Abstract
Stem cell transplantation represents a critical approach for the treatment of many malignant and non-malignant diseases. The foundation for these approaches is the ability to cryopreserve marrow cells for future use. This technique is routinely employed in all autologous settings and is critical for cord blood transplantation. A variety of cryopreservatives have been used with multiple freezing and thawing techniques as outlined in the later chapters. Freezing efficiency has been proven repeatedly and the ability of long-term stored marrow to repopulate has been established. Standard approaches outlined here are used in many labs as the field continues to evolve.
Keywords: stem cell, marrow, cryopreservative, freezing methods
INTRODUCTION
The role of hematopoietic stem cell transplantation in the treatment of hematologic and nonhematologic malignancies is rapidly expanding. In certain situations fresh stem cells can be employed in the setting of allogeneic transplantation. If the transfer from donor to recipient can be established within 72 hr, protocols for preliminary storage at suprafreezing temperatures are in place. However, the current therapeutic strategies demand that the progenitor cells are cryopreserved for virtually all autologous and many allogeneic transplants. This strategy has been proven to be safe and not associated with significant adverse outcomes regarding failure to engraft, graft versus host disease (GVHD), or engraftment failure [1].
The cryopreservation process is of importance for all types of stem cell collection, but is perhaps particularly critical for umbilical cord blood (UCB). The actual transplant is here harvested at the time of birth and used at a later point in time for a yet, at the time point of the harvest, often indeterminate recipient. The UCB is usually stored by either public or private cord blood banks. Public cord blood banks are usually nonprofit organizations, which offer the donor unit to matching recipients via national or international registries to potential recipients in need [2]. Cord blood banks store a donor specimen for the donor or in the case of public banks for an unknown recipient for an indeterminate time span. There are now about 170,000 frozen units in 37 cord-blood registries in 21 countries. Two thousand nine hundred units have been transplanted to date, with adults having received about one third of those units [3].
The cryopreservation process entails the following general components:
Harvesting of the donor cells, which entails the actual collection of the specimen and the reduction of bulk.
Addition of cryopreservatives
The actual freezing procedure
Assessment of the viability of the frozen unit after about 72 hr
The thawing procedure
The washing and conditioning of the donor unit prior to transplant
No single cryopreservation method has been universally used. Variations in technique occur between different transplant centers. Our review revealed that slight changes have been observed over the last 15 years [4]. At our institution we use a standardized NIH protocol for the preservation of allogeneic and autologous peripheral hematopoietic stem cell and bone marrow transplant specimens.
We collect Hematopoietic Progenitor Stem Cells in a minimally manipulated fashion as defined by the Foundation for the Accreditation of Hematopoietic Cell Therapy (FAHCT) with a minimal cell dose of at least 2.5 × 106–5.0 × 106 CD34(+) cells/kg body weight, as currently considered standard [5]. The specimen is then centrifuged to develop the cell rich pellet. In autologous transplants donor plasma is used. The supernatant out of this cryoprecipitation process is used for the reliquidification of the pellet cells, after the addition of a solution of heparinized Plasmalyte solution and 10% DMSO (Dimethylsulfoxide). This usually eventuates into a cellular concentration of 500 × 10−6 cells in the cryopreservate. We store the bone marrow or peripheral blood stem cell product at initially −4°C [6]. Then the sample is frozen down to the target temperature of −156°C (when stored in the vapor phase) to −196°C (when stored in the liquid phase), depending on where in the container the specimen is stored. To guarantee the integrity of the donor unit prior to myeloablative therapy, a viability reassessment of the unit is performed using a Trypan Blue assay, and if this is equivocal a propidium iodide assay. Prior to the actual stem cell infusion the sample is rapidly thawed in a 37°C water bath.
Several elements of the cryopreservative procedure are still matter of debate. Algorithms differing in freezing temperature, freezing rate, cryopreservatives, durability, thawing temperature, and thawing rate have been published. This article will review the status of the literature regarding some of those elements [4,7,8].
TEMPERATURE
The temperatures used for the cryopresevation of hemopoeitic stem cells over the past fifteen years has been −196, −156, or −80°C, reflecting the storage temperatures in liquid and vapor phase nitrogen and in cryopreservation mechanical freezers, respectively. The development of the use of cryopreservative temperatures was from lower temperatures of around −196°C in the 1980s to around −80°C in the 1990s [2,9–16]. For umbilical cord isolated stem cells similar trends are observed. The standard temperatures currently in use are −196 to −80°C [17–19]. Secondary to the recent reports about the spread of infectious agents (i.e., aspergillus as well as viral spread), through the liquid phase of the nitrogen tanks, the currently recommended optimal storage conditions are in the vapor nitrogen phase, at −156°C. Mechanical freezers might represent a viable alternative.
Also, several studies examined the possibility to store HSCs at suprafreezing temperature, at 4°C. A preclinical study that examined PBSCs mobilized in autologous plasma with post-storage clonogenic and viability assays suggested that a storage up to five days is safe [20]. A small case series by Ruiz-Arguelles et al. successfully used PBPCs after 96 hr storage at 4°C for rescue after high dose chemotherapy [21].
FREEZING RATE
The rate of freezing was widely debated in the literature. The controlled rate freezing technique is still considered standard, mainly due to the fact that the heat liberation at the transition or eutactic point (about 4°C) is deemed detrimental to the stem cell population. At this point the water molecules within the frozen unit are in a precise molecular order, what eventuates in the thermodynamic liberation of fusion heat.
In controlled rate freezing, the concentrated stem cells are frozen down at a rate of 1–2°C/min up to a temperature point of about −40°C. Then, the freezing process down to a target of −120°C is performed at a faster pace, about 3–5°C/min. For umbilical cord stem cells, bone marrow, and PBSCs the controlled rate freezing process is considered standard [22–24], and was in different reports found to be superior to uncontrolled freezing approaches. This procedure is time consuming and requires staff with a specific expertise. Hence, the use of uncontrolled rate freezing in which the specimen is first cooled down to −4°C and then directly deposited into a freezer at −80°C or put into liquid phase nitrogen has been evaluated. Several reports [9,12,13] established that the uncontrolled method is safe and reveals comparable results to the controlled rate process for BM and PBSCs. A controlled study performed by Perez-Oteyza et al. [14] showed that the controlled and uncontrolled rate freezing approach are comparable in terms of viability testing and that only a statistically significant decrease in the CFU-GM clonality assay could be detected in the uncontrolled freezing situation. Recent studies suggested that uncontrolled freezing is also a viable approach for UCB stem cells [25,26]. No consensus exists about the relevance of the compensation for fusion heat during the freezing procedure [6]. However, a study of Balint et al. outlined the importance of this intervention, comparing five different freezing protocols [6]. The protocols using a five step controlled freezing approach compensating for the fusion heat achieved better post thawing viability.
DURABILITY
The actual durability, defined as the time that stem cells can be preserved, is still unclear. The viability of the stem cells in cryopreservation has been questioned in different studies after a time course of cryopreservation of 6 months [10]. Further studies have demonstrated a prolonged freezing time with complete preservation of stem cell function.
