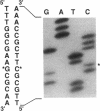
Abstract
The formation of 2-succinyl-6-hydroxy-2,4-cyclohexadiene-1-carboxylic acid (SHCHC), the first identified intermediate in the menaquinone biosynthetic pathway, requires two reactions. They are the decarboxylation of alpha-ketoglutarate by an alpha-ketoglutarate decarboxylase, which results in the formation of succinic semialdehyde-thiamine PPi (TPP) anion, and the addition of the succinic semialdehyde-TPP anion to isochorismate carried out by the enzyme SHCHC synthase. Evidence is provided to support the conclusion that both enzymatic activities are encoded by an extended menD gene which is capable of generating a bifunctional 69-kDa protein. Consistent with the requirement for TPP in the decarboxylation of alpha-ketoglutarate, the translated amino acid sequence contains the characteristic TPP-binding motif present in all well-characterized TPP-requiring enzymes.
Full text
PDF
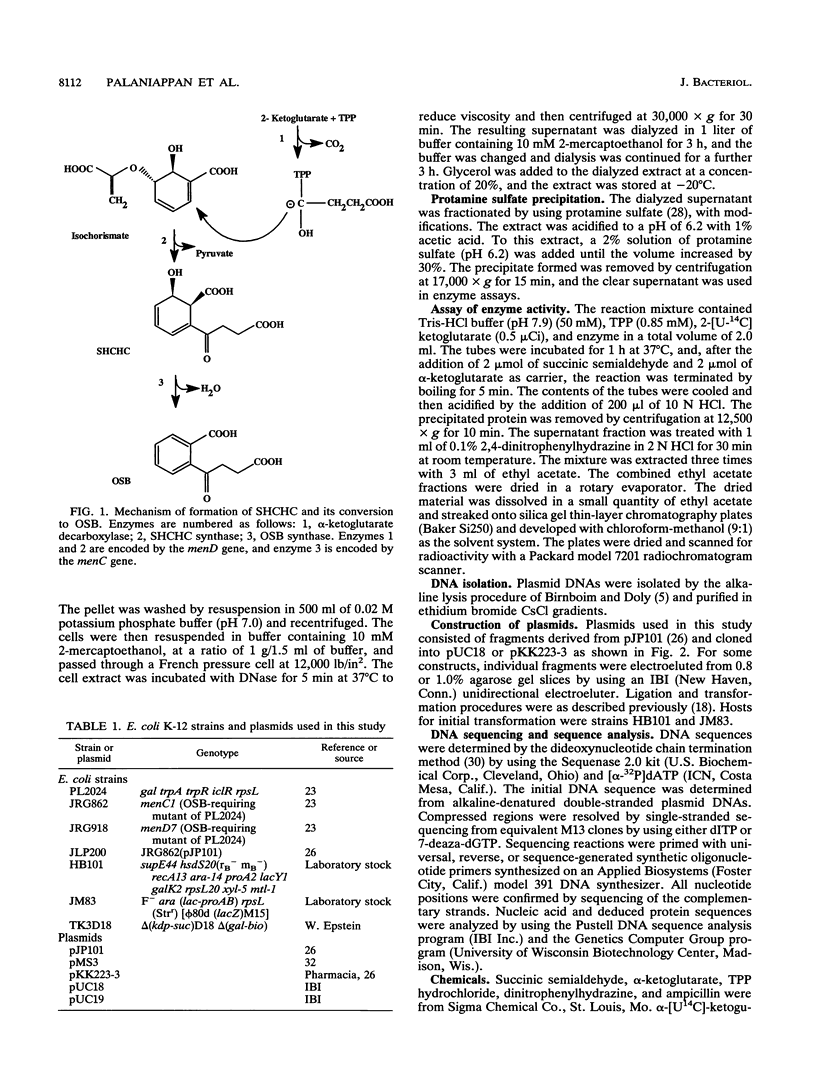
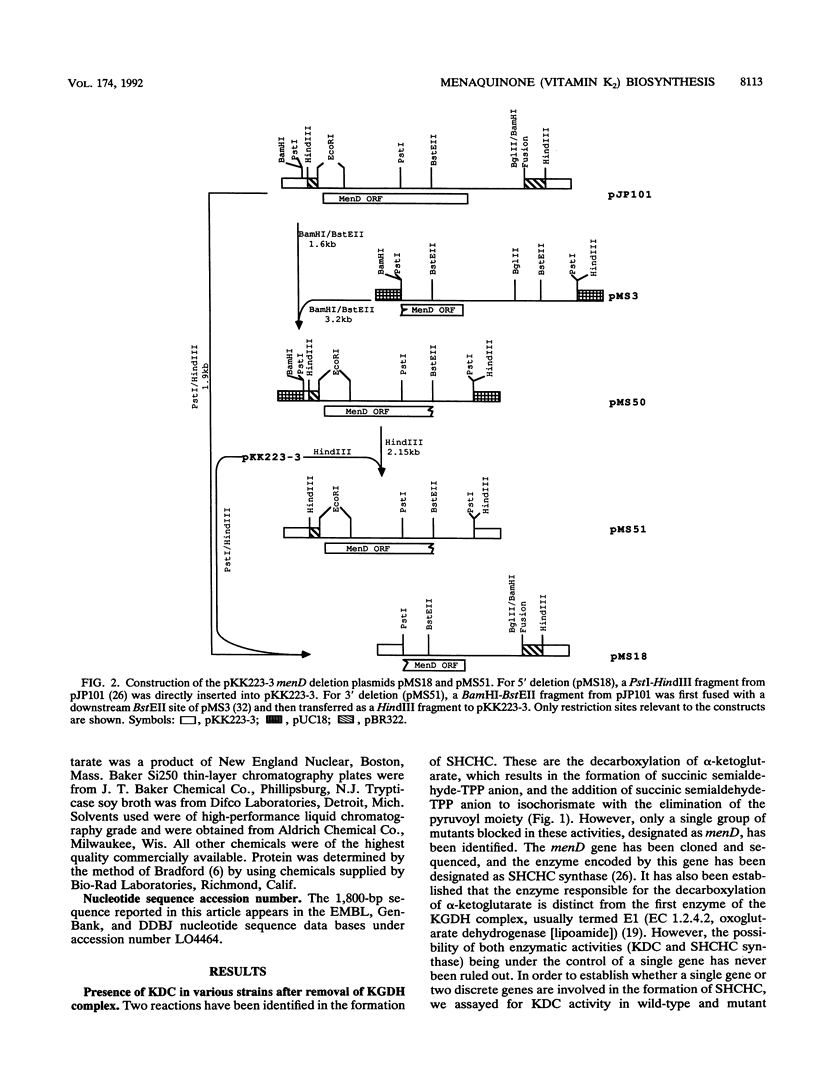
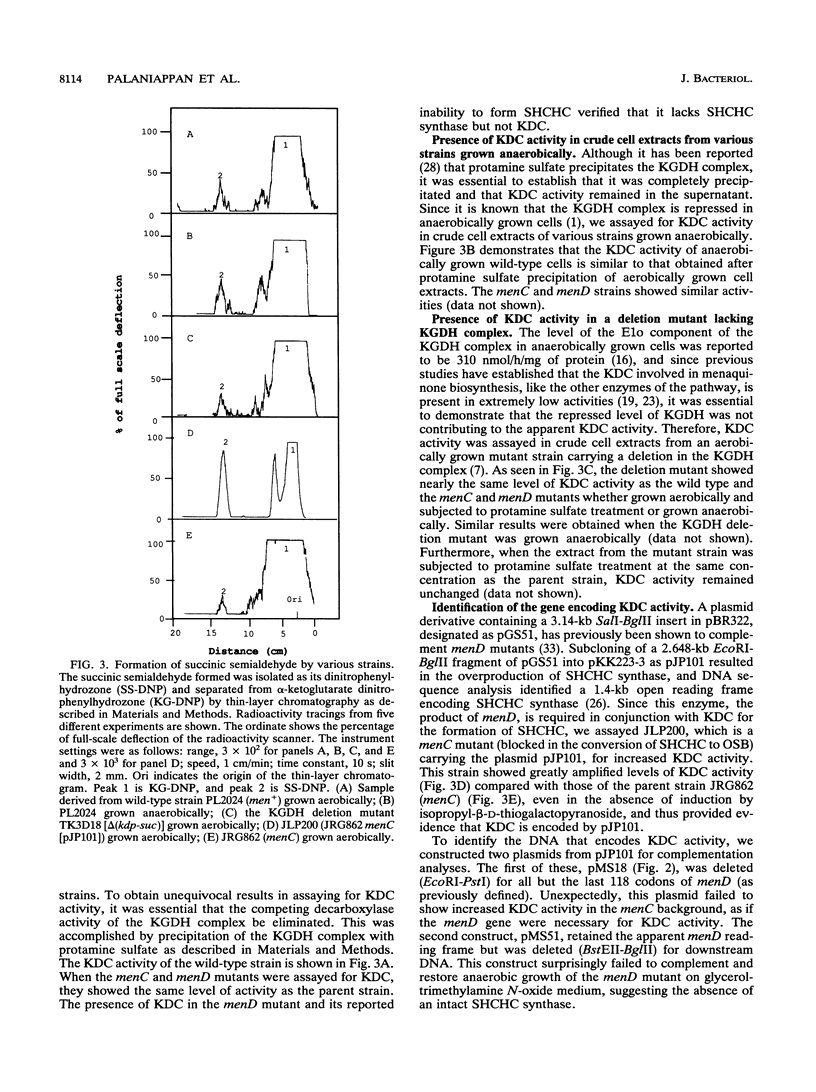
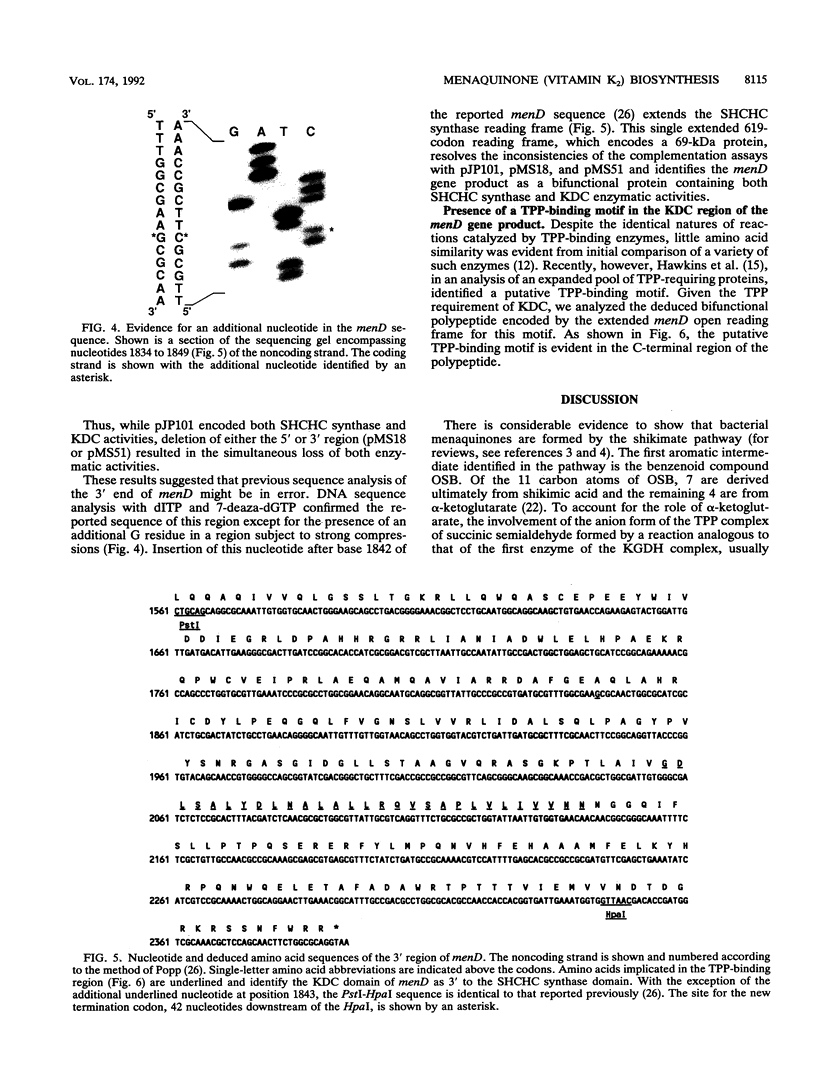



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amarasingham C. R., Davis B. D. Regulation of alpha-ketoglutarate dehydrogenase formation in Escherichia coli. J Biol Chem. 1965 Sep;240(9):3664–3668. [PubMed] [Google Scholar]
