Abstract
Extracellular concentrations of the predominant excitatory neurotransmitter, glutamate, and related excitatory amino acids are maintained at relatively low levels to ensure an appropriate signal-to-noise ratio and to prevent excessive activation of glutamate receptors that can result in cell death. The later phenomenon is known as ‘excitotoxicity’ and has been associated with a wide range of acute and chronic neurodegenerative disorders, as well as disorders that result in the loss of non-neural cells such as oligodendroglia in multiple sclerosis. Unfortunately clinical trials with glutamate receptor antagonists that would logically seem to prevent the effects of excessive receptor activation have been associated with untoward side effects or little clinical benefit. In the mammalian CNS, the extracellular concentrations of glutamate are controlled by two transporters; these include a family of Na+-dependent transporters and a cystine-glutamate exchange process, referred to as system Xc−. In this review, we will focus primarily on the Na+-dependent transporters. A brief introduction to glutamate as a neurotransmitter will be followed by an overview of the properties of these transporters, including a summary of the presumed physiologic mechanisms that regulate these transporters. Many studies have provided compelling evidence that impairing the function of these transporters can increase the sensitivity of tissue to deleterious effects of aberrant activation of glutamate receptors. Over the last decade, it has become clear that many neurodegenerative disorders are associated with a change in localization and/or expression of some of the subtypes of these transporters. This would suggest that therapies directed toward enhancing transporter expression might be beneficial. However, there is also evidence that glutamate transporters might increase the susceptibility of tissue to the consequences of insults that result in a collapse of the electrochemical gradients required for normal function such as stroke. In spite of the potential adverse effects of upregulation of glutamate transporters, there is recent evidence that up-regulation of one of the glutamate transporters, GLT-1 (also called EAAT2), with β-lactam antibiotics attenuates the damage observed in models of both acute and chronic neurodegenerative disorders. While it seems somewhat unlikely that antibiotics specifically target GLT-1 expression, these studies identify a potential strategy to limit excitotoxicity. If successful, this type of approach could have widespread utility given the large number of neurodegenerative diseases associated with decreases in transporter expression and excitotoxicity. However, given the massive effort directed at developing glutamate receptor agents during the 1990s and the relatively modest advances to date, one wonders if we will maintain the patience needed to carefully understand the glutamatergic system so that it will be successfully targeted in the future.
1. Introduction
1.1 Glutamate as a Neurotransmitter and Neurotoxin
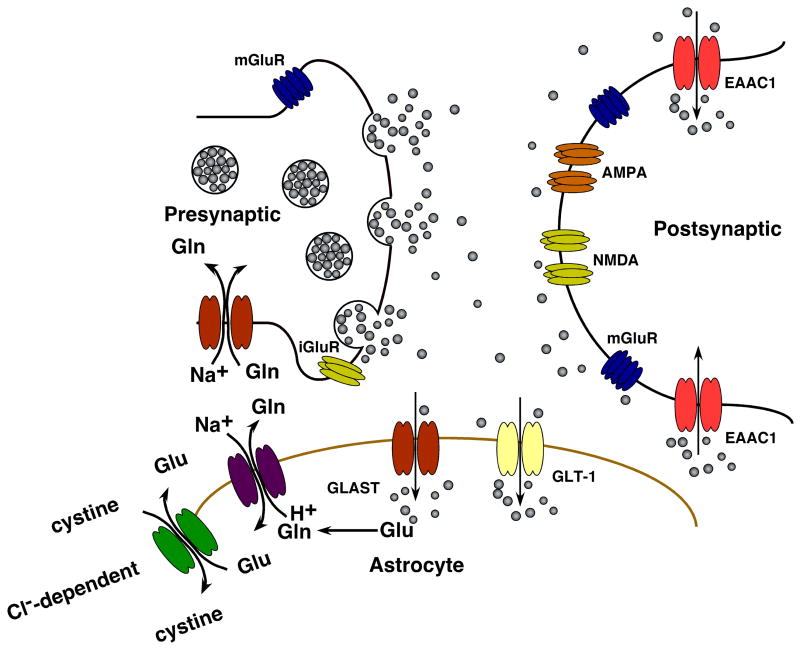
Glutamate is the predominant excitatory neurotransmitter in the mammalian CNS. Glutamate activates a family of ligand gated ion channels that were originally named for exogenous agonists that are selective for each subtype and include α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA), kainate, and N-methyl-D-aspartate (NMDA). Each of these receptors are multimeric assemblies of one or more subunits, and there are considerable numbers of variants of the subunits related to either alternate splicing of the RNA transcripts or editing of the RNA that results in changes in a single base and a corresponding change in a single amino acid (for reviews, see Monaghan, et al., 1989, Seeburg, 1993, Hollman and Heinemann, 1994). In addition, glutamate activates a family of G-protein coupled receptors, referred to as metabotropic glutamate receptors (for reviews, see Conn and Patel, 1994, Nakanishi, 1994). Both families of receptors are situated on various aspects of an excitatory synapse, including the pre-synaptic terminal, the post-synaptic terminal, and astrocytes that sheath the synapse (Fig. 1) (for reviews, see Schoepp and Conn, 1993, Engelman and MacDermott, 2004). In addition, these receptors are found on other cells in the CNS. The various localizations of some of these receptors are consistent with the notion that some of these receptors function as sensors to an accumulation of glutamate. For example, activation of some of the presynaptic receptors dampens release of glutamate. In addition, there is now pretty clear evidence that excitatory cell-to-cell signaling is not restricted to that observed between neurons; astrocytes release glutamate and express glutamate receptors (for reviews, see Carmignoto, 2000, Haydon and Carmignoto, 2006). Oligodendroglia also express glutamate receptors (for review, see Butt, 2006).
Figure 1.
Schematic of an excitatory synapse. Glutamate released from pre-synaptic terminals activates ionotropic and metabotropic glutamate receptors. Glutamate uptake is mediated by a family of Na+-dependent glutamate transporters. Glial cells possess glutamine synthetase, enabling them to convert transported glutamate into glutamine, which can then be shuttled to neurons via glutamine transporters and converted to glutamate (reviewed in Danbolt, 2001, Hertz, 2006). System Xc− exchanges glutamate for cystine, providing cysteine as a precursor for glutathione synthesis. Adapted from (González and Robinson, 2004).
Compared to essentially all other neurotransmitters, the levels of glutamate are extremely high in the mammalian CNS approaching 5–10 mmol/Kg (Butcher and Hamberger, 1987); these levels are ~1000-fold higher than those of many other important neurotransmitters, such as dopamine, norepinephrine, and serotonin. The levels of aspartate are nearly as high as those for glutamate (1–5 mmol/Kg). The notion that aspartate may be a neurotransmitter (for review, see Robinson and Coyle, 1987) has fallen out of favor because it is not a substrate for the cloned vesicular transporters that package glutamate into vesicles (Bellocchio, et al., 2000, Takamori, et al., 2000) nor is it a substrate for the vesicular uptake measured in brain tissue (Naito and Ueda, 1985). However, aspartate is excitatory and does activate at least some of the glutamate receptors (for review, see McDonald and Johnston, 1990). The extracellular concentrations of glutamate, aspartate, and other endogenous excitatory amino acids need to be kept low to limit tonic activation of receptors and to ensure that the depolarization-evoked release of glutamate is accompanied by a sufficient increase in glutamate receptor activation and subsequent signaling. In addition, it has long been recognized that excessive activation of glutamate receptors can kill the cells that express these receptors (for reviews, see Meldrum and Garthwaite, 1990, Choi, 1992). Many of the receptors are activated by low micromolar (1–10 μM) concentrations of these amino acids, and somewhat higher concentrations of these amino acids (10–100 μM) easily kills neurons maintained in culture (Choi, et al., 1987).
1.2 Transporters and clearance of excitatory amino acids
There is no evidence for extracellular metabolism of glutamate or aspartate (for review, see Schousboe, 1981). This implies that these amino acids need to be cleared from the extracellular space by transporters, and it is generally thought that these amino acids are cleared by a family of Na+-dependent ‘high-affinity’ transporters (for reviews, see Gegelashvili and Schousboe, 1997, Seal and Amara, 1999, Danbolt, 2001, Shigeri, et al., 2004). The rationale is based on several observations. First, Na+-independent transport does not contribute more than a few percent of total accumulation of radiolabeled substrate in brain membrane fractions or in cultures prepared from brain tissue (Weiler, et al., 1979; for review, see Robinson, 1998). Second, the capacity for Na+-dependent transport can be quite high in both brain membrane preparations and in primary culture with Vmax values of up to 15 nmol/mg protein per min (Garlin, et al., 1995). Third, the densities of the Na+-dependent transporters are remarkably high (Chaudhry, et al., 1995, Lehre, et al., 1995). Finally, as will be described throughout this review, manipulations (pharmacologic or genetic) that decrease transporter activity have profound effects in the nervous system.
Although there is some variation in the Km values reported for both glutamate and aspartate, they are generally in the low μM range (1–10 μM) for the high-affinity process (for review, see Robinson and Dowd, 1997). This implies that these transporters will be most effective at responding to changes in glutamate concentrations in this range. These transporters function as symporters co-transporting 2 or 3 molecules of Na+ and a proton with each molecule of glutamate (or aspartate). To complete the cycle a K+ ion is counter-transported resulting in reorientation of the transporter such that the glutamate binding site is once again accessible to the extracellular environment (Zerangue and Kavanaugh, 1996, Levy, et al., 1998). There is also evidence that there are low-affinity uptake systems with Km values in the low mM range (Logan and Snyder, 1972), but this system may be Na+-independent (Bennett, et al., 1973) and a specific gene product that mediates this activity may not have been identified (for discussion, see Danbolt, 2001). In addition, there is a transport system that mediates the exchange of glutamate for cystine (Bannai, 1986, Ishii, et al., 1992). Under physiologic conditions, this transporter provides a source of cysteine to cells that need to produce glutathione. This process, termed system Xc−, seems to be a relatively low capacity system, but inhibitors of this transport system substantially decrease extracellular glutamate in microdialysis studies (Baker, et al., 2002). It was proposed that this process controls extrasynaptic glutamate. This system does not transport aspartate, so this system would not prevent a toxic accumulation of this potential excitotoxin (for discussion, see Gochenauer and Robinson, 2001).
1.3 A family of transporters and their localization
Five different Na+-dependent high-affinity glutamate transporters have been identified; these transporters share approximately 50–60% amino acid sequence similarity. Based on the recent elucidation of the crystal structure of a bacterial glutamate transporter homologue (Yernool, et al., 2004), the transporters are predicted to have 8 transmembrane domains with intracellular carboxyl and amino termini and most likely exist as trimers (Yernool, et al., 2003, Koch and Larsson, 2005). Two of these transporters are called GLAST (Storck, et al., 1992) and GLT-1 (Pines, et al., 1992); these are also called EAAT1 or EAAT2, respectively (Arriza, et al., 1994). There are several variants of GLT-1 that originate from alternate splicing of mRNA and these variants differ in their carboxyl- and amino-terminal sequences (Reye, et al., 2002a, Reye, et al., 2002b, Rauen, et al., 2004, Sullivan, et al., 2004). Both GLT-1 and GLAST are found primarily on astrocytes (Rothstein, et al., 1994, Lehre, et al., 1995). Two lines of evidence suggest that these two glial transporters are not uniformly distributed on the astrocytic membrane. First, GLT-1 protein co-localizes with the synaptic vesicle protein synaptophysin (Minelli, et al., 2001). Second, quantitative electron microscopic analysis shows that GLT-1 and GLAST are enriched on astrocytic processes near synaptic termini, suggesting that they are targeted to portions of the membrane that are near the synapse (Chaudhry, et al., 1995, Lehre, et al., 1995). These transporters are also found on many other cells, such as oligodendroglia and macrophages (Domercq, et al., 1999, Gras, et al., 2003). There is also evidence that GLT-1 can be expressed by neurons during development, in the adult nervous system, and in primary cultures (Schmitt, et al., 1996, Furuta, et al., 1997a, Brooks-Kayal, et al., 1998, Mennerick, et al., 1998, Sullivan, et al., 2004, Berger, et al., 2005); at least some of this protein is found on presynaptic nerve terminals (Schmitt, et al., 2002, Chen, et al., 2004). Two members of the transporter family, EAAC1 (also called EAAT3) and EAAT4, are generally considered neuronal transporters (Rothstein, et al., 1994, Furuta, et al., 1997b), but on many neurons they appear to localize to post-synaptic elements, suggesting that they do not participate in transmitter recycling in a fashion analogous to that observed for monoamine transporters (for review, see Blakely and Bauman, 2000, Torres, et al., 2003). EAAC1 is unevenly distributed on the post-synaptic spine with lower levels in the post-synaptic density (enriched in some glutamate receptors) and with higher levels in perisynaptic regions (He, et al., 2000, He, et al., 2001). Although expression of EAAC1 is enriched in pyramidal neurons of the hippocampus and cortex, it is also expressed by many other glutamatergic neurons, by GABAergic neurons (Rothstein, et al., 1994), and by oligodendroglia (Conti, et al., 1998). There is some evidence that low levels of EAAC1 are observed in astrocytes (Conti, et al., 1998, Schlag, et al., 1998). EAAT4 expression is generally thought to be restricted to GABAergic Purkinje cell neurons of the cerebellum in opposition to terminals that release glutamate (Fairman, et al., 1995, Furuta, et al., 1997b), but can also be found in astrocytes (Hu, et al., 2003). Finally, EAAT5 expression seems to be restricted to rod photoreceptor and bipolar cells of the retina (Arriza, et al., 1997).
1.4 Functions and relative contributions of these transporters
In considering the role of transporters in controlling excitatory synaptic transmission, it is important to remember that the time required for a transporter to complete the translocation of glutamate across the membrane is between 10 and 75 msec (Wadiche, et al., 1995b, Bergles and Jahr, 1997). This is much slower than the time course for many of the fast excitatory responses recorded throughout the nervous system; these can be as fast as 10 msec. Therefore, other mechanisms probably contribute to the rapid time-course, including desensitization of some types of glutamate receptors (Tang, et al., 1989). However, the density of transporters situated near the synapse is sufficient to diminish the amount of released glutamate that is available for activation of glutamate receptors; they appear to essentially act as buffers with binding to the transporters being sufficient to limit receptor activation (Tong and Jahr, 1994, Diamond and Jahr, 1997; for reviews, see Conti and Weinberg, 1999, Huang and Bergles, 2004).
Under physiological conditions, it appears that neuronal and glial glutamate uptake effectively limits glutamate spillover, so that most synapses function independently, and only a small amount of glutamate is able to diffuse away to activate neighboring synapses (reviewed in Vizi, 2000, Vizi and Mike, 2006). However, extrasynaptic signaling appears important under different conditions, for instance in area CA1 of the hippocampus there is intersynaptic spillover during synchronous activity. In addition, there is evidence that NMDA receptors located both intra- and extrasynaptically can be activated by glutamate released from more than one synapse during long-term potentiation. Glutamate can also activate presynaptic receptors to modulate transmitter release. Furthermore, there is some tonic activation of extrasynaptic glutamate receptors by ambient transmitter levels.
Based on several lines of evidence, it appears the GLT-1/EAAT2 has the predominant role in clearing glutamate throughout the neuroaxis (for review, see Robinson, 1999, Danbolt, 2001). In fact, there is reasonable evidence that GLT-1 may represent up to 1% of brain protein (for review, see Danbolt, 2001). It has been somewhat easier to define roles for GLT-1 because relatively selective pharmacological inhibitors have been identified, such as dihydrokainate and WAY-855 (Arriza, et al., 1994 for reviews, see Robinson and Dowd, 1997, Bridges and Esslinger, 2005, Dunlop, 2006). Although it has been more difficult to define a precise role for GLAST, the density of this transporter approaches that of GLT-1 (Lehre, et al., 1995) and is enriched even further in some specialized locations (Bergmann glia, glia in the vestibular end organ, and Muller cells of the retina) (for review, see Huang and Bergles, 2004). Understanding the role of the neuronal transporters has also been somewhat difficult. There is evidence that EAAC1 transports glutamate into GABA-ergic nerve terminals, providing glutamate as a precursor for GABA synthesis (Sepkuty, et al., 2002, Mathews and Diamond, 2003). A post-synaptic neuronal transporter has been implicated in controlling the spillover of glutamate between synapses in hippocampus (Rothstein, et al., 1994, Diamond, 2001). Since EAAC1 is essentially the only transporter found in this location (Rothstein, et al., 1994), it suggests that the intermixing of EAAC1 and post-synaptic receptors may locally regulate receptor activation. At least a few of these transporters can also transport cysteine (the reduced form of cystine), suggesting that they might also subserve a role in providing this important precursor for the synthesis of the endogenous anti-oxidant, glutathione (for review, see McBean, 2002). In this regard, mice deleted of EAAC1 display a neurodegenerative process that is associated with decreased brain glutathione levels and is reversed by providing an alternate source of intracellular cysteine, N-acetylcysteine (Aoyama, et al., 2006). In addition to stoichiometrically transporting substrate, the binding of substrate to these transporters results in activation of a Cl− channel that is apparently intrinsic to the transporters themselves. The largest currents observed are associated with neuronal transporters (Fairman, et al., 1995, Wadiche, et al., 1995a, Arriza, et al., 1997 for reviews, see Slotboom, et al., 2001). In fact, EAAT4 appears to be relatively inefficient at translocating substrate with a very slow turnover number, suggesting that the more prominent role of EAAT4 may be to serve as a ligand-gated anion channel (Mim, et al., 2005). Similarly, a recent study provides compelling evidence that EAAT5 on the rod bipolar cells of the retina may control transmitter release by hyperpolarizing the presynaptic nerve terminal (Veruki, et al., 2006), but it also has a role in controlling extracellular glutamate in the retina (Hasegawa, et al., 2006).
1.5 Regulation of these transporters
Like many proteins, these transporters are regulated by both transcriptional and post-transcriptional mechanisms. These regulatory mechanisms have been the topic of many recent reviews (Gegelashvili and Schousboe, 1997, Danbolt, 2001, González and Robinson, 2004, Beart and O’Shea R, 2006, Robinson, 2006); therefore we will only highlight the examples of some of the mechanisms that may be relevant to understanding the alterations in glutamate transporters that have been observed under pathological conditions. Approximately a decade ago, several groups realized that GLT-1 is essentially not expressed by astrocytes in culture even though it is easily detected in brain tissue. Co-culturing neurons with astrocytes induces expression of GLT-1 in astrocytes (Gegelashvili, et al., 1997, Swanson, et al., 1997, Schlag, et al., 1998). At least part of this effect can be mimicked using conditioned media from neuronal cultures (Gegelashvili, et al., 1997) or by maintaining neurons on a semipermeable membrane over a monolayer of astrocytes (Schlag, et al., 1998). This suggests that secreted factors contribute to induction of GLT-1. Based on the observation that the level of induction of GLT-1 expression caused by conditioned media or in the transwells is quite small, it seems likely that both contact and secreted factors contribute to the regulation of GLT-1. Although a number of exogenously applied factors mimic the effects of neurons, it is still not clear which of these factors are sufficient to induce GLT-1 expression to the levels observed in vivo (Eng, et al., 1997, Swanson, et al., 1997, Schlag, et al., 1998, Figiel and Engele, 2000, Zelenaia, et al., 2000, Aronica, et al., 2003, Rodriguez-Kern, et al., 2003, Zschocke, et al., 2005), but some of these factors activate the GLT-1 promoter (Su, et al., 2003, Sitcheran, et al., 2005). Excitotoxic destruction of neurons in mixed cultures of neurons and astrocytes results in decreased expression of GLT-1 and a robust increase in GLAST expression in the remaining astrocytes within 7 days (Schlag, et al., 1998). Similarly, lesioning of projection neurons or deafferentation reduces expression of GLT-1 in target areas in vivo; decreases in GLAST expression were also observed (Ginsberg, et al., 1995, Levy, et al., 1995, Ginsberg, et al., 1996). Based on this data, it seems highly likely that neurons contribute to induction and to the maintenance of GLT-1 expression both in vitro and in vivo. These observations have potential implications for understanding the mechanisms that might contribute to decreased expression of GLT-1 and/or GLAST that accompanies many different neurodegenerative disorders (see below). It also seems that some of the decreases in GLT-1 expression described below may simply be an epiphenomenon of neurodegeneration rather than causative.
In addition to transcriptional mechanisms, there is evidence that the activity of many of the glutamate transporters can be acutely (within min) regulated by mechanisms that are independent of changes in the expression of the transporters (for review, see Robinson, 2002, Beart and O’Shea R, 2006). Some of these effects are associated with a redistribution of the various transporters to or from the plasma membrane in response to activation of a variety of signaling molecules (Davis, et al., 1998, Duan, et al., 1999, Sims, et al., 2000, Kalandadze, et al., 2002, Levenson, et al., 2002, Najimi, et al., 2002, Zhou and Sutherland, 2004, Guillet, et al., 2005, Najimi, et al., 2005, O’Shea, et al., 2006). In addition, there is reasonable evidence that the catalytic efficiency or turnover number of the transporters might be regulated, but at present it is not clear how this might be accomplished (for a recent discussion, see Robinson, 2006). While these acute effects represent mechanisms that may be altered under pathologic conditions, most analyses have understandably focused on measuring protein and/or mRNA levels in human autopsy tissue from individuals with neurodegenerative diseases and in animal models of these diseases. Therefore, we are even further from linking this type of regulation to neurodegenerative diseases than we are from linking possible transcriptional events.
