Abstract
Ceramide is generated in response to numerous stress-inducing stimuli and has been implicated in the regulation of diverse cellular responses, including cell death, differentiation, and insulin sensitivity. Recent evidence indicates that ceramide may regulate these responses by inhibiting the stimulus-mediated activation of protein kinase B (PKB), a key determinant of cell fate and insulin action. Here we show that inhibition of this kinase involves atypical PKCζ, which physically interacts with PKB in unstimulated cells. Insulin reduces the PKB-PKCζ interaction and stimulates PKB. However, dissociation of the kinase complex and the attendant hormonal activation of PKB were prevented by ceramide. Under these circumstances, ceramide activated PKCζ, leading to phosphorylation of the PKB-PH domain on Thr34. This phosphorylation inhibited phosphatidylinositol 3,4,5-trisphosphate (PIP3) binding to PKB, thereby preventing activation of the kinase by insulin. In contrast, a PKB-PH domain with a T34A mutation retained the ability to bind PIP3 even in the presence of a ceramide-activated PKCζ and, as such, expression of PKB T34A mutant in L6 cells was resistant to inhibition by ceramide treatment. Inhibitors of PKCζ and a kinase-dead PKCζ both antagonized the inhibitory effect of ceramide on PKB. Since PKB confers a prosurvival signal and regulates numerous pathways in response to insulin, suppressing its activation by a PKCζ-dependent process may be one mechanism by which ceramide promotes cell death and induces insulin resistance.
Protein kinase B (PKB), also known as c-Akt, is a serine/threonine kinase that has been implicated in the control of diverse cellular functions, including glucose metabolism, gene transcription, cell proliferation, and apoptosis (16, 27, 34, 48). Three PKB isoforms (α, β, and γ) have been identified, and these can be activated rapidly in response to insulin and growth factors in a phosphoinositide 3-kinase (PI3K)-dependent manner. PI3K activation results in the increased production of 3-phosphoinositides, e.g., phosphatidylinositol 3,4,5-trisphosphate [PtdIns(3,4,5)P3] and phosphatidylinositol 3,4-bisphosphate, which play a key role in the recruitment of PKB to the plasma membrane (5). The N-terminal domain of all three PKB isoforms contains a pleckstrin homology (PH) domain, which is considered critical in allowing the kinase to interact with 3-phosphoinositides and possibly other signaling proteins (13, 16, 19). Binding of 3-phosphoinositides to the PH domain of PKB is also thought to induce conformational changes in the kinase that expose two key regulatory sites, Thr308 and Ser473 (3), allowing them to be phosphorylated by two upstream kinases. One of these, 3-phosphoinositide-dependent kinase-1 (PDK1), phosphorylates Thr308 (4, 44), whereas the identity of the second kinase that phosphorylates Ser473 (putatively termed PDK2) remains unknown, although a number of potential candidates have recently been proposed (for a review, see reference 15).
The activation of PKB elicited by insulin and growth factors can be reduced dramatically by stimuli such as tumor necrosis factor alpha (TNF-α) and saturated fatty acids, such as palmitate (41), which have been implicated strongly in the pathogenesis of insulin resistance. Both TNF-α and palmitate, as well as numerous other stress-inducing stimuli, such as heat shock, UV radiation, and oxidants, have been shown to promote an increase in the production of ceramide, a sphingomyelin-derived lipid molecule (28, 32). Cell-permeant analogues of ceramide have been shown to exert a profound inhibitory effect on insulin-stimulated glucose transport in muscle and fat cells (26, 46), and there is mounting evidence to support the idea that the lipid also acts as an intracellular effector molecule that promotes cell death (32). We and others have shown that ceramide inhibits insulin-stimulated glucose transport by suppressing the hormonal activation of PKB in L6 muscle cells and 3T3-L1 adipocytes (26, 46). In these cell types, ceramide blocks the insulin-dependent recruitment of PKB to the plasma membrane despite an increase in cellular 3-phosphoinositides, suggesting that ceramide may target additional elements that may either be required or regulate the translocation and activation of PKB. Ceramide is known to regulate both directly and indirectly the activity of numerous signaling molecules, such as mitogen-activated protein kinases (MAPKs), stress-activated protein kinases, phosphatases, and members of the PKC family, such as atypical PKCζ (10, 36, 37). The latter is of particular interest given that a number of studies have shown that PKCζ can interact with and inhibit PKB (9, 22, 33, 35). Moreover, the observation that a dominant-negative PKCζ attenuates ceramide's ability to inhibit PKB activation in smooth muscle cells (9) and that inhibition of PKCζ in neuroblastoma cells suppresses apoptosis induced by ceramide analogues (8) provides a strong basis for suggesting that the lipid may dysregulate PKB-directed signaling via PKCζ. In the present study we tested this proposition with a view to ascertaining the mechanism by which PKCζ may modulate PKB signaling in response to ceramide.
MATERIALS AND METHODS
Materials.
α-Minimal essential medium, fetal bovine serum, and antimycotic-antibiotic solution were from Life Technologies. All other reagent-grade chemicals, insulin, DAPI (4′,6′-diamidino-2-phenylindole), and protamine-agarose beads were obtained from Sigma-Aldrich (Poole, United Kingdom). PKCζ and the PKCα myristoylated-pseudosubstrate inhibitors, Ro 31.8220, SB-203580, rapamycin, dihydroceramide, and phorbol 12-myristate 13-acetate (PMA), were purchased from Calbiochem-Novabiochem, Ltd. (Nottingham, United Kingdom), and ceramide was obtained from Tocris (Bristol, United Kingdom). SB-212963 was a gift from GlaxoSmithKline (Harlow, United Kingdom). Antibodies against the PH domain of PKBα, peptide substrates for kinase assays, and recombinant PKBα-ΔPH protein were provided by Philip Cohen (MRC Protein Phosphorylation Unit, University of Dundee, Dundee, Scotland). Antibodies to phospho-PKCζ410, constructs encoding glutathione S-transferase (GST)-PKCζ, kinase GST-dead GST-PKCζ, PKB, and the isolated PH domain of PKB were provided by Dario Alessi (MRC Protein Phosphorylation Unit, University of Dundee). A semipurified isolated PKB PH domain protein was provided by Dan Van Aalten (University of Dundee). sn-2-Stearoyl-3-arachidonyl d-PtdIns(3,4,5)P3 (PIP3) and phosphatidylserine (PS) were gifts from Peter Downes (University of Dundee). Antibodies to p38 MAPK, PKBα, phospho-PKB308, and phospho-PKB473 were from New England Biolabs (Herts, United Kingdom); anti-phospho-GSK3 was from Life Signaling Technologies; anti-c-myc was from Sigma-Aldrich; and anti-PKCζ was purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.). Horseradish peroxidase (HRP)-conjugated to anti-rabbit immunoglobulin G (IgG), anti-mouse IgG, and anti-sheep/goat IgG were obtained from the Scottish Antibody Production Unit (Law Hospital, Carluke, Lanarkshire, Scotland). Protein A-Sepharose beads were purchased from ICN Biomedicals (Basingstoke, Hants, United Kingdom). ATP and Hyperfilm MP autoradiography film were purchased from Amersham Biosciences). Fugene 6 transfection reagent, antihemagglutinin (anti-HA) antibody, and Complete protein phosphatase inhibitor tablets were purchased from Boehringer-Roche Diagnostics (Basel, Switzerland).
Tissue culture and cellular fractionation.
L6 muscle cells were grown as described previously in α-minimal essential medium containing 2% (vol/vol) fetal bovine serum and 1% (vol/vol) antimycotic-antibiotic solution at 37°C in an atmosphere of 5% CO2 and 95% air (25). Plasma membranes were isolated from L6 cells as described previously (25). The protein content of the isolated membrane fractions was determined by the Bradford assay (11).
L6 cell transfection.
Subconfluent L6 myoblasts were transfected with 1 μg of pCMV5-PKCζ, pCMV5-kinase dead PKCζ, or the PCMV5 vector alone by using Fugene 6 transfection reagent. After 48 h, muscle cells were treated with 100 μM C2-ceramide and 100 nM insulin prior to lysis. Cell lysates were subsequently subjected to analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting.
Phospho-PKBT34 antibody.
A phosphospecific antibody recognizing PKBα phosphorylated at Thr34 was raised in sheep against the peptide LKNDGTFIGYK (corresponding to residues 29 to 39 of full-length human PKBα; the underlined residue is phosphothreonine). The antibodies were affinity purified on activated Sepahrose covalently coupled to the phosphorylated peptide.
SDS-PAGE and immunoblotting.
Cell lysates (50 μg) and isolated membrane fractions from L6 cells (20 μg) were subjected to SDS-PAGE on 10% resolving gels and transferred onto Immobilon-P or Hybond-C membranes (Millipore, Harts, United Kingdom) as described previously (25). Membranes were probed with primary antibodies to proteins of interest. For analysis of PKCζ phosphorylation on its activation loop residue (Thr410), cell lysates (250 μg of protein) were initially subjected to incubation with protamine-agarose beads to “pull down” and enrich PKCζ as described previously (6). PKCζ associated with protamine beads was resuspended in SDS sample buffer and resolved by SDS-PAGE prior to immunoblotting with an phospho-specific antibody directed against Thr410. Detection of primary antibodies was performed with either HRP-labeled anti-rabbit IgG, HRP-labeled anti-mouse IgG, or HRP-labeled anti-sheep/goat IgG and then visualized by using enhanced chemiluminescence (Amersham Biosciences) on Kodak X-Omat film (Eastman-Kodak, Rochester, United Kingdom).
Analysis of PKBα activity in L6 cells.
