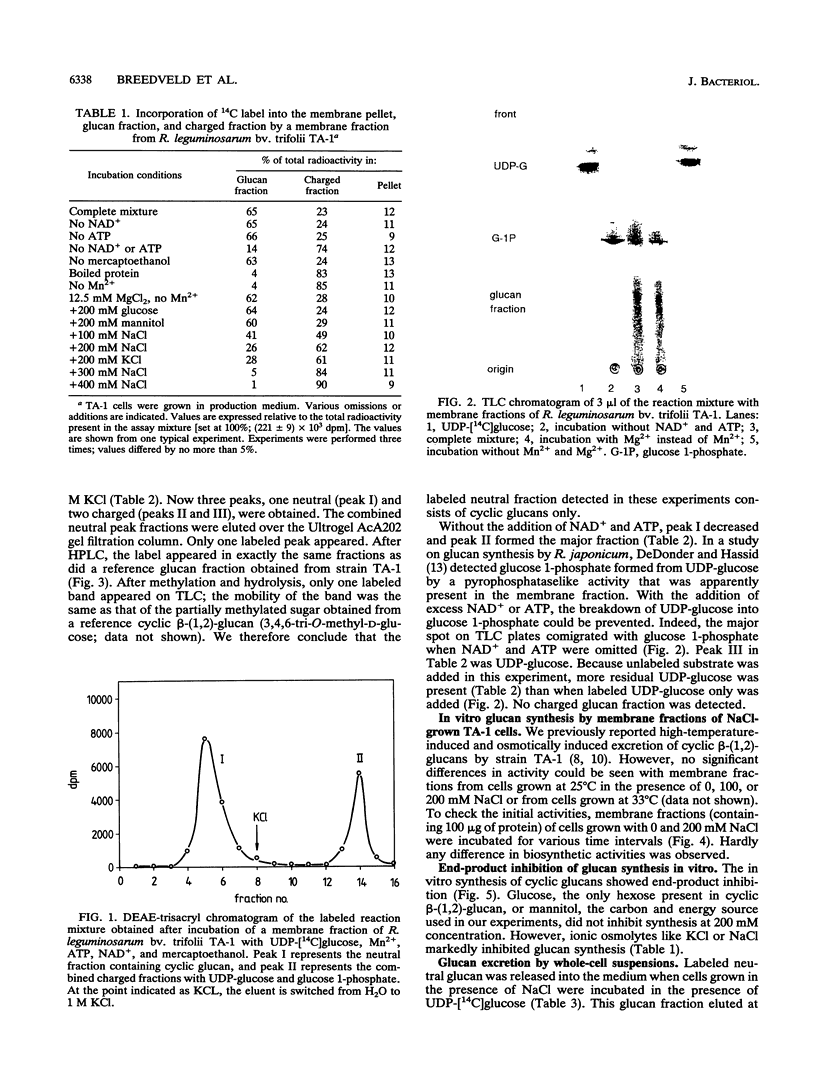
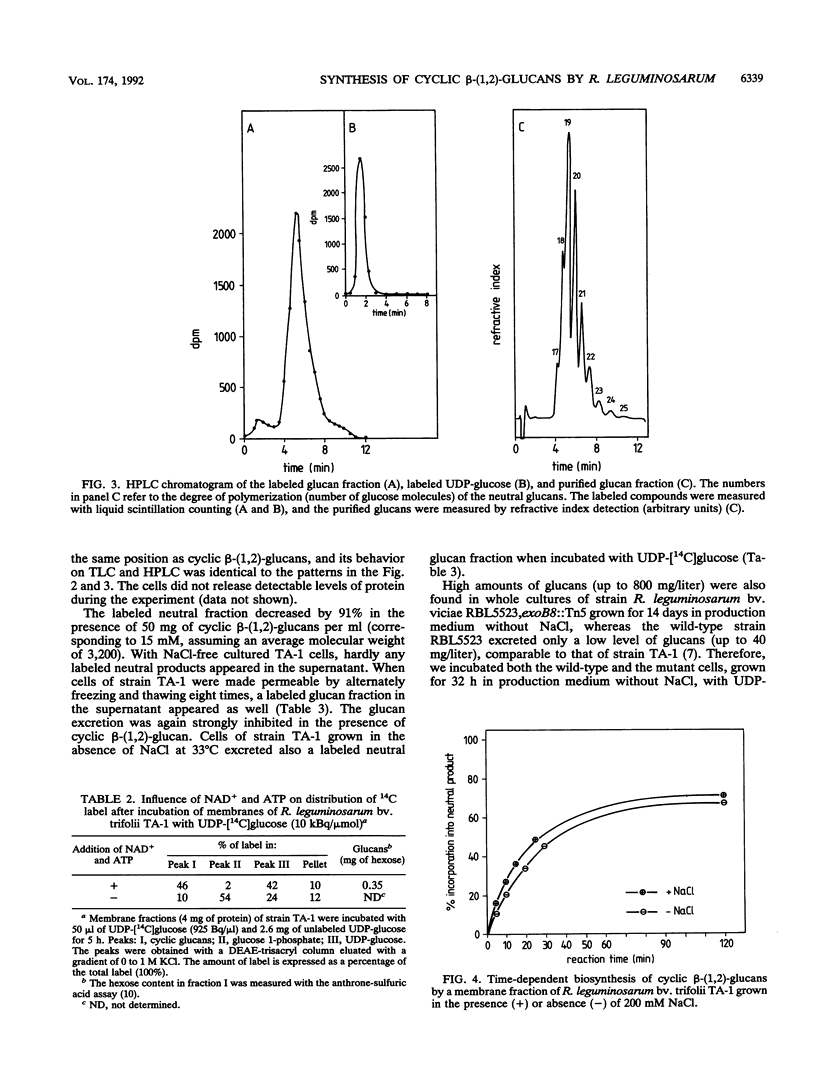
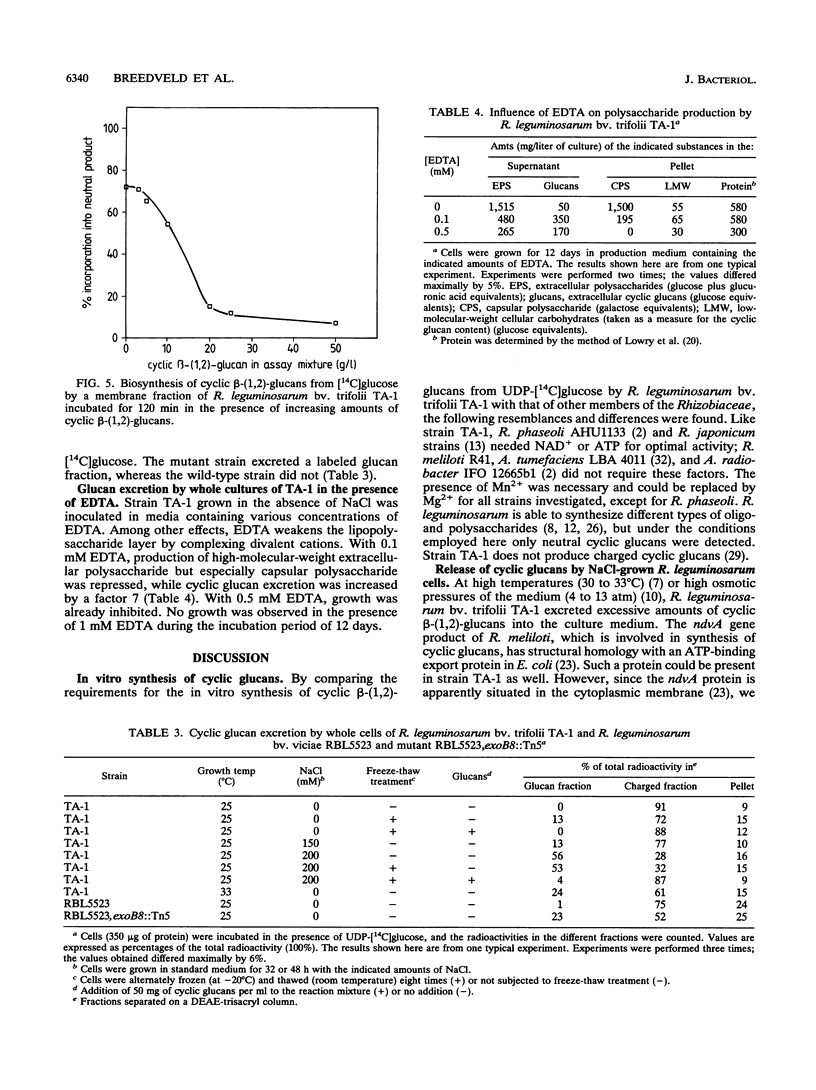
Abstract
The synthesis of cyclic beta-(1,2)-glucans from UDP-[14C]glucose by a crude membrane preparation and whole cells of Rhizobium leguminosarum bv. trifolii TA-1 was investigated. The crude membrane system needed Mn2+, ATP, and NAD+ for optimal activity. Hardly any difference in biosynthetic activity between membrane fractions of TA-1 cells grown in the presence (200 mM) or absence of NaCl was observed. Whole TA-1 cells grown in the presence of NaCl excreted labeled, neutral cyclic beta-(1,2)-glucan during incubation with added UDP-[14C]glucose. With NaCl-free cultured TA-1 cells, no excretion was observed; however, after these cells were alternately frozen and thawed eight times, they excreted glucans. Glucan formation in vitro and glucan excretion by whole cells were strongly inhibited in the presence of 50 mg of cyclic glucan per ml (about 15 mM), indicating that biosynthesis of cyclic beta-(1,2)-glucans in strain TA-1 is controlled by end-product inhibition. These observations indicate that TA-1 cells become more permeable to cyclic glucans at high NaCl concentrations. The constant loss of glucans from cells grown in the presence of 200 mM NaCl prevented end-product inhibition and resulted in glucan accumulation of up to 1,600 mg/liter in the medium.