Abstract
A new class of cold shock-induced proteins that may be involved in an adaptive process required for cell viability at low temperatures or may function as antifreeze proteins in Escherichia coli and Saccharomyces cerevisiae has been identified. We purified a small Bacillus subtilis cold shock protein (CspB) and determined its amino-terminal sequence. By using mixed degenerate oligonucleotides, the corresponding gene (cspB) was cloned on two overlapping fragments of 5 and 6 kb. The gene encodes an acidic 67-amino-acid protein (pI 4.31) with a predicted molecular mass of 7,365 Da. Nucleotide and deduced amino acid sequence comparisons revealed 61% identity to the major cold shock protein of E. coli and 43% identity to a family of eukaryotic DNA binding proteins. Northern RNA blot and primer extension studies indicated the presence of one cspB transcript that was initiated 119 bp upstream of the initiation codon and was found to be induced severalfold when exponentially growing B. subtilis cell cultures were transferred from 37 degrees C to 10 degrees C. Consistent with this cold shock induction of cspB mRNA, a six- to eightfold induction of a cspB-directed beta-galactosidase synthesis was observed upon downshift in temperature. To investigate the function of CspB, we inactivated the cold shock protein by replacing the cspB gene in the B. subtilis chromosome with a cat-interrupted copy (cspB::cat) by marker replacement recombination. The viability of cells of this mutant strain, GW1, at freezing temperatures was strongly affected. However, the effect of having no CspB in GW1 could be slightly compensated for when cells were preincubated at 10 degrees C before freezing. These results indicate that CspB belongs to a new type of stress-inducible proteins that might be able to protect B. subtilis cells from damage caused by ice crystal formation during freezing.
Full text
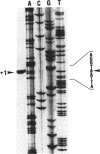
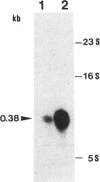
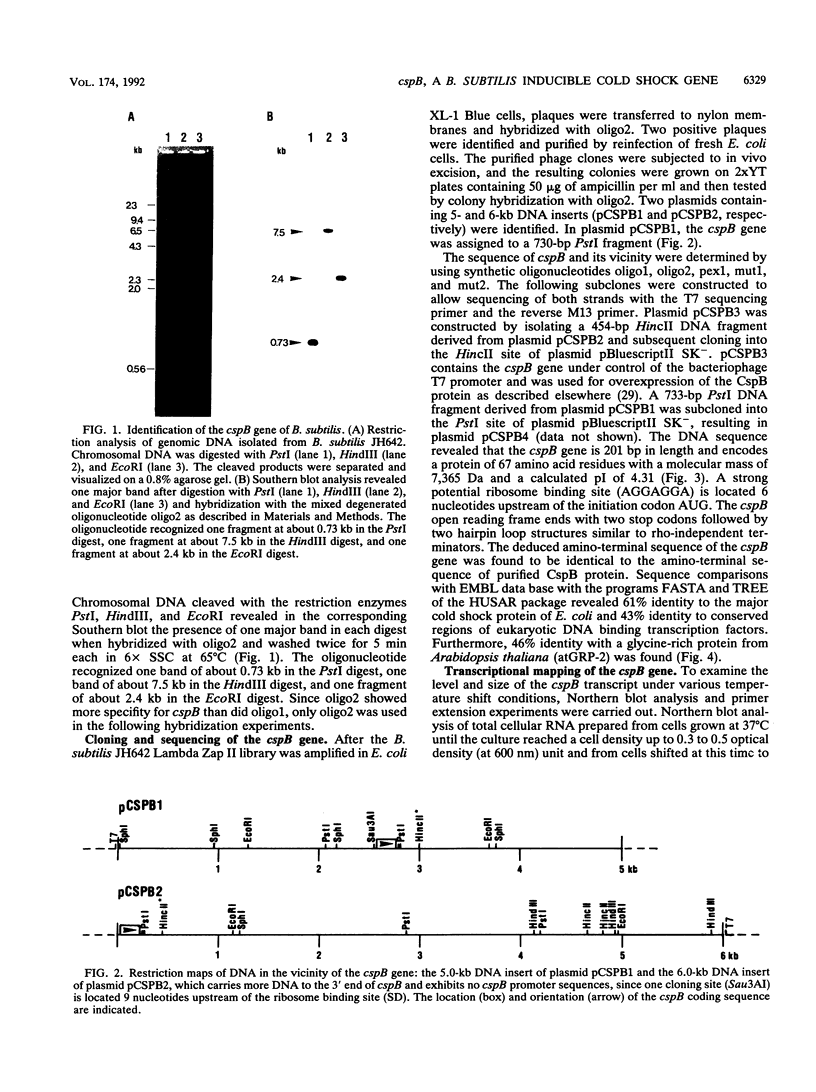
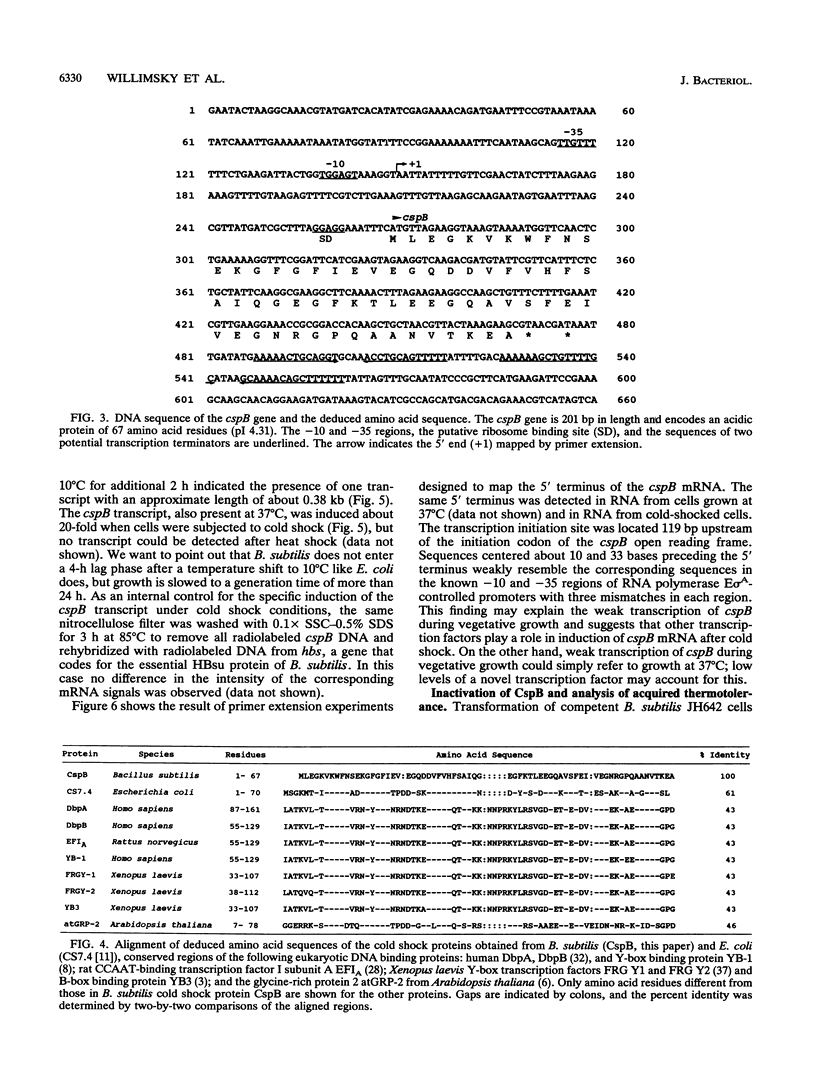
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broeze R. J., Solomon C. J., Pope D. H. Effects of low temperature on in vivo and in vitro protein synthesis in Escherichia coli and Pseudomonas fluorescens. J Bacteriol. 1978 Jun;134(3):861–874. doi: 10.1128/jb.134.3.861-874.