Abstract
The role of interneurones in the control of sympathetic activity has been somewhat of a mystery since, for many years, it was difficult to target these cells for study. Recently scientists have started to unravel the action potential properties of these neurones, where they receive their inputs from and where they project to. This review looks at the information known to date about sympathetic interneurones. The locations of these neurones and their local axonal ramifications suggest that they play a more widespread function than previously thought. Therefore the data to support such a theory are also examined.
Autonomic control of the cardiovascular system and many other end organs is a major contributor to homeostasis in all organisms. A principle means by which this is achieved is through a complex interplay between the central and peripheral nervous systems. A vital connection between the CNS and the rest of the body is provided by the sympathetic nervous system, one branch of the autonomic nervous system, which influences virtually all tissues. By learning more precise means of controlling the activity of sympathetic outflow from the CNS, we can help to restore homeostasis during stressful situations such as local hypoxia or ischaemia.
Our understanding of the role that spinal cord circuits play in controlling sympathetic outflow from the CNS is perpetually changing as we unravel the complexities of these circuits. We now know that the spinal cord acts as much more than a relay for the transmission of information between the brain and periphery as it integrates, manipulates and even generates activity using intrinsic complex neuronal circuits. This review focuses firstly on the role of local interneurones in the spinal cord circuitry and how they may contribute to sympathetic outflow to the end organs. This is still an area of controversy since many believe that such interneurones are of minor importance unless some level of spinal cord damage has occurred, whilst others consider that they are vital components in the many, varied and specific autonomic responses in both physiological and pathophysiological states. Considering the importance of the autonomic nervous system in maintaining homeostasis, our understanding of this circuitry, particularly the role of interneurones, is far from complete. Perhaps a more controversial and thought-provoking idea is that such interneurones may in fact be crucial not only for control and shaping of sympathetic activity but also for determining and synchronizing the spinal responses, beyond autonomic, that are associated with pain or locomotion. With this last idea, perhaps the time has come for us to stop trying to compartmentalize our thinking on neurones, depending on our own area of interest, and broaden our outlook regarding the function of such interneurones. Only then are we likely to be able to understand the complexities of spinal cord circuitry.
Evidence that interneurones comprise a component in sympathetic circuitry
To start this section, first a reminder of how sympathetic activity is controlled at the level of the spinal cord. The outflow from the spinal cord in the control of sympathetic activity arises from sympathetic preganglionic neurones (SPNs) that provide the singular gateway between the CNS and the peripheral nervous system in sympathetic control. These are innervated by supraspinal inputs, which in some circuits utilize interneurones as the final contact with the SPNs, and by indirect pathways from afferent inputs. Astonishingly, it is only in the last 15–20 years that we have been able to pinpoint the location of interneurones involved in sympathetic control and thus start to target these interneurones for study. This is because it has been difficult to identify these cells within the heterogeneous population of neurones in the spinal cord. Studies utilizing transneuronal tracers that cross the synapses between SPNs and interneurones after injections into various end organs have overcome these problems and reveal the presence of interneurones in laminae V, VII and X and also in the intermediolateral cell column region where the majority of SPNs are themselves located (Strack et al. 1989a,b; Strack & Loewy, 1990; Cabot et al. 1994; Jansen et al. 1995; Tang et al. 2004). Further, Deuchars et al. (2005) demonstrate that some transneuronally labelled interneurones in lamina X are inhibitory as they are GABAergic (see Fig. 1). These interneurones provide ongoing drive to SPNs that is observed in spinal cord slices in the form of EPSPs and IPSPs (Dun & Mo, 1989; Krupp & Feltz, 1993; Lewis et al. 1993; Spanswick et al. 1994).
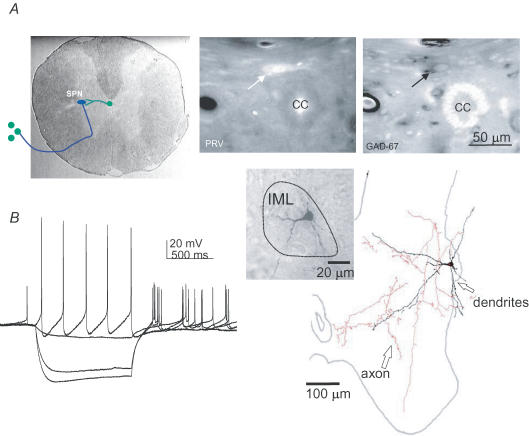
Figure 1. Identification of interneurones projecting to SPNs.
