed a dose-dependent effect on membrane potential (Fig. 2C). Two out of eight neurons did not respond to 30 μm and 100 μm PAR2-AP. Superfusion of the PAR2 agonist trypsin (300 nm) also evoked slow depolarizations (mean peak amplitude = 3.8 ± 0.53 mV; n = 7).
The effects of PAR2-AP on action potential duration and slope were also evaluated in a separate series of experiments, using short-duration depolarizing pulses (30 ms). Following PAR2 activation, the mean action potential duration (n = 4) at one-half the peak amplitude (Fig. 3) was longer at all time points (3–15 min; n = 6) and statistically significant at 15 min (0 min mean = 3.18 ± 0.53 versus 15 min mean = 3.48 ± 0.50; P < 0.05, n = 6). The action potential duration was unchanged in control neurons (n = 4) over a similar time period (0 min mean = 2.49 ± 0.49 ms versus 10 min mean = 2.49 ± 0.46 ms). The rising slope of the action potential was measured up to 10 min following PAR2 activation (n = 5), and was not altered (0 min mean = 133.41 ± 11.43 mV ms−1versus 0 min mean = 121.77 ± 17.63 mV ms−1).
Effect of PAR2 activation on K+ and Na+ currents
Numerous studies, including our own (Stewart et al. 2003; Beyak & Vanner, 2005), have shown that intestinal inflammation and specific inflammatory mediators causes a significant increase in TTX-resistant Na+ currents (Nav1.8) and/or a suppression of transient IA, and/or delayed rectifier IK potassium currents. We therefore examined whether changes in these currents may underlie the effects of PAR2 activation on neuronal excitability.
IA and IK currents
In the whole-cell voltage-clamp mode we observed a frequent run-down in K+ membrane currents over a 5–10 min period. Preliminary studies using the perforated-patch-clamp mode demonstrated that, unlike in whole-cell mode, current run-down did not occur in perforated control neurons over the 15 min time period studied. The transient IA current was blocked by 4-aminopyridine (1 mm; n = 3), as previously described (Stewart et al. 2003).
Using the perforated-patch technique, superfusion of PAR2-AP (100 μm) (n = 7, 3 Fast Blue-labelled, 4 unlabelled neurons) caused a significant decrease in IK at 3 min (mean = 23.7%; P < 0.05) (Fig. 4). This persisted for the duration of the recording period and was maximal by 10 min (mean = 51.5%). No differences were observed between Fast Blue-labelled and non-labelled neurons. In contrast, PAR2 activation had no effect on IA currents (Fig. 4). Similarly, superfusion of PAR2-RP (100 μm) had no effect on IA or IK (Fig. 4). Correlation of the current–voltage relationship (Fig. 4D) for IK demonstrated PAR2 significantly decreased currents near −40 mV, suggesting this effect was active in the physiological range of membrane potentials.
Voltage-dependence of activation and inactivation (see Methods) were compared, to determine if changes in these parameters could contribute to the reduced current density observed following PAR2 activation. There were no significant differences in the activation curves for the IA or IK currents following superfusion of PAR2-AP (100 μm; t = 10 min) (n = 7 for activation curves, 3 Fast Blue-labelled cells) or reverse peptide (100 μm) (n = 8, 3 Fast Blue-labelled cells). We also found no significant difference in IA, or IK inactivation curves (Fig. 4) between cells superfused with PAR2-AP (100 μm) (IA and IKn = 5, unlabelled cells) and PAR2-RP. The voltage of half-activation and inactivation (V50) and corresponding slope factors (k) were determined from the mean of the individual curve fits and are summarized in Table 1. No significant difference was found between the PAR2-AP and corresponding PAR2-RP values for V50 and k.
Table 1.
The effect of PAR2 activation on V50 and k-values
| PAR2-AP | PAR2-RP | |||
|---|---|---|---|---|
| Experiment | V50 (mV) | k | V50 (mV) | k |
| IA | ||||
| Activation | −22.7 ± 6.8 | 27.9 ± 9.2 | −15.2 ± 4.4 | 21.8 ± 4.9 |
| Inactivation | −59.2 ± 1.5 | −12.7 ± 1.3 | −59.6 ± 1.7 | −11.9 ± 1.4 |
| IK | ||||
| Activation | −4.3 ± 5.2 | 19.8 ± 4.9 | 10.1 ± 5.4 | 26.6 ± 3.8 |
| Inactivation | −49.7 ± 2.8 | −18.2 ± 3.6 | −52.1 ± 3.4 | −18.5 ± 4.0 |
| TTX-R Nav | ||||
| Activation | −43.4 ± 1.2 | 3.8 ± 1.0 | −41.5 ± 1.0 | 3.9 ± 0.8 |
| Inactivation | −29.9 ± 1.5 | −5.7 ± 1.3 | −31.3 ± 0.5 | −5.3 ± 0.4 |
PAR2 activation did not alter voltage of half-activation/inactivation (V50) or the slope (k) in IA, IK, or TTX-resistant Na+ currents.