1.6 Extracellular accumulation of glutamate causes toxicity
For over 40 years, it has been recognized that glutamate and glutamate receptor agonists can cause toxicity in the nervous system (for reviews, see Olney, 1989, Choi, 1992, Coyle and Puttfarcken, 1993, Greene and Greenamyre, 1996, Doble, 1999). Although acidic amino acids are not thought to readily cross the blood brain barrier, they do seem to enter the brain when the blood brain barrier is either not fully formed, such as is observed early in development, or when the blood brain barrier is compromised by injury as would occur with trauma or swelling. In fact, one of the first studies to demonstrate evidence for glutamate-mediated toxicity was performed in young mice with peripheral injections of glutamate (Olney, 1969). During the subsequent 20 years, it became clear that several different non-endogenous excitatory amino acids cause neurotoxicity and the patterns of damage including regional and cellular specificity resembled that observed in neurodegenerative diseases, such as Huntington’s disease (Beal, et al., 1986, Storey, et al., 1992). In addition, several studies demonstrated that acute insults such as stroke or traumatic injury are associated with increases in the extracellular concentrations of glutamate and aspartate (Butcher, et al., 1987, Faden, et al., 1989). Finally, in the late 1980s, it was demonstrated that glutamate receptor antagonists can attenuate the damage observed in animal models of these same acute insults (for review, see Chen and Lipton, 2006). These observations prompted several years of drug discovery efforts directed toward the development of glutamate receptor antagonists. Based on these efforts, there is very strong evidence that excessive activation of glutamate receptors can cause cell death both in vitro and in vivo. It is not completely clear why these efforts were not successful, although some of these agents have neurotoxic effects (for review, see Ikonomidou and Turski, 2002). In addition, some have psychotomimetic effects by blocking the NMDA subtype of glutamate receptor.
In addition to causing cell death through excessive activation of glutamate receptors, an extracellular accumulation of glutamate can also cause toxicity through an interaction with system Xc− (Murphy, et al., 1990). As indicated above, this transporter is thought to normally stoichiometrically exchange extracellular cystine for intracellular glutamate, so as to provide a source of cysteine for the synthesis of glutathione. Lewerenz et al. demonstrated that glutamate neurotoxicity in the HT22 neuronal cell line results from excess extracellular glutamate blocking system Xc− (Lewerenz, et al., 2006). Over-expression of an Xc− subunit and EAAT3 cooperatively protects against glutamate toxicity by decreasing the amount of extracellular glutamate available to block Xc−, thereby preventing glutathione depletion. This type of damage is clearly attenuated by anti-oxidants.
1.7 Transporters and vulnerability to excitotoxicity
Many of the original inhibitors of these transporters are substrates and are often referred to as ‘substrate-inhibitors’ (Arriza, et al., 1994 for review, see Campiani, et al., 2003, Bridges and Esslinger, 2005). As the name implies, these compounds are translocated by the transporters, much like glutamate or aspartate. There are several problems with using these types of compounds in vivo and even in culture. Particularly in vivo the capacity for clearance is apparently so high that the compounds cannot diffuse very far from where they are injected (for discussion, see Garthwaite, 1985). This means that the concentrations of inhibitor need to be higher than is required to acutely block glutamate transporters because of the continuous clearance; this could at least theoretically result in non-specific effects through interactions with other extracellular targets (e.g. receptors). In addition, these compounds will likely accumulate in the cells that express high levels of transporters, potentially leading to additional non-specific effects through interactions with intracellular targets. In fact, original in vivo studies failed to demonstrate a role for glutamate transporters in excitotoxicity, but these studies were dependent upon using these substrate inhibitors (Mangano and Schwarcz, 1983, Massieu, et al., 1995). In a cell culture model, one of the more selective substrate-inhibitors potentiates glutamate toxicity (Robinson, et al., 1993) and increases the sensitivity of neurons to oxygen-glucose deprivation (Dugan, et al., 1995). Similarly, the substrate inhibitor trans-pyrrolidine-2,4-dicarboxylate (t-PDC) causes both NMDA-dependent neurotoxicity and NMDA-independent gliotoxicity in hippocampal mixed cultures (Guiramand, et al., 2005). The mechanism of trans-pyrrolidine-2,4-dicarboxylate induced gliotoxicity remains unclear, but it is also independent of oxidative stress and glutathione deficiency. As mentioned above, transportable inhibitors have the potential to affect intracellular targets, which is one possible explanation to account for gliotoxicity. On the other hand, blockade of glutamate transporters by the non-transportable glutamate uptake inhibitor threo-β-benzyloxyaspartate (TBOA) is neurotoxic through activation of NMDA receptors but is not toxic to glia (Guiramand, et al., 2005). Glutamate transporters also play an important role in protecting against necrotic death. In a series of experiments, Bonde and colleagues have shown that blocking glutamate transporters with threo-β-benzyloxyaspartate under normal conditions in rat hippocampal slice cultures results in marked necrotic neurodegeneration, presumably due to increased glutamate in the synaptic cleft, as the effect is blocked by glutamate receptor antagonists (Bonde, et al., 2005). In addition, threo-β-benzyloxyaspartate exacerbates ischemia in rat hippocampus (Selkirk, et al., 2005b). In culture, uptake into glia has a dramatic effect on the sensitivity of neurons to excitotoxic insults (Rosenberg, et al., 1992, Dugan, et al., 1995). With the cloning of the transporters, several additional tools became available to manipulate glutamate transporter activity. Using anti-sense knock-down, Rothstein and his colleagues demonstrated that impaired glutamate transporter expression was associated with neurodegeneration in normal animals (Rothstein, et al., 1996). In addition, mice deleted of GLT-1 display markedly diminished transport activity, seizures, and increased sensitivity to neurotoxicity (Tanaka, et al., 1997), convincingly demonstrating that impaired glutamate transport can cause neurodegeneration in an otherwise normal setting. However, the extent to which glutamate transport needs to be impaired to cause CNS damage is not clear. Although this is only based on intuition, it seems somewhat unlikely that a 10% (or maybe even 20%) decrease in transporter expression would necessarily lead to neurodegeneration, unless larger localized changes accounted for the rather modest reduction in total expression. With that in mind, alterations in glutamate transporters have been reported for several neurodegenerative disorders including amyotrophic lateral sclerosis, Huntington’s disease, Parkinson’s disease, and Alzheimer’s disease. It is not clear in most cases whether glutamate dysfunction contributes to pathogenesis, or results from the disease pathology. It is important to determine whether the onset of neurodegeneration precedes or follows glutamate transporter alterations. In any case, decreases in transporter expression could contribute to ongoing pathology by making the tissue more vulnerable to excitotoxicity.
2.1 Glutamate transporters in Amyotrophic Lateral Sclerosis (ALS)
Amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig’s disease, is the most common motor neuron disease observed in adults with a prevalence of 2–3 per 100,000 individuals. It is characterized by a progressive loss of motor neurons and is generally fatal within 1–5 years of onset (for review, see Cleveland and Rothstein, 2001). The degeneration is more pronounced in the motor neurons of the spinal cord and brainstem, but degeneration is also observed in the motor cortex. The majority of cases appear to be independent of clear genetic linkage (referred to as sporadic) with a small percentage (5–10%) linked to inheritance as a dominant trait (familial). A subgroup of familial cases are related to gain-of-function mutations in an enzyme that converts superoxide anions to hydrogen peroxide and oxygen called Cu2+/Zn2+ superoxide dismutase (SOD1).
Altered expression of glutamate transporters in ALS is perhaps one of the best studied examples linking neuropathology to glutamate transporter dysfunction (reviewed in Rattray and Bendotti, 2006). In a series of studies in the early 1990s, Rothstein and his colleagues found evidence for increases in cerebrospinal fluid glutamate levels (Rothstein, et al., 1990), decreases in functional transport activity (Rothstein, et al., 1992), and decreased levels of GLT-1/EAAT2 protein (Rothstein, et al., 1995) in patients with ALS compared to a control or reference population of patients who died of ‘non-neurologic’ diseases. The levels of transporter activity are ~33% of that observed in the control patients and the levels of GLT-1 protein are reduced to a comparable extent. The pattern of alterations in transporter function and protein levels roughly correlates with the pattern of damage observed in these patients; the largest alterations are observed near motor neurons of the spinal cord. This finding has now been replicated and extended in additional studies (Medina, et al., 1996, Fray, et al., 1998). Rat and mouse models of ALS have been made by introducing variants of SOD1 that resemble those observed in patients with heritable ALS. With exception of one study (Deitch, et al., 2002), several groups have documented reduced levels of transport activity and/or GLT-1 in spinal cord from these animals (Bruijn, et al., 1997, Bendotti, et al., 2001, Howland, et al., 2002, Dunlop, et al., 2003). A mouse model of the related neurological disorder, ALS-Parkinsonism dementia complex (ALS-PDC), has been made by feeding mice washed cycad flour, the major epidemiological link to ALS-PDC (Wilson and Shaw, 2006). These mice display progressive motor, cognitive, and sensory behavioral deficits, and levels of GLT-1 protein are also decreased. For many of the diseases of the nervous system associated with altered glutamate transporter expression, activity (and in some cases protein levels) have been measured in peripheral tissues since there is evidence that many of these Na+-dependent transporters are not exclusively found in the nervous system. In fact, Na+-dependent transport activity is lower in platelets from patients with ALS and plasma glutamate levels are higher (Ferrarese, et al., 2001). However, a recent report suggests that levels of EAAT2 are normal in platelets from patients with ALS, while glutamine synthetase expression is increased (Bos, et al., 2006). Therefore, glutamine synthetase may represent a peripheral marker of the disease.
It is still not clear why the levels of GLT-1/EAAT2 protein are lower in patients with ALS. Although one mutation in the coding region of GLT-1 that results in reduced transporter activity has been linked to ALS (Aoki, et al., 1998, Trotti, et al., 2001), a reasonably large study involving 151 patients failed to identify mutations in the coding region of GLT-1 that change the primary amino acid sequence (Jackson, et al., 1999). Since there is evidence that the levels of GLT-1 expression are dependent upon neurons and these effects are associated with changes in GLT-1 mRNA (see Section 1.5), it seems possible that the loss of GLT-1 is due to damage to or loss of neurons that precedes the loss of GLT-1. However, in at least some of the studies conducted with rodent models, lower levels of GLT-1 were observed in animals prior to evidence of clear neurodegeneration (Howland, et al., 2002). Furthermore, the levels of GLT-1 mRNA are not different in patients with ALS compared to ‘controls’ (Bristol and Rothstein, 1996). Finally, if the loss of activity in platelets is due to decreases in GLT-1 protein, it would be hard to understand how the death of neurons results in decreased transport activity in circulating cells. While this does not rule out the possibility that the loss of GLT-1 is secondary to damage to neurons, these studies seem to suggest that the loss of GLT-1 may not be related to decreased transcription. Lin and colleagues suggested that while the levels of total GLT-1 mRNA are not different, specimens from patients with ALS have higher levels of variants of GLT-1 that originate from altered RNA processing (Lin, et al., 1998). These mRNA species either retain specific introns or lack specific exons that are found in ‘normal’ GLT-1 transcripts. These protein products can function as dominant-negative inhibitors of ‘wild-type’ protein by presumably preventing processing/assembly of transporter in the endoplasmic reticulum (for discussion, see Kalandadze, et al., 2004). Although several studies have observed fairly high levels of these ‘aberrant’ GLT-1 transcripts, the levels of these transcripts were found to be comparable in samples from control patients and patients with ALS (Meyer, et al., 1998, Nagai, et al., 1998, Meyer, et al., 1999, Flowers, et al., 2001). However, in a more recent study using quantitative RT-PCR, the levels of these transcripts were found to be quite low in control subjects (Lauriat, et al., 2006). The linkage of SOD1 to ALS has prompted Trotti and his colleagues to study the effects of co-expression of mutants of SOD1 with GLT-1. They have found that GLT-1 is selectively vulnerable to inactivation by these mutants (Trotti, et al., 2001). In more recent studies, this same group has linked SOD1 to activation of caspase 3 and cleavage of the carboxyl terminal of GLT-1 (Boston-Howes, et al., 2006). Therefore, it seems that the loss of GLT-1 may be attributed to selective inactivation of GLT-1 in at least some of the patients with ALS.
2.2 Glutamate transporters in Alzheimer’s disease
Glutamate toxicity has also been posited to play a role in neurodegeneration in Alzheimer’s disease (AD). Two pathological hallmarks of AD are extracellular amyloid plaques, consisting mostly of the β-amyloid peptide (Aβ), and intraneuronal aggregates of the protein tau (for review see Citron, 2002). There are also structural disparities of the glutamatergic system in AD brain tissue. Dystrophic bulbous glutamatergic terminals have been reported in AD brain tissue, and the dystrophic neurites are located near amyloid plaques (Bell, et al., 2006). As is the case with ALS, reduced glutamate transporter expression and glutamate uptake are associated with AD. Glutamate uptake is decreased in brains (Masliah, et al., 1996) and platelets (Ferrarese, et al., 2000) harvested from patients with AD. Measuring glutamate uptake in platelets from patients may be indicative of glutamate uptake in brain, as platelets exhibit high-affinity glutamate uptake with similar kinetics to that seen in synaptosomes and express many of the same transporters (Mangano and Schwarcz, 1981; Ferrarese, et al., 2001). Glutamate transporter expression appears to decline with age and decline even more rapidly in AD. Compared to younger controls, older humans have decreased glutamate uptake and EAAT1 expression in platelets or cultured fibroblasts (Zoia, et al., 2004, Zoia, et al., 2005). When one controls for age, patients with AD have decreased EAAT1 expression and glutamate uptake in platelets or fibroblasts compared to a control reference population, suggesting that decreased EAAT1 and therefore glutamate clearance may contribute to neurodegeneration associated with AD (Ferrarese, et al., 2000, Zoia, et al., 2004, Zoia, et al., 2005).
Glutamate transporters are also decreased in animal models of AD. In transgenic mice expressing a mutant form of human amyloid precursor protein (the precursor protein for α-amyloid peptide or β-amyloid peptide), aspartate binding, GLAST protein, and GLT-1 protein are significantly reduced compared to littermate controls (Masliah, et al., 2000). However mRNA levels are similar, suggesting a post-translational mechanism for reducing glutamate transporter protein expression. β-amyloid peptide (Aβ), the primary constituent of senile plaques found in AD brain, inhibits glutamate uptake in rat cortical synaptosomes (Keller, et al., 1997b, Lauderback, et al., 1999) and cultured astrocytes (Harris, et al., 1995, Harris, et al., 1996), although the mechanism for this effect is unclear. Neuronal and glial inclusions of the protein tau are another hallmark of AD and other tauopathies (Lee, et al., 2001). Transgenic mice expressing tau protein in astrocytes under the control of the astrocytic-specific glial fibrillary acidic protein (GFAP) promoter develop age-dependent accumulations of tau inclusions (Forman, et al., 2005). These mice display decreased expression of GLT-1 and GLAST proteins in the spinal cord, brainstem, and cortex at 12 and 24 months of age and reduced glutamate transport in synaptosomes. These changes correlate with motor impairments, but precede tau pathology (Dabir, et al., 2006). Therefore, glial glutamate transporter dysfunction may play a role in tauopathy pathogenesis.
In addition to decreased expression of glial glutamate transporters in AD, oxidation of GLT-1 has been hypothesized as a potential mechanism leading to GLT-1 inactivation and glial dysfunction. GLT-1 can be covalently modified by a lipid peroxidation product, 4-hydroxynonenal (HNE), and GLT-1 immunoprecipitated from AD brain has significantly higher levels of HNE modified protein compared to aged-matched controls (Lauderback, et al., 2001). In addition, adding Aβ to rat synaptosomes results in HNE-modified GLT-1. Therefore, GLT-1 inactivation by HNE modification may be a mechanism whereby glutamate clearance is inhibited, resulting in glutamate toxicity (reviewed in Butterfield, et al., 2002).
Another phenomenon associated with AD is aberrant expression of glial glutamate transporters in neurons (Scott, et al., 2002, Thai, 2002). EAAT1 is co-expressed with the neurofibrillary marker, tau, in cortical pyramidal neurons in AD post-mortem brain, while age-matched controls exhibit no or rare EAAT1 cortical neuronal expression (Scott, et al., 2002). Similarly, AD-specific expression of EAAT2 is found in the cytoplasm and neurites of distinct types of neurons from post-mortem tissue (Thai, 2002). EAAT2-expressing neurons exhibit shrunken, condensed nuclei and intracellular accumulation of tau protein. It is still unknown what role increased neuronal expression of EAAT2 may play in AD pathology. It is tempting to propose that neuronal expression of EAAT2 represents a neuroprotective mechanism to combat excitotoxicity. On the other hand, aberrant glial glutamate transporter expression in neurons may somehow lead to tangle formation or simply result from tau pathology.
Although treatments for AD designed to target glutamate transporters for upregulation are not currently in use, one treatment option acts to decrease glutamatergic activation of NMDA receptors (for reviews see Plosker and Lyseng-Williamson, 2005, Heinen-Kammerer, et al., 2006). Memantine is an NMDA receptor partial antagonist that protects against glutamate-induced neurotoxicity without preventing physiological activation of NMDA receptors, and has been shown to significantly reduce functional and cognitive decline in patients with AD. Therefore it is conceivable that treatments aimed at upregulating glutamate transporters could also have beneficial effects for AD patients by decreasing excitotoxicity.
2.3 Glutamate transporters in Huntington’s disease
Huntington’s disease (HD) is a genetic disease caused by expansion of CAG codons for glutamine, resulting in a polyglutamine (polyQ) tract of at least thirty residues in the protein huntingtin (for review, see Borrell-Pages, et al., 2006). This mutation leads to degeneration of the striatum and deep cortical layers, and eventually, the hippocampus and hypothalamus. Within the striatum, medium spiny projection neurons are most sensitive to neurodegeneration. There are a variety of ways that mutant huntingtin could result in neurodegeneration, such as formation of protein aggregates, effects on exocytosis and endocytosis, effects on the brain-derived neurotrophic factor system, and interaction with other proteins (for review see Smith, et al., 2005). Mutant huntingtin has also been reported to affect the glutamatergic system. Mutant huntingtin with the polyQ expansion displays decreased binding to post-synaptic density protein 95 (PSD-95), freeing PSD-95 to bind NMDA receptors at the cell surface, thereby stabilizing NMDA receptors and increasing receptor activation (Sun, et al., 2001). Striatal mixed cultures, which are extremely sensitive to glutamate toxicity, express comparatively low cell surface levels of GLT-1 and GLAST along with low levels of total EAAC1 (Brustovetsky, et al., 2004). Therefore, medium spiny projection neurons may be especially susceptible to increased glutamate due to a combination of abundant NMDA receptors, low levels of total EAAC1 expression, and low levels of cell surface glial glutamate transporters.
Interestingly, EAAT2 mRNA measured by in situ hybridization is reduced in striatum of post-mortem brains of HD patients (Arzberger, et al., 1997); in addition, mutant huntingtin accumulates in glial nuclei in post-mortem brains obtained from patients with HD (Shin, et al., 2005). Mutant huntingtin also forms intranuclear aggregates in glia from the R6/2 mouse model of HD, in which mice express ~150 CAG repeats in the sequence for huntingtin (Mangiarini, et al., 1997). Interestingly, glial aggregates of mutant huntingtin correlate with decreased GLT-1 protein expression in these mice (Shin, et al., 2005). R6/2 mice display motor impairment by 5 weeks of age (Carter, et al., 1999), neurological symptoms by 8 weeks, and frequently die after 12 weeks (Davies, et al., 1997). At 4 weeks of age, GLT-1 mRNA in R6/2 mice is unchanged compared to littermate controls but progressively decreases in the striatum and cerebral cortex from 8 to 12 weeks, while GLAST and EAAC1 mRNA remain unchanged (Lievens, et al., 2001). GLT-1 protein is reduced in cortex and striatum in R6/2 mice at 12 weeks compared to littermate controls, and R6/2 mice display functional consequences of decreased GLT-1 expression. Aspartate binding is a potential surrogate marker of glutamate transporter activity. Aspartate binding in cortex and striatum, as well as glutamate uptake in synaptosomes prepared from 12-week old R6/2 mice are lower compared to littermate controls. Reductions in GLT-1 protein do not appear to be the primary event in HD however, since it occurs after the appearance of polyQ huntingtin aggregates (Davies, et al., 1997, Li, et al., 1999) and motor impairment (Carter, et al., 1999), but may still contribute to chronic excitotoxicity. Mutant huntingtin expressed by adenoviral vectors reduces glutamate uptake and GLT-1 protein in cultured astrocytes without evidence of gliotoxicity, suggesting that glial dysfunction caused by mutant huntingtin may contribute to neuronal excitotoxicity (Shin, et al., 2005). In addition, astrocytes cultured from R6/2 mice are significantly less protective against neurotoxicity compared to wild type astrocytes. Therefore, mutant huntingtin expressed in glial cells appears to contribute to glial dysfunction; this could result in excitotoxicity and neuronal dysfunction. Interestingly, medium spiny projection neurons receive many glutamatergic inputs (Parent and Hazrati, 1995). Therefore, it is possible that the combination of increased NMDA receptor stabilization and decreased glutamate uptake may make them especially vulnerable to excitotoxic cell death.
In a Drosophila model of HD expressing the first exon of human huntingtin with 20, 48, or 93 CAG repeats in a subset of glia, Q48 and Q93 flies die prematurely and display huntingtin nuclear inclusions in glia without loss of glial cells (Lievens, et al., 2005). This is associated with decreased Drosophila EAAT1 (dEAAT1) promoter activity and dEAAT1 protein, indicating that inclusions may inhibit transcription of dEAAT1. In addition, the presence of mutant huntingtin in glial cells inhibits epidermal growth factor receptor activated upregulation of dEAAT1 fused to GFP (dEAAT1-GFP). Thus, decreased dEAAT1 transcription may be associated with lethargy and early death, possibly resulting from glial dysfunction rather than glial death; decreased dEAAT1 should result in reduced glutamate uptake and increased glutamate excitotoxicity.
