Abstract
Prestin, a member of the solute carrier (SLC) family SLC26A, is the molecular motor that drives the somatic electromotility of mammalian outer hair cells (OHCs). Its closest reported homologue, zebrafish prestin (zprestin), shares ∼70% strong amino acid sequence similarity with mammalian prestin, predicting an almost identical protein structure. Immunohistochemical analysis now shows that zprestin is expressed in hair cells of the zebrafish ear. Similar to mammalian prestin, heterologously expressed zprestin is found to generate voltage-dependent charge movements, giving rise to a non-linear capacitance (NLC) of the cell membrane. Compared with mammalian prestin, charge movements mediated by zprestin display a weaker voltage dependence and slower kinetics; they occur at more positive membrane voltages, and are not associated with electromotile responses. Given this functional dissociation of NLC and electromotility and the structural similarity with mammalian prestin, we anticipate that zprestin provides a valuable tool for tracing the molecular and evolutionary bases of prestin motor function.
Active mechanical feedback assists hearing in vertebrates and invertebrates (Manley, 2000; Göpfert & Robert, 2003; Göpfert et al. 2005, 2006; for a review, see Manley, 2001; Geleoc & Holt, 2003). A prominent actuator of such amplificatory feedback is the somatic electromotility of outer hair cells (OHCs), which, besides active hair bundle motility (Chan & Hudspeth, 2005; Kennedy et al. 2005; for a review, see Fettiplace, 2006), is widely thought to promote cochlear amplification in mammalian ears (Jia & He, 2005; for a review, see Fettiplace & Hackney, 2006). The molecular basis of OHC somatic electromotility is formed by the unconventional motor protein named prestin (Zheng et al. 2000; Liberman et al. 2002). Prestin molecules are densely packed into the OHC lateral membrane, where they may form supramolecular motor complexes (Navaratnam et al. 2005). These elementary motors are thought to respond to changes in membrane potential with concerted conformational changes that eventually build up to an overall motion of the OHC soma. The underlying elementary event, i.e. the conformational transition of the single prestin molecules between an expanded and a contracted state, involves the translocation of a charged voltage sensor across the membrane. Prestin motor activity is thus inherently electrogenic, with the protein converting voltage changes into movements, much like the piezoelectric crystal that was initially proposed by Gold (1948) to be at work in the cochlea. The voltage-dependent charge movement conferred by prestin's voltage sensor can be measured as a non-linear capacitance (NLC) of the cell membrane. Since this NLC is linked to motility and can easily be assayed experimentally, it is often used as a substitute for direct measurements of the somatic motility of outer hair cells and prestin-transfected cells (for a review, see Dallos & Fakler, 2002).
Molecularly, prestin is affiliated to the highly versatile SLC26 family of anion transporters (for a review, see Mount & Romero, 2004). Presently, 10 mammalian SLC26A members (A1–A10) are known. The closest reported homologue to mammalian prestin (SLC26A5), as assessed by over-all sequence identity, however, is found in zebrafish (Weber et al. 2003). The zebrafish prestin orthologue, zprestin, shares ∼50% amino acid identity with mammalian prestin, compared with an amino acid identity of only ∼37% between mammalian prestin and its closest paralogue PAT-1 (SLC26A6). This molecular kinship with the mammalian prestin motor, along with the presence of zprestin transcripts in the zebrafish auditory organ (Weber et al. 2003), have raised the principal question whether zprestin constitutes a hair cell motor as well (Weber et al. 2003), thus challenging the generally held view that prestin-mediated electromotility is a unique property of mammalian OHCs (He et al. 2003). To test this hypothesis, we systematically explored the molecular and functional properties of zprestin, including its genomic structure, its expression, and its putative motor function, whereby the latter was assayed by probing for NLC and electromotility in zprestin-transfected cells. A parallel study investigated the role of zprestin in anion transport (Schaechinger & Oliver, in press).
Methods
All experiments conformed to the European Community guiding principles on the care and use of animals (86/609/CEE) and were in accordance with the institutional guidelines for the use of animals in research.
Animals
Wildtype larvae and adult fish of the AB strain were used for immunohistochemistry and in situ hybridizations. Larvae were produced by pair-wise breeding and raised in embryo medium (5 mm NaCl, 0.17 mm KCl, 0.33 mm CaCl2, 0.33 mm MgSO4) at 30°C. Adult males were taken from laboratory stocks. Zebrafish were maintained and staged as described elsewhere (Kimmel et al. 1995).
Cloning of zprestin
For measurements of NLC and sequence analysis the zebrafish full-length cDNA was cloned from zebrafish utricle and lagena. Procedures to isolate mRNA are described in Weber et al. (2003). The 3′-end of zebrafish prestin was determined using the GeneRacer Kit (Invitrogen) according to manufacturer's instructions, using the specific primers Dan3, 5′-GCTGTCAACGATAC-GCTACGTAACG-3′, and Dan3nested, 5′-CGCTACGTA-ACGGCATGACAGTG-3′. The full-length cDNA was amplified with the forward primer DanF1, 5′-TGCAGC-CATGGAGCACGTAACTGTTAG-3′, and the reverse primers DanR5, 5′-TTGTGTTCAGTGGATGTTTGGG-TGGAC-3′, respectively, DanR7, 5′-GTTCCAGC-ACAACGCCATCCATATTAG-3′ and cloned into the pCRII TOPO vector (Invitrogen) following manufacturer's instructions. To generate a GFP fusion-protein the stop codon of the full-length clone was deleted by PCR using the primers DanPres_USP_ApaI, 5′-TCCGGGCCCGCCATGGAGCACGTAACTGTTAG-CGAGG-3′, and DanPres_DSP_BamHI, 5′-CGCGGAT-CCGTGGATGTTTGGGTGGACGGGAAG-3′. The PCR product was cloned into the ApaI and BamHI sites of the expression vector pEGFP-N3 (BD Biosciences). Correct orientation and reading frame were confirmed by sequence analysis.
