Abstract
A single episode of status epilepticus (se) induced in rodents by the convulsant pilocarpine, produces, after a latent period of ≥ 2 weeks, a chronic epileptic condition. During the latent period of epileptogenesis, most CA1 pyramidal cells that normally fire in a regular pattern, acquire low-threshold bursting behaviour, generating high-frequency clusters of 3–5 spikes as their minimal response to depolarizing stimuli. Recruitment of a Ni2+- and amiloride-sensitive T-type Ca2+ current (ICaT), shown to be up-regulated after se, plays a critical role in burst generation in most cases. Several lines of evidence suggest that ICaT driving bursting is located in the apical dendrites. Thus, bursting was suppressed by focally applying Ni2+ to the apical dendrites, but not to the soma. It was also suppressed by applying either tetrodotoxin or the KV7/M-type K+ channel agonist retigabine to the apical dendrites. Severing the distal apical dendrites ∼150 μm from the pyramidal layer also abolished this activity. Intradendritic recordings indicated that evoked bursts are associated with local Ni2+-sensitive slow spikes. Blocking persistent Na+ current did not modify bursting in most cases. We conclude that se-induced increase in ICaT density in the apical dendrites facilitates their depolarization by the backpropagating somatic spike. The ICaT-driven dendritic depolarization, in turn, spreads towards the soma, initiating another backpropagating spike, and so forth, thereby creating a spike burst. The early appearance and predominance of ICaT-driven low-threshold bursting in CA1 pyramidal cells that experienced se most probably contribute to the emergence of abnormal network discharges and may also play a role in the circuitry reorganization associated with epileptogenesis.
A variety of brain insults can induce the manifestation of temporal lobe epilepsy, the most common human epileptic syndrome (Engel et al. 1997). The processes underlying temporal lobe epileptogenesis have been studied most extensively in models of status epilepticus (se) (Morimoto et al. 2004). In these animal models, a single episode of se evoked by chemical or electrical stimulation of mesial temporal lobe structures, causes, after a latent period of several weeks, the emergence of spontaneous seizures. Once these appear, they are sustained for the rest of the animal's life. During the latent period of epileptogenesis, the animals appear behaviourally normal, though electroencephalographic (EEG) recordings disclose the appearance of interictal ‘spikes’ (Stewart & Leung, 2003). Numerous long-lasting changes in excitatory and inhibitory synaptic functions have been associated with epileptogenesis following se (Dudek et al. 2002; Morimoto et al. 2004). In addition, intrinsic neuronal properties are also persistently altered by se. Thus, se induced by the convulsant pilocarpine (pilocarpine-se) causes a dramatic and enduring increase in the propensity of CA1 (Sanabria et al. 2001), subicular (Wellmer et al. 2002) and layer V neocortical pyramidal neurons (Sanabria et al. 2002) to fire in burst mode. In CA1 cells, where this plasticity phenomenon was first discovered, many regular-firing pyramidal cells convert to a low-threshold bursting mode, generating high-frequency clusters of 3–5 spikes as their minimal response to depolarizing stimuli or even spontaneously (Sanabria et al. 2001). The spontaneous bursters, which comprise ∼10% of the total neuronal population, serve as the initiators and pacemakers of spontaneous epileptiform bursts, during which the entire CA1 network is engaged in repetitive discharge (Sanabria et al. 2001).
Which ionic mechanisms underlie intrinsic bursting in pyramidal neurons that experienced se (se-experienced)? A somatic burst is generated when the spike afterdepolarization (ADP) is sufficiently large to attain spike threshold and trigger a second spike, which is also followed by a large ADP, and so forth (Jensen et al. 1996). In ordinary adult CA1 pyramidal cells, the spike ADP and associated bursting are driven predominantly by persistent Na+ current (INaP), as evidenced by their sensitivity to blockers of this current and refractoriness to blockers of Ca2+ currents (Azouz et al. 1996; Su et al. 2001; Yue et al. 2005). Yet, intrinsic bursting in most se-experienced neurons was readily suppressed by low concentrations (50–100 μm) of Ni2+, implicating a Ni2+-sensitive Ca2+ current in its generation (Sanabria et al. 2001; Su et al. 2002). In agreement with this, we have shown a marked and selective up-regulation of the low-voltage-activated (T-type) Ca2+ current (ICaT) in se-experienced neurons (Su et al. 2002), assigning ICaT a critical role in bursting. However, the possible contribution of INaP to this discharge mode was not explored.
Here we combined several experimental approaches to further elucidate the ionic mechanisms underlying the de novo intrinsic bursting in se-experienced CA1 pyramidal cells. Three issues were examined regarding this aberrant activity: (i) the contribution of ICaTversus that of the high-voltage-activated R-type Ca2+ current (ICaR); (ii) the subcellular localization of the underlying ICaT; and (iii) the contribution of INaP. Our data show conclusively that bursts arise via activation of ICaT in the apical dendrites by backpropagating somatic spikes. Whereas a secondary contribution of ICaR to bursting cannot be excluded, activation of INaP does not play a role in this activity in most cases.
Methods
Induction of SE
All animal experiments were conducted in accordance with the guidelines of the Animal Care Committee of the Hebrew University. Male Sabra rats (150–200 g) were injected with the muscarinic agonist pilocarpine (300–380 mg kg−1i.p.), which induced se in most (∼80%) animals (Turski et al. 1983; Sanabria et al. 2001). Peripheral muscarinic effects were reduced by prior administration of methylscopolamine (1 mg kg−1s.c.; 30 min before injecting pilocarpine). Diazepam (0.1 mg kg−1s.c.) was administered to all animals 2 h after the pilocarpine injection. It terminated the convulsions in the responsive rats and sedated all animals. Within 24 h after pilocarpine injection, the rats appeared behaviourally normal. Both the animals that experienced se (se-experienced rats; n=49) and those which did not (control rats; n=12) were used 7–21 days after drug treatment. We have shown previously that hippocampal slices from se-experienced rats, but not from control rats, manifest signs of pyramidal cell hyperexcitability (Sanabria et al. 2001).
Hippocampal slices
The rats were decapitated under deep isoflurane anaesthesia, and transverse hippocampal slices (400 μm) were prepared with a vibrating microslicer (Leica, Germany) and transferred to a storage chamber perfused with oxygenated (95% O2–5% CO2) artificial cerebrospinal fluid (ACSF) containing (mm): NaCl 124, KCl 3.5, MgCl2 2, CaCl2 2, NaHCO3 26 and d-glucose 10, pH 7.4, osmolarity 305 mosmol l−1, where they were maintained at room temperature (20-22°C). Slices were placed one at a time in an interface chamber (33.5°C) and perfused with oxygenated ACSF. In some experiments, as indicated, a deep cut was made in stratum radiatum about 150 μm from, and parallel to, stratum pyramidale (see Fig. 8A), using a broken pipette or a razor blade chip propelled by a micromanipulator (Yue et al. 2005; Golomb et al. 2006). The slices were allowed to recover in the chamber for at least 1 h before initiating a recording session. In most experiments 6-cyano-7-nitro-quinoxaline-2,3-dione (CNQX; 15 μm), 2-amino-5-phosphono-valeric acid (APV; 50 μm) and picrotoxin (100 μm) were added to the ACSF to block fast excitatory and inhibitory synaptic transmission. Other drugs were added to the ACSF as indicated.
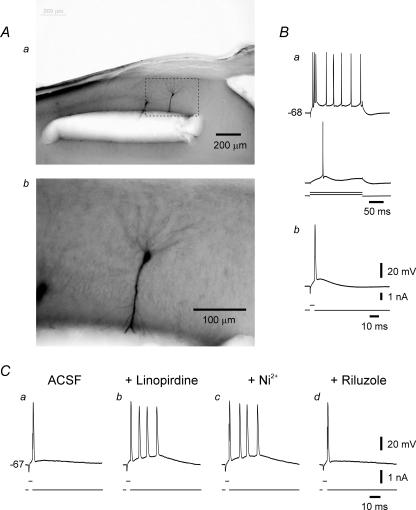
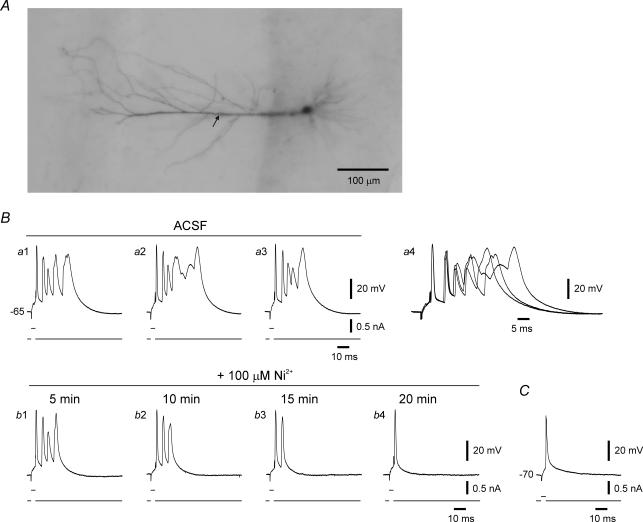
Figure 8. Firing pattern of an se-experienced CA1 pyramidal cell after truncation of its apical dendrites.
