Abstract
A 58-year-old man was admitted with symptoms of lethargy and easy bruising for four months duration. Peripheral blood (PB) analysis revealed a white blood cell count (WBC) of 15.9 × 109/l with monocytes 5.4 × 109/l. Bone marrow (BM) was hypercellular with 15% blasts, monocytosis and trilineage dysplasia. Conventional cytogenetic analysis (G-banding) detected an apparently normal male karyotype (46,XY). A diagnosis of chronic myelomonocytic leukaemia (CMML) was made. After 3 years, PB analysis revealed a WBC count of 22 × 109/l and a predominance of blasts. BM aspirate analysis also revealed 89% myeloid blasts and G-banding detected the emergence of an abnormal clone harbouring an extra copy of chromosomes 13 and 15. A diagnosis of disease transformation to acute myeloid leukaemia (AML) was made. Post chemotherapy BM aspirate was very hypocellular and the abnormal +13,+15 clone was still present suggesting primary refractory disease. A second course of chemotherapy was only administered for 24 hours due to complications. The abnormal +13,+15 clone was still present and it was decided that no further treatment apart from palliative care could be offered. The patient died 11 weeks later, five months after AML transformation. This is the first description of a cytogenetically normal CMML patient transforming to AML with the emergence of a unique +13, +15 double trisomy resulting in an adverse outcome.
INTRODUCTION
CMML is a clonal myeloid BM stem cell disorder accounting for 10-15% of all myelodysplastic syndromes (MDS)1. The specific aetiology of the disease is unknown but exposure to occupational and environmental carcinogens, ionising radiation and cytotoxic agents have all been implicated2. The French-American-British (FAB) scheme classified CMML as a myelodysplastic syndrome because dysplastic changes were commonly found in both PB and BM3. The World Health Organisation (WHO) classification has now placed CMML in a new MDS/MPD category along with juvenile myelomonocytic leukaemia and atypical chronic myeloid leukaemia4. The WHO classification has also recommended that PB and BM blast counts (includes myeloblasts, monoblasts and promonocytes) be used to distinguish between CMML-I (<5% in PB or <10% in BM) and CMML-II (5-19% in PB or 10-19% in BM) sub-types4,5.
Prognosis is extremely variable in CMML. The median survival time is in the order of 12 to 24 months with transformation to AML occurring in about 15-20% of patients6,7. In the majority of prognostic studies carried out, the percentage of blasts in PB and BM appears to be the most important factor in determining survival6,8. Other factors contributing to survival of CMML patients include clonal chromosome abnormalities, WBC, circulating levels of haemoglobin, platelets, marrow erythroid cells, serum lactate dehydrogenase and β2-microglobulin6,7. Clonal chromosome abnormalities are found in 20-40% of patients but none are classified as being disease-specific6.
In the present case, G-banding and interphase FISH techniques were used to monitor the karyotype status of a patient who transformed to AML after a 3 year history of cytogenetically normal CMML. Upon transformation the patient acquired a unique double trisomy for chromosomes 13 and 15 and had a poor outcome.
METHODS
Case description
A 58-year-old man was admitted to hospital in March 1999 presenting with symptoms of lethargy and easy bruising for approximately four months duration. On examination no abnormality was detected. PB analysis revealed haemoglobin 13.2g/dl, WBC 15.9 × 109/l with monocytes 5.4 × 109/l, neutrophils 6.7 × 109/l, neutrophil band forms 0.6 × 109/l, lymphocytes 3.2 × 109/l and platelets 48.0 × 109/l. BM was markedly hypercellular with 15% blasts, monocytosis and trilineage dysplasia. G-banding and interphase FISH analyses of his BM aspirate demonstrated an apparently normal male karyotype. In particular there was no evidence of a Philadelphia chromosome by G-banding or a BCR/ABL gene rearrangement by interphase FISH. A diagnosis of CMML was made.
In February 2002 he became progressively anaemic with a rising WBC and progressive splenomegaly. PB examination confirmed the increase in his WBC to be secondary to transformation to AML. On admission to hospital PB analysis revealed haemoglobin 8.7g/dl, WBC 22 × 109/l (predominantly blasts) and platelets 6.0 × 109/l. BM aspirate analysis revealed 89% myeloid blasts with CD7, CD13, CD33, CD34 and CD117 positivity. G-banding and interphase FISH analyses detected an abnormal clonal cell population harbouring an extra copy of chromosomes 13 and 15. A diagnosis of disease transformation to AML (M2 FAB-type) was made.