L6 myotubes grown in 10-cm dishes were exposed to 5 μM Ro 31.8220 for 2.5 h, 100 μM C2-ceramide for 2 h, and 100 nM insulin for 10 min. Cells were then harvested in lysis buffer (50 mM Tris-HCl [pH 7.5], 1 mM EDTA, 1 mM EGTA, 1% [vol/vol] Triton X-100, 1 mM Na3VO4, 10 mM sodium β-glycerophosphate, 50 mM NaF, 5 mM Na4P2O7, 1 μM microcystin-LR, 270 mM sucrose, 1 mM benzamidine, 10 μg of leupeptin/ml, Complete proteinase inhibitor cocktail [one tablet per 25 ml], and 0.1% [vol/vol] 2-mercaptoethanol). PKBα was immunoprecipitated from lysates by using an antibody against the C-terminal domain, and kinase activity assayed by using the synthetic peptide substrate “crosstide” as described previously (17).
In vitro protein phosphorylation.
Phosphorylation assays were performed by incubating 1 μg of recombinant PKBα PH domain protein with 30 μl of assay buffer (20 mM morpholinepropanesulfonic acid [pH 7.2], 25 mM β-glycerophosphate, 1 mM sodium orthovanadate, 1 mM dithiothreitol, 1 mM CaCl2, 5 mM MgCl2, 1 μM ATP, 5 μCi of [32P]ATP, 4 pg of PS/ml) for 10 min at 30°C in the absence or presence of 30 ng of recombinant PKCζ (Upstate Biotech) and 100 μM ceramide. The reaction was stopped by the addition of Laemmli buffer; phosphorylated PH peptides and PKCζ were then resolved by SDS-PAGE, and bands visualized by exposure to Amersham Hyperfilm MP film. Alternatively, resolved samples were immunoblotted with antibodies to the PH domain and PKCζ as described above.
PKCζ/PKBα overlay assay.
Protein-protein overlay assays were carried out by using recombinant GST-PKCζ, GST-PKBα, and GST-PKBα lacking the PH domain (PKBΔPH). Briefly, 0.1 μg of GST-PKCζ and 50 μg of L6 lysate were subjected to SDS-10% PAGE and then immunoblotted. Immobilon membranes were next incubated overnight at 4°C with 0.1 μg of recombinant PKB and PKBΔPH proteins, as well as 500 μg of L6 cell lysate. Membranes were then incubated with antibodies against PKCζ, PKBα, and c-myc and probed with HRP-conjugated anti-mouse and anti-rabbit antibodies, and immunoreactivity was visualized by enhanced chemiluminescence as described above.
Protein-lipid overlay assay.
To assess whether C2-ceramide and PKCζ affect the phospholipid-binding properties of PKB, a protein-lipid overlay assay was carried out with GST fusion proteins of PKCζ and PKBα as described previously (23, 26). Briefly, 1 μl of PIP3 (500 pM stock) dissolved in chloroform-methanol-water (1:2:0.8) was spotted onto Hybond C-Extra membrane (Amersham Pharmacia Biotech, Amersham, United Kingdom) and allowed to dry for 1 h at room temperature. Membranes were blocked with 5% (wt/vol) bovine serum albumin in Tris-buffered saline-Tween (TBST; 10 mM Tris-HCl [pH 8.0], 150 mM NaCl, 0.1% [vol/vol] Tween 20) for 1 h at room temperature. Membranes were then incubated overnight at 4°C in TBST containing 5% (wt/vol) bovine serum albumin, recombinant proteins, and C2-ceramide at the concentrations indicated in the figure legends. After this incubation period, membranes were washed with TBST before incubation with an anti-GST antibody (1:1,000). Membranes were then probed with HRP-conjugated anti-mouse antibody, and the immunoreactivity was visualized by enhanced chemiluminescence.
PKBα PH domain mutagenesis.
Site-directed mutagenesis was carried out by using the QuikChange kit (Stratagene). 3′ and 5′ primers (3′-CCTGAAAAACGACGGAGCATTTATAGGTTAC and 5′-GTAACCTATAAATGCTCCGTCGTTTTTCAGG) were used to mutate Thr34 to an Ala in a pGEX4.1 construct encoding wild-type PKBα PH domain. The resulting plasmid was transformed into XL1-Blue cells and then harvested by using a Qiagen plasmid Miniprep kit according to the manufacturer's protocol. The sequence of the mutated PH domain was verified by using an automated DNA sequencer (model 373; Applied Biosystems).
Analysis of cell viability.
To assess cell viability in response to ceramide, subconfluent L6 myoblasts were transfected with 1 μg of the indicated constructs by using the Fugene 6 system as described above. After 48 h, muscle cells were incubated in the absence or presence of either 100 μM C2-ceramide or C2-ceramide plus 5 μM Ro 31.8220. Viable cells were classified as those that were adherent, displayed trypan blue exclusion, and stained positively with DAPI. Stained cells were visualized by using an Axiovert 200 fluorescence microscope and quantitated by counting individual nuclei from several randomly chosen visual fields.
Statistical analyses.
One-way analysis of variance, followed by a Newman-Keuls posttest, was used to assess statistical significance. The data analysis was performed by using GraphPad Prism software and considered statistically significant at P values of <0.05.
RESULTS
Ceramide inhibits the insulin-mediated phosphorylation and activation of PKB: effect of kinase inhibitors.
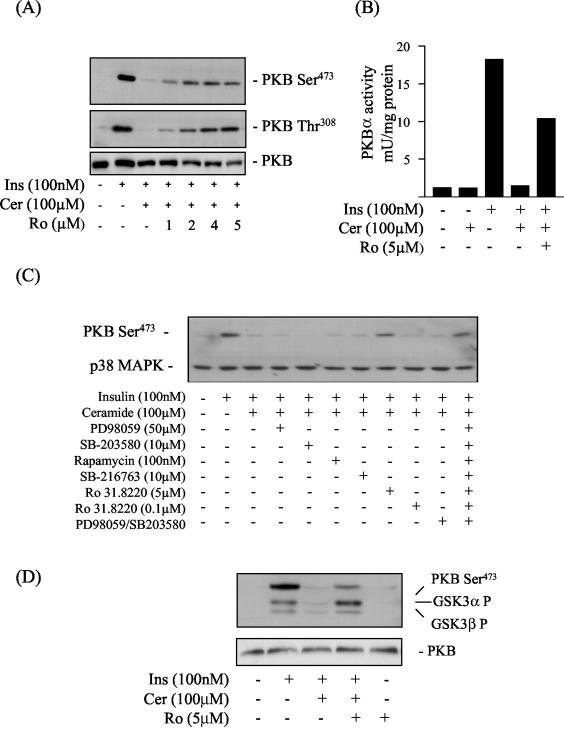
In accord with our previous work (26), Fig. 1A and B show that ceramide treatment abolishes completely the insulin-induced phosphorylation and activation of PKB. As a first step in attempting to establish whether ceramide mediates this inhibition via PKCs, we investigated the effects of Ro 31.8220, a bisindolemaleimide, that potently inhibits conventional and novel PKCs in an ATP-competitive manner with 50% inhibitory concentration values in the submicromolar range (2, 43) and atypical PKCs in the micromolar range (43). At submicromolar concentrations, Ro 31.8220 had no significant effect on the loss in PKB activation elicited by ceramide. However, this inhibition was progressively reversed when muscle cells were incubated with increasing concentrations of Ro 31.8220 in the micromolar range that inhibited atypical PKCs (Fig. 1A and B). Similar results were obtained with GF 109203X, another structurally unrelated bisindolemaleimide (data not shown).
FIG. 1.
Representative blots showing the effects of ceramide and kinase inhibitors on the insulin-mediated phosphorylation of PKB and GSK3. L6 myotubes were incubated in the absence or presence of C2-ceramide (Cer, 100 μM) for 2 h. In some experiments, cells were preincubated with Ro 31.8220 (Ro, at the concentrations indicated) for 30 min prior to incubation with ceramide. At the end of this incubation period cells were incubated with insulin (Ins, 100 nM) for a further 10 min before being lysed. (A and B) Cell lysates were then immunoblotted with a phospho-specific antibody directed against PKB-Ser473, PKB-Thr308, or PKB (A) and used for assaying PKB activity as described in the text (B). (C) L6 myotubes were incubated in the absence or presence (singularly or in combination) of kinase inhibitors at the indicated concentrations for 30 min prior to incubation with C2-ceramide (100 μM) for 2 h and with insulin (100 nM) for 10 min. Cell lysates were immunoblotted with a phospho-specific antibody directed against PKB-Ser473, with blots being reprobed with an antibody against p38 MAPK (which was used as a marker for protein loading). (D) L6 myotubes were pretreated with Ro 31.8220 (Ro, 5 μM) for 30 min prior to incubation with C2-ceramide (100 μM) for 2 h and insulin (Ins, 100 nM) for 10 min. Cell lysates were immunoblotted with phospho-specific antibodies directed against either GSK3α-Ser21, GSK3β-Ser7, PKB-Ser473, or PKB.
The selectivity of both Ro 31.8220 and GF109203X has been questioned in recent years, since evidence exists that these inhibitors also suppress the activity of other kinases, including, for example, S6K1, GSK3β, MSK1, and MAPKAP-K1 (2, 20). To assess the possible involvement of these kinases in the ceramide-induced inhibition of PKB, we preincubated L6 myotubes with Ro 31.8220, PD 98059 (which inhibits the classical MAPK [Erk] pathway), SB-203580 (which inhibits the p38 MAPK pathway), rapamycin (which inhibits the mTOR pathway), SB-216763 (which selectively inhibits GSK3 subtypes), and PD 98059 and SB-203580 together (which suppress activation of MSK1) (20). The ability of these compounds to inhibit their respective target pathways in our cell system was verified in separate experiments (data not shown). Figure 1C shows that, with the exception of Ro 31.8220 (when used at 5 μM), none of the inhibitors tested could prevent the loss in PKB activation elicited by ceramide.