The bulk of work on the role of glutamate transporters and HD suggests that a reduction in glutamate transporters may aggravate HD symptoms. However, it is unlikely that altered expression of EAATs cause HD; rather mutant huntingtin may decrease glutamate transporter levels, resulting in decreased glutamate uptake and exacerbating excitotoxicity.
2.4 Glutamate transporters in Parkinson’s disease
The hallmarks of Parkinson’s disease (PD) are striking degeneration of dopaminergic nigrostriatal neurons and the presence of Lewey bodies; the loss of nigrostriatal neurons leads to motor dysfunction including tremor, rigidity, and bradykinesia (reviewed in Tuite and Riss, 2003). One hypothesis is that overactivation of glutamate receptors on nigrostriatal neurons may contribute to excitotoxic death. Glutamatergic neurons of the subthalamic nucleus projecting to the substantia nigra display increased firing and bursting patterns in PD patients and experimental models of PD (Bergman, et al., 1994, Hassani, et al., 1996, Hutchison, et al., 1998, Benazzouz, et al., 2002). As is observed in AD, glutamate uptake is reduced in platelets from patients with PD (Ferrarese, et al., 1999). Therefore, impaired glutamate uptake may exacerbate an already heightened state of glutamate receptor activation. Neurotoxins used to simulate PD-like conditions, such as 6-hydroxydopamine (6-OHDA), N-methyl-4-phenylpyridinium (MPP+), and rotenone all decrease glutamate uptake in PC12 cells, and glutamate transport is impaired in synaptosomes from the 6-OHDA PD rat model (Yang, et al., 2005). MPP+ also inhibits glutamate uptake in C6 cells (Yao, et al., 2004) and cultured astrocytes as a consequence of energy failure (Hazell, et al., 1997, Di Monte, et al., 1999).
The involvement of glutamate toxicity in PD is highlighted by reports indicating glutamate receptor blockers or drugs that increase glutamate uptake have beneficial effects in models of PD. NMDA antagonists provide short-term protection after MPP+ administration in rat substantia nigra (Turski, et al., 1991). Likewise, the NMDA antagonist MK801 prevents development of PD syndrome induced by MPP+ in primates (Zuddas, et al., 1992). ATP-sensitive potassium (KATP) channel openers, such as iptakalim, attenuate MPP+-induced increases in extracellular glutamate and cell death in PC12 cells, SH-SY5Y cells, and synaptosomes prepared from a rat model of PD (Hu, et al., 2005, Yang, et al., 2005). Iptakalim treatment in SH-SY5Y cells, which endogenously express EAAT1, 2, and 3, reverses inhibition of glutamate uptake induced by MPP+ (Hu, et al., 2005); therefore, the mechanism of neuroprotection in models of PD exerted by iptakalim may work in part by increasing glutamate uptake. There are a few ways iptakalim may increase glutamate uptake; iptakalim opens mitochondrial KATP channels, regulating mitochondrial volume, preserving intermembrane architecture and allowing efficient energy transfer between the cellular ATPase and mitochondria. Activation of mitochondrial KATP channels also activates protein kinase C (Kis, et al., 2003); in other systems EAAC1 activity and trafficking to the cell surface are increased by activation of protein kinase C (Dowd and Robinson, 1996, Davis, et al., 1998).
The major treatment for PD is L-3,4-dihydroxyphenylalanine (L-DOPA), to replace the progressive loss of dopamine; however repeated L-DOPA treatment leads to motor complications. Chronic L-DOPA treatment in rat models of PD leads to dyskinesias associated with increased extracellular glutamate levels in the basal ganglia (Robelet, et al., 2004) and increased GLT-1 mRNA expression (Robelet, et al., 2004, St-Hilaire, et al., 2005). Therefore, the L-DOPA-dependent increase in GLT-1 expression may represent a compensatory mechanism to protect against glutamate excitotoxicity, in response to high levels of extracellular glutamate.
2.5 Glutamate transporters in epilepsy
Epilepsy is a common neurologic disorder; by 20 years of age, 1% of the population will have developed epilepsy, and the incidence increases to 3% by 75 years of age. A multitude of circumstances exist that can contribute to the development of diverse phenotypes of epilepsy (for review, see Stafstrom, 2006). In addition to perturbations to the GABA-ergic system, alterations in the glutamatergic system have been hypothesized to play a role in the development of seizures and epilepsy. Many laboratories have sought to determine the function/dysfunction of glutamate transporters in relation to developing epileptic pathologies. The non-transportable pan glutamate transport inhibitor threo-β-benzyloxyaspartate (Shimamoto, et al., 1998) prolongs epileptiform activity at low concentrations because it blocks glutamate uptake, sustaining glutamate receptor activation (Campbell and Hablitz, 2005). Threo-β-benzyloxyaspartate also causes alternating periods of bursting activity and hypoactivity in newborn rat (Milh, et al., 2007). GLT-1 knockout mice have lethal seizures, underscoring the importance for effective glutamate uptake in preventing severe seizures (Tanaka, et al., 1997), and GLAST knockout mice also display high seizure susceptibility (Ueda, et al., 2002). Therefore, bulk glial glutamate transport must be crucial in preventing hyperexcitability and seizure generation. Although EAAC1 knockout does not produce a seizure phenotype, antisense knockdown of EAAC1 in hippocampus of adult rat results in an absence-seizure like phenotype, indicating that other glutamate transporters most likely compensate for the loss of EAAC1 in the knockout mice (Rothstein, et al., 1996). Interestingly, it appears that the resultant seizure phenotype in EAAC1-knockdown mice is due in part to decreased GABA-ergic signaling resulting from a reduction in new GABA synthesis; as mentioned in the introduction, EAAC1 provides a source of glutamate for GABA synthesis (Sepkuty, et al., 2002, Mathews and Diamond, 2003).
In humans a mutation in EAAT1 has been found in one patient with epilepsy. When this mutant is expressed in the COS7 mammalian cell line, it displays decreased glutamate uptake compared to cells expressing wild-type EAAT1, suggesting that impaired glutamate homeostasis may be a factor contributing to this patient’s epilepsy (Jen, et al., 2005). As described earlier, multiple mRNA splice variants of EAAT2/GLT-1 exist. The levels of two mRNA splice variants of EAAT2 are significantly elevated in neocortical specimens from patients with temporal lobe epilepsy compared to controls (Hoogland, et al., 2004). Interestingly, these are the same mRNA splice variants potentially associated with amyotrophic lateral sclerosis (ALS) (Lin, et al., 1998).
Further studies using animal models of epilepsy support the role of glutamate transporters in preventing seizure phenotypes. Astrocyte-specific gene inactivation of tuberous sclerosis complex protein 1 in mice (TSC1 conditional knock-out mice) results in progressive epilepsy; these mice express decreased levels of GLT-1 and GLAST protein and display decreased glutamate transport in hippocampal slices and astrocyte cultures (Wong, et al., 2003). Therefore, TSC1 inactivation in astrocytes may result in disrupted glutamate homeostasis, leading to seizures. Reductions in glial glutamate transporters have also been reported in parietal cortex and hippocampus of the EL epileptic mouse model (Ingram, et al., 2001). Similarly, SOD2(−/+) mice display an age-related decrease in GLT-1 and GLAST expression, and increased spontaneous and handling-induced seizures (Liang and Patel, 2004). In addition, idiopathic, epileptic Shetland sheepdogs with lethal seizures have increased levels of extracellular glutamate that are associated with decreased levels of GLT-1 protein in the cerebral cortex and thalamus (Morita, et al., 2005). EAAC1 expression is reduced within hours following kainic acid-induced seizures in hippocampus (Simantov, et al., 1999, Furuta, et al., 2003). Interestingly, an early effect of kainic acid-induced status epilepticus is internalization of EAAC1, mostly in pyramidal neurons, and decreased electrogenic transporter currents (Furuta, et al., 2003), suggesting that kainic acid inhibits neuronal glutamate transport in the early stages of epilepsy. Recently, a mechanism for inducing internalization of EAAC1 in response to kainic acid has been elucidated. A member of the SNARE family of proteins, syntaxin 1A, enhances kainic acid -induced sorting of EAAC1 to endosomes and lysosomes, leading to degradation in C6 glioma cells, while depletion of syntaxin 1 by siRNA blocks kainic acid -induced EAAC1 degradation (Yu, et al., 2006). However, the role of EAAC1 internalization remains unclear. Decreased cell surface EAAC1 in pyramidal cells could limit excessive uptake of glutamate, and therefore limit reverse uptake and epileptic injury to pyramidal cells (Furuta, et al., 2003). On the other hand, if EAAC1 cell surface expression is also decreased on GABA-ergic neurons, it could lead to decreased GABA production and perhaps increase excitability (Yu, et al., 2006).
In contrast to reports of decreased levels of glutamate transporters, increased levels of glutamate transporters have also been described in animal models of epilepsy. Given that glutamate transporter knockout or knockdown leads to seizures, it seems likely that increases in glutamate transporters might represent a compensatory neuroprotective mechanism, rather than contribute to epileptogenesis. In a recent finding, Voutsinos-Porche et al. reported that EAAC1 expression increases in rat pyramidal neurons of the hippocampus and the olfactory tubule during an acute period of induced status epilepticus (Voutsinos-Porche, et al., 2006). Levels return to normal in most of the hippocampus during the latent period following status epilepticus, except EAAC1 is still strongly expressed in the dentate gyrus, and also in the olfactory tubule, cerebral cortex and striatum. Several other groups have also reported increases in glutamate transporters in response to status epilepticus. Zhang et al. found an increase in EAAC1 protein and a decrease in the glutamate receptor subunit GluR2 mRNA and protein following status epilepticus (Zhang, et al., 2004), consistent with a potential mechanism to restore glutamatergic homeostasis. Similarly, Crino et al. found increased EAAC1/EAAT3 mRNA associated with temporal lobe epilepsy in dentate granule cells from rats and humans (Crino, et al., 2002). GLT-1 and EAAC1 protein levels are increased in the cortex of seizure susceptible GLAST −/− mice, possibly as a compensatory mechanism (Ueda, et al., 2002). However, GLT-1 and EAAC1 expression remain unchanged in the hippocampus, indicating a possible lack of compensation, which may be indicative of impaired glutamate uptake in the hippocampus of these mice.
Thus far, experimental work supports the notion that downregulation of glutamate transporters may contribute to seizure phenotypes and upregulation could be a compensatory neuroprotective mechanism. Therefore, would drugs targeted at increasing glutamate transporter activity be therapeutic for epileptic patients? Several studies suggest that some drugs used to treat epilepsy have effects on glutamate transporters, in addition to effects on GABA receptors. GLAST protein expression in hippocampus is downregulated in an animal model of FeCl3-induced spontaneous recurring seizures; however, the benzodiazepine clobazam restores GLAST expression in hippocampus and increases GLAST expression in frontal cortex of saline-injected animals (Doi, et al., 2005). Clobazam may act indirectly to upregulate GLAST expression as a consequence of restoring the brain to a non-epileptic state. However, since it also increases GLAST in control animals, it may have a more direct effect to upregulate GLAST expression. Zonisamide, which blocks voltage-sensitive Na+ channels to prevent repetitive neuronal firing, increases EAAC1 protein in the hippocampus and cortex and decreases GABA transporter 1 (GAT-1) protein in the FeCl3-induced spontaneous seizure model and control animals. Therefore, the antiepileptic effects of zonisamide may be mediated in part by increased extracellular GABA and decreased extracellular glutamate (Ueda, et al., 2003). Since zonisamide increases EAAC1 in both control and FeCl3 animals, upregulation of EAAC1 may result from zonisamide treatment rather than the more indirect effect of rescuing the brain from hyperexcitability. Another common antiepileptic drug, carbamazepine, enhances endogenous EAAC1 activity in C6 cells and oocytes expressing EAAC1 (Lee, et al., 2005). The anticonvulsant topiramate also decreases extracellular levels of glutamate in rat hippocampus (Cross, 2004), perhaps in part by increasing levels of glutamate transporters, as topiramate treatment increases cell surface expression of GLAST and overall expression of GLT-1 protein in rat co-cultures of astrocytes and neurons (Poulsen, et al., 2006).
In summary, blocking glutamate transporters or decreasing expression with molecular tools results in seizure phenotypes, suggesting normal glutamate transport function is important for preventing hyperexcitability. In addition, glutamate transporters are downregulated in a number of animal models of epilepsy. However, glutamate transporters are upregulated in other models of epilepsy, possibly as a compensatory mechanism. Several antiepileptic drugs cause upregulation of glutamate transporters in addition to their effects on the GABA-ergic system. However, as will be discussed in a later section, targeting glutamate transporters pharmacologically for upregulation should be approached with caution, as global increases in glutamate transporter expression may actually have deleterious effects for other disorders, such as stroke.
2.6 Glutamate transporters in cerebral ischemia
Stroke is the third leading cause of death in the U.S., and the predominant type of stroke is cerebral ischemia. There is reasonable evidence that glutamate-mediated excitotoxicity, inflammation, and cell death contribute to the neurodegeneration observed after stroke (for reviews see Dirnagl, et al., 1999, Lo, et al., 2005). In animal models of cerebral ischemia, delayed hippocampal neuronal death following a transient ischemic insult is a well-documented phenomenon but is still not fully understood. Glutamate transporters operate in a reverse direction under conditions of energy failure, when the Na+/K+ electrochemical gradient collapses due to decreased ATP, resulting in the release of glutamate into the extracellular space (Longuemare and Swanson, 1995, Phillis, et al., 2000). Therefore, in addition to Ca2+-dependent vesicular release of glutamate and swelling-activated anion channels, reversal of glutamate transporters plays an important role in acutely elevating levels of extracellular glutamate and resulting excitotoxicity. However, presumably once the electrochemical gradients are restored, glutamate transporters are important once again for limiting excitotoxicity. Alterations in glutamate transporter expression following ischemia have been proposed as a potential mechanism contributing to the delayed neuronal death in hippocampus. In a rat model of cerebral ischemia, neuronal death in CA1 begins at day 4 and continues for up to 21 days post-reperfusion (Bruhn, et al., 2000). However, alterations in glutamate transporters manifest as early as one day post-reperfusion. On day one, there is a loss of GLT-1 mRNA throughout the hippocampus, with losses highest in CA1. This decrease is still present in CA1 at day 4, when GLT-1 protein expression is also reduced. This effect does not appear to be a general dysfunction in astrocytic protein synthesis, as the astrocyte-specific marker glial fibrillary acidic protein (GFAP) is increased at 4 and 21 days post-ischemia. Other studies suggest an even more rapid regulation of glutamate transporters, with decreased GLT-1 protein and mRNA in CA1 in as little as 3–6 hours post-reperfusion (Torp, et al., 1995, Chen, et al., 2005, Yeh, et al., 2005). This decrease could be dependent upon activation of group III metabotropic glutamate receptors (mGluR), which are linked to adenylate cyclase inhibition. Pretreatment with group III mGluR antagonists prevents the decrease in GLT-1 and neuronal damage in CA1 (Chen, et al., 2005). Likewise, after global cerebral ischemia in gerbils, GLT-1 and EAAC1 protein are significantly decreased in the hippocampus compared to sham animals one day post-reperfusion, which precedes neuronal death (Rao, et al., 2000). Since glutamate uptake via EAAC1 has been posited to provide a source for GABA synthesis, decreases in EAAC1 may lead to decreased GABA-ergic activity. Since the reduction in glutamate transporters precedes neuronal death, resultant increased extracellular glutamate and decreased GABA may lead to overexcitation and delayed hippocampal neuronal death. A complementary approach to measuring glutamate transporter changes that occur as a result of ischemia is to alter glutamate transporter expression to determine the effect on neuronal survival post-ischemia. Antisense knockdown of GLT-1 increases infarct volume and neuronal damage in cortex and striatum after transient focal cerebral ischemia in rat (Rao, et al., 2001b). This result argues that uptake of glutamate by GLT-1 plays a neuroprotective role after ischemia.
In humans that experience stroke, the concentration of glutamate in the plasma and cerebrospinal fluid (CSF) is significantly elevated in patients with a large cerebral infarct (Castillo, et al., 1996, Castillo, et al., 1997). However, glutamate concentrations do not necessarily correlate with initial stroke severity, suggesting that there may be some glutamate susceptibility that is unique among individuals. Interestingly, bioinformatics analysis of the EAAT2 promoter yielded several potential regulatory transcription factor-binding elements that may contribute to EAAT2 regulation (Su, et al., 2003). A polymorphism exists in the EAAT2 promoter that has recently been associated with higher levels of plasma glutamate concentrations after a stroke and higher frequency of early neurological deterioration (Mallolas, et al., 2006). This polymorphism changes a consensus site for activator protein-2 (AP2) to a site for GC-binding factor 2 (GCF2). Upon transfection of the mutant promoter into rat astrocytes, promoter activity is decreased compared to wild type promoter activity. In addition co-transfection with AP2 upregulates wild-type promoter activity only, while co-transfection of GCF2 downregulates mutant promoter activity only. Even more interesting, cerebral ischemia in rat increases GCF2 expression at both 2 hrs and 24 hrs post-reperfusion in the cortex and striatum; this transcription factor is not detectable in control animals. These results suggest a mechanism whereby a polymorphism in the EAAT2 promoter may predispose a subset of patients to downregulation of EAAT2 expression after stroke, presumably leading to higher extracellular glutamate and poorer outcome.
Another well-known observation is that preconditioning with sublethal ischemia is consequently neuroprotective against a subsequent ischemic insult (reviewed in Davis and Patel, 2003). Ischemic preconditioning produces an upregulation of GLT-1 and EAAC1 protein, compared to rats that do not undergo preconditioning. Upregulation of EAAC1 appears dependent on tumor necrosis factor receptor 1 (TNFR1) signaling, since anti-TNFα antibody or antisense knockdown of TNFR1 inhibits the effect (Pradillo, et al., 2006). Interestingly, dihydrokainate dose-dependently blocks the protection induced by ischemic preconditioning, suggesting that upregulation of GLT-1 may play a role in the protective mechanism of preconditioning (Zhang, et al., 2007). GLT-1 upregulation appears to occur via the actions of the anti-inflammatory peroxisome proliferator-activated receptor, as an antagonist to this receptor inhibits ischemic preconditioning-induced tolerance and the increase in GLT-1 expression (Romera, et al., 2007). In addition, an agonist for this receptor, rosiglitazone, increases GLT-1 mRNA and protein expression and reduces oxygen-glucose deprivation-induced glutamate release and cell death. Six peroxisome proliferator-activated receptor response elements have been identified in the promoter of GLT-1, and rosiglitazone increases GLT-1 promoter activity. Therefore, increased levels of glutamate transporters may be neuroprotective against subsequent glutamate excitotoxicity. In addition to upregulation of EAAC1, ischemic preconditioning causes a redistribution of EAAC1 away from the cytosol toward the plasma membrane (Pradillo, et al., 2006). Similarly, acute hypoxia stimulates glutamate transport in isolated rat retinal cells, while blockade of actin polymerization inhibits the effect, suggesting that the increase in transport is due to increased trafficking of glutamate transporters (Payet, et al., 2004). Therefore, acute hypoxia may result in rapid mobilization of glutamate transporters, representing another possible neuroprotective mechanism. In neonatal rat brain, increased GLT-1 protein due to hypoxic preconditioning is correlated with increased estrogen receptor alpha (ERα) protein expression (Cimarosti, et al., 2005). Estrogens may be neuroprotective against brain ischemia, although it is unclear if the effects are in part exerted through EAATs, as estrogens have been shown to both augment (Keller, et al., 1997a, Liang, et al., 2002) and inhibit glutamate uptake (Sato, et al., 2003); presumably estrogens also regulate many other targets.
On the other hand, an association between glutamate transporter downregulation and ischemic preconditioning has been reported. There are likely to be many events taking place during preconditioning, and some may depend on the model of preconditioning being used. Yamada and colleagues demonstrated that a nitric oxide synthase (NOS)-dependent decrease in extracellular glutamate during ischemia is associated with downregulation of GLT-1 in co-cultures of neurons and astrocytes (Yamada, et al., 2006). Preconditioning causes an attenuation in the rise of extracellular glutamate during a subsequent ischemic insult, while inhibition of NOS reverses this decrease. In this model, GLT-1 protein is downregulated after preconditioning in an NOS-dependent manner. Therefore, it appears that NOS-derived nitric oxide produced during preconditioning contributes to GLT-1 downregulation, and this is associated with increased survival of neurons in mixed cultures. Presumably, in this model, decreased GLT-1 is neuroprotective through decreased release of glutamate via GLT-1 reversal.
2.7 Glutamate transporters in other neurological disorders
Decreased levels of glutamate transporters seem to be a common observation in many neurodegenerative diseases. Niemann-Pick disease symptoms range from ataxia and dystonia to dementia, and are due to mutations in genes for proteins that affect intracellular transport of cholesterol, leading to accumulations of cholesterol in the endosomes/lysosome, neuronal swelling and ultimately neuronal death (for review, see Vanier and Suzuki, 1998). In a mouse model of the disease, NPC −/− mice exhibit increased GABA transporter 3 (GAT3) protein and decreased EAAC1 protein in the hippocampus compared to wild type mice, suggesting that decreased GABA-ergic signaling combined with increased glutamatergic signaling may contribute to the neurodegenerative pathology to some extent (Byun, et al., 2006).
