Abstract
Many different receptors can stimulate cAMP synthesis in the heart, but not all elicit the same functional responses. For example, it has been recognized for some time that prostaglandins such as PGE1 increase cAMP production and activate PKA, but they do not elicit responses like those produced by β-adrenergic receptor (βAR) agonists such as isoproterenol (isoprenaline), even though both stimulate the same signalling pathway. In the present study, we confirm that isoproterenol, but not PGE1, is able to produce cAMP-dependent stimulation of the L-type Ca2+ current in guinea pig ventricular myocytes. This is despite finding evidence that these cells express EP4 prostaglandin receptors, which are known to activate Gs-dependent signalling pathways. Using fluorescence resonance energy transfer-based biosensors that are either freely diffusible or bound to A kinase anchoring proteins, we demonstrate that the difference is due to the ability of isoproterenol to stimulate cAMP production in cytosolic and caveolar compartments of intact cardiac myocytes, while PGE1 only stimulates cAMP production in the cytosolic compartment. Unlike other receptor-mediated responses, compartmentation of PGE1 responses was not due to concurrent activation of a Gi-dependent signalling pathway or phosphodiesterase activity. Instead, compartmentation of the PGE1 response in cardiac myocytes appears to be due to transient stimulation of cAMP in a microdomain that can communicate directly with the bulk cytosolic compartment but not the caveolar compartment associated with βAR regulation of L-type Ca2+ channel function.
The second messenger cAMP is involved in regulating a variety of responses in virtually every cell in our bodies. In the heart, multiple receptors are coupled to the activation of adenylyl cyclase and cAMP production through the stimulatory G protein (Gs). However, much of what we know about this signalling pathway has come from studying β1-adrenergic receptor (β1AR) responses, because it is production of cAMP following activation of this receptor that is responsible for the acute effects associated with sympathetic regulation of cardiac electrical, mechanical and metabolic activity (Bers, 2001).
Although many different receptors can stimulate cAMP synthesis in the heart, not all elicit the same responses. For example, prostaglandins such as PGE1 increase cAMP production and activate PKA, but they do not elicit acute functional responses like those produced by βAR agonists, even though both stimulate the same signalling pathway (Keely, 1977, 1979; Brunton et al. 1979, 1981; Hayes et al. 1979, 1980). While βAR agonists enhance contractility and promote glycogen metabolism, PGE1 produces neither of these effects. This can be attributed to the ability of βAR agonists, but not PGE1, to stimulate PKA-dependent phosphorylation of specific proteins, such as glycogen phosphorylase and troponin I.
The disparity in cAMP-dependent responses produced by various agonists has been attributed to the ability of different receptors to stimulate cAMP production that is localized to distinct subcellular compartments (Steinberg & Brunton, 2001). This conclusion is supported by work using biochemical methods to demonstrate that βAR activation stimulates cAMP production and PKA activation in both particulate (membrane associated) and soluble (cytosolic) fractions of homogenized cardiac preparations, while PGE1 only stimulates cAMP production and PKA activation in the soluble fraction (Hayes et al. 1979, 1980; Hayes & Brunton, 1982; Buxton & Brunton, 1983). Yet how or even if this relates to what is happening in intact, living myocytes is not known.
The idea that an agonist does not lead to uniform production of cAMP throughout the cell might seem intuitively obvious, yet it is still not entirely understood how it is achieved. One impediment to a clearer understanding of PGE1 responses in cardiac myocytes has been the lack of information on the exact type of receptor(s) involved. PGE1 can produce effects through the activation of E-type prostaglandin (EP) receptors, of which there are four subtypes (Bos et al. 2004). EP2 and EP4 receptors are typically associated with Gs-dependent production of cAMP, while EP1 receptors activate Gq, and EP3 receptors activate Gi signalling pathways. There is also evidence that the EP4 receptor may actually couple to both Gs and Gi signalling pathways (Fujino & Regan, 2006). This is similar to the β2AR, which is also known to produce compartmentation of cAMP-dependent responses in cardiac myocytes. In fact, it is the coupling to Gi that has been proposed to be responsible for the compartmentation of β2AR responses (Xiao, 2001). Although PGE1 production of cAMP is likely to involve either EP2 or EP4 receptors, it is not known if compartmentation involves the parallel activation of Gi through either the EP3 or EP4 receptor. Phosphodiesterases (PDEs) are also known to restrict free diffusion of cAMP in cardiac myocytes (Jurevicius et al. 2003; Mongillo et al. 2004), and it has been proposed that PDE activity plays a role in compartmentation of responses to PGE1 (Steinberg & Brunton, 2001).
Another impediment to better understanding cAMP signalling mechanisms has been the absence of tools capable of monitoring changes in cAMP activity in different subcellular compartments of intact cardiac myocytes. This problem has recently been addressed by the development of genetically encoded biosensors that respond directly to changes in cAMP levels (Zaccolo et al. 2000; Rich et al. 2001; Nikolaev et al. 2004). The present study demonstrates that it is possible to distinguish between the effects of prostaglandin and βAR stimulation in intact cardiac myocytes by using fluorescence resonance energy transfer (FRET)-based biosensors that are either freely diffusible throughout the cytoplasm or anchored to signalling domains within the cell. We then tested the hypothesis that acute functional responses are associated with changes in cAMP activity specifically within these signalling domains and that the inability of PGE1 to elicit such responses is due to compartmentation of cAMP involving a Gi-dependent signalling pathway and/or PDE activity.
Methods
Isolation and primary culture of cardiac myocytes
Hearts from adult Hartley guinea pigs were used to isolate cardiac ventricular myocytes as previously described (Belevych et al. 2001; Warrier et al. 2005). The methods were in accordance with The Guide for the Care and Use of Laboratory Animals, as adopted by the National Institutes of Health and approved by the Institutional Animal Care and Use Committee at Case Western Reserve University. Briefly, guinea pigs were anaesthetized by intraperitoneal injection of pentobarbital (150 mg kg−1). Hearts were quickly excised and the coronary arteries perfused via the aorta with physiological salt solution (PSS) containing (mm): NaCl 140, KCl 5.4, MgCl2 2.5, CaCl2 1.5, glucose 11 and Hepes 5.5 (pH 7.4). After perfusion with nominally Ca2+-free PSS for 5 min, enough collagenase (Type II; Worthington) was added to achieve a final concentration of ∼0.5 mg ml−1. After 20–35 min of digestion at 37°C, the ventricles were removed and placed in a Kraft–Brühe solution. The tissue was then minced and single myocytes were obtained by filtering through a 100 μm nylon mesh.