Tissue preparation and histology
For immunohistochemistry, adult male fish were over-anaesthetized with 0.02% 3-aminobenzoic acid ethyl ester until complete cessation of movements. The anterior part of the animal was subsequently dissected and fixed with 4% paraformaldehyde in PBS for 24 h at 4°C. The specimen was then rinsed with PBS and decalcified with 0.5 m EDTA in PBS (pH 7.8) for 7 days at 4°C. Prior to embedding in TissueTek OCT compound (Sakura, Zoeterwounde, the Netherlands) the specimen was immersed in 25% sucrose in PBS for 12 h at 4°C. After freezing the specimen at −20°C, cross sections, 25 μm in thickness, were made on a Reichert-Jung 2800 Frigocut E (Heidelberg, Germany), collected on Superfrost/Plus slides (Menzel, Braunschweig, Germany), dried for 1 h at room temperature (RT) and stored at −20°C until used.
For standard histology, specimens were fixed and embedded as described above. Cryosections were cut to a thickness of 10 μm and dried for 2 h. Sections were washed in deionized water, treated with potassium permanganate and oxalic acid, followed by staining with Eosin Y, Orange G and toluidine blue, dehydration and mounting.
Generation of zprestin-specific antisera
To produce polyclonal primary antibodies against zprestin (Accession No. NP_958881), rabbits were immunized with synthetic peptides corresponding to portions of the N- or C- terminal domain of the zebrafish prestin (N-terminal residues 32–46 (LHKRKKTPKPYKLR) for antibody anti-N-zprestin and residues 617–637 (NGPQKPKHVHTNGQMTEKHIE) for antibody anti-C-zprestin). Antibodies were affinity purified against corresponding peptides.
Immunohistochemistry
Cross sections of the zebrafish ear were thawed and dried for 1 h at RT. The sections were briefly washed in PBS, permeabilized with 0.5% Triton X-100 in PBS for 5 min, and blocked for 1 h with blocking solution (5% normal goat serum, 5% bovine serum albumin, in PBS). Goat-polyclonal anti-GFP antibodies were purchased from Santa Cruz Biotechnology Inc. (sc-5384 and sc-5385). Primary antibodies were diluted in the reaction buffer (2% NaCl, 0.5% normal goat serum, 0.25% bovine serum albumin, 0.1% Triton X-100 in PBS) and incubated overnight at 4°C. Subsequently, sections were washed 3 × 10 min with PBS and incubated with the secondary antibody AlexaFluor 633 goat anti-rabbit (Molecular Probes, Eugene, USA) at a dilution of 1: 1000 in reaction buffer for 1 h at RT. After a brief wash with PBS, the sections were counterstained with AlexaFluor 488 phalloidin (Molecular Probes) at a concentration of 2.5 mg ml−1, washed 3 × 10 min with PBS, mounted and imaged using a Zeiss 510 LSM Meta (Oberkochen, Germany) using a 40 × oil lens.
Riboprobe synthesis and in situ hybridization
zprestin specific riboprobes were in vitro transcribed following standard protocols. Whole-mount in situ hybridization procedures were performed according a modified method (Harland, 1991; Thisse et al. 1993), with the following further modifications. The described hybridization buffer was replaced with microarray-hyb-buffer (Amersham Bioscience Buckinghamshire, UK), and a different blocking solution (Roche, Grenzach-Wyhlen, Germany) was used.
Electrophysiology
For electrophysiological experiments, the expression plasmids pEGFP-N1-rprestin or pEGFP-N3-zprestin were transfected into CHO cells using the Metafectene (Biontex; Munich, Germany) or JetPEI (Polyplus transfection; Illkirch, France) transfection reagent according to the manufacturers' instructions. Whole-cell patch-clamp recordings were carried out at RT (22–24°C) with an EPC10 patch clamp amplifier (Heka; Lambrecht, Germany) 24–48 h after transfection. Electrodes were pulled from quartz glass to resistances of 1.5–2.5 MΩ and coated with Sylgard. Whole-cell series resistances ranged from 2 to 8 MΩ. Electrodes were filled with a solution containing (mm): 135 KCl, 3.5 MgCl2, 0.1 CaCl2, 5 K2EGTA, 5 Hepes, 2.5 Na2ATP (pH 7.3). In some experiments a simplified pipette solution (160 CsCl, 1 Hepes, 1 K2EGTA, pH 7.3) was used, yielding identical results. Extracellular solution was (mm): 144 NaCl, 5.8 KCl, 1.3 CaCl2, 0.9 MgCl2, 10 Hepes, 0.7 Na2HPO4, 5.6 glucose, adjusted to pH 7.4 with NaOH.
Current and capacitance recordings were made with the Patchmaster acquisition software (Heka). For recordings of charge transfer, all linear current components were subtracted using a P/−8 protocol with negative P/over pulses at −100 mV for zprestin and positive P/over pulses at +50 mV for rat prestin (rprestin) (for a more detailed description, please refer to Armstrong & Bezanilla, 1974). Currents were low-pass filtered at cut-off frequencies of 3 or 5 kHz and sampled at 50 kHz. Kinetics of charge transfer were determined by fitting the time courses of the current relaxations with monoexponential curves. For these recordings, serial resistance (Rs) compensation was applied to speed up the voltage clamp yielding clamp time constants < 20 μs (Cm=7–15 pF, Rs=2.2–3.6 MΩ), and currents were low-pass filtered with a cut-off at 10 kHz. Charge was obtained by integration of the averaged transient currents over time and plotted versus membrane potential. Charge–voltage plots were fitted with a two-state Boltzmann function,
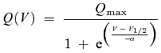 |
(1) |
where V is membrane potential, Qmax is maximum voltage sensor charge moved through the membrane electrical field, V1/2 is the voltage at half-maximum charge transfer and α is the slope factor describing the voltage dependence.
Voltage-dependent capacitance was measured using the lock-in extension of Patchmaster in the sine+DC mode with current filter set to 10 kHz. Voltage ramps from −150 mV (or −130) to +100 mV (duration: 0.5 s) were summed to the sinusoid command during capacitance measurements to obtain voltage dependence. Capacitance traces were plotted versus membrane voltage and fitted with the derivative of a Boltzmann function (Deak et al. 2005),
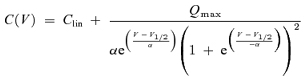 |
(2) |
where Clin is the residual linear membrane capacitance. Processing and fitting of data was performed with IgorPro (Wavemetrics, Lake Oswego, OR, USA). All values are given as mean ± standard deviation (s.d.).
