Abstract
Background Context
Spinal manipulation (SM) is a form of manual therapy used clinically to treat patients with low back and neck pain. The most common form of this maneuver is characterized as a high velocity (duration < 150ms), low amplitude (segmental translation < 2mm, rotation < 4°, and applied force 220-889N) impulse thrust (HVLA-SM). Clinical skill in applying an HVLA-SM lies in the practitioner's ability to control the duration and magnitude of the load (i.e., the rate of loading), the direction in which the load is applied, and the contact point at which the load is applied. Control over its mechanical delivery presumably related to its clinical effects. Biomechanical changes evoked by an HVLA-SM are thought to have physiological consequences caused, at least in part, by changes in sensory signaling from paraspinal tissues.
Purpose
If activation of afferent pathways does contribute to the effects of an HVLA-SM, it seems reasonable to anticipate that neural discharge might increase or decrease in a non-linear fashion as the thrust duration thrust approaches a threshold value. We hypothesized that the relationship between the duration of an impulsive thrust to a vertebra and paraspinal muscle spindle discharge would be non-linear with an inflection near the duration of an HVLA-SM delivered clinically (<150ms). In addition, we anticipated that muscle spindle discharge would be more sensitive to larger amplitude thrusts.
Study Design/Setting
A neurophysiological study of spinal manipulation using the lumbar spine of a feline model.
Methods
Impulse thrusts (duration: 12.5, 25, 50, 100, 200, and 400 ms; amplitude 1 or 2mm posterior to anterior) were applied to the spinous process of the L6 vertebra of deeply anesthetized cats while recording single unit activity from dorsal root filaments of muscle spindle afferents innervating the lumbar paraspinal muscles. A feedback motor was used in displacement control mode to deliver the impulse thrusts. The motor's drive arm was securely attached to the L6 spinous process via a forceps.
Results
As thrust duration became shorter the discharge of the lumbar paraspinal muscle spindles increased in a curvilinear fashion. A concave up inflection occurred near the 100ms duration eliciting both a higher frequency discharge compared to the longer durations and a substantially faster rate of change as thrust duration was shortened. This pattern was evident in paraspinal afferents with receptive fields both close and far from the midline. Paradoxically, spindle afferents were almost twice as sensitive to the 1mm compared to the 2mm amplitude thrust (6.2 vs 3.3 spikes/s/mm/s). This latter finding may be related to the small vs large signal range properties of muscle spindles.
Conclusions
. The results indicate that the duration and amplitude of a spinal manipulation elicits a pattern of discharge from paraspinal muscle spindles different from slower mechanical inputs. Clinically, these parameters may be important determinants of an HVLA-SM's therapeutic benefit.
Keywords: lumbar spine, spinal manipulation, chiropractic, osteopathy, paraspinal muscles, muscle spindle
INTRODUCTION
Spinal manipulation is a form of body-based therapy (1) often used clinically to treat patients with low back pain or neck pain (2-4). This form of therapy is typically delivered by osteopaths, physical therapists, and chiropractors. In the United States, chiropractors deliver more than 90% of spinal manipulations during nearly 200 million patient visits annually (5;6). Experimentally, a number of studies indicate that spinal manipulation increases pain tolerance and pain threshold (7-9), inhibits the H reflex [(10;11) but see (12)], increases muscle force (13-15), and increases passive and active spinal range of motion (16;17). Despite the fact that clinical evidence supports the use of spinal manipulation for low back and neck pain (3;4;18), the biological mechanisms underlying its effects remain elusive.
Spinal manipulation is a mechanical intervention by its very nature. This maneuver's most common form is characterized as a high velocity, low amplitude (HVLA) mechanical impulse. The clinician delivers an HVLA to a vertebral segment through a short lever arm by manually contacting the skin overlying the spinous, transverse, or mammillary process of the vertebra or the lamina or articular pillar (19). The load-time profile of an HVLA spinal manipulation (HVLA-SM) can be divided into 3 phases: preload, thrust, and resolution (20). The preload phase typically last up to 5 seconds as the practitioner brings the vertebra being manipulated to the end point of its physiological range of motion. The preload force can comprise up to 25% of the thrust force although this percentage may vary greatly. Inspection of applied loading profiles from a number of studies (21-23) indicates the combined thrust and resolution phases of a manually delivered HVLA-SM can be likened to a half sine wave. For an HVLA-SM applied to the thoracic and lumbar regions the thrust phase rises to a peak load in less than 150ms (21;22;24). Peak forces typically range between 220 to 889N (21-23;25) and force rates range between 500 to 3000 N/s (20;24). Intervertebral motions are small. Translation within a cardinal plane is typically less than 2 mm with rotation about an axis less than 4° (26-30). In the lumbar spine, an HVLA-SM may increase the synovial space of the facet joints by up to 0.7mm (31) remaining increased beyond the duration of the manipulation itself thus likely stretching tissues that cross the joint.
Biomechanical changes evoked by the manipulation are thought to have physiological consequences caused, at least in part, by affecting sensory signaling from paraspinal tissues (32). Mechanoreceptive endings from a variety of primary afferent neurons are likely stimulated although this idea has received little direct investigation (33). If activation of afferent pathways does contribute to the effects of an HVLA-SM, it seems reasonable to anticipate that an unexpected change in neural discharge might occur (e.g., a non-linear increase or decrease) as the duration of the thrust or resolution phases approaches a threshold value. In recent studies, single unit recordings from low threshold mechanoreceptors in lumbar paraspinal tissues exposed to impulse loads simulating a spinal manipulation under force control provided initial evidence that the relationship between neural discharge and impulse duration is not linear (34;35). Neural discharge increases disproportionately as the impulse duration becomes similar to that given during an HVLA-SM. In the present study we sought to extend these findings by determining how lumbar paraspinal muscle spindles respond under displacement control where the L6 vertebra is translated 1 and 2 mm and where thrust duration is slower, faster, and similar to those of an HVLA-SM. We hypothesized that the relationship between thrust duration and spindle discharge would be non-linear with an observable inflection near the thrust duration of an HVLA-SM delivered clinically. In addition, we anticipated that muscle spindle discharge would be more sensitive to the larger amplitude thrust. Evidence is provided that the animal model used to test these hypotheses kept the spine biomechanically sound. An additional objective was to determine if the animal model we used to test these hypotheses kept the spine biomechanically sound.
METHODS
General
Experiments were performed on 54 deeply anesthetized adult cats. All cats were treated in accordance with the Guiding Principles in the Care and Use of Animals approved by the American Physiological Society. The methods have been described previously (34-36) and are presented here in brief form. Anesthesia was induced with halothane and maintained with sodium pentobarbital (35 mg/kg, iv) after placing catheters in the common carotid artery and external jugular vein to monitor blood pressure and introduce fluids. A Harvard Respirator (model 681; Harvard Apparatus, South Natick, MA) mechanically ventilated each cat after tracheal intubation. Additional Nembutal (5mg/kg) was administered when the cat demonstrated a withdrawal reflex to noxious pinching of the toe pad, when mean arterial pressure increased above 120mmHg, or when the cat exhibited a pressure response to surgical manipulation. Arterial pH, PCO2, and PO2 were monitored every 60-90 minutes using an i-Stat pH/blood gas analyzer (i-Stat Corp., East Windsor, NJ) and were maintained within the normal range (pH 7.32 to 7.43; PCO2, 32-37 mm Hg; PO2, >85 mm Hg).
The lumbar spinal column was prepared to obtain neural recordings from the dorsal roots and for manipulating the L6 vertebra. A bilateral laminectomy including the bottom half of L4 and all of L5 exposed the L5 and L6 spinal cord. Paraspinal muscles side were removed on the left side of L4 and L5 but remained intact on the right side except for their dorsal vertebral attachments. The intervertebral discs and facet joints between L5 - L6 and between L6 - L 7 remained intact. In addition, the L6 and L7 vertebrae and associated paraspinal tissues remained intact bilaterally including the lumbodorsal fascia and the multifidus, longissimus, and iliocostalis muscles. The lumbar spine was mechanically secured at the L4 spinous process and the iliac crests using a Kopf spinal unit. Skin margins surrounding the lumbar spine were elevated forming a pool to bathe the paraspinal tissues in warmed (37°C) mineral oil. The dura mater was slit longitudinally. The L6 sensory nerve root was identified and cut close to its entrance into the spinal cord and placed on a small platform. Thin filaments from the root were teased apart using forceps under a dissecting microscope until impulse activity from a single unit with a receptive field in the paraspinal muscles could be identified. Due to the relatively small laminectomy, it was not possible to access the L6 ventral roots without potentially injuring the spinal cord. Therefore, the ventral roots were not cut. Because Nembutal anesthesia was maintained at a deep level based upon blood pressure and the absence of a cardiovascular or motor response to noxious pinch, γ- motoneuron discharge was considered depressed and not labile (37). Nerve activity was amplified using standard electrophysiological equipment and parameters (36) and recorded by a PC based data acquisition system (Spike 2, Cambridge Electronic Design, Great Britain) for off-line analysis.