Myocytes were maintained in serum-free, Dulbecco's modified Eagle's medium (DMEM; Gibco-Invitrogen) and infected using an MOI of 50–100 for each virus. Experiments were conducted 48–72 h after infection. For experiments involving the use of membrane-permeable Ht31 or Ht31P peptides, cells expressing the PKA-based sensor were pretreated with peptide (20 μm) for at least 45 min at 37°C before use. For experiments involving PTX, cells were treated with the toxin (2 μg ml−1) for at least 2 h at 37°C before use.
mRNA profile analysis
RNA was prepared from isolated ventricular myocytes using Tripure RNA isolation reagent (Roche). PCR was carried out using 0.2 μg of total RNA and M-MuLV reverse transcriptase (New England Biolabs). The resulting cDNA was amplified by 35 polymerase chain reaction (PCR) cycles using an annealing temperature of 60°C with primer sets specific for each EP receptor. As the guinea pig EP receptors have not been cloned, previously published primer sequences for mouse receptors were used (Ma et al. 2001). The 5′- and 3′-sequences used were as follows: EP1, 5′-CGCAGGGTTCACGCACACGA-3′, 5′-CACTGTGCCGGGAACTAC-GC-3′; EP2, 5′-AGGA-CTTCGATGGCAGAGAGAC-3′, 5′-CAGCCCCTTACA-CTTCTC-CAATG-3′; EP3, 5′-GGTATGCCAGCCACAA-TGAAGAC-3′, 5′-CAAGATCTGGTTCAG-CGAAGCC-3′; and EP4, 5′-TTCCGCTCGTGGTGCGAGTGTTC-3′, 5′-GAGGTGGTGTC-TGCTTGGGTCAG-3′. RT-PCR of EP receptor mRNA from the kidney, which is known to express all four EP receptor isoforms (Breyer & Breyer, 2000) was used as a positive control.
Electrophysiology
Whole-cell L-type Ca2+ currents were recorded using the conventional patch clamp technique, as previously described (Belevych et al. 2001). Microelectrodes had resistances of 1–2 MΩ when filled with an intracellular solution containing (mm): CsCl 130, TEA-Cl 20, EGTA 5, MgATP 5, TrisGTP 0.06 and Hepes 5.5 (pH 7.2). Cells were bathed in a potassium-free extracellular solution that contained (mm): NaCl 140, CsCl 5.4, CaCl2 2.5, MgCl2 0.5, glucose 11 and Hepes 5.5 (pH 7.4) The potassium-free solutions containing TEA and Cs ensured the elimination of currents due to potassium channel activity. The voltage clamp protocol employed a holding potential of −80 mV. A 50 ms prepulse to −30 mV was used to inactivate Na+ channels. Changes in the magnitude of the Ca2+ current were monitored by applying a 100 ms test pulse to 0 mV once every 15–20 s. The amplitude of the Ca2+ current was determined by measuring the absolute magnitude of the peak inward current during the step depolarization to 0 mV.
Preparation of adenoviruses
Adenoviruses encoding the recombinant subunits of type II PKA tagged to cyan fluorescent protein (CFP) and yellow fluorescent protein (YFP) were created using the Clontech Adeno-X expression system (BD Biosciences), as previously described (Warrier et al. 2005). The pcDNA3-EYFP-Epac2(murine)-ECFP vector (Nikolaev et al. 2004) was used to construct an adenovirus expressing the exchange protein activated by cAMP (Epac)-based probe. Viruses were amplified in HEK 293 cells, purified by CsCl gradient centrifugation, and titres determined by standard plaque assay.
Fluorescence imaging
Images were obtained using an inverted microscope (Olympus IX70) equipped with a 40 × water immersion objective (1.3 NA, Olympus) and a CCD camera (Hamamatsu Orca ER). CFP excitation (0.5–2 s) was achieved using a 175 W xenon arc lamp (Lambda DG-4, Sutter Instruments) and a D436/20 bandpass filter with a 455DCLP dichroic mirror. CFP and YFP (FRET) emissions were measured using D480/30 and D535/40 bandpass filters, respectively. For ratiometric FRET experiments, CFP and YFP emissions were measured simultaneously using a Dual View Micro-Imager (Optical Insights) equipped with 505CXR beamsplitter. Fluorescence images were acquired using 2 × 2 binning and analysed using Simple PCI imaging software (Compix Inc.). Changes in cAMP activity were defined as the relative change in the ratio of the background corrected fluorescence intensity at the emission wavelength for CFP and YFP measured over the area of the entire cell.
Drugs and peptides
PGE1 (Cayman Chemicals) and IBMX (3-isobutyl-1-methylxanthine, Calbiochem) were prepared as stock solutions in DMSO. Stearated Ht31 peptide, control Ht31P peptide (Promega, Inc.), PTX (List Biochemicals), and isoproterenol bitartrate (Sigma RBI) were prepared as aqueous stock solutions. Isoproterenol stock and working solutions were prepared fresh daily. Ascorbic acid was added to the solutions to prevent oxidation.
Statistics
All data are expressed as the mean ± s.e.m. of the results obtained from the indicated number (n) of cells. Statistical significance between groups was defined by paired Student's t test with P values < 0.05.
Results
Which EP receptors are expressed in ventricular myocytes?
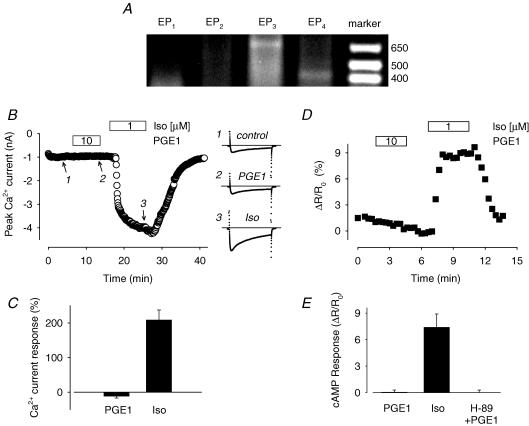
PGE1 has been reported to increase levels of cAMP in cardiac tissue from various species including the guinea pig (Hayes et al. 1980). However, the identity of the receptor(s) mediating this response is unknown. We searched for some evidence that EP receptors capable of regulating cAMP production are expressed in guinea pig ventricular myocytes. RT-PCR analysis was performed using mRNA obtained from isolated guinea pig ventricular myocytes; mRNA isolated from the kidney was used as a positive control. Amplification products of the size expected for all four receptor subtypes (EP1, 334 bp; EP2, 400 bp; EP3, 600 bp; and EP4, 420 bp) were detected in the kidney samples (data not shown). However, there was only evidence for expression of EP3 and EP4 receptor message in the samples isolated from ventricular myocytes (Fig. 1A). This suggests that EP4 receptor activation of Gs is most probably responsible for any increase in cAMP produced by exposure of these cells to PGE1. However, these results also leave open the possibility that activation of Gi, by either the EP3 receptor or the EP4 receptor itself, could modify the cAMP response.