A, transneuronal tracing (depicted in left panel) using pseudorabies viral injections into the adrenal gland labels a neurone in lamina X of the spinal cord (arrow, middle panel) that contains the mRNA for GAD67 (arrow, right panel). B, this interneurone was recorded in the intermediolateral cell column (IML) and the voltage responses to hyperpolarizing and depolarizing current pulses are shown in the left panel. It was then filled and a drawing of the filled neurone is shown on the right with axon and dendrites extending throughout the spinal cord. The inset shows the photomontage of the filled soma and proximal dendrites.
What influences the activity of these interneurones?
Sympathetic interneurones are innervated by both supraspinal and afferent pathways which are key players in maintaining sympathetic outflow. Stimulation of the lateral funiculus, which contains descending fibres that innervate the autonomic regions of the spinal cord (Dembowsky et al. 1985), elicits monosynaptic excitatory and inhibitory responses in interneurones (Deuchars et al. 2001a; Brooke et al. 2004). Axons from the rostral ventrolateral medulla and corticospinal tract closely appose both SPNs and interneurones and these may be influential in amplifying or modulating the response at a spinal level (Pan et al. 2005). In fact, interneurones may be the main relay from the medial prefrontal cortex (which, when stimulated, decreases blood pressure and heart rate) since axons from this region are targeted to the central region of the spinal cord (Bacon & Smith, 1993) where there are few SPNs but many GABAergic presympathetic interneurones that are likely to be the source of the inhibitory input to SPNs (Deuchars et al. 2005).
If these interneurones are to be considered as critical links in particular reflex responses to maintain homeostasis, then it would be expected that activation of specific neuromodulatory receptors known to be influential in descending pathways would also influence the activity of the interneurones. This is indeed the case since activation of receptors for neuromodulators such as substance P (Dun & Mo, 1988; Cammack & Logan, 1996); adrenaline (epinephrine) (Miyazaki et al. 1989); oxytocin and vasopressin (Sermasi & Coote, 1994), angiotensin II (Lewis & Coote, 1993) and 5-HT (Lewis et al. 1993) increase inhibitory and in some cases also excitatory synaptic activity onto SPNs via interneurones.
The actual contribution of spinal interneurones in bulbospinal control of SPNs is unknown but they may help to maintain and synchronize a particular input by innervating a number of SPNs to amplify and prolong the effect. Equally, the extensive arborization of interneuronal dendrites suggests that one interneurone may receive convergent information from both descending and afferent regions, and filtering of information at the interneuronal level will shape the final action potential discharge patterns of SPNs.
Sympathetic activity is also influenced heavily by incoming information from the somatic nervous system and both innocuous and noxious stimulation will elicit autonomic changes that have a spinal component. Interneurones are vital components in these circuits since there is no direct pathway from the primary afferents onto SPNs (Petras & Cummings, 1972; Todd, 2002). Afferent stimulation can excite some presumptive sympathetic interneurones whilst others are inhibited with a similar time course to the inhibition of SPNs, suggesting disfacilitation of SPNs through inhibition of excitatory interneurones (Wyszogrodski & Polosa, 1973). In the lower thoracic spinal cord, noxious somatic stimulation elicits responses in both IML and dorsal horn interneurones which are excitatory or inhibitory depending on the dermatome stimulated (Chau et al. 2000).
The role of spinal interneurones seems to alter and indeed increase after any degree of spinal cord injury when many of the supraspinal inputs are lost and autonomic responses become exaggerated and/or inappropriate. Interneurones immunoreactive for GAP43 (a marker for reactive sprouting) increase with time after spinal cord lesions (Weaver et al. 1997) and interneurones exhibit exaggerated responses to somatic stimulation (Krassioukov et al. 2002). There is a larger increase in numbers of inhibitory over excitatory inputs onto SPNs after spinalization (Llewellyn-Smith & Weaver, 2001) suggesting that inhibitory interneurones play a prominent role in sympathetic control after spinal cord injury.