A, photomicrograph (Aa) shows two neighbouring neurons injected with biocytin through the recording microelectrode about 1 h after severing the apical dendrites by a cut made in stratum radiatum about 150 μm from the pyramidal layer. The biocytin stains the somata, the basal dendrites and the proximal stumps of the apical dendrites of these neurons. The area with in the dashed rectangle in Aa is enlarged in Ab. B, recordings from the truncated neuron shown in Ab show high-threshold bursting behaviour (Ba). Brief stimuli evoke single spikes in this neuron (Bb). C, in another similarly truncated se-experienced neuron, single spikes (Ca) are converted to bursts of 4 spikes about 20 min after adding 10 μm linopirdine to the ACSF (Cb). The linopirdine-induced bursting is resistant to 100 μm Ni2+ applied for 30 min (Cc), but is readily suppressed by 10 μm riluzole (Cd).
Electrophysiological recordings
Intracellular recordings were obtained using sharp glass microelectrodes containing 4 m potassium acetate (90–110 MΩ) and an amplifier (Axoclamp 2B, Axon Instruments, Union City, CA, USA) that allowed simultaneous injection of current and measurement of membrane potential. The bridge balance was carefully monitored and adjusted before each measurement. The intracellular signals were filtered on-line at 10 kHz, digitized at a sampling rate of 10 kHz or more, and stored on a personal computer using a data acquisition system (Digidata 1322A) and pCLAMP software (Axon Instruments).
Drug applications
Drugs were applied to the whole slice by bath application or to selected locations in the slice using a puff application system. Because the spread of drugs in slices maintained in an interface chamber is predominantly by diffusion, the onset and offset of drug effects are slow (of the order of tens of minutes). For all bath-applied drugs used in this study, maximal effects were obtained within 20–30 min of adding them to the ACSF.
For focal drug applications we used, as in previous studies (Chen et al. 2005; Yue et al. 2005; Yue & Yaari, 2006), a puffing system consisting of a pneumatic pump (Picospritzer III, General Valve, Fairfield, NJ, USA) connected to a patch pipette (tip diameter, ∼10 μm). The pipette was filled with ACSF containing the drug to be applied at 10-fold higher concentration than that required in bath-application experiments. The tip of the pipette touched the upper surface of the slice. Drugs were applied using pressure pulses of 5 bars lasting 20 ms. These pulses produced a drop that covered a circular area ∼50 μm in diameter when ejected onto the surface of the slice (visualized by including fast-green dye in the pipette solution). To apply drugs to the region of the soma and axon initial segment (a region we refer to here as axo-soma though it may include to some extent also proximal apical and basilar dendrites), the puffing pipette was positioned in stratum pyramidale about 25 μm from the recording microelectrode (lodged in the soma of the neuron). To apply drugs to the distal apical dendrites, the puffing pipette was positioned in stratum radiatum about 200–300 μm away from, and vertical to, the stratum pyramidale. Likewise, to apply drugs to the basal dendrites, the puffing pipette was positioned in stratum oriens about 100 μm away from, and vertical to, the stratum pyramidale. This experimental arrangement is shown below in Fig. 4A. The effects of focally applied drugs, when present, appeared usually within 3 min of puffing, and reached a maximum within 8–10 min. These delays are undoubtedly due to slow diffusional spread of the drugs from their superficial site of application into the slice.
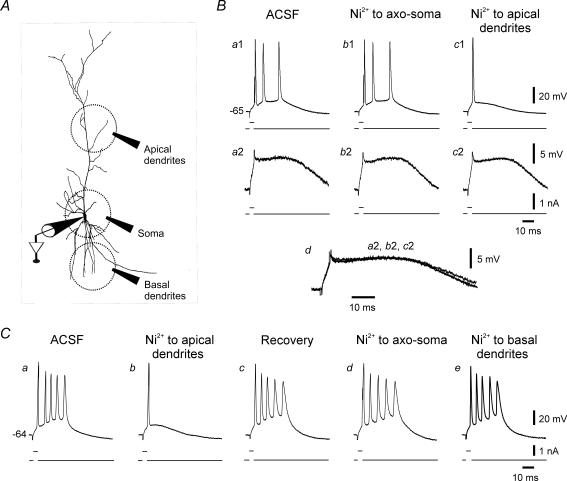
Figure 4. Effects of focal applications of Ni2+ on bursting.
A, scheme of experimental arrangement for focal pressure application of drugs (see Methods). B, this neuron generated a burst of 3 spikes in response to brief stimuli (Ba1). Brief, threshold-straddling pulses that failed to elicit a spike evoked a prolonged SDP (Ba2). Focal application of Ni2+ to the axo-soma had no effect on any of the spiking or subthreshold responses (Bb1 and b2). In contrast, when Ni2+ was applied to the apical dendrites, bursting was suppressed after 3 min (Bc1). Yet at the same time, the SDP was unchanged (Bc2; see also Bd in which portions from traces Ba2, Bb2 and Bc2 are expanded and overlaid). C, in another neuron firing in bursts of 5 spikes (Ca), Ni2+ was focally applied in the reversed order. When puffed on the apical dendrites, Ni2+ promptly suppressed bursting (Cb). This effect recovered after 10 min (Cc). Then Ni2+ was applied to the axo-soma (Cd) and after another 10 min to the basal dendrites (Ce). Neither of the latter applications suppressed bursting.
Chemicals and drugs
Stock solutions of 4β-phorbol 12,13-dibutyrate (PDB; 10 mm) and riluzole (10 mm) were prepared in dimethyl sulfoxide (DMSO) and stored at −20°C. They were usually diluted at 1: 1000 when added to the ACSF. The ACSF contained equal amounts of DMSO (0.001%), which by itself had no effects on the measured parameters. All other drugs were added to the ACSF from aqueous stock solutions. Chemicals and drugs were obtained from Sigma (Petach-Tikva, Israel), except for SNX-482 (Alomone Laboratories, Jerusalem, Israel).
Cell staining
Cell somata or putative apical dendrites of CA1 pyramidal cells were injected with biocytin. Following the experiment, the slices were fixed overnight in 4% paraformaldehyde, cut into thin (120 μm) slices and incubated with avidin-biotin complex (Vectastain ABC Elite kit, Vector).
Analysis
To measure passive membrane properties, the pyramidal cells were injected with small (0.1–0.5 nA) 200 ms negative current pulses. The apparent input resistance was provided by the slope of the linear regression line fitted through the linear portion of the steady-state voltage–current amplitude plot. Spike threshold was defined as the membrane potential where the slope of the voltage trace increased abruptly during membrane charging induced by prolonged positive current pulses. The fast spike afterhyperpolarization (fAHP) was measured as the potential attained at the end of the spike downstroke. The size of the subthreshold depolarizing potential (SDP) was measured as the integrated ‘area under the curve’ between the end of the stimulus artifact and the point at which membrane voltage returned to resting potential.
Averaged data are expressed as mean ± s.e.m. The significance of the differences between the measured parameters was evaluated using paired Student's t test with a significance level of 0.05.
Terminology
In this study of bursting we focus only on neurons that generate a burst as their minimal response to depolarizing current pulses. We have previously referred to these neurons as ‘low-threshold’ bursters, to differentiate them from neurons that generate burst-like responses when stimulated with 2–3 times threshold depolarizing current pulses (referred to as ‘high-threshold’ bursters; Jensen et al. 1994, 1996; Su et al. 2001). High-threshold bursting is found in both normal and se-experienced CA1 pyramidal cells and its mechanism has been thoroughly investigated (Yue et al. 2005).
Results
De novo bursting in SE-experienced CA1 pyramidal cells
The se-experienced and control rats were killed for in vitro experiments 7–21 days after drug treatment. The data here were collected from 32 se-experienced rats (98 neurons in 80 slices) and seven control rats (22 neurons in 15 slices). The neurons were impaled with sharp glass microelectrodes for current-clamp recordings of passive and active neuronal membrane properties. The mean values in the se-experienced and control neurons, respectively (n=20 in both groups), of resting membrane potential (66.3 ± 0.8 and 65.8 ± 0.9 mV) and apparent input resistance (34.2 ± 1.2 and 36.2 ± 1.2 mΩ), as well as of spike threshold (−53.3 ± 1.1 and −53.1 ± 1.2 mV), amplitude (90.2 ± 2.0 and 90.4 ± 1.4 mV) and width (1.9 ± 0.2 and 2.0 ± 0.1 ms), were similar. In all neurons, spikes evoked by brief (4 ms) threshold-straddling depolarizing current pulses repolarized to a membrane potential about 10 mV more positive than resting potential (−53.3 ± 1.2 and −54.7 ± 1.4 mV in se-experienced and control neurons, respectively; n=20); the so-called fast afterhyperpolarization (fAHP). Following the fAHP the membrane potential declined monotonically to baseline or, in most cases, first repolarized again and then declined slowly to baseline, creating an ‘active’ spike ADP.