Figure 1. Guinea pig ventricular myocytes express EP receptors, but PGE1 produces no detectable responses.
A, message for EP3 and EP4 prostaglandin receptors identified by RT-PCR. Representative result from one of three preparations. B, time course of changes in amplitude of the L-type Ca2+ current and examples of current traces under control conditions (1), during exposure to 10 μm PGE1 (2), and during exposure to 1 μM Iso (3). C, average increase in Ca2+ current amplitude recorded in the presence of 10 μM PGE and following subsequent exposure to 1 μM Iso (n = 5). D, time course of changes in the CFP/YFP emission intensity ratio produced by the PKA-based biosensor during exposure of an isolated myocyte to 10 μm PGE1 and 1 μm Iso. E, average change in CFP/YFP emission intensity ratio produced by 10 μM PGE1 and subsequent exposure to 1 μM Iso in untreated cells (n = 5).
Effect of PGE1 on L-type Ca2+ channel responses
PKA-dependent phosphorylation and subsequent stimulation of L-type Ca2+ channel activity plays a pivotal role in regulating cardiac electrical and mechanical activity (Bers, 2001). Thus, monitoring changes in the L-type Ca2+ current is an ideal means of evaluating the effect of cAMP signalling on functional responses in single cardiac myocytes. So we looked at the response to what should be a maximally effective concentration of PGE1 (Buxton & Brunton, 1983) and compared it to the response elicited by a maximally stimulating concentration of the βAR agonist isoproterenol (Iso). Exposure to 10 μm PGE1 had no effect (−12 ± 4.8%, P > 0.5, n = 5), despite the fact that in the same cells, 1 μm Iso increased the magnitude of Ca2+ current by 208 ± 29% (P < 0.005) over baseline (Fig. 1B and C). This result confirms the idea that the lack of PGE1 effect on functional responses in the heart includes cAMP-dependent regulation of the L-type Ca2+ channel in ventricular myocytes (Alloatti et al. 1991).
Does PGE1 produce compartmentation of cAMP in intact myocytes?
For direct evidence that PGE1 stimulates cAMP production in these cells, we first used a PKA-based cAMP biosensor (Fig. 1D and E). This probe consists of the catalytic subunit of PKA tagged with enhanced yellow fluorescent protein (Cat-YFP) and the type II regulatory subunit of PKA tagged with enhanced cyan fluorescent protein (RII-CFP) (Zaccolo & Pozzan, 2002). In the absence of cAMP, FRET occurs. However, when cAMP binds to the regulatory subunit, the catalytic subunit is released, resulting in a loss of FRET. As a result, an increase in cAMP levels produces an increase in the CFP/YFP emission ratio that can be used to monitor changes in cAMP activity in intact myocytes.
The constructs for this probe were introduced into acutely isolated, adult guinea pig ventricular myocytes using adenoviruses. Previously, we demonstrated that this system is capable of detecting changes in cAMP activity in response to physiological levels of βAR stimulation. Furthermore, the relative magnitude of the agonist-induced cAMP responses detected using this system were shown to correspond directly with the magnitude of agonist-induced Ca2+ channel responses (Warrier et al. 2005). Consistent with the fact that PGE1 had no effect on the Ca2+ current in these cells, we found that exposure to PGE1 at concentrations of up to 10 μm did not produce a detectable change in the emission ratio (0.047 ± 0.25%, P > 0.5, n = 5), even though subsequent exposure of the same cells to 1 μm Iso elicited a significant increase (7.4 ± 1.5%, P < 0.005). To rule out the possibility that this lack of response to PGE1 was due to an increase in PKA activity associated with overexpression of the catalytically active probe, these experiments were repeated in the presence of the PKA inhibitor H-89. However, there was still no significant change in the emission ratio upon exposure to 10 μm PGE1 (−0.004 ± 0.29, P > 0.5, n = 7).
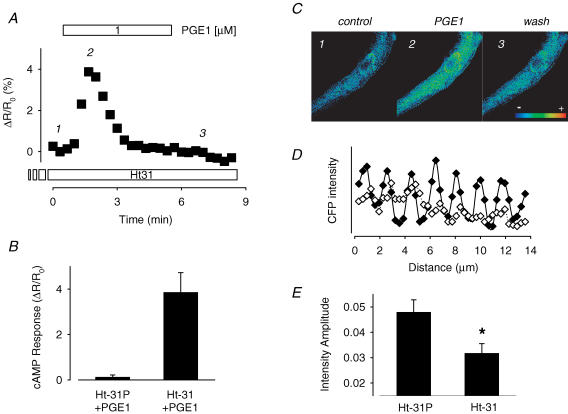
Although the lack of a detectable response was not unexpected, the question still remained as to whether or not PGE1 stimulates cAMP production at all. If it does, the reason we may not have detected it using this PKA-based probe is because this sensor was constructed using the RII subunit of PKA, which binds to A kinase anchoring proteins (AKAPs). AKAPs are known to create signalling complexes that bring PKA together with target proteins such as the Ca2+ channel (Hulme et al. 2003). This explains the localization of the PKA-based probe along the Z-line of cardiac myocytes (Zaccolo & Pozzan, 2002; Warrier et al. 2005), just like endogenous type II PKA (Yang et al. 1998). Furthermore, type II PKA is specifically associated with the particulate fraction of cardiac myocytes (Corbin & Keely, 1977). Since PGE1 has been reported to stimulate cAMP production in the soluble fraction of cardiac preparations and not the particulate fraction, this would explain why we did not detect a response. To test this hypothesis, we exposed our cells to a membrane-permeable form of the Ht31 peptide, which mimics the region of the AKAP protein known to bind RII subunits and prevents PKA anchoring (Zaccolo & Pozzan, 2002). We verified that treatment with the Ht31 peptide disrupts localization of the PKA-based probe by analysing the CFP fluorescence intensity profile of the exogenous RII subunit along the length of the cell (Fig. 2D and E). Control cells were treated with the Ht31P peptide, which contains a proline substitution that prevents its interaction with AKAP binding domains. In these cells, the CFP fluorescence intensity profile exhibited a sinusoidal pattern reflecting the concentration of the RII subunit of the probe along the Z-lines, as previously described (Warrier et al. 2005). Cells treated with the Ht31 peptide exhibited a similar pattern. However, the absolute amplitude of the peak fluorescence intensity at the Z-lines was significantly smaller than that observed in cells treated with Ht31P (P < 0.05). This indicates that a significant fraction of the probe was released from its anchoring sites along the Z-lines.
Figure 2. Blocking AKAP-interaction reveals PGE1-induced changes in cAMP activity detected by the PKA-based sensor.
