Abstract
In several neuronal types of the CNS, glutamate and GABA receptors mediate a persistent current which reflects the presence of a low concentration of transmitters in the extracellular space. Here, we further characterize the tonic current mediated by ambient glutamate in rat hippocampal slices. A tonic current of small amplitude (53.99 ± 6.48 pA at +40 mV) with the voltage dependency and the pharmacology of NMDA receptors (NMDARs) was detected in virtually all pyramidal cells of the CA1 and subiculum areas. Manipulations aiming at increasing d-serine or glycine extracellular concentrations failed to modify this current indicating that the glycine binding sites of the NMDARs mediating the tonic current were saturated. In contrast, non-transportable inhibitors of glutamate transporters increased the amplitude of this tonic current, indicating that the extracellular concentration of glutamate primarily regulates its magnitude. Neither AMPA/kainate receptors nor metabotropic glutamate receptors contributed significantly to this tonic excitation of pyramidal neurons. In the presence of glutamate transporter inhibitors, however, a significant proportion of the tonic conductance was mediated by AMPA receptors. The tonic current was unaffected when inhibiting vesicular release of transmitters from neurons but was increased upon inhibition of the enzyme converting glutamate in glutamine in glial cells. These observations indicate that ambient glutamate is mainly of glial origin. Finally, experiments with the use-dependent antagonist MK801 indicated that NMDARs mediating the tonic conductance are probably extra-synaptic NMDARs.
Concentration of transmitters in the extracellular space of the central nervous system is determined by a balance between release, degradation and uptake mechanisms. During fast synaptic transmission, vesicular release of neurotransmitters such as glutamate and GABA leads to a rapid rise of neurotransmitter concentration which reaches the millimolar range within the synaptic cleft (Clements, 1996). Diffusion and efficient uptake by membrane-bound transporters ensure a rapid decay of the transmitter concentration in the cleft and a minimal spread of transmitter to neighbouring synapses (for review see Bergles et al. 1999; Attwell & Gibb, 2005). It is thus generally assumed that between each episode of synaptic activation the concentration of transmitter within and outside the cleft is maintained at a very low level thereby preventing continuous activation or desensitization of receptors. However, microdialysis experiments suggest that in vivo the ambient concentration of amino acids such as glutamate, glycine and GABA reaches a low micromolar range, i.e. a concentration value sufficiently high to activate several types of glutamate and GABA receptors (Cavelier et al. 2005). Accordingly, tonic currents mediated by the activation of GABAA receptors have been recorded in different cerebellar and cortical neurons (Semyanov et al. 2004; Farrant & Nusser, 2005). This tonic conductance shows cell-type specific differences in magnitude and pharmacology, changes during postnatal development and is mediated by extrasynaptic receptors. In vitro but also in vivo electrophysiological studies indicate that tonic GABAA receptor-mediated inhibition influences synaptic integration during sensory processing (Hamann et al. 2002; Chadderton et al. 2004). In comparison, the role of ambient glutamate has been much less studied. Yet, tonic activation of NMDA receptors (NMDARs) by ambient glutamate has been observed in pyramidal and granule cells of the hippocampus in vitro (Sah et al. 1989; Dalby & Mody, 2003; Angulo et al. 2004; Cavelier & Attwell, 2005). Moreover, blocking glutamate uptake in organotypic cultures (Jabaudon et al. 1999) and in acute slices (Cavelier & Attwell, 2005) of the hippocampus unmask a similar excitatory tonic current which most likely results from a glial release of glutamate. Although the identification of the release mechanism awaits for the development of more specific pharmacological tools (Cavelier & Attwell, 2005), the presence of ambient glutamate raises several questions on its physiological or pathological roles. On the one hand, Sah and co-workers proposed that this tonic current modulates the input/output function of CA1 neurons (Sah et al. 1989). On the other hand, if the receptors mediating this tonic excitation are extra-synaptic, as those mediating tonic inhibition (see above), they may play a crucial role in triggering cell death (Hardingham et al. 2002). In the present study, we aimed at further characterizing the receptors responsible for the tonic excitatory current observed in CA1 pyramidal cells and the source of the ambient glutamate activating these receptors.
Methods
Slice preparation
All experiments followed European Union and institutional guidelines for the care and use of laboratory animals (Council directive 86/609EEC). Fourteen- to 29-day-old Wistar rats were either anaesthetized with an intraperitoneal injection of a mixture of ketamine (65 mg kg−1) and xylazine (14 mg kg−1) or humanely killed by cervical dislocation and decapitated. Transverse hippocampal slices (400 μm) were cut in an oxygenated ice-cold solution containing (mm): 235 sucrose, 2.5 KCl, 1.25 NaH2PO4, 0.5 CaCl2, 7 MgCl2, 20 glucose, 26 NaHCO3, 5 pyruvate. They were incubated at 34°C for 30 min and then maintained at room temperature for 0.5–4 h in an oxygenated physiological solution containing (mm): 126 NaCl, 2.5 KCl, 1.25 NaH2PO4, 2 CaCl2, 1 MgCl2, 20 glucose, 26 NaHCO3, 5 pyruvate. Finally the slices were transferred into a recording chamber perfused at 2.5 ml min−1 with the same solution. Recordings were performed at room temperature except during some experiments performed at 35°C as indicated in the Results section.
For experiments aiming at inhibiting the vacuolar H+-ATPase the slices were incubated for 2.5 h at 34°C in the physiological solution containing 4 μm of bafilomycin A1; the control slices were from the same rat and were incubated in the same conditions but without bafilomycin A1. The same procedure was followed for experiments aiming at inhibiting the glutmamine synthase with l-methionine sulfoximine (MSO; 5 mm) except that the incubation time varied from 1 to 3 h.
Electrophysiology
Pyramidal neurons from the subiculum or the CA1 regions were visually identified by means of infrared videomicroscopy. Whole-cell recordings were performed at a holding potential of +40 mV with an intracellular solution containing (mm): 100 caesium gluconate, 10 TEACl, 4 NaCl, 1 MgCl2, 10 Hepes, 10 BAPTA, 5 phosphocreatine, 2 ATP, 0.3 GTP; the pH was adjusted to 7.3 with CsOH. For recordings performed at −60 mV the intracellular solution contained (mm): 130 potassium gluconate, 10 Hepes, 4 NaCl, 1 MgCl2, 10 phosphocreatine, 4 ATP, 0.3 GTP, 0.2 EGTA; and the pH was adjusted to 7.3. With these intracellular solutions patch pipettes had a resistance of 3–5 MΩ. All potentials were corrected for a junction potential of −10 mV.
