Abstract
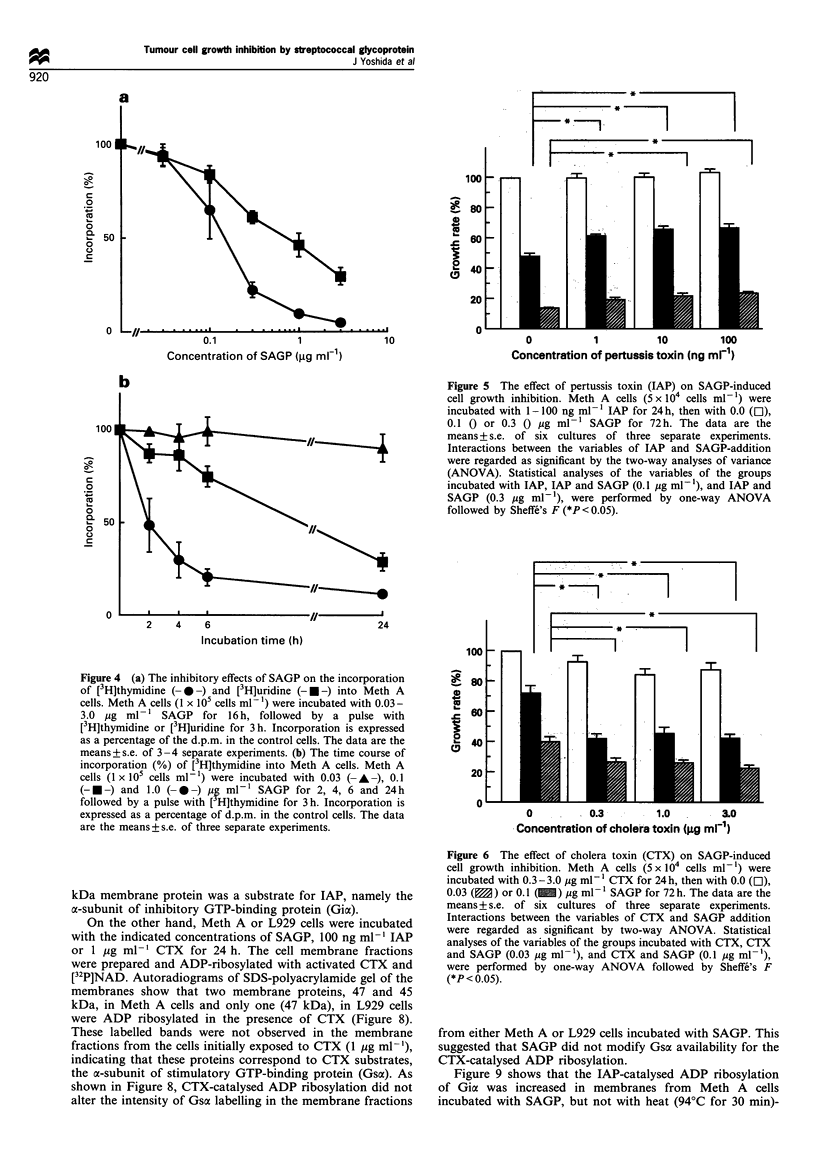
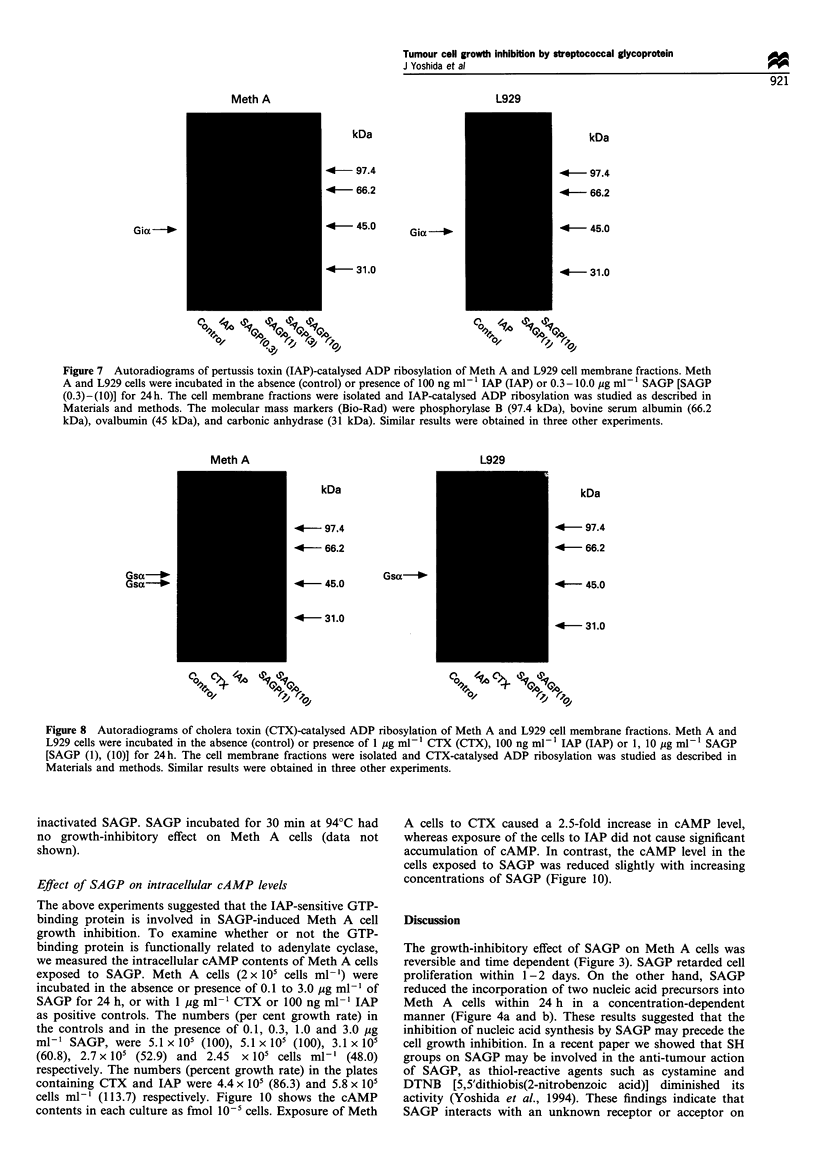
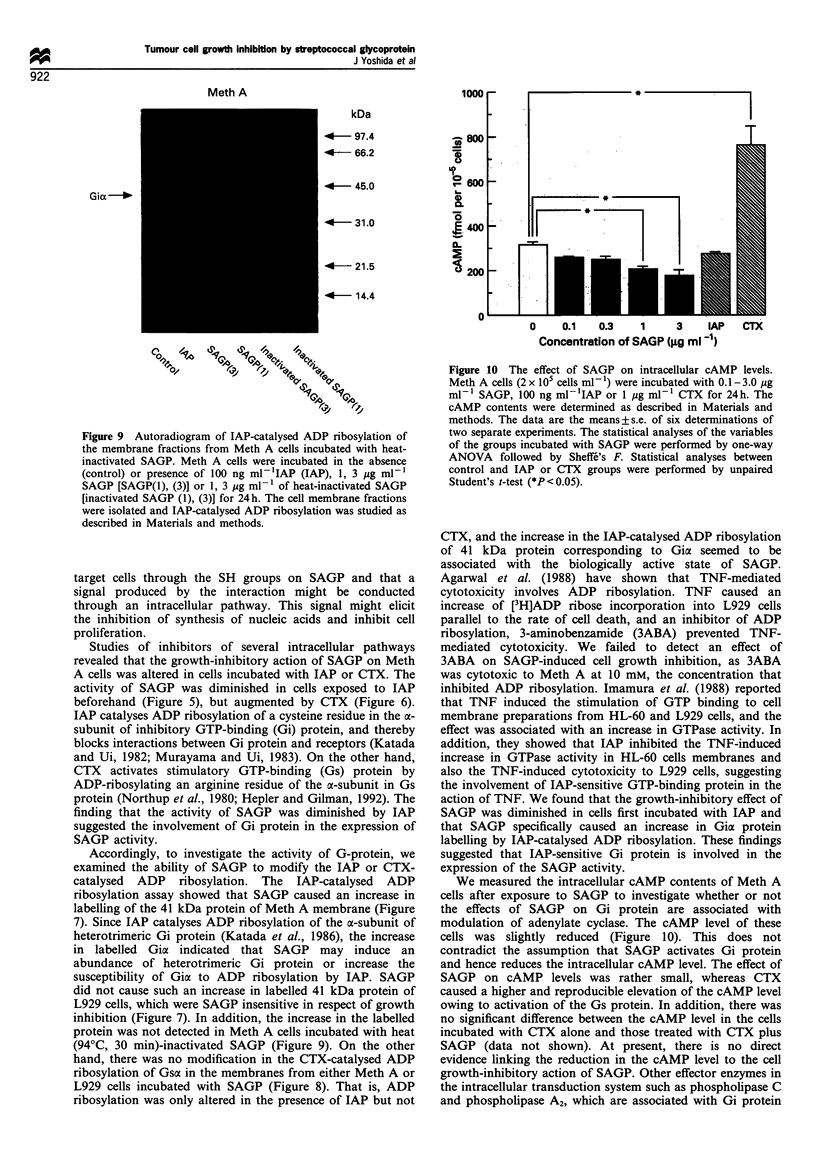
We studied the mechanism of anti-tumour action of sulphydryl glycoprotein (SAGP) purified from an extract of Streptococcus pyogenes in vitro. SAGP rapidly inhibited the incorporation of nucleic acid precursors into murine fibrosarcoma (Meth A) cells before it inhibited the cell growth. SAGP-induced cell growth inhibition was diminished by incubating the cells with pertussis toxin (IAP), whereas the SAGP activity was augmented by incubating the cells with cholera toxin (CTX). Meth A cells exposed to SAGP underwent an increase in labelling of the alpha-subunit of an inhibitory guanine nucleotide-binding (Gi) protein in a subsequent IAP-catalysed [32P]ADP ribosylation of the cell membrane fraction. Gi alpha labelling was not increased either in the membrane from the Meth A cells exposed to heat-inactivated SAGP or in the membrane from L929 cells exposed to SAGP, in which growth was also unaffected. By contrast, SAGP caused no alteration in labelling the alpha-subunit of stimulatory guanine nucleotide-binding (Gs) protein in a subsequent CTX-catalysed ADP ribosylation of membrane fractions of Meth A and L929 cells. The amount of intracellular cAMP was decreased slightly in Meth A cells incubated with SAGP. Although the precise roles of Gs protein and adenylate cyclase in the cell growth inhibition induced by SAGP are not clear, these findings suggested that the modulation of Gi protein is involved in such SAGP-induced cellular events as the inhibition of nucleic acid synthesis and cell growth inhibition.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal S., Drysdale B. E., Shin H. S. Tumor necrosis factor-mediated cytotoxicity involves ADP-ribosylation. J Immunol. 1988 Jun 15;140(12):4187–4192. [PubMed] [Google Scholar]
