Abstract
Understanding the influence of maternal exposures on gestational age and birth weight is essential given that pre-term and/or low birth weight infants are at risk for increased mortality and morbidity. We performed a retrospective analysis of a cohort exposed to polybrominated biphenyls (PBB) through accidental contamination of cattle feed and polychlorinated biphenyls (PCB) through residual contamination in the geographic region. Our study population consisted of 444 mothers and their 899 infants born between 1975 and 1997. Using restricted maximum likelihood estimation, no significant association was found between estimated maternal serum PBB at conception or enrollment PCB levels and gestational age or infant birth weight in unadjusted models or in models that adjusted for maternal age, smoking, parity, infant gender, and decade of birth. For enrollment maternal serum PBB, no association was observed for gestational age. However, a negative association with high levels of enrollment maternal serum PBB and birth weight was suggested. We also examined the birth weight and gestational age among offspring of women with the highest (10%) PBB or PCB exposure, and observed no significant association. Because brominated compounds are currently used in consumer products and therefore, are increasingly prevalent in the environment, additional research is needed to better understand the potential relationship between in utero exposure to brominated compounds and adverse health outcomes.
Keywords: maternal exposures, infant health, environmental toxicants, gestational age, birth weight, PBB, PCB
INTRODUCTION
Infant gestational age and birth weight are reflective of developmental progression from the time of conception to birth, and also are associated with various health outcomes in later life. For those infants born pre-term or of low birth weight, there is a heightened risk of morbidities, including developmental disabilities and chronic conditions, and mortality (McCormick, 1985; Kramer et al., 2000; Kramer, 2003). While the proportion of infants born of low birth weight in developed countries has declined (Kramer, 2003), the overall number of pre-term births is on the rise in industrialized nations (Johnston et al., 2001).
Maternal exposures during gestation can influence the structural and functional development of the fetus. A wide range of factors, including low maternal socioeconomic status, lack of prenatal care, and smoking, have been associated with infants born pre-term or of low birth weight (Kramer, 2003; Knackstedt et al., 2005). Due to the heightened sensitivity of the fetal life stage, the fetus is extremely vulnerable to environmental challenges, whether endogenous to the mother, external biological agents, or synthetic compounds.
Brominated flame retardants are relatively ubiquitous in the environment. These halogenated organic compounds are supplemental agents in a variety of consumer products, including electronic appliances, automotive parts, furniture, textiles, and plastic foams, intended to reduce fire-related injuries and property damage (Birnbaum and Staskal, 2004). Although these compounds have been recognized for their utility, the possible consequences for human health have been described in the scientific literature (Anderson et al., 1979; Landrigan et al., 1979; Meester, 1979; Rosenman et al., 1979; Silva et al., 1979; Stross et al., 1979; Valciukas et al., 1979; Wolff et al., 1979a; Wolff et al., 1979b; Bahn et al., 1980; Weil et al., 1981; Chanda et al., 1982; Kreiss et al., 1982; Eyster et al., 1983; Seagull, 1983; Jacobson et al., 1984; Lipson, 1987; Hoque et al., 1998; Blanck et al., 2000a; Blanck et al., 2000b; Sweeney et al., 2001; Blanck et al., 2002; Birnbaum and Staskal, 2004). Moreover, production of these compounds has increased dramatically over the last 20 years, and there is an increasing prevalence of exposure to brominated flame retardants worldwide (Birnbaum and Staskal, 2004).
Polybrominated biphenyls (PBB) constitute a class of compounds that were once marketed for use as a flame retardant. A mixture of persistent organic congeners with lipophilic properties (ATSDR, 2004), PBB congeners are resistant to degradation and are stored in adipose tissue. In 1973, this halogenated organic mixture contaminated the food source of numerous Michigan communities due to an inadvertent replacement of a nutritional supplement, NutriMaster, with FireMaster in cattle feed.
The production of PBB ceased in 1979; however, the environmental and human health consequences of exposure are still being documented. Because PBB congeners added to consumer products are not covalently bound (ATSDR, 2004), PBB congeners are continually released into the environment and have the potential to contaminate ecosystems years after their production has ceased. Exposure to PBB has been associated with adverse effects on the endocrine, immune and neurological systems (Landrigan et al., 1979; Meester, 1979; Silva et al., 1979; Weil et al., 1981; Lipson, 1987; Hoque et al., 1998; Blanck et al., 2000a; Blanck et al., 2000b; Blanck et al., 2002).
Polychlorinated biphenyls (PCB) are related halogenated organic compounds, comprised of over 200 congeners, once used as lubricants or coolants in electrical equipment. Not unlike the cessation of PBB production, manufacturing of PCB was also banned in North America and other industrialized countries in the 1970s (ATSDR, 2000). The primary pathway of exposure to PCB in the general population is through the food chain, with the most noted being fish consumption. Fish consumed from the PCB-contaminated waters of the Great Lakes have been identified as a heightened source of dietary exposure (ATSDR, 2000). The adverse human health outcomes associated with PCB exposure include dermal anomalies, endocrine disruption, neurobehavioral disorders, and an impaired immune system (for review see (Ross, 2004)).
Several experimental animal studies indicate that PBB and PCB can cross the placental barrier (Eyster et al., 1983; Jacobson et al., 1984; Guvenius et al., 2003; ATSDR, 2004) and in utero PBB exposure results in reduced fetal birth weight and shortened gestational period (Corbett et al., 1975; Harris et al., 1978; Lambrecht et al., 1978; Aulerich and Ringer, 1979; McCormack et al., 1981). Evidence from studies of related halogenated organics, such as PCB, and DDT (1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane), suggests that in utero exposure to these agents is associated with infants born pre-term (Longnecker et al., 2001) and of low birth weight (Fein et al., 1984; Baibergenova et al., 2003). Thus, there is reason to believe that exposures to PBB in utero may adversely affect the developing infant. Given the temporal trends in premature births, the ubiquitous presence of halogenated organics, the present study examines the potential association between exposure to PBB and PCB in utero and infant birth weight and gestational age.
RESEARCH METHODS AND STUDY DESIGN
Study Population
The contamination of the Michigan food chain with the fire retardant, FireMaster, exposed thousands of residents to PBB. In an effort to understand the consequences of this exposure, the Michigan Department of Community Health (MDCH), in collaboration with the U.S. Public Health Service, organized a prospective cohort study in 1976, which enrolled almost 4,000 men, women and children who had consumed contaminated food products. Information regarding the incident and the registry cohort has been previously described (Carter, 1976; Landrigan et al., 1979; Meester, 1979; Fries, 1985). Follow-up interviews by telephone, termed the 1997 Michigan Female Health Study, were conducted from 1997 to 1998 with 1,185 women at least 18 years of age from the original cohort (Thomas et al., 2001; Kaiser et al., 2003). Five hundred seventy eight of these participants were mothers who consented to release birth certificate information for their children. Of these women, 493 conceived and gave birth from 1973 to 1997 to infants who were potentially exposed in utero. A serum PBB measure was available for 450 of these mothers.
