Abstract
We have proposed a model of intestinal glucose absorption in which transport by SGLT1 induces rapid insertion and activation of GLUT2 in the apical membrane by a PKC βII-dependent mechanism. Since PKC βII requires Ca2+ and glucose is depolarizing, we have investigated whether glucose absorption is regulated by the entry of dietary Ca2+ through Cav1.3 in the apical membrane. When rat jejunum was perfused with 75 mm glucose, Ca2+-deplete conditions, or perfusion with the L-type antagonists nifedipine and verapamil strongly diminished the phloretin-sensitive apical GLUT2, but not the phloretin-insensitive SGLT1 component of glucose absorption. Western blotting showed that in each case there was a significant decrease in apical GLUT2 level, but no change in SGLT1 level. Inhibition of apical GLUT2 absorption coincided with inhibition of unidirectional 45Ca2+ entry by nifedipine and verapamil. At 10 mm luminal Ca2+, 45Ca2+ absorption in the presence of 75 mm glucose was 2- to 3-fold that in the presence of 75 mm mannitol. The glucose-induced component was SGLT1-dependent and nifedipine-sensitive. RT-PCR revealed the presence of Cavβ3 in jejunal mucosa; Western blotting and immunocytochemistry localized Cavβ3 to the apical membrane, together with Cav1.3. We conclude that in times of dietary sufficiency Cav1.3 may mediate a significant pathway of glucose-stimulated Ca2+ entry into the body and that luminal supply of Ca2+ is necessary for GLUT2-mediated glucose absorption. The integration of glucose and Ca2+ absorption represents a complex nutrient-sensing system, which allows both absorptive pathways to be regulated rapidly and precisely to match dietary intake.
We have proposed a new model for intestinal sugar absorption. When rat jejunum is challenged with glucose, the facilitative transporter, GLUT2, is rapidly inserted into the apical membrane (Kellett & Helliwell, 2000; Helliwell et al. 2000a; Affleck et al. 2003). In addition, the intrinsic activity of GLUT2 is rapidly up-regulated (Kellett & Helliwell, 2000; Helliwell et al. 2000b). Since GLUT2 is a high Km, high capacity transporter compared with the Na+–glucose cotransporter, SGLT1, GLUT2 can provide a facilitated component several times greater than the active component at high glucose concentrations. Apical GLUT2 therefore provides a cooperative mechanism by which absorptive capacity is rapidly and precisely matched to dietary intake (Kellett, 2001; Kellett & Brot-Laroche, 2005). In this model, fructose absorption across the apical membrane is mediated by GLUT5 and GLUT2 (Cheeseman, 1993; Corpe et al. 1996; Helliwell et al. 2000a, 2000b). When intestine of wild-type, unanaesthetized mouse is challenged with fructose, glucose or sucrose by gastric intubation, large increases in GLUT2-mediated fructose absorption occur within minutes; the increase does not occur in GLUT2-null mice and the difference is attributable entirely to rapid, apical insertion of GLUT2 (Gouyon et al. 2003). Apical GLUT2 insertion is increased in response to enteroendocrine sensing (Au et al. 2002), energy sensing (Walker et al. 2004), experimental diabetes (Corpe et al. 1996; Marks et al. 2003), long-term dietary carbohydrate (Gouyon et al. 2003) and refeeding after phase 3 starvation (Habold et al. 2005). GLUT2 is present in the apical membrane of the midgut of larvae (Caccia et al. 2005) and increases in the apical membrane of rat after birth (Baba et al. 2005).
Two observations in particular point to a role for Ca2+ in apical GLUT2 regulation. First, regulation involves a PKC βII-dependent pathway, which is activated by glucose transport through SGLT1 and forms part of a sugar-sensing mechanism (Kellett & Helliwell, 2000; Helliwell et al. 2000a, Helliwell et al. 2003). PKC βII is a conventional PKC isoform dependent on Ca2+ for activity (Hug & Sarre, 1993). Second, Ca2+ is essential for the cytoskeletal rearrangement of the enterocyte accompanying glucose entry (Madara & Pappenheimer, 1987; Turner, 2000). It follows that there must be an apical mechanism for Ca2+ entry capable of operating under conditions of sustained depolarization.
However, these observations are not readily explained by the current view of transepithelial intestinal Ca2+ transport. Thus, active (transcellular) Ca2+ transport comprises three steps. In duodenum, absorption from the lumen across the apical membrane by epithelial Ca2+ channels TRPV5 (ECaC) and TRPV6 (CaT1) is strongly favoured by the electrochemical gradient (Ward & Boyd, 1980; Sharp & Debnam, 1994). Cytosolic diffusion of Ca2+ is enhanced by binding to calbindin-D9K (Bronner et al. 1986; Feher et al. 1992). Finally, Ca2+ is transported actively across the basolateral membrane by the plasma membrane Ca2+-dependent ATPase (Bronner, 2003). TRPV5/6 are present predominantly in duodenum (Hoenderop et al. 2000; Zhuang et al. 2002), where there is little active absorption of glucose. Moreover, TRPV5/6 are activated by hyperpolarization, since they lack the S4 voltage sensor of L-type channels (Hoenderop et al. 1999, 2001; Peng et al. 1999). In contrast, apical GLUT2 insertion is concerned with Ca2+ absorption in jejunum under depolarizing conditions in the presence of high concentrations of nutrient at the apical membrane. Yet it is widely asserted that L-type channels are not present in intestine (Favus & Angeid-Backman, 1985; Fox & Green, 1986). Finally, we should note also that although active, saturable Ca2+ transport predominates in duodenum, the consensus is that at high Ca2+ concentrations the saturable component in the rest of the intestine is small compared with a non-saturable route of low permeability, which is attributed to paracellular flow (Pansu et al. 1983). When Ca2+ supply is plentiful, the active route apparently accounts only for ∼15% of overall absorption.
We have reported that the non-classical, neuroendocrine L-type channel, Cav1.3, is present in the apical membrane of rat intestine (Morgan et al. 2003). The level of Cav1.3 is negligible in duodenum, caecum and colon and maximal in distal jejunum and proximal ileum, that is, it is located in precisely the right region to play an important role in Ca2+ absorption during digestion and the associated generation of depolarizing nutrients. Cav1.3 is capable of operation under conditions of sustained, weak depolarization at low voltage thresholds and is therefore potentially ideal for intestine (Koschak et al. 2001; Lipscombe et al. 2004). Furthermore, in jejunum, we were able to monitor significant rates of Ca2+ absorption with L-type characteristics. Thus, unidirectional lumen-to-mucosa transport of 1.25 mm Ca2+ (the same as plasma free Ca2+) at 20 mm glucose was inhibited by nifedipine, Mg2+ and by repolarization of the membrane induced by blocking of glucose absorption with phloridzin; Ca2+ absorption was also activated by Bay K 8644. None of these four quite different conditions affect TRPV5/6. In the absence of evidence for any other known channel, we assume that Cav1.3 is functional and provides a significant route of transcellular absorption operating in times of dietary sufficiency.
We now report here that Ca2+ absorption across the apical membrane mediated by an L-type channel does indeed play an important role in the regulation of intestinal glucose absorption by controlling apical GLUT2 insertion. Furthermore, glucose stimulates Ca2+ absorption, most probably through Cav1.3.
Methods
Animals
All procedures used conformed to the UK Animals (Scientific Procedures) Act 1986. Male Wistar rats (240–270 g) were maintained on standard Bantin and Kingman (Hull, UK) rat and mouse diet ad libitum with free access to water.