- Birnbaumer L., Abramowitz J., Brown A. M. Receptor-effector coupling by G proteins. Biochim Biophys Acta. 1990 May 7;1031(2):163–224. doi: 10.1016/0304-4157(90)90007-y. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L. Receptor-to-effector signaling through G proteins: roles for beta gamma dimers as well as alpha subunits. Cell. 1992 Dec 24;71(7):1069–1072. doi: 10.1016/s0092-8674(05)80056-x. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990 Nov 8;348(6297):125–132. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Hepburn A., Boeynaems J. M., Fiers W., Dumont J. E. Modulation of tumor necrosis factor-alpha cytotoxicity in L929 cells by bacterial toxins, hydrocortisone and inhibitors of arachidonic acid metabolism. Biochem Biophys Res Commun. 1987 Dec 16;149(2):815–822. doi: 10.1016/0006-291x(87)90440-2. [DOI] [PubMed] [Google Scholar]
- Hepler J. R., Gilman A. G. G proteins. Trends Biochem Sci. 1992 Oct;17(10):383–387. doi: 10.1016/0968-0004(92)90005-t. [DOI] [PubMed] [Google Scholar]
- Imamura K., Sherman M. L., Spriggs D., Kufe D. Effect of tumor necrosis factor on GTP binding and GTPase activity in HL-60 and L929 cells. J Biol Chem. 1988 Jul 25;263(21):10247–10253. [PubMed] [Google Scholar]
- Kahn R. A., Gilman A. G. ADP-ribosylation of Gs promotes the dissociation of its alpha and beta subunits. J Biol Chem. 1984 May 25;259(10):6235–6240. [PubMed] [Google Scholar]
- Kanaoka M., Fukita Y., Taya K., Kawanaka C., Negoro T., Agui H. Antitumor activity of streptococcal acid glycoprotein produced by Streptococcus pyogenes Su. Jpn J Cancer Res. 1987 Dec;78(12):1409–1414. [PubMed] [Google Scholar]
- Katada T., Oinuma M., Ui M. Two guanine nucleotide-binding proteins in rat brain serving as the specific substrate of islet-activating protein, pertussis toxin. Interaction of the alpha-subunits with beta gamma-subunits in development of their biological activities. J Biol Chem. 1986 Jun 25;261(18):8182–8191. [PubMed] [Google Scholar]
