Abstract
Changes in the cytosolic Ca2+ concentration ([Ca2+]c) are essential for triggering neurotransmitter release from presynaptic nerve terminals. Calcium-induced Ca2+ release (CICR) from the endoplasmic reticulum (ER) may amplify the [Ca2+]c signals and facilitate neurotransmitter release in sympathetic neurons. In adrenal chromaffin cells, functional triads are formed by voltage-operated Ca2+ channels (VOCCs), CICR sites and mitochondria. In fact, mitochondria take up most of the Ca2+ load entering the cells and are essential for shaping [Ca2+]c signals and exocytosis. Here we have investigated the existence of such functional triads in sympathetic neurons. The mitochondrial Ca2+ concentration ([Ca2+]m) in soma and neurites of individual mouse superior cervical ganglion (SCG) neurons was monitored by bioluminescence imaging of targeted aequorins. In soma, Ca2+ entry through VOCCs evoked rapid, near millimolar [Ca2+]m increases in a subpopulation of mitochondria containing about 40% of the aequorin. Caffeine evoked a similar [Ca2+]m increase in a mitochondrial pool containing about 30% of the aequorin and overlapping with the VOCC-sensitive pool. These observations suggest the existence of functional triads similar to the ones described in chromaffin cells. In neurites, mitochondria were able to buffer [Ca2+]c increases resulting from activation of VOCCs but not those mediated by caffeine-induced Ca2+ release from the ER. The weaker Ca2+ buffering by mitochondria in neurites could contribute to facilitate Ca2+-induced exocytosis at the presynaptic sites.
Calcium signals govern important processes in neurons, including neurotransmitter release, membrane excitability, neurite outgrowth and neurodegeneration (Clapham, 1995; Ghosh & Greenberg, 1995). The cytosolic Ca2+ concentration ([Ca2+]c) increases upon activation of plasma membrane Ca2+ channels or Ca2+ release from intracellular stores (Berridge, 1998). In addition, Ca2+ signals can be amplified by release of Ca2+ from the endoplasmic reticulum (ER) through Ca2+-induced Ca2+ released (CICR; Thayer et al. 1988; Hernández-Cruz et al. 1990; Friel & Tsien, 1992), mediated by ryanodine receptors (RyRs). Cytoplasmic organelles also participate in Ca2+ signalling (Alvarez et al. 1999). It is becoming increasingly clear that, besides its central role in cell energetics (Robb-Gaspers et al. 1998), mitochondria modulate cytosolic Ca2+ homeostasis (Herrington et al. 1996). Mitochondria can take up Ca2+ through the mitochondrial Ca2+ uniporter (Gunter & Pfeiffer, 1990; Kirichok et al. 2004), a high-capacity, low-affinity Ca2+ transport that activates exponentially with the increase of [Ca2+] in the surrounding cytosol (Gunter & Pfeiffer, 1990; Rizzuto et al. 1993; Montero et al. 2000). It has been reported that mitochondria can take up Ca2+ with relatively modest increases of [Ca2+] in the bulk cytosol (Herrington et al. 1996; Villalobos et al. 2002). In particular, mitochondria seem able to buffer physiological Ca2+ loads in neurons, and activation is paralleled by the increase of mitochondrial calcium concentration ([Ca2+]m; Werth & Thayer, 1994; Babcock et al. 1997). This probably results from the generation of subcellular high-Ca2+ domains at sites of Ca2+ entry or Ca2+ release (Rizzuto et al. 1993; Xu et al. 1997; Duchen, 1999; Montero et al. 2000), and mitochondrial uptake is much faster at these high-Ca2+ microdomains. Thus, Ca2+ uptake by mitochondria is favoured in mitochondria located strategically near Ca2+ hot spots, such as the mouth of Ca2+ channels or ryanodine and/or inositol 1,4,5-trisphosphate (IP3) receptors. We have reported that, in chromaffin cells, a subpopulation of mitochondria is strategically and functionally coupled to both Ca2+ entry through voltage-operated Ca2+ channels (VOCCs) and Ca2+ release from intracellular stores, whereas the remaining mitochondria are located far away from these Ca2+ hot spots (Montero et al. 2000). The former mitochondrial subpopulation takes up larger Ca2+ loads, approaching millimolar levels, during cell stimulation (Montero et al. 2000; Villalobos et al. 2001). In fact, quantitative analysis of Ca2+ redistribution among different cytoplasmic compartments in chromaffin cells has demonstrated that most of the Ca2+ entering through VOCCs or released from ER by CICR is, in the short term, removed from the cytosol by mitochondria rather than by the other Ca2+ extruding systems (Villalobos et al. 2002). Consistently, prevention of mitochondrial Ca2+ uptake enhanced both the [Ca2+]c rise and exocytosis (Montero et al. 2000). Therefore, strategically located mitochondria modulate the [Ca2+]c signals and make possible the coexistence of high subplasmalemmal Ca2+ levels favouring exocytosis with a much lower [Ca2+]c at the cell core. This is attained by avoiding spread of the entering Ca2+ load towards the cell core. In addition, intramitochondrial Ca2+ stimulates cellular respiration and tunes ATP synthesis to the increased requirements during the active state. The functional units or triads formed by VOCCs, RyRs and mitochondria seem essential for correct organization of the secretory process in adrenal chromaffin cells (Montero et al. 2000). Whether such functional units are present in neurons has not been investigated, perhaps owing to the difficulty of monitoring aequorin bioluminescence in single neurons. Here we combine the superb selectivity of targeted aequorin, the high expression induced by a viral vector (Alonso et al. 1998) and the high sensitivity provided by a photon-counting camera (Frawley et al. 1994; Rutter et al. 1996; Villalobos et al. 2002) to resolve changes of [Ca2+]m at the single-cell level and even at the subcellular level (cell body and neurites).