We hypothesized that if Ro 31.8220 was able to suppress the inhibitory effects of ceramide on PKB activation, then the inhibitor should also reinstate PKB signaling to downstream targets, such as GSK3 (17). Figure 1D shows that insulin induces phosphorylation of PKB and both GSK3 isoforms but that this was reduced substantially in ceramide-treated cells. However, this reduction was attenuated significantly when muscle cells were preincubated with 5 μM Ro 31.8220 prior to treatment with ceramide (Fig. 1D).
Classical and novel PKC isoforms are unlikely to mediate the loss in PKB activation by ceramide.
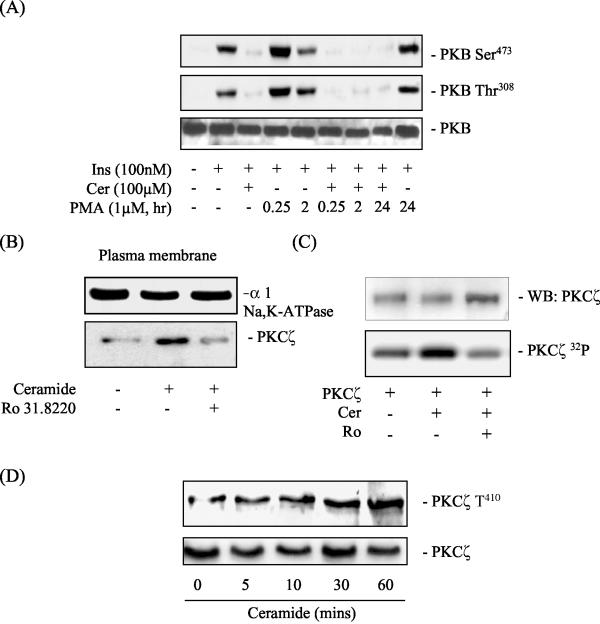
In order to exclude the involvement of the classical and novel PKC isoforms as potential players in the regulation of PKB by ceramide, we incubated L6 cells with 1 μM PMA for 15 min, 2 h, and 20 h prior to incubation with ceramide. Short-term PMA treatment (up to 2 h) stimulates classical and novel PKC isoforms, whereas long-term PMA exposure (24 h) downregulates their expression (18, 39). Figure 2A shows that neither short nor long-term incubation of muscle cells with PMA had any detectable effect on ceramide's ability to inhibit the hormonal activation of PKB. This finding suggests that it is highly unlikely that classical or novel PKC isoforms are utilized by ceramide as effector molecules that regulate PKB activation.
FIG. 2.
Effects of insulin, ceramide, and PMA on PKB and PKCζ phosphorylation in L6 myotubes. (A) L6 myotubes were pretreated with 1 μM PMA for the times indicated prior to incubation with C2-ceramide (Cer, 100 μM) for 2 h and with insulin (Ins, 100 nM) for 10 min. Cells lysates were immunoblotted with a phospho-specific antibody directed against PKB-Ser473 or PKB-Thr308. (B) L6 myotubes were incubated in the absence or presence of Ro 31.8220 (Ro, 5 μM) for 30 min prior to treatment with C2-ceramide (Cer, 100 μM) for 10 min. Myotubes were harvested, and plasma membranes were isolated by subcellular fractionation as described in the text. Plasma membranes (20 μg of protein) were subjected to SDS-PAGE and immunoblotted with antibodies against the α1 subunit of the Na/K-ATPase (a plasma membrane marker) and PKCζ. (C) In vitro activation of PKCζ was assessed by incubating 0.1 μg of recombinant PKCζ protein with C2-ceramide (Cer, 100 μM) and Ro 31.8220 (Ro, 5 μM) in the presence of [γ-32P]ATP, PS (4 pg/ml), and 5 mM MgCl2 at 30°C for 20 min as described in the text. PKCζ was subsequently resolved by SDS-PAGE and subjected to analysis by autoradiography. Loading of PKCζ on SDS gels was assessed by probing the transfer membrane with an antibody directed against PKCζ. (D) L6 myotubes were incubated with C2-ceramide (Cer, 100 μM) for the times indicated and then lysed. Cells lysates were immunoblotted with a phospho-specific antibody directed against PKCζ-Thr410 or PKCζ.
Ceramide activates PKCζ.
The data presented in Fig. 1 and 2A provide prima face evidence that ceramide may suppress the hormonal activation of PKB via atypical PKCs. To test this possibility further, we determined whether ceramide activates PKCζ in L6 myotubes. Braiman et al. (12) have previously shown that activation of PKCζ in cultured rat skeletal muscle cells involves its increased association with the plasma membrane. We therefore isolated plasma membranes from L6 myotubes after treatment with ceramide and/or Ro 31.8220 and then assessed PKCζ abundance by immunoblotting. Figure 2B shows that ceramide increased the plasma membrane abundance of PKCζ but that this was reduced in cells that had been pretreated with 5 μM Ro 31.8220. In contrast, the abundance of the α1 subunit of the Na/K-ATPase, a plasma membrane marker, was unaltered. The observation that Ro 31.8220, an ATP competitive inhibitor, suppresses ceramide-induced recruitment to the plasma membrane implies that the basal activity of PKCζ may be required to support its translocation and subsequent activation at the cell surface. This suggestion is consistent with a previous study reporting that autophosphorylation of PKCζ on Thr560 is crucial for supporting the activation of the kinase in response to stimuli such as insulin (7). Since ceramide has been shown to directly activate PKCζ in vitro (10) and the kinase is autophosphorylated upon activation (42), we also assessed whether ceramide enhanced 32P incorporation into PKCζ in vitro. Figure 2C shows that incubation of PKCζ with ceramide led to increased 32P labeling, which was suppressed significantly in the presence of Ro 31.8220. In addition to autophosphorylation, activation of PKCζ requires phosphorylation of its T-loop residue (Thr410). A phospho-specific antibody directed against this residue revealed detectable phosphorylation of this site even in unstimulated muscle cells, which was enhanced upon ceramide treatment of cells in a time-dependent manner (Fig. 2D).
A myristoylated PKCζ pseudosubstrate inhibitor and cellular expression of a kinase-dead PKCζ (kd-PKCζ) overcome the loss in PKB activation elicited by ceramide.
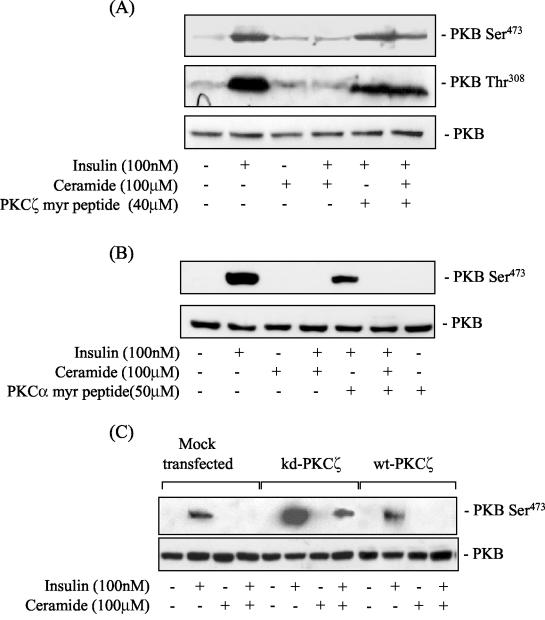
To further establish the involvement of PKCζ as a ceramide effector molecule, we utilized a myristoylated PKCζ pseudosubstrate peptide, which has been shown to specifically inhibit PKCζ activity (43). Pretreatment of muscle cells with the peptide prior to incubation with ceramide suppressed the ceramide-mediated loss in PKB phosphorylation on both Ser473 and Thr308 normally elicited by insulin (Fig. 3A). In contrast, no such protection against the inhibitory effects of ceramide was afforded in cells preincubated with a myristoylated PKCα pseudosubstrate peptide inhibitor (Fig. 3B). Collectively, the data obtained by using the bisindolemaleimide compounds and the peptide inhibitors implies that the catalytic activity of PKCζ is required for mediating the inhibitory effects of ceramide on PKB activation. To strengthen this proposition, we investigated whether the expression of a kd-PKCζ would antagonize the inhibition exerted by ceramide. Figure 3C shows that, compared to cells transfected with the expression vector alone or overexpressing wild-type PKCζ, cells expressing the kd-PKCζ displayed enhanced phosphorylation of PKB Ser473 in response to insulin. Furthermore, although ceramide treatment abolished insulin's ability to promote PKB phosphorylation in cells expressing the empty vector or the wild-type PKCζ, the hormone was still capable of inducing PKB phosphorylation in cells expressing kd-PKCζ. The transfection efficiency in these studies was ∼60%. Consequently, the hormonal activation of PKB would still be susceptible to the inhibition by ceramide in a significant proportion of the cells not expressing the kd-PKCζ, which would account for the slightly lower intensity of the Ser473 phosphorylation signal on the immunoblot.
FIG. 3.
A myristoylated PKCζ pseudosubstrate peptide inhibitor and expression of a kinase-inactive PKCζ mutant attenuate the inhibitory effects of ceramide on the insulin-induced phosphorylation of PKB in muscle cells. (A) L6 myotubes were pretreated with PKCζ myr-pseudosubstrate peptide (40 μM) or (B) with PKCα myr-pseudosubstrate peptide (50 μM) for 30 min prior to incubation with C2-ceramide (100 μM) for 2 h and with insulin (100 nM) in the penultimate 10-min period prior to cell lysis. Cells lysates were immunoblotted with a phospho-specific antibody directed against PKB-Ser473 or PKB-Thr308. (C) L6 myoblasts were transfected with pCMV5 lacking or containing cDNA encoding kd-PKCζ or wild-type PKCζ as described in the text. Cells were then incubated with C2-ceramide (100 μM) for 2 h prior to treatment with insulin (100 nM) for 10 min. Cell lysates were subsequently immunoblotted with a phospho-specific antibody to PKB-Ser473 or with an antibody to PKB.