Manipulating the L6 Vertebra
Impulse loads were applied to the L6 vertebra in a dorsal-ventral direction. The output from an arbitrary waveform generator commanded an electronic feedback control system (Aurora Scientific, Lever System Model 310, Ontario, Canada) under displacement control. The control system was attached to the L6 spinous process via a pair of adjustable tissue forceps (1×2 teeth) which were clamped tightly onto the lateral surfaces of the L6 spinous process. The forceps were narrow requiring only a narrow slit (approximately 2 mm long) along either side of the L6 spinous process for attachment. Only a small portion of the multifidus muscle was detached from the vertebra using this method because most of the muscle fibers attach to the spinous process via a tendinous insertion onto its caudal edge (38). The output from the feedback control system measured the delivered displacement and force.
Spinal manipulative thrusts were delivered as ¼ sine waves and with durations that simulated and encompassed the loading profile of an HVLA-SM given by clinicians (see Introduction). Thus, the arbitrary waveform generator was programmed to deliver thrusts of 12.5, 25, 50, 100, 200, and 400 ms in duration and displacing the L6 vertebra 1 and 2mm dorsal-ventral. The presentation order of the 6 thrust durations was randomized to minimize potential ordering effects introduced by the mechanical non-linearity inherent in soft tissue. Presentation of each impulse was separated by 10 minutes to allow recovery. Prior to each impulse the L6 vertebra was positioned identically. The motor was positioned so that force upon the spine was 0 N during the preload phase.
Electrophysiological Recordings and their Classification. Activity from a putative muscle spindle in the lumbar spine was first identified by gently compressing the lumbar paraspinal tissues or by manually moving the L6 vertebra ventralward which evoked a high frequency. Only afferents whose discharge was highest in response to vertebral movement or probing the back muscles compared with probing the gluteal, hip, or leg regions were used. Muscle spindle endings were classified as primary or secondary based upon afferent responses to ramp and hold stretch described by Scott (39). Primary endings show 1) a sharp increase in firing level at the start of ramp followed by a leveling off of firing rate despite continuation of the ramp; 2) a sharp reduction in firing rate at the end of ramp-up as the hold begins; 3) the absence of firing at the beginning of ramp-down as the hold is released. Secondary endings show 1) a slow continuous increase in IF during ramp-up; 2) slow adaptation at the end of ramp-up; 3) continued firing at the beginning or ramp-down. Intermediate endings show mixed characteristics. The presence or absence of an initial burst was not considered as a criteria because it usually was generally not present (37 of 53 afferents) and when present it was small. With the lumbodorsal fascia intact, a ramp (1mm/s) and hold was applied delivered to the paraspinal muscles by translating the L6 vertebra ventralward to a magnitude that loaded the paraspinal tissues with 50-60% of the cat's body weight (BW). This value was determined empirically in that higher loads often tore dorsal root filaments from the electrode.
After delivering the 6 manipulative thrusts, we opened the intact lumbodorsal fascia and used a variety of approaches to confirm that the single unit was from a lumbar paraspinal muscle spindle. The sacrocaudalis dorsalis lateralis (lumbococcygeus) muscle, which lies between the lumbar multifidus and longissimus muscles, was removed to improve the mechanical isolation of the latter two muscles. First and second sacral nerves innervate the lumbococcygeus (40) muscle so none of the afferent recordings were lost by its removal. Von Frey hairs were applied to determine the most sensitive area for mechanically activating the afferent.
Two methods were used to confirm that neural activity was from a muscle spindle: decreased discharge to a muscle twitch and an increased discharge to succinylcholine that was maintained for at least 30 seconds (100-300 μg/kg, ia). An afferent's response to muscle twitch was determined using needle electrodes inserted into the muscle on either side of the receptive field. Because deep paraspinal muscle nerves are short and difficult to isolate, the afferent's response to twitch was determined by direct muscle stimulation (0.12-6mA, 0.02-1s) using a constant current stimulus isolation unit (Grass Instrument, PSIU6, West Warwick, RI) and a square wave stimulator (Grass Instrument, S88). Two needle electrodes were typically inserted into either side of the most sensitive portion of the afferent's receptive field.
Nerve conduction velocity was obtained by stimulating the L6 spinal nerve after inserting 2 needle electrodes in the vicinity of the L6-7 intervertebral foramina (where the L6 posterior ramus joins the spinal nerve) and recording the action potential at the dorsal root filament as it was not possible to isolate the muscle nerve. The conduction distance was divided by the conduction time to obtain conduction velocity. Conduction distance was determined by measuring the length of a thin thread extending from the recording electrode along the dorsal root and spinal nerve to its entrance at the intervertebral foramen. Typically conduction times were ≤1 ms and conduction distances approximately 45mm. At these short conduction times, a 10% error in conduction distance or utilization time could miscalculate conduction velocity by ∼5 m/s.
Data Reduction and Analysis
Muscle spindle activity was converted to instantaneous frequency (IF) by taking the reciprocal of the time interval between consecutive spikes. The response of a muscle spindle to a manipulative thrust was calculated as a difference by subtracting mean IF during the preload phase (10 seconds) from the mean IF during the thrust phase and is therefore reported as a change in mean instantaneous discharge frequency (ΔMIF). Instantaneous discharge frequency as opposed to mean frequency was used because on a moment-to-moment basis, it is the timing between a neuron's action potentials that directly affects the time course of post-synaptic potentials in 2nd order neurons.
A descriptive relationship between thrust duration and ΔMIF for each afferent is shown using profile plots. Because thrusts were given under displacement control we also compared the effect of thrust velocity on ΔMIF. With the repeated measure design on velocity we used response features analysis (41) by calculating the slope of the relationship between ΔMIF and thrust velocity for each muscle spindle afferent and then compared these slopes using a two-way analysis of variance (ANOVA) [2 levels of displacement (1 vs 2mm) and 3 levels of afferent ending (primary, secondary, and intermediate)]. Slope represented spindle sensitivity measured as spikes/s/mm/s.
In a small subset of cats (n=6) we determined how the surgical procedure affected the spine's mechanical response to the manipulations. Average spinal stiffness during each thrust was calculated as the change in force divided by the change in displacement from the beginning to the end of the thrust. Spinal stiffness was compared using a two-way ANOVA (thrust velocity × surgery) with repeated measures on velocity. Whenever the ANOVA detected a significant difference at p< 0.05, Bonferroni t-tests adjusted for multiple comparisons were used for pairwise comparisons. Data are expressed as mean (standard deviation) values unless otherwise indicated. Statistical analyses were performed using SigmaStat v3.3 software.
RESULTS
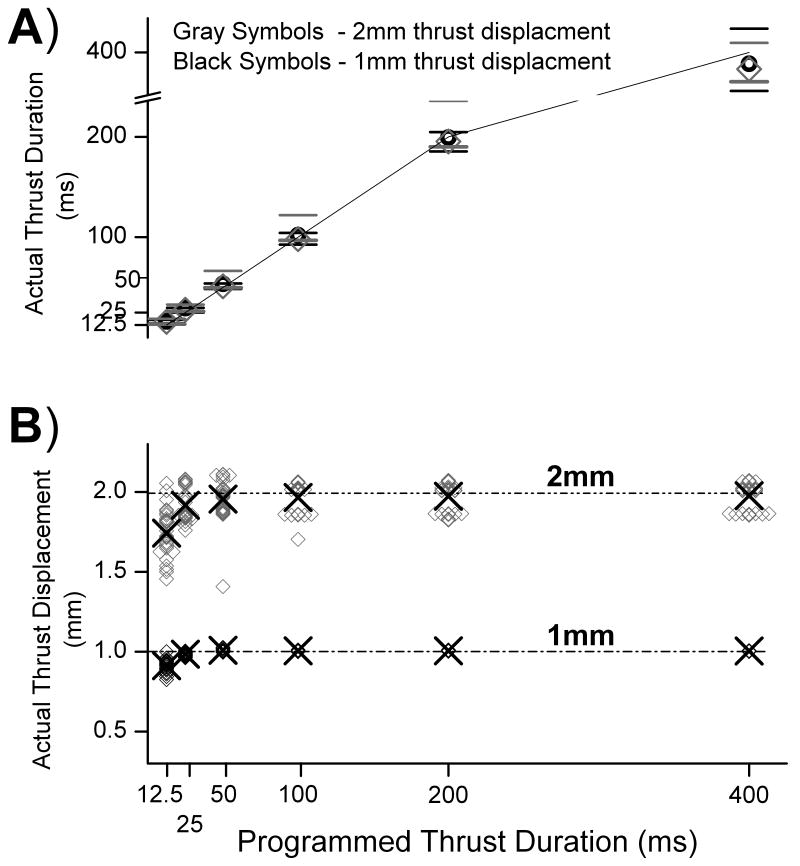
Figure 1 compares the programmed with the actual thrust displacements and durations delivered to the L6 vertebra. At the shortest durations the motor did not consistently reach the programmed displacement of 1 and 2 mm. Nonetheless, the average of the larger vertebral displacement was nearly twice that of the smaller displacement at each thrust duration (1.91x, 1.95x, 1.94x, 1.95x, 1.96x, 1.97x at 12.5, 25, 50, 100, 200 and 400 ms, respectively). Conversely, the average actual thrust durations for the 3 shortest impulses were slightly longer than the programmed durations (16 vs 12.5, 29 vs 25, and 54 vs 50 ms) whereas the actual thrust durations for the 3 longest impulses (100, 200 and 400ms) were within ±3% of the programmed durations. Consequently, at each programmed duration thrust velocities were consistently nearly twice as fast (range: 1.85 -1.96x, Table 1) for the 2 mm compared with the 1 mm thrust displacement. Peak applied forces at the spinous process of the L6 vertebra were greater than 9N but less than 30 N and were greater for the 2mm compared with the 1mm thrusts for each thrust duration. Average force rates were nearly 2.5 greater for the 2mm compared with the 1mm thrusts. Average force rate during the 1mm impulse ranged from 24 – 607 N/s and from 62 - 1419 N/s during the 2mm thrust (longest to shortest thrust duration).