The current–voltage relationship of the tonic current was obtained by subtracting the mean of three current responses to a voltage ramp from +40 to −80 mV recorded in the presence of d-AP5 (50 μm) to that recorded just before the application of the antagonist. In experiments aimed at studying evoked synaptic currents, a surgical cut was made between CA3 and CA1. Evoked EPSCs recorded at +40 mV in the presence of 100 μm picrotoxin and 10 μm NBQX were elicited by a monopolar electrode placed in a patch pipette filled with the extracellular solution and positioned in the stratum radiatum. Short (100 μs) current pulses were delivered at a rate of 0.1 Hz and stimulus intensity was adjusted to maintain the EPSC amplitude in the range 50–150 pA.
Data collection and analysis
Membrane currents were recorded using an Axopatch 200B (Axon Instruments) amplifier. They were filtered at 2–5 kH, digitized at 5–20 kHz with a 1322A Digidata (Axon Instruments). Series resistance was not compensated but was regularly monitored throughout the experiment using a −1 mV step and recordings showing unstable (> 20%) series resistance were rejected. Acquisitions and off-line analysis were performed using pClamp9 softwares (Axon Instruments). The amplitude of the synaptic currents was determined by measuring the difference between the mean of the current during 5 ms just before stimulation artefacts and the mean of the current during a 5 ms period around the peak of the EPSCs. When the effects of a drug on the synaptic and on the tonic NMDAR-mediated currents had to be compared, responses were normalized to the amplitude of the currents blocked by a saturating concentration of d-AP5 or MK801. The effect of MK801 on synaptic current was determined by comparing the mean amplitude of the 10 last EPSCs in control conditions to the amplitude of the first EPSC after stimulation resumption.
For evaluating statistical differences between two samples Student's t test was performed; when more than two samples were compared an ANOVA test was used, followed by Dunnett's or Tukey tests. Differences were considered to be significant if P < 0.05. Values are given as mean ± s.e.m., and n refers to the number of cells.
Drugs
Tetrodotoxin (TTX) was purchased from Latoxan (Valence, France). Sarcosine, picrotoxin and l-methionine sulfoximine (MSO) were purchased from Sigma. [±]-cis-1-[Phenanthren-2yl-carbonyl]piperazine-2,3-dicarboxylic acid (PPDA) was a generous gift from D. T. Monaghan. This competitive antagonist of NMDARs has a slightly better affinity for NMDAR2C- or 2D-containing receptors (Lozovaya et al. 2004; Feng et al. 2004). [(R)-[(S)-1-(4-Bromo-phenyl)-ethylamino] - (2,3-dioxo-1,2,3,4-tetrahydroquinoxalin-5-yl)-methyl]-phosphonic acid (NVP-AAM077) was a generous gift from Novartis. At a concentration of 0.1 μm this compound is selective for NMDAR2A-containing NMDARs (Auberson et al. 2002; Liu et al. 2004; Berberich et al. 2005). (2S)-2-Amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-(xanth-9-yl) propanoic acid (LY341495), 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[F]quinoxaline-7-sulphonamine disodium salt (NBQX disodium salt), 7-chloro-4-hydroxyquinoline-2-carboxilic acid (7-chlorokynurenic acid), d-(−)-2-amino-5-phosphonopentanoic acid (d-AP5), (5S,10R)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate (MK801), d-serine, 6-chloro-3,4-dihydro-3 - (5-norbornen-2-yl) - 2H-1,2,4-benzothiazidianzine-7-sulphonamide-1,1-dioxide (cyclothiazide), dl-threo-β-benzyloxyaspartic acid (TBOA), 2S,3S,4R - 2 - carboxy - 4 - isopropyl - 3 - pyrrolidineacetic acid (DHK, dihydrokainic acid), bafilomycin A1 and 2-(4-benzylpiperidino) - 1 - (4 - hydroxyphenyl) - 1 - propanol hemitartrate (ifenprodil hemitartrate) were purchased from Tocris Cookson (Bristol, UK).
Results
Tonic activation of NMDARs in hippocampal pyramidal neurons
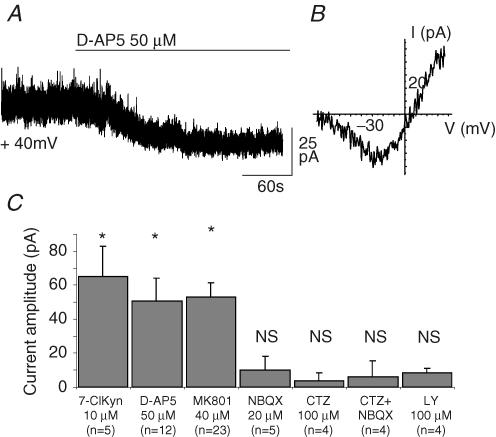
We recorded pyramidal cells of the CA1 and subiculum regions in acute slices of the rat hippocampus. At a holding potential of +40 mV and in the presence of extracellular Mg2+, TTX (0.5–1 μm) and picrotoxin (100 μm), application of a saturating concentration of the NMDAR antagonist d-AP5 (50 μm) blocked a tonic current that had a mean amplitude of 50.8 ± 13.4 pA (n=12, Fig. 1A). Application of two other NMDAR blockers, MK801 (40 μm) and 7-Cl-KYN (10 μm), revealed a tonic current comparable to that observed with d-AP5 (respectively 53.2 ± 8.3 pA, n=23 and 65.3 ± 17.7 pA, n=5, Fig. 1C). This pharmacological profile suggests that the tonic current results from the activation of NMDARs by ambient glutamate rather than from spontaneous openings of these channels. Indeed, competitive antagonists, such as d-AP5, are less efficient at reducing single-channel currents due to spontaneous openings than ligand-activated currents (Turecek et al. 1997). The tonic current reversed at +5.18 ± 4.43 mV (n=6) and showed the expected inward rectification of NMDA channels at membrane potentials between −80 and −30 mV (Fig. 1B). Near resting membrane potential, the tonic current was barely detectable whereas it reached −27.6 ± 6.46 pA at −30 mV (n=6). Neither NBQX (40 μm, n=5), which blocks both AMPA and kainate ionotropic receptors, nor LY341495 (100 μm, n=4), a non-selective antagonist at all glutamate metabotropic receptors, decreased the amplitude of the tonic current. Since AMPARs could be fully desensitized by prolonged applications of glutamate we also reduced desensitization of these receptors by applying cyclothiazide (100 μm). However, neither cyclothiazide alone nor NBQX applied in the presence of cyclothiazide (n=4) had any effect on the holding current of pyramidal cells (Fig. 1C). These results indicate that NMDARs were the only glutamate receptor type contributing to the tonic current recorded in pyramidal cells.
