Abstract
The aim of this study was to analyse the directional coding of two-dimensional limb movements by cutaneous afferents from skin areas covering a multidirectional joint, the ankle. The activity of 89 cutaneous afferents was recorded in the common peroneal nerve, and the mean discharge frequency of each unit was measured during the outward phase of ramp and hold movements imposed in 16 different directions. Forty-two afferents responded to the movements in the following decreasing order (SA2, n=24/27; FA2, n=13/17; FA1, n=3/24; SA1, n=2/21). All the units activated responded to a specific range of directions, defining their ‘preferred sector’, within which their response peaked in a given direction, their ‘preferred direction’. Based on the distribution of the preferred directions, two populations of afferents, and hence two skin areas were defined: the anterior and the external lateral parts of the leg. As the directional tuning of each population was cosine shaped, the neuronal population vector model was applied and found to efficiently describe the movement direction encoded by cutaneous afferents, as it has been previously reported for muscle afferents. The responses of cutaneous afferents were then considered with respect to those of the afferents from the underlying muscles, which were previously investigated, and an almost perfect matching of directional sensitivity was observed. It is suggested that the common movement-encoding characteristics exhibited by cutaneous and muscle afferents, as early as the peripheral level, may facilitate the central co-processing of their feedbacks subserving kinaesthesia.
Kinaesthesia involves multiple sensory messages arising from mechanoreceptors located in the joints themselves and in all the surrounding tissues. The relative contributions of joint, muscle and cutaneous information to kinaesthesia are still a much-debated issue because the corresponding receptors are concurrently subjected to mechanical constraints during the performance of movements (see Gandevia, 1996).
Joint inputs contribute to kinaesthesia, as intracapsular anaesthesia impairs subjects' ability to evaluate the velocity of passive finger movements (Ferrell et al. 1987), and intraneural microstimulation applied to joint afferents induces illusory sensations of joint displacement (Macefield et al. 1990). The information arising from these receptors might, however, operate only at the extremes of joint displacement ranges (Burke et al. 1988; Clark et al. 1989).
As far as the muscle inputs are concerned, nerve block reversibly removing these inputs impairs the subjects' ability to detect passive movements (Clark et al. 1985), while vibration induces clearly perceptible illusory sensations of movement (Goodwin et al. 1972) by strongly activating primary muscle spindle endings, as shown by microneurographic recordings performed on humans (Burke et al. 1976a,b; Roll & Vedel, 1982; Roll et al. 1989). Tendon vibration causes the sensation of an illusory movement, the direction of which is that of the real movement which would have stretched the receptor-bearing muscle. It was initially suggested that the sensory messages arising from the lengthened muscle contributed mainly to kinaesthesia (Roll & Vedel, 1982). More recently it was established, however, that muscle feedback originating not only from the antagonist-lengthened muscle, but also from the agonist muscle, co-contribute to the encoding of unidirectional movements (Ribot-Ciscar & Roll, 1998). This assumption was then extended to the case of multidirectional movements, based on the finding that all the muscle spindle information arising from all the muscles surrounding a joint contributes together to the coding of two-dimensional movement parameters under both passive and active conditions (Bergenheim et al. 2000; Roll et al. 2000; Jones et al. 2001; Ribot-Ciscar et al. 2002, 2003).
Cutaneous information seems to also contribute to kinaesthesia since anaesthesia of the digital nerves impairs finger-movement detection (Brown et al. 1954; Gandevia & McCloskey, 1976; Refshauge et al. 1998). In addition, transcutaneous electrical stimulation applied to the hand induces illusory sensations of movement (Collins & Prochazka, 1996), as does stretching of the skin over the hand (Edin & Abbs, 1991; Edin & Johansson, 1995; Collins & Prochazka, 1996), elbow and knee joints (Collins et al. 2005). Lastly, vibratory stimulation applied to the plantar sole, which was liable to stimulate the cutaneous mechanoreceptors, was found to induce illusory perceptions of orientated whole-body leaning (Roll et al. 2002).
Although muscle spindle and tactile afferent feedbacks contribute separately to kinaesthesia, evidence has been accumulated during the last decade that these sensory inputs are co-processed and contribute to movement perception (Collins et al. 2000) and erect stance maintenance (Kavounoudias et al. 2001). By combining muscle-vibration and skin-stretching stimuli, Collins et al. (2005) observed, for example, that the amplitude of the illusory movements induced was larger when muscle spindle and tactile receptors were activated simultaneously rather than separately. These data add to the findings made in the pioneer study by Collins et al. (2000) showing that cutaneous feedback from the fingers does not facilitate the sensations resulting from muscle receptor activation, but may actually help the subject to identify which finger is moving (see also Refshauge et al. 2003).
If integrated muscle and cutaneous inputs provide kinaesthetic information, these two sensory modalities can be expected to share some general encoding characteristics. As regards the direction of movements and muscle proprioceptive information, it was recently established that each muscle surrounding a particular joint encodes a specific range of movement directions, which has been called the muscle's preferred sensory sector, and particularly one movement direction, its preferred sensory direction (Bergenheim et al. 2000). This study showed in addition that movement direction is encoded by populations of afferents originating from all the muscles subjected to deformation, in keeping with the neuronal population vector model (Georgopoulos et al. 1986). Since this population-encoding process was also found to reflect the directional encoding of forces applied to periodontal and finger-tip mechanoreceptors (Trulsson et al. 1992; Birznieks et al. 2001), the aim of the present study was to determine whether cutaneous afferents with receptive fields surrounding a multidirectional joint, the ankle, encode the direction of two-dimensional movements in keeping with the same population vector model.
