Abstract
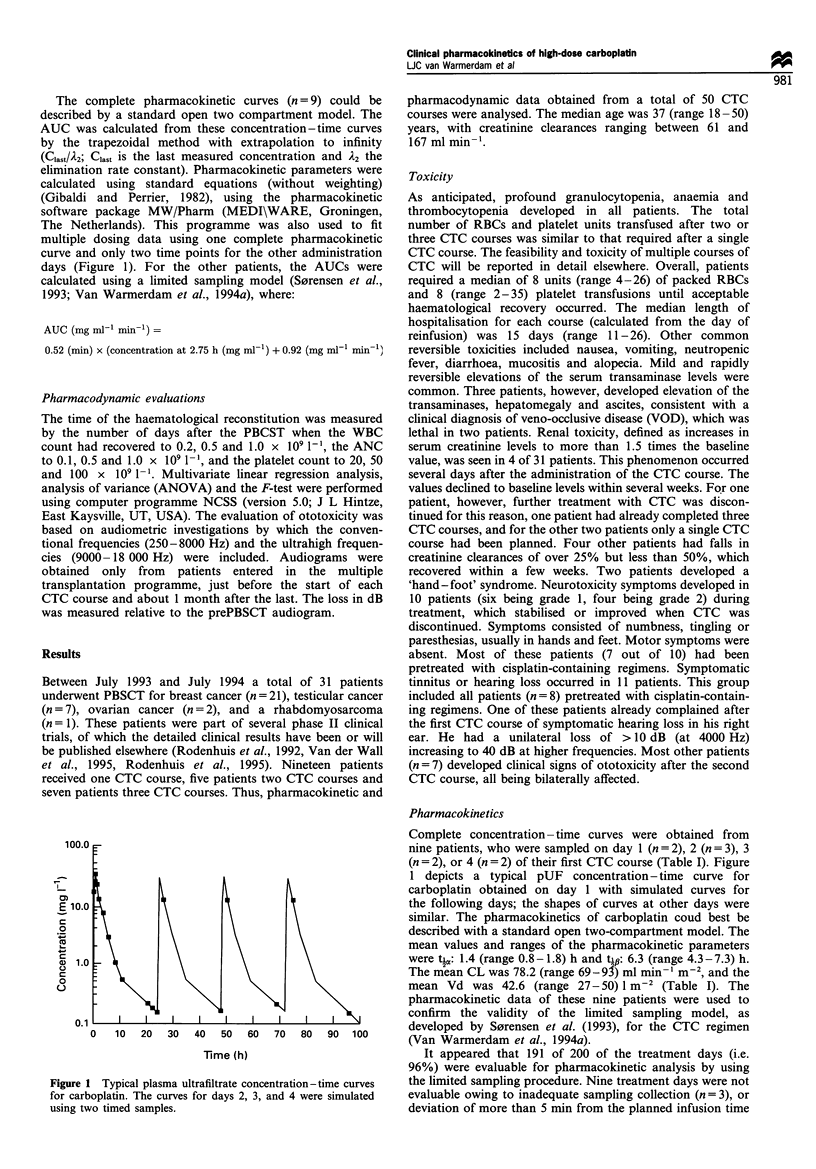
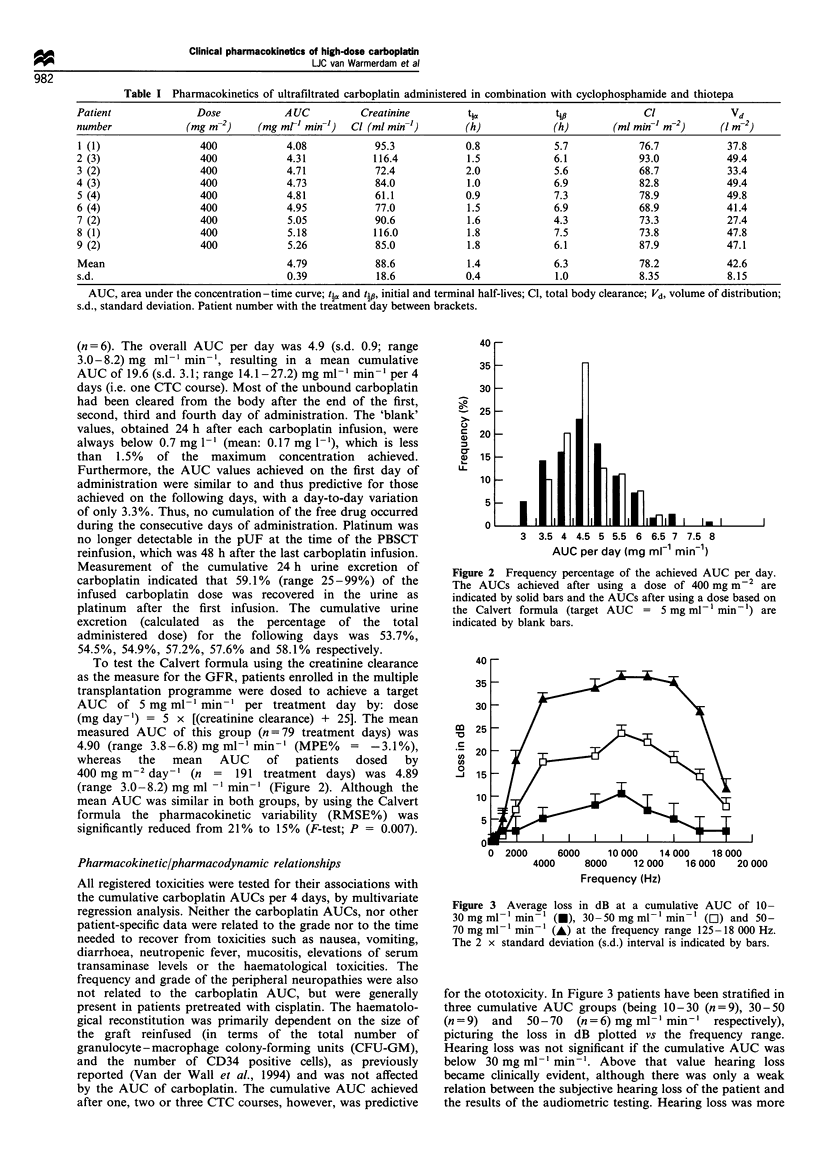
The aim of this pharmacokinetic/pharmacodynamic study was to define the relationships of the carboplatin exposure with the toxicity in patients treated with high dose carboplatin (400 mg m-2 day-1), cyclophosphamide (1500 mg m-2 day-1) and thiotepa (120 mg m-2 day-1) for four consecutive days, followed by peripheral stem cell transplantation. Exposure to carboplatin was studied in 200 treatment days by measuring the area under the carboplatin plasma ultrafiltrate (pUF) concentration vs time curve (AUC). The AUC was obtained by using a previously validated limited sampling model. A total of 31 patients was studied who received one, two or three courses of this high-dose chemotherapy regimen. The unbound, plasma ultrafiltrate carboplatin was almost completely cleared from the body before each next treatment day in a course; the day-to-day AUC variation was 3.3%. The mean cumulative AUC over 4 days was 19.6 (range 14.1-27.2) mg ml-1 min-1. In 97 treatment days the carboplatin dose was calculated using the Calvert formula with the creatinine clearance as the measure for the glomerular filtration rate (GFR). For these courses, the inter-patient variability in pharmacokinetics was significantly reduced from 21% to 15% (P = 0.007) in comparison with the schemes where it was given as a fixed dose of 400 mg m-2. There were no relationships found between toxicity and the AUC of carboplatin, which may be due to the influence of overlapping toxicities of cyclophosphamide and thiotepa. However, the ototoxicity was strongly related to the cumulative carboplatin AUC. This toxicity was dose limiting for carboplatin in this schedule. It appeared that the carboplatin pharmacokinetics in these regimens were similar to those reported at conventional dosages. To reduce the inter-patient variation, the carboplatin dose can be calculated using the Calvert-formula with the creatinine clearance as the measure for the GFR.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antman K., Ayash L., Elias A., Wheeler C., Hunt M., Eder J. P., Teicher B. A., Critchlow J., Bibbo J., Schnipper L. E. A phase II study of high-dose cyclophosphamide, thiotepa, and carboplatin with autologous marrow support in women with measurable advanced breast cancer responding to standard-dose therapy. J Clin Oncol. 1992 Jan;10(1):102–110. doi: 10.1200/JCO.1992.10.1.102. [DOI] [PubMed] [Google Scholar]