He was treated with intensive chemotherapy. Soon after commencement he became ill with septic shock but eventually recovered to complete his 10-day course of chemotherapy. He remained pancytopenic. His 35 day post chemotherapy BM aspirate was very hypocellular and still showed evidence of the abnormal +13,+15 clone suggesting primary refractory disease. A second course of chemotherapy was planned but after the first day of Fludarabine treatment he developed septic shock, became hypotensive and required inotropic support. Chemotherapy was therefore abandoned and he made some recovery but remained in a very poor state and was not fit to complete the course of chemotherapy. The patient remained pancytopenic with no recovery of his blood counts. G-banding and interphase FISH analyses still confirmed the presence of the abnormal +13,+15 clone. No further treatment apart from palliative care could be offered. The patient died 11 weeks later, five months after transforming to AML.
Conventional Cytogenetics (G-banding) and Interphase FISH i(FISH)
Chromosome spreads and interphase nuclei were examined from 24-hour unstimulated BM cells at CMML diagnosis, transformation to AML and after each course of chemotherapy using G-banding and i(FISH) techniques respectively. Chromosomes were Giemsa-Trypsin-Giemsa (GTG) banded and karyotypes were described according to ISCN 20059. i(FISH) analyses were carried out using a BCR/ABL dual fusion probe (at diagnosis only) to screen for a t(9;22) rearrangement and chromosome 13, 15 and Y enumeration probes to assess ploidy status at CMML diagnosis (retrospectively), transformation to AML and after each course of chemotherapy according to the protocols provided by the manufacturer (Abbott Vysis, UK). Y chromosome ploidy status was monitored because of the apparent age-related link between trisomy 15 (+15) and Y chromosome loss (−Y), a phenomenon that has previously been reported in the literature10.
RESULTS
i(FISH) analysis detected a normal Y chromosome ploidy level at CMML diagnosis, AML transformation and after each course of chemotherapy. There was therefore no evidence of Y chromosome loss during the course of the disease.
CMML diagnosis (March 1999)
G-banded analysis detected an apparently normal male karyotype with no evidence of a Philadelphia chromosome (Fig 1A). i(FISH) analysis did not detect an underlying t(9;22) translocation or aneuploidies involving chromosomes 13 and/or 15 (Fig 1A).
fig 1.
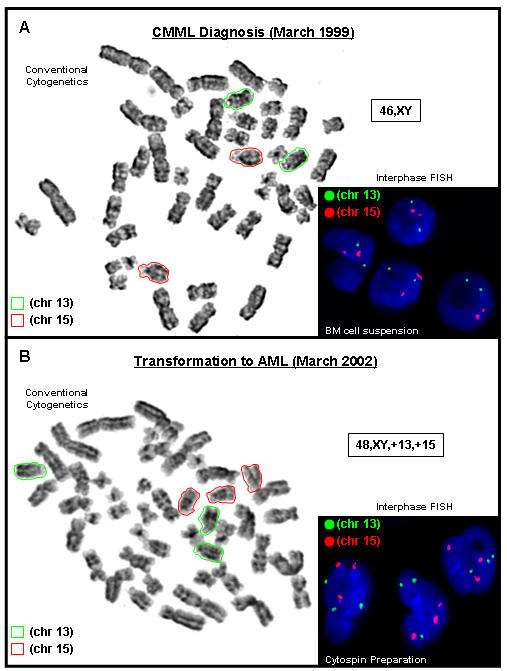
Conventional cytogenetic analysis using G-banded metaphase spreads and interphase FISH analysis from A) unstimulated bone marrow cell suspensions at CMML diagnosis (March 1999) and B) unstimulated bone marrow cell suspensions (conventional cytogenetic analysis only) and a cytospin preparation (interphase FISH only) at transformation to AML (March 2002). Chromosome 13 is highlighted (green) and chromosome 15 (red). 46,XY karyotype indicated in (A). 48,XY,+13,+15 karyotype indicated in (B).
