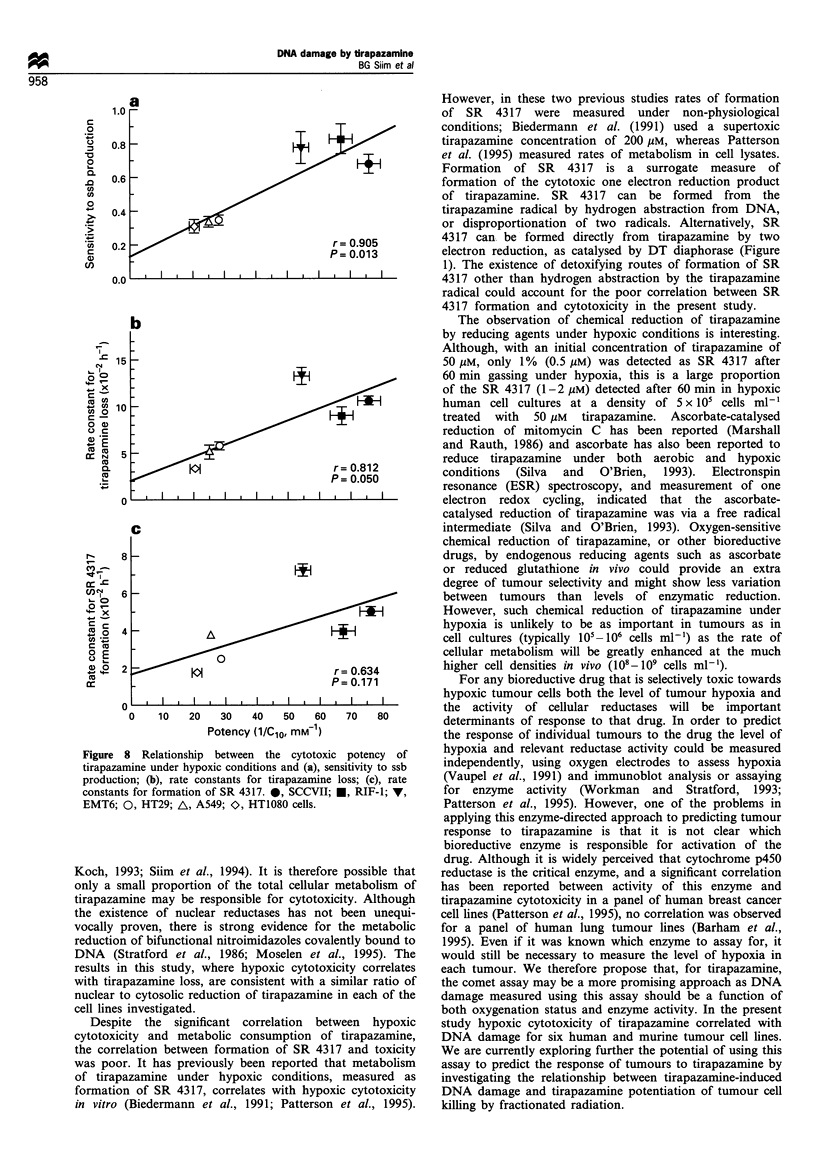
Abstract
Tirapazamine (SR 4233), a bioreductive drug selectively toxic towards hypoxic cells, is presently in phase II clinical trials. Since it would not be expected that all tumours would respond equally to the drug, we are exploring ways of predicting the response of individual tumours. In this study we have tested whether the comet assay, which measures DNA damage in individual cells, can provide a simple, surrogate end point for cell killing by tirapazamine. We examined the relationship between the cytotoxicity of tirapazamine under hypoxic conditions and tirapazamine-induced DNA strand breaks in murine (SCCVII, EMT6, RIF-1) and human (HT1080, A549, HT29) tumour cell lines. These results were compared with the relationship between tirapazamine cytotoxicity and another measure of the ability of cells to metabolise tirapazamine; high-performance liquid chromatography (HPLC) analysis of tirapazamine loss or formation of the two electron reduction product SR 4317. The correlation between the hypoxic cytotoxic potency of tirapazamine and DNA damage was highly significant (r = 0.905, P = 0.013). A similar correlation was observed for hypoxic potency and tirapazamine loss (r = 0.812, P = 0.050), while the correlation between hypoxic potency and SR 4317 formation was not significant (r = 0.634, P = 0.171). The hypoxic cytotoxicity of tirapazamine in vitro can therefore be predicted by measuring tirapazamine-induced DNA damage using the comet assay. This approach holds promise for predicting the response of individual tumours to tirapazamine in the clinic.