What shapes the activity of sympathetic interneurones?
From extracellular recordings in vivo and patch clamp recordings in vitro the action potential properties and discharge patterns of interneurones involved in autonomic control have been partially characterized. Some of these interneurones discharge action potentials at high frequencies (Deuchars et al. 2001b), which may be due in part to the presence of the Kv3.1b voltage-gated potassium channel subunit in interneurones but not SPNs (Deuchars et al. 2001b; Brooke et al. 2002). In vivo experiments show that these interneurones discharge action potentials in correlation with sympathetic activity (Chau et al. 2000; Miller et al. 2001; Tang et al. 2003) and can also exhibit action potential firing that correlates with the cardiac cycle (Gebber & McCall, 1976; Barman & Gebber, 1984). Interneurones in the intermediolateral cell column region of the spinal cord can be reflexly inhibited by raised blood pressure and activated by stimulation of sympathoexcitatory medullary regions suggesting a sympathoexcitatory role (Gebber & McCall, 1976; Barman & Gebber, 1984). Those in the intermediomedial cell column are excited by stimulation of the depressor region of the NTS indicating an inhibitory role (McCall et al. 1977). It is also possible to deduce whether interneurones are sympathoexcitatory or inhibitory due to their pattern of correlation to sympathetic nerve activity (Chau et al. 2000; Miller et al. 2001; Tang et al. 2003) and thus it was noted that there were higher proportions of sympathoexcitatory over sympathoinhibitory interneurones at the T10 level of the spinal cord.
So these data suggest that sympathetic interneurones are widespread throughout the spinal cord and may play a prominent role in sympathetic control; however, should we restrict our search for their function to the autonomic nervous system?
A wider role for sympathetic interneurones?
It is always too easy to focus your mind just on questions that seem pertinent to your area of interest and so we study neurones in isolation or in the context of one control system. For example, many studies have focused on the roles of spinal interneurones exclusively in the control of either motor or autonomic outflow but have not necessarily considered whether such interneurones may allow or facilitate interplay between the two output systems. This is especially significant when considering the organism's response to pain or when preparing for defence against another organism, when changes in cardiovascular and respiratory variables accompany movement or preparation for movement. Indeed in any form of exercise, there must be close co-ordination of muscle control with that of autonomically mediated redistribution of blood flow and changes in cardiorespiratory variables. Thus, as the intensity of a motor task increases, so the autonomic responses are increased to match demand. This is thought to involve both higher centre command regions, that co-ordinate motor and autonomic responses and muscle reflexes that are triggered by the muscle activity and feedback to increase autonomic outflow (Kramer et al. 2000). To date, studies have focused on identifying these rostral sites of synchronization or central command regions and have identified a number of neurones in the pontomedullary reticular formation that may simultaneously target gastrocnemius motoneurones and adrenal SPNs via direct collaterals (Kerman et al. 2003). Other neurones have been identified in the pedunculopontine tegmental nucleus (PPN) and lateral hypothalamus that were retrogradely double labelled after injections of two different transneuronal tracers into the motor cortex and stellate ganglion and these have been classified as central commanders of autonomic and motor outflow (Jansen et al. 1995; Krout et al. 2003). However, here I would like to consider evidence for a role of spinal interneurones in co-ordinating outflows and suggest possible candidates for such a role.