Methods
Primary culture of sympathetic neurons from adult mouse superior cervical ganglion
The cell culture method was a modification of a previously described protocol (Martínez et al. 2002). Adult (8–12 weeks old) male Balb/c mice were fed ad libitum and maintained under a standard 12 h light–12 h dark photoregime. Experiments were conducted following the Guidelines of the Animal Care Committee and the Commission d'Ethique d'Experimentation Animale of the University of Lovain School of Medicine. Groups of four or five animals were killed by cervical dislocation and their superior cervical ganglia (SCG) rapidly removed under sterile conditions and transferred to a Petri dish containing ice-cold, Ca2+- and Mg2+-free Hank's balanced salt solution (HBSS). Ganglia were incubated for 10 min in HBSS containing collagenase (1.6 mg ml−1) at 37°C, washed and incubated again in HBSS containing trypsin (1 mg ml−1) for 15 min at 37°C. After gentle mechanical disruption with a siliconized, fire-polished Pasteur pipette, the cells were suspended in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS), washed twice and suspended in culture medium (DMEM supplemented with 10% FBS and 50 ng ml−1 of nerve growth factor, NGF). Aliquots of the cell suspension (25 μl) were plated on poly l-lysine-coated 12 mm diameter glass coverslips. On average, we obtained the same number of cell-containing coverslips as ganglia dispersed. Cells were allowed to attach for 1 h and then an additional 300 μl of culture medium was added to each well and culture was continued for 2 days before the experiments.
Measurements of cytosolic [Ca2+] by fluorescence microscopy
The cells were loaded with fura-2 by incubation with fura-2 AM (4 μm) for about 1 h at room temperature in a standard medium of the following composition (mm): NaCl, 145; KCl, 5; MgCl2, 1; CaCl2, 1; glucose, 10; and sodium-Hepes, 10; pH 7.4 (adjusted with NaCH). Cells were then washed with the same medium, placed in a thermostatically controlled (37°C) chamber on the stage of an inverted microscope (Nikon Diaphot) and perfused with standard medium, prewarmed at 37°C. Cells were epi-illuminated alternately at 340 and 380 nm, and light emitted above 520 nm was recorded using a Magical Image processor (Applied Imaging, Newcastle, UK). Pixel-by-pixel ratios of consecutive frames were captured, and [Ca2+]c values were estimated from these ratios by comparison with fura-2 standards. Other details were as reported previously (Núñez & García-Sancho, 1996; Villalobos et al. 2002).
Expression of aequorins
Mitochondria-targeted wild-type aequorin (mitAEQ) and low-Ca2+-affinity, mutated aequorin (mitmutAEQ) were delivered using defective herpes simplex virus type 1 (HSV-1) amplicons prepared, packed and titrated as previously described (Alonso et al. 1998; Montero et al. 2000). The range of Ca2+ sensitivies for these aequorins has been published elsewhere (Montero et al. 2000). The native aequorin can discriminate different Ca2+ concentrations within the 0.1–10 μm range, whereas the low-Ca2+-affinity, mutated aequorin can distinguish different Ca2+ concentrations from the tens of micromolar to the low millimolar range when used in combination with celenterazine n. Cells were infected with 1 × 103 to 3 × 103 infectious virus particles per coverslip of the HSV-1 containing the corresponding aequorin gene and cultured for 18 h before measurements. Infection efficiency ranged between 50 and 85% (mean ± s.e.m., 67 ± 3%; 120 cells from 18 experiments). The correct targeting of aequorins to mitochondria has been reported elsewhere (Montero et al. 2000) and was confirmed here (results not shown).
Imaging of aequorin bioluminescence
Cells expressing the apoaequorins were incubated for 1–2 h at room temperature with 1 μm coelenterazine. Coelenterazine n was used in some experiments for reconstitution of the low-Ca2+-affinity mutmitAEQ. Cells were placed into a perfusion chamber thermostatically regulated to 37°C under a Zeiss Axiovert 100 TV microscope and perfused at 5–10 ml min−1 with the test solutions, based on the standard perfusing solution described above, prewarmed at 37°C. At the end of each experiment, cells were permeabilized with 0.1 mm digitonin in 10 mm CaCl2 to release all the residual aequorin counts. Bioluminescence images were taken with a Hamamatsu VIM photon counting camera handled with an Argus-20 image processor and integrated for 10 s periods. Photons per cell in each image were quantified using the Hamamatsu Aquacosmos software. Total counts per cell ranged between 2 × 103 and 2 × 105 and noise was (mean ±s.d.) 1 ± 1 counts s−1 per typical cell area (2000 pixels). Data were first quantified as rates of photoluminescence emission divided by the total counts remaining at each time and divided by the integration period (L/Ltotal in s−1). Emission values of less than 4 counts s−1 were not used for calculations. Calibrations in terms of [Ca2+]m were performed using the constant's values published previously (Alvarez & Montero, 2001). A bright field image was also taken at the beginning of each experiment. Further details have been reported previously (Villalobos et al. 2001, 2005).
Confocal microscopy
To identify endoplasmic reticulum and mitochondria in living sympathetic neurons, we first transiently expressed a fused green fluorescent protein (GFP) targeted to the ER. Endoplasmic reticulum–GFP contain in-frame fusion of enhanced green fluorescent protein (EGFP) and a Lys-Asp-Glu-Leu (KDEL) ER retention sequence in the herpes simplex virus plasmid (pHSV) amplicon vector. Expression was achieved by infecting cells with a defective HSV-1 containing the ER–GFP–aequorin gene and cultured for 18 h before measurements. Then, cells were loaded with Mitotracker Red® (200 nm) for 30 min at 37°C and washed with standard incubation medium. Confocal images were obtained using a Bio-Rad laser scanning system (Radiance 2100) coupled to a Nikon eclipse TE2100U inverted microscope with ×63 oil immersion objective (N.A.=1.4). Green fluorescent protein was excited by the 488 nm laser line and emission was collected through a 500–560 nm bandpass filter. Mitotracker Red® was excited at 543 nm, and emitted light was collected through a 590 nm long-pass filter.
Results
In a first series of experiments, we studied the effects of both Ca2+ entry from the extracellular medium and Ca2+ release from the intracellular stores on [Ca2+]m. Calcium entry was induced by stimulation with high-K+ solution, which depolarizes the plasma membrane and opens VOCCs. Calcium release from the ER was induced by stimulation of the ryanodine receptors with caffeine (Thayer et al. 1988; Hernández-Cruz et al. 1995). In all cases, we measured the changes of the [Ca2+]c using fura-2 (Villalobos et al. 2002) and the changes of [Ca2+]m with different aequorins (Montero et al. 2000). Both measurements were performed in the soma of the sympathetic neurons.