TTX-resistant Na+ currents (Nav1.8)
TTX-resistant Na+ currents were obtained using whole-cell recording techniques. In TTX (1 μμ), superfusion of cells with PAR2-AP (100 μm) for 3 min had no effect on TTX-resistant Na+ currents (Fig. 5; n = 5 Fast Blue-labelled cells). Similarly, currents were not altered by the reverse peptide (100 μm) (n = 4, 3 Fast Blue-labelled cells). The time course of these currents was similar to those described for slow-inactivating Nav1.8 currents as apposed to ultra-slow-inactivating Nav1.9 currents (Beyak et al. 2004). Previous studies have shown that ATP enhances TTX-resistant Na+ currents in rat DRG neurons (Park et al. 2004). Therefore, we also examined whether ATP alters these currents in mouse DRG neurons. When ATP (1 μm) was superfused for 3 min, TTX-resistant Na+ currents were significantly increased by 3 min following the drug application (285.5%; n = 3 Fast Blue-labelled cells; Fig. 5). Further studies were also conducted using perforated-patch-clamp mode to provide an additional measure that effects of PAR2 activation were not masked in the whole-cell mode. Using the perforated-patch technique, mean TTX-resistant Na+ currents were not altered by PAR2 activation (0 min mean = 2374.2 ± 148.9 pA versus 10 min 1797.1 ± 352.0 pA).
Figure 5. The effect of PAR2 activation on DRG TTX-R sodium currents.
PAR2 activation did not alter TTX-resistant sodium currents (TTX-R) in DRG neurons. A, representative Na+ currents elicited using 30 ms steps from −80 to +40 mV. TTX- resistant Na+ currents were isolated with tetrodotoxin (TTX; 1 μm) applied for 3 min. PAR2-AP (100 μm) was superfused for 3 min and recordings obtained at t = 3, 5 and 10 min. PAR2-AP had no effect on TTX-R Na+ currents. B, summary of change in peak Na+ current evoked by application of PAR2-AP (n = 5) or PAR2-RP (n = 4). Using the protocol in A, cells were also superfused with ATP (1 μm; n = 3). ATP caused a significant increase in TTX-R Na+ currents. *P < 0.05, two-way repeated ANOVA followed by Bonferroni post tests. C, PAR2-AP had no effect on TTX-R-Na+ activation and inactivation kinetics. TTX-R Na+ current inactivation curves were generated using a two-pulse protocol, with a 1 s prepulse between −120 and 0 mV, followed by a 30 ms test pulse to 0 mV. Normalized current (I/Imax) was plotted against prepulse voltage and fitted to a negative Boltzmann function.
Activation curves for TTX-resistant Na+ currents were not altered (Fig. 5) by prior application of PAR2-AP (100 μm; n = 5, Fast Blue-labelled) compared to cells superfused with PAR2-RP (100 μm) (n = 4, 3 Fast Blue-labelled cells). Similarly, inactivation curves were not altered (Fig. 5) (PAR2-AP; n = 3, all Fast Blue-labelled; PAR2-RP; n = 4, all Fast Blue-labelled). No significant differences were observed between V50 and k values obtained with PAR2-AP compared to PAR2-RP (Table 1). The threshold for activation of these Nav1.8 currents is more hyperpolarized than that described for other species, as previously reported (Beyak et al. 2004).
PAR2 activation of PKC and ERK1/2
ERK1/2 and PKC antagonists block PAR2-induced neuronal excitability
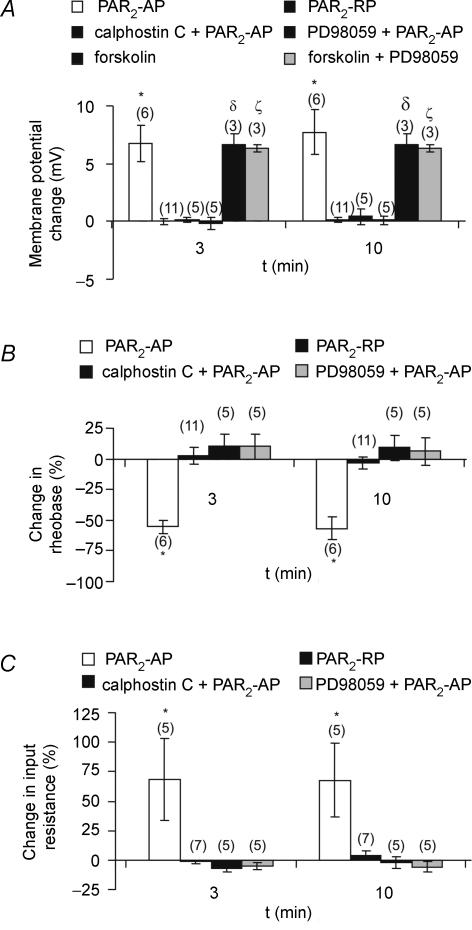
To investigate whether the observed changes in DRG neuronal excitability were mediated by the activation of PKC and ERK1/2-dependent pathways, the effects of the PKC inhibitor calphostin C (1 μm) and the ERK1/2 inhibitor PD98059 (100 μm) were studied. Calphostin C and PD98059 were added to the intracellular pipette solution and superfused in the extracellular medium during electrophysiological recordings. Neurons were also incubated in PD98059 for 3 h prior to recordings.