Full text
PDF

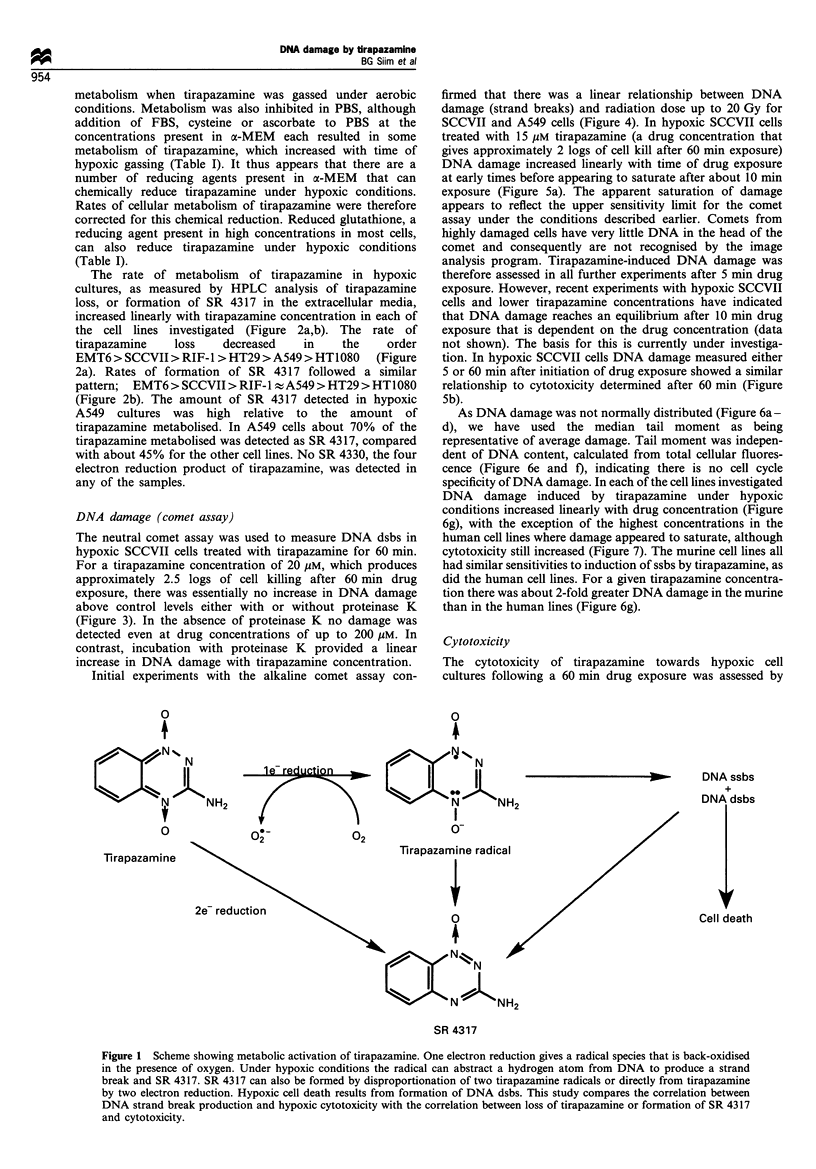
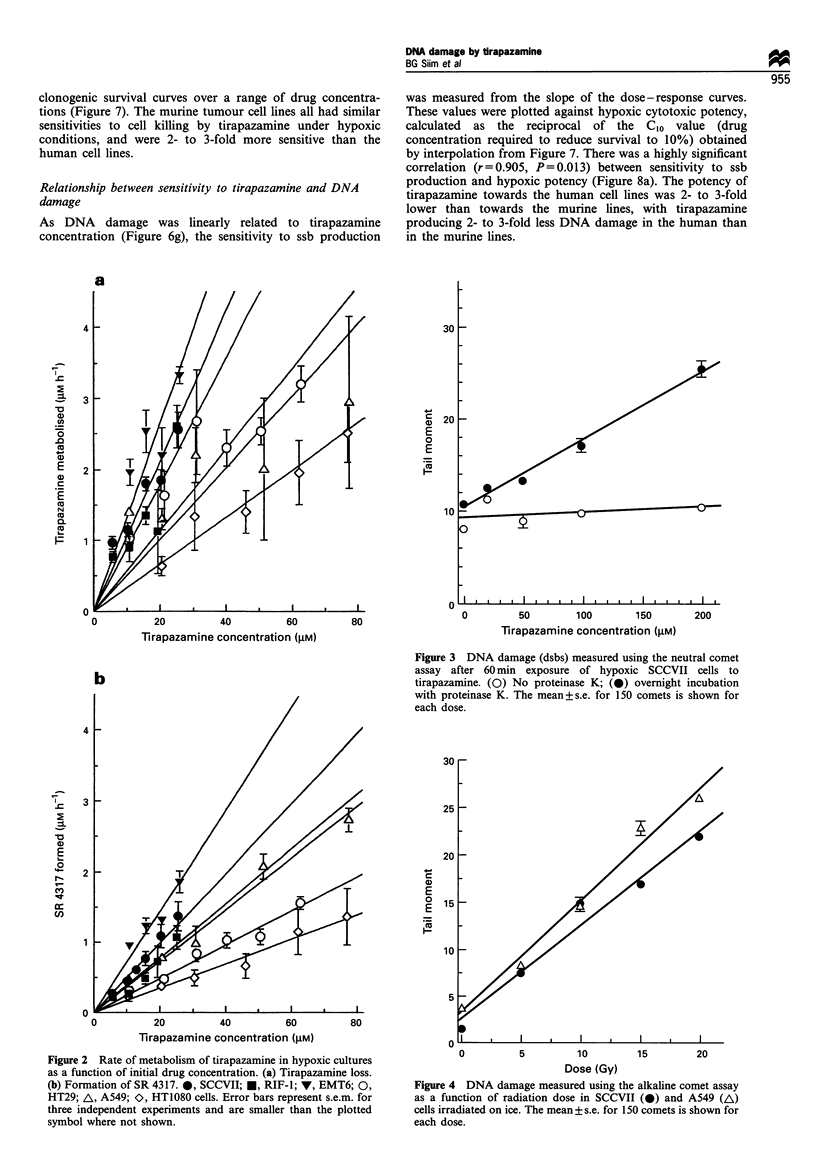
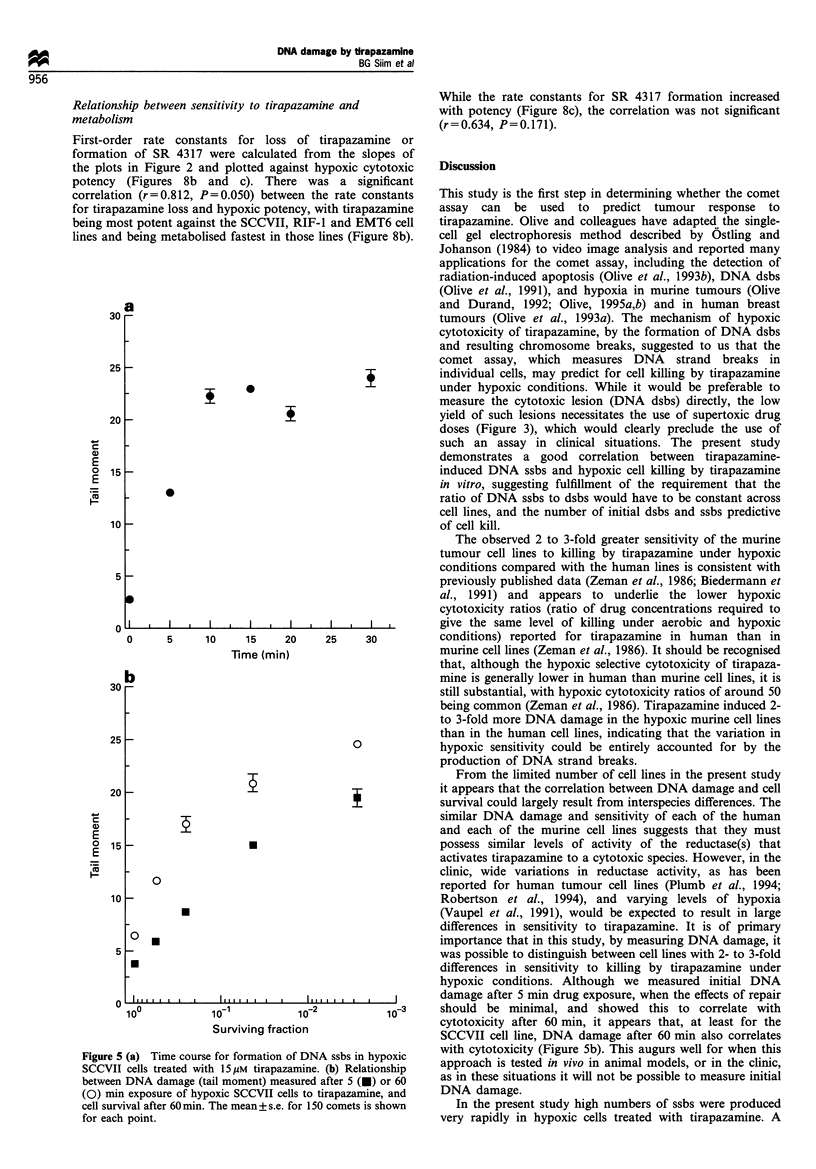
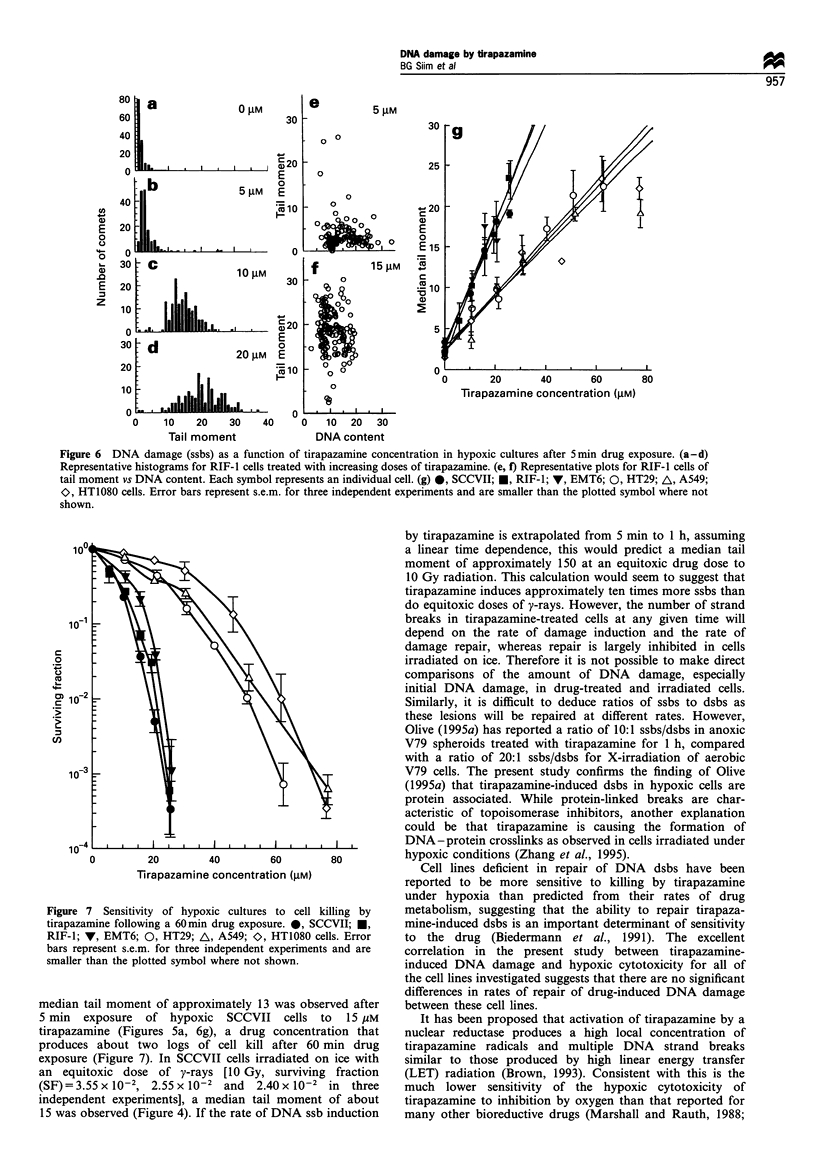


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biedermann K. A., Wang J., Graham R. P., Brown J. M. SR 4233 cytotoxicity and metabolism in DNA repair-competent and repair-deficient cell cultures. Br J Cancer. 1991 Mar;63(3):358–362. doi: 10.1038/bjc.1991.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. M., Giaccia A. J. Tumour hypoxia: the picture has changed in the 1990s. Int J Radiat Biol. 1994 Jan;65(1):95–102. doi: 10.1080/09553009414550131. [DOI] [PubMed] [Google Scholar]
- Brown J. M., Koong A. Therapeutic advantage of hypoxic cells in tumors: a theoretical study. J Natl Cancer Inst. 1991 Feb 6;83(3):178–185. doi: 10.1093/jnci/83.3.178. [DOI] [PubMed] [Google Scholar]
- Brown J. M., Lemmon M. J. Potentiation by the hypoxic cytotoxin SR 4233 of cell killing produced by fractionated irradiation of mouse tumors. Cancer Res. 1990 Dec 15;50(24):7745–7749. [PubMed] [Google Scholar]
- Brown J. M., Lemmon M. J. Tumor hypoxia can be exploited to preferentially sensitize tumors to fractionated irradiation. Int J Radiat Oncol Biol Phys. 1991 Mar;20(3):457–461. doi: 10.1016/0360-3016(91)90057-b. [DOI] [PubMed] [Google Scholar]
- Brown J. M. SR 4233 (tirapazamine): a new anticancer drug exploiting hypoxia in solid tumours. Br J Cancer. 1993 Jun;67(6):1163–1170. doi: 10.1038/bjc.1993.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush R. S., Jenkin R. D., Allt W. E., Beale F. A., Bean H., Dembo A. J., Pringle J. F. Definitive evidence for hypoxic cells influencing cure in cancer therapy. Br J Cancer Suppl. 1978 Jun;3:302–306. [PMC free article] [PubMed] [Google Scholar]