Electronic birth certificate information was obtained for 925 infants born to the 450 mothers. Twenty six infants were excluded due to the following: gestational age less than 28 weeks (n=1), congenital anomalies (n=3), twins (n=19, one stillborn), or race other than white (n=3). The final sample includes 899 infants and 444 mothers.
The Institutional Review Board at Emory University, Atlanta, GA, the Centers for Disease Control and Prevention, Atlanta, GA, and the Michigan Department of Community Health approved the protocols, and the participants gave informed consent.
Exposure Assessment
Serum PBB concentrations were measured at enrollment in the cohort (1976 – 1978) and during follow-up (1978 – 1993). Quantitative analyses for PBB were carried out using gas chromatography with electron capture detection. The MCDH laboratories conducted all of the analyses. Quantification was based on FireMaster FF-1 at a limit of detection (LOD) of 1.0 part per billion. The main congener of FF-1 is 2,2′4,4′5,5′ hexa-bromobiphenyl (PBB-153). An estimate of maternal serum PBB concentration at the time of conception was extrapolated from PBB measurements taken at the date of enrollment with an exponential decay model developed for PBB (Blanck et al., 2000b). The correlation between predicted and measured levels of PBB using this model was 0.92 (Blanck et al., 2000b). Serum PCB concentrations were also measured from samples collected between 1976 and 1986. Quantification was based on Aroclor 1254, with a LOD of 5.0 ppb.
The coefficients of variation for PBB quantification ranged from 7.1 to 14.0%, and recovery ranged from 80 to 90% (Needham et al., 1981). For PCB, the coefficients of variation ranged from 12 to 30%, and the average recovery was 82% (Burse et al., 1980; Needham et al., 1981). Participants were not asked to fast prior to sample collection and serum lipids were not measured.
Assessment of birth weight, gestational age and covariates
Electronic birth certificate information was obtained for infants born to mothers enrolled in the Michigan PBB registry. Information from the birth certificates used in the current study included: infant birth weight, gestational age, year of the birth, paternal age at time of birth, maternal medical risk factors such as hypertension and diabetes, month prenatal care began, maternal level of education obtained, the race of the infant, and congenital anomalies.
The Michigan Female Health Study interview provided information on: smoking in the year prior to pregnancy, consumption of alcohol in the first trimester, parity, gravidity, multiple vs. singleton birth, as well as infant gestational age, infant birth date, and infant gender. For each infant, two variables were created, maternal age at time of birth and the diagnosis of thyroid disorder in the year of or prior to the pregnancy.
Gestational age was calculated based on the last menstrual period (LMP) taken from the electronic birth certificate file. If the day of the LMP was missing (n=77), the 15th day of the month was assigned and the gestational age calculated based on this assigned LMP. If the entire LMP date was missing (n=19), the gestational age was taken from the Michigan Female Health Study interview unless the value was outside the range of 28 to 46 weeks (n=1) in which case gestational age was considered missing.
Statistical Analyses
Birth weight and gestational age were categorized for an initial assessment of statistical relationships between outcomes and covariates using a Chi-Square test. Categories for birth weight were ≤ 2,500 grams, > 2,500 to ≤ 3,800 grams, and > 3,800 grams based on the common clinical cut points defining low birth weight, average birth weight, and high birth weight. Categories for gestational age were defined as < 37 weeks, ≥ 37 weeks to 42 weeks, and ≥ 42 weeks in accordance with the clinical definitions for pre-term, term, and post-term. Weight for gestational age was defined based on percentiles of a standard distribution of birth weight by gestational age and sex for singleton births in the U. S. Weight for gestational age was categorized as: in the smallest 10% of infants in the standard distribution (small for gestational age, SGA), within 10% to < 90% of the standard distribution (average for gestational age, AGA), and larger than ≥ 90% of the infants in the standard distribution (large for gestational age, LGA) (Alexander et al., 1996). Both birth weight and gestational age were approximately normally distributed and modeled as continuous variables using restricted maximum likelihood estimation (RMLE) accounting for the correlation among infants born to the same mother. Additional analyses used GEE for binary outcomes (PROC GENMOD) to model categories of gestational age (pre-term or post-term vs. term,), birth weight (low birth weight or high birth weight vs. average birth weight), and weight for gestational age (SGA or LGA vs. AGA).
Categories for maternal serum PBB at the time of conception were categorized as at or below the LOD and the median of the estimated serum PBB concentrations of those above the LOD for the remaining infants (≤ 1.0 ppb, > 1.0 to ≤ 3.38 ppb, > 3.38 ppb). Maternal serum PBB concentrations at the time of enrollment were categorized as at or below the LOD and the median value for PBB concentrations above the LOD (≤ 1.0 ppb, > 1.0 to ≤ 3.0 ppb, > 3.0 ppb). PBB exposure categories were also redefined to assess the top 10% exposure levels for both estimated PBB levels (≤ 1.0 ppb, > 1.0 to < 7.55 ppb, ≥ 7.55 ppb) and enrollment PBB levels (≤ 1.0 ppb, > 1.0 to < 14.0 ppb, ≥ 14.0 ppb). Maternal serum PCB levels at the time of enrollment were dichotomized as below the LOD and at or above the LOD for the quantitative analysis method used (5.0 ppb). PCB categories were also redefined to assess the top 10% exposure levels (< 5.0 ppb, ≥ 5.0 to < 11.0 ppb, ≥11.0 ppb).
Covariates considered as potential confounders included: paternal age, maternal age, smoking during the year prior to pregnancy, alcohol ingestion in the first trimester (yes vs. no), maternal education level (high school graduate or beyond), prenatal care (1st trimester, 2nd–3rd trimesters, or none), gravidity (number of previous pregnancies − 0, 1, 2+) or parity (the number of previous live births − 0, 1, 2+), and gender of the infant. In addition, we examined potential confounding by maternal medical risk factors including diabetes, hypertension, or thyroid dysfunction. To assess potential confounding, the association between each covariate and the outcomes (birth weight or gestational age), as well as with the exposures, was examined using ANOVA (cut-off p < 0.1).
We used restricted maximum likelihood to account for the lack of independence among pregnancies for the same woman (PROC MIXED, SAS Version 9.0). An exchangeable correlation structure was chosen because it appeared both biologically plausible and consistent with observed correlations between pregnancies in this data. Robust variances were used to account for potential deviance from the assumptions made about the correlation structure. Interactions between the main exposures, maternal serum PBB or PCB levels, and infant gender or maternal smoking in the year prior to pregnancy were assessed. Covariates that were significantly associated with the outcome or that changed the effect estimates by more than 10% were retained in the final model. Statistical significance was determined using a Wald test yielding a p-value less than 0.05.
To assess the stability of estimated regression coefficients, the model was run with the full data set (n=899 infants) and also a truncated data set that retained only first births (n=444 infants). Models were assembled to account for the cluster-specific, random effects of individual mothers. The association between gestational age or birth weight and PBB was considered utilizing either estimated serum PBB levels at conception or serum PBB levels at enrollment. Regression models for birth weight were explored, both with and without adjustment for gestational age (as a continuous variable). Sensitivity analyses were also performed to consider potential deviations caused by estimating gestational age with combined information from the birth certificate and telephone interview versus the birth certificate file only.