FIGURE 1.
Comparison of programmed and actual thrust A) durations and B) displacements. In A symbols denote means of all cats and horizontal bars denote the minimum and maximum actual thrust duration. Solid line is the line of identity between actual and programmed thrust duration. In B symbols denote thrust displacements for each cat and “X” denotes their means. Overlapping data result in thicker symbols.
Table 1.
Loading characteristics of the spinal manipulative-like impulses for thrust displacements of 1 and 2mm.
| VELOCITY [mm/s] | PEAK FORCE [N] | AVERAGE FORCE RATE [%BW/s] | ||||||
|---|---|---|---|---|---|---|---|---|
| PROGRAMMED THRUST DURATION | Mean (SD) | VELOCITY RATIO | Mean (SD) | Mean (SD) | FORCE RATE RATIO | |||
| (ms) | 1mm | 2mm | 1mm | 2mm | 1mm | 2mm | ||
| 12.5 | 58.0 | 108.9 | 1.88 | 9.7 | 23.2 | 1727 | 3923 | 2.3 |
| (5.5) | (15.9) | (2.8) | (9.0) | (426) | (1388) | |||
| 25 | 35.5 | 65.6 | 1.85 | 10.7 | 26.3 | 1084 | 2444 | 2.3 |
| (1.8) | (5.9) | (3.0) | (12.0) | (264) | (1014) | |||
| 50 | 19.8 | 37.1 | 1.87 | 10.6 | 26.1 | 590 | 1329 | 2.3 |
| (0.5) | (2.5) | (2.8) | (13.1) | (143) | (609) | |||
| 100 | 10.3 | 19.4 | 1.88 | 10.0 | 24.3 | 291 | 651 | 2.2 |
| (0.3) | (1.1) | (2.8) | (12.9) | (78) | (327) | |||
| 200 | 5.2 | 10.0 | 1.92 | 9.5 | 24.9 | 138 | 345 | 2.5 |
| (0.17) | (0.6) | (2.8) | (12.9) | (39) | (179) | |||
| 400 | 2.6 | 5.1 | 1.96 | 9.1 | 24.5 | 67 | 173 | 2.6 |
| (0.1) | (0.27) | (2.8) | (12.5) | (20) | (90) | |||
Ratios represents 2mm displacement relative to 1mm displacement. N,newtons;BW,body weight.
Single unit recordings were obtained from afferents that were responsive to dorsal-ventral movement of the L6 vertebra. The receptive field for each of 53 afferents was located in the lumbar paraspinal muscles and 1 receptive field was located over the iliac fossa. The longissimus muscle contained the receptive field of 41 afferents. For 13 of these 41 afferents (32%) the most sensitive region of the receptive field was located near a facet joint, either L6-7 or L7-S1 which likely represented an area close to the muscle's attachment at the accessory process. For 20 of these 41 afferents (49%) the most sensitive region was located along the medial border of the longissimus where the lumbococcygeus had been removed. For the remaining 8 afferents (19%) the most sensitive region was located on the dorsal surface of the longissimus. The multifidus muscle contained the receptive field of 8 afferents. For 3 of these 8 afferents (38%) the most sensitive region of the receptive field was located near the L7-S1 facet joint in the area of the muscle's attachment to the S1 mammillary process; for 1 of these 8 afferents (12%) it was located near the muscle's attachment at the L6 spinous process. For the remaining 4 afferents (50%) the most sensitive region was located in the belly of the multifidus. The iliocostalis muscle contained 1 afferent's receptive field. For 2 afferents each receptive field was located deep in the sulcus between the longissimus and iliocostalis muscles, and a location specific to either muscle could not be distinguished. Similarly the receptive field of 1 afferent could not be distinguished between the longissimus and multifidus muscles.
All 54 afferents were unloaded by muscle twitch. Only 48 of the 54 afferents were responsive succinylcholine. Three afferent endings were vascularly inaccessible to succinylcholine because a depolarizing, non-lethal injection of concentrated KCl (3M, ia) did not activate the afferents but did increase arterial blood pressure. Succinylcholine was not administered to 3 afferents. These latter 3 afferents had low mechanical thresholds, (2.0, 1.4 and 2.0 gm) and high resting discharge rates (22, 32 and 46 Hz, respectively) and were therefore considered muscle spindles. The vascularly inaccessible afferents had low mechanical thresholds (2.0, 2.0, and 4.0 gm) but none had a resting discharge. Therefore 51 muscle spindle afferents were included in the analysis.
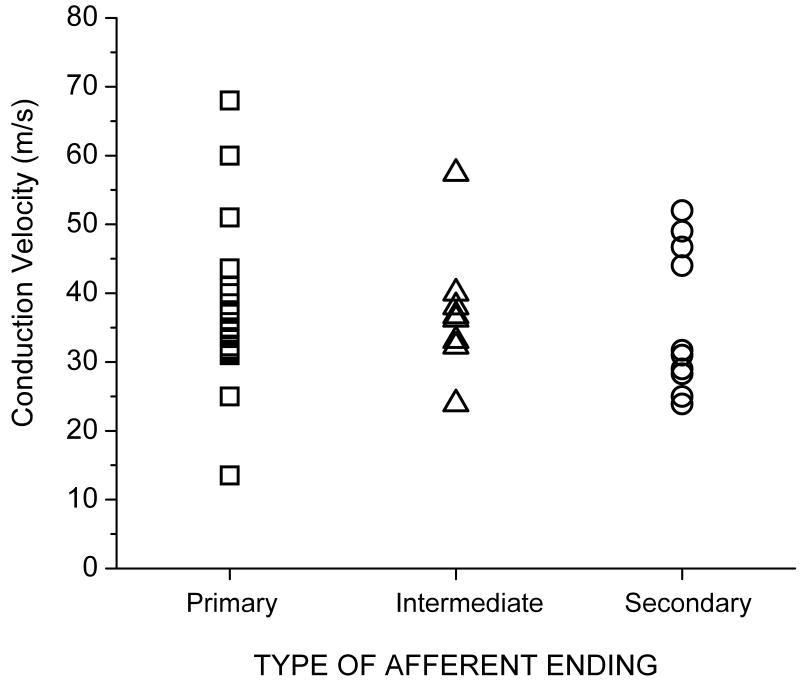
Mechanical thresholds obtained using nylon monofilaments (Stoelting Co, Wood Dale, IL, USA) ranged between 0.2 and 60grams (g) [6.2 (11.6)g]. Most afferents had low mechanical thresholds as might be expected from muscle spindles, especially if they were located superficially. Only 2 afferents had mechanical thresholds greater than 24g, one near the L6-7 facet joint and one in the region of the longissimus and iliocostalis. Twenty-four spindle afferents were classified as primary (47%), 17 as secondary (33%), and 10 as intermediate (20%). Conduction velocities were obtained for 42 of the 51 afferents and ranged from 13.5 to 68.0 m/s. Their distribution (Figure 2) was unimodal and not skewed (mean = 36.8 m/s and median = 36.0 m/s). Mean conduction velocities for primary, secondary and intermediate afferents were similar to each other [37.4 (11.2), 35.1 (10.7), and 37.6 (9.0) m/s, respectively, mean(SD)]. No relationship was evident between conduction velocity and whether afferents were classified as primary, secondary, or intermediate (Figure 2). The range of conduction velocities tended to be narrower for afferents ordered from primary to intermediate, to secondary.
FIGURE 2.
Relationship between conduction velocity and the type of lumbar paraspinal muscle spindle afferent based upon classification of its receptive ending to a ramp and hold stretch (see Methods).
Forty-six of the 51 muscle spindle afferents had a resting discharge at the start of each protocol [28.5 spikes/s (11.1)]. Neither the mean resting discharge between the 1 and 2mm displacement protocols [26.6 (9.3) vs 30.6 (12.4) spikes/s, respectively] nor their median coefficients of variation [4.8 vs 3.2, respectively] were statistically different (p=0.23 and p=0.46, respectively) using Student's unpaired t-test and Mann Whitney Rank Sum test (coefficients of variation were not normally distributed), respectively. Four of the muscle spindle afferents had no resting discharge and 1 afferent had no resting discharge for 5 of the 6 protocols but developed a low frequency discharge (5 spikes/s) at the start of one manipulation protocol.