- Cole S., Stratford I. J., Fielden E. M., Adams G. E., Leopold W., Elliott W., Suto M., Sebolt-Leopold J. Dual function nitroimidazoles less toxic than RSU 1069: selection of candidate drugs for clinical trial (RB 6145 and/or PD 130908. Int J Radiat Oncol Biol Phys. 1992;22(3):545–548. doi: 10.1016/0360-3016(92)90872-f. [DOI] [PubMed] [Google Scholar]
- Denny W. A., Wilson W. R. Bioreducible mustards: a paradigm for hypoxia-selective prodrugs of diffusible cytotoxins (HPDCs). Cancer Metastasis Rev. 1993 Jun;12(2):135–151. doi: 10.1007/BF00689806. [DOI] [PubMed] [Google Scholar]
- Doherty N., Hancock S. L., Kaye S., Coleman C. N., Shulman L., Marquez C., Mariscal C., Rampling R., Senan S., Roemeling R. V. Muscle cramping in phase I clinical trials of tirapazamine (SR 4233) with and without radiation. Int J Radiat Oncol Biol Phys. 1994 May 15;29(2):379–382. doi: 10.1016/0360-3016(94)90293-3. [DOI] [PubMed] [Google Scholar]
- Dorie M. J., Brown J. M. Tumor-specific, schedule-dependent interaction between tirapazamine (SR 4233) and cisplatin. Cancer Res. 1993 Oct 1;53(19):4633–4636. [PubMed] [Google Scholar]
- Durand R. E. The influence of microenvironmental factors during cancer therapy. In Vivo. 1994 Nov-Dec;8(5):691–702. [PubMed] [Google Scholar]
- Gatenby R. A., Kessler H. B., Rosenblum J. S., Coia L. R., Moldofsky P. J., Hartz W. H., Broder G. J. Oxygen distribution in squamous cell carcinoma metastases and its relationship to outcome of radiation therapy. Int J Radiat Oncol Biol Phys. 1988 May;14(5):831–838. doi: 10.1016/0360-3016(88)90002-8. [DOI] [PubMed] [Google Scholar]
