Abstract
Mutations in the expanded gene act as hyperplastic tumor suppressors, interfere with cell competition, and elevate Dpp signaling. Unlike Dpp overexpression, ex causes few patterning defects. Our data suggest that patterning effects are partly masked by antagonistic roles of other signaling pathways that are also activated. ex causes proliferation of cells in the posterior eye disc that are normally postmitotic. ex mutations elevate Wg signaling, but Dpp signaling antagonizes patterning effects of Wg. By contrast, if Dpp signaling is blocked in ex mutant cells, the elevated Wg signaling preserves an immature developmental state and prevents retinal differentiation. An effect of ex mutations on vesicle transport is suggested by evidence for altered sterol distribution. Mutations in ft show effects on proliferation, Wg signaling, and sterols very similar to those of ex mutations. During disc growth, ex was largely epistatic to ft, and the Warts pathway mutation hippo largely epistatic to ex. Our data suggest that ft and ex act partially through the Warts pathway.
Introduction
The growth of an organism or tissue is the net product of mechanisms that control cell growth, cell proliferation and cell death (Conlon and Raff, 1999; Hipfner and Cohen, 2004). The coordinated regulation of these processes requires integration of multiple pathways that control size, and patterning and cell fate allocation. Growth is regulated at the organismal level, and within tissues by signals that are also involved in morphogenesis and differentiation. The Notch, Wingless, Decapentaplegic, Hedgehog and EGF signaling pathways are all implicated in the control of the cell cycle, growth, apoptosis and differentiation (Brachmann and Cagan, 2003; Dominguez and Casares, 2005; Edgar et al., 2001; Firth and Baker, 2005; Hipfner and Cohen, 2004; Johnston and Sanders, 2003; Lee and Orr-Weaver, 2003; Martin et al., 2004; Voas and Rebay, 2004). In addition, the Warts pathway might also be regulated by extracellular signals. The Warts pathway includes the cytoplasmic protein Sav and the kinase Hpo, which together phosphorylate Wts, in the presence of Mats. Activated Wts phosphorylates and inactivates Yorkie, a transcriptional regulator of genes involved in proliferation and survival (Edgar, 2006) (Hariharan, 2006).
The expanded (ex) and fat (ft) genes both encode negative growth regulators. Clones of cells that are mutated for either grow more rapidly, but have little effect on differentiation, although both are also involved in planar polarity signaling (Blaumueller and Mlodzik, 2000; Boedigheimer and Laughon, 1993; Bryant et al., 1988; Mahoney et al., 1991; Rawls et al., 2002; Yang et al., 2002). ex encodes a cytoplasmic protein localized to the apical junctions of epithelial cells (Boedigheimer and Laughon, 1993; Boedigheimer et al., 1997). ft encodes a large atypical cadherin (Mahoney et al., 1991).
Ex is a member of the FERM (4.1, Ezrin, Radixin, Moesin) domain protein superfamily, a family of proteins that provide regulated linkages between membrane proteins and cytoplasmic proteins or the actin cytoskeleton (Bretscher et al., 2002). The effects of loss of ex on growth and cell competition reflect a major perturbation in growth control (Blaumueller and Mlodzik, 2000; Boedigheimer and Laughon, 1993). Ex interacts with the related protein Merlin and the double mutant phenotype is still more severe (McCartney et al., 2000). It has been proposed that Ex and Mer together regulate the Warts pathway (Hamaratoglu et al., 2006). It has also been proposed that Ex and Mer together restrain endocytosis of multiple transmembrane receptors and so reduce their signaling (Maitra et al., 2006).
The fat gene shares with ex similar phenotypes that indicate requirement in regulating both planar polarity and growth (Blaumueller and Mlodzik, 2000; Boedigheimer and Laughon, 1993; Bryant et al., 1988; Mahoney et al., 1991; Rawls et al., 2002; Yang et al., 2002). ft encodes a large atypical cadherin with a large number of extracellular interaction domains, including with 34 cadherin repeats, two laminin G and five EGF-like domains, and a single transmembrane domain (Mahoney et al., 1991). Ft appears to function as a cell surface receptor, signaling through its intracellular domain, which is necessary and sufficient for the functions of ft in growth and polarity control (Matakatsu and Blair, 2006; Saburi and McNeill, 2005).
We obtained new loss-of-function alleles of both ex and ft in a screen for mutations that permit the survival of Minute heterozygous clones in the presence of wild type cells in the eye (Tyler et al., 2007). It is thought that competition between wild type and Minute heterozygous cells reflects competition for the growth factor Dpp (Moreno et al., 2002). Consistent with this, both ex and ft mutations increased Dpp signal transduction, as measured by expression levels in the wing of Spalt-major and Brinker, two targets of Dpp signaling, although they did not detectably affect levels of Mad phosphorylation (Tyler et al., 2007). However, the ex and ft mutant phenotypes differ from that of elevated Dpp signaling in not much affecting pattern. In addition, ex mutations were able to rescue M/+ clones even when mutated for Mad (Tyler et al., 2007). These findings suggest that ex and ft affect other pathways in addition to Dpp signaling. To investigate this further we studied the effects of ex and ft mutations on eye development, a process in which the roles of many signaling pathways have been studied (Voas and Rebay, 2004). We also sought to determine whether ex and ft affect the Warts pathway, because these mutations also affected cell competition and affect growth like ex and ft do (Edgar, 2006) (Hariharan, 2006).
Methods
Drosophila strains
The following Drosophila strains were used:
exNY1, ftNY2, ftNY1 are EMS-induced alleles that were recovered in a screen for mutations involved in cell competition (Tyler et al., 2007). exNY1 and ftNY1 behave as null alleles when compared with exe1 (Boedigheimer and Laughon, 1993) and ftG-rv (Mahoney et al., 1991) ftNY2 behaves as a strong hypomorph. UAS-ft was kindly provided by D. Strutt. exe1 is an enhancer-trap insertion (Boedigheimer and Laughon, 1993).
nkd-lacZ (l(3)4869), UAS-nkd-myc (Zeng et al., 2000); FRT82 sav3, FRT82 wtsMGH1 (Tapon et al., 2002); mad12 (Sekelsky et al., 1995); IAP-lacZ (P[lacWj5C8]th) (Ryoo et al., 2002); act>CD2>Gal4 (Pignoni and Zipursky, 1997); UAS-hpo (Pantalacci et al., 2003); Ey-FLP (Newsome et al., 2000); UAS-P35 (Hay et al., 1994); FRT42 hpoMGH4 (Harvey et al., 2003); GMR-GAL4 (Freeman, 1996); tkva12 (Burke and Basler, 1996a); UAS-ex (Boedigheimer et al., 1997); UAS-axin (Willert et al., 1999)
Induction of mosaics
Homozygous mutant clones were generated using FRT/FLP-mediated recombination using hs-Flp122 or ey-FLP as a recombinase source (Newsome et al., 2000; Xu and Rubin, 1993).
Genes were over-expressed in clones of cells using the GAL4-UAS system and an Actin FLP-on transgene (Brand and Perrimon, 1993; Pignoni and Zipursky, 1997)
The MARCM technique was used to expressed particular genes in mutant cells (Lee and Luo, 1999). Genotypes of MARCM experiments were as follows:
y w hs-FLP UAS-GFP; Tub-GAL80 FRT40 / X FRT40; Tub-GAL4 / UAS-Y
where X was exe1, ftG-rv or exe1 mad12 and Y one of UAS-ex, UAS-ft, UAS-hpo UAS-nkd-myc or UAS-axin.
or
y w hs-FLP UAS-GFP; FRT42D Tub-GAL80/ FRT42D hpoMGH4; Tub-GAL4 /UAS-Y
Clone size measurements
Larvae of all genotypes were heat-shocked and dissected in parallel to ensure that clones were of identical age. Eggs were collected for 48h, and heat-shocked for 20min at 37?C after a further 48h. Wandering third instar larvae were selected 48h after heat shock for dissection and labeling. Cell numbers in clones located in the wing pouch were counted in at least 5 discs per genotype. 16-30 clones were counted for each genotype.
Immunohistochemistry
Imaginal discs were dissected on 0.1M sodium phosphate (pH7.2) and fixed in PLP (Tomlinson and Ready, 1987) for 45’ at 4°C. Antibodies were diluted and washes performed in PDT (0.1M sodium phosphate, 0.3% sodium deoxycholate, 0.3% Triton X-100), PBT (0.1M sodium phosphate, 0.1% BSA, 0.2% Triton X-100) or NSG (0.1M sodium phosphate, 5% Normal Goat Serum, 0.1% saponin (Sigma catalog # S-1252)
Primary antibodies used were: ELAV (Robinow and White, 1991); Cyclin B (mAb F2F4) (Knoblich and Lehner, 1993); Cyclin D (gift of N. Dyson); Discs large (mAb 4F3) (Woods et al., 1997); Cubitus interruptus (Motzny and Holmgren, 1995); Fas III (Patel et al., 1987); Dlp (Lum et al., 2003); Flotillin (Galbiati et al., 1998); Salm (Kuhnlein et al., 1994); Wingless (Brook and Cohen, 1996); engrailed (mAb 4D9) (Patel et al., 1989) Senseless (Nolo et al., 2000); Cyclin E (Richardson et al., 1995); anti-active Caspase 3(CM1) (Srinivasan et al., 1998; Yu et al., 2002); Eyes absent (Bonini et al., 1993); Homothorax (Casares and Mann, 1998); Teashirt (Wu and Cohen, 2000); Rabbit anti-beta-galactosidase (Cappell); anti-GFP (Invitrogen); anti-BrdU (Becton-Dickinson).; anti-Boca (Culi and Mann, 2003). Secondary antibodies were multiply subtracted whole IgG conjugated to cyanine dyes (Jackson Immunoresearch). For epistasis experiments, GFP was visualized directly without antibody labeling. Discs were fixed 20′ at room temperature in 4% formaldehyde, washed in phosphate buffer and mounted in 75% glycerol/2% n-propyl gallate. Images were collected on a Radiance 2000 confocal microscope (Biorad) and processed using ImageJ (NIH) and Photoshop (Adobe Systems).
BrdU incorporation assays were performed essentially as described (Negre et al., 2003). Pupae were aged at 25°C: dissection was performed 24-28h after puparium formation for CM1 labeling; 38-42h after puparium formation for anti-Dlg labeling. Pupal retinas were fixed for 30′ in 4% formaldehyde in 0.1M phosphate buffer, then treated as for imaginal discs.
For Filipin labeling, formaldehyde-fixed imaginal discs were incubated with 0.05 mg/ml filipin in PBS for 2 h (adapted from (Blanchette-Mackie et al., 1988).
To detect GPI-linked proteins, fixed imaginal dics were incubated for 30 min at room temperature in 10 nM fluorescently-labeled proaerolysin (FLAER; Protox Biotech) or 1μg/ml fluorescently-labeled cholera toxin B subunit (Molecular Probes), and washed in 0.1 M buffered phosphate.
Results
ex limits cell cycle activity in the developing eye
Loss of function mutations of ex were isolated that prevented cell competition (Tyler et al., 2007). Since previous studies have linked cell competition to Dpp signaling (Moreno et al., 2002), we sought to understand how ex function is related to Dpp signaling, which is elevated in ex mutant cells (Tyler et al., 2007). We used eye development to gain insight into the pathways with which ex interacts, because the signaling pathways that control differentiation, proliferation, and survival in the Drosophila eye have been much studied (Firth and Baker, 2005; Lee and Treisman, 2002; Pappu and Mardon, 2002; Voas and Rebay, 2004).