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Bentley R., Meganathan R. Biosynthesis of vitamin K (menaquinone) in bacteria. Microbiol Rev. 1982 Sep;46(3):241–280. doi: 10.1128/mr.46.3.241-280.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buck D., Spencer M. E., Guest J. R. Cloning and expression of the succinyl-CoA synthetase genes of Escherichia coli K12. J Gen Microbiol. 1986 Jun;132(6):1753–1762. doi: 10.1099/00221287-132-6-1753. [DOI] [PubMed] [Google Scholar]
- COX G. B., GIBSON F. BIOSYNTHESIS OF VITAMIN K AND UBIQUINONE. RELATION TO THE SHIKIMIC ACID PATHWAY IN ESCHERICHIA COLI. Biochim Biophys Acta. 1964 Oct 9;93:204–206. doi: 10.1016/0304-4165(64)90285-5. [DOI] [PubMed] [Google Scholar]
- Campbell I. M., Coscia C. J., Kelsey M., Bentley R. Origin of the aromatic nucleus in bacterial menaquinones. Biochem Biophys Res Commun. 1967 Jul 10;28(1):25–29. doi: 10.1016/0006-291x(67)90400-7. [DOI] [PubMed] [Google Scholar]
- Dansette P., Azerad R. A new intermediate in naphthoquinone and menaquinone biosynthesis. Biochem Biophys Res Commun. 1970 Sep 10;40(5):1090–1095. doi: 10.1016/0006-291x(70)90906-x. [DOI] [PubMed] [Google Scholar]
- Darlison M. G., Spencer M. E., Guest J. R. Nucleotide sequence of the sucA gene encoding the 2-oxoglutarate dehydrogenase of Escherichia coli K12. Eur J Biochem. 1984 Jun 1;141(2):351–359. doi: 10.1111/j.1432-1033.1984.tb08199.x. [DOI] [PubMed] [Google Scholar]
