Abstract
Steroid receptor coactivator 3 (SRC-3/p/CIP/AIB1/ACTR/RAC3/TRAM-1) is a member of the p160 family of nuclear receptor coactivators, which includes SRC-1 (NCoA-1) and SRC-2 (TIF2/GRIP1/NCoA2). Previous studies indicate that SRC-3 is required for normal animal growth and is often amplified or overexpressed in many cancers, including breast and prostate cancers. However, the mechanisms of SRC-3-mediated growth regulation remain unclear. In this study, we show that overexpression of SRC-3 stimulates cell growth to increase cell size in prostate cancer cell lines. Furthermore, our results indicate that overexpression of SRC-3 can modulate the AKT signaling pathway in a steroid-independent manner, which results in the activation of AKT/mTOR signaling concomitant with an increase in cell size. In contrast, down-regulation of SRC-3 expression in cells by small interfering RNA decreases cell growth, leading to a smaller cell size. Similarly, in SRC-3 null mutant mice, AKT signaling is down-regulated in normally SRC-3-expressing tissues. Taken together, these results suggest that SRC-3 is an important modulator for mammalian cell growth.
Nuclear receptors (NRs) are ligand-inducible transcription factors that require coregulators (coactivators or corepressors) to regulate target genes involved in metabolism, development, and reproduction (32-34, 38, 50). The steroid receptor coactivators (SRCs), also known as p160 proteins, were among the first factors identified that interact with NRs and enhance their transactivation in a ligand-dependent manner (7). Members of the SRC family, which includes SRC-1/NCoA-1, SRC-2/GRIP1/TIF2/NCoA2, and SRC-3/p/CIP/AIB1/ACTR/RAC3/TRAM-1 (2, 9, 20, 24, 30, 36, 45, 46, 49, 54), have been shown to play important roles as NR coactivators, at least in part by recruiting histone acetyltransferase (e.g., CBP/p300 and p/CAF) (49) and histone methyltransferase (8). The three members of the SRC family have 40% overall sequence similarity, and in vitro transfection assays show that they can enhance the activity of many NRs in a similar manner, which suggests a potential functional redundancy between members of the SRC family (32). However, there is evidence indicating that they have different physiological functions. SRC-1 knockout mice exhibit a partial resistance to steroid hormones and a reduction in growth of steroid target organs in response to hormonal stimulation (60). Elimination of SRC-2 revealed that it plays a critical role in mouse reproductive functions (16). Unlike SRC-1 and SRC-2, SRC-3 exhibits greater promiscuity in enhancing transcriptional activity of a number of different activators (49). Consistent with this observation, ablation of SRC-3 in mice results in growth retardation from embryonic day 13.5 through adulthood. SRC-3-null mice are small in size and exhibit delayed puberty, reduced female reproductive function, and reduced mammary gland development (56, 59). In addition, SRC-3 is often overexpressed and sometime amplified in many cancer cells and primary tumors, including breast cancers, ovarian cancers (2, 31), endometrial carcinomas (17), gastric cancers (39), and prostate cancers (18). These observations, taken together, suggest that SRC-3 is required for normal somatic growth and may play a role in oncogenesis. However, so far little is known regarding the mechanisms involved in SRC-3's function.
Overall difference in size between animals can be accounted for by difference in cell size, cell number, or both. Cell numbers depend on both cell division and cell death, while cell sizes depend on both intracellular and extracellular signaling molecules that regulate these programs. In response to nutrient, mitogen, or growth factor stimulation, eukaryotic cells go through growth processes, which correspond to an accumulation of cell mass and increased size. At a molecular level, growth is intimately linked to an increase in de novo RNA and protein synthesis that is largely dedicated to the assembly of ribosomes (44). In animals, coordinated increases in cell number (cell proliferation) and cell size (cell growth) contribute to the growth of an organ and of the whole body (11). To remain constant in size, most proliferating cells grow (i.e., mass and size increase) and duplicate their contents through increased macromolecular biosynthesis before they divide. Various studies have established that cell growth (increases in cell mass and size) and cell proliferation (increases in cell number) are two separable, distinct yet coupled processes that are governed by different mechanisms (11, 23). It has been known that growth factors, such as insulin and insulin-like growth factor-1 (IGF-1), can stimulate cell growth and that the components of the insulin-phosphatidylinositol 3-kinase (PI3K)-AKT pathway are important for cell growth control (27, 44, 57). Members of this pathway include the positive regulators, such as insulin receptor, insulin receptor substrate, PI3K, 3-phosphorylation-dependent protein kinase 1, AKT, and TOR (target of rapamycin) and its downstream targets, S6 kinase (S6K) and initiation factor 4E binding protein (4E-BP), and the negative regulators, such as phosphatases with tensin domain (PTEN) and tuberous sclerosis (TSC). In Drosophila, overexpression of these positive regulators or mutation of negative regulators increases cell size and/or cell number, whereas hypomorphic or null mutation of the positive regulators results in marked decreases in cell size (27, 37, 44, 57). Similarly, in mice and mammalian cells, factors such as PI3K (12), AKT (4, 43, 52), 3-phosphorylation-dependent protein kinase 1 (29), PTEN (12, 28), TSC (21), and Raptor (26), as well as mTOR and its downstream targets, S6K and eukaryote initiation factor 4E (eIF4E) (14), have been implicated in cell growth control, suggesting the existence of an evolutionary functional conservation of cell growth machinery in higher eukaryotes.
In SRC-3−/− mice, a significant decrease in the IGF-1 level was observed (56, 59). In addition, SRC-3−/− embryonic fibroblasts and liver cells show a cell-autonomous defect in response to IGF-1 stimulation, suggesting that the IGF-1 signaling pathway is impaired by the disruption of SRC-3 in mice. In fact, further study showed that the expression levels of most nuclear receptor target genes in SRC−/− mice appear to be normal (56), indicating that SRC-3's role in somatic growth may not be mediated through nuclear receptors but instead may be mediated through other transactivators. However, the mechanisms involved still remain unclear.
Requirement of SRC-3 for normal animal growth and the fact that its expression is up-regulated in tumors suggest that SRC-3 may play an important role for cell growth, cell proliferation, and tumorigenesis. To understand the mechanisms through which SRC-3 contributes to cell growth and cell proliferation, especially its role in the oncogenesis of prostate cancers, we studied the effect of overexpression of SRC-3 in prostate cancer cells using both an inducible gene expression system and transient transfection assays. Here we show that overexpression of SRC-3 induced cell growth to increase the cell size. Furthermore, we found that SRC-3 can modulate AKT activity, leading to an activation of AKT/mTOR signaling and consequently increased cell growth. In contrast, down-regulation of SRC-3 in cells lead to decrease AKT signaling and cell growth. Finally, we demonstrate that AKT signaling was reduced in tissues of SRC-3 null mice. Taken together, these results suggest that SRC-3 is an important modulator of cell growth machinery in mammalian cells.
MATERIALS AND METHODS
Materials.
Reagents were obtained from the following sources: mifepristone and R1881 were from BIOMOL Research Laboratories Inc; LY294002 and PD98059 were from Cell Signaling; Rapamycin was from Calbiochem; lipofectamine and lipofectamine 2000 were from Life Technologies/Invitrogen; RNase A was from Roche. The nitrocellulose membrane was from Bio-Rad. All other chemicals were from Sigma.
Antibodies.
Anti-SRC-3 antibody was kindly provided by Jieming Wong (Baylor College of Medicine, Houston, Tex.); Phospho-S473 AKT/PKB, Phospho-S473 AKT, Phospho-Thr308 AKT, total AKT, Phospho-T389 S6K1, Phospho-GSK3, and mitogen-activated protein kinase (MAPK) antibodies were purchased from Cell Signaling; hemagglutinin (HA) monoclonal antibody was from Roche; Tubulin, actin, and Flag monoclonal antibodies were from Sigma. Horseradish peroxidase-conjugated antimouse and antirabbit secondary antibodies were from Santa Cruz Biotechnology; fluorescein isothiocyanate (FITC)- and Cy3-conjugated antirabbit secondary antibodies were from Jackson ImmunoResearch Laboratories.
Plasmids.
pCMX-SRC-3 expression vector was obtained from Don Chen (University of Massachusetts Medical School). The regulator and SRC-3 target gene expression vector were constructed using a two-vector inducible transcription system that has been described previously (6, 51). Briefly, the regulator, which consists of the GAL4 DNA binding domain, the progesterone receptor ligand binding domain lacking the last 19 amino acids at the C terminus and the p65 activation domain, is under the control of a cytomegalovirus promoter. For SRC-3 reporter construction, a full-length SRC-3 cDNA fragment was inserted behind a 17(x 4) Gal4-TATA promoter, which was further cloned into the gkNeo vector to generate 17(x 4)Gal4-TATA-SRC-3.pgkneo.
Cell culture and generation of SRC-3 stable cell lines.