- Hirst D. G., Brown J. M., Hazlehurst J. L. Effect of partition coefficient on the ability of nitroimidazoles to enhance the cytotoxicity of 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea. Cancer Res. 1983 May;43(5):1961–1965. [PubMed] [Google Scholar]
- Hughes C. S., Shen J. W., Subjeck J. R. Resistance to etoposide induced by three glucose-regulated stresses in Chinese hamster ovary cells. Cancer Res. 1989 Aug 15;49(16):4452–4454. [PubMed] [Google Scholar]
- Höckel M., Knoop C., Schlenger K., Vorndran B., Baussmann E., Mitze M., Knapstein P. G., Vaupel P. Intratumoral pO2 predicts survival in advanced cancer of the uterine cervix. Radiother Oncol. 1993 Jan;26(1):45–50. doi: 10.1016/0167-8140(93)90025-4. [DOI] [PubMed] [Google Scholar]
- Höckel M., Schlenger K., Knoop C., Vaupel P. Oxygenation of carcinomas of the uterine cervix: evaluation by computerized O2 tension measurements. Cancer Res. 1991 Nov 15;51(22):6098–6102. [PubMed] [Google Scholar]
- Kim I. H., Brown J. M. Reoxygenation and rehypoxiation in the SCCVII mouse tumor. Int J Radiat Oncol Biol Phys. 1994 Jun 15;29(3):493–497. doi: 10.1016/0360-3016(94)90444-8. [DOI] [PubMed] [Google Scholar]
- Koch C. J. Unusual oxygen concentration dependence of toxicity of SR-4233, a hypoxic cell toxin. Cancer Res. 1993 Sep 1;53(17):3992–3997. [PubMed] [Google Scholar]
- Marshall R. S., Rauth A. M. Modification of the cytotoxic activity of mitomycin C by oxygen and ascorbic acid in Chinese hamster ovary cells and a repair-deficient mutant. Cancer Res. 1986 Jun;46(6):2709–2713. [PubMed] [Google Scholar]
- Marshall R. S., Rauth A. M. Oxygen and exposure kinetics as factors influencing the cytotoxicity of porfiromycin, a mitomycin C analogue, in Chinese hamster ovary cells. Cancer Res. 1988 Oct 15;48(20):5655–5659. [PubMed] [Google Scholar]