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Breedveld M. W., Zevenhuizen L. P., Zehnder A. J. Excessive excretion of cyclic beta-(1,2)-glucan by Rhizobium trifolii TA-1. Appl Environ Microbiol. 1990 Jul;56(7):2080–2086. doi: 10.1128/aem.56.7.2080-2086.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cangelosi G. A., Martinetti G., Nester E. W. Osmosensitivity phenotypes of Agrobacterium tumefaciens mutants that lack periplasmic beta-1,2-glucan. J Bacteriol. 1990 Apr;172(4):2172–2174. doi: 10.1128/jb.172.4.2172-2174.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canter Cremers H. C., Batley M., Redmond J. W., Eydems L., Breedveld M. W., Zevehuizen L. P., Pees E., Wijffelman C. A., Lugtenberg B. J. Rhizobium leguminosarum exoB mutants are deficient in the synthesis of UDP-glucose 4'-epimerase. J Biol Chem. 1990 Dec 5;265(34):21122–21127. [PubMed] [Google Scholar]
- DEDONDER R. A., HASSID W. Z. THE ENZYMATIC SYNTHESIS OF A (BETA-I,2-)-LINKED GLUCAN BY AN EXTRACT OF RHIZOBIUM JAPONICUM. Biochim Biophys Acta. 1964 Aug 19;90:239–248. doi: 10.1016/0304-4165(64)90187-4. [DOI] [PubMed] [Google Scholar]
- Dylan T., Helinski D. R., Ditta G. S. Hypoosmotic adaptation in Rhizobium meliloti requires beta-(1----2)-glucan. J Bacteriol. 1990 Mar;172(3):1400–1408. doi: 10.1128/jb.172.3.1400-1408.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger O., Weissborn A. C., Kennedy E. P. Biosynthesis and excretion of cyclic glucans by Rhizobium meliloti 1021. J Bacteriol. 1991 May;173(9):3021–3024. doi: 10.1128/jb.173.9.3021-3024.