PKCζ interacts with PKB in vitro and in vivo.
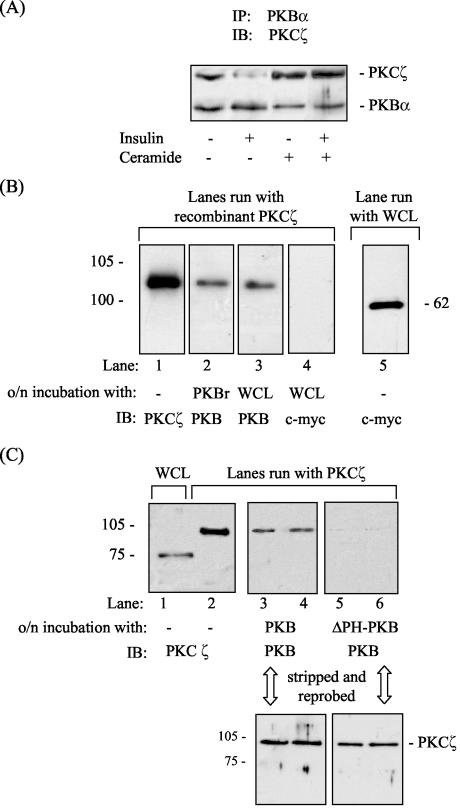
To understand the mechanism by which PKCζ may negatively regulate PKB activation in response to ceramide, we assessed whether the two kinases could interact with each other. Immunoprecipitation of PKB from unstimulated L6 myotubes revealed that PKCζ could be coprecipitated (Fig. 4A). Interestingly, when PKBα was immunoprecipitated from cells after incubation with insulin, the amount of PKCζ coprecipitated was significantly less, indicating that insulin promotes dissociation of the PKB-PKCζ complex. However, the interaction between the two kinases was enhanced after treatment of cells with ceramide and, more importantly, the lipid abolished insulin's ability to induce dissociation of the kinase complex (Fig. 4A).
FIG. 4.
PKB and PKCζ physically interact with each other both in vivo and in vitro, and this interaction requires the PH domain of PKB. (A) L6 myotubes were incubated in the absence or presence of C2-ceramide (100 μM) for 2 h and/or with insulin (100 nM) in the penultimate 10-min period prior to cell lysis. PKBα was immunoprecipitated from cell lysates and resolved by SDS-PAGE prior to immunoblotting with antibodies to PKB or PKCζ. (B) Far-Western analysis was performed by resolving GST-tagged PKCζ (0.1 μg of protein) and whole-cell lysates from L6 cells (WCL, 50 μg of protein) by SDS-PAGE, followed by immobilization on polyvinylidene difluoride (PVDF) membranes. Membranes weresubsequently incubated overnight at 4°C with either 0.1 μg of recombinant PKB or 250 μg of whole-cell lysates prior to immunoblotting with antibodies directed against PKCζ (lane 1) or PKB (lanes 2 and 3). As a negative control, membranes retaining PKCζ were also probed with an antibody directed to c-myc (lane 4) after overnight incubation with whole-cell lysates. Expression of c-myc in L6 lysates was confirmed by probing whole-cell lysates with a c-myc antibody (lane 5). (C) To assess the importance of the PKB-PH domain for kinase interaction, far-Western analysis was performed by resolving 50 μg of whole-cell lysates and 0.1 μg of GST-PKCζ protein by SDS-PAGE, followed by immobilization of PKCζ on PVDF membranes. Membranes were subsequently incubated overnight at 4°C with either 0.1 μg of recombinant PKB or 0.1 μg of recombinant PKB lacking its PH domain (PKBΔPH) prior to immunoblotting with antibodies to PKCζ (lanes 1 and 2) or PKB (lanes 3 to 6). To confirm the presence of PKCζ on the membranes, lanes 3 to 6 were subsequently stripped and probed with an antibody to PKCζ.
In addition to the interaction observed in vivo, we also observed that PKB and PKCζ can interact in vitro, as assessed by using a far-Western (protein-protein overlay) assay. Recombinant PKB or PKB present within whole-cell lysates was capable of associating with PKCζ (Fig. 4B, lanes 2 and 3). In contrast, c-myc did not show any detectable binding to PKCζ, suggesting that the observed interaction between PKB and PKCζ was unlikely to be nonspecific (Fig. 4B, lane 5). Our in vivo and in vitro data are consistent with previous work showing that PKCζ interacts with PKB via its Akt homology domain (9, 22, 33, 35). Since ceramide inhibits the cell surface recruitment of the isolated PH domain of PKB in fibroblasts (45), we investigated whether the interaction between the two kinases requires the PH domain of PKB. Far-Western analysis revealed that only PKB possessing its PH domain was capable of interacting with PKCζ (Fig. 4C).
Activated PKCζ suppresses the binding of PIP3 to the PH-domain of PKB.
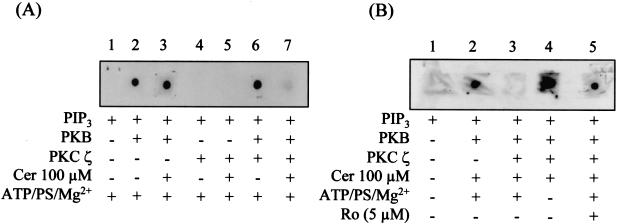
Since the PH domain of PKB plays a critical role in 3-phosphoinositide binding, it is plausible that the association of PKCζ with this domain inhibits PIP3 binding, resulting in loss of PKB activation. A protein-lipid overlay assay, which allows a qualitative assessment of the binding between PIP3 and PKB (23), was performed to test this possibility. Figure 5A shows that PKB binds to PIP3 spotted on nitrocellulose filters and that this interaction was not affected by the presence of ceramide (lane 3), an observation in line with previous work (26). PKCζ did not bind PIP3, and this interaction was not promoted even in the presence of ceramide (Fig. 5A, lanes 4 and 5). Moreover, the presence of PKCζ in the in vitro assay did not suppress the binding of PKB to PIP3. However, in vitro activation of PKCζ, achieved by inclusion of ceramide in the incubation solution, led to a dramatic loss in PKB binding to PIP3 (Fig. 5A, lane 7). To further substantiate that activation of PKCζ interferes with the PKB-PIP3 binding, additional experimental controls were performed in which ATP, PS and Mg2+ (required to support PKCζ activation) were either excluded, or Ro 31.8220 (PKC inhibitor) was added to the incubation solution. Under these conditions, the inclusion of ceramide did not inhibit the interaction between PKB and PIP3 (Fig. 5B, lanes 4 and 5).
FIG. 5.
Protein-lipid overlay assay showing that in vitro activation of PKCζ by ceramide leads to a loss in PIP3 binding to PKB. (A) Nitrocellulose membranes were spotted with 1 μl of 500 pM PIP3. Membranes were then incubated overnight at 4°C in TBST buffer containing 1 μM ATP, 4 pg of PS/ml, and 5 mM MgCl2. This buffer also contained or lacked GST-PKB (0.5 μg/ml), GST-PKCζ (0.5 μg/ml), and/or C2-ceramide (Cer, 100 μM) as indicated. Membranes were subsequently washed, and bound PKB was detected by probing with an anti-GST antibody. (B) To assess the importance of PKCζ activation in influencing the binding of PIP3 to PKB, the experiment in panel A was repeated but with TBST buffer that either lacked ATP, PS, and MgCl2 or which had been supplemented with Ro 31.8220 (Ro, 5 μM), as indicated.
PKCζ phosphorylates the PH domain of PKB.
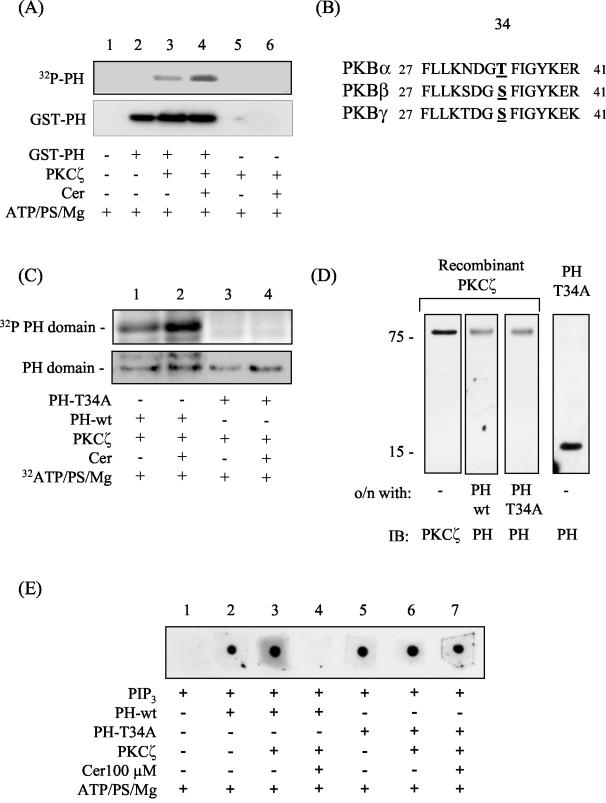
Since the interaction between PKCζ and PKB requires the PH domain of PKB and binding of PIP3 to this domain can be suppressed by ceramide-activated PKCζ, we hypothesized that PKCζ may phosphorylate the PH domain. To test this hypothesis, we assessed whether PKCζ phosphorylates the isolated PKB PH domain in vitro. Figure 6A shows that, in the absence of ceramide but in the presence of ATP, PS, and MgCl2, PKCζ induces a detectable incorporation of labeled phosphate into the PH domain (lane 3). The incorporation of label was increased by ∼3-fold upon activation of PKCζ by inclusion of ceramide in the assay mixture (lane 4).