Motility measurements
HEK293 cells were transiently transfected with the expression plasmids for zebrafish prestin (pEGFP-N3-zprestin) or gerbil prestin (pEGFP-N2-gprestin; see Zheng et al. 2000 for details). To confirm proper membrane targeting of zprestin, transfected HEK cells were probed for voltage-dependent charge movements and a corresponding non-linear capacitance of the cell membrane with an AC technique that has been described elsewhere (Santos-Sacchi et al. 1998). In brief, it utilized a continuous high-resolution (2.56 ms) two-sine voltage stimulus protocol (10 mV peak at both 390.6 and 781.2 Hz), with subsequent FFT-based admittance analysis. The high frequency sinusoids were superimposed on voltage ramp stimuli. Capacitance data were fitted to the first derivative of a two-state Boltzmann function eqn (2). The resting membrane potentials of zprestin-transfected HEK cells were measured by whole-cell patch-clamp recordings in a separate subset of cells that was not subjected to motility experiments. Values were taken immediately after rupture of the cell membrane (−8.1 ± 4.1 mV, N=17) and are thus likely to reflect the membrane potentials of unruptured cells (as used for the motility experiments). Electromotility was probed in the microchamber configuration. A detailed description of the microchamber technique is given elsewhere (He et al. 1994). In brief, a suction pipette or microchamber was used to both mechanically hold the cell and deliver voltage commands. Microchambers were fabricated from 1.5 mm thin-wall glass tubes (WPI, Inc.) by a Flaming/Brown Micropipette Puller (Sutter Instrument Company, Model P-97) and heat-polished to an aperture diameter of ∼13–15 μm. The microchamber, with a series resistance of approximately 0.3–0.4 mΩ, was mounted in an electrode holder, which was held by a Leitz 3-D micromanipulator (Leitz, Germany). By moving the microchamber, cells in the bath could be picked up easily. The inserted cell and the microchamber formed a resistive seal (3–4mΩ) that was mechanically stable. Motility was measured and calibrated by a photodiode-based measurement system mounted on the Leica microscope (Jia & He, 2005). The magnified image of the edge of the cell was projected onto a photodiode through a rectangular slit. Somatic length changes, evoked by voltage stimuli, modulated the light influx to the photodiode. The photocurrent response was calibrated to displacement units by moving the slit a fixed distance (0.5 μm) with the image of the cell in front of the photodiode. After amplification, the photocurrent signal was low-pass filtered by an anti-aliasing filter before being digitized by a 16-bit A/D board (Digidata 1322A, Axon Instrument). The photodiode system had a cutoff (3 dB) frequency of 1100 Hz. The sampling frequency was 5 kHz. With an averaging of 400 trials and low-pass filtering set at 400 Hz, movement amplitudes as low as 3 nm could be detected. The electrical stimulus was a sinusoidal voltage burst of 100 ms duration. Voltage commands of 380 mV (peak to peak) were used. Since the cells were approximately 60% inserted into the microchamber, the resultant voltage drops on the extruded segment were estimated to be 60% of the voltage applied, or 228 mV (cf. Dallos et al. 1991).
Results
Exonic and molecular structure
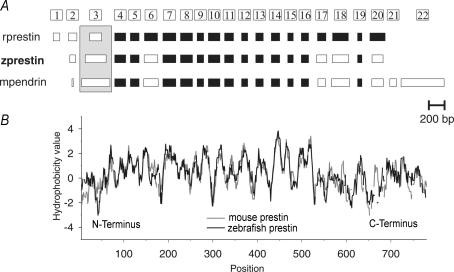
Functional diversification within a given gene family is deemed to rely on sequential, genomic duplication events (Hughes, 2005). Comparisons of genomic and molecular structures can therefore be used to gain insights into the evolutionary relations within families of genes. We compared the exonic organization of zprestin with some of its mammalian SLC26A homologues (rprestin, mpendrin; Fig. 1A) using the exon structure of mouse prestin as a template The sizes of exons 4–5, and 7–16 are conserved between all four SLC26A members. The lengths of exons 6, 17, 18 and 20, as well as the presence of two untranslated exons are conserved among the mammalian prestin genes but not shared by zprestin and mpendrin. The termination of zprestin after exon 20, which results in 18 coding exons only, however, is a unique feature of zebrafish and mammalian prestin. Sequence alignment between zprestin and mouse prestin (not shown) reveals an identity of 52.1%, and a strong similarity (i.e. substitution with identical or physico-chemically similar amino acids; alignment calculated with the CustalW program (Chenna et al. 2003)) of 71.7%. Hydrophobicity analysis indicates that this sequence similarity extends to protein topology (Fig. 1B). The hydrophobic core regions of mouse and zebrafish prestin, where sequence conservation is especially high, closely match (Fig. 1B). Presumably, these central parts of the proteins correspond to regions of transmembrane segments (TMs). In line with this, computational algorithms yield the same number of TMs for both prestin orthologues (predictions of web-based programs: TMHMM ver.1.0: 10 TMs for both mouse and zebrafish prestin, LOOPP ver.3.0: 12 TMs for both mouse and zebrafish prestin; for more information on prestin membrane topologies see Oliver et al. 2001; Zheng et al. 2001; Deak et al. 2005; Navaratnam et al. 2005). Although the actual membrane topology has not yet been solved for any of the SLC26A members experimentally, exonic and molecular analyses further document the close relationship between zebrafish and mammalian prestin, suggesting that zebrafish and mammalian prestin evolved from a prestin-like ancestor in the course of SLC26 evolution.
Figure 1. Exonic and molecular analysis of zprestin.