We employed the same strategy to assess associations with categories of birth weight, gestational age, or weight for gestational age. Covariates included in these models (except models for low birth weight) were maternal age, parity, smoking in the year prior to pregnancy, infant gender and decade of birth. Because of the small number of low birth weight infants, only the main effect was included in the models.
RESULTS
This study included 899 infants born to 444 mothers. Table 1 shows the demographic characteristics and pregnancy-related history for this cohort. The average age of mothers at the time of birth was 26 years, ranging in years from 15 to 44. The age of fathers ranged from 16 to 54, with a mean age of 28 years. The majority of the mothers had completed a high school education (51.1%). Eighty one percent of the pregnancies for these women included prenatal care in the first trimester. First births represented only 30% of the infants born in this study.
Table 1.
Characteristics of Parents and Infants According to Outcomes in the Michigan PBB Registry (1975–1997).
| Gestational Age (weeks) | Birth Weight (grams) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | N | % | Mean i | S.E. | p-value | N | Mean i | S.E. | p-value |
| Age of mother at time of birth | 0.1722 | 0.0102 | |||||||
| 15–19 | 67 | 7.5 | 39.57 | 0.24 | 67 | 3,511 | 60.61 | ||
| 20–24 | 296 | 32.9 | 40.08 | 0.12 | 296 | 3,493 | 31.90 | ||
| 25–29 | 331 | 36.8 | 39.83 | 0.11 | 331 | 3,535 | 30.32 | ||
| 30–34 | 162 | 18.0 | 39.72 | 0.16 | 162 | 3,659 | 39.81 | ||
| 35–44 | 43 | 4.8 | 39.62 | 0.31 | 43 | 3,617 | 78.51 | ||
| Age of father at time of birth | 0.3352 | 0.1018 | |||||||
| 16–20 | 42 | 4.7 | 40.17 | 0.31 | 42 | 3,460 | 74.12 | ||
| 21–29 | 481 | 53.5 | 39.93 | 0.10 | 481 | 3,515 | 27.47 | ||
| 30–34 | 220 | 24.5 | 39.86 | 0.14 | 220 | 3,597 | 35.53 | ||
| 35–54 | 116 | 12.9 | 39.57 | 0.19 | 116 | 3,626 | 49.38 | ||
| missing | 40 | 4.4 | 39.59 | 0.32 | 40 | 3,520 | 80.76 | ||
| Maternal paritya | 0.8282 | <0.0001 | |||||||
| 0 | 322 | 35.8 | 39.86 | 0.11 | 322 | 3,455 | 28.41 | ||
| 1 | 300 | 33.4 | 39.90 | 0.12 | 300 | 3,601 | 29.20 | ||
| 2+ | 277 | 30.8 | 39.80 | 0.13 | 277 | 3,612 | 32.33 | ||
| Maternal gravidity b | 0.5818 | <0.0001 | |||||||
| 0 | 271 | 30.1 | 39.86 | 0.12 | 271 | 3,440 | 30.43 | ||
| 1 | 274 | 30.5 | 39.95 | 0.12 | 274 | 3,573 | 30.25 | ||
| 2+ | 354 | 39.4 | 39.79 | 0.11 | 354 | 3,621 | 29.51 | ||
| Maternal smoking statusC | 0.6698 | 0.0040 | |||||||
| No | 710 | 79.0 | 39.85 | 0.08 | 710 | 3,582 | 24.75 | ||
| Yes | 186 | 20.7 | 39.93 | 0.16 | 186 | 3,426 | 43.88 | ||
| missing | 3 | 0.3 | 37.00 | 4.00 | 3 | 3,648 | 148.44 | ||
| Maternal alcohol ingestion d | 0.5102 | 0.3401 | |||||||
| No | 785 | 87.3 | 39.87 | 0.08 | 785 | 3,558 | 23.38 | ||
| Yes | 109 | 12.1 | 39.73 | 0.20 | 109 | 3,504 | 53.32 | ||
| missing | 5 | 0.6 | 40.40 | 0.93 | 5 | 3,044 | 233.83 | ||
| Maternal prenatal care e | 0.2055 | 0.2928 | |||||||
| 1st trimester | 729 | 81.1 | 39.82 | 0.08 | 729 | 3,554 | 23.78 | ||
| >1st trimester or none | 161 | 17.9 | 40.04 | 0.16 | 161 | 3,510 | 40.64 | ||
| missing | 9 | 1.0 | 39.44 | 0.91 | 9 | 3,468 | 238.72 | ||
| Maternal education | 0.1902 | 0.8959 | |||||||
| ≤H.S. | 459 | 51.1 | 39.96 | 0.10 | 459 | 3,551 | 29.77 | ||
| >H.S. | 438 | 48.7 | 39.76 | 0.11 | 438 | 3,545 | 30.99 | ||
| missing | 2 | 0.2 | 37.00 | 3.00 | 2 | 2,863 | 482.00 | ||
| Maternal diagnosis of hyptertension | 0.9775 | 0.2061 | |||||||
| No | 889 | 98.9 | 39.86 | 0.08 | 889 | 3550 | 22.43 | ||
| Yes | 10 | 1.1 | 39.84 | 0.63 | 10 | 3325 | 155.06 | ||
| Maternal diagnosis of diabetes | 0.0864 | 0.8553 | |||||||
| No | 888 | 98.8 | 39.87 | 0.08 | 888 | 3547 | 22.47 | ||
| Yes | 11 | 1.2 | 38.60 | 0.62 | 11 | 3577 | 155.69 | ||
| Maternal diagnosis of thyroid disorder | 0.9635 | 0.6596 | |||||||
| No | 844 | 93.9 | 39.86 | 0.08 | 844 | 3545 | 23.09 | ||
| Yes | 55 | 6.1 | 39.87 | 0.29 | 55 | 3589 | 83.64 | ||
| Infant gender | 0.1276 | <0.0001 | |||||||
| male | 495 | 55.1 | 39.77 | 0.10 | 495 | 3,636 | 25.88 | ||
| female | 404 | 44.9 | 39.97 | 0.10 | 404 | 3,442 | 27.35 | ||
| Infant gestation | <0.0001 | ||||||||
| Pre-term (<37 weeks) | 36 | 4.0 | - | - | 36 | 2,927 | 79.31 | ||
| Term (37 to <42 weeks) | 714 | 79.4 | - | - | 714 | 3,560 | 22.59 | ||
| Post-Term (≥ 42 weeks) | 149 | 16.6 | - | - | 149 | 3,647 | 39.96 | ||
| Infant birth weight | <0.0001 | ||||||||
| ≤ 2,500 grams | 30 | 3.3 | 37.06 | 0.35 | - | - | - | ||