FIG. 6.
In vitro activation of PKCζ by ceramide results in the phosphorylation of the isolated PH domain of PKB on Thr34 with important consequences for PIP3 binding. (A) The isolated PH domain of PKB (1 μg) was incubated in the absence or presence of 30 ng of PKCζ and/or C2-ceramide (Cer, 100 μM) in buffer containing [γ-32P]ATP and cofactors required to support kinase activation for 20 min at 30°C as described in the text. Phosphorylated proteins were then resolved by SDS-PAGE and transferred to PVDF membranes prior to autoradiography and immunoblotting with anti-PH antibodies. (B) To identify putative PKCζ phosphorylation sites within the isolated PH domain of all three PKBisoforms, a web-based motif-scanning analysis tool was used (49). The aligned peptide sequences of all three PKB isoforms are shown and highlight the putative PKCζ phosphorylation site. (C) In vitro phosphorylation of wild-type (PH-wt) and T34A mutant PH (PH-T34A) domains was performed by incubating 30 ng of recombinant PKCζ with the appropriate PH peptide (1 μg of protein) in the presence of 1 μΜ [γ-32P]ATP and cofactors required for supporting kinase activation but in the absence or presence of C2-ceramide (Cer, 100 μM) for 20 min at 30°C as indicated. PH peptides were then resolved by SDS-PAGE and subjected to analysis by autoradiography. Protein loading was subsequently assessed with an antibody directed against the PKB-PH domain. (D) Far-Western analysis was performed by resolving recombinant PKCζ (0.1 μg of protein) by SDS-PAGE and transferring it onto PVDF membranes. The membranes were then incubated overnight at 4°C with either 0.1 μg of PH-wt (lane 2) or 0.1 μg of PH-T34A peptide (lane 3) prior to immunoblotting them with antibodies to PKCζ (lane 1) or the PH domain of PKB (lanes 2 to 4). (E) Protein-lipid overlay was performed to assess PIP3 binding to the PH-wt and T34A-PH peptides. Nitrocellulose membranes were spotted with 1 μl of PIP3 and subsequently incubated overnight at 4°C in TBST buffer containing 1 μM ATP, 4 pg of PS/ml, and 5 mM MgCl2. The incubation buffer either contained or lacked 0.5 μg of the appropriate PH peptide/ml, 0.5 μg of PKCζ/ml, and C2-ceramide (Cer, 100 μM) as indicated. Membranes were washed, and bound PH protein was detected by probing samples with an anti-PH domain antibody.
To identify the site(s) phosphorylated within the PH domain, we utilized a peptide library-based searching algorithm that identifies sequence motifs likely to be phosphorylated by specific protein kinases (49). High-stringency analysis revealed a single surface-accessible PKCζ phosphorylation site in all three PKB isoforms at residue 34 (Fig. 6B, Thr in PKBα and Ser in PKBβ and PKBγ). To establish whether this site was phosphorylated in vitro by PKCζ, we mutated Thr34 in the PH domain of PKBα to an alanine. Figure 6C shows that ceramide-activated PKCζ phosphorylates the wild-type PH peptide but not the T34A mutant peptide. Since the mutant peptide still interacts with PKCζ, the lack of any detectable phosphorylation cannot be attributed to a loss in its physical interaction with the kinase (Fig. 6D). This finding implies that, although Thr34 is phosphorylated by PKCζ, it is unlikely to play a critical role in supporting the interaction between the two kinases.
Since activation of PKCζ inhibits PIP3 binding to PKB (presumably due to phosphorylation of site 34 within the PH domain), we investigated whether the T34A-PH protein retains the capacity to bind PIP3 in the presence of active PKCζ. Figure 6E shows that the wild-type PH protein binds PIP3 (lanes 2 and 3), but its ability to do so is lost in the presence of ceramide-activated PKCζ (lane 4). Τhe T34A-PH protein also binds PIP3 (Fig. 6E, lanes 5 and 6) but, unlike the wild-type protein fragment, it retains the ability to bind PIP3 even in the presence of an active PKCζ (Fig. 6E, compare lanes 4 and 7). It is plausible that the T34A-PH peptide may have a higher binding affinity for PIP3 than the wild-type PH fragment, which could potentially explain the observed binding of PIP3 to the mutated PH fragment in the presence of activated PKCζ. However, using a sensitive FRET-based assay that monitors the displacement of biotinylated-PIP3 from GST-tagged PH peptide by nonbiotinylated lipid (24), we could not detect any significant differences in the PIP3 displacement profiles between the wild type and the T34A PH peptide (data not shown).
PKB is phosphorylated on Thr34 in response to ceramide in vivo.
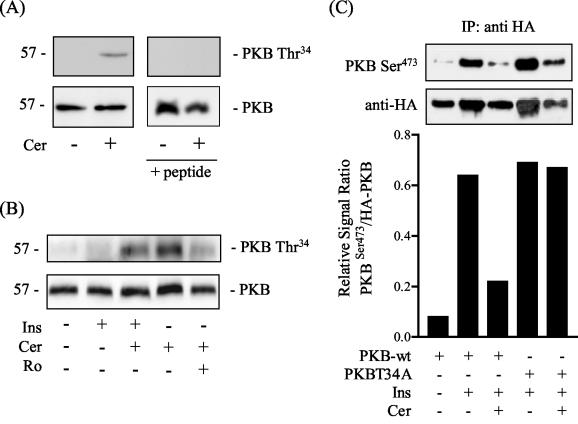
To establish whether ceramide induces phosphorylation of PKB on Thr34 in intact cells, we probed cell lysates with a phospho-specific antibody against this site. Figure 7A shows that the antibody detected an immunoreactive protein band corresponding to PKB from ceramide-treated cells but not from untreated cells. The specificity of this signal was verified by demonstrating that antisera that had been preadsorbed with the antigenic phospho-peptide failed to detect the phosphorylated kinase. We subsequently investigated whether the ceramide-induced phosphorylation of this site could be influenced by insulin and Ro 31.8220. Figure 7B shows that insulin per se had no effect on Thr34 and, moreover, does not appear to antagonize phosphorylation of this site in response to ceramide. However, incubation of cells with 5 μM Ro 31.8220 prior to treatment with ceramide led to a noticeable reduction in the phosphorylation of Thr34.
FIG. 7.
Ceramide induces phosphorylation of PKB on Thr34 in intact cells and a PKB T34A mutant is ceramide resistant. (A) L6 myotubes were incubated in the absence or presence of C2-ceramide (Cer, 100 μM) for 2 h prior to cell lysis. Cell lysates were resolved by SDS-PAGE prior to immunoblotting with an antibody to PKB, a phospho-specific antibody to PKB-Thr34, or anti-PKB-Thr34 that had been preadsorbed the antigenic phospho-peptide (100 μg/ml). (B) Myotubes were treated as in panel A but, in addition, were also exposed to insulin (Ins, 100 nM for 10 min) or pretreated with Ro 31.8220 (Ro, 5 μM) prior to incubation with Cer. Cells were lysed and immunoblotted with antibodies to PKB or PKBThr34. (C) HA-tagged PKB (wild type) and HA-tagged PKB T34A were transiently transfected into L6 cells as described. Cells were exposed to C2-ceramide (Cer, 100 μM, 2 h) and or insulin (Ins, 100 nM, 10 min) prior to cell lysis and immunoprecipitation with an anti-HA antibody. Precipitated kinases were resolved by SDS-PAGE and immunoblotted with antibodies to PKB-Ser473 or anti-HA. Phospho-PKB-Ser473- and HA-immunoreactive bands were quantified and are expressed as a ratio (lower panel).
We postulated that if phosphorylation of PKB on Thr34 contributes to its inhibition by ceramide, then a PKB T34A mutant should be resistant to ceramide. To test this, we transiently expressed HA-tagged wild-type PKB and HA-tagged PKB T34A into L6 cells. Cells expressing these kinases were then incubated with ceramide and/or insulin prior to immunoprecipitation and immunoblotting with an antibody to PKB-Ser473 or HA. Figure 7C shows that wild-type PKB is phosphorylated on Ser473 in response to insulin but that this was reduced by incubation of cells with ceramide. The PKB T34A mutant was also phosphorylated on Ser473 by insulin treatment but, in contrast to the wild-type kinase, phosphorylation of this site was retained in cells incubated with ceramide. Owing to slight variations in the amounts of the HA-tagged kinases that were immunoprecipitated among the different experimental treatments, the immunoblot data were quantified and normalized by expressing them as ratios of the signal intensities of Ser473 to HA (Fig. 7C, lower bar panel).
Physiological implications.