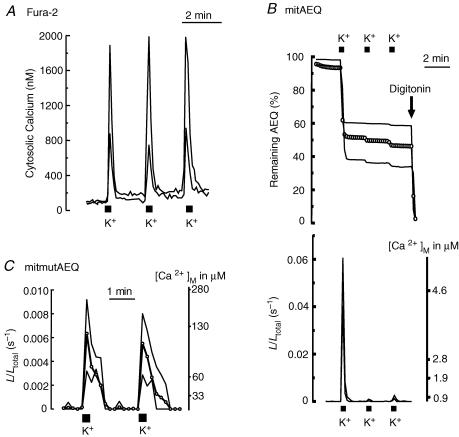
Figure 1 summarizes the effects of repetitive stimulation with high K+. Use of fura-2 produced reproducible increases of [Ca2+]c, whose size varied between 800 and 2000 nm from cell to cell (Fig. 1A). In contrast, bioluminescence imaging of mitochondria-targeted aequorin (Montero et al. 2000; Villalobos et al. 2001) produced very different effects for the first stimulation and for subsequent ones (Fig. 1B). Since aequorin is burned out during the high-[Ca2+] periods (Shimomura et al. 1993; Alvarez & Montero, 2001; Villalobos et al. 2001), aequorin consumption provides a realistic record of what has happened each time, not biased by any calculation. The first stimulus consumed 44 ± 3% of the total photonic emissions (mean ± s.e.m. of 35 cells in 17 experiments). Aequorin consumption could be prevented by collapsing the mitochondrial membrane potential with the protonophore carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone (FCCP) (data not shown), confirming that it results from mitochondrial Ca2+ uptake. The second and subsequent K+ pulses released only 3 ± 1% of the total photons (Fig. 1B). The remaining photonic emissions could be released easily by permeabilizing the plasma membrane with digitonin and perfusing these permeabilized cells with solutions containing high levels of Ca2+ (> 20 μm; Fig. 1B and results not shown). The bottom part of Fig. 1B shows the calibrated [Ca2+]m values. Apparently, the first stimulus produced a large [Ca2+]m increase, whereas subsequent ones had a much smaller effect.
Figure 1. Effects of repeated stimulation of sympathetic neurons by depolarization with high K+ on cytosolic and mitochondrial [Ca2+].
Cells were repeatedly stimulated by perfusion with high K+ solution (150 mm; 10 s pulses every 2 min). A, sympathetic neurons loaded with fura-2. [Ca2+]c traces from two representative neurons (large and small response) are shown. Sixty-four consecutive fura-2 fluorescence images taken at either 340 or 380 nm excitation were captured, averaged and ratioed by hardware every 5 s. The image size was 256 × 256 pixels and the spatial resolution was 1 μm per pixel. Results are representative of 15 similar experiments. B, sympathetic neurons infected with mitAEQ and subjected to photon counting imaging. The upper traces show AEQ consumption in two representative neurons (large and small response), and open circles show the average value of 7 neurons present in the same microscopic field. The lower traces show calibrated signals (two single cells) from the same experiment. Data are representative of 17 similar experiments. C, sympathetic neurons were infected with mitmutAEQ and reconstituted with coelenterazine n (the low-Ca2+-affinity system; see Methods). Data were corrected for the pool size (44% of the total AEQ counts). The traces corresponding to two extreme individual cells (large and small response) and the average of 5 cells present in the same microscope field (open circles) are shown. Only photonic emissions from the neuron cell body were counted for quantification of [Ca2+]m. Data are representative of 7 similar experiments.
The outcome of these experiments is similar to previous findings in chromaffin cells (Montero et al. 2000; Villalobos et al. 2002), and we interpret them in terms of the existence of two mitochondrial pools with different spatial locations. The first pool, probably closer to the mouth of the Ca2+ channels at the plasma membrane, senses the high [Ca2+] concentrations built up in the high-Ca2+ microdomains. These mitochondria take up large amounts of Ca2+ that burns up all the aequorin very quickly during the first stimulus. During subsequent stimuli, this subplasmalemmal mitochondrial pool does not emit photoluminescence because all the aequorin contained therein was burned during the first stimulus. The second mitochondrial pool is closer to the cell core and would sense smaller [Ca2+]c increases, of the same order of magnitude as the ‘averaged’ values found using fura-2. These mitochondria take up smaller amounts of Ca2+ and emit a much weaker photoluminescence signal, which is concealed during the first stimulus by the signal coming from the first mitocondrial pool. During the second and third stimuli, when all the aequorin from the first mitochondrial pool has burned out, the smaller signals coming from the core mitochondria show up (Fig. 1B).
In order to test the validity of this interpretation, we studied the effects of the same sequential stimuli in cells expressing mitmutAEQ reconstituted with coelenterazine n. This low-Ca2+-affinity aequorin (Montero et al. 2000) would not be burned out completely during the first stimulus, and should then report the real and reproducible [Ca2+]m responses to repetitive stimuli. Confirmation of this prediction is shown in Fig. 1C. This panel shows the calibrated [Ca2+]m recordings of two representative individual cells (the ones with the strongest and the smallest response) and the average value of five cells present in the same microscopic field. Note that the responses were similar during the first and second stimuli, reaching [Ca2+]m values that ranged between 100 and 300 μm. Note also that at such high Ca2+ concentrations the wild-type aequorin would be fully burned within a few seconds, since the half-consumption time is below 1 s at these saturating Ca2+ concentrations (Shimomura et al. 1993; Alvarez & Montero, 2001). The [Ca2+]m peaks reached in the core mitochondrial pool, as shown by the wild-type aequorin during the second and the third high-K+ stimuli, were much smaller (about 1 μm; Fig. 1B) and therefore should not be sensed by the low-Ca2+-affinity mutmitAEQ.