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Cohen I., Reynolds W. F. The Xenopus YB3 protein binds the B box element of the class III promoter. Nucleic Acids Res. 1991 Sep 11;19(17):4753–4759. doi: 10.1093/nar/19.17.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad B., Mount D. W. Microcomputer programs for DNA sequence analysis. Nucleic Acids Res. 1982 Jan 11;10(1):31–38. doi: 10.1093/nar/10.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988 Nov 25;16(22):10881–10890. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig E. A., Gross C. A. Is hsp70 the cellular thermometer? Trends Biochem Sci. 1991 Apr;16(4):135–140. doi: 10.1016/0968-0004(91)90055-z. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didier D. K., Schiffenbauer J., Woulfe S. L., Zacheis M., Schwartz B. D. Characterization of the cDNA encoding a protein binding to the major histocompatibility complex class II Y box. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7322–7326. doi: 10.1073/pnas.85.19.7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman J., Horwath K. The role of hemolymph proteins in the cold tolerance of insects. Annu Rev Physiol. 1983;45:261–270. doi: 10.1146/annurev.ph.45.030183.001401. [DOI] [PubMed] [Google Scholar]
- Feng D. F., Doolittle R. F. Progressive alignment and phylogenetic tree construction of protein sequences. Methods Enzymol. 1990;183:375–387. doi: 10.1016/0076-6879(90)83025-5. [DOI] [PubMed] [Google Scholar]
- Goldstein J., Pollitt N. S., Inouye M. Major cold shock protein of Escherichia coli. Proc Natl Acad Sci U S A. 1990 Jan;87(1):283–287. doi: 10.1073/pnas.87.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryczan T. J., Dubnau D. Construction and properties of chimeric plasmids in Bacillus subtilis. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1428–1432. doi: 10.1073/pnas.75.3.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igo M. M., Losick R. Regulation of a promoter that is utilized by minor forms of RNA polymerase holoenzyme in Bacillus subtilis. J Mol Biol. 1986 Oct 20;191(4):615–624. doi: 10.1016/0022-2836(86)90449-3. [DOI] [PubMed] [Google Scholar]
- Jones P. G., Cashel M., Glaser G., Neidhardt F. C. Function of a relaxed-like state following temperature downshifts in Escherichia coli. J Bacteriol. 1992 Jun;174(12):3903–3914. doi: 10.1128/jb.174.12.3903-3914.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. G., VanBogelen R. A., Neidhardt F. C. Induction of proteins in response to low temperature in Escherichia coli. J Bacteriol. 