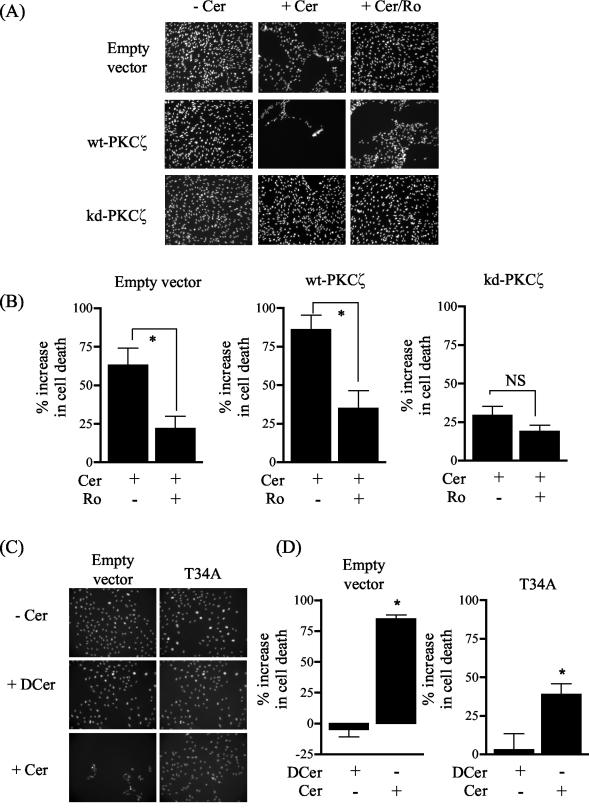
Previous work has shown that PKCζ plays a key role in inducing growth arrest in vascular smooth muscle cells in response to ceramide and that a key feature of this mechanism is a loss in PKB activation (9). L6 myotubes are terminally differentiated syncytia and, as such, display minimal cell cycle or growth activity. Thus, to assess the importance of PKCζ in ceramide-induced cell death, we transfected wild-type PKCζ, kd-PKCζ, and the PKB T34A mutant into L6 myoblasts. PKB activation is also diminished by ceramide in mononucleated muscle cells (data not shown) but myoblasts, unlike myotubes, exhibit significant cell cycle and growth activity and display a far greater sensitivity to ceramide; consequently, they are more suited for cell viability studies. Based on nuclear counting of adherent viable cells in several randomly chosen visual fields, ceramide treatment led to an ∼60% loss in the number of myoblasts expressing the empty expression vector compared to an untreated cell population (Fig. 8A and B). The sensitivity to ceramide was enhanced in cells overexpressing the wild-type PKCζ on the basis that cell viability was reduced further (∼80%, Fig. 8A and B). However, the increase in cell loss was not apparent when control (empty vector) or wild-type expressing cells were incubated with the inactive C2-dihydroceramide analogue (data not shown). Moreover, the effects of ceramide could be significantly reduced by pretreating cells with 5 μM Ro 31.8220 (Fig. 8A and B). Interestingly, expression of the kd-PKCζ in L6 myoblasts conferred significant resistance to cell death induced by ceramide (Fig. 8A and B). These findings are consistent with the notion that PKCζ plays an important role in reducing cell survival rate in response to ceramide. Since PKB activation would be suppressed under circumstances when PKCζ is activated by ceramide (Fig. 1), we subsequently assessed whether expression of a PKB T34A mutant offered any protection against the death-inducing effects of ceramide. Figure 8C and D show that the ability of ceramide to promote cell loss was lower in cells expressing the PKB T43A mutant compared to that of a control cell population transfected with the empty expression vector. Interestingly, we also noted in separate experiments that myoblasts stably expressing a constitutively active membrane-targeted PKB that is resistant to inhibition by ceramide (26, 46) also exhibit a higher survival potential in the presence of ceramide (data not shown).
FIG. 8.
Effects of ceramide on muscle cell viability. L6 myoblasts transiently transfected with pCMV5 lacking or containing cDNA encoding wild-type PKCζ, kd-PKCζ, or PKB T34A were incubated with either vehicle alone (dimethyl sulfoxide), C2-ceramide (Cer, 100 μM, 2 h), or C2-ceramide (100 μM, 2 h) plus Ro 31.8220 (5 μM, added 15 min prior to ceramide). Ceramide promotes detachment and death of myoblasts, and thus viable cells were those that remained adherent, displayed trypan blue exclusion, and stained positively for DAPI. (A and C) Representative images of DAPI-stained L6 cells; (B and D) quantitative analysis of viable cells from five randomly chosen visual fields. Cell loss was expressed as a percentage change in cell number relative to that from the appropriate untreated experimental cell population. Asterisks signify significant changes (P < 0.05) between the indicated bars or compared to an untreated cell population, as determined by one-way analysis of variance.
DISCUSSION
Previous work from our group has shown that C2-ceramide inhibits the hormonal activation of PKB in L6 muscle cells by suppressing its cell surface recruitment despite the fact that insulin enhances cellular PIP3 levels (26). This finding is in agreement with a similar study showing that ceramide blocks the stimulus-induced recruitment of the PH domain of PKB in NIH 3T3 fibroblasts (45). Collectively, these observations suggest that ceramide may either directly regulate PKB recruitment via interaction with its PH domain or alternatively targets an ancillary molecule(s) involved in regulating its translocation and activation. There is no evidence supporting the former possibility in the literature and, indeed, we have shown previously that ceramide does not associate with the PH domain of PKB nor does it compete or interfere with PIP3 binding to the kinase (26). Here we show that another member of the AGC family of kinases, atypical PKCζ, interacts with the PH domain of PKB and that it is activated by ceramide. We present novel data showing that activation of PKCζ results in phosphorylation of Thr34 in the PH domain of PKBα and that this leads to a loss in PIP3 binding to the kinase PH domain in vitro. Suppressing PKCζ activation by using chemical or peptide-based inhibitors or by expressing a dominant-interfering kd-PKCζ, alleviates the inhibitory effect of ceramide on PIP3 binding to the PH domain of PKB and its activation by insulin.
The finding that PKCζ interacts with PKB is not unprecedented. Konishi et al. first reported this interaction in COS-7 cells nearly a decade ago (33). Subsequently, work by other investigators has shown that these two kinases can form stable complexes in CHO (22), breast cancer (35), and vascular smooth muscle (9) cells and that, within these complexes, PKCζ appears to negatively regulate PKB activity. The interaction between the two kinases would be expected to be regulated in order to allow the positive flow of signals through the PI3K/PKB pathway in response to stimuli such insulin and growth factors. This proposition is indeed supported by the finding that insulin and platelet-derived growth factor induce dissociation of PKB and PKCζ in L6 (Fig. 4A) and COS-1 cells (22), respectively. Precisely how dissociation of the complex is achieved is understood poorly, but it has been suggested that PKB activity is a key requirement for this event, whereas activation of PKCζ appears not to be crucial in this regard (22). Nevertheless, it is difficult to negate the possibility that PKCζ activity may serve to stabilize or prevent dissociation of the complex based on the observation that ceramide, which activates PKCζ, not only enhances the association between the two kinases but also blocks insulin's ability to dissociate the complex (Fig. 4A). In addition, the demonstration that PKC inhibitors and the expression of an inactive PKCζ alleviate the negative regulation of PKB strengthens the idea that PKCζ activity exerts a powerful influence on PKB signaling (Fig. 1 and 3) (22, 35).
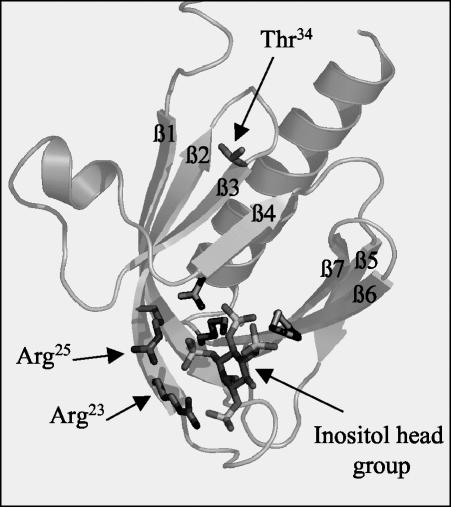
A key issue that has remained poorly understood concerns the mechanism by which ceramide inhibits PKB activation. In some cell types, such as PC12 and C2C12 cells, ceramide activates a protein phosphatase 2A-like activity, which dephosphorylates PKB on Thr308 and Ser473 in an okadaic acid-sensitive manner (14, 40). However, in L6 muscle cells and 3T3-L1 adipocytes, okadaic acid does not antagonize ceramide action on PKB (26, 46). The data presented here indicate that ceramide suppresses PKB activation by modulating the activity of PKCζ and by stabilizing its interaction with PKB. Recently, both mutational analysis and a high-resolution crystal structure of the isolated PKB-PH domain complexed to PIP3 have revealed that the key amino acid residues that interact with the inositol head group of PIP3 are localized within the first two beta sheets of the PH domain (47). Since PKCζ also binds to this region (33), it is conceivable that its association with PKB results in the competitive exclusion of PIP3 with a subsequent loss of PKB activation. However, our lipid-overlay data (Fig. 5A) indicate that in the absence of ceramide, PKCζ, and PIP3 do not compete for PKB binding. Moreover, previous work has shown that PKB molecules can form homotypic complexes via interactions formed between their PH domains at regions that overlap with those important for 3-phosphoinositide binding (19). Despite the apparent overlap in the lipid and protein binding regions, it has been proposed that these complexes can be activated, implying that their formation per se is unlikely to hinder PIP3 binding to the PH domain (16, 19). However, when PKB and PKCζ were incubated in the presence of ceramide there was a dramatic loss in PIP3 binding to PKB (Fig. 5A). Our findings indicate that this loss stems from ceramide's ability to activate PKCζ, which then phosphorylates the PKB PH domain at Thr34. The significance of this phosphorylation in regulating PIP3 binding to PKB is underscored by the finding that a T34A PH domain mutant retains the ability to bind PIP3 and to be activated by insulin in intact cells even in the presence of ceramide (Fig. 6E and 7C). Since binding of PIP3 to PKB is considered a prerequisite for recruiting the kinase to the plasma membrane prior to phosphorylation by PDK1 and PDK2, the inability to bind this lipid in ceramide treated cells would account for the loss in cell surface PKB recruitment previously reported (26). Thr34 is located within a region of the PH domain that is five and seven amino acids downstream, respectively, from Arg25 and Arg23, which play a critical role in PIP3 binding (47) (Fig. 9). Initial analysis of the crystal structure of the PKB-PH domain suggests that Thr34 is sufficiently removed from these critical arginine residues to have any direct effect on PIP3 binding. However, it is plausible that phosphorylation of Thr34 may instigate changes in the conformation of the PKB-PH domain that either prevent PIP3 binding through steric exclusion or, alternatively, affects the affinity of the kinase for the 3-phosphoinositide as a result of space-charge repulsions from the negative phosphate groups.
