Abstract
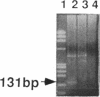
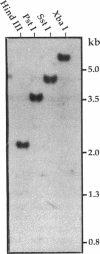
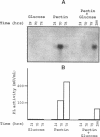
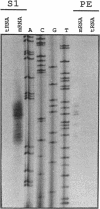
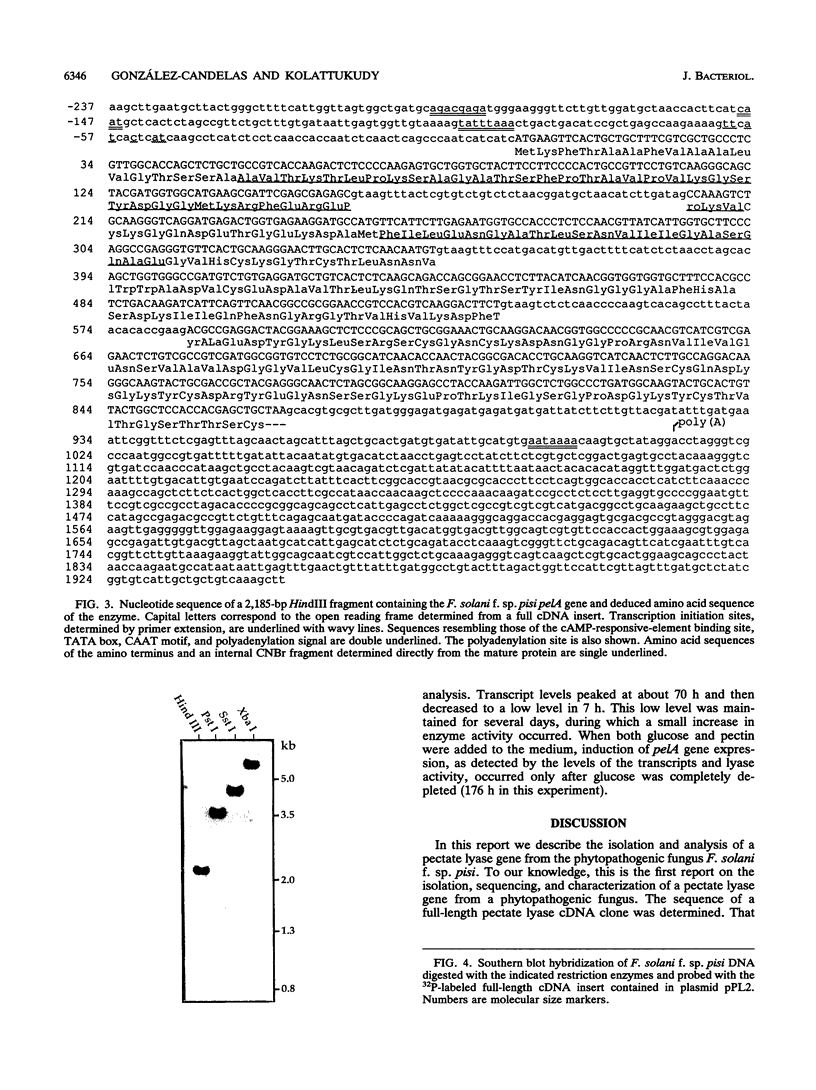
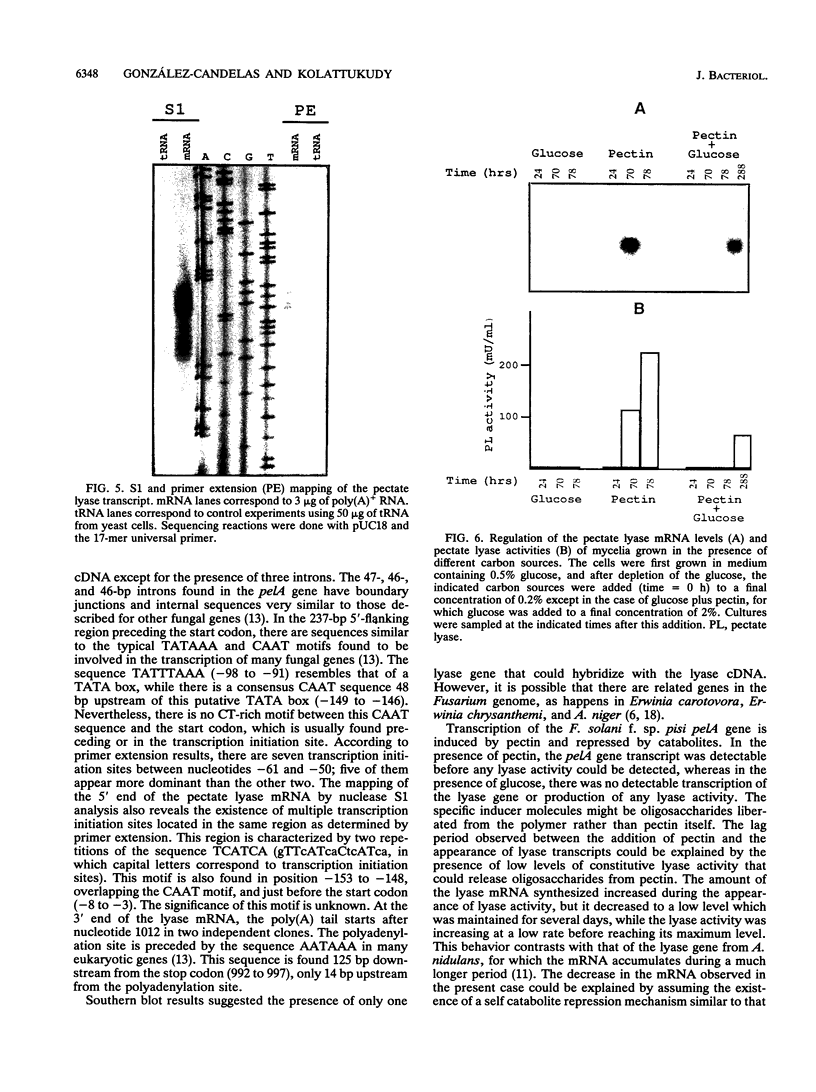
A pectate lyase produced by Fusarium solani f. sp. pisi (Nectria haematococca, mating population VI) was previously shown to be essential for host infection (M. S. Crawford and P. E. Kolattukudy, Arch. Biochem. Biophys. 258:196-205, 1987). Pectate lyase genes have not been cloned from any phytopathogenic fungi. A gene, designated pelA, encoding an inducible pectate lyase was isolated from F. solani f. sp. pisi. A probe was synthesized by polymerase chain reaction with oligonucleotide primers based on the known amino acid sequences of two regions of the mature protein and first-strand cDNA as template. Both cDNA and the gene were isolated and sequenced. That the cloned cDNA represents the previously purified pectate lyase is shown by the complete match of the sequences of the N-terminal 38 amino acid residues and the 20 amino acid residues of an internal peptide with the sequence deduced from the cDNA sequence. This lyase sequence shows little homology to those of other pectolytic enzymes. The pelA gene shows standard characteristics with respect to promoter, intron, and polyadenylation sequences. As determined by primer extension and nuclease S1 analysis of the origin of the transcription, there are multiple initiation sites clustered in a region of 12 nucleotides located about 55 bp upstream of the start codon. Northern (RNA) blot analysis showed a single band of mRNA at about 1 kb. The pelA gene mRNA was detected only when F. solani f. sp. pisi was grown with pectin, and there was no detectable transcript accumulation when the fungus