- Emmons G. T., Campbell I. M., Bentley R. Vitamin K (menaquinone) biosynthesis in bacteria: purification and probable structure of an intermediate prior to o-succinylbenzoate. Biochem Biophys Res Commun. 1985 Sep 16;131(2):956–960. doi: 10.1016/0006-291x(85)91332-4. [DOI] [PubMed] [Google Scholar]
- Guest J. R. Anaerobic growth of Escherichia coli K12 with fumarate as terminal electron acceptor. Genetic studies with menaquinone and fluoroacetate-resistant mutants. J Gen Microbiol. 1979 Dec;115(2):259–271. doi: 10.1099/00221287-115-2-259. [DOI] [PubMed] [Google Scholar]
- Hawkins C. F., Borges A., Perham R. N. A common structural motif in thiamin pyrophosphate-binding enzymes. FEBS Lett. 1989 Sep 11;255(1):77–82. doi: 10.1016/0014-5793(89)81064-6. [DOI] [PubMed] [Google Scholar]
- Langley D., Guest J. R. Biochemical genetics of the alpha-keto acid dehydrogenase complexes of Escherichia coli K12: genetic characterization and regulatory properties of deletion mutants. J Gen Microbiol. 1978 May;106(1):103–117. doi: 10.1099/00221287-106-1-103. [DOI] [PubMed] [Google Scholar]
- Marley M. G., Meganathan R., Bentley R. Menaquinone (vitamin K2) biosynthesis in Escherichia coli: synthesis of o-succinylbenzoate does not require the decarboxylase activity of the ketoglutarate dehydrogenase complex. Biochemistry. 1986 Mar 25;25(6):1304–1307. doi: 10.1021/bi00354a017. [DOI] [PubMed] [Google Scholar]