Figure 1. Tonic activation of NMDA receptors in hippocampal neurons.
A, bath application of the NMDAR antagonist d-AP5 (50 μm) blocks a tonic current in a CA1 pyramidal neuron held at +40 mV. B, current–voltage relationship of the d-AP5-sensitive tonic current. C, NMDAR antagonists 7-chlorokynurenate (7-ClKyn, 10 μm), d-AP5 (50 μm), and MK801 (40 μm) significantly reduce the tonic current while the AMPA/kainate receptor antagonist NBQX (20 μm), the inhibitor of the AMPA receptor desensitization cyclothiazide (CTZ, 100 μm), and the metabotropic glutamate receptor antagonist LY 341495 (LY, 100 μm) have no effect on the holding current. In all figures, the holding potential was +40 mV and * indicates a P < 0.05.
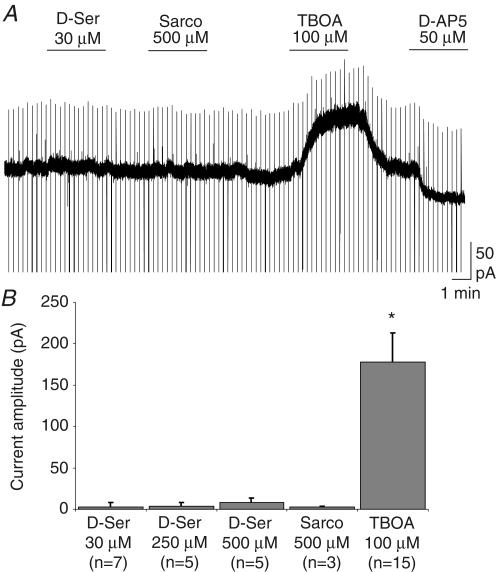
The activation of NMDARs requires the binding of two co-agonists, i.e. glutamate and glycine or d-serine (Johnson & Ascher, 1987; Mothet et al. 2000). We therefore tested whether changes in the ambient concentration of glycine or d-serine might modulate the amplitude of the tonic current. Figure 2 shows that neither the application of d-serine (30 μm, n=7; 250 μm, n=5; 500 μm, n=5) nor that of sarcosine (500 μm, n=3), which inhibits the uptake of glycine (Lopez-Corcuera et al. 1998), changed the amplitude of the holding current. As exemplified in Fig. 2A, after wash-out of these two compounds, we checked for the presence of the tonic current by applying a glutamate transporter blocker (TBOA, see below) and/or d-AP5. We also tested the effect of d-serine on evoked NMDAR-mediated EPSCs elicited by the stimulation of the Schaffer collaterals. We observed that d-serine (30 μm) did not change the mean amplitude of these EPSCs both at room temperature (+14.2 ± 8.3%; P > 0.5; n=4) and at 34°C (+7.0 ± 8.3%; P > 0.5; n=8; data not shown). These observations indicate that the glycine binding sites of NMDARs mediating the tonic and the synaptic current are fully saturated.
Figure 2. The NMDAR-mediated tonic current is not sensitive to increases in the concentration of ambient glycine or D-serine.
A, applications of d-serine (D-Ser, 30 μm) and of the glycine transporter inhibitor sarcosine (Sarco, 500 μm) had no effect on the holding current while the glutamate transporter inhibitor TBOA (100 μm) induced a large tonic current. B, histogram of the effects of d-serine (30, 250, 500 μm), sarcosine (500 μm) and TBOA (100 μm) on the holding current of pyramidal cells.
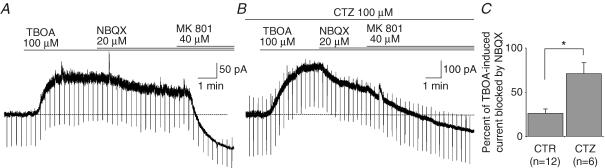
The selective activation of NMDARs is probably due to a level of ambient glutamate too low to activate substantially other types of receptors. One way to test this hypothesis is to increase the concentration of ambient glutamate by blocking its uptake with TBOA (Shimamoto et al. 1998). As shown in Figs 2A and 3A, TBOA (100 μm) greatly increased the tonic current (ITBOA=177.8 ± 35.1 pA; n=15). The other non-transportable blocker of glutamate uptake, DHK (300 μm), also increased the tonic current but to a lesser extent (IDHK=63.8 ± 8.95 pA, n=3; data not shown) and we therefore concentrated on the effect of TBOA. NBQX (20 μm), which had no effect on the basal tonic current (Fig. 1C), had a variable but significant effect on the current induced by TBOA (Fig. 3A). On average this antagonist of AMPA/kainate receptors blocked 26.0 ± 4.76% (n=12; Fig. 3C) of the TBOA-induced current. We then tested whether this effect of NBQX was enhanced during inhibition of AMPAR desensitization by cyclothiazide. As shown in Fig. 3B, the percentage block of the TBOA-induced current by NBQX was increased to an average value of 70.9 ± 12.5% (n=6; Fig. 3C) in the presence of cyclothiazide. In control as well as in cyclothiazide-treated slices, the part of the TBOA-induced current left unblocked by NBQX was entirely suppressed by MK801, which also blocked a basal tonic current (Fig. 3A and B). These results therefore indicate that the ambient concentration of glutamate is normally too low to activate AMPARs, even when their desensitization is blocked, and that a contribution of these receptors is observed only when the extracellular glutamate concentration rises upon blockade of glutamate transporters.
Figure 3. Tonic activation of AMPARs upon blockade of glutamate transporters.
A, effects on the holding current of TBOA (100 μm), NBQX (20 μm) and MK801 (40 μm). Note that NBQX slightly reduced the amplitude of the tonic current induced by TBOA. B, in another pyramidal cell the effect of NBQX was more pronounced in the presence of cyclothiazide (CTZ, 100 μm). Note that in A and B the holding current in MK801 reached a lower level than before TBOA indicating the presence of a basal tonic current. C, percent of the TBOA-induced current blocked by NBQX in the absence (CTR) and in the presence of cyclothiazide (CTZ).