Prostate cancer cell lines LNCaP and PC3 were used throughout this study; LNCaP cells were cultured in RPMI 1640 medium (GIBCO/Invitrogen), PC3 cells were maintained in Dulbecco's modified Eagle medium-F12 medium (DMEM/F12) (Life Technologies/Invitrogen). Both media contained 10% fetal bovine serum (FBS) (Life Technologies/Invitrogen) supplemented with 100 U of penicillin/ml and 100 μg of streptomycin/ml. All cells were maintained as monolayers in a humidified atmosphere containing 5% CO2 at 37°C and passaged at confluence by trypsinization. The SRC-inducible LNCaP and PC3 cell lines, LNCaP-SRC-3 and PC3-SRC-3, were generated using the two-plasmid inducible transcription system developed in our laboratory in which a mifepristone-regulated SRC-3 gene is activated in the presence of mifepristone (6, 51). To generate a parent cell line containing the GLp65 regulator, LNCaP and PC3 cells were transfected with GLp65, and stable clones were selected with hygromycin B (200 μg/ml). Pools of hygromycin-resistant clones were further transfected with 17× 4Gal4-TATA-SRC-3.pgkneo and selected with G418 (400 μg/ml). The final clones resistant to both G418 and hygromycin were chosen to test for SRC-3 inducibility in the presence of mifepristone.
Hormone-deprived medium was prepared by addition of 10% charcoal-stripped serum instead of untreated whole FBS to the medium. The hormone deprivation protocol was initiated by plating LNCaP cells at about 30% confluence in serum- and phenol red-free medium for 48 h. Next, cells were continuously cultured in stripped medium in the presence or absence of 2 nM R1881 for at least 48 h before further drug treatments.
DNA and small interfering RNA (siRNA) transfection.
For transient transfection, 106 asynchronously growing cells were plated per 10-cm dish in the medium containing 10% FBS the day before transfection. Cells were transfected with 10 μg of DNA [4 μg of 17(x 4)Gal4-TATA-wild-type SRC-3 or 17(x 4)Gal4-TATA-mutant SRC-3 together with 4 μg of GLp65 regulator and 1 μg of green fluorescent protein (GFP) vector] using lipofectamine transfection reagent in Opti-MEM media (Life Technologies) without serum. Five hours posttransfection, the medium was replaced with fresh serum-free Opti-MEM medium. After an additional 36 h, the medium was replaced with DMEM/F12 containing 2.5% FBS. Mifepristone (10−8 M) was present in all media. Twenty-four hours later, the cell size of GFP-positive cells in the G1 cell population was determined by forward light-scatter fluorescence-activated cell sorter (FACS) analysis as described below.
For siRNA transfection, 21 nucleotide-complementary RNAs for the luciferase gene and target gene were obtained from Dharmacon Research Inc. Sequence of siRNA for SRC-3 is (5′-AGACUCCUUAGGACCGCUUdTdT-3′). Annealed oligonucleotides were transfected at a 40 nM concentration with Lipofectamine 2000 into PC3 cells cultured in Opti-MEMI media without serum. Five hours after transfection, the medium was replaced with fresh serum-free Opti-MEMI medium for an additional 36 h. The medium was then replaced with DMEM/F12 containing 2.5% FBS. Twenty-four hours later, the cell size of the G1 cell population was determined by FACS analysis as described below.
Cell lysis and immunoblotting.
Cells were washed twice with phosphate-buffered saline (PBS) buffer, and cell pellets were frozen at −80°C until use. Treated cells were lysed in lysis buffer (20 mM Tris-Cl [pH 8.0], 125 mM NaCl, 1% Triton X-100, 2 mM EDTA, 0.2 mM NaF, 100 μg of PMSF/ml, protease inhibitor cocktail), and tissues were homogenized and lysed in RIPA buffer plus proteinase inhibitor cocktail for 30 min on ice with constant vigorous vortexing. The debris was cleared by centrifugation at 12,000 rpm for 10 min at 4°C. Lysates were boiled in gel loading buffer and subjected to sodium dodecyl sulfate-8% polyacrylamide gel electrophoresis. Proteins were electrotransferred to nitrocellulose membranes and immunoblotted with the indicated primary antibodies overnight at 4°C followed by horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. All blots were developed with Supersignal substrate (Pierce, Rockford, Ill.) and visualized by enhanced chemiluminescence. Subsequent probing with different antibodies was made possible by stripping the membranes with stripping buffer (62.5 mM Tris-HCl [pH 6.8], 2% sodium dodecyl sulfate, 100 mM β-mercaptoethanol) at 55°C for 30 min. Bradford assays were used to determine protein content (Bio-Rad).
Histology and immunohistochemistry.
Cultured cells were fixed in 4% paraformaldehyde (PFA) in PBS (pH 7.5) for 30 min. PFA-prefused livers from heterozygous or SRC-3−/− null mutant mice were dissected immediately and fixed in 4% PFA overnight at 4°C, washed with PBS, dehydrated, embedded in paraffin, and sectioned at 5 μm thick. Primary antibodies were used at the following dilutions: anti-AKT, 1:100; anti-phospho-AKT (Ser473), 1:500. Primary incubations were performed at room temperature for 30 min (culture cells) or overnight at 4°C (tissues). Primary antibodies were detected either by immunofluorescence labeling with FITC-conjugated (1:400) or Cy3-conjugated (1:800) antirabbit secondary antibodies or by immunoperoxidase using a Vectastain ABC kit (Vector Labs).
Cell size analysis.
A Becton Dickinson FACSCalibur flow cytometer with Cell Quest software was used to determine DNA content and cell size. Briefly, treated cells were gently scraped off the plates, transferred to 15-ml conical tubes, pipetted several times to break cell clumps, centrifuged for 5 min at 1,000 rpm, and washed twice in PBS. The cells were resuspended in 0.5 ml of PBS and fixed by adding 5 ml of ice-cold 88% enthanol (80% final) for at least 30 min at −20°C. Immediately before analysis on the flow cytometer, the fixed cells were centrifuged at 2,000 rpm for 5 min, washed once in PBS, and then incubated at 37°C for 30 min in propidium iodide-RNase A solution (10 μg of propidium iodide/ml and 250 μg of RNase A/ml in PBS). For FACS analysis of cells, 10,000 single cells were collected. Single cells with a DNA content of 2N (the G1 population) were gated, and forward scatter height (FSC-H) was calculated. The analysis was performed with similar results on three independent experiments.
For FACS analysis of transiently transfected cells, cells (60 h posttransfection) were fixed in 4% PFA in PBS for 10 min and 80% ethanol for at 30 min, followed by propidium iodide staining in the presence of RNase A, as described above. The forward scatter height of the GFP-positive transfected cells with a 2N DNA content (the G1 population) was analyzed.
RESULTS
Establishment of inducible cell lines overexpressing SRC-3.
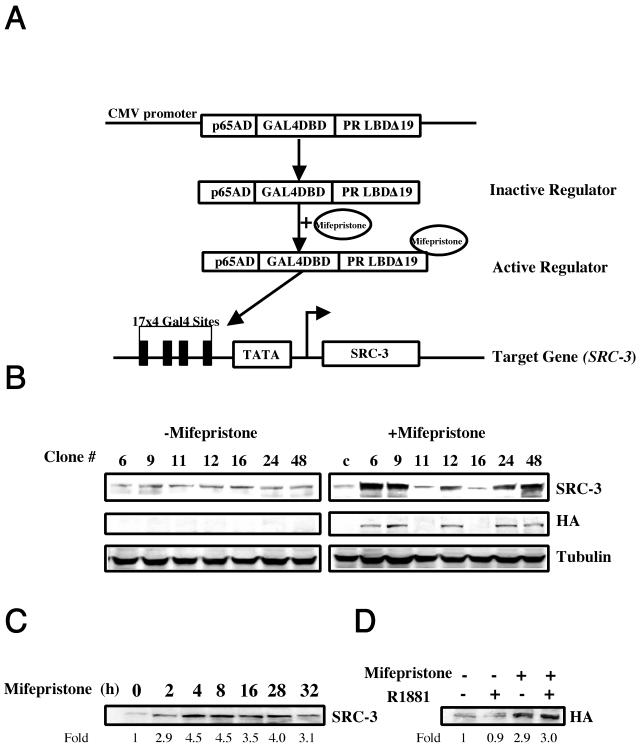
To determine the effects of SRC-3 overexpression on cell growth and the possible role of SRC-3 in prostate cancer development, we used the mifepristone-inducible gene expression system developed in our laboratory (6, 51) to establish stable cell lines from LNCaP and PC3 cells. This system includes two plasmids: a regulator and a target (Fig. 1A). The regulator is a fusion protein consisting of three functional domains: a DNA-binding domain derived from the Gal4 DNA-binding domain, a p65 transactivation domain, and a mutant progesterone receptor ligand-binding domain that binds to mifepristone but not to endogenous ligands as described in Materials and Methods. The target construct contains SRC-3 cDNA placed under the control of a TATA minimal promoter and four copies of 17mer Gal4 DNA binding sites. As shown in Fig. 1A, in the absence of mifepristone, the regulator is not active. When mifepristone is administered, it binds to the regulator and induces its conformational change, resulting in activation of the regulator, which subsequently binds to the Gal4 DNA binding sites and activates SRC-3 expression. To establish stable cell lines, both regulator and target plasmids were transfected into LNCaP cells. After selection, we successfully obtained several clones that express higher levels of SRC-3 than parental LNCaP cells in the presence of mifepristone treatment. Western blotting using antibodies against both SRC-3 and HA tag (exogenous SRC-3 gene is HA tagged) showed that a twofold to fivefold induction of SRC-3 expression is present in positive clones, such as clones 6, 9, 12, 24, and 48 (Fig. 1B). Furthermore, the induction of SRC-3 expression can be seen as early as 2 h after mifepristone treatment and remains at elevated levels for at least 24 h (Fig. 1C). To investigate whether the induction of SRC-3 by mifepristone is dependent on androgen or other steroid signaling, we cultured the stable cells in 10% stripped serum and treated them with mifepristone in the presence or absence of androgen agonist R1881. Our results showed that SRC-3 expression can be induced in the presence or absence of R1881, indicating that the induction of SRC-3 expression is hormone independent (Fig. 1D).