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P. J., Henry R. J., Blakeney A. B., Stone B. A. An improved procedure for the methylation analysis of oligosaccharides and polysaccharides. Carbohydr Res. 1984 Apr 2;127(1):59–73. doi: 10.1016/0008-6215(84)85106-x. [DOI] [PubMed] [Google Scholar]
- Kennedy E. P., Rumley M. K. Osmotic regulation of biosynthesis of membrane-derived oligosaccharides in Escherichia coli. J Bacteriol. 1988 Jun;170(6):2457–2461. doi: 10.1128/jb.170.6.2457-2461.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Miller K. J., Kennedy E. P., Reinhold V. N. Osmotic adaptation by gram-negative bacteria: possible role for periplasmic oligosaccharides. Science. 1986 Jan 3;231(4733):48–51. doi: 10.1126/science.3941890. [DOI] [PubMed] [Google Scholar]
- Miller K. J., Reinhold V. N., Weissborn A. C., Kennedy E. P. Cyclic glucans produced by Agrobacterium tumefaciens are substituted with sn-1-phosphoglycerol residues. Biochim Biophys Acta. 1987 Jul 10;901(1):112–118. doi: 10.1016/0005-2736(87)90262-8. [DOI] [PubMed] [Google Scholar]
- Stanfield S. W., Ielpi L., O'Brochta D., Helinski D. R., Ditta G. S. The ndvA gene product of Rhizobium meliloti is required for beta-(1----2)glucan production and has homology to the ATP-binding export protein HlyB. J Bacteriol. 1988 Aug;170(8):3523–3530. doi: 10.1128/jb.170.8.3523-3530.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Rauch B., Roseman S. Periplasmic space in Salmonella typhimurium and Escherichia coli. J Biol Chem. 1977 Nov 10;252(21):7850–7861. [PubMed] [Google Scholar]