Glial origin of the ambient glutamate
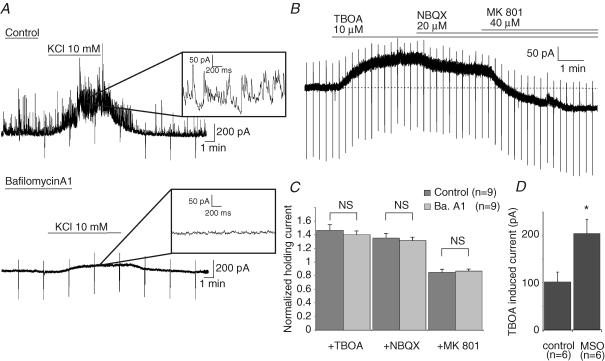
We next asked whether ambient glutamate concentration was dependent upon neuronal activity. We therefore compared the amplitude and the pharmacological profile of the tonic current in control slices and in slices pre-treated with bafilomycin A1, an inhibitor of the vacuolar H+-ATPases. This pump establishes the proton gradient across vesicular membrane which drives transmitter uptake into synaptic vesicles (Drose & Altendorf, 1997). Pre-treatment of the slices for 2.5 h at 34°C with 4 μm bafilomycin A1 (see Methods) totally abolishes spontaneous synaptic currents as well as those evoked by raising extracellular potassium concentration (Fig. 4A). In these conditions, bath application of TBOA (100 μm) still induced an increase of the tonic current which did not differ from that induced in control slices (Fig. 4B and C). Moreover, the effect of NBQX and MK801 on the TBOA-induced current did not differ between control and bafylomycin-treated slices (Fig. 4C). Importantly, as described for control slices, application of MK801 significantly reduced the holding current revealing the presence of a NMDAR-mediated tonic current in bafylomycin A1-pre-treated slices (Fig. 4C). At a holding potential of +40 mV, the average amplitude of this basal tonic current was 65.19 ± 20.99 pA (n=9), a value not significantly different from that of the tonic current recorded in control slices (50.34 ± 11.74 pA; n=9). Therefore, the amplitude of the basal and TBOA-induced currents mediated by NMDARs was not dependent upon vesicular release of glutamate.
Figure 4. Glial origin of the ambient glutamate mediating the tonic activation of NMDARs.
A, the frequency of the synaptic currents recorded in a pyramidal neuron in a control slice bathed in normal extracellular solution was greatly enhanced by the application of 10 mm KCl (top). Pre-incubating the slices with the H+-ATPase inhibitor bafilomycin A1 (4 μm, 2.5 h, 34°C) inhibited spontaneous and KCl-evoked synaptic currents (bottom). Insets show a portion of each recording at a faster time scale. B, the trace shows the effect of TBOA (100 μm), NBQX (20 μm) and MK801 (40 μm) on the tonic current of a pyramidal neuron recorded in bafilomycin A1-treated slice. C, the histogram shows that the effects of TBOA, NBQX and MK801 do not differ significantly between control (dark grey bars) and bafilomycin A1-treated slices (light grey bars). Values of the tonic currents are normalized to the amplitude of the baseline holding current (i.e. before the application of TBOA). D, incubation of the slices in presence of the glutamine synthase inhibitor l-methionine sulfoximine (MSO, 5 mm, 1–3 h, 34°C) increased the TBOA-induced current.
We then tested whether the inhibition of the glutamine synthase modulated the amplitude of the tonic current. This enzyme is responsible for the conversion of glutamate into glutamine in glial cells (Purich, 1998). Inhibiting this enzyme by l-methionine sulfoximine (MSO) in hippocampal slice cultures induces a two-fold decrease of glutamate-like immunoreactivity in neuronal terminal and four-fold increase in glia (Laake et al. 1995). After incubating the slices for 1–3 h in MSO (5 mm; see Methods), however, the amplitude of the basal tonic current was not significantly changed (32.4 ± 2.7 pA in control, 34.7 ± 3.8 pA after incubation in MSO; n=6 in both conditions, P=0.62). This observation could be related to the ability of glutamate transporters to buffer an increase in the extracellular glutamate concentration. In keeping with this hypothesis the amplitude of the current induced by TBOA doubled in slices treated with MSO (109.0 ± 13.4 pA in control versus 213.2 ± 3.79 pA after MSO; n=6 in both conditions; P=0.006, Fig. 4D). These observations indicate that the release of glutamate is increased in MSO-treated slices and thus confirm previous observations indicating that ambient glutamate originates mainly from glial cells (Jabaudon et al. 1999; Cavelier & Attwell, 2005).
Extrasynaptic NMDA receptors mediate tonic current
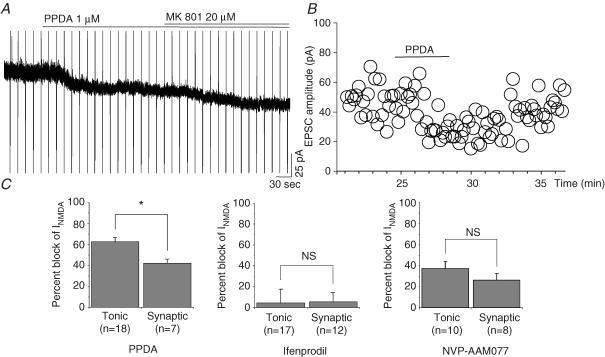
Pharmacological and physiological studies of GABAA receptors mediating the tonic inhibition of cerebellar and hippocampal neurons have provided evidence that these receptors are extra-synaptic and have a subunit composition which differs from that of synaptic GABAA receptors (Semyanov et al. 2004; Farrant & Nusser, 2005). We thus compared the effects of different subunit-selective NMDAR antagonists on the synaptic and tonic currents recorded in CA1 pyramidal neurons. However, the results obtained with PPDA (a NMDAR2C- and D-preferring antagonist), ifenprodil (a NMDAR2B antagonist) and NVP-AAM077 (a NMDAR2A antagonist; see Methods) did not reveal clear different pharmacological profile between synaptic and tonic currents (Fig. 5). PPDA inhibited more the tonic than the synaptic currents (62.7 ± 4.2%, n=18 versus 42.3 ± 3.7%, n=7, respectively; P=0.0092). The larger percentage block of the tonic current by this competitive antagonist could result from a more important contribution of NMDAR2D subunits to this current or from a lower concentration of the glutamate mediating the tonic current relative to that responsible for the synaptic currents. Ifenprodil left unaffected the amplitude of both tonic and synaptic currents (4.5 ± 13.3%, n=19; 5.6 ± 8.9%, n=12, respectively). NVP-AAM077, the NMDAR2A-preferring antagonist, inhibits tonic and synaptic currents in the same proportions (37.0 ± 6.77, n=10; 26.1 ± 6.37%, n=8, respectively; P=0.2647).