Glial dysfunction seems to contribute to neurodegeneration and ataxia in a mouse model of cerebellar ataxia 7, in which mutant ataxin containing a polyQ expansion is specifically expressed in Bergmann glia of the cerebellum (Custer, et al., 2006). Functional glutamate uptake and GLAST protein expression are reduced in these transgenic mice. This decrease in GLAST expression and glutamate uptake is associated with Purkinje cell degeneration; therefore a polyQ mutation in ataxin may lead to glial dysfunction and decreased glutamate uptake, resulting in neurodegeneration. Another type of ataxia, spinocerebellar ataxia type 5 (SCA5), can be caused by a mutation in β-III spectrin (Ikeda, et al., 2006). β-III spectrin is expressed in Purkinje neurons, where it stabilizes EAAT4 at the cell surface (Jackson, et al., 2001). However mutant β-III spectrin co-expressed with eGFP-EAAT4 in HEK293 cells does not stabilize eGFP-EAAT4 and lateral mobility in the plasma membrane is increased (Ikeda, et al., 2006). In addition, EAAT4 protein expression is reduced relative to calbindin (a Purkinje cell-specific control protein) in cerebellar autopsy tissue from patients with SCA5, and localization of EAAT4 to synaptosomal fractions is also decreased, suggesting that glial dysfunction and decreased glutamate uptake may also be contributing to this neurodegenerative disorder through glutamatergic overactivation.
Glutamate transporter dysfunction may also contribute to HIV-associated dementia (HAD). HIV infection of astrocytes induces astrogliosis and glutamate excitotoxicity (Sabri, et al., 2003) and results in transcriptional down-regulation of EAAT2 and decreased glutamate uptake in astrocytes (Doble, 1995, Wang, et al., 2003). Astrocyte elevated gene (AEG)-1 expression is increased in HIV-infected astrocytes, and ectopic expression of astrocyte elevated gene-1 inhibits EAAT2 promoter activity, suggesting that HIV-1 reduces EAAT2 expression and glutamate uptake via increased astrocyte elevated gene-1 expression, leading to overactivation of the glutamatergic system (Kang, et al., 2005).
Glutamate transporter alterations have also been implicated in different models of CNS injury. Controlled cortical impact significantly decreases GLT-1 mRNA and protein in hippocampus compared to sham controls 24–72 hours post-traumatic brain injury (TBI) (Rao, et al., 2001a). In addition, antisense knockdown of GLT-1 in rat exacerbates neuronal damage following controlled cortical impact. Therefore, it appears that decreased GLT-1 could lead to impaired glutamate uptake and more severe neuronal loss post-TBI (reviewed in Yi and Hazell, 2006). However, another study describes early losses of the GLT-1 splice variant, GLT-1v, after lateral fluid percussion injury but an increase in the predominant splice variant, GLT-1α, in hippocampus 6–24 hrs post-TBI, possibly as a compensatory neuroprotective mechanism (Yi, et al., 2005). For many years it has been known that there is an efflux of glutamate from brain to blood (Berl, et al., 1961), and glutamate transporters are present on brain blood vessels (Danbolt, 2001). Blood glutamate scavengers, such as oxaloacetate, increase the brain to blood efflux of glutamate by decreasing blood glutamate levels (Gottlieb, et al., 2003). Interestingly, intravenous administration of oxaloacetate provides neuroprotection following TBI (Zlotnik, et al., 2007), suggesting that glutamate shuttling via transporters present on brain blood vessels lowers extracellular levels of glutamate in the brain, thereby offering neuroprotection. Glutamate transporter alterations are also present in models of peripheral nerve injury. GLT-1, GLAST, and EAAC1, are initially upregulated 1–4 days after peripheral nerve injury; however, all three are significantly downregulated by days 7–14 (Sung, et al., 2003, Bear, et al., 2004). Wang and colleagues investigated one possible mechanism behind downregulation of glutamate transporters in the spinal cord dorsal horn induced by peripheral nerve injury (Wang, et al., 2006). They found that chronic constriction nerve injury in rat induces a significant downregulation of EAAC1 protein expression 7 days post-operative within the ipsilateral spinal cord dorsal horn. Glucocorticoid receptor antagonists or infusion of antisense glucocorticoid receptor oligonucleotides for six days post-operative significantly diminish the downregulation. These results suggest that glucocorticoid receptor activation may play a role in the regulation of EAAC1 expression after peripheral nerve injury.
Alterations in glutamate transporters have also been reported in some psychiatric illnesses, including schizophrenia. Schizophrenia is a debilitating mental illness characterized by positive symptoms, such as delusions and hallucinations, as well as negative symptoms, such as decreased affect (for reviews, see Laruelle, et al., 2003, Jarskog, et al., 2006). Decreased glutamatergic signaling in prefrontal cortex has been hypothesized to contribute to schizophrenia, possibly through changes in glutamate receptors (Tsai and Coyle, 2002). In support, phencyclidine (PCP), which blocks NMDA receptors and decreases glutamatergic signaling in prefrontal cortical circuits, has psychotomimetic effects. EAAT1 and EAAT2 mRNA transcripts are increased in thalamus of post-mortem schizophrenic tissue (Smith, et al., 2001). Similarly, GLT-1 mRNA and protein are increased in non-medicated schizophrenic patients compared to controls (Matute, et al., 2005). However medicated patients display no significant changes in EAAT mRNA transcripts compared to controls (Matute, et al., 2005, Lauriat, et al., 2006). In rat, chronic treatment with the antipsychotic clozapine reduces cortical GLT-1 expression and function; likewise clozapine treatment decreases glutamate uptake and GLT-1 expression in rat cultured astrocytes (Melone, et al., 2001, Vallejo-Illarramendi, et al., 2005). Therefore, increased GLT-1 expression and function may represent a mechanism whereby glutamatergic signaling is dysfunctional in schizophrenic patients, and antipsychotic medications may work, in part, by normalizing glutamate transporters.
On the other hand, increased glutamatergic signaling may contribute to symptoms of obsessive-compulsive disorder (OCD). Riluzole treatment reduces glutamatergic transmission by inhibiting glutamate release, and it also inactivates Na+ channels and blocks GABA uptake (Jehle, et al., 2000, Urbani and Belluzzi, 2000). Riluzole augmentation helps alleviate obsessive-compulsive disorder symptoms in treatment-resistant patients (Coric, et al., 2005). Conversely therefore, increased glutamatergic signaling is expected to contribute to obsessive-compulsive disorder symptoms. Also of interest two groups recently reported that statistically significant transmission of obsessive-compulsive disorder through males is associated with the genetic locus 9p24, which codes for EAAT3 (Arnold, et al., 2006, Dickel, et al., 2006).
3.1 Reversal of glutamate transporters
A common theme emerging from many of the neurological disorders discussed is that decreased expression and function of glutamate transporters may contribute to excitotoxicity associated with many of these disorders. Therefore, would treatment targeted towards upregulating glutamate transporters offer neuroprotection? And are there any possible deleterious side effects of over-expression of glutamate transporters? As previously mentioned, glutamate transporters reverse when the Na+/K+ electrochemical gradient collapses due to decreased ATP, releasing glutamate and aspartate into the extracellular space (Longuemare and Swanson, 1995, Phillis, et al., 2000, reviewed in Campiani, et al., 2003). Pharmacologically inducing glutamate transporter reversal using trans-pyrrolidine-2,4-dicarboxylate first activates microglia, followed by neuronal loss and astrogliosis (de Yebra, et al., 2006). A number of studies have focused on identifying the source of extracellular glutamate following energy failure using inhibitors of glutamate transport. The pan glutamate transporter inhibitor trans-pyrrolidine-2,4-dicarboxylate blocks the Ca2+-independent (non-vesicular) release of glutamate in neonatal rat hippocampal slices during K+ depolarization, but has no effect in adult slices (Katsumori, et al., 1999). EAAC1 levels are higher during the neonatal period, and the levels of GLT-1 and GLAST are low early in development (Furuta, et al., 1997a), suggesting that reversal is occurring primarily through the neuronal glutamate transporter, EAAC1. In hippocampal slices from adult rats, the GLT-1 selective inhibitor dihydrokainate potentiates the Ca2+-independent release of glutamate, suggesting that reversal of EAAC1 in adult rats may be masked by uptake via GLT-1 (Katsumori, et al., 1999). On the other hand, glutamate and aspartate levels measured by microdialysis during forebrain ischemia are attenuated by dihydrokainate, suggesting that reversal of GLT-1 contributes to the extracellular accumulation of excitatory amino acids during ischemia in vivo (Seki, et al., 1999). Rossi and colleagues attempted to determine the relative contributions of glutamate transporters to reversal during conditions mimicking severe ischemia in hippocampal slices by measuring anoxic depolarization currents (Rossi, et al., 2000). After an ischemic insult, a glutamate receptor-gated, large inward current can be measured in CA1 pyramidal cells. This current, referred to as an anoxic depolarization current, represents a surrogate measure of extracellular glutamate accumulation. It is Ca2+- independent and also independent of swelling-activated anion channels; in addition trans-pyrrolidine-2,4-dicarboxylate blocks or attenuates the current in hippocampal slices, suggesting that glutamate transporter reversal is the predominant cause of anoxic depolarization. Of interest, dihydrokainate has no effect on the anoxic depolarization current under conditions mimicking severe ischemia in hippocampal slices, suggesting that reversal of GLT-1 is not the source of extracellular glutamate.
The levels of glutamate are generally thought to be higher in neurons since astrocytes are selectively endowed with glutamine synthetase, suggesting that reverse operation of neuronal transporters could be a richer source of glutamate released in this mode. In fact, anoxic depolarization currents measured in hippocampal slices from neonatal GLT-1 knock-out mice following simulated ischemia display similar latency to onset and amplitude as currents measured in wild-type mice, suggesting that reversal of GLT-1 does not to contribute to extracellular glutamate early on during ischemia (Hamann, et al., 2002). Interestingly, the onset to anoxic depolarization following hypoxia in hippocampal slices is significantly delayed in EAAC1 knock-out mice compared to wild-type mice, supporting reversal of EAAC1 as an important source of extracellular glutamate following hypoxia (Gebhardt, et al., 2002). However, the anoxic depolarization current is still present, suggesting that other mechanisms must also contribute to the current.
3.2 Would manipulation of glutamate transporters be beneficial in the treatment of neurological disorders?
Several different approaches have been used to increase glutamate transporter expression with the goal of determining if over-expression might be therapeutic, including transgenic mouse models overexpressing glutamate transporters, pharmacological upregulation, and transduction of various cell types with exogenous glutamate transporters using viral vectors. Double transgenic mice created from crossing the ALS mouse model to a mouse model overexpressing EAAT2 display delayed grip strength decline and motor neuron loss and increased life expectancy (Sutherland, et al., 2001, Sutherland, et al., 2002, Guo, et al., 2003, Scholz and Sutherland, 2003). Similarly, EAAT2-overexpressing transgenic mice display less severe seizures following kainic acid or pilocarpine administration, as well as a reduction in cell death and increased survival (Martinowich, et al., 2001, McMinn, et al., 2003). Recently, Rothstein and colleagues discovered that the β-lactam class of antibiotics stimulates GLT-1 expression and glutamate uptake through increased transcription of GLT-1 (Rothstein, et al., 2005). Interestingly, the β-lactam class antibiotic ceftriaxone is neuroprotective in vitro in a model of motor neuron degeneration and in vivo in a mouse model of ALS. Ceftriaxone treatment delays neuronal death and muscle strength loss and increases survival in these mice. Furthermore, GPI-1046, a synthetic neuroimmunophilin ligand that selectively increases GLT-1 expression and activity, protects motor neurons against excitotoxicity in vitro and increases the survival rate of transgenic ALS mice (Ganel, et al., 2006). In addition, HEK cells engineered to overexpress EAAT2 protect cultured motor neurons exposed to glutamate toxicity (Wisman, et al., 2003). Therefore, multiple studies support the notion that upregulation of GLT-1 is neuroprotective in models of ALS. However, there is some dispute as to whether ceftriaxone would benefit ALS patients clinically, since it did not substantially improve muscle strength and disability scores in an open trial of 108 ALS patients (Beghi, et al., 2006), though this trial did not meet today’s standards in that it was not double blind and had no placebo group.
Likewise, upregulation of GLT-1 may be neuroprotective in cerebral ischemia. Pretreatment for 48 hours with the β-lactam class antibiotic ceftriaxone before oxygen-glucose deprivation in cultured neurons is neuroprotective, and at a level similar to neuroprotection observed with ischemic preconditioning. In addition, ceftriaxone treatment in rats before transient focal ischemia upregulates GLT-1 mRNA and protein and reduces infarct volume (Chu, et al., 2007). The neuroprotective cytokine, ciliary neurotrophic factor, may exert some of its neuroprotective effects through alterations to glutamate transporters (Escartin, et al., 2006). Over-expression of ciliary neurotrophic factor in rat striatum using lentivirus leads to highly glycosylated forms of GLT-1 and GLAST in activated astrocytes; in addition, the glial transporters are redistributed to lipid raft subdomains of the plasma membrane. Interestingly, levels of extracellular glutamate are significantly attenuated after quinolinic acid-evoked glutamate release in lenti-ciliary neurotrophic factor treated striatum, suggesting that ciliary neurotrophic factor may be neuroprotective in part by increasing glutamate uptake. Also of interest, a common anesthetic, isoflurane, causes a PKCα-dependent increase in glutamate uptake and EAAC1 expression at the plasma membrane, suggesting the possibility that this mechanism may also offer protection via increased glutamate uptake (Huang and Zuo, 2005),
Pharmacological and viral-vector mediated manipulations of glutamate transporters carry possible caveats, however. For instance, pharmacologic agents may have unwanted non-specific side effects, or viral-vector mediated over-expression of transporters could result in altered trafficking or targeting if these events are dependent upon interactions with other proteins that are present in limiting amounts. In addition, since glutamate transporters reverse under conditions of energy failure, upregulation may actually exacerbate ischemic insults. The glutamate transporter inhibitor threo-β-benzyloxyaspartate, when used at a concentration which is non-toxic but effectively inhibits reversed glutamate transport in neuronal or astrocytic cultures (Anderson, et al., 2001), reduces oxygen-glucose deprivation-induced damage to area CA1 pyramidal hippocampal neurons (Bonde, et al., 2003a). Likewise, upregulation of GLAST and GLT-1 expression by glial cell line-derived neurotrophic factor (GDNF) pre-treatment increases oxygen-glucose deprivation-induced cell death in rat hippocampal slice cultures, which may be due to increased reverse glutamate transport (Bonde, et al., 2003b). Similarly, adeno-associated viral (AAV) mediated aberrant over-expression of EAAT2 in neurons results in increased neuronal damage to area CA1 of the hippocampus following acute exposure to exogenous glutamate (Selkirk, et al., 2005a).
Mitani and Tanaka attempted to define the role of GLT-1 by measuring levels of extracellular glutamate in wild type and mutant mice lacking GLT-1 using in vivo brain microdialysis during 5 or 20 min of ischemia (Mitani and Tanaka, 2003). Interestingly, glutamate levels are higher in GLT-1 knock-out mice compared to wild type mice during 5 min of ischemia, which is followed by delayed neuronal death in CA1 of the knock-out mice only. However, the reverse is true during longer periods of ischemia; glutamate levels are higher in wild type mice compared to GLT-1 knock-out mice during the second half of a 20 min ischemic insult, and this is accompanied by acute neuronal death in CA1 of wild type mice. So it appears that uptake of glutamate via GLT-1 is neuroprotective early during ischemic insult, but longer insults leading to increased extracellular glutamate, probably by GLT-1 reversal, may contribute to acute neurotoxicity. It is evident that contradictory results exist regarding the role of glutamate transporters in neuroprotection versus contribution to neurotoxicity during ischemic insults. Although glutamate transporters prevent excitotoxicity under normal conditions, their reversal during acute conditions of energy failure may be a mechanism that exacerbates neuronal toxicity.
Conclusion
Alterations in glutamate transporter expression and/or function have been implicated increasingly in a variety of neurological disorders. It is important to attempt to determine whether these changes contribute to pathogenesis or result from existing pathology. In many cases, the role of glutamate transporters in pathogenesis is still unclear, due to conflicting results, possibly due to differing methodology. Although their reversal can actually lead to elevated extracellular concentrations of glutamate and aspartate acutely, glutamate transporters are undeniably important for terminating glutamatergic transmission and preventing glutamate-induced toxicity. In some cases, there is convincing evidence that changes in glutamate transporters precede neurodegeneration, whereas in other disorders alterations to glutamate transporters may result from neurodegeneration. In the latter case, glutamate transporter alterations, such as upregulation, may represent a compensatory neuroprotective mechanism; on the other hand, downregulation may contribute to and exacerbate the continuing pathology. Therefore, treatments aimed at returning glutamate transporters to normal levels of expression and function may be therapeutic; in fact some anticonvulsants and antipsychotics appear to work in part by reversing pathologic alterations to glutamate transporters and restoring glutamatergic homeostasis. In addition β-lactam antibiotics and neuroimmunophilin ligands offer a promising new avenue of treatment against excitotoxicity. However, global manipulations of glutamate transporters may also have harmful effects; for instance widespread upregulation of glutamate transporters may aggravate stroke due to reversal of glutamate transport. The benefits of glutamate transporter upregulation may still outweigh the risks, especially when dealing with fatal diseases.