A, comparison of the exonic structures of zebrafish prestin (GenBank Acc. No. AAH54604), rat prestin (AJ303372), and mouse pendrin (AF167411) using the exon structure of mouse prestin (AF529192) as a template. Exon sizes identical to mouse prestin are highlighted in black, differing exons are marked in white. The first coding exon is boxed. Note that exon sizes of zprestin more closely resemble those of mpendrin, whereas the number of translated exons is identical to that of mammalian prestins. B, aligned hydrophobicity profiles of zebrafish and mouse prestin (Kyte–Doolittle, window=9). The strong sequence similarity between mouse and zebrafish prestin (71.7%) extends to the hydrophobicity profile. The mutual agreement is particularly high in the central hydrophobic core region, presumably corresponding to transmembrane segments.
Heterologously expressed zprestin is targeted to the plasma membrane
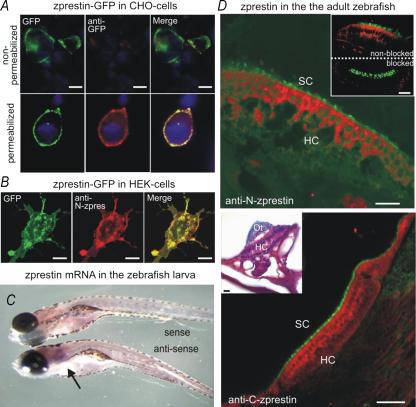
The motor function of mammalian prestin crucially relies on its integration into the cell membrane. Such membrane localization was observed for zprestin when a zprestin–GFP fusion protein (C-terminal GFP) was expressed in HEK cells (Fig. 2B). Cells transfected with the empty GFP expression vector displayed only cytoplasmic fluorescence (not shown). Labelling of zprestin-transfected cells with a GFP-specific antibody was only successful after permeabilization of the cells (Fig. 2A, lower middle and right). Apparently, zprestin, as also suggested by topology predictions, shares the intracellular localization of the C-terminus previously determined for mammalian prestin (Ludwig et al. 2001; Zheng et al. 2001).
Figure 2. Expression analysis of zprestin.
A, in accordance with zprestin topology predictions GFP fluorescence is distributed around the plasma membrane of zprestin–GFP transfected CHO cells (Left). Without permeabilization, zprestin–GFP is not detected by antibodies directed against the C-terminal GFP tag of the zprestin–GFP fusion protein (upper middle). After permeabilization, immunohistochemistry with anti-GFP antibodies produces a clear signal (red) that localizes to the cell membrane (lower middle). Merged images of the GFP (green) and anti-GFP (red) fluorescence channels of non-permeabilized (upper right) and permeabilized (lower right) cells showing co-localization of GFP and anti-GFP fluorescent signals in permeabilized cells (yellow in lower right). Cells were counterstained with DAPI (blue) (bars=10 μm). B, zprestin immunoreactivity co-localizes with the GFP signal in zprestin–GFP transfected HEK cells. C, in situ hybridization with a zprestin-specific riboprobe detects transcripts in the ear of wildtype larvae (arrow). No such signals were detected after hybridization with the sense probe. D, double fluorescence labelling of F-actin (green, phalloidin) and zprestin (red) with anti-zprestin antibodies directed against the N-terminus (upper) and C-terminus (lower) of the protein. F-actin localizes to the stereocilia; zprestin appears distributed throughout the hair cells. No signals were seen in peptide blocked controls (upper inset). Toluidine blue/Eosine Y/Orange G overview staining of a cross section depicting a row of zebrafish hair cells and overlying remains of the otolith (lower inset). HC, hair cell; SC, stereocilia; Ot, otolith (all bars=20 μm).
zprestin is expressed in hair cells of the zebrafish ear
To test whether zprestin occurs in hair cells of the zebrafish ear, its expression was probed by in situ hybridization and immunohistochemical analysis. Whole-mount in situ hybridizations with a zprestin-specific riboprobe detected transcripts in the ears of wildtype larvae (Fig. 2C, lower). Hybridization with the sense probe did not reveal any positive signals (Fig. 2C, upper). Two antibodies, one directed against the N-terminus and the other directed against the C-terminus of the protein, were generated and used to assay the localization of zprestin in hair cells of the zebrafish ear. Hair cells were identified by phalloidin counter staining of their actin-rich hair bundles. The two antibodies produced signals in auditory hair cells (Fig. 2D). These signals were not seen in peptide-blocked controls (Fig. 2D, upper inset). Interestingly, the antibody stainings indicate that zprestin is distributed throughout the hair cells, hampering the estimation of the degree of membrane localization. This subcellular staining pattern contrasts with the exclusive membrane localization of prestin in mammalian OHCs, but resembles the somatic localization of prestin reported for mammalian vestibular hair cells (Adler et al. 2003). Since structural predictions and heterologous expression analysis unambiguously identify both mammalian and zebrafish prestin as membrane proteins, the cytoplasmic distributions are unexpected. Whether they are related to protein recycling/processing or result from a degradation of membrane proteins in the course of tissue preparation, remains unclear.
zprestin confers NLC to transfected cells
A hallmark of electromotility mediated by mammalian prestin is a voltage-dependent transfer of charges across the cell membrane that is associated with electromotility. Such charge transfer can be measured directly as a transient current at the onset of a voltage pulse (similar to an ion channel's gating current) or as the resultant non-linear capacitance (NLC) of the cell membrane (Santos-Sacchi, 1991). We tested whether a similar charge transfer is mediated by zprestin. CHO cells transfected with GFP-tagged zprestin and displaying strong membrane fluorescence were selected for the electrophysiological measurements. When depolarized by a voltage step, zprestin-transfected cells displayed a transient current that increased with the amplitude of the voltage step (Fig. 3A, left). Repolarization produced an inverse current of similar amplitude. Integration of these transient currents over time yielded the amount of translocated charge. The charges translocated upon the onset and offset of the stimulus were equal, demonstrating a full reversibility of the charge movement (Fig. 3B). The relation between charge and step potential was well described by a two-state Boltzmann function (Fig. 3C). This behaviour qualitatively agrees with that of mammalian prestin (Fig. 3A, right), yet quantitative differences exist: first, charge transfer by zprestin occurred at much more positive potentials: voltage at half-maximal charge transfer, V1/2, was +96 ± 27 mV (n=5) as opposed to −74 ± 9 mV for rprestin; second, voltage dependence was less pronounced for zprestin, where Boltzmann fits yielded about 53 mV for an e-fold change in charge transfer (slope α ≈ 34 mV for rprestin; Fig. 3B). Saturated charge transfer obtained from the fits was 65 ± 26 fC for zprestin and 95 ± 27 fC for rat prestin. Membrane fluorescence in zprestin–GFP transfected cells was usually somewhat stronger than in cells transfected with GFP-tagged rprestin as assessed visually by wide field fluorescence microscopy. We thus did not find any evidence for a substantially lower expression level of zprestin compared with its mammalian counterpart.