FIG. 1.
Inducible expression of SRC-3 in LNCaP stable cell lines. (A) Diagram of the mifepristone-inducible gene expression system. (B) Western blot of different LNCaP stable clones in the presence and absence of mifepristone treatment. Note that SRC-3 expression was induced in clones 6, 9, 12, 24, and 48 after mifepristone treatment (10−8 M) for 24 h, while no induced SRC-3 expression was seen in clones 11, 16, and the control parental cell line. c, control. Similar induction was also shown with a HA antibody, since exogenously transfected SRC-3 is HA tagged. (C) Time course of SRC-3 induction shown by Western blots (clone 12). SRC-3 expression was induced as early as 2 h upon mifepristone treatment and remained at elevated levels for at least 24 h in the stable clones; (D) Androgen-independent induction of SRC-3 shown by Western blotting using a HA antibody (clone 48). Mifepristone (10−8 M) and 2 nM R1881 were used to treat cells for 24 h under stripped serum culture conditions. Similar results were obtained from other stable clones.
Overexpression of SRC-3 increases cell growth (size).
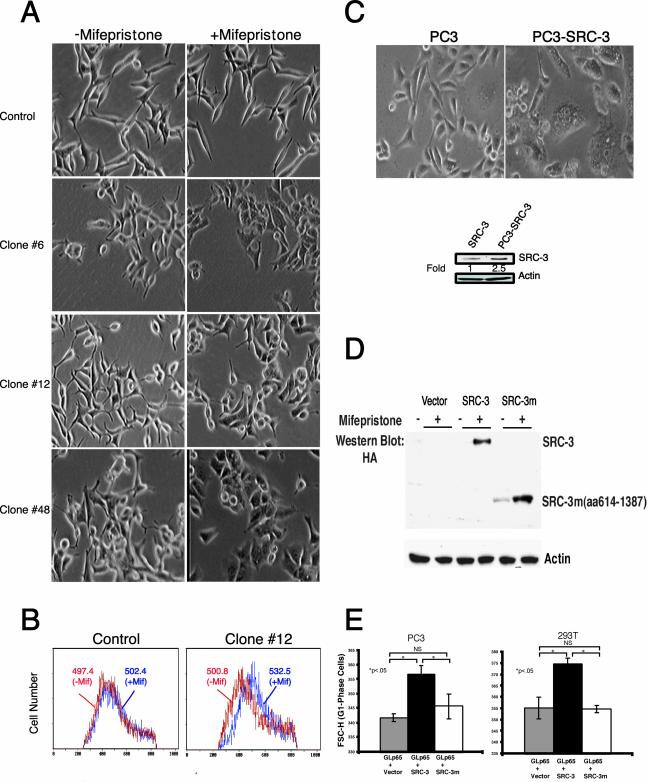
After obtaining SRC-3 stable LNCaP cells, we first examined the effects of SRC-3 overexpression on cell growth. As shown in Fig. 2A, upon mifepristone treatment, morphological changes characterized by an increase in cell size were observed in the stable LNCaP cell lines, whereas changes in cell size were not detected in the control parental LNCaP cells or the noninducible clones, such as clone no. 11 (data not shown). To measure the size increase, we analyzed cells with flow cytometry (see Materials and Methods). The mifepristone-treated and untreated LNCaP cells were gated and analyzed on a flow cytometer for DNA content and for cell size using the mean FSC-H parameter (FSC-H), which is a measure of relative cell size. Consistent with our observation, mifepristone treatment led to a rightward shift in the mean FSC-H histograms of G1-phase LNCaP-SRC-3 cells when compared with control cells, indicating an increase in cell size (Fig. 2B). In addition to LNCaP cells, we also established SRC-3 stable cells using the mifepristone-inducible system in PC3 cells. Interestingly, the clones obtained were already significantly larger than the control cells even in the absence of mifepristone treatment, which is presumably due to the leaky (higher) expression of SRC-3 in the PC3-SRC-3 stable cells in the absence of mifepristone (Fig. 2C).
FIG. 2.
Overexpression of SRC-3 enhances cell growth (size). (A) Morphology of LNCaP-SRC-3 stable cell lines and control parental cells after the treatment with mifepristone (10−8 M) for 24 h. Shown is phase contrast microscopy, indicating that mifepristone treatment promotes cell growth (size increase) in LNCaP-SRC-3 stable cell lines. (B) FSC-H (cell size) from the control and clone no. 12 before and after mifepristone treatment (P < 0.005). The mean FSC-H values for each histogram curve are indicated. The mean ± standard deviation FSC-H values in control cells are as follows: 497.4 ± 5.8 (n = 4), without mifepristone treatment (-Mif); 502.4 ± 6.1 9 (n = 5), treated with mifepristone (+Mif). The mean ± SD FSC-H values for clone no. 12 are as follows: 500.8 ± 3.5 (n = 4), without mifepristone treatment (−Mif); 532.8 ± 5.1 (n = 5), treated with mifepristone (+Mif). (C) Cell morphology of PC-3-SRC-3 stable clones and control parental cells. Stable clones were isolated from PC-3 cellstransfected with both regulator and target (SRC-3) (as shown Fig. 1A) vectors and selected with G418 and hygromycin. (D) The expression of wild-type and mutant SRC-3 proteins in 293T cells. Amino acids from 614 to 1387 were deleted in the mutant SRC-3 construct. Two micrograms of 17(x 4)Gal4-TATA-wild-type SRC-3 or 17(x 4)Gal4-TATA-mutant SRC-3 together with 2 μg of GLp65 regulator were transfected into cells in a 6-well plate. (E) FSC-H (cell size) of GFP-positive cells from triplicate transfections in PC3 and 293T cells. Cells were cotransfected with the indicated plasmids and GFP expression construct in the presence of mifepristone.
To further substantiate this observation and eliminate the possibility of artifacts attributable to a nonspecific toxic phenomenon by overexpression of recombinant protein in selected cell lines, we used transient-transfection assays to test the effect of overexpression of SRC-3 or its mutant protein on cell growth. For this purpose, we constructed an SRC-3 C-terminal deletion mutant (from amino acids 614 to 1,387) in a mifepristone-inducible vector. Deletion of this sequence abolishes the coactivation activity of SRC-3 on steroid receptors (data not shown). As shown in Fig. 2D, when equal amount of wild-type and mutant SRC-3 constructs were transfected into 293T cells, the mutant construct showed a slightly higher level of expression than the wild-type after mifepristone induction. We next cotransfected GFP plasmid together with wild-type SRC-3 plus regulator or mutant vector plus regulator into PC3 and 293T cells in the presence of mifepristone. Seventy-two hours later, cells were harvested and subjected to flow cytometry analysis for the cell size of GFP-positive cells. We observed that cells transfected with wild-type SRC-3 plasmids are larger than cells transfected with mutant SRC-3 (Fig. 2E), indicating that the expression of wild-type SRC-3 has a positive impact on cell growth. Since the analysis was restricted to cells in the G1 phase, these effects are not due to differential distribution of cells in different cell cycle stages. Taken together, our results from both stable and transient-transfection assays strongly suggest that overexpression of SRC-3 promotes cell growth to increase cell size. In addition, since 293T cells have a wild-type copy of PTEN, our finding of SRC-3-induced cell growth is not restricted to PTEN mutant cells, such as LNCaP and PC3 cells.
Overexpression of SRC-3 induces AKT activity.