Neurons were superfused for 3 min with the PAR2-AP (100 μm) or PAR2-RP (100 μm), and recordings were made at 3 and 10 min (Fig. 6). In the presence of either calphostin C (n = 5) or PD98059 (n = 5), superfusion of PAR2-AP had no effect on resting membrane potential, rheobase, or input resistance. These measurements of the membrane properties following PAR2 activation (i.e. in the presence of inhibitors) did not differ from those obtained following the superfusion of the reverse peptide (100 μm) alone (P > 0.05), implying that the effects of PAR2 proceed down PKC and ERK1/2-mediated pathways.
Figure 6. Calphostin C and PD98059 blocked PAR2-evoked DRG neuronal hyperexcitability.
A, summary of mean changes in membrane potential evoked by PAR2-AP at 3 and 10 min either alone or in presence of PKC inhibitor calphostin C (1 μm; applied for 3 min by superfusion and in pipette) or the ERK1/2 inhibitor PD98059 (100 μm, applied for 3 h to cultures prior to application of PAR2-AP and in pipette). Depolarizations evoked by the PKA agonist forskolin (1 μm) were not inhibited by PK98059, supporting a selective action of this antagonist. B, summary of effects of inhibitors on rheobase. C, summary of effects on input resistance. For experimental protocol see Figs 2 and 3. The numbers in parentheses indicate the number of cells for every experiment. *, δ and ζ indicate P < 0.05 versus the PAR2-RP; two-way repeated ANOVA followed by Bonferroni post tests.
To examine the selectivity of PD98059 in DRG neurons, the PKA activator forskolin (1 μm) was studied to determine if PKA-mediated depolarizations were altered by the ERK1/2 inhibitor PD98059 (Fig. 6). In these studies forskolin (1 μm) was superfused for 3 min, and changes in membrane potential were recorded at 3 and 10 min. Forskolin depolarized these neurons (n = 3, 2 Fast Blue-labelled) at t = 3 min (mean = 6.7 mV) and 10 min (mean = 6.7 mV). In separate cells, when forskolin was applied in the presence of PD98059 (100 μm; n = 3, 2 Fast Blue-labelled neurons), depolarizations were not substantively different (mean = 6.3 mV at t = 3 min, mean = 6.3 mV at t = 10 min) compared to those obtained with forskolin alone.
PAR2 agonists activate ERK1/2 and induce phosphorylation of ERK1/2 at the plasma membrane
We have previously reported that PAR2 agonists activate ERK1/2 in transfected cell lines and in enterocytes that naturally express this receptor (Defea et al. 2000). Since ERK1/2 can phosphorylate and inhibit Kv4.2 in the CNS and thereby enhance neuronal excitability (Adams et al. 2000), we determined if PAR2 agonists phosphorylate and thus activate ERK1/2 in cultured DRG neurons. In mouse DRG neurons, there were low levels of immunoreactive pERK1/2 in the unstimulated state (Fig. 7). When neurons were incubated with PMA (1 μm, 5 min), as a positive control for ERK1/2 activation, pERK1/2 was observed in the cytosol of neurons, with the most intense signals in close proximity to the plasma membrane (Fig. 7B). Incubation of neurons with PAR2-RP (100 μm, 1–15 min), a control agonist that does not activate PAR2, had no effect on levels of detectable pERK1/2 (Fig. 7C). However, when neurons were incubated with PAR2-AP (100 μm, 1–15 min), pERK1/2 was detected in the cytosol of many neurons. PAR2-AP induced the appearance of pERK1/2 within 1–5 min (not shown), and by 15 min there were strong signals in the cytosol in close proximity to the plasma membrane (Fig. 7D). Since we have previously reported that PAR2 activators cause hyperexcitability of DRG neurons in rats, we also determined if they activate ERK1/2 in DRG neurons from this species. There were low levels of detectable immunoreactive pERK1/2 in unstimulated rat DRG neurons (Fig. 7E). When neurons were incubated with PMA (1 μm) or PAR2-AP (100 μm) for 1–15 min, pERK1/2 was detected in the cytosol of many neurons in close proximity to the plasma membrane (PMA, not shown, PAR2-AP 15 min, Fig. 7F). As a control, PAR2-RP had no effect on levels of pERK1/2 in rat DRG neurons (not shown). Together, these results suggest that PAR2 agonists stimulate activation of ERK1/2 in the region of the plasma membrane, where ERK1/2 may phosphorylate and thereby control the activity of ion channels that regulate neuronal excitability.
Figure 7. Localization of pERK1/2 in cultured DRG neurons from mice (A–D) and rats (E and F).