AML transformation (March 2002)
G-banded analysis detected 3 copies of chromosomes 13 and 15 in 5 out of 7 cells analysed (Fig 1B). i(FISH) analysis using a cytospin preparation detected 3 copies of chromosomes 13 and 15 in 79 out of 100 nuclei examined. These results demonstrate the emergence of an abnormal clone harbouring an extra copy of chromosomes 13 and 15.
Post Chemotherapy (Course 1)
G-banded analysis detected 3 copies of chromosomes 13 and 15 in 9 out of 30 cells analysed. i(FISH) analysis detected 3 copies of chromosomes 13 and 15 in 9 out of 100 nuclei examined. There was therefore evidence of the same aneuploidy involving chromosomes 13 and 15 detected at AML transformation.
Post Chemotherapy (Course 2)
Chemotherapy was abandoned after 24 hours due to complications. G-banded analysis detected 3 copies of chromosomes 13 and 15 in all 5 cells analysed. i(FISH) analysis detected 3 copies of chromosomes 13 and 15 in 18 out of 100 nuclei examined. There was therefore still evidence of the aneuploidy involving chromosomes 13 and 15 (table I).
Table I.
The number of cells detected with 3 copies of chromosomes 13 and 15 using conventional cytogenetics (G-banding) and interphase FISH techniques (expressed as the number detected out of the total number of cells analysed).
| STAGE OF DISEASE | Number of cells with 3 copies of chromosomes 13 and 15 | |
|---|---|---|
| G-Banding | Interphase FISH | |
| CMML diagnosis (March 1999) | 0/10 | 0/100 |
| AML transformation (March 2002) | 5/7 | 79/100 |
| Post Course 1 Chemotherapy | 9/30 | 9/100 |
| Post Course 2 Chemotherapy | 5/5 | 18/100 |
DISCUSSION
We report a male patient having had a three year history of cytogenetically normal CMML before transforming to AML. Disease transformation coincided with the emergence of a unique +13,+15 double trisomy in a proportion of the cells suggesting further evolution of the malignant stem cells. The WHO classification uses the following criteria to make a diagnosis of CMML11:
Persistent PB monocytosis (> 1 × 109/L),
No Philadelphia chromosome or BCR/ABL fusion gene,
< 20% blasts (includes myeloblasts, monoblasts and promonocytes) in PB or BM,
Dysplasia in one or more myeloid lineages.
Furthermore, if myelodysplasia is absent or minimal, CMML can still be diagnosed if the criteria mentioned above are met and an acquired chromosome abnormality is detected in the BM or monocytosis has been persistent for at least 3 months and all other causes of monocytosis have been excluded11. Our patient met all of the above criteria having a monocyte count of 5.4 × 109/l, no Philadelphia chromosome or BCR/ABL gene rearrangement, 15% blasts in the BM and trilineage dysplasia.
The number of blasts usually account for <5% of WBC in PB and <10% of the nucleated marrow cell at the time of diagnosis. A higher blast count can identify patients who may be at risk of having a poor prognosis or who may undergo rapid transformation to an acute leukemia8,12,13. Interestingly, our patient, according to the WHO classification4,5, had a CMML-II sub-type (i.e. 15% BM blasts) with an overall survival (∼40 months) that was not only better than the 12 to 24 months median survival time reported in the literature6,7 but also transformed to AML 36 months after initial disease diagnosis.
Clonal chromosome abnormalities are found in 20-40% of CMML cases but none have been classified as being disease-specific6. The most frequent recurring chromosome abnormalities include trisomy 8, monosomy 7, deletions of part of the long arm of chromosome 7, structural abnormalities of the short arm of chromosome 12 and complex karyotypes. CMML patients with a clonal chromosome abnormality usually have a shorter survival time compared to cytogenetically normal CMML patients6. Furthermore, it has not yet been possible to stratify the recurring clonal chromosome abnormalities into any particular risk category. However, a rare t(5;12)(q33;p13) chromosome translocation found in 1-2% of CMML patients has been associated with a number of chronic MPD/MDS disorders, often categorised as eosinophilic leukaemia or as CMML with eosinophilia14,15. The molecular mechanism of leukaemogenesis is fusion of the Platelet-Derived Growth Factor Receptor-β (PDGFRβ) gene at 5q33 with the ETV6 (previously known as TEL) gene at 12p1314,16. The identification of patients with this PDGFRβ gene rearrangement is of major prognostic importance, not because of any particular impact on the natural course of CMML, but in view of their reported responsiveness to treatment with the tyrosine kinase inhibitor imatinib mesylate17,18.