- Calvert A. H., Newell D. R., Gumbrell L. A., O'Reilly S., Burnell M., Boxall F. E., Siddik Z. H., Judson I. R., Gore M. E., Wiltshaw E. Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol. 1989 Nov;7(11):1748–1756. doi: 10.1200/JCO.1989.7.11.1748. [DOI] [PubMed] [Google Scholar]
- Cheson B. D., Lacerna L., Leyland-Jones B., Sarosy G., Wittes R. E. Autologous bone marrow transplantation. Current status and future directions. Ann Intern Med. 1989 Jan 1;110(1):51–65. doi: 10.7326/0003-4819-110-1-51. [DOI] [PubMed] [Google Scholar]
- Jodrell D. I., Egorin M. J., Canetta R. M., Langenberg P., Goldbloom E. P., Burroughs J. N., Goodlow J. L., Tan S., Wiltshaw E. Relationships between carboplatin exposure and tumor response and toxicity in patients with ovarian cancer. J Clin Oncol. 1992 Apr;10(4):520–528. doi: 10.1200/JCO.1992.10.4.520. [DOI] [PubMed] [Google Scholar]
- Motzer R. J., Gulati S. C., Tong W. P., Menendez-Botet C., Lyn P., Mazumdar M., Vlamis V., Lin S., Bosl G. J. Phase I trial with pharmacokinetic analyses of high-dose carboplatin, etoposide, and cyclophosphamide with autologous bone marrow transplantation in patients with refractory germ cell tumors. Cancer Res. 1993 Aug 15;53(16):3730–3735. [PubMed] [Google Scholar]
- Newell D. R., Siddik Z. H., Gumbrell L. A., Boxall F. E., Gore M. E., Smith I. E., Calvert A. H. Plasma free platinum pharmacokinetics in patients treated with high dose carboplatin. Eur J Cancer Clin Oncol. 1987 Sep;23(9):1399–1405. doi: 10.1016/0277-5379(87)90126-x. [DOI] [PubMed] [Google Scholar]
- Ozols R. F., Ostchega Y., Curt G., Young R. C. High-dose carboplatin in refractory ovarian cancer patients. J Clin Oncol. 1987 Feb;5(2):197–201. doi: 10.1200/JCO.1987.5.2.197. [DOI] [PubMed] [Google Scholar]