Several substitute assays are used to estimate the functional hematopoietic reconstitutive capacity of the first frozen and then thawed specimens. While BFU-E and CFU-GM appear to be compromised earlier in the course of cryopreservation, the recovery of nucleated cells (NC) and CD34+ cells and the actual engraftment in NOD/SCID mice seems to be preserved for a longer period of time [2,9,12,14 15,27,28]. Those observations have been initially made in bone marrow and PBSC, and similar observations have been made with UBC stem cells [18,29–33]. The NOD/SCID mouse assay is currently considered the most valuable assay to assess the functionality for hematologic recovery of HSC preparations [34–37], but is not routinely practical. After Kobylka et al. [38] and Mugishima et al. [39] proved the durability after 12 and 15 years with flow cytometric and clonogenic assays, respectively, and Broxmeyer et al. [30] performed a reassessment of his long-term preserved CB units, a durability of up to 15 years was established by using hematopoietic reconstitution in NOD/SCID mice. Clinical validity of preclinical studies was documented in anecdotal reports when successful trilineage engraftment was achieved with BM, stored for 7 years [26]. A systematic review evaluating the combined experience of the Brigham and Women’s Hospital and the EBMT Group [11] noticed that HSC can be effectively cryopreserved for up to 11 years. A retrospective study from Seattle revealed full trilineage recovery in patients receiving HSC, stored for up to 7.8 years without consistent detrimental effects [27].
CELL CONCENTRATION
Reinfusion of cryopreserved cells has been associated with varying toxicities, which were partially attributed to the total volume and the cryopreservatives in the solution [7,8,40]. Concern was raised in the past that a high cell concentration in the cryopreservate can eventuate in toxicity to the cells. Hence, the initially proposed concentration of cryopreserved cells was suggested to be not over 2 × 10−7/ml NC [11,41]. This would eventuate in a cryopreservation volume of about 7 l [10] per patient. The storage space needed and the labor to wash the graft prior to reinfusion would be immense [4].
After initial murine models were found safe, Rowley et al. established that high cell concentrations (up to 5.6 × 10−8 cells/ml) in the cryopreservate are well tolerated, not associated with significant adverse effect to the cells, and resulted in good clinical outcomes [4]. Similar conclusions were drawn from subsequent studies by Kawano et al. [42] and Cabezudo et al. [43]. For practical purposes a cell concentration of 200 × 10−6/ml appears achievable [4,44,45].
CRYOPRESERVATIVES
Cryopreservatives are necessary additives to stem cell concentrates, since they inhibit the formation of intra and extracellular crystals and hence cell death. The standard cryoprotectant is DMSO, which prevents freezing damage to living cells [46]. It was initially introduced into medical use as an anti-inflammatory reagent and is still occasionally used in auto-immune disorders [47,48]. Usually it is used at concentrations of 10% combined with normal saline and serum albumin [2,9,13,49]. This was established to be a safe and non-stem cell toxic agent [49]. However, DMSO is associated with a clinically significant side effect profile. Nausea, vomiting, and abdominal cramps occur in about half of all the cases [50]. Other side effects encompass cardiovascular [7], respiratory [51,52], CNS [8,53–55], renal, hemolytic [56], and hepatotoxic presentations. Case fatalities attributed to DMSO toxicities have been reported [57].
A recent multinational questionnaire based survey, including data from 97 EBMT transplant centers, revealed that DMSO related toxicities other than nausea and vomiting are observed in about one out of 50 transplants with a mean incidence of 2.2% of all administered units. Cardiovascular side effects were the most frequently observed group of adverse events witnessed in 27% of the participating centers. Respiratory events were observed in 17%, CNS toxicities, including seizures, in 5%, and renal adverse effects in 5% [58].
Based on these toxicity considerations, newer approaches have been tried. Lower dosages of DMSO, varying from 2.2 to 6% [9,10,12,59,60] have been established to be efficacious in bone marrow and PBSCs as well as for UCB. On the contrary, a Yugoslavian study compared the 10% DMSO concentration to lower concentrations with different freezing rates. The 10% DMSO cryopreservation proved to be superior to lower concentrations in this in vitro study [6]. To enhance the effect of the cryopreservative, the combination of DMSO and the extracellular cryoprotectant hydroxyethyl starch (HES) has been used with success in PBSCs and bone marrow grafts [12,13] and UCB cells.
Alternative preservation methods for cryopreservation are propylene glycol, a combination of alpha tocopherol, catalase, and ascorbic acid and the glucose dimer trehalose as intra and extracellular cryoprotectant [28,32,61].
Interesting preclinical data from Germany suggests that activation of caspases, particularly during the thawing process, can induce apoptosis and hence contribute to the cryoinjury to transplant grafts. Addition of the caspase inhibitor zVAD-fmk as cryopreservative presents an intriguing future perspective [62].
THAWING
Several techniques for the thawing procedure have been proposed. The standard method is warming in a water bath at 37°C until all ice crystals disappear [13]. A German study compared the thawing of cryopreserved units in a warm water bath with dry heat applied by gel pads at 37°C. The viability and clonogenic potential were comparable, with a trend towards less infectious contamination in the dry method [63]. Different studies examined the preservation of function when thawed units were incubated at 0–37°C [13,31]. No significant differences were detected in a study by Yang et al. who compared an incubation of the thawed unit at 0, 20, and 37°C for 20 min [31]. The used cryopreservative proved to be nontoxic to the stem cells during the cryopreservation process, as already established by previous studies [4,49]. Reducing the DMSO content at thawing temperature is an intriguing concept, because of the clinical toxicity profile of this cryopreservative. Hence, the effect of reducing the DMSO content in the thawing solution by virtue of washing or dilution has been explored [59,64]. Minor or no effect on the stem cell viability has been observed. An automated method to wash out the cryopreservative has proven feasible in pre-clinical models [65].
WASHING PROCEDURE
For stem cells of cord, peripheral blood, and bone marrow origin, the process of washing out the cryopreservative after the thawing can still be considered standard [19,32], since the DMSO is assumed to have toxic effect on the stem cells. This has been questioned by several more recent reports, which suggested stem cell resistance against DMSO exposure [13,49]. The wash out of the most popular cryopreservative has conceivable benefits for the recipient, i.e. reduction of toxicity, since the degree of DMSO toxicity is proportional to the amount of DMSO contained in the infused stem cell solution [66]. It was also suggested that wash out of DMSO can enhance engraftment [67]. This has been disputed [68].
The current standard washing protocol follows the New York Blood Center protocol [19], in which the two step dilution of the thawed stem cell unit with 2.5% human serum albumin and 5% dextran 40 is followed by centrifugation at 10°C for 10 min. The supernatant is then removed and HSA and dextran solution is again added twice to a final DMSO concentration of less then 1.7%. The washed solution is infused as soon as possible. Although this procedure has been established to be safe and associated with a reasonable recovery of NC and progenitor assays [69], it is also very labor intensive and not free of cell loss [70,71]. Recently new automated cell washing devices have been introduced with promising results [50,65,72,73].
CONTAINERS FOR STORAGE
The International Society for Cellular Therapy (ISCT) described on its supplier information website for cryocontainers nine different cryostorage container products. Six of them are Ethinyl Vinyl Acetate (EVA) based, usually gamma irradiated.
Trademarks are
Cryocyte/Baxter
CellFlex/Maco Pharma
Cell Freeze™ Charter Medical
Pall Medical Freezing Bag 791-05
Cryostore EVA/Origen Biomedical Inc.