The association of trisomy of various chromosomes with haematological malignancies is well established19. The presence of some single acquired autosomal trisomies is also supportive of diagnosis and indicative of prognosis in haematological disorders. Single autosomal trisomies for chromosomes 13 and 15 are very rare in AML20. Trisomy 13 as the sole cytogenetic abnormality is found in less than 1% of AML cases and to the best of our knowledge there have been only 70 cases reported in the literature20. Patients are usually elderly males presenting with M0 or M1 AML and having a generally poor response to treatment21. These patients not only have a low rate of complete remission22 but also remain in complete remission for a short period of time23,24. The molecular mechanism whereby trisomy 13 confers neoplastic potential on a clone is not understood but candidate genes include the FMS-like tyrosine kinase (FLT) proto-oncogene (mapped to 13q12) and the retinoblastoma gene (mapped to 13q14).
Trisomy 15 as the sole autosomal abnormality is also very rare in leukaemia being found in both myeloid and lymphoid leukaemias25,26. To the best of our knowledge there have been only 11 cases of trisomy 15 as the sole autosomal trisomy in AML reported in the literature20. The significance of trisomy 15 in haematological neoplasia and the molecular mechanism whereby trisomy 15 confers neoplastic potential on a clone is again not understood. To date there are no known genes/proteins associated with trisomy 15 and haematological malignancy25. However, disruption of candidate genes on chromosome 15 known to contribute to transformation of myeloid cells include the PML gene (mapped to 15q22) in acute promyelocytic leukaemia (M3 AML)27 and the AF15q14 gene (mapped to 15q14) fused to the MLL gene (mapped to 11q23) in AML with a t(11;15)(q23;q14) translocation28.
Although the presence of a clonal chromosome abnormality in BM is usually indicative of haematological malignancy, not all apparent chromosome abnormalities can be taken as proof of malignancy. Loss of the Y chromosome in BM of elderly males has been accepted as being an age-related phenomenon29. There is also evidence in the literature suggesting that individual trisomies for chromosomes such as 7 and 15 may not be malignant10,30. An apparent link involving trisomy 15 and loss of the Y chromosome in relation to an age-related phenomenon has even been suggested10. Trisomy 15 has also been observed as a minor clone in patients with anaemia, MDS and Non-Hodgkin's Lymphoma (NHL) but as a major clone in patients with AML31. Although the significance of trisomy 15 as a single autosomal abnormality remains unclear, it may, like loss of the Y chromosome, be unhelpful as a marker of malignancy.
i(FISH) analysis was therefore carried out on all samples received for cytogenetic investigation using a chromosome Y enumeration probe in conjunction with the 13 and 15 chromosome enumeration probes in order to investigate this ‘age-related association’ involving chromosomes 15 and Y. i(FISH) analysis did not detect Y chromosome loss in any of the samples examined. This immediately indicates there was no age–related Y chromosome loss. This result also demonstrates the lack of an apparent age-related link between chromosome 15 and Y since the extra copy of chromosome 15 was only ever found in the presence of an extra copy of chromosome 13 in the samples analysed from the time of disease transformation to AML. This would therefore suggest that the extra copy of chromosomes 13 and 15 may both play a role in the malignant transformation to AML.
The case presented here is therefore notable for the emergence of a novel +13, +15 double trisomy cell population in a patient with AML having had a 3 year history of cytogenetically normal CMML. Both trisomy 13 and trisomy 15 as sole autosomal trisomies are known to be very rare in haematological disorders. This case demonstrates that the coexistence of a +13, +15 double trisomy is not only an extremely rare phenomenon but also may have contributed to a particularly poor outcome in this patient.