Two signal transduction pathways have emerged as critical effectors of cell growth response, the Ras/MAPK pathway and the PI3K/AKT pathway (44). To investigate whether the MAPK pathway is involved in the cell growth response in stable LNCaP-SRC-3 cell lines, we determined whether MAPK activity is affected by SRC-3 overexpression by using Western blotting with an antibody against phospho-MAPK. As shown in Fig. 3A, no detectable phosphorylation of MAPK was detected after treatment of SRC-3 stable cell lines with mifepristone, suggesting that the MAPK pathway is not activated in the LNCaP-SRC-3 cell lines. In addition, PD98059, a MAPK signaling inhibitor, was also used to confirm that the MAPK pathway is indeed not involved in cell growth response in the LNCaP-SRC-3 cell lines (see Fig. 5B, lane 7, and Fig. 6). Next, we examined the possible role of AKT signaling in cell growth response to SRC-3 overexpression. Figure 3B shows that the total AKT and phospho-Ser473 and -Thr308 AKT (active form of AKT) are induced in several LNCaP-SRC-3 stable cell lines following treatment with mifepristone. As a negative control, no such changes are detected in parental LNCaP cells.
FIG. 3.
Overexpression of SRC-3 induces AKT activity. (A) MAPK signaling is not affected by SRC-3 overexpression in LNCaP-SRC-3 stable cells, as measured by immunoblotting with a phospho-specific antibody for Erk1 and Erk2, which detected activated MAPK. 468 and Siha cell extracts serve as a positive control. (B) Treatment with mifepristone (10−8M) induces AKT activity in LNCaP-SRC-3 stable cell lines. Phosphorylation of AKT (both Ser473 and Thr 308) and total AKT are increased by mifepristone treatment in stable clones. Shown are the results from clones no. 6 and 12, which are characteristic examples of the results seen for other stable clones. (C) Immunostaining of PC3 cells transiently transfected with Flag-tagged SRC-3 and its deletion mutant. Seventy-two hours after transfection, cells were stained with both anti-Flag (a, d, and g) and anti-AKT (b) or anti-phospho-Ser473 AKT (e and h) antibodies. Panels a to c show the same section, as do panels d to f and g to i. Flag-tagged SRC-3 (a and d), its deletion (amino acids 614 to 1,387) mutant (g), AKT (b), and phospho-AKT (e and h) are shown by FITC- or Cy3-labeled secondary antibodies, respectively. Note that high levels of SRC-3 expression (green) are colocalized with high levels of AKT and phospho-AKT (red) in wild-type SRC-3 but not in its deletion mutant expression cells.
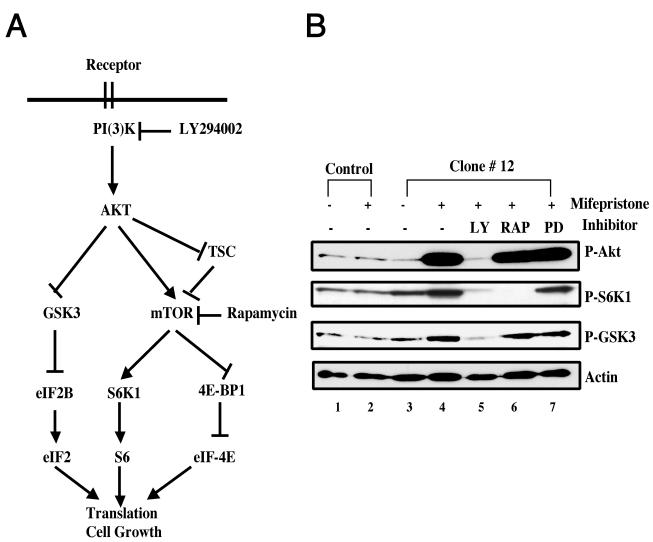
FIG. 5.
Signaling pathways of SRC-3-induced cell growth in stable LNCaP-SRC-3 cells. (A) Schematic overview of the PI3K/AKT/mTOR signaling cascade, linking receptor tyrosine kinase-derived signals to protein synthesis and cell growth regulatory mechanisms. (B) Activation of the PI3K/AKT/mTOR pathway by treatment with mifepristone with LNCaP-SRC-3 stable cells. Immunoblot analysis of clone no. 12 and parental LNCaP cells showed that stimulation with mifepristone (10−8 M) for 12 h increased the phosphorylation of AKT, S6K1, and GSK3 (lane 4) compared with controls (lanes 1 to 3). Treatment of SRC-3 stable cell lines with kinase inhibitors for PI3K (10 μM LY294002 [LY]) (lane 5), mTOR inhibitor (2 ng/ml rapamycin [RAP]) (lane 6), and an inhibitor of Erk1/2, PD98059 (PD) (lane 7) is shown. Similar results are also seen for other stable clones.
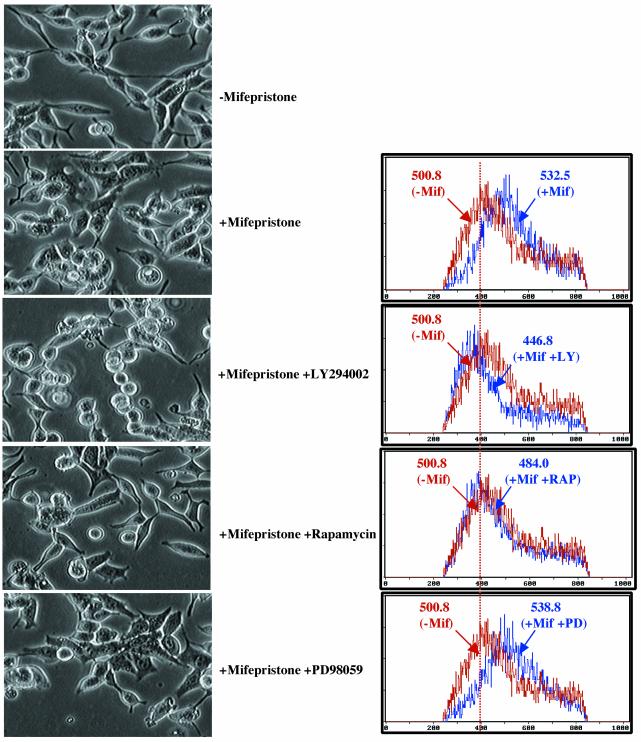
FIG. 6.
AKT/mTOR signaling is required for cell growth induced by overexpression of SRC-3 in stable LNCaP cells. Left, morphology of LNCaP-SRC-3 stable cell lines (clone no. 12) after the treatment with mifepristone (10−8 M) for 24 h in the presence of different kinase inhibitors. Shown is phase-contrast microscopy (×100), indicating that mifepristone-induced cell growth (increased cell size) was inhibited by the PI3K inhibitor LY294002 and the mTOR inhibitor rapamycin but not by the MAPK inhibitor PD98059. Right, FSC-H (cell size) from clone no. 12 before and after mifepristone treatment in the presence of different kinase inhibitors. The mean FSC-H values for each histogram curve are indicated. The mean ± standard deviation FSC-Hs are as follows: 500.8 ± 3.5 (n = 4), treated without mifepristone (-Mif); 532.8 ± 5.1 (n = 5), treated with mifepristone (+Mif); 446.8 ± 5.4 (n = 5), treated with mifepristone plus LY294002 (+Mif+LY); 484 ± 6.3 (n = 5), treated with mifepristone plus rapamycin (+Mif+RAP); 538.8 ± 6.1, treated with mifepristone plus PD98059 (+Mif+PD). Similar results are also seen for other stable clones.
To further confirm that the overexpression of SRC-3 can modulate AKT signaling, we transiently transfected a Flag-tagged SRC-3 cDNA or its deletion (amino acid 614 to 1387) mutant into PC-3 cells. Thirty-six hours later, cells were fixed and stained with antibodies against the Flag tag, total AKT, and phospho-AKT and analyzed by fluorescence microscopy. The results showed that cells with higher levels of Flag (SRC-3) staining also have higher expression of both total and phospho-AKT in wild-type SRC-3 but not in its mutant transfection (Fig. 3C). These results are consistent with the notion that overexpression of SRC-3 can augment AKT signaling in both PC3 and LNCaP cells.
Steroid-independent and cell-autonomous activation of AKT activity by SRC-3 overexpression.