1987 May;169(5):2092–2095. doi: 10.1128/jb.169.5.2092-2095.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney T. J., Moran C. P., Jr Genetic evidence for interaction of sigma A with two promoters in Bacillus subtilis. J Bacteriol. 1991 Jun;173(11):3282–3290. doi: 10.1128/jb.173.11.3282-3290.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo K., Inouye M. TIP 1, a cold shock-inducible gene of Saccharomyces cerevisiae. J Biol Chem. 1991 Sep 15;266(26):17537–17544. [PubMed] [Google Scholar]
- La Teana A., Brandi A., Falconi M., Spurio R., Pon C. L., Gualerzi C. O. Identification of a cold shock transcriptional enhancer of the Escherichia coli gene encoding nucleoid protein H-NS. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10907–10911. doi: 10.1073/pnas.88.23.10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Marahiel M. A., Zuber P., Czekay G., Losick R. Identification of the promoter for a peptide antibiotic biosynthesis gene from Bacillus brevis and its regulation in Bacillus subtilis. J Bacteriol. 1987 May;169(5):2215–2222. doi: 10.1128/jb.169.5.2215-2222.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micka B., Groch N., Heinemann U., Marahiel M. A. Molecular cloning, nucleotide sequence, and characterization of the Bacillus subtilis gene encoding the DNA-binding protein HBsu. J Bacteriol. 1991 May;173(10):3191–3198. doi: 10.1128/jb.173.10.3191-3198.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., VanBogelen R. A., Vaughn V. The genetics and regulation of heat-shock proteins. Annu Rev Genet. 1984;18:295–329. doi: 10.1146/annurev.ge.18.120184.001455. [DOI] [PubMed] [Google Scholar]
- Ozer J., Faber M., Chalkley R., Sealy L. Isolation and characterization of a cDNA clone for the CCAAT transcription factor EFIA reveals a novel structural motif. J Biol Chem. 1990 Dec 25;265(36):22143–22152. [PubMed] [Google Scholar]
- Pain R. H. Protein structure. Helices of antifreeze. Nature. 1988 May 19;333(6170):207–208. doi: 10.1038/333207a0. [DOI] [PubMed] [Google Scholar]
- Penn M. D., Thireos G., Greer H. Temporal analysis of general control of amino acid biosynthesis in Saccharomyces cerevisiae: role of positive regulatory genes in initiation and maintenance of mRNA derepression. Mol Cell Biol. 1984 Mar;4(3):520–528. doi: 10.1128/mcb.4.3.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggot P. J., Hoch J. A. Revised genetic linkage map of Bacillus subtilis. Microbiol Rev. 1985 Jun;49(2):158–179. doi: 10.1128/mr.49.2.158-179.