Abstract
Diabetes mellitus is a growing epidemic with severe cardiovascular complications. Although much is known about mechanical and electrical cardiac dysfunction in diabetes, few studies have investigated propagation of the electrical signal in the diabetic heart and the associated changes in intercellular gap junctions. This study was designed to investigate these issues, using hearts from control and diabetic rats. Diabetic conditions were induced by streptozotocin (STZ), given i.v. 7–14 days before experiments. Optical mapping with the voltage-sensitive dye di-4-ANEPPS, using hearts perfused on a Langendorff apparatus, showed little change in baseline conduction velocity in diabetic hearts, reflecting the large reserve of function. However, both the gap junction uncoupler heptanol (0.5–1 mm) and elevated potassium (9 mm, to reduce cell excitability) produced a significantly greater slowing of impulse propagation in diabetic hearts than in controls. The maximal action potential upstroke velocity (an index of the sodium current) and resting potential was similar in single ventricular myocytes from control and diabetic rats, suggesting similar electrical excitability. Immunoblotting of connexin 43 (Cx43), a major gap junction component, showed no change in total expression. However, immunofluorescence labelling of Cx43 showed a significant redistribution, apparent as enhanced Cx43 lateralization. This was quantified and found to be significantly larger than in control myocytes. Labelling of two other gap junction proteins, N-cadherin and β-catenin, showed a (partial) loss of co-localization with Cx43, indicating that enhancement of lateralized Cx43 is associated with non-functional gap junctions. In conclusion, conduction reserve is smaller in the diabetic heart, priming it for impaired conduction upon further challenges. This can desynchronize contraction and contribute to arrhythmogenesis.
Diabetes mellitus is a growing epidemic with wide-ranging implications (Zimmet et al. 2001; Mooradian, 2003). Cardiovascular complications often develop (Mooradian, 2003; Fang et al. 2004; Fonarow, 2005). A distinct cardiomyopathy (Fang et al. 2004) makes diabetes an independent risk factor for heart failure (Fonarow, 2005). Diabetic cardiac dysfunction is evident as mechanical (Severson et al. 2003) and electrical (Abo et al. 1996) abnormalities, leading to a higher incidence of cardiac arrhythmias and sudden death (El-Atat et al. 2004). Diabetes (types 1 and 2) is associated with electrocardiogram (ECG) abnormalities, including QT prolongation and increased QT dispersion (Christensen et al. 2000; Rossing et al. 2001). These recognized risks for lethal arrhythmias reflect abnormal repolarization and prolongation of the ventricular action potential, caused partially by attenuation of repolarizing K+ currents (Shimoni et al. 1998; Xu et al. 2002). Earlier work provided indirect evidence suggesting that the diabetic heart may also develop abnormalities in propagation of the cardiac impulse.
Normal ventricular function depends on a very rapid spread of electrical activation, ensuring synchronous contraction. Intercellular propagation of the electrical impulse depends on excitability and on electrical coupling between cells (Shaw & Rudy, 1997; Kleber & Rudy, 2004). Propagation is facilitated by a very large and rapidly activating sodium current (Shaw & Rudy, 1997), and by specialized, tightly regulated intercellular connections called gap junctions (Kanno & Saffitz, 2001; Laird, 2005; Turner et al. 2005). A major component of ventricular gap junctions is the protein connexin (Cx), with Cx43 as the dominant mammalian isoform (van Rijen et al. 2004; Laird, 2005). Pathological alterations in Cx abundance or function can lead to slowing of conduction (Danik et al. 2004; Turner et al. 2005; Akar et al. 2005; Zeevi-Levin et al. 2005). Impaired propagation, reflected in a broadening of the QRS complex of the ECG, reduces coordinated ventricular contraction and forms a substrate for cardiac arrhythmias (Akar et al. 2005; Zeevi-Levin et al. 2005), as observed in cardiac-restricted Cx43 ‘knockout’ mice (Danik et al. 2004).
In humans and in animal models, various cardiac diseases lead to gap junction remodelling and changes in connexin (Kanno & Saffitz, 2001; Kostin et al. 2003; Teunissen et al. 2004; Turner et al. 2005; Akar et al. 2005; Zeevi-Levi et al. 2005). A reduced expression of Cx43 may occur together with enhanced Cx45 or Cx40 expression (Yamada et al. 2003).
In the presence of diabetic conditions, several studies in animal models (Inoguchi et al. 2001; Okruhlicova et al. 2002; Howarth et al. 2005) and in humans (Yang et al. 1990; Celiker et al. 1994) described prolonged QRS durations in the ECG, suggesting impaired conduction of the electrical impulse. Some studies also found a reduction in Cx43 expression in hearts of STZ-diabetic rats, with an increased susceptibility to ventricular fibrillation (Inoguchi et al. 2001; Okruhlicova et al. 2002). Further animal studies found that high glucose levels reduce intercellular communication (Kuroki et al. 1998; Inoguchi et al. 2001; De Mello, 2006). The mechanisms underlying these changes are not known, although a possible contribution of an activated renin–angiotensin system has been suggested (De Mello, 2006).
The heart has a very large ‘conduction reserve’ (van Veen et al. 2005; van Rijen et al. 2005). Severe alterations in gap junctions (reduced coupling) or a substantial reduction in excitability or sodium current are required to produce significant changes in conduction velocity (Shaw & Rudy, 1997; Danik et al. 2004; van Veen et al. 2005). This is evident in heterozygous Cx43 ‘knockout’ mice, in which conduction slowing is either absent (Morley et al. 1999; van Rijen et al. 2004) or modest (Guerrero et al. 1997). Some pathologies may induce only moderate reduction in cellular coupling and/or in excitability. This would not be expected to produce significant changes in baseline conduction, although it may reduce the conduction reserve, making propagation more sensitive to perturbation. For example, in the Scn5a knockout mouse a 50% reduction in expression of Scn5a, the major pore-forming α-subunit of the sodium channel, only slightly affects conduction. However, substantial conduction impairment is observed when the reduced Scn5a expression is accompanied by fibrosis in aged Scn5a knockout mice (van Veen et al. 2005). In the present work, we demonstrate a similar phenomenon in the diabetic heart. Reduced intercellular coupling due to connexin redistribution does not in itself result in significant conduction impairment. However, a substantial slowing of conduction is observed in diabetic hearts when excitability is reduced in response to elevated extracellular potassium concentrations, a known clinical complication of diabetes (Jarman & Mather, 2003). No studies to date have directly measured impulse propagation in the diabetic heart, although changes in propagation of the cardiac impulse could potentially be a significant but underestimated complication of diabetes. The present study explored these issues.
Methods
This study conforms to the NIH Guide for the Care and Use of Laboratory Animals, and was approved by the University of Calgary Animal Care Committee
Animals
Age-matched male Sprague-Dawley rats (250–300 g) were used as controls or 7–14 days following i.v. injection of STZ (100 mg kg−1) to induce diabetes. The diabetic state was confirmed by measuring (non-fasting) blood glucose levels with a glucometer (One Touch Ultra, LifeScan Inc, Milpitas, CA, USA). In diabetic rats (n=12) glucose levels were higher than 28 mm (in most rats glucose concentrations were > 33 mm, the saturating level of the glucometer). The non-fasting glucose level in control rats was 15.1 ± 1.2 mm (mean ± s.e.m., n=11).
Several groups of experiments were conducted relating to functional and structural changes in the diabetic hearts. Rats were anaesthetized by CO2 inhalation and killed by cervical dislocation. Hearts were removed, mounted on a Langendorff apparatus by aortic cannulation, and perfused (at 37°C, constant flow rate). The experiments involved using whole hearts for imaging, or enzymatically isolated ventricular cells for immunolabelling, Western blotting and action potential recordings. Thin tissue sections were also used for labelling.
Voltage-sensitive dye imaging
We used voltage-sensitive dye imaging technology (as in, e.g., Efimov et al. 2004) to quantify the pattern of activation and conduction velocity in the left ventricular free wall. Voltage-sensitive dyes emit, when illuminated at appropriate wavelengths, a fluorescence signal that is proportional to the voltage across the cell membrane. By recording this signal with a high-speed video camera one can obtain recordings of electrical activation of the heart. Hearts were perfused (as in Nygren et al. 2000, 2003) at 8–13 ml min−1 (depending on heart size). Perfusion pressure and temperature were monitored (Nygren et al. 2000). After a 20 min stabilization period, the solution was switched to one containing 1 μm di-4-ANEPPS (Molecular Probes, Eugene, OR, USA) for 5 min.
Image data processing
Data were processed offline (Nygren et al. 2000, 2003). The activation time for each pixel in an image was detected as the time of maximum rate-of-rise of the fluorescence signal (Nygren et al. 2000). Activation maps were computed for individual cycles with activation times referenced to the stimulus pulse. Signal-averaged activation maps were then computed by averaging the activation times for individual pixels over all cycles. Activation maps were signal-averaged over 5 s of recording (20–25 cycles). An overall measure (‘activation time’) of the speed of activation was obtained by constructing a histogram of all individual pixel activation times from each activation map. The width of this histogram (defined as width at 20% of peak level) was taken as an overall measure (‘activation time’) of the speed of activation. By comparing this activation time for the same region of interest in the same preparation before and after an intervention, we were able to obtain a normalized measure of the change in conduction velocity with each individual preparation serving as its own control. Activation times were computed for paced recordings in normal and test solutions. All activation times were normalized to the value obtained in normal Krebs solution. This measure was therefore not influenced by the exact region of interest chosen or by the size of the preparation. No pharmacological ‘motion blockers’ were used in this study, due to the known side-effects of these agents on intercellular coupling (Baker et al. 2004). The presence of motion artifacts in our recordings required us to assess changes in conduction velocity in terms of the activation time measure defined above, rather than measuring local conduction velocities directly. The advantages of the activation time measure over local conduction velocities in the presence of motion artifacts are discussed in more detail in the online Supplemental material (Appendix B).
The preparation was paced at a cycle length of 200 ms, except in a few cases where the spontaneous rate was higher. In those cases, a cycle length of 180 ms was used (see below for effects of rate of stimulation). A unipolar stimulation electrode was located at the base of the left ventricle. This produced an approximately straight activation wavefront, travelling from base to apex of the free wall.