A, time course of changes in CFP/YFP emission intensity ratio during exposure of an Ht31-treated myocyte to 1 μM PGE1. The intensity ratio is an average of the values measured across the entire cell. B, average change in CFP/YFP emission intensity ratio produced by 1 μM PGE1 in cells treated with inactivate, Ht31P peptide (n = 4) or active Ht31 peptide (n = 4). C, pseudocolour images illustrating the CFP/YFP emission ratio in a cell treated with Ht31 peptide before (1) and during the peak (2) and steady-state response (3) to 1 μM PGE1. D, fluorescence intensity profile of CFP emission measured across a 3 μm × 14 μm region of a cell treated with control Ht31P (♦) or active Ht31 peptide (⋄). E, average peak amplitude of the CFP fluorescence measured at the Z-lines in a 3 μm × 14 μm region of cells treated with control Ht31P (n = 4) or active Ht31 peptide (n = 4).
In cells pretreated with Ht31, subsequent exposure to 1–10 μm PGE1 produced a significant albeit transient response (Fig. 2A–C). The average peak increase in the CFP/YFP emission ratio produced by 1 μm PGE1 was 3.9 ± 0.87% (P < 0.5, n = 5) over baseline. No such response was detected in experiments using cells exposed to the control Ht31P peptide (P > 0.5, n = 4). These results are consistent with the idea that a significant fraction of the probe had indeed been released from its anchoring sites and diffused to the location where cAMP was being produced.
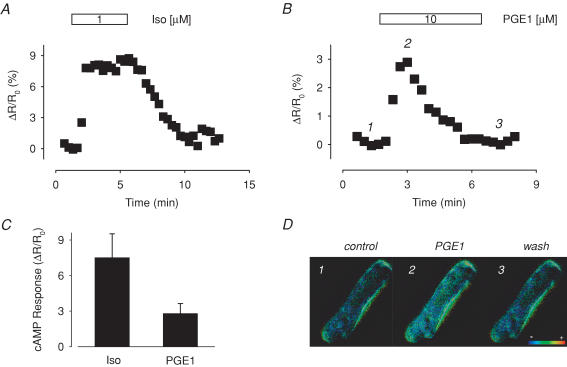
To confirm the above observation, we examined the response to PGE1 using a FRET-based probe that was created using the exchange protein activated by cAMP (Epac) (Nikolaev et al. 2004) (Fig. 3). CFP and YFP are attached to the carboxy and amino termini of the cAMP binding domain. The structure of this construct is such that FRET occurs when cAMP is not bound, and there is a loss of FRET when cAMP binds. So again, the CFP/YFP emission ratio can be used to detect changes in cAMP activity. However, this Epac-based probe does not contain any of the anchoring sequences found in the full length protein, so that it can freely diffuse throughout the cell. Therefore, we predicted that this probe should detect a response to PGE1 that is similar to the one sensed by the PKA-based probe in cells treated with the Ht31 peptide.
Figure 3. PGE1 induced changes in cAMP detected by the Epac-based sensor.
A, time course of changes in the CFP/YFP intensity ratio during exposure of an isolated myocyte to 1 μm Iso. B, time course of changes in CFP/YFP intensity ratio during exposure of an isolated myocyte to 10 μm PGE1. The intensity ratio is an average of the values measured across the entire cell. C, average change in CFP/YFP emission intensity ratio produced by exposure to 1 μM Iso (n = 8) and 10 μM PGE1 (n = 8) D, pseudocolour images illustrating the CFP/YFP emission ratio before (1) and during the (2) and steady-state response (3) to 10 μm PGE1.
In cells infected with adenovirus encoding the Epac-based sensor, exposure to Iso produced a significant increase in the CFP/YFP emission ratio that reversed upon washout of the agonist. In the presence of 1 μm Iso, the emission ratio increased by 7.5 ± 0.22% over baseline (P < 0.05, n = 9). Furthermore, exposure to PGE1 also produced a significant effect. Like the response detected by the PKA-based probe in cells treated with Ht31 peptide, the PGE1 response sensed by the Epac-based probe was transient. In the presence of 10 μm PGE1, the magnitude of the peak response was 2.8 ± 0.84% (P < 0.005, n = 8). These results clearly indicate that PGE1 does stimulate cAMP production in isolated ventricular myocytes, but that some means of compartmentation prevents it from eliciting functional responses, such as those associated with regulation of L-type Ca2+ channel activity. The question then is what is responsible for that compartmentation?
Is Gi activation involved in compartmentation of cAMP responses produced by PGE1?
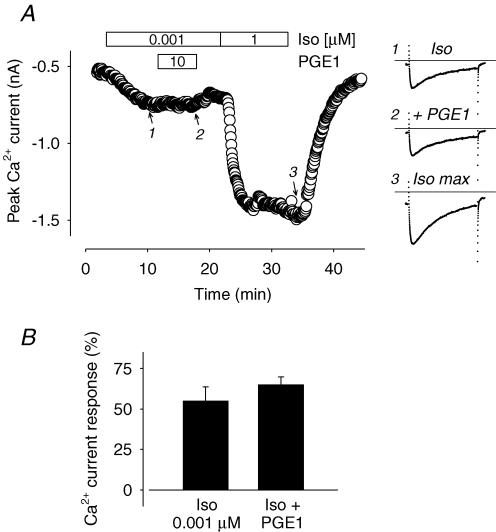
As indicated by the mRNA profile analysis, there is evidence for the expression of Gi coupled EP receptor subtypes in guinea pig ventricular myocytes (see Fig. 1A). Therefore, it is possible that PGE1 is able to produce a Gi-dependent inhibitory effect that masks any stimulatory response that might otherwise be detected as a change in Ca2+ channel function or fluorescence emission intensity ratio of the PKA-based cAMP sensor. If this is occurring, exposure to PGE1 should antagonize or inhibit the stimulatory response to βAR activation. Consistent with this possibility, PGE1 has been reported to inhibit cAMP-dependent regulation of Ca2+ channel function in rabbit atrial myocytes (Yamamoto et al. 1999). However, we found that exposure to 10 μm PGE1 did not inhibit the stimulatory response to a submaximal concentration of Iso (Fig. 4). Exposure to 1 nm Iso alone stimulated the Ca2+ current by 55 ± 8.7% (n = 5) over baseline, and subsequent addition of 10 μm PGE1 produced no inhibitory effect. The magnitude of the Ca2+ current recorded in the presence of Iso plus PGE1 was 65 ± 4.8% (P > 0.5) over baseline.
Figure 4. PGE1 does not produce a Gi-dependent inhibitory effect on βAR stimulation of the L-type Ca2+ current.
A, time course of changes in the amplitude of the L-type Ca2+ current and example of current traces recorded during exposure to 0.001 μm Iso (1), 10 μm PGE1 plus 0.001 μm Iso (2), and 1 μm Iso (3). B, average increase in amplitude of the L-type Ca2+ current recorded in the presence of 0.001 μm Iso and upon subsequent addition of 10 μm PGE1 (n = 5).