Thermogenesis Corp./Freezing bag 80346-0
Other, not EVA based products are
American Fluoroseal/FEP(Teflon)
Fresenius Hemocare/Hemofreeze(Teflon,Kaplon)
Origen Biomedical Inc./Permalife Bag, FEP/Polyimide
Other approaches have been undertaken. A Czech group published their successful experience with a stainless steel cryopreservation container specifically designed for PBSC [74]. In the US, the most commonly used cryocontainer is an EVA freezing bag (Yang, 2005 [75]), [76].
The use of specific containers, PVC and polyolefin plastic bags and polyethelene cryostorage vials, achieved different results regarding the viability of the stored specimen. PVC and polyolefin bags achieved satisfactory results, while polyethelene cryostorage vials did not in one study [2]. A group from Boston suggested that polyolefin cryobags achieve a longer functional duration than PVC bags.
INFECTIOUS CONSIDERATIONS
Microbial contamination of transplants represents a significant hazard to the severely immunosuppressed recipient. The FDA estimates that seven transplant related deaths per year could be avoided by elimination of infection related to donor cell infusions [77]. The overall demonstrated microbiological contamination rates are 0–4.5% [27,78–83].
The major parts of the cultured bacteria are skin flora and commensal bacteria. The remainder is mainly made up out of enteric bacteria. Of note is that bone marrow derived stem cell cryounits are much more likely to be contaminated by pathogens, which is explained by the harvesting process. The rates of contamination between PBSC and bone marrow differ significantly by up to a factor of sixteen (0.23% for PBSC and 3.8% for BM) [84].
Table I displays the incidence with which different pathogens were cultured from donor units in four different studies addressing the bacterial contamination of stem cell products [78,82–84]:
TABLE I.
Organisms Cultured/Overall Incidence of Positive Cultures
| Organisms cultured | Overall incidence of positive cultures (%) |
|---|---|
| Staph. epidermidis and other coagulase negative Staphylococcus (CNS) | 3–11.7 |
| Propionibacterium acni | 0.6–2.2 |
| Staphylococcus aureus | 0–1.6 |
| Bacillus cereus and other Bacillus spec. | 0.06–0.35 |
| Pseudomonas spec.(aeruginosa, putida and fluoresces) | 0.1–0.8 |
| Corynebacterium spec. | 0–0.3 |
| Aspergillus fumigatus | 0–0.3 |
| Mixed cultures | 0.1–1.6 |
The cryopreservation process was associated with reduction of detectable microorganisms. In one German study the detection of Staphylococcus epidermidis was reduced by an average of 9.3% and Escherichia coli by 18.1% [85]. Also, several studies reported the occurrence of positive cultures post thawing [27,83,86]. This suggests the risk of contamination of the culture bottles (i.e., not induced by the donor graft).
The incidence of severe sepsis upon infusion of stem cells, which cultured positive for commensals and skin flora bacteria, is low and most of the febrile episodes developing after their infusion are treatable with antibiotics [83].
The stability of viruses in liquid nitrogen has been documented [87]. An English source published an epidemic outbreak of hepatitis B in autologous bone marrow recipients [88], which was subsequently linked by nucleotide sequence analysis to another cryopreservative stored in the same container [89]. Subsequent analysis of debris in the liquid nitrogen phase of the same container demonstrated spread of the pathogen via the liquid phase. Similar outbreaks have been reported [90]. To prevent such incidences we store infectious conserves separately and provide protective sleeves around the cryopreservative bags, as reported to be efficacious previously [91].
To prevent infectious complications by the infusion of donor stem cells the following measures should be employed:
Processing in clean areas and scrupulous microbiologic monitoring of all stages of the stem cell preservation procedure according to current standards [92,93].
Detection of microbiologic contamination prior to infusion. This has been successfully done in an automated manner for other blood products [86].
Screening of donors, even in the autologous setting as circulated in regular guidelines [92]. Upon detection of an infectious graft there should be separate or protected storage [91].
EMBRYONIC STEM CELLS
Embryonic stem cells portray a different biologic behavior under cryopreservation. Because of the enormous potential of Embryonic Stem (ES) cells for transplantation therapy, recent studies have evaluated the manner in which these fragile cells are stored. One difficulty with the cryopreservation of these cells is their extreme fragility resulting in poor survival of the cells under standard freezing procedures, usually in the range of 1% [93]. Not only do the cells have a poor yield with the standard freezing protocols, the cells are also induced into differentiation. Ware et al. investigated a method using a very slow controlled rate freezing procedure and 10% DMSO. Along with the very slow freezing rate, a rapid thaw was found to be critical for a successful storage [93]. A study from Wisconsin identified HES (hydroxy ethyl starch) as a valuable cryoadditive during slow rate freezing and vitrification procedures [94]. Some methods have been derived in which the ES cells are frozen in 24-well plates with minimal media and 10% DMSO at −80°C. The importance of rapid thawing by adding minimal media at body temperature was emphasized. In a study by Ure et al., all 227 clones tested grew successfully, although molecular and phenotypic studies were not done in this instance to prove the cell lineage [95]. Adams et al. successfully cryopreserved primary hepatocytes in a University of Wisconsin solution containing FBS and DMSO for 8 months with the preservation of viability and key phenotypic properties [96]. A recent preclinical study by Milosevic et al. highlighted the role for caspase inhibitors as additives to cryopreservative in embryonic murine neural precursors, an approach that was previously undertaken in hematopoietic precursors [62]. A 60–70% viability was achieved by adding the caspase inhibitor zVAD-fmk to different cryopreservatives after five days [99].
One theory as to the cause of cell death is ice crystal formation in the cytoplasm during freezing [98]. The process of vitrification makes attempts of freezing the ES cells while avoiding the ice formation. Reubinoff was the first to implement this method using an open pulled straw vitrification method, which had previously been successful in the cryopreservation of embryos. This procedure, which evaluated the more fragile human ES cells, proved a 100% viability of the ES cell clumps that all generated colonies compared to a 70% recovery post thaw and 16% differentiation using standard methods [99]. The test cells also had normal karyotypes, OCT-4 expression, and developed teratomas in xenografts of SCID mice [100]. Since this experiment, others have looked at closed seal straw vitrification or alternative freezing media and a simplified vitrification method [100,101] that showed similar results.
FUNCTIONAL SUBSTITUTE ASSAYS
The most commonly used clonogenic assays are CFU-Sd12 [6], a murine assay, MRA (CFU-GM) [6,27,32,38,66,102], CFU-GEMM [6,103], BFU-E [27,38,66,103], and the long term culture initiating cell [104] (LTC-IC) assay (Fig. 1). Still, in spite of the availability of these assays to quantitatively and qualitatively assess the clonogenic potential of the hemopoietic cell in suspension, the eventual evaluation of the engraftment potential relies on the evidence of hematopoietic reconstitution in myeloablated mammals.
Fig. 1.