A, unstimulated neurons; B, PMA, 1 μm, 5 min; C, PAR2-RP, 100 μm, 15 min; D, PAR2-AP, 100 μm, 15 min; E, unstimulated neurons; F, PAR2-AP (100 μm, 15 min). When neurons were incubated with PMA or PAR2-AP, pERK1/2 were detected in the cytosol in close proximity to the plasma membrane (arrows), whereas PAR2-RP (control) had no effect on the levels of pERK1/2. Representative images are shown from experiments with neurons prepared from n > 3 animals. Scale bar = 20 μm.
Discussion
This study demonstrates that PAR2 agonists applied to neurons from mouse DRG caused a remarkably sustained hyperexcitability of these neurons. Several steps were taken to further clarify the relevance of this finding. Firstly, small neurons were targeted in these studies because they express properties associated with nociceptors (i.e. TTX-resistant action potentials, TRPV1 receptors, substance P and CGCP immunoreactivity) (Gold et al. 1996a; Yoshimura & de Groat, 1999; Moore et al. 2002; Stewart et al. 2003; Beyak et al. 2004; Beyak & Vanner, 2005). Secondly, electrophysiological recordings were obtained in neurons labelled with retrograde tracers injected into the wall of the colon, confirming that these findings were characteristic of nociceptors innervating the colon. Finally, we also demonstrated that labelled neurons from the colon expressed PAR2 immunoreactivity. Together, these findings demonstrate that activation of PAR2 on the plasma membrane of nociceptive DRG neurons innervating the mouse colon leads to sustained hyperexcitability.
Sustained neuronal hyperexcitability
The long-lasting changes in membrane potential and rheobase observed in this study lasted up to 1 h after a brief application of the PAR2 agonist. We have observed similar sustained effects on enteric neurons (Reed et al. 2003) and other studies of unidentified rat DRG neurons (Amadesi et al. 2004). The specificity of the PAR2 agonists used in the current study has been carefully demonstrated in our previous studies (Reed et al. 2003; Amadesi et al. 2004) and others (Nystedt et al. 1994; Dai et al. 2004). Consistent with these observations, in the present study we found that PAR2-AP had a dose-dependent effect, which was mimicked by trypsin, whereas the reverse peptide, which lacks biological activity at PAR2, had no effect. This specificity has also been observed in studies demonstrating that PAR2-AP directly activates intestinal afferents in anaesthetized animals (Kirkup et al. 2003).
Ionic mechanism(s) underlying sustained hyperexcitability
There is considerable evidence that voltage-gated Na+ and K+ channels underlying action potential electrogenesis are modulated by visceral inflammation or specific inflammatory mediators (Gold et al. 1996a; Yoshimura & de Groat, 1999; Gebhart et al. 2002; Beyak et al. 2004; Dang et al. 2004; Beyak & Vanner, 2005). The most common findings are that TTX-resistant Na+ currents (Nav1.8) are increased and/or voltage-gated K+ currents, namely IA and IK, are suppressed. However, the specific channel(s) affected may be dependent on the nature of the inflammation (Beyak & Vanner, 2005c) and/or the inflammatory mediator.
In the current study, mouse colonic DRG neurons exhibited transient IA and sustained delayed-rectifier IK voltage-gated K+ currents (see Fig. 4) characteristic of other DRG neurons (McFarlane & Cooper, 1991; Stewart et al. 2003; Dang et al. 2004; Beyak & Vanner, 2005). The voltage and 4-AP sensitivity (Stewart et al. 2003) of the transient K+ currents were typical of IA currents, and the kinetics of the IK currents match those described in other species (Stewart et al. 2003; Dang et al. 2004). We found that the hyperexcitability following PAR2 activation was associated with a profound suppression of the IK currents (>50%) but not of IA. The effect was active in the physiological range of membrane potentials (Fig. 4) (i.e. around the threshold for action potential electrogenesis), and therefore may contribute to the decrease in rheobase observed with PAR2 activation. However, models of visceral inflammation in the ileum, stomach and bladder (Yoshimura & de Groat, 1999; Stewart et al. 2003;Dang et al. 2004) have also shown that IA is suppressed in models of chronic inflammation, supporting the notion that individual mediators may signal preferentially to selective K+ channels. Previous studies of the biophysical and pharmacological properties of IK currents suggest these currents may comprise three distinct currents (Gold et al. 1996b). The molecular determinants of the channels underlying these IK currents are currently unknown although studies suggest candidates of the α-subunits include KV 2.1/2.2 and 3.1/3.2 (Song, 2002; Kim et al. 2002).
In addition to the observed changes in IK currents, our findings also suggest that PAR2 activation affects other K+ currents. The increases in membrane depolarization and input resistance following PAR2 activation cannot be accounted for by IK currents, because they were not open at the resting membrane potential. It is probable that these changes result from closure of background or ‘leak’ K+-selective channels. These channels may be members of the two, and possibly four, transmembrane segment K+ channels (Patel & Honore, 2001), which are important targets of neurotransmitters and paracrine agents.