| > 2,500 to ≤ 3,800 grams | 597 | 66.4 | 39.76 | 0.08 | - | - | - | ||
| > 3,800 grams | 272 | 30.3 | 40.40 | 0.12 | - | - | - | ||
| Maternal serum PCB level f | 0.3047 | 0.5135 | |||||||
| < 5.0 ppb | 358 | 39.8 | 39.88 | 0.12 | 358 | 3,566 | 35.37 | ||
| ≥ 5.0 ppb | 456 | 50.7 | 39.91 | 0.11 | 456 | 3,548 | 31.66 | ||
| missing | 85 | 9.5 | 39.51 | 0.24 | 85 | 3,475 | 70.67 | ||
| Estimated maternal serum PBB levelg | 0.1694 | 0.4193 | |||||||
| ≤ 1.0 ppb | 500 | 55.6 | 39.74 | 0.10 | 500 | 3,553 | 28.72 | ||
| >1.0 to 3.38 ppb | 200 | 22.3 | 40.07 | 0.15 | 200 | 3,581 | 42.18 | ||
| > 3.38 ppb | 199 | 22.1 | 39.94 | 0.16 | 199 | 3,502 | 45.74 | ||
| Maternal serum PBB level h | 0.4732 | 0.0871 | |||||||
| ≤ 1.0 ppb | 290 | 32.3 | 39.85 | 0.13 | 290 | 3,576 | 38.61 | ||
| >1.0 to 3.0 ppb | 298 | 33.2 | 39.97 | 0.13 | 298 | 3,589 | 38.61 | ||
| > 3.0 ppb | 311 | 34.6 | 39.75 | 0.13 | 311 | 3,479 | 38.54 | ||
Number of previous live births
Total number of prior pregnancies
Maternal smoking status in the year prior to pregnancy
Maternal alcohol ingestion in the first trimester of pregnancy
Month prenatal care began
Maternal serum PCB levels at the time of enrollment
Maternal serum PBB levels at the time of conception
Maternal serum PBB level at the time of enrollment
Means are adjusted for the correlated data
Mothers reported smoking in the year prior to pregnancy for approximately 20% of the pregnancies, and drinking alcohol during the first trimester for 12% of the pregnancies. A small percentage of pregnancies had diagnoses of maternal hypertension (1.1%), diabetes (1.2%), or thyroid disorder (6.1%). The maximum enrollment maternal serum PBB level among the 444 women was 1490 ppb and 32% of the levels were at or below the limit of detection (1.0 ppb). The maximum estimated serum PBB levels at the time of conception was 1090 ppb and 56% were at or below the limit of detection (1.0 ppb). The maternal serum PCB levels at the time of enrollment ranged from below the limit of detection to 78.0 ppb, with 40% of the infants born to mothers whose PCB level was below the limit of detection (5.0 ppb). Fifty five percent of infants were male.
Gestational Age
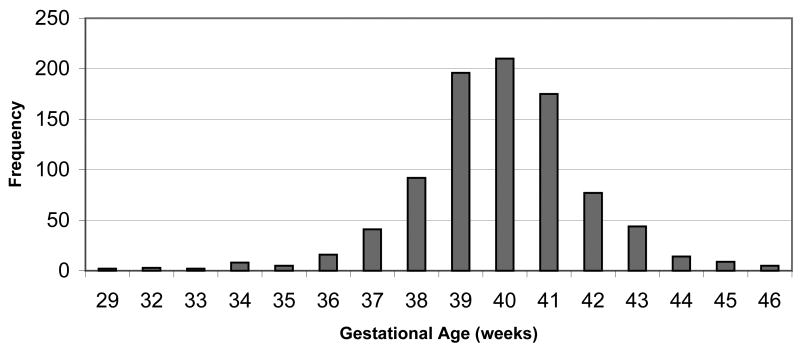
Gestational age for the infants ranged from 29 to 46 weeks with a mean of 39.8 weeks (std. dev. = 2.0) (Figure 1). Four percent of these infants were born pre-term (< 37 weeks). Mean gestational age of infants did not differ by maternal PBB or PCB exposure levels (Table 1). No significant differences were observed in the mean gestational age of infants in relation to paternal age, maternal age, parity, smoking status, education, or diagnoses of hypertension, diabetes, or thyroid disorders.
Figure 1.
Gestational age of infants born 1975–1997 to mothers in the Michigan PBB Study registry (N=899).
Birth Weight
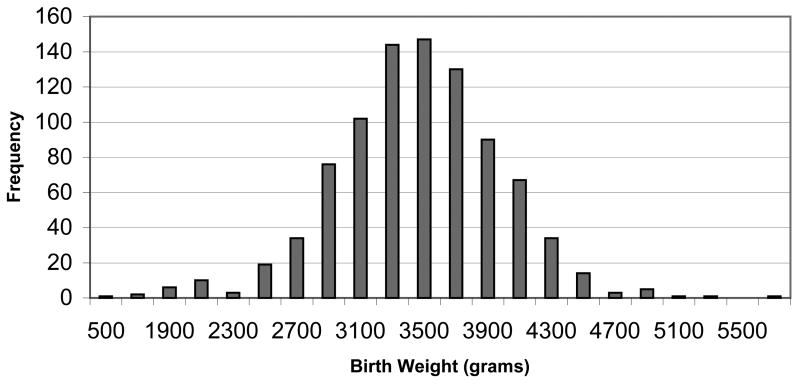
The mean birth weight for infants was 3,551 grams (std. dev. = 533), ranging from 508 to 5,868 grams (Figure 2), and 3.3% of infants were low birth weight. Birth weight was significantly associated with maternal smoking, parity, gravidity, infant gestational age, gender, and maternal age in unadjusted analyses (Table 1). Specifically, the mean infant birth weight was lower if the mother reported smoking in the year prior to pregnancy. The mean infant birth weight increased with a rise in maternal parity, gravidity or infant gestational age. The mean birth weight of female infants was significantly lower than the mean birth weight of males. The mean infant birth weight among infants differed according to maternal age at the time of birth. Finally, the mean infant birth weight was lowest among women with enrollment serum PBB levels above 3.0 ppb.
Figure 2.
Birth weight of infants born 1975–1997 to mothers in the Michigan PBB Study registry (N=899).
Regression Models
No significant association was found between estimated maternal serum PBB levels and gestational age in the models that adjusted for maternal age, smoking, parity, infant gender, and the decade of birth (Table 2). Likewise, maternal serum PBB levels at the time of enrollment were not associated with infant gestational age. Maternal serum PCB levels also showed no relationship with infant gestational age (Table 2). Women with missing PCB exposure data were compared to women with measured exposure levels, and no significant differences were found with regard to maternal characteristics (data not shown).
Table 2.