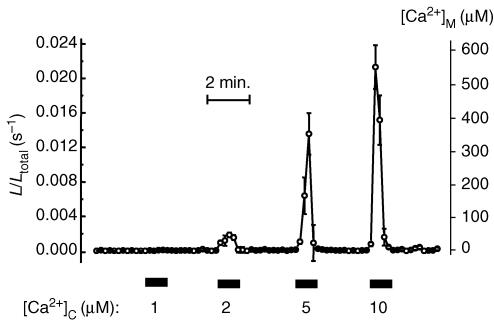
Experiments to assess the gross properties of the mitochondrial Ca2+ uniporter in these neurons were performed in digitonin-permeabilized cells perfused with intracellular-like solutions containing different Ca2+ concentrations (Montero et al. 2000). Representative results are shown in Fig. 2. The low-Ca2+-affinity aequorin (mitmutAEQ) almost failed to detect noticeable mitochondrial uptake at Ca2+ concentrations below 2 μm, but emitted quickly and uniformly more than 90% of the photonic emissions at 5 μm [Ca2+], and this was prevented by FCCP (not shown). Thus, although mitochondrial Ca2+ uptake at cytosolic Ca2+ concentrations below 2 μm might occur, it would not be detected with the low-Ca2+-affinity aequorin with a sensitivity limit for [Ca2+] in the low micromolar range. As we have discussed elsewhere, the steepness of the Ca2+ dependence of the mitochondrial Ca2+ uniporter, together with the second-order dependence of the light emission on [Ca2+], stresses the abruptness of the transition from non-perceptible to massive mitochondrial uptake (Alonso et al. 2006). When high-Ca2+-affinity aequorin is used, some Ca2+ uptake is measured also at 1 μm [Ca2+]c and even below (Villalobos et al. 2002). In any case, these results indicate that the properties of the mitochondrial Ca2+ uniporter in sympathetic neurons are similar to those reported in other tissues (Gunter & Pfeiffer, 1990; Villalobos et al. 2002) and that high-Ca2+ microdomains stimulate mitochondrial uptake (Villalobos et al. 2002). Even more important, the fact that all the aequorin is consumed uniformly and homogeneously in the permeabilized cells indicates that the two mitochondrial pools, the subplasmalemmal and the core-located one, are both equally able to take up Ca2+ and that the differences seen in the intact cells do not result from their Ca2+ transport properties but from their spatial location.
Figure 2. Ca2+ dependence of mitochondrial Ca2+ uptake in permeabilized sympathetic neurons.
Sympathetic neurons infected with mitmutAEQ and reconstituted with coelenterazine n were permeabilized by treatment with 20 μm digitonin over 1 min at 37°C in Ca2+-free (containing 0.2 mm EGTA) intracellular-like medium (composition, in mm: NaCl, 5; KCl, 130; MgCl2, 2; KH2PO4, 2; Mg-ATP, 0.2; and potassium-Hepes, 20; pH 7.0 adjusted with KOH). Then the cells were perfused with ‘intracellular-like’ medium containing different [Ca2+] values, as shown in the figure. The different [Ca2+] values were obtained using buffers containing 1 mm EGTA or 1 mm N-(hydroxyethyl) ethylenediamine triacetic acid (HEDTA) and adjusting Ca2+ and Mg2+ as required (Patton et al. 2004). Values represent the mean ± s.e.m. of 5 different cells present in the same microscopic field. Results are representative of 5 similar experiments.
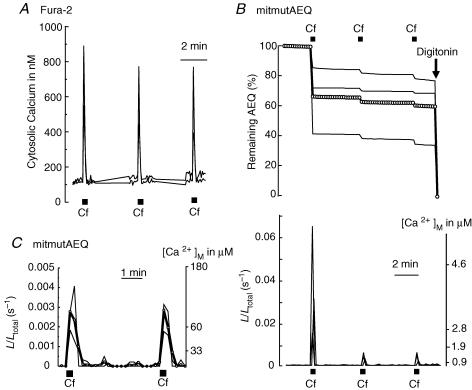
Next, we studied the effects of repetitive stimulation with caffeine. Caffeine opens ER channels by activation of RyRs and promotes Ca2+ release to the cytosol. Repetitive stimulation with caffeine (1 stimulus every 4 min) produced sharp and reproducible [Ca2+]c peaks ranging, from cell to cell, between 500 and 900 nm in size (Fig. 3A; measured with fura-2). Figure 3B illustrates the results obtained in neurons expressing the wild-type mitochondrial aequorin. The first stimulus released 32 ± 4% of total photonic emissions (mean ± s.e.m. of 25 cells from 12 experiments). In contrast, the second and subsequent caffeine stimuli released only 2 ± 1% of the total photons. These results are similar to those obtained with sequential stimulation using high K+ (Fig. 1). Likewise, we interpret these results in terms of two mitochondrial pools with different spatial locations, the first one close and the second one away from the sites of Ca2+ release (i.e. RyRs at ER). This first mitochondrial pool would take up a larger amount of Ca2+, thus burning up all its aequorin very quickly. To confirm this view, we carried out new measurements using the mutated, low-Ca2+-affinity aequorin (mitmutAEQ reconstituted with coelenterazine n). Figure 3C shows that now each caffeine stimulus produced a similar output that, once corrected for the pool size (32%), revealed an increase of [Ca2+]m of about 150 μm, a value close to the one achieved in the mitochondria sensing the VOCCs upon high-K+ stimulation. These results suggest that Ca2+ release from ER by activation of ryanodine receptors generates subcellular high-[Ca2+] microdomains that are sensed only by a subpopulation of mitochondria, while the remainder sense much smaller Ca2+ concentrations.
Figure 3. Effects of repeated stimulation with caffeine on [Ca2+]c and [Ca2+]m.
Sympathetic neurons were repeatedly stimulated with caffeine (Cf; 50 mm for 10 s every 4 min). A, effects on [Ca2+]c measured with fura-2. Data are representative of 16 similar experiments. B, effects on [Ca2+]m measured with mitAEQ (high-Ca2+-affinity system). Data are representative of 12 similar experiments. C, effect on [Ca2+]m measured with mutmitAEQ and coelenterazine n (low-Ca2+-affinity system). Data are representative of 6 similar experiments. Other details as in Fig. 1.