Perfusion of jejunal loops
Rats were anaesthetized by an intraperitoneal injection of a mixture of 1.0 ml Hypnorm (Janssen Animal Health, High Wycombe, Bucks, UK) and 0.5 ml Hypnovel (Roche Diagnostics, Welwyn Garden City, Herts, UK) per kilogram body weight. Tail pinch, foot pinch and corneal reflexes were carefully monitored throughout the duration of the perfusion. Additional anaesthetic was administered by intramuscular injection of a mixture of 0.4 ml Hypnorm and 0.2 ml Hypnovel per kilogram body weight when required. Rats were humanely killed by exsanguination under anaesthetic at the conclusion of the experiment. A mid-to-distal loop of jejunum was cannulated at 10 and 35 cm from the Ligament of Treitz and perfused in vivo in single-pass mode with perfusate comprising nutrient at the stated concentration in modified Krebs–Henseleit buffer (KHB) containing 120 mm NaCl, 4.5 mm KCl, 1.0 mm MgSO4, 1.8 mm Na2HPO4, 0.2 mm NaH2PO4, 1.25 mm CaCl2, 25 mm NaHCO3 gassed (19: 1, O2: CO2) to pH 7.4 before use (Kellett & Helliwell, 2000). 45Ca2+ (0.35 kBq ml−1) and 3H-inulin (0.70 kBq ml−1) were also added for the determination of Ca2+ and water fluxes: the counting protocol automatically corrected for channel crossover. The flow rate of perfusate was 0.37 ml min−1 and that of gas 0.19 ml min−1. The perfusate flow rate of 0.37 ml min−1 was determined by the need to measure both glucose and unidirectional 45Ca2+ fluxes in a single experiment, when the rate of glucose absorption was some 70-fold greater than that of Ca2+. Thus Δc, the concentration difference across the loop, for 45Ca2+ disappearance was measurable, yet glucose was not depleted by more than 25% in order to maintain apical membrane depolarization. For studies using a high calcium concentration (10.0 mm), we used a phosphate- and Mg2+-free buffer containing (mm): 140 NaCl, 3.4 KCl, 12 NaHCO3 and 10.0 CaCl2 (Auchere et al. 1997).
To test the effect of drugs on glucose and Ca2+ absorption, or to resolve the contributions of SGLT1 and GLUT2 to glucose absorption by selective inhibition of GLUT2 with phloretin, the loop was perfused with glucose for a control period of 0–40 min and then switched to perfusion with glucose and drug for an experimental period of 40–90 min. Perfusions were viable over a period of 90 min. All drugs were from Sigma (UK). The concentration of solvent (ethanol or DMSO) used for drugs or phloretin ranged from 0.1 to 0.3% v/v and solvent alone had no effect on any components of absorption. The unidirectional absorption of Ca2+ from lumen to mucosa was determined by disappearance of 45Ca2+ from the lumen as described elsewhere (Auchere et al. 1998). Total Ca2+ was determined by ICP-OES (inductively coupled plasma optical emission spectroscopy) using a Spectro CIROS CCD instrument (Spectro Analytical Instruments, Kleve, Germany) and glucose was determined as previously described (Kellett & Helliwell, 2000). Perfusion rates for both glucose and Ca2+ were expressed in μmol min−1 (g dry wt)−1.
PCR of β-subunits
RT-PCR was performed for the four known auxiliary β-subunits using primers designed to rat subunit-specific sequences and cDNA from rat brain (Origene Technologies, Inc.) and a rat jejunal mucosal cDNA preparation provided by Dr D. Meredith, University of Oxford. Primers for rat β-subunits were designed using GenBank accession NM_017346 (Cacnb1), NM_053851 (Cacnb2), NM_012828 (Cacnb3) and XM_215742 (Cacnb4). The β1-subunit primers were sense (5′-GAC TGG TGG ATC GGG AGG C-3′) and antisense (5′-CAA CCA GCT GCA AGG TCC GG-3′); β2-subunit primers were sense (5′-ATC CAT CAC AAG AGT CAC TGC-3′) and antisense (5′-GGT GGG GCT CAG AGG TAA AG-3′); β3-subunit primers were sense (5′-TCA GCC GAC TCC TAC ACC AG-3′) and antisense (5′-GAC GCG GGT GAT GGA GAT C-3′); and β4-subunit primers were sense (5′-CGG AAG TAC AGA GTG AAA TTG AA-3′) and antisense (5′-ATA CGG TGA GAG AGC TGT GGA-3′). Cycling conditions were 95°C for 10 min followed by 35 cycles of 95°C for 1 min, 60°C (β1) or 55°C (β2, β3, β4) for 1 min and 72°C for 2 min with final extension at 72°C for 10 min using the Expand High Fidelity PCR System (Roche) and 1.25 mm Mg2+. Predicted PCR product sizes for β-subunits were 531 bp (β1), 525 bp (β2), 602 bp (β3), and 409 bp (β4). All PCR products were ligated directly into the pCR4.1-TOPO vector (Invitrogen) and then sequenced by the Oxford Biochemistry DNA sequencing facility to verify their identity.
Immunocytochemistry
Immunocytochemistry of Cav1.3 and β3-subunits was performed as previously described. Briefly, unperfused jejunum or jejunum perfused as described above (75 mm glucose and 1.25 mm Ca2+) was fixed with paraformaldehyde lysine periodate fixative in vivo. Squares of tissue from the midpoint of the segment were fixed, cryoprotected and probed as previously described (Affleck et al. 2003). For Cav1.3, the primary antibody supplied by Calbiochem (1: 100 dilution) was an affinity purified rabbit antibody to residues 809–825 (DNKVTIDDYQEEAEDKD) recognizing all rat isoforms. For the β3-subunit, the primary antibody supplied by Chemicon International (1: 40 dilution) was raised to residues 463–477 (DRNWQRNRPWPKDSY). The secondary antibody in each case was FITC-conjugated goat anti-rabbit IgG (1: 100 dilution, Sigma). In order to demonstrate specificity of labelling, some sections were treated with antibody that had been neutralized by incubation for 1 h with excess of antigenic peptide (antibody to peptide 1: 1 v/v, peptide 50 μg ml−1). Fluorescence micrographs were taken using a Zeiss LSM 510 confocal microscope (Carl Zeiss GmbH). The intensities of apical membrane staining were quantified with LSM 510 Examiner software (v3.3).
Western blotting of membrane vesicles and nuclei
Brush-border and basolateral membrane (BBM and BLM, respectively) vesicles were prepared as previously described; the enrichments of sucrase and Na+/K+-ATPase were 20-fold and 14-fold, respectively (Corpe et al. 1996; Helliwell et al. 2000a). Every stage of the preparation was performed at 0–4°C to prevent changes in trafficking of proteins after the intestine had been excised. Enrichment of sucrase activity in these highly purified brush-border preparations ranged from 16- to 20-fold; there was no significant enrichment of Na+/K+-ATPase activity. For Western blots, protein (20 μg) was separated by SDS-PAGE using either 7.5% or 10% gels and transblotted onto polyvinylidene difluoride (PVDF): Cav1.3 and β3-subunits were detected with the same antibodies as for immunocytochemistry; GLUT2, SGLT1 and PKC βII were detected by enhanced chemiluminescence (ECL) as previously described (Helliwell et al. 2000a). The same loading of protein was used for all samples: comparison of relative levels of GLUT2 was made on a protein basis in order to minimize potential complications that might be caused by the trafficking of other proteins in response to the same stimuli that affect GLUT2 trafficking. Specificity of staining was checked by neutralization of antibody with excess antigenic peptide as for immunocytochemistry. The major protease inhibitor in membrane preparation was phenyl methane sulphonyl fluoride (PMSF): the use of a commercial protease inhibitor cocktail (Sigma UK, P8340) did not produce any significant changes in banding patterns.