Methods
Experiments were conducted on 20 healthy volunteers (mean age 23 years), who all gave their written informed consent to the experimental conditions, as required by the Declaration of Helsinki. This study was approved by the local ethics committee (CCPPRB, Marseille I).
The subjects were comfortably seated in an armchair, with their legs positioned in cushioned grooves so that a standardized relaxed position could be maintained throughout the experiment. The knee joint was placed at an angle of about 120–130 deg, and the feet were resting on supports. The right foot lay on a stationary plate and the left foot was attached to a movable pedal connected to a computer-controlled machine used to impose two-dimensional movements on the ankle joint (Bergenheim et al. 2000; Roll et al. 2000; Ribot-Ciscar et al. 2003). These movements consisted of passive ramp-and-hold movements imposed in 16 directions from the same starting position (as illustrated in Fig. 2A). The movement amplitude was 25 mm at the tip of the foot, and the movement velocity was 18 mm s−1. The various movements were imposed in a randomized order and the various positions reached were maintained for 2 s before the foot pedal was returned to the starting position.
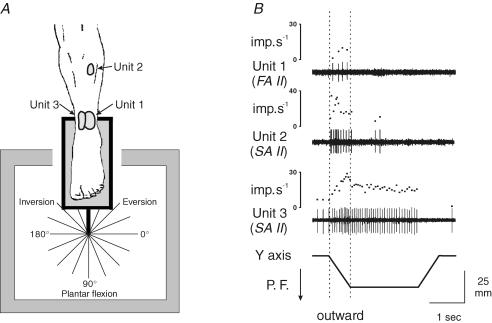
Figure 2. Typical patterns of cutaneous response to ramp-and-hold movements.
A, location of the receptive fields of three cutaneous afferents tested in three subjects and diagram showing the 16 directions tested, where 270 deg corresponds to a dorsiflexion and 90 deg to a plantar flexion movement. B, response of these afferents during ramp-and-hold movements imposed in the direction of a plantar flexion (P.F.). The activity of each unit is illustrated by its spike train and the corresponding instantaneous frequency curve in response to movements along the vertical y axis. For further analysis, the mean discharge frequency was measured only during the outward movement (dotted vertical bars).
Microneurographic recordings
Cutaneous afferent activity was recorded using the microneurographic technique (Vallbo & Hagbarth, 1968; Bergenheim et al. 1999), with an insulated tungsten microelectrode (Frederick Haer & Co. Bowdoinham, ME USA; impedance 300 kΩ to 1 MΩ tested at 1 kHz, tip diameter around 5–8 μm, length 30 mm). The electrode was inserted manually into the common peroneal nerve at the popliteal fossa. The recordings were monitored on an oscilloscope and a loudspeaker. The neural activity was amplified, 300–3000 Hz filtered, and sampled at 10 kHz.
Once the microelectrode had reached an intraneural location, the subjects usually reported that they experienced a short-lasting localized paraesthesia within the innervated territory. While a second experimenter was palpating large skin areas, the microelectrode was then moved in minute steps until the activity of a single unit was isolated and the corresponding receptor field was then located. The force threshold was defined, using Semmes-Weinstein nylon monofilaments (Stoetling Co., IL, USA), as the calibrated force of the weakest filament that evoked a reliable response. The force threshold of spontaneously active units was defined as the smallest force that produced a clear-cut change in the ongoing activity. The receptive field was mapped using a monofilament that delivered four times the force threshold. Only afferents that responded to gentle pinching of the relevant skin area and showed receptive fields that maintained their relations to the skin surface when the skin was laterally translated were taken to be cutaneous afferents (see Edin & Abbs, 1991).
Receptor adaptation properties were determined by pressing a monofilament delivering four times the force threshold onto the hot spot, and keeping it there for several seconds. Units showing sustained activity in response to these maintained pressures were classified as slowly adapting (SA) units, whereas those producing on–off responses were classified as fast adapting (FA) units. Lastly, type I units with small receptive fields and clearly defined boundaries were distinguished from type II units with larger receptive fields and obscure boundaries (Vallbo & Johansson, 1984). In the present study, no distinction was made between the SA I units in which the frequency of the responses to sustained skin indentation was irregular (pure SA I units) and those where the frequency of the responses was regular (SA III units, see Edin, 2001).
Data processing
The x and y components of each movement were sampled at 100 Hz. The x and y displacements of the servo-controlled machine and the unitary afferent activity were stored on a digital tape recorder (DTR 1802, Biologic, Lyon, France). The data were processed offline using ‘Spike 2’ software (CED Ltd, Cambridge, UK).