Fibre orientation
The fibre architecture of the heart is very complex. Fibre orientation is different at different depths through the ventricular walls, rotating through a full 180 deg from epicardium to endocardium. This has been clearly illustrated by Scollan et al. (2000; their Fig. 7) in the rabbit heart. The results obtained by these authors show that the epicardial fibre orientation in the centre of the left ventricle (LV) free wall is parallel to the long axis, rotating so that it is perpendicular to the long axis mid-wall, and parallel to the long axis on the endocardial surface. Since the myocardium is electrically coupled in all directions, including transmurally, propagation from base to apex in response to our stimulation protocol (even if observed on the epicardial surface) will include contributions from multiple fibre orientations. Our measurements thus provide an assessment of overall conduction velocity changes in both the longitudinal and transverse directions. No attempt has been made in this study to distinguish longitudinal and transverse conduction velocity.
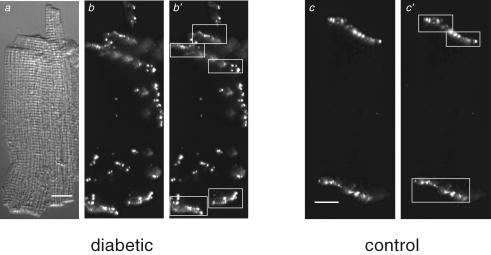
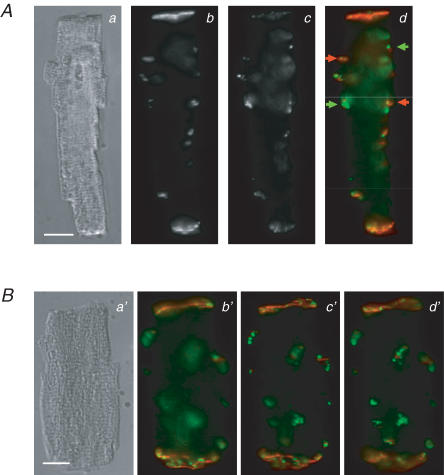
Figure 7. Analysis of lateralization of Cx43.
Panels a–b′, differential interference contrast (a) and immunofluorescence (b and b′) images of an isolated ventricular myocyte from an STZ-diabetic rat heart labelled with anti-Cx43. Panels c and c′: immunofluorescence images of an isolated ventricular myocyte from a non-diabetic (control) rat heart labelled with anti-Cx43. White rectangles in b′ and c′ surround the intercalated discs. To identify the intercalated discs in images, the images were first temporarily rescaled (using Photoshop software) so that the entire cell could be seen in an image. This made the disc regions more evident. The disc regions were then surrounded by boxes (panels b′ and c′) and the pixel intensities within the boxes were set to the background level for the analysis of lateralization (using locally written software) as described in the text. To rule out possible biasing in choosing the cellular regions associated with the intercalated discs in the images, the same set of images was analysed separately by two individuals. Results from the two separate analyses were not significantly different from one another. Scale bars in panels a and c represent 10 μm. Cells were labelled with anti-Cx43 primary antibody and secondary antibody conjugated to Alexa 488.
Limitations of voltage-sensitive dye imaging
It is important to recognize that the optically recorded action potential represents the average of the membrane potential in a volume of cells (the size of which is determined by the spatial resolution of the system). Due to the time required for the activation wavefront to travel through this volume, the optical action potential upstroke will be ‘smoothed’ (low-pass filtered), in particular for low conduction velocities. The rate of rise of the optical action potential therefore depends on the conduction velocity, in addition to the true rate of rise of a single cell action potential inside the volume (Girouard et al. 1996). The rate of rise of the action potential was therefore measured in separate experiments on single myocytes (below) rather than from the imaging data.
Action potential recordings in isolated cells
Ventricular myocytes were obtained by enzymatic dispersion, as described recently (Shimoni et al. 2006). In brief, hearts were perfused for 5–6 min (at 37°C, bubbled with 100% O2) with a solution consisting of (mm): 113 NaCl; 4.7 KCl; 1.2 MgSO4; 0.6 KH2PO4; 0.6 Na2HPO4; 12 NaHCO3; 12 KHCO3; 5.5 glucose, 10 Hepes; 30 taurine; 10 2,3-butanedione monoxime (BDM). This was followed by the same solution containing the digestive enzymes Liberase Blendzyme (Roche; 0.02–0.25 mg ml−1) and trypsin (0.14 mg ml−1), and 12.5 μm CaCl2. After 7–8 min the free wall of the right ventricle was cut into pieces. After shaking, the tissue was filtered through a mesh and a suspension of cells collected and stored at room temperature in a solution containing no enzymes, 20 mm taurine, 5 mg ml−1 albumin, and 0.1 mm CaCl2.
Cells were placed on the stage of an inverted microscope and perfused (at 21–22°C) with a solution containing (mm): 150 NaCl; 5.4 KCl; 1 CaCl2; 1 MgCl2; 5 Hepes; 5 glucose (pH 7.4, NaOH). Action potentials were recorded (at a stimulation rate of 1 Hz) using the current clamp method. Traces were digitized at a 10 kHz sampling rate. Recording pipettes (2–3 MΩ resistance) contained solutions consisting of (mm): 120 potassium aspartate; 30 KCl; 4 Na2ATP; 10 Hepes; 10 EGTA; 1 CaCl2; 1 MgCl2 (pH 7.2, KOH). The maximum upstroke velocity was measured as an index of the sodium current, and compared in cells from control and diabetic rats. Cell capacitance was measured in the voltage clamp mode by integrating current traces (over time), obtained by giving 5 mV depolarizing steps from –80 mV (digitized at 10 kHz).
Immunofluorescence
Isolated ventricular myocytes and thin sections were prepared for immunofluorescence microscopy as previously described (Shimoni et al. 2005, 2006). Briefly, isolated cell suspensions or tissue sections on glass slides were fixed with 1% formaldehyde and permeabilized with 1% Triton X-100. Primary antibody (polyclonal anti-Cx43, Sigma, St Louis, MO, USA) was added to aliquots (50 or 100 μl) of a cell suspension or pipetted onto the tissue sections in a humid chamber (non-specific binding was blocked with 2% BSA + 5% normal goat serum). The sections or cell suspensions were incubated overnight (at 4°C) in primary antibody, washed 3 times (in the case of cell suspensions by low-speed centrifugation and re-suspension of the resulting pellet) and then exposed to secondary antibody conjugated to a fluorochrome (Alexa fluor. 488, Molecular Probes, Eugene, OR, USA; or Cy3, Jackson Laboratories, West Grove, PA, USA) and incubated at room temperature for 1–1.5 h. Aliquots of cell suspensions (∼5 μl) were washed 3 times and pipetted into a drop of mounting medium on glass slides, covered with coverslips and sealed with clear nail polish. For tissue sections, mounting medium was pipetted onto a slide and the slide sealed with a coverslip. Control experiments with secondary antibody alone verified antibody specificity and determined background fluorescence. All sections or cells suspensions were labelled at the same time under the same conditions and images were collected using the same camera settings. Cells labelled with the Alexa 488- or the Cy3-conjugated secondary antibody were both included in the analysis, to rule out possible artifacts due to choice of secondary antibody.
Dual labelling experiments were carried out following the same basic procedure used for the single labelling experiments with the following modifications. After overnight incubation with one of the primary antibodies, the cells were washed 3 times using low-speed centrifugation and re-suspension of the resulting pellet. Following the third wash step, the cells were re-suspended in buffer with the second primary antibody and incubated for 1–1.5 h at room temperature. Following this, the cells were again washed 3 times and then incubated with both secondary antibodies (at the same time) for 1–1.5 h at room temperature. The remaining steps in the incubation and mounting procedures were the same as those used for the single labelling experiments. Monoclonal anti-N-cadherin and anti-β-catein were purchased from Zymed Laboratories (San Francisco, CA, USA) and BD. Biosciences (Mississauga, ON, Canada), respectively.
Quantification of connexin distribution
For this analysis, ∼20 individual cell immunofluorescence images were collected from each cell preparation labelled with anti-Cx43. Background fluorescence for the images was first determined in one of two ways: (1) as the maximum fluorescence in images of cells exposed to secondary antibody only; (2) as the light emanating from cellular regions that were clearly not labelled with the primary antibody. Both methods produced similar results. In each image, the total light intensity above background was determined. This was taken as a measure of the total fluorescence from all of the labelled Cx43 in the image. The total fluorescence from Cx43 was then compared to the fluorescence in the same image after all of the fluorescence from Cx43 associated with the intercalated discs was subtracted from the image. This was done by identifying the intercalated discs (see panels b′ and c′ in Fig. 7) and reducing the pixel intensities from the discs to the background level. The fraction of total cell fluorescence associated with the intercalated discs and the fraction emanating from Cx43 not associated with the intercalated discs (lateralized Cx43) were then determined for each cell imaged.
Western blotting
SDS-PAGE was performed to measure expression levels of Cx43 in cell lysates from control and diabetic hearts. Proteins from cell lysates were separated by SDS-PAGE and transferred to nitrocellulose membranes. The transferred proteins were first blotted with polyclonal anti-Cx43 (Sigma, St Louis, MO, USA). The Cx43 bands were labelled with secondary antibody conjugated to horseradish peroxidase and visualized on Kodak X-Omat AR film using the enhanced chemiluminescence (ECL) detection system (Amersham Biosciences, UK; instructions provided by the manufacturer). The blots were then stained with amido black to quantify the total protein in each lane on the blot. Digital images of the Cx43 bands and of the amido black-stained blot were inverted (black-to-white) and analysed as follows. A measure of the total protein in each lane of the amido black-stained blot was determined by computing the mean light intensity in the lane using Photoshop software. This value was used to normalize the mean light intensity of the Cx43 bands from images of the X-ray film. Using total protein rather than an individual marker protein for normalization reduced the possibility that diabetes-induced changes in the expression of any individual marker protein would bias the results.