Statistical analysis
Values are presented as means ± s.e.m. and were tested for significance using paired or unpaired Student's t test as appropriate.
Results
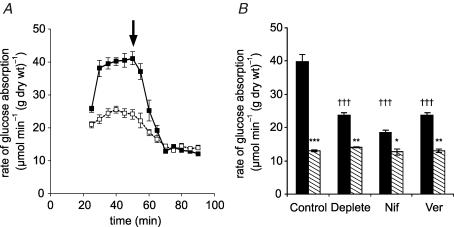
To test the hypothesis that an apical Ca2+ entry mechanism is important for the increased glucose absorption capacity at high (75 mm) glucose concentrations, rat mid to distal jejunum was perfused luminally in single-pass mode with KHB prepared with and without addition of Ca2+. The concentration of Ca2+ in modified KHB is normally 1.25 mm. Ca2+ chelators were not added to the Ca2+-free solution, as these might disrupt the integrity of the tight-junctions; this solution is therefore referred to as ‘Ca2+ deplete’. The intestine was pre-perfused for 20 min with 75 mm mannitol to allow any added effector time to act prior to perfusion with 75 mm glucose and effector; subsequent experiments, however, have shown this pre-perfusion to be unnecessary. To determine if any effects were indeed associated with the GLUT2 component, 1 mm phloretin, which inhibits GLUT2 but not SGLT1 in whole intestine, was added after an initial steady-state rate of glucose absorption was achieved (Fig. 1A). Experiments were also conducted without addition of phloretin to confirm that the steady state could be maintained for the duration of the experiment and was not affected by addition of solvent vehicle (data not shown). For control (1.25 mm Ca2+) perfusions, the initial steady-state rate was 39.9 ± 0.5 μmol min−1 (g dry wt)−1 and the SGLT1 component was 13.0 ± 0.3 μmol min−1 (g dry wt)−1 (Fig. 1A and B). These values are very similar to those previously reported; we have further established that use of cytochalasin B, or phloridzin or replacement of Na+ with choline (no inhibitors or solvents) give the same quantitative data (Kellett & Helliwell, 2000; Helliwell & Kellett, 2002). It is clear that the Ca2+ deplete condition affects the initial steady-state rate (24.2 ± 0.4 μmol min−1 (g dry wt)−1, P < 0.001, n=4), while the phloretin-insensitive rate is unaffected (13.9 ± 0.2 μmol min−1 (g dry wt)−1). Hence luminal Ca2+ concentration regulates the GLUT2- but not the SGLT1-mediated component.
Figure 1. The effect of luminal Ca2+ and L-type effectors on glucose absorption.
A, a representative time course showing the effect of Ca2+. Rat mid and distal jejunum was pre-perfused in vivo in single-pass mode with modified KHB + 100 mm mannitol containing either 1.25 mm Ca2+ (▪) or no Ca2+ (□, Ca2+-deplete perfusate); after 20 min the perfusate was switched to 75 mm glucose + 25 mm mannitol to achieve an initial steady state (30–50 min) before the addition of 1 mm phloretin at 50 min (arrow) to determine the phloretin-insensitive (SGLT1) steady-state rate (60–90 min). B, the effect of L-type effectors, which were present from the start of perfusions. Total (black bars) and phloretin-insensitive (hatched bars) steady-state rates were obtained for control, Ca2+ deplete, nifedipine (10 μm, Nif) and verapamil (100 μm, Ver) perfusions (n=4, each case). Rates are means ± s.e.m. expressed in μmol min−1 (g dry wt)−1. Student's t test was used to determine statistical significance. †††P < 0.001, unpaired test for the comparison of the initial steady-state rates of the Ca2+ deplete, nifedipine and verapamil to that of the control. ***P < 0.001, **P < 0.01, *P < 0.05, paired test comparing the initial steady-state rate with its phloretin-insensitive rate. The phloretin-insensitive rates are not statistically different from one another.
We have previously demonstrated that the non-classical L-type channel Cav1.3 is located in the apical membrane of jejunum, and have detected L-type activity pharmacologically in the presence of 20 mm glucose. We wished therefore to determine whether an L-type channel could provide the entry mechanism responsible for the Ca2+ effects at high glucose concentration. The rates of glucose and 45Ca2+ absorption were determined in the presence and absence of two L-type channel inhibitors, nifedipine (10 μm) and verapamil (100 μm). The concentration of nifedipine was the same as that used for studies of Cav1.3 in INS-1 cells (Huang et al. 2004), while that of verapamil was the same as that widely used in the study of Ca2+ channels, such as TRPV5/6 (Peng et al. 2000). Figure 2 shows that both inhibitors caused significant reductions in both glucose absorption (51.8 ± 8.6% and 45.3 ± 1.0% for nifedipine and verapamil, respectively; n=4, P < 0.001 each) and 45Ca2+ absorption (40.8 ± 4.5% and 38.1 ± 0.3%, P < 0.001). To confirm that the verapamil and nifedipine effects were associated solely with the GLUT2 component, paired perfusions with phloretin (1 mm) were also conducted. These show clearly that initial steady-state rates were reduced to a similar extent to that of the Ca2+ deplete experiments (Fig. 1B, 36.9 ± 2.4%, 50.4 ± 1.8% and 40.6 ± 3.0% for Ca2+ deplete, nifedipine and verapamil, respectively, P < 0.001), while the phloretin-insensitive rate was unaffected. Thus the GLUT2-mediated component of glucose absorption is regulated by Ca2+, while the SGLT1 component is not.
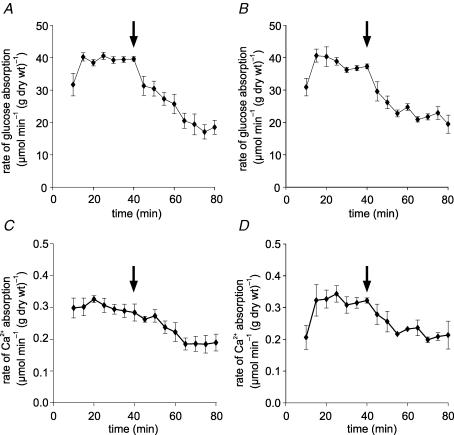
Figure 2. Inhibition of glucose absorption occurs concomitantly with inhibition of 45Ca2+ absorption by L-type channel antagonists.
Jejunum was perfused in vivo with perfusate comprising modified KHB containing 75 mm glucose, 1.25 mm Ca2+ and 45Ca2+ (0.35 kBq ml−1) as a tracer to determine unidirectional lumen-to-mucosa 45Ca2+ absorption. After a control period, the perfusion was switched (arrow) to an experimental period in which perfusate contained an L-type antagonist. Time courses are shown for: (A) the rate of glucose absorption when (C) 45Ca2+ absorption was inhibited by nifedipine (10 μm) and for (B) the rate of glucose absorption when (D) 45Ca2+ absorption was inhibited by verapamil (100 μm). Absorption rates are presented as μmol min−1 (g dry wt)−1 and means ± s.e.m., n=4.