Calculating the preferred direction
The response of each afferent to each movement was determined by measuring its mean discharge frequency during the outward phase of the movement. The preferred direction of each afferent was calculated as follows (see Bergenheim et al. 2000). The unitary activity was expressed by a vector, the direction of which was that of the outward movement, while its modulus was the corresponding mean firing rate. This procedure was repeated with each outward movement, and 16 vectors were thus obtained. All these vectors were then summed to calculate a sum vector giving the preferred direction of the afferent under consideration (illustrated in Fig. 3B).
Figure 3. Responses of a SA II afferent to the 16 test directions.
A, each diagram illustrates the response of a SA II unit to a given movement direction. From top to bottom, the diagram shows the instantaneous discharge frequency of the unit, its spike train, and the x and y coordinates of the movement. Arrows give the preferred sector of the unit. B, the receptive field of this unit was located on the belly of the extensor digitorum longus muscle. In each movement direction, the unit response is given by vectors (thin lines), the length of which corresponds to the mean discharge frequency. These vectors were then summed, giving a sum vector (bold line) indicating the preferred direction of the unit, i.e. 51.2 deg in the present case. For printing convenience, the modulus of the sum vector has been truncated.
Population vector analysis
To determine whether the direction of a movement was encoded on the basis of feedback from populations of cutaneous afferents, the neuronal population vector model was applied (Georgopoulos, 1990). This model is based on the idea that the neuronal coding can be analysed in terms of a series of population vectors and by finally calculating a sum vector. In the present case, each population vector gives the contribution of all the cutaneous afferents from one directionally tuned skin area, i.e. each population vector points in the preferred direction of a given skin area, and its length gives the mean firing rate of all the afferents recorded from this skin area during a movement in one of the 16 directions tested.
This model requires that the mean tuning curves of each skin area afferent population should be cosine shaped (see Bergenheim et al. 2000; Ribot-Ciscar et al. 2003). This point was checked by performing multiple regression analysis to determine the constants b0, b1, and b2 required for the tuning equation:
F=b0 + b1sinθ + b2θ, where F is the mean discharge frequency and θ corresponds to the angle, i.e. the direction of the tested movement. Note that as the present study focused only on the encoding of the movement direction, the response of each single afferent was normalized by taking its largest response in any direction to be equal to 1.
Statistical analyses
Two circular statistical analyses were carried out in this study (Batschelet, 1981). The clustering of all the preferred directions of all the units belonging to a given skin area was tested using the Rayleigh test. The accuracy of the sum vectors giving the actual directions of the ongoing movements was tested using the V test.
Results
Recordings were carried out on 89 cutaneous afferents having receptive fields spread over the anterior part of the leg from 6 cm below the knee joint to the toes, and the external lateral part of the leg from 5 cm below the popliteal fossa to the lateral malleolus.
The centres of these receptive fields were plotted on a standardized leg, as illustrated in Fig. 1, where circles stand for SA and squares for FA afferents, and symbols are black or white depending on whether the afferent was responsive to movements or not. As can be seen in this figure, type II units, the receptive fields of which are shown by large symbols, were the most responsive to movements, i.e. they amounted to 84% as against 11% of the type I units tested (see also Table 1). The receptive fields of the afferents activated by movements were distributed along the whole leg: this means that even afferents having receptive fields located near the knee joint were still responsive to ankle joint movements. The cutaneous afferents showed specific patterns of response, as most of them were activated only during ramp movements (see units 1 and 2 in Fig. 2B). Some others remained activated during the hold phase (see unit 3 in Fig. 2B). Conversely, most of the units innervating the foot or toes did not respond to ankle movements, but some of them (type II units) responded to manually imposed orientated movements of the toe joints (data not shown).
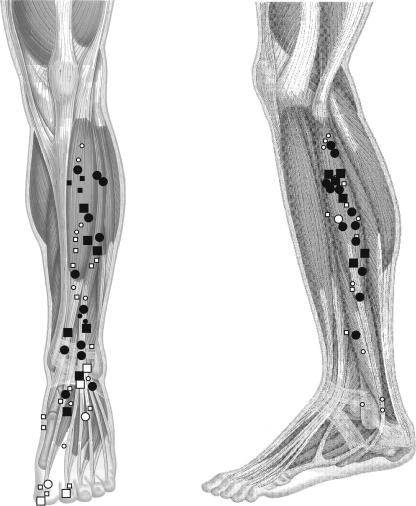
Figure 1. Location of the centres of the afferent receptive fields and their responsiveness to movements.
In the whole population of cutaneous afferents tested, the centres of the receptive fields were plotted on a standardized leg (left: anterior view; right: lateral view). The units were classified into slow- (circles) and fast- (squares) adapting mechanoreceptors. The receptive fields of type I units are given by small symbols and those of type II units are given by large symbols. Dark symbols correspond to units responsive to the imposed movements, and open symbols to non-responsive units.
Table 1.
Distribution, characteristics and responsiveness to movements of the whole population of cutaneous afferents tested
| Anterior skin area | Lateral skin area | |||||
|---|---|---|---|---|---|---|
| Unit class | Mean receptive field size (mm2) | Threshold force range (mN) | Responsive to movements (n) | Not responsive to movements (n) | Responsive to movements (n) | Not responsive to movements (n) |
| SA I | 25 | 1.5–147 | 2 | 9 | 0 | 10 |
| SA II | 86 | 0.4–1764 | 12 | 2 | 12 | 1 |
| FA I | 31 | 0.1–147 | 3 | 15 | 0 | 6 |
| FA II | 52 | 0.1–588 | 7 | 4 | 6 | 0 |
SA and FA, slow- and fast-adapting mechanoreceptors, respectively.