Figure 5. Effects of subunits preferring antagonists on the tonic and the synaptic NMDA receptor-mediated currents.
A, PPDA (1 μm), an NMDAR antagonist more selective for NMDAR2C- and NMDAR2D-containing receptors, inhibited a significant portion of the basal NMDAR-mediated tonic current. B, effect of PPDA (1 μm) on the peak amplitude of EPSCs evoked by the stimulation of Schaffer collaterals. C, mean percentage inhibition of PPDA (1 μm), ifenprodil (10 μm), and NVP-AAM077 (0.1 μm) on tonic and evoked synaptic NMDAR-mediated currents (normalization relative to the current blocked by 20 μm MK801 or by 50 μmd-AP5).
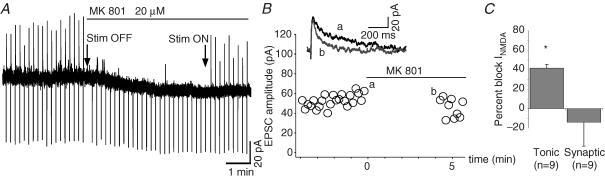
We then tested whether the synaptic and tonic currents were mediated by the same pool of receptors by taking advantage of the use-dependent NMDAR blocker MK801 that has the ability to block only the open NMDAR channels. Evoked EPSCs were collected during a control period (at least 20 min long) to obtain a stable response then the synaptic stimulation was stopped and a saturating concentration of MK801 was bath applied (Fig. 6A). After about 5 min of superfusion, MK801 reduced the tonic current (Fig. 6A and C) indicating that a significant proportion of tonically activated NMDARs had been blocked. At this time, the synaptic stimulation was resumed and the amplitude of the first evoked EPSCs was compared with that of the last EPSCs elicited before the application of MK801 (see Methods). For the cell in Fig. 6A and B, the amplitude of the first EPSC in MK801 was 91.5% of the average response in control condition revealing that in the time lapse of MK801 application, the majority of synaptic NMDARs were closed and thus unaffected by MK801 while a significant proportion of NMDARs mediating the tonic current had been inhibited by MK801. We did not wait until complete block of the tonic current by MK801 before testing the synaptic response to reduce the probability that spontaneous quantal release of glutamate from presynaptic neurons would activate synaptic receptors therefore leading to their blockade by MK801. For nine such experiments, the amplitude of the first EPSC evoked after 5 min in MK801 was not significantly changed (n=9, Fig. 6C) while at the same time the tonic current was reduced on average by 54.9 ± 3.8% (n=9, Fig. 6C). It is worth noting that the decay time constant of the first synaptic currents recorded in the presence of MK801 was faster than in control (inset in Fig. 6B): on average the decay time constant changed from 105.4 ± 21.5 ms in control to 52.6 ± 9.6 ms in MK801 (n=3), as expected from the shortening of the NMDA average open time by the use-dependent channel blocker. These results strongly support the hypothesis that NMDARs responsible for the tonic current are extrasynaptic.
Figure 6. Extrasynaptic NMDARs mediate the tonic current.
Effect of MK801 (20 μm) on the tonic current (A) and on the peak amplitude of the NMDAR-mediated EPSCs evoked by Schaffer collateral stimulations (B) in a pyramidal cell. Note that the synaptic stimulation was ceased (Stim OFF) during the first 4.5 min application of the use-dependent blocker and then resumed (Stim ON), as indicated in A. MK801 failed to affect the amplitude of the first evoked EPSC while progressively inhibited the subsequent ones (B). Time 0 corresponds to stim OFF and MK801 application. The inset shows the superposition of the last EPSC evoked before MK801 application (a) and of the first EPSC elicited after 4.5 min MK801 (b). Note the faster decay time of the EPSC in b. C, comparison of percentage inhibition produced by 4–5 min application of MK801 on the tonic and synaptic NMDAR-mediated currents. Currents are normalized to the amplitude of the currents fully blocked by MK801 or by addition of 50 μm d-AP5.
Discussion
Our results confirm previous reports indicating that ambient glutamate of glial origin tonically activate NMDARs in the hippocampus (Sah et al. 1989; Jabaudon et al. 1999; Dalby & Mody, 2003; Angulo et al. 2004; Cavelier & Attwell, 2005). In addition, the data presented here demonstrate that NMDARs are the only glutamatergic receptor contributing to the basal tonic current and that glutamate, and not glycine or d-serine, concentration controls the amplitude of the NMDAR-mediated tonic current. The results are also consistent with the involvement of extrasynaptic NMDARs mediating this tonic current.
The amplitude of the NMDAR-mediated tonic conductance recorded in our experiments (∼1 nS) falls in between those reported by Sah and co-workers (∼5 nS; Sah et al. 1989) and by Cavelier and Attwell (∼0.15 nS; Cavelier & Attwell, 2005) in CA1 pyramidal cells. These variations may originate partly from differences in the thickness of the slices (400 μm in our case; 225 μm for Cavelier & Attwell; mostly 400 μm for Sah et al. who also tested 100 μm thick slices) and from the depth at which the neurons were recorded. Sah et al. (1989) observed that the tonic current measured in thin slices was smaller than in thick slices and that the current recorded near the surface of thick slices was similar to that recorded in thin slices. Another difference between the experimental conditions of these studies is the age of the animals: Cavelier & Attwell (2005) used young rats (P11 to P13) whereas we used animals between P14 and P29 but most of our recordings were obtained around P18 (the age of the animals used by Sah et al. was not specified). In this context, it is worth noting that the expression of glutamate transporters is not completed before P20 (Kugler & Schleyer, 2004) and that the total concentration of glutamate as determined in vivo by means of NMR spectroscopy doubles between P14 and P21 (and stabilizes thereafter) in the hippocampus (Tkac et al. 2003).