- Moselen J. W., Hay M. P., Denny W. A., Wilson W. R. N-[2-(2-methyl-5-nitroimidazolyl)ethyl]-4-(2-nitroimidazolyl)butanamide (NSC 639862), a bisnitroimidazole with enhanced selectivity as a bioreductive drug. Cancer Res. 1995 Feb 1;55(3):574–580. [PubMed] [Google Scholar]
- Mueller-Klieser W., Schlenger K. H., Walenta S., Gross M., Karbach U., Hoeckel M., Vaupel P. Pathophysiological approaches to identifying tumor hypoxia in patients. Radiother Oncol. 1991;20 (Suppl 1):21–28. doi: 10.1016/0167-8140(91)90182-g. [DOI] [PubMed] [Google Scholar]
- Olive P. L., Banáth J. P., Durand R. E. Heterogeneity in radiation-induced DNA damage and repair in tumor and normal cells measured using the "comet" assay. Radiat Res. 1990 Apr;122(1):86–94. [PubMed] [Google Scholar]
- Olive P. L. Detection of hypoxia by measurement of DNA damage in individual cells from spheroids and murine tumours exposed to bioreductive drugs. I. Tirapazamine. Br J Cancer. 1995 Mar;71(3):529–536. doi: 10.1038/bjc.1995.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive P. L. Detection of hypoxia by measurement of DNA damage in individual cells from spheroids and murine tumours exposed to bioreductive drugs. II. RSU 1069. Br J Cancer. 1995 Mar;71(3):537–542. doi: 10.1038/bjc.1995.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive P. L., Durand R. E. Detection of hypoxic cells in a murine tumor with the use of the comet assay. J Natl Cancer Inst. 1992 May 6;84(9):707–711. doi: 10.1093/jnci/84.9.707. [DOI] [PubMed] [Google Scholar]