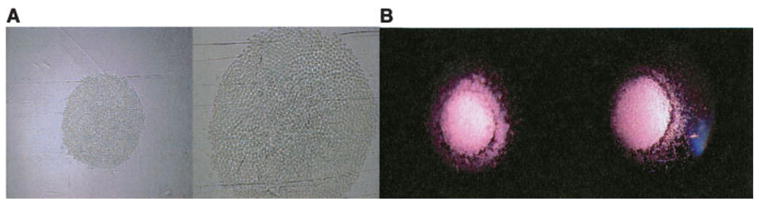
Clonogenic assays. Cells are plated in soft agar and incubated. At set time periods, the cell colonies are counted; this provides an in vitro surrogate of hematopoietic reconstitution potential. (A) CFU/GM (B) CFU/GEMMA.
Different techniques that have been used for human cells are cell counting for NC [32,66,105] and CD34+ cells [61,105], tryptan blue exclusion for viability [94,103], 7-Aminoactinomycin [29,31], engraftment in NOD/SCID mice, and clonogenic assays [27].
Though no absolute consensus is reached as to the optimal method to assess the functionality of a donor graft, several substitute assays have been used to estimate the functional hematopoietic reconstitutive capacity of the first frozen and then thawed specimen. The broad categories of assays used for this purpose are cell counting assays, viability assays, clonogenic experiments, and the engraftment of donor cells in NOD/SCID mice.
| 1. | Cell counting assays | Count of NC [32,66,105]
Flow cytometry of CD34+ cells [61,105] |
| 2. | Viability assays | Tryptan blue [93,103]
7-Aminoactinomycin [29,31] Propidium iodide [106,107] |
| 3. | Clonogenic assays | CFU-Sd12 [6] assay in mice
CFU-GM [6,27,32,38,66,102] CFU-GEMM [6,32] BFU-E [27,38,66,102] LTC-IC [104] |
| 4. | Direct engraftment experiments | NOD/SCID mice [2,9,12,14,15,27] |
While the BFU-E and CFU-GM appear to be compromised earlier in the course of cryopreservation, the recovery of NC and CD 34+ cells and the actual engraftment in NOD/SCID mice seems to be preserved for a longer period of time [2,9,12,14,15,27,28]. These observations have been made in bone marrow and PBSC, and for UBC the same observations were made [18,29–33].
Yang et al. evaluated two different functional assays, the CD 34 count and the CFU-GM, by correlating the pre and post thawing assay outcome with engraftment in 52 patients. The pre and post thawing assay correlated well with each other and with the actual clinical engraftment (Yang et al., 2005, [24]). A general summary of a standard cryopreservation technique is presented in Figure 2.
Fig. 2.
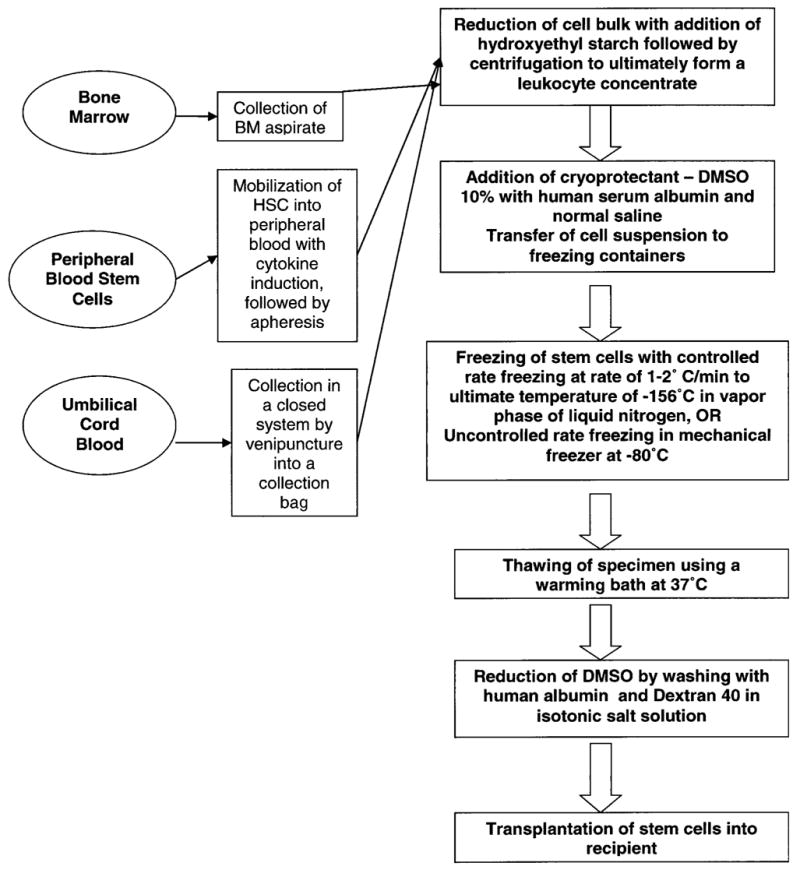
The cryopreservation process for bone marrow, peripheral blood stem cells, and UCB.
Footnotes
Grant sponsor: NIH; Contract grant numbers: 1P20RR018757, 5R01, DK61858, 5KO DK064980.
References
- 1.Stockschlaeder M, Hassan HT, Krog C. Long term follow up of leukemic patients after related cryopreserved bone marrow transplantation. Br J Haematol. 1997;96:382–386. doi: 10.1046/j.1365-2141.1997.d01-2032.x. [DOI] [PubMed] [Google Scholar]
- 2.Valeri CR, Pivacek LE. Effects of the temperature, the duration of frozen storage, and the freezing container on in vitro measurements in human peripheral blood mononuclear cells. Transfusion. 1996;36:303–308. doi: 10.1046/j.1537-2995.1996.36496226141.x. [DOI] [PubMed] [Google Scholar]
- 3.Sanz MA. Cord-blood transplantation in patients with leukemia—a real alternative for adults. N Engl J Med. 2004;351:2328–2330. doi: 10.1056/NEJMe048275. [DOI] [PubMed] [Google Scholar]
- 4.Rowley SD, Bensinger WI, Gooley TA, Buckner CD. Effect of cell concentration on bone marrow and peripheral blood stem cell cryopreservation. Blood. 1994;83:2731–2736. [PubMed] [Google Scholar]
- 5.Rebulla P. Cord blood banking 2002: 112,010 of 7,914,773 chances. Transfusion. 2002;42:1246–1248. doi: 10.1046/j.1537-2995.2002.00256.x. [DOI] [PubMed] [Google Scholar]
- 6.Balint B, Ivanovic Z, Petakov M, et al. The cryopreservation protocol optimal for progenitor recovery is not optimal for preservation of marrow repopulating ability. Bone Marrow Transplant. 1999;23:613–619. doi: 10.1038/sj.bmt.1701623. [DOI] [PubMed] [Google Scholar]
- 7.Davis JM, Rowley SD, Braine HG, Piantadosi S, Santos GW. Clinical toxicity of cryopreserved bone marrow graft infusion. Blood. 1990;75:781–786. [PubMed] [Google Scholar]
- 8.Hoyt R, Szer J, Grigg A. Neurological events associated with the infusion of cryopreserved bone marrow and/or peripheral blood progenitor cells. Bone Marrow Transplant. 2000;25:1285–1287. doi: 10.1038/sj.bmt.1702443. [DOI] [PubMed] [Google Scholar]
- 9.Cilloni D, Garau D, Regazzi E, Sammarelli G, et al. Primitive hematopoietic progenitors within mobilized blood are spared by uncontrolled rate freezing. Bone Marrow Transplant. 1999;23:497–503. doi: 10.1038/sj.bmt.1701601. [DOI] [PubMed] [Google Scholar]
- 10.Galmes A, Besalduch J, Bargay J, et al. Long-term storage at −80°C of hematopoietic progenitor cells with 5% dimethyl sulfoxide as the sole cryoprotectant. Transfusion. 1999;39:70–73. doi: 10.1046/j.1537-2995.1999.39199116897.x. [DOI] [PubMed] [Google Scholar]
- 11.Aird W, Labopin M, Gorin NC, Antin JH. Long-term cryopreservation of human stem cells. Bone Marrow Transplant. 1992;9:487–490. [PubMed] [Google Scholar]
- 12.Halle P, Tournilhac O, Knopinska-Posluszny W, et al. Uncontrolled-rate freezing and storage at −80°C, with only 3.5% DMSO in cryoprotective solution for 109 autologous peripheral blood progenitor cell transplantations. Transfusion. 2001;41:667–673. doi: 10.1046/j.1537-2995.2001.41050667.x. [DOI] [PubMed] [Google Scholar]
- 13.Katayama Y, Yano T, Bessho A, et al. The effects of a simplified method for cryopreservation and thawing procedures on peripheral blood stem cells. Bone Marrow Transplant. 1997;19:283–287. doi: 10.1038/sj.bmt.1700644. [DOI] [PubMed] [Google Scholar]