Most differentiation occurs normally in ex null mutant clones. We looked at markers of neuronal differentiation, including senseless and ELAV, and found that these were expressed with wild-type timing and patterning (Figure 1A, B). This was in accordance with previous reports that only minor differentiation defects occur in ex mutant clones, with the exception of abnormal planar polarity (Blaumueller and Mlodzik, 2000).
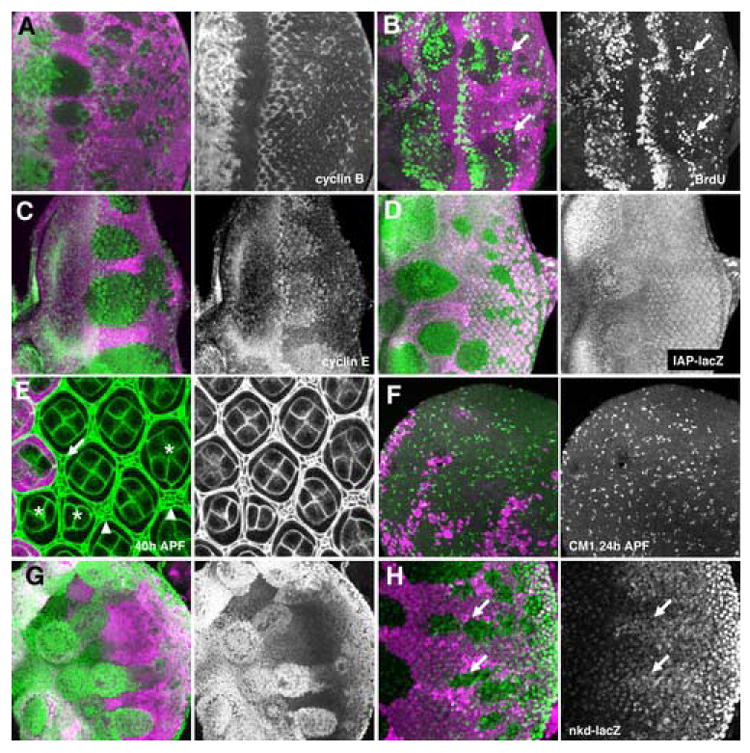
Figure 1. Differentiation and cell cycle in ex mutant clones.
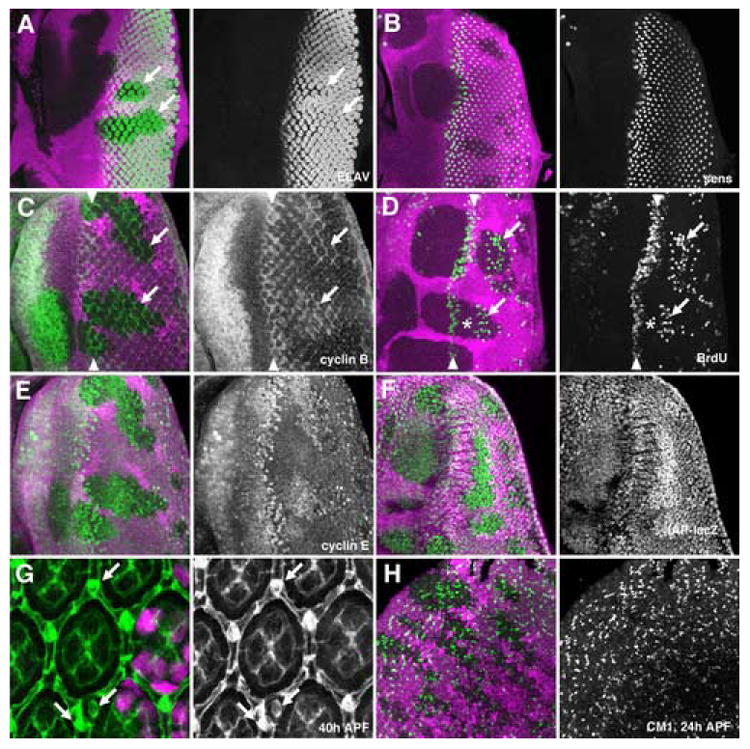
All figures show clones of homozygous ex mutant cells that lack the magenta lineage marker. Green labelling is ELAV (A), Sens (B), Cyclin B (C), BrdU (D), Cyclin E (E), IAP:LacZ (F), Discs Large (G), and CM1 (H).
A) No difference in photoreceptor differentiation was visible between ex mutant and wild-type tissue. ELAV-expressing nuclei sometimes appear to be more widely-spaced in mutant tissue, perhaps because of the proliferation of intervening cells, but are the same size as in wild type.
B) R8 photoreceptor differentiation occurred normally in ex mutant tissue, although a slight delay was sometimes apparent.
C) Cyclin B expression marks cells in S-, G2-, and early M-phases of the cell cycle. Cycling cells can be seen at the SMW (arrowheads). In exNY1 mutant clones there are further interommatidial cells in the cell cycle iposterior to the SMW (arrows).
D) S-phase cells are labeled in the SMW (arrowheads). Additional S-phases occured in exNY1 mutant clones (arrows). There was a gap (indicated by asterisk). between the normal S-phases of the SMW (asterisks) and later ectopic S-phases.
E) Cyclin E protein levels were elevated in exNY1 mutant clones
F) dIAP-LacZ transcription was elevated in exNY1 mutant clones
G) anti-Discs large highlights cell membranes in pupal retina. The number and morphology of photoreceptor, cone pigment cells are the same in wild-type tissue and exNY1 mutant clones. There are ectopic bristle cells and duplicated bristles in ex mutant tissue (arrows).
H) Similar amounts of pupal apoptosis occured in exNY1 mutant clones an in wild type retina.
Proliferation of eye imaginal disc cells is also highly patterned and regulated (Firth and Baker, 2005; Wolff and Ready, 1993). Early in development, eye precursor cells undergo continuous, unpatterned proliferation. As the wave of differentiation sweeps across the disc, the first photoreceptors are selected within the “morphogenetic furrow”, during a G1 phase arrest. Cells that have not been allocated any neural fate later re-enter the cell cycle in the “Second Mitotic Wave” (SMW) before becoming postmitotic (Baker and Yu, 2001; Thomas et al., 1994).
We looked at cyclin B expression to monitor cell cycle phases. Cyclin B accumulates in S and G2 phases and is degraded in M phase (Evans et al., 1983; Knoblich and Lehner, 1993). ex mutations had little effect on the cell cycle until after the SMW, when ex mutant inter-ommatidial cells continue to express cyclin B after wild-type cells have ceased to do so. This indicated persistent ectopic cell cycle progression (Figure 1C). In addition, cell cycle arrest anterior to the furrow was sometimes slightly delayed in ex mutants (Figure 1C).
BrdU incorporation was used to monitor S-phases in ex mutant tissue directly. Ectopic S-phases were found in mutant cells posterior to the second mitotic wave (Figure 1D). S-phase cells were not detected throughout mutant tissue, but instead were found several columns posterior to the SMW, suggesting that there is a refractory period after the SMW before S-phase can be re-initiated. The accumulation of Cyclin E protein, which promotes transition from G1 to S phase, was also affected by ex mutations. Cyclin E protein levels were higher in ex mutant tissue (Figure 1E).
Because many signals that regulate growth and proliferation also regulate apoptosis (Hay and Guo, 2003; Hipfner and Cohen, 2004; Lowe et al., 2004) we examine the effects of ex mutations on IAP1 transcription, using an enhancer trap insertion in the dIAP1 locus (Ryoo et al., 2002). We found that dIAP1-lacZ transcription was increased in ex mutant tissue (Figure 1F). This finding complements previous reports that overexpression of ex induces apoptosis (Blaumueller and Mlodzik, 2000).
In order to determine the fate of extra cells generated in ex mutant eye discs, we dissected retinas from pupae with ex clones, and found that ex mutant tissue contained a normal number of inter-ommatidial pigment cells (Figure 1G). We did find, however, that there were extra bristle cells in ex mutant tissue. Wild-type eyes contain one bristle per ommatidium; the hexagonal arrangement of ommatidia therefore means that each has bristles at three of its six vertices (Wolff and Ready, 1993). We found that not only did some ex mutant ommatidia contact as many as five bristles, some vertices also contained multiple bristle structures (Figure 1G). The extra bristles seen in ex mosaic retinas were found in both mutant and nearby wild-type tissue. There were also non-autonomous effects on ommatidial cell number; ommatidia containing extra cone cells were observed in both mutant and wild-type tissue (data not shown).
During normal development, superfluous inter-ommatidial cells are eliminated by a wave of apoptosis that occurs around 24h after puparium formation (Wolff and Ready, 1993). We labeled retinas of this age with an antibody against activated caspases, to detect dying cells. We found that levels of apoptosis were similar in ex mutant and wild-type tissue (Figure 1G). Thus ex mutations did not prevent cell death at the pupal stage, which may eliminate many of the extra cells generated during larval development of ex mutants. As a result, the effects of ex mutations are less extreme than reported for Mer ex double mutants (Hamaratoglu et al., 2006; McCartney et al., 2000).
dpp-independent roles of ex affect cell cycle and differentiation
The effects of ex on eye development did not resemble those caused by loss or gain of function of known extracellular signaling pathways. For example, ectopic expression of Dpp in the eye disc results in the suppression of S-phases, does not affect Cyclin E levels (Horsfield et al., 1998), but accelerates the morphogenetic furrow (Baonza and Freeman, 2001; Pignoni and Zipursky, 1997). In contrast, loss of ex promoted proliferation and slightly delayed the morphogenetic furrow (Figure 1).
Because no target gene is known that is as sensitive to Dpp signaling during eye development as Salm is during wing development (Tyler et al., 2007), we generated recombinant chromosomes doubly mutant for ex and either the Dpp signal transducer Mad, or the type I receptor tkv to characterize the Dpp-independent effects of ex in more detail. Such cells should have little response to Dpp (Massague, 1996). Mad or tkv single mutant cells differentiate quite normally, but grow poorly and can not normally be recovered in eye imaginal discs without being given a growth advantage using the Minute technique (Burke and Basler, 1996b). By contrast to Mad mutant clones, ex Mad clones were recovered at normal frequencies and were hyperplastic and larger than their twin spots (Figure 2). Neither Mad nor ex Mad clones are excluded from the eye disc to the extent that Mad clones are excluded from parts of the developing wing disc (Gibson and Perrimon, 2005; Shen and Dahmann, 2005) (Figure 3, and data not shown).
Figure 2. Differentiation and cell cycle without ex and Dpp signaling.
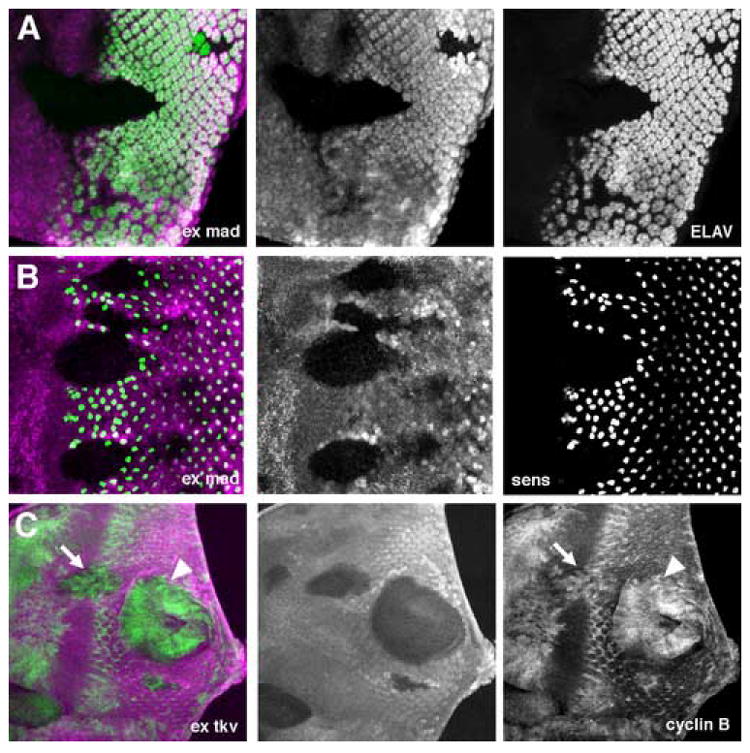
Clones of homozygous ex Mad mutant cells (A,B) or ex tkv mutant cells (C) lack the magenta lineage marker. Green labelling is ELAV (A), Sens (B), Cyclin B (C).