- Olive P. L., Durand R. E., Le Riche J., Olivotto I. A., Jackson S. M. Gel electrophoresis of individual cells to quantify hypoxic fraction in human breast cancers. Cancer Res. 1993 Feb 15;53(4):733–736. [PubMed] [Google Scholar]
- Olive P. L., Frazer G., Banáth J. P. Radiation-induced apoptosis measured in TK6 human B lymphoblast cells using the comet assay. Radiat Res. 1993 Oct;136(1):130–136. [PubMed] [Google Scholar]
- Olive P. L., Wlodek D., Banáth J. P. DNA double-strand breaks measured in individual cells subjected to gel electrophoresis. Cancer Res. 1991 Sep 1;51(17):4671–4676. [PubMed] [Google Scholar]
- Olive P. L., Wlodek D., Durand R. E., Banáth J. P. Factors influencing DNA migration from individual cells subjected to gel electrophoresis. Exp Cell Res. 1992 Feb;198(2):259–267. doi: 10.1016/0014-4827(92)90378-l. [DOI] [PubMed] [Google Scholar]
- Ostling O., Johanson K. J. Microelectrophoretic study of radiation-induced DNA damages in individual mammalian cells. Biochem Biophys Res Commun. 1984 Aug 30;123(1):291–298. doi: 10.1016/0006-291x(84)90411-x. [DOI] [PubMed] [Google Scholar]
- Patterson A. V., Barham H. M., Chinje E. C., Adams G. E., Harris A. L., Stratford I. J. Importance of P450 reductase activity in determining sensitivity of breast tumour cells to the bioreductive drug, tirapazamine (SR 4233). Br J Cancer. 1995 Nov;72(5):1144–1150. doi: 10.1038/bjc.1995.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumb J. A., Gerritsen M., Workman P. DT-diaphorase protects cells from the hypoxic cytotoxicity of indoloquinone EO9. Br J Cancer. 1994 Dec;70(6):1136–1143. doi: 10.1038/bjc.1994.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson N., Haigh A., Adams G. E., Stratford I. J. Factors affecting sensitivity to EO9 in rodent and human tumour cells in vitro: DT-diaphorase activity and hypoxia. Eur J Cancer. 1994;30A(7):1013–1019. doi: 10.1016/0959-8049(94)90134-1. [DOI] [PubMed] [Google Scholar]