In a spinal cord preparation, 5-HT and NMDA will induce fictive locomotor activity and 5-HT has differing effects on commissural interneurones that are implicated in the co-ordination of left–right movements during locomotion (Zhong et al. 2006). What is of particular interest to this review is that spinal application of NMDA and 5-HT also has profound effects on autonomic outflow by inducing rhythmic sympathetic activity in intact and spinalized rats (Madden & Morrison, 2006; Marina et al. 2006). However, what we need is evidence that motor and automatic rhythmic activity can be coupled in certain circumstances at the spinal level. This evidence is provided in an elegant study using the spinal cord hind limb preparation when application of NMDA induced co-ordinated activity in motor and autonomic outflow (Fig. 2) measured as nerve activity since the preparation was paralysed, thus removing interference from activation of muscle reflexes (Chizh et al. 1998). The fact that this co-ordinated activity occurs in a spinal cord hind limb preparation shows clearly that the higher centres are not always necessary for such synchronization and that the spinal cord can generate coupling of outflows. In fact, it is likely that there are components of the sympathetic/motor circuitry in the spinal cord that are crucial in mediating this co-ordination of outflows. Thus, neurones that contribute to synchronization of outputs are also likely to exist in the spinal cord, and interneurones in lamina X and the intermediolateral cell column of the spinal cord may be perfect candidates to fulfil this role (Fig. 3). In our own laboratory, we have preliminary evidence that some of the labelled interneurones in these regions have axons ramifying in regions containing ventral horn motoneurones and SPNs (see Fig. 1). Furthermore direct synaptic contacts between a single filled interneurone and ChAT-positive structures have been observed in both ventral horn and intermediolateral cell column region (S. A. Deuchars, B. Frater and J. Deuchars, unpublished observations).
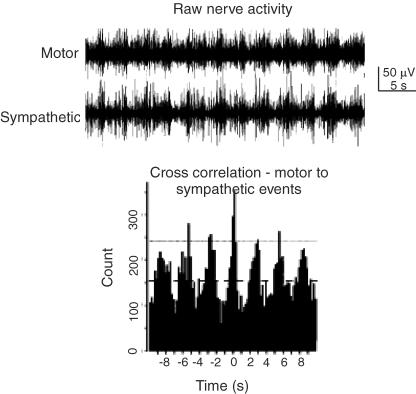
Figure 2. Motor and sympathetic outflows can be correlated at the spinal level.
Top trace, raw nerve activity showing correlation between activity in the femoral motor nerve and abdominal sympathetic chain during application of NMDA. Bottom trace, cross correlogram of sympathetic activity triggered from the peaks of motor activity shows clearly the degree of correlation between the 2 outflows. Adapted from Chizh et al. (1998).
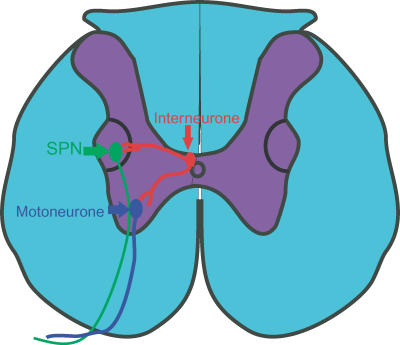
Figure 3.
Line drawing showing putative interneurones innervating SPNs and motoneurones to synchronize outflow.
We need other evidence to support such an idea of spinal ‘synchronizing’ neurones in the spinal cord and this is provided by both direct and indirect findings. A study injected abductor caudae dorsalis muscle with transneuronal tracers and combined this with a sympathectomy that will prevent labelling of neurones in sympathetic pathways innervating blood vessels in the muscle whilst preserving labelling of neurones in the spinal cord that are exclusively involved in the control of outflow to muscle fibres. In this study some degree of labelling of interneurones in autonomic regions of the thoracic spinal cord remained (Jasmin et al. 1997). These may form a subset of interneurones involved in both autonomic and motor outflow that have long axon collaterals travelling caudally to motoneurones. Further indirect evidence comes from Kerman et al. (2003) who noted neurones in the rostral ventrolateral medulla and A5 pontine region that were double labelled after longer exposures to pseudorabies virus (PRV) injections into both gastrocnemius muscle and adrenal gland. Longer exposure times suggest a less direct pathway involving other neurones – a possibility may be infection of an interneurone in the spinal cord that is common to both pathways. Some interesting support for a wider role for lamina X interneurones comes from Wilson et al. (2005) who identified interneurones that were positive for homeodomain protein, Hb9. This transcription factor is important during neuronal development to consolidate motoneurone identity; however, a small group of interneurones (located in the medial portion of lamina VIII) also expresses this protein. These interneurones display rhythmic activity synchronous with ventral root rhythmic activity and were classified as part of the circuit involved in locomotor activity (Hinckley et al. 2005). Of particular interest to this review was the presence of a group of interneurones in lamina X around the central canal located more rostrally at thoracic levels (Wilson et al. 2005), which would equate to the position of presympathetic interneurones (Deuchars et al. 2005). If (as is likely) Hb9-positive interneurones are associated with the generation of rhythmic locomotor activity, then these Hb9-positive neurones in lamina X are perfectly placed to generate rhythm not only in motor outflow but also in sympathetic activity.