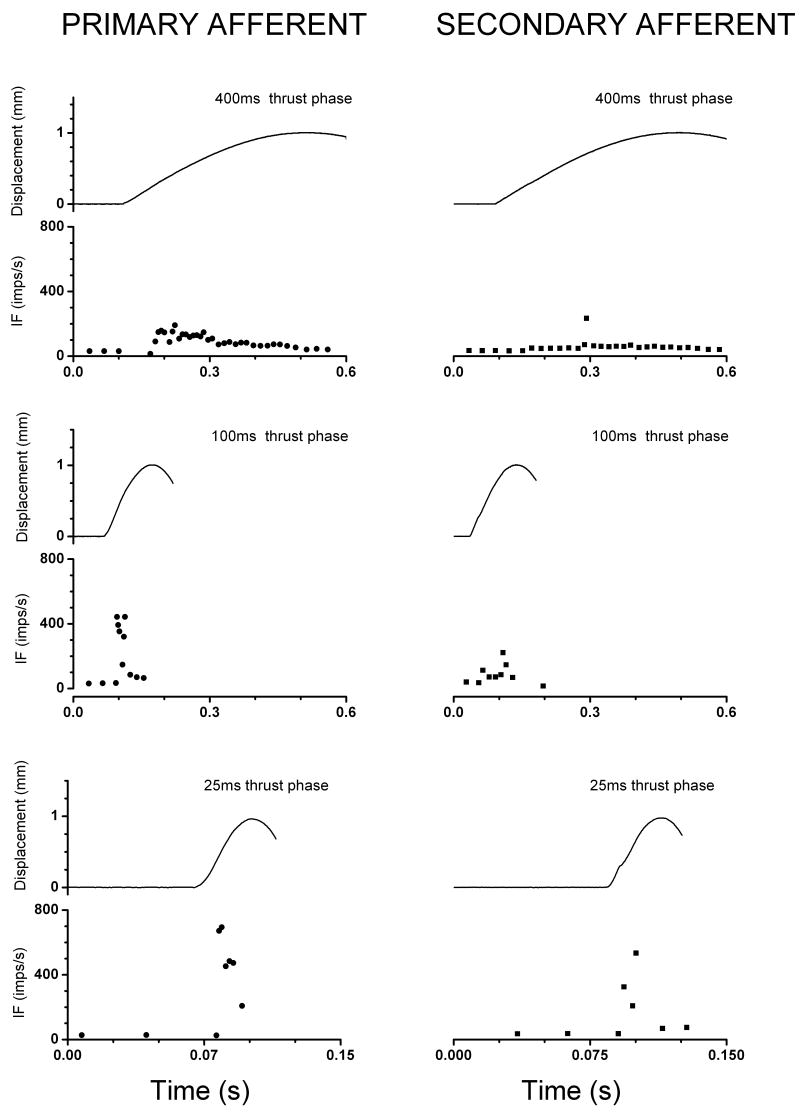
Figure 3 shows representative patterns of instantaneous discharge frequency obtained from a primary and a secondary muscle spindle afferent to each of 3 thrust durations (400, 100 and 25 ms). As the duration of the manipulative thrust shortened, neuronal discharge became higher in frequency reaching upwards of 400Hz. The time interval over which the discharges occurred decreased as the thrust duration decreased and they did not extend beyond the duration of the imposed vertebral displacement. A portion of the highest discharge rate in the primary afferent may reflect the initial burst as the afferent responds to the fast acceleration.
FIGURE 3.
Examples of responses from primary (left column) and secondary (right column) lumbar paraspinal muscle spindle afferents for the 400 (top row), 200 (middle row) and 100ms (bottom row) manipulative thrust durations. Each panel shows the displacement caused by the thrust (top trace) and instantaneous discharge plots obtained from original recordings of action potentials (bottom plot). The starting time of the x-axis is arbitrary; all recordings were preceded by a 10 second control period. Note, for the 25ms thrust the x-axis is expanded.
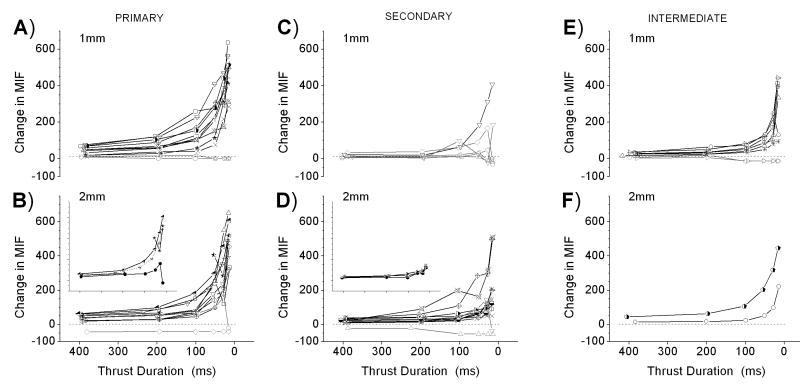
The means of the response trajectories for each paraspinal muscle spindle afferent as a function of thrust duration, type of afferent, and displacement magnitude are shown in Figure 4. Mean instantaneous discharge frequency generally increased as thrust duration decreased. The increase was disproportionate to the decrease in duration, i.e., MIF increased in a non-linear fashion with an inflection near the 100ms thrust duration for both the 1mm and 2mm impulses. During the 1mm displacement the discharge of 15 of the 26 afferents (58%) increased relative to each of the next longest thrust durations. For the remaining 11 afferents (42%), the pattern was similar except at the 2 shortest thrust durations. The discharge of 2 spindle afferents slightly decreased at either, but not both, the 12.5 or the 25ms duration. Even though the remaining 9 afferents had previously responded to the ramp and hold displacement used to characterize dynamic responsiveness 6 were unresponsive and 3 were silenced by the 25 and/or the 50ms thrust durations (plotted as 0 spikes/s and negative values, respectively in Figure 4). Two of these 9 afferents were also unresponsive or silenced to the longer thrust durations as well. During the 2mm impulse, a similar pattern was observed. The discharge of 19 of 25 (76%) muscle spindle afferents increased relative to each of the next longest thrust durations. Two afferents at the 2 shortest thrust durations showed variations on this pattern, their discharge slightly decreasing at either the 12.5 or the 50 ms duration compared with the next longest thrust duration. Two afferents were completely unloaded by the 12.5 ms thrust duration and 2 were completely unloaded by all thrust durations.
FIGURE 4.
Effect of thrust duration on paraspinal muscle spindle discharge during 1mm (top row) and 2mm (bottom row) displacements of the L6 vertebra. Y-axis represents the difference between mean instantaneous discharge frequency during the thrust compared to the prior 10 second control period. Grayed symbols represent the response of those afferents (n=13) whose discharge did not consistently increase as thrust duration shortened (see text). See last paragraph of Results for description of inset. Inset x-axis scale identical to larger plots.
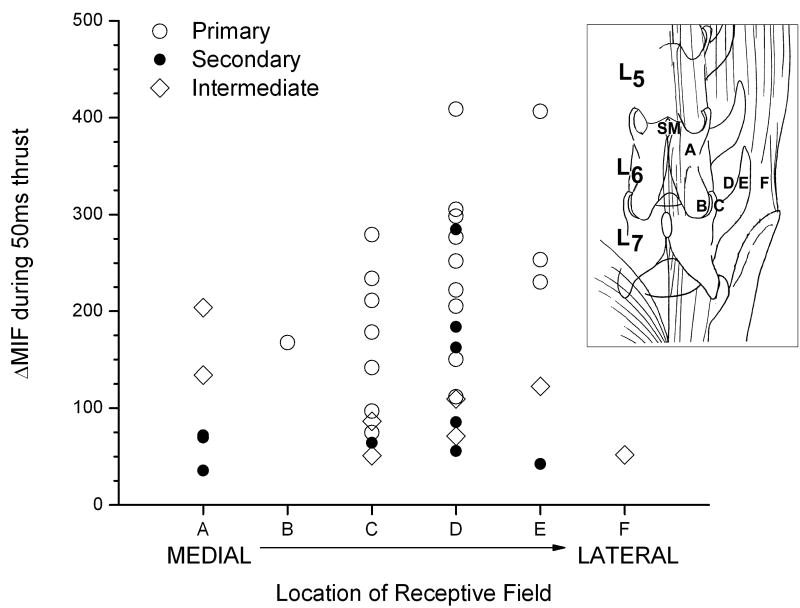
Only those afferents whose discharge increased in response to all thrust durations (n=38) were further analyzed (black symbols in Figure 4) whereas the 13 afferents that were either not responsive or silenced by at least one duration (gray symbols in Figure 4) were excluded from further analysis. Despite the receptive endings of these afferents being widely distributed throughout the region of the low back, the mechanical stimulation provided by the thrust appears to have been transmitted throughout the region as shown in Figure 5 for the 50ms thrust duration. There appeared to be no systematic influence of spindle location on the afferent responses to the thrust.
FIGURE 5.