was grown with glucose as the sole carbon source. When both carbon sources were present, the pelA gene was transcribed only after glucose was completely depleted, indicating carbon catabolite repression. Moreover, the levels of transcription decreased rapidly prior to maximal enzyme accumulation, suggesting a mechanism of self catabolite repression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bajar A., Podila G. K., Kolattukudy P. E. Identification of a fungal cutinase promoter that is inducible by a plant signal via a phosphorylated trans-acting factor. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8208–8212. doi: 10.1073/pnas.88.18.8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H. S., Li B., Lin Z., Huang E., Liu A. Y. cAMP and cAMP-dependent protein kinase regulate the human heat shock protein 70 gene promoter activity. J Biol Chem. 1991 Jun 25;266(18):11858–11865. [PubMed] [Google Scholar]
- Collmer A., Bateman D. F. Impaired induction and self-catabolite repression of extracellular pectate lyase in Erwinia chrysanthemi mutants deficient in oligogalacturonide lyase. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3920–3924. doi: 10.1073/pnas.78.6.3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford M. S., Kolattukudy P. E. Pectate lyase from Fusarium solani f. sp. pisi: purification, characterization, in vitro translation of the mRNA, and involvement in pathogenicity. Arch Biochem Biophys. 1987 Oct;258(1):196–205. doi: 10.1016/0003-9861(87)90336-5. [DOI] [PubMed] [Google Scholar]
- Dean R. A., Timberlake W. E. Regulation of the Aspergillus nidulans pectate lyase gene (pelA). Plant Cell. 1989 Mar;1(3):275–284. doi: 10.1105/tpc.1.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
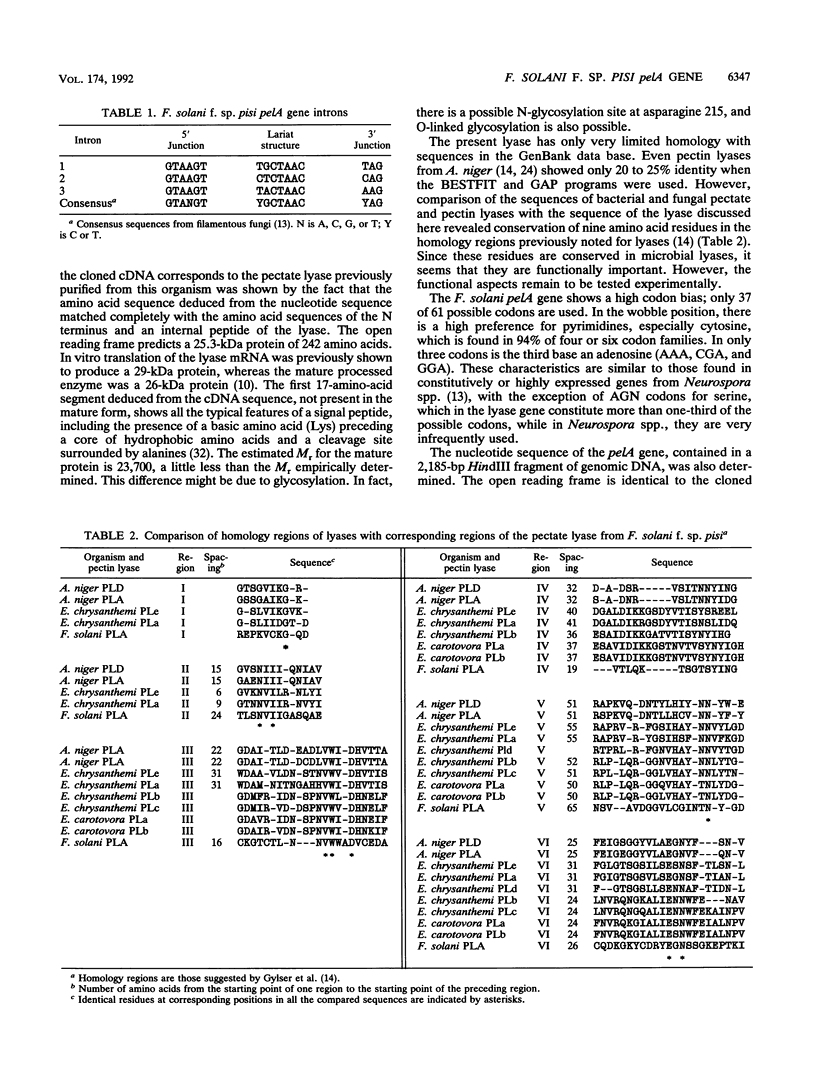