The authors have no conflict of interest
REFERENCES
- 1.Aul C, Gattermann N, Schneider W. Epidemiological and etiological aspects of myelodysplastic syndromes. Leuk Lymphoma. 1995;16(3–4):247–62. doi: 10.3109/10428199509049764. [DOI] [PubMed] [Google Scholar]
- 2.Aul C, Bowen DT, Yoshida Y. Pathogenesis, etiology and epidemiology of myelodysplastic syndromes. Haematologica. 1998;83(1):71–86. [PubMed] [Google Scholar]
- 3.Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol. 1982;51(2):189–99. [PubMed] [Google Scholar]
- 4.Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, et al. The World Health Organization classification of hematological malignancies: report of the Clinical Advisory Committee meeting - Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17(12):3835–49. doi: 10.1200/JCO.1999.17.12.3835. [DOI] [PubMed] [Google Scholar]
- 5.Bennett JM. WHO-classification of the acute leukemias and myelodysplastic syndromes. Int J Hematol. 2000;72(2):131–3. [PubMed] [Google Scholar]
- 6.Onida F, Kantarjian HM, Smith TL, Ball G, Keating MJ, Estey EH, et al. Prognostic factors and scoring systems in chronic myelomonocytic leukemia: a retrospective analysis of 213 patients. Blood. 2002;99(3):840–9. doi: 10.1182/blood.v99.3.840. [DOI] [PubMed] [Google Scholar]
- 7.Germing U, Kundgen A, Gattermann N. Risk assessment in chronic myelomonocytic leukemia (CMML) Leuk Lymphoma. 2004;45(7):1311–8. doi: 10.1080/1042819042000207271. [DOI] [PubMed] [Google Scholar]
- 8.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079–88. [PubMed] [Google Scholar]
- 9.Shaffer LG, Tommerup N, editors. ISCN 2005. An International System For Human Cytogenetic Nomenclature. Basel: S Karger; 2005. [Google Scholar]
- 10.Sinclair EJ, Potter AM, Watmore AE, Fitchett M, Ross F. Trisomy 15 associated with loss of the Y chromosome in bone marrow: a possible new aging effect. Cancer Genet Cytogenet. 1998;105(1):20–3. doi: 10.1016/s0165-4608(98)00003-x. [DOI] [PubMed] [Google Scholar]
- 11.Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2001. World Health Organization Classification of Tumours. [Google Scholar]
- 12.Fenaux P, Jouet JP, Zandecki M, Lai JL, Simon M, Pollet JP, et al. Chronic and subacute myelomonocytic leukaemia in the adult: a report of 60 cases with special reference to prognostic factors. Br J Haematol. 1988;65(1):101–6. doi: 10.1111/j.1365-2141.1987.tb06142.x. [DOI] [PubMed] [Google Scholar]
- 13.Fenaux P, Beuscert R, Lai JL, Jouet JP, Bauters F. Prognostic factors in adult chronic myelomonocytic leukemia: an analysis of 107 cases. J Clin Oncol. 1988;6(9):1417–24. doi: 10.1200/JCO.1988.6.9.1417. [DOI] [PubMed] [Google Scholar]
- 14.Bain BJ. Eosinophilic leukaemias and the idiopathic hypereosinophilic syndrome. Br J Haematol. 1996;95(1):2–9. [PubMed] [Google Scholar]
- 15.Bain BJ. An overview of translocation-related oncogenesis in the chronic myeloid leukaemias. Acta Haematol. 2002;107(2):57–63. doi: 10.1159/000046634. [DOI] [PubMed] [Google Scholar]
- 16.Wlodarska I, Mecucci C, Baens M, Marynen P, van den Berghe H. ETV-6 gene rearrangements in hematopoietic malignant disorders. Leuk Lymphoma. 1996;23(3–4):287–95. doi: 10.3109/10428199609054831. [DOI] [PubMed] [Google Scholar]
