Abstract
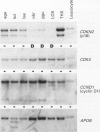
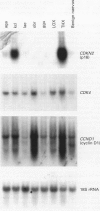
Biopsies from 61 sporadic metastatic malignant melanomas and five melanoma cell lines were examined for homozygous deletions and mutations in the CDKN2 gene (p16). As the p16 protein is involved in a cell cycle regulatory pathway consisting of at least pRb, cdk4 and cyclin D1, the tumours were also screened for amplifications of the last two genes. Moreover, the transcript levels of the genes were determined and the results compared with the immunohistochemically assessed expression of pRb. Altogether, homozygous deletions of CDKN2 were found in seven tumours (11%) and two of five cell lines, whereas a mutation was detected in only one biopsy, indicating that in sporadic melanomas the former mechanism is predominant for inactivating this gene. Notably, in total 59% of the metastatic lesions lacked detectable expression of p16 mRNA, whereas all the biopsies were found to express pRb. In accordance with the postulated negative feedback loop between p16 and pRb, one melanoma cell line showed overexpression of CDKN2 mRNA together with very low levels of the Rb protein. Amplification of the other two genes may not be important in the tumorigenesis of melanomas, as only one CDK4 and no CCND1 amplification was observed. However, highly elevated CDK4 mRNA levels, compared with that seen in a panel of normal tissues, were observed in 76% of the tumours, accompanied in 71% of the cases by high expression of the CCND1 cyclin activator. Although a low frequency of CDKN2 DNA aberrations was observed, the high number of tumours that lacked CDKN2 expression but showed overexpression of CDK4 and/or CCND1, suggest that functional inactivation of pRb through this pathway may be involved in the development or progression of sporadic human melanomas.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aagaard L., Lukas J., Bartkova J., Kjerulff A. A., Strauss M., Bartek J. Aberrations of p16Ink4 and retinoblastoma tumour-suppressor genes occur in distinct sub-sets of human cancer cell lines. Int J Cancer. 1995 Mar 29;61(1):115–120. doi: 10.1002/ijc.2910610120. [DOI] [PubMed] [Google Scholar]
- Baldin V., Lukas J., Marcote M. J., Pagano M., Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993 May;7(5):812–821. doi: 10.1101/gad.7.5.812. [DOI] [PubMed] [Google Scholar]
- Bartkova J., Lukas J., Strauss M., Bartek J. Cyclin D1 oncoprotein aberrantly accumulates in malignancies of diverse histogenesis. Oncogene. 1995 Feb 16;10(4):775–778. [PubMed] [Google Scholar]
- Buckley M. F., Sweeney K. J., Hamilton J. A., Sini R. L., Manning D. L., Nicholson R. I., deFazio A., Watts C. K., Musgrove E. A., Sutherland R. L. Expression and amplification of cyclin genes in human breast cancer. Oncogene. 1993 Aug;8(8):2127–2133. [PubMed] [Google Scholar]
- Cairns P., Mao L., Merlo A., Lee D. J., Schwab D., Eby Y., Tokino K., van der Riet P., Blaugrund J. E., Sidransky D. Rates of p16 (MTS1) mutations in primary tumors with 9p loss. Science. 1994 Jul 15;265(5170):415–417. doi: 10.1126/science.8023167. [DOI] [PubMed] [Google Scholar]
- Cairns P., Tokino K., Eby Y., Sidransky D. Homozygous deletions of 9p21 in primary human bladder tumors detected by comparative multiplex polymerase chain reaction. Cancer Res. 1994 Mar 15;54(6):1422–1424. [PubMed] [Google Scholar]
- Cannon-Albright L. A., Goldgar D. E., Meyer L. J., Lewis C. M., Anderson D. E., Fountain J. W., Hegi M. E., Wiseman R. W., Petty E. M., Bale A. E. Assignment of a locus for familial melanoma, MLM, to chromosome 9p13-p22. Science. 1992 Nov 13;258(5085):1148–1152. doi: 10.1126/science.1439824. [DOI] [PubMed] [Google Scholar]