- 14.Perez-Oteyza J, Bornstein R, Corral M, et al. Controlled-rate versus uncontrolled-rate cryopreservation of peripheral blood progenitor cells: A prospective multicenter study. Group for cryobiology and biology of bone marrow transplantation (CBTNO), Spain. Haematologica. 1998;83:1001–1005. [PubMed] [Google Scholar]
- 15.Matsumoto N, Yoshizawa H, Kagamu H, et al. Successful liquid storage of peripheral blood stem cells at subzero non-freezing temperature. Bone Marrow Transplant. 2002;30:777–784. doi: 10.1038/sj.bmt.1703692. [DOI] [PubMed] [Google Scholar]
- 16.Humpe A, Riggert J, Vehmeyer K, et al. Comparison of CD34+ cell numbers and colony growth before and after cryopreservation of peripheral blood progenitor and stem cell harvests: Influence of prior chemotherapy. Transfusion. 1997;37:1050–1057. doi: 10.1046/j.1537-2995.1997.371098016444.x. [DOI] [PubMed] [Google Scholar]
- 17.Wang SY, Hsu ML, Huang MZ, et al. The activity in ex vivo expansion of cord blood myeloid progenitor cells before and after cryopreservation. Acta Haematol. 2001;105:38–44. doi: 10.1159/000046531. [DOI] [PubMed] [Google Scholar]
- 18.Rubinstein P, Rosenfield RE, Adamson JW, Stevens CE. Stored placental blood for unrelated bone marrow reconstitution. Blood. 1993;81:1679–1690. [PubMed] [Google Scholar]
- 19.Rubinstein P, Dobrila L, Rosenfield RE, et al. Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proc Natl Acad Sci USA. 1995;92:10119–10122. doi: 10.1073/pnas.92.22.10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hechler G, Weide R, Heymanns J, Koppler H, Havemann K. Storage of noncryopreserved periphered blood stem cells for transplantation. Ann Hematol. 1996;72:303–306. doi: 10.1007/s002770050176. [DOI] [PubMed] [Google Scholar]
- 21.Ruiz-Arguelles GJ, Ruiz-Arguelles A, Perez-Romano B, Marin-Lopez A, Larregina-Diez A, Apreza-Molina MG. Filgrastim-mobilized peripheral-blood stem cells can be stored at 4°C and used in autografts to rescue high-dose chemotherapy. Am J Hematol. 1995;48:100–103. doi: 10.1002/ajh.2830480206. [DOI] [PubMed] [Google Scholar]
- 22.Lewis JP, Passovoy M, Conti SA, McFate PA, Trobaugh FE., Jr The effect of cooling regimens on the transplantation potential of marrow. Transfusion. 1967;7:17–32. doi: 10.1111/j.1537-2995.1967.tb04826.x. [DOI] [PubMed] [Google Scholar]
- 23.Meryman HT, Williams RJ, Douglas MS. Freezing injury from “solution effects” and its prevention by natural or artificial cryoprotection. Cryobiology. 1977;14:287–302. doi: 10.1016/0011-2240(77)90177-8. [DOI] [PubMed] [Google Scholar]
- 24.Ketheesan N, Whiteman C, Malczewski AB, Hirst RG, La Brooy JT. Effect of cryopreservation on the immunogenicity of umbilical cord blood cells. Transfus Apher Sci. 2004;30:47–54. doi: 10.1016/j.transci.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Paczkowska E. Freezing of umbilical blood cells in mechanical freezers (−80°C) Ann Acad Med Stetin. 2002;48:117–133. [PubMed] [Google Scholar]
- 26.Walter Z, Szostek M, Weglarska D, et al. Methods for freezing, thawing and viability estimation of hemopoietic stem cells. Przegl Lek. 1999;56:34–39. [PubMed] [Google Scholar]
- 27.Attarian H, Feng Z, Buckner CD, MacLeod B, Rowley SD. Long-term cryopreservation of bone marrow for autologous transplantation. Bone Marrow Transplant. 1996;17:425–430. [PubMed] [Google Scholar]
- 28.Buchanan SS, Gross SA, Acker JP, Toner M, Carpenter JF, Pyatt DW. Cryopreservation of stem cells using trehalose: Evaluation of the method using a human hematopoietic cell line. Stem Cells Dev. 2004;13:295–305. doi: 10.1089/154732804323099226. [DOI] [PubMed] [Google Scholar]
- 29.Xiao M, Dooley DC. Assessment of cell viability and apoptosis in human umbilical cord blood following storage. J Hematother Stem Cell Res. 2003;12:115–122. doi: 10.1089/152581603321210190. [DOI] [PubMed] [Google Scholar]
- 30.Broxmeyer HE, Srour EF, Hangoc G, Cooper S, Anderson SA, Bodine DM. High-efficiency recovery of functional hematopoietic progenitor and stem cells from human cord blood cryopreserved for 15 years. Proc Natl Acad Sci USA. 2003;100:645–650. doi: 10.1073/pnas.0237086100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang H, Acker JP, Hannon J, Miszta-Lane H, Akabutu JJ, McGann LE. Damage and protection of UC blood cells during cryopreservation. Cytotherapy. 2001;3:377–386. doi: 10.1080/146532401753277193. [DOI] [PubMed] [Google Scholar]
- 32.Woods EJ, Liu J, Derrow CW, Smith FO, Williams DA, Criser JK. Osmometric and permeability characteristics of human placental/umbilical cord blood CD34+ cells and their application to cryopreservation. J Hematother Stem Cell Res. 2000;9:161–173. doi: 10.1089/152581600319379. [DOI] [PubMed] [Google Scholar]
- 33.Zhang XB, Li K, Yau KH, et al. Trehalose ameliorates the cryopreservation of cord blood in a preclinical system and increases the recovery of CFUs, long-term culture-initiating cells, and non-obese diabetic-SCID repopulating cells. Transfusion. 2003;43:265–272. doi: 10.1046/j.1537-2995.2003.00301.x. [DOI] [PubMed] [Google Scholar]
- 34.Vormoor J, Lapidot T, Pflumio F, et al. Immature human cord blood progenitors engraft and proliferate to high levels in severe combined immunodeficient mice. Blood. 1994;83:2489–2497. [PubMed] [Google Scholar]