FIG. 9.
Structure of PKBαPH complexed to Ins(1,3,4,5)P4. A ribbon structure of the PH domain of PKBα, depicting the localization of Thr34 relative to that of Arg25 and Arg23, which play a critical role in the binding of the inositol head group, is shown. Also labeled are the seven β strands (labeled β1 to β7). The ribbon drawing is based on the crystal structure proposed by Thomas et al. (47).
Our observation (Fig. 4A) and that of others (9, 22) that PKCζ can form stable complexes with PKB prompts us to wonder what relevance this interaction may have in unstimulated cells. One recent study has proposed that this interaction may be important for recruiting a “PDK2-like” activity to this complex, which phosphorylates the regulatory Ser residue in the C terminus of PKB (29). If this model of PKB activation is accepted, then PKCζ may normally serve to deliver PKB to the cell surface and place it in close proximity to its upstream kinases prior to dissociating from the complex. However, this “delivery” process may become impaired when PKCζ is activated by ceramide as a result of its ability to block the interaction between PIP3 and the PKB-PH domain, a step, which, as already indicated, is essential for recruiting PKB to the cell surface. The finding that ceramide inhibits the cell surface recruitment of PKB but fails to suppress the activity of a membrane-targeted form of the kinase in muscle and fat cells is consistent with such a proposition (26, 46).
Reports showing that PKCζ can be activated by insulin and growth factors in a PI3K-dependent manner (1, 43) support a positive role for the kinase in cell signaling. However, evidence presented here and by others (9, 22) suggests that PKCζ can also regulate cell signaling in a negative fashion. Such diversity in PKCζ function has been documented previously, and there is now growing recognition that the kinase acts as a molecular switch which, depending on the activating stimulus, either promotes or inhibits cell signaling (37). Since the ability of PKCζ to modulate PKB signaling is stimulus dependent, establishing the mechanism by which different stimuli modulate PKCζ, including their site of action, is likely to be important for understanding the control of PKB-regulated cell functions. Ceramide production at the plasma membrane can be enhanced significantly in response to numerous stress-inducing stimuli such as cytokines (e.g., TNF-α), heat shock, and oxidants (32). Indeed, the localization of the TNF-α receptor and sphingomyelinases within caveola-like microdomains in the plasma membrane (30) provides for an extremely effective way of producing a highly localized concentration of ceramide (21). Approximately 70% of the cellular ceramide is thought to be produced in such membrane domains (21), which may serve to localize proteins that bind the lipid with high affinity, such as PKCζ (37). This proposition is supported by evidence that PKCζ interacts with caveolin and that PKC isoforms can accumulate in caveolae (38). Thus, it is tempting to speculate that an increase in ceramide production in caveola-like microdomains may not only help localize and activate PKCζ but also sequester the PKB-PKCζ complex. If the complex does localize to caveola-like domains and is spatially segregated from the upstream PKB kinases, then this may provide a mechanism for maintaining PKB in a repressed state. Testing these possibilities will be important investigative goals for future work.
Ceramide's ability to suppress PKB activation has important implications for numerous cellular responses. It is well documented, for example, that ceramide has negative effects on cell growth and survival (36), which in many cell types has been linked to an inhibition in PKB activation (26, 40, 41, 46, 50). In L6 skeletal muscle cells and in vascular smooth muscle cells (9), this inhibition is mediated via activation of PKCζ by ceramide with important consequences for cell viability. Indeed, the ability of PKC inhibitors and of ceramide-resistant forms of PKB to impede ceramide-induced cell death (Fig. 8) implies that the regulation of PKB by PKCζ is likely to be of physiological significance especially under circumstances when intracellular ceramide synthesis may be modulated (e.g., in response to TNF-α, free fatty acids, hyperosmotic stress, and UV radiation, as well as certain anti-cancer drug therapies) (31, 36, 51). Thus, a greater understanding of the physical interplay between PKB and PKCζ is likely to be of significant value for developing novel strategies aimed at controlling cell growth, survival, and death.
In summary, the work presented here demonstrates that in our experimental system PKB interacts with PKCζ via its PH domain and that this association is reduced upon treating muscle cells with insulin. However, when muscle cells are incubated with C2-ceramide not only is insulin's capacity to dissociate this kinase complex impaired, but the hormone fails to induce activation of PKB. Under these circumstances, ceramide stimulates PKCζ, which phosphorylates the PH domain of PKB on Thr34. Phosphorylation of this site suppresses the binding of PIP3 to the PH domain of PKB, thereby severely limiting its activation in response to insulin. Since PKB is thought to play a key role in regulating cell survival and insulin action, the ability of ceramide to suppress PKB signaling via regulation of PKCζ provides one potential mechanism by which the lipid may promote cell death and induce insulin resistance.
Acknowledgments
We are grateful to Dario Alessi, Philip Cohen, and Peter Downes for making available some of the reagents used in our studies. We thank Daan van Aalten for providing the structural image of the isolated PH domain of PKB in complex with the head group of PIP3, and we thank Alex Gray (DSTT) and Maria Deak (MRC phosphorylation unit) for technical input in some of the experimental work.
This work was supported by the MRC, the Diabetes Research and Wellness Foundation, and Diabetes UK.
REFERENCES
- 1.Akimoto, K., R. Takahashi, S. Moriya, N. Nishioka, J. Takayanagi, K. Kimura, Y. Fukui, S. Osada, K. Mizuno, S. Hirai, A. Kazlauskas, and S. Ohno. 1996. EGF or PDGF receptors activate atypical PKCλ through phosphatidylinositol 3-kinase. EMBO J. 15:788-798. [PMC free article] [PubMed] [Google Scholar]
- 2.Alessi, D. R. 1997. The protein kinase C inhibitors RO318220 and GF109203X are equally potent inhibitors of MAPKAP kinase-1β (Rsk-2) and p70 s6 kinase. FEBS Lett. 402:121-123. [DOI] [PubMed] [Google Scholar]
- 3.Alessi, D. R., and P. Cohen. 1998. Mechanism of activation and function of protein kinase B. Curr. Opin. Genet. Dev. 8:55-62. [DOI] [PubMed] [Google Scholar]
- 4.Alessi, D. R., S. R. James, C. P. Downes, A. B. Holmes, P. R. Gaffney, C. B. Reese, and P. Cohen. 1997. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr. Biol. 7:261-269. [DOI] [PubMed] [Google Scholar]
- 5.Andjelkovic, M., D. R. Alessi, R. Meier, A. Fernandez, N. J. C. Lamb, M. Frech, P. Cron, P. Cohen, J. M. Lucocq, and B. A. Hemmings. 1997. Role of translocation in the activation and function of protein kinase B. J. Biol. Chem. 272:31515-31524. [DOI] [PubMed] [Google Scholar]
- 6.Balendran, A., G. R. Hare, A. Kieloch, M. R. Williams, and D. R. Alessi. 2000. Further evidence that 3-phosphoinositide-dependent protein kinase-1 (PDK1) is required for the stability and phosphorylation of protein kinase C (PKC) isoforms. FEBS Lett. 484:217-223. [DOI] [PubMed] [Google Scholar]
- 7.Bandyopadhyay, G., M. L. Standaert, M. P. Sajan, L. M. Karnitz, L. Cong, M. J. Quon, and R. V. Farese. 1999. Dependence of insulin-stimulated glucose transporter 4 translocation on 3-phosphoinositide-dependent protein kinase-1 and its target threonine-410 in the activation loop of protein kinase Cζ. Mol. Endocrinol. 13:1766-1772. [DOI] [PubMed] [Google Scholar]
- 8.Bieberich, E., T. Kawaguchi, and R. K. Yu. 2000. N-acylated serinol is a novel ceramide mimic inducing apoptosis in neuroblastoma cells. J. Biol. Chem. 275:177-181. [DOI] [PubMed] [Google Scholar]
- 9.Bourbon, N. A., L. Sandirasegarane, and M. Kester. 2002. Ceramide-induced inhibition of Akt is mediated through protein kinase Cζ: implications for growth arrest. J. Biol. Chem. 277:3286-3292. [DOI] [PubMed] [Google Scholar]
- 10.Bourbon, N. A., J. Yun, and M. Kester. 2000. Ceramide directly activates protein kinase C ζ to regulate a stress-activated protein kinase signaling complex. J. Biol. Chem. 275:35617-35623. [DOI] [PubMed] [Google Scholar]
- 11.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 12.Braiman, L., A. Alt, T. Kuroki, M. Ohba, A. Bak, T. Tennenbaum, and S. R. Sampson. 2001. Activation of protein kinase C ζ induces serine phosphorylation of VAMP2 in the GLUT4 compartment and increases glucose transport in skeletal muscle. Mol. Cell. Biol. 21:7852-7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brazil, D. P., J. Park, and B. A. Hemmings. 2002. PKB binding proteins: getting in on the Akt. Cell 111:293-303. [DOI] [PubMed] [Google Scholar]