Cell length and area
The images of isolated myocytes used for the immunofluorescence studies were also used to analyse cell length and area. Cell lengths were determined by manually drawing a line along the long axis of the cells using Spot software (V32, Diagnostic Instruments Inc. Sterling Heights, MI, USA). The area measurements were made by either drawing a region-of-interest around the cell in an image using Photoshop software and then setting the intensity of all pixels within the region to 100 and the intensity of all pixels outside of the region to 0 or by adjusting the image histogram to determine a lower threshold intensity that eliminated all of the light in the image outside of the cell area. The number of pixels in the images above 0 (in the first method) or above threshold (for the second method) was then determined using locally written software. For the ×60 images that were analysed, pixels were square and had an area of 0.016 μm2. There were no differences in the results obtained when the two methods of determining cell area were compared.
Collagen content
For comparing the collagen content of control and diabetic ventricles, tissue sections were labelled with antibodies to either collagen I or collagen III (Abcam, with either Cy3 or Alexa 488 as secondary antibodies) using the methods described above. Images of the sections were collected as described above and analysed as follows. A lower threshold for each image was first determined to eliminate all of the pixel intensities in the image that could not be attributed to tissue. The number of pixels in the image above this lower threshold was a measure of the total area of tissue in the image. A second threshold was then determined so that only light from the labelled collagen in the image was above threshold. The number of pixels above this higher threshold was used as a measure of the area in the image occupied by collagen. To reduce error, 10 randomly selected fields were examined for each control and diabetic section and multiple sections (cut from different hearts and labelled with different secondary antibodies) were analysed. To further reduce the possibility of error, some image sets were reanalysed with independently determined upper and lower thresholds and results obtained using images collected with ×10 and ×40 objectives were also compared. Total tissue area and area occupied by collagen were determined using locally written software and results were expressed as the percentage of tissue area occupied by collagen. Results obtained from the analysis of images collected with the ×40 objective were the same as those obtained from the analysis of images collected with the ×10 objective.
Results
Propagation of the cardiac impulse
Our first experiments examined whether the diabetic rat heart exhibits impairment of conduction of the ventricular action potential, as suggested by the broadening of the QRS interval of the ECG in some studies (Inoguchi et al. 2001; Okruhlicova et al. 2002). Using optical mapping with a voltage-sensitive dye, we measured the activation time of the left ventricular free wall following paced stimulation at the base of the ventricle (see Methods) as an indication of conduction velocity. Our measurements under normal conditions showed that the activation time in diabetic hearts was not strikingly different from control hearts (see Discussion). This is concordant with the existence of a robust cardiac reserve of function, protecting the heart from abnormal propagation.
Effect of reduced intercellular coupling
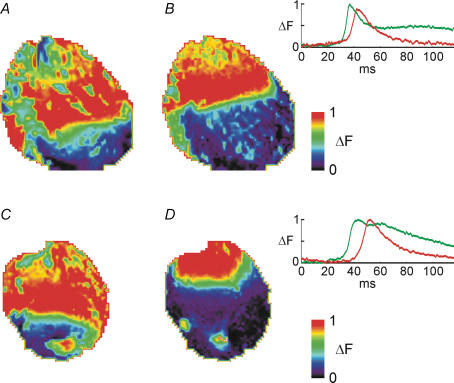
Hearts were exposed to (0.2–1 mm) heptanol, a gap junction uncoupler (Weingart & Bukauskas, 1998; Rodriguez-Sinovas et al. 2004). This procedure unmasked significant differences between hearts from control and diabetic rats. Whereas conduction slowing occurred in both groups, this was much more prominent in the diabetic group. For example, with 0.5 mm heptanol, activation time was increased by a factor of 1.12 ± 0.01 in eight control hearts, and by 1.32 ± 0.03 in nine diabetic hearts (P < 0.01). Figure 1 shows fluorescence images of the activation wavefront at the same poststimulus interval in a control (upper panels) and diabetic heart (lower panels), before and after 15 min in heptanol. The inset shows signal-averaged optically recorded action potentials from the hearts illustrated, indicating the considerably larger delay in response obtained in the diabetic heart following heptanol. Animations of the entire activation sequences from which the ‘snapshots’ in Fig. 1 were taken are available in the online Supplemental material.
Figure 1.
Fluorescence images showing the activation wavefronts during activation in response to pacing near the base of the left ventricle (top of image) in a control heart in normal Krebs solution (A); the same control heart in the presence of 0.5 mm heptanol (B); a diabetic heart in normal Krebs solution (C); and the same diabetic heart in the presence of 0.5 mm heptanol (D). All images show the state of activation 23.6 ms after the onset of the stimulus pulse. Note the considerably more pronounced slowing in response to heptanol in the diabetic heart (C and D) compared to the control heart (A and B). The colour scale shows fluorescence after background subtraction, a measure of membrane potential, with red corresponding to the most depolarized potentials. All data were signal averaged over approximately 20 activation cycles (see Supplemental material, Appendix A). The insets show optically recorded action potentials (APs) from a pixel near the apex of the left ventricle of the two hearts shown. Green lines show APs in normal Krebs solution, and red lines show APs in the presence of 0.5 mm heptanol. (Upper panel, APs in the control heart; lower panel, APs in the diabetic heart.) Note the considerably larger delay in response to heptanol in the diabetic heart compared to the control heart. Data were signal averaged over approximately 20 cycles.
Washout of heptanol (15 min) reversed its effect on activation time (not shown). Superimposed action potentials from a selected pixel near the apex are shown before and after perfusion of 0.5 mm heptanol.
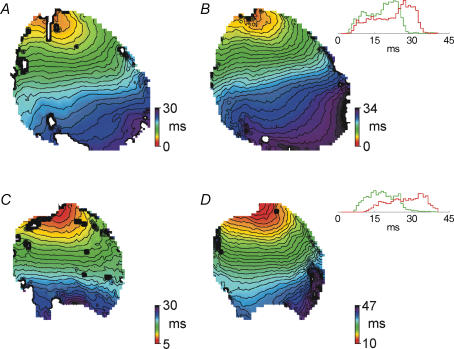
To further illustrate this result and the methods used for analysis, Fig. 2 shows isochronal maps of activation times in response to pacing. Control and diabetic hearts are shown in the top and bottom panels, respectively, in the absence of (Fig. 2A and C) or following (Fig. 2B and D) heptanol (0.5 mm, 15 min). The insets in Fig. 2 also show the histograms constructed from activation times at individual points in these hearts. The widths of these histograms were used to calculate the activation times. The isochronal maps show the larger effect of heptanol on activation times in hearts from diabetic rats (lower conduction velocity is manifest as more closely spaced isochrones).
Figure 2.
Isochronal maps (1 ms isochrones) showing activation in response to pacing near the base of the left ventricle (top of image) in a control heart in normal Krebs solution (A); the same control heart in the presence of 0.5 mm heptanol (B); a diabetic heart in normal Krebs solution (C); and the same diabetic heart in the presence of 0.5 mm heptanol (D). Insets in B and D show histograms constructed from the times of activation for individual points on the preparation (the data shown in the colour maps). Green histograms represent activation in normal Krebs solution and red histograms activation in the presence of 0.5 mm heptanol. The widths of these histograms at 20% of peak levels were used as a measure of the total activation time of the preparation. Note the considerably more pronounced broadening of the histogram in response to heptanol in the diabetic heart (inset in D) compared to the control heart (inset in B). The colour scale in the isochronal maps shows the time of activation at each point on the preparation, with red corresponding to the points activated the earliest. All times are with reference to the onset of the stimulus pulse for example (a point with time of activation=5 ms is activated 5 ms after the onset of the stimulus pulse). Activation data were averaged over approximately 20 activation cycles.
These results suggest that even though baseline conduction velocity may not be significantly slower in hearts from short-term diabetic rats due to the large conduction reserve, these hearts are compromised, so that an additional challenge such as exposure to the gap junction uncoupler heptanol causes greater slowing in diabetic than in control hearts.
Earlier work by Weingart & Bukauskas (1998) showed that long chain n-alkanols reduce intercellular conductance with increasing potency as chain length increases. In control experiments we found that hexanol affected propagation significantly less than heptanol. For example, 0.75 mm heptanol (n=4) slowed conduction velocity by a factor of 1.39 ± 0.08, while 0.75 mm hexanol (n=3) slowed conduction by 1.11 ± 0.05 (P < 0.03 compared with heptanol). While small non-specific effects of heptanol cannot be ruled out completely, this observation is consistent with the work of Weingart & Bukauskas (1998) and provides some evidence that the slowing of conduction in response to heptanol in this work is primarily due to the gap junction-blocking effects of heptanol.
The propagation of the cardiac impulse depends on a combination of parameters involving cell excitability as well as gap junction properties. We therefore tested whether, in addition to the greater effects on conduction (in diabetic hearts) following exposure to gap junction uncouplers, there were also differences when cell excitability was reduced.
Effect of reduced excitability
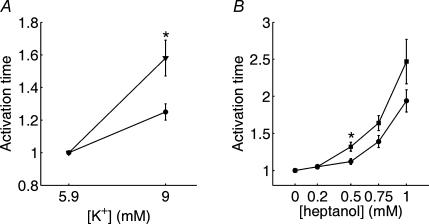
The subsequent set of experiments compared the effects of reducing cell excitability by elevated potassium on propagation in hearts from control and diabetic rats. In these experiments, we compared the effects of changing potassium from 5.9 to 9 mm. As anticipated, this procedure caused a slowing of activation time in six control hearts by a factor of 1.25 ± 0.05. In seven diabetic hearts, this procedure caused a slowing of activation time by a factor of 1.58 ± 0.11, which is significantly (P < 0.02) larger. The effect of elevated potassium on activation time was completely reversible (not shown). Since an established complication of diabetes is an abnormal elevation of potassium levels (Jarman & Mather, 2003), these effects of elevated potassium on propagation in the diabetic heart are of importance for further understanding of diabetes-related cardiac pathology. Our results show that two independent manoeuvres, one affecting intercellular communication and one affecting cellular excitability, reveal impairment in the propagation of the cardiac impulse in the diabetic heart. Data showing effects on activation times of elevated potassium and heptanol are summarized in Fig. 3.
Figure 3.