Each cutaneous afferent responds to a preferred movement direction
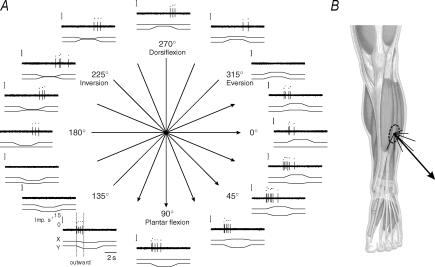
Forty-two afferents were found to respond to the movements imposed on the ankle joint. As a representative example, Fig. 3 shows the response of a SA II unit with its receptive field located on the belly of the extensor digitorum longus muscle during movements imposed in 16 different directions. This unit responded to several outward movement directions ranging from 337.5 deg to 112.5 deg (arrows in Fig. 3A) and became silent for directions ranging from 135 deg to 315 deg. Note that within the latter sector, the unit was responsive to inward movements; this means that the afferent activation occurring in the 337.5–112.5 deg sector was again exhibited. The afferent discharge corresponding to each outward movement direction was then expressed by a vector, and the sum of all these vectors yielded a sum vector reflecting the preferred direction of that afferent, i.e. 51.2 deg (Fig. 3B).
The preferred directions of the 41 other units responsive to movements were calculated in the same way; the results are given in Fig. 4A and C. As can be seen, although each unit had its own preferred direction, two populations of afferents may be distinguished depending on the distribution of their preferred directions. The directional distribution was relatively homogeneous within each population and seems to depend on the location of the afferents' receptive fields. By statistically clustering the distributions of the preferred directions using the Rayleigh test, two skin areas were therefore defined. The anterior skin area included all the afferents with receptive fields spreading over the areas covering the ankle dorsiflexor muscles (r=0.90, n=24, P < 0.001, Fig. 4A), and the external lateral skin area included the afferents with receptive fields spreading mostly over the areas covering the peroneus lateralis muscle (r=0.95, n=18, P < 0.001, Fig. 4C). Both the distribution and the mean value of the preferred directions differed between the afferents from the two skin areas. The preferred directions ranged from 10 deg to 110 deg, averaging 71 deg, in the case of the afferents originating from the anterior skin area and from 271 deg to 332 deg, averaging 311 deg, in that of the afferents originating from the lateral skin area (Fig. 4B and D, respectively).
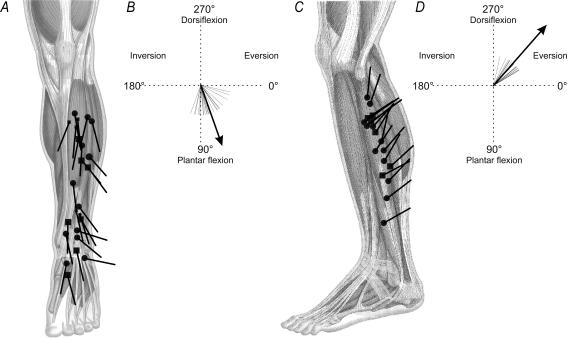
Figure 4. The preferred directions of the cutaneous afferents responsive to movements.
A and C, starting from the centre of its receptive field, the preferred direction of each cutaneous afferent is given by a line. For the meaning of symbols, see the legends to Fig. 1B and D, calculating the preferred direction in each skin area. Each cutaneous afferent has a preferred direction illustrated by a short, fine line. A population vector based on these individual vectors, gives the mean preferred direction (long, thick line) of the skin on the anterior part of the leg (B) and that on the external lateral part (D). Note that the data given in this figure involve only the angle of the preferred direction.
Each skin area encodes a specific range of movement directions
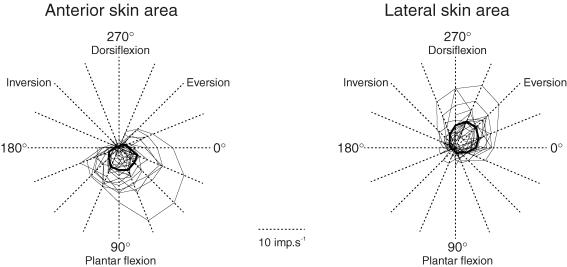
As the units responded to several directions on both sides of their preferred directions, a preferred sector was defined for each unit and then for each skin area. This is illustrated in Fig. 5, where the connected points correspond to the mean discharge frequency of each afferent during the outward part of the movements in each particular direction (thin lines) and for the population of afferents (bold lines). This figure shows that for each unit and each population of afferents, the response decreased as the movement direction went further from the preferred direction. The mean preferred sectors were 22.5–157.5 deg and 247.5–0 deg for the anterior and lateral skin areas, respectively.
Figure 5. Individual and population preferred sectors.
The response of each afferent to each movement direction was plotted on the axis of the corresponding movement. The points plotted were then connected by drawing thin lines forming a sector which corresponds to that afferent's preferred sector. Delimited by thick lines, the mean preferred sector of all afferents originating from the anterior skin area (left part) differs from that of the afferents from the lateral skin area (right part).