Restricted Maximum Likelihood Estimation Models for Gestational Age and Infant Birth Weighta
| Gestational Age
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimated PBB at Conception
|
Enrollment PBB
|
||||||||
| Variable
|
Est. Coefficient | 95% C. I. | p-value | Est. Coefficient | 95% C. I. | p-value | |||
| Intercept | 39.81 | 39.30 | 40.33 | <.0001 | 39.80 | 39.28 | 40.33 | <.0001 | |
| PBB b | |||||||||
| ≤ LOD | ref | 0.00 | - | - | - | 0.00 | - | - | - |
| Moderate | 0.20 | −0.26 | 0.66 | 0.3802 | 0.17 | −0.18 | 0.52 | 0.3451 | |
| High | 0.06 | −0.29 | 0.42 | 0.7259 | −0.11 | −0.47 | 0.25 | 0.5340 | |
| PCB c | |||||||||
| < 5.0 ppb | ref | 0.00 | - | - | - | 0.00 | - | - | - |
| ≥ 5.0 ppb | −0.02 | −0.34 | 0.29 | 0.8898 | −0.01 | −0.33 | 0.30 | 0.9369 | |
| missing | −0.35 | −0.82 | 0.12 | 0.1431 | −0.37 | −0.84 | 0.10 | 0.1210 | |
|
| |||||||||
|
Birth Weight
|
|||||||||
| Estimated PBB at Conception
|
Enrollment PBB
|
||||||||
| Variable
|
Est. Coefficient | 95% C. I. | p-value | Est. Coefficient | 95% C. I. | p-value | |||
| Intercept | 3353.18 | 3243.42 | 3462.93 | <.0001 | 3375.44 | 3253.26 | 3497.62 | <.0001 | |
| PBB b | |||||||||
| ≤ LOD | ref | 0.00 | - | - | - | 0.00 | - | - | - |
| Moderate | −11.31 | −110.91 | 88.28 | 0.8200 | 5.94 | −92.77 | 104.64 | 0.9060 | |
| High | −57.64 | −165.07 | 49.79 | 0.2855 | −98.84 | −197.01 | −0.68 | 0.0484 | |
| PCB c | |||||||||
| < 5.0 ppb | ref | 0.00 | - | - | - | 0.00 | - | - | - |
| ≥ 5.0 ppb | −27.26 | −110.23 | 55.72 | 0.5189 | −25.63 | −108.85 | 57.59 | 0.5453 | |
| missing | −69.80 | −250.04 | 110.43 | 0.4470 | −74.38 | −254.76 | 105.99 | 0.4181 | |
Adjusted models controlling for maternal age, smoking, parity, infant gender, and decade of birth. Birthweight models also adjusted for gestational age.
PBB represents maternal serum PBB levels at the time of conception or the time of enrollment
PCB represents maternal serum PCB levels at the time of enrollment
Estimated PBB level categories => LOD :≤ 1.0, MODERATE: > 1.0 to 3.38, HIGH: > 3.38
Enrollment PBB level categories => LOD :≤ 1.0, MODERATE: > 1.0 to 3.0, HIGH: > 3.0
Neither estimated maternal serum PBB levels at the time of conception, enrollment serum PBB nor PCB levels at the time of enrollment were associated with infant birth weight in unadjusted models (data not shown). No consistent relationship was observed between PBB exposure and birth weight in analyses adjusting for maternal age, smoking, parity, infant gender, decade of birth, or gestational age (Table 2). There was a suggestion of an association of high PBB exposure with lower birth weight but this association was only apparent for maternal enrollment PBB and not for estimated PBB at conception.
When maternal serum PBB levels were categorized to examine potential associations between the top 10% exposure levels and birth weight or gestational age, no relationship was observed for either estimated PBB levels at conception (birth weight β = 27.25 C.I. (−110.05, 164.55) and gestational age β = 0.224 C.I. (−0.295, 0.743)) or PBB levels at the time of enrollment (birth weight β = 115.69 C.I. (−33.35, 264.73) and gestational age β = 0.206 C.I. (−0.324, 0.735)), after adjusting for the aforementioned covariates.
Finally, we evaluated a potential relationship between PBB or PCB and the tails of the outcome distributions using GEE models. Again, no significant relationship was observed between PBB or PCB exposure and gestational age, birth weight, or weight for gestational age (Table 3).
Table 3.
Generalized Estimating Equation Models for Gestational Age, Infant Birth Weight, and Weight for Gestational Age a
| Gestational Age
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-Term vs. Term
|
Post-Term vs. Term
|
||||||||
| Variable
|
Odds Ratio | 95% C. I | p-value | Odds Ratio | 95% C. I. | p-value | |||
| PBB b | |||||||||
| ≤ 1.0 ppb | ref | 1.00 | - | - | - | 1.00 | - | - | - |
| >1.0 to 3.38 ppb | 1.63 | 0.624 | 4.258 | 0.3182 | 1.43 | 0.889 | 2.295 | 0.1410 | |
| > 3.38 ppb | 0.60 | 0.182 | 1.963 | 0.3956 | 0.87 | 0.537 | 1.424 | 0.5903 | |
| PCB c | |||||||||
| < 5.0 ppb | ref | 1.00 | - | - | - | 1.00 | - | - | - |
| ≥ 5.0 ppb | 0.64 | 0.284 | 1.459 | 0.2913 | 0.97 | 0.658 | 1.423 | 0.8663 | |
| missing | 1.64 | 0.564 | 4.741 | 0.3650 | 0.66 | 0.323 | 1.335 | 0.2451 | |
|
| |||||||||
|
Birth Weight
|
|||||||||
| Low Birth Weight vs. Average Birth Weight
|
High Birth Weight vs. Average Birth Weight
|
||||||||
| Variable
|
Odds Ratio | 95% C. I. | p-value | Odds Ratio | 95% C. I. | p-value | |||
| PBB b | |||||||||
| ≤ 1.0 ppb | ref | 1.00 | - | - | - | 1.00 | - | - | - |
| >1.0 to 3.38 ppb | 1.70 | 0.69 | 4.23 | 0.2500 | 1.32 | 0.87 | 1.99 | 0.1899 | |
| > 3.38 ppb | 1.38 | 0.56 | 3.40 | 0.4838 | 0.99 | 0.62 | 1.59 | 0.9652 | |
| PCB c | |||||||||
| < 5.0 ppb | ref | 1.00 | - | - | - | 1.00 | - | - | - |
| > 5.0 ≥ppb | 0.87 | 0.36 | 2.11 | 0.7620 | 0.90 | 0.629 | 1.288 | 0.5643 | |
| missing | 1.69 | 0.56 | 5.11 | 0.3488 | 0.88 | 0.443 | 1.751 | 0.7180 | |
|
| |||||||||
|
Weight for Gestational Age
|
|||||||||
| SGA vs. AGA
|
LGA vs. AGA
|
||||||||
| Variable
|
Odds Ratio | 95% C. I. | p-value | Odds Ratio | 95% C. I. | p-value | |||
| PBB b | |||||||||
| ≤ 1.0 ppb | ref | 1.00 | - | - | - | 1.00 | - | - | - |
| >1.0 to 3.38 ppb | 1.13 | 0.47 | 2.72 | 0.7881 | 1.18 | 0.73 | 1.90 | 0.5106 | |
| > 3.38 ppb | 1.96 | 0.89 | 4.35 | 0.0968 | 0.78 | 0.44 | 1.37 | 0.385 | |
| PCB c | |||||||||
| < 5.0 ppb | ref | 1.00 | - | - | - | 1.00 | - | - | - |
| ≥ 5.0 ppb | 1.89 | 0.90 | 3.98 | 0.0933 | 0.98 | 0.62 | 1.53 | 0.9173 | |
| missing | 3.02 | 1.07 | 8.55 | 0.0368 | 1.13 | 0.50 | 2.55 | 0.7702 | |
Adjusted models controlling for maternal age, smoking, parity, infant gender, and decade of birth. Birthweight models also adjusted for gestational age.