- 35.Vormoor J, Lapidot T, Pflumio F, et al. SCID mice as an in vivo model of human cord blood hematopoiesis. Blood Cells. 1994;20:316–320. discussion 320–322. [PubMed] [Google Scholar]
- 36.Bock TA, Orlic D, Dunbar CE, Broxmeyer HE, Bodine DM. Improved engraftment of human hematopoietic cells in severe combined immunodeficient (SCID) mice carrying human cytokine transgenes. J Exp Med. 1995;182:2037–2043. doi: 10.1084/jem.182.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orazi A, Braun SE, Broxmeyer HE. Commentary: Immunohistochemistry represents a useful tool to study human cell engraftment in SCID mice transplantation models. Blood Cells. 1994;20:323–330. [PubMed] [Google Scholar]
- 38.Kobylka P, Ivanyi P, Breur-Vriesendorp BS. Preservation of immunological and colony-forming capacities of long-term (15 years) cryopreserved cord blood cells. Transplantation. 1998;65:1275–1278. doi: 10.1097/00007890-199805150-00024. [DOI] [PubMed] [Google Scholar]
- 39.Mugishima H, Harada K, Chin M, et al. Effects of long-term cryopreservation on hematopoietic progenitor cells in umbilical cord blood. Bone Marrow Transplant. 1999;23:395–396. doi: 10.1038/sj.bmt.1701580. [DOI] [PubMed] [Google Scholar]
- 40.Kessinger A, Schmit-Pokorny K, Smith D, Armitage J. Cryopreservation and infusion of autologous peripheral blood stem cells. Bone Marrow Transplant. 1990;5 (Suppl 1):25–27. [PubMed] [Google Scholar]
- 41.Silberstein L, Jefferies L. Placental-blood banking—A new frontier in transfusion medicine. N Engl J Med. 1996;335:199–201. doi: 10.1056/NEJM199607183350310. [DOI] [PubMed] [Google Scholar]
- 42.Kawano Y, Lee CL, Watanabe T, et al. Cryopreservation of mobilized blood stem cells at a higher cell concentration without the use of a programmed freezer. Ann Hematol. 2004;83:50–54. doi: 10.1007/s00277-003-0817-8. [DOI] [PubMed] [Google Scholar]
- 43.Cabezudo E, Dalmases C, Ruz M, et al. Leukapheresis components may be cryopreserved at high cell concentrations without additional loss of HPC function. Transfusion. 2000;40:1223–1227. doi: 10.1046/j.1537-2995.2000.40101223.x. [DOI] [PubMed] [Google Scholar]
- 44.Villalon L, Odriozola J, Ramos P, Ramos ML, Herrera P, de Oteyza JP. Cryopreserving with increased cellular concentrations of peripheral blood progenitor cells: Clinical results. Haematologica. 2002;87:ELT06. [PubMed] [Google Scholar]
- 45.Gluckman E. Hematopoietic stem cell transplants using umbilical cord blood. N Engl J Med. 2001;344:1860–1861. doi: 10.1056/NEJM200106143442410. [DOI] [PubMed] [Google Scholar]
- 46.Lovelock JE, Bishop MW. Prevention of freezing damage to living cells by dimethyl sulphoxide. Nature. 1959;183:1394–1395. doi: 10.1038/1831394a0. [DOI] [PubMed] [Google Scholar]
- 47.Eberhardt R, Zwingers T, Hofmann R. DMSO in patients with active gonarthrosis. A double-blind placebo controlled phase III study. Fortschr Med. 1995;113:446–450. [PubMed] [Google Scholar]
- 48.Albanell J, Baselga J. Systemic therapy emergencies. Semin Oncol. 2000;27:347–361. [PubMed] [Google Scholar]
- 49.Branch DR, Calderwood S, Cecutti MA, Herst R, Solh H. Hematopoietic progenitor cells are resistant to dimethyl sulfoxide toxicity. Transfusion. 1994;34:887–890. doi: 10.1046/j.1537-2995.1994.341095026975.x. [DOI] [PubMed] [Google Scholar]
- 50.Zambelli A, Poggi G, Da Prada G, et al. Clinical toxicity of cryopreserved circulating progenitor cells infusion. Anticancer Res. 1998;18(6B):4705–4708. [PubMed] [Google Scholar]
- 51.Benekli M, Anderson B, Wentling D, Bernstein S, Czuczman M, McCarthy P. Severe respiratory depression after dimethylsulphoxide-containing autologous stem cell infusion in a patient with AL amyloidosis. Bone Marrow Transplantation. 2000;25:1299–1301. doi: 10.1038/sj.bmt.1702452. [DOI] [PubMed] [Google Scholar]
- 52.Miniero R, Vai S, Giacchino M, Giubellino C, Madon E. Severe respiratory depression after autologous bone marrow infusion. Haematologica. 1992;77:98–99. [PubMed] [Google Scholar]
- 53.Hequet O, Dumontet C, El Jaafari-Corbin A, et al. Epileptic seizures after autologous peripheral blood progenitor infusion in a patient treated with high-dose chemotherapy for myeloma. Bone Marrow Transplant. 2002;29:544. doi: 10.1038/sj.bmt.1703383. [DOI] [PubMed] [Google Scholar]
- 54.Higman MA, Port JD, Beauchamp NJ, Jr, Chen AR. Reversible leukoencephalopathy associated with re-infusion of DMSO preserved stem cells. Bone Marrow Transplant. 2000;26:797–800. doi: 10.1038/sj.bmt.1702589. [DOI] [PubMed] [Google Scholar]
- 55.Ferrucci PF, Martinoni A, Cocorocchio E, et al. Evaluation of acute toxicities associated with autologous peripheral blood progenitor cell reinfusion in patients undergoing high-dose chemotherapy. Bone Marrow Transplant. 2000;25:173–177. doi: 10.1038/sj.bmt.1702120. [DOI] [PubMed] [Google Scholar]
- 56.Burger J, Gilmore MJ, Jackson B, Prentice HG. Acute haemoglobinaemia associated with the reinfusion of bone marrow buffy coat for autologous bone marrow transplantation. Bone Marrow Transplant. 1991;7:322–324. [PubMed] [Google Scholar]
- 57.Zenhausern R, Tobler A, Leoncini L, Hess OM, Ferrari P. Fatal cardiac arrhythmia after infusion of dimethylsulfoxide cryopreserved hematopoietic stem cells in a patient with severe primary cardiac amyloidosis and end-stage renal failure. Ann Hematol. 2000;79:523–526. doi: 10.1007/s002770000186. [DOI] [PubMed] [Google Scholar]