Summary data showing the effect of increased [K+] (A) and increasing concentrations of heptanol on the activation time (width of histograms shown in Fig. 3) (B). Activation times for each preparation were normalized to the value obtained in normal Krebs solution to account for differences in heart size. *Statistical significance (t test with P < 0.05). Slowing in response to elevated [K+], as well as heptanol application, was significantly more pronounced in diabetic hearts than in control hearts under the same conditions. Data shown are based on n=6–7 for [K+] (A) and n=8–9 for heptanol concentrations of 0.5 mm or less (B). Data for heptanol concentrations greater than 0.5 mm are based on n=4–6 due to lower success rate (complete conduction failure in some preparations) at these higher concentrations.
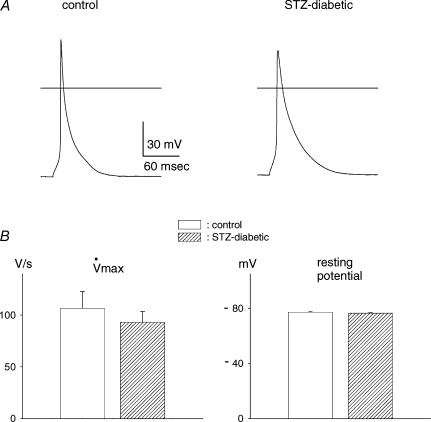
Conduction velocity depends on a number of factors (Shaw & Rudy, 1997; Kanno & Saffitz, 2001), including cellular excitability. Excitability depends largely on sodium current. In the next series of experiments we compared the maximal rate of rise (upstroke velocity) of the action potential (V˙max), an index of the sodium current, in single myocytes from control and diabetic rats. Action potential durations are longer in myocytes from diabetic rats due to attenuated potassium currents (e.g. Shimoni et al. 1998). However, maximal upstroke velocities were found to be comparable, indicating that there was no significant difference in sodium current amplitude. The mean values were 106.7 ± 16.0 V s−1 (n=12) in control cells, and 92.9 ± 10.6 V s−1 (n=15) in cells from diabetic rats (P > 0.45). This result, as well as sample action potentials, is shown in Fig. 4.
Figure 4.
A, sample action potentials recorded from single ventricular myocytes obtained from a control (left) and a diabetic (right) rat. Horizontal lines depict 0 mV level. B, summary data for maximal upstroke rate (left) and resting potentials (right) for cells from control (open columns) and diabetic (hatched columns) rats. No significant differences were measured in either parameter.
In these cells, there were no differences in the resting potential, which may also affect excitability. Mean values were –77.1 ± 0.8 mV for control and –76.5 ± 0.4 mV in cells from diabetic rats. Earlier work also showed that a further factor affecting excitability, the inward rectifier K+ current IK1, is not altered in the diabetic state (Shimoni et al. 1998).
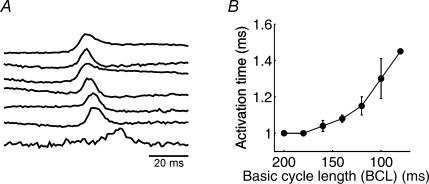
Finally, we investigated the dependence of activation time on the rate of stimulation, to determine whether full recovery from refractoriness occurs during our measurements. In these experiments we used diabetic rat hearts, in which the longer action potentials (Shimoni, 2001) are more likely to interfere with conduction at high stimulation rates. The results show that activation times are unaffected until cycle lengths approach 150 ms, with a delay in onset of activation at shorter cycle lengths. Figure 5A shows one example, with similar time to onset of activation at cycle lengths of 200, 180 and 160 ms (top 3 traces). Activation onset is increasingly delayed at cycle lengths of 140–80 ms (lower traces). Figure 5B shows the mean activation times at these cycle lengths.
Figure 5. Effect of stimulation rate on onset of activation.
A, action potentials (in a heart from a diabetic rat) obtained from one pixel at cycle lengths of 200–80 ms (top to bottom, 20 ms intervals). The first discernible delay occurs at 140 ms. B, summary data obtained from 3 hearts.
Gap junction organization
The results presented so far suggest that functional conduction reserve is compromised in the diabetic hearts. We next proceeded to investigate whether there is an underlying structural disruption of gap junction organization. We investigated several aspects of gap junction organization, comparing hearts from control and diabetic rats. The focus of study was connexin 43 (Cx43), the major component of mammalian ventricular gap junctions.
Connexin 43
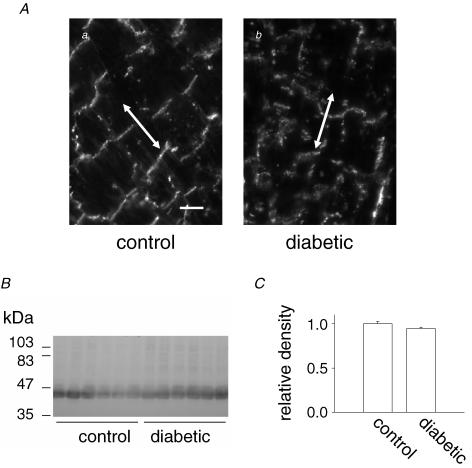
The first experiments were done on thin ventricular sections from control and diabetic rats, labelled with anti-Cx43. As shown in Fig. 6A, the organization of intercalated discs was significantly altered 7 days following STZ injection. The number of discrete discs was reduced, and Cx43 redistributed along the sides of cells, rather than at the ends, a process termed lateralization (Kostin et al. 2003; Akar et al. 2005). Interestingly, Western blotting (Fig. 6B and C) indicated that following this short duration of diabetes total Cx43 expression was not altered.
Figure 6.
A, thin sections of the left ventricle from a non-diabetic (left panel) and STZ-diabetic (right panel) rat heart showing the structural changes associated with diabetes. Sections were labelled with anti-Cx43 (primary antibody) and secondary antibody conjugated to Cy3. Scale bar in right panel represents 20 μm; arrows are drawn parallel to the long axis of the cells in these sections. B, Western blot of ventricular cardiac myocyte homogenates from control and diabetic rats labelled with anti-Cx43. Molecular mass is shown to the left. C, analysis of the Western blot shown in B. Densities of the bands labelled with anti-Cx43 were normalized to the total protein transferred to the blot (determined from densitometric analysis of the blot after labelling of all proteins with amido black). Results are expressed relative to the mean normalized density of the Cx43 bands from the control samples. The relative density of the Cx43 bands from the control and diabetic samples were not significantly different from one another (P=0.11).
In previous reports relating to other cardiac pathologies, changes in Cx43 distribution were quantified by measuring Cx43 area relative to total myocyte or tissue area (Saffitz et al. 2000; Kostin et al. 2003), or by measuring co-localization with another gap junction component, N-cadherin (Akar et al. 2005). However, the first method depends on a reduction in total Cx43, as indeed occurs in diverse cardiac pathologies (Kostin et al. 2003; Akar et al. 2005). The second method depends on the response of other proteins to the pathological conditions. In our study, total Cx43 expression is unchanged. Instead, substantial disorganization develops, apparent as lateralization of Cx43. The complex morphology of the cardiac tissue from diabetic animals (evident in the thin section shown in Fig. 6) makes it difficult to examine the extent of lateralization in thin sections, due to difficulty in identification of cell boundaries in these sections. We therefore devised an independent method of quantifying lateralization (as described in Methods), and compared it in isolated cells from control and diabetic rats. Examples of cells from control and diabetic ventricles are shown in Fig. 7. Intercalated discs are surrounded by white rectangles, as shown in panels b′ and c′.
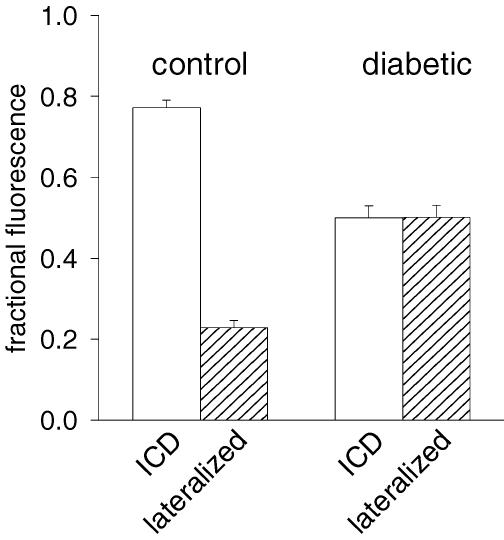
Figure 8 shows results obtained by comparing cells from control (40 cells) and from diabetic (41 cells) rats. Whereas the fraction of fluorescence associated with lateralized Cx43 is low in control conditions (0.22 ± 0.02), this fraction is very significantly (P < 0.0005) augmented (to 0.50 ± 0.03) in diabetic conditions, as shown in Fig. 8.
Figure 8. Summary of analysis of lateralization.
The fraction of anti-Cx43 immunofluorescence associated with the intercalated discs and that associated with lateralized Cx43 was determined as described in the text. Results shown were compiled from two separate cell preparations from non-diabetic and two from diabetic rats. Forty individual control cell images and 41 STZ cell images were analysed. The fraction of lateralized Cx43 fluorescence was significantly higher in the STZ cells than that in the control cells (P < 0.0005).
Earlier work suggested that laterally located Cx43 can still form functional intercellular connections. Thus, an important issue to determine was whether the redistribution of Cx43 we measured maintains functional gap junctions, or whether this process is at least partially responsible for the functional impairment in propagation properties.
A number of studies using long-term cultures of adult or neonatal rat cardiac myocytes have provided evidence that the adhesion proteins N-cadherin and the catenins (including β-catenin) form complexes at the developing intercalated discs that proceed, and are required for, the subsequent formation of functional gap junctions (see Hertig et al. 1996a,b; Toyofuku et al. 1998; Kostin et al. 1999; Wu et al. 2003 and references therein). It was also recently reported that alteration of N-cadherin–catenin complexes disrupts gap junctional communication in the intact heart (Li et al. 2005). If the lateralized Cx43 in myocytes from diabetic rat hearts is associated with functional gap junctions, one would thus expect N-cadherin and connexins to co-localize with the lateralized Cx43. To test this, we carried out dual label immunofluorescence experiments in which we examined the distribution of N-cadherin and Cx43, as well as β-catenin and Cx43 in myocytes from diabetic rats. The results of these experiments are shown in Figs 9 and 10. It is evident in these figures that, although some lateralized Cx43 co-localizes with N-cadherin (red arrows in Fig. 9Ad) and β-catenin (red arrows in Fig. 10Ad), there is more lateralized Cx43 that is not co-localized with N-cadherin or β-catenin (green arrows in Fig. 9Ad and Fig. 10Ad). Conventional (rather than confocal) immunofluorescence images were captured to increase the depth of field along the Z-axis. This reduced the possibility that Cx43 did not appear to be co-localized with N-cadherin or β-catenin because the latter proteins were labelled but out of the plane of focus in the images. To further rule out this possibility, we collected multiple image planes from the dual-labelled cells. As can be seen in Figs 9B and 10B, even when multiple image planes were examined, some of the lateralized Cx43 was not associated with N-cadherin or β-catenin.