Figure 3. Voltage-dependent charge movement by zprestin.
A, transient currents measured from a CHO cell expressing zprestin–GFP (left) or rprestin–GFP (right) were elicited by a series of depolarizing voltage steps of 3 ms duration and 10 mV increments starting at −30 mV (zprestin) and −130 mV (rprestin), as are illustrated in the lower panels (dashed lines indicate that larger voltage steps are omitted for clarity). Any residual linear – capacitive or resistive – currents were eliminated using a P/−8 protocol. Each trace is the average of 10 consecutive recordings. Note the inverse transient current at return to the initial voltage. Non-transfected cells had no detectable transient currents (data not shown). B, charge measured from a zprestin-expressing cell at the end of a voltage step (OFF) is plotted against the charge at the onset of the step (ON). A line of unity slope well described the relation, indicating equality of ON- and OFF-charges. C, charge measured as in A is plotted against step voltage. Data from individual cells were fitted with a 2-state Boltzmann function and normalized to saturating charge. The plot shows mean normalized data (±s.d.) from 5 cells (zprestin) and 4 cells (rprestin). Continuous lines show the best fits to the averaged data with V1/2=94.7 mV, α=53.2 mV for zprestin and V1/2=−74.6 mV, α=34.0 mV for rprestin. D, NLC measured from the same CHO cell expressing zprestin–GFP with the different stimulus frequencies indicated. Continuous lines are the best fits to the first derivative of a 2-state Boltzmann function. For fit results, see Results. Capacitance recordings are presented with linear membrane capacitance subtracted. E, NLC measured from a rprestin–GFP-expressing cell as described in (C). Fits (continuous lines) yielded peak NLC of 0.75 ± 0.35 pF, Qmax of 111 ± 47 fC, V1/2 of −69 ± 14 mV and slope values of 35.5 ± 1.2 mV (at 2 kHz; n=6). Inset shows the lack of detectable NLC in a representative control cell (recordings for all four frequencies are superimposed). F, frequency dependence of NLC in cells expressing zprestin: peak NLC obtained with the various stimulation frequencies was normalized to peak NLC at 0.5 kHz stimulation frequency for each cell (n=7). G, transient currents measured in response to voltage steps (from −140 to −20 mV, rprestin; from −80 to +120 mV, zprestin). Traces are averaged from 4 and 10 repetitive recordings, respectively, and normalized to peak current. Superimposed curves show monoexponential fits to the current relaxation yielding time constants (τON) of 26 μs (rprestin) and 120 μs (zprestin). For recording conditions, see Methods. H, time constants of charge transfer for a range of step potentials were obtained as in G. Data are from n=3 and 6 cells expressing rprestin and zprestin, respectively.
Given that zprestin mediates charge transfer, we next tested for a non-linear capacitance (NLC) of the cell membrane, which directly results from voltage-dependent charge transfer and is widely used to characterize the function of mammalian prestin (for a review, see Dallos & Fakler, 2002). As shown in Fig. 3D, zprestin-tranfected cells displayed a NLC similar to the bell-shaped NLC conferred by mammalian prestin (Fig. 3E). NLC apparently resulted from the insertion of zprestin into the plasma membrane of the cells, as it was never observed in non-transfected cells (Fig. 3E, inset). The amplitude of NLC generated by zprestin strongly decreased with increasing stimulus frequency used for detection, vanishing into the noise floor for frequencies greater than ca. 5 kHz (Fig. 3D and F). Such frequency dependence indicates that the charge transfer underlying the NLC is too slow to follow high-frequency stimulation. A drop of NLC amplitudes as the stimulus sinusoids approach the charge transition rates has been observed in OHCs, where the effect was found to occur above 10 kHz (Gale & Ashmore, 1997). In agreement with these observations, NLC generated by rprestin showed up in our experiments at all stimulus frequencies (Fig. 3E), whereby only a slight high-frequency drop of the NLC amplitude was found. As mammalian prestin operates without attenuation up to at least 20 kHz (Ludwig et al. 2001), this slight drop is likely to reflect the frequency limits of our instrumentation rather than those of prestin-mediated charge tranfer.
To test more directly whether the charge-transfer kinetics of zprestin is slower than that of mammalian prestin, we measured current relaxations in response to voltage steps in transfected cells. For rprestin-mediated charge transfer, time constants of charge relaxation were about 25 μs, reflecting the temporal limitations set by low-pass filtering by the amplifier and the voltage-clamp speed. For zprestin-mediated charge transfer, current transients were substantially slower. Time constants (around 130 μs, Fig. 3H) were well above the clamp and filter time constants and, thus, are likely to reflect the actual kinetics of the charge transfer. A time constant of 130 μs corresponds to a corner frequency of 1.2 kHz, which is in reasonable agreement with the observed roll-off of the NLC in the kilohertz range.