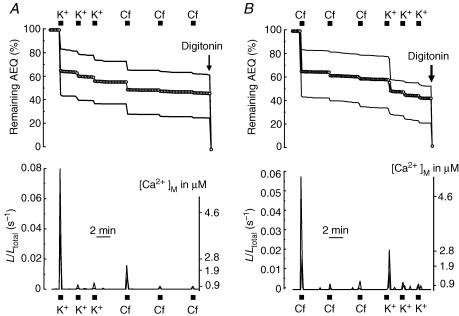
We then asked whether the pool of mitochondria that sense Ca2+ entry and the one sensing Ca2+ release is the same or a different pool. To this end, neurons expressing the high-Ca2+-affinity mitochondrial aequorin were subjected to photon counting imaging for [Ca2+]m measurements after sequential stimulation, first with high K+ and then with caffeine (Fig. 4A) or the same stimuli in the reverse order (Fig. 4B). After stimulation with high K+ the effects of caffeine on mitAEQ consumption were very small, only 6 ± 1% of all the photons (mean ± s.e.m. of 17 cells from 7 experiments; Fig. 4A). When the protocol was performed in the reverse order, first stimulation with caffeine and then with high K+, the consumption during the first K+ stimulus was only 15 ± 2% of the total counts (21 cells in 12 experiments; Fig. 4B). This value is larger than with caffeine, but still much smaller than the one of about 40% obtained during the first stimulation with high K+ when there was not previous stimulation with caffeine (Figs 1B and 4A). These results indicate that the subpopulation of mitochondria sensing the plasma membrane Ca2+ entry and the ER Ca2+ release have a large degree of overlap.
Figure 4. Overlap between the mitochondrial pools sensing Ca2+ entry through the plasma membrane and Ca2+ release from ER.
Sympathetic neurons expressing the high-Ca2+-affinity mitAEQ were repeatedly stimulated first with high K+ and then with caffeine (Cf; A) or first with caffeine and then with high K+ (B), as shown. The upper traces show aequorin consumption and lower traces show the calibrated signal. Values obtained in two extreme cells (large and small responses) and the average of all the cells present in the same microscope fields (circles) are shown. Data are representative of 7 (A) and 12 independent experiments (B).
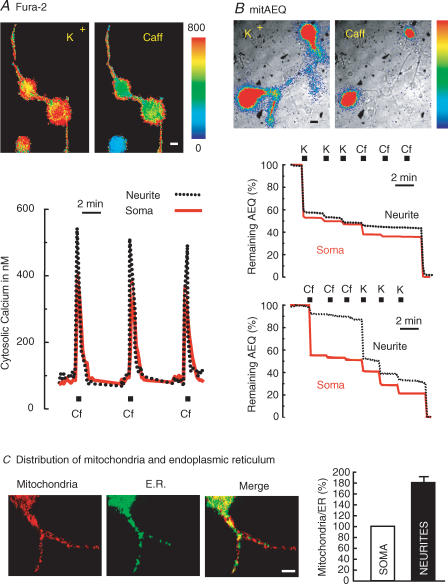
All the above observations refer to the mitochondria located in the soma. We then asked whether mitochondria located in the neurites would behave in a similar manner. To this end, we collected photonic emissions simultaneously in both neurites and soma of sympathetic neurons subjected to stimulation with high K+ and/or caffeine. Figure 5A summarizes the effects on [Ca2+]c, measured with fura-2. The [Ca2+]c increase induced by high K+ was similar in soma and neurites (Fig. 5A, K+ panel, and results not shown). Interestingly, the size of the [Ca2+]c increases induced by caffeine was significantly larger in the neurites than in the soma (Fig. 5A, Caff panel and recordings). In four independent experiments, the mean Δ[Ca2+]c values obtained after stimulation with caffeine were (mean ± s.e.m. of 6 cells) 325 ± 8 nm in the soma and 435 ± 27 nm in the neurites, respectively. The differences were statistically significant (P < 0.05; Student's paired t test). However, the duration of the peak and the rates of [Ca2+]c increase and decline were similar in the neurites and the soma.
Figure 5. Differential effects of stimulation with high K+ and caffeine in the cell body and the neurites.
A, effects on [Ca2+]c measured with fura-2 as described in Methods. Sixty-four 256 × 256 pixel images at alternate excitation wavelengths (340 and 380 nm) were averaged and ratioed by hardware every 5 s. Spatial resolution was of 1 μm per pixel. Results are representative of 4 similar experiments. B, effects on [Ca2+]m measured with mitAEQ (high-Ca2+-affinity system). The first high-K+ stimulation released 37 ± 4% of the total photonic emissions in neurites; the second and subsequent stimuli released 3 ± 1% (mean ± s.e.m. of 20 cells from 15 experiments). The first caffeine stimulus induced a very small aequorin consumption in neurites (5 ± 1% of total photonic emissions; mean ± s.e.m. of 22 cells from 16 experiments); the subsequent stimulation with high K+ consumed 21 ± 2% of aequorin. Regions of interest for measurements were defined selectively either in soma or neurites. Other details are as in Figs 1 and 2. C, analysis of relative content of mitochondria and ER in soma and neurites of sympathetic neurons. A sample of confocal slicing of a neuron expressing ER-GFP (green) and loaded with Mitotracker Red® (red) and the merged image are shown. The right-hand panel compares the contents of soma and neurites for 12 neurons (mean ± s.e.m.). The values are expressed as mitochondria/ER ratio and were normalized to 100% for the soma content in each case (*P < 0.001). Bars in images represent 10 μm.
In another set of experiments, the effects of sequential and repetitive stimulation with high K+ and caffeine on [Ca2+]m were measured using the mitochondrial aequorin (Fig. 5B). The outcome with high K+ was not surprising. As already shown for the soma in another experiment (Fig. 3B), the second and third stimuli were scarcely effective, and previous stimulation with K+ ‘occluded’ the further effect of caffeine. The behaviour in the neurites in response to stimulation with high K+ was similar (Fig. 5B). The results with caffeine were, however, most unexpected, since the effect of caffeine on [Ca2+]m was much smaller in the neurites than in the soma (Fig. 5B). Taken together, these results indicate that mitochondria in neurites are scarcely able to take up and buffer the Ca2+ released from the ER, even though they efficiently buffer the Ca2+ entering though VOCCs.