In principle, inhibition of glucose and 45Ca2+ absorption in in vivo perfusions could have arisen if Ca2+ channel inhibitors reduced water absorption, so increasing the volume of the perfusate in the experimental phase (containing L-type effectors) relative to that of the control phase and resulting in the reduction of luminal glucose and Ca2+ concentrations. A reduction in average luminal glucose concentration might act to repolarize the apical membrane and inhibit Ca2+ absorption. Water absorption is indeed inhibited (nifedipine 0.062 ± 0.006 ml min−1 (g dry wt)−1 and verapamil 0.05 ± 0.02 ml min−1 (g dry wt)−1 compared with 0.151 ± 0.02 ml min−1 (g dry wt)−1 in the control period; P < 0.001). In the control period of perfusions with an initial concentration of 75 mm glucose, the average concentration in the luminal effluent from single-pass experiments was 66.6 ± 0.5 mm and the average value throughout the loop was therefore 70.8 mm. However, inhibition of water absorption did not cause a reduction in the effluent glucose concentration. Indeed, the concentration was slightly increased in the presence of nifedipine and remained unaltered in the case of verapamil (70.8 ± 0.6 and 66.8 ± 1.4 mm, respectively, so that the average concentrations through the loop were 72.9 and 70.9 mm, respectively). Similarly, total [Ca2+] determined by ICP-OES remained constant within experimental error between the experimental and control periods. Changes in glucose and unidirectional Ca2+ absorption could not therefore be caused by changes in luminal concentration as a secondary consequence of effects on water transport. This conclusion is in agreement with that of Younoszai & Nathan (1985), who showed that the 5-fold increase in water absorption on switching from an isotonic and a hypotonic solution had little effect on Ca2+ absorption in rat jejunum.
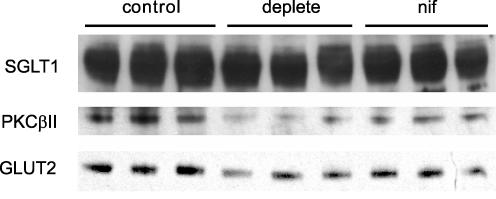
Brush-border membrane vesicles were then prepared to determine whether the Ca2+ effects were associated with alterations in GLUT2 trafficking to the apical membrane (Fig. 3). Vesicles were prepared from rat jejunum initially perfused in vivo with 75 mm glucose in modified KHB (1.25 mm Ca2+). At 20 min, perfusates were switched to 75 mm glucose in KHB containing either 1.25 mm Ca2+ (control), 0 mm Ca2+ (Ca2+ deplete), or 1.25 mm Ca2+ containing nifedipine (10 μm). Vesicles were then immunoblotted to determine the relative levels of GLUT2, PKC βII and SGLT1; while a representative blot is shown in Fig. 3, we have quantified the data from three separate blots. There were significant reductions in apical membrane levels relative to control: GLUT2 49.7 ± 3.5% (Ca2+ deplete, P < 0.01), 37.3 ± 9.7% (nifedipine, P < 0.05) and PKC βII 44.1 ± 2.1% (Ca2+ deplete, P < 0.01), 44.9 ± 4.2% (nifedipine, P < 0.01). As expected the levels of SGLT1 remained unaltered. This confirms that inhibition of Ca2+ entry inhibits both the activation of PKC βII and the trafficking of GLUT2 to the apical membrane.
Figure 3. Dependence of GLUT2, PKC βII and SGLTI levels on luminal Ca2+ and nifedipine.
Brush-border membrane vesicles were prepared from rat jejunum initially perfused in vivo with 75 mm glucose in modified KHB (1.25 mm Ca2+). At 20 min, perfusates were switched to 75 mm glucose in KHB containing either 1.25 mm Ca2+ (control), 0 mm Ca2+ (Ca2+ deplete), or 1.25 mm Ca2+ containing nifedipine (10 μm). Vesicle protein (20 μg) was then separated by SDS-PAGE (10% gels), transblotted on to PVDF and Western blotted for GLUT2, SGLT1 and PKC βII. For full details, see Methods.
Since apical Ca2+ entry involves a channel with sensitivity to L-type effectors, it would be expected that membrane depolarization by Na+–glucose cotransport should increase Ca2+ absorption. This is very clearly demonstrated with 10 mm Ca2+ in the lumen, that is, when there is a substantial transepithelial gradient. In paired comparisons made within a single perfusion, 75 mm glucose was perfused for 0–40 min and was then substituted with 75 mm mannitol for 40–80 min; Fig. 4A shows that the rate of 45Ca2+ absorption with glucose was 2-fold that with mannitol (P < 0.001, n=4). In unpaired comparisons, that is, when mannitol and glucose were used alone in separate perfusions (Fig. 4B), glucose increased the steady-state rate of 45Ca2+ absorption 3-fold (P < 0.001, n=8).
Figure 4. Activation of 45Ca2+ absorption by glucose.
A, the rates of 45Ca2+ absorption in the presence of either mannitol or glucose were compared within a single perfusion (paired comparison): rat jejunum was perfused for a control period of 40 min with 75 mm glucose and 10 mm Ca2+ (n=4). After 40 min (arrow), perfusion was continued for an experimental period in which 75 mm mannitol was substituted for 75 mm glucose. B, separate perfusions (unpaired comparison): rat jejunum was perfused with 75 mm glucose in the presence of 10 mm Ca2+ for 40 min only (n=8); separate perfusions were undertaken in which mannitol replaced glucose (n=8). P < 0.001 for comparison of the effects of glucose and mannitol in A and B (†††).
Figure 5 confirms that the channel operating at 75 mm glucose and 10 mm Ca2+ is under the control of SGLT1 and has L-type characteristics, that is, no new channel appears to have come into play. Thus 1 mm phloridzin inhibited 45Ca2+ absorption by 72%, the phloridzin-insensitive component being similar to the rate of absorption in the presence of mannitol alone (Fig. 5C); note that glucose absorption was concomitantly inhibited by 80%, as phloridzin inhibits SGLT1 directly and also indirectly blocks that part of GLUT2 which rapidly traffics away from the membrane through inhibition of Ca2+ absorption (Fig. 5A; see Kellett & Helliwell, 2000). At 10 mm Ca2+, 10 μm nifedipine inhibited 45Ca2+ absorption by 69% (Fig. 5D); glucose absorption was also concomitantly inhibited by 43% to give in Fig. 5B a rate of 20.13 ± 0.56 μmol min−1 (g dry wt)−1, similar to that at 1.25 mm Ca2+ seen under Ca2+-deplete conditions or with nifedipine or verapamil (Fig. 1); the nifedipine-insensitive component represents absorption mediated by SGLT1 plus that part of apical GLUT2 which does not traffic readily away from the membrane (see Fig. 3). This has been confirmed by showing that in the presence of nifedipine, phloretin diminishes the rate of glucose absorption from 21.45 ± 0.33 to 10.31 ± 0.26 μmol min−1 (g dry wt)−1 (P < 0.001, n=3); for comparison, phloretin diminishes the rate of glucose absorption at 75 mm glucose and 10 mm Ca2+ from 34.99 ± 0.68 to 9.98 ± 0.82 μmol min−1 (g dry wt)−1 (P < 0.001, n=3), the insensitive component being assigned to SGLT1. The magnitudes of the components of glucose absorption with either 20 mm or 75 mm glucose are not significantly different at 10 mm compared with 1.25 mm Ca2+.
Figure 5. Ca2+ absorption at high Ca2+ concentration also displays L-type characteristics.
A and C, jejunum was perfused for a control period of 40 min with 75 mm glucose and 10 mm Ca2+ (n=4). After 40 min (arrow), perfusion was continued for an experimental period with 1 mm phloridzin also present in the perfusate. A and C show the glucose and Ca2+ fluxes, respectively. B and D, jejunum was perfused for a control period of 40 min with 75 mm glucose and 10 mm Ca2+ (n=4). After 40 min (arrow), perfusion was continued for an experimental period with 10 μm nifedipine also present in the perfusate. B and D show the glucose and Ca2+ fluxes, respectively. Rates are presented as μmol min−1 (g dry wt)−1.