Voltage dependence of NLC of mammalian prestin is well characterized by the first derivative of the Boltzmann function that describes charge movement (Santos-Sacchi, 1991). Similarly for zprestin, NLC was bell-shaped and was described by a Boltzmann derivative (Fig. 3D). However, because only part of the voltage range (below 100 mV) covered by NLC was accessible to capacitance measurements at a useful recording quality, the fit parameters were somewhat noisy and some uncertainty remains as to whether a 2-state Boltzmann function is the most appropriate description of the voltage dependence of zprestin. Parameter values yielded by the fits, however, were in reasonable agreement with those obtained from direct measurements of charge movement: for stimulus frequencies of 0.5, 1 and 2 kHz, Boltzmann fits yielded V1/2 values of 84 ± 21, 85 ± 16 and 93 ± 13 mV, slopes of 79 ± 15, 80 ± 14 and 71 ± 20 mV, and peak NLCs of 0.43 ± 0.16, 0.26 ± 0.12 and 0.12 ± 0.07 pF, respectively (n=7). Hence, charge movement characterizes zprestin as a voltage-sensitive SLC26A member, the voltage dependence of which is rather broad and shifted to potentials largely beyond physiological membrane voltages. According to our data, the molecular kinetics underlying the charge transfer conferred by zprestin is substantially slower than that of mammalian prestin.
zprestin NLC is not associated with motile responses
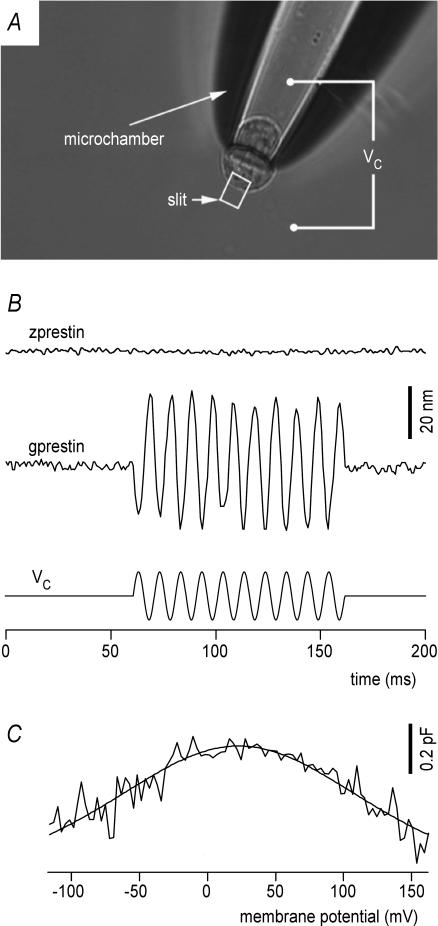
Zprestin is expressed in hair cells and mediates NLC. As the NLC of mammalian prestin is mechanistically linked to electromotility, zprestin transfected HEK cells were probed for motility using a microchamber configuration. For detachment, cells were treated with Ca2+-free medium. Whereas the cells were initially spherical, preventing effective surface area changes at constant volume, drawing them into a suction pipette, or microchamber (Dallos et al. 1991) forced them into a dumbbell or hour-glass shape (Fig. 4A). Sinusoidal voltage bursts of ∼168 mV effective peak-to-peak amplitude and 100 ms duration were delivered to the cells via the microchamber. We measured 51 randomly selected zprestin-transfected cells, none of which showed any measurable motile response (an example of lack of motility is shown in Fig. 4B, upper). When HEK cells were transfected with gerbil prestin (gprestin), in contrast, motile responses were obtained from 7 of 31 cells tested (Fig. 4, middle). Additional 23 zprestin–GFP transfected cells, all showing clear membrane labelling, were probed for electromotility using larger sinusoidal voltage bursts of 228 mV (peak to peak) and stimulation frequencies of 100 Hz and 500 Hz (not shown). Given a resting membrane potential of around −10 mV, a sinusoidal stimulus of 228 mV peak-to-peak amplitude would result in membrane voltage swings from −124 mV to +104 mV. Such large potential changes should be sufficient to evoke motility (if any) even though the V1/2 of the zprestin-mediated NLC is shifted towards more positive potentials as compared with mammalian prestin. Signs of electromotility, however, were not detected in any of the zprestin-transfected cells.
Figure 4. zprestin-transfected cells do not display somatic electromotility.
A, transfected HEK cells were probed for electromotility in a microchamber configuration, which allows simultaneous electrical stimulation and displacement recording. The rectangular slit indicates the area that was projected onto a photodiode for motility measurements. The stimulus command voltage (Vc) was applied between bath and microchamber compartment. Proportional to their degree of insertion into the microchamber, the cell's resultant membrane voltage swing was calculated to be ∼60% of Vc. B, lack of somatic motility in a zprestin-transfected HEK cell (upper trace). A 380 mV (peak-to-peak) voltage burst (lower trace) was applied via the microchamber. The voltage drop on the extruded segment was estimated to be 228 mV (peak-to-peak). The cell's resting membrane potentials normally were around −10 mV. Therefore, the effective membrane voltage was estimated to vary from −124 to +104 mV. Note that in contrast, robust electromotiltiy was observed in gprestin-transfected cells (middle trace). C, HEK cells transfected with zprestin display NLC confirming proper membrane targeting of zprestin in HEK cells. Voltage-dependent capacitance was measured using a 2-sine-wave method.
Discussion
Zebrafish prestin is the closest reported relative of mammalian prestin. Like its mammalian orthologue, zprestin is expressed in hair cells of the ear and confers NLC to the membranes of transfected cells, similar to the characteristic electrogenic charge movement that accompanies the prestin-mediated somatic electromotility of mammalian OHCs. Comparable voltage-dependent charge movements are absent from cells transfected with the closest paralogues of mammalian prestin, SLC26A4 (pendrin; Zheng et al. 2000) and SLC26A6 (Oliver et al. 2001), identifying zprestin as the first SLC26 member besides mammalian prestin that generates NLC. Notwithstanding these parallels, it seems unlikely that zprestin gives rise to a somatic motility of hair cells in the zebrafish ear. First, the distributed expression pattern of zprestin in zebrafish hair cells is different from the exclusive membrane localization of prestin in mammalian OHCs, but rather resembles the cytoplasmic distribution reported for prestin in mammalian vestibular hair cells that neither display somatic electromotility nor NLC. Second, although our expression analysis and electrophysiological data show that zprestin properly localizes to the cell membrane upon heterologous expression, it nonetheless fails to generate electromotile responses of the transfected cells. Hence, though displaying a prestin-like voltage sensitivity, zprestin does not seem to be a prestin-like motor, supporting the general view that a prestin-mediated somatic electromotility is a unique feature of mammalian OHCs.