- Pollera C. F., Marolla P., Nardi M., Ameglio F., Cozzo L., Bevere F. Very high-dose cisplatin-induced ototoxicity: a preliminary report on early and long-term effects. Cancer Chemother Pharmacol. 1988;21(1):61–64. doi: 10.1007/BF00262741. [DOI] [PubMed] [Google Scholar]
- Reed E., Janik J., Bookman M. A., Rothenberg M., Smith J., Young R. C., Ozols R. F., VanderMolen L., Kohn E., Jacob J. L. High-dose carboplatin and recombinant granulocyte-macrophage colony-stimulating factor in advanced-stage recurrent ovarian cancer. J Clin Oncol. 1993 Nov;11(11):2118–2126. doi: 10.1200/JCO.1993.11.11.2118. [DOI] [PubMed] [Google Scholar]
- Reyno L. M., Egorin M. J., Canetta R. M., Jodrell D. I., Swenerton K. D., Pater J. L., Burroughs J. N., Novak M. J., Sridhara R. Impact of cyclophosphamide on relationships between carboplatin exposure and response or toxicity when used in the treatment of advanced ovarian cancer. J Clin Oncol. 1993 Jun;11(6):1156–1164. doi: 10.1200/JCO.1993.11.6.1156. [DOI] [PubMed] [Google Scholar]
- Rodenhuis S., van der Wall E., ten Bokkel Huinink W. W., Schornagel J. H., Richel D. J., Vlasveld L. T. Pilot study of a high-dose carboplatin-based salvage strategy for relapsing or refractory germ cell cancer. Cancer Invest. 1995;13(4):355–362. doi: 10.3109/07357909509031915. [DOI] [PubMed] [Google Scholar]
- Shea T. C., Flaherty M., Elias A., Eder J. P., Antman K., Begg C., Schnipper L., Frei E., 3rd, Henner W. D. A phase I clinical and pharmacokinetic study of carboplatin and autologous bone marrow support. J Clin Oncol. 1989 May;7(5):651–661. doi: 10.1200/JCO.1989.7.5.651. [DOI] [PubMed] [Google Scholar]
- Shea T. C., Mason J. R., Storniolo A. M., Bissent E., Breslin M., Mullen M., Taetle R. High-dose carboplatin chemotherapy with GM-CSF and peripheral blood progenitor cell support: a model for delivering repeated cycles of dose-intensive therapy. Cancer Treat Rev. 1993;19 (Suppl 100):11–20. doi: 10.1016/0305-7372(93)90043-q. [DOI] [PubMed] [Google Scholar]
- Siegert W., Beyer J., Strohscheer I., Baurmann H., Oettle H., Zingsem J., Zimmermann R., Bokemeyer C., Schmoll H. J., Huhn D. High-dose treatment with carboplatin, etoposide, and ifosfamide followed by autologous stem-cell transplantation in relapsed or refractory germ cell cancer: a phase I/II study. The German Testicular Cancer Cooperative Study Group. J Clin Oncol. 1994 Jun;12(6):1223–1231. doi: 10.1200/JCO.1994.12.6.1223. [DOI] [PubMed] [Google Scholar]