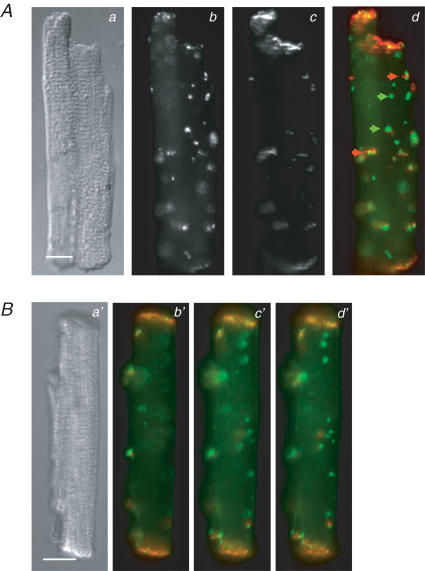
Figure 9. Distribution of N-cadherin and connexin 43 in isolated left ventricular cardiac myocytes from STZ-diabetic rats.
A, differential interference contrast (DIC) micrograph of an isolated myocyte (a) and immunofluorescence images showing the distribution of N-cadherin (b) and Cx43 (c) in the myocyte. Panel d is an overlay of the images in b (pseudo-coloured red) and c (pseudo-coloured green). Green arrows in c point to lateralized Cx43 labelling that is not co-localized with N-cadherin; red arrows point to lateralized Cx43 labelling that is co-localized with N-cadherin labelling. B, DIC micrograph (a′) and three image planes (b′–d′) of a second cell dual-labelled with anti-connexin 43 (green) and anti-N-cadherin (red). Cells were dual-labelled as described in Methods; scale bars in a and a′ represent 10 μm. Cells were dual-labelled with polyclonal anti-connexin 43 (secondary antibody was conjugated to Alexa fluor 488) and monoclonal anti-N-cadherin (secondary antibody was conjugated to Cy3).
Figure 10. Distribution of β-catenin and Cx43 in isolated left ventricular cardiac myocytes from STZ-diabetic rats.
A, DIC micrograph of an isolated myocyte (a) and immunofluorescence images showing the distribution of β-catenin (b) and Cx43 (c) in the myocyte. Panel d is an overlay of the images in b (pseudo-coloured red) and c (pseudo-coloured green). Green arrows in c point to lateralized Cx43 labelling that is not co-localized with β-catenin; red arrows point to lateralized Cx43 labelling that is co-localized with β-catenin labelling. B, DIC micrograph (a′) and three image planes (b′–d′) of a second cell dual-labelled with anti-Cx43 (green) and anti-β-catenin (red). Cells were dual-labelled as described in Methods; scale bars in a and a′ represent 10 μm. Cells were dual-labelled with polyclonal anti-Cx43 (secondary antibody was conjugated to Alexa fluor 488) and monoclonal anti-β-catenin (secondary antibody was conjugated to Cy3).
These results indicate that our observation of functional impairment in propagation is due at least in part to the lateralization of non-functional Cx43 that reduces the pool of this protein at the intercalated discs.
Contribution of additional factors to conduction
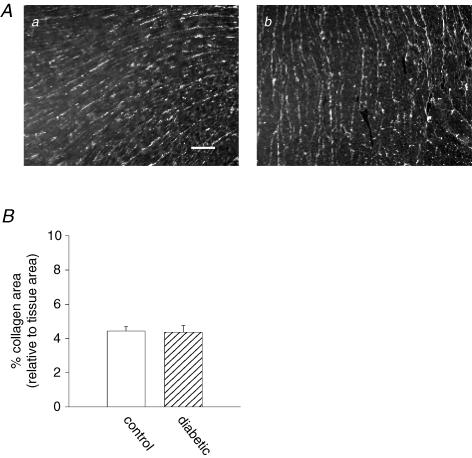
In the final part of this work, we considered the potential contribution of additional factors that impact propagation of the cardiac impulse. An important determinant is the extracellular resistivity, determined by the composition of the interstitial matrix. It is well-documented that among the complications of diabetes is the development of cardiac fibrosis (Westermann et al. 2006; Loganathan et al. 2006), a major contributor to cardiac dysfunction in diabetes (Bollano et al. 2006). However, fibrosis was measured after 8–12 weeks in these studies. We investigated whether changes in collagen could be detected as early as 7–14 days after STZ injection, which would contribute to reduction in conduction reserve in the diabetic hearts. Ventricular tissue from control and diabetic rats was labelled with antibodies directed against collagen I and III, and analysis of immunofluorescence labelling was performed, as described in Methods. Figure 11 shows that after 1–2 weeks, no differences could be detected in collagen III labelling. Similar results were obtained with collagen I (not shown).
Figure 11. Analysis of the collagen content of ventricular tissue from control and diabetic rats.
Aa, immunofluorescence image of a thin section from a control rat ventricle labelled with anti-collagen III; Ab, similarly labelled image from a diabetic rat ventricle (scale bar in a, 100 μm). B, summary of analysis of images of anti-collagen III-labelled ventricular sections. Results are expressed as percentage of tissue area occupied by collagen III. Area occupied by collagen in the control sections was not significantly different from that occupied by collagen in the diabetic sections (P=0.9). Thirty control and 30 diabetic images were analysed (10 of each were collected with a ×40 objective and 20 with a ×10 objective).
A final consideration was given to changes in cell size. Cell geometry has been shown to be one of the determinants of conduction velocity (Spach et al. 2004). However, the relationship is complex, with reports showing either an increase or a decrease in velocity with hypertrophy (McIntyre & Fry, 1997: Toyoshima et al. 1982). The direction of change in velocity, if present, appears to depend on concomitant changes in the distribution and conduction of gap junctions (Spach et al. 2004). In the present study, we compared the area and length of myocytes isolated from the left ventricles of control and diabetic rats (7–14 days after STZ). The mean cell area of control myocytes (see Methods) was 2484 ± 93 μm2 (n=110), whereas in cells from diabetic rats the mean area was 2156 ± 81 μm2 (n=100, P < 0.009). The mean cell length of myocytes from control rats was 97 ± 2 μm (n=111), with a mean cell length of 84 ± 2 μm (n=114) in cells from diabetic rats (P < 0.0005). Thus, both area and lengths of myocytes from diabetic animals were significantly lower that those of myocytes from control animals. Concordant with these results, measurements of cell capacitance (see Methods) showed a reduction in cells from diabetic hearts, compared to controls. Mean capacitance of control cells was 82.3 ± 2.3 pF (n=108), whereas 11–14 days after the onset of diabetes, mean cell capacitance was 65.5 ± 1.5 pF (n=99, P < 0.00001).
Discussion
Summary of results
The present study provides the first detailed investigation of propagation of the cardiac impulse in diabetes. The study shows that in a rat model of (short-term) insulin-dependent diabetes, conduction reserve is impaired. Although there is no substantial reduction in baseline conduction velocity, both exposure to the gap junction uncoupler heptanol and elevation of extracellular potassium levels (reduced cellular excitability) produce a greater slowing of propagation in diabetic, in comparison to control, hearts (Figs 1–3). These observations are associated with substantial changes in the organization of Cx43, a key component of gap junctions (Figs 7–9). The main feature of these changes is the enhanced lateralization of Cx43, which has also been reported in other cardiac pathologies such as heart failure (Kostin et al. 2003). This enhanced lateralization of Cx43 is evident even after only a short duration (7–14 days) of diabetic conditions, a time at which total Cx43 expression is unchanged. The partial dissociation between lateralized Cx43 and two other major components of gap junctions, N-cadherin and β-catenin (Figs 9 and 10), suggests that lateralization results in non-functional junctions and an overall reduction in intercellular coupling. The similarity of action potential upstroke in cells from control and diabetic hearts (Fig. 4) suggests that no major differences exist in sodium current.
Interpretation of results
Despite the lack of demonstrable change in baseline conduction velocity, hearts from diabetic rats were clearly more susceptible to perturbations of either intercellular coupling or excitability. The gap junction uncoupler heptanol (0.5 mm) caused significantly greater slowing in diabetic hearts than in control hearts. Application of hexanol produced a much smaller conduction slowing than that produced by the same concentration of heptanol. This supports the assumption that the primary effect of heptanol under our experimental conditions is uncoupling of gap junctions (rather than other non-specific effects of n-alkanols), since Weingart & Bukauskas (1998) showed hexanol to be much less effective as an uncoupler than heptanol. Similarly, elevation of extracellular potassium concentration to 9 mm caused a significantly greater conduction slowing in diabetic hearts than in controls. Our interpretation is that this reduced conduction reserve is due to reduced intercellular coupling, caused by disorganization (lateralization) of Cx43. Since the lateralized Cx43 appears to at least partially dissociate from other functionally important junction proteins, much of the lateralized Cx43 probably does not form functional intercellular connections. Lateralization of Cx43 in the diabetic heart is thus likely to result in an overall reduction in intercellular coupling, but unlikely to specifically enhance intercellular coupling in the transverse direction despite the increased lateral presence of Cx43. Consistent with this hypothesis, the measurements reported here assess changes in overall conduction velocity without regard to fibre orientation.