An array of inflammatory mediators including adenosine, 5-HT, ATP, PGE2 and NGF have been found to enhance TTX-resistant Na+ currents (Nav1.8) in nociceptive DRG neurons (Gold et al. 1996a; Cardenas et al. 2001; Zhang et al. 2002; Park et al. 2004; Beyak & Vanner, 2005). In models of established inflammation in the stomach, ileum, colon, and bladder (Yoshimura & de Groat, 1999; Gebhart et al. 2002; Stewart et al. 2003; Beyak et al. 2004) these currents were also found to be increased in neurons innervating the inflamed segments. Somewhat surprisingly, in our study PAR2 activation had no effect on this current. To demonstrate we could induce current increases in our neurons, we also tested ATP and found that this mediator enhanced currents, as predicted by other studies (Park et al. 2004). Recent studies have also suggested TTX-sensitive Na+ currents (Nav1.7) may also be modulated by inflammation (Black et al. 2004). We cannot conclude that PAR2 activation does not modulate these currents because they were not examined in our study. However, this seems unlikely given that previous studies examining the effects of inflammation either failed to observe an effect on TTX-sensitive Na+ current (Yoshimura & de Groat, 1999; Gebhart et al. 2002; Beyak et al. 2004) or, in the recent study, suggested an effect on TTX-sensitive Na+ currents (Black et al. 2004), which paralleled the effects on TTX-resistant Na+ currents. We also did not examine the possibility that TTX-resistant Nav1.9 currents were modulated by PAR2 activation. However our previous studies (Beyak et al. 2004) suggest these currents are not frequently found in nociceptive DRG neurons innervating the mouse colon, and furthermore other studies suggest that measurement of pharmacological effects on these currents can be extremely difficult (Maruyama et al. 2004). Finally, although our findings strongly suggest that PAR2 activation does not directly modulate Nav1.8 currents, this may occur through indirect mechanisms in the whole animal because PAR2 stimulates PGE2 and CGRP release from nerve terminals (Steinhoff et al. 2000; Vergnolle et al. 2003) and these agents in turn can enhance the Na+ currents (Baker, 2005; Natura et al. 2005).
PAR2 signal transduction mechanisms of hyperexcitability in colonic DRG neurons
The intracellular mechanism(s) that underlie the sustained hyperexcitability evoked by PAR2 activation on DRG neurons described in this study and others is unknown. Our findings suggest ERK1/2 signalling may play a major role because PAR2 activation stimulates phosphorylation of ERK1/2 at the plasma membrane (Fig. 7) where it could modulate ion channels, and the highly selective ERK1/2 inhibitor (PD98059) (Thomas & Huganir, 2004) blocked the PAR2-mediated effects on neuronal excitability (Fig. 6). We were specifically interested in examining the potential role of ERK1/2 signalling because recent reports in the CNS (Thomas & Huganir, 2004) suggest that ERK1/2 signals not only to nuclear pathways regulating gene expression but also to cytoplasmic proteins including voltage-gated K+ channels. Moreover, phosphorylation of these channels in an ERK-dependent manner in the hippocampus underlies some forms of sustained excitability. Several recent studies have also demonstrated activation of ERK1/2 in peripheral DRG neurons (Seino et al. 2006; Takahashi et al. 2006).
The signal transduction pathway by which PAR2 agonists activate ERK1/2 in neurons also remains to be defined. However, possible mechanisms include the transactivation of growth factor receptors, activation of protein kinase C, and β-arrestin-dependent activation (Defea et al. 2000; Darmoul et al. 2004). We did observe that calphostin, a PKC blocker, also inhibited the PAR2-induced neuronal hyperexcitability (see Fig. 6), but further studies are needed to examine the interaction of PKC and ERK1/2. β-arrestin-dependent mechanisms may be of particular importance in targeting activated EERK1/2 to the plasma membrane, where they may phosphorylate and regulate ion channels (Defea et al. 2000; Luttrell & Lefkowitz, 2002). Agonists of many G-protein-coupled receptors, including PAR2, stimulate the translocation of β-arrestins from the cytosol to phosphorylate receptors at the plasma membrane (Dery et al. 1999; Luttrell & Lefkowitz, 2002). β-Arrestins act to uncouple receptors from heterotrimeric G-proteins and thereby desensitize G-protein signalling, couple G-protein-coupled receptors to clathrin and AP2, and thereby mediate receptor endocytosis. β-Arrestins are also molecular scaffolds that recruit and organize components of the MAP kinase pathway to activated G-protein-coupled receptors, thereby determining the subcellular location and function of activated ERK1/2. We have shown that agonists of PAR2 promote the assembly of a stable signalling complex in cells that includes PAR2, β-arrestins, raf-1, MEKK and pERK1/2 (Defea et al. 2000). This complex acts to prevent the trafficking of activated ERK1/2 to the nucleus and instead retain ERK1/2 within the cytosol, where it could phosphorylate and regulate other targets, including ion channels. Whether this mechanism occurs in neurons and permits ERK1/2 to regulate ion channels that mediate PAR2-induced hyperexcitability, such as those underlying IK currents, remains to be determined.