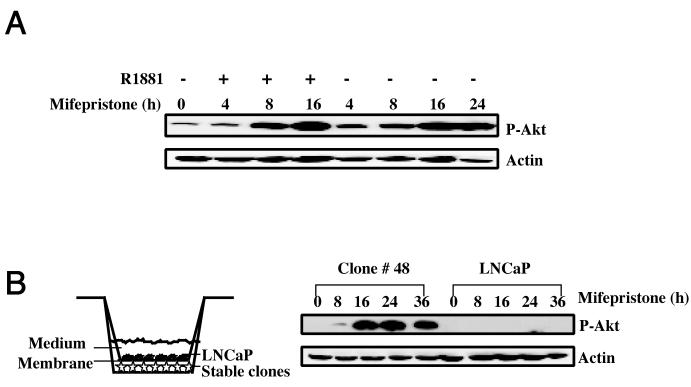
It is known that steroids and steroid receptors, such as androgen and androgen receptor (AR), play an important role in prostate cell growth, proliferation, and prostate cancer development (19). Given that SRC-3 is a coactivator of AR (47), it raises the possibility that the activation of AKT signaling by SRC-3 in LNCaP stable cell lines is mediated through androgen-AR or other steroid signaling pathways. To test this possibility, stable LNCaP-SRC-3 cells were cultured in medium with 10% stripped serum and then treated with mifepristone in the presence or absence of R1881. As shown in Fig. 4A, additional phosphorylation of AKT is not observed in the presence of the androgen agonist R1881 (Fig. 4A), suggesting that androgen does not influence the activation of AKT by SRC-3. This result, together with the fact that SRC-3 can activate AKT signaling in stripped serum (Fig. 4A), indicates that SRC-3 functions through steroid-independent pathways to modulate AKT activity.
FIG. 4.
Steroid-independent and cell-autonomous activation of AKT activity by SRC-3 overexpression. (A) Steroid-independent induction of AKT activity by SRC-3 overexpression. Immunoblot analysis with the phospho-Ser473 antibody for AKT, which detects activated AKT. Clone no. 48 of LNCaP-SRC-3 cultured in the medium with 10% charcoal-stripped serum was treated with mifepristone (10−8 M) in the presence or absence of the androgen agonist R1881 (2 nM). (B) Cell-autonomous induction of AKT activity by SRC-3 overexpression. Immunoblot analysis of clone no. 48 and parental LNCaP cells cocultured in the medium separated by a 0.45-μm membrane which is permeable to growth factors. Similar results are also seen for other stable clones.
Since activation of AKT generally involves the activation of PI3K by upstream receptor protein-tyrosine kinases and their cognate ligands, we examined whether SRC-3 activates the AKT through the synthesis of growth factors and whether these factors subsequently serve as paracrine or autocrine signals to activate PI3K. To investigate this possibility, we used a coculture system in which stable LNCaP-SRC-3 cells and parental LNCaP cells were both grown in the same culture wells but separated by a 0.45-μm membrane that is permeable to medium and growth factors. If SRC-3 activates AKT through a paracrine pathway, we would expect that AKT signaling in parental LNCaP cells would be induced in this coculture system after treatment with mifepristone. However, our results show that increased AKT phosphorylation occurred only in LNCaP-SRC-3 stable cells but not in parental LNCaP cells, indicating that paracrine signaling is unlikely to be responsible for the activation of AKT signaling by SRC-3. Together, these results suggest that activation of AKT by SRC-3 overexpression in stable LNCaP-SRC-3 cells is steroid independent and involves a cell-autonomous mechanism that is independent of the stimulation by extracellular signals.
AKT/mTOR signaling is required for cell growth induced by overexpression of SRC-3 in stable LNCaP-SRC-3 cells.
Cell growth is a process of synthesis of macromolecules, which results in increased cell mass or size. AKT participates in cell growth control and promotes protein synthesis in several ways. One of them is through mTOR, a serine/threonine kinase that serves as a molecular sensor to regulate protein synthesis through its downstream targets S6K1 and 4E-BP1 on the basis of the availability of growth factors and/or nutrients (1, 40). It is known that AKT can modulate mTOR activity either by directly targeting mTOR itself (41) or by phosphorylating its repressor TSC (21). Subsequent activation of S6K1 by mTOR leads to phosphorylation of the 40S ribosomal protein S6 that ultimately drives translation of 5′ polypyrimidine tract mRNAs, which are a small family of abundant transcripts that primarily encode ribosomal proteins and components of the translational apparatus. Similarly, phosphorylation of 4E-BP1 by mTOR releases it from inhibiting the translation initiation factor eIF4E, thereby permitting the binding of eIF4E to eIF4G and promoting the initiation of protein translation. As a consequence of controlling rRNA translation and translation initiation, mTOR upregulates the translational machinery to promote protein synthesis and cell growth (Fig. 5A). In addition, phosphorylation of glycogen synthase kinase 3β (GSK-3β) by AKT can also lead to GSK-3β inhibition and the upregulation of protein synthesis (22).
To investigate whether activation of AKT by overexpression of SRC-3 contributes to increased cell growth in stable LNCaP-SRC-3 cells, we first examined the phosphorylation of other components in the AKT signaling pathway. Consistent with its role in AKT signaling, the activation of AKT by induction of SRC-3 overexpression in stable LNCaP-SRC-3 cells results in increased phosphorylation of AKT downstream targets, such as S6K1 and GSK-3 (Fig. 5B, lane 4). This finding demonstrates that the AKT pathway and its downstream targets are activated by SRC-3 overexpression in stable LNCaP-SRC-3 cells.
To determine whether the PI3K/AKT/mTOR pathway was required for SRC-3-induced cell growth, we used kinase inhibitors to inhibit the PI3K/AKT/mTOR pathway. As shown in Fig. 5B, the PI3K inhibitor LY294002 blocked all measurable activation of phosphorylation in the pathway from AKT to S6K1 and GSK3 (lane 5). As a result of LY294002 inhibition of PI3K, the increase in cell size induced by SRC-3 overexpression in the presence of mifepristone was abolished, and in fact cell size was decreased compared with the control (Fig. 6). The downstream inhibitor rapamycin, a specific inhibitor for mTOR, was also used in our study. As expected, treatment with rapamycin did not alter the phosphorylation of AKT itself or GSK-3, but it effectively blocked mTOR downstream targets, such as phosphorylation of S6K1 (Fig. 5B, lane 6). More importantly, the treatment with rapamycin also completely prevented the cell growth induced by SRC-3 overexpression (Fig. 6). It should be noted that the enhanced effect seen with LY294002 compared to that of rapamycin suggests that other branches of this pathway, such as GSK3 signaling, might also contribute to cell growth. As a control for specificity, an inhibitor of the ERK signaling pathway, PD98059, was used. We found that PD98059 had no effect on both the PI3K/AKT/mTOR phospho-activation cascade (Fig. 5B, lane 7) and cell growth induced by SRC-3 overexpression (Fig. 6). Taken together, theses results suggest that the AKT/mTOR pathway is activated and required for cell growth induced by SRC-3 in stable LNCaP-SRC-3 cells.
Down-regulation of SRC-3 expression reduces the cell size.
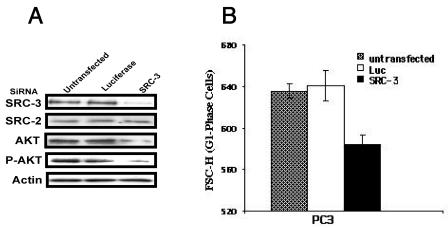
To further investigate the role of SRC-3 in cell growth, we used siRNA (13) to decrease endogenous levels of SRC-3 in PC3 cells and measured cell growth in response to serum stimulation. As a control, siRNAs for luciferase were also transfected into cells. Figure 7 shows that SRC-3 siRNA specifically inhibits more than 80% of its target gene expression while it exerts no effect on expression of SRC-2. Consistent with a role for SRC-3 in the AKT signaling pathway, we found that PC3 cells transfected with SRC-3 siRNA had decreased AKT signaling compared with untransfected cells or control cells transfected with luciferase siRNA (Fig. 7A). As expected, the average size of PC3 cells was also reduced due to the reduction of SRC-3 expression by siRNA. SRC-3 siRNA-mediated reductions in cell size are significant (P < 0.001) and appear to be unrelated to cell cycle progression, since the size analysis of PC3 cells was restricted to cells in the G1 phase. Together, these results indicate that SRC-3 is necessary for maintaining normal cell growth by regulating cell size.
FIG. 7.
SRC-3 participates in regulating cell size. PC3 cells transfected with siRNAs were analyzed by immunoblotting for indicated proteins, and cell size were determined using flow cytometry. PC3 cells transfected with siRNA were grown under conditions as described in Materials and Methods. (A) Immunoblot analyses show that SRC-3 siRNAs specifically down-regulate their SRC-3 gene expression. Note that in cells transfected with SRC-3 siRNA, AKT and phospo-AKT are also decreased. (B) The mean FSC-H values of mock-transfected cells (only in the presence of lipofectamine 2000) and of cells transfected with the indicated siRNAs. Luc, luciferase siRNA; SRC-3, SRC-3 siRNA. The means of cell sizes (FSC-H) ± standard deviation (n = 5) are shown.
Decreased AKT signaling in SRC-3−/− mutant mice.
