Abstract
AMPK (adenosine monophosphate-activated protein kinase), a key regulator of cellular energy metabolism and whole-body energy balance, is present in brown adipose tissue but its role in regulating the acute metabolic state and chronic thermogenic potential of this metabolically unique tissue is unknown. To address this, the AMPK signalling system in brown and white adipose tissue was studied in C57Bl/6 mice under control conditions, during acute and chronic cold exposure, and during chronic adrenergic stimulation. In control mice AMPK activity in brown adipose tissue was higher than in any tissue yet reported (3-fold the level in liver) secondary to a high level of expression of the α1 isoform. During the first day of cold, a time of intense non-shivering thermogenesis, AMPK activity remained at basal levels. However, chronic (>7 days) cold caused a progressive increase in brown adipose tissue AMPK activity secondary to increased expression of the α1 isoform. To investigate the signalling pathway involved, noradrenaline (norepinephrine) and the β3-adrenergic-specific agonist CL 316, 243 were given for 14 days. This increased uncoupling protein-1 content in brown adipose tissue, but not AMPK activity. In white adipose tissue 15 days of cold increased α1 AMPK activity 98 ± 20%, an effect reproduced by chronic noradrenaline or CL 316 243. We conclude that chronic cold not only increases AMPK activity in brown and white adipose tissue, but that it does so via distinct signalling pathways. Our data are consistent with AMPK acting primarily as a regulator of chronic thermogenic potential in brown adipose tissue, and not in the acute activation of non-shivering thermogenesis.
Adenosine monophosphate-activated protein kinase (AMPK) is a highly conserved serine/threonine kinase that regulates multiple aspects of cellular metabolism via its effects on expression and activity of metabolic enzymes (Carling, 2004). While AMPK was initially viewed primarily as a sensor of acute intracellular energetic stress (decreased ratio of ATP to AMP), it has now become clear that AMPK has a much broader function in the long-term regulation of whole-body energy balance, including roles in hypothalamic sensation of hunger and mitochondrial biogenesis in skeletal muscle (Andersson et al. 2004; Minokoshi et al. 2004; Lee et al. 2006).
Given the importance of AMPK in regulating whole-body energy balance, surprisingly little is known about the physiological roles of AMPK in brown and white adipose tissue, or how AMPK activity is regulated in these two tissues. For example, while recent data suggest that the sympathetic nervous system may regulate AMPK activity in some cells types in vitro, the extent to which this occurs in brown and white adipose tissue in vivo is unknown (Moule & Denton, 1998; Kishi et al. 2000). Understanding the role of AMPK in regulating brown adipose tissue (BAT) metabolism is of particular interest because BAT has the unique ability to dissipate calories as heat via uncoupled metabolism. Thus it has been suggested that controlling the amount and/or metabolic rate of BAT might allow for some degree of therapeutic control of body weight (Himms-Hagen et al. 1994, 2000; Tiraby & Langin, 2003). While in humans BAT is thought to be most important during the neonatal period, chronic cold exposure appears to activate BAT even in adults (Joy, 1963; Kang et al. 1970; Huttunen et al. 1981; Asakura, 2004).
Accordingly, our goal was to examine the regulation and physiological role of AMPK in BAT. To accomplish this we first characterized the AMPK signalling system in BAT under euthermic conditions by comparing it to the well described AMPK system in liver. Next, we defined the time course by which cold exposure affects AMPK activity in mice. Finally, we investigated the role of sympathetic activation, such as occurs during cold stress, in regulating AMPK activity in brown and white adipose tissue. Our results provide novel insights not only into the physiological role of AMPK in BAT, but also into the regulation of AMPK activity, a subject of intense interest due to its pathophysiological importance in diseases including obesity, diabetes, cancer, and heart disease (Blair et al. 2001; Gollob et al. 2001; Arad et al. 2002; Luo et al. 2005; Rattan et al. 2005).
Methods
Animals
Male C57Bl/6 mice were housed at a University of Wisconsin Animal Care Facility. Experiments were performed on mice aged 10–12 weeks that had unrestricted access to food and water. The facilities and research protocols were approved by the University of Wisconsin Institutional Animal Care and Use Committee. Mice were killed via cervical dislocation. Liver, an epididymal fat pad, and interscapular BAT were rapidly collected, frozen in liquid nitrogen, and stored at −80°C until analysed.
For the cold-exposure studies, mice were studied over a range of lengths of cold exposure. Cold exposure was performed in a controlled-temperature room at the University of Wisconsin Biotron facility (Madison, WI, USA). Mice were acclimatized to this room for 3 days at 22°C before the temperature of the room was reduced to 4°C. For each cold-exposure study, tissues were collected from a control group immediately prior to the reduction in room temperature.