Implications of PAR2-evoked sustained hyperexcitability of nociceptive DRG neurons in the colon
There is growing evidence that the release of proteases from mast cells plays an important role in the genesis of visceral hyperalgesia in functional bowel disorders such as IBS (Barbara et al. 2004; Spiller & Campbell, 2006; Barbara et al. 2006). Studies have demonstrated that intestinal mast cell numbers are increased, at least in subsets of patients, and that there is evidence of increased degranulation and tissue content of mast cell tryptase. Moreover, preliminary studies have shown that tryptase in the supernatant of colonic biopsies from IBS patients can stimulate calcium signals in DRG neurons (Cenac et al. 2005), suggesting activation of these neurons. The proximity of mast cells to nerve terminals in the intestine has also been correlated with the severity of abdominal pain (Barbara et al. 2004). Numerous in vitro and in vivo animal studies have also shown that proteases, such as tryptase, released from degranulating mast cells have similar actions to those induced by the PAR2 agonists used in this study. When taken together, the findings of the current study combined with these previous studies suggest it is possible that the sustained excitability of nociceptive DRG neurons following PAR2 activation could be important in the genesis of abdominal pain in patients with IBS, and that repeated mast cell degranulation may lead to ongoing ‘re-priming’ of these neurons.
Acknowledgments
We thank Lorna Divino and Iva Kosatka for excellent technical support. This work was supported by grants from NIH (DK57840 and D43207, NWB), CIHR (SV) and CCFC (SV).
References
- Adams JP, Anderson AE, Varga AW, Dineley KT, Cook RG, Pfaffinger PJ, Sweatt JD. The A-type potassium channel Kv4.2 is a substrate for the mitogen-activated protein kinase ERK. J Neurochem. 2000;75:2277–2287. doi: 10.1046/j.1471-4159.2000.0752277.x. [DOI] [PubMed] [Google Scholar]
- Amadesi S, Nie J, Vergnolle N, Cottrell GS, Grady EF, Trevisani M, Manni C, Geppetti P, McRoberts JA, Ennes H, Davis JB, Mayer EA, Bunnett NW. Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. J Neurosci. 2004;24:4300–4312. doi: 10.1523/JNEUROSCI.5679-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker MD. Protein kinase C mediates up-regulation of tetrodotoxin-resistant, persistent Na+ current in rat and mouse sensory neurones. J Physiol. 2005;567:851–867. doi: 10.1113/jphysiol.2005.089771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbara G, De Stanghellini V, GR Corinaldesi R. Functional gastrointestinal disorders and mast cells: implications for therapy. Neurogastroenterol Motil. 2006;18:6–17. doi: 10.1111/j.1365-2982.2005.00685.x. [DOI] [PubMed] [Google Scholar]
- Barbara G, De Stanghellini VGR, Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate AM, Grady EF, Bunnett NW, Collins SM, Corinaldesi R. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693–702. doi: 10.1053/j.gastro.2003.11.055. [DOI] [PubMed] [Google Scholar]
- Beyak MJ, Ramji N, Krol KM, Kawaja MD, Vanner SJ. Two TTX-resistant Na+ currents in mouse colonic dorsal root ganglia neurons and their role in colitis-induced hyperexcitability. Am J Physiol Gastrointest Liver Physiol. 2004;287:G845–G855. doi: 10.1152/ajpgi.00154.2004. [DOI] [PubMed] [Google Scholar]
- Beyak MJ, Vanner S. Inflammation-induced hyperexcitability of nociceptive gastrointestinal DRG neurones: the role of voltage-gated ion channels. Neurogastroenterol Motil. 2005;17:175–186. doi: 10.1111/j.1365-2982.2004.00596.x. [DOI] [PubMed] [Google Scholar]
- Black JA, Liu S, Tanaka M, Cummins TR, Waxman SG. Changes in the expression of tetrodotoxin-sensitive sodium channels within dorsal root ganglia neurons in inflammatory pain. Pain. 2004;108:237–247. doi: 10.1016/j.pain.2003.12.035. [DOI] [PubMed] [Google Scholar]
- Cardenas LM, Cardenas CG, Scroggs RS. 5HT increases excitability of nociceptor-like rat dorsal root ganglion neurons via cAMP-coupled TTX-resistant Na+ channels. J Neurophysiol. 2001;86:241–248. doi: 10.1152/jn.2001.86.1.241. [DOI] [PubMed] [Google Scholar]
- Cenac N, Chapman K, Andrade-Gordon P, Ferrez J, Andrews C, Schaffer E, Vergnolle N. Role for proteases and protease-activated receptor-2 (PAR2) in pain associated with irritable bowel syndrome (IBS) Gastroenterol. 2005;128(Suppl. 2):P-20. [Google Scholar]