- 17.Apperley JF, Gardembas M, Melo JV, Russell-Jones R, Bain BJ, Baxter EJ, et al. Response to imatinib mesylate in patients with chronic myeloproliferative diseases with rearrangements of the platelet-derived growth factor receptor beta. N Eng J Med. 2002;347(7):481–7. doi: 10.1056/NEJMoa020150. [DOI] [PubMed] [Google Scholar]
- 18.Pitini V, Arrigo C, Teti D, Barresi G, Righi M, Alo G. Response to STI571 in chronic myelomonocytic leukemia with platelet derived growth factor beta receptor involvement: a new case report. Haematologica. 2003;88(5) ECR18. [PubMed] [Google Scholar]
- 19.United Kingdom Cancer Cytogenetics Group (UKCCG) Primary, single autosomal trisomies associated with haematological disorders. Leuk Res. 1992;16(9):841–51. doi: 10.1016/0145-2126(92)90030-b. [DOI] [PubMed] [Google Scholar]
- 20.Mitelman F, Johansson B, Mertens F, editors. Mitelman Database of Chromosome Aberrations in Cancer. Bethesda: National Cancer Institute; 2006. [Google Scholar]
- 21.Mehta AB, Bain BJ, Fitchett M, Shah S, Secker-Walker LM. Trisomy 13 and myeloid malignancy: characteristic blast cell morphology. A United Kingdom Cancer Cytogenetics Group survey. Br J Haematol. 1998;101(4):745–52. doi: 10.1046/j.1365-2141.1998.00760.x. [DOI] [PubMed] [Google Scholar]
- 22.Baer MR, Bloomfield CD. Trisomy 13 in acute leukaemia. Leuk Lymphoma. 1992;7(1–2):1–6. doi: 10.3109/10428199209053596. [DOI] [PubMed] [Google Scholar]
- 23.Cuneo A, Ferrant A, Michaux JL, Bosly A, Chatelain B, Stul M, et al. Cytogenetic and clinicobiological features of acute leukemia with stem cell phenotype: study of nine cases. Cancer Genet Cytogenet. 1996;92(1):31–6. doi: 10.1016/s0165-4608(96)00127-6. [DOI] [PubMed] [Google Scholar]
- 24.Dohner H, Arthur DC, Ball ED, Sobol RE, Davey FR, Lawrence D, et al. Trisomy 13: a new recurring chromosome abnormality in acute leukaemia. Blood. 1990;76(8):1614–21. [PubMed] [Google Scholar]
- 25.Sinclair EJ, Potter AM. +15 or trisomy 15 (as sole autosomal abnormality) Atlas of Genet Cytogenet Oncol Haematol Online journal. 1999 May; Available from: http://www.atlasgeneticsoncology.org/Anomalies/tri15ID1144.html. Last accessed May 2007.
- 26.Smith SR, Rowe D. Trisomy 15 in hematological malignancies: six cases and review of the literature. Cancer Genet Cytogenet. 1996;89(1):27–30. doi: 10.1016/0165-4608(96)00020-9. [DOI] [PubMed] [Google Scholar]
- 27.Huret JL, Chomienne C. t(15;17)(q22;q21) Atlas of Genet Cytogenet Oncol Haematol Online journal. 1998 Apr; Available from: http://www.atlasgeneticsoncology.org/Anomalies/t1517ID1035.html. Last accessed May 2007.
- 28.Huret JL, Charrin C. t(11;15)(q23;q14) Atlas of Genet Cytogenet Oncol Haematol Online journal. 2000 Mar; Available from: http://www.atlasgeneticsoncology.org/Anomalies/t1115ID1199.html. Last accessed May 2007.
- 29.United Kingdom Cancer Cytogenetics Group (UKCCG) Loss of the Y chromosome from normal and neoplastic bone marrow. Genes Chromosomes Cancer. 1992;5(1):83–8. doi: 10.1002/gcc.2870050112. [DOI] [PubMed] [Google Scholar]
- 30.Johansson B, Heim S, Mandahl N, Mertens F, Mitelman F. Trisomy 7 in nonneoplastic cells. Genes Chromsomes Cancer. 1993;6(4):199–205. doi: 10.1002/gcc.2870060402. [DOI] [PubMed] [Google Scholar]
- 31.Batanian JR, Slovak ML, Mohamed A, Dobin S, Luthardt FW, Keitges EA. Trisomy 15 is frequently observed as a minor clone in patients with Anemia/MDS/NHL and as a major clone in patients with AML. Cancer Genet Cytogenet. 2000;121(2):186–9. doi: 10.1016/s0165-4608(00)00253-3. [DOI] [PubMed] [Google Scholar]