For studies of chronic drug administration, Alzet micro-osmotic pumps (model 1002) were implanted subcutaneously in the mid-back of each mouse (n=6–8 per group). This surgery was conducted at a depth of general anaesthesia sufficient to eliminate the pedal withdrawal reflex (2–3% isoflurane in oxygen, inhaled via nose cone). Pumps contained approximately 100 μl of either vehicle (0.2% ascorbic acid), noradrenaline or the β3 adrenergic agonist CL 316 243 (Sigma-Aldrich). Concentrations of the adrenergic agonists in the micro-osmotic pumps were set to provide doses of noradrenaline of 3 or 12 mg (kg body weight)−1 day−1, or 1 mg (kg body weight)−1 day−1 of CL 316 243. After 14 days of drug infusion, mice were killed and tissue harvested.
AMPK activity assay
AMPK activity was measured as previously described (Mulligan et al. 2005) and expressed as pmol min−1 (mg homogenate protein)−1. Briefly, tissue was homogenized in the presence of protease inhibitors and phosphatase inhibitors. Fifty micrograms of total protein from the resulting supernatant was immunoprecipitated with protein A/G agarose beads (Santa Cruz Biotech) and antibodies against either the α1 or α2 (Upstate) catalytic subunits of AMPK for 2 h at 4°C. The beads containing the immunoprecipitated AMPK were washed, resuspended in reaction buffer with 0.2 mm SAMS peptide (Davies et al. 1989) and 0.2 mm [γ-32P]ATP and incubated for 10 min at 37°C in a thermomixer in the presence of various concentrations of AMP. When not indicated, AMPK activity was measured in the presence of 0.2 mm AMP. After incubation, the beads were quickly pelleted and a portion of each supernatant was spotted on P-81 phosphocellulose paper, washed in 1% phosphoric acid, then washed in acetone and air-dried. The incorporated radioactivity was counted in a TriCarb 3000 beta scintillation counter.
Western blots
Previously snap-frozen tissues were homogenized in 50 mm MOPS supplemented with (mm): 250 sucrose, 2 EDTA, 2 EGTA, 50 NaF, 5 NaPPi, 1 Na3VO4 and 5 μl ml−1 protease inhibitor mixture (Sigma). The homogenate was centrifuged for 10 min at 10 000 g for AMPK blots, and at 600 × g for ACC, and UCP-1 blots. Supernatant protein was resolved on SDS-polyacrylamide (PAGE) gels and transferred to Immobilon-P membranes (Millipore). Blots were blocked in 5% milk, 0.1% Tween-20, 50 mm NaF in TBS. Primary antibodies diluted in the blocking solution were as follows: AMPK α1 (Upstate, Cat. no. 07-350), AMPK α2 (raised against the AMPK α2 peptide, C-DDSAMHIPPGLKPHP. Primary antibodies for UCP-1 and phospho-specific blots were incubated in 5% BSA, 0.1% Tween-20, 50 mm NaF in TBS and include the following: UCP-1 (Alpha Diagnostics Cat. no. UCP11-A) and phospho-acetyl-CoA carboxylase (ACC) (Ser-79; Upstate, Cat. no. 07–303). All secondary incubations were with HRP-conjugated goat antirabbit IgG (Pierce, Cat. no. 31402) in 5% milk, 0.1% Tween-20/50 mm NaF in TBS. After blocking, blots for total ACC were incubated for 15 min in HRP-conjugated streptavidin (Pierce, Cat. no. 21126). Immunoreactive bands were detected by ECL-Plus chemiluminescense (Amersham Biosciences) and quantified by densitometry using Molecular Analyst (Bio-Rad). The large difference in ACC expression between liver and BAT prevented reliable quantification of these blots (Fig. 3A and B).
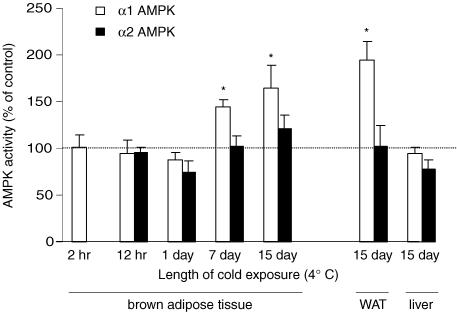
Figure 3. Effect of varying lengths of cold exposure on α1 and α2 AMPK activities in mouse tissue.
Mice were housed at 4°C for the indicated times before tissue collection. Each cold-exposure group had its own control group that was maintained at 22°C until being killed. AMPK activity was measured in homogenates of BAT following immunoprecipitation with an α1- or α2-specific antibody. Data are expressed as mean ± s.e.m. for n=5–6 per group, *P ≤ 0.05 relative to control group. Note that the cold-induced increase in AMPK activity is both isoform and tissue specific.