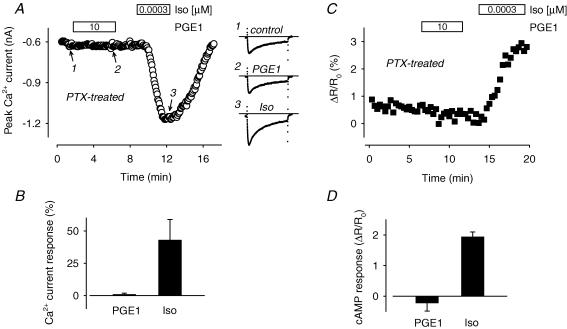
Even though there was no inhibition of βAR responses by PGE1, a Gi-mediated pathway could still conceivably restrict PGE1 production of cAMP to a compartment separate from where type II PKA and L-type Ca2+ channels are found. If this is true then pretreatment of cells with pertussis toxin (PTX), which inactivates the inhibitory G protein Gi, should remove this restriction. However, pretreatment with PTX did not unveil any stimulatory effect of PGE1 that could be detected by L-type Ca2+ channels or the PKA-based cAMP sensor (Fig. 5). In the presence of 10 μm PGE1, the Ca2+ current deviated from baseline by only 1.0 ± 0.94% (P > 0.5, n = 5) and the CFP/YFP emission ratio changed by only −0.23 ± 0.26% (P > 0.05, n = 3). Despite the lack of change in response to PGE1, PTX treatment was clearly effective. We have previously demonstrated that PTX treatment shifts the sensitivity of these cells to βAR stimulation (Belevych et al. 2001). Consistent with this known effect of PTX, the same cells that did not react to PGE1, did respond to a concentration of Iso that is normally subthreshold. Exposure to 0.3 nm Iso increased the Ca2+ current magnitude by 43 ± 16% (P < 0.05) over baseline and the CFP/YFP emission ratio by 1.9 ± 0.15% (P < 0.05) over baseline. These results further support the suggestion that activation of Gi is not responsible for compartmentation of PGE1 production of cAMP.
Figure 5. Pertussis toxin (PTX) inhibition of Gi does not reveal a PGE1 stimulatory effect.
A, time course of changes in the amplitude of the L-type Ca2+ current and examples of current traces recorded before (1) and during exposure of a PTX-treated myocyte to 10 μm PGE1 (1) and a normally subthreshold concentration (0.0003 μm) of Iso (3). B, average increase in the amplitude of the L-type Ca2+ current produced by 10 μm PGE1 and subsequent exposure to a subthreshold concentration of Iso (0.0003 μm) in PTX-treated myocytes (n = 5). C, time course of changes in the CFP/YFP emission intensity ratio during exposure of a PTX-treated myocyte to 10 μm PGE1 and 0.0003 μm Iso. D, average change in CFP/YFP emission intensity ratio produced by 10 μm PGE1 and subsequent exposure to 0.0003 μm Iso in PTX-treated cells (n = 3).
Is PDE activity involved in compartmentation of cAMP responses produced by PGE1?
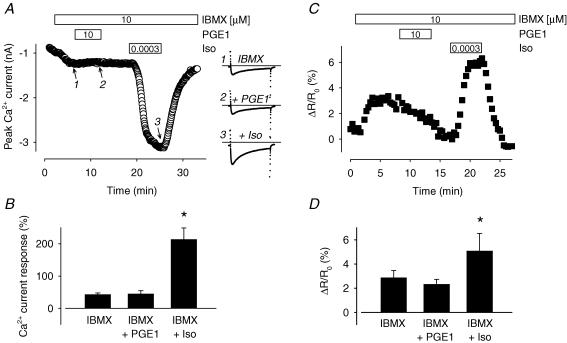
In isolated cardiac myocytes, βAR activation normally regulates Ca2+ channels that are in close proximity to the receptor. However, inhibition of PDE activity enables activation of at least certain receptors to regulate Ca2+ channels at more distant sites (Jurevicius et al. 2003). This indicates that PDE activity can prevent free diffusion of cAMP throughout cardiac myocytes. Such a mechanism could also explain the inability of cAMP produced by PGE1 in a microdomain associated with the cytosolic fraction of cardiac myocytes from producing a functional response in a microdomain associated with the membrane fraction of these cells. To evaluate this possibility, we looked for PGE1-induced responses in myocytes exposed to IBMX, a non-selective phosphodiesterase inhibitor (Fig. 6). We used a concentration of IBMX that would significantly inhibit all isoforms of PDE capable of hydrolysing cAMP in guinea pig ventricular myocytes without producing a maximal response (Bethke et al. 1992). Application of 10 μm IBMX by itself produced a submaximal increase of the L-type Ca2+ current (43 ± 4.7% increase over baseline) as well as the CFP/YFP emission intensity ratio in cells expressing the PKA-based probe (2.9 ± 0.59% increase over baseline). However, subsequent addition of PGE1 produced no significant effect. In the presence of IBMX plus 10 μm PGE1, the Ca2+ current magnitude was still only 45 ± 9.4% (P > 0.05) over baseline and the CFP/YFP emission intensity ratio was 2.3 ± 0.40% (P > 0.05) over baseline. Although there was a slight decrease in the magnitude of the PKA-based probe response during exposure to PGE1, the change was not statistically significant and it continued even after washout. This suggests that there was a slight time-dependent decay in the response to IBMX that was independent of exposure to PGE1. However, there was no indication that this masked any potential stimulatory effect, as shown by the ability of subsequent exposure to a normally subthreshold concentration of Iso to produce a near-maximal response. In the presence of IBMX plus 0.3 nm Iso, the Ca2+ current increased to 213 ± 35% (P < 0.5) over baseline and the CFP/YFP emission intensity ratio increased to 5.1 ± 1.4% (P < 0.05). These results suggest that PDE activity is not responsible for compartmentation of PGE1 responses.
Figure 6. Phosphodiesterase inhibition does not reveal a PGE1 stimulatory effect.
A, time course of changes in the amplitude of the L-type Ca2+ current and examples of current traces recorded during exposure to 10 μm IBMX (1), IBMX plus 10 μm PGE1 (2), and IBMX plus a normally subthreshold concentration (0.0003 μm) of Iso. B, average increase in L-type Ca2+ current amplitude during exposure to 10 μm IBMX, IBMX plus 10 μm PGE1, and IBMX plus a subthreshold concentration of Iso (0.0003 μm; n = 8). C, time course of changes in the CFP/YFP emission intensity ratio during exposure to 10 μm IBMX, IBMX plus 10 μm PGE1, and IBMX plus 0.0003 μm Iso. D, average change in CFP/YFP emission intensity ratio during exposure to 10 μm IBMX, IBMX plus 10 μm PGE1, and IBMX plus 0.0003 μm Iso (n = 3).