Relationship between responsiveness of paraspinal muscle spindles during a 50ms spinal manipulative thrust and the distance of their receptive fields from the point of thurst (denoted by “SM”). The incrementing letters refer to the relative location of the receptive fields grouped by spinal region (inset): “A”, multifidus muscle;, “B”, multifidus muscle near the L6-7 or L7-S1 facet joint; “C”, longissumus muscle near the L6-7 or L7-S1 facet joint; “D”, medial portion of longissimus: “E”, lateral portion of longissimus; and “F”, iliocostalis muscle.
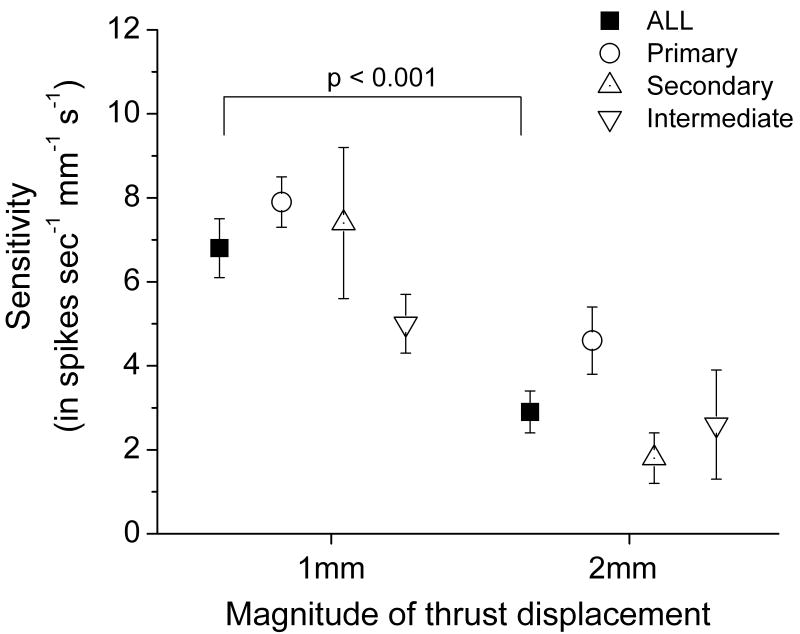
Transforming thrust duration to thrust velocity using measured displacements provided a relatively linear fit [r= 0.96 (0.06)] for describing the relationship between each afferent's response and increasing thrust velocity. Muscle spindle sensitivity to thrust velocity depended on the magnitude of the displacement in an unexpected manner. Two way ANOVA revealed a significant effect for the magnitude of thrust displacement (F1, 32 = 20.968, p<0.001) as shown in Figure 6. Spindle afferents as a population were more than twice as sensitive to the 1mm compared to the 2mm amplitude thrust during a spinal manipulation [6.8 (SEM 0.7) vs 2.9 (SEM 0.5) spikes/s/ mm/s, respectively]. The type of afferent had a significant effect on the sensitivity to velocity (F2, 32 = 3.996, p=0.028) where post-hoc comparisons indicated primary afferents were significantly more responsive to velocity than intermediate afferents (p<0.05, not shown in Figure 6). Displacement magnitude had no effect on any of these relationships, i.e. there was no significant interaction (F2, 32 = 0.873, p=0.427).
FIGURE 6.
Effect of the magnitude of thrust displacement on the sensitivity of lumbar paraspinal muscle spindles to the thrust velocity of an impulse load. Shown as mean ±1 SEM.
The paradoxical finding that the paraspinal muscle spindles were more sensitive to the spinal manipulation velocity during smaller compared to the larger displacements led us to consider the possibility that our metric using mean instantaneous frequency (MIF) gave rise to this unexpected result based upon the following consideration: at comparable thrust velocities, the duration over which the larger displacement occurred was nearly twice as long as over the smaller displacement. As a result, if the larger displacements had produced higher peak instantaneous discharge frequencies (PIF) followed by subsequent slowing of the instantaneous discharge frequency, then averaging over the longer time interval could result in a lower MIF for the 2mm compared to the 1mm displacement. Therefore, we also used PIF in the response feature analysis. A linear fit also provided a good description of the relationship between PIF and thrust velocity for the 38 afferents [r=0.89 (0.13)]. As shown in Table 2 the two way ANOVA demonstrated a significant effect of thrust displacement on the relationship between PIF and thrust velocity (F1, 32 = 9.726, p= 0.004). Similar to using MIF as the outcome measure, spindle afferents were more than twice as sensitive to the velocity of a 1mm compared to a 2mm amplitude thrust using PIF as the outcome measure (6.7 vs 3.2 spikes/s/mm/s). Neither the type afferent nor the interaction between displacement and afferent type significantly affected the slope relationship.
Table 2.
Sensitivity of lumbar paraspinal muscle spindles PIF to the thrust velocity of a spinal manipulation as a function of 2 thrust displacements.
| SPINDLE CLASSIFICATION | VELOCITY SENSITIVITY
(in spikes s-1 mm-1 s-1) |
|
|---|---|---|
| 1 mm displacement | 2mm displacement | |
| All afferents | 6.7 (0.9)* | 3.2 (0.7) |
| Primary afferents | 8.5 (0.8) | 4.6 (0.8) |
| Secondary afferents | 6.4 (2.4) | 1.9 (0.8) |
| Intermediate afferents | 5.1 (1.0) | 3.2 (1.7) |
PIF, peak instantaneous discharge frequency ; mean (SEM);
p=0.004 compared to 2mm thrust, PIF, peak instantaneous discharge frequency
Biomechanical Integrity
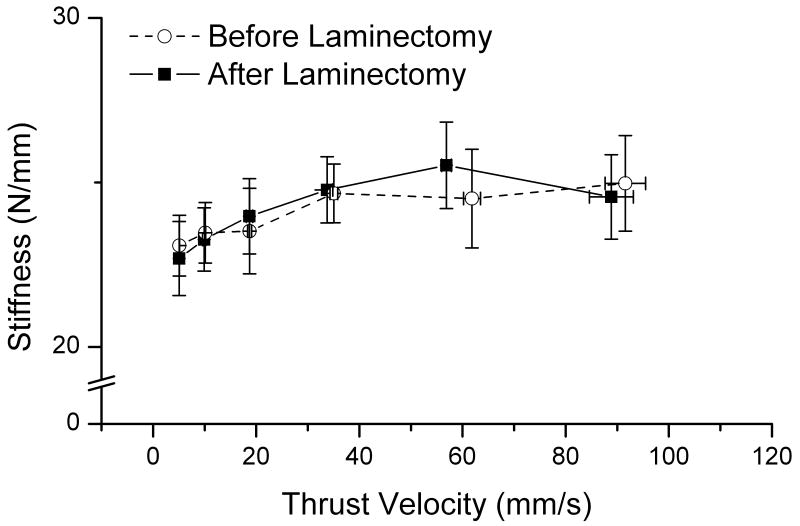
The methods necessary to perform this study required a surgical procedure that potentially altered the spine's mechanical integrity. While the articulating structures (i.e., facet joints and intervertebral discs) between L5-6 and L6-7 remained intact, paraspinal muscles overlying the L5 vertebra had to be removed or detached and the L5 lamina removed bilaterally. To assess the spine's mechanical integrity we compared the average spinal stiffness before and after the surgical procedure in 6 cats. Average spinal stiffness was calculated during a manipulation as the change in force from the initial to the peak load divided by the change in displacement. Stiffness was calculated for each of the 6 thrust durations only for the larger of the two thrust displacements, i.e. 2mm. Muscle spindle recordings from these 6 cats after the surgical procedure are shown in the inset of Figure 4. The response trajectory from these spindles was similar to the larger population suggesting the surgical preparation in these 6 cats was not different from the remaining 48 cats. Average spinal stiffness before and after the surgical procedure was not significantly different using a repeated measures two-way analysis of variance for factors thrust displacement and thrust velocity (F1,5 =0.167, p=0.700). Average spinal stiffness measured at L6 vertebra ranged between 22.7 and 25.5 N/mm (Figure 7). Average spinal stiffness increased slightly but significantly as thrust velocity increased (F5,25 = 10.171, p<0.001) indicating the time-dependent properties of this viscoelastic system.
FIGURE 7.
Effect of the surgical preparation on the average spinal stiffness in response to the manipulative thrust velocity. Shown as mean ±1 SE.