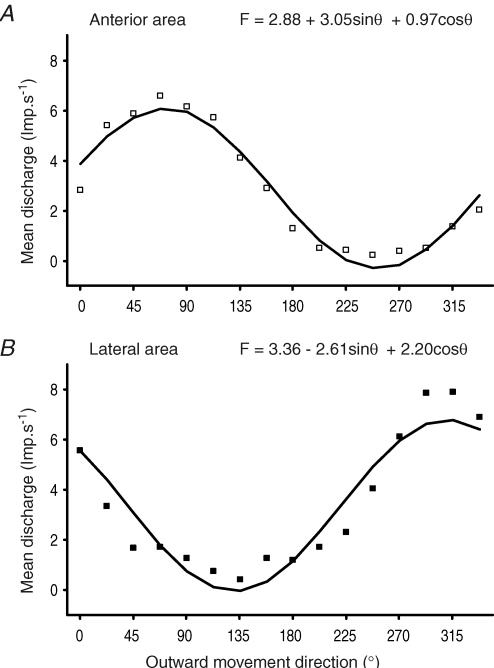
Figure 6 gives the tuning curves of the afferent population in each skin area. The mean discharge frequency (ordinates) is plotted here versus each movement direction (abscissa). As can be seen, the directional tuning of each population was cosine shaped, as a cosine function was fitted to the data (anterior area: R2=0.98, Fig. 6A; lateral area: R2=0.94, Fig. 6B). These cosine-tuned functions allow us to apply the neuronal population vector model.
Figure 6. Population tuning curves.
Each tuning curve gives the averaged response of each population of afferents responsive to movements to the 16 directions tested. Each population coded the various orientated movements according to a specific cosine-tuned function, the equation for which is given on top of each graph. Cosine-shaped curves mean that it is possible to apply the neuronal population vector model.
The neuronal population vector model describes the encoding of movement direction by cutaneous afferents
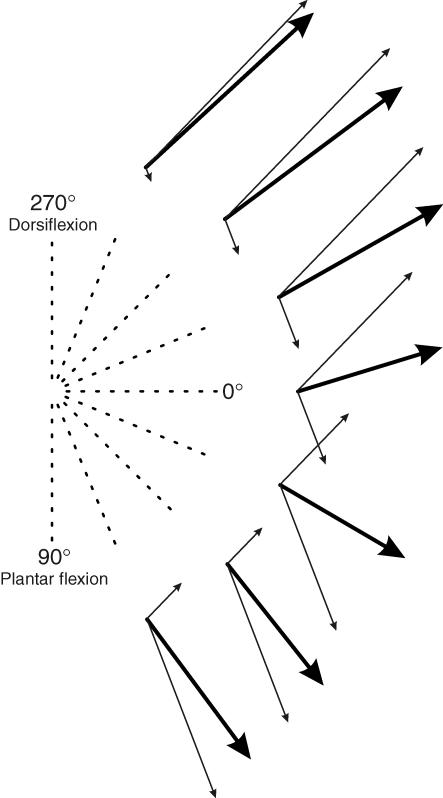
In each directionally tuned skin area, the contribution of all the cutaneous afferents to the coding of each movement direction was given by a vector, the direction of which corresponds to the preferred direction of the skin area under consideration, while the length of the vector reflects the mean firing rate of all the afferents originating from this skin area. This yielded two population vectors (Fig. 7, thin lines) which were summed together giving a specific sum vector for each movement direction (Fig. 7, thick lines). This processing was performed for movement directions ranging between the two preferred directions of each skin area, i.e. from 292.5 deg to 67.5 deg. As can be seen, the direction of these sum vectors was found to reorient depending on the movement direction and to almost point in the actual direction of the ongoing movements, as confirmed by statistical analysis (u=5.50, P < 0.01, V test).
Figure 7. The neuronal population vector model.
Each diagram gives the result obtained in one movement direction: the thin arrows give the population vector corresponding to each skin area and the thick arrow gives the sum vector calculated. As can be seen, the sum vectors calculated point approximately in the actual directions of the ongoing movements.
Discussion
The aim of the present study was to analyse the directional coding of two-dimensional limb movements by cutaneous afferents. The results showed that the actual direction of movement is encoded by the activity of populations of cutaneous afferents originating from the skin areas subjected to deformation. In addition, the movement direction is encoded in line with the neuronal population vector model.
Cutaneous afferents from a given skin area exhibit a specific directional sensitivity
As in the case of the skin covering the palm (Knibestöl, 1975; Hulliger et al. 1979), slow- and fast-adapting type II units were found here to be the most responsive to movement. As suggested by previous authors, ‘the cutaneous mechanoreceptors, and particularly the SA II and FA II units, may provide not only exteroceptive but also proprioceptive information’ (Johansson & Vallbo, 1983). The slowly adapting cutaneous afferents have even been reported to show a similar positional and velocity sensitivity to that observed in muscle spindle afferents, which means that they are liable to encode the kinematic components of movements (Edin, 1992, 2001; Grill & Hallett, 1995). The sensitivity of these units was such that despite the relatively small amplitude of the imposed movements (25 mm at the tip of the foot), many of the units with receptive fields located quite far from the ankle joint were still responsive. Note that half of the afferents recorded in the present study were not responsive to the imposed movements. However, among these afferents some became responsive when ankle movements with a larger amplitude were imposed. The small amplitude of the movements may thus explain why a lower rate of responsiveness was observed here among the whole population of cutaneous afferents tested than in previous studies (Hulliger et al. 1979; Edin & Abbs, 1991; Edin, 2001).