As an alternative, it is possible that changes in cellular excitability could underlie the greater susceptibility of diabetic hearts to conduction slowing. However, the similarity of the maximal rates of action potential upstroke in myocytes from control and diabetic hearts suggests that sodium currents are not greatly altered in short-term diabetes. As suggested by Shaw & Rudy (1997) and shown by van Veen et al. (2005), only very severe attenuation of sodium currents will affect propagation. Our earlier work (Shimoni et al. 1998) showed that the inward rectifier K+ current IK1, another determinant of excitability, is unchanged in diabetes. In combination, these results show that the baseline excitability of cells is unaltered by short-term diabetic conditions.
Conduction velocity also depends on extracellular resistivity, which can change following development of fibrosis (Candido et al. 2003). However, we detected no changes in collagen I or III after 7–14 days of diabetes (Fig. 11), suggesting no fibrosis and no significant changes in extracellular resistivity. The involvement of fibrosis in longer-term diabetes will be examined in future studies. Our morphological measurements showed that 7–14 days after onset of diabetes there is a reduction in cell length and cross-sectional area. Reduced cell length would increase the number of gap junctions per unit length, and thus increase overall intracellular resistance per unit length. An increase in intracellular resistance, whether it is due to reduced intercellular coupling or reduced cell length, will result in reduced conduction reserve or even reduced baseline conduction velocity (Spach et al. 2004). We cannot, based on the measurements reported here, rule out a contribution from reduced cell length to the reduced conduction reserve.
Redistribution of gap junction proteins
The changes in gap junction organization, expressed as an enhancement in lateralization of Cx43, seem to be a common feature of different pathologies. This suggests that common factors such as oxidative stress may be underlying triggering mechanisms, although this was not directly tested. Our method of quantifying lateralization has some advantage over previously used methods in that it does not depend on an overall reduction in Cx43 expression (as in Kostin et al. 2003), or on a comparison with other junction proteins (as in Akar et al. 2005). Although lateral Cx43 may enable functional intercellular communication, our results obtained by co-labelling Cx43 with either N-cadherin or with β-catenin show that some of the lateralized Cx43 cannot be part of functional gap junctions. This suggests that lateralization of Cx43, resulting in non-functional gap junctions and an overall reduction in intercellular coupling, is a major contributor to the reduced conduction reserve in the diabetic heart.
Potential clinical significance
The observation that conduction velocity in diabetic hearts is more sensitive to conditions known to reduce intercellular coupling is of great importance since a wide variety of disruptions in cellular homeostasis reduce gap junction coupling. These include hypoxia, elevated calcium, acidosis, etc. (Shaw & Rudy, 1997; Turner et al. 2005; Zeevi-Levin et al. 2005; Dakin et al. 2005), perturbations which are common in ischaemia. Presumably, based on the results with heptanol, such conditions would slow propagation in the diabetic heart more than in controls. Earlier work (animal and human studies) showing longer QRS durations (and reduced Cx43 expression) in diabetic conditions was done after longer durations than in the present study (Inoguchi et al. 2001; Okruhlicova et al. 2002). Given the reduced conduction reserve demonstrated in the present work, it is possible that a longer duration of diabetes would produce a more substantial slowing of conduction in comparison to controls. This could arise due to development of longer term processes such as fibrosis (Candido et al. 2003) or to cumulative progressive damage caused, for example by advanced glycation products (Schmidt & Stern, 2000).
Our results also show that a reduction in excitability by elevation of extracellular potassium levels has more profound effects on the diabetic heart. Elevated potassium levels, which are a common feature of diabetes (Jarman & Mather, 2003), produce a larger slowing of conduction in diabetic hearts, compared to controls. This result may have wider implications. For example, physical stress such as exercise often entails elevation in potassium levels (Paterson, 1996). Some of the possible deleterious effects of elevated potassium are normally offset by catecholamines (Paterson, 1996). However, abundant evidence suggests diminished release of, and response to, catecholamines in diabetes (Casis & Echevarria, 2004). Unopposed elevation in potassium could thus pose an additional risk under diabetic conditions, leading to an enhanced slowing of propagation, compared to control hearts. This could partly underlie reports of exercise-induced cardiac dysfunction in diabetes (Scognamiglio et al. 2000).
The fact that a substantial change in normal gap junction organization does not attenuate baseline conduction velocity (or increase activation time, the parameter used in this study) supports earlier ideas regarding a large ‘conduction reserve’ (Shaw & Rudy, 1997; van Rijen et al. 2005; van Veen et al. 2005). Regardless of the underlying causes, an augmented slowing of propagation in the diabetic heart would interfere with the synchronized activation of cardiac contraction. This would contribute to the reduction in cardiac output associated with diabetes. Furthermore, gap junction disruption can lead to re-entrant ventricular arrhythmias (Peters et al. 1997), even with small, localized changes (Spach & Josephson, 1994). This may contribute to the increased incidence of arrhythmias observed in diabetes.
Reduced cell size
Changes in cell dimensions in the context of diabetes are a complex issue. In general, diabetes leads to apoptosis and a reduction in LV mass (Ghosh et al. 2005; Fiordaliso et al. 2006). Body weight also decreases (or increases at a slower than normal rate). The LV mass/body weight ratio is often used as an index of cardiac pathology. This index does not change during the first 2 weeks after STZ injection (Hoit et al. 1999), which coincides with our experimental protocol. Reports indicating that, in the longer term, there is some increase in cell volume compensating for cell loss (Fiordaliso et al. 2006) are not applicable to the present study.
In addition, effects of type 1 diabetes are complicated by the concurrence of hyperglycaemia and insulin deficiency. Lack of cardiac insulin receptors reduces cell size, indicating the trophic effects of insulin (Belke et al. 2002). The insulin deficiency in the STZ model may thus be a major contributor to the decrease in LV mass and cell size. In our experiments, the reduction in cell size 1 week after STZ injection was assessed by two different methods – as a reduction in cell capacitance and by morphometry. It is possible that longer term diabetic conditions would lead to some compensatory hypertrophy. This will have to be examined in future studies.
Reduced cell length would result in more gap junctions per unit length of myocardium. Assuming that the gap junctional resistance remains constant, this would translate to a higher intracellular resistance per unit length, and would be expected to lead to reduced conduction reserve or conduction velocity slowing. As discussed by Spach et al. (2004) prediction of conduction velocity changes in myocardial remodelling requires knowledge of both changes in gap junctional conductance and cell size. Intracellular resistance is the sum of the resistance of the cytoplasm inside the cells, and that of the gap junctions connecting the cells. A change in intracellular resistance can come about through a change in either (or both) of these components. Since we are unable to manipulate cell size during an experiment, it is not possible to distinguish the two based on measurements of overall conduction velocity (activation time). Our immunofluorescence results clearly indicate that there is a reorganization of Cx43 in the diabetic hearts, which we believe is a major contributor to the reduced conduction reserve. However, we cannot rule out that there is also a significant contribution from the reduced cell size.
Limitations
Great care must be taken in applying results from animal studies to human disease. However, many of the pathological aspects of the diabetic heart have been found to be common to patients and animal models. These include electrocardiographic changes such as QT and QRS prolongation (Yang et al. 1990; Celiker et al. 1994; Christensen et al. 2000; Rossing et al. 2001; Inoguchi et al. 2001), as well as activation of cardiac autocrine systems, such as the renin–angiotensin system (Frustaci et al. 2000; Shimoni, 2001). It is possible that the lack of a convincing change in baseline activation time in diabetic hearts is due to methodological considerations. Diabetic hearts were on average smaller than controls, which would tend to reduce activation time. Our methodology accounts for this difference in size by normalizing activation times for each preparation to the value obtained in normal Krebs solution. However, in the process, any small differences that may exist between diabetic and control hearts may be masked.
In conclusion, the diabetic heart has a substantially smaller conduction reserve than the healthy heart, leaving it vulnerable to conduction disturbances and arrhythmias caused by challenges such as ischaemia or exercise. Our results suggest that the underlying mechanism is compromised intercellular coupling in the diabetic heart due to disorganization (enhanced lateralization) of Cx43 and its dissociation from other junction proteins. Our results suggest a potentially major complication of diabetes, which has been insufficiently recognized to date.
Acknowledgments
Y.S. and G.K. were supported by grants from the Canadian Institutes of Health Research (CIHR, grant nos 68907 and 12557). A.N. was supported by an operating grant from the Natural Sciences and Engineering Research Council (NSERC). Y.S., G.K. and A.N. were also supported by grants from the Heart and Stroke Foundation of Canada. We would like to thank Dr R. Clark for his valuable and helpful suggestions.
Supplemental material
The online version of this paper can be accessed at:
DOI: 10.1113/jphysiol.2006.123729
http://jp.physoc.org/cgi/content/full/jphysiol.2006.123729/DC1 and contains 3 files of supplemental material as follows. (1) A Word file consisting of
Animations
Analysis method
Data recorded from a diabetic heart in normal Krebs solution
Simulated data illustrating the robustness of the activation time method. (2) AVI animation file: Control animation. (3) AVI animation file: Diabetic animation.