DISCUSSION
This study was designed to investigate the relationship between paraspinal muscle spindle discharge and both the duration and amplitude of a displacement-controlled impulse load that simulated a spinal manipulation. Before discussing the neurophysiological findings, we consider the potential for biomechanical compromise created by the laminectomy. Even though the facet joints remained intact in this experimental preparation, the surgical procedure we performed one segment above the manipulated vertebra could have altered the region's mechanical properties. Despite the laminectomy, however, the region's stiffness was not different compared with the completely intact preparation without laminectomy. That spinal stiffness can remain stable in the presence of a laminectomy where the facet joints remain intact is supported by a recent study in the canine lumbar spine (42). Hemilaminectomies and bilateral laminectomies were performed at both single and multiple levels. Neither lumbar stiffness nor range of motion was affected by any of these procedures except for a consistent decrease in the neutral zone's stiffness. With a single level laminectomy the neutral zone's stiffness decreased by 30%. The dorsal-ventral regional stiffness we measured in the cat spine (20N/mm) was higher but similar to that in the rat lumbar spine [14.4 N/mm (43)] and the lumbar spine of healthy human volunteers [∼11 to 17 N/mm (44) and 14.05 to 16.41 N/mm (45) depending upon the segmental level]. In contrast, dorsal-ventral stiffness of the sheep lumbar spine is 0.3 to 0.5 fold lower (46) depending upon the rate of loading (5.4 to 11.8 N/mm between sinusoidal loading frequencies of 2.0 and 11.7 Hz). It seems reasonable that in the present experiments the neurophysiological responses to spinal manipulative-like loads were obtained from a lumbar region whose biomechanical integrity simulated the intact spine.
The mechanical stimulus of the manipulative thrust, when applied to a single vertebra at its spinous process, activated paraspinal muscle spindles throughout the lumbar region, including multifidus, longissimus and iliocostalis muscles. The effectiveness of the stimulus did not appear to depend upon the distance between its application at the spinous process and the afferent's receptive field. The relationship between the thrust's duration and the discharge of lumbar paraspinal muscle spindles was curvilinear. A concave up inflection occurred near the 50 to 100ms thrust duration which elicited both a higher frequency discharge compared to the longer durations and a substantially faster rate of change as thrust duration became shorter. This curvilinear relationship and the impulse duration near the inflection point does not appear unique to our having controlled displacement because impulsive thrusts under force control evoke a similar discharge pattern from muscle spindles (34) and other low threshold mechanoreceptors (35). Pickar and Kang (34) suggested that the duration at which the inflection occurs reflects an inherent discharge property of muscle spindles originally described by Matthews and Stein [see reference (47)]. A spindle's responsiveness to the dynamics of length change can predominate over its responsiveness to the magnitude of length change when a sinusoidal mechanical input is sufficiently fast (47). In the hindlimb soleus muscle of the cat Matthews and Stein found a corner sinusoidal frequency between 1 and 2.5Hz. In the present experiments the 100ms, ¼ sine wave thrust represents a full sinewave of 2.5Hz. Clinically, skilled application of HVLA-SM to the lumbar spine is typically delivered with thrust durations shorter than 150ms (21;22;24) and with applied force rates of 500 - 3000 N/s (20;24). Assuming a 70 kg human (686 N) the range of force rates normalize to 72% - 437 %BW/s. The normalized force rates we achieved while controlling thrust displacement were similar to or higher than those used clinically (20;24). In the cat, our force rates at the 100ms thrust duration averaged 281 and 651 %BW/s using 1 and 2mm displacements, respectively. However, our force rates were substantially higher (1700 and 3000 %BW/s, respectively) at the fastest thrust duration.
The higher frequency discharges occurring in primary versus either secondary, or intermediate spindle afferents as thrust duration became shorter (seen in Figures 3 and 4) suggest a contribution from the “initial burst”. This short-lasting, high frequency discharge at the onset of muscle stretch from a rest position has been considered a response to acceleration (48). While this response predominantly occurs in primary afferents (48), secondary paraspinal spindle afferents could also respond with an initial burst at fast manipulative durations (Figure 3). Interestingly, Matthews also reported that the initial burst is not present when intrafusal muscle fibers are contracting (48). In the present experiments, the intrafusal fibers were not likely contracting due to the deep level of anesthesia. From a clinical perspective, practitioners seek to have their patients relaxed as much as possible prior to the delivery of a manipulation, a state in which gamma-motoneuron tone is low and intrafusal contractions minimal or absent. We speculate that besides serving as a means to decrease spinal stiffness and prevent tissue injury, this relaxation has a neurophysiological consequence. Spinal manipulation may evoke proprioceptive discharge at a frequency that is not developed during either voluntary or self-imposed passive spinal movement. Because the initial burst represents a response to acceleration, spindle responses are likely different between mobilization interventions which use cyclic movements and an HVLA-SM where the thrust is typically delivered as an impulse from a rest position.
In clinical use, the magnitude of an HVLA-SM's preload phase can vary greatly constituting 1 - 25% of the total applied force (21-23). Our experimental design employed no preload, however it seems reasonable to assume that this did not bias the results. First, muscle spindles are thought to reset as muscle is stretched from a new resting length because spindles retain their responsiveness even at longer muscle lengths (47;49). Second, in the present experiment the number of action potentials during the 100ms thrust ranged from 2-12 spikes (e.g. see Figure 1). Similarly, original recordings [see inset in Figure 7 of reference (50)] show that manipulation also increased muscle spindle discharge by 12 spikes even when a preload preceded the thrust phase.
While the preload phase may not affect muscle spindle responses to an HVLA-SM, this may not be true for all paraspinal mechanoreceptive endings (33). A number of biomechanical studies indicate that mechanoreceptive endings of groups III and IV muscle afferents as well as slowly adapting Type I skin mechanoreceptive endings of A-β fibers are most sensitive to stress when under compression, with no apparent resetting because their response saturates (51;52). In clinical application, an HVLA-SM is applied either manually or using an instrument with contact made orthogonal to the body surface over the paraspinal muscles. Thus, both the preload and thrust phases provide substantial compressive loading to paraspinal tissues. While muscle spindles are known to monitor muscle length, the local mechanical state to which they respond may be better related to compressive stress. It has been conjectured that depressions within the sarcolemmal surface of intrafusal fibers into which primary and secondary endings are embedded cannot sustain much longitudinal deformation and actually flatten and compress the receptive endings (53). This consideration highlights a limitation of the present study where contact was made, not with the paraspinal muscles, but directly to the vertebra via a clamp attached to the spinous process. The preload and thrust phases of an HVLA-SM applied by contact to the muscle may produce spindle responses different in either pattern or magnitude compared to those measured in the present experiment. Additional work is needed to clarify this issue.
In the hindlimb of the cat, distinguishing between primary (group Ia) and secondary (group II) muscle spindle afferents is frequently based upon conduction velocity. The distribution of conduction velocities is bimodal with 72m/s being the modal trough (54). The distributions overlap, however, and muscle spindle afferents conducting between 60 and 80 m/s are typically not classified based upon conduction velocity alone. In lumbar paraspinal muscles of the cat a bimodal distribution was not evident, a finding similar to spindle afferents from biventer cervicis and complexus in the cervical spine (55). In addition, lumbar afferents with primary, secondary, and intermediate response properties could not be distinguished based upon conduction velocity. Unlike that in leg muscles, the overlapping conduction velocities between the functional divisions of lumbar paraspinal muscle spindle afferents would lead to similar central arrival times for their sensory information. The consequences for neuromuscular control mechanisms of the spine compared to the appendicular muscles are unknown.
The greater responsiveness that paraspinal muscle spindles displayed to thrust duration as thrust amplitude increased (Figure 4) may reflect, in part, the non-linear properties of spindles originally described by Matthews and Stein (47). When muscle stretch is large (greater than about 100 μm), spindle sensitivity falls substantially compared with small stretch. Primary spindle endings are more sensitive than secondary endings to very small stretches whereas each type of ending is equally sensitive to large stretches. Due to the technical requirements of the preparation, vertebral displacement was controlled rather than the amount of paraspinal muscle stretch. Because paraspinal muscles lie predominately in the longitudinal plane and because impulse loads were delivered orthogonally to this plane, paraspinal muscles were likely stretched by a fraction of the vertebral displacement. Using the law of cosines for plane triangles, 1 and 2mm vertical displacements would stretch a 50mm long muscle (the approximate length of 2 vertebral segments in the cat) by 87 and 174μm, respectively if the muscle were inclined 5° relative to the longitudinal plane. This approximation and the higher velocity sensitivity of primary afferents to the 1mm compared with the 2mm thrust is consistent with the idea that the 1mm but not the 2mm thrust stretched the spindle apparatus in its small signal range. On the other hand, secondary afferents also showed more velocity sensitivity to the 1mm compared with the 2mm thrust despite being considered less sensitive to small signal changes (47). Consequently, factors other than or in addition to the spindle's small signaling range may contribute to their increased velocity sensitivity at smaller amplitude manipulations. Whatever the underlying mechanism were, impulse loads simulating a spinal manipulation produced distinctive effects related to both thrust duration and amplitude. Clinically, these parameters of an HVLA-SM may be important determinants of its therapeutic benefit.
The mechanisms underlying the effects of spinal manipulation are often thought to include neural changes, yet little is known regarding its direct effects on the nervous system (32). Based upon the present study we suggest that one effect of spinal manipulation is the generation of a high frequency discharge in paraspinal primary sensory afferents and we speculate that this discharge may give rise to both immediate and longer-lasting neural consequences. Nearly 2 decades ago several laboratories (56;56-58) showed that synaptic efficacy is affected by the history of high frequency bursting from group Ia and group II muscle afferents whose effect lasts beyond the duration of the burst itself. In α-motoneurons, bursts of action potentials with short interspike intervals affect the magnitude of post-synaptic potentials differently from longer interspike intervals. α-Motoneurons are bistable and can sustain plateau potentials. Brief periods of excitation can switch them into a period of self-sustained firing (59) with apparent consequences for the normal production of muscle force (60). Similarly, high frequency stimulation of smaller diameter A-δ and C-fibers also affects spinal synaptic transmission. Both long-term potentiation as well as depression may be produced (61). The effects can last up to 1 hour after the initial sensory barrage (61;62). These considerations highlight the need for further investigation into the effects of spinal manipulation on both primary afferents as well as higher order central neurons.