We also know that changes in motor and autonomic outflow are often triggered in response to painful or aversive stimuli; in fact, many interneurones involved in either motor control or autonomic control respond to noxious stimulation. In the lower thoracic spinal cord, noxious somatic stimulation elicits responses that are more varied and more complicated in intermediolateral interneurones than dorsal horn interneurones and the extent and nature of their receptive fields are more complex (Chau et al. 2000). Perhaps some of these interneurones also couple the two outflows in a co-ordinated response to an aversive stimulus? Exercise and response to noxious stimuli also involve the co-ordination of respiratory and cardiovascular variables and much research has been carried out to identify regions of cardiorespiratory integration. At the upper thoracic spinal cord level, interneurones, both those that exhibit respiratory-related activity (Qin et al. 2002) and those that were not classified as underlying a particular function (Euchner-Wamser et al. 1993, 1994), respond to painful visceral and somatic stimuli and these are located in the regions of the spinal cord where sympathetic interneurones have been identified. An attractive conjecture is that these project both to motoneurones involved in respiration and SPNs.
Conclusion
This review has considered evidence that interneurones form a vital link in the spinal cord circuitry involved in autonomic control. It seems that the impact that interneurones have in co-ordinating and controlling both autonomic and perhaps motor activity is yet to be fully appreciated and will form the basis for many future studies. Such an understanding may lead to clearer paths for control of aberrant responses when our vital homeostatic processes are disturbed.
Acknowledgments
I would like to thank Jim Deuchars for his critical input to this chapter and to all the people, past and present, who have contributed to our research on interneurones. The generous support of the British Heart Foundation (grant number PG/04/125/17971) and The Wellcome Trust is gratefully acknowledged.
References
- Bacon SJ, Smith AD. A monosynaptic pathway from an identified vasomotor centre in the medial prefrontal cortex to an autonomic area in the thoracic spinal cord. Neuroscience. 1993;54:719–728. doi: 10.1016/0306-4522(93)90242-8. [DOI] [PubMed] [Google Scholar]
- Barman SM, Gebber GL. Spinal interneurons with sympathetic nerve-related activity. Am J Physiol. 1984;247:R761–R767. doi: 10.1152/ajpregu.1984.247.5.R761. [DOI] [PubMed] [Google Scholar]
- Brooke RE, Deuchars J, Deuchars SA. Input-specific modulation of neurotransmitter release in the lateral horn of the spinal cord via adenosine receptors. J Neurosci. 2004;24:127–137. doi: 10.1523/JNEUROSCI.4591-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke RE, Pyner S, McLeish P, Buchan S, Deuchars J, Deuchars SA. Spinal cord interneurones labelled transneuronally from the adrenal gland by a GFP-herpes virus construct contain the potassium channel subunit Kv3.1b. Auton Neurosci. 2002;98:45–50. doi: 10.1016/s1566-0702(02)00030-9. [DOI] [PubMed] [Google Scholar]
- Cabot JB, Alessi V, Carroll J, Ligorio M. Spinal cord lamina V and lamina VII interneuronal projections to sympathetic preganglionic neurons. J Comp Neurol. 1994;347:515–530. doi: 10.1002/cne.903470404. [DOI] [PubMed] [Google Scholar]
- Cammack C, Logan SD. Excitation of rat sympathetic preganglionic neurones by selective activation of the NK1 receptor. J Auton Nerv Syst. 1996;57:87–92. doi: 10.1016/0165-1838(95)00103-4. [DOI] [PubMed] [Google Scholar]