In 42 of 65 sampled se-experienced neurons (65%), the spike ADP was sufficiently large to trigger additional spikes, thus producing a spike burst. The fraction of bursting neurons was about the same in slices obtained during the second week (7–14 days: 23 of 35; 66%) and third week (15–21 days: 19 of 30; 64%) after pilocarpine-se. The number of intraburst spikes varied between 3 and 5, averaging 3.6 ± 0.1 spikes. The intraburst firing frequency ranged between 144.1 and 377.2 Hz, averaging 231.2 ± 9.9 Hz (n=30). In many of these neurons, such as the one illustrated in Fig. 1, the timing of individual intraburst spikes displayed a jitter, which was more pronounced in the late part of long (> 3 intraburst spikes) bursts (Fig. 1A–C and overlaid traces in Fig. 1D). In some neurons, failure of one of the late spikes revealed an underlying small subthreshold ‘hump’ (see arrows in Fig. 1B and C). In contrast to the se-experienced neurons, none of the control neurons generated a spike burst when similarly depolarized.
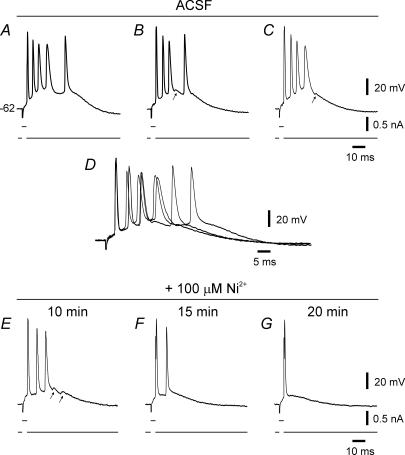
Figure 1. Low-threshold bursting in an se-experienced CA1 pyramidal cell.
The experiment was performed 7 days after pilocarpine-se. In this and in subsequent figures, the bottom trace in each panel depicts the stimulus waveform, whereas the top trace shows the voltage response. The resting potential of the neuron is indicated to the left of the uppermost voltage trace. Brief stimuli evoked bursts of 4–5 spikes (A–C; these traces are expanded and overlaid in D to facilitate comparison). Note jitter in spike timing, particularly of later spikes. Spike failure during a burst sometimes revealed a small subthreshold ‘hump’ (see arrows in B, C and E). Adding 100 μm Ni2+ to the ACSF caused progressive suppression of bursting (E–G).
Adding 100 μm Ni2+ to the ACSF progressively converted bursting to single spiking in eight of 10 bursting se-experienced neurons (Fig. 1E–G). In the remaining two neurons, bursting persevered though the number of intraburst spikes was reduced from 3 to 2 spikes in one neuron. Raising the Ni2+ concentration to 1 mm also did not suppress bursting in these two neurons (data not shown). Altogether, Ni2+ reduced the number of intraburst spikes from 3.9 ± 0.3 to 1.4 ± 0.3 (n=10; P < 0.005). These findings confirm that bursting emerging early in epileptogenesis is driven by a Ni2+-sensitive Ca2+ current.
Effects of amiloride and SNX-482
Low concentrations (50–100 μm) of Ni2+ block ICaT, as well as ICaR (Williams et al. 1999; Lee et al. 1999). We have previously shown that the density of ICaT is strongly up-regulated early after pilocarpine-se, whereas that of ICaR is unchanged. Moreover, the up-regulated ICaT is highly sensitive to Ni2+ (IC50 ∼25 μm; Su et al. 2002). Of the three α1 subunits generating ICaT (CaV3.1, CaV3.2 and CaV3.3), CaV3.2 is 20-fold more sensitive to Ni2+ (IC50, 13 μm) than the other two isoforms (Lee et al. 1999). Together, these findings suggested that CaV3.2 T-type Ca2+ channels provide the critical depolarization for bursting in se-experienced neurons. To further assess the roles of ICaT and ICaR in this discharge behaviour, we monitored the effects of amiloride, known to block ICaT preferentially over high-voltage activated Ca2+ currents (Tang et al. 1988; Takahashi et al. 1989; Scroggs & Fox, 1992; Williams et al. 1997; Todorovic & Lingle, 1998; Kim & Chung, 1999; Ikeda & Matsumoto, 2003), and to block CaV3.2 (IC50, 167 μm; Williams et al. 1999) preferentially over CaV3.1 (IC50 value in the millimolar range; Lacinová et al. 2000). It is important to note that amiloride (up to 500 μm) was ineffective in blocking ICaR, while strongly blocking ICaT in the same neurons (Hilaire et al. 1997). In 4 out of 5 se-experienced neurons, as illustrated in Fig. 2A, adding 300 μm amiloride to the ACSF converted bursting to single spiking within 20 min. In the fifth neuron, the number of intraburst spikes actually increased from 4 to 5 spikes. Altogether, amiloride reduced the average number of intraburst spikes from 3.6 ± 0.2 to 1.8 ± 0.8 (n=5; P < 0.05).
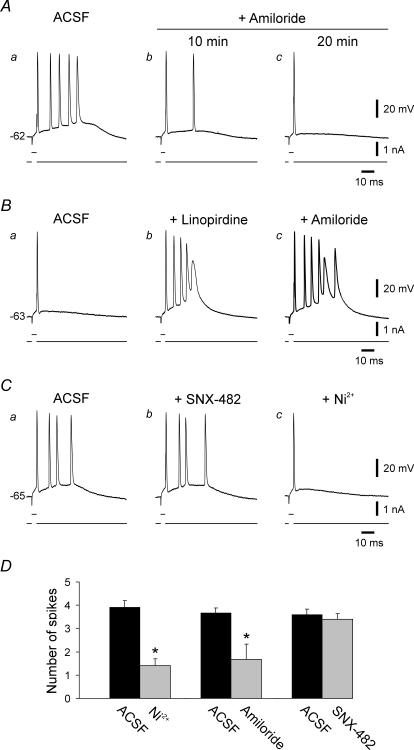
Figure 2. Effects of amiloride and SNX-482 on bursting.
A, exposure of a bursting se-experienced neuron (five intraburst spikes; Aa) to 300 μm amiloride gradually suppressed bursting; after 20 min exposure the neuron generated a solitary spike (Ab and c). B, exposure of regular firing control neuron (Ba) to ACSF containing 10 μm linopirdine induced bursting (five intraburst spikes) within 20 min (Bb). Addition of 300 μm amiloride to the ACSF enhanced bursting (six intraburst spikes; Bc). Bursting persisted throughout the 45 min exposure to this drug combination. C, exposure of another bursting se-experienced neuron (four intraburst spikes; Ca) to 1 μm SNX-482 for 45 min did not modify it bursting mode (Cb). Subsequent addition of 100 μm Ni2+ to the ACSF converted bursting to single spiking within 20 min (Cc). D, bar histogram summarizes the effects of 100 μm Ni2+, 300 μm amiloride and 1 μm SNX-482 on bursting se-experienced neurons. Both Ni2+ and amiloride caused significant suppression of bursting (indicated with asterisks).
The disadvantage of using amiloride to characterize the function of ICaT is that it also blocks Na+–H+ and Na+–Ca2+ exchangers (Kleyman & Cragoe, 1988) and acid-activated ion channels (Waldman et al. 1997). To evaluate the possibility that these actions may be responsible for amiloride-induced burst suppression in se-experienced neurons, we examined its effects in four control neurons on bursting induced by linopirdine (Yue & Yaari, 2004), a selective blocker of KV7 (KCNQ) channels that generate neuronal M-type K+ current (IM; Robbins, 2001). As shown in Fig. 2B, linopirdine-induced bursting (Fig. 2Ba and b) was not suppressed by amiloride (up to 45 min exposure) and was even enhanced in this particular neuron (Fig. 2Bc), as well as in another one. Altogether, amiloride increased the average number of intraburst spikes from 4.0 ± 0.4 to 4.5 ± 0.6, but this effect was not statistically significant.
We also examined the effects of SNX-482 on bursting in se-experienced neurons. This spider toxin variably reduces ICaR in different types of brain neurons by blocking several isoforms of CaV2.3 subunits (Wilson et al. 2000; Tottene et al. 2000; Sochivko et al. 2002). Perfusion with ACSF containing 1 μm SNX-482 (which blocks ∼50% of ICaR in rat CA1 pyramidal cells; Sochivko et al. 2003) for up to 45 min did not affect bursting in 4 out of 5 neurons (Fig. 2Ca and Cb). In the fifth neuron, the number of intraburst spikes decreased from 5 to 4 spikes. In three of the neurons, 100 μm Ni2+ was added following exposure to SNX-482, resulting in burst suppression (Fig. 2Cc). Overall, there were no statistically significant effects of SNX-482 on the number of intraburst spikes (3.6 ± 0.2 before versus 3.4 ± 0.2 after exposure to SNX-482).