Acknowledgments
The authors are supported by grants from the National Institutes of Health (A.L.S. NS39011 and MH071008; M.B.R NS39011, NS36465, and NS 29868).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson CM, Bridges RJ, Chamberlin AR, Shimamoto K, Yasuda-Kamatani Y, Swanson RA. Differing effects of substrate and non-substrate transport inhibitors on glutamate uptake reversal. J Neurochem. 2001;79:1207–16. doi: 10.1046/j.1471-4159.2001.00668.x. [DOI] [PubMed] [Google Scholar]
- Aoki M, Lin C-LG, Rothstein JD, Geller BA, Hosler BA, Munsat TL, Horbitz HR, Brown RH. Mutations in the glutamate transporter EAAT2 gene do not cause abnormal EAAT2 transcripts in amyotrophic lateral sclerosis. Ann Neurol. 1998;43:645–653. doi: 10.1002/ana.410430514. [DOI] [PubMed] [Google Scholar]
- Aoyama K, Suh SW, Hamby AM, Liu J, Chan WY, Chen Y, Swanson RA. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat Neurosci. 2006;9:119–26. doi: 10.1038/nn1609. [DOI] [PubMed] [Google Scholar]
- Arnold PD, Sicard T, Burroughs E, Richter MA, Kennedy JL. Glutamate transporter gene SLC1A1 associated with obsessive-compulsive disorder. Arch Gen Psychiatry. 2006;63:769–76. doi: 10.1001/archpsyc.63.7.769. [DOI] [PubMed] [Google Scholar]
- Aronica E, Gorter JA, Ljlst-Keizsers H, Rozemuller AJ, Yankaya B, Leenstra S, Troost D. Expression and functional role of mGluR3 and mGluR5 in human astrocytes and glioma cells: opposite regulation of glutamate transporter proteins. Eur J Neurosci. 2003;17:2106–2118. doi: 10.1046/j.1460-9568.2003.02657.x. [DOI] [PubMed] [Google Scholar]
- Arriza JL, Eliasof S, Kavanaugh MP, Amara SG. Excitatory amino acid transporter 5, a retinal glutamate transporter coupled to a chloride conductance. Proceedings of the National Academy of Sciences USA. 1997;94:4155–4160. doi: 10.1073/pnas.94.8.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriza JL, Fairman WA, Wadiche JI, Murdoch GH, Kavanaugh MP, Amara SG. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J Neurosci. 1994;14:5559–5569. doi: 10.1523/JNEUROSCI.14-09-05559.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzberger T, Krampfl K, Leimgruber S, Weindl A. Changes of NMDA receptor subunit (NR1, NR2B) and glutamate transporter (GLT1) mRNA expression in Huntington’s disease--an in situ hybridization study. J Neuropathol Exp Neurol. 1997;56:440–54. doi: 10.1097/00005072-199704000-00013. [DOI] [PubMed] [Google Scholar]
- Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo glutamate. J Neurosci. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannai S. Exchange of cystine and glutamate across plasma membrane of human fibroblasts. J Biol Chem. 1986;261:2256–2263. [PubMed] [Google Scholar]
- Beal MF, Kowall NW, Ellison DW, Mazurek MF, Swartz KJ, Martin JB. Replication of the neurochemical characteristics of Huntington’s disease by quinolinic acid. Nature. 1986;321:168–171. doi: 10.1038/321168a0. [DOI] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–7. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Beart PM, O’Shea RD. Transporters for L-glutamate: An update on their molecular pharmacology and pathological involvement. Br J Pharmacol. 2006 doi: 10.1038/sj.bjp.0706949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beghi E, Bendotti C, Mennini T. New ideas for therapy in ALS: critical considerations. Amyotroph Lateral Scler. 2006;7:126–7. doi: 10.1080/14660820510012040. discussion 127. [DOI] [PubMed] [Google Scholar]
- Bell KF, Ducatenzeiler A, Ribeiro-da-Silva A, Duff K, Bennett DA, Claudio Cuello A. The amyloid pathology progresses in a neurotransmitter-specific manner. Neurobiol Aging. 2006;27:1644–57. doi: 10.1016/j.neurobiolaging.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Bellocchio EE, Reimer RJ, Fremeau RT, Jr, Edwards RH. Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science. 2000;289:957–60. doi: 10.1126/science.289.5481.957. [DOI] [PubMed] [Google Scholar]
- Benazzouz A, Breit S, Koudsie A, Pollak P, Krack P, Benabid AL. Intraoperative microrecordings of the subthalamic nucleus in Parkinson’s disease. Mov Disord. 2002;17(Suppl 3):S145–9. doi: 10.1002/mds.10156. [DOI] [PubMed] [Google Scholar]
- Bendotti C, Tortarolo M, Suchak SK, Calvaresi N, Carvelli L, Bastone A, Rizzi M, Rattray M, Mennini T. Transgenic SOD1 G93A mice develop reduced GLT-1 in spinal cord without alterations in cerebrospinal fluid glutamate levels. J Neurochem. 2001;79:737–746. doi: 10.1046/j.1471-4159.2001.00572.x. [DOI] [PubMed] [Google Scholar]
- Bennett JP, Logan WJ, Snyder SH. Amino acids as central nervous system transmitters: The influence of ions, amino acid analogues, and ontogeny on transport systems for L-glutamic and L-aspartic acids and glycine into central nervous synaptosomes of the rat. J Neurochem. 1973;21:1533–1550. doi: 10.1111/j.1471-4159.1973.tb06037.x. [DOI] [PubMed] [Google Scholar]
- Berger UV, DeSilva TM, Chen W, Rosenberg PA. Cellular and subcellular mRNA localization of glutamate transporter isoforms GLT1a and GLT1b in rat brain by in situ hybridization. J Comp Neurol. 2005;492:78–89. doi: 10.1002/cne.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles DE, Jahr CE. Synaptic activation of glutamate transporters in hippocampal astrocytes. Neuron. 1997;19:1297–1308. doi: 10.1016/s0896-6273(00)80420-1. [DOI] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol. 1994;72:507–20. doi: 10.1152/jn.1994.72.2.507. [DOI] [PubMed] [Google Scholar]
- Berl S, Lajtha A, Waelsch H. Amino acid and protein metabolism-VI cerebral compartments of glutamic acid metabolism. J Neurochem. 1961;7:186–197. doi: 10.1111/j.1471-4159.1959.tb12638.x. [DOI] [PubMed] [Google Scholar]
- Blakely RD, Bauman AL. Biogenic amine transporters: regulation in flux. Curr Opin Neurobiol. 2000;10:328–336. doi: 10.1016/s0959-4388(00)00088-x. [DOI] [PubMed] [Google Scholar]
- Bonde C, Noraberg J, Noer H, Zimmer J. Ionotropic glutamate receptors and glutamate transporters are involved in necrotic neuronal cell death induced by oxygen-glucose deprivation of hippocampal slice cultures. Neuroscience. 2005;136:779–94. doi: 10.1016/j.neuroscience.2005.07.020. [DOI] [PubMed] [Google Scholar]
- Bonde C, Sarup A, Schousboe A, Gegelashvili G, Zimmer J, Noraberg J. Neurotoxic and neuroprotective effects of the glutamate transporter inhibitor DL-threo-beta-benzyloxyaspartate (DL-TBOA) during physiological and ischemia-like conditions. Neurochem Int. 2003a;43:371–80. doi: 10.1016/s0197-0186(03)00024-x. [DOI] [PubMed] [Google Scholar]
- Bonde C, Sarup A, Schousboe A, Gegelashvili G, Noraberg J, Zimmer J. GDNF pre-treatment aggravates neuronal cell loss in oxygen-glucose deprived hippocampal slice cultures: a possible effect of glutamate transporter up-regulation. Neurochem Int. 2003b;43:381–8. doi: 10.1016/s0197-0186(03)00025-1. [DOI] [PubMed] [Google Scholar]
- Borrell-Pages M, Zala D, Humbert S, Saudou F. Huntington’s disease: from huntingtin function and dysfunction to therapeutic strategies. Cell Mol Life Sci. 2006;63:2642–60. doi: 10.1007/s00018-006-6242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos IW, Hoogland G, Meine Jansen CF, Willigen G, Spierenburg HA, van den Berg LH, de Graan PN. Increased glutamine synthetase but normal EAAT2 expression in platelets of ALS patients. Neurochem Int. 2006;48:306–11. doi: 10.1016/j.neuint.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Boston-Howes W, Gibb SL, Williams EO, Pasinelli P, Brown RH, Jr, Trotti D. Caspase-3 cleaves and inactivates the glutamate transporter EAAT2. J Biol Chem. 2006;281:14076–84. doi: 10.1074/jbc.M600653200. [DOI] [PubMed] [Google Scholar]
- Bridges RJ, Esslinger CS. The excitatory amino acid transporters: pharmacological insights on substrate and inhibitor specificity of the EAAT subtypes. Pharmacol Ther. 2005;107:271–85. doi: 10.1016/j.pharmthera.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Bristol LA, Rothstein JD. Glutamate transporter gene expression in amyotropohic lateral sclerosis motor cortex. Ann Neurol. 1996;39:676–679. doi: 10.1002/ana.410390519. [DOI] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Munir M, Jin H, Robinson MB. The glutamate transporter, GLT-1, is expressed in cultured hippocampal neurons. Neurochem Int. 1998;33:95–100. doi: 10.1016/s0197-0186(98)00018-7. [DOI] [PubMed] [Google Scholar]
- Bruhn T, Levy LM, Nielsen M, Christensen T, Johansen FF, Diemer NH. Ischemia induced changes in expression of the astrocyte glutamate transporter GLT1 in hippocampus of the rat. Neurochem Int. 2000;37:277–85. doi: 10.1016/s0197-0186(00)00029-2. [DOI] [PubMed] [Google Scholar]
- Bruijn LI, Becher MW, Lee MK, Anderson KL, Jenkins NA, Copeland NG, Sisodia SS, Rothstein JD, Borchelt DR, Price DL, Cleveland DW. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 1997;18:327–338. doi: 10.1016/s0896-6273(00)80272-x. [DOI] [PubMed] [Google Scholar]
- Brustovetsky T, Purl K, Young A, Shimizu K, Dubinsky JM. Dearth of glutamate transporters contributes to striatal excitotoxicity. Exp Neurol. 2004;189:222–30. doi: 10.1016/j.expneurol.2004.03.021. [DOI] [PubMed] [Google Scholar]
- Butcher SP, Hamberger A. In vivo studies of the extracellular, and veratridine-releasable, pools of endogenous amino acids in the rat striatum: Effects of corticostriatal deafferentation and kainic acid lesion. J Neurochem. 1987;48:713–721. doi: 10.1111/j.1471-4159.1987.tb05575.x. [DOI] [PubMed] [Google Scholar]
- Butcher SP, Sandberg M, Hagberg H, Hamberger A. Cellular origins of endogenous amino acids released into the extracellular fluid of the rat striatum during severe insulin-induced hypoglycemia. J Neurochem. 1987;48:722–728. doi: 10.1111/j.1471-4159.1987.tb05576.x. [DOI] [PubMed] [Google Scholar]
- Butt AM. Neurotransmitter-mediated calcium signalling in oligodendrocyte physiology and pathology. Glia. 2006;54:666–75. doi: 10.1002/glia.20424. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Castegna A, Lauderback CM, Drake J. Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer’s disease brain contribute to neuronal death. Neurobiol Aging. 2002;23:655–64. doi: 10.1016/s0197-4580(01)00340-2. [DOI] [PubMed] [Google Scholar]
- Byun K, Kim J, Cho SY, Hutchinson B, Yang SR, Kang KS, Cho M, Hwang K, Michikawa M, Jeon YW, Paik YK, Lee B. Alteration of the glutamate and GABA transporters in the hippocampus of the Niemann-Pick disease, type C mouse using proteomic analysis. Proteomics. 2006;6:1230–6. doi: 10.1002/pmic.200500412. [DOI] [PubMed] [Google Scholar]
- Campbell S, Hablitz JJ. Modification of epileptiform discharges in neocortical neurons following glutamate uptake inhibition. Epilepsia. 2005;46(Suppl 5):129–33. doi: 10.1111/j.1528-1167.2005.01020.x. [DOI] [PubMed] [Google Scholar]
- Campiani G, Fattorusso C, De Angelis M, Catalanotti B, Butini S, Fattorusso R, Fiorini I, Nacci V, Novellino E. Neuronal high-affinity sodium-dependent glutamate transporters (EAATs): targets for the development of novel therapeutics against neurodegenerative diseases. Curr Pharm Des. 2003;9:599–625. doi: 10.2174/1381612033391261. [DOI] [PubMed] [Google Scholar]
- Carmignoto G. Astrocyte-neurone crosstalk: variants of the same language? Trends Pharmacol Sci. 2000;21:373–5. doi: 10.1016/s0165-6147(00)01547-9. [DOI] [PubMed] [Google Scholar]
- Carter RJ, Lione LA, Humby T, Mangiarini L, Mahal A, Bates GP, Dunnett SB, Morton AJ. Characterization of progressive motor deficits in mice transgenic for the human Huntington’s disease mutation. J Neurosci. 1999;19:3248–57. doi: 10.1523/JNEUROSCI.19-08-03248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo J, Davalos A, Noya M. Progression of ischaemic stroke and excitotoxic aminoacids. Lancet. 1997;349:79–83. doi: 10.1016/S0140-6736(96)04453-4. [DOI] [PubMed] [Google Scholar]
- Castillo J, Davalos A, Naveiro J, Noya M. Neuroexcitatory amino acids and their relation to infarct size and neurological deficit in ischemic stroke. Stroke. 1996;27:1060–5. doi: 10.1161/01.str.27.6.1060. [DOI] [PubMed] [Google Scholar]
- Chaudhry FA, Lehre KP, Campagne MVL, Ottersen OP, Danbolt NC, Storm-Mathisen J. Glutamate transporters in glial plasma membranes: Highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron. 1995;15:711–720. doi: 10.1016/0896-6273(95)90158-2. [DOI] [PubMed] [Google Scholar]
- Chen HS, Lipton SA. The chemical biology of clinically tolerated NMDA receptor antagonists. J Neurochem. 2006;97:1611–26. doi: 10.1111/j.1471-4159.2006.03991.x. [DOI] [PubMed] [Google Scholar]
- Chen JC, Hsu-Chou H, Lu JL, Chiang YC, Huang HM, Wang HL, Wu T, Liao JJ, Yeh TS. Down-regulation of the glial glutamate transporter GLT-1 in rat hippocampus and striatum and its modulation by a group III metabotropic glutamate receptor antagonist following transient global forebrain ischemia. Neuropharmacology. 2005;49:703–14. doi: 10.1016/j.neuropharm.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Chen W, Mahadomrongkul V, Berger UV, Bassan M, DeSilva T, Tanaka K, Irwin N, Aoki C, Rosenberg PA. The glutamate transporter GLT1a is expressed in excitatory terminals of mature hippocampal neurons. J Neurosci. 2004;24:1136–1148. doi: 10.1523/JNEUROSCI.1586-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW. Excitotoxic cell death. J Neurobiol. 1992;23:1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- Choi DW, Maulucci-Gedde M, Kriegstein AR. Glutamate neurotoxicity in cortical cell culture. J Neurosci. 1987;7:357–368. doi: 10.1523/JNEUROSCI.07-02-00357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu K, Lee ST, Sinn DI, Ko SY, Kim EH, Kim JM, Kim SJ, Park DK, Jung KH, Song EC, Lee SK, Kim M, Roh JK. Pharmacological Induction of Ischemic Tolerance by Glutamate Transporter-1 (EAAT2) Upregulation. Stroke. 2007;38:177–82. doi: 10.1161/01.STR.0000252091.36912.65. [DOI] [PubMed] [Google Scholar]
- Cimarosti H, Jones NM, O’Shea RD, Pow DV, Salbego C, Beart PM. Hypoxic preconditioning in neonatal rat brain involves regulation of excitatory amino acid transporter 2 and estrogen receptor alpha. Neurosci Lett. 2005;385:52–7. doi: 10.1016/j.neulet.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Citron M. Alzheimer’s disease: treatments in discovery and development. Nat Neurosci. 2002;5(Suppl):1055–7. doi: 10.1038/nn940. [DOI] [PubMed] [Google Scholar]
- Cleveland DW, Rothstein JD. From charcot to lou gehrig: Deciphering selective motor neuron death in ALS. Nature Reviews Neuroscience. 2001;2:806–819. doi: 10.1038/35097565. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Patel J. The metabotropic glutamate receptors. Totowa, NJ: Humana Press; 1994. [Google Scholar]
- Conti F, Weinberg RJ. Shaping excitation at glutamatergic synapses. Trends Neurosci. 1999;22:451–458. doi: 10.1016/s0166-2236(99)01445-9. [DOI] [PubMed] [Google Scholar]
- Conti F, DiBiasi S, Minelli A, Rothstein JD, Melone M. EAAC1, a high-affinity glutamate transporter, is localized to astrocytes and gabaergic neurons besides pyramidal cells in the rat cerebral cortex. Cereb Cortex. 1998;8:108–116. doi: 10.1093/cercor/8.2.108. [DOI] [PubMed] [Google Scholar]
- Coric V, Taskiran S, Pittenger C, Wasylink S, Mathalon DH, Valentine G, Saksa J, Wu YT, Gueorguieva R, Sanacora G, Malison RT, Krystal JH. Riluzole augmentation in treatment-resistant obsessive-compulsive disorder: an open-label trial. Biol Psychiatry. 2005;58:424–8. doi: 10.1016/j.biopsych.2005.04.043. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–95. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- Crino PB, Jin H, Shumate MD, Robinson MB, Coulter DA, Brooks-Kayal AR. Increased expression of the neuronal glutamate transporter (EAAT3/EAAC1) in hippocampal and neocortical epilepsy. Epilepsia. 2002;43:211–8. doi: 10.1046/j.1528-1157.2002.35001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross JH. Topiramate. In: Shorvan S, Perucca E, Fish D, Dodsan E, editors. The Treatment of Epilepsy. 2. Blackwell Publications; Oxford: 2004. pp. 515–527. [Google Scholar]
- Custer SK, Garden GA, Gill N, Rueb U, Libby RT, Schultz C, Guyenet SJ, Deller T, Westrum LE, Sopher BL, La Spada AR. Bergmann glia expression of polyglutamine-expanded ataxin-7 produces neurodegeneration by impairing glutamate transport. Nat Neurosci. 2006;9:1302–11. doi: 10.1038/nn1750. [DOI] [PubMed] [Google Scholar]
- Dabir DV, Robinson MB, Swanson E, Zhang B, Trojanowski JQ, Lee VM, Forman MS. Impaired glutamate transport in a mouse model of tau pathology in astrocytes. J Neurosci. 2006;26:644–54. doi: 10.1523/JNEUROSCI.3861-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Davies SW, Turmaine M, Cozens BA, DiFiglia M, Sharp AH, Ross CA, Scherzinger E, Wanker EE, Mangiarini L, Bates GP. Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell. 1997;90:537–48. doi: 10.1016/s0092-8674(00)80513-9. [DOI] [PubMed] [Google Scholar]
- Davis DP, Patel PM. Ischemic preconditioning in the brain. Curr Opin Anaesthesiol. 2003;16:447–52. doi: 10.1097/00001503-200310000-00002. [DOI] [PubMed] [Google Scholar]
- Davis KE, Straff DJ, Weinstein EA, Bannerman PG, Correale DM, Rothstein JD, Robinson MB. Multiple signaling pathways regulate cell surface expression and activity of the excitatory amino acid carrier 1 subtype of Glu transporter in C6 glioma. J Neurosci. 1998;18:2475–2485. doi: 10.1523/JNEUROSCI.18-07-02475.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Yebra L, Malpesa Y, Ursu G, Pugliese M, Lievens JC, Goff LK, Mahy N. Dissociation between hippocampal neuronal loss, astroglial and microglial reactivity after pharmacologically induced reverse glutamate transport. Neurochem Int. 2006;49:691–7. doi: 10.1016/j.neuint.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Deitch JS, Alexander GM, Del Valle L, Heiman-Patterson TD. GLT-1 glutamate transporter levels are unchanged in mice expressing G93A human mutant SOD1. J Neurol Sci. 2002;193:117–26. doi: 10.1016/s0022-510x(01)00656-6. [DOI] [PubMed] [Google Scholar]
- Di Monte DA, Tokar I, Langston JW. Impaired glutamate clearance as a consequence of energy failure caused by MPP(+) in astrocytic cultures. Toxicol Appl Pharmacol. 1999;158:296–302. doi: 10.1006/taap.1999.8717. [DOI] [PubMed] [Google Scholar]
- Diamond JS. Neuronal glutamate transporters limit activation of NMDA receptors by neurotransmitter spillover on CA1 pyramidal cells. J Neurosci. 2001;21:8328–8338. doi: 10.1523/JNEUROSCI.21-21-08328.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond JS, Jahr CE. Transporters buffer synaptically released glutamate on a submillisecond time scale. J Neurosci. 1997;17:4672–4687. doi: 10.1523/JNEUROSCI.17-12-04672.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickel DE, Veenstra-VanderWeele J, Cox NJ, Wu X, Fischer DJ, Van Etten-Lee M, Himle JA, Leventhal BL, Cook EH, Jr, Hanna GL. Association testing of the positional and functional candidate gene SLC1A1/EAAC1 in early-onset obsessive-compulsive disorder. Arch Gen Psychiatry. 2006;63:778–85. doi: 10.1001/archpsyc.63.7.778. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–7. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Doble A. Excitatory amino acid receptors and neurodegeneration. Therapie. 1995;50:319–37. [PubMed] [Google Scholar]
- Doble A. The role of excitotoxicity in neurodegenerative disease: Implications for therapy. Pharmacology and Therapeutics. 1999;81:163–221. doi: 10.1016/s0163-7258(98)00042-4. [DOI] [PubMed] [Google Scholar]
- Doi T, Ueda Y, Tokumaru J, Willmore LJ. Molecular regulation of glutamate and GABA transporter proteins by clobazam during epileptogenesis in Fe(+++)-induced epileptic rats. Brain Res Mol Brain Res. 2005;142:91–6. doi: 10.1016/j.molbrainres.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Domercq M, Sanchez-Gomez MV, Areso P, Matute C. Expression of glutamate transporters in rat optic nerve oligodendrocytes. Eur J Neurosci. 1999;11:2226–36. doi: 10.1046/j.1460-9568.1999.00639.x. [DOI] [PubMed] [Google Scholar]
- Dowd LA, Robinson MB. Rapid stimulation of EAAC1-mediated Na+-dependent L-glutamate transport activity in C6 glioma by phorbol ester. J Neurochem. 1996;67:508–516. doi: 10.1046/j.1471-4159.1996.67020508.x. [DOI] [PubMed] [Google Scholar]