A. Most exe1mad12 mutant cells fail to differentiate as photoreceptors.
B. Most exe1mad12 mutant clones fail to specify R8 photoreceptors.
C. exe1tkva12 double mutant cells fail to arrest in G1 anterior to the morphogenetic furrow (arrow) or in the posterior eye disc (arrowhead).
Figure 3. Eye specification in ex Mad mutant cells.
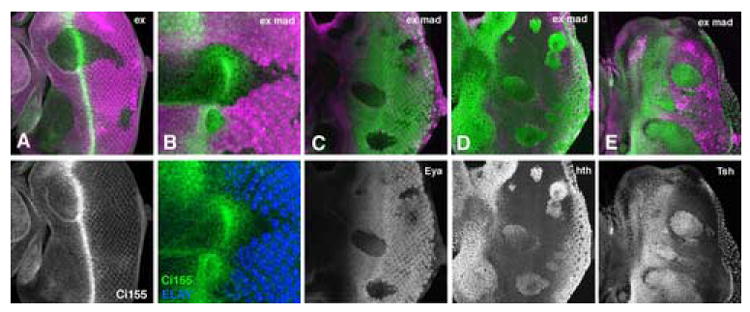
Clones of homozygous ex (A) or ex Mad (B-E) mutant cells lack the magenta lineage marker.
A. Ci155 accumulates almost normally in ex mutant clones (Ci155 protein in green). Thus, a wave of Hh signal transduction passes through ex mutant clones, as in wild type.
B. A wave of Ci155 accumulation is somewhat delayed in ex Mad mutant clones compared to wild type (Ci155 protein in green). Thus, a wave of Hh signal transduction passes through ex Mad mutant clones. Delay may reflect the greater distance to photoreceptor cells that are the source of Hh secretion, as these differentiate only in wild type regions (ELAV protein labelling of photoreceptor cells shown in blue). Note that the distance between photoreceptor cells and peak Ci155 accumulation is similar in wild type and ex Mad mutant regions. Panel B is shown at 2x greater magnification than other panels.
C. Eya protein (green) is not induced in exe1Mad12 mutant clones. Eya is necessary for eye specification.
D. Hth protein (green) accumulates to high levels in exe1mad12 clones.
E. Tsh protein (green) accumulates to high levels in exe1mad12 clones.
There was another striking difference from any of the single mutant phenotypes. ex Mad and ex tkv mutant cells largely failed to differentiate. We labeled discs with antibodies against ELAV to characterize photoreceptor differentiation and antibodies against Senseless, which are specific for the R8 photoreceptor cells. The majority of cells in large ex Mad clones fail to express ELAV, or Senseless (Figure 2A,B). Differentiation sometimes occurred in smaller clones and in the posterior portion of larger clones. Such differentiation could reflect nonautonomy, or perdurance of Ex or Mad proteins in cells that have undergone few cell cycles since clone induction.
ex tkv clones fail to withdraw from the cell cycle ahead of the morphogenetic furrow. Cyclin B is expressed in all cells throughout ex tkv clones both in the furrow and posterior to the SMW. It has previously been reported that tkv null clones show a delayed G1 arrest anterior to the furrow but are subsequently arrested by Hedgehog (Hh) signaling (Firth and Baker, 2005; Horsfield et al., 1998; Penton et al., 1997). Dpp and Hh can each induce G1 arrest and differentiation, but Dpp has a longer range and is required for timely G1 arrest. Inactivation of both Dpp and Hh signaling causes failure to differentiation as well as continued proliferation (Curtiss and Mlodzik, 2000; Firth and Baker, 2005 ; Greenwood and Struhl, 1999). The phenotype of ex tkv mutant cells is therefore distinct from that of tkv alone, and resembles that of smo Mad or smo tkv clones in which both Dpp and Hh are downregulated, both with respect differentiation and cell proliferation. The similarities raised the question of whether ex was required for Hh signaling. To test this we labeled clones with an antibody against the unprocessed form of the Ci protein, Ci155, which accumulates in response to Hh signaling at the morphogenetic furrow (Ma et al., 1993; Motzny and Holmgren, 1995). If ex was required for Hh signaling, we would expect that ex would be required for Ci155 accumulation. Contrary to this prediction, Ci155 accumulation was unaffected in ex mutant clones (Figure 3A). Sometimes Ci155 accumulation was delayed in ex tkv and ex Mad double mutant clones, however (Figure 3B, and data not shown). The cause may be indirect; because Hh protein is secreted by photoreceptor cells, which are largely absent from ex Mad clones, Hh protein may have to diffuse further to reach the center of ex Mad clones. Consistent with this interpretation, Ci155 protein does accumulate in ex Mad clones at an appropriate distance from the source of Hh (Figure 3B). These data show that Hh signaling occurs in both ex and ex Mad cells, so loss of Hh signal activity is not responsible for the loss of differentiation in ex mad clones.
Induction of photoreceptor differentiation by the Dpp and Hh signals requires the prior expression of a hierarchy of eye specification genes (Silver and Rebay, 2005). Because ex Mad mutant clones did not differentiate in response to Hh signaling, they might already differ from normal cells by the time Hh and Dpp signaling occurs in the morphogenetic furrow. We looked at the expression of eye specification genes to try to understand at what level in this hierarchy the differentiation process is interrupted. The transcription factor Eya is required for differentiation of photoreceptor cells (Bonini et al., 1993). Eya antibody labeling was greatly reduced in ex Mad cells (Figure 3C). It has been reported that Eya can be repressed by the overexpression of the transcription factors Tsh and Hth in combination (Bessa et al., 2002). Hth and Tsh are expressed in overlapping domains in the anterior part of the eye and together with Eyeless restrict the expression of downstream genes that promote differentiation (Bessa et al., 2002; Singh et al., 2002). We found that both Hth and Tsh proteins expression was maintained posterior to their usual expression domains in ex Mad clones (Figure 3D,E). Some derepression of Hth and Tsh occurs in Mad mutant clones (Bessa et al., 2002) (L. Firth and N.E.B., unpublished results), but dereperession in ex Mad clones was more complete and greater than can be accounted for by loss of Dpp signaling alone. These findings support the notion that ex Mad or ex tkv cells maintain a primitive status, normally characteristic of the most anterior part of the eye imaginal disc, which does not respond to Hh and Dpp signaling by cell cycle arrest or differentiation.
ex limits wg signaling
Although the ex Mad phenotype did not resemble the loss of any known signaling pathway, it resembled the effects of ectopic Wg signaling. Activation of Wg signaling by removal of axin maintains cells in an undifferentiated, proliferative state where they continue to express Hth and Eya is not expressed (Baonza and Freeman, 2002; Lee and Treisman, 2001; Singh et al., 2002). We hypothesized that ex Mad clones might activate Wg signaling. If this was correct, we would predict that downstream targets of Wg signaling would be upregulated, and that differentiation would be restored by blocking Wg signal transduction in the clones.
To determine whether ex affects wingless signaling, we used a lacZ P-element insertion in the naked cuticle (nkd) locus that acts as a reporter of Wg signaling (Zeng et al., 2000) (Fang et al., 2006). nkd-lacZ is expressed in a gradient from posterior to anterior in the eye disc. Because of the perdurance of the beta galactosidase protein, nkd-LacZ may report expression of nkd over time. We found that expression of nkd-lacZ was increased in cells mutant for ex, consistent with increased Wg signaling in these cells (Figure 4A). No ectopic Wg expression was observed, indicating an effect on Wg reception and/or signal transduction (data not shown, but see Supplementary Figure 1).
Figure 4. Elevated Wg signaling in ex mutant clones.
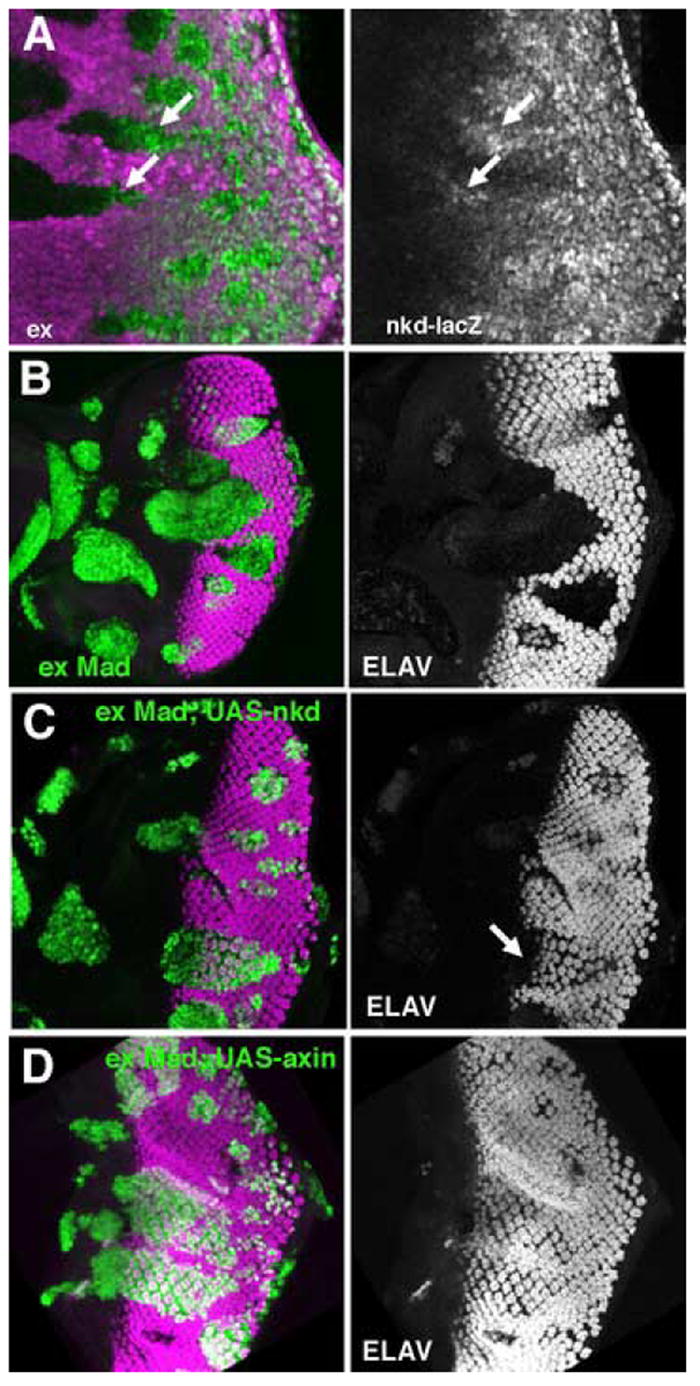
In panel A, clones of ex mutant cells lack the magenta lineage marker. In panels B-C, clones of ex Mad mutant cells express GFP (Green).