Finally, we attempted to quantify the relative contents of mitochondria and ER in both soma and neurites. To this end, sympathetic neurons were infected with a defective HSV-1 virus containing ER-targeted GFP. After 18 h of culture, these cells were loaded with Mitotracker Red® to selectively stain mitochondria and analysed by double-channel laser scanning confocal fluorescence microscopy (Fig. 5C). The amount of mitochondria relative to ER in both soma and neurites was quantified by dividing the Mitotracker Red® fluorescence signal coming from mitochondria by the green GFP fluorescence coming from the ER. The first two images in Fig. 5C show the labelling of mitochondria and ER, respectively, in two serial 1 μm optical sections (data representative of 18 cells). Mitochondria and ER were present in both the soma and the neurites. These results are in agreement with the ability to release aequorin photonic emissions from mitochondria-targeted aequorin both in the soma and in the neurites. The presence of ER at both locations, soma and neurites, is consistent with the ability of caffeine to increase [Ca2+]c at both locations (Fig. 5A). The relative abundance of mitochondria and ER in soma and neurites was, however, not uniform. Mitochondria seemed to be more abundant in neurites, as shown by the more extensive red labelling at this location (see merged image in Fig. 5C). The average of red/green fluorescence ratio in soma and neurites in 10 independent experiments is summarized in the bar graph in Fig. 5C. The values have been normalized for each neuron with regard to the value in the soma. On average, the mitochondria/ER ratio in the neurites was almost twice as large as in the soma (P < 0.001).
Discussion
Single-cell photon counting imaging of targeted aequorins reveals that mitochondria take up large amounts of Ca2+ during stimulation of mouse sympathetic neurons. Since mitochondrial aequorin is consumed when [Ca2+]m increases, the history of the different mitochondrial pools that contribute to Ca2+ accumulation during repetitive stimulation could be traced (Montero et al. 2000; Villalobos et al. 2002). As in chromaffin cells (Montero et al. 2000), most of the Ca2+ uptake takes place in a subpopulation of mitochondria, probably the ones closest to the sites of Ca2+ entry or Ca2+ release. The applicable dimensions have been estimated between 0.2 and 2 μm in adrenal chromaffin cells (Villalobos et al. 2002). These mitochondria would sense Ca2+ microdomains in which high enough concentrations are achieved to activate the low-affinity mitochondrial Ca2+ uniporter for fast transport inside the mitochondrial matrix. The progression of the Ca2+ wave towards the cell core is effectively shielded by this mitochondrial pool in such a way that the other mitochondrial pool senses and takes up much smaller amounts of Ca2+ (Figs 1 and 3). These results agree with previous studies using X-ray microanalysis, in which stimulated and rapidly frozen frog sympathetic neurons contained two different subpopulations of mitochondria with different Ca2+ levels (Pivovarova et al. 1999). This black and white view is somewhat artificial. A continuum of mitochondria with different Ca2+ uptakes must exist, but the transition between both ends of the population must be extremely steep because the rate of mitochondrial Ca2+ uptake depends exponentially on [Ca2+]c (Gunter & Pfeiffer, 1990; Rizzuto et al. 1993; Montero et al. 2000). This real bimodality is further exaggerated by aequorin, whose luminescence emission also depends exponentially on [Ca2+] (Alonso et al. 2006). Thus, the low-affinity aequorin system senses almost exclusively the high-Ca2+ mitochondrial pool, whereas the high-affinity aequorin system is quickly burned in this pool and, after a while, only detects the low-Ca2+ mitochondrial pool. In this way, by buffering the Ca2+ load, mitochondria can shape the cytosolic Ca2+ signal and exocytosis (Herrington et al. 1996; Montero et al. 2000; Friel, 2000; Villalobos et al. 2002; David & Barrett, 2003). Previous observations showing that mitochondrial depolarization, a manoeuvre that limits mitochondrial Ca2+ uptake, results in increased [Ca2+]c spikes induced by electric stimulation in sympathetic neurons (Peng, 1998; Friel, 2000) are consistent with this view. The mitochondrial pool sensing Ca2+ entry through VOCCs overlapped with the one that accumulated Ca2+ after Ca2+ release through RyRs. These results suggest that, as in chromaffin cells (Montero et al. 2000), plasma membrane channels, ER release sites and mitochondria group spatially in triads or functional units that activate jointly, regardless of whether the Ca2+ originates extra- or intracellularly. Consistent with this view, local Ca2+ release events evoked by low caffeine concentrations have recently been reported to take place near the plasma membrane in the soma of superior cervical ganglion neurons (Yao et al. 2006).
It has been proposed that, in addition to Ca2+ fluxes through Ca2+ channels at the plasma membrane or the ER, other modulatory processes may contribute to generate Ca2+ signals with specific spatial location (Johenning et al. 2002). In dorsal root ganglion (DRG) neurons, mitochondria seem to buffer Ca2+ entering through plasmalemmal channels more efficiently than Ca2+ released from the ER (Svichar et al. 1997). We have investigated here whether redistribution of Ca2+ among different subcellular compartments is similar in the soma and neurites inside single sympathetic neurons. Our bioluminescence imaging approach enables photonic emissions from mitochondria in soma and neurites to be dissected. As in the soma, the behaviour in neurites was not homogeneous with regard to VOCCs, and we identified two different populations of mitochondria. One pool accumulated large amounts of Ca2+, whereas the other took up much smaller amounts. In contrast, the mitochondria in neurites differed from those in the soma in that they were not able to sense Ca2+ release induced by caffeine, as revealed by the high-Ca2+-affinity aequorin (Fig. 5B). This did not result from a lack of RyRs in neurites, since caffeine was able to increase [Ca2+]c at this location (Fig. 5A), and previous reports have also demonstrated the presence of functional RyRs in the presynaptic terminals of sympathetic neurons (Peng, 1996). In contrast, ER-directed GFP was also expressed in neurites (Fig. 5C). Thus, even though mitochondria are more abundant in neurites than in the soma (Fig. 5C), mitochondria in neurites are more loosely coupled to ER and do not efficiently buffer the Ca2+ released through RyRs. This may explain why the [Ca2+]c signal induced by caffeine is larger in the neurites than in the soma (Fig. 5A). Interestingly, other authors have also reported that Ca2+ signals may be larger in neurites than in the soma in a variety of cell models (Svichar et al. 1997; Koizumi et al. 1999; Johenning et al. 2002; Yao et al. 2006). For example, interference of mitochondrial Ca2+ uptake with carbonyl cyanide m-chlorophenylhyrazone (CCCP) modulated [Ca2+]c transients induced by Ca2+ entry through VOCC channels, but not those resulting from caffeine-induced release of Ca2+ from ER in DRG neurons (Svichar et al. 1997). In addition, the somatic Ca2+ response to carbachol in differentiated PC12 cells displayed a much shallower slope and smaller amplitude than the response obtained in the neurites (Johenning et al. 2002). These differences were not attributable to differences in the Ca2+ contents of the ER in the neurites and soma, and the authors suggested that additional, unrecognized modulatory processes are necessary to generate spatially specific calcium signals. Global Ca2+ signals in neurons are made of elementary signals, which have been studied in great detail in both differentiated PC12 cells (Koizumi et al. 1999) and superior cervical ganglion neurons (Yao et al. 2006). In fact, the so-called elementary events (Ca2+ sparks and glows) were reported to be larger and more frequent in the neurites than in the soma of these neurons (Yao et al. 2006). The larger responses of these neurites could be at least partly explained by the smaller Ca2+-buffering capacity of the mitochondria in the neurites. Our data are consistent with previous suggestions (Svichar et al. 1997) attributing the smaller buffering capacity to peculiar localization of mitochondria, far away from the ER release sites, rather than to functional differences in Ca2+ handling by the mitochondrial uniporter. Calcium-induced Ca2+ release is believed to play an important role in the action potential-evoked release of neurotransmitter in postganglionic sympathetic nerve terminals (Smith & Cunnane, 1996) and presynaptic neuromuscular terminals (Narita et al. 2000). We can speculate that the lack of mitochondria close enough to ER could result in local higher [Ca2+]c increases at the synaptic sites. Thus, strategic location of mitochondria may shape cytosolic Ca2+ signals differentially in soma and neurites.