- Katada T., Ui M. Direct modification of the membrane adenylate cyclase system by islet-activating protein due to ADP-ribosylation of a membrane protein. Proc Natl Acad Sci U S A. 1982 May;79(10):3129–3133. doi: 10.1073/pnas.79.10.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurachi Y., Ito H., Sugimoto T., Shimizu T., Miki I., Ui M. Arachidonic acid metabolites as intracellular modulators of the G protein-gated cardiac K+ channel. Nature. 1989 Feb 9;337(6207):555–557. doi: 10.1038/337555a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Murayama T., Ui M. Loss of the inhibitory function of the guanine nucleotide regulatory component of adenylate cyclase due to its ADP ribosylation by islet-activating protein, pertussis toxin, in adipocyte membranes. J Biol Chem. 1983 Mar 10;258(5):3319–3326. [PubMed] [Google Scholar]
- Nakahata N., Abe M. T., Matsuoka I., Ono T., Nakanishi H. Adenosine inhibits histamine-induced phosphoinositide hydrolysis mediated via pertussis toxin-sensitive G protein in human astrocytoma cells. J Neurochem. 1991 Sep;57(3):963–969. doi: 10.1111/j.1471-4159.1991.tb08244.x. [DOI] [PubMed] [Google Scholar]
- Neer E. J., Clapham D. E. Roles of G protein subunits in transmembrane signalling. Nature. 1988 May 12;333(6169):129–134. doi: 10.1038/333129a0. [DOI] [PubMed] [Google Scholar]
- Northup J. K., Sternweis P. C., Smigel M. D., Schleifer L. S., Ross E. M., Gilman A. G. Purification of the regulatory component of adenylate cyclase. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6516–6520. doi: 10.1073/pnas.77.11.6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old L. J. Tumor necrosis factor (TNF). Science. 1985 Nov 8;230(4726):630–632. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- REILLY H. C. Microbiology and cancer therapy; a review. Cancer Res. 1953 Dec;13(12):821–834. [PubMed] [Google Scholar]