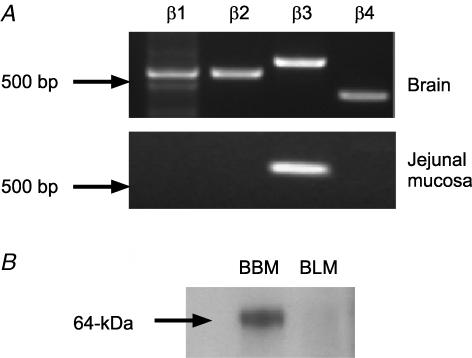
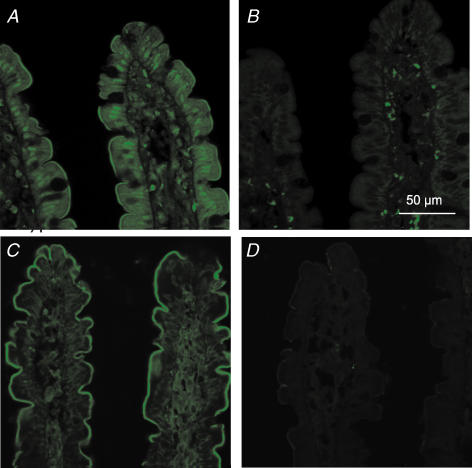
We have reported that the α1 pore-forming subunit of the CaV1.3 channel is located in the apical membrane of rat jejunum (Morgan et al. 2003). It was therefore important to identify and localize the auxiliary β-subunit(s) in jejunum. The primary screen was by homology-based RT-PCR; the primers were designed to all four known rat β-subunits and spanned intronic sequences. As shown in Fig. 6, PCR detected all four β-subunit transcripts expressed in brain cDNA (Ludwig et al. 1997); sequencing confirmed their 100% identity with the reported rat sequences. A single β3 PCR product of the correct size was detected in the rat jejunal mucosal cDNA preparation. Sequencing confirmed its 100% identity with the reported rat β3 sequence. PCR screening revealed exclusive expression of the β3-subunit transcript in jejunal mucosa. To determine the localization of β3, Western blots of BBM and BLM vesicles were prepared after perfusion of jejunum with 75 mm glucose and probed with β3 antibody. β3 was detected only at the apical membrane as a single band of ∼64 kDa, consistent with the reported molecular mass of 67 kDa in neuronal cells (Pichler et al. 1997). Immunocytochemistry of jejunum confirmed that both Cav1.3 and Cavβ3 are located at the apical membrane of rat jejunum (Fig. 7). Neutralization of antibody with excess peptide confirms that labelling is specific. There appears also to be significant intracellular labelling of Cav1.3 at the nucleus and in the cytosol, as well as labelling of both proteins within the lamina propria. Similar results were obtained whether jejunum was perfused or unperfused.
Figure 6. Rat jejunal mucosa expresses β3 mRNA and β3 protein is located in the apical membrane.
A, RT-PCR was performed for the four known auxiliary Cavβ subunits using primers designed to rat specific sequences and cDNA derived from rat brain and rat jejunal mucosa. B, jejunum was perfused for 30 min with 75 mm glucose; brush-border membrane (BBM) and basolateral membrane (BLM) vesicles were then prepared and Western blotted for β3. The BBM band was eliminated by neutralization of antibody with excess peptide (data not shown).
Figure 7. Immunocytochemical localization of Cav1.3 and β3-subunit in the proximal jejunum.
Sections of unperfused jejunum were labelled with a rabbit antibody that recognizes all forms of rat Cav1.3 (A and B) and with a rabbit antibody which specifically recognizes the β3 subunit (C and D). The secondary antibody was FITC-conjugated goat anti-rabbit IgG. The peptide controls were sections treated with antibody to Cav1.3 (B) and β3 (D) that had been pre-absorbed with excess antigenic peptide; non-specific staining in the lamina propria of these sections was seen with the secondary alone. All sections are at 63 × magnification and were taken at the same settings with a Zeiss LSM 510 confocal microscope.
Discussion
Regulation of glucose absorption by Ca2+
When Ca2+ was omitted from the luminal perfusate at high glucose concentrations, glucose absorption was inhibited (Fig. 1A). Inhibition occurred even though Ca2+ supply from the blood was still maintained in perfusions in vivo. Selective inhibition with phloretin showed that only the apical GLUT2 component of glucose absorption was inhibited, by 60%; the SGLT1 component was unaffected. Apical GLUT2 levels were decreased to a similar extent, but SGLT1 levels remained unaltered (Fig. 3). A possible reason for the lack of an effect on SGLT1 is that the chow diet used to feed the rats up-regulates SGLT1 (Kellett & Barker, 1989). That GLUT2 trafficking and absorption depend on luminal Ca2+ is consistent with the observation that nifedipine and verapamil inhibited the GLUT2 component of absorption and apical GLUT2 insertion in a manner very similar to that caused by omission of luminal Ca2+; there was again no effect on SGLT1 (Figs 2 and 3). Moreover, nifedipine and verapamil inhibited over 40% of 45Ca2+ absorption from the lumen at 75 mm glucose and the inhibition of 45Ca2+ absorption was concomitant with that of glucose absorption (Fig. 2).
The link between Ca2+ entry and glucose absorption must be cytosolic Ca2+, but there appears to be no way of measuring this directly during a perfusion in vivo. Fortunately, there are two established biochemical markers, which show that increased Ca2+ absorption goes with increased cytosolic Ca2+ and increased glucose absorption at high concentrations. Thus, apical GLUT2 insertion correlates with activation of PKC βII, which requires an increase in cytosolic Ca2+. Moreover, glucose induces cytoskeletal rearrangement of the enterocyte, which is dependent on an increase in cytosolic Ca2+ to stimulate myosin phosphorylation (Turner et al. 1997). Figure 3 shows that PKC βII is inactivated in Ca2+-deplete conditions or by luminal application of nifedipine. The accompanying paper shows that these two conditions, as well as replacement of glucose by mannitol or Ca2+-deplete conditions, block myosin phosphorylation in the terminal web and hence cytoskeletal rearrangement and apical GLUT2 insertion (Mace et al. 2007).
We have already reported that at 20 mm luminal glucose in vivo a significant pathway of jejunal Ca2+ absorption is mediated by a channel with L-type characteristics (Morgan et al. 2003). Although L-type effectors, namely nifedpine, Mg2+ and Bay K 8644, caused marked changes in Ca2+ absorption, there was little change in glucose absorption. In addition, repolarization of the membrane with phloridzin rapidly inhibited 45Ca2+ absorption; this observation is consistent with the fact that the apparent Km of glucose transport by SGLT1 is 23–26 mmin vivo (Debnam & Levin, 1975; Kellett & Helliwell, 2000). In those experiments therefore it appeared that glucose regulated Ca2+ absorption, but not that Ca2+ regulated glucose absorption. The reason is that at low glucose (20 mm and below, or mannitol), there is a basal level of GLUT2; insertion of additional GLUT2 only occurs at 30 mm and above. This statement applies only to fed rats maintained on Bantin & Kingman (UK) chow diet; the situation is different in other conditions, for example, overnight starved mice maintained on different chow diets (Gouyon et al. 2003). In the absence of luminal Ca2+ (Ca2+ deplete), only the basal level of GLUT2 is observed at 75 mm glucose, when addition of 1.25 mm Ca2+ to the lumen increases insertion (Figs 1 and 3).
We therefore conclude that the presence of luminal Ca2+ is a prerequisite for apical GLUT2 insertion in vivo. However, we note that apical GLUT2 insertion is not detectable in our experiments until 30 mm glucose, whereas repolarization of the membrane with phloridzin inhibited 45Ca2+ absorption at 20 mm glucose (Morgan et al. 2003). Also, increasing luminal Ca2+ from 1.25 mm to 10 mm increases the total rate of Ca2+ absorption almost 8-fold, but does not affect the rate of glucose absorption or the two components, either at 75 mm (see above) or at 20 mm glucose (Morgan et al. 2003). Luminal Ca2+ is therefore a necessary, but not a sufficient, requirement for insertion; a second, unknown signal is required at high glucose concentrations.