- Cattoretti G., Becker M. H., Key G., Duchrow M., Schlüter C., Galle J., Gerdes J. Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB 1 and MIB 3) detect proliferating cells in microwave-processed formalin-fixed paraffin sections. J Pathol. 1992 Dec;168(4):357–363. doi: 10.1002/path.1711680404. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Flørenes V. A., Oyjord T., Holm R., Skrede M., Børresen A. L., Nesland J. M., Fodstad O. TP53 allele loss, mutations and expression in malignant melanoma. Br J Cancer. 1994 Feb;69(2):253–259. doi: 10.1038/bjc.1994.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountain J. W., Karayiorgou M., Ernstoff M. S., Kirkwood J. M., Vlock D. R., Titus-Ernstoff L., Bouchard B., Vijayasaradhi S., Houghton A. N., Lahti J. Homozygous deletions within human chromosome band 9p21 in melanoma. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10557–10561. doi: 10.1073/pnas.89.21.10557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich D. W., Lee W. H. Molecular characterization of the retinoblastoma susceptibility gene. Biochim Biophys Acta. 1993 May 25;1155(1):43–61. doi: 10.1016/0304-419x(93)90021-4. [DOI] [PubMed] [Google Scholar]
- Gruis N. A., Weaver-Feldhaus J., Liu Q., Frye C., Eeles R., Orlow I., Lacombe L., Ponce-Castaneda V., Lianes P., Latres E. Genetic evidence in melanoma and bladder cancers that p16 and p53 function in separate pathways of tumor suppression. Am J Pathol. 1995 May;146(5):1199–1206. [PMC free article] [PubMed] [Google Scholar]
- He J., Allen J. R., Collins V. P., Allalunis-Turner M. J., Godbout R., Day R. S., 3rd, James C. D. CDK4 amplification is an alternative mechanism to p16 gene homozygous deletion in glioma cell lines. Cancer Res. 1994 Nov 15;54(22):5804–5807. [PubMed] [Google Scholar]
- Hinds P. W., Dowdy S. F., Eaton E. N., Arnold A., Weinberg R. A. Function of a human cyclin gene as an oncogene. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):709–713. doi: 10.1073/pnas.91.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz J. M., Park S. H., Bogenmann E., Cheng J. C., Yandell D. W., Kaye F. J., Minna J. D., Dryja T. P., Weinberg R. A. Frequent inactivation of the retinoblastoma anti-oncogene is restricted to a subset of human tumor cells. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2775–2779. doi: 10.1073/pnas.87.7.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovig E., Smith-Sørensen B., Brøgger A., Børresen A. L. Constant denaturant gel electrophoresis, a modification of denaturing gradient gel electrophoresis, in mutation detection. Mutat Res. 1991 Jan;262(1):63–71. doi: 10.1016/0165-7992(91)90108-g. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. A comparative study of the peroxidase-antiperoxidase method and an avidin-biotin complex method for studying polypeptide hormones with radioimmunoassay antibodies. Am J Clin Pathol. 1981 May;75(5):734–738. doi: 10.1093/ajcp/75.5.734. [DOI] [PubMed] [Google Scholar]
- Hunter T., Pines J. Cyclins and cancer. II: Cyclin D and CDK inhibitors come of age. Cell. 1994 Nov 18;79(4):573–582. doi: 10.1016/0092-8674(94)90543-6. [DOI] [PubMed] [Google Scholar]
- Hussussian C. J., Struewing J. P., Goldstein A. M., Higgins P. A., Ally D. S., Sheahan M. D., Clark W. H., Jr, Tucker M. A., Dracopoli N. C. Germline p16 mutations in familial melanoma. Nat Genet. 1994 Sep;8(1):15–21. doi: 10.1038/ng0994-15. [DOI] [PubMed] [Google Scholar]
- Jiang W., Kahn S. M., Tomita N., Zhang Y. J., Lu S. H., Weinstein I. B. Amplification and expression of the human cyclin D gene in esophageal cancer. Cancer Res. 1992 May 15;52(10):2980–2983. [PubMed] [Google Scholar]
- Kamb A. Cell-cycle regulators and cancer. Trends Genet. 1995 Apr;11(4):136–140. doi: 10.1016/s0168-9525(00)89027-7. [DOI] [PubMed] [Google Scholar]
- Kamb A., Gruis N. A., Weaver-Feldhaus J., Liu Q., Harshman K., Tavtigian S. V., Stockert E., Day R. S., 3rd, Johnson B. E., Skolnick M. H. A cell cycle regulator potentially involved in genesis of many tumor types. Science. 1994 Apr 15;264(5157):436–440. doi: 10.1126/science.8153634. [DOI] [PubMed] [Google Scholar]