- Chau D, Johns DG, Schramm LP. Ongoing and stimulus-evoked activity of sympathetically correlated neurons in the intermediate zone and dorsal horn of acutely spinalized rats. J Neurophysiol. 2000;83:2699–2707. doi: 10.1152/jn.2000.83.5.2699. [DOI] [PubMed] [Google Scholar]
- Chizh BA, Headley PM, Paton JF. Coupling of sympathetic and somatic motor outflows from the spinal cord in a perfused preparation of adult mouse in vitro. J Physiol. 1998;508:907–918. doi: 10.1111/j.1469-7793.1998.907bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembowsky K, Czachurski J, Seller H. An intracellular study of the synaptic input to sympathetic preganglionic neurones of the third thoracic segment of the cat. J Auton Nerv Syst. 1985;13:201–244. doi: 10.1016/0165-1838(85)90012-8. [DOI] [PubMed] [Google Scholar]
- Deuchars SA, Brooke RE, Deuchars J. Adenosine A1 receptors reduce release from excitatory but not inhibitory synaptic inputs onto lateral horn neurons. J Neurosci. 2001a;21:6308–6320. doi: 10.1523/JNEUROSCI.21-16-06308.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuchars SA, Brooke RE, Frater B, Deuchars J. Properties of interneurones in the intermediolateral cell column of the rat spinal cord: role of the potassium channel subunit Kv3.1. Neuroscience. 2001b;106:433–446. doi: 10.1016/s0306-4522(01)00277-9. [DOI] [PubMed] [Google Scholar]
- Deuchars SA, Milligan CJ, Stornetta RL, Deuchars J. GABAergic neurons in the central region of the spinal cord: a novel substrate for sympathetic inhibition. J Neurosci. 2005;25:1063–1070. doi: 10.1523/JNEUROSCI.3740-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun NJ, Mo N. In vitro effects of substance P on neonatal rat sympathetic preganglionic neurones. J Physiol. 1988;399:321–333. doi: 10.1113/jphysiol.1988.sp017083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun NJ, Mo N. Inhibitory postsynaptic potentials in neonatal rat sympathetic preganglionic neurones in vitro. J Physiol. 1989;410:267–281. doi: 10.1113/jphysiol.1989.sp017532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euchner-Wamser I, Meller ST, Gebhart GF. A model of cardiac nociception in chronically instrumented rats: behavioral and electrophysiological effects of pericardial administration of algogenic substances. Pain. 1994;58:117–128. doi: 10.1016/0304-3959(94)90191-0. [DOI] [PubMed] [Google Scholar]
- Euchner-Wamser I, Sengupta JN, Gebhart GF, Meller ST. Characterization of responses of T2–T4 spinal cord neurons to esophageal distension in the rat. J Neurophysiol. 1993;69:868–883. doi: 10.1152/jn.1993.69.3.868. [DOI] [PubMed] [Google Scholar]
- Gebber GL, McCall RB. Identification and discharge patterns of spinal sympathetic interneurons. Am J Physiol. 1976;231:722–733. doi: 10.1152/ajplegacy.1976.231.3.722. [DOI] [PubMed] [Google Scholar]
- Hinckley CA, Hartley R, Wu L, Todd A, Ziskind-Conhaim L. Locomotor-like rhythms in a genetically distinct cluster of interneurons in the mammalian spinal cord. J Neurophysiol. 2005;93:1439–1449. doi: 10.1152/jn.00647.2004. [DOI] [PubMed] [Google Scholar]
- Jansen AS, Wessendorf MW, Loewy AD. Transneuronal labeling of CNS neuropeptide and monoamine neurons after pseudorabies virus injections into the stellate ganglion. Brain Res. 1995;683:1–24. doi: 10.1016/0006-8993(95)00276-v. [DOI] [PubMed] [Google Scholar]
- Jasmin L, Carstens E, Basbaum AI. Interneurons presynaptic to rat tail-flick motoneurons as mapped by transneuronal transport of pseudorabies virus: few have long ascending collaterals. Neuroscience. 1997;76:859–876. doi: 10.1016/s0306-4522(96)00384-3. [DOI] [PubMed] [Google Scholar]