- Gysler C., Harmsen J. A., Kester H. C., Visser J., Heim J. Isolation and structure of the pectin lyase D-encoding gene from Aspergillus niger. Gene. 1990 Apr 30;89(1):101–108. doi: 10.1016/0378-1119(90)90211-9. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hankin L., Kolattukudy P. E. Metabolism of a plant wax paraffin (n-nonacosane) by a soil bacterium (Micrococcus cerificans). J Gen Microbiol. 1968 May;51(3):457–463. doi: 10.1099/00221287-51-3-457. [DOI] [PubMed] [Google Scholar]
- Harmsen J. A., Kusters-van Someren M. A., Visser J. Cloning and expression of a second Aspergillus niger pectin lyase gene (pelA): indications of a pectin lyase gene family in A. niger. Curr Genet. 1990 Aug;18(2):161–166. doi: 10.1007/BF00312604. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Kusters-van Someren M. A., Harmsen J. A., Kester H. C., Visser J. Structure of the Aspergillus niger pelA gene and its expression in Aspergillus niger and Aspergillus nidulans. Curr Genet. 1991 Sep;20(4):293–299. doi: 10.1007/BF00318518. [DOI] [PubMed] [Google Scholar]
- MacDonald R. J., Swift G. H., Przybyla A. E., Chirgwin J. M. Isolation of RNA using guanidinium salts. Methods Enzymol. 1987;152:219–227. doi: 10.1016/0076-6879(87)52023-7. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Craig J. S., Panaccione D. G., Cervone F., Walton J. D. Endopolygalacturonase is not required for pathogenicity of Cochliobolus carbonum on maize. Plant Cell. 1990 Dec;2(12):1191–1200. doi: 10.1105/tpc.2.12.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliday C. L., Dickman M. B., Kolattukudy P. E. Structure of the cutinase gene and detection of promoter activity in the 5'-flanking region by fungal transformation. J Bacteriol. 1989 Apr;171(4):1942–1951. doi: 10.1128/jb.171.4.1942-1951.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]