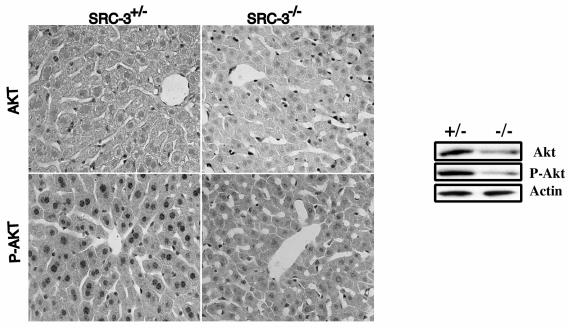
Our results from in vitro-cultured cells suggest that SRC-3 is an important component implicated in the control of cell growth, which is consistent with the observed impaired growth in SRC-3 knockout mice (56). To further investigate mechanisms through which SRC-3 regulates cell growth and confirm the role of AKT in SRC-3 signaling, we evaluated the expression level of phosphorylated AKT and total AKT in SRC-3 null mice and their control littermates. To this end, mouse livers, which express a moderate level of SRC-3 (59; our unpublished results), were used for immunohistochemical staining with antibodies against both phosphorylated AKT and total AKT. Hepatocytes from both SRC-3 null mice and control littermates showed a low level of expression of total AKT. However, we observed a consistently higher expression level of AKT for heterozygous littermates than for the homozygous mutant (Fig. 8). Similarly, phosphorylated AKT levels in hepatocytes were also significantly lower in SRC-3−/− mutant livers than for the heterozygous littermates (Fig. 8). These results were further confirmed by using Western blotting, which indicated a reduced expression of total AKT as well as phosphorylated AKT in homozygous (SRC-3−/−) livers in comparison to the heterozygous (SRC-3+/−) littermates (Fig. 8). Taken together, these findings demonstrate that SRC-3 is involved in the regulation of AKT activity and deletion of SRC-3 down-regulates AKT levels and its signaling activity in vivo.
FIG. 8.
Decreased AKT signaling in SRC-3 null mice. Left panel, immunohistochemical staining of liver paraffin sections from 2-month-old SRC+/− and SRC-3−/− littermate mice with antibodies against AKT and phospho-AKT (ser473). Right panel, immunoblot analysis of liver extracts from the mice as in the left panel, using antibodies against AKT, phospho-AKT (ser473), and phospho-GSK3 antibodies.
DISCUSSION
The fact that SRC-3 null mice are smaller than control littermates provides genetic evidence suggesting that SRC-3 is required for normal animal growth (56, 59). However, the mechanisms of SRC-3-mediated growth effect in vivo are not clear. The small size of mutant animals could potentially be attributed to reduced cell growth, decreased cell proliferation, or increased apoptosis in developing tissues. Here, we provide direct evidence suggesting that SRC-3 plays a role in regulation of cell growth. Our results show that overexpression of SRC-3 in different prostate cancer cell lines can increase cell size to induce cell growth, whereas down-regulation of SRC-3 expression decreases cell growth. Furthermore, our data indicate that AKT and its downstream effectors, including mTOR and S6K1, are important mediators of cell growth induced by SRC-3 overexpression. Thus, the effect of SRC-3 on AKT may provide one of the mechanisms for the in vivo growth effect of SRC-3, and decreased AKT activity in vivo may contribute to the phenotypes of growth retardation exhibited by SRC-3 null mice.
Consistent with this observation, the IGF-1 signaling pathway appears to be impaired in SRC-3 null mice (56). Studies on the control of cell growth in Drosophila and mammalian cells indicate that the insulin/insulin receptor-PI3K-AKT-S6K and TOR pathways are major signaling pathways coordinating cell growth. Deregulation of any member in this pathway has been shown to affect cell growth (4, 14, 21, 26-29, 37, 43, 44, 52, 57). For instance, AKT1 null mice are small in size and show a growth retardation phenotype (10). It is believed that downstream effectors of the AKT pathway, such as S6K and mTOR, regulate rRNA translation and protein translation initiation, contributing to overall protein synthesis and cell growth. In support of this, our results also showed that in addition to cell size, protein synthesis was also inhibited by the down-regulation of SRC-3 expression with siRNA in PC-3 cells (data not shown).
It is still unknown whether SRC-3 regulates AKT directly or through other effectors. Although we show that overexpression of SRC-3 in our LNCaP-SRC-3 stable cell lines can activate AKT signaling through a steroid-independent pathway (Fig. 4), the mechanisms involved remain largely undefined. Since SRC-3 is a coactivator of many transcription factors, it remains highly possible that SRC-3 may modulate the expression of AKT mRNA through potentiation of the functional activity of its transcription regulators. In addition, the induction of phosphorylation of AKT sometimes appears to precede the induction of total AKT in our stable cell lines (Fig. 3B), raising the possibility that SRC-3 may be able to target AKT phosphorylation before inducing AKT protein synthesis and stability.
The observation that AKT signaling is involved in SRC-3 biological functions is very important. In addition to cell growth, AKT signaling has been implicated in many other processes, such as antiapoptosis, cell survival, and cell proliferation (53). Because of its involvement in many diverse biological functions, AKT is emerging as a central player in tumorigenesis. Many components of the PI3K-AKT signaling pathway have been identified as being dysregulated (amplified, overexpressed, or mutated) in a wide spectrum of human cancers including breast and prostate cancers. Gain- or loss-of-function mutants of several components of this pathway usually lead to neoplastic transformation in model systems (25, 35, 48, 53). One important feature of SRC-3 is that amplification and overexpression of SRC-3 is frequently observed in breast cancers, ovarian cancers, and prostate cancers (2, 18, 32; our unpublished results), which suggest that SRC-3 may play a role during tumorigenesis. The fact that SRC-3 is overexpressed in tumors derived from steroid hormone target organs, such as breast and prostate, and the result from one study suggesting that amplification of SRC-3 is correlated with ER and progesterone receptor expression in primary breast tumors (3), lead us to suspect that SRC-3 may function through modulating steroid receptor signaling in these tumors. However, more-recent studies have shown that overexpression of SRC-3 does not correlate with ER expression but instead correlates with the expression of the Her2/neu oncogene in breast cancers (5), suggesting that SRC-3 is involved in signaling pathways other than that of steroid receptor signaling during the development of breast cancers. It has been shown that SRC-3 exhibits greater promiscuity than other SRC family members by enhancing the transcriptional activity of a number of non-NR activators, including cAMP regulatory element binding protein (CREB) and NF-κB (49, 58). In fact, it has been reported that the overexpression of SRC-3 also occurs in tumors derived from organs that are not targeted by steroid hormones, such as gastric cancers (39) and liver cancer (55). In SRC-3 null mice, it appears that SRC-3 is dispensable for most steroid receptor target gene expression. Instead, it is required for maximal expression of a subset of genes critical for growth factor regulatory pathways that regulate physiological patterns of somatic growth (56). In support of these hypotheses, our results demonstrate that SRC-3 indeed regulates growth factor signaling pathways by modulating AKT activity (Fig. 3). Furthermore, our data show that its ability to enhance AKT signaling is not dependent on steroid hormones (Fig. 4). Taken together, these findings highlight the importance of SRC-3 as a nonsteroid receptor coactivator in cell growth, cell proliferation, and tumor development.
The correlative finding of SRC-3 and Her2/neu overexpression in breast cancers raises important questions regarding possible cross talks between different signaling pathways involved in these two factors. It has been shown that SRC-3 is a phospho-protein that is regulated by different protein kinases, such as MAPK (15) and IκB kinase (58). It is reasonable to postulate that overexpression of Her2/neu can regulate SRC-3 through its downstream targets, such as MAPK, to enhance SRC-3 functional activity in breast cancers. On the other hand, our results show that SRC-3 itself can target AKT, another downstream target of Her2/neu signaling, suggesting that SRC-3 may synergize with the Her2/neu pathway to reinforce its biological functions involved in cell growth, cell proliferation, and tumorigenesis.
Finally, despite the oncogenic role of SRC-3 as suggested by its overexpression in different tumors and its possible activation of AKT signaling, the attempts to establish stable cell lines constitutively overexpressing SRC-3 have not been successful in our laboratory, suggesting that the levels of SRC-3 in cells may be tightly controlled and too much or too little SRC-3 expression could be detrimental to the survival of the expressing cells. In fact, in our LNCaP-SRC-3 stable cell lines, induction of SRC-3 expression by mifepristone stimulates cell growth, but cell cycle progression is inhibited in normal 10% serum culture condition (data not shown). This observation may explain why it is difficult to generate constitutively expressed stable cell lines. Interestingly, in addition to its amplification and overexpression, loss of heterozygosity of SRC-3 was also found in breast cancers (42). Since losses of heterozygosity happen to most of the tumor suppressors, such as p53, RB, etc., it raises the possibility that SRC-3 has both oncogenic and antioncogenic roles for the development of cancers. However, so far, mechanisms involved in the possible antiproliferation role of SRC-3 are still unknown.