What might be the reason for the dissociation of NLC and electromotility in zprestin-transfected cells? The area motor principle (Iwasa, 2001) generally thought to underlie the prestin-mediated electromotility posits that this protein undergoes two sequential molecular events: First a charged voltage sensor responds to changes in membrane potential by moving across the membrane (Sensing) (Oliver et al. 2001; for a review, see Dallos & Fakler, 2002). This, in turn, must trigger a gross conformational change of the molecule, resulting in the generation of force (Acting). Finally, these elementary electromechanical responses of large arrays of prestin molecules translate into whole-cell motility. This latter translation from molecular to cellular behaviour may involve interactions between prestin monomers (Navaratnam et al. 2005) and/or interactions between prestin and the cytoskeleton (Coupling). Our data show that zprestin responds to changes in membrane potential with the translocation of charge. This ‘Sensing’ capacity of zprestin, despite quantitative differences, qualitatively resembles that of mammalian prestin. Whether the charge movements generated by zprestin and mammalian prestin result from the same molecular events remains to be examined. The absence of cellular electromotility in the presence of voltage-dependent charge movements observed for zprestin suggests that either the voltage-induced charge transfer is not followed by equivalent conformational reorientations (no ‘Acting’), or, alternatively, that these conformational changes are not efficiently coupled into the overall mechanics of the cell (no ‘Coupling’).
As zprestin does not seem to be a motor, what then is its function? With the exception of mammalian prestin, members of the SLC26 protein family have been shown to be bona fide anion transporters (for a review, see Mount & Romero, 2004). Based on the results presented here, the transport function of zprestin has been tested in a parallel study, revealing that this protein is an electrogenic transporter that exchanges sulphate (or oxalate) for chloride (Schaechinger & Oliver, unpublished results). Chloride ions, in turn, have been shown to interfere with both the voltage dependence and the motor function of the mammalian prestin motor (Oliver et al. 2001), suggesting that the molecular events that lead to electromotility are mechanistically related to anion transport processes (Oliver et al. 2001, 2006). This idea has recently found support by modelling work suggesting that the motor function of mammalian prestin is driven by an electrogenic anion transport cycle (Muallem & Ashmore, 2006). Mediating anion transport and voltage-dependent charge movements (although quantitatively distinct from those of mammalian prestin) but no associated electromotile activity, zprestin can thus be regarded as the ‘missing link’ that may help us understand the sequence of events that has led to the emergence of a novel type of motor protein in the course of SLC26 evolution. How the zprestin-mediated voltage sensitivity described here relates to anion transport and whether it affects the function of zebrafish hair cells under physiological conditions remains to be seen.
The discovery of voltage-dependent charge movements conferred by zprestin, together with the demonstration of its electrogenic transport function, make this protein a good candidate for exploring the common grounds of charge movement, electromotility and anion transport. We anticipate that using zprestin as a non-motor background will help to trace the molecular peculiarities that bring about the motor function of mammalian prestin.
Acknowledgments
We thank Sigrun Korsching for providing animals (‘Thanks for all the fish’) and Karin Rohbock for technical assistance. This work was supported by grants from the Volkswagen-Foundation (J.T.A., O.H., M.C.G.), the Deutsche Forschungsgemeinschaft DFG Kn316/4-1, the Interdisciplinary Center of Clinical Research Tübingen (M.K.), the National Institute of Health NIH R01 DC 004696 (D.Z.Z.H.), and the European Union, LSHG-CT-20054-512063 (‘Eurohear’, D.O.).
References
- Adler HJ, Belyantseva IA, Merritt RC, Jr, Frolenkov GI, Dougherty GW, Kachar B. Expression of prestin, a membrane motor protein, in the mammalian auditory and vestibular periphery. Hear Res. 2003;184:27–40. doi: 10.1016/s0378-5955(03)00192-8. [DOI] [PubMed] [Google Scholar]
- Armstrong CM, Bezanilla F. Charge movement associated with the opening and closing of the activation gates of the Na channels. J Gen Physiol. 1974;63:533–552. doi: 10.1085/jgp.63.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DK, Hudspeth AJ. Ca2+ current-driven nonlinear amplification by the mammalian cochlea in vitro. Nat Neurosci. 2005;8:149–155. doi: 10.1038/nn1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, Higgins DG, Thompson JD. Multiple sequence alignmen with the Clustal series of programs. Nucl Acids Res. 2003;31:3497–3500. doi: 10.1093/nar/gkg500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P, Evans NE, Hallworth R. Nature of the motor element in electrokinetic shape changes of cochlear outer hair cells. Nature. 1991;350:155–157. doi: 10.1038/350155a0. [DOI] [PubMed] [Google Scholar]
- Dallos P, Fakler B. Prestin, a new type of motor protein. Nat Rev Mol Cell Biol. 2002;3:104–111. doi: 10.1038/nrm730. [DOI] [PubMed] [Google Scholar]
- Deak L, Zheng J, Orem A, Du GG, Aguinaga S, Matsuda K, Dallos P. Effects of cyclic nucleotides on the function of prestin. J Physiol. 2005;563:483–496. doi: 10.1113/jphysiol.2004.078857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fettiplace R. Active hair bundle movements in auditory hair cells. J Physiol. 2006;576:29–36. doi: 10.1113/jphysiol.2006.115949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fettiplace R, Hackney CM. The sensory and motor roles of auditory hair cells. Nat Rev Neurosci. 2006;7:19–29. doi: 10.1038/nrn1828. [DOI] [PubMed] [Google Scholar]
- Gale JE, Ashmore JF. An intrinsic frequency limit to the cochlear amplifier. Nature. 1997;389:63–66. doi: 10.1038/37968. [DOI] [PubMed] [Google Scholar]