- Dai Y, Moriyama T, Higashi T, Togashi K, Kobayashi K, Yamanaka H, Tominaga M, Noguchi K. Proteinase-activated receptor 2-mediated potentiation of transient receptor potential vanilloid subfamily 1 activity reveals a mechanism for proteinase-induced inflammatory pain. J Neurosci. 2004;24:4293–4299. doi: 10.1523/JNEUROSCI.0454-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang K, Bielefeldt K, Gebhart GF. Gastric ulcers reduce A-type potassium currents in rat gastric sensory ganglion neurons. Am J Physiol Gastrointest Liver Physiol. 2004;286:G573–G579. doi: 10.1152/ajpgi.00258.2003. [DOI] [PubMed] [Google Scholar]
- Darmoul D, Gratio V, Devaud H, Laburthe M. Protease-activated receptor 2 in colon cancer: trypsin-induced MAPK phosphorylation and cell proliferation are mediated by epidermal growth factor receptor transactivation. J Biol Chem. 2004;279:20927–20934. doi: 10.1074/jbc.M401430200. [DOI] [PubMed] [Google Scholar]
- Defea KA, Zalevsky J, Thoma MS, Dery O, Mullins RD, Bunnett NW. beta-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol. 2000;148:1267–1281. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dery O, Thoma MS, Wong H, Grady EF, Bunnett NW. Trafficking of proteinase-activated receptor-2 and beta-arrestin-1 tagged with green fluorescent protein. beta-Arrestin-dependent endocytosis of a proteinase receptor. J Biol Chem. 1999;274:18524–18535. doi: 10.1074/jbc.274.26.18524. [DOI] [PubMed] [Google Scholar]
- gado-Aros S, Camilleri M. Visceral hypersensitivity. J Clin Gastroenterol. 2005;39:S194–S203. doi: 10.1097/01.mcg.0000156114.22598.1b. [DOI] [PubMed] [Google Scholar]
- Gebhart GF, Bielefeldt K, Ozaki N. Gastric hyperalgesia and changes in voltage gated sodium channel function in the rat. Gut. 2002;51(Suppl. 1):i15–i18. doi: 10.1136/gut.51.suppl_1.i15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MS, Reichling DB, Shuster MJ, Levine JD. Hyperalgesic agents increase a tetrodotoxin-resistant Na+ current in nociceptors. Proc Natl Acad Sci U S A. 1996a;93:1108–1112. doi: 10.1073/pnas.93.3.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MS, Shuster MJ, Levine JD. Characterization of six voltage-gated K+ currents in adult rat sensory neurons. J Neurophysiol. 1996b;75:2629–2646. doi: 10.1152/jn.1996.75.6.2629. [DOI] [PubMed] [Google Scholar]
- Kim DS, Choi JO, Rim HD, Cho HJ. Downregulation of voltage-gated potassium channel alpha gene expression in dorsal root ganglia following chronic constriction injury of the rat sciatic nerve. Brain Res Mol Brain Res. 2002;105:146–152. doi: 10.1016/s0169-328x(02)00388-1. [DOI] [PubMed] [Google Scholar]
- Kirkup AJ, Jiang W, Bunnett NW, Grundy D. Stimulation of proteinase-activated receptor 2 excites jejunal afferent nerves in anaesthetised rats. J Physiol. 2003;552:589–601. doi: 10.1113/jphysiol.2003.049387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembo A, Ameen VZ, Drossman DA. Irritable bowel syndrome: toward an understanding of severity. Clin Gastroenterol Hepatol. 2005;3:717–725. doi: 10.1016/s1542-3565(05)00157-6. [DOI] [PubMed] [Google Scholar]
- Lomax AE, O'Hara JR, Hyland NP, Mawe GM, Sharkey KA. Persistent alterations to enteric neural signalling in guinea pig colon following resolution of colitis. Am J Physiol Gastrointest Liver Physiol. Am J Physiol Gastrointest Liver Physiol. 2006;292:G482–4891. doi: 10.1152/ajpgi.00355.2006. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Lefkowitz RJ. The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J Cell Sci. 2002;115:455–465. doi: 10.1242/jcs.115.3.455. [DOI] [PubMed] [Google Scholar]
- Maruyama H, Yamamoto M, Matsutomi T, Zheng T, Nakata Y, Wood JN, Ogata N. Electrophysiological characterization of the tetrodotoxin-resistant Na+ channel, Nav1.9, in mouse dorsal root ganglion neurons. Pflugers Arch. 2004;449:76–87. doi: 10.1007/s00424-004-1315-0. [DOI] [PubMed] [Google Scholar]
- Mawe GM, Collins SM, Shea-Donohue T. Changes in enteric neural circuitry and smooth muscle in the inflamed and infected gut. Neurogastroenterol Motil. 2004;16(Suppl. 1):133–136. doi: 10.1111/j.1743-3150.2004.00489.x. [DOI] [PubMed] [Google Scholar]
- McFarlane S, Cooper E. Kinetics and voltage dependence of A-type currents on neonatal rat sensory neurons. J Neurophysiol. 1991;66:1380–1391. doi: 10.1152/jn.1991.66.4.1380. [DOI] [PubMed] [Google Scholar]