- Sørensen B. T., Strömgren A., Jakobsen P., Jakobsen A. A limited sampling method for estimation of the carboplatin area under the curve. Cancer Chemother Pharmacol. 1993;31(4):324–327. doi: 10.1007/BF00685679. [DOI] [PubMed] [Google Scholar]
- Wagstaff A. J., Ward A., Benfield P., Heel R. C. Carboplatin. A preliminary review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in the treatment of cancer. Drugs. 1989 Feb;37(2):162–190. doi: 10.2165/00003495-198937020-00005. [DOI] [PubMed] [Google Scholar]
- van Warmerdam L. J., Rodenhuis S., van Tellingen O., Maes R. A., Beijnen J. H. Validation of a limited sampling model for carboplatin in a high-dose chemotherapy combination. Cancer Chemother Pharmacol. 1994;35(2):179–181. doi: 10.1007/BF00686644. [DOI] [PubMed] [Google Scholar]
- van Warmerdam L. J., ten Bokkel Huinink W. W., Maes R. A., Beijnen J. H. Limited-sampling models for anticancer agents. J Cancer Res Clin Oncol. 1994;120(7):427–433. doi: 10.1007/BF01240143. [DOI] [PubMed] [Google Scholar]
- van der Vijgh W. J. Clinical pharmacokinetics of carboplatin. Clin Pharmacokinet. 1991 Oct;21(4):242–261. doi: 10.2165/00003088-199121040-00002. [DOI] [PubMed] [Google Scholar]
- van der Wall E., Nooijen W. J., Baars J. W., Holtkamp M. J., Schorangel J. H., Richel D. J., Rutgers E. J., Slaper-Cortenbach I. C., van der Schoot C. E., Rodenhuis S. High-dose carboplatin, thiotepa and cyclophosphamide (CTC) with peripheral blood stem cell support in the adjuvant therapy of high-risk breast cancer: a practical approach. Br J Cancer. 1995 Apr;71(4):857–862. doi: 10.1038/bjc.1995.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wall E., Richel D. J., Holtkamp M. J., Slaper-Cortenbach I. C., van der Schoot C. E., Dalesio O., Nooijen W. J., Schornagel J. H., Rodenhuis S. Bone marrow reconstitution after high-dose chemotherapy and autologous peripheral blood progenitor cell transplantation: effect of graft size. Ann Oncol. 1994 Nov;5(9):795–802. doi: 10.1093/oxfordjournals.annonc.a059007. [DOI] [PubMed] [Google Scholar]