A. nkd-lacZ expression (green), a reporter of Wg signal transduction, is increased in exNY1 mutant clones.
B. exe1mad12 clones marked by the expression of GFP (green) do not differentiate. Photoreceptor neurons labeled for ELAV (magenta).
C. Differentiation is rescued by UAS-nkd:myc expression in exe1mad12 clones. Photoreceptor neurons labeled for ELAV (magenta).
D. Dfferentiation is rescued in exe1mad12 clones.expressing UAS-axin. Photoreceptor neurons labeled for ELAV (magenta).
To test whether increased Wg signaling is responsible for the failure of ex Mad cells to differentiate, we employed the MARCM system (Lee and Luo, 1999) to express antagonists of Wg signaling in ex Mad clones. nkd and axin encode negative regulators of Wg signal transduction (Willert et al., 1999; Zeng et al., 2000). We found that expression of UAS-nkd-myc in ex Mad cells reverted the loss of differentiation, so that ex Mad, UAS-nkd-myc cells differentiated whereas ex Mad clones expressing GFP did not (Figure 4B, C). Photoreceptor differentiation was slightly delayed in such rescued clones, similar to a delay reported previously for tkv mutant clones (Figure 4C) (Burke and Basler, 1996b). Similar results were obtained using UAS-axin to antagonize wg signaling in ex Mad clones (Figure 4D). Expression of UAS-nkd-myc and UAS-axin had no effect on photoreceptor differentiation in ex mutant cells (data not shown). Taken together, these results establish that Wg signal transduction is elevated in ex Mad clones, and is responsible for their failure to differentiate.
phenotypic similarities between ex and ft
The atypical cadherin ft shares some phenotypes with ex (Blaumueller and Mlodzik, 2000; Boedigheimer and Laughon, 1993; Bryant et al., 1988; Mahoney et al., 1991; Rawls et al., 2002; Yang et al., 2002). ft and ex have similar effects on planar polarity, and both mutations have been characterized as tumor suppressors. Furthermore, both ex and ft were recovered in the same screen for mutations that affect cell competition, and both elevated Dpp signaling, as assessed by Salm expression in wing imaginal discs (Tyler et al., 2007)
To determine whether ex and ft also share cell cycle and apoptosis phenotypes, we labeled ft mutant clones with cell cycle markers. As with ex, we found that ectopic cyclin B accumulation occurred in ft mutant cells posterior to the SMW, indicative of persistent cell cycling (Figure 5A). Consistent with this, ectopic S-phases were detected in ft mutant tissue by BrdU incorporation (Figure 5B), and Cyclin E protein levels were elevated (Figure 5C). Thus ft and ex share similar phenotypes with respect to the control of the cell cycle.
Figure 5. Differentiation, cell cycle, and eye specification in ft mutant clones.
Clones of homozygous ft (A-F, H) or ft tkv (G) mutant cells lack the magenta lineage marker.
A. ft mutant clones display similar cell cycle defects to ex. Cyclin B (green) is elevated in posterior cells in the eye disc.
B. BrdU incorporation (green) shows ectopic S-phases in ft mutant clones, posterior to the furrow (arrow).
C. Cyclin E protein (green) accumulates to higher levels in ft mutant clones.
D. diap-lacZ expression (green) is elevated to a lesser degree in ftNY1 mutant clones than was seen for ex mutant clones (compare Figure 1F), and apparent only anterior to the morphogenetic furrow.
E. Discs Large protein outlines cells in the pupal retina (green). ft mutant clones contain a few supernumary pigment cells(arrows), and ectopic and duplicated bristles (arrowheads). Some ft mutan ommatidia have abnormal numbers of cone cells; 2, 3 or 5 cells compared to 4 in wild-type (asterisks).
F. Pupal apoptosis (CM1 antibody labelling in green) occurs normally in ft mutant clones.
G. Hth protein expression (green) is maintained in ft tkv mutant clones.
H. nkd-lacZ expression (green), a reporter of Wg signal transduction, is increased in ftNY1 mutant clones.
As for ex, we found that IAP transcription was also elevated in ft mutant cells, as indicated by elevated levels of the IAP-LacZ reporter (Figure 5D). We observed extra secondary and tertiary pigment cells, as well as duplicated bristles, remaining at 40h after puparium formation (Figure 5E). We also noticed that ft mutant ommatidia had numbers of cone cells that varied between 2 and 5, whereas wild-type ommatidia have 4 cone cells. Apoptosis in pupal retinas occurred at levels similar to that of wild type tissue in ft mutant clones in pupal retinas (Figure 5F). Thus ft mutant clones differed somewhat from ex mutant clones in that more of the supernumary cells generated by proliferation in the eye disc were not subsequently removed by apoptosis. The number of such surviving supernumerary cells was still much less than described for mutations in some other genes, such as components of the Warts pathway, for example (Hamaratoglu et al., 2006; Maitra et al., 2006).
As for ex, ft mutations did not alter levels of Mad phosphorylation in the eye disc (Supplementary Figure 1). In order to test whether ft affected development independently of Dpp signaling, clones of ft tkv mutant cells were examined. Such ft tkv double mutant clones resembled ex tkv clones or ex Mad clones in their failure to differentiate, and continued proliferation, associated with sustained expression of Tsh and Hth proteins (Figure 5G, and data not shown). nkd-lacZ expression was elevated in ft clones, indicating an increase in Wg signaling (Figure 5H). This was not associated with ectopic Wg expression (Supplementary Figure 1). These findings support the view that ft clones, like ex, elevate Wg signaling in the eye in addition to Dpp signaling in the wing.
ex and ft affect intracellular membrane properties
It has been reported that ex and Mer redundantly regulate endocytosis of cell surface receptors (Maitra et al., 2006). Neither we nor others have detected effects of ex single mutants on receptor levels or internalization (Maitra et al., 2006)(data not shown). However, we have uncovered an effect of ex and ft mutations on membrane properties. During immunochemistry, detergents are added to fixed preparations to render cellular membranes permeable to antibodies. Surprisingly, fixed cells homozygous for ex or ft mutations had nuclear membranes that were not rendered permeable by saponins, unlike neighboring wild type or heterozygous cells. Similar observations were made in both eye and wing imaginal discs. Nuclear antigens, including Engrailed, Spalt Major, Groucho, or Cyclin E, were not detected in saponin-treated mutant nuclei, except in mitotic cells where the nuclear membrane has broken down (Figure 6A, B and data not shown). These nuclear antigens were readily detected in the mutant cells when detergents such as Triton X-100 or deoxycholate were used (Figure 6C, and data not shown). By contrast, cytoplasmic antigens such as the intracellular domain of EGF receptor, or the intracellular domain of Notch were detected similarly regardless of detergent, indicating that the plasma membrane is permeable to saponin. In addition, whereas both nuclear and cytoplasmic Cyclin D proteins were detected in cells permeabilized with Triton X-100 and deoxycholate, or in wild type cells permeabilized with saponin, Cyclin D was detected only in the cytoplasm of ex mutant cells permeabilized with saponin (data not shown). The ER membrane may also be abnormal in ex or ft mutant cells, as levels of a lumenal ER protein, Boca (Culi and Mann, 2003), appeared normal in ex or ft cells permeabilized with Triton, but was only detected in wild type cells using saponin (Figure 6D and data not shown). Boca and nuclear antigens such as Engrailed were detected normally in wts mutant cells, regardless of the detergent used, so not all tumor suppressor mutants affect internal membrane properties (Figure 6E).
Figure 6. Detergent permeabilization of fixed cells.
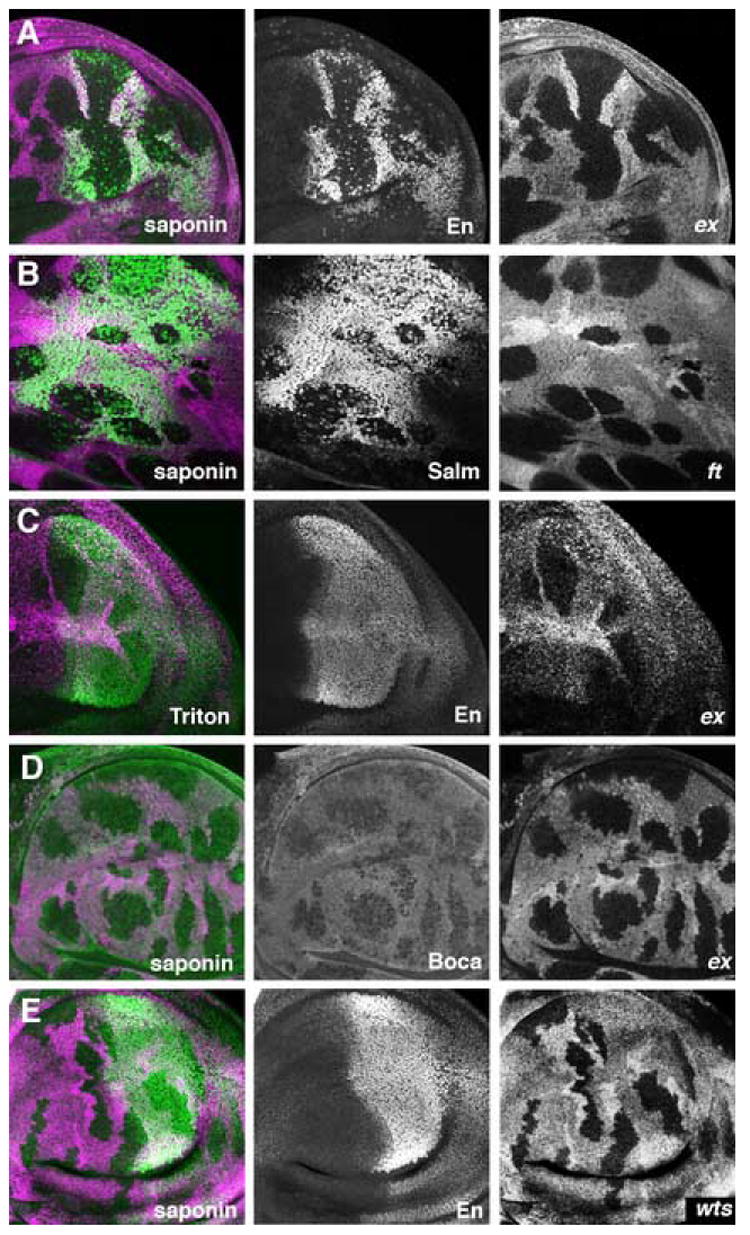
Clones of homozygous ft or ex mutant cells lack the magenta lineage marker in the wing disc.
A. Nuclear En protein (green) is not detected in ex mutant cells permeabilized with saponin. Scattered mitotic cells are labeled within the clones, as expected from nuclear membrane breakdown. En is normally expressed in posterior compartments of the wing disc.