In summary, we studied the effect of spinal impulse loading on neural activity from lumbar paraspinal muscle spindles. Distinctive neural responses were observed which related to both the impulse thrust's duration and its displacement amplitude. Short duration thrusts simulating a high velocity, low amplitude spinal manipulation produced a higher frequency discharge compared with longer thrust durations. The relationship between thrust duration and neural discharge was curvilinear with a concave-up inflection occurring near the 50 to 100 ms duration. This pattern was evident in muscle spindles located in both medial and lateral paraspinal muscles and in all 3 types of spindle afferents: primary, secondary and intermediate. Paradoxically, spindle afferents were almost twice as sensitive to a 1 mm compared to a 2 mm displacement amplitude. The high velocity and low amplitude characteristics of a spinal manipulation appear to take advantage of inherent signaling properties of muscle spindles including their high sensitivity to very rapid changes in length and their non-linear response to small stretches. The data suggest that, in the clinical practice of spinal manipulation, control of both thrust duration and thrust amplitude are determinants of the level to which paraspinal muscle spindles are activated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dr. Joel G. Pickar, Palmer Center Chiropractic Research Davenport, IA UNITED STATES.
Paul S. Sung, Palmer Center for Chiropractic Research, and University of Iowa, drpsung@yahoo.com.
Yu-Ming Kang, Palmer Center for Chiropractic Research, and University of Iowa, ykang36@yahoo.com.
Weiqing Ge, Palmer College of Chiropractic, Palmer Center for Chiropractic Research, Davenport, IA, Weiqing.Ge@palmer.edu.
REFERENCE LIST
- 1.National Center for Complementary and Alternative Medicine. Expanding horizons of health care, strategic plan 2005-2009. NIH Publication; 2004. #04-5568- [Google Scholar]
- 2.Bronfort G, Haas M, Evans RL, Bouter LM. Efficacy of spinal manipulation and mobilization for low back pain and neck pain: a systematic review and best evidence synthesis. Spine J. 2004;4(3):335–356. doi: 10.1016/j.spinee.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Shekelle PG. A critical appraisal of the use of medications and spinal manipulation for idiopathic low back pain. In: Weinstein JN, Gordon SL, editors. Low Back Pain, A Scientific and Clinical Overview. Rosemont: American Academy of Orthopaedic Surgeons; 1996. pp. 439–446. [Google Scholar]
- 4.Bigos S, Bowyer O, Braen G, et al. Acute low-back problems in adults. 1994. AHCPR Pub No 95-0643- [Google Scholar]
- 5.Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, Van Rompay M, Kessler RC. Trends in alternative medicine use in the United States, 1990-1997: Results of a follow-up national survery. Journal of the American Medical Assocationof. 1998;280(18):1569–1575. doi: 10.1001/jama.280.18.1569. [DOI] [PubMed] [Google Scholar]
- 6.Shekelle PG, Adams AH, Chassin MR, Hurwitz EL, Brook RH. Spinal manipulation for low-back pain. Ann Intern Med. 1992;117(7):590–598. doi: 10.7326/0003-4819-117-7-590. [DOI] [PubMed] [Google Scholar]
- 7.Terrett ACJ, Vernon HT. Manipulation and pain tolerance: a controlled study of the effect of spinal manipulation on paraspinal cutaneous pain tolerance levels. Am J Phys Med. 1984;63(5):217–225. [PubMed] [Google Scholar]
- 8.Vernon HT. Pressure pain threshold evaluation of the effect of spinal manipulation on chronic neck pain: a single case study. J Can Chiro Assoc. 1988;32(4):191–194. [Google Scholar]
- 9.Song XJ, Gan Q, Cao JL, Wang ZB, Rupert RL. Spinal manipulation reduces pain and hyperalgesia after lumbar intervertebral foramen inflammation in the rat. J Manipulative Physiol Ther. 2006;29(1):5–13. doi: 10.1016/j.jmpt.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Dishman JD, Bulbulian R. Spinal reflex attenuation associated with spinal manipulation. Spine. 2000;25(19):2519–2525. doi: 10.1097/00007632-200010010-00015. [DOI] [PubMed] [Google Scholar]
- 11.Murphy BA, Dawson NJ, Slack JR. Sacroiliac joint manipulation decreases the H-reflex. Electromyogr Clin Neurophysiol. 1995;35:87–94. [PubMed] [Google Scholar]
- 12.Suter E, McMorland G, Herzog W. Short-term effects of spinal manipulation on H-reflex amplitude in healthy and symptomatic subjects. J Manipulative Physiol Ther. 2005;28(9):667–672. doi: 10.1016/j.jmpt.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Suter E, McMorland G, Herzog W, Bray R. Conservative lower back treatment reduces inhibition in knee-extensor muscles: a randomized controlled trial. J Manipulative Physiol Ther. 2000;23(2):76–80. [PubMed] [Google Scholar]
- 14.Keller TS, Colloca CJ. Mechanical force spinal manipulation increases trunk muscle strength assessed by electromyography: a comparative clinical trial. J Manipulative Physiol Ther. 2000;23(9):585–595. doi: 10.1067/mmt.2000.110947. [DOI] [PubMed] [Google Scholar]
- 15.Dishman JD, Ball KA, Burke J. First Prize: Central motor excitability changes after spinal manipulation, a transcranial magnetic stimulation study. J Manipulative Physiol Ther. 2002;25(1):1–9. [PubMed] [Google Scholar]
- 16.Nansel DD, Cremata E, Carlson J, Szlazak M. Effect of unilateral spinal adjustments on goniometrically-assessed cervical lateral-flexion end-range asymmetries in otherwise asymptomatic subjects. J Manipulative Physiol Ther. 1989;12(6):419–427. [PubMed] [Google Scholar]
- 17.Whittingham W, Nilsson N. Active range of motion in the cervical spine increases after spinal manipulation (toggle recoil) J Manipulative Physiol Ther. 2001;24(9):552–555. doi: 10.1067/mmt.2001.118979. [DOI] [PubMed] [Google Scholar]
- 18.Bronfort G, Haas M, Evans RL, Bouter LM. Efficacy of spinal manipulation and mobilization for low back pain and neck pain: a systematic review and best evidence synthesis. Spine J. 2004;4(3):335–356. doi: 10.1016/j.spinee.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Bergmann TF. High-velocity low-amplitude manipulative techniques. In: Haldeman S, Dagenais S, Budgell B, Grunnet-Nilsson N, Hooper PD, Meeker WC, et al., editors. Principles and Practice of Chiropractic. New York: McGraw-Hill; 2005. pp. 755–766. [Google Scholar]
- 20.Herzog W. The mechanical, neuromuscular, and physiological effects produced by spinal manipulation. In: Herzog W, et al., editors. Clinical Biomechanics of Spinal Manipulation. New York: Churchill Livingstone; 2000. pp. 191–207. [Google Scholar]
- 21.Herzog W, Conway PJ, Kawchuk GN, Zhang Y, Hasler EM. Forces exerted during spinal manipulative therapy. Spine. 1993;18(9):1206–1212. doi: 10.1097/00007632-199307000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Hessell BW, Herzog W, Conway PJW, McEwen MC. Experimental measurement of the force exerted during spinal manipulation using the Thompson technique. J Manipulative Physiol Ther. 1990;13(8):448–453. [PubMed] [Google Scholar]
- 23.Triano J, Schultz AB. Loads transmitted during lumbosacral spinal manipulative therapy. Spine. 1997;22(17):1955–1964. doi: 10.1097/00007632-199709010-00003. [DOI] [PubMed] [Google Scholar]
- 24.Triano JJ. Biomechanics of spinal manipulative therapy. Spine J. 2001;1(2):121–130. doi: 10.1016/s1529-9430(01)00007-9. [DOI] [PubMed] [Google Scholar]
- 25.Conway PJW, Herzog W, Zhang Y, Hasler EM, Ladly K. Forces required to cause cavitation during spinal manipulation of the thoracic spine. Clin Biomech. 1993;8:210–214. doi: 10.1016/0268-0033(93)90016-B. [DOI] [PubMed] [Google Scholar]
- 26.Ianuzzi A, Khalsa PS. High loading rate during spinal manipulation produces unique facet joint capsule strain patterns compared with axial rotations. J Manipulative Physiol Ther. 2005;28(9):673–687. doi: 10.1016/j.jmpt.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 27.Smith DB, Fuhr AW, Davis BP. Skin accelerometer displacement and relative bone movement of adjacent vertebrae in response to chiropractic percussion thrusts. J Manipulative Physiol Ther. 1989;12(1):26–37. [PubMed] [Google Scholar]