Discussion
The diffusible second messenger cAMP plays a central role in regulating a variety of functional responses in cardiac myocytes. Yet despite this fact, or maybe because of it, not all agonists that stimulate cAMP production elicit the same responses. The capacity of βAR agonists but not prostaglandins to regulate myocardial contractility is a classic example (Steinberg & Brunton, 2001). This has been attributed to the fact that only βAR agonists stimulate PKA-dependent phosphorylation of proteins involved in the regulation of contraction, such as troponin I (Brunton et al. 1979). The present study confirms that the disparity in the functional responses produced by these two signalling pathways also includes PKA-dependent regulation of the L-type Ca2+ channel.
While it makes sense that different receptors are stimulating cAMP production in specific and sometimes distinct subcellular compartments, the number of different microdomains that exist and what keeps cAMP produced in one from reaching another is still not well understood. Conventional biochemical methods used previously demonstrate that Iso, but not PGE1, stimulates cAMP production in the particulate fraction of homogenized cardiac preparations (Hayes et al. 1980; Buxton & Brunton, 1983). Yet how that relates to what is happening in an intact myocyte is not entirely obvious. Our present results clearly demonstrate that it is possible to directly measure changes in cAMP activity in distinct subcellular domains of intact cardiac myocytes. Only Iso was able to stimulate cAMP activity that could be detected by the FRET-based probe constructed from type II PKA, which is associated specifically with the particulate or membrane fraction of cardiac myocytes (Corbin et al. 1977). On the other hand, only the probe constructed from the freely diffusible cAMP binding domain of Epac was able to detect responses to PGE1. The fact that the Epac-based probe could detect a response to PGE1, when the anchored PKA-based probe could not, is not due to differences in cAMP binding affinity. The PKA-based probe, which has an EC50 of ∼300 nm (Mongillo et al. 2004), is actually more sensitive to changes in cAMP activity than the Epac-based probe, which has an EC50 of ∼1 μm (Nikolaev et al. 2004). These results support the idea that the Epac-based sensor can detect responses in the bulk cytoplasmic compartment, which correlates with the soluble fraction of homogenized cardiac preparations.
The ability of Iso to produce a response that can be detected by the Epac-based probe as well as the PKA-based probe suggests that βAR responses in these cells are not compartmentalized. However, it should be pointed out that in guinea pig ventricular myocytes, these responses are most likely due specifically to activation of β1ARs (Hool & Harvey, 1997). PGE1 produced a response that could be detected by the Epac-based probe but not the anchored PKA-based probe. These observations, together with the fact that Iso but not PGE1 is able to stimulate the L-type Ca2+ current, support the conclusion that there is a close correlation between β1AR activation of the PKA-based sensor and the regulation of Ca2+ channels. These results support the idea that even though β1AR stimulation appears to produce an increase in cAMP throughout the entire cell, Ca2+ channel responses are due specifically to an increase in cAMP in a microdomain where type II PKA is located.
While the present results clearly demonstrate that there are at least two compartments in which cAMP can be produced in intact myocytes, the explanation for why they do not readily communicate with one another is still not fully resolved. We investigated two popular hypotheses. The first is the possibility that the functional separation somehow involves activation of a parallel, Gi-dependent signalling pathway. Such a mechanism is believed to explain the ability of β2AR activation to compartmentalize its cAMP-dependent responses (Xiao, 2001). Although the signalling mechanism responsible has not been fully elucidated, it has been suggested to involve stimulation of phosphatase activity and dephosphorylation of selected target proteins (Kuschel et al. 1999). Despite evidence that these cardiac myocytes express EP receptors potentially capable of activating Gi, we found no indication that this occurs. Furthermore, if the inability of PGE1 to regulate Ca2+ channel function was not due to localization of cAMP, but instead activation of a phosphatase by a parallel signalling pathway, one would still have expected to see activation of the anchored PKA-based probe, but we did not.
Another potential explanation for compartmentation of cAMP is that PDE activity acts as a functional barrier to prevent free diffusion. The idea that PDE activity is capable of regulating free diffusion of cAMP in cardiac myocytes has been clearly demonstrated (Zaccolo & Pozzan, 2002; Jurevicius et al. 2003). However, our results suggest that PDE activity does not prevent cAMP diffusion specifically between the compartment where PGE1 receptors stimulate cAMP production and the compartment where type II PKA regulates L-type Ca2+ channels. The concentration of IBMX used in our experiments should have completely or significantly inhibited all PDE isoforms involved in hydrolysis of cAMP in our cells (Bethke et al. 1992). While clearly altering the response to βAR activation, IBMX had no effect on the ability of PGE1 to stimulate cAMP in microdomains where regulation of L-type Ca2+ channels occurs. This observation confirms more recent work demonstrating that selective inhibition of PDE3 and/or PDE4 activity does not enable PGE1 to stimulate the Ca2+ current in rat ventricular myocytes (Rochais et al. 2006). Taken together, these results support the conclusion that PDE activity alone is not directly responsible for the inability of PGE1 to increase cAMP levels in subcellular microdomains were effectors such as the L-type Ca2+ channel are found.
So then why can't cAMP produced in response to PGE1 reach the compartment that is associated with the particulate fraction of cardiac myocytes? One possible explanation is that the two compartments are separated by a barrier that physically blocks diffusion between them. The complex morphology of cardiac ventricular myocytes consists of restricted spaces that are known to limit access to some cytosolic domains (Parfenov et al. 2006). This may explain how cAMP can remain associated with the membrane fraction of homogenized myocyte preparations. Yet, while this may significantly slow diffusion, it is unlikely to completely block movement between compartments. Our experiments with the Ht31 peptide are consistent with this idea. The fact that treatment of cells with this peptide resulted in the ability of the PKA-based probe to detect a response to PGE1, suggests that unanchored probe was able to diffuse to the location where cAMP was being produced. Therefore, even though it is likely that there is some physical basis for restricted or limited diffusion between compartments, upon inhibition of PDE activity, cAMP should have reached detectable levels in all compartments.
An alternative explanation is that there are actually more than two distinct compartments for cAMP signalling within the cell. One is associated with responses that can be detected by the PKA-based probe. It is linked with type II PKA and the functional responses that are associated with βAR stimulation. While the present results suggest that this compartment reflects the particulate or membrane fraction of cardiac myocytes, it is likely to represent a more specific microdomain that includes the caveolar membrane fraction. This conclusion is supported by the fact that type II PKA is found specifically in caveolar membrane fractions (Rybin et al. 2000). This is also where L-type Ca2+ channels and signalling complexes associated with βARs are found (Ostrom & Insel, 2004; Scriven et al. 2005; Balijepalli et al. 2006). A second compartment is associated with PGE1 responses that can be detected by the Epac- but not the PKA-based probe. Consistent with this idea, there is evidence that EP receptors are expressed in extracaveolar domains (Ostrom & Insel, 2004). While our results suggest that this reflects the bulk cytosolic compartment, it does not tell us if that represents a single compartment in which EP receptors are expressed uniformly. The fact that PGE1 did not affect L-type Ca2+ channel activity, even when Gi and PDE activity were inhibited, suggests that the EP receptors may be located in a separate microdomain that has direct contact with the bulk cytoplasmic compartment, but not the caveolar domain.