- Rockwell S. C., Kallman R. F., Fajardo L. F. Characteristics of a serially transplanted mouse mammary tumor and its tissue-culture-adapted derivative. J Natl Cancer Inst. 1972 Sep;49(3):735–749. [PubMed] [Google Scholar]
- Siim B. G., Atwell G. J., Wilson W. R. Oxygen dependence of the cytotoxicity and metabolic activation of 4-alkylamino-5-nitroquinoline bioreductive drugs. Br J Cancer. 1994 Oct;70(4):596–603. doi: 10.1038/bjc.1994.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J. M., O'Brien P. J. Molecular mechanisms of SR 4233-induced hepatocyte toxicity under aerobic versus hypoxic conditions. Br J Cancer. 1993 Sep;68(3):484–491. doi: 10.1038/bjc.1993.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford I. J., O'Neill P., Sheldon P. W., Silver A. R., Walling J. M., Adams G. E. RSU 1069, a nitroimidazole containing an aziridine group. Bioreduction greatly increases cytotoxicity under hypoxic conditions. Biochem Pharmacol. 1986 Jan 1;35(1):105–109. doi: 10.1016/0006-2952(86)90566-6. [DOI] [PubMed] [Google Scholar]
- Stratford I. J., Stephens M. A. The differential hypoxic cytotoxicity of bioreductive agents determined in vitro by the MTT assay. Int J Radiat Oncol Biol Phys. 1989 Apr;16(4):973–976. doi: 10.1016/0360-3016(89)90898-5. [DOI] [PubMed] [Google Scholar]
- Tannock I. Cell kinetics and chemotherapy: a critical review. Cancer Treat Rep. 1978 Aug;62(8):1117–1133. [PubMed] [Google Scholar]
- Twentyman P. R., Brown J. M., Gray J. W., Franko A. J., Scoles M. A., Kallman R. F. A new mouse tumor model system (RIF-1) for comparison of end-point studies. J Natl Cancer Inst. 1980 Mar;64(3):595–604. [PubMed] [Google Scholar]