- Rook G. Tumours and Coley's toxins. Nature. 1992 Jun 18;357(6379):545–545. doi: 10.1038/357545a0. [DOI] [PubMed] [Google Scholar]
- Spriggs D. R., Sherman M. L., Imamura K., Mohri M., Rodriguez C., Robbins G., Kufe D. W. Phospholipase A2 activation and autoinduction of tumor necrosis factor gene expression by tumor necrosis factor. Cancer Res. 1990 Nov 15;50(22):7101–7107. [PubMed] [Google Scholar]
- Starnes C. O. Coley's toxins in perspective. Nature. 1992 May 7;357(6373):11–12. doi: 10.1038/357011a0. [DOI] [PubMed] [Google Scholar]
- Yoshida J., Takamura S., Suzuki S. A simplified method for purification of an antitumor acidic glycoprotein from Streptococcus pyogenes (Su strain) by immunoadsorbent chromatography. J Antibiot (Tokyo) 1989 Mar;42(3):448–453. doi: 10.7164/antibiotics.42.448. [DOI] [PubMed] [Google Scholar]
- Yoshida J., Takamura S., Suzuki S. Antitumor action of an acidic glycoprotein (SAGP) from Streptococcus pyogenes in mice. Biotherapy. 1991;3(4):331–336. doi: 10.1007/BF02221325. [DOI] [PubMed] [Google Scholar]
- Yoshida J., Takamura S., Suzuki S. Cell growth-inhibitory action of SAGP, an antitumor glycoprotein from Streptococcus pyogenes (Su strain). Jpn J Pharmacol. 1987 Oct;45(2):143–147. doi: 10.1254/jjp.45.143. [DOI] [PubMed] [Google Scholar]
- Yoshida J., Takamura S., Suzuki S. Evidence for the involvement of sulfhydryl groups in the expression of antitumor activity of streptococcal acid glycoprotein (SAGP) purified from crude extract of Streptococcus pyogenes. Anticancer Res. 1994 Sep-Oct;14(5A):1833–1837. [PubMed] [Google Scholar]
- Yoshida J., Yoshimura M., Takamura S., Kobayashi S. Purification and characterization of an antitumor principle from Streptococcus hemolyticus, Su strain. Jpn J Cancer Res. 1985 Mar;76(3):213–223. [PubMed] [Google Scholar]