- Kerman IA, Enquist LW, Watson SJ, Yates BJ. Brainstem substrates of sympatho-motor circuitry identified using trans-synaptic tracing with pseudorabies virus recombinants. J Neurosci. 2003;23:4657–4666. doi: 10.1523/JNEUROSCI.23-11-04657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JM, Plowey ED, Beatty JA, Little HR, Waldrop TG. Hypothalamus, hypertension, and exercise. Brain Res Bull. 2000;53:77–85. doi: 10.1016/s0361-9230(00)00311-7. [DOI] [PubMed] [Google Scholar]
- Krassioukov AV, Johns DG, Schramm LP. Sensitivity of sympathetically correlated spinal interneurons, renal sympathetic nerve activity, and arterial pressure to somatic and visceral stimuli after chronic spinal injury. J Neurotrauma. 2002;19:1521–1529. doi: 10.1089/089771502762300193. [DOI] [PubMed] [Google Scholar]
- Krout KE, Mettenleiter TC, Loewy AD. Single CNS neurons link both central motor and cardiosympathetic systems: a double-virus tracing study. Neuroscience. 2003;118:853–866. doi: 10.1016/s0306-4522(02)00997-1. [DOI] [PubMed] [Google Scholar]
- Krupp J, Feltz P. Synaptic- and agonist-induced chloride currents in neonatal rat sympathetic preganglionic neurones in vitro. J Physiol. 1993;471:729–748. doi: 10.1113/jphysiol.1993.sp019925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DI, Coote JH. Angiotensin II in the spinal cord of the rat and its sympatho-excitatory effects. Brain Res. 1993;614:1–9. doi: 10.1016/0006-8993(93)91010-p. [DOI] [PubMed] [Google Scholar]
- Lewis DI, Sermasi E, Coote JH. Excitatory and indirect inhibitory actions of 5-hydroxytryptamine on sympathetic preganglionic neurones in the neonate rat spinal cord in vitro. Brain Res. 1993;610:267–275. doi: 10.1016/0006-8993(93)91410-t. [DOI] [PubMed] [Google Scholar]
- Llewellyn-Smith IJ, Weaver LC. Changes in synaptic inputs to sympathetic preganglionic neurons after spinal cord injury. J Comp Neurol. 2001;435:226–240. doi: 10.1002/cne.1204. [DOI] [PubMed] [Google Scholar]
- McCall RB, Gebber GL, Barman SM. Spinal interneurons in the baroreceptor reflex arc. Am J Physiol. 1977;232:H657–H665. doi: 10.1152/ajpheart.1977.232.6.H657. [DOI] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Serotonin potentiates sympathetic responses evoked by spinal NMDA. J Physiol. 2006;577:525–537. doi: 10.1113/jphysiol.2006.116574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marina N, Taheri M, Gilbey MP. Generation of a physiological sympathetic motor rhythm in the rat following spinal application of 5-HT. J Physiol. 2006;571:441–450. doi: 10.1113/jphysiol.2005.100677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CO, Johns DG, Schramm LP. Spinal interneurons play a minor role in generating ongoing renal sympathetic nerve activity in spinally intact rats. Brain Res. 2001;918:101–106. doi: 10.1016/s0006-8993(01)02965-1. [DOI] [PubMed] [Google Scholar]
- Miyazaki T, Coote JH, Dun NJ. Excitatory and inhibitory effects of epinephrine on neonatal rat sympathetic preganglionic neurons in vitro. Brain Res. 1989;497:108–116. doi: 10.1016/0006-8993(89)90976-1. [DOI] [PubMed] [Google Scholar]
- Pan B, Kim EJ, Schramm LP. Increased close appositions between corticospinal tract axons and spinal sympathetic neurons after spinal cord injury in rats. J Neurotrauma. 2005;22:1399–1410. doi: 10.1089/neu.2005.22.1399. [DOI] [PubMed] [Google Scholar]