- Vaupel P., Schlenger K., Knoop C., Höckel M. Oxygenation of human tumors: evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res. 1991 Jun 15;51(12):3316–3322. [PubMed] [Google Scholar]
- Wang J., Biedermann K. A., Brown J. M. Repair of DNA and chromosome breaks in cells exposed to SR 4233 under hypoxia or to ionizing radiation. Cancer Res. 1992 Aug 15;52(16):4473–4477. [PubMed] [Google Scholar]
- Workman P., Stratford I. J. The experimental development of bioreductive drugs and their role in cancer therapy. Cancer Metastasis Rev. 1993 Jun;12(2):73–82. doi: 10.1007/BF00689802. [DOI] [PubMed] [Google Scholar]
- Zeman E. M., Brown J. M., Lemmon M. J., Hirst V. K., Lee W. W. SR-4233: a new bioreductive agent with high selective toxicity for hypoxic mammalian cells. Int J Radiat Oncol Biol Phys. 1986 Jul;12(7):1239–1242. doi: 10.1016/0360-3016(86)90267-1. [DOI] [PubMed] [Google Scholar]
- Zeman E. M., Brown J. M. Pre- and post-irradiation radiosensitization by SR 4233. Int J Radiat Oncol Biol Phys. 1989 Apr;16(4):967–971. doi: 10.1016/0360-3016(89)90897-3. [DOI] [PubMed] [Google Scholar]
- Zeman E. M., Hirst V. K., Lemmon M. J., Brown J. M. Enhancement of radiation-induced tumor cell killing by the hypoxic cell toxin SR 4233. Radiother Oncol. 1988 Jul;12(3):209–218. doi: 10.1016/0167-8140(88)90263-0. [DOI] [PubMed] [Google Scholar]
- Zhang H., Koch C. J., Wallen C. A., Wheeler K. T. Radiation-induced DNA damage in tumors and normal tissues. III. Oxygen dependence of the formation of strand breaks and DNA-protein crosslinks. Radiat Res. 1995 May;142(2):163–168. [PubMed] [Google Scholar]