B. Nuclear Salm protein (green) is not detected in ft mutant cells permeabilized with saponin.
C. Nuclear En protein (green) is unaffected by ex mutations when Triton X-100 is used to permeabilize the preparation.
D. The luminal ER protein Boca (green) is detected at reduced levels in ex mutant cells permeabilized with saponin.
E. Nuclear En protein (green) is readily detected in wts mutant cells permabilized with saponin.
Saponins are thought to permeabilize membranes by forming complexes with sterol molecules (Sclosser and Wulff, 1969). Higher concentrations of saponin are required to permeabilize internal membranes because sterol concentrations are much lower there than in the plasma membrane (Colbeau et al., 1971) (Wassler et al., 1987). Our results are consistent with further reduced sterol content in the nuclear and ER membranes of ex or ft mutant cells compared to wild type or wts mutant cells. We attempted to assess sterol levels using filipin, a fluorescent compound that binds to sterols (Drabikowski et al., 1973) (Bornig and Geyer, 1974). Similar levels of filipin binding were observed to plasma membranes of wild type and ex mutant cells, but internal sterols were undetectable in all cases (data not shown). These findings suggest that ex and ft mutations lack gross effects on cellular sterol content, but are consistent with an altered intracellular distribution of these molecules. We also looked at the GPI-linked proteins Dlp and Fasc III, flotillin, which may be involved in lipid-raft mediated endocytosis, and used aerolysin and cholera toxin B subunit as reagents to detect GPI-linked proteins and CM1 gangliosides respectively, without finding obvious differences between wild type and ex mutant cells (data not shown).
Over-expression of ft or ex affect growth and survival
Over-expression was used to compare the gain-of-function phenotypes of ft and ex (Figure 7). We overexpressed Ft and Ex in clones of cells using an actin > CD2 > GAL4 flp-out cassette. We found that clones of cells over-expressing ft (ie act>ft) clones could be recovered using this technique, but not act>ex clones (data not shown). This could be because overexpression of ex prevents growth more effectively than ft, or it could be due to effects on apoptosis. To separate the roles of growth suppression and apoptotic induction, we also co-expressed the apoptotic inhibitor p35 (Hay et al., 1994). We induced clones of cells co-expressing p35 and either ft or ex, and counted the cells in the clones 48h later (Figure 7). We found that that act>p35, ex clones were recovered, so the previous failure to recover act> ex clones can be attributed to apoptosis. We found that that both UAS-ex and UAS-ft suppressed growth of cells with respect to controls expressing p35 alone (Figure 7). There was a quantitative difference between the effects of ex and ft. Expression of ex reduced cell number 2.5-fold, whereas expression of ft reduced cell number 1.8-fold. We cannot say whether this is because of a difference in the activity of these proteins or because of differences in the efficiency of induction of the proteins. We conclude that overexpression of ex and ft suppresses growth, and ex also induces cell death.
Figure 7. Growth inhibition by ectopic ex or ft.
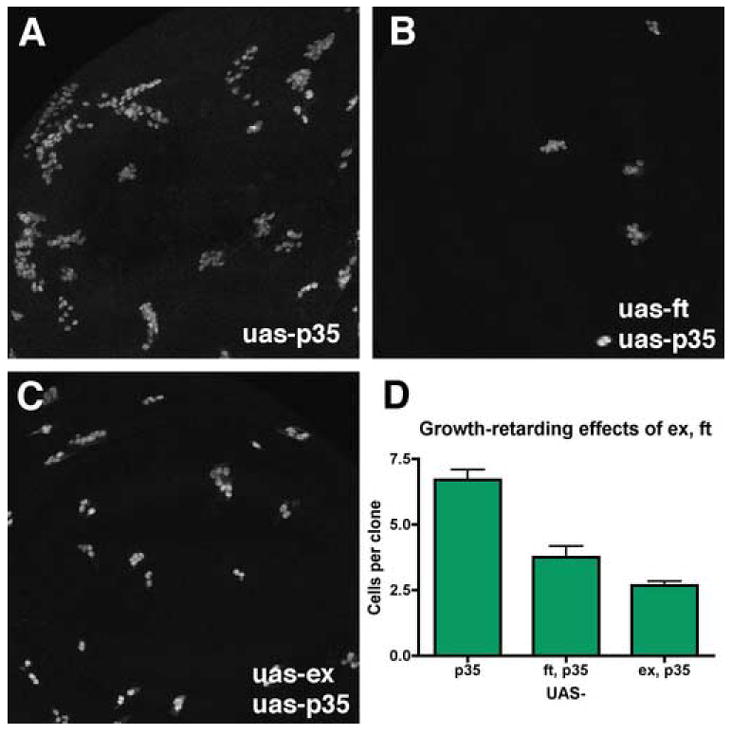
Panels A-C show clones of UAS:GFP UAS:p35-expressing cells induced by FLP-induced recombination to activate an Act:Gal4 transgene.
B. Simultaneous overexpression of ft from a UAS:ft transgene reduces clonal growth 1.8-fold.
C. Simultaneous overexpression of ex form aUAS:ex transgene reduces clonal growth 2.5-fold.
D. Quantification of the results (p35, N=23; p35 ft, N=16; p35 ex, N=40).
Epistatic relationship of Fat , Expanded, and the Warts Pathway
Similar eye phenotypes of ft and ex mutations suggested that these genes might share a common pathway. We hypothesised that ft might function as a receptor at the cell surface, with the ex gene acting as a transducer for the signal. It was possible to test whether the effects of ex anf ft on growth of undifferentiated imaginal discs were also related, making use of the contrasting effects of ft and ex over-expression with their loss-of-function phenotypes to test their epistatic relationship.
MARCM (Lee and Luo, 1999) was used to combine gain- and loss-of-function of ex and ft. Clones mutant for ex or ft (FRT ex or FRT ft clones) were larger and rounder than gratuitously marked control clones (compare Figures 8E, F with 8A). Overexpression of ex driven by Tub:Gal4 (UAS-ex) resulted in a dramatic reduction in size (Figure 8B). UAS-ft clones are also smaller than controls (Figure 8C). It is likely that ectopic ex clones were not completely eliminated in this MARCM experiment, unlike the experiment described above using Act:Gal4, because Tub:Gal4 drives less ectopic expression. We labeled MARCM clones of cells expressing UAS-ft or UAS-ex with CM1 antibody to detect apoptosis. We found that UAS-ex inducedCM1 labeling both in cells overexpressing ex and in wild-type neighbors (Figure 8D). No cell death was associated with cells expressing ft (data not shown).
Figure 8. Epistasis studies of ft , ex and hpo mutants and overexpression.
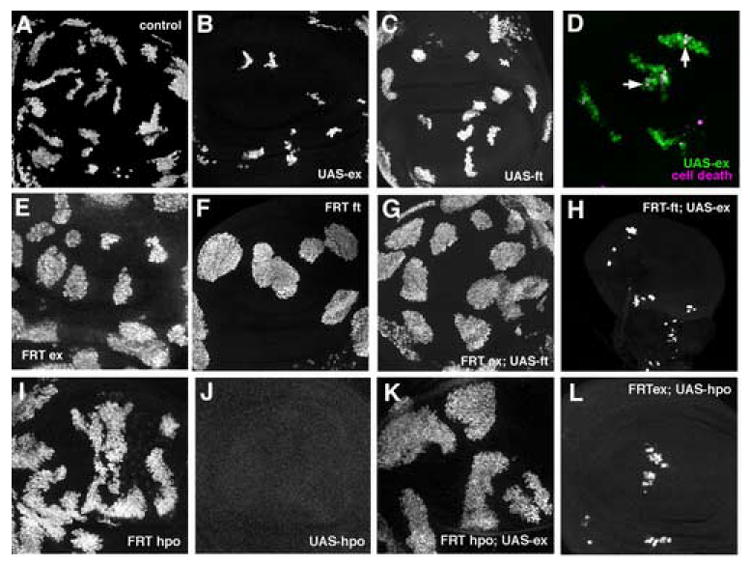
MARCM was used to combine overexpression of GFP, and either ft ,ex, or hpo, with mitotic clones of mutant chromosomes in the wing imaginal disc. GFP expression marks the clones (green in panel B).
A. Otherwise wild type, GFP-expressing control clones induced in parallel with panels C-H.
B. ex overexpressing clones
C. ft overexpressing clones
D. Ex overexpression induced cell death both within and nearby the clones. Cell death identified by CM1 labelling (magenta). Dying, Ex-expressing cells appear white (eg vertical arrow); dying, non-expressing cells appear magenta (eg horizontal arrow).
E. ex mutant clones.
F. ft mutant clones
G. ft overexpression in ex mutant clones.
H. ex overexpression in ft mutant clones. Magnification is the same as other panels; small disc size seems to be a non-autonomous effect
I. hpo mutant clones
J. hpo overexpressing clones (clones were not recovered).
K. ex overexpression in hpo mutant clones.
L. hpo overexpression in ex mutant clones.
In combination, we found that FRT ex; UAS-ft clones were indistinguishable from FRT ex clones (compare Figures 8G and 8E), whereas FRT ft; UAS-ex clones resemble UAS-ex (compare Figures 8H and 8B). This indicated that ex was required for the growth-suppressing activity of ft, whereas ft was not required for ex activity. Unexpectedly, overall disc growth was reduced by the presence of FRT ft; UAS-ex clones (Figure 8H). This suggests some role of ft independent of ex. As the UAS-ft transgene encodes the entire Ft protein, it is not certain whether this nonautonomous effect depends on signal transduction through the Ft intracellular domain, or on interactions of the extracellular domain with other proteins (Matakatsu and Blair, 2006).
Relationship to the wts signaling pathway
Because mutations in tumor suppressors of the Warts pathway resembled ft and ex in affecting cell competition and Dpp signaling (Tyler et al., 2007), we performed further epistasis experiments to assess the relationship to the Warts Pathway gene hpo. We used the MARCM system again to combine gain- and loss-of-function of ex with hpo. Clones mutant for hpo grew larger than controls (Figure 8I). By contrast, UAS-hpo clones were eliminated (Figure 8J). We found that FRT hpo; UAS-ex clones were indistinguishable from FRT hpo clones, consistent with a requirement for hpo in the growth effects of ex (Figure 8K). However, although growth of FRT ex, UAS-hpo clones was severely diminished, such clones survived better than UAS-hpo alone, suggesting some contribution of ex to hpo-induced elimination (Figure 8L).
Discussion
Role ex and ft in growth control
The ex gene acts as a hyperplastic tumor suppressor, and is thought to have overlapping function with the related gene Mer (Hamaratoglu et al., 2006; Maitra et al., 2006; McCartney et al., 2000). We now find that ex null mutations by themselves are sufficient to cause cell cycle entry by cells in the posterior eye, but that they do not prevent apoptosis as ex Mer double mutants do. Mutations in ft resemble ex. We suggest that ft and ex act together on multiple signal transduction pathways, including the Warts Pathway.
Interactions between ex and ft and morphogen signaling
The ft and ex genes encode negative growth regulators thought to play little role in developmental patterning. We report elsewhere that ex and ft mutations rescue M/+ clones that are thought to be competing for Dpp, and elevate expression of the Dpp target gene salm in the developing wing (Tyler et al., 2007) . As ex and ft mutations affect the Dpp target genes Salm and Brk during wing development, even though Mad phosphorylation is little affected (Tyler et al., 2007) (Supplementary Figure 1 and unpublished results), they may affect Dpp subtly, or downstream of Mad phosphorylation. Because Dpp signaling has many patterning roles that seemed not to depend on ex or ft, and also because M/+ clones are also rescued by ex in the absence of Mad, we reasoned that ex and ft might affect other pathways in addition to Dpp, and that such pathways make more significant contributions to the ex and ft mutant phenotypes. Our main results are that both genes antagonize Wg signaling, and that patterning depends on the balance of Wg and Dpp signals, which is not sufficiently perturbed in ex or ft mutants by themselves.
Mutations in ex and ft affect eye development similarly to one another. Neither accelerated the morphogenetic furrow or arrested the cell cycle, as is seen when Dpp signaling is elevated (Baonza and Freeman, 2001; Horsfield et al., 1998; Pignoni and Zipursky, 1997). Instead ft and ex cause only minor patterning defects in addition to the planar polarity effects described previously. However, both mutations elevated Cyclin E expression and DIAP1 transcription, and led to additional cell cycles posterior to the Second Mitotic Wave, a stage at which wild type cells are postmitotic. The supernumerary cells produced were largely eliminated by cell death during the pupal stage, although some supernumerary pigment cells remained in the case of ft.