- 14.Cazzolli, R., L. Carpenter, T. J. Biden, and C. Schmitz-Peiffer. 2001. A role for protein phosphatase 2A-like activity, but not atypical protein kinase Cζ, in the inhibition of protein kinase B/Akt and glycogen synthesis by palmitate. Diabetes 50:2210-2218. [DOI] [PubMed] [Google Scholar]
- 15.Chan, T. O., and P. N. Tsichlis. 2001. PDK2: a complex tail in one Akt. Science STKE 2001:E1. [DOI] [PubMed] [Google Scholar]
- 16.Coffer, P. J., J. Jin, and J. R. Woodgett. 1998. Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem. J. 335:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cross, D. A. E., D. R. Alessi, P. Cohen, M. Andjelkovic, and B. A. Hemmings. 1995. Inhibition of glycogen synthase kinase-3 by insulin-mediated protein kinase B. Nature 378:785-789. [DOI] [PubMed] [Google Scholar]
- 18.Das, M., K. R. Stenmark, L. J. Ruff, and E. C. Dempsey. 1997. Selected isozymes of PKC contribute to augmented growth of fetal and neonatal bovine PA adventitial fibroblasts. Am. J. Physiol. 273:L1276-L1284. [DOI] [PubMed] [Google Scholar]
- 19.Datta, K., T. F. Franke, T. O. Chan, A. Makris, S. I. Yang, D. R. Kaplan, D. K. Morrison, E. A. Golemis, and P. N. Tsichlis. 1995. AH/PH domain-mediated interaction between Akt molecules and its potential role in Akt regulation. Mol. Cell. Biol. 15:2304-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davies, S. P., H. Reddy, M. Caivano, and P. Cohen. 2000. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 351:95-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dobrowsky, R. T. 2000. Sphingolipid signalling domains floating on rafts or buried in caves? Cell Signal 12:81-90. [DOI] [PubMed] [Google Scholar]
- 22.Doornbos, R. P., M. Theelen, P. C. van der Hoeven, W. J. van Blitterswijk, A. J. Verkleij, and van Bergen en Henegouwen PM. 1999. Protein kinase Cζ is a negative regulator of protein kinase B activity. J. Biol. Chem. 274:8589-8596. [DOI] [PubMed] [Google Scholar]
- 23.Dowler, S., G. Kular, and D. R. Alessi. 2002. Protein lipid overlay assay. Science STKE 2002:L6. [DOI] [PubMed] [Google Scholar]
- 24.Gray, A., H. Olsson, I. H. Batty, L. Priganica, and C. P. Downes. 2003. Nonradioactive methods for the assay of phosphoinositide 3-kinases and phosphoinositide phosphatases and selective detection of signaling lipids in cell and tissue extracts. Anal. Biochem. 313:234-245. [DOI] [PubMed] [Google Scholar]
- 25.Hajduch, E., D. R. Alessi, B. A. Hemmings, and H. S. Hundal. 1998. Constitutive activation of protein kinase Bα (PKBα) by membrane targeting promotes glucose and system A amino acid transport, protein synthesis and GSK3 inactivation in L6 muscle cells. Diabetes 47:1006-1013. [DOI] [PubMed] [Google Scholar]
- 26.Hajduch, E., A. Balendran, I. H. Batty, G. J. Litherland, A. S. Blair, C. P. Downes, and H. S. Hundal. 2001. Ceramide impairs the insulin-dependent membrane recruitment of protein kinase B leading to a loss in downstream signalling in L6 skeletal muscle cells. Diabetologia 44:173-183. [DOI] [PubMed] [Google Scholar]
- 27.Hajduch, E., G. J. Litherland, and H. S. Hundal. 2001. Protein kinase B: a key regulator of glucose transport? FEBS Lett. 492:199-203. [DOI] [PubMed] [Google Scholar]
- 28.Hannun, Y. A., and L. M. Obeid. 2002. The Ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J. Biol. Chem. 277:25847-25850. [DOI] [PubMed] [Google Scholar]
- 29.Hodgkinson, C. P., E. M. Sale, and G. J. Sale. 2002. Characterization of PDK2 activity against protein kinase B gamma. Biochemistry 41:10351-10359. [DOI] [PubMed] [Google Scholar]
- 30.Ko, Y. G., J. S. Lee, Y. S. Kang, J. H. Ahn, and J. S. Seo. 1999. TNF-alpha-mediated apoptosis is initiated in caveolae-like domains. J. Immunol. 162:7217-7223. [PubMed] [Google Scholar]
- 31.Kolesnick, R. 2002. The therapeutic potential of modulating the ceramide/sphingomyelin pathway. J. Clin. Investig. 110:3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolesnick, R. N., and M. Kronke. 1998. Regulation of ceramide production and apoptosis. Annu. Rev. Physiol. 60:643-665. [DOI] [PubMed] [Google Scholar]
- 33.Konishi, H., S. Kuroda, and U. Kikkawa. 1994. The pleckstrin homology domain of RAC protein kinase associates with the regulatory domain of protein kinase C ζ. Biochem. Biophys. Res. Commun. 205:1770-1775. [DOI] [PubMed] [Google Scholar]
- 34.Lawlor, M. A., and D. R. Alessi. 2001. PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J. Cell Sci. 114:2903-2910. [DOI] [PubMed] [Google Scholar]
- 35.Mao, M., X. Fang, Y. Lu, R. Lapushin, J. R. Bast, and G. B. Mills. 2000. Inhibition of growth-factor-induced phosphorylation and activation of protein kinase B/Akt by atypical protein kinase C in breast cancer cells. Biochem. J. 352:475-482. [PMC free article] [PubMed] [Google Scholar]
- 36.Mathias, S., L. A. Pena, and R. N. Kolesnick. 1998. Signal transduction of stress via ceramide. Biochem. J. 335:465-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muller, G., M. Ayoub, P. Storz, J. Rennecke, D. Fabbro, and K. Pfizenmaier. 1995. PKCζ is a molecular switch in signal transduction of TNF-α, bifunctionally regulated by ceramide and arachidonic acid. EMBO J. 14:1961-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oka, N., M. Yamamoto, C. Schwencke, J. Kawabe, T. Ebina, S. Ohno, J. Couet, M. P. Lisanti, and Y. Ishikawa. 1997. Caveolin interaction with protein kinase C: isoenzyme-dependent regulation of kinase activity by the caveolin scaffolding domain peptide. J. Biol. Chem. 272:33416-33421. [DOI] [PubMed] [Google Scholar]
- 39.Puceat, M., R. Hilal-Dandan, B. Strulovici, L. L. Brunton, and J. H. Brown. 1994. Differential regulation of protein kinase C isoforms in isolated neonatal and adult rat cardiomyocytes. J. Biol. Chem. 269:16938-16944. [PubMed] [Google Scholar]
- 40.Salinas, M., R. Lopez-Valdaliso, D. Martin, A. Alvarez, and A. Cuadrado. 2000. Inhibition of PKB/Akt1 by C2-ceramide involves activation of ceramide-activated protein phosphatase in PC12 cells. Mol. Cell. Neurosci. 15:156-169. [DOI] [PubMed] [Google Scholar]
- 41.Schmitz-Peiffer, C., D. L. Craig, and T. J. Biden. 1999. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J. Biol. Chem. 274:24202-24210. [DOI] [PubMed] [Google Scholar]
- 42.Standaert, M. L., G. Bandyopadhyay, Y. Kanoh, M. P. Sajan, and R. V. Farese. 2001. Insulin and PIP3 activate PKC-ζ by mechanisms that are both dependent and independent of phosphorylation of activation loop (T410) and autophosphorylation (T560) sites. Biochemistry 40:249-255. [DOI] [PubMed] [Google Scholar]
- 43.Standaert, M. L., L. Galloway, P. Karnam, G. Bandyopadhyay, J. Moscat, and R. V. Farese. 1997. Protein kinase C-ζ as a downstream effector of phosphatidylinositol 3-kinase during insulin stimulation in rat adipocytes: potential role in glucose transport. J. Biol. Chem. 272:30075-30082. [DOI] [PubMed] [Google Scholar]
- 44.Stokoe, D., L. R. Stephens, T. Copeland, P. R. J. Gaffney, C. B. Reese, G. F. Painter, A. B. Holmes, F. McCormick, and P. T. Hawkins. 1997. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science 277:567-570. [DOI] [PubMed] [Google Scholar]
- 45.Stratford, S., D. B. Dewald, and S. A. Summers. 2001. Ceramide dissociates 3′-phosphoinositide production from pleckstrin homology domain translocation. Biochem. J. 354:359-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Summers, S. A., L. A. Garza, H. Zhou, and M. J. Birnbaum. 1998. Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol. Cell. Biol. 18:5457-5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas, C. C., M. Deak, D. R. Alessi, and D. M. van Aalten. 2002. High-resolution structure of the pleckstrin homology domain of protein kinase B/akt bound to phosphatidylinositol-(3,4,5)-trisphosphate. Curr. Biol. 12:1256-1262. [DOI] [PubMed] [Google Scholar]
- 48.Whiteman, E. L., H. Cho, and M. J. Birnbaum. 2002. Role of Akt/protein kinase B in metabolism. Trends Endocrinol. Metab. 13:444-451. [DOI] [PubMed] [Google Scholar]
- 49.Yaffe, M. B., G. G. Leparc, J. Lai, T. Obata, S. Volinia, and L. C. Cantley. 2001. A motif-based profile scanning approach for genome-wide prediction of signaling pathways. Nat. Biotechnol. 19:348-353. [DOI] [PubMed] [Google Scholar]
- 50.Zhou, H., S. A. Summers, M. J. Birnbaum, and R. N. Pittman. 1998. Inhibition of Akt kinase by cell-permeable ceramide and its implications for ceramide-induced apoptosis. J. Biol. Chem. 273:16568-16575. [DOI] [PubMed] [Google Scholar]
- 51.Zundel, W., and A. Giaccia. 1998. Inhibition of the anti-apoptotic PI(3)K/Akt/Bad pathway by stress. Genes Dev. 12:1941-1946. [DOI] [PMC free article] [PubMed] [Google Scholar]