- Duan S, Anderson CM, Stein BA, Swanson RA. Glutamate induces rapid upregulation of glutamate transport and cell-surface expression of GLAST. J Neurosci. 1999;19:10193–10200. doi: 10.1523/JNEUROSCI.19-23-10193.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugan LL, Bruno VMG, Amagasu SM, Giffard RG. Glia modulate the response of murine cortical neurons to excitotoxicity: Glia exacerbate AMPA neurotoxicity. J Neurosci. 1995;15:4545–4555. doi: 10.1523/JNEUROSCI.15-06-04545.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop J. Glutamate-based therapeutic approaches: targeting the glutamate transport system. Curr Opin Pharmacol. 2006;6:103–7. doi: 10.1016/j.coph.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Dunlop J, McIvain HB, She Y, Howland DS. Impaired spinal cord glutamate transport capacity and reduced sensitivity to riluzole in a transgenic superoxide dismutase mutant rat model of amytrophic lateral sclerosis. J Neurosci. 2003;23:1688–1696. doi: 10.1523/JNEUROSCI.23-05-01688.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng DL, Lee YL, Lal PG. Expression of glutamate uptake transporters after dibutyryl cyclic AMP differentiation and traumatic injury in cultured astrocytes. Brain Res. 1997;778:215–221. doi: 10.1016/s0006-8993(97)01093-7. [DOI] [PubMed] [Google Scholar]
- Engelman HS, MacDermott AB. Presynaptic ionotropic receptors and control of transmitter release. Nat Rev Neurosci. 2004;5:135–45. doi: 10.1038/nrn1297. [DOI] [PubMed] [Google Scholar]
- Escartin C, Brouillet E, Gubellini P, Trioulier Y, Jacquard C, Smadja C, Knott GW, Kerkerian-Le Goff L, Deglon N, Hantraye P, Bonvento G. Ciliary neurotrophic factor activates astrocytes, redistributes their glutamate transporters GLAST and GLT-1 to raft microdomains, and improves glutamate handling in vivo. J Neurosci. 2006;26:5978–89. doi: 10.1523/JNEUROSCI.0302-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faden AI, Demediuk P, Panter SS, Vink R. The role of excitatatory amino acids and NMDA receptors in traumatic brain injury. Science. 1989;244:798–800. doi: 10.1126/science.2567056. [DOI] [PubMed] [Google Scholar]
- Fairman WA, Vandenberg RJ, Arriza JL, Kavanaugh MP, Amara SG. An excitatory amino acid transporter with properties of a ligand-gated chloride channel. Nature (Lond) 1995;375:599–603. doi: 10.1038/375599a0. [DOI] [PubMed] [Google Scholar]
- Ferrarese C, Begni B, Canevari C, Zoia C, Piolti R, Frigo M, Appollonio I, Frattola L. Glutamate uptake is decreased in platelets from Alzheimer’s disease patients. Ann Neurol. 2000;47:641–3. [PubMed] [Google Scholar]
- Ferrarese C, Zoia C, Pecora N, Piolti R, Frigo M, Bianchi G, Sala G, Begni B, Riva R, Frattola L. Reduced platelet glutamate uptake in Parkinson’s disease. J Neural Transm. 1999;106:685–92. doi: 10.1007/s007020050189. [DOI] [PubMed] [Google Scholar]
- Ferrarese C, Sala G, Riva R, Begni B, Zoia C, Tremolizzo L, Galimberti G, Millul A, Bastone A, Mennini T, Balzarini C, Frattola L, Beghi E. Decreased platelet glutamate uptake in patients with amyotrophic lateral sclerosis. Neurology. 2001;56:270–2. doi: 10.1212/wnl.56.2.270. [DOI] [PubMed] [Google Scholar]
- Figiel M, Engele J. Pituitary adenylate cyclase-activating polypeptide (PACAP), a neuron-derived peptide regulating glial glutamate transport and metabolism. J Neurosci. 2000;20:3596–3605. doi: 10.1523/JNEUROSCI.20-10-03596.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers JM, Powell JF, Leigh PN, Andersen P, Shaw CE. Intron 7 retention and exon 9 skipping EAAT2 mRNA variants are not associated with amyotrophic lateral sclerosis. Ann Neurol. 2001;49:643–649. [PubMed] [Google Scholar]
- Forman MS, Lal D, Zhang B, Dabir DV, Swanson E, Lee VM, Trojanowski JQ. Transgenic mouse model of tau pathology in astrocytes leading to nervous system degeneration. J Neurosci. 2005;25:3539–50. doi: 10.1523/JNEUROSCI.0081-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fray AE, Ince PG, Banner SJ, Milton ID, Usher PA, Cookson MR, Shaw PJ. The expression of the glial glutamate transporter protein EAAT2 in motor neuron disease: an immunohistochemical study. Eur J Neurosci. 1998;10:2481–2489. doi: 10.1046/j.1460-9568.1998.00273.x. [DOI] [PubMed] [Google Scholar]
- Furuta A, Rothstein JD, Martin LJ. Glutamate transporter protein subtypes are expressed differentially during rat central nervous system development. J Neurosci. 1997a;17:8363–8375. doi: 10.1523/JNEUROSCI.17-21-08363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta A, Martin LJ, Lin C-LG, Dykes-Hoberg M, Rothstein JD. Cellular and synaptic localization of the neuronal glutamate transporters EAAT3 and EAAT4. Neuroscience. 1997b;81:1031–1042. doi: 10.1016/s0306-4522(97)00252-2. [DOI] [PubMed] [Google Scholar]
- Furuta A, Noda M, Suzuki SO, Goto Y, Kanahori Y, Rothstein JD, Iwaki T. Translocation of glutamate transporter subtype excitatory amino acid carrier 1 protein in kainic acid-induced rat epilepsy. Am J Pathol. 2003;163:779–87. doi: 10.1016/S0002-9440(10)63705-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganel R, Ho T, Maragakis NJ, Jackson M, Steiner JP, Rothstein JD. Selective up-regulation of the glial Na+-dependent glutamate transporter GLT1 by a neuroimmunophilin ligand results in neuroprotection. Neurobiol Dis. 2006;21:556–67. doi: 10.1016/j.nbd.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Garlin AB, Sinor AD, Sinor JD, Jee SH, Grinspan JB, Robinson MB. Pharmacology of sodium-dependent high-affinity L-[3H]glutamate transport in glial cultures. J Neurochem. 1995;64:2572–2580. doi: 10.1046/j.1471-4159.1995.64062572.x. [DOI] [PubMed] [Google Scholar]
- Garthwaite J. Cellular uptake disguises action of L-glutamate on N-methyl-D-aspartate receptors. Br J Pharmacol. 1985;85:297–307. doi: 10.1111/j.1476-5381.1985.tb08860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt C, Körner R, Heinemann U. Delayed anoxic depolarizations in hippocampal neurons of mice lacking the excitatory amino acid carrier 1. Journal of Cerebral Blood Flow and Metabolism. 2002;22:569–575. doi: 10.1097/00004647-200205000-00008. [DOI] [PubMed] [Google Scholar]
- Gegelashvili G, Schousboe A. High affinity glutamate transporters: regulation of expression and activity. Mol Pharmacol. 1997;52:6–15. doi: 10.1124/mol.52.1.6. [DOI] [PubMed] [Google Scholar]
- Gegelashvili G, Danbolt NC, Schousboe A. Neuronal soluble factors differentially regulate the expression of the GLT1 and GLAST glutamate transporters in cultured astroglia. J Neurochem. 1997;69:2612–5. doi: 10.1046/j.1471-4159.1997.69062612.x. [DOI] [PubMed] [Google Scholar]
- Ginsberg SD, Martin LJ, Rothstein JD. Regional deafferentation down-regulates subtypes of glutamate transporter proteins. J Neurochem. 1995;65:2800–2803. doi: 10.1046/j.1471-4159.1995.65062800.x. [DOI] [PubMed] [Google Scholar]
- Ginsberg SD, Rothstein JD, Price DL, Martin LJ. Fimbria-fornix transections selectively down-regulate subtypes of glutamate transporter and glutamate receptor proteins in septum and hippocampus. J Neurochem. 1996;67:1208–1216. doi: 10.1046/j.1471-4159.1996.67031208.x. [DOI] [PubMed] [Google Scholar]
- Gochenauer GE, Robinson MB. Dibutyryl-cAMP (dbcAMP) up-regulates astrocytic chloride-dependent L-[3H]glutamate transport and expression of both system Xc− subunits. J Neurochem. 2001;78:276–286. doi: 10.1046/j.1471-4159.2001.00385.x. [DOI] [PubMed] [Google Scholar]
- González MI, Robinson MB. Protein Kinase C-Dependent Remodeling of Glutamate Transporter Function. Mol Interventions. 2004;4:48–58. doi: 10.1124/mi.4.1.48. [DOI] [PubMed] [Google Scholar]
- Gottlieb M, Wang Y, Teichberg VI. Blood-mediated scavenging of cerebrospinal fluid glutamate. J Neurochem. 2003;87:119–26. doi: 10.1046/j.1471-4159.2003.01972.x. [DOI] [PubMed] [Google Scholar]
- Gras G, Chretien F, Vallat-Decouvelaere AV, Le Pavec G, Porcheray F, Bossuet C, Leone C, Mialocq P, Dereuddre-Bosquet N, Clayette P, Le Grand R, Creminon C, Dormont D, Rimaniol AC, Gray F. Regulated expression of sodium-dependent glutamate transporters and synthetase: a neuroprotective role for activated microglia and macrophages in HIV infection? Brain Pathol. 2003;13:211–22. doi: 10.1111/j.1750-3639.2003.tb00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene JG, Greenamyre JT. Bioenergetics and glutamate excitotoxicity. Prog Neurobiol. 1996;48:613–634. doi: 10.1016/0301-0082(96)00006-8. [DOI] [PubMed] [Google Scholar]
- Guillet BA, Velly LJ, Canolle BFMM, Nieoullon AL, Pisano P. Differential regulation by protein kinases of activity and cell surface expression of glutamate transporters in neuron-enriched cultures. Neurochem Int. 2005;46:337–346. doi: 10.1016/j.neuint.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Guiramand J, Martin A, de Jesus Ferreira MC, Cohen-Solal C, Vignes M, Recasens M. Gliotoxicity in hippocampal cultures is induced by transportable, but not by nontransportable, glutamate uptake inhibitors. J Neurosci Res. 2005;81:199–207. doi: 10.1002/jnr.20557. [DOI] [PubMed] [Google Scholar]
- Guo H, Lai L, Butchbach MER, Stockinger MP, Shan X, Bishop GA, Lin C-LG. Increased expression of the glial glutamate transporter EAAT2 modulates excitotoxicity and delays the onset but not the outcome of ALS in mice. Hum Mol Genet. 2003;12:2519–2532. doi: 10.1093/hmg/ddg267. [DOI] [PubMed] [Google Scholar]
- Hamann M, Rossi DJ, Marie H, Attwell D. Knocking out the glial glutamate transporter GLT-1 reduces glutamate uptake but does not affect hippocampal glutamate dynamics in early simulated ischemia. Eur J Neurosci. 2002;15:308–314. doi: 10.1046/j.0953-816x.2001.01861.x. [DOI] [PubMed] [Google Scholar]
- Harris ME, Wang YNW, Pedigo J, Hensley K, Butterfield DA, Carney JM. Amyloid β Peptide (25–35) inhibits Na+-dependent glutamate uptake in rat hippocampal astrocyte cultures. J Neurochem. 1996;67:277–286. doi: 10.1046/j.1471-4159.1996.67010277.x. [DOI] [PubMed] [Google Scholar]
- Harris ME, Carney JM, Cole PS, Hensley K, Howard BJ, Martin L, Bummer P, Wang Y, Pedigo NW, Jr, Butterfield DA. beta-Amyloid peptide-derived, oxygen-dependent free radicals inhibit glutamate uptake in cultured astrocytes: implications for Alzheimer’s disease. Neuroreport. 1995;6:1875–9. doi: 10.1097/00001756-199510020-00013. [DOI] [PubMed] [Google Scholar]
- Hasegawa J, Obara T, Tanaka K, Tachibana M. High-density presynaptic transporters are required for glutamate removal from the first visual synapse. Neuron. 2006;50:63–74. doi: 10.1016/j.neuron.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Hassani OK, Mouroux M, Feger J. Increased subthalamic neuronal activity after nigral dopaminergic lesion independent of disinhibition via the globus pallidus. Neuroscience. 1996;72:105–15. doi: 10.1016/0306-4522(95)00535-8. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–31. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- Hazell AS, Itzhak Y, Liu H, Norenberg MD. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) decreases glutamate uptake in cultured astrocytes. J Neurochem. 1997;68:2216–9. doi: 10.1046/j.1471-4159.1997.68052216.x. [DOI] [PubMed] [Google Scholar]
- He Y, Janssen WGM, Rothstein JD, Morrison JH. Differential synaptic locallization of the glutamate transporter EAAC1 and glutamate receptor subunit GluR2 in the rat hippocampus. J Comp Neurol. 2000;418:255–269. [PubMed] [Google Scholar]
- He Y, Hof PH, Janssen WGM, Rothstein JD, Morrison JH. Differential synaptic localization of GluR2 and EAAC1 in the macaque monkey entorhinal cortex: a postembedding immunogold study. Neurosci Lett. 2001;311:161–164. doi: 10.1016/s0304-3940(01)02180-2. [DOI] [PubMed] [Google Scholar]
- Heinen-Kammerer T, Rulhoff H, Nelles S, Rychlik R. Added therapeutic value of memantine in the treatment of moderate to severe Alzheimer’s disease. Clin Drug Investig. 2006;26:303–14. doi: 10.2165/00044011-200626060-00001. [DOI] [PubMed] [Google Scholar]
- Hertz L. Glutamate, a neurotransmitter--and so much more. A synopsis of Wierzba III. Neurochem Int. 2006;48:416–425. doi: 10.1016/j.neuint.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Hollman M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Hoogland G, van Oort RJ, Proper EA, Jansen GH, van Rijen PC, van Veelen CW, van Nieuwenhuizen O, Troost D, de Graan PN. Alternative splicing of glutamate transporter EAAT2 RNA in neocortex and hippocampus of temporal lobe epilepsy patients. Epilepsy Res. 2004;59:75–82. doi: 10.1016/j.eplepsyres.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Howland DS, Liu J, She Y, Goad B, Maragakis NJ, Kim B, Erickson J, Kulik J, DeVito L, Psaltis G, DeGennaro LJ, Cleveland DW, Rothstein JD. Focal loss of the glutamate transporter EAAT2 in a transgenic rat model of SOD1 mutant-mediated amyotrophic lateral sclerosis (ALS) Proceedings of the National Academy of Sciences USA. 2002;99:1604–1609. doi: 10.1073/pnas.032539299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu LF, Wang S, Shi XR, Yao HH, Sun YH, Ding JH, Liu SY, Hu G. ATP-sensitive potassium channel opener iptakalim protected against the cytotoxicity of MPP+ on SH-SY5Y cells by decreasing extracellular glutamate level. J Neurochem. 2005;94:1570–9. doi: 10.1111/j.1471-4159.2005.03306.x. [DOI] [PubMed] [Google Scholar]
- Hu WH, Walters WM, Xia XM, Karmally SA, Bethea JR. Neuronal glutamate transporter EAAT4 is expressed in astrocytes. Glia. 2003;44:13–25. doi: 10.1002/glia.10268. [DOI] [PubMed] [Google Scholar]
- Huang Y, Zuo Z. Isoflurane induces a protein kinase Ca-dependent increase in cell surface protein level and activity of glutamate transporter type 3. Mol Pharmacol. 2005;67:1522–1533. doi: 10.1124/mol.104.007443. [DOI] [PubMed] [Google Scholar]
- Huang YH, Bergles DE. Glutamate transporters bring competition to the synapse. Curr Opin Neurobiol. 2004;14:346–352. doi: 10.1016/j.conb.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Hutchison WD, Allan RJ, Opitz H, Levy R, Dostrovsky JO, Lang AE, Lozano AM. Neurophysiological identification of the subthalamic nucleus in surgery for Parkinson’s disease. Ann Neurol. 1998;44:622–8. doi: 10.1002/ana.410440407. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Dick KA, Weatherspoon MR, Gincel D, Armbrust KR, Dalton JC, Stevanin G, Durr A, Zuhlke C, Burk K, Clark HB, Brice A, Rothstein JD, Schut LJ, Day JW, Ranum LP. Spectrin mutations cause spinocerebellar ataxia type 5. Nat Genet. 2006;38:184–90. doi: 10.1038/ng1728. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Turski L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic injury?? Lancet Neurology. 2002;1:383–386. doi: 10.1016/s1474-4422(02)00164-3. [DOI] [PubMed] [Google Scholar]
- Ingram EM, Wiseman JW, Tessler S, Emson PC. Reduction of glial glutamate transporters in the parietal cortex and hippocampus of the EL mouse. J Neurochem. 2001;79:564–75. doi: 10.1046/j.1471-4159.2001.00612.x. [DOI] [PubMed] [Google Scholar]
- Ishii T, Sato H, Miura K, Sagara J, Bannai S. Induction of cystine transport activity by stress. Annals New York Academy of Sciences. 1992;663:497–498. doi: 10.1111/j.1749-6632.1992.tb38714.x. [DOI] [PubMed] [Google Scholar]
- Jackson M, Steers G, Leigh PN, Morrison KE. Polymorphisms in the glutamate transporter gene EAAT2 in European ALS patients. J Neurol. 1999;246:1140–4. doi: 10.1007/s004150050532. [DOI] [PubMed] [Google Scholar]
- Jackson M, Song W, Liu M-Y, Jin L, Dykes-Hoberg M, Lin C-LG, Bowers WJ, Federoff HJ, Sternwels PC, Rothstein JD. Modulation of the neuronal glutamate transporter EAAT4 by two interacting proteins. Nature. 2001;410:89–93. doi: 10.1038/35065091. [DOI] [PubMed] [Google Scholar]
- Jarskog LF, Miyamoto S, Lieberman JA. Schizophrenia: New Pathological Insights and Therapies. Annu Rev Med. 2006 doi: 10.1146/annurev.med.58.060904.084114. [DOI] [PubMed] [Google Scholar]
- Jehle T, Bauer J, Blauth E, Hummel A, Darstein M, Freiman TM, Feuerstein TJ. Effects of riluzole on electrically evoked neurotransmitter release. Br J Pharmacol. 2000;130:1227–34. doi: 10.1038/sj.bjp.0703424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jen JC, Wan J, Palos TP, Howard BD, Baloh RW. Mutation in the glutamate transporter EAAT1 causes episodic ataxia, hemiplegia, and seizures. Neurology. 2005;65:529–34. doi: 10.1212/01.wnl.0000172638.58172.5a. [DOI] [PubMed] [Google Scholar]
- Kalandadze A, Wu Y, Robinson MB. Protein kinase C activation decreases cell surface expression of the GLT-1 subtype of glutamate transporter. Requirement of a carboxyl-terminal domain and partial dependence on serine 486. J Biol Chem. 2002;277:45741–45750. doi: 10.1074/jbc.M203771200. [DOI] [PubMed] [Google Scholar]
- Kalandadze A, Wu Y, Fournier K, Robinson MB. Identification of motifs involved in endoplasmic reticulum retention-forward trafficking of the GLT-1 subtype of glutamate transporter. J Neurosci. 2004;24:5183–5192. doi: 10.1523/JNEUROSCI.0839-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DC, Su ZZ, Sarkar D, Emdad L, Volsky DJ, Fisher PB. Cloning and characterization of HIV-1-inducible astrocyte elevated gene-1, AEG-1. Gene. 2005;353:8–15. doi: 10.1016/j.gene.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Katsumori H, Baldwin RA, Wasterlain CG. Reverse transport of glutamate during depolarization in immature hippocampal slices. Brain Res. 1999;819:160–4. doi: 10.1016/s0006-8993(98)01352-3. [DOI] [PubMed] [Google Scholar]
- Keller JN, Germeyer A, Begley JG, Mattson MP. 17Beta-estradiol attenuates oxidative impairment of synaptic Na+/K+-ATPase activity, glucose transport, and glutamate transport induced by amyloid beta-peptide and iron. J Neurosci Res. 1997a;50:522–30. doi: 10.1002/(SICI)1097-4547(19971115)50:4<522::AID-JNR3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Keller JN, Pang Z, Geddes JW, Begley JG, Germeyer A, Waeg G, Mattson MP. Impairment of glucose and glutamate transport and induction of mitochondrial oxidative stress and dysfunction in synaptosomes by amyloid β-peptide: role of the lipid peroxidation product 4-hydroxynonenal. J Neurochem. 1997b;69:273–284. doi: 10.1046/j.1471-4159.1997.69010273.x. [DOI] [PubMed] [Google Scholar]
- Kis B, Rajapakse NC, Snipes JA, Nagy K, Horiguchi T, Busija DW. Diazoxide induces delayed pre-conditioning in cultured rat cortical neurons. J Neurochem. 2003;87:969–80. doi: 10.1046/j.1471-4159.2003.02072.x. [DOI] [PubMed] [Google Scholar]
- Koch HP, Larsson HP. Small-scale molecular motions accomplish glutamate uptake in human glutamate transporters. J Neurosci. 2005;25:1730–6. doi: 10.1523/JNEUROSCI.4138-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laruelle M, Kegeles LS, Abi-Dargham A. Glutamate, dopamine, and schizophrenia: from pathophysiology to treatment. Ann N Y Acad Sci. 2003;1003:138–58. doi: 10.1196/annals.1300.063. [DOI] [PubMed] [Google Scholar]
- Lauderback CM, Harris-White ME, Wang Y, Pedigo NW, Jr, Carney JM, Butterfield DA. Amyloid beta-peptide inhibits Na+-dependent glutamate uptake. Life Sci. 1999;65:1977–81. doi: 10.1016/s0024-3205(99)00459-2. [DOI] [PubMed] [Google Scholar]
- Lauderback CM, Hackett JM, Huang FF, Keller JN, Szweda LI, Markesbery WR, Butterfield DA. The glial glutamate transporter, GLT-1, is oxidatively modified by 4-hydroxy-2-nonenal in the Alzheimer’s disease brain: the role of Abeta1-42. J Neurochem. 2001;78:413–6. doi: 10.1046/j.1471-4159.2001.00451.x. [DOI] [PubMed] [Google Scholar]
- Lauriat TL, Dracheva S, Chin B, Schmeidler J, McInnes LA, Haroutunian V. Quantitative analysis of glutamate transporter mRNA expression in prefrontal and primary visual cortex in normal and schizophrenic brain. Neuroscience. 2006;137:843–51. doi: 10.1016/j.neuroscience.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Lee G, Huang Y, Washington JM, Briggs NW, Zuo Z. Carbamazepine enhances the activity of glutamate transporter type 3 via phosphatidylinositol 3-kinase. Epilepsy Research. 2005;66:145–53. doi: 10.1016/j.eplepsyres.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–59. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- Lehre KP, Levy LM, Ottersen OP, Storm-Mathisen J, Danbolt NC. Differential expression of two glial glutamate transporters in the rat brain: Quantitative and immunocytochemical observations. J Neurosci. 1995;15:1835–1853. doi: 10.1523/JNEUROSCI.15-03-01835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson J, Weeber E, Selcher JC, Kategaya LS, Sweatt JD, Eskin A. Long-term potentiation and contextual fear conditioning increase neuronal glutamate uptake. Nat Neurosci. 2002;5:155–161. doi: 10.1038/nn791. [DOI] [PubMed] [Google Scholar]