The most parsimonious conclusion from these data, summarized in Fig. 2D, is that ICaT, particularly CaV3.2, provides a critical depolarizing drive for bursting in se-experienced CA1 pyramidal cells. However, because SNX-482 only partially blocks ICaR in these neurons (Sochivko et al. 2003), we cannot exclude an auxiliary contribution of ICaR to this aberrant activity.
Effects of PDB and riluzole
Another current that may contribute to bursting is INaP (French et al. 1990; Yue et al. 2005). In normal CA1 pyramidal cells, INaP drives bursting induced by high-K+ or low-Ca2+ ACSFs (Azouz et al. 1996; Su et al. 2001) or by blocking M-type K+ channels (Yue & Yaari, 2006). This current also contributes critically to bursting in developing CA1 pyramidal cells in synergy with various Ca2+ currents (Chen et al. 2005; Metz et al. 2005).
We assessed the contribution of INaP by monitoring the effects of the protein kinase C activator PDB and the neuroprotective drug riluzole. In CA1 pyramidal cells these drugs block INaP completely in doses that only mildly reduce transient Na+ current (Yue et al. 2005). To validate the block of INaP by PDB and riluzole in these experiments, we monitored not only spiking activity, but also the subthreshold depolarizing potentials (SDPs) evoked by brief threshold-straddling stimuli that fail to trigger spikes (Azouz et al. 1996; Su et al. 2001). These potentials, lasting 50–100 ms, are probably driven by INaP because they are readily suppressed by tetrodotoxin (TTX), as well as by more-selective INaP blockers, but are resistant to blockade of voltage-gated Ca2+ currents (Azouz et al. 1996; Su et al. 2001).
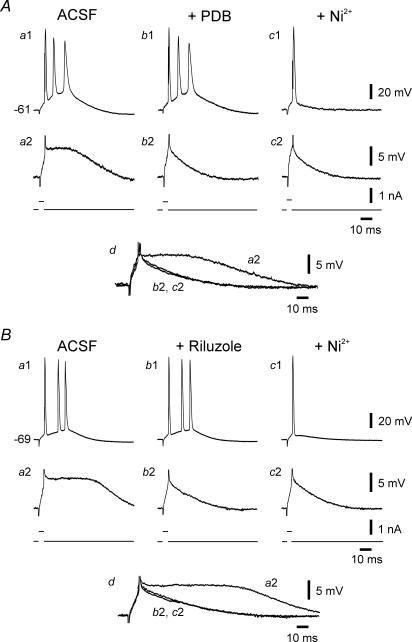
As illustrated in Fig. 3A, bursting in an se-experienced neuron was unaffected by 5 μm PDB applied in the ACSF for up to 1 h (Fig. 3Aa1 and b1). Yet, within 12 min of exposure to PDB, the SDP was reduced to a passive membrane response (Fig. 3Aa2 and b2 and overlaid traces in Fig. 3Ad). Subsequent addition of 100 μm Ni2+ to the PDB-containing ACSF converted bursting (three intraburst spikes) to single spiking within 15 min (Fig. 3Ac1). Similar results were obtained in 4 out of 5 neurons exposed sequentially to 5 μm PDB and Ni2+. In the exceptional neuron, exposure to 5 μm PDB converted the neuron from bursting (three intraburst spikes) to single spiking (data not shown). Altogether, PDB suppressed the SDPs by 45.4 ± 6.1% (from 216.6 ± 25.7 to 94.1 ± 8.1 mV ms; P < 0.005). Adding 10 μm riluzole to the ACSF also did not affect bursting in 4 out of 4 se-experienced neurons (Fig. 3Ba1 and b1), while suppressing the SDPs in all of them by 53.7 ± 5.7% (from 191.1 ± 10.2 to 104.3 ± 5.7 mV ms; P < 0.005; Fig. 3Ba2 and b2). Again, subsequent addition of 100 μm Ni2+ converted bursting to single spiking in all cases (Fig. 3Bc1).
Figure 3. Bursting in se-experienced neurons is resistant to 4β-phorbol 12,13-dibutyrate (PDB) and riluzole.
A, exposure of the first burster (three intraburst spikes; Aa1) to 5 μm PDB for 30 min exerted no effect on the bursting (Ab1), even though the large SDP (Aa2) was suppressed (Ab2). Addition of 100 μm Ni2+ to the ACSF within 25 min converted bursting to single spiking (Ac1), while the SDP was not further affected (Ac2). The traces of the SDPs (Aa2, b2 and c2) are expanded and overlaid in Ad to facilitate comparison. B, exposure of another bursting neuron (Ba1) to 10 μm riluzole for 30 min exerted no effect on the bursting behaviour (three intraburst spikes) of the neuron (Bb1) even though the large SDP (Ba2) was suppressed (Bb2). Addition of 100 μm Ni2+ to the ACSF within 20 min converted bursting to single spiking (Bc1), while the SDP was not further affected (Bc2). The traces of the SDPs (Ba2, b2 and c2) are expanded and overlaid in Bd to facilitate comparison.
Together, these data indicate that INaP activation is not mandatory for bursting in most cases. Nonetheless, in the small fraction of bursting neurons (∼20%) that are not suppressed by Ni2+, INaP may play a dominant role in burst production.
Focal applications of Ni2+
In ordinary adult CA1 pyramidal cells, ICaT is located predominantly in the distal apical dendrites, as it disappears entirely by truncating them at a distance of 150 μm from the soma (Karst et al. 1993). We speculated that the up-regulated ICaT driving bursting in se-experienced neurons is also located in the distal apical dendrites. To test this notion, we first monitored the effects of Ni2+ focally applied to various parts of bursting neurons. The experimental arrangement is illustrated in Fig. 4A (see Methods; Chen et al. 2005; Yue et al. 2005; Yue & Yaari, 2006). The application pipette was filled with ACSF containing 5 mm Ni2+. Two representative experiments are shown in Fig. 4 B and C. In the first experiment, recordings were made from a neuron generating bursts of 3 spikes in response to brief depolarizing current pulses. Applying Ni2+ to the axo-soma had no effect on bursting (Fig. 4Ba1 and b1). By contrast, 3 min after puffing Ni2+ onto the apical dendrites at a distance ∼300 μm from the soma, bursting was suppressed (Fig. 4Bc1). Similar results were obtained in 5 out of 6 experiments. In the sixth experiment, focal applications of Ni2+, followed by bath application of 100 μm Ni2+, exerted no effects on bursting (data not shown). The SDPs were unaffected by the focal applications of Ni2+ (Fig. 4Ba2–c2 and overlaid traces in Fig. 4Bd).
In another series of three experiments we applied Ni2+ sequentially to three regions: apical dendrites, axo-soma and basal dendrites. As shown in Fig. 4C, bursting (five intraburst spikes; Fig. 4Ca) was converted to single spiking 3 min after puffing Ni2+ onto the apical dendrites (Fig. 4Cb). This blocking effect subsided after another 10 min and the neuron recovered its bursting behaviour (Fig. 4Cc). The application pipette was then moved proximally. Applying Ni2+ to the axo-soma had no effect on bursting (Fig. 4Cd). After another 10 min the application pipette was moved to stratum oriens at ∼100 μm from the recording microelectrode and Ni2+ was reapplied. Again, no change was seen (Fig. 4Ce).
Simply puffing ACSF on the apical dendrites of se-experienced neurons had no effect on bursting during the subsequent 15 min observation period (n=4; data not shown). Together, these observations strongly suggest that burst generation requires activation of ICaT in the distal apical dendrites.