To provide proprioceptive information, cutaneous afferents have to be responsive to movements, as well as being capable of directional sensitivity. Previous studies on this topic have focused so far on the skin surrounding unidirectional joints, and the results have shown that cutaneous afferents are particularly sensitive to a given orientation (Knibestöl, 1975; Johansson, 1978; Edin & Abbs, 1991; Edin, 1992,2001). In the present study, this picture was enlarged by investigating a multidirectional joint. Each afferent is responsive to a specific range of movement directions, its preferred sector, and which one particular direction, the preferred direction, gives the strongest response. As the distribution of the preferred directions was found to be homogeneous depending on the location of the receptive fields, two populations of afferents, and hence two distinct skin areas were defined: those located on the anterior and external lateral parts of the leg. Each of these populations responded to movement directions in keeping with a specific cosine-tuned function.
The encoding of movement directions by cutaneous afferents obeys the neuronal population vector model
As two distinct populations of cutaneous afferents were defined, and as their sensitivity to movement fitted a cosin-tuned function, it seemed to be worth analysing the directional encoding of movements by cutaneous afferents using the neuronal population vector model (Georgopoulos et al. 1986), as previously done in the case of muscle spindle afferents (Bergenheim et al. 2000; Roll et al. 2000; Jones et al. 2001). Since the cutaneous afferents recorded from the common peroneal nerve belonged to the skin on the anterior and external lateral parts of the leg, this analysis was applied only to movements ranging from plantar flexion to foot eversion.
The results showed that the sum vectors pointed significantly in the actual directions of the ongoing movements, despite some small skews (see Fig. 7). These slight discrepancies are probably due to the relatively small size of the afferent populations, and thus to the imperfectly cosine-shaped tuning curves (Deneve et al. 1999). Furthermore, only two skin areas were explored here, but other unexplored areas probably contributed to this multidirectional coding. All in all, these findings on tactile sensitivity extend the scope of the neuronal population vector model as a means of describing the directional encoding of limb movements, as previously done in connection with the directional encoding of forces applied to the fingertips (Birznieks et al. 2001).
Kinaesthesia: when skin afferents act like muscle afferents
The directional encoding characteristics described here in cutaneous afferents turned out to be very similar to those previously described in ankle and wrist muscle afferents (Bergenheim et al. 2000; Roll et al. 2000; Jones et al. 2001). Like muscle afferents, cutaneous afferents show strong directional sensitivity: they respond to a preferred range of movement directions within which their response peaks in a given direction and decreases in keeping with a cosine tuning function as the movement departs from this preferred direction. Like muscle afferents, cutaneous afferents seem also to encode limb movement directions in line with the neuronal population vector model (Georgopoulos et al. 1986).
It was possible to compare cutaneous and muscle afferent data more closely here, since both sets of data were obtained with the same movements imposed at the level of the same joint (see Bergenheim et al. 2000). The main point that emerged from these comparisons was the almost perfect match between the skin areas defined here and the underlying muscles. More specifically, the skin afferents and the muscle afferents located in the underlying muscles show the same specific directional sensitivity in terms of both the preferred sectors and the preferred directions: the 22–157 deg sector and 71 deg mean preferred direction found to correspond to the anterior skin area are very similar to the values of 0–180 deg and 76 deg previously obtained on the ankle dorsiflexor muscle afferents, namely those in the tibialis anterior, extensor hallucis longus, and extensor digitorum longus muscles. As regards the external lateral skin area, the 247–0 deg sector and 311 deg mean preferred direction are similar to the values of 247–45 deg and 311 deg previously obtained on the peroneus lateralis muscle afferents. This coincidence is probably due to the fact that the muscles and the covering skin are concurrently subjected to congruent directional constraints. There therefore exists a clear-cut parallel between cutaneous and muscle afferent encoding of movement direction. As the afferent messages from both the lengthened and contracted muscles contribute to kinaesthesia (Albert et al. 2006), the cutaneous messages from both stretched and relaxed skin regions also contribute to kinaesthesia (Collins et al. 2000). However, only some cutaneous afferents are involved in movement encoding, whereas all the muscle afferents participate.
To conclude, the fact that cutaneous and muscle afferents show common general movement-encoding characteristics as early as the peripheral level may facilitate the central co-processing of the feedback information subserving kinaesthesia.
Acknowledgments
This research was supported by grants from Association Française contre les Myopathies, CNRS, and ACI Jeunes chercheurs.