- Levy LM, Warr O, Attwell D. Stoichiometry of the glial glutamte transporter GLT-1 expressed inducibly in a chinese hamster ovary cell line selected for low endogenous Na+-dependent glutamate uptake. J Neurosci. 1998;18:9620–9628. doi: 10.1523/JNEUROSCI.18-23-09620.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy LM, Lehre KP, Walaas SI, Storm-Mathisen J, Danbolt NC. Down-regulation of glial glutamate transporters after glutamatergic denervation in the rat brain. Eur J Neurosci. 1995;7:2036–2041. doi: 10.1111/j.1460-9568.1995.tb00626.x. [DOI] [PubMed] [Google Scholar]
- Lewerenz J, Klein M, Methner A. Cooperative action of glutamate transporters and cystine/glutamate antiporter system Xc- protects from oxidative glutamate toxicity. J Neurochem. 2006;98:916–25. doi: 10.1111/j.1471-4159.2006.03921.x. [DOI] [PubMed] [Google Scholar]
- Li H, Li SH, Cheng AL, Mangiarini L, Bates GP, Li XJ. Ultrastructural localization and progressive formation of neuropil aggregates in Huntington’s disease transgenic mice. Hum Mol Genet. 1999;8:1227–36. doi: 10.1093/hmg/8.7.1227. [DOI] [PubMed] [Google Scholar]
- Liang LP, Patel M. Mitochondrial oxidative stress and increased seizure susceptibility in Sod2(−/+) mice. Free Radic Biol Med. 2004;36:542–54. doi: 10.1016/j.freeradbiomed.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Liang Z, Valla J, Sefidvash-Hockley S, Rogers J, Li R. Effects of estrogen treatment on glutamate uptake in cultured human astrocytes derived from cortex of Alzheimer’s disease patients. J Neurochem. 2002;80:807–14. doi: 10.1046/j.0022-3042.2002.00779.x. [DOI] [PubMed] [Google Scholar]
- Lievens JC, Rival T, Iche M, Chneiweiss H, Birman S. Expanded polyglutamine peptides disrupt EGF receptor signaling and glutamate transporter expression in Drosophila. Hum Mol Genet. 2005;14:713–24. doi: 10.1093/hmg/ddi067. [DOI] [PubMed] [Google Scholar]
- Lievens JC, Woodman B, Mahal A, Spasic-Boscovic O, Samuel D, Kerkerian-Le Goff L, Bates GP. Impaired glutamate uptake in the R6 Huntington’s disease transgenic mice. Neurobiol Dis. 2001;8:807–21. doi: 10.1006/nbdi.2001.0430. [DOI] [PubMed] [Google Scholar]
- Lin C-LG, Bristol LA, Jin L, Dykes-Hoberg M, Crawford T, Clawson L, Rothstein JD. Aberrant RNA processing in a neurodegenerative disease: the cause for absent EAAT2, a glutamate transporter, in amyotrophic lateral sclerosis. Neuron. 1998;20:589–602. doi: 10.1016/s0896-6273(00)80997-6. [DOI] [PubMed] [Google Scholar]
- Lo EH, Moskowitz MA, Jacobs TP. Exciting, radical, suicidal: how brain cells die after stroke. Stroke. 2005;36:189–92. doi: 10.1161/01.STR.0000153069.96296.fd. [DOI] [PubMed] [Google Scholar]
- Logan WJ, Snyder SH. High affinity uptake systems for glycine, glutamic and aspartic acids in synaptosomes of rat central nervous tissues. Brain Res. 1972;42:413–431. doi: 10.1016/0006-8993(72)90540-9. [DOI] [PubMed] [Google Scholar]
- Longuemare MC, Swanson RA. Excitatory amino acid release from astrocytes during energy failure by reversal of sodium-dependent uptake. J Neurosci Res. 1995;40:379–386. doi: 10.1002/jnr.490400312. [DOI] [PubMed] [Google Scholar]
- Mallolas J, Hurtado O, Castellanos M, Blanco M, Sobrino T, Serena J, Vivancos J, Castillo J, Lizasoain I, Moro MA, Davalos A. A polymorphism in the EAAT2 promoter is associated with higher glutamate concentrations and higher frequency of progressing stroke. J Exp Med. 2006;203:711–7. doi: 10.1084/jem.20051979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangano RM, Schwarcz R. The human platelet as a model for the glutamatergic neuron: platelet uptake of L-glutamate. J Neurochem. 1981;36:1067–76. doi: 10.1111/j.1471-4159.1981.tb01701.x. [DOI] [PubMed] [Google Scholar]
- Mangano RM, Schwarcz R. Chronic infusion of endogenous excitatory amino acids into rat striatum and hippocampus. Brain Res Bull. 1983;10:47–51. doi: 10.1016/0361-9230(83)90073-4. [DOI] [PubMed] [Google Scholar]
- Mangiarini L, Sathasivam K, Mahal A, Mott R, Seller M, Bates GP. Instability of highly expanded CAG repeats in mice transgenic for the Huntington’s disease mutation. Nat Genet. 1997;15:197–200. doi: 10.1038/ng0297-197. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Mendelowitz DS, Williams SH, Sutherland ML. EAAT2 overexpression plays a neuroprotective role in models of temporal lobe epilepsy. Society for Neuroscience Abstract 2001 [Google Scholar]
- Masliah E, Alford M, DeTeresa R, Mallory M, Hansen L. Deficient glutamate transport is associated with neurodegeneration in Alzheimer’s disease. Ann Neurol. 1996;40:759–766. doi: 10.1002/ana.410400512. [DOI] [PubMed] [Google Scholar]
- Masliah E, Alford M, Mallory M, Rockenstein E, Moechars D, Van Leuven F. Abnormal glutamate transport function in mutant amyloid precursor protein transgenic mice. Exp Neurol. 2000;163:381–7. doi: 10.1006/exnr.2000.7386. [DOI] [PubMed] [Google Scholar]
- Massieu L, Morales-Villagran A, Tapia R. Accumulation of extracellular glutamate by inhibition of its uptake is not sufficient for inducing neuronal damage: An in vivo microdialysis study. J Neurochem. 1995;64:2262–2272. doi: 10.1046/j.1471-4159.1995.64052262.x. [DOI] [PubMed] [Google Scholar]
- Mathews GC, Diamond JS. Neuronal glutamate uptake contributes to GABA synthesis and inhibitory synaptic strength. J Neurosci. 2003;23:2040–2048. doi: 10.1523/JNEUROSCI.23-06-02040.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute C, Melone M, Vallejo-Illarramendi A, Conti F. Increased expression of the astrocytic glutamate transporter GLT-1 in the prefrontal cortex of schizophrenics. Glia. 2005;49:451–5. doi: 10.1002/glia.20119. [DOI] [PubMed] [Google Scholar]
- McBean GJ. Cerebral cystine uptake: a tale of two transporters. Trends Pharmacol Sci. 2002;23:299–302. doi: 10.1016/s0165-6147(02)02060-6. [DOI] [PubMed] [Google Scholar]
- McDonald JW, Johnston MV. Physiological and pathophysiological roles of excitatory amino acids during central nervous system development. Brain Res Rev. 1990;15:41–70. doi: 10.1016/0165-0173(90)90011-c. [DOI] [PubMed] [Google Scholar]
- McMinn MT, Williams SH, Sutherland ML. The role of enhanced glutamate transport in a pilocarpine model of temporal lobe epilepsy. Society for Neuroscience Abstract. 2003:688.2. [Google Scholar]
- Medina L, Figueredo-Cardenas G, Rothstein JD, Reiner A. Differential abundance of glutamate transporter subtypes in Amyotrophic Lateral Scleroso (ALS)-vulnerable versus ALS-resistant brain stem motor cell groups. Exp Neurol. 1996;142:287–295. doi: 10.1006/exnr.1996.0198. [DOI] [PubMed] [Google Scholar]
- Meldrum B, Garthwaite J. Excitatory amino acid neurotoxicity and neurodegenerative disease. Trends Pharmacol Sci. 1990;11:379–387. doi: 10.1016/0165-6147(90)90184-a. [DOI] [PubMed] [Google Scholar]
- Melone M, Vitellaro-Zuccarello L, Vallejo-Illarramendi A, Perez-Samartin A, Matute C, Cozzi A, Pellegrini-Giampietro DE, Rothstein JD, Conti F. The expression of glutamate transporter GLT-1 in the rat cerebral cortex is down-regulated by the antipsychotic drug clozapine. Mol Psychiatry. 2001;6:380–6. doi: 10.1038/sj.mp.4000880. [DOI] [PubMed] [Google Scholar]
- Mennerick S, Dhond RP, Benz A, Xu W, Rothstein JD, Danbolt NC, Isenberg KE, Zorumski CF. Neuronal expression of the glutamate transporter GLT-1 in hippocampal microcultures. J Neurosci. 1998;18:4490–4499. doi: 10.1523/JNEUROSCI.18-12-04490.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T, Munch C, Knappenberger B, Liebau S, Volkel H, Ludolph AC. Alternative splicing of the glutamate transporter EAAT2 (GLT-1. Neurosci Lett. 1998;241:68–70. doi: 10.1016/s0304-3940(97)00973-7. [DOI] [PubMed] [Google Scholar]
- Meyer T, Fromm A, Munch C, Schwalenstocker B, Fray AE, Ince PG, Stamm S, Gron G, Ludolph AC, Shaw PJ. The RNA of the glutamate transporter EAAT2 is variably spliced in amyotrophic lateral sclerosis and normal individuals. J Neurol Sci. 1999;170:45–50. doi: 10.1016/s0022-510x(99)00196-3. [DOI] [PubMed] [Google Scholar]
- Milh M, Becq H, Villeneuve N, Ben-Ari Y, Aniksztejn L. Inhibition of glutamate transporters results in a “suppression-burst” pattern and partial seizures in the newborn rat. Epilepsia. 2007;48:169–74. doi: 10.1111/j.1528-1167.2006.00839.x. [DOI] [PubMed] [Google Scholar]
- Mim C, Balani P, Rauen T, Grewer C. The glutamate transporter subtypes EAAT4 and EAATs 1–3 transport glutamate with dramatically different kinetics and voltage dependence but share a common uptake mechanism. J Gen Physiol. 2005;126:571–89. doi: 10.1085/jgp.200509365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minelli A, Barbaresi P, Reimer RJ, Edwards RH, Conti F. The glial glutamate transporter GLT-1 is localized both in the vicinity of and at distance from axon terminals in the rat cerebral cortex. Neuroscience. 2001;108:51–9. doi: 10.1016/s0306-4522(01)00375-x. [DOI] [PubMed] [Google Scholar]
- Mitani A, Tanaka K. Functional changes of glial glutamate transporter GLT-1 during ischemia: An in vivo study in the hippocampal CA1 of normal mice and mutant mice lacking GLT-1. J Neurosci. 2003;23:7176–7182. doi: 10.1523/JNEUROSCI.23-18-07176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan DT, Bridges RJ, Cotman CW. The excitatory amino acid receptors: Their classes, pharmacology, and distinct properties in the function of the nervous system. Annu Rev Pharmacol Toxicol. 1989;29:365–402. doi: 10.1146/annurev.pa.29.040189.002053. [DOI] [PubMed] [Google Scholar]
- Morita T, Takahashi M, Takeuchi T, Hikasa Y, Ikeda S, Sawada M, Sato K, Shibahara T, Shimada A. Changes in extracellular neurotransmitters in the cerebrum of familial idiopathic epileptic shetland sheepdogs using an intracerebral microdialysis technique and immunohistochemical study for glutamate metabolism. J Vet Med Sci. 2005;67:1119–26. doi: 10.1292/jvms.67.1119. [DOI] [PubMed] [Google Scholar]
- Murphy TH, Schnaar RL, Coyle JT. Immature cortical neurons are uniquely sensitive to glutamate toxicity by inhibtion of cystine uptake. FASEB J. 1990;4:1624–1633. [PubMed] [Google Scholar]
- Nagai M, Abe K, Okamoto K, Itoyama Y. Identification of alternative splicing forms of GLT-1 mRNA in the spinal cord of amyotrophic lateral sclerosis patients. Neurosci Lett. 1998;244:165–8. doi: 10.1016/s0304-3940(98)00158-x. [DOI] [PubMed] [Google Scholar]
- Naito S, Ueda T. Characterization of glutamate uptake into synaptic vesicles. J Neurochem. 1985;44:99–109. doi: 10.1111/j.1471-4159.1985.tb07118.x. [DOI] [PubMed] [Google Scholar]
- Najimi M, Maloteaux JM, Hermans E. Cytoskeleton-related trafficking of the EAAC1 glutamate transporter after activation of the G(q/11)-coupled neurotensin receptor NTS1. FEBS Lett. 2002;523:224–228. doi: 10.1016/s0014-5793(02)02981-2. [DOI] [PubMed] [Google Scholar]
- Najimi M, Maloteaux J-M, Hermans E. Pertussis toxin-sensitive modulation of glutamate transport by endothelin-1 type A receptors in glioma cells. Biochimica et Biophysica Acta. 2005;1668:195–202. doi: 10.1016/j.bbamem.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Nakanishi S. Metabotropic glutamate receptors: Synaptic transmission, modulation, and plasticity. Neuron. 1994;13:1031–1037. doi: 10.1016/0896-6273(94)90043-4. [DOI] [PubMed] [Google Scholar]
- O’Shea RD, Lau CL, Farso MC, Diwakarla S, Zagami CJ, Svendsen BB, Feeney SJ, Callaway JK, Jones NM, Pow DV, Danbolt NC, Jarrott B, Beart PM. Effects of lipopolysaccharide on glial phenotype and activity of glutamate transporters: Evidence for delayed up-regulation and redistribution of GLT-1. Neurochem Int. 2006;48:604–10. doi: 10.1016/j.neuint.2005.12.028. [DOI] [PubMed] [Google Scholar]
- Olney JW. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science. 1969;164:719–21. doi: 10.1126/science.164.3880.719. [DOI] [PubMed] [Google Scholar]
- Olney JW. Excitatory amino acids and neuropsychiatric disorders. Biological Psychiatry. 1989;26:505–525. doi: 10.1016/0006-3223(89)90072-3. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- Payet O, Maurin L, Bonne C, Muller A. Hypoxia stimulates glutamate uptake in whole rat retinal cells in vitro. Neurosci Lett. 2004;356:148–50. doi: 10.1016/j.neulet.2003.11.036. [DOI] [PubMed] [Google Scholar]
- Phillis JW, Ren J, O’Regan MH. Transporter reversal as a mechanism of glutamate release from the ischemic rat cerebral cortex: studies with DL-threo-beta-benzyloxyaspartate. Brain Res. 2000;880:224. doi: 10.1016/s0006-8993(00)02755-4. [DOI] [PubMed] [Google Scholar]
- Pines G, Danbolt NC, Bjørås M, Zhang Y, Bendahan A, Eide L, Koepsell H, Storm-Mathisen J, Seeberg E, Kanner BI. Cloning and expression of a rat brain L-glutamate transporter. Nature. 1992;360:464–467. doi: 10.1038/360464a0. [DOI] [PubMed] [Google Scholar]
- Plosker GL, Lyseng-Williamson KA. Memantine: a pharmacoeconomic review of its use in moderate-to-severe Alzheimer’s disease. Pharmacoeconomics. 2005;23:193–206. doi: 10.2165/00019053-200523020-00010. [DOI] [PubMed] [Google Scholar]
- Poulsen CF, Schousboe I, Sarup A, White HS, Schousboe A. Effect of topiramate and dBcAMP on expression of the glutamate transporters GLAST and GLT-1 in astrocytes cultured separately, or together with neurons. Neurochem Int. 2006;48:657–61. doi: 10.1016/j.neuint.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Pradillo JM, Hurtado O, Romera C, Cardenas A, Fernandez-Tome P, Alonso-Escolano D, Lorenzo P, Moro MA, Lizasoain I. TNFR1 mediates increased neuronal membrane EAAT3 expression after in vivo cerebral ischemic preconditioning. Neuroscience. 2006;138:1171–8. doi: 10.1016/j.neuroscience.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Rao VL, Dogan A, Bowen KK, Todd KG, Dempsey RJ. Antisense knockdown of the glial glutamate transporter GLT-1 exacerbates hippocampal neuronal damage following traumatic injury to rat brain. Eur J Neurosci. 2001a;13:119–28. [PubMed] [Google Scholar]
- Rao VLR, Rao AM, Dogan A, Bowen KK, Hatcher J, Rothstein JD, Dempsey RJ. Glial glutamate transporter GLT-1 down-regulation precedes delayed neuronal death in gerbil hippocampus following transient global ischemia. Neurochem Int. 2000;36:531–537. doi: 10.1016/s0197-0186(99)00153-9. [DOI] [PubMed] [Google Scholar]
- Rao VLR, Dogan A, Todd JG, Bowen KK, Kim B-T, Rothstein JD, Dempsey RJ. Antisense knockdown of the glial glutamate transporter GLT-1, but not the neuronal glutamate transporter EAAC1, exacerbates transient focal cerebral ischemia-induced neuronal damage in rat brain. J Neurosci. 2001b;21:1876–1883. doi: 10.1523/JNEUROSCI.21-06-01876.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattray M, Bendotti C. Does excitotoxic cell death of motor neurons in ALS arise from glutamate transporter and glutamate receptor abnormalities? Exp Neurol. 2006;201:15–23. doi: 10.1016/j.expneurol.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Rauen T, Wiessner M, Sullivan R, Lee A, Pow DV. A new GLT1 splice variant: cloning and immunolocalization of GLT1c in the mammalian retina and brain. Neurochem Int. 2004;45:1095–106. doi: 10.1016/j.neuint.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Reye P, Sullivan R, Pow DV. Distribution of two splice variants of the glutamate transporter GLT-1 in the developing rat retina. J Comp Neurol. 2002a;447:323–30. doi: 10.1002/cne.10218. [DOI] [PubMed] [Google Scholar]
- Reye P, Sullivan R, Scott H, Pow DV. Distribution of two splice variants of the glutamate transporter GLT-1 in rat brain and pituitary. Glia. 2002b;38:246–55. doi: 10.1002/glia.10059. [DOI] [PubMed] [Google Scholar]
- Robelet S, Melon C, Guillet B, Salin P, Kerkerian-Le Goff L. Chronic L-DOPA treatment increases extracellular glutamate levels and GLT1 expression in the basal ganglia in a rat model of Parkinson’s disease. Eur J Neurosci. 2004;20:1255–66. doi: 10.1111/j.1460-9568.2004.03591.x. [DOI] [PubMed] [Google Scholar]
- Robinson MB. Examination of glutamate transporter heterogeneity using synaptosomal preprations. Methods Enzymol. 1998;296:189–202. doi: 10.1016/s0076-6879(98)96015-3. [DOI] [PubMed] [Google Scholar]
- Robinson MB. The family of sodium-dependent glutamate transporters: A focus on the GLT-1/EAAT2 subtype. Neurochem Int. 1999;33:479–491. doi: 10.1016/s0197-0186(98)00055-2. [DOI] [PubMed] [Google Scholar]
- Robinson MB. Regulated trafficking of neurotransmitter transporters: Common notes different melodies. J Neurochem. 2002;80:1–11. doi: 10.1046/j.0022-3042.2001.00698.x. [DOI] [PubMed] [Google Scholar]
- Robinson MB. Acute regulation of sodium-dependent glutamate transporters: A focus on constitutive and regulated trafficking. Handbook of Experimental Pharmacology. 2006;175:251–275. doi: 10.1007/3-540-29784-7_13. [DOI] [PubMed] [Google Scholar]
- Robinson MB, Coyle JT. Glutamate and related acidic excitatory neurotransmitters: from basic science to clinical application. Faseb J. 1987;1:446–55. doi: 10.1096/fasebj.1.6.2890549. [DOI] [PubMed] [Google Scholar]
- Robinson MB, Dowd LA. Heterogeneity and functional properties of subtypes of sodium-dependent glutamate transporters in the mammalian central nervous system. Advances in Pharmacology. 1997;37:69–115. doi: 10.1016/s1054-3589(08)60948-5. [DOI] [PubMed] [Google Scholar]
- Robinson MB, Djali S, Buchhalter JR. Inhibition of glutamate uptake with L-trans-pyrrolidine-2,4-dicarboxylate potentiates glutamate toxicity in primary hippocampal cultures. J Neurochem. 1993;61:2099–2103. doi: 10.1111/j.1471-4159.1993.tb07447.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Kern A, Gegelashvili M, Schousboe A, Zhang J, Sung L, Gegelashvili G. Beta-amyloid and brain-derived neurotrophic factor, BDNF, up-regulate the expression of glutamate transporter GLT-1/EAAT2 via different signaling pathways utilizing transcription factor NF-kappaB. Neurochem Int. 2003;43:363–70. doi: 10.1016/s0197-0186(03)00023-8. [DOI] [PubMed] [Google Scholar]
- Romera C, Hurtado O, Mallolas J, Pereira MP, Morales JR, Romera A, Serena J, Vivancos J, Nombela F, Lorenzo P, Lizasoain I, Moro MA. Ischemic preconditioning reveals that GLT1/EAAT2 glutamate transporter is a novel PPARgamma target gene involved in neuroprotection. J Cereb Blood Flow Metab. 2007 doi: 10.1038/sj.jcbfm.9600438. [DOI] [PubMed] [Google Scholar]
- Rosenberg PA, Amin S, Leitner M. Glutamate uptake disguises neurotoxic potency of glutamate agonists in cerebral cortex in dissociated cell culture. J Neurosci. 1992;12:56–61. doi: 10.1523/JNEUROSCI.12-01-00056.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DJ, Oshima T, Attwell D. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature. 2000;403:316–321. doi: 10.1038/35002090. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Martin LJ, Kuncl RW. Decreased glutamate transport by the brain and spinal cord in amyotrophic lateral sclerosis. New Engl J Med. 1992;326:1464–1468. doi: 10.1056/NEJM199205283262204. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Van Kammen M, Levey AI, Martin LJ, Kuncl RW. Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Ann Neurol. 1995;38:73–84. doi: 10.1002/ana.410380114. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Tsai G, Kuncl RW, Clawson L, Cornblath DR, Drachman DB, Pestronk A, Stauch BL, Coyle JT. Abnormal excitatory amino acid metabolism in amyotrophic lateral sclerosis. Ann Neurol. 1990;28:18–25. doi: 10.1002/ana.410280106. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger M, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–7. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Sabri F, Titanji K, De Milito A, Chiodi F. Astrocyte activation and apoptosis: their roles in the neuropathology of HIV infection. Brain Pathol. 2003;13:84–94. doi: 10.1111/j.1750-3639.2003.tb00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Matsuki N, Ohno Y, Nakazawa K. Estrogens inhibit l-glutamate uptake activity of astrocytes via membrane estrogen receptor alpha. J Neurochem. 2003;86:1498–505. doi: 10.1046/j.1471-4159.2003.01953.x. [DOI] [PubMed] [Google Scholar]