By comparing the amplitude of the tonic current with that of the response induced by a saturating concentration of agonist and using the dose–response curve for NMDARs obtained from cultured neurons (Hill coefficient, nH, of 1.5 and EC50 of 2.3 μm; Patneau & Mayer, 1990), Cavelier & Attwell (2005) estimated an ambient glutamate concentration of 27–33 nm at 25°C, and 77–89 nm at 35°C. Using the value of the tonic current recorded in our experiments and following the same reasoning (for the details of the procedure see the Appendix in Cavelier & Attwell, 2005), we estimated the extracellular concentration of glutamate to reach 83–87 nm at 25°C. These values are far below those reported in vivo by glutamate-sensitive microelectrode array and microdialysis experiments which measured low micromolar (∼2 μm) concentrations of extracellular glutamate (Baker et al. 2002; Montiel et al. 2005). This important built up of ambient glutamate concentration in vivo may be a consequence of the higher synaptic activity observed in vivo than in vitro (Pare et al. 1998). In keeping with this hypothesis, extracellular glutamate measured with glutamate-sensitive electrodes is highly dependent on neuronal action potential propagation (Day et al. 2006). However, microdialysis measurements in vivo led to a different picture in which the ambient glutamate is not dependent on action potential activity (Baker et al. 2002). Therefore, the relative contribution of neuronal activity-dependent and -independent mechanisms in establishing the level of ambient glutamate in vivo remains unclear, while in the in vitro slice preparation the tonic current induced by ambient glutamate is totally independent of neuronal activity (see Results). Finally, it is difficult to ascertain that extracellular glutamate is not artefactually low in the slice preparation or that it is artefactually high when measured in vivo with large probes that might damage the tissue and compromise the integrity of the blood–brain barrier (Westergren et al. 1995; see also Cavelier et al. 2005).
Nevertheless, both in in vivo (Day et al. 2006) and in vitro experiments (Jabaudon et al. 1999; Angulo et al. 2004; Cavelier & Attwell, 2005; see also Arnth-Jensen et al. 2002), TBOA increases the concentration of ambient glutamate, indicating that the sodium-dependent glutamate uptake plays an important role in controlling the functional consequences of the tonic release of glutamate. The amplitude of the tonic current recorded in the presence of TBOA was nearly four times larger than in control conditions. This would correspond (see above) to an extracellular glutamate concentration of ∼300 nm. Although still low, this concentration appeared to be sufficient to activate some AMPARs. This relatively high affinity of AMPARs expressed by pyramidal neurons compares favourably with that of GluR1 homomeric receptors expressed in oocytes which start to be activated with glutamate concentrations as low as 100 nm (Dawson et al. 1990; Sakimura et al. 1990). When glutamate uptake is not inhibited, however, neither NBQX nor cyclothiazide have any effect on the tonic current, confirming that the ambient glutamate concentration is below 100 nm in the slices.
The activation of NMDARs requires the presence of two co-agonists, glutamate and glycine or d-serine (Johnson & Ascher, 1987; Mothet et al. 2000). Although manipulating the ambient glutamate concentration with inhibitors of the glutamate uptake readily modulates the tonic current, inhibitors of glycine uptake or applications of exogenous d-serine or glycine do not increase the amplitude of the tonic current. The saturation of the glycine sites of the NMDARs mediating the tonic current might have pointed to a difference between these receptors and those mediating synaptic responses. Indeed, Martina et al. (2003) have shown that the NMDA component of the synaptic responses evoked by the Schaffer collateral stimulation in rat hippocampal slices is potentiated by adding exogenous d-serine. However, in our recording conditions d-serine failed to increase the amplitude of NMDAR-mediated Schaffer collateral EPSCs. The reason of this discrepancy is not clear but our results are in good agreement with those of Ballard et al. (2002) and Scimemi et al. (2004) who also reported that saturating concentrations of d-serine do not increase the amplitude of the synaptic and extra-synaptic NMDAR-mediated responses in mouse CA1 pyramidal neurons.
Pyramidal neurons of CA1 express NR1, NR2A, NR2B and NR2D subunits of the NMDARs (Monyer et al. 1994; Thompson et al. 2002). Our results with NVP-AAM077 and PPDA show that the NMDARs mediating the tonic and the synaptic currents most probably contain NR2A and NR2D subunits. The higher percentage block of the tonic current by PPDA may indicate that NR2D-containing NMDARs contribute more to the tonic current than to the synaptic responses. However, the results obtained with this competitive antagonist may also simply reflect the difference in the glutamate concentrations mediating the tonic and the synaptic currents. Finally, it is difficult to exclude totally a contribution of NR2B subunits to NMDARs mediating the tonic current on the ground of the observation that ifenprodil does not inhibit this current. Indeed, the selectivity of this ‘subunit-selective’ antagonist is not known for all types of NMDARs and the effects of ifenprodil depend also on the glutamate concentration (Neyton & Paoletti, 2006). Thus, our results do not support a difference in the subunit composition of the NMDARs mediating the synaptic and the tonic currents although the experiments with MK801 point toward an extra-synaptic localization of the receptors activated by ambient glutamate.
The tonic current studied here shares many common features with slow transient currents recorded in the same neurons and resulting from a release of glutamate by astrocytes. In particular, both types of currents are mediated by the activation of NMDARs and are independent of neuronal vesicular release (Angulo et al. 2004; Fellin et al. 2004; Perea & Araque, 2005). These similarities suggest that the glutamate originates in both cases from the same source, i.e. astroglial cells. In keeping with this hypothesis, inhibition of the astrocyte-specific enzyme glutamine synthetase (Norenberg & Martinez-Hernandez, 1979), which converts glutamate into glutamine (Ottersen et al. 1992), increases the tonic current observed upon inhibition of glutamate transporters by TBOA, both in acute slices (our present results and Cavelier et al. 2005) and in organotypic cultures (Jabaudon et al. 1999) of the hippocampus. In addition, a similar tonic current mediated by NMDARs has been observed in CA1 pyramidal cells when astrocytic purinergic receptors are activated (Fellin et al. 2006).
Our results are in keeping with the idea that ambient glutamate predominantly originates from glial cells and tonically activates extrasynaptic NMDARs but the physiological role of this ambient glutamate remains unclear. The magnesium block of NMDARs near resting membrane potential implies that the tonic current will operate only at depolarizing potentials. Sah and co-workers showed that blocking the tonic current reduces the action potential firing frequency and the functional coupling between dendritic and somatic compartments of CA1 neurons (Sah et al. 1989). However, in our experimental conditions, i.e. with a 5–10 times smaller tonic current, bath application of d-AP5 failed to significantly change the action potential discharge of CA1 neurons (see Supplementary Fig. 1). Further experiments are therefore needed to test whether the level of ambient glutamate reported here or by Cavelier & Attwell (2005) finely tunes dendritic processing of synaptic inputs. Finally, it has been shown that activation of extrasynaptic receptors can selectively trigger cell death (Hardingham et al. 2002). Our results showing that ambient glutamate primarily originates from astrocytes and targets extrasynaptic NMDARs therefore suggest that astrocytes play a pivotal role in many excitotoxicity-related pathologies.