This material can also be found as part of the full-text HTML version available from http://www.blackwell-synergy.com
References
- Abo K, Ishida Y, Yoshida R, Hozumi T, Ueno H, Shiotani H, Matsunaga K, Kazumi T. Torsade de pointes in NIDDM with long QT intervals. Diabetes Care. 1996;19:1010. doi: 10.2337/diacare.19.9.1010. [DOI] [PubMed] [Google Scholar]
- Akar FG, Spragg DD, Tunin RS, Kass DA, Tomaselli GF. Mechanisms underlying conduction slowing and arrhythmogenesis in nonischemic dilated cardiomyopathy. Circ Res. 2005;95:717–725. doi: 10.1161/01.RES.0000144125.61927.1c. [DOI] [PubMed] [Google Scholar]
- Baker LC, Wolk R, Choi BR, Watkins S, Plan P, Shah A, Salama G. Effects of mechanical uncouplers, diacetyl monoxime, and cytochalasin-D on the electrophysiology of perfused mouse hearts. Am J Physiol Heart Circ Physiol. 2004;287:H1771–H1779. doi: 10.1152/ajpheart.00234.2004. [DOI] [PubMed] [Google Scholar]
- Belke DD, Betuing S, Tuttle MJ, Graveleau C, Young ME, Pham M, Zhang D, Cooksey RC, McClain DA, Litwin SE, Taegtmeyer H, Severson D, Kahn CR, Abel ED. J Clin Invest. 2002;109:629–639. doi: 10.1172/JCI13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollano E, Omerovic E, Svensson H, Waagstein F, Fu M. Cardiac remodelling rather than disturbed myocardial energy metabolism is associated with cardiac dysfunction in diabetic rats. Int J Cardiol. 2006;114:195–201. doi: 10.1016/j.ijcard.2006.01.027. [DOI] [PubMed] [Google Scholar]
- Candido R, Srivastava P, Cooper ME, Burrell LM. Diabetes mellitus: a cardiovascular disease. Curr Opin Invest Drugs. 2003;4:1088–1094. [PubMed] [Google Scholar]
- Casis O, Echevarria E. Diabetic cardiomyopathy: electromechanical cellular alterations. Curr Vasc Pharmacol. 2004;2:237–248. doi: 10.2174/1570161043385655. [DOI] [PubMed] [Google Scholar]
- Celiker A, Akinci A, Ozin B. The signal-averaged electrocardiogram in diabetic children. Int J Cardiol. 1994;44:271–274. doi: 10.1016/0167-5273(94)90291-7. [DOI] [PubMed] [Google Scholar]
- Christensen PK, Gall MA, Major-Pedersen A, Sato A, Rossing P, Breum L, Pietersen A, Kastrup J, Parving HH. QTc interval length and QT dispersion as predictors of mortality in patients with non-insulin-dependent diabetes. Scand J Clin Lab Invest. 2000;60:323–332. doi: 10.1080/003655100750046486. [DOI] [PubMed] [Google Scholar]
- Dakin K, Zhao Y, Li WH. LAMP, a new imaging assay of gap junctional communication unveils that Ca2+ influx inhibits cell coupling. Nat Methods. 2005;2:55–62. doi: 10.1038/nmeth730. [DOI] [PubMed] [Google Scholar]
- Danik SB, Liu F, Zhang J, Suk HJ, Morley GE, Fishman GI, Gutstein DE. Modulation of cardiac gap junction expression and arrhythmic susceptibility. Circ Res. 2004;95:1035–1041. doi: 10.1161/01.RES.0000148664.33695.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mello WC. Impaired cell communication in the diabetic heart. The role of the renin angiotensin system. Mol Cell Biochem. 2006 doi: 10.1007/s11010-006-9297-1. 10.1007/s11010-006-9297-1. [DOI] [PubMed] [Google Scholar]
- Efimov IR, Nikolski VP, Salama G. Optical imaging of the heart. Circ Res. 2004;95:21–33. doi: 10.1161/01.RES.0000130529.18016.35. [DOI] [PubMed] [Google Scholar]
- El-Atat FA, McFarlane SI, Sowers JR, Bigger JT. Sudden cardiac death in patients with diabetes. Curr Diab Rep. 2004;4:187–193. doi: 10.1007/s11892-004-0022-8. [DOI] [PubMed] [Google Scholar]
- Fang ZY, Prins JB, Marwick TH. Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr Rev. 2004;25:543–567. doi: 10.1210/er.2003-0012. [DOI] [PubMed] [Google Scholar]
- Fiordaliso F, Cuccovillo I, Bianchi R, Bai A, Doni M, Salio M, De Angelis N, Ghezzi P, Latini R, Masson S. Cardiovascular oxidative stress is reduced by an ACE inhibitor in a rat model of streptozotocin-induced diabetes. Life Sci. 2006;79:121–129. doi: 10.1016/j.lfs.2005.12.036. [DOI] [PubMed] [Google Scholar]
- Fonarow GC. An approach to heart failure and diabetes mellitus. Am J Cardiol. 2005;96:47E–52E. doi: 10.1016/j.amjcard.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, Nadal-Ginard B, Anversa P. Myocardial cell death in human diabetes. Circ Res. 2000;87:1123–1132. doi: 10.1161/01.res.87.12.1123. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Pulinilkunnil T, Yuen G, Kewalramani G, An D, Qi D, Abrahani A, Rodrigues B. Cardiomyocyte apoptosis induced by short-term diabetes requires mitochondrial GSH depletion. Am J Physiol Heart Circ Physiol. 2005;289:H768–H776. doi: 10.1152/ajpheart.00038.2005. [DOI] [PubMed] [Google Scholar]
- Girouard SD, Laurita KR, Rosenbaum DS. Unique properties of cardiac action potentials recorded with voltage-sensitive dyes. J Cardiovasc Electrophysiol. 1996;7:1024–1038. doi: 10.1111/j.1540-8167.1996.tb00478.x. [DOI] [PubMed] [Google Scholar]
- Guerrero PA, Schuessler RB, Davis LM, Beyer EC, Johnson CM, Yamada KA, Saffitz JE. Slow ventricular conduction in mice heterozygous for a connexin43 null mutation. J Clin Invest. 1997;99:1991–1998. doi: 10.1172/JCI119367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertig CM, Butz S, Koch S, Eppenberger-Eberhardt M, Kemler R, Eppenberger HM. N-cadherin in adult rat cardiomyocytes in culture. II. Spatio-temporal appearance of proteins involved in cell-cell contact and communication. Formation of two distinct N-cadherin/catenin complexes. J Cell Sci. 1996b;109:11–20. doi: 10.1242/jcs.109.1.11. [DOI] [PubMed] [Google Scholar]
- Hertig CM, Eppenberger-Eberhardt M, Koch S, Eppenberger HM. N-cadherin in adult rat cardiomyocytes in culture. I. Functional role of N-cadherin and impairment o cell-cell contact by a truncated N-cadherin mutant. J Cell Sci. 1996a;109:1–10. doi: 10.1242/jcs.109.1.1. [DOI] [PubMed] [Google Scholar]
- Hoit BD, Castro C, Bultron G, Knight S, Matlib MA. Noninvasive evaluation of cardiac dysfunction by echocardiography in streptozotocin-induced diabetic rats. J Card Fail. 1999;5:324–333. doi: 10.1016/s1071-9164(99)91337-4. [DOI] [PubMed] [Google Scholar]
- Howarth FC, Jacobson M, Shafiullah M, Adeghate E. Long-term effects of streptozotocin-induced diabetes on the electrocardiogram, physical activity and body temperature in rats. Exp Physiol. 2005;90:827–835. doi: 10.1113/expphysiol.2005.031252. [DOI] [PubMed] [Google Scholar]
- Inoguchi T, Yu HY, Imamura M, Kakimoto M, Kuroki T, Maruyama T, Nawata H. Altered gap junction activity in cardiovascular tissues of diabetes. Med Electron Microsc. 2001;34:86–91. doi: 10.1007/s007950170002. [DOI] [PubMed] [Google Scholar]
- Jarman PR, Mather HM. Diabetes may be independent risk factor for hyperkalemia. Br Med J. 2003;327:147–149. doi: 10.1136/bmj.327.7418.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno S, Saffitz JE. The role of myocardial gap junctions in electrical conduction and arrhythmogenesis. Cardiovasc Pathol. 2001;10:169–177. doi: 10.1016/s1054-8807(01)00078-3. [DOI] [PubMed] [Google Scholar]
- Kleber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev. 2004;88:431–488. doi: 10.1152/physrev.00025.2003. [DOI] [PubMed] [Google Scholar]
- Kostin S, Hein S, Bauer PE, Schaper J. Spatiotemporal development and distribution of intercellular junctions in adult rat cardiomyocytes in culture. Circ Res. 1999;85:154–167. doi: 10.1161/01.res.85.2.154. [DOI] [PubMed] [Google Scholar]
- Kostin S, Rieger M, Dammer S, Hein S, Richter M, Klovekorn WP, Bauer EP, Schaper J. Gap junction remodeling and altered connexin43 expression in the failing human heart. Mol Cell Biochem. 2003;242:135–144. [PubMed] [Google Scholar]
- Kuroki T, Inoguchi T, Umeda F, Ueda F, Nawata H. High glucose induces alteration of gap junction permeability and phosphorylation of connexin-43 in cultured aortic smooth muscle cells. Diabetes. 1998;47:931–936. doi: 10.2337/diabetes.47.6.931. [DOI] [PubMed] [Google Scholar]
- Laird DW. Connexin phosphorylation as a regulatory event linked to gap junction internalization and degradation. Biochim Biophys Acta. 2005;1711:172–182. doi: 10.1016/j.bbamem.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Li J, Patel VV, Kostetskii I, Xiong Y, Chu AF, Jacobson JT, Yu C, Morley GE, Molkentin JD, Radice GL. Cardiac-specific loss of N-cadherin leads to alteration in connexins with conduction slowing and arrhythmogenesis. Circ Res. 2005;97:474–481. doi: 10.1161/01.RES.0000181132.11393.18. [DOI] [PubMed] [Google Scholar]
- Loganathan R, Bilgen M, Al-Hafez B, Smirnov IV. Characterization of alterations in diabetic myocardial tissue using high resolution MRI. Int J Cardiovasc Imaging. 2006;22:81–90. doi: 10.1007/s10554-005-5386-6. [DOI] [PubMed] [Google Scholar]
- McIntyre H, Fry CH. Abnormal action potential conduction in isolated human hypertrophied left ventricular myocardium. J Cardiovasc Electrophysiol. 1997;8:887–894. doi: 10.1111/j.1540-8167.1997.tb00850.x. [DOI] [PubMed] [Google Scholar]
- Mooradian AD. Cardiovascular disease in type 2 diabetes mellitus. Arch Int Med. 2003;163:33–40. doi: 10.1001/archinte.163.1.33. [DOI] [PubMed] [Google Scholar]