- Schlag BD, Vondrasek JR, Munir M, Kalandadze A, Zelenaia OA, Rothstein JD, Robinson MB. Regulation of the glial Na+-dependent glutamate transporters by cyclic AMP analogs and neurons. Mol Pharmacol. 1998;53:355–369. doi: 10.1124/mol.53.3.355. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Asan E, Lesch KP, Kugler P. A splice variant of glutamate transporter GLT1/EAAT2 expressed in neurons: Cloning and localization in rat nervous system. Neuroscience. 2002;109:45–61. doi: 10.1016/s0306-4522(01)00451-1. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Asan E, Puschel B, Jons T, Kugler P. Expression of the glutamate transporter GLT1 in neural cells of the rat central nervous system: non-radioactive in situ hybridization and comparative immunocytochemistry. Neuroscience. 1996;71:989–1004. doi: 10.1016/0306-4522(95)00477-7. [DOI] [PubMed] [Google Scholar]
- Schoepp DD, Conn PJ. Metabotropic glutamate receptors in brain function and pathology. Trends Pharmacol Sci. 1993;14:13–20. doi: 10.1016/0165-6147(93)90107-u. [DOI] [PubMed] [Google Scholar]
- Scholz RE, Sutherland ML. The role of enhanced glutamate transport in two mouse models of ALS. Society for Neuroscience Abstract. 2003;33:96.8. [Google Scholar]
- Schousboe A. Transport and metabolism of glutamate and GABA in neurons and glial cells. Int Rev Neurobiol. 1981;22:1–45. doi: 10.1016/s0074-7742(08)60289-5. [DOI] [PubMed] [Google Scholar]
- Scott HL, Pow DV, Tannenberg AE, Dodd PR. Aberrant expression of the glutamate transporter excitatory amino acid transporter 1 (EAAT1) in Alzheimer’s disease. J Neurosci. 2002;22:RC206. doi: 10.1523/JNEUROSCI.22-03-j0004.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal RP, Amara SG. Excitatory amino acid transporters: A family in flux. Annual Reviews in Pharmacology and Toxicology. 1999;39:431–456. doi: 10.1146/annurev.pharmtox.39.1.431. [DOI] [PubMed] [Google Scholar]
- Seeburg PH. The TINS/TiPS Lecture. The molecular biology of mammalian glutamate receptor channels. Trends Neurosci. 1993;16:359–65. doi: 10.1016/0166-2236(93)90093-2. [DOI] [PubMed] [Google Scholar]
- Seki Y, Feustel PJRW, Keller J, Tranmer BI, Kimelberg HK. Inhibition of ischemia-induced glutamate release in rat striatum by dihydrokainate and an anion channel blocker. Stroke. 1999;30:433–440. doi: 10.1161/01.str.30.2.433. [DOI] [PubMed] [Google Scholar]
- Selkirk JV, Stiefel TH, Stone IM, Naeve GS, Foster AC, Poulsen DJ. Over-expression of the human EAAT2 glutamate transporter within neurons of mouse organotypic hippocampal slice cultures leads to increased vulnerability of CA1 pyramidal cells. Eur J Neurosci. 2005a;21:2291–6. doi: 10.1111/j.1460-9568.2005.04059.x. [DOI] [PubMed] [Google Scholar]
- Selkirk JV, Nottebaum LM, Vana AM, Verge GM, Mackay KB, Stiefel TH, Naeve GS, Pomeroy JE, Petroski RE, Moyer J, Dunlop J, Foster AC. Role of the GLT-1 subtype of glutamate transporter in glutamate homeostasis: the GLT-1-preferring inhibitor WAY-855 produces marginal neurotoxicity in the rat hippocampus. Eur J Neurosci. 2005b;21:3217–28. doi: 10.1111/j.1460-9568.2005.04162.x. [DOI] [PubMed] [Google Scholar]
- Sepkuty JP, Cohen AS, Eccles C, Rafiq A, Behar K, Ganel R, Coulter DA, Rothstein JD. A neuronal glutamate transporter contributes to neurotransmitter GABA synthesis and epilepsy. J Neurosci. 2002;22:6372–6379. doi: 10.1523/JNEUROSCI.22-15-06372.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeri Y, Seal RP, Shimamoto K. Molecular pharmacology of glutamate transporters, EAATs and VGLUTs. Brain Res Rev. 2004;45:250–265. doi: 10.1016/j.brainresrev.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Shimamoto K, Lebrun B, Yasuda-Kamatani Y, Sakaitani M, Shigeri Y, Yumoto N, Nakajima T. DL-threo-beta-benzyloxyaspartate, a potent blocker of excitatory amino acid transporters. Mol Pharmacol. 1998;53:195–201. doi: 10.1124/mol.53.2.195. [DOI] [PubMed] [Google Scholar]
- Shin JY, Fang ZH, Yu ZX, Wang CE, Li SH, Li XJ. Expression of mutant huntingtin in glial cells contributes to neuronal excitotoxicity. J Cell Biol. 2005;171:1001–12. doi: 10.1083/jcb.200508072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simantov R, Crispino M, Hoe W, Broutman G, Tocco G, Rothstein JD, Baudry M. Changes in expression of neuronal and glial glutamate transporters in rat hippocampus following kainate-induced seizure activity. Brain Res Mol Brain Res. 1999;65:112–23. doi: 10.1016/s0169-328x(98)00349-0. [DOI] [PubMed] [Google Scholar]
- Sims KD, Straff DJ, Robinson MB. Platelet-derived growth factor rapidly increases activity and cell surface expression of the EAAC1 subtype of glutamate transporters through activation of phosphatidylinositol 3-kinase. J Biol Chem. 2000;274:5228–5327. doi: 10.1074/jbc.275.7.5228. [DOI] [PubMed] [Google Scholar]
- Sitcheran R, Gupta P, Fisher PB, Baldwin AS. Positive and negative regulation of EAAT2 by NF-kappaB: a role for N-myc in TNFalpha-controlled repression. EMBO J. 2005;24:510–20. doi: 10.1038/sj.emboj.7600555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotboom DJ, Konings WN, Lolkema JS. Glutamate transporters combine transporter- and channel-like features. Trends Biochem Sci. 2001;26:534–9. doi: 10.1016/s0968-0004(01)01925-9. [DOI] [PubMed] [Google Scholar]
- Smith R, Brundin P, Li JY. Synaptic dysfunction in Huntington’s disease: a new perspective. Cell Mol Life Sci. 2005;62:1901–12. doi: 10.1007/s00018-005-5084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RE, Haroutunian V, Davis KL, Meador-Woodruff JH. Expression of excitatory amino acid transporter transcripts in the thalamus of subjects with schizophrenia. Am J Psychiatry. 2001;158:1393–9. doi: 10.1176/appi.ajp.158.9.1393. [DOI] [PubMed] [Google Scholar]
- St-Hilaire M, Landry E, Levesque D, Rouillard C. Denervation and repeated L-DOPA induce complex regulatory changes in neurochemical phenotypes of striatal neurons: implication of a dopamine D1-dependent mechanism. Neurobiol Dis. 2005;20:450–60. doi: 10.1016/j.nbd.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Stafstrom CE. Epilepsy: a review of selected clinical syndromes and advances in basic science. J Cereb Blood Flow Metab. 2006;26:983–1004. doi: 10.1038/sj.jcbfm.9600265. [DOI] [PubMed] [Google Scholar]
- Storck T, Schulte S, Hofmann K, Stoffel W. Structure, expression, and functional analysis of a Na+-dependent glutamate/aspartate transporter from rat brain. Proceedings of the National Academy of Sciences USA. 1992;89:10955–10959. doi: 10.1073/pnas.89.22.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey E, Kowall NW, Finn SF, Mazurek MF, Beal MF. The cortical lesion of Huntington’s disease: further neurochemical characterization, and reproduction of some of the histological and neurochemical features by N-methyl-D-aspartate lesions of rat cortex. Ann Neurol. 1992;32:526–534. doi: 10.1002/ana.410320408. [DOI] [PubMed] [Google Scholar]
- Su ZZ, Leszczyniecka M, Kang DC, Sarkar D, Chao W, Volsky DJ, Fisher PB. Insights into glutamate transport regulation in human astrocytes: cloning of the promoter for excitatory amino acid transporter 2 (EAAT2) Proceedings of the National Academy of Sciences USA. 2003;100:1955–1960. doi: 10.1073/pnas.0136555100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R, Rauen T, Fischer F, Wiessner M, Grewer C, Bicho A, Pow DV. Cloning, transport properties, and differential localization of two splice variants of GLT-1 in the rat CNS: implications for CNS glutamate homeostasis. Glia. 2004;45:155–69. doi: 10.1002/glia.10317. [DOI] [PubMed] [Google Scholar]
- Sun Y, Savanenin A, Reddy PH, Liu YF. Polyglutamine-expanded huntingtin promotes sensitization of N-methyl-D-aspartate receptors via post-synaptic density 95. J Biol Chem. 2001;276:24713–8. doi: 10.1074/jbc.M103501200. [DOI] [PubMed] [Google Scholar]
- Sung B, Lim G, Mao J. Altered expression and uptake activity of spinal glutamate transporters after nerve injury contribute to the pathogenesis of neuropathic pain in rats. J Neurosci. 2003;23:2899–910. doi: 10.1523/JNEUROSCI.23-07-02899.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland ML, Martinowich K, Rothstein JD. EAAT2 overexpression plays a neuroprotective role in the SOD1 G93A model of amyotrophic lateral sclerosis. Society for Neuroscience Abstract. 2001:607.6, 309. [Google Scholar]
- Sutherland ML, Potter E, Scholz RE, Martinowich K, Rothstein JD. Enhancement of glutamate transport in the SOD1 G93A model of ALS delays disease onset. Society for Neuroscience Abstract. 2002:44.2. [Google Scholar]
- Swanson RA, Liu J, Miller JW, Rothstein JD, Farrell K, Stein BA, Longuemare MC. Neuronal regulation of glutamate transporter subtype expression in astrocytes. J Neurosci. 1997;17:932–40. doi: 10.1523/JNEUROSCI.17-03-00932.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature. 2000;407:189–94. doi: 10.1038/35025070. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- Tang CM, Dichter M, Morad M. Quisqualate activates a rapidly inactivating high conductance ionic channel in hippocampal neurons. Science. 1989;243:1474–7. doi: 10.1126/science.2467378. [DOI] [PubMed] [Google Scholar]
- Thai DR. Excitatory amino acid transporter EAAT-2 in tangle-bearing neurons in Alzheimer’s disease. Brain Pathol. 2002;12:405–11. doi: 10.1111/j.1750-3639.2002.tb00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong G, Jahr CE. Block of glutamate transporters potentiates postsynaptic excitation. Neuron. 1994;13:1195–1203. doi: 10.1016/0896-6273(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Torp R, Lekieffre D, Levy LM, Haug FM, Danbolt NC, Meldrum BS, Ottersen OP. Reduced postischemic expression of a glial glutamate transporter, GLT-1, in the rat hippocampus. Exp Brain Res. 1995;103:51–58. doi: 10.1007/BF00241964. [DOI] [PubMed] [Google Scholar]
- Torres GE, Gainetdinov RR, Caron MG. Plasma membrane monoamine transporters: Structure, regulation and function. Nature Reviews Neuroscience. 2003;4:13–25. doi: 10.1038/nrn1008. [DOI] [PubMed] [Google Scholar]
- Trotti D, Aoki M, Pasine P, Berger UV, Danbolt NC, Brown RH, Hediger MA. Amyotrophic lateral schlerosis-linked glutamate transporter mutant has impaired glutamate clearance capacity. J Biol Chem. 2001;276:576–582. doi: 10.1074/jbc.M003779200. [DOI] [PubMed] [Google Scholar]
- Tsai G, Coyle JT. Glutamatergic mechanisms in schizophrenia. Annu Rev Pharmacol Toxicol. 2002;42:165–79. doi: 10.1146/annurev.pharmtox.42.082701.160735. [DOI] [PubMed] [Google Scholar]
- Tuite P, Riss J. Recent developments in the pharmacological treatment of Parkinson’s disease. Expert Opin Investig Drugs. 2003;12:1335–52. doi: 10.1517/13543784.12.8.1335. [DOI] [PubMed] [Google Scholar]
- Turski L, Bressler K, Rettig KJ, Loschmann PA, Wachtel H. Protection of substantia nigra from MPP+ neurotoxicity by N-methyl-D-aspartate antagonists. Nature. 1991;349:414–8. doi: 10.1038/349414a0. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Doi T, Tokumaru J, Willmore LJ. Effect of zonisamide on molecular regulation of glutamate and GABA transporter proteins during epileptogenesis in rats with hippocampal seizures. Brain Res Mol Brain Res. 2003;116:1–6. doi: 10.1016/s0169-328x(03)00183-9. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Doi T, Tsuru N, Tokumaru J, Mitsuyama Y. Expression of glutamate transporters and ionotropic glutamate receptors in GLAST knockout mice. Brain Res Mol Brain Res. 2002;104:120–6. doi: 10.1016/s0169-328x(02)00325-x. [DOI] [PubMed] [Google Scholar]
- Urbani A, Belluzzi O. Riluzole inhibits the persistent sodium current in mammalian CNS neurons. Eur J Neurosci. 2000;12:3567–74. doi: 10.1046/j.1460-9568.2000.00242.x. [DOI] [PubMed] [Google Scholar]
- Vallejo-Illarramendi A, Torres-Ramos M, Melone M, Conti F, Matute C. Clozapine reduces GLT-1 expression and glutamate uptake in astrocyte cultures. Glia. 2005;50:276–9. doi: 10.1002/glia.20172. [DOI] [PubMed] [Google Scholar]
- Vanier MT, Suzuki K. Recent advances in elucidating Niemann-Pick C disease. Brain Pathol. 1998;8:163–74. doi: 10.1111/j.1750-3639.1998.tb00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veruki ML, Morkve SH, Hartveit E. Activation of a presynaptic glutamate transporter regulates synaptic transmission through electrical signaling. Nat Neurosci. 2006;9:1388–96. doi: 10.1038/nn1793. [DOI] [PubMed] [Google Scholar]
- Vizi ES. Role of high-affinity receptors and membrane transporters in nonsynaptic communication and drug action in the central nervous system. Pharmacol Rev. 2000;52:63–89. [PubMed] [Google Scholar]
- Vizi ES, Mike A. Nonsynaptic receptors for GABA and glutamate. Curr Top Med Chem. 2006;6:941–8. doi: 10.2174/156802606777323782. [DOI] [PubMed] [Google Scholar]
- Voutsinos-Porche B, Koning E, Clement Y, Kaplan H, Ferrandon A, Motte J, Nehlig A. EAAC1 glutamate transporter expression in the rat lithium-pilocarpine model of temporal lobe epilepsy. J Cereb Blood Flow Metab. 2006;26:1419–30. doi: 10.1038/sj.jcbfm.9600295. [DOI] [PubMed] [Google Scholar]
- Wadiche JI, Amara SG, Kavanaugh MP. Ion fluxes associated with excitatory amino acid transport. Neuron. 1995a;15:721–728. doi: 10.1016/0896-6273(95)90159-0. [DOI] [PubMed] [Google Scholar]
- Wadiche JI, Arriza JL, Amara SG, Kavanaugh MP. Kinetics of a human glutamate transporter. Neuron. 1995b;14:1019–1027. doi: 10.1016/0896-6273(95)90340-2. [DOI] [PubMed] [Google Scholar]
- Wang S, Lim G, Yang L, Sung B, Mao J. Downregulation of spinal glutamate transporter EAAC1 following nerve injury is regulated by central glucocorticoid receptors in rats. Pain. 2006;120:78–85. doi: 10.1016/j.pain.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Wang Z, Pekarskaya O, Bencheikh M, Chao W, Gelbard HA, Ghorpade A, Rothstein JD, Volsky DJ. Reduced expression of glutamate transporter EAAT2 and impaired glutamate transport in human primary astrocytes exposed to HIV-1 or gp120. Virology. 2003;312:60–73. doi: 10.1016/s0042-6822(03)00181-8. [DOI] [PubMed] [Google Scholar]
- Weiler CT, Nyström B, Hamberger A. Characteristics of glutamate vs glutamate transport in isolated glia and synaptosomes. J Neurochem. 1979;32:559–565. doi: 10.1111/j.1471-4159.1979.tb00384.x. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Shaw CA. Late appearance of glutamate transporter defects in a murine model of ALS-parkinsonism dementia complex. Neurochem Int. 2006 doi: 10.1016/j.neuint.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Wisman LA, van Muiswinkel FL, de Graan PN, Hol EM, Bar PR. Cells over-expressing EAAT2 protect motoneurons from excitotoxic death in vitro. Neuroreport. 2003;14:1967–70. doi: 10.1097/00001756-200310270-00017. [DOI] [PubMed] [Google Scholar]
- Wong M, Ess KC, Uhlmann EJ, Jansen LA, Li W, Crino PB, Mennerick S, Yamada KA, Gutmann DH. Impaired glial glutamate transport in a mouse tuberous sclerosis epilepsy model. Ann Neurol. 2003;54:251–6. doi: 10.1002/ana.10648. [DOI] [PubMed] [Google Scholar]
- Yamada T, Kawahara K, Kosugi T, Tanaka M. Nitric oxide produced during sublethal ischemia is crucial for the preconditioning-induced down-regulation of glutamate transporter GLT-1 in neuron/astrocyte co-cultures. Neurochem Res. 2006;31:49–56. doi: 10.1007/s11064-005-9077-4. [DOI] [PubMed] [Google Scholar]
- Yang YL, Meng CH, Ding JH, He HR, Ellsworth K, Wu J, Hu G. Iptakalim hydrochloride protects cells against neurotoxin-induced glutamate transporter dysfunction in in vitro and in vivo models. Brain Res. 2005;1049:80–8. doi: 10.1016/j.brainres.2005.04.073. [DOI] [PubMed] [Google Scholar]
- Yao HH, Ding JH, He HR, Hu G. Inhibitory effects of 1-methyl-4-phenylpyridinium on glutamate uptake into cultured C6 glioma cells. Acta Pharmacol Sin. 2004;25:855–60. [PubMed] [Google Scholar]
- Yeh TH, Hwang HM, Chen JJ, Wu T, Li AH, Wang HL. Glutamate transporter function of rat hippocampal astrocytes is impaired following the global ischemia. Neurobiol Dis. 2005;18:476–83. doi: 10.1016/j.nbd.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Yernool D, Boudker O, Folta-Stogniew E, Gouaux E. Trimeric subunit stoichiometry of the glutamate transporters from Bacillus caldotenax and Bacillus stearothermophilus. Biochemistry. 2003;42:12981–8. doi: 10.1021/bi030161q. [DOI] [PubMed] [Google Scholar]
- Yernool D, Boudker O, Jin Y, Gouaux E. Structure of a glutamate transporter homologue from Pyrococus horikoshii. Nature. 2004;431:811–818. doi: 10.1038/nature03018. [DOI] [PubMed] [Google Scholar]
- Yi JH, Hazell AS. Excitotoxic mechanisms and the role of astrocytic glutamate transporters in traumatic brain injury. Neurochem Int. 2006;48:394–403. doi: 10.1016/j.neuint.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Yi JH, Pow DV, Hazell AS. Early loss of the glutamate transporter splice-variant GLT-1v in rat cerebral cortex following lateral fluid-percussion injury. Glia. 2005;49:121–33. doi: 10.1002/glia.20099. [DOI] [PubMed] [Google Scholar]
- Yu YX, Shen L, Xia P, Tang YW, Bao L, Pei G. Syntaxin 1A promotes the endocytic sorting of EAAC1 leading to inhibition of glutamate transport. J Cell Sci. 2006;119:3776–87. doi: 10.1242/jcs.03151. [DOI] [PubMed] [Google Scholar]
- Zelenaia O, Schlag BD, Gochenauer GE, Ganel R, Song W, Beesley JS, Grinspan JB, Rothstein JD, Robinson MB. Epidermal growth factor receptor agonists increase expression of glutamate transporter GLT-1 in astrocytes through pathways dependent on phosphatidylinositol 3-kinase and transcription factor NF-κB. Mol Pharmacol. 2000;57:667–678. doi: 10.1124/mol.57.4.667. [DOI] [PubMed] [Google Scholar]
- Zerangue N, Kavanaugh MP. Flux coupling in a neuronal glutamate transporter. Nature. 1996;383:634–637. doi: 10.1038/383634a0. [DOI] [PubMed] [Google Scholar]
- Zhang G, Raol YS, Hsu FC, Brooks-Kayal AR. Long-term alterations in glutamate receptor and transporter expression following early-life seizures are associated with increased seizure susceptibility. J Neurochem. 2004;88:91–101. doi: 10.1046/j.1471-4159.2003.02124.x. [DOI] [PubMed] [Google Scholar]
- Zhang M, Li WB, Geng JX, Li QJ, Sun XC, Xian XH, Qi J, Li SQ. The upregulation of glial glutamate transporter-1 participates in the induction of brain ischemic tolerance in rats. J Cereb Blood Flow Metab. 2007 doi: 10.1038/sj.jcbfm.9600441. [DOI] [PubMed] [Google Scholar]
- Zhou J, Sutherland ML. Glutamate transporter cluster formation in astrocytic processes regulates glutamate uptake activity. J Neurosci. 2004;24:6301–6306. doi: 10.1523/JNEUROSCI.1404-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik A, Gurevich B, Tkachov S, Maoz I, Shapira Y, Teichberg VI. Brain neuroprotection by scavenging blood glutamate. Exp Neurol. 2007;203:213–20. doi: 10.1016/j.expneurol.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Zoia C, Cogliati T, Tagliabue E, Cavaletti G, Sala G, Galimberti G, Rivolta I, Rossi V, Frattola L, Ferrarese C. Glutamate transporters in platelets: EAAT1 decrease in aging and in Alzheimer’s disease. Neurobiol Aging. 2004;25:149–57. doi: 10.1016/s0197-4580(03)00085-x. [DOI] [PubMed] [Google Scholar]
- Zoia CP, Tagliabue E, Isella V, Begni B, Fumagalli L, Brighina L, Appollonio I, Racchi M, Ferrarese C. Fibroblast glutamate transport in aging and in AD: correlations with disease severity. Neurobiol Aging. 2005;26:825–32. doi: 10.1016/j.neurobiolaging.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Zschocke J, Bayatti N, Clement AM, Witan H, Figiel M, Engele J, Behl C. Differential promotion of glutamate transporter expression and function by glucocorticoids in astrocytes from various brain regions. J Biol Chem. 2005;280:34924–32. doi: 10.1074/jbc.M502581200. [DOI] [PubMed] [Google Scholar]
- Zuddas A, Oberto G, Vaglini F, Fascetti F, Fornai F, Corsini GU. MK-801 prevents 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism in primates. J Neurochem. 1992;59:733–9. doi: 10.1111/j.1471-4159.1992.tb09429.x. [DOI] [PubMed] [Google Scholar]