Acknowledgments
This work was supported by an Inserm Avenir grant (E.A.). K.L.M. is supported by a studentship from the Ministère de l'Education Nationale, de la Recherche et de la Technologie and M.G. was supported by an Inserm postdoctoral fellowship.
Supplemental material
The online version of this paper can be accessed at:
DOI: 10.1113/jphysiol.2006.123570
http://jp.physoc.org/cgi/content/full/jphysiol.2006.123570/DC1 and contains supplemental material showing that d-AP5 does not change the excitability of CA1 pyramidal cells.
This material can also be found as part of the full-text HTML version available from http://www.blackwell-synergy.com
References
- Angulo MC, Kozlov AS, Charpak S, Audinat E. Glutamate released from glial cells synchronizes neuronal activity in the hippocampus. J Neuroscience. 2004;24:6920–6927. doi: 10.1523/JNEUROSCI.0473-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnth-Jensen N, Jabaudon D, Scanziani M. Cooperation between independent hippocampal synapses is controlled by glutamate uptake. Nat Neurosci. 2002;5:325–331. doi: 10.1038/nn825. [DOI] [PubMed] [Google Scholar]
- Attwell D, Gibb A. Neuroenergetics and the kinetic design of excitatory synapses. Nat Rev Neurosci. 2005;6:841–849. doi: 10.1038/nrn1784. [DOI] [PubMed] [Google Scholar]
- Auberson YP, Allgeier H, Bischoff S, Lingenhoehl K, Moretti R, Schmutz M. 5-Phosphonomethylquinoxaline-diones as competitive NMDA receptor antagonists with a preference for the human 1A/2A, rather than 1A/2B receptor composition. Bioorg Med Lett. 2002;12:1099–1102. doi: 10.1016/s0960-894x(02)00074-4. [DOI] [PubMed] [Google Scholar]
- Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neuroscience. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard TM, Pauly-Evers M, Higgins GA, Ouagazzal AM, Mutel V, Borroni E, Kemp JA, Bluethmann H, Kew JN. Severe impairment of NMDA receptor function in mice carrying targeted point mutations in the glycine binding site results in drug-resistant nonhabituating hyperactivity. J Neuroscience. 2002;22:6713–6723. doi: 10.1523/JNEUROSCI.22-15-06713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berberich S, Punnakkal P, Jensen V, Pawlak V, Seeburg PH, Hvalby O, Kohr G. Lack of NMDA receptor subtype selectivity for hippocampal long-term potentiation. J Neuroscience. 2005;25:6907–6910. doi: 10.1523/JNEUROSCI.1905-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles DE, Diamond JS, Jahr CE. Clearance of glutamate inside the synapse and beyond. Curr Opin Neurobiol. 1999;9:293–298. doi: 10.1016/s0959-4388(99)80043-9. [DOI] [PubMed] [Google Scholar]
- Cavelier P, Attwell D. Tonic release of glutamate by a DIDS-sensitive mechanism in rat hippocampal slices. J Physiol. 2005;564:397–410. doi: 10.1113/jphysiol.2004.082131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavelier P, Hamann M, Rossi D, Mobbs P, Attwell D. Tonic excitation and inhibition of neurons: ambient transmitter sources and computational consequences. Prog Biophys Mol Biol. 2005;87:3–16. doi: 10.1016/j.pbiomolbio.2004.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadderton P, Margrie TW, Hausser M. Integration of quanta in cerebellar granule cells during sensory processing. Nature. 2004;428:856–860. doi: 10.1038/nature02442. [DOI] [PubMed] [Google Scholar]
- Clements JD. Transmitter timecourse in the synaptic cleft: its role in central synaptic function. Trends Neurosciences. 1996;19:163–171. doi: 10.1016/s0166-2236(96)10024-2. [DOI] [PubMed] [Google Scholar]
- Dalby NO, Mody I. Activation of NMDA receptors in rat dentate gyrus granule cells by spontaneous and evoked transmitter release. J Neurophysiol. 2003;90:786–797. doi: 10.1152/jn.00118.2003. [DOI] [PubMed] [Google Scholar]
- Dawson TL, Nicholas RA, Dingledine R. Homomeric GluR1 excitatory amino acid receptors expressed in Xenopus oocytes. Mol Pharmacol. 1990;38:779–784. [PubMed] [Google Scholar]
- Day BK, Pomerleau F, Burmeister JJ, Huettl P, Gerhardt GA. Microelectrode array studies of basal and potassium-evoked release of 1-glutamate in the anesthetized rat brain. J Neurochem. 2006;96:1626–1635. doi: 10.1111/j.1471-4159.2006.03673.x. [DOI] [PubMed] [Google Scholar]
- Drose S, Altendorf K. Bafilomycins and concanamycins as inhibitors of V-ATPases and P-ATPases. J Exp Biol. 1997;200:1–8. doi: 10.1242/jeb.200.1.1. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA ceptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron. 2004;43:729–743. doi: 10.1016/j.neuron.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Fellin T, Pozzan T, Carmignoto G. Purinergic receptors mediate two distinct glutamate release pathways in hippocampal astrocytes. J Biol hem. 2006;281:4274–4284. doi: 10.1074/jbc.M510679200. [DOI] [PubMed] [Google Scholar]
- Feng B, Tse HW, Skifter DA, Morley R, Jane DE, Monaghan DT. Structure-activity analysis of a novel NR2C/NR2D-preferring NMDA receptor antagonist: 1-(phenanthrene-2-carbonyl) piperazine-2,3-dicarboxylic acid. Br J Pharmacol. 2004;141:508–516. doi: 10.1038/sj.bjp.0705644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann M, Rossi DJ, Attwell D. Tonic and spillover inhibition of granule cells control information flow through cerebellar cortex. Neuron. 2002;33:625–633. doi: 10.1016/s0896-6273(02)00593-7. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Jabaudon D, Shimamoto K, Yasuda-Kamatani Y, Scanziani M, Gahwiler BH, Gerber U. Inhibition of uptake unmasks rapid extracellular turnover of glutamate of nonvesicular origin. Proc Natl Acad Sci U S A. 1999;96:8733–8738. doi: 10.1073/pnas.96.15.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Kugler P, Schleyer V. Developmental expression of glutamate transporters and glutamate dehydrogenase in astrocytes of the postnatal rat hippocampus. Hippocampus. 2004;14:975–985. doi: 10.1002/hipo.20015. [DOI] [PubMed] [Google Scholar]