Real-time quantitative PCR
Total RNA was isolated from frozen tissue using the RNA-Bee reagent (Tel. Test, Inc.) and treated with DNase I (New England Biolabs) to remove contaminating DNA. First-strand cDNA synthesis was performed with random hexamer primers using TaqMan Reverse Transcription Reagents (Applied Biosystems). Real-time PCR was performed on the ABI Prism 7700 Sequence Detection System (Applied Biosystems) using SYBR Green PCR Master Mix (Applied Biosystems). AMPK α1-specific primers were designed using Primer Express software (Applied Biosystems) and span intron-exon junctions to avoid amplification of genomic DNA; forward: 5′-AAGCCGACCCAATGACATCA-3′, reverse: 5′-CTTCCTTCGTACACGCAAAT-3′. Primers for 18S rRNA were purchased from Applied Biosystems. The relative amounts of mRNAs were calculated using the comparative CT method. 18S rRNA was used as a reference. Final values are taken from at least two replicates that were performed at the cDNA synthesis step. Dissociation plots indicated a single PCR product in all cases.
Statistics
Group comparisons were made by either Student's t test or one-way ANOVA with Tukey's post hoc. A P value <0.05 was considered statistically significant. All data are expressed as mean ± s.e.m.
Results
High AMPK activity in BAT
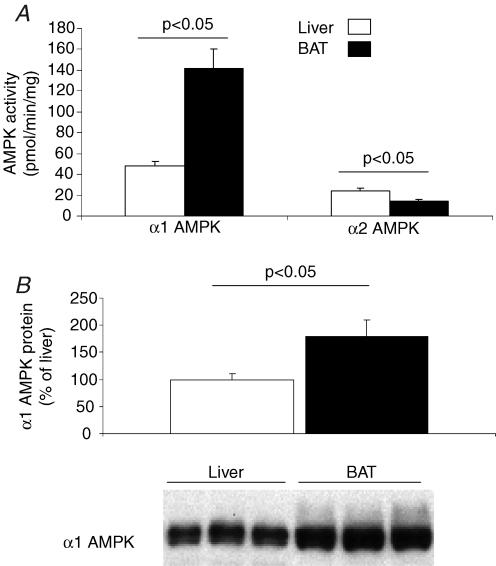
We found that the activity of α1 AMPK in mice was approximately three-fold higher in BAT than in liver (Fig. 1A, P < 0.05). Consistent with what has been reported for white adipose tissue (WAT), α1 appears to be the dominant isoform of AMPK in BAT (Matejkova et al. 2004; Sponarova et al. 2005). To determine the molecular basis for the high α1 AMPK activity in mouse BAT, AMPK protein and mRNA levels were measured and compared to those in liver. We found that levels of α1 AMPK mRNA and protein were 480 ± 30% and 80 ± 30% greater in BAT than liver, respectively (Figs 1B, P < 0.05). Thus our data indicate that the high α1 AMPK activity in BAT is caused primarily by a high expression of AMPK protein.
Figure 1. Comparison of AMPK activity in mouse BAT and liver.
A, activities of α1 AMPK and α2 AMPK were measured in homogenates of BAT and liver from mice, following immunoprecipitation with isoform-specific antibodies. Activities were determined in the presence of 200 μm AMP. Data are expressed as mean ± s.e.m. for n=5 in each group. B, Western blots were performed on homogenates of BAT and liver from mice. Immunoblots were probed with antibodies directed against α1 AMPK protein.
High levels of ACC and phosphorylated ACC in BAT
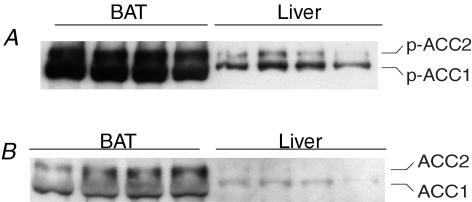
To investigate the downstream effect of the high AMPK activity in mouse BAT, the abundance of ACC phosphorylated at Ser79 (phospho-ACC) was determined in BAT. ACC was used as a representative AMPK target because it is an important control point for synthesis and oxidation of free fatty acids that is phosphorylated at Ser79 exclusively by AMPK (Saha & Ruderman, 2003). Consistent with a high AMPK activity in BAT, both isoforms of phosphoACC were more abundant in BAT than in liver (Fig. 2A). This high level of phosphoACC in BAT was associated with a large amount of total ACC protein in this tissue (Fig. 2B). Since phosphorylation inhibits ACC, these data suggest that a large pool of ACC in BAT is maintained in the inhibited form by α1 AMPK.
Figure 2. Western blots on homogenates of mouse BAT and liver.
Immunoblots were probed with antibodies directed against phospho-(Ser79)-ACC (A) or total ACC (B). Compared to liver, BAT contains a large amount of total ACC protein, as well as phosphorylated (inhibited) ACC.
Effect of cold exposure on tissue levels of AMPK activity
To determine how AMPK activity in BAT is altered during non-shivering thermogenesis, mice were studied after cold exposure (4°C) ranging from 2 h to 15 days. Each cold-exposure group had its own control group of mice that were maintained at 22°C until they were killed. As shown in Fig. 3, 2–24 h of cold exposure did not significantly affect AMPK activity in BAT. However, after 7 days of cold exposure α1 AMPK activity was significantly elevated above control levels (45%, P < 0.05), with a further elevation noted after 15 days of cold exposure (65% above baseline, P < 0.05). The cold-induced increase in AMPK activity was isoform-specific as α1, but not α2 AMPK activity increased.