PBB represents estimated maternal serum PBB levels at the time of conception
PCB represents maternal serum PCB levels at the time of enrollment
DISCUSSION
Halogenated organic compounds have been associated with risk of preterm birth or low birth weight in several epidemiological studies (Fein et al., 1984; Longnecker et al., 2001; Baibergenova et al., 2003) as well as in animal models (Corbett et al., 1975; Harris et al., 1978; Lambrecht et al., 1978; Aulerich and Ringer, 1979; McCormack et al., 1981; Faroon et al., 2001). Other studies have found no relationship between maternal exposure to halogenated organics and birth weight or gestational age (Longnecker et al., 2005; Khanjani and Sim, 2006). We did not observe a consistent association between maternal exposure to PBB and infant birth weight or gestational age in this cohort. Though there was a significant, negative relationship between a high level of maternal enrollment PBB and infant birth weight, this result was not supported by analyses using estimated maternal PBB at conception, with subsequent analyses of the highest 10% of those exposed, nor in GEE models to assess the tails of the outcome distributions. Thus, the association noted between maternal enrollment PBB and infant birth weight could be due to chance. Moreover, our findings of no association were similar when we limited our analyses to firstborn infants, and when we accounted for the random effect of mothers. Therefore, our findings suggest no association between maternal exposure to PCB or PBB during the gestational period and infant birth weight or gestational age.
Although substantial information regarding risk factors was available, not all potential risk factors could be addressed due to the fact that this was a retrospective analysis. For example, no reliable data was available regarding maternal characteristics such as pre-pregnancy BMI, socioeconomic status at the time of each pregnancy, or additional exposures such as bacterial vaginosis, other infections during pregnancy, or stress. There was also limited data available for smoking during pregnancy, which could be a potential source of bias. However, we found no association between smoking in the year prior to pregnancy and PBB or PCB exposure. Thus, smoking status is unlikely to be a confounder unless unexposed women were more likely than exposed women to quit smoking. Finally, it is difficult to assess how well the results of the present study can be generalized, as the majority of this cohort was white and well educated.
Utilizing gestational age as an outcome variable has certain limitations (Kramer et al., 1988) This is largely because gestational age is calculated from a mother’s recollection of the date of her last menstrual period (LMP). A measurement of gestational age that is obtained using ultrasound technology during the first trimester, comparing fetal development to a standardized, graded scale is considered the gold standard (Kramer, 2003). Though we had access to two sources (mother’s recall and electronic birth certificate data), which were significantly correlated (R = 0.58, p < 0.01), neither of these sources employed ultrasound technology to obtain the estimated gestational age, therefore some misclassification of this outcome is likely. However, there were a few infants (n=16) with gestational ages calculated from the LMP provided by the birth certificate file that were outside the range of biological plausibility for a viable birth in which case the mother’s recall of gestational length was more plausible.
The potential relationships between exposures to halogenated organic compounds during gestational development and infant health outcomes addressed herein are extremely relevant given the temporal trends in the U.S. over the last 30 years suggesting increased exposure to polybrominated diphenyl ethers (PBDE) through detection of congeners in human blood and breast milk (Schecter et al., 2005). Because the Michigan Long Term PBB Study has been prospectively collecting detailed information on cohort participants for over 30 years, this cohort provides an excellent opportunity to examine this relationship. A strength of this study is that two sources of information were available for infant gestational age, self-reported by the mother and from the birth certificate file. A considerable amount of information regarding lifestyle and health outcomes has also been obtained through telephone interviews with these women. Moreover, medical records were obtained for many of the pregnancies in this cohort, making it possible to verify medical complications, though reported conditions were rare. Birth certificate files also provide an objective and reliable source for birth weight.
Our findings do not indicate a significant association between infant gestational age or birth weight and maternal exposure to PBB or PCB. These findings add to the conflicting reports of associations for halogenated compounds and infant health outcomes. As previously proposed, it is quite plausible that the inconsistent relationships between such gestational exposures and infant health outcomes reported to date can be attributed to the diverse and complex mixtures of halogenated organic compounds considered as exposures, uncontrolled confounding, or perhaps a threshold effect on infant health outcomes (Sagiv et al., 2006 ). Although not directly comparable due to the biological matrices assessed, the analytical method of quantification, and mixture of congeners, the PCB exposure levels in this cohort were on the lower end of the exposure spectrum in relation to PCB levels evaluated among other study populations (Longnecker et al., 2003). However, this cohort study provides a unique opportunity to specifically address maternal PBB exposure levels and gestational age or birth weight. Thus, there exists a continued need for surveillance and additional research studies to address the complexities of such compounds and potential health effects, particularly among the most highly exposed.
Acknowledgments
Funding for this research was provided by US EPA (R 825300), NIEHS (RO1 ES08341, R01 ES012014), and by CDC cooperative agreement U37/CCU500392. We thank the staff of the Michigan Long-Term PBB Study, Michigan Department of Community Health, for providing historical and laboratory data on the cohort, and, with the Michigan Public Health Institute, assisting in carrying out the Michigan Female Health Study. We thank Glenn Copeland and the Division of Vital Records and Health Statistics, Michigan Department of Community Health, for creating the birth records linkage, and assisting in its editing and interpretation. We thank Anne Sweeney of Texas A & M University for providing the resources for Vital Records to link the females of the PBB Study registry to the birth certificate files for births 1975–1994.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the CDC.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- Anderson HA, Wolff MS, Lilis R, Holstein EC, Valciukas JA, Anderson KE, Petrocci M, Sarkozi L, Selikoff IJ. Symptoms and clinical abnormalities following ingestion of polybrominated-biphenyl-contaminated food products. Ann N Y Acad Sci. 1979;320:684–702. doi: 10.1111/j.1749-6632.1979.tb56644.x. [DOI] [PubMed] [Google Scholar]
- ATSDR. Toxicological Profile for Polychlorinated Biphenyls (PCBs) USDHHS; 2000. [PubMed] [Google Scholar]
- ATSDR. Toxicological Profile for Polybrominated Biphenyls and Polybrominated Diphenyl Ethers (PBBs and PBDEs) 2006. 2004 September 2004; from http://www.atsdr.cdc.gov/toxprofiles/tp68.html.