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakura H., Maekawa T., Imamoto F., Yasuda K., Ishii S. Two human genes isolated by a novel method encode DNA-binding proteins containing a common region of homology. Gene. 1988 Dec 20;73(2):499–507. doi: 10.1016/0378-1119(88)90514-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin H., Herrler M., Willimsky G., Marahiel M. A., Heinemann U. Overproduction, crystallization, and preliminary X-ray diffraction studies of the major cold shock protein from Bacillus subtilis, CspB. Proteins. 1992 Sep;14(1):120–124. doi: 10.1002/prot.340140113. [DOI] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streips U. N., Polio F. W. Heat shock proteins in bacilli. J Bacteriol. 1985 Apr;162(1):434–437. doi: 10.1128/jb.162.1.434-437.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafuri S. R., Wolffe A. P. Xenopus Y-box transcription factors: molecular cloning, functional analysis and developmental regulation. Proc Natl Acad Sci U S A. 1990 Nov;87(22):9028–9032. doi: 10.1073/pnas.87.22.9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe H., Goldstein J., Yang M., Inouye M. Identification of the promoter region of the Escherichia coli major cold shock gene, cspA. J Bacteriol. 1992 Jun;174(12):3867–3873. doi: 10.1128/jb.174.12.3867-3873.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wistow G. Cold shock and DNA binding. Nature. 1990 Apr 26;344(6269):823–824. doi: 10.1038/344823c0. [DOI] [PubMed] [Google Scholar]
- Wu L., Welker N. E. Temperature-induced protein synthesis in Bacillus stearothermophilus NUB36. J Bacteriol. 1991 Aug;173(15):4889–4892. doi: 10.1128/jb.173.15.4889-4892.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D. S., Sax M., Chakrabartty A., Hew C. L. Crystal structure of an antifreeze polypeptide and its mechanistic implications. Nature. 1988 May 19;333(6170):232–237. doi: 10.1038/333232a0. [DOI] [PubMed] [Google Scholar]
- Zuber P., Losick R. Role of AbrB in Spo0A- and Spo0B-dependent utilization of a sporulation promoter in Bacillus subtilis. J Bacteriol. 1987 May;169(5):2223–2230. doi: 10.1128/jb.169.5.2223-2230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira D. E., Seurinck J., Inzé D., Van Montagu M., Botterman J. Differential expression of five Arabidopsis genes encoding glycine-rich proteins. Plant Cell. 1990 May;2(5):427–436. doi: 10.1105/tpc.2.5.427. [DOI] [PMC free article] [PubMed] [Google Scholar]