In summary, we have shown the importance of SRC-3 in controlling cell growth and the potential cross talk between AKT and SRC-3 signaling pathways. However, much still remains to be explored to determine the mechanisms involved. It should be also noted that in addition to the PI3K/AKT/mTOR pathway, there are other possible signaling pathways that may play a role in cell growth mediated by SRC-3 expression. Like other coactivators, SRC family members appear to be subjected to modifications by different kinase-mediated cellular signaling pathways. Such modulations influence the recruitment of coactivator into active transcriptional complexes at distinct promoters in different tissues (33). On the other hand, we now provide evidence that SRC-3 itself can modulate these cellular signaling pathways. Taken together, our studies identify SRC-3 as a component of the diverse signaling pathways that can integrate multiple stimuli into an appropriate cellular response. Through AKT and other downstream effectors, SRC-3 might play critical roles in many biological processes, especially in cell growth control.
Acknowledgments
This work is supported by NIH grants to S.Y.T. (DK57743, project 3) and M.-J.T. (DK62821 and CA58204). G.Z. is supported by a Fellowship of DOD for Prostate Cancer Research (DAMD 17-99-1-9509).
We thank Francesco J. DeMayo, Debra E. Bramblett, Sue-Hwa Lin, David M. Lonard, Xiaotao Li, and Ray-chang Wu for discussions and helpful comments on the manuscript. We are also grateful to Liliang Wang and Baolin Liu for providing technical assistance.
REFERENCES
- 1.Abraham, R. T. 2002. Identification of TOR Signaling Complexes. More TORC for the cell growth engine. Cell 111:9-12. [DOI] [PubMed] [Google Scholar]
- 2.Anzick, S. L., J. Kononen, R. L. Walker, D. O. Azorsa, M. M. Tanner, X. Y. Guan, G. Sauter, O. P. Kallioniemi, J. M. Trent, and P. S. Meltzer. 1997. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science 277:965-968. [DOI] [PubMed] [Google Scholar]
- 3.Bautista, S., H. Valles, R. L. Walker, S. Anzick, R. Zeillinger, P. Meltzer, and C. Theillet. 1998. In breast cancer, amplification of the steroid receptor coactivator gene AIB1 is correlated with estrogen and progesterone receptor positivity. Clin. Cancer Res. 4:2925-2929. [PubMed] [Google Scholar]
- 4.Bodine, S. C., T. N. Stitt, M. Gonzalez, W. O. Kline, G. L. Stover, R. Bauerlein, E. Zlotchenko, A. Scrimgeour, J. C. Lawrence, D. J. Glass, and G. D. Yancopoulos. 2001. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 3:1014-1019. [DOI] [PubMed] [Google Scholar]
- 5.Bouras, T., M. C. Southey, and D. J. Venter. 2001. Overexpression of the steroid receptor coactivator AIB1 in breast cancer correlates with the absence of estrogen and progesterone receptors and positivity for p53 and HER2/neu. Cancer Res. 61:903-907. [PubMed] [Google Scholar]
- 6.Burcin, M. M., G. Schiedner, S. Kochanek, S. Y. Tsai, and B. W. O'Malley. 1999. Adenovirus-mediated regulable target gene expression in vivo. Proc. Natl. Acad. Sci. USA 96:355-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavailles, V., S. Dauvois, P. S. Danielian, and M. G. Parker. 1994. Interaction of proteins with transcriptionally active estrogen receptors. Proc. Natl. Acad. Sci. USA 91:10009-10013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, D., H. Ma, H. Hong, S. S. Koh, S. M. Huang, B. T. Schurter, D. W. Aswad, and M. R. Stallcup. 1999. Regulation of transcription by a protein methyltransferase. Science 284:2174-2177. [DOI] [PubMed] [Google Scholar]
- 9.Chen, H., R. J. Lin, R. L. Schiltz, D. Chakravarti, A. Nash, L. Nagy, M. L. Privalsky, Y. Nakatani, and R. M. Evans. 1997. Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell 90:569-580. [DOI] [PubMed] [Google Scholar]
- 10.Chen, W. S., P. Z. Xu, K. Gottlob, M. L. Chen, K. Sokol, T. Shiyanova, I. Roninson, W. Weng, R. Suzuki, K. Tobe, T. Kadowaki, and N. Hay. 2001. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 15:2203-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conlon, I., and M. Raff. 1999. Size control in animal development. Cell 96:235-244. [DOI] [PubMed] [Google Scholar]
- 12.Crackower, M. A., G. Y. Oudit, I. Kozieradzki, R. Sarao, H. Sun, T. Sasaki, E. Hirsch, A. Suzuki, T. Shioi, J. Irie-Sasaki, R. Sah, H. Y. Cheng, V. O. Rybin, G. Lembo, L. Fratta, A. J. Oliveira-dos-Santos, J. L. Benovic, C. R. Kahn, S. Izumo, S. F. Steinberg, M. P. Wymann, P. H. Backx, and J. M. Penninger. 2002. Regulation of myocardial contractility and cell size by distinct PI3K-PTEN signaling pathways. Cell 110:737-749. [DOI] [PubMed] [Google Scholar]
- 13.Elbashir, S. M., J. Harborth, W. Lendeckel, A. Yalcin, K. Weber, and T. Tuschl. 2001. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411:494-498. [DOI] [PubMed] [Google Scholar]
- 14.Fingar, D. C., S. Salama, C. Tsou, E. Harlow, and J. Blenis. 2002. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 16:1472-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Font de Mora, J., and M. Brown. 2000. AIB1 is a conduit for kinase-mediated growth factor signaling to the estrogen receptor. Mol. Cell. Biol. 20:5041-5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gehin, M., M. Mark, C. Dennefeld, A. Dierich, H. Gronemeyer, and P. Chambon. 2002. The function of TIF2/GRIP1 in mouse reproduction is distinct from those of SRC-1 and p/CIP. Mol. Cell. Biol. 22:5923-5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glaeser, M., T. Floetotto, B. Hanstein, M. W. Beckmann, and D. Niederacher. 2001. Gene amplification and expression of the steroid receptor coactivator SRC3 (AIB1) in sporadic breast and endometrial carcinomas. Horm. Metab. Res. 33:121-126. [DOI] [PubMed] [Google Scholar]
- 18.Gnanapragasam, V. J., H. Y. Leung, A. S. Pulimood, D. E. Neal, and C. N. Robson. 2001. Expression of RAC 3, a steroid hormone receptor co-activator in prostate cancer. Br. J. Cancer 85:1928-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heinlein, C. A., and C. Chang. 2002. Androgen receptor (AR) coregulators: an overview. Endocr. Rev. 23:175-200. [DOI] [PubMed] [Google Scholar]
- 20.Hong, H., K. Kohli, A. Trivedi, D. L. Johnson, and M. R. Stallcup. 1996. GRIP1, a novel mouse protein that serves as a transcriptional coactivator in yeast for the hormone binding domains of steroid receptors. Proc. Natl. Acad. Sci. USA 93:4948-4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inoki, K., Y. Li, T. Zhu, J. Wu, and K. L. Guan. 2002. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 4:648-657. [DOI] [PubMed] [Google Scholar]
- 22.Jefferson, L. S., J. R. Fabian, and S. R. Kimball. 1999. Glycogen synthase kinase-3 is the predominant insulin-regulated eukaryotic initiation factor 2B kinase in skeletal muscle. Int. J. Biochem. Cell Biol. 31:191-200. [DOI] [PubMed] [Google Scholar]
- 23.Johnston, G. C., J. R. Pringle, and L. H. Hartwell. 1977. Coordination of growth with cell division in the yeast Saccharomyces cerevisiae. Exp. Cell Res. 105:79-98. [DOI] [PubMed] [Google Scholar]
- 24.Kamei, Y., L. Xu, T. Heinzel, J. Torchia, R. Kurokawa, B. Gloss, S. C. Lin, R. A. Heyman, D. W. Rose, C. K. Glass, and M. G. Rosenfeld. 1996. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell 85:403-414. [DOI] [PubMed] [Google Scholar]
- 25.Katso, R., K. Okkenhaug, K. Ahmadi, S. White, J. Timms, and M. D. Waterfield. 2001. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu. Rev. Cell Dev. Biol. 17:615-675. [DOI] [PubMed] [Google Scholar]
- 26.Kim, D. H., D. Sarbassov dos, S. M. Ali, J. E. King, R. R. Latek, H. Erdjument-Bromage, P. Tempst, and D. M. Sabatini. 2002. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110:163-175. [DOI] [PubMed] [Google Scholar]