References
- Albert F, Bergenheim M, Ribot-Ciscar E, Roll JP. The Ia afferent feedback of a given movement evokes the illusion of the same movement when returned to the subject via muscle tendon vibration. Exp Brain Res. 2006;172:163–174. doi: 10.1007/s00221-005-0325-2. [DOI] [PubMed] [Google Scholar]
- Batschelet E. Circular Statistics in Biology. London: Academic Press; 1981. [Google Scholar]
- Bergenheim M, Ribot-Ciscar E, Roll JP. Proprioceptive population coding of two-dimensional limb movements in humans. I. Muscle spindle feedback during spatially oriented movements. Exp Brain Res. 2000;134:301–310. doi: 10.1007/s002210000471. [DOI] [PubMed] [Google Scholar]
- Bergenheim M, Roll JP, Ribot-Ciscar E. Microneurography in humans. In: Windhorst U, Johansson H, editors. Modern Techniques in Neuroscience Research. Berlin Heidelberg New York: Springer; 1999. pp. 801–819. [Google Scholar]
- Birznieks I, Jenmalm P, Goodwin AW, Johansson RS. Encoding of direction of fingertip forces by human tactile afferents. J Neurosci. 2001;21:8222–8237. doi: 10.1523/JNEUROSCI.21-20-08222.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K, Lee J, Ring PA. The sensation of passive movement at the metatarso-phalangeal joint of the great toe in man. J Physiol. 1954;126:448–458. doi: 10.1113/jphysiol.1954.sp005221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Gandevia SC, Macefield VG. Responses to passive movement of receptors in joint, skin and muscle of the human hand. J Physiol. 1988;402:347–361. doi: 10.1113/jphysiol.1988.sp017208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Hagbarth KE, Lofstedt L, Wallin BG. The responses of human muscle spindle endings to vibration during isometric contraction. J Physiol. 1976a;261:695–711. doi: 10.1113/jphysiol.1976.sp011581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Hagbarth KE, Lofstedt L, Wallin BG. The responses of human muscle spindle endings to vibration of non-contracting muscles. J Physiol. 1976b;261:673–693. doi: 10.1113/jphysiol.1976.sp011580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark FJ, Burgess RC, Chapin JW, Lipscomb WT. Role of intramuscular receptors in the awareness of limb position. J Neurophysiol. 1985;54:1529–1540. doi: 10.1152/jn.1985.54.6.1529. [DOI] [PubMed] [Google Scholar]
- Clark FJ, Grigg P, Chapin JW. The contribution of articular receptors to proprioception with the fingers in humans. J Neurophysiol. 1989;61:186–193. doi: 10.1152/jn.1989.61.1.186. [DOI] [PubMed] [Google Scholar]
- Collins DF, Prochazka A. Movement illusions evoked by ensemble cutaneous input from the dorsum of the human hand. J Physiol. 1996;496:857–871. doi: 10.1113/jphysiol.1996.sp021733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DF, Refshauge KM, Gandevia SC. Sensory integration in the perception of movements at the human metacarpophalangeal joint. J Physiol. 2000;529:505–515. doi: 10.1111/j.1469-7793.2000.00505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DF, Refshauge KM, Todd G, Gandevia SC. Cutaneous receptors contribute to kinesthesia at the index finger, elbow and knee. J Neurophysiol. 2005;94:1699–1706. doi: 10.1152/jn.00191.2005. [DOI] [PubMed] [Google Scholar]
- Deneve S, Latham PE, Pouget A. Reading population codes: a neural implementation of ideal observers. Nat Neurosci. 1999;2:740–745. doi: 10.1038/11205. [DOI] [PubMed] [Google Scholar]
- Edin BB. Quantitative analysis of static strain sensitivity in human mechanoreceptors from hairy skin. J Neurophysiol. 1992;67:1105–1113. doi: 10.1152/jn.1992.67.5.1105. [DOI] [PubMed] [Google Scholar]
- Edin BB. Cutaneous afferents provide information about knee joint movements in humans. J Physiol. 2001;531:289–297. doi: 10.1111/j.1469-7793.2001.0289j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edin BB, Abbs JH. Finger movement responses of cutaneous mechanoreceptors in the dorsal skin of the human hand. J Neurophysiol. 1991;65:657–670. doi: 10.1152/jn.1991.65.3.657. [DOI] [PubMed] [Google Scholar]
- Edin BB, Johansson N. Skin strain patterns provide kinaesthetic information to the human central nervous system. J Physiol. 1995;487:243–251. doi: 10.1113/jphysiol.1995.sp020875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell WR, Gandevia SC, McCloskey DI. The role of joint receptors in human kinaesthesia when intramuscular receptors cannot contribute. J Physiol. 1987;386:63–71. doi: 10.1113/jphysiol.1987.sp016522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC. Kinesthesia: role for afference signals and motor commands. In: Rowel L, Sheperd J, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. New York: Oxford University Press; 1996. pp. 128–172. [Google Scholar]