- Khatib Z. A., Matsushime H., Valentine M., Shapiro D. N., Sherr C. J., Look A. T. Coamplification of the CDK4 gene with MDM2 and GLI in human sarcomas. Cancer Res. 1993 Nov 15;53(22):5535–5541. [PubMed] [Google Scholar]
- Koh J., Enders G. H., Dynlacht B. D., Harlow E. Tumour-derived p16 alleles encoding proteins defective in cell-cycle inhibition. Nature. 1995 Jun 8;375(6531):506–510. doi: 10.1038/375506a0. [DOI] [PubMed] [Google Scholar]
- Lammie G. A., Fantl V., Smith R., Schuuring E., Brookes S., Michalides R., Dickson C., Arnold A., Peters G. D11S287, a putative oncogene on chromosome 11q13, is amplified and expressed in squamous cell and mammary carcinomas and linked to BCL-1. Oncogene. 1991 Mar;6(3):439–444. [PubMed] [Google Scholar]
- Lewis D. C., Warren N., Shukla V. K., Grimshaw D., Laidler P., Padua R. A. Gross rearrangements and deletions of the retinoblastoma gene are rare in malignant melanoma. Acta Derm Venereol. 1993 Jun;73(3):236–236. doi: 10.2340/0001555573236. [DOI] [PubMed] [Google Scholar]
- Li Y., Nichols M. A., Shay J. W., Xiong Y. Transcriptional repression of the D-type cyclin-dependent kinase inhibitor p16 by the retinoblastoma susceptibility gene product pRb. Cancer Res. 1994 Dec 1;54(23):6078–6082. [PubMed] [Google Scholar]
- Liu Q., Neuhausen S., McClure M., Frye C., Weaver-Feldhaus J., Gruis N. A., Eddington K., Allalunis-Turner M. J., Skolnick M. H., Fujimura F. K. CDKN2 (MTS1) tumor suppressor gene mutations in human tumor cell lines. Oncogene. 1995 Mar 16;10(6):1061–1067. [PubMed] [Google Scholar]
- Lukas J., Parry D., Aagaard L., Mann D. J., Bartkova J., Strauss M., Peters G., Bartek J. Retinoblastoma-protein-dependent cell-cycle inhibition by the tumour suppressor p16. Nature. 1995 Jun 8;375(6531):503–506. doi: 10.1038/375503a0. [DOI] [PubMed] [Google Scholar]
- Maelandsmo G. M., Berner J. M., Flørenes V. A., Forus A., Hovig E., Fodstad O., Myklebost O. Homozygous deletion frequency and expression levels of the CDKN2 gene in human sarcomas--relationship to amplification and mRNA levels of CDK4 and CCND1. Br J Cancer. 1995 Aug;72(2):393–398. doi: 10.1038/bjc.1995.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushime H., Roussel M. F., Ashmun R. A., Sherr C. J. Colony-stimulating factor 1 regulates novel cyclins during the G1 phase of the cell cycle. Cell. 1991 May 17;65(4):701–713. doi: 10.1016/0092-8674(91)90101-4. [DOI] [PubMed] [Google Scholar]
- Medema R. H., Herrera R. E., Lam F., Weinberg R. A. Growth suppression by p16ink4 requires functional retinoblastoma protein. Proc Natl Acad Sci U S A. 1995 Jul 3;92(14):6289–6293. doi: 10.1073/pnas.92.14.6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo A., Herman J. G., Mao L., Lee D. J., Gabrielson E., Burger P. C., Baylin S. B., Sidransky D. 5' CpG island methylation is associated with transcriptional silencing of the tumour suppressor p16/CDKN2/MTS1 in human cancers. Nat Med. 1995 Jul;1(7):686–692. doi: 10.1038/nm0795-686. [DOI] [PubMed] [Google Scholar]
- Motokura T., Arnold A. Cyclin D and oncogenesis. Curr Opin Genet Dev. 1993 Feb;3(1):5–10. doi: 10.1016/s0959-437x(05)80334-x. [DOI] [PubMed] [Google Scholar]
- Nobori T., Miura K., Wu D. J., Lois A., Takabayashi K., Carson D. A. Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers. Nature. 1994 Apr 21;368(6473):753–756. doi: 10.1038/368753a0. [DOI] [PubMed] [Google Scholar]
- Ohta M., Nagai H., Shimizu M., Rasio D., Berd D., Mastrangelo M., Singh A. D., Shields J. A., Shields C. L., Croce C. M. Rarity of somatic and germline mutations of the cyclin-dependent kinase 4 inhibitor gene, CDK4I, in melanoma. Cancer Res. 1994 Oct 15;54(20):5269–5272. [PubMed] [Google Scholar]
- Otterson G. A., Khleif S. N., Chen W., Coxon A. B., Kaye F. J. CDKN2 gene silencing in lung cancer by DNA hypermethylation and kinetics of p16INK4 protein induction by 5-aza 2'deoxycytidine. Oncogene. 1995 Sep 21;11(6):1211–1216. [PubMed] [Google Scholar]