Unexpectedly, ex Mad, ex tkv, or ft tkv mutant clones were dramatically different from ex or ft clones in that they largely failed to differentiate as retina, instead continuing to proliferate, and differentiating as head cuticle. This was due to Wg signaling, which we found was elevated in ex or ft mutant cells
Ft is a negative regulator of Wg expression in the proximal developing wing (Cho et al., 2006), but we found no effect of ft or ex mutations on Wg expression in eye antennal discs (Supplementary Figure 1 and data not shown). We note that Wg signaling is repressed by ft in distal wing tissues without any effect on Wg expression, and agree with other authors that ft, and ex, may primarily affect Wg signaling (Jaiswal et al., 2006). Wg signaling could affect Wg expression secondarily, in those tissues where Wg expression is wg-dependent, as it is in the proximal wing (Rodriguez et al., 2002).
The data indicate that the Dpp and Wg signaling that occur in ex or ft mutant cells antagonize one another so that there is little effect on patterning. When Dpp signaling is prevented by mutation of tkv or Mad, the elevated Wg signaling then has significant patterning effects. The ectopic Wg signaling is not caused by tkv or Mad mutations, which do not block differentiation by themselves. Instead a reporter of Wg signaling activity was elevated in ex and ft mutant cells. The ex or ft mutant cells differentiate, however, and this must be attributed to their ability to respond to Dpp, because such differentiation depends on the tkv and Mad genes. Wg and Dpp are thought to act antagonistically in normal eye differentiation (Hazelett et al., 1998; Lee and Treisman, 2001). Interestingly, ex has previously been shown to enhance dominantly the planar polarity phenotype of overexpressed dishevelled (dsh) (Blaumueller and Mlodzik, 2000). As Dsh is involved in wg signaling, the interaction with ectopic Dsh might also be explained by the elevated Wg signaling that occurs in ex clones.
Mechanism of ex and ft function
It was recently reported that multiple signaling pathways are upregulated in ex, Merlin double mutant clones through reduced receptor clearance from the cell surface (Maitra et al., 2006). It has proven difficult to demonstrate effect on receptor distribution in the single mutants ((Maitra et al., 2006)and our unpublished results). We find that Dpp and Wg signaling are elevated in ex single mutants, and in mutants for ft.
Endocytosis is thought to contribute positively to signaling by Dpp, Wg, N and receptor tyrosine kinases (Fischer et al., 2006) How would mutations reducing endocytosis elevate signaling, as found for ex Mer? The ex and ft mutations may affect the distribution of membrane sterols. If ex and ft affect the sterol-rich lipid raft compartment, or the sorting of proteins into such compartments, then this may explain signaling pathway activation, because although clathrin-mediated endocytosis is positively required for many signaling pathways, caveolin/lipid raft mediated endocytosis leads to receptor degradation (Di Guglielmo et al., 2003; Polo and Di Fiore, 2006). Although we have not studied Mer mutations, Merlin protein is associated with both lipid rafts and endosomes, and is relocalized between different lipid raft domains when it switches between its inactive and its active, growth-suppressive states (Stickney et al., 2004). We hypothesize that elevated signaling, and altered properties of intracellular membranes, are both consequences of a change in sterol-rich membrane domains in ex or ft mutations. Further studies will be required to identify whether ex and ft affect endocytosis because they modulate membrane sterols, or whether the distribution of membrane sterols is affected secondary to lipid raft-mediated endocytosis, or whether ex and ft act through other mechanisms.
ex and ft in growth regulation
ex and ft mutations have similar effects on growth control and planar polarity (Blaumueller and Mlodzik, 2000; Boedigheimer and Laughon, 1993; Bryant et al., 1988; Mahoney et al., 1991; Rawls et al., 2002; Yang et al., 2002). We find they also have similar effects on cyclin E and DIAP expression, on Dpp and Wg signaling, and on membrane permeability. They differ in that ectopic ex caused apoptosis, which we did not detect from ectopic ft, and in that ft mutations have additional nonautonomous effects on the SMW (Figure 5B and data not shown). We suggest that ex and ft might act on a common pathway.
Epistasis data suggest that ex acts downstream of ft, at least with respect to growth. We found that growth retarding effects of ectopic ft expression required the ex gene, whereas ectopic ex retarded growth independently of ft. Ft has also been proposed to signal through atrophin, whose mutations mimic a subset of ft phenotypes including effects on planar polarity but not growth, and through dachs, which appears to mediate effects of ft on growth and the expression of target genes including wg and rotund (Cho and Irvine, 2004; Fanto et al., 2003). Because ex affects planar polarity and growth, ex may act before the dachs- and atrophin- mediated pathways diverge, close to ft.
It is now thought that ex, along with another FERM domain protein, Merlin, modulates the Warts pathway (Edgar, 2006; Hamaratoglu et al., 2006) (Figure 8). Our results suggest that ft encodes a transmembrane protein that regulates the Warts Pathway through Ex and Mer to control growth. While this paper has been under review, others reached similar conclusions (Cho et al., 2006) (Bennett and Harvey, 2006; Willecke et al., 2006) (Silva et al., 2006). Our studies add to this recent work, which assayed gene expression in the proximal wing, and gene expression and ectopic proliferation in the eye disc posterior to the morphogenetic furrow, by directly showing that ft depends on ex and ex depends on hpo to regulate clonal growth in undifferentiated imaginal discs. In addition, particular results (Figure 8) (Cho et al., 2006), suggest that ex might also affect growth partially independently of hpo, and that ft may affect growth partially independently of the cell autonomous effects on ex (Figure 8) (Cho et al., 2006)(our unpublished data). We also found that wts mutations did not affect membrane properties as ex and ft did (Figure 6). Taken together, these data suggest that although ft and ex affect Warts Pathway activity, the relationship may not be entirely linear, and that ex and ft affect growth and survival by other mechanisms in addition, including Wg, Dpp, and perhaps other signaling pathways.
Supplementary Material
Acknowledgments
We thank J. Axelrod, I. Hariharan, K. Irvine, M. Mlodzik, DJ Pan, M. A. Simon, D. Strutt, N. Tapon, K. Wharton, and the Drosophila Stock Center at Bloomington Indiana for Drosophila strains. Antibodies were provided by H. Bellen, N. Dyson, R. Mann, B. Mollereau, H. Richardson, and the Developmental Studies Hybridoma Bank, maintained by the University of Iowa, Department of Biological Sciences, Iowa City IA52242, USA under contract N01-HD-7-3263 from the NICHD. We are particularly grateful to I. Hariharan, K. Irvine, H. MacNeill, B. Pellock, and D. Strutt for communicating results and reagents in advance of publication, to L. Firth and M. Cammer and the AECOM Analytical Imaging Center for help and advice, and to M. Keilian and M. Lisanti for useful discussions. We thank members of our lab and J. Treisman for comments on the manuscript. NEB is a Scholar of the Irma T. Hirschl Trust. Supported by grant GM61230 from the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker NE. Transcription of the segment polarity gene wingless in the imaginal discs of Drosophila, and the phenotype of a pupal-lethal wg mutation. Development. 1988;102:489–497. doi: 10.1242/dev.102.3.489. [DOI] [PubMed] [Google Scholar]
- Baker NE, Yu SY. The EGF receptor defines domains of cell cycle progression and survival to regulate cell number in the developing Drosophila eye. Cell. 2001;104:699–708. doi: 10.1016/s0092-8674(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Baonza A, Freeman M. Notch signalling and the initiation of neural development in the Drosophila eye. Development. 2001;128:3889–98. doi: 10.1242/dev.128.20.3889. [DOI] [PubMed] [Google Scholar]
- Baonza A, Freeman M. Control of Drosophila eye specification by Wingless signalling. Development. 2002;129:5313–22. doi: 10.1242/dev.00096. [DOI] [PubMed] [Google Scholar]
- Bennett FC, Harvey KF. Fat cadherin modulates organ size in Drosophila via the Salvador/Warts/Hippo signaling pathway. Curr Biol. 2006;16:2101–2110. doi: 10.1016/j.cub.2006.09.045. [DOI] [PubMed] [Google Scholar]
- Bessa J, Gebelein B, Pichaud F, Casares F, Mann RS. Combinatorial control of Drosophila eye development by eyeless, homothorax, and teashirt. Genes Dev. 2002;16:2415–27. doi: 10.1101/gad.1009002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchette-Mackie EJ, Dwyer NK, Amende LM, Kruth HS, Butler JD, Sokol J, Comly ME, Vanier MT, August JT, Brady RO, Pentchev PG. Type-C Niemann-Pick disease: Low density lipoprotein uptake is associated with premature cholesterol accumulation in the Golgi complex and excessive cholesterol storage in lysosomes. Proc Natl Acad Sci U S A. 1988;85:8022–8026. doi: 10.1073/pnas.85.21.8022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaumueller CM, Mlodzik M. The Drosophila tumor suppressor expanded regulates growth, apoptosis, and patterning during development. Mech Dev. 2000;92:251–62. doi: 10.1016/s0925-4773(00)00246-x. [DOI] [PubMed] [Google Scholar]
- Boedigheimer M, Laughon A. Expanded: a gene involved in the control of cell proliferation in imaginal discs. Development. 1993;118:1291–301. doi: 10.1242/dev.118.4.1291. [DOI] [PubMed] [Google Scholar]
- Boedigheimer MJ, Nguyen KP, Bryant PJ. Expanded functions in the apical cell domain to regulate the growth rate of imaginal discs. Dev Genet. 1997;20:103–10. doi: 10.1002/(SICI)1520-6408(1997)20:2<103::AID-DVG3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Bonini NM, Leiserson WM, Benzer S. The eyes absent gene: genetic control of cell survival and differentiation in the developing Drosophila eye. Cell. 1993;72:379–95. doi: 10.1016/0092-8674(93)90115-7. [DOI] [PubMed] [Google Scholar]
- Bornig H, Geyer G. Staining of cholesterol with the fluorescent antibiotic “filipin”. Acta Histochem. 1974;174:110–115. [PubMed] [Google Scholar]