- Meganathan R., Bentley R. Biosynthesis of o-succinylbenzoic acid in a men- Escherichia coli mutant requires decarboxylation of L-glutamate at the C-1 position. Biochemistry. 1981 Sep 1;20(18):5336–5340. doi: 10.1021/bi00521a038. [DOI] [PubMed] [Google Scholar]
- Meganathan R., Bentley R. Thiamine pyrophosphate requirement for o-succinylbenzoic acid synthesis in Escherichia coli and evidence for an intermediate. J Bacteriol. 1983 Feb;153(2):739–746. doi: 10.1128/jb.153.2.739-746.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meganathan R. Enzymes from Escherichia coli synthesize o-succinylbenzoic acid, an intermediate in menaquinone (vitamin K2) biosynthesis. J Biol Chem. 1981 Sep 25;256(18):9386–9388. [PubMed] [Google Scholar]
- Meganathan R., Schrementi J. Tetrahydrothiophene 1-oxide as an electron acceptor for Escherichia coli. J Bacteriol. 1987 Jun;169(6):2862–2865. doi: 10.1128/jb.169.6.2862-2865.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp J. L., Berliner C., Bentley R. Vitamin K (menaquinone) biosynthesis in bacteria: high-performance liquid chromatographic assay of the overall synthesis of o-succinylbenzoic acid and of 2-succinyl-6-hydroxy-2,4-cyclohexadiene-1-carboxylic acid synthase. Anal Biochem. 1989 May 1;178(2):306–310. doi: 10.1016/0003-2697(89)90643-x. [DOI] [PubMed] [Google Scholar]