Regulation of Ca2+ absorption by glucose
As noted, previous work indicated that glucose regulated Ca2+ absorption. In addition, Western blotting and immunocytochemistry revealed Cav1.3 in the apical membrane of jejunum (see also Fig. 7). Since no other known channel in intestine can explain these observations, the L-type activity was attributed to Cav1.3 (Morgan et al. 2003). The present perfusion data at 75 mm glucose confirm the existence of an apical route of Ca2+ entry with L-type characteristics. Moreover, following identification of transcripts for Cavβ3 L-type channel subunits in rat jejunal mucosal cDNA, Cavβ3 was localized to the apical membrane by Western blotting and immunocytochemistry (Figs 6 and 7). This first demonstration of the presence of Cav1.3 and its auxiliary subunit Cavβ3 provides strong evidence for the existence of an L-type channel in the apical membrane of the mucosal epithelium. Auxiliary β-subunits direct α1-subunits to the plasma membrane by concealing an endoplasmic reticulum retention signal (Bichet et al. 2000) and appear important for functional expression of the α1-subunit at the cell surface (Gao et al. 1999). Co-expression studies in oocytes show that four different β isoforms, including Cavβ3, enhanced Ca2+ currents compared with Cav1.3 alone, but had no effect on channel activation threshold (Xu & Lipscombe, 2001).
Perfusion of 75 mm glucose at 10 mm Ca2+ increased the total rate of 45Ca2+ absorption up to 3-fold compared with 75 mm mannitol (Fig. 4B). Thus, when there was a large transepithelial gradient of Ca2+ under these conditions, there was a large glucose-induced 45Ca2+ flux, which was SGLT1-dependent since it was abolished by phloridzin. In principal, such a flux could have arisen by paracellular flow. However, the glucose-induced 45Ca2+ flux was also abolished by nifedipine, indicating that it was mediated by an L-type channel. Moreover, at 20 mm glucose, nifedipine inhibits absorption of 10 mm Ca2+ in the lumen with a time course identical to that at 1.25 mm Ca2+ (Morgan et al. 2003). In the latter case, any absorption by paracellular flow should be minimal because plasma free Ca2+ is 1.25 mm. Of particular interest, our data suggest a signalling role for Cav1.3 in intestine similar to that in pancreatic β cells. Thus the cultured cell line INS-1 contains both Cav1.2 and Cav1.3, yet glucose-stimulated insulin secretion is preferentially coupled to Cav1.3 (Liu et al. 2003); moreover, glucose also induces a nifedipine-sensitive Ca2+ influx through Cav1.3 (Huang et al. 2004). The nifedipine- and phloridzin-insensitive component of Ca2+ absorption is most likely channel-mediated, probably by TRPV5/6 (Mace et al. 2007).
Nutrient sensing: the integration of glucose and Ca2+ absorption
Glucose regulates Ca2+ absorption and Ca2+ regulates glucose absorption. The signalling and absorptive roles of Ca2+ and glucose are not distinct, rather they are integrated. The primary signal for regulation of both glucose and Ca2+ absorption is glucose. High concentrations depolarize the apical membrane by transport through the Na+–glucose cotransporter, which exerts an important regulatory role, so that Ca2+ absorption is increased up to 3-fold by induction of a nifedipine-sensitive component through Cav1.3. Binding of Ca2+ to calbindin-D9K prevents the increase in Ca2+ absorption from flooding the absorptive cell and stimulates transcellular transport, which would in principle act to attenuate potential increases in cytosolic Ca2+. However, studies in kidney have revealed that the kinetics of Ca2+ binding to calbindin-D28K are slow relative to the initiation of the rise in cytosolic Ca2+ (Koster et al. 1995). Ca2+ is therefore able to exert a signalling role with respect to glucose absorption in intestine, despite the presence of calbindin-D9K, which is necessary for normal absorptive function.
Although we have focused on glucose and Ca2+, the transport of other nutrients, such as Na+-dependent amino acids or H+-dependent peptides, may also depolarize the apical membrane. There exists, therefore, a network of nutrient interactions beyond glucose, which has the potential to modulate Ca2+ absorption. This does not mean that these other nutrients are necessarily capable of promoting apical GLUT2 insertion. Their ability to do so will depend ultimately on whether they are also able to provide the second signal, which glucose does so effectively at high but not low concentrations, and also on their rate of delivery to the jejunum. Potential nutrient interactions are also modulated by long-term diet. Maintenance of mice on a low carbohydrate/high protein diet prevents apical GLUT2 formation and blocks its induction by a bolus of sugar (Gouyon et al. 2003). This observation may explain why patients with glucose galactose absorption syndrome, who are required to avoid dietary sugar, cannot absorb glucose even in the presence of amino acids.
Our observations emphasize the importance of studying the absorption of one nutrient in the presence of another. Nevertheless, the number of papers in the literature in which the effect of glucose on Ca2+ absorption has been studied are small (Younoszai & Nathan, 1985; Carroll et al. 1988). The majority of work on Ca2+ absorption has been done on starved rats in the absence of nutrient, conditions which cause membrane hyperpolarization and diminish the contribution of L-type channels, so that they may not be functionally detectable with L-type antagonists (Favus & Angeid-Backman, 1985; Fox & Green, 1986). Other possible reasons why the role of Cav1.3 has been overlooked include the fact that the majority of work has been in duodenum, where active sugar transport and Cav1.3 are low, but TRPV5/6 are high. In addition, Cav1.3 is 10-fold less sensitive to L-type channel blockers than are classical channels (Xu & Lipscombe, 2001).
The physiological significance of the regulatory and absorptive mechanisms described seems clear. Ca2+ is normally plentiful when food is plentiful, so that the soluble Ca2+ concentration in the lumen is about 5–10 mm after a meal (Bronner, 2003). The initial digestion products along with free and complexed dietary Ca2+ pass into the duodenum, where active transport of glucose is low, as are the level and activity of Cav1.3. Even though the activities of TRPV5/6 in duodenum are at their highest in the gastrointestinal tract, Ca2+ absorption is very restricted because the transit time through the duodenum in rat is just 2.5 min (Bronner, 2003). In contrast, the transit time from the proximal jejunum to the mid-ileum, where apical Cav1.3 is located (Morgan et al. 2003), is 126 min. In this region, the final digestion products, namely glucose, amino acids and oligopeptides, are generated in high local concentrations at the apical membrane by membrane-bound hydrolases. The transport of these products depolarizes the apical membrane and induces the nifedipine-sensitive component of Ca2+ absorption through Cav1.3, helping to clear most of the free or loosely complexed Ca2+. Glucose absorption is up-regulated by Ca2+-dependent apical GLUT2 insertion, which seems to require another signal in addition to Ca2+. As glucose is absorbed, the process is reversed; Ca2+ absorption is down-regulated as the apical membrane is repolarized and glucose absorption is down-regulated by loss of apical GLUT2. The integration of glucose and Ca2+ absorption represents a complex nutrient sensing system, which allows both absorptive pathways to be regulated rapidly and precisely to match dietary intake.
Acknowledgments
This work was supported by The Wellcome Trust. E.L.M. was the recipient of a BBSRC studentship and is now a BBSRC fellow. O.J.M. was the recipient of a BBSRC studentship.