To determine the molecular basis for the cold-induced increase in α1 AMPK activity in BAT, α1 AMPK mRNA and protein were measured. The amount of mRNA for α1 AMPK increased by 2.1-fold from day 0 to day 15 of cold (n=5, P < 0.05). Likewise, the level of α1 AMPK protein increased 2.1-fold after 15 days in the cold (P < 0.05). These findings strongly suggest that the cold-induced increase in α1 AMPK activity is caused by increased expression of the α1 AMPK protein.
To determine if the cold-induced increase in AMPK activity was specific to BAT, AMPK activity was measured in liver and epididymal white adipose tissue (WAT) from control and 15 day cold-exposed mice. As shown in Fig. 3, α1 AMPK activity approximately doubled in WAT in response to chronic cold. As was the case with BAT, this effect was isoform specific. In liver, AMPK activity was unaffected by chronic cold exposure.
Effect of chronic in vivo adrenergic stimulation on AMPK activity in brown and white adipose tissue
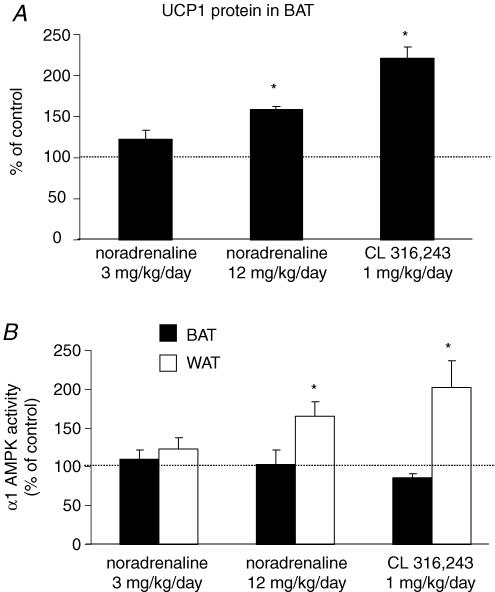
To evaluate the signalling pathway by which chronic cold exposure increases α1 AMPK activity in brown and white adipose tissue, mice were subjected to pharmacological stimulation of adrenergic receptors for 14 days with either noradrenaline, or the β3-adrenergic-specific agonist CL 316 243. Consistent with previous reports, administration of this β3-adrenergic agonist decreased the weight of the epididymal fat pad without significantly altering BAT weight or body weight (Table 1) (Himms-Hagen et al. 1994, 2000). A noradrenaline dose of 3 mg kg−1 day−1 did not significantly affect any of the parameters in Table 1 or the amount of UCP1 protein in BAT (Fig. 4A). In contrast, a higher dose of noradrenaline (12 mg kg−1 day−1) significantly decreased the size of the epididymal fat pad and also significantly increased the amount of interscapular BAT. While this dose of noradrenaline was sufficient to cause morphological changes in brown and white adipose tissue, it did not induce a significant cardiac hypertrophy as indicated by the ratio of heart weight to body weight (Table 1). The higher dose of noradrenaline and the β3-adrenergic stimulation both caused an increase in UCP-1 in BAT as expected (Fig. 4A).
Table 1.
Effects of 14 days of noradrenaline and CL 316 243 treatment in mice.
| Control (n=6) | Noradrenaline (n=6) 3 mg kg−1 day−1 | Noradrenaline (n=6) 12 mg kg−1 day−1 | CL 316 243 (n=6) 1 mg kg−1 day−1 | |
|---|---|---|---|---|
| Initial body weight (g) | 26.6 ± 0.4 | 26.4 ± 0.6 | 25.7 ± 1.0 | 26.1 ± 0.5 |
| Final body weight (g) | 28.5 ± 0.6 | 28.4 ± 0.6 | 28.2 ± 0.6 | 28.8 ± 0.6 |
| Heart weight/body weight (mg g−1) | 4.98 ± 0.18 | 5.00 ± 0.12 | 5.28 ± 0.14 | 5.03 ± 0.04 |
| Interscapular BAT weight (mg) | 59.1 ± 5.5 | 53.7 ± 3.2 | 92.3 ± 5.3* | 55.7 ± 3.1 |
| Epididymal white adipose tissue weight (mg) | 130.9 ± 11.4 | 110.0 ± 11.1 | 69.3 ± 3.9* | 82.2 ± 9.4* |
Values are mean ± s.e.m.
Significantly different from control group (P < 0.05).
Figure 4. Effects of 14 days of treatment with adrenergic agonists on UCP1 protein levels in BAT (A), and α1 AMPK activity in BAT and WAT.
(B) Despite the expected increases in BAT UCP-1, α1 AMPK activity in BAT was not increased by β3-adrenergic stimulation. In contrast, both the higher dose of noradrenaline and β3-adrenergic stimulation significantly increased α1 AMPK activity in WAT. Data are expressed as mean ± s.e.m. for n=5–6 per group; *P ≤ 0.05 compared to the control group that received vehicle.