- Popp J. L. Sequence and overexpression of the menD gene from Escherichia coli. J Bacteriol. 1989 Aug;171(8):4349–4354. doi: 10.1128/jb.171.8.4349-4354.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins D. J., Campbell I. M., Bentley R. Glutamate--a precusor for the naphthalene nucleus of bacterial menaquinones. Biochem Biophys Res Commun. 1970;39(6):1081–1086. doi: 10.1016/0006-291x(70)90669-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V., Suvarna K., Meganathan R., Hudspeth M. E. Menaquinone (vitamin K2) biosynthesis: nucleotide sequence and expression of the menB gene from Escherichia coli. J Bacteriol. 1992 Aug;174(15):5057–5062. doi: 10.1128/jb.174.15.5057-5062.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weische A., Johanni M., Leistner E. Biosynthesis of o-succinylbenzoic acid. I: Cell free synthesis of o-succinylbenzoic acid from isochorismic acid in enzyme preparations from vitamin K producing bacteria. Arch Biochem Biophys. 1987 Jul;256(1):212–222. doi: 10.1016/0003-9861(87)90439-5. [DOI] [PubMed] [Google Scholar]
- Wookey P. J., Pittard A. J. DNA sequence of the gene (tyrP) encoding the tyrosine-specific transport system of Escherichia coli. J Bacteriol. 1988 Oct;170(10):4946–4949. doi: 10.1128/jb.170.10.4946-4949.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]