References
- Affleck J, Helliwell PA, Kellett GL. Immunocytochemical detection of GLUT2 at the rat intestinal brush-border membrane. J Histochem Cytochem. 2003;51:1567–1574. doi: 10.1177/002215540305101116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au A, Gupta A, Schembri P, Cheeseman CI. Rapid insertion of GLUT2 into the rat jejunal brush-border membrane promoted by glucagon-like peptide 2. Biochem J. 2002;367:247–254. doi: 10.1042/BJ20020393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auchere D, Tardivel S, Gounelle JC, Drueke T, Lacour B. Role of transcellular pathway in ileal Ca2+ absorption: stimulation by low-Ca2+ diet. Am J Physiol. 1998;275:G951–G956. doi: 10.1152/ajpgi.1998.275.5.G951. [DOI] [PubMed] [Google Scholar]
- Auchere D, Tardivel S, Gounelle JC, Lacour B. Stimulation of ileal transport of calcium by sorbitol in in situ perfused loop in rats. Gastroenterol Clin Biol. 1997;21:960–966. [PubMed] [Google Scholar]
- Baba R, Yamami M, Sakuma Y, Fujita M, Fujimoto S. Relationship between glucose transporter and changes in the absorptive system in small intestinal absorptive cells during the weaning process. Med Mol Morph. 2005;38:47–53. doi: 10.1007/s00795-004-0275-y. [DOI] [PubMed] [Google Scholar]
- Bichet D, Lecomte C, Sabatier JM, Felix R, De Waard M. Reversibility of the Ca2+ channel α1–β subunit interaction. Biochem Biophys Res Commun. 2000;277:729–735. doi: 10.1006/bbrc.2000.3750. [DOI] [PubMed] [Google Scholar]
- Bronner F. Mechanisms of intestinal calcium absorption. J Cell Biochem. 2003;88:387–393. doi: 10.1002/jcb.10330. [DOI] [PubMed] [Google Scholar]
- Bronner F, Pansu D, Stein WD. Analysis of calcium transport in rat intestine. Adv Exp Med Biol. 1986;208:227–234. doi: 10.1007/978-1-4684-5206-8_28. [DOI] [PubMed] [Google Scholar]
- Caccia S, Leonardi MG, Casartelli M, Grimaldi A, de Eguileor M, Pennacchio F, Giordana B. Nutrient absorption by Aphidius ervi larvae. J Insect Physiol. 2005;51:1183–1192. doi: 10.1016/j.jinsphys.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Wood RJ, Chang EB, Rosenberg IH. Glucose enhancement of transcellular calcium transport in the intestine. Am J Physiol. 1988;255:G339–G345. doi: 10.1152/ajpgi.1988.255.3.G339. [DOI] [PubMed] [Google Scholar]
- Cheeseman CI. GLUT2 is the transporter for fructose across the rat intestinal basolateral membrane. Gastroenterology. 1993;105:1050–1056. doi: 10.1016/0016-5085(93)90948-c. [DOI] [PubMed] [Google Scholar]
- Corpe CP, Basaleh MM, Affleck J, Gould G, Jess TJ, Kellett GL. The regulation of GLUT5 and GLUT2 activity in the adaptation of intestinal brush-border fructose transport in diabetes. Pflugers Arch. 1996;432:192–201. doi: 10.1007/s004240050124. [DOI] [PubMed] [Google Scholar]
- Debnam ES, Levin RJ. An experimental method of identifying and quantifying the active transfer electrogenic component from the diffusive component during sugar absorption measured in vivo. J Physiol. 1975;246:181–196. doi: 10.1113/jphysiol.1975.sp010885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favus MJ, Angeid-Backman E. Effects of 1,25(OH)2D3 and calcium channel blockers on cecal calcium transport in the rat. Am J Physiol. 1985;248:G676–G681. doi: 10.1152/ajpgi.1985.248.6.G676. [DOI] [PubMed] [Google Scholar]
- Feher JJ, Fullmer CS, Wasserman RH. Role of facilitated diffusion of calcium by calbindin in intestinal calcium absorption. Am J Physiol. 1992;262:C517–C526. doi: 10.1152/ajpcell.1992.262.2.C517. [DOI] [PubMed] [Google Scholar]
- Fox J, Green DT. Direct effects of calcium channel blockers on duodenal calcium transport in vivo. Eur J Pharmacol. 1986;129:159–164. doi: 10.1016/0014-2999(86)90347-x. [DOI] [PubMed] [Google Scholar]
- Gao T, Chien AJ, Hosey MM. Complexes of the α1C and β subunits generate the necessary signal for membrane targeting of class C L-type calcium channels. J Biol Chem. 1999;274:2137–2144. doi: 10.1074/jbc.274.4.2137. [DOI] [PubMed] [Google Scholar]
- Gouyon F, Caillaud L, Carriere V, Klein C, Dalet V, Citadelle D, Kellett GL, Thorens B, Leturque A, Brot-Laroche E. Simple-sugar meals target GLUT2 at enterocyte apical membranes to improve sugar absorption: a study in GLUT2-null mice. J Physiol. 2003;552:823–832. doi: 10.1113/jphysiol.2003.049247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habold C, Foltzer-Jourdainne C, Le Maho Y, Lignot JH, Oudart H. Intestinal gluconeogenesis and glucose transport according to body fuel availability in rats. J Physiol. 2005;566:575–586. doi: 10.1113/jphysiol.2005.085217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell PA, Kellett GL. The active and passive components of glucose absorption in rat jejunum under low and high perfusion stress. J Physiol. 2002;544:579–589. doi: 10.1113/jphysiol.2002.028209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell PA, Richardson M, Affleck J, Kellett GL. Stimulation of fructose transport across the intestinal brush-border membrane by PMA is mediated by GLUT2 and dynamically regulated by protein kinase C. Biochem J. 2000a;350:149–154. [PMC free article] [PubMed] [Google Scholar]
- Helliwell PA, Richardson M, Affleck J, Kellett GL. Regulation of GLUT5, GLUT2 and intestinal brush-border fructose absorption by the extracellular signal-regulated kinase, p38 mitogen-activated kinase and phosphatidylinositol 3-kinase intracellular signalling pathways: implications for adaptation to diabetes. Biochem J. 2000b;350:163–169. [PMC free article] [PubMed] [Google Scholar]
- Helliwell PA, Rumsby MG, Kellett GL. Intestinal sugar absorption is regulated by phosphorylation and turnover of protein kinase C βII mediated by phosphatidylinositol 3-kinase- and mammalian target of rapamycin-dependent pathways. J Biol Chem. 2003;278:28644–28650. doi: 10.1074/jbc.M301479200. [DOI] [PubMed] [Google Scholar]
- Hoenderop JG, Hartog A, Stuiver M, Doucet A, Willems PH, Bindels RJ. Localization of the epithelial Ca2+ channel in rabbit kidney and intestine. J Am Soc Nephrol. 2000;11:1171–1178. doi: 10.1681/ASN.V1171171. [DOI] [PubMed] [Google Scholar]
- Hoenderop JG, van der Kemp AW, Hartog A, van de Graaf SF, van Os CH, Willems PH, Bindels RJ. Molecular identification of the apical Ca2+ channel in 1, 25-dihydroxyvitamin D3-responsive epithelia. J Biol Chem. 1999;274:8375–8378. doi: 10.1074/jbc.274.13.8375. [DOI] [PubMed] [Google Scholar]