- Gandevia SC, McCloskey DI. Joint sense, muscle sense, and their combination as position sense, measured at the distal interphalangeal joint of the middle finger. J Physiol. 1976;260:387–407. doi: 10.1113/jphysiol.1976.sp011521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos AP. Neural coding of the direction of reaching and a comparison with saccadic eye movements. Cold Spring Harb Symp Quant Biol. 1990;55:849–859. doi: 10.1101/sqb.1990.055.01.080. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP, Schwartz AB, Kettner RE. Neuronal population coding of movement direction. Science. 1986;233:1416–1419. doi: 10.1126/science.3749885. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, McCloskey DI, Matthews PBC. The contribution of muscle afferents to kinaesthesia shown by vibration induced illusions of movement and by the effects of paralysing joint afferents. Brain. 1972;95:705–748. doi: 10.1093/brain/95.4.705. [DOI] [PubMed] [Google Scholar]
- Grill SE, Hallett M. Velocity sensitivity of human muscle spindle afferents and slowly adapting type II cutaneous mechanoreceptors. J Physiol. 1995;489:593–602. doi: 10.1113/jphysiol.1995.sp021075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulliger M, Nordh E, Thelin AE, Vallbo AB. The responses of afferent fibres from the glabrous skin of the hand during voluntary finger movements in man. J Physiol. 1979;291:233–249. doi: 10.1113/jphysiol.1979.sp012809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson RS. Tactile sensibility in the human hand: receptive field characteristics of mechanoreceptive units in the glabrous skin area. J Physiol. 1978;281:101–125. doi: 10.1113/jphysiol.1978.sp012411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson H, Vallbo AB. Tactile sensory coding in the glabrous skin of the human hand. Trends Neurosci. 1983;6:27–32. [Google Scholar]
- Jones KE, Wessberg J, Vallbo AB. Directional tuning of human forearm muscle afferents during voluntary wrist movements. J Physiol. 2001;536:635–647. doi: 10.1111/j.1469-7793.2001.0635c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavounoudias A, Roll R, Roll JP. Foot sole and ankle muscle inputs contribute jointly to human erect posture regulation. J Physiol. 2001;532:869–878. doi: 10.1111/j.1469-7793.2001.0869e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knibestöl M. Stimulus-response functions of slowly adapting mechanoreceptors in the human glabrous skin area. J Physiol. 1975;245:63–80. doi: 10.1113/jphysiol.1975.sp010835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macefield VG, Gandevia SC, Burke D. Perceptual responses to microstimulation of single afferents innervating joints, muscles and skin of the human hand. J Physiol. 1990;429:113–129. doi: 10.1113/jphysiol.1990.sp018247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refshauge KM, Collins DF, Gandevia SC. The detection of human finger movement is not facilitated by input from receptors in adjacent digits. J Physiol. 2003;551:371–377. doi: 10.1113/jphysiol.2003.045997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refshauge KM, Kilbreath SL, Gandevia SC. Movement detection at the distal joint of the human thumb and fingers. Exp Brain Res. 1998;122:85–92. doi: 10.1007/s002210050494. [DOI] [PubMed] [Google Scholar]
- Ribot-Ciscar E, Bergenheim M, Albert F, Roll JP. Proprioceptive population coding of limb position in humans. Exp Brain Res. 2003;149:512–519. doi: 10.1007/s00221-003-1384-x. [DOI] [PubMed] [Google Scholar]
- Ribot-Ciscar E, Bergenheim M, Roll JP. The preferred sensory direction of muscle spindle primary endings influences the velocity coding of two-dimensional limb movements in humans. Exp Brain Res. 2002;145:429–436. doi: 10.1007/s00221-002-1135-4. [DOI] [PubMed] [Google Scholar]
- Ribot-Ciscar E, Roll JP. Ago-antagonist muscle spindle inputs contribute together to joint movement coding in man. Brain Res. 1998;791:167–176. doi: 10.1016/s0006-8993(98)00092-4. [DOI] [PubMed] [Google Scholar]
- Roll JP, Bergenheim M, Ribot-Ciscar E. Proprioceptive population coding of two-dimensional limb movements in humans. II. Muscle-spindle feedback during ‘drawing-like’ movements. Exp Brain Res. 2000;134:311–321. doi: 10.1007/s002210000472. [DOI] [PubMed] [Google Scholar]
- Roll R, Kavounoudias A, Roll JP. Cutaneous afferents from human plantar sole contribute to body posture awareness. Neuroreport. 2002;13:1957–1961. doi: 10.1097/00001756-200210280-00025. [DOI] [PubMed] [Google Scholar]
- Roll JP, Vedel JP. Kinaesthetic role of muscle afferents in man, studied by tendon vibration and microneurography. Exp Brain Res. 1982;47:177–190. doi: 10.1007/BF00239377. [DOI] [PubMed] [Google Scholar]
- Roll JP, Vedel JP, Ribot E. Alteration of proprioceptive messages induced by tendon vibration in man: a microneurographic study. Exp Brain Res. 1989;76:213–222. doi: 10.1007/BF00253639. [DOI] [PubMed] [Google Scholar]
- Trulsson M, Johansson RS, Olsson KA. Directional sensitivity of human periodontal mechanoreceptive afferents to forces applied to the teeth. J Physiol. 1992;447:373–389. doi: 10.1113/jphysiol.1992.sp019007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth KE. Activity from skin mechanoreceptors recorded percutaneously in awake human subjects. Exp Neurol. 1968;21:270–289. doi: 10.1016/0014-4886(68)90041-1. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Johansson RS. Properties of cutaneous mechanoreceptors in the human hand related to touch sensation. Hum Neurobiol. 1984;3:3–14. [PubMed] [Google Scholar]