Another factor that is likely to be important in explaining compartmentation of PGE1 responses is the transient nature of the cAMP production. If PGE production of cAMP were sustained, one would expect it to eventually reach significant levels even in the caveolar compartment. However, because cAMP production is transient, a brief pulse of cAMP that diffuses from an extracaveolar domain into a larger bulk cytoplasmic compartment could dissipate before reaching the caveolar domain and producing a functional response. The reason for the transient response to PGE1 is not known for certain. Activation of EP receptors has been shown to produce transient responses in other preparations as well (Rich et al. 2001). It has been suggested that feedback stimulation of PDE4 activity may be involved. However, EP4 receptors may also undergo rapid desensitization (Bos et al. 2004).
The present study addresses the observation that not all agonists that stimulate cAMP production in cardiac myocytes produce the same functional responses. In comparing the effects of β-adrenergic and prostaglandin receptor activation, we demonstrate that cAMP production can be delimited to certain subcellular microdomains of intact adult ventricular myocytes. Furthermore, our results suggest that compartmentation of PGE1 responses is not necessarily a function of Gi-dependent signalling mechanisms or PDE activity. Instead, production of cAMP in an extracaveolar region that is not in direct communication with a caveolar domain, together with the transient nature of the response, may play a more critical role in subcellular localization of prostaglandin effects. These results support the idea that multiple microdomains of cAMP signalling exist within cardiac myocytes. While AKAPs are important in targeting PKA-dependent responses (Dodge-Kafka et al. 2006), our results clearly demonstrate that localization of cAMP production also plays a critical role, and that functional responses like those involving regulation of L-type Ca2+ channels are due to an increase in cAMP activity within a specific subcellular compartment that is associated with AKAP-dependent anchoring of type II PKA.
Acknowledgments
This work was supported by grants from the NIH and the American Heart Association. The authors thank Radu Iancu for many helpful discussions.
References
- Alloatti G, Serazzi L, Levi RC. Prostaglandin I2 (PGI2) enhances calcium current in guinea-pig ventricular heart cells. J Mol Cell Cardiol. 1991;23:851–860. doi: 10.1016/0022-2828(91)90218-b. [DOI] [PubMed] [Google Scholar]
- Balijepalli RC, Foell JD, Hall DD, Hell JW, Kamp TJ. Localization of cardiac L-type Ca2+ channels to a caveolar macromolecular signaling complex is required for β2-adrenergic regulation. Proc Natl Acad Sci U S A. 2006;103:7500–7505. doi: 10.1073/pnas.0503465103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belevych AE, Sims C, Harvey RD. ACh-induced rebound stimulation of L-type Ca2+ current in guinea-pig ventricular myocytes, mediated by Gβγ-dependent activation of adenylyl cyclase. J Physiol. 2001;536:677–692. doi: 10.1111/j.1469-7793.2001.00677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. 2. Kluwer: Dordrecht; 2001. [Google Scholar]
- Bethke T, Meyer W, Schmitz W, Scholz H, Stein B, Thomas K, Wenzlaff H. Phosphodiesterase inhibition in ventricular cardiomyocytes from guinea-pig hearts. Br J Pharmacol. 1992;107:127–133. doi: 10.1111/j.1476-5381.1992.tb14474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos CL, Richel DJ, Ritsema T, Peppelenbosch MP, Versteeg HH. Prostanoids and prostanoid receptors in signal transduction. Int J Biochem Cell Biol. 2004;36:1187–1205. doi: 10.1016/j.biocel.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Breyer MD, Breyer RM. Prostaglandin E receptors and the kidney. Am J Physiol Renal Physiol. 2000;279:F12–F23. doi: 10.1152/ajprenal.2000.279.1.F12. [DOI] [PubMed] [Google Scholar]
- Brunton LL, Hayes JS, Mayer SE. Hormonally specific phosphorylation of cardiac troponin I and activation of glycogen phosphorylase. Nature. 1979;280:78–80. doi: 10.1038/280078a0. [DOI] [PubMed] [Google Scholar]
- Brunton LL, Hayes JS, Mayer SE. Functional compartmentation of cyclic AMP and protein kinase in heart. Adv Cyclic Nucleotide Res. 1981;14:391–397. [PubMed] [Google Scholar]
- Buxton IL, Brunton LL. Compartments of cyclic AMP and protein kinase in mammalian cardiomyocytes. J Biol Chem. 1983;258:10233–10239. [PubMed] [Google Scholar]
- Corbin JD, Keely SL. Characterization and regulation of heart adenosine 3′:5′-monophosphate-dependent protein kinase isozymes. J Biol Chem. 1977;252:910–918. [PubMed] [Google Scholar]
- Corbin JD, Sugden PH, Lincoln TM, Keely SL. Compartmentalization of adenosine 3′:5′-monophosphate and adenosine 3′:5′-monophosphate-dependent protein kinase in heart tissue. J Biol Chem. 1977;252:3854–3861. [PubMed] [Google Scholar]
- Dodge-Kafka KL, Langeberg L, Scott JD. Compartmentation of cyclic nucleotide signaling in the heart: the role of A-kinase anchoring proteins. Circ Res. 2006;98:993–1001. doi: 10.1161/01.RES.0000218273.91741.30. [DOI] [PubMed] [Google Scholar]
- Fujino H, Regan JW. EP4 prostanoid receptor coupling to a pertussis toxin-sensitive inhibitory G protein. Mol Pharmacol. 2006;69:5–10. doi: 10.1124/mol.105.017749. [DOI] [PubMed] [Google Scholar]
- Hayes JS, Brunton LL. Functional compartments in cyclic nucleotide action. J Cyclic Nucleotide Res. 1982;8:1–16. [PubMed] [Google Scholar]
- Hayes JS, Brunton LL, Brown JH, Reese JB, Mayer SE. Hormonally specific expression of cardiac protein kinase activity. Proc Natl Acad Sci U S A. 1979;76:1570–1574. doi: 10.1073/pnas.76.4.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JS, Brunton LL, Mayer SE. Selective activation of particulate cAMP-dependent protein kinase by isoproterenol and prostaglandin E1. J Biol Chem. 1980;255:5113–5119. [PubMed] [Google Scholar]