- Brachmann CB, Cagan RL. Patterning the fly eye: the role of apoptosis. Trends Genet. 2003;19:91–6. doi: 10.1016/S0168-9525(02)00041-0. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002;3:586–99. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- Brook WJ, Cohen SM. Antagonistic interactions between wingless and decapentaplegic responsible for dorsal-ventral pattern in the Drosophila Leg. Science. 1996;273:1373–7. doi: 10.1126/science.273.5280.1373. [DOI] [PubMed] [Google Scholar]
- Bryant PJ, Huettner B, Held LI, Jr, Ryerse J, Szidonya J. Mutations at the fat locus interfere with cell proliferation control and epithelial morphogenesis in Drosophila. Dev Biol. 1988;129:541–54. doi: 10.1016/0012-1606(88)90399-5. [DOI] [PubMed] [Google Scholar]
- Burke R, Basler K. Dpp receptors are autonomously required for cell proliferation in the entire developing Drosophila wing. Development. 1996a;122:2261–9. doi: 10.1242/dev.122.7.2261. [DOI] [PubMed] [Google Scholar]
- Burke R, Basler K. Hedgehog-dependent patterning in the Drosophila eye can occur in the absence of Dpp signaling. Dev Biol. 1996b;179:360–8. doi: 10.1006/dbio.1996.0267. [DOI] [PubMed] [Google Scholar]
- Casares F, Mann RS. Control of antennal versus leg development in Drosophila. Nature. 1998;392:723–6. doi: 10.1038/33706. [DOI] [PubMed] [Google Scholar]
- Cho E, Feng Y, Rauskolb C, Maitra S, Fehon RG, Irvine KD. Delineation of a Fat tumor suppressor pathway. Nature Genetics. 2006;38:1142–1150. doi: 10.1038/ng1887. [DOI] [PubMed] [Google Scholar]
- Cho E, Irvine KD. Action of fat, four-jointed, dachsous and dachs in distal-to-proximal wing signaling. Development. 2004;131:4489–500. doi: 10.1242/dev.01315. [DOI] [PubMed] [Google Scholar]
- Colbeau A, Nachbaur J, Vignais PM. Enzymic characterization and lipid composition of rat liver subcellular membranes. Biochim Biophys Acta. 1971;249:462–492. doi: 10.1016/0005-2736(71)90123-4. [DOI] [PubMed] [Google Scholar]
- Conlon I, Raff M. Size control in animal development. Cell. 1999;96:235–44. doi: 10.1016/s0092-8674(00)80563-2. [DOI] [PubMed] [Google Scholar]
- Culi J, Mann RS. Boca, an endoplasmic reticulum protein required for wingless signaling and trafficking of LDL receptor family members in Drosophila. Cell. 2003;112:343–354. doi: 10.1016/s0092-8674(02)01279-5. [DOI] [PubMed] [Google Scholar]
- Curtiss J, Mlodzik M. Morphogenetic furrow initiation and progression during eye development in Drosophila: the roles of decapentaplegic, hedgehog and eyes absent. Development. 2000;127:1325–1336. doi: 10.1242/dev.127.6.1325. [DOI] [PubMed] [Google Scholar]
- Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat Cell Biol. 2003;5:410–21. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- Dominguez M, Casares F. Organ specification-growth control connection: new insights from the Drosophila eye-antennal disc. Dev Dyn. 2005;232:673–684. doi: 10.1002/dvdy.20311. [DOI] [PubMed] [Google Scholar]
- Drabikowski W, Lagwinska E, Sarzala MG. Filipin as a fluorescent probe for the location of cholesterol in the membranes of fragmented sarcoplasmic reticulum. Biochim Biophys Acta. 1973;291:61–70. doi: 10.1016/0005-2736(73)90060-6. [DOI] [PubMed] [Google Scholar]
- Edgar BA. From cell structure to transcription: Hippo forges a new path. Cell. 2006;124:267–273. doi: 10.1016/j.cell.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Edgar BA, Britton JS, de l Cruz AF, Johnston LA, Lehman DA, Martin-Castellanos C, Prober D. Pattern- and growth-linked cell cycles in Drosophila development. Novartis Foundation Symposium. 2001;237:3–12. doi: 10.1002/0470846666.ch2. [DOI] [PubMed] [Google Scholar]
- Evans T, Rosenthal ET, Youngblom J, Hunt T. Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell. 1983;33:389–396. doi: 10.1016/0092-8674(83)90420-8. [DOI] [PubMed] [Google Scholar]
- Fang M, Li J, Blauwkamp T, Campbell N, Cadigan KM. C-terminal-binding protein directly activates and represses Wnt transcriptional targets in Drosophila. Embo J. 2006;25:2735–2745. doi: 10.1038/sj.emboj.7601153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanto M, Clayton L, Meredith J, Hardiman K, Charroux B, Kerridge S, McNeill H. The tumor-suppressor and cell adhesion molecule Fat controls planar polarity via physical interactions with Atrophin, a transcriptional co-repressor. Development. 2003;130:763–74. doi: 10.1242/dev.00304. [DOI] [PubMed] [Google Scholar]
- Firth LC, Baker NE. Extracellular signals responsible for spatially regulated proliferation in the differentiating Drosophila eye. Dev Cell. 2005;8:541–51. doi: 10.1016/j.devcel.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Fischer JA, Eun SH, Doolan BT. Endocytosis, edosome trafficking, and the regulation of Drosophila development. Annu Rev Cell Dev Biol. 2006;22:181–206. doi: 10.1146/annurev.cellbio.22.010605.093205. [DOI] [PubMed] [Google Scholar]
- Freeman M. Reiterative use of the EGF receptor triggers differentiation of all cell types in the Drosophila eye. Cell. 1996;87:651–60. doi: 10.1016/s0092-8674(00)81385-9. [DOI] [PubMed] [Google Scholar]
- Galbiati F, Volonte D, Golz JS, Steele Z, Sen J, Jurcsak J, Stein D, Stevens L, Lisanti MP. Identification, sequence and developmental expression of invertebrate flotillins from Drosophila melanogaster. Gene. 1998;210:229–237. doi: 10.1016/s0378-1119(98)00064-x. [DOI] [PubMed] [Google Scholar]
- Gibson MC, Perrimon N. Extrusion and death of DPP/BMP-compromised epithelial cells in the developing Drosophila wing. Science. 2005;307:1785–9. doi: 10.1126/science.1104751. [DOI] [PubMed] [Google Scholar]
- Greenwood S, Struhl G. Progression of the morphogenetic furrow in the Drosophila eye: the roles of Hedgehog, Decapentaplegic and the Raf pathway. Development. 1999;126:5795–808. doi: 10.1242/dev.126.24.5795. [DOI] [PubMed] [Google Scholar]
- Hamaratoglu F, Willecke M, Kango-Singh M, Nolo R, Hyun E, Tao C, Jafar-Nejad H, Halder G. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol. 2006;8:27–36. doi: 10.1038/ncb1339. [DOI] [PubMed] [Google Scholar]
- Hariharan IK. Growth regulation: a beginning for the Hippo Pathway. Curr Biol. 2006;16:R1037–R1039. doi: 10.1016/j.cub.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Harvey KF, Pfleger CM, Hariharan IK. The Drosophila Mst Ortholog, hippo, Restricts Growth and Cell Proliferation and Promotes Apoptosis. Cell. 2003;114:457–67. doi: 10.1016/s0092-8674(03)00557-9. [DOI] [PubMed] [Google Scholar]
- Hay BA, Guo M. Coupling cell growth, proliferation, and death. Hippo weighs in. Dev Cell. 2003;5:361–3. doi: 10.1016/s1534-5807(03)00270-3. [DOI] [PubMed] [Google Scholar]
- Hay BA, Wolff T, Rubin GM. Expression of baculovirus P35 prevents cell death in Drosophila. Development. 1994;120:2121–9. doi: 10.1242/dev.120.8.2121. [DOI] [PubMed] [Google Scholar]
- Hazelett DJ, Bourouis M, Walldorf U, Treisman JE. decapentaplegic and wingless are regulated by eyes absent and eyegone and interact to direct the pattern of retinal differentiation in the eye disc. Development. 1998;125:3741–51. doi: 10.1242/dev.125.18.3741. [DOI] [PubMed] [Google Scholar]
- Hipfner DR, Cohen SM. Connecting proliferation and apoptosis in development and disease. Nat Rev Mol Cell Biol. 2004;5:805–15. doi: 10.1038/nrm1491. [DOI] [PubMed] [Google Scholar]
- Horsfield J, Penton A, Secombe J, Hoffman FM, Richardson H. decapentaplegic is required for arrest in G1 phase during Drosophila eye development. Development. 1998;125:5069–78. doi: 10.1242/dev.125.24.5069. [DOI] [PubMed] [Google Scholar]
- Jaiswal M, Agrawal N, Sinha P. Fat and Wingless signaling oppositely regulate epithelial cell-cell adhesion and distal wing development in Drosophila. Development. 2006;133:925–935. doi: 10.1242/dev.02243. [DOI] [PubMed] [Google Scholar]
- Johnston LA, Sanders AL. Wingless promotes cell survival but constrains growth during Drosophila wing development. Nat Cell Biol. 2003;5:827–33. doi: 10.1038/ncb1041. [DOI] [PubMed] [Google Scholar]
- Knoblich JA, Lehner CF. Synergistic action of Drosophila cyclins A and B during the G2-M transition. Embo J. 1993;12:65–74. doi: 10.1002/j.1460-2075.1993.tb05632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnlein RP, Frommer G, Friedrich M, Gonzalez-Gaitan M, Weber A, Wagner-Bernholz JF, Gehring WJ, Jackle H, Schuh R. spalt encodes an evolutionarily conserved zinc finger protein of novel structure which provides homeotic gene function in the head and tail region of the Drosophila embryo. Embo J. 1994;13:168–79. doi: 10.1002/j.1460-2075.1994.tb06246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JD, Treisman JE. The role of Wingless signaling in establishing the anteroposterior and dorsoventral axes of the eye disc. Development. 2001;128:1519–29. doi: 10.1242/dev.128.9.1519. [DOI] [PubMed] [Google Scholar]
- Lee JD, Treisman JE. Regulators of the morphogenetic furrow. Results Prob Cell Differen. 2002;37:21–33. doi: 10.1007/978-3-540-45398-7_3. [DOI] [PubMed] [Google Scholar]
- Lee LA, Orr-Weaver TL. Regulation of cell cycles in Drosophila development: intrinsic and extrinsic cues. Annu Rev Genet. 2003;37:545–78. doi: 10.1146/annurev.genet.37.110801.143149. [DOI] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–61. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- Lowe SW, Cepero E, Evan G. Intrinsic tumour suppression. Nature. 2004;432:307–15. doi: 10.1038/nature03098. [DOI] [PubMed] [Google Scholar]
- Lum L, Yao S, Mozer B, Rovescalli A, Von Kessler D, Nirenberg M, Beachy PA. Identification of Hedgehog pathway components by RNA in Drosophila cultured cells. Science. 2003;299:2039–2045. doi: 10.1126/science.1081403. [DOI] [PubMed] [Google Scholar]
- Ma C, Moses K. Wingless and patched are negative regulators of the morphogenetic furrow and can affect tissue polarity in the developing Drosophila compound eye. Development. 1995;121:2279–2289. doi: 10.1242/dev.121.8.2279. [DOI] [PubMed] [Google Scholar]
- Ma C, Zhou Y, Beachy PA, Moses K. The segment polarity gene hedgehog is required for progression of the morphogenetic furrow in the developing Drosophila eye. Cell. 1993;75:927–38. doi: 10.1016/0092-8674(93)90536-y. [DOI] [PubMed] [Google Scholar]
- Mahoney PA, Weber U, Onofrechuk P, Biessmann H, Bryant PJ, Goodman CS. The fat tumor suppressor gene in Drosophila encodes a novel member of the cadherin gene superfamily. Cell. 1991;67:853–68. doi: 10.1016/0092-8674(91)90359-7. [DOI] [PubMed] [Google Scholar]
- Maitra S, Kulikauskas R, Gavilan H, Fehon R. The tumor suppressors Merlin and expanded function cooperatively to modulate receptor endocytosis and signaling. Curr Biol. 2006;16:702–709. doi: 10.1016/j.cub.2006.02.063. [DOI] [PubMed] [Google Scholar]