- Geleoc GS, Holt JR. Auditory amplification, outer hair cells pres the issue. Trends Neurosci. 2003;26:115–117. doi: 10.1016/S0166-2236(03)00030-4. [DOI] [PubMed] [Google Scholar]
- Gold T. Hearing. II. The physical basis of the action of the cochlea. Proc R Soc London Series B. 1948;135:492–498. [Google Scholar]
- Göpfert MC, Albert JT, Nadrowski B, Kamikouchi A. Specification of auditory sensitivity by Drosophila TRP channels. Nat Neurosci. 2006;9:999–1000. doi: 10.1038/nn1735. [DOI] [PubMed] [Google Scholar]
- Göpfert MC, Humphris ADL, Albert JT, Robert D, Hendrich O. Power gain exhibited by motile mechanosensory neurons in Drosophila ears. Proc Natl Acad Sci U S A. 2005;102:325–330. doi: 10.1073/pnas.0405741102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göpfert MC, Robert D. Motion generation by Drosophila mechanosensory neurons. Proc Natl Acad Sci U S A. 2003;100:5514–5519. doi: 10.1073/pnas.0737564100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harland RM. In situ hybridization: an improved whole-mount method for Xenopus embryos. Meth Cell Biol. 1991;36:685–695. doi: 10.1016/s0091-679x(08)60307-6. [DOI] [PubMed] [Google Scholar]
- He DZ, Beisel KW, Chen L, Ding DL, Jia S, Fritzsch B, Salvi R. Chick hair cells do not exhibit voltage-dependent somatic motility. J Physiol. 2003;546:511–520. doi: 10.1113/jphysiol.2002.026070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He DZ, Evans BN, Dallos P. First appearance and development of electromotility in neonatal gerbil outer hair cells. Hear Res. 1994;78:77–90. doi: 10.1016/0378-5955(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Hughes AL. Gene duplication and the origin of novel proteins. Proc Natl Acad Sci U S A. 2005;102:8791–8792. doi: 10.1073/pnas.0503922102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasa KH. A two-state piezoelectric model for outer hair cell motility. Biophys J. 2001;81:2495–2506. doi: 10.1016/S0006-3495(01)75895-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia S, He DZ. Motility-associated hair-bundle motion in mammalian outer hair cells. Nature Neurosci. 2005;8:1028–1034. doi: 10.1038/nn1509. [DOI] [PubMed] [Google Scholar]
- Kennedy HJ, Crawford AC, Fettiplace R. Force generation by mammalian hair bundles supports a role in cochlear amplification. Nature. 2005;433:880–883. doi: 10.1038/nature03367. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Gao J, He DZ, Wu X, Jia S, Zuo J. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature. 2002;419:300–304. doi: 10.1038/nature01059. [DOI] [PubMed] [Google Scholar]
- Ludwig J, Oliver D, Frank G, Klocker N, Gummer AW, Fakler B. Reciprocal electromechanical properties of rat prestin: the motor molecule from rat outer hair cells. Proc Natl Acad Sci U S A. 2001;98:4178–4183. doi: 10.1073/pnas.071613498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley GA. Cochlear mechanisms from a phylogenetic viewpoint. Proc Natl Acad Sci U S A. 2000;97:11736–11743. doi: 10.1073/pnas.97.22.11736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley GA. Evidence for an active process and a cochlear amplifier in nonmammals. J Neurophysiol. 2001;86:541–549. doi: 10.1152/jn.2001.86.2.541. [DOI] [PubMed] [Google Scholar]
- Mount DB, Romero MF. The SLC26 gene family of multifunctional anion exchangers. Pflugers Arch. 2004;447:710–721. doi: 10.1007/s00424-003-1090-3. [DOI] [PubMed] [Google Scholar]
- Muallem DR, Ashmore JF. An anion antiporter model of prestin, the outer hair cell motor protein. Biophys J. 2006;90:4035–4045. doi: 10.1529/biophysj.105.073254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navaratnam D, Bai JP, Samaranayake H, Santos-Sacchi J. N-terminal-mediated homomultimerization of prestin, the outer hair cell motor protein. Biophys J. 2005;89:3345–3352. doi: 10.1529/biophysj.105.068759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D, He DZ, Klocker N, Ludwig J, Schulte U, Waldegger S, Ruppersberg JP, Dallos P, Fakler B. Intracellular anions as the voltage sensor of prestin, the outer hair cell motor protein. Science. 2001;292:2340–2343. doi: 10.1126/science.1060939. [DOI] [PubMed] [Google Scholar]
- Oliver D, Schaechinger T, Fakler B. Interaction of prestin (SLC26A5) with monovalent intracellular anions. Novartis Found Symp. 2006;273:244–253. [PubMed] [Google Scholar]
- Santos-Sacchi J. Reversible inhibition of voltage-dependent outer hair cell motility and capacitance. J Neurosci. 1991;11:3096–3110. doi: 10.1523/JNEUROSCI.11-10-03096.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J, Kakehata S, Takahashi S. Effecs of membrane potential on the voltage dependence of motility-related charge in outer hair cells of the guinea-pig. J Physiol. 1998;510:225–235. doi: 10.1111/j.1469-7793.1998.225bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaechinger TJ, Oliver D. Non-mammalian orthologs of prestin (SLC26A5) are electrogenic divalent/chloride anion exchangers (in press) doi: 10.1073/pnas.0608583104. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thisse C, Thisse B, Schilling TF, Postlethwait JH. Structure of the zebrafish snail1 gene and its expression in wild-type, spadetail and no tail mutant embryos. Development. 1993;119:1203–1215. doi: 10.1242/dev.119.4.1203. [DOI] [PubMed] [Google Scholar]
- Weber T, Göpfert MC, Winter H, Zimmermann U, Kohler H, Meier A, Hendrich O, Rohbock K, Robert D, Knipper M. Expression of prestin-homologous solute carrier (SLC26). in auditory organs of nonmammalian vertebrates and insects. Proc Natl Acad Sci U S A. 2003;100:7690–7695. doi: 10.1073/pnas.1330557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Long KB, Shen W, Madison LB, Dallos P. Prestin topology, localization of protein epitopes in relation to the plasma membrane. Neuroreport. 2001;12:1929–1935. doi: 10.1097/00001756-200107030-00032. [DOI] [PubMed] [Google Scholar]
- Zheng J, Shen W, He DZ, Long KB, Madison LD, Dallos P. Prestin is the motor protein of cochlear outer hair cells. Nature. 2000;405:149–155. doi: 10.1038/35012009. [DOI] [PubMed] [Google Scholar]