- Hoenderop JG, Vennekens R, Muller D, Prenen J, Droogmans G, Bindels RJ, Nilius B. Function and expression of the epithelial Ca2+ channel family: comparison of mammalian ECaC1 and 2. J Physiol. 2001;537:747–761. doi: 10.1111/j.1469-7793.2001.00747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Bhattacharjee A, Taylor JT, Zhang M, Keyser BM, Marrero L, Li M. [Ca2+]i regulates trafficking of Cav1.3 (α1D Ca2+ channel) in insulin-secreting cells. Am J Physiol Cell Physiol. 2004;286:C213–C221. doi: 10.1152/ajpcell.00346.2003. [DOI] [PubMed] [Google Scholar]
- Hug H, Sarre TF. Protein kinase C isoenzymes: divergence in signal transduction? Biochem J. 1993;291:329–343. doi: 10.1042/bj2910329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellett GL. The facilitated component of intestinal glucose absorption. J Physiol. 2001;531:585–595. doi: 10.1111/j.1469-7793.2001.0585h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellett GL, Barker ED. The stimulation of glucose absorption and metabolism in rat jejunum by bradykinin: dependence on the composition of commercial diets. Biochim Biophys Acta. 1989;992:128–130. doi: 10.1016/0304-4165(89)90059-7. [DOI] [PubMed] [Google Scholar]
- Kellett GL, Brot-Laroche E. Apical GLUT2: a major pathway of intestinal sugar absorption. Diabetes. 2005;54:3056–3062. doi: 10.2337/diabetes.54.10.3056. [DOI] [PubMed] [Google Scholar]
- Kellett GL, Helliwell PA. The diffusive component of intestinal glucose absorption is mediated by the glucose-induced recruitment of GLUT2 to the brush-border membrane. Biochem J. 2000;350:155–162. [PMC free article] [PubMed] [Google Scholar]
- Koschak A, Reimer D, Huber I, Grabner M, Glossmann H, Engel J, Striessnig J. α1D (Cav1.3) subunits can form L-type Ca2+ channels activating at negative voltages. J Biol Chem. 2001;276:22100–22106. doi: 10.1074/jbc.M101469200. [DOI] [PubMed] [Google Scholar]
- Koster HP, Hartog A, Van Os CH, Bindels RJ. Calbindin-D28K facilitates cytosolic calcium diffusion without interfering with calcium signaling. Cell Calcium. 1995;18:187–196. doi: 10.1016/0143-4160(95)90063-2. [DOI] [PubMed] [Google Scholar]
- Lipscombe D, Helton TD, Xu W. L-type calcium channels: the low down. J Neurophysiol. 2004;92:2633–2641. doi: 10.1152/jn.00486.2004. [DOI] [PubMed] [Google Scholar]
- Liu G, Dilmac N, Hilliard N, Hockerman GH. Cav1.3 is preferentially coupled to glucose-stimulated insulin secretion in the pancreatic β-cell line INS-1. J Pharmacol Exp Ther. 2003;305:271–278. doi: 10.1124/jpet.102.046334. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Flockerzi V, Hofmann F. Regional expression and cellular localization of the α1 and β subunit of high voltage-activated calcium channels in rat brain. J Neurosci. 1997;17:1339–1349. doi: 10.1523/JNEUROSCI.17-04-01339.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mace OJ, Morgan EL, Affleck JA, Lister N, Kellett GL. Calcium absorption by Cav1.3 induces terminal web myosin II phosphorylation and apical GLUT2 insertion in rat intestine. J Physiol. 2007;580:605–616. doi: 10.1113/jphysiol.2006.124784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madara JL, Pappenheimer JR. Structural basis for physiological regulation of paracellular pathways in intestinal epithelia. J Membr Biol. 1987;100:149–164. doi: 10.1007/BF02209147. [DOI] [PubMed] [Google Scholar]
- Marks J, Carvou NJ, Debnam ES, Srai SK, Unwin RJ. Diabetes increases facilitative glucose uptake and GLUT2 expression at the rat proximal tubule brush border membrane. J Physiol. 2003;553:137–145. doi: 10.1113/jphysiol.2003.046268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EL, Mace OJ, Helliwell PA, Affleck J, Kellett GL. A role for Cav1.3 in rat intestinal calcium absorption. Biochem Biophys Res Commun. 2003;312:487–493. doi: 10.1016/j.bbrc.2003.10.138. [DOI] [PubMed] [Google Scholar]
- Pansu D, Bellaton C, Roche C, Bronner F. Duodenal and ileal calcium absorption in the rat and effects of vitamin D. Am J Physiol. 1983;244:G695–G700. doi: 10.1152/ajpgi.1983.244.6.G695. [DOI] [PubMed] [Google Scholar]
- Peng JB, Chen XZ, Berger UV, Vassilev PM, Tsukaguchi H, Brown EM, Hediger MA. Molecular cloning and characterization of a channel-like transporter mediating intestinal calcium absorption. J Biol Chem. 1999;274:22739–22746. doi: 10.1074/jbc.274.32.22739. [DOI] [PubMed] [Google Scholar]
- Peng JB, Chen XZ, Berger UV, Weremowicz S, Morton CC, Vassilev PM, Brown EM, Hediger MA. Human calcium transport protein CaT1. Biochem Biophys Res Commun. 2000;278:326–332. doi: 10.1006/bbrc.2000.3716. [DOI] [PubMed] [Google Scholar]
- Pichler M, Cassidy TN, Reimer D, Haase H, Kraus R, Ostler D, Striessnig J. β Subunit heterogeneity in neuronal L-type Ca2+ channels. J Biol Chem. 1997;272:13877–13882. doi: 10.1074/jbc.272.21.13877. [DOI] [PubMed] [Google Scholar]
- Sharp PA, Debnam ES. The role of cyclic AMP in the control of sugar transport across the brush-border and basolateral membranes of rat jejunal enterocytes. Exp Physiol. 1994;79:203–214. doi: 10.1113/expphysiol.1994.sp003753. [DOI] [PubMed] [Google Scholar]
- Turner JR. Show me the pathway! Regulation of paracellular permeability by Na+-glucose cotransport. Adv Drug Deliv Rev. 2000;41:265–281. doi: 10.1016/s0169-409x(00)00046-6. [DOI] [PubMed] [Google Scholar]
- Turner JR, Rill BK, Carlson SL, Carnes D, Kerner R, Mrsny RJ, Madara JL. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am J Physiol. 1997;273:C1378–C1385. doi: 10.1152/ajpcell.1997.273.4.C1378. [DOI] [PubMed] [Google Scholar]
- Walker J, Jijon HB, Diaz H, Salehi P, Churchill T, Madsen K. 5-aminoimidazole-4-carboxamide riboside (AICAR) enhances GLUT2-dependent jejunal glucose transport: a possible role for AMPK. Biochem J. 2004;385:485–491. doi: 10.1042/BJ20040694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward MR, Boyd CA. Intracellular study of ionic events underlying intestinal membrane transport of oligopeptides. Nature. 1980;287:157–158. doi: 10.1038/287157a0. [DOI] [PubMed] [Google Scholar]
- Xu W, Lipscombe D. Neuronal Cav1.3α1 L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J Neurosci. 2001;21:5944–5951. doi: 10.1523/JNEUROSCI.21-16-05944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younoszai MK, Nathan R. Intestinal calcium absorption is enhanced by d-glucose in diabetic and control rats. Gastroenterology. 1985;88:933–938. doi: 10.1016/s0016-5085(85)80010-x. [DOI] [PubMed] [Google Scholar]
- Zhuang L, Peng JB, Tou L, Takanaga H, Adam RM, Hediger MA, Freeman MR. Calcium-selective ion channel, CaT1, is apically localized in gastrointestinal tract epithelia and is aberrantly expressed in human malignancies. Laboratory Invest. 2002;82:1755–1764. doi: 10.1097/01.lab.0000043910.41414.e7. [DOI] [PubMed] [Google Scholar]