- 27.Kozma, S. C., and G. Thomas. 2002. Regulation of cell size in growth, development and human disease: PI3K, PKB and S6K. Bioessays 24:65-71. [DOI] [PubMed] [Google Scholar]
- 28.Kwon, C. H., X. Zhu, J. Zhang, L. L. Knoop, R. Tharp, R. J. Smeyne, C. G. Eberhart, P. C. Burger, and S. J. Baker. 2001. Pten regulates neuronal soma size: a mouse model of Lhermitte-Duclos disease. Nat. Genet. 29:404-411. [DOI] [PubMed] [Google Scholar]
- 29.Lawlor, M. A., A. Mora, P. R. Ashby, M. R. Williams, V. Murray-Tait, L. Malone, A. R. Prescott, J. M. Lucocq, and D. R. Alessi. 2002. Essential role of PDK1 in regulating cell size and development in mice. EMBO J. 21:3728-3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li, H., P. J. Gomes, and J. D. Chen. 1997. RAC3, a steroid/nuclear receptor-associated coactivator that is related to SRC-1 and TIF2. Proc. Natl. Acad. Sci. USA 94:8479-8484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.List, H. J., R. Reiter, B. Singh, A. Wellstein, and A. T. Riegel. 2001. Expression of the nuclear coactivator AIB1 in normal and malignant breast tissue. Breast Cancer Res. Treat. 68:21-28. [DOI] [PubMed] [Google Scholar]
- 32.McKenna, N. J., R. B. Lanz, and B. W. O'Malley. 1999. Nuclear receptor coregulators: cellular and molecular biology. Endocr. Rev. 20:321-344. [DOI] [PubMed] [Google Scholar]
- 33.McKenna, N. J., and B. W. O'Malley. 2002. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 108:465-474. [DOI] [PubMed] [Google Scholar]
- 34.McKenna, N. J., and B. W. O'Malley. 2002. Minireview: nuclear receptor coactivators-an update. Endocrinology 143:2461-2465. [DOI] [PubMed] [Google Scholar]
- 35.Nicholson, K. M., and N. G. Anderson. 2002. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 14:381-395. [DOI] [PubMed] [Google Scholar]
- 36.Onate, S. A., S. Y. Tsai, M. J. Tsai, and B. W. O'Malley. 1995. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science 270:1354-1357. [DOI] [PubMed] [Google Scholar]
- 37.Potter, C. J., H. Huang, and T. Xu. 2001. Drosophila Tsc1 functions with Tsc2 to antagonize insulin signaling in regulating cell growth, cell proliferation, and organ size. Cell 105:357-368. [DOI] [PubMed] [Google Scholar]
- 38.Rosenfeld, M. G., and C. K. Glass. 2001. Coregulator codes of transcriptional regulation by nuclear receptors. J. Biol. Chem. 276:36865-36868. [DOI] [PubMed] [Google Scholar]
- 39.Sakakura, C., A. Hagiwara, R. Yasuoka, Y. Fujita, M. Nakanishi, K. Masuda, A. Kimura, Y. Nakamura, J. Inazawa, T. Abe, and H. Yamagishi. 2000. Amplification and over-expression of the AIB1 nuclear receptor co- activator gene in primary gastric cancers. Int. J. Cancer 89:217-223. [PubMed] [Google Scholar]
- 40.Schmelzle, T., and M. N. Hall. 2000. TOR, a central controller of cell growth. Cell 103:253-262. [DOI] [PubMed] [Google Scholar]
- 41.Sekulic, A., C. C. Hudson, J. L. Homme, P. Yin, D. M. Otterness, L. M. Karnitz, and R. T. Abraham. 2000. A direct linkage between the phosphoinositide 3-kinase-AKT signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res. 60:3504-3513. [PubMed] [Google Scholar]
- 42.Shibata, A., Y. Hayashi, T. Imai, H. Funahashi, A. Nakao, and H. Seo. 2001. Somatic gene alteration of AIB1 gene in patients with breast cancer. Endocr. J. 48:199-204. [DOI] [PubMed] [Google Scholar]
- 43.Shioi, T., J. R. McMullen, P. M. Kang, P. S. Douglas, T. Obata, T. F. Franke, L. C. Cantley, and S. Izumo. 2002. Akt/protein kinase B promotes organ growth in transgenic mice. Mol. Cell. Biol. 22:2799-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stocker, H., and E. Hafen. 2000. Genetic control of cell size. Curr. Opin. Genet. Dev. 10:529-535. [DOI] [PubMed] [Google Scholar]
- 45.Suen, C. S., T. J. Berrodin, R. Mastroeni, B. J. Cheskis, C. R. Lyttle, and D. E. Frail. 1998. A transcriptional coactivator, steroid receptor coactivator-3, selectively augments steroid receptor transcriptional activity. J. Biol. Chem. 273:27645-27653. [DOI] [PubMed] [Google Scholar]
- 46.Takeshita, A., G. R. Cardona, N. Koibuchi, C. S. Suen, and W. W. Chin. 1997. TRAM-1, a novel 160-kDa thyroid hormone receptor activator molecule, exhibits distinct properties from steroid receptor coactivator-1. J. Biol. Chem. 272:27629-27634. [DOI] [PubMed] [Google Scholar]
- 47.Tan, J. A., S. H. Hall, P. Petrusz, and F. S. French. 2000. Thyroid receptor activator molecule, TRAM-1, is an androgen receptor coactivator. Endocrinology 141:3440-3450. [DOI] [PubMed] [Google Scholar]
- 48.Testa, J. R., and A. Bellacosa. 2001. AKT plays a central role in tumorigenesis. Proc. Natl. Acad. Sci. USA 98:10983-10985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Torchia, J., D. W. Rose, J. Inostroza, Y. Kamei, S. Westin, C. K. Glass, and M. G. Rosenfeld. 1997. The transcriptional co-activator p/CIP binds CBP and mediates nuclear-receptor function. Nature 387:677-684. [DOI] [PubMed] [Google Scholar]
- 50.Tsai, M. J., and B. W. O'Malley. 1994. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu. Rev. Biochem. 63:451-486. [DOI] [PubMed] [Google Scholar]
- 51.Tsai, S. Y., B. W. O'Malley, F. J. DeMayo, Y. Wang, and S. S. Chua. 1998. A novel RU486 inducible system for the activation and repression of genes. Adv. Drug Deliv. Rev. 30:23-31. [DOI] [PubMed] [Google Scholar]
- 52.Tuttle, R. L., N. S. Gill, W. Pugh, J. P. Lee, B. Koeberlein, E. E. Furth, K. S. Polonsky, A. Naji, and M. J. Birnbaum. 2001. Regulation of pancreatic beta-cell growth and survival by the serine/threonine protein kinase Akt1/PKBalpha. Nat. Med. 7:1133-1137. [DOI] [PubMed] [Google Scholar]
- 53.Vivanco, I., and C. L. Sawyers. 2002. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat. Rev. Cancer 2:489-501. [DOI] [PubMed] [Google Scholar]
- 54.Voegel, J. J., M. J. Heine, C. Zechel, P. Chambon, and H. Gronemeyer. 1996. TIF2, a 160 kDa transcriptional mediator for the ligand-dependent activation function AF-2 of nuclear receptors. EMBO J. 15:3667-3675. [PMC free article] [PubMed] [Google Scholar]
- 55.Wang, Y., M. C. Wu, J. S. Sham, W. Zhang, W. Q. Wu, and X. Y. Guan. 2002. Prognostic significance of c-myc and AIB1 amplification in hepatocellular carcinoma. A broad survey using high-throughput tissue microarray. Cancer 95:2346-2352. [DOI] [PubMed] [Google Scholar]
- 56.Wang, Z., D. W. Rose, O. Hermanson, F. Liu, T. Herman, W. Wu, D. Szeto, A. Gleiberman, A. Krones, K. Pratt, R. Rosenfeld, C. K. Glass, and M. G. Rosenfeld. 2000. Regulation of somatic growth by the p160 coactivator p/CIP. Proc. Natl. Acad. Sci. USA 97:13549-13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weinkove, D., and S. J. Leevers. 2000. The genetic control of organ growth: insights from Drosophila. Curr. Opin. Genet. Dev. 10:75-80. [DOI] [PubMed] [Google Scholar]
- 58.Wu, R. C., J. Qin, Y. Hashimoto, J. Wong, J. Xu, S. Y. Tsai, M. J. Tsai, and B. W. O'Malley. 2002. Regulation of SRC-3 (pCIP/ACTR/AIB-1/RAC-3/TRAM-1) coactivator activity by I kappa B kinase. Mol. Cell. Biol. 22:3549-3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu, J., L. Liao, G. Ning, H. Yoshida-Komiya, C. Deng, and B. W. O'Malley. 2000. The steroid receptor coactivator SRC-3 (p/CIP/RAC3/AIB1/ACTR/TRAM-1) is required for normal growth, puberty, female reproductive function, and mammary gland development. Proc. Natl. Acad. Sci. USA 97:6379-6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu, J., Y. Qiu, F. J. DeMayo, S. Y. Tsai, M. J. Tsai, and B. W. O'Malley. 1998. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science 279:1922-1925. [DOI] [PubMed] [Google Scholar]