Focal applications of TTX
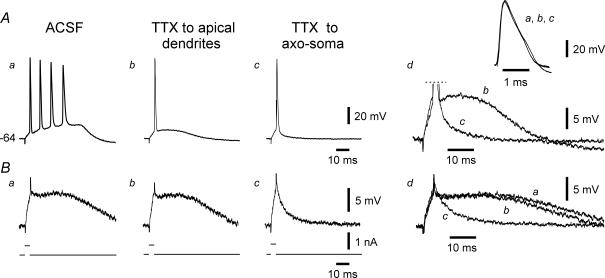
Recruitment of Ca2+ channels in apical dendrites by a spike initiated at the proximal axon is facilitated by its active backpropagation (Spruston et al. 1995). Hence, blocking spike backpropagation should interfere with bursting that depends on apical dendritic Ca2+ currents (Magee & Carruth, 1999; Chen et al. 2005; Yue & Yaari, 2006). We tested this prediction in five se-experienced bursting neurons by puffing TTX on their apical dendrites. The application pipette was filled with 50 nm TTX. A representative experiment is shown in Fig. 5. Within 5 min of TTX application, bursting (four intraburst spikes) was converted to single spiking (Fig. 5Aa and Ab). At the same time, the SDPs evoked by identical stimuli, were only minimally affected (Fig. 5Ba and Bb, and overlaid races in Fig. 5Bd). Likewise, the rise time and amplitude of the primary spike were unchanged (inset in Fig. Ad). The latter observations confirm that TTX blocked bursting by acting at distal portions of the neuron. The application pipette was then moved to the axo-soma and TTX was re-applied. After 4 min, the spike ADP (Fig. 5Ac, and overlaid traces in Fig. 5Ad) and the SDPs were strongly suppressed (Fig. 5Bc, and overlaid races in Fig. 5Bd). These latter effects concurred before any noticeable change in the rise time or amplitude of the primary spike (inset in Fig. 5Ad), suggesting that they are due to block of INaP (Yue et al. 2005), which is 4-fold more sensitive to TTX than the transient Na+ current generating the fast spike (Hammarstrom & Gage, 1998). Several minutes later, however, the primary spike also decreased and eventually was blocked entirely. Similar results were obtained in 4 out of 5 experiments. In the fifth experiment, dendritic applications of TTX failed to affect bursting; subsequent axo-somatic application suppressed bursting before diminishing the primary spike (data not shown). On average, the SDPs were unaffected by dendritic applications of TTX (224.0 ± 22.0 versus 221.8 ± 20.4 mV ms), but were reduced to 40.9 ± 3.5% after its axo-somatic application (88.8 ± 6.6 mV ms; n=5; P < 0.005).
Figure 5. Effects of focal applications of TTX on bursting.
This neuron generated a burst of 4 spikes in response to brief stimuli (Aa). Brief, threshold-straddling pulses that failed to elicit a spike evoked a prolonged SDP (Ba). Focal application of TTX to the distal apical dendrites within 5 min suppressed bursting (Ab), but not the SDP (Bb). When applied to the axo-soma, TTX within 4 min further reduced the spike ADP (Ac; see also Ad in which portions from traces Ab and Ac are expanded and overlaid for comparison) and suppressed the SDP (Bc; see also Bd in which portions from traces Ba, Bb and Bc are expanded and overlaid). These latter effects concurred without a significant change in the rise time or amplitude of the primary spike (Ad, inset, showing expanded and overlaid traces from Aa, Ab and Ac). After 5 min, all spiking was blocked (not shown).
These data suggest that somatic spike backpropagation into the apical dendrites is a critical step in burst electrogenesis in se-experienced neurons. It is unlikely that TTX suppressed bursting by blocking critical INaP in the apical dendrites, because, as shown above (Fig. 3), the INaP blockers PDB and riluzole applied to the entire neuron in the superfusing ACSF did not affect this firing mode.
Focal applications of retigabine
To further assess the role of dendritic Ca2+-dependent electrogenesis in bursting, we tested the effects of retigabine applied to the distal apical dendrites. Retigabine is an agonist of KV7/M-type K+ channels, enhancing Im by strongly shifting its activation curve to more negative potentials (Main et al. 2000; Wickenden et al. 2000; Tatulian et al. 2001). We have shown previously that retigabine applied to the apical dendrites of normal CA1 pyramidal cells locally suppresses dendritic Ca2+ spikes and Ca2+-dependent bursting induced by 4-aminopyridine without affecting spike generation in the axo-soma (Yue & Yaari, 2006).
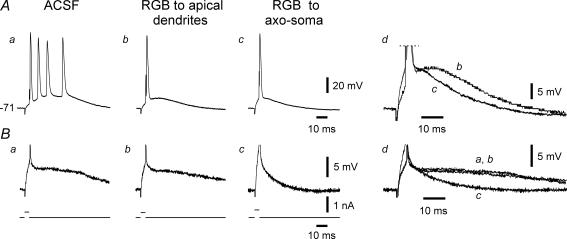
The application pipette was filled with 100 μm retigabine. A representative experiment is shown in Fig. 6. After 6 min of applying retigabine to the apical dendrites, bursting (four intraburst spikes) was converted to single spiking (Fig. 6Aa and Ab). Despite the suppression of bursting, the SDPs were unaffected (Fig. 6Ba and Bb, and overlaid races in Fig. 6Bd). Likewise, resting potential and apparent input resistance were unaffected by distally applied retigabine (Yue & Yaari, 2006). The application pipette was then moved to the axo-soma and retigabine was re-applied. This application hyperpolarized the neuron from −71.0 to −75.4 mV within 5 min. We counteracted this hyperpolarization by steadily injecting appropriate positive current. Under this condition the spike ADP was further reduced (Fig. 6Ac, and overlaid traces in Fig. 6Bd) and the SDPs were markedly suppressed (Fig. 6Bc, and overlaid traces in Fig. 6Bd). Similar results were obtained in 4 out of 4 experiments. On average, the SDPs were unaffected by dendritic applications of retigabine (234.3 ± 30.0 versus 218.4 ± 25.1 mV ms), but were reduced to 43.3 ± 5.0% after its axo-somatic application (92.2 ± 8.2 mV ms; n=4; P < 0.05).
Figure 6. Effects of focal applications of retigabine on bursting.
This neuron generated a burst of 4 spikes in response to brief stimuli (Aa). Brief, threshold-straddling pulses that failed to elicit a spike evoked a prolonged SDP (Ba). Focal application of retigabine (RGB) to the distal apical dendrites within 6 min suppressed bursting (Ab), but not the SDP (Bb). When applied to the axo-soma, retigabine further reduced the spike ADP (Ac; see also Ad in which portions from traces Ab and Ac are expanded and overlaid for comparison) and suppressed the SDP (Bc; see also Dd in which portions from traces Ba, Bb and Bc are expanded and overlaid).
These data confirm that bursting in se-experienced neurons requires interplay between axo-somatic and apical dendritic conductances, and can be suppressed by increasing Im conductance in the apical dendrites.
Intradendritic recordings
To further assess the role of apical dendrites in bursting, we performed intradendritic recordings from putative apical dendrites of se-experienced and of control CA1 pyramidal cells. Cells were impaled in stratum radiatum about 200 μm from the cell body layer, and biocytin was injected via the recording microelectrode for post hoc histological verification of cell type. Successful staining was obtained in 6 out of 8 and 3 out of 4 impalements from se-experienced and control neurons, respectively. In all cases the stained neuron had its soma in the pyramidal layer and manifested characteristic pyramidal cell morphology (Fig. 7A). In 3 out of 6 confirmed intradendritic recordings from se-experienced neurons, the primary fast spike was followed by a series of fast and slow spikes. A representative example of such complex spiking is shown in Fig. 7B. It can be seen in the three sequential responses (Fig. 7Ba1–a3, overlaid in Fig. 7Ba4) that the late slow spikes (presumed to be Ca2+ spikes) show considerable jitter from one response to another. Addition of 100 μm Ni2+ to the ACSF progressively reduced the burst responses until they were reduced to a single spike (Fig. 7Bb1–b4). In the other three recordings from the se-experienced neurons (Fig. 7C), as well as in the three confirmed intradendritic recordings from control neurons, brief depolarizing current pulses evoked a solitary spike that was followed by a monotonically declining ADP.
Figure 7. Intradendritic recordings from se-experienced and control CA1 pyramidal cell.
A, photomicrograph shows a biocytin-stained se-experienced neuron, clearly a pyramidal cell, that was impaled in stratum radiatum at about 200 μm from the pyramidal layer. The approximate site of recording is indicated with arrow. B, recordings from the neuron shown in A. Brief stimuli evoked bursts of three fast spikes followed by several irregular slow spikes (a1–a3; these traces are expanded and overlaid in a4 to facilitate comparison). Note marked jitter in timing of slow spikes. Adding 100 μm Ni2+ to the ACSF first suppressed the slow spikes and eventually reduced the burst to a single spike (b1–b4). C, intradendritic recordings from a control neuron, identified by biocytin staining as a pyramidal cell. The neuron fired single spikes in response to brief stimuli.
Our findings are consistent with previous studies showing that backpropagating spikes do not normally evoke bursts or Ca2+ spikes in the apical dendrites (Spruston et al. 1995; Magee & Carruth, 1999). However, we show here that early after pilocarpine-se, these dendrites manifest a heightened propensity for generating Ca2+-dependent bursts and Ca2+ spikes upon their invasion by backpropagating fast spikes.
Truncation of distal apical dendrites
As a final test of the active role played by the apical dendrites in the bursting behaviour of se-experienced neurons, we examined the consequences of truncating these dendrites. Somatic intracellular recordings were made > 1 h after surgically cutting the apical dendrites at about 150 μm from the soma (Fig. 8A; see Methods). We have shown previously that truncated CA1 pyramidal cells remain viable and normally excitable (Yue et al. 2005). Of 22 truncated se-experienced neurons, only one (5%) neuron fired bursts as its minimal spike output (compared to 65% in intact se-experienced neurons), whereas all other neurons generated a solitary spike (Fig. 8B). Notwithstanding, the latter neurons readily converted to bursting mode after linopirdine (10 μm) was added to the ACSF (n=4; data not shown), as shown for normal intact neurons (Yue & Yaari, 2004), as well as for control intact neurons in this study (see above, Fig. 2Ba and b). These results further support our hypothesis the bursts in se-experienced neurons result from interplay between somatic and apical dendritic conductances.