- Moore BA, Stewart TM, Hill C, Vanner SJ. TNBS ileitis evokes hyperexcitability and changes in ionic membrane properties of nociceptive DRG neurons. Am J Physiol Gastrointest Liver Physiol. 2002;282:G1045–G1051. doi: 10.1152/ajpgi.00406.2001. [DOI] [PubMed] [Google Scholar]
- Natura G, von Banchet GS, Schaible HG. Calcitonin gene-related peptide enhances TTX-resistant sodium currents in cultured dorsal root ganglion neurons from adult rats. Pain. 2005;116:194–204. doi: 10.1016/j.pain.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Nystedt S, Emilsson K, Wahlestedt C, Sundelin J. Molecular cloning of a potential proteinase activated receptor. Proc Natl Acad Sci U S A. 1994;91:9208–9212. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Kim HI, Shin YK, Lee CS, Park M, Song JH. Modulation of sodium currents in rat sensory neurons by nucleotides. Brain Res. 2004;1006:168–176. doi: 10.1016/j.brainres.2004.01.061. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honore E. Properties and modulation of mammalian 2P domain K+ channels. Trends Neurosci. 2001;24:339–346. doi: 10.1016/s0166-2236(00)01810-5. [DOI] [PubMed] [Google Scholar]
- Rae J, Cooper K, Gates P, Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Meth. 1991;37:15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Reed DE, Barajas-Lopez C, Cottrell G, Velazquez-Rocha S, Dery O, Grady EF, Bunnett NW, Vanner SJ. Mast cell tryptase and proteinase-activated receptor 2 induce hyperexcitability of guinea-pig submucosal neurons. J Physiol. 2003;547:531–542. doi: 10.1113/jphysiol.2002.032011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Bunnett NW. Protease-activated receptors: regulation of neuronal function. Neuromolecular Med. 2005;7:79–99. doi: 10.1385/NMM:7:1-2:079. [DOI] [PubMed] [Google Scholar]
- Sandler RS, Drossman DA, Nathan HP, McKee DC. Symptom complaints and health care seeking behavior in subjects with bowel dysfunction. Gastroenterology. 1984;87:314–318. [PubMed] [Google Scholar]
- Seino D, Tokunaga A, Tachibana T, Yoshiya S, Dai Y, Obata K, Yamanaka H, Kobayashi K, Noguchi K. The role of ERK signaling and the P2X receptor on mechanical pain evoked by movement of inflamed knee joint. Pain. 2006;123:193–203. doi: 10.1016/j.pain.2006.02.032. [DOI] [PubMed] [Google Scholar]
- Song WJ. Genes responsible for native depolarization-activated K+ currents in neurons. Neurosci Res. 2002;42:7–14. doi: 10.1016/s0168-0102(01)00305-4. [DOI] [PubMed] [Google Scholar]
- Spiller R, Campbell E. Post-infectious irritable bowel syndrome. Curr Opin Gastroenterol. 2006;22:13–17. doi: 10.1097/01.mog.0000194792.36466.5c. [DOI] [PubMed] [Google Scholar]
- Steinhoff M, Vergnolle N, Young SH, Tognetto M, Amadesi S, Ennes HS, Trevisani M, Hollenberg MD, Wallace JL, Caughey GH, Mitchell SE, Williams LM, Geppetti P, Mayer EA, Bunnett NW. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat Med. 2000;6:151–158. doi: 10.1038/72247. [DOI] [PubMed] [Google Scholar]
- Stewart T, Beyak MJ, Vanner S. Ileitis modulates potassium and sodium currents in guinea pig dorsal root ganglia sensory neurons. J Physiol. 2003;552:797–807. doi: 10.1113/jphysiol.2003.046409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Kikuchi S, Shubayev VI, Campana WM, Myers RR. TNF-alpha and phosphorylation of ERK in DRG and spinal cord: insights into mechanisms of sciatica. Spine. 2006;31:523–529. doi: 10.1097/01.brs.0000201305.01522.17. [DOI] [PubMed] [Google Scholar]
- Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- Vergnolle N, Ferazzini M, D'Andrea MR, Buddenkotte J, Steinhoff M. Proteinase-activated receptors: novel signals for peripheral nerves. Trends Neurosci. 2003;26:496–500. doi: 10.1016/S0166-2236(03)00208-X. [DOI] [PubMed] [Google Scholar]
- Vergnolle N, Wallace JL, Bunnett NW, Hollenberg MD. Protease-activated receptors in inflammation, neuronal signaling and pain. Trends Pharmacol Sci. 2001;22:146–152. doi: 10.1016/s0165-6147(00)01634-5. [DOI] [PubMed] [Google Scholar]
- Yoshimura N, de Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. J Neurosci. 1999;19:4644–4653. doi: 10.1523/JNEUROSCI.19-11-04644.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Vasko MR, Nicol GD. Ceramide, a putative second messenger for nerve growth factor, modulates the TTX-resistant Na+ current and delayed rectifier K+ current in rat sensory neurons. J Physiol. 2002;544:385–402. doi: 10.1113/jphysiol.2002.024265. [DOI] [PMC free article] [PubMed] [Google Scholar]