- York W. S., McNeil M., Darvill A. G., Albersheim P. Beta-2-linked glucans secreted by fast-growing species of Rhizobium. J Bacteriol. 1980 Apr;142(1):243–248. doi: 10.1128/jb.142.1.243-248.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zevenhuizen L. P., Scholten-Koerselman H. J. Surface carbohydrates of Rhizobium. I. Beta-1, 2-glucans. Antonie Van Leeuwenhoek. 1979;45(2):165–175. doi: 10.1007/BF00418581. [DOI] [PubMed] [Google Scholar]
- Zevenhuizen L. P., van Veldhuizen A., Fokkens R. H. Re-examination of cellular cyclic beta-1,2-glucans of Rhizobiaceae: distribution of ring sizes and degrees of glycerol-1-phosphate substitution. Antonie Van Leeuwenhoek. 1990 Apr;57(3):173–178. doi: 10.1007/BF00403952. [DOI] [PubMed] [Google Scholar]
- Zorreguieta A., Cavaignac S., Geremia R. A., Ugalde R. A. Osmotic regulation of beta(1-2) glucan synthesis in members of the family Rhizobiaceae. J Bacteriol. 1990 Aug;172(8):4701–4704. doi: 10.1128/jb.172.8.4701-4704.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorreguieta A., Tolmasky M. E., Staneloni R. J. The enzymatic synthesis of beta 1-2 glucans. Arch Biochem Biophys. 1985 May 1;238(2):368–372. doi: 10.1016/0003-9861(85)90176-6. [DOI] [PubMed] [Google Scholar]
- Zorreguieta A., Ugalde R. A., Leloir L. F. An intermediate in cyclic beta 1-2 glucan biosynthesis. Biochem Biophys Res Commun. 1985 Jan 16;126(1):352–357. doi: 10.1016/0006-291x(85)90613-8. [DOI] [PubMed] [Google Scholar]