Discussion
In this study we further explore the mechanism of low-threshold bursting that emerges in CA1 pyramidal cells after pilocarpine-se (Sanabria et al. 2001). We show that this aberrant firing mode, which greatly amplifies the spike output of se-experienced neurons, predominates already during the second week after pilocarpine-se before the emergence of spontaneous seizures (> 2 weeks after pilocarpine-se; Turski et al. 1983; Priel et al. 1996). We confirm the predominant and critical role of ICaT in bursting and show that bursts are the product of interplay between backpropagating Na+ spikes and ICaT-driven depolarizations in the distal apical dendrites. A comparable ‘ping-pong’ mechanism underlies low-threshold bursting that appears in CA1 pyramidal cells during the second and third postnatal weeks (Chen et al. 2005). Thus, our findings highlight a similarity between epileptogenesis and ontogenesis, suggesting that the emergence of bursting in se-experienced neurons may be a pathological replay of a normal developmental programme.
Critical role of ICaT in bursting
We have shown previously that bursting in se-experienced CA1 pyramidal cells (examined 2–6 weeks after pilocarpine-se) is suppressed in most cases by inhibiting voltage-gated Ca2+ currents (Sanabria et al. 2001). Further experiments with type-selective Ca2+ channel blockers indicated that bursting is unaffected by blockers of L-, N-, P- and Q-type Ca2+ currents, but is readily suppressed by 50 or 100 μm Ni2+ (Su et al. 2002). At these low concentrations, Ni2+ blocks ICaT (particularly the CaV3.2 isoform), as well as ICaR (Williams et al. 1999; Lee et al. 1999). The latter finding is replicated here also in neurons examined 1 week after pilocarpine-se (Fig. 1).
Several experimental findings point to the predominant role of ICaT in the bursting of se-experienced CA1 pyramidal cells. Firstly, the density of ICaT is markedly (∼3-fold) up-regulated after pilocarpine-se, while that of ICaR is unchanged (Su et al. 2002). Secondly, modest depolarization, which causes steady-state inactivation of ICaT but not of ICaR (Randall & Tsien, 1997), abolishes bursting (Su et al. 2002). Thirdly, as shown above (Fig. 2), the ICaT blocker amiloride suppresses bursting, whereas the IR blocker SNX-482 does not. However, because ICaR is only partially blocked by SNX-482 (Sochivko et al. 2003), our results do not exclude an auxiliary role for ICaR in bursting.
In normal adult CA1 pyramidal cells, the spike ADP is smaller than in se-experienced neurons and is insensitive to Ni2+ (Su et al. 2002; Yue et al. 2005), indicating that ICaT is not involved in its generation. Rather, INaP furnishes the main driving force for the spike ADP (Yue et al. 2005). Likewise, INaP drives bursting induced acutely in these neurons by various experimental manipulations that augment the spike ADP (Azouz et al. 1996; Alroy et al. 1997; Su et al. 2001; Yue & Yaari, 2006). Nevertheless, we show here that despite the critical role of INaP in shaping the firing mode of normal adult neurons, PDB and riluzole, at concentrations that block INaP entirely (Yue et al. 2005), do not modify bursting in most se-experienced neurons (Fig. 3). Thus, INaP activation generally is not required for bursting. Only in the small subset of bursters (∼20%) that do not respond to Ni2+, INaP probably provides the main driving force for bursting.
‘Ping-pong’ mechanism of bursting
The fact that ICaT and INaP can independently drive bursting in the same type of neuron, albeit in different conditions, is likely to be due to their differential spatial distribution. Whereas T-type Ca2+ channels are expressed mainly in the distal trunk and oblique processes of apical dendrites (Karst et al. 1993; Christie et al. 1995; Frick et al. 2003), persistent Na+ channels are expressed largely in the proximal axo-soma (Yue & Yaari, 2006; Golomb et al. 2006), perhaps only in the initial axon segment (Astman et al. 2006). We have shown previously, that INaP-driven bursting commences in the axo-soma and persists in truncated neurons (Yue et al. 2005; Yue & Yaari, 2006; Golomb et al. 2006). Here we provide ample evidence that the ICaT-driven bursting involves interplay between electrical events in axo-soma and apical dendrites. Firstly, focal application of Ni2+ was effective in suppressing bursting when targeted to the distal apical dendrites, but not to the axo-soma (Fig. 4). Secondly, bursting was suppressed by distally applied TTX to block backpropagating spikes (Fig. 5). Thirdly, bursting was suppressed by distally applied retigabine to decrease the excitability of the apical dendrites (Fig. 6; Yue & Yaari, 2006). Fourthly, intradendritic recordings revealed Ni2+-sensitive slow spikes associated with bursting (Fig. 7). Finally, severing the distal apical dendrites eliminated bursting (Fig. 8). These data support a ‘ping-pong’ mechanism of bursting, which commences when the somatic spike backpropagates into the apical dendrites, causing a local depolarization, or even a regenerative slow spike, by recruiting ICaT. The distal ICaT-driven depolarization, in turn, spreads back to the axo-soma, boosting the spike ADP and triggering additional fast spikes, which also backpropagate to the dendrites, reinforcing their depolarization, and so forth. This interplay continues until opposing slow K+ currents repolarize the neuron (Golomb et al. 2006; Sipila et al. 2006).
A similar ‘ping-pong’ mechanism of bursting can be invoked in ordinary adult CA1 pyramidal cells by treating them with millimolar levels of 4-aminopyridine (Magee & Carruth, 1999). In this case, the formation of a Ca2+ spike in the apical dendrites largely by ICaT is attributed to block of A-type K+ current (IA) that normally limits the extent of dendritic spike invasion (Hoffman et al. 1997). Interestingly, it was shown recently that active spike invasion of apical dendrites is more extensive in se-experienced CA1 pyramidal cells from chronically epileptic rats (6 ± 2 months after pilocarpine-se), perhaps due to down-regulation of IA (Bernard et al. 2004). If such a change occurs early in epileptogenesis, it would complement the increase in ICaT density in driving apical dendritic Ca2+ spikes and bursting.
Implications for epileptogenesis
The factors that couple pilocarpine-se to the increase in ICaT density and hence to the emergence of bursting are unknown, but are likely to involve transcriptional and/or post-translational increases in CaV3.2 expression. It is noteworthy that during the second and third weeks of postnatal development, most CA1 pyramidal cells transiently express a bursting phenotype that also involves recruitment of apical dendritic Ni2+-sensitive Ca2+ channels by the backpropagating somatic spikes (Chen et al. 2005). Intrigingly intrinsic bursting behaviour in these developing neurons emerges after a period of intense population bursting (Garaschuk et al. 1998). It would be interesting to determine whether this natural seizure-like activity has a causative role in the subsequent emergence of low-threshold bursting. If that is the case, then the induction of bursting behaviour by pilocarpine-se may be a pathological replay of a normal development process (Cohen et al. 2003).
A large body of evidence suggests that intrinsic bursters are pivotal to the generation of interictal-like population bursts that occur in between and during epileptic seizures (Yaari & Beck, 2002). In particular, spontaneously bursting neurons are critically important in entraining other neurons into synchronized population bursts, and thus may serve as the initiators and pacemakers of epileptic discharges (Traub et al. 1987; Chagnac-Amitai & Connors, 1989; Jensen & Yaari, 1997). We have shown previously in the pilocarpine-se model, that bursting neurons drive the entire CA1 network into interictal-like population bursts (Sanabria et al. 2001). It is therefore very likely that the early emergence of intrinsic bursters, perhaps in conjunction with reduced GABAergic inhibition (Dudek et al. 2002), fosters the appearance of interictal EEG ‘spikes’ early in epileptogenesis (Stewart & Leung, 2003).
Although in this study we have used brief depolarizing current injections to evoke burst discharges, threshold-straddling excitatory postsynaptic potentials (EPSPs) similarly evoke full-blown bursts in bursting neurons (Su et al. 2002). Because postsynaptic bursts induce long-term potentiation of EPSPs (Thomas et al. 1998; Pike et al. 1999), the emergence of bursting early after pilocarpine-se, may secondarily induce persistent increases in excitatory synaptic transmission that will further lower the threshold for seizure generation (Staley et al. 2005).