- Hool LC, Harvey RD. Role of β1- and β2-adrenergic receptors in regulation of Cl− and Ca2+ channels in guinea pig ventricular myocytes. Am J Physiol. 1997;273:H1669–H1676. doi: 10.1152/ajpheart.1997.273.4.H1669. [DOI] [PubMed] [Google Scholar]
- Hulme JT, Lin TW, Westenbroek RE, Scheuer T, Catterall WA. β-Adrenergic regulation requires direct anchoring of PKA to cardiac CaV1.2 channels via a leucine zipper interaction with A kinase-anchoring protein 15. Proc Natl Acad Sci U S A. 2003;100:13093–13098. doi: 10.1073/pnas.2135335100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurevicius J, Skeberdis VA, Fischmeister R. Role of cyclic nucleotide phosphodiesterase isoforms in cAMP compartmentation following β2-adrenergic stimulation of ICa,L in frog ventricular myocytes. J Physiol. 2003;551:239–252. doi: 10.1113/jphysiol.2003.045211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keely SL. Activation of cAMP-dependent protein kinase without a corresponding increase in phosphorylase activity. Res Commun Chem Pathol Pharmacol. 1977;18:283–290. [PubMed] [Google Scholar]
- Keely SL. Prostaglandin E1 activation of heart cAMP-dependent protein kinase: apparent dissociation of protein kinase activation from increases in phosphorylase activity and contractile force. Mol Pharmacol. 1979;15:235–245. [PubMed] [Google Scholar]
- Kuschel M, Zhou YY, Cheng H, Zhang SJ, Chen Y, Lakatta EG, Xiao RP. Gi protein-mediated functional compartmentalization of cardiac β2-adrenergic signaling. J Biol Chem. 1999;274:22048–22052. doi: 10.1074/jbc.274.31.22048. [DOI] [PubMed] [Google Scholar]
- Ma H, Hara A, Xiao CY, Okada Y, Takahata O, Nakaya K, Sugimoto Y, Ichikawa A, Narumiya S, Ushikubi F. Increased bleeding tendency and decreased susceptibility to thromboembolism in mice lacking the prostaglandin E receptor subtype EP3. Circulation. 2001;104:1176–1180. doi: 10.1161/hc3601.094003. [DOI] [PubMed] [Google Scholar]
- Mongillo M, McSorley T, Evellin S, Sood A, Lissandron V, Terrin A, Huston E, Hannawacker A, Lohse MJ, Pozzan T, Houslay MD, Zaccolo M. Fluorescence resonance energy transfer-based analysis of cAMP dynamics in live neonatal rat cardiac myocytes reveals distinct functions of compartmentalized phosphodiesterases. Circ Res. 2004;95:67–75. doi: 10.1161/01.RES.0000134629.84732.11. [DOI] [PubMed] [Google Scholar]
- Nikolaev VO, Bunemann M, Hein L, Hannawacker A, Lohse MJ. Novel single chain cAMP sensors for receptor-induced signal propagation. J Biol Chem. 2004;279:37215–37218. doi: 10.1074/jbc.C400302200. [DOI] [PubMed] [Google Scholar]
- Ostrom RS, Insel PA. The evolving role of lipid rafts and caveolae in G protein-coupled receptor signaling: implications for molecular pharmacology. Br J Pharmacol. 2004;143:235–245. doi: 10.1038/sj.bjp.0705930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfenov AS, Salnikov V, Lederer WJ, Lukyanenko V. Aqueous diffusion pathways as a part of the ventricular cell ultrastructure. Biophys J. 2006;90:1107–1119. doi: 10.1529/biophysj.105.071787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich TC, Fagan KA, Tse TE, Schaack J, Cooper DM, Karpen JW. A uniform extracellular stimulus triggers distinct cAMP signals in different compartments of a simple cell. Proc Natl Acad Sci U S A. 2001;98:13049–13054. doi: 10.1073/pnas.221381398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochais F, Abi-Gerges A, Horner K, Lefebvre F, Cooper DM, Conti M, Fischmeister R, Vandecasteele G. A specific pattern of phosphodiesterases controls the cAMP signals generated by different Gs-coupled receptors in adult rat ventricular myocytes. Circ Res. 2006;98:1081–1088. doi: 10.1161/01.RES.0000218493.09370.8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybin VO, Xu X, Lisanti MP, Steinberg SF. Differential targeting of β-adrenergic receptor subtypes and adenylyl cyclase to cardiomyocyte caveolae. A mechanism to functionally regulate the cAMP signaling pathway. J Biol Chem. 2000;275:41447–41457. doi: 10.1074/jbc.M006951200. [DOI] [PubMed] [Google Scholar]
- Scriven DR, Klimek A, Asghari P, Bellve K, Moore ED. Caveolin-3 is adjacent to a group of extradyadic ryanodine receptors. Biophys J. 2005;89:1893–1901. doi: 10.1529/biophysj.105.064212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg SF, Brunton LL. Compartmentation of G protein-coupled signaling pathways in cardiac myocytes. Annu Rev Pharmacol Toxicol. 2001;41:751–773. doi: 10.1146/annurev.pharmtox.41.1.751. [DOI] [PubMed] [Google Scholar]
- Warrier S, Belevych AE, Ruse M, Eckert RL, Zaccolo M, Pozzan T, Harvey RD. β-Adrenergic and muscarinic receptor induced changes in cAMP activity in adult cardiac myocytes detected using a FRET based biosensor. Am J Physiol Cell Physiol. 2005;289:C455–C461. doi: 10.1152/ajpcell.00058.2005. [DOI] [PubMed] [Google Scholar]
- Xiao RP. β-Adrenergic signaling in the heart: dual coupling of the β2-adrenergic receptor to Gs and Gi proteins. Sci STKE. 2001 doi: 10.1126/stke.2001.104.re15. RE15. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Habuchi Y, Tanaka H, Suto F, Morikawa J, Kashima K, Yoshimura M. EP receptor-mediated inhibition by prostaglandin E1 of cardiac L-type Ca2+ current of rabbits. Am J Physiol. 1999;277:H1369–H1374. doi: 10.1152/ajpheart.1999.277.4.H1369. [DOI] [PubMed] [Google Scholar]
- Yang J, Drazba JA, Ferguson DG, Bond M. A-kinase anchoring protein 100 (AKAP100) is localized in multiple subcellular compartments in the adult rat heart. J Cell Biol. 1998;142:511–522. doi: 10.1083/jcb.142.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccolo M, De Giorgi F, Cho CY, Feng L, Knapp T, Negulescu PA, Taylor SS, Tsien RY, Pozzan T. A genetically encoded, fluorescent indicator for cyclic AMP in living cells. Nat Cell Biol. 2000;2:25–29. doi: 10.1038/71345. [DOI] [PubMed] [Google Scholar]
- Zaccolo M, Pozzan T. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science. 2002;295:1711–1715. doi: 10.1126/science.1069982. [DOI] [PubMed] [Google Scholar]