- Aulerich RJ, Ringer RK. Toxic effects of dietary polybrominated biphenyls on mink. Arch Environ Contam Toxicol. 1979;8:487–498. doi: 10.1007/BF01056355. [DOI] [PubMed] [Google Scholar]
- Bahn AK, Mills JL, Snyder PJ, Gann PH, Houten L, Bialik O, Hollmann L, Utiger RD. Hypothyroidism in workers exposed to polybrominated biphenyls. N Engl J Med. 1980;302:31–33. doi: 10.1056/NEJM198001033020105. [DOI] [PubMed] [Google Scholar]
- Baibergenova A, Kudyakov R, Zdeb M, Carpenter DO. Low birth weight and residential proximity to PCB-contaminated waste sites. Environ Health Perspect. 2003;111:1352–1357. doi: 10.1289/ehp.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum LS, Staskal DF. Brominated flame retardants: cause for concern? Environ Health Perspect. 2004;112:9–17. doi: 10.1289/ehp.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanck HM, Marcus M, Hertzberg V, Tolbert PE, Rubin C, Henderson AK, Zhang RH. Determinants of polybrominated biphenyl serum decay among women in the Michigan PBB cohort. Environ Health Perspect. 2000a;108:147–152. doi: 10.1289/ehp.00108147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanck HM, Marcus M, Rubin C, Tolbert PE, Hertzberg VS, Henderson AK, Zhang RH. Growth in girls exposed in utero and postnatally to polybrominated biphenyls and polychlorinated biphenyls. Epidemiology. 2002;13:205–210. doi: 10.1097/00001648-200203000-00016. [DOI] [PubMed] [Google Scholar]
- Blanck HM, Marcus M, Tolbert PE, Rubin C, Henderson AK, Hertzberg VS, Zhang RH, Cameron L. Age at menarche and tanner stage in girls exposed in utero and postnatally to polybrominated biphenyl. Epidemiology. 2000b;11:641–647. doi: 10.1097/00001648-200011000-00005. [DOI] [PubMed] [Google Scholar]
- Burse VW, Needham LL, Liddle JA, Bayse DD, Price HA. Interlaboratory comparison for results of analyses for polybrominated biphenyls in human serum. J Anal Toxicol. 1980;4:22–26. doi: 10.1093/jat/4.1.22. [DOI] [PubMed] [Google Scholar]
- Carter LJ. Michigan’s PBB Incident: Chemical Mix-Up Leads to Disaster. Science. 1976;192:240–243. doi: 10.1126/science.192.4236.240. [DOI] [PubMed] [Google Scholar]
- Chanda JJ, Anderson HA, Glamb RW, Lomatch DL, Wolff MS, Voorhees JJ, Selikoff IJ. Cutaneous effects of exposure to polybrominated biphenyls (PBBs): the Michigan PBB incident. Environ Res. 1982;29:97–108. doi: 10.1016/0013-9351(82)90011-1. [DOI] [PubMed] [Google Scholar]
- Corbett TH, Beaudoin AR, Cornell RG, Anver MR, Schumacher R, Endres J, Szwabowska M. Toxicity of polybrominated biphenyls Firemaster BP-6 in rodents. Environ Res. 1975;10:390–396. doi: 10.1016/0013-9351(75)90034-1. [DOI] [PubMed] [Google Scholar]
- Eyster JT, Humphrey HE, Kimbrough RD. Partitioning of polybrominated biphenyls (PBBs) in serum, adipose tissue, breast milk, placenta, cord blood, biliary fluid, and feces. Arch Environ Health. 1983;38:47–53. doi: 10.1080/00039896.1983.10543978. [DOI] [PubMed] [Google Scholar]
- Faroon OM, Keith S, Jones D, de Rosa C. Effects of polychlorinated biphenyls on development and reproduction. Toxicol Ind Health. 2001;17:63–93. doi: 10.1191/0748233701th097oa. [DOI] [PubMed] [Google Scholar]
- Fein GG, Jacobson JL, Jacobson SW, Schwartz PM, Dowler JK. Prenatal exposure to polychlorinated biphenyls: effects on birth size and gestational age. J Pediatr. 1984;105:315–320. doi: 10.1016/s0022-3476(84)80139-0. [DOI] [PubMed] [Google Scholar]
- Fries GF. The PBB episode in Michigan: an overall appraisal. Crit Rev Toxicol. 1985;16:105–156. doi: 10.3109/10408448509056268. [DOI] [PubMed] [Google Scholar]
- Guvenius DM, Aronsson A, Ekman-Ordeberg G, Bergman A, Noren K. Human prenatal and postnatal exposure to polybrominated diphenyl ethers, polychlorinated biphenyls, polychlorobiphenylols, and pentachlorophenol. Environ Health Perspect. 2003;111:1235–1241. doi: 10.1289/ehp.5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SJ, Cecil HC, Bitman J. Embryotoxic effects of polybrominated biphenyls (PBB) in rats. Environ Health Perspect. 1978;23:295–300. doi: 10.1289/ehp.7823295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoque A, Sigurdson AJ, Burau KD, Humphrey HE, Hess KR, Sweeney AM. Cancer among a Michigan cohort exposed to polybrominated biphenyls in 1973. Epidemiology. 1998;9:373–378. [PubMed] [Google Scholar]
- Jacobson JL, Fein GG, Jacobson SW, Schwartz PM, Dowler JK. The transfer of polychlorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs) across the human placenta and into maternal milk. Am J Public Health. 1984;74:378–379. doi: 10.2105/ajph.74.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RB, Jr, Williams MA, Hogue CJ, Mattison DR. Overview: new perspectives on the stubborn challenge of preterm birth. Paediatr Perinat Epidemiol. 2001;15(Suppl 2):3–6. doi: 10.1046/j.1365-3016.2001.00003.x. [DOI] [PubMed] [Google Scholar]
- Kaiser R, Marcus M, Blanck HM, Naughton M, Zhang RH, Henderson AK, Tolbert PE, Rubin CH, Hertzberg VS. Polybrominated Biphenyl Exposure and Benign Breast Disease in a Cohort of US Women. Annals of Epidemiology. 2003;13:16–23. doi: 10.1016/s1047-2797(02)00256-9. [DOI] [PubMed] [Google Scholar]
- Khanjani N, Sim MR. Maternal contamination with PCBs and reproductive outcomes in an Australian population. J Expo Sci Environ Epidemiol. 2006 doi: 10.1038/sj.jes.7500495. June 14, 2006, Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Knackstedt MK, Hamelmann E, Arck PC. Mothers in stress: consequences for the offspring. Am J Reprod Immunol. 2005;54:63–69. doi: 10.1111/j.1600-0897.2005.00288.x. [DOI] [PubMed] [Google Scholar]
- Kramer MS. The epidemiology of adverse pregnancy outcomes: an overview. J Nutr. 2003;133:1592S–1596S. doi: 10.1093/jn/133.5.1592S. [DOI] [PubMed] [Google Scholar]
- Kramer MS, Demissie K, Yang H, Platt RW, Sauve R, Liston R. The contribution of mild and moderate preterm birth to infant mortality. Fetal and Infant Health Study Group of the Canadian Perinatal Surveillance System. Jama. 2000;284:843–849. doi: 10.1001/jama.284.7.843. [DOI] [PubMed] [Google Scholar]
- Kramer MS, McLean FH, Boyd ME, Usher RH. The validity of gestational age estimation by menstrual dating in term, preterm, and postterm gestations.[see comment] JAMA. 1988;260:3306–3308. [PubMed] [Google Scholar]