In summary, we show that within a few days after pilocarpine-se, the intrinsic firing mode of 65% of CA1 pyramidal cells shifts from regular firing to bursting. This dramatic change is accounted for mainly by up-regulation of ICaT, whose recruitment in the apical dendrites by backpropagating somatic spikes boosts the somatic spike ADPs to the point of bursting. The appearance of such a large proportion of abnormally firing, or ‘epileptic’, neurons during the latent period of epileptogenesis, undoubtedly contributes directly and indirectly to the eventual development of a chronic epileptic condition. T-type Ca2+ channels (particularly CaV3.2) are thus identified as potential targets for pharmacological and molecular treatments aimed at halting epileptogenesis during its latent phase.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft SFB TR3, the German–Israel collaborative research programme of the Bundesministerium für Bildung und Forschung (BMBF) and the Ministry of Science and Technology (MOST), the Binational US–Israel Science Foundation grant, and the Henri J. and Erna D. Leir Chair for Research in Neurodegenerative Diseases.
References
- Astman N, Gutnick MJ, Fleidervish IA. Persistent sodium current in layer 5 neocortical neurons is primarily generated in the proximal axon. J Neurosci. 2006;26:3465–3473. doi: 10.1523/JNEUROSCI.4907-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azouz R, Jensen MS, Yaari Y. Ionic basis of spike after-depolarization and burst generation in adult rat hippocampal CA1 pyramidal cells. J Physiol. 1996;492:211–223. doi: 10.1113/jphysiol.1996.sp021302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard C, Anderson A, Becker A, Poolos NP, Beck H, Johnston D. Acquired dendritic channelopathy in temporal lobe epilepsy. Science. 2004;305:532–535. doi: 10.1126/science.1097065. [DOI] [PubMed] [Google Scholar]
- Chagnac-Amitai Y, Connors BW. Synchronized excitation and inhibition driven by intrinsically bursting neurons in neocortex. J Neurophysiol. 1989;62:1149–1162. doi: 10.1152/jn.1989.62.5.1149. [DOI] [PubMed] [Google Scholar]
- Chen S, Yue C, Yaari Y. A transitional period of Ca2+-dependent bursting triggered by spike backpropagation into apical dendrites in developing rat CA1 neurons. J Physiol. 2005;567:79–93. doi: 10.1113/jphysiol.2005.084590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie BR, Eliot LS, Ito K, Miyakawa H, Johnston D. Different Ca2+channels in soma and dendrites of hippocampal pyramidal neurons mediate spike-induced Ca2+ influx. J Neurophysiol. 1995;73:2553–2557. doi: 10.1152/jn.1995.73.6.2553. [DOI] [PubMed] [Google Scholar]
- Cohen I, Navarro V, Le Duigou C, Miles R. Mesial temporal lobe epilepsy: a pathological replay of developmental mechanisms? Biol Cell. 2003;95:329–333. doi: 10.1016/s0248-4900(03)00081-9. [DOI] [PubMed] [Google Scholar]
- Dudek FE, Hellier JL, Williams PA, Ferraro DJ, Staley KJ. The course of cellular alterations associated with the development of spontaneous seizures after status epilepticus. Prog Brain Res. 2002;135:53–65. doi: 10.1016/S0079-6123(02)35007-6. [DOI] [PubMed] [Google Scholar]
- Engel J, Jr, Williamson PD, Wieser H-G. Mesial temporal epilepsy. In: Engel J Jr, Pedley TA, editors. Epilepsy: a Comprehensive Textbook. Philadelphia: Lippincott-Taven Publishers; 1997. pp. 2417–2426. [Google Scholar]
- French CR, Sah P, Buckett KJ, Gage PW. A voltage-dependent persistent sodium current in mammalian hippocampal neurons. J Gen Physiol. 1990;95:1139–1157. doi: 10.1085/jgp.95.6.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick A, Magee J, Koester HJ, Migliore M, Johnston D. Normalization of Ca2+ signals by small oblique dendrites of CA1 pyramidal neurons. J Neurosci. 2003;23:3243–3250. doi: 10.1523/JNEUROSCI.23-08-03243.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaschuk O, Hanse E, Konnerth A. Developmental profile and synaptic origin of early network oscillations in the CA1 region of rat neonatal hippocampus. J Physiol. 1998;507:219–236. doi: 10.1111/j.1469-7793.1998.219bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb D, Yue C, Yaari Y. Contribution of persistent Na+ current and M-type K+ current to somatic bursting in CA1 pyramidal cells: combined experimental and modeling study. J Neurophysiol. 2006;96:1912–1926. doi: 10.1152/jn.00205.2006. [DOI] [PubMed] [Google Scholar]
- Hammarstrom AK, Gage PW. Inhibition of oxidative metabolism increases persistent sodium current in rat CA1 hippocampal neurons. J Physiol. 1998;510:735–741. doi: 10.1111/j.1469-7793.1998.735bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilaire C, Diochot S, Desmadryl G, Richard S, Valmier J. Toxin-resistant calcium currents in embryonic mouse sensory neurons. Neurosci. 1997;80:267–276. doi: 10.1016/s0306-4522(97)00101-2. [DOI] [PubMed] [Google Scholar]
- Hoffman DA, Magee JC, Colbert CM, Johnston D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 1997;387:869–875. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Matsumoto S. Classification of voltage-dependent Ca2+ channels in trigeminal ganglion neurons from neonatal rats. Life Sci. 2003;73:1175–1187. doi: 10.1016/s0024-3205(03)00414-4. [DOI] [PubMed] [Google Scholar]
- Jensen MS, Azouz R, Yaari Y. Variant firing patterns in rat hippocampal pyramidal cells modulated by extracellular potassium. J Neurophysiol. 1994;71:831–839. doi: 10.1152/jn.1994.71.3.831. [DOI] [PubMed] [Google Scholar]
- Jensen MS, Azouz R, Yaari Y. Spike after-depolarization and burst generation in adult rat hippocampal CA1 pyramidal cells. J Physiol. 1996;492:199–210. doi: 10.1113/jphysiol.1996.sp021301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MS, Yaari Y. Role of intrinsic burst firing, potassium accumulation, and electrical coupling in the elevated potassium model of hippocampal epilepsy. J Neurophysiol. 1997;77:1224–1233. doi: 10.1152/jn.1997.77.3.1224. [DOI] [PubMed] [Google Scholar]
- Karst H, Joels M, Wadman WJ. Low-threshold calcium current in dendrites of the adult rat hippocampus. Neurosci Lett. 1993;164:154–158. doi: 10.1016/0304-3940(93)90880-t. [DOI] [PubMed] [Google Scholar]
- Kim HC, Chung MK. Voltage-dependent sodium and calcium currents in acutely isolated adult rat trigeminal root ganglion neurons. J Neurophysiol. 1999;81:1123–1134. doi: 10.1152/jn.1999.81.3.1123. [DOI] [PubMed] [Google Scholar]
- Kleyman TR, Cragoe EJ., Jr Amiloride and its analogs as tools in the study of ion transport. J Membr Biol. 1988;105:1–21. doi: 10.1007/BF01871102. [DOI] [PubMed] [Google Scholar]
- Lacinová L, Klugbauer N, Hofmann F. Regulation of the calcium channel α1G subunit by divalent cations and organic blockers. Neuropharmacology. 2000;39:1254–1266. doi: 10.1016/s0028-3908(99)00202-6. [DOI] [PubMed] [Google Scholar]
- Lee JH, Gomora JC, Cribbs LL, Perez-Reyes E. Nickel block of three cloned T-type calcium channels: low concentrations selectively block α1H. Biophys J. 1999;77:3034–3042. doi: 10.1016/S0006-3495(99)77134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC, Carruth M. Dendritic voltage-gated ion channels regulate the action potential firing mode of hippocampal CA1 pyramidal neurons. J Neurophysiol. 1999;82:1895–1901. doi: 10.1152/jn.1999.82.4.1895. [DOI] [PubMed] [Google Scholar]
- Main MJ, Cryan JE, Dupere JR, Cox B, Clare JJ, Burbidge SA. Modulation of KCNQ2/3 potassium channels by the novel anticonvulsant retigabine. Mol Pharmacol. 2000;58:253–262. doi: 10.1124/mol.58.2.253. [DOI] [PubMed] [Google Scholar]
- Morimoto K, Fahnestock M, Racine RJ. Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog Neurobiol. 2004;73:1–60. doi: 10.1016/j.pneurobio.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Newcomb R, Szoke B, Palma A, Wang G, Chen X, Hopkins W, et al. Selective peptide antagonist of the class E calcium channel from the venom of the tarantula Hysterocrates gigas. Biochemistry. 1998;37:15353–15362. doi: 10.1021/bi981255g. [DOI] [PubMed] [Google Scholar]