- 58.Windrum P, Morris TC, Drake MB, Niederwieser D, Ruutu T. Variation in dimenthyl sulfoxide use in stem cell transplantation: A survey of EBMT centres. Bone Marrow Transplant. 2005;36:601–603. doi: 10.1038/sj.bmt.1705100. [DOI] [PubMed] [Google Scholar]
- 59.Syme R, Bewick M, Stewart D, Porter K, Chadderton T, Gluck S. The role of depletion of dimethyl sulfoxide before autografting: On hematologic recovery, side effects, and toxicity. Biol Blood Marrow Transplant. 2004;10:135–141. doi: 10.1016/j.bbmt.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 60.Bakken AM, Bruserud O, Abrahamsen JF. No differences in colony formation of peripheral blood stem cells frozen with 5% or 10% dimethyl sulfoxide. J Hematother Stem Cell Res. 2003;12:351–358. doi: 10.1089/152581603322023089. [DOI] [PubMed] [Google Scholar]
- 61.Sasnoor LM, Kale VP, Limaye LS. Supplementation of conventional freezing medium with a combination of catalase and trehalose results in better protection of surface molecules and functionality of hematopoietic cells. J Hematother Stem Cell Res. 2003;12:553–564. doi: 10.1089/152581603322448268. [DOI] [PubMed] [Google Scholar]
- 62.Stroh C, Cassens U, Samraj AK, Sibrowski W, Schulze-Osthoff K, Los M. The role of caspases in cryoinjury: Caspase inhibition strongly improves the recovery of cryopreserved hematopoietic and other cells. FASEB J. 2002;16:1651–1653. doi: 10.1096/fj.02-0034fje. [DOI] [PubMed] [Google Scholar]
- 63.Rollig C, Babatz J, Wagner I, et al. Thawing of cryopreserved mobilized peripheral blood-comparison between water bath and dry warming device. Cytotherapy. 2002;4:551–555. doi: 10.1080/146532402761624719. [DOI] [PubMed] [Google Scholar]
- 64.Moroff G, Seetharaman S, Kurtz JW, et al. Retention of cellular properties of PBPCs following liquid storage and cryopreservation. Transfusion. 2004;44:245–252. doi: 10.1111/j.1537-2995.2004.00630.x. [DOI] [PubMed] [Google Scholar]
- 65.Calmels B, Houze P, Hengesse JC, Ducrot T, Malenfant C, Chabannon C. Preclinical evaluation of an automated closed fluid management device: Cytomate, for washing out DMSO from hematopoietic stem cell grafts after thawing. Bone Marrow Transplant. 2003;31:823–828. doi: 10.1038/sj.bmt.1703905. [DOI] [PubMed] [Google Scholar]
- 66.Shlebak AA, Marley SB, Roberts IA, Davidson RJ, Goldman JM, Gordon MY. Optimal timing for processing and cryopreservation of umbilical cord haematopoietic stem cells for clinical transplantation. Bone Marrow Transplant. 1999;23:131–136. doi: 10.1038/sj.bmt.1701551. [DOI] [PubMed] [Google Scholar]
- 67.Kurtzberg J, Laughlin M, Graham ML, et al. Placental blood as a source of hematopoietic stem cells for transplantation into unrelated recipients. N Engl J Med. 1996;335:157–166. doi: 10.1056/NEJM199607183350303. [DOI] [PubMed] [Google Scholar]
- 68.Nagumura-Inoue T, Shioya M, Sugo M, et al. Wash-out of DMSO does not improve the speed of engraftment of cord blood transplantation: Follow-up of 46 adult patients with units shipped from a single cord blood bank. Transfusion. 2003;43:1285–1295. doi: 10.1046/j.1537-2995.2003.00486.x. [DOI] [PubMed] [Google Scholar]
- 69.Beaujean F, Hartmann O, Kuentz M, Le Forestier C, Divine M, Duedari N. A simple, efficient washing procedure for cryopreserved human hematopoietic stem cells prior to reinfusion. Bone Marrow Transplant. 1991;8:291–294. [PubMed] [Google Scholar]
- 70.Zingsem J, Strasser E, Weisbach V, et al. Cord blood processing with an automated and functionally closed system. Transfusion. 2003;43:806–813. doi: 10.1046/j.1537-2995.2003.00398.x. [DOI] [PubMed] [Google Scholar]
- 71.Ayello J, Hesdorffer C, Reiss RF. A semi-automated technique for volume reduction of stem cell suspensions for auto transplantation. J Hematother. 1995;4:545–549. doi: 10.1089/scd.1.1995.4.545. [DOI] [PubMed] [Google Scholar]
- 72.Rodriguez L, Azqueta C, Azzalin S, Garcia J, Querol S. Washing of cord blood grafts after thawing: High cell recovery using an automated and closed system. Vox Sang. 2004;87:165–172. doi: 10.1111/j.1423-0410.2004.00550.x. [DOI] [PubMed] [Google Scholar]
- 73.Rodriguez L, Velasco B, Garcia J, Martin-Henao GA. Evaluation of an automated cell processing device to reduce the dimethyl sulfoxide from hematopoietic grafts after thawing. Transfusion. 2005;45:1391–1397. doi: 10.1111/j.1537-2995.2005.00213.x. [DOI] [PubMed] [Google Scholar]
- 74.Mericka P, Schustr P, Vins M, et al. Containers for freezing and storage of bone marrow stem cells. Sb Ved Pr Lek Fak Karlovy University Hradci Kralove. 1991;34(4):367–387. [PubMed] [Google Scholar]
- 75.Yang H, Acker JP, Cabuhat M, Letcher B, Larratt L, McGann LE. Association of post-thaw viable CD34+ cells and CFU-GM with time to hematopoietic engraftment. Bone Marrow Transplant. 2005;35:881–887. doi: 10.1038/sj.bmt.1704926. [DOI] [PubMed] [Google Scholar]
- 76.Hubel A, Carlquist D, Clay M, McCullough J. Liquid storage, shipment, and cryopreservation of cord blood. Transfusion. 2004;44:518–525. doi: 10.1111/j.1537-2995.2004.03238.x. [DOI] [PubMed] [Google Scholar]
- 77.FDA. Current good tissue practice for manufacturers of human cellular tissue based products. Inspection and enforcement; Proposed rule. 2001 Available at www.access.gpo.gov/su_docs/aces140.html.