- Morley GE, Vaidya D, Samie FH, Lo C, Delmar M, Jalife J. Characterization of conduction in the ventricles of normal and heterozygous Cx43 knockout mice using optical mapping. J Cardiovasc Electrophysiol. 1999;10:1361–1375. doi: 10.1111/j.1540-8167.1999.tb00192.x. [DOI] [PubMed] [Google Scholar]
- Nygren A, Clark RB, Belke DD, Kondo C, Giles WR, Witkowski FX. Voltage-sensitive dye mapping of activation and conduction in adult mouse hearts. Ann Biomed Eng. 2000;28:958–967. doi: 10.1114/1.1308501. [DOI] [PubMed] [Google Scholar]
- Nygren A, Kondo C, Clark RB, Giles WR. Voltage-sensitive dye mapping in Langendorff-perfused rat hearts. Am J Physiol Heart Circ Physiol. 2003;284:H892–H902. doi: 10.1152/ajpheart.00648.2002. [DOI] [PubMed] [Google Scholar]
- Okruhlicova L, Tribulova N, Misejkova M, Kucka M, Stetka R, Slezak J, Manoach M. Gap junction remodelling is involved in the susceptibility of diabetic rats to hypokalemia-induced ventricular fibrillation. Acta Histochem. 2002;104:387–391. doi: 10.1078/0065-1281-00675. [DOI] [PubMed] [Google Scholar]
- Paterson DJ. Role of potassium in the regulation of systemic physiological function during exercise. Acta Physiol Scand. 1996;156:287–294. doi: 10.1046/j.1365-201X.1996.190000.x. [DOI] [PubMed] [Google Scholar]
- Peters NS, Corimilas J, Severs NJ, Wit AL. Disturbed Cx43 gap junction distribution correlates with the location of reentrant circuits in the epicardial border zone of healing canine infarcts that cause ventricular tachycardia. Circulation. 1997;95:988–996. doi: 10.1161/01.cir.95.4.988. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Sinovas A, Garcia-Dorado D, Ruiz-Maena M, Soler-Soler J. Enhanced effect of gap junction uncouplers on macroscopic electrical properties of reperfused myocardium. J Physiol. 2004;559:245–257. doi: 10.1113/jphysiol.2004.065144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossing P, Breum L, Major-Pedersen A, Sato A, Winding H, Pietersen A, Kastrup J, Parving HH. Prolonged QTc interval predicts mortality in patients with Type 1 diabetes mellitus. Diabet Med. 2001;18:199–205. doi: 10.1046/j.1464-5491.2001.00446.x. [DOI] [PubMed] [Google Scholar]
- Saffitz JE, Green KG, Kraft WJ, Schechtman KB, Yamada K. Effects of diminished expression of connexin43 on gap junction number and size in ventricular myocardium. Am J Physiol Heart Circ Physiol. 2000;278:H1662–H1670. doi: 10.1152/ajpheart.2000.278.5.H1662. [DOI] [PubMed] [Google Scholar]
- Schmidt AM, Stern DM. RAGE: a new target for the prevention and treatment of the vascular and inflammatory complications of diabetes. Trends Endocrinol Metab. 2000;11:368–375. doi: 10.1016/s1043-2760(00)00311-8. [DOI] [PubMed] [Google Scholar]
- Scognamiglio R, Casara D, Avogaro A. Myocardial dysfunction and adrenergic innervation in patients with Type 1 diabetes mellitus. Diabetes Nutr Metab. 2000;13:346–349. [PubMed] [Google Scholar]
- Scollan DF, Holmes A, Zhang J, Winslow RL. Reconstruction of cardiac ventricular geometry and fiber orientation using magnetic resonance imaging. Ann Biomed Eng. 2000;28:934–944. doi: 10.1114/1.1312188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson DL, Aasum E, Belke DD, Larsen TS, Semeniuk LM, Shimoni Y. Atherosclerosis, Hypertension and Diabetes. Boston, USA: Kluwer Academic Publishers; 2003. Diabetes and cardiac dysfunction. [Google Scholar]
- Shaw RM, Rudy Y. Ionic mechanisms of propagation in cardiac tissue. Circ Res. 1997;81:727–741. doi: 10.1161/01.res.81.5.727. [DOI] [PubMed] [Google Scholar]
- Shimoni Y. Inhibition of the formation or action of angiotensin II reverses attenuated K+ currents in type 1 and type 2 diabetes. J Physiol. 2001;537:83–92. doi: 10.1111/j.1469-7793.2001.0083k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoni Y, Ewart HS, Severson D. Type I and II models of diabetes produce different modifications of K+ currents in rat heart: role of insulin. J Physiol. 1998;505:485–496. doi: 10.1111/j.1469-7793.1998.485bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoni Y, Hunt D, Chen KY, Emmett T, Kargacin G. Differential autocrine modulation of atrial and ventricular potassium currents and of oxidative stress in diabetic rats. Am J Physiol Heart Circ Physiol. 2006;290:H1879–H1888. doi: 10.1152/ajpheart.01045.2005. [DOI] [PubMed] [Google Scholar]
- Shimoni Y, Hunt D, Chuang M, Chen KY, Kargacin G, Severson D. Modulation of potassium currents by angiotensin and oxidative stress in cardiac cells from the diabetic rat. J Physiol. 2005;567:177–190. doi: 10.1113/jphysiol.2005.090639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spach MS, Heidlage JF, Barr RC, Dolber PC. Cell size and communication: role in structural and electrical development and remodeling of the heart. Heart Rhythm. 2004;4:500–515. doi: 10.1016/j.hrthm.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Spach MS, Josephson ME. The role of nonuniform anisotropy in small circuits. J Cardiovasc Electrophysiol. 1994;5:182–209. doi: 10.1111/j.1540-8167.1994.tb01157.x. [DOI] [PubMed] [Google Scholar]
- Teunissen BE, Jongsma HJ, Bierhuizen MF. Regulation of myocardial connexins during hypertrophic remodeling. Eur Heart J. 2004;25:1979–1989. doi: 10.1016/j.ehj.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Hori M, Tada M. Direct association of the gap junction protein connexin-43 with ZO-1 in cardiac myocytes. J Biol Chem. 1998;273:12725–12731. doi: 10.1074/jbc.273.21.12725. [DOI] [PubMed] [Google Scholar]
- Toyoshima H, Park YD, Ishikawa Y, Nagata S, Hirata Y, Sakakibara H, Shimomura K, Nakayama R. Effect of ventricular hypertrophy on conduction velocity of activation front in the ventricular myocardium. Am J Cardiol. 1982;49:1938–1945. doi: 10.1016/0002-9149(82)90213-2. [DOI] [PubMed] [Google Scholar]
- Turner MS, Haywood GA, Andreka P, You L, Martin PE, Evans H, Webster KA, Bishopric NH. Reversible connexin 43 dephosphorylation during hypoxia and reoxygenation is linked to cellular ATP levels. Circ Res. 2005;95:726–733. doi: 10.1161/01.RES.0000144805.11519.1e. [DOI] [PubMed] [Google Scholar]
- van Rijen HVM, de Bakker JMT, van Veen TAB. Hypoxia, electrical uncoupling, and conduction slowing: role of conduction reserve. Cardiovasc Res. 2005;66:9–11. doi: 10.1016/j.cardiores.2005.02.003. [DOI] [PubMed] [Google Scholar]
- van Rijen HV, Eckardt D, Degen J, Theis M, Ott T, Willecke K, Jongsma HJ, Opthof T, de Bakker JM. Slow conduction and enhanced anisotropy increase the propensity for ventricular tachyarrhythmias in adult mice with induced deletion of connexin43. Circulation. 2004;109:1048–1055. doi: 10.1161/01.CIR.0000117402.70689.75. [DOI] [PubMed] [Google Scholar]
- van Veen TAB, Stein M, Royer A, Quang KL, Charpentier F, Colledge WH, Huang CLH, Wilders R, Grace AA, Escande D, de Bakker JMT, van Rijen HVM. Impaired impulse propagation in Scn5a-knockout mice. Circulation. 2005;112:1927–1935. doi: 10.1161/CIRCULATIONAHA.105.539072. [DOI] [PubMed] [Google Scholar]
- Weingart R, Bukauskas FF. Long-chain n-alkanols and arachidonic acid interfere with the Vm-sensitive gating mechanism of gap junction channels. Pflugers Arch. 1998;435:310–319. doi: 10.1007/s004240050517. [DOI] [PubMed] [Google Scholar]
- Westermann D, Rutschow S, Van Linthout S, Linderer A, Bucker-Gartner C, Sobirey M, Riad A, Pauschinger M, Schultheiss HP, Tschope C. Inhibition of p38 mitogen-activated protein kinase attenuates left ventricular dysfunction by mediating pro-inflammatory cardiac cytokine levels in a mouse model of diabetes mellitus. Diabetologia. 2006;49:2507–2513. doi: 10.1007/s00125-006-0385-2. [DOI] [PubMed] [Google Scholar]
- Wu JC, Tsai RY, Chung TH. Role of catenins in the development of gap junctions in rat cardiomyocytes. J Cell Biochem. 2003;88:823–835. doi: 10.1002/jcb.10390. [DOI] [PubMed] [Google Scholar]
- Xu Z, Patel KP, Lou MF, Rozanski GJ. Up-regulation of K+ channels in diabetic rat ventricular myocytes by insulin and glutathione. Cardiovasc Res. 2002;53:80–88. doi: 10.1016/s0008-6363(01)00446-1. [DOI] [PubMed] [Google Scholar]
- Yamada KA, Rogers JG, Sundset R, Steinberg TH, Saffitz JE. Up-regulation of connexin45 in heart failure. J Cardiovasc Electrophysiol. 2003;14:1205–1212. doi: 10.1046/j.1540-8167.2003.03276.x. [DOI] [PubMed] [Google Scholar]
- Yang Q, Kiyoshige K, Fujimoto T, Katayama M, Fujino K, Saito K, Nakaya Y, Mori H. Signal-averaging electrocardiogram in patients with diabetes mellitus. Jpn Heart J. 1990;31:25–33. doi: 10.1536/ihj.31.25. [DOI] [PubMed] [Google Scholar]
- Zeevi-Levin N, Barac YD, Reisner Y, Reiter I, Yaniv G, Meiry G, Abassi Z, Kostin S, Schaper J, Rosen MR, Resnick N, Binah O. Gap junctional remodeling by hypoxia in cultured neonatal rat ventricular myocytes. Cardiovasc Res. 2005;66:64–73. doi: 10.1016/j.cardiores.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Animations
Analysis method
Data recorded from a diabetic heart in normal Krebs solution
Simulated data illustrating the robustness of the activation time method. (2) AVI animation file: Control animation. (3) AVI animation file: Diabetic animation.