- Laake JH, Slyngstad TA, Haug FM, Ottersen OP. Glutamine from glial cells is essential for the maintenance of the nerve terminal pool of glutamate: immunogold evidence from hippocampal slice cultures. J Neurochem. 1995;65:871–881. doi: 10.1046/j.1471-4159.1995.65020871.x. [DOI] [PubMed] [Google Scholar]
- Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- Lopez-Corcuera B, Martinez-Maza R, Nunez E, Roux M, Supplisson S, Aragon C. Differential properties of two stably expressed brain-specific glycine transporters. J Neurochem. 1998;71:2211–2219. doi: 10.1046/j.1471-4159.1998.71052211.x. [DOI] [PubMed] [Google Scholar]
- Lozovaya NA, Grebenyuk SE, Tsintsadze TS, Feng B, Monaghan DT, Krishtal OA. Extrasynaptic NR2B and NR2D subunits of NMDA receptors shape ‘superslow’ afterburst EPSC in rat hippocampus. J Physiol. 2004;558:451–463. doi: 10.1113/jphysiol.2004.063792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina M, Krasteniakov NV, Bergeron R. d-Serine differently modulates NMDA receptor function in rat CA1 hippocampal pyramidal cells and interneurons. J Physiol. 2003;548:411–423. doi: 10.1113/jphysiol.2002.037127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montiel T, Camacho A, Estrada-Sanchez AM, Massieu L. Differential effects of the substrate inhibitor 1-trans-pyrrolidine-2,4-dicarboxylate (PDC) and the non-substrate inhibitor DL-threo-beta-benzyloxyaspartate (DL-TBOA) of glutamate transporters on neuronal damage and extracellular amino acid levels in rat brain in vivo. Neuroscience. 2005;133:667–678. doi: 10.1016/j.neuroscience.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Mothet JP, Parent AT, Wolosker H, Brady RO, Jr, Linden DJ, Ferris CD, Rogawski MA, Snyder SH. d-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proc Natl Acad Sci U S A. 2000;97:4926–4931. doi: 10.1073/pnas.97.9.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyton J, Paoletti P. Relating NMDA receptor function to receptor subunit composition: limitations of the pharmacological approach. J Neuroscience. 2006;26:1331–1333. doi: 10.1523/JNEUROSCI.5242-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norenberg MD, Martinez-Hernandez A. Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Res. 1979;161:303–310. doi: 10.1016/0006-8993(79)90071-4. [DOI] [PubMed] [Google Scholar]
- Ottersen OP, Zhang N, Walberg F. Metabolic compartmentation of glutamate and glutamine: morphological evidence obtained by quantitative immunocytochemistry in rat cerebellum. Neuroscience. 1992;46:519–534. doi: 10.1016/0306-4522(92)90141-n. [DOI] [PubMed] [Google Scholar]
- Pare D, Shink E, Gaudreau H, Destexhe A, Lang EJ. Impact of spontaneous synaptic activity on the resting properties of cat neocortical pyramidal neurons In vivo. J Neurophysiol. 1998;79:1450–1460. doi: 10.1152/jn.1998.79.3.1450. [DOI] [PubMed] [Google Scholar]
- Patneau DK, Mayer ML. Structure-activity relationships for amino acid transmitter candidates acting at N-methyl-D-aspartate and quisqualate receptors. J Neuroscience. 1990;10:2385–2399. doi: 10.1523/JNEUROSCI.10-07-02385.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea G, Araque A. Properties of synaptically evoked astrocyte calcium signal reveal synaptic information processing by astrocytes. J Neuroscience. 2005;25:2192–2203. doi: 10.1523/JNEUROSCI.3965-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purich DL. Advances in the enzymology of glutamine synthesis. Adv Enzymol Relat Areas Mol Biol. 1998;72:9–42. doi: 10.1002/9780470123188.ch2. [DOI] [PubMed] [Google Scholar]
- Sah P, Hestrin S, Nicoll RA. Tonic activation of NMDA receptors by ambient glutamate enhances excitability of neurons. Science. 1989;246:815–818. doi: 10.1126/science.2573153. [DOI] [PubMed] [Google Scholar]
- Sakimura K, Bujo H, Kushiya E, Araki K, Yamazaki M, Yamazaki M, Meguro H, Warashina A, Numa S, Mishina M. Functional expression from cloned cDNAs of glutamate receptor species responsive to kainate and quisqualate. FEBS Lett. 1990;272:73–80. doi: 10.1016/0014-5793(90)80452-o. [DOI] [PubMed] [Google Scholar]
- Scimemi A, Fine A, Kullmann DM, Rusakov DA. NR2B-containing receptors mediate cross talk among hippocampal synapses. J Neuroscience. 2004;24:4767–4777. doi: 10.1523/JNEUROSCI.0364-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Shimamoto K, Lebrun B, Yasuda-Kamatani Y, Sakaitani M, Shigeri Y, Yumoto N, Nakajima T. DL-threo-beta-benzyloxyaspartate, a potent blocker of excitatory amino acid transporters. Mol Pharmacol. 1998;53:195–201. doi: 10.1124/mol.53.2.195. [DOI] [PubMed] [Google Scholar]
- Thompson CL, Drewery DL, Atkins HD, Stephenson FA, Chazot PL. Immunohistochemical localization of N-methyl-D-aspartate receptor subunits in the adult murine hippocampal formation: evidence for a unique role of the NR2D subunit. Brain Res Mol Brain Res. 2002;102:55–61. doi: 10.1016/s0169-328x(02)00183-3. [DOI] [PubMed] [Google Scholar]
- Tkac I, Rao R, Georgieff MK, Gruetter R. Developmental and regional changes in the neurochemical profile of the rat brain determined by in vivo 1H NMR spectroscopy. Magn Reson Med. 2003;50:24–32. doi: 10.1002/mrm.10497. [DOI] [PubMed] [Google Scholar]
- Turecek R, Vlachova V, Vyklicky L., Jr Spontaneous openings of NMDA receptor channels in cultured rat hippocampal neurons. Eur J Neurosci. 1997;9:1999–2008. doi: 10.1111/j.1460-9568.1997.tb01368.x. [DOI] [PubMed] [Google Scholar]
- Westergren I, Nystrom B, Hamberger A, Johansson BB. Intracerebral dialysis and the blood–brain barrier. J Neurochem. 1995;64:229–234. doi: 10.1046/j.1471-4159.1995.64010229.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.