Acknowledgments
This work was supported by grants from Junta de Castilla y León (VA071/02; VA022A05), Ministerio de Educación y Ciencia (MEC; BFU2004-02764), Fondo de Investigaciones Sanitarias FIS 04/1510 and Ministerio de Sanidad, Instituto de Salud Carlos III, Red de Terapia Celular. L.N. is fellow of the Ramón y Cajal Program (MEC and FEDER-FSE). L.S. is the recipient of a predoctoral fellowship from MEC. Pablo Chamero held a predoctoral fellowship from the Basque Government.
References
- Alonso MT, Barrero MJ, Carnicero E, Montero M, García-Sancho J, Alvarez J. Functional measurements of [Ca2+] in the endoplasmic reticulum using a herpes virus to deliver targeted aequorin. Cell Calcium. 1998;24:87–96. doi: 10.1016/s0143-4160(98)90076-8. [DOI] [PubMed] [Google Scholar]
- Alonso MT, Villalobos C, Chamero P, Alvarez J, García-Sancho J. Calcium microdomains in mitochondria and nucleus. Cell Calcium. 2006;40:513–525. doi: 10.1016/j.ceca.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Alvarez J, Montero M. Ca2+ measurements with luminescent proteins in the endoplasmic reticulum. In: Petersen OH, editor. Measuring Calcium and Calmodulin Inside and Outside Cells. Springer Verlag Berlin: Springer Laboratory Manual; 2001. pp. 147–163. [Google Scholar]
- Alvarez J, Montero M, García-Sancho J. Subcellular Ca2+ dynamics. News Physiol Sci. 1999;14:161–168. [PubMed] [Google Scholar]
- Babcock DF, Herrington J, Park YB, Hille B. Mitochondrial participation in the intracellular Ca2+ network. J Cell Biol. 1997;136:833–843. doi: 10.1083/jcb.136.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- Clapham DE. Calcium signaling. Cell. 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- David G, Barrett EF. Mitochondrial Ca2+ uptake prevents desynchronization of quantal release and minimizes depletion during repetitive stimulation of mouse motor nerve terminals. J Physiol. 2003;548:425–438. doi: 10.1113/jphysiol.2002.035196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen MR. Contributions of mitochondria to animal physiology: from homeostatic sensor to calcium signaling and cell death. J Physiol. 1999;516:1–17. doi: 10.1111/j.1469-7793.1999.001aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frawley LS, Faught WJ, Nicholson J, Moomaw B. Real time measurement of gene expression in living endocrine cells. Endocrinology. 1994;135:468–471. doi: 10.1210/endo.135.1.8013386. [DOI] [PubMed] [Google Scholar]
- Friel DD. Mitochondria as regulators of stimulus-evoked calcium signals in neurons. Cell Calcium. 2000;28:307–316. doi: 10.1054/ceca.2000.0172. [DOI] [PubMed] [Google Scholar]
- Friel DD, Tsien RW. A caffeine- and ryanodine-sensitive Ca2+ store in bullfrog sympathetic neurons modulates effects of Ca2+ entry on [Ca2+] J Physiol. 1992;450:217–246. doi: 10.1113/jphysiol.1992.sp019125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Greenberg ME. Calcium signaling in neurons: molecular mechanisms and cellular consequences. Science. 1995;268:239–247. doi: 10.1126/science.7716515. [DOI] [PubMed] [Google Scholar]
- Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. Am J Physiol Cell Physiol. 1990;258:C755–C786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- Hernández-Cruz A, Díaz-Muñoz M, Gómez-Chavarin M, Canedo-Merino R, Protti DA, Escobar AL, Sierralta J, Súarez-Isla BA. Properties of the ryanodine-sensitive release channels that underlie caffeine-induced Ca2+ mobilization from intracellular stores in mammalian sympathetic neurons. Eur J Neurosci. 1995;7:1684–1699. doi: 10.1111/j.1460-9568.1995.tb00690.x. [DOI] [PubMed] [Google Scholar]
- Hernández-Cruz A, Sala F, Adams PR. Subcellular Ca2+ transients visualizad by confocal microscopy in a voltage-clamped vertebrate neuron. Science. 1990;247:858–862. doi: 10.1126/science.2154851. [DOI] [PubMed] [Google Scholar]
- Herrington J, Park YB, Babcock DF, Hille B. Dominant role of mitochondria in clearance of large Ca2+ loads from rat adrenal chromaffin cells. Neuron. 1996;16:219–228. doi: 10.1016/s0896-6273(00)80038-0. [DOI] [PubMed] [Google Scholar]
- Johenning FW, Zochowski M, Conway SJ, Holmes AB, Koulen P, Ehrlich BE. Distinct intracellular calcium transients in neurites and somata integrate neuronal signals. J Neurosci. 2002;22:5344–5353. doi: 10.1523/JNEUROSCI.22-13-05344.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- Koizumi S, Bootman MD, Bobanovic LK, Schell MJ, Berridge MJ, Lipp P. Characterization of elementary Ca2+ release signals in NGF-differentiated PC12 cells and hippocampal neurons. Neuron. 1999;22:125–137. doi: 10.1016/s0896-6273(00)80684-4. [DOI] [PubMed] [Google Scholar]
- Martínez JA, Lamas JA, Gallego R. Calcium current components in intact and dissociated adult mouse sympathetic neurons. Brain Res. 2002;951:227–236. doi: 10.1016/s0006-8993(02)03165-7. [DOI] [PubMed] [Google Scholar]
- Montero M, Alonso MT, Carnicero E, Cuchillo-Ibáñez I, Albillos A, García AA, García-Sancho J, Alvarez J. Chromaffin-cell stimulation triggers fast millimolar mitochondrial Ca2+ transients that modulate secretion. Nature Cell Biol. 2000;2:57–61. doi: 10.1038/35000001. [DOI] [PubMed] [Google Scholar]