- 28.Fuhr AW, Smith DC. Accuracy of piezoelectric accelerometers measuring displacement of a spinal adjusting instrument. J Manipulative Physiol Ther. 1986;9(1):15–21. [PubMed] [Google Scholar]
- 29.Nathan M, Keller TS. Measurement and analysis of the in vivo posteroanterior impulse response of the human thoracolumbar spine: a feasibility study. J Manipulative Physiol Ther. 1994;17(7):431–441. [PubMed] [Google Scholar]
- 30.Gal J, Herzog W, Kawchuk G, Conway PJ, Zhang YT. Movements of vertebrae during manipulation thrusts to unembalmed human cadavers. J Manipulative Physiol Ther. 1997;20:30–40. [PubMed] [Google Scholar]
- 31.Cramer GD, Gregerson DM, Knudsen JT, Hubbard BB, Ustas LM, Cantu JA. The effects of side-posture positioning and spinal adjusting on the lumbar Z joints: a randomized controlled trial with sixty-four subjects. Spine. 2002;27(22):2459–2466. doi: 10.1097/00007632-200211150-00008. [DOI] [PubMed] [Google Scholar]
- 32.Pickar JG. Neurophysiological effects of spinal manipulation. Spine J. 2002:2357–371. doi: 10.1016/s1529-9430(02)00400-x. [DOI] [PubMed] [Google Scholar]
- 33.Gillette RG. A speculative argument for the coactivation of diverse somatic receptor populations by forceful chiropractic adjustments. Manual Med. 1987;3:1–14. [Google Scholar]
- 34.Pickar JG, Kang YM. Paraspinal muscle spindle responses to the duration of a spinal manipulation under force control. J Manipulative Physiol Ther. 2006;29(1):22–31. doi: 10.1016/j.jmpt.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 35.Sung PS, Kang YM, Pickar JG. Effect of spinal manipulation duration on low threshold mechanoreceptors in lumbar paraspinal muscles: a preliminary report. Spine. 2005;30(1):115–122. doi: 10.1097/01.brs.0000147800.88242.48. [DOI] [PubMed] [Google Scholar]
- 36.Pickar JG. An in vivo preparation for investigating neural responses to controlled loading of a lumbar vertebra in the anesthetized cat. J Neurosci Methods. 1999;89:87–96. doi: 10.1016/s0165-0270(99)00060-6. [DOI] [PubMed] [Google Scholar]
- 37.Collins JG, Kendig JJ, Mason P. Anesthetic actions within the spinal cord: contributions to the state of general anesthesia. Trends Neurosci. 1995;18(12):549–553. doi: 10.1016/0166-2236(95)98377-b. [DOI] [PubMed] [Google Scholar]
- 38.Bogduk N. The dorsal lumbar muscles of the cat. Acta Anz, Jena. 1980;148:55–67. [PubMed] [Google Scholar]
- 39.Scott JJ. Classification of muscle spindle afferents in the peroneus brevis muscle of the cat. Brain Res. 1990;509(1):62–70. doi: 10.1016/0006-8993(90)90309-y. [DOI] [PubMed] [Google Scholar]
- 40.Bogduk N. The innervation of the lumbar spine. Spine. 1983;8:286–293. doi: 10.1097/00007632-198304000-00009. [DOI] [PubMed] [Google Scholar]
- 41.Crowder MJ. Analysis of repeated measures. London: Chapman and Hall; 1990. [Google Scholar]
- 42.Corse MR, Renberg WC, Friis EA. In vitro evaluation of biomechanical effects of multiple hemilaminectomies on the canine lumbar vertebral column. Am J Vet Res. 2003;64(9):1139–1145. doi: 10.2460/ajvr.2003.64.1139. [DOI] [PubMed] [Google Scholar]
- 43.Henderson CNR, DeVocht JW, Kirstukas SJ, Cramer GD. In vivo biomechanical assessment of a small animal model of the vertebral subluxation. Proceedings of the 2000 International Conference on Spinal Manipulation; 2000. pp. 193–195. [Google Scholar]
- 44.Lee M, Liversidge K. Posteroanterior stiffness at three locations in the lumbar spine. J Manipulative Physiol Ther. 1994;17(8):511–516. [PubMed] [Google Scholar]
- 45.Viner A, Lee M, Adams R. Posteroanterior stiffness in the lumbosacral spine. The correlation between adjacent vertebral levels. Spine. 1997;22(23):2724–2730. doi: 10.1097/00007632-199712010-00004. [DOI] [PubMed] [Google Scholar]
- 46.Keller TS, Colloca CJ. Dynamic dorsoventral stiffness assessment of the ovine lumbar spine. J Biomech. 2005 Dec 21; doi: 10.1016/j.jbiomech.2005.10.037. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 47.Matthews PBC, Stein RB. The sensitivity of muscle spindle afferents to small sinusoidal changes of length. J Physiol. 1969:200723–743. doi: 10.1113/jphysiol.1969.sp008719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matthews PBC. The response of de-efferented muscle spindle receptors to stretching at different velocities. J Physiol. 1963;168:660–678. doi: 10.1113/jphysiol.1963.sp007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hasan Z, Houk JC. Transition in sensitivity of spindle receptors that occurs when muscle is stretched more than a fraction of a millimeter. J Neurophysiol. 1975;38(3):673–689. doi: 10.1152/jn.1975.38.3.673. [DOI] [PubMed] [Google Scholar]
- 50.Pickar JG, Wheeler JD. Response of muscle proprioceptors to spinal manipulative-like loads in the anesthetized cat. J Manipulative Physiol Ther. 2001;24(1):2–11. doi: 10.1067/mmt.2001.112017. [DOI] [PubMed] [Google Scholar]
- 51.Ge W, Khalsa PS. Encoding of compressive stress during indentation by slowly adapting type I mechanoreceptors in rat hairy skin. J Neurophysiol. 2002;87(4):1686–1693. doi: 10.1152/jn.00414.2001. [DOI] [PubMed] [Google Scholar]
- 52.Ge W, Khalsa PS. Encoding of compressive stress during indentation by group III and IV muscle mechano-nociceptors in rat gracilis muscle. J Neurophysiol. 2003;89(2):785–792. doi: 10.1152/jn.00624.2002. [DOI] [PubMed] [Google Scholar]
- 53.Banks RW, Barker D, Stacey MJ. Form and distribution of sensory terminals in cat hindlimb muscle spindles. Philos Trans R Soc Lond B Biol Sci. 1982;299(1096):329–364. doi: 10.1098/rstb.1982.0136. [DOI] [PubMed] [Google Scholar]
- 54.Hunt CC. Relation of function to diameter in afferent fibers of muscle nerves. J Gen Physiol. 1954;38:448–451. doi: 10.1085/jgp.38.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Richmond FJR, Abrahams VC. Physiological properties of muscle spindles in dorsal neck muscles of the cat. J Neurophysiol. 1979;42(2):604–615. doi: 10.1152/jn.1979.42.2.604. [DOI] [PubMed] [Google Scholar]
- 56.Davis BM, Collins WF, Mendell LM. Potentiation of transmission at Ia-motoneuron connections induced by repeated short bursts of afferent activity. J Neurophysiol. 1985;54(6):1541–1552. doi: 10.1152/jn.1985.54.6.1541. [DOI] [PubMed] [Google Scholar]
- 57.Luscher HR, Ruenzel PW, Henneman E. Effects of impulse frequency, PTP, and temperature on responses elicited in large populations of mononeurons by impulses in single Ia-fibers. J Neurophysiol. 1983;50(5):1045–1058. doi: 10.1152/jn.1983.50.5.1045. [DOI] [PubMed] [Google Scholar]
- 58.Collins WF, III, Honig MG, Mendell LM. Heterogeneity of group Ia synapses on homonymous alpha-motoneurons as revealed by high-frequency stimulation of Ia afferent fibers. J Neurophysiol. 1984;52(5):980–993. doi: 10.1152/jn.1984.52.5.980. [DOI] [PubMed] [Google Scholar]
- 59.Hounsgaard J, Hultborn H, Kiehn O. Transmitter-controlled properties of alpha-motoneurones causing long-lasting motor discharge to brief excitatory inputs. Prog Brain Res. 1986;64:39–49. doi: 10.1016/S0079-6123(08)63398-1. [DOI] [PubMed] [Google Scholar]
- 60.Collins DF, Burke D, Gandevia SC. Sustained contractions produced by plateau-like behaviour in human motoneurones. J Physiol. 2002;538(Pt 1):289–301. doi: 10.1113/jphysiol.2001.012825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Randic M, Jiang MC, Cerne R. Long-term potentiation and long-term depression of primary afferent neurotransmission in the rat spinal cord. J Neurosci. 1993;13(12):5228–5241. doi: 10.1523/JNEUROSCI.13-12-05228.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ikeda H, Asai T, Murase K. Robust changes of afferent-induced excitation in the rat spinal dorsal horn after conditioning high-frequency stimulation. J Neurophysiol. 2000;83:2412–2420. doi: 10.1152/jn.2000.83.4.2412. [DOI] [PubMed] [Google Scholar]