- Pollock P. M., Yu F., Qiu L., Parsons P. G., Hayward N. K. Evidence for u.v. induction of CDKN2 mutations in melanoma cell lines. Oncogene. 1995 Aug 17;11(4):663–668. [PubMed] [Google Scholar]
- Ranade K., Hussussian C. J., Sikorski R. S., Varmus H. E., Goldstein A. M., Tucker M. A., Serrano M., Hannon G. J., Beach D., Dracopoli N. C. Mutations associated with familial melanoma impair p16INK4 function. Nat Genet. 1995 May;10(1):114–116. doi: 10.1038/ng0595-114. [DOI] [PubMed] [Google Scholar]
- Reed J. A., Loganzo F., Jr, Shea C. R., Walker G. J., Flores J. F., Glendening J. M., Bogdany J. K., Shiel M. J., Haluska F. G., Fountain J. W. Loss of expression of the p16/cyclin-dependent kinase inhibitor 2 tumor suppressor gene in melanocytic lesions correlates with invasive stage of tumor progression. Cancer Res. 1995 Jul 1;55(13):2713–2718. [PubMed] [Google Scholar]
- Reifenberger G., Reifenberger J., Ichimura K., Meltzer P. S., Collins V. P. Amplification of multiple genes from chromosomal region 12q13-14 in human malignant gliomas: preliminary mapping of the amplicons shows preferential involvement of CDK4, SAS, and MDM2. Cancer Res. 1994 Aug 15;54(16):4299–4303. [PubMed] [Google Scholar]
- Serrano M., Gómez-Lahoz E., DePinho R. A., Beach D., Bar-Sagi D. Inhibition of ras-induced proliferation and cellular transformation by p16INK4. Science. 1995 Jan 13;267(5195):249–252. doi: 10.1126/science.7809631. [DOI] [PubMed] [Google Scholar]
- Serrano M., Hannon G. J., Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993 Dec 16;366(6456):704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- Spruck C. H., 3rd, Gonzalez-Zulueta M., Shibata A., Simoneau A. R., Lin M. F., Gonzales F., Tsai Y. C., Jones P. A. p16 gene in uncultured tumours. Nature. 1994 Jul 21;370(6486):183–184. doi: 10.1038/370183a0. [DOI] [PubMed] [Google Scholar]
- Sun Y., Hildesheim A., Lanier A. E., Cao Y., Yao K. T., Raab-Traub N., Yang C. S. No point mutation but decreased expression of the p16/MTS1 tumor suppressor gene in nasopharyngeal carcinomas. Oncogene. 1995 Feb 16;10(4):785–788. [PubMed] [Google Scholar]
- Tam S. W., Shay J. W., Pagano M. Differential expression and cell cycle regulation of the cyclin-dependent kinase 4 inhibitor p16Ink4. Cancer Res. 1994 Nov 15;54(22):5816–5820. [PubMed] [Google Scholar]
- Tam S. W., Theodoras A. M., Shay J. W., Draetta G. F., Pagano M. Differential expression and regulation of Cyclin D1 protein in normal and tumor human cells: association with Cdk4 is required for Cyclin D1 function in G1 progression. Oncogene. 1994 Sep;9(9):2663–2674. [PubMed] [Google Scholar]
- Wadayama B., Toguchida J., Shimizu T., Ishizaki K., Sasaki M. S., Kotoura Y., Yamamuro T. Mutation spectrum of the retinoblastoma gene in osteosarcomas. Cancer Res. 1994 Jun 1;54(11):3042–3048. [PubMed] [Google Scholar]
- Weinberg R. A. The retinoblastoma protein and cell cycle control. Cell. 1995 May 5;81(3):323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- Wölfel T., Hauer M., Schneider J., Serrano M., Wölfel C., Klehmann-Hieb E., De Plaen E., Hankeln T., Meyer zum Büschenfelde K. H., Beach D. A p16INK4a-insensitive CDK4 mutant targeted by cytolytic T lymphocytes in a human melanoma. Science. 1995 Sep 1;269(5228):1281–1284. doi: 10.1126/science.7652577. [DOI] [PubMed] [Google Scholar]
- Xu H. J., Hu S. X., Cagle P. T., Moore G. E., Benedict W. F. Absence of retinoblastoma protein expression in primary non-small cell lung carcinomas. Cancer Res. 1991 May 15;51(10):2735–2739. [PubMed] [Google Scholar]
- Yeager T., Stadler W., Belair C., Puthenveettil J., Olopade O., Reznikoff C. Increased p16 levels correlate with pRb alterations in human urothelial cells. Cancer Res. 1995 Feb 1;55(3):493–497. [PubMed] [Google Scholar]