- Martin FA, Perez-Garijo A, Moreno E, Morata G. The brinker gradient controls wing growth in Drosophila. Development. 2004;131:4921–30. doi: 10.1242/dev.01385. [DOI] [PubMed] [Google Scholar]
- Massague J. TGFbeta signaling: receptors, transducers, and Mad proteins. Cell. 1996;85:947–50. doi: 10.1016/s0092-8674(00)81296-9. [DOI] [PubMed] [Google Scholar]
- Matakatsu H, Blair SS. Separating the adhesive and signaling functions of the Fat and Dachsous protocadherins. Development. 2006;133:2315–2324. doi: 10.1242/dev.02401. [DOI] [PubMed] [Google Scholar]
- McCartney BM, Kulikauskas RM, LaJeunesse DR, Fehon RG. The neurofibromatosis-2 homologue, Merlin, and the tumor suppressor expanded function together in Drosophila to regulate cell proliferation and differentiation. Development. 2000;127:1315–24. doi: 10.1242/dev.127.6.1315. [DOI] [PubMed] [Google Scholar]
- Moreno E, Basler K, Morata G. Cells compete for decapentaplegic survival factor to prevent apoptosis in Drosophila wing development. Nature. 2002;416:755–9. doi: 10.1038/416755a. [DOI] [PubMed] [Google Scholar]
- Motzny CK, Holmgren R. The Drosophila cubitus interruptus protein and its role in the wingless and hedgehog signal transduction pathways. Mech Dev. 1995;52:137–50. doi: 10.1016/0925-4773(95)00397-j. [DOI] [PubMed] [Google Scholar]
- Negre N, Ghysen A, Martinez AM. Mitotic G2-arrest is required for neural cell fate determination in Drosophila. Mech Dev. 2003;120:253–65. doi: 10.1016/s0925-4773(02)00419-7. [DOI] [PubMed] [Google Scholar]
- Newsome TP, Asling B, Dickson BJ. Analysis of Drosophila photoreceptor axon guidance in eye-specific mosaics. Development. 2000;127:851–60. doi: 10.1242/dev.127.4.851. [DOI] [PubMed] [Google Scholar]
- Nolo R, Abbott LA, Bellen HJ. Senseless, a Zn finger transcription factor, is necessary and sufficient for sensory organ development in Drosophila. Cell. 2000;102:349–62. doi: 10.1016/s0092-8674(00)00040-4. [DOI] [PubMed] [Google Scholar]
- Pantalacci S, Tapon N, Leopold P. The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat Cell Biol. 2003;5:921–7. doi: 10.1038/ncb1051. [DOI] [PubMed] [Google Scholar]
- Pappu K, Mardon G. Retinal specification and determination in Drosophila. Res Problems Cell Differen. 2002;37:5–20. doi: 10.1007/978-3-540-45398-7_2. [DOI] [PubMed] [Google Scholar]
- Patel NH, Martin-Blanco E, Coleman KG, Poole SJ, Ellis MC, Kornberg TB, Goodman CS. Expression of engrailed proteins in arthropods, annelids, and chordates. Cell. 1989;58:955–968. doi: 10.1016/0092-8674(89)90947-1. [DOI] [PubMed] [Google Scholar]
- Patel NH, Snow PM, Goodman CS. Characterization and cloning of fasciclin III: a glycoprotein expressed on a subset of neurons and axon pathways in Drosophila. Cell. 1987;48:975–988. doi: 10.1016/0092-8674(87)90706-9. [DOI] [PubMed] [Google Scholar]
- Penton A, Selleck SB, Hoffmann FM. Regulation of cell cycle synchronization by decapentaplegic during Drosophila eye development. Science. 1997;275:203–6. doi: 10.1126/science.275.5297.203. [DOI] [PubMed] [Google Scholar]
- Pignoni F, Zipursky SL. Induction of Drosophila eye development by decapentaplegic. Development. 1997;124:271–8. doi: 10.1242/dev.124.2.271. [DOI] [PubMed] [Google Scholar]
- Polo S, Di Fiore PP. Endocytosis conducts the cell signaling orchestra. Cell. 2006;124:897–900. doi: 10.1016/j.cell.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Rawls AS, Guinto JB, Wolff T. The cadherins fat and dachsous regulate dorsal/ventral signaling in the Drosophila eye. Curr Biol. 2002;12:1021–6. doi: 10.1016/s0960-9822(02)00893-x. [DOI] [PubMed] [Google Scholar]
- Richardson H, O’Keefe LV, Marty T, Saint R. Ectopic cyclin E expression induces premature entry into S phase and disrupts pattern formation in the Drosophila eye imaginal disc. Development. 1995;121:3371–9. doi: 10.1242/dev.121.10.3371. [DOI] [PubMed] [Google Scholar]
- Robinow S, White K. Characterization and spatial distribution of the ELAV protein during Drosophila melanogaster development. J Neurobiol. 1991;22:443–61. doi: 10.1002/neu.480220503. [DOI] [PubMed] [Google Scholar]
- Rodriguez DDA, Terriente J, Galindo MI, Couso JP, Diaz-Benjumea FJ. Different mechanisms initiate and maintain wingless expression in the Drosophila wing hinge. Development. 2002;129:3995–4004. doi: 10.1242/dev.129.17.3995. [DOI] [PubMed] [Google Scholar]
- Ryoo HD, Bergmann A, Gonen H, Ciechanover A, Steller H. Regulation of Drosophila IAP1 degradation and apoptosis by reaper and ubcD1. Nat Cell Biol. 2002;4:432–8. doi: 10.1038/ncb795. [DOI] [PubMed] [Google Scholar]
- Saburi S, McNeill H. Organising cells into tissues: new roles for cell adhesion molecules in planar cell polarity. Curr Opin Cell Biol. 2005 doi: 10.1016/j.ceb.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Sclosser E, Wulff G. Structural specificity of saponin hemolysis. I. Triterpene saponins and aglycones. Z Naturforschung B. 1969;24:1284–1290. [PubMed] [Google Scholar]
- Sekelsky JJ, Newfeld SJ, Raftery LA, Chartoff EH, Gelbart WM. Genetic characterization and cloning of mothers against dpp, a gene required for decapentaplegic function in Drosophila melanogaster. Genetics. 1995;139:1347–58. doi: 10.1093/genetics/139.3.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Dahmann C. Extrusion of cells with inappropriate Dpp signaling from Drosophila wing disc epithelia. Science. 2005;307:1789–90. doi: 10.1126/science.1104784. [DOI] [PubMed] [Google Scholar]
- Silva E, Tsatskis Y, Gardano L, Tapon N, McNeill H. The tumour suppressor gene fat controls tissue growth upstream of Expanded in the Hippo signalling pathway. Curr Biol. 2006;16:2081–2089. doi: 10.1016/j.cub.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Silver SJ, Rebay I. Signaling circuitries in development: insights from the retinal determination gene network. Development. 2005;132:3–13. doi: 10.1242/dev.01539. [DOI] [PubMed] [Google Scholar]
- Singh A, Kango-Singh M, Sun YH. Eye suppression, a novel function of teashirt, requires Wingless signaling. Development. 2002;129:4271–80. doi: 10.1242/dev.129.18.4271. [DOI] [PubMed] [Google Scholar]
- Srinivasan A, Roth KA, Sayers RO, Shindler KS, Wong AM, Fritz LC, Tomaselli K. In situ immunodetection of activated caspase-3 in apoptotic neurons in the developing nervous system. Cell Death and Differentiation. 1998;5:1004–1016. doi: 10.1038/sj.cdd.4400449. [DOI] [PubMed] [Google Scholar]
- Stickney JT, Bacon WC, Rojas M, Ratner N, Ip W. Activation of the tumor suppressor merlin modulates its interaction with lipid rafts. Cancer Res. 2004;64:2717–24. doi: 10.1158/0008-5472.can-03-3798. [DOI] [PubMed] [Google Scholar]
- Tapon N, Harvey K, Bell D, Wahrer D, Schiripo T, Haber D, Hariharan I. salvador Promotes Both Cell Cycle Exit and Apoptosis in Drosophila and Is Mutated in Human Cancer Cell Lines. Cell. 2002;110:467. doi: 10.1016/s0092-8674(02)00824-3. [DOI] [PubMed] [Google Scholar]
- Thomas BJ, Gunning DA, Cho J, Zipursky L. Cell cycle progression in the developing Drosophila eye: roughex encodes a novel protein required for the establishment of G1. Cell. 1994;77:1003–14. doi: 10.1016/0092-8674(94)90440-5. [DOI] [PubMed] [Google Scholar]
- Tomlinson A, Ready DF. Neuronal differentiation in the Drosophila ommatidium. Dev Biol. 1987;120:336–376. doi: 10.1016/0012-1606(87)90239-9. [DOI] [PubMed] [Google Scholar]
- Treisman JE, Rubin GM. wingless inhibits morphogenetic furrow movement in the Drosophila eye disc. Development. 1995;121:3519–3527. doi: 10.1242/dev.121.11.3519. [DOI] [PubMed] [Google Scholar]
- Tyler D, Li W, Zhou N, Pellock B, Baker NE. Genes affecting cell competition in Drosophila melanogaster. Genetics. 2007 doi: 10.1534/genetics.106.061929. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voas MG, Rebay I. Signal integration during development: insights from the Drosophila eye. Dev Dyn. 2004;229:162–75. doi: 10.1002/dvdy.10449. [DOI] [PubMed] [Google Scholar]
- Vrailas AD, Moses K. Smoothened, thickveins and the genetic control of cell cycle and cell fate in the developing Drosophila eye. Mech Dev. 2006;123:151–165. doi: 10.1016/j.mod.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Wassler M, Jonasson I, Persson R, Fries E. Differential permeabilization of membranes by saponin treatment of isolated rat hepatocytes. Release of secretory proteins. Biochem J. 1987;247:407–415. doi: 10.1042/bj2470407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willecke M, Hamaratoglu F, Kango-Singh M, Uden R, Chen CI, Tao C, Zhang X, Halder G. The Fat cadherin acts through the Hippo tumor-suppressor pathway to regulate tissue size. Curr Biol. 2006;16:2090–2100. doi: 10.1016/j.cub.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Willert K, Logan CY, Arora A, Fish M, Nusse R. A Drosophila Axin homolog, Daxin, inhibits Wnt signaling. Development. 1999;126:4165–73. doi: 10.1242/dev.126.18.4165. [DOI] [PubMed] [Google Scholar]
- Wolff T, Ready DF. Pattern formation in the Drosophila retina. In: Bate M, Martinez Arias A, editors. The Development of Drosophila melanogaster. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1993. pp. 1277–1326. [Google Scholar]
- Woods DF, Wu JW, Bryant PJ. Localization of proteins to the apico-lateral junctions of Drosophila epithelia. Dev Genet. 1997;20:111–8. doi: 10.1002/(SICI)1520-6408(1997)20:2<111::AID-DVG4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Wu J, Cohen SM. Proximal distal axis formation in the Drosophila leg: distinct functions of teashirt and homothorax in the proximal leg. Mech Dev. 2000;94:47–56. doi: 10.1016/s0925-4773(00)00311-7. [DOI] [PubMed] [Google Scholar]
- Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–37. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- Yang CH, Axelrod JD, Simon MA. Regulation of Frizzled by fat-like cadherins during planar polarity signaling in the Drosophila compound eye. Cell. 2002;108:675–88. doi: 10.1016/s0092-8674(02)00658-x. [DOI] [PubMed] [Google Scholar]
- Yu SY, Yoo SJ, Yang L, Zapata C, Srinivasan A, Hay BA, Baker NE. A pathway of signals regulating effector and initiator caspases in the developing Drosophila eye. Development. 2002;129:3269–78. doi: 10.1242/dev.129.13.3269. [DOI] [PubMed] [Google Scholar]
- Zeng W, Wharton KA, Jr, Mack JA, Wang K, Gadbaw M, Suyama K, Klein PS, Scott MP. naked cuticle encodes an inducible antagonist of Wnt signalling. Nature. 2000;403:789–95. doi: 10.1038/35001615. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