- Pike FG, Meredith RM, Olding AW, Paulsen O. Postsynaptic bursting is essential for ‘Hebbian’ induction of associative long-term potentiation at excitatory synapses in rat hippocampus. J Physiol. 1999;518:571–576. doi: 10.1111/j.1469-7793.1999.0571p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priel MR, dos Santos NF, Cavalheiro EA. Developmental aspects of the pilocarpine model of epilepsy. Epilepsy Res. 1996;26:115–121. doi: 10.1016/s0920-1211(96)00047-2. [DOI] [PubMed] [Google Scholar]
- Randall AD, Tsien RW. Contrasting biophysical and pharmacological properties of T-type and R-type calcium channels. Neuropharmacology. 1997;36:879–893. doi: 10.1016/s0028-3908(97)00086-5. [DOI] [PubMed] [Google Scholar]
- Robbins J. KCNQ potassium channels: physiology, pathophysiology, and pharmacology. Pharmacol Ther. 2001;90:1–19. doi: 10.1016/s0163-7258(01)00116-4. [DOI] [PubMed] [Google Scholar]
- Sanabria ER, da Silva AV, Spreafico R, Cavalheiro EA. Damage, reorganization, and abnormal neocortical hyperexcitability in the pilocarpine model of temporal lobe epilepsy. Epilepsia. 2002;43(Suppl. 5):96–106. doi: 10.1046/j.1528-1157.43.s.5.31.x. [DOI] [PubMed] [Google Scholar]
- Sanabria ER, Su H, Yaari Y. Initiation of network bursts by Ca2+-dependent intrinsic bursting in the rat pilocarpine model of temporal lobe epilepsy. J Physiol. 2001;532:205–216. doi: 10.1111/j.1469-7793.2001.0205g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scroggs RS, Fox AP. Calcium current variation between acutely isolated adult rat dorsal root ganglion neurons of different size. J Physiol. 1992;445:639–658. doi: 10.1113/jphysiol.1992.sp018944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipila ST, Huttu K, Voipio J, Kaila K. Intrinsic bursting of immature CA3 pyramidal neurons and consequent giant depolarizing potentials are driven by a persistent Na+ current and terminated by a slow Ca2+-activated K+ current. Eur J Neurosci. 2006;23:2330–2338. doi: 10.1111/j.1460-9568.2006.04757.x. [DOI] [PubMed] [Google Scholar]
- Sochivko D, Chen J, Becker A, Beck H. Blocker-resistant Ca2+ currents in rat CA1 hippocampal pyramidal neurons. Neuroscience. 2003;116:629–638. doi: 10.1016/s0306-4522(02)00777-7. [DOI] [PubMed] [Google Scholar]
- Sochivko D, Pereverzev A, Smyth N, Gissel C, Schneider T, Beck H. The CaV2.3 Ca2+ channel subunit contributes to R-type Ca2+ currents in murine hippocampal and neocortical neurones. J Physiol. 2002;542:699–710. doi: 10.1113/jphysiol.2002.020677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruston N, Schiller Y, Stuart G, Sakmann B. Activity-dependent action potential invasion and calcium influx into hippocampal CA1 dendrites. Science. 1995;268:297–300. doi: 10.1126/science.7716524. [DOI] [PubMed] [Google Scholar]
- Staley K, Hellier JL, Dudek FE. Do interictal spikes drive epileptogenesis? Neuroscientist. 2005;11:272–276. doi: 10.1177/1073858405278239. [DOI] [PubMed] [Google Scholar]
- Stewart LS, Leung LS. Temporal lobe seizures alter the amplitude and timing of rat behavioral rhythms. Epilepsy Behav. 2003;4:153–160. doi: 10.1016/s1525-5050(03)00006-4. [DOI] [PubMed] [Google Scholar]
- Su H, Alroy G, Kirson ED, Yaari Y. Extracellular calcium modulates persistent sodium current-dependent intrinsic bursting in rat hippocampal neurons. J Neurosci. 2001;21:4173–4182. doi: 10.1523/JNEUROSCI.21-12-04173.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, Sochivko D, Becker A, Chen J, Jiang Y, Yaari Y, Beck H. Upregulation of a T-type Ca2+ channel causes a long-lasting modification of neuronal firing mode after status epilepticus. J Neurosci. 2002;22:3645–3655. doi: 10.1523/JNEUROSCI.22-09-03645.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Wakamori M, Akaike N. Hippocampal CA1 pyramidal cells of rats have four voltage-dependent calcium conductances. Neurosci Lett. 1989;104:229–234. doi: 10.1016/0304-3940(89)90359-5. [DOI] [PubMed] [Google Scholar]
- Tang CM, Presser F, Morad M. Amiloride selectively blocks the low threshold (T) calcium channel. Science. 1988;240:213–215. doi: 10.1126/science.2451291. [DOI] [PubMed] [Google Scholar]
- Tatulian L, Delmas P, Abogadie FC, Brown DA. Activation of expressed KCNQ potassium currents and native neuronal M-type potassium currents by the anticonvulsant drug retigabine. J Neurosci. 2001;21:5535–5545. doi: 10.1523/JNEUROSCI.21-15-05535.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MJ, Watabe AM, Moody TD, Makhinson M, O'Dell TJ. Postsynaptic complex spike bursting enables the induction of LTP by theta frequency synaptic stimulation. J Neurosci. 1998;18:7118–7126. doi: 10.1523/JNEUROSCI.18-18-07118.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorovic SM, Lingle CJ. Pharmacological properties of T-type Ca2+ current in adult rat sensory neurons: effects of anticonvulsant and anesthetic agents. J Neurophysiol. 1998;79:240–252. doi: 10.1152/jn.1998.79.1.240. [DOI] [PubMed] [Google Scholar]
- Tottene A, Volsen S, Pietrobon D. α1E subunits form the pore of three cerebellar R-type calcium channels with different pharmacological and permeation properties. J Neurosci. 2000;20:171–178. doi: 10.1523/JNEUROSCI.20-01-00171.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Miles R, Wong RK. Models of synchronized hippocampal bursts in the presence of inhibition. I. Single population events. J Neurophysiol. 1987;58:739–751. doi: 10.1152/jn.1987.58.4.739. [DOI] [PubMed] [Google Scholar]
- Turski WA, Cavalheiro EA, Schwarz M, Czuczwar SJ, Kleinrok Z, Turski L. Limbic seizures produced by pilocarpine in rats: behavioural, electroencephalographic and neuropathological study. Behav Brain Res. 1983;9:315–335. doi: 10.1016/0166-4328(83)90136-5. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- Wellmer J, Su H, Beck H, Yaari Y. Long-lasting modification of intrinsic discharge properties in subicular neurons following status epilepticus. Eur J Neurosci. 2002;16:259–266. doi: 10.1046/j.1460-9568.2002.02086.x. [DOI] [PubMed] [Google Scholar]
- Wickenden AD, Yu W, Zou A, Jegla T, Wagoner PK. Retigabine, a novel anti-convulsant, enhances activation of KCNQ2/Q3 potassium channels. Mol Pharmacol. 2000;58:591–600. doi: 10.1124/mol.58.3.591. [DOI] [PubMed] [Google Scholar]
- Williams ME, Washburn MS, Hans M, Urrutia A, Brust PF, Prodanovich P, Harpold MM, Stauderman KA. Structure and functional characterization of a novel human low-voltage activated calcium channel. J Neurochem. 1999;72:791–799. doi: 10.1046/j.1471-4159.1999.0720791.x. [DOI] [PubMed] [Google Scholar]
- Williams S, Serafin M, Muhlethaler M, Bernheim L. Distinct contributions of high- and low-voltage-activated calcium currents to afterhyperpolarizations in cholinergic nucleus basalis neurons of the guinea pig. J Neurosci. 1997;17:7307–7315. doi: 10.1523/JNEUROSCI.17-19-07307.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Toth PT, Oh SB, Gillard SE, Volsen S, Ren D, Philipson LH, Lee EC, Fletcher CF, Tessarollo L, Copeland NG, Jenkins NA, Miller RJ. The status of voltage-dependent calcium channels in α1E knock-out mice. J Neurosci. 2000;20:8566–8571. doi: 10.1523/JNEUROSCI.20-23-08566.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaari Y, Beck H. ‘Epileptic neurons’ in temporal lobe epilepsy. Brain Pathol. 2002;12:234–239. doi: 10.1111/j.1750-3639.2002.tb00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue C, Remy S, Su H, Beck H, Yaari Y. Proximal persistent Na+ channels drive spike afterdepolarizations and associated bursting in adult CA1 pyramidal cells. J Neurosci. 2005;25:9704–9720. doi: 10.1523/JNEUROSCI.1621-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue C, Yaari Y. KCNQ/M channels control spike afterdepolarization and burst generation in hippocampal neurons. J Neurosci. 2004;24:4614–4624. doi: 10.1523/JNEUROSCI.0765-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue C, Yaari Y. Axo-somatic and apical dendritic Kv7/M channels differentially regulate the intrinsic excitability of adult rat CA1 pyramidal cells. J Neurophysiol. 2006;95:3480–3495. doi: 10.1152/jn.01333.2005. [DOI] [PubMed] [Google Scholar]