- Petras JM, Cummings JF. Autonomic neurons in the spinal cord of the Rhesus monkey: a correlation of the findings of cytoarchitectonics and sympathectomy with fiber degeneration following dorsal rhizotomy. J Comp Neurol. 1972;146:189–218. doi: 10.1002/cne.901460205. [DOI] [PubMed] [Google Scholar]
- Qin C, Chandler MJ, Foreman RD, Farber JP. Upper thoracic respiratory interneurons integrate noxious somatic and visceral information in rats. J Neurophysiol. 2002;88:2215–2223. doi: 10.1152/jn.00120.2002. [DOI] [PubMed] [Google Scholar]
- Sermasi E, Coote JH. Oxytocin acts at V1 receptors to excite sympathetic preganglionic neurones in neonate rat spinal cord in vitro. Brain Res. 1994;647:323–332. doi: 10.1016/0006-8993(94)91331-5. [DOI] [PubMed] [Google Scholar]
- Spanswick D, Pickering AE, Gibson IC, Logan SD. Inhibition of sympathetic preganglionic neurons by spinal glycinergic interneurons. Neuroscience. 1994;62:205–216. doi: 10.1016/0306-4522(94)90325-5. [DOI] [PubMed] [Google Scholar]
- Strack AM, Loewy AD. Pseudorabies virus: a highly specific transneuronal cell body marker in the sympathetic nervous system. J Neurosci. 1990;10:2139–2147. doi: 10.1523/JNEUROSCI.10-07-02139.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack AM, Sawyer WB, Hughes JH, Platt KB, Loewy AD. A general pattern of CNS innervation of the sympathetic outflow demonstrated by transneuronal pseudorabies viral infections. Brain Res. 1989a;491:156–162. doi: 10.1016/0006-8993(89)90098-x. [DOI] [PubMed] [Google Scholar]
- Strack AM, Sawyer WB, Platt KB, Loewy AD. CNS cell groups regulating the sympathetic outflow to adrenal gland as revealed by transneuronal cell body labeling with pseudorabies virus. Brain Res. 1989b;491:274–296. doi: 10.1016/0006-8993(89)90063-2. [DOI] [PubMed] [Google Scholar]
- Tang X, Neckel ND, Schramm LP. Locations and morphologies of sympathetically correlated neurons in the T10 spinal segment of the rat. Brain Res. 2003;976:185–193. doi: 10.1016/s0006-8993(03)02601-5. [DOI] [PubMed] [Google Scholar]
- Tang X, Neckel ND, Schramm LP. Spinal interneurons infected by renal injection of pseudorabies virus in the rat. Brain Res. 2004;1004:1–7. doi: 10.1016/j.brainres.2004.01.016. [DOI] [PubMed] [Google Scholar]
- Todd AJ. Anatomy of primary afferents and projection neurones in the rat spinal dorsal horn with particular emphasis on substance P and the neurokinin 1 receptor. Exp Physiol. 2002;87:245–249. doi: 10.1113/eph8702351. [DOI] [PubMed] [Google Scholar]
- Weaver LC, Cassam AK, Krassioukov AV, Llewellyn-Smith IJ. Changes in immunoreactivity for growth associated protein-43 suggest reorganization of synapses on spinal sympathetic neurons after cord transection. Neuroscience. 1997;81:535–551. doi: 10.1016/s0306-4522(97)00151-6. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Hartley R, Maxwell DJ, Todd AJ, Lieberam I, Kaltschmidt JA, Yoshida Y, Jessell TM, Brownstone RM. Conditional rhythmicity of ventral spinal interneurons defined by expression of the Hb9 homeodomain protein. J Neurosci. 2005;25:5710–5719. doi: 10.1523/JNEUROSCI.0274-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyszogrodski I, Polosa C. The inhibition of sympathetic preganglionic neurons by somatic afferents. Can J Physiol Pharmacol. 1973;51:29–38. doi: 10.1139/y73-005. [DOI] [PubMed] [Google Scholar]
- Zhong G, Diaz-Rios M, Harris-Warrick RM. Intrinsic and functional differences among commissural interneurons during fictive locomotion and serotonergic modulation in the neonatal mouse. J Neurosci. 2006;26:6509–6517. doi: 10.1523/JNEUROSCI.1410-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]