- Narita K, Akita T, Hachisuka J, Huang S, Ochi K, Kuba K. Functional coupling of Ca2+ channels to ryanodine receptors at presynaptic terminals. Amplification of exocytosis and plasticity. J Gen Physiol. 2000;115:519–532. doi: 10.1085/jgp.115.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Núñez L, García-Sancho J. Two different constituents of plasma increase cytosolic calcium selectively in neurons or glia of primary rat cerebellar cultures. J Physiol. 1996;490:577–583. doi: 10.1113/jphysiol.1996.sp021168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton C, Thompson S, Epel D. Some precautions in using chelators to buffer metals in biological solutions. Cell Calcium. 2004;35:427–431. doi: 10.1016/j.ceca.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Peng Y. Ryanodine-sensitive component of calcium transients evoked by nerve firing at presynaptic nerve terminals. J Neurosci. 1996;16:6703–6712. doi: 10.1523/JNEUROSCI.16-21-06703.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YY. Effects of mitochondrion on calcium transients at intact presynaptic terminals depend on frequency of nerve firing. J Neurophysiol. 1998;80:186–195. doi: 10.1152/jn.1998.80.1.186. [DOI] [PubMed] [Google Scholar]
- Pivovarova NB, Hongpaisan J, Andrews SB, Friel DD. Depolarization-induced mitochondrial Ca2+ accumulation in sympathetic neurons: spatial and temporal characteristics. J Neurosci. 1999;19:6372–6384. doi: 10.1523/JNEUROSCI.19-15-06372.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Brini M, Murgia M, Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262:744–747. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- Robb-Gaspers LD, Burnett P, Rutter GA, Denton RM, Rizzuto R, Thomas AP. Integrating cytosolic calcium signals into mitochondrial metabolic responses. EMBO J. 1998;17:4987–5000. doi: 10.1093/emboj/17.17.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter GA, Burnett P, Rizzuto R, Brini M, Murgia M, Pozzan T, Tavaré JM, Denton RM. Subcellular imaging of intramitochondrial Ca2+ with recombinant targeted aequorin: significance for the regulation of pyruvate dehydrogenase activity. Proc Natl Acad Sci U S A. 1996;93:5489–5494. doi: 10.1073/pnas.93.11.5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura O, Musicki B, Kishi Y, Inouye S. Light-emitting properties of recombinant semi-synthetic aequorins and recombinant fluorescein-conjugated aequorin for measuring cellular calcium. Cell Calcium. 1993;14:373–378. doi: 10.1016/0143-4160(93)90041-4. [DOI] [PubMed] [Google Scholar]
- Smith AB, Cunnane TC. Ryanodine-sensitive calcium stores involved in neurotransmitter release from sympathetic nerve terminals of the guinea-pig. J Physiol. 1996;497:657–664. doi: 10.1113/jphysiol.1996.sp021797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svichar N, Kostyuk P, Verkhratsky A. Mitochondria buffer Ca2+ entry but not intracellular Ca2+ release in mouse DRG neurons. Neuroreport. 1997;8:3929–3932. doi: 10.1097/00001756-199712220-00017. [DOI] [PubMed] [Google Scholar]
- Thayer SA, Hirning LD, Miller RJ. The role of caffeine-sensitive calcium stores in the regulation of the intracellular free calcium concentration in rat sympathetic neurons in vitro. Mol Pharmacol. 1988;34:664–673. [PubMed] [Google Scholar]
- Villalobos C, Nadal A, Núñez L, Quesada I, Chamero P, Alonso MT, García-Sancho J. Bioluminescence imaging of nuclear calcium oscillations in intact pancreatic islets of Langerhans from the mouse. Cell Calcium. 2005;38:131–139. doi: 10.1016/j.ceca.2005.06.029. [DOI] [PubMed] [Google Scholar]
- Villalobos C, Núñez L, Chamero P, Alonso MT, García-Sancho J. Mitochondrial [Ca2+] oscillations driven by local high [Ca2+] domains generated by spontaneous electric activity. J Biol Chem. 2001;276:40293–40297. doi: 10.1074/jbc.C100465200. [DOI] [PubMed] [Google Scholar]
- Villalobos C, Núñez L, Montero M, García AG, Alonso MT, Chamero P, Alvarez J, García-Sancho J. Redistribution of Ca2+ among cytosol and organella during stimulation of bovine chromaffin cells. FASEB J. 2002;16:343–353. doi: 10.1096/fj.01-0630com. [DOI] [PubMed] [Google Scholar]
- Werth JL, Thayer SA. Mitochondria buffer physiological calcium loads in cultured rat dorsal root ganglion neurons. J Neurosci. 1994;14:346–356. doi: 10.1523/JNEUROSCI.14-01-00348.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Naraghi M, Kang H, Neher E. Kinetic studies of Ca2+ binding and Ca2+ clearance in the cytosol of adrenal chromaffin cells. Biophys J. 1997;73:532–545. doi: 10.1016/S0006-3495(97)78091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Wang G, Ou-Yang K, Wei C, Wang X, Wang S, Yao W, Huang H, Luo J, Wu C, Liu J, Zhuan Z, Cheng H. Ca2+ sparks and Ca2+ glows in superior cervical ganglion neurons. Acta Pharmacol Sin. 2006;7:848–852. doi: 10.1111/j.1745-7254.2006.00402.x. [DOI] [PubMed] [Google Scholar]