- 78.Jestice HK, Farrington M, Hunt C, Matthews I, Scott MA, Foreman J, Marcus RE. Bacterial contamination of peripheral blood progenitor cells for transplantation. Transfus Med. 1996;6:103–110. doi: 10.1046/j.1365-3148.1996.d01-57.x. [DOI] [PubMed] [Google Scholar]
- 79.Padley D, Koontz F, Trigg ME, Gingrich R, Strauss RG. Bacterial contamination rates following processing of bone marrow and peripheral blood progenitor cell preparations. Transfusion. 1996;36:53–56. doi: 10.1046/j.1537-2995.1996.36196190515.x. [DOI] [PubMed] [Google Scholar]
- 80.Espinosa MT, Fox R, Creger RJ, Lazarus HM. Microbiologic contamination of peripheral blood progenitor cells collected for hematopoietic cell transplantation. Transfusion. 1996;36:789–793. doi: 10.1046/j.1537-2995.1996.36996420754.x. [DOI] [PubMed] [Google Scholar]
- 81.Webb IJ, Coral FS, Andersen JW, et al. Sources and sequelae of bacterial contamination of hematopoietic stem cell components: Implications for the safety of hematotherapy and graft engineering. Transfusion. 1996;36:782–788. doi: 10.1046/j.1537-2995.1996.36996420753.x. [DOI] [PubMed] [Google Scholar]
- 82.Schwella N, Zimmermann R, Heuft HG, et al. Microbiologic contamination of peripheral blood stem cell autografts. Vox Sang. 1994;67:32–35. doi: 10.1111/j.1423-0410.1994.tb05034.x. [DOI] [PubMed] [Google Scholar]
- 83.Prince HM, Page SR, Keating A, et al. Microbial contamination of harvested bone marrow and peripheral blood. Bone Marrow Transplant. 1995;15:87–91. [PubMed] [Google Scholar]
- 84.Attarian H, Bensinger WI, Buckner CD, McDonald DL, Rowley SD. Microbial contamination of PBSC collections. Bone Marrow Transplant. 1996;17:699–702. [PubMed] [Google Scholar]
- 85.Kipp F, Linnemann E, Fischer RJ, Sibrowski W, Cassens U. Cryopreservation reduces the concentration of detectable bacteria in contaminated peripheral blood progenitor cell products. Transfusion. 2004;44:1098–1103. doi: 10.1111/j.1537-2995.2004.03392.x. [DOI] [PubMed] [Google Scholar]
- 86.Brecher ME, Means N, Jere CS, Heath D, Rothenberg S, Stutzman LC. Evaluation of an automated culture system for detecting bacterial contamination of platelets: An analysis with 15 contaminating organisms. Transfusion. 2001;41:477–482. doi: 10.1046/j.1537-2995.2001.41040477.x. [DOI] [PubMed] [Google Scholar]
- 87.Schafer TW, Everett J, Silver GH, Came PE. Biohazard: Virus-contaminated liquid nitrogen, Scientific Letter. Science. 1976;191:25–26. doi: 10.1126/science.191.4222.24-c. No. 4222. [DOI] [PubMed] [Google Scholar]
- 88.Tedder RS, Zuckerman MA, Goldstone AH, et al. Hepatitis B transmission from contaminated cryopreservation tank. Lancet. 1995;346:137–140. doi: 10.1016/s0140-6736(95)91207-x. [DOI] [PubMed] [Google Scholar]
- 89.Hawkins AE, Zuckerman MA, Briggs M, et al. Hepatitis B nucleotide sequence analysis: Linking an outbreak of acute hepatitis B to contamination of a cryopreservation tank. J Virol Methods. 1996;60:81–88. doi: 10.1016/0166-0934(96)02048-4. [DOI] [PubMed] [Google Scholar]
- 90.Fountain D, Ralston M, Higgins N, et al. Liquid nitrogen freezers: A potential source of microbial contamination of hematopoietic stem cell components. Transfusion. 1997;37:585–591. doi: 10.1046/j.1537-2995.1997.37697335152.x. [DOI] [PubMed] [Google Scholar]
- 91.Husebekk A, Skaug K, Kostad A, Dahl IM, Gutteberg T, Skogen B. Hepatitis B virus-infected peripheral blood progenitor cell harvests in liquid nitrogen freezer containing non-infectious products. Transfusion. 2004;44:942–943. doi: 10.1111/j.1537-2995.2004.00379.x. [DOI] [PubMed] [Google Scholar]
- 92.Voak D, Cann R, Finney RD, et al. Guidelines for administration of blood products: Transfusion of infants and neonates. British Committee of Standards in Haematology Blood Transfusion Task Force Transfus Med. 1994;4:63–69. doi: 10.1111/j.1365-3148.1994.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 93.Ware CB, Nelson AM, Blau CA. Controlled-rate freezing of human ES cells. Biotechniques. 2005;38:879–880. 882–883. doi: 10.2144/05386ST01. [DOI] [PubMed] [Google Scholar]
- 94.He J, Liu JH, Jiang K, Zhu FM, Yan LX. Assessment on effect of short-term cryopreservation of cord blood hematopoietic cells. Zhongguo Shi Yan Xue ye Xue za Zhi. 2004;12:375–377. [PubMed] [Google Scholar]
- 95.Ure JM, Fiering S, Smith AG. A rapid and efficient method for freezing and recovering clones of embryonic stem cells. Trends Genet. 1992;8:6. doi: 10.1016/0168-9525(92)90004-n. [DOI] [PubMed] [Google Scholar]
- 96.Adams RM, Wang M, Crane AM, Brown B, Darlington GJ, Ledley FD. Effective cryopreservation and long-term storage of primary human hepatocytes with recovery of viability, differentiation, and replicative potential. Cell Transplant. 1995;4:579–586. doi: 10.1177/096368979500400607. [DOI] [PubMed] [Google Scholar]
- 97.Milosevic J, Storch A, Schwarz J. Cryopreservation does not affect proliferation and multipotency of murine neural precursor cells. Stem Cells. 2005;23:681–688. doi: 10.1634/stemcells.2004-0135. [DOI] [PubMed] [Google Scholar]
- 98.Dobrinksy JR. Cellular approach to cryopreservatiion of embryos. Theriogenology. 1996;45:17–26. [Google Scholar]
- 99.Reubinoff BE, Pera MF, Vajta G, Trounson AO. Effective cryopreservation of human embryonic stem cells by the open pulled straw vitrification method. Hum Reprod. 2001;16:2187–2194. doi: 10.1093/humrep/16.10.2187. [DOI] [PubMed] [Google Scholar]
- 100.Fujioka T, Yasuchika K, Nakamura Y, Nakatsuji N, Suemori H. A simple and efficient cryopreservation method for primate embryonic stem cells. Int J Dev Biol. 2004;48:1149–1154. doi: 10.1387/ijdb.041852tf. [DOI] [PubMed] [Google Scholar]
- 101.Zhou CQ, Mai QY, Li T, Zhuang GL. Cryopreservation of human embryonic stem cells by vitrification. Chin Med J (Engl) 2004;117:1050–1055. [PubMed] [Google Scholar]
- 102.Perseghin P, Epis R, Vigano M, Malacrida A, Pastorini A, Camerone G. Satisfactory recovery and viability of stem cells cryopreserved at high cell concentration. Transfus Sci. 1997;18:399–403. doi: 10.1016/S0955-3886(97)00037-4. [DOI] [PubMed] [Google Scholar]
- 103.Liu K, Gao Z, Jiang Y, Dong W, et al. Collection, processing and cryopreservation of placental cord blood hematopoietic stem cells. Beijing Da Xue Bao. 2003;35:119–122. [PubMed] [Google Scholar]
- 104.Barker JN, Wagner JE. Umbilical-cord blood transplantation for the treatment of cancer. Nat Rev Cancer. 2003;3:526–532. doi: 10.1038/nrc1125. [DOI] [PubMed] [Google Scholar]
- 105.Donnenberg AD, Koch EK, Griffin DL, et al. Viability of cryopreserved BM progenitor cells stored for more than a decade. Cytotherapy. 2002;4:157–163. doi: 10.1080/146532402317381866. [DOI] [PubMed] [Google Scholar]
- 106.Nichols J, Zevnik B, Anastassiadis K, et al. Formation of pluri-potent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 107.Yin AH, Miraglia S, Zanjani ED, et al. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–5012. [PubMed] [Google Scholar]