- Kreiss K, Roberts C, Humphrey HE. Serial PBB levels, PCB levels, and clinical chemistries in Michigan’s PBB cohort. Arch Environ Health. 1982;37:141–147. doi: 10.1080/00039896.1982.10667553. [DOI] [PubMed] [Google Scholar]
- Lambrecht LK, Barsotti DA, Allen JR. Responses of nonhuman primates to a polybrominated biphenyl mixture. Environ Health Perspect. 1978;23:139–145. doi: 10.1289/ehp.7823139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan PJ, Wilcox KR, Jr, Silva J, Jr, Humphrey HE, Kauffman C, Heath CW., Jr Cohort study of Michigan residents exposed to polybrominated biphenyls: epidemiologic and immunologic findings. Ann N Y Acad Sci. 1979;320:284–294. doi: 10.1111/j.1749-6632.1979.tb56611.x. [DOI] [PubMed] [Google Scholar]
- Lipson SM. Effect of polybrominated biphenyls on the growth and maturation of human peripheral blood lymphocytes. Clin Immunol Immunopathol. 1987;43:65–72. doi: 10.1016/0090-1229(87)90157-7. [DOI] [PubMed] [Google Scholar]
- Longnecker MP, Klebanoff MA, Brock JW, Guo X. Maternal levels of polychlorinated biphenyls in relation to preterm and small-for-gestational-age birth. Epidemiology. 2005;16:641–647. doi: 10.1097/01.ede.0000172137.45662.85. [DOI] [PubMed] [Google Scholar]
- Longnecker MP, Klebanoff MA, Zhou H, Brock JW. Association between maternal serum concentration of the DDT metabolite DDE and preterm and small-for-gestational-age babies at birth. Lancet. 2001;358:110–114. doi: 10.1016/S0140-6736(01)05329-6. [DOI] [PubMed] [Google Scholar]
- Longnecker MP, Wolff MS, Gladen BC, Brock JW, Grandjean P, Jacobson JL, Korrick SA, Rogan WJ, Weisglas-Kuperus N, Hertz-Picciotto I, Ayotte P, Stewart P, Winneke G, Charles MJ, Jacobson SW, Dewailly E, Boersma ER, Altshul LM, Heinzow B, Pagano JJ, Jensen AA. Comparison of polychlorinated biphenyl levels across studies of human neurodevelopment. Environ Health Perspect. 2003;111:65–70. doi: 10.1289/ehp.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack KM, Lepper LF, Wilson DM, Hook JB. Biochemical and physiological sequelae to perinatal exposure to polybrominated biphenyls: a multigeneration study in rats. Toxicol Appl Pharmacol. 1981;59:300–313. doi: 10.1016/0041-008x(81)90202-7. [DOI] [PubMed] [Google Scholar]
- McCormick MC. The contribution of low birth weight to infant mortality and childhood morbidity. N Engl J Med. 1985;312:82–90. doi: 10.1056/NEJM198501103120204. [DOI] [PubMed] [Google Scholar]
- Meester WD. The effect of polybrominated biphenyls on man: the Michigan PBB disaster. Vet Hum Toxicol. 1979;21(Suppl):131–135. [PubMed] [Google Scholar]
- Needham LL, Burse VW, Price HA. Temperature-programmed gas chromatographic determination of polychlorinated and polybrominated biphenyls in serum. J Assoc Off Anal Chem. 1981;64:1131–1137. [PubMed] [Google Scholar]
- Rosenman KD, Anderson HA, Selikoff IJ, Wolff MS, Holstein E. Spermatogenesis in men exposed to polybrominated biphenyl (PBB) Fertil Steril. 1979;32:209–213. [PubMed] [Google Scholar]
- Ross G. The public health implications of polychlorinated biphenyls (PCBs) in the environment. Ecotoxicol Environ Saf. 2004;59:275–291. doi: 10.1016/j.ecoenv.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Sagiv SK, Tolbert PE, Altshul LM, Korrick SA. Polychlorinated biphenyl and organochlorine pesticide exposure during pregnancy and measures of infant size at birth. Epidemiology. 2006 doi: 10.1097/01.ede.0000249769.15001.7c. in press. [DOI] [PubMed] [Google Scholar]
- Schecter A, Papke O, Tung KC, Joseph J, Harris TR, Dahlgren J. Polybrominated diphenyl ether flame retardants in the U.S. population: current levels, temporal trends, and comparison with dioxins, dibenzofurans, and polychlorinated biphenyls. Journal of Occupational & Environmental Medicine. 2005;47:199–211. doi: 10.1097/01.jom.0000158704.27536.d2. [DOI] [PubMed] [Google Scholar]
- Seagull EA. Developmental abilities of children exposed to polybrominated biphenyls (PBB) Am J Public Health. 1983;73:281–285. doi: 10.2105/ajph.73.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J, Kauffman CA, Simon DG, Landrigan PJ, Humphrey HE, Heath CW, Jr, Wilcox KR, Jr, VanAmburg G, Kaslow RA, Ringel A, Hoff K. Lymphocyte function in humans exposed to polybrominated biphenyls. J Reticuloendothel Soc. 1979;26:341–347. [PubMed] [Google Scholar]
- Stross JK, Nixon RK, Anderson MD. Neuropsychiatric findings in patients exposed to polybrominated biphenyls. Ann N Y Acad Sci. 1979;320:368–372. doi: 10.1111/j.1749-6632.1979.tb56618.x. [DOI] [PubMed] [Google Scholar]
- Sweeney AM, Symanski E, Burau KD, Kim YJ, Humphrey HE, Smithci MA. Changes in serum PBB and PCB levels over time among women of varying ages at exposure. Environ Res. 2001;86:128–139. doi: 10.1006/enrs.2001.4261. [DOI] [PubMed] [Google Scholar]
- Thomas AR, Marcus M, Zhang RH, Blanck HM, Tolbert PE, Hertzberg V, Henderson AK, Rubin C. Breast-feeding among women exposed to polybrominated biphenyls in Michigan. Environmental Health Perspectives. 2001;109:1133–1137. doi: 10.1289/ehp.01109133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valciukas JA, Lilis R, Anderson HA, Wolff MS, Petrocci M. The neurotoxicity of polybrominated biphenyls: results of a medical field survey. Ann N Y Acad Sci. 1979;320:337–367. doi: 10.1111/j.1749-6632.1979.tb56617.x. [DOI] [PubMed] [Google Scholar]
- Weil WB, Spencer M, Benjamin D, Seagull E. The effect of polybrominated biphenyl on infants and young children. J Pediatr. 1981;98:47–51. doi: 10.1016/s0022-3476(81)80531-8. [DOI] [PubMed] [Google Scholar]
- Wolff MS, Anderson HA, Camper F, Nikaido MN, Daum SM, Haymes N, Selikoff IJ, Aubrey B. Analysis of adipose tissue and serum from PBB (polybrominated biphenyl)-exposed workers. J Environ Pathol Toxicol. 1979a;2:1397–1411. [PubMed] [Google Scholar]
- Wolff MS, Anderson HA, Rosenman KD, Selikoff IJ. Equilibrium of polybrominated biphenyl (PBB) residues in serum and fat of Michigan residents. Bull Environ Contam Toxicol. 1979b;21:775–781. doi: 10.1007/BF01685504. [DOI] [PubMed] [Google Scholar]