Somewhat surprisingly, neither noradrenaline nor β3-adrenergic stimulation caused any change in AMPK activity in BAT. In fact, β3-adrenergic stimulation caused a modest decrease in α1 AMPK activity in BAT (P=0.051, Fig. 4B). Since the protein content in the BAT was increased upon β3-adrenergic stimulation, the decrease in AMPK activity (expressed per mg protein) probably indicates that, rather than being actively downregulated, AMPK is simply not among the proteins that are upregulated by β3-adrenergic stimulation.
In contrast to BAT, α1 AMPK activity was significantly increased in WAT by both β3 adrenergic stimulation and the higher dose of noradrenaline (Fig. 4B). In the liver, which has few if any β3-adrenergic receptors, CL 316 243 had no effect on α1 AMPK activity (data not shown).
Discussion
Here we report for the first time that AMPK activity is significantly increased by chronic cold exposure in white and brown adipose tissue. This finding not only indicates the presence of a previously unrecognized temperature-responsive signalling pathway regulating AMPK activity in these tissues, but also strongly suggests that AMPK plays a role in thermoregulation. Our investigation of the signalling pathway linking cold exposure and AMPK activity revealed that selective β3-adrenergic stimulation is sufficient to upregulate AMPK activity in WAT, suggesting this as the mechanism of cold-induced increases in WAT AMPK activity. The β3-adrenergic-induced increase in AMPK activity in WAT also demonstrates the existence of ‘cross-talk’ between these two stress response systems in WAT. In BAT, adrenergic stimulation did not recapitulate the cold-induced increase in AMPK activity, a surprising finding given the prevailing view that all cold-induced changes in BAT are mediated by the sympathetic nervous system. Thus our data suggest the existence of a non-adrenergic pathway by which cold exposure alters BAT biology. Regarding the physiological role of AMPK in BAT, we found that cold exposure does not increase AMPK activity in BAT until well after the initial acute period of intense non-shivering thermogenesis. Thus the cold-induced increases in BAT free fatty acid oxidation are not dependent on an increased AMPK activity as has been suggested by some in vitro data (Hutchinson et al. 2005; Inokuma et al. 2005). Taken as a whole, our data support the view that AMPK plays a role in the chronic maintenance of thermogenic potential of BAT.
AMPK activity in BAT during euthermic conditions
AMPK is ubiquitously expressed throughout the body with the previously highest reported levels in liver (Davies et al. 1989). Given the large number of cellular processes influenced by AMPK, the high level of activity in BAT suggests an important role for AMPK in controlling BAT metabolism. The presence of this level of AMPK activity in BAT is intriguing, since AMPK is regarded as an ATP-conserving enzyme, whereas BAT is an ATP-‘wasting’ tissue in that its uncoupled metabolism produces heat instead of ATP (Cannon & Nedergaard, 2004). The molecular basis for this high level of AMPK activity in BAT is not likely to be related to tissue levels of AMP since the assay of AMPK activity is performed under conditions of fixed AMP concentration (200 μm). Additionally, AMP levels would be expected to influence the activities of both the α1 and α2 isoforms of AMPK, whereas in BAT only α1 AMPK activity was elevated (Salt et al. 1998; Gonzalez et al. 2004a,b; Mulligan et al. 2005). Our data instead indicate that the high level of α1 AMPK activity in BAT is caused primarily, if not entirely, by a high level of expression of α1 AMPK protein. A large amount of α1 AMPK activity relative to α2 AMPK activity was present, and while this dominance of the α1 isoform contrasts with what is seen in other mitochondria-rich tissue such as heart, it is similar to what has been reported in WAT (Gonzalez et al. 2004a,b; Matejkova et al. 2004).
AMPK activity in BAT during first 24 h of cold exposure
During acute cold exposure, sympathetically mediated activation of BAT increases its metabolic rate, resulting in oxidation of large quantities of free fatty acids (Carneheim et al. 1989; Martins et al. 1991; Golozoubova et al. 2001; Cannon & Nedergaard, 2004). The metabolic control system that permits this high level of caloric expenditure by BAT is of great interest in understanding regulation of body weight (Cannon & Nedergaard, 2004). Accordingly, we tested the hypothesis that entry of BAT into the thermogenic state would be associated with a rapid increase in AMPK activity. This hypothesis was based on the increased need for catabolism of metabolic substrates (primarily free fatty acids) by BAT during non-shivering thermogenesis (Guerra et al. 1998), and the established role of AMPK in other tissue types as a key regulator of this process (Carling, 2004). We found that AMPK activity in BAT was not significantly altered at the 2, 12 or 24 h time points of cold exposure in mice. Since this is a time of intense non-shivering thermogenesis, our data demonstrate that an increase in AMPK activity is not obligatory for the cold-induced increase in free fatty acid oxidation or increases in BAT metabolic rate that occur during non-shivering thermogenesis. We cannot rule out the possibility that a transient increase in AMPK activity may occur during the early minutes of cold exposure. In vitro, pharmacological stimulation of adrenergic receptors has been reported to increase AMPK activity and/or phosphorylation of AMPK in BAT within a few minutes (Hutchinson et al. 2005; Inokuma et al. 2005). However, the extent to which a transient sympathetically mediated increase in AMPK activity occurs in BAT during the first hour of cold exposure in vivo remains to be determined.
Chronic cold exposure and adrenergic stimulation in BAT
In addition to acute activation of non-shivering thermogenesis, cold exposure causes brown adipose tissue to chronically adapt in ways that increase its thermogenic potential (Cannon & Nedergaard, 2004). During this more chronic time frame, we found that AMPK activity progressively increased. This increase in AMPK activity was selective for the α1 isoform, indicating that the cause is not canonical activation such as would occur with an increased concentration of AMP or a general increase in transcription of AMPK subunits. Instead, the cold-induced increase in AMPK activity was caused by a selective increase in expression of α1 AMPK. By what mechanism does chronic cold exposure cause this isoform-specific increase in AMPK expression in BAT? Since most cold-induced changes in BAT (i.e. upregulation of UCP-1 expression) are caused by the sympathetic nervous system, we tested the hypothesis that in vivo pharmacological stimulation with noradrenaline would mimic the cold-induced increase in AMPK activity. Despite the fact that noradrenaline caused the expected upregulation of UCP-1, it did not increase AMPK activity in BAT. Thus it appears that the cold-induced increase in AMPK activity is caused by a non-sympathetic mechanism. Identifying the signalling pathway(s) that causes the cold-induced increase in AMPK expression will provide a novel opportunity to study how AMPK expression, a poorly understood processes, is regulated. The slow time course for the activation of AMPK by cold exposure suggests that AMPK is not involved in the acute thermogenic response of BAT, instead it probably plays a role in coordinating the long-term control of thermogenic potential in BAT. Recent data demonstrating a close link between AMPK activity and peroxisomal proliferator activated receptor gamma coactivator 1 alpha (PGC1α)-mediated mitochondrial biogenesis (Zong et al. 2002; Suwa et al. 2003; Lee et al. 2006) raise the possibility that AMPK activity in BAT plays a role in maintaining the high mitochondrial density of BAT during euthermia, and increasing it during chronic cold exposure.
Chronic cold exposure and adrenergic stimulation in white adipose tissue
In addition to affecting BAT, cold-induced sympathetic activation is know to have important physiological effects on WAT (Rayner, 2001; Koska et al. 2002). Consistent with this we found that chronic cold exposure caused an isoform-specific increase in AMPK activity in WAT that was similar in magnitude to that observed in BAT. In contrast to BAT, chronic administration of noradrenaline increased α1 AMPK activity in WAT. To further define the adrenergic signalling pathway responsible for this adrenergic-mediated increase in WAT α1 AMPK, the β3-specific adrenergic agonist CL 316 243 was administered for 14 days. This resulted in a robust increase in white, but not brown adipose tissue α1 AMPK activity. It is well established that chronic β3-adrenergic stimulation causes a ‘remodelling’ of WAT that includes a decrease in fat pad mass, increase in protein concentration, and sometimes the appearance of multilocular mitochondria-rich cells that resemble brown adipocytes (Himms-Hagen et al. 1994, 2000). Whether β3-adrenergic stimulation increased the amount of AMPK activity in WAT by increasing the level of AMPK activity within mature white adipocytes, or by shifting the cellular composition of WAT towards a cell type containing higher levels of AMPK activity remains to be determined.
Acknowledgments
We would like to thank Raquel Sancho for her technical support, and Dr Elizabeth Kersteen for her astute editorial assistance. This work was supported by National Institute on Ageing grant AG-00908 and National Heart Lung and Blood Institute grant HL-07936.
References
- Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, Carling D, Small CJ. AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem. 2004;279:12005–12008. doi: 10.1074/jbc.C300557200. [DOI] [PubMed] [Google Scholar]
- Arad M, Benson DW, Perez-Atayde AR, McKenna WJ, Sparks EA, Kanter RJ, McGarry K, Seidman JG, Seidman CE. Constitutively active AMP kinase mutations cause glycogen storage disease mimicking hypertrophic cardiomyopathy. J Clin Invest. 2002;109:357–362. doi: 10.1172/JCI14571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura H. Fetal and neonatal thermoregulation. J Nippon Med Sch. 2004;71:360–370. doi: 10.1272/jnms.71.360. [DOI] [PubMed] [Google Scholar]
- Blair E, Redwood C, Ashrafian H, Oliveira M, Broxholme J, Kerr B, Salmon A, Ostman-Smith I, Watkins H. Mutations in the γ2 subunit of AMP-activated protein kinase cause familial hypertrophic cardiomyopathy: evidence for the central role of energy compromise in disease pathogenesis. Hum Mol Genet. 2001;10:1215–1220. doi: 10.1093/hmg/10.11.1215. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Carling D. The AMP-activated protein kinase cascade – a unifying system for energy control. Trends Biochem Sci. 2004;29:18–24. doi: 10.1016/j.tibs.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Carneheim C, Cannon B, Nedergaard J. Rare fatty acids in brown fat are substrates for thermogenesis during arousal from hibernation. Am J Physiol Regul Integr Comp Physiol. 1989;256:R146–R154. doi: 10.1152/ajpregu.1989.256.1.R146. [DOI] [PubMed] [Google Scholar]
- Davies SP, Carling D, Hardie DG. Tissue distribution of the AMP-activated protein kinase, and lack of activation by cyclic-AMP-dependent protein kinase, studied using a specific and sensitive peptide assay. Eur J Biochem. 1989;186:123–128. doi: 10.1111/j.1432-1033.1989.tb15185.x. [DOI] [PubMed] [Google Scholar]
- Gollob MH, Seger JJ, Gollob TN, Tapscott T, Gonzales O, Bachinski L, Roberts R. Novel PRKAG2 mutation responsible for the genetic syndrome of ventricular preexcitation and conduction system disease with childhood onset and absence of cardiac hypertrophy. Circulation. 2001;104:3030–3033. doi: 10.1161/hc5001.102111. [DOI] [PubMed] [Google Scholar]
- Golozoubova V, Hohtola E, Matthias A, Jacobsson A, Cannon B, Nedergaard J. Only UCP1 can mediate adaptive nonshivering thermogenesis in the cold. FASEB J. 2001;15:2048–2050. doi: 10.1096/fj.00-0536fje. [DOI] [PubMed] [Google Scholar]
- Gonzalez AA, Kumar R, Mulligan JD, Davis AJ, Saupe KW. Effects of aging on cardiac and skeletal muscle AMPK activity: basal activity, allosteric activation, and response to in vivo hypoxemia in mice. Am J Physiol Regul Integr Comp Physiol. 2004a;287:R1270–R1275. doi: 10.1152/ajpregu.00409.2004. [DOI] [PubMed] [Google Scholar]
- Gonzalez AA, Kumar R, Mulligan JD, Davis AJ, Weindruch R, Saupe KW. Metabolic adaptations to fasting and chronic caloric restriction in heart, muscle, and liver do not include changes in AMPK activity. Am J Physiol Endocrinol Metab. 2004b;287:E1032–E1037. doi: 10.1152/ajpendo.00172.2004. [DOI] [PubMed] [Google Scholar]
- Guerra C, Koza RA, Walsh K, Kurtz DM, Wood PA, Kozak LP. Abnormal nonshivering thermogenesis in mice with inherited defects of fatty acid oxidation. J Clin Invest. 1998;102:1724–1731. doi: 10.1172/JCI4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himms-Hagen J, Cui J, Danforth E, Jr, Taatjes DJ, Lang SS, Waters BL, Claus TH. Effect of CL-316,243, a thermogenic beta 3-agonist, on energy balance and brown and white adipose tissues in rats. Am J Physiol regul Integr Comp Physiol. 1994;266:R1371–R1382. doi: 10.1152/ajpregu.1994.266.4.R1371. [DOI] [PubMed] [Google Scholar]
- Himms-Hagen J, Melnyk A, Zingaretti MC, Ceresi E, Barbatelli G, Cinti S. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am J Physiol Cell Physiol. 2000;279:C670–C681. doi: 10.1152/ajpcell.2000.279.3.C670. [DOI] [PubMed] [Google Scholar]
- Hutchinson DS, Chernogubova E, Dallner OS, Cannon B, Bengtsson T. beta-Adrenoceptors, but not alpha-adrenoceptors, stimulate AMP-activated protein kinase in brown adipocytes independently of uncoupling protein-1. Diabetologia. 2005;48:2386–2395. doi: 10.1007/s00125-005-1936-7. [DOI] [PubMed] [Google Scholar]
- Huttunen P, Hirvonen J, Kinnula V. The occurrence of brown adipose tissue in outdoor workers. Eur J Appl Physiol Occup Physiol. 1981;46:339–345. doi: 10.1007/BF00422121. [DOI] [PubMed] [Google Scholar]
- Inokuma K, Ogura-Okamatsu Y, Toda C, Kimura K, Yamashita H, Saito M. Uncoupling protein 1 is necessary for norepinephrine-induced glucose utilization in brown adipose tissue. Diabetes. 2005;54:1385–1391. doi: 10.2337/diabetes.54.5.1385. [DOI] [PubMed] [Google Scholar]
- Joy RJ. Responses of cold-acclimatized men to infused norepinephrine. J Appl Physiol. 1963;18:1209–1212. doi: 10.1152/jappl.1963.18.6.1209. [DOI] [PubMed] [Google Scholar]
- Kang BS, Han DS, Paik KS, Park YS, Kim JK, Kim CS, Rennie DW, Hong SK. Calorgigenic action of norepinephrine in the Korean women divers. J Appl Physiol. 1970;29:6–9. doi: 10.1152/jappl.1970.29.1.6. [DOI] [PubMed] [Google Scholar]
- Kishi K, Yuasa T, Minami A, Yamada M, Hagi A, Hayashi H, Kemp BE, Witters LA, Ebina Y. AMP-Activated protein kinase is activated by the stimulations of G(q)-coupled receptors. Biochem Biophys Res Commun. 2000;276:16–22. doi: 10.1006/bbrc.2000.3417. [DOI] [PubMed] [Google Scholar]
- Koska J, Ksinantova L, Sebokova E, Kvetnansky R, Klimes I, Chrousos G, Pacak K. Endocrine regulation of subcutaneous fat metabolism during cold exposure in humans. Ann N Y Acad Sci. 2002;967:500–505. doi: 10.1111/j.1749-6632.2002.tb04308.x. [DOI] [PubMed] [Google Scholar]
- Lee WJ, Kim M, Park HS, Kim HS, Jeon MJ, Oh KS, Koh EH, Won JC, Kim MS, Oh GT, Yoon M, Lee KU, Park JY. AMPK activation increases fatty acid oxidation in skeletal muscle by activating PPARalpha and PGC-1. Biochem Biophys Res Commun. 2006;340:291–295. doi: 10.1016/j.bbrc.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Luo Z, Saha AK, Xiang X, Ruderman NB. AMPK, the metabolic syndrome and cancer. Trends Pharmacol Sci. 2005;26:69–76. doi: 10.1016/j.tips.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Martins R, Atgie C, Gineste L, Nibbelink M, Ambid L, Ricquier D. Increased GDP binding and thermogenic activity in brown adipose tissue mitochondria during arousal of the hibernating garden dormouse (Eliomys quercinus L.) Comp Biochem Physiol A. 1991;98:311–316. doi: 10.1016/0300-9629(91)90538-n. [DOI] [PubMed] [Google Scholar]
- Matejkova O, Mustard KJ, Sponarova J, Flachs P, Rossmeisl M, Miksik I, Thomason-Hughes M, Grahame Hardie D, Kopecky J. Possible involvement of AMP-activated protein kinase in obesity resistance induced by respiratory uncoupling in white fat. FEBS Lett. 2004;569:245–248. doi: 10.1016/j.febslet.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- Moule SK, Denton RM. The activation of p38 MAPK by the beta-adrenergic agonist isoproterenol in rat epididymal fat cells. FEBS Lett. 1998;439:287–290. doi: 10.1016/s0014-5793(98)01392-1. [DOI] [PubMed] [Google Scholar]
- Mulligan JD, Gonzalez AA, Kumar R, Davis AJ, Saupe KW. Aging elevates basal AMPK activity and eliminates hypoxic activation of AMPK in mouse liver. J Gerontol A Biol Sci Med Sci. 2005;60:21–27. doi: 10.1093/gerona/60.1.21. [DOI] [PubMed] [Google Scholar]
- Rattan R, Giri S, Singh AK, Singh I. 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside inhibits cancer cell proliferation in vitro and in vivo via AMP-activated protein kinase (AMPK) J Biol Chem. 2005;280:29582–395893. doi: 10.1074/jbc.M507443200. [DOI] [PubMed] [Google Scholar]
- Rayner DV. The sympathetic nervous system in white adipose tissue regulation. Proc Nutr Soc. 2001;60:357–364. doi: 10.1079/pns2001101. [DOI] [PubMed] [Google Scholar]
- Saha AK, Ruderman NB. Malonyl-CoA and AMP-activated protein kinase: an expanding partnership. Mol Cell Biochem. 2003;253:65–70. doi: 10.1023/a:1026053302036. [DOI] [PubMed] [Google Scholar]
- Salt I, Celler JW, Hawley SA, Prescott A, Woods A, Carling D, Hardie DG. AMP-activated protein kinase: greater AMP dependence, and preferential nuclear localization, of complexes containing the alpha2 isoform. Biochem J. 1998;334:177–187. doi: 10.1042/bj3340177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sponarova J, Mustard KJ, Horakova O, Flachs P, Rossmeisl M, Brauner P, Bardova K, Thomason-Hughes M, Braunerova R, Janovska P, Hardie DG, Kopecky J. Involvement of AMP-activated protein kinase in fat depot-specific metabolic changes during starvation. FEBS Lett. 2005;579:6105–6110. doi: 10.1016/j.febslet.2005.09.078. [DOI] [PubMed] [Google Scholar]
- Suwa M, Nakano H, Kumagai S. Effects of chronic AICAR treatment on fiber composition, enzyme activity, UCP3, and PGC-1 in rat muscles. J Appl Physiol. 2003;95:960–968. doi: 10.1152/japplphysiol.00349.2003. [DOI] [PubMed] [Google Scholar]
- Tiraby C, Langin D. Conversion from white to brown adipocytes: a strategy for the control of fat mass? Trends Endocrinol Metab. 2003;14:439–441. doi: 10.1016/j.tem.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, Shulman